Abstract
OBJECTIVE
Given temporal changes in diabetes prevalence and stroke incidence, this study investigated age, race, and sex differences in the diabetes–stroke association in a contemporary prospective cohort, the REasons for Geographic and Racial Differences in Stroke (REGARDS) Study.
RESEARCH DESIGN AND METHODS
We included 23,002 non-Hispanic black and white U.S. adults aged ≥45 years without prevalent stroke at baseline (2003–2007). Diabetes was defined as fasting glucose ≥126 mg/dL, random glucose ≥200 mg/dL, or use of glucose-lowering medication. Incident stroke events were expert adjudicated and available through September 2017.
RESULTS
The prevalence of diabetes was 19.1% at baseline. During follow-up, 1,018 stroke events occurred. Among adults aged <65 years, comparing those with diabetes to those without diabetes, the risk of stroke was increased for white women (hazard ratio [HR] 3.72 [95% CI 2.10–6.57]), black women (HR 1.88 [95% CI 1.22–2.90]), and white men (HR 2.01 [95% CI 1.27–3.27]) but not black men (HR 1.27 [95% CI 0.77–2.10]) after multivariable adjustment. Among those aged ≥65 years, diabetes increased the risk of stroke for white women and black men, but not black women (HR 1.05 [95% CI 0.74–1.48]) or white men (HR 0.86 [95% CI 0.62–1.21]).
CONCLUSIONS
In this contemporary cohort, the diabetes–stroke association varied by age, race, and sex together, with a more pronounced effect observed among adults aged <65 years. With the recent increase in the burden of diabetes complications at younger ages in the U.S., additional efforts are needed earlier in life for stroke prevention among adults with diabetes.
Introduction
Diabetes is an established risk factor for stroke (1) and is included in cardiovascular risk prediction models (2,3). However, it is unclear what impact age, sex, and race may have on the association of diabetes with stroke risk, as several prior studies have reported different findings. In the Greater Cincinnati/Northern Kentucky Stroke Study (GCNKSS), the risk of stroke associated with diabetes was greater among adults aged <65 years compared with those aged ≥65 years. Similarly, a stronger association at younger ages was reported in the recently updated Framingham Stroke Risk Function (3). For sex, no differences were reported in several prospective cohort studies (4–6), whereas two meta-analyses indicated the stroke risk associated with diabetes was greater among women than men (7,8). In contrast, the recently updated Framingham Stroke Risk Function reported that the magnitude of the diabetes–stroke association was stronger among men than women (3). For race, no racial differences in the diabetes–stroke association were reported in an initial investigation (1987–1995) from the Atherosclerosis Risk in Communities (ARIC) study (9); however, an updated analysis with additional follow-up found that the diabetes–stroke association was stronger among black adults than white adults (10,11). In contrast, the GCNKSS in the 1990s reported that the diabetes–stroke association was stronger among black adults than white adults (12), whereas by the mid-2000s, the association was stronger among white adults than black adults (13).
Given the observed increase in diabetes prevalence over the last few decades (14,15) and decline in stroke incidence (11) and a recent resurgence in stroke incidence among younger adults with diabetes (16), the objective of this study was to investigate whether the diabetes–stroke association was jointly modified by age, race, and sex in a contemporary prospective cohort of black and white adults in the U.S.
Research Design and Methods
Study Population
The REasons for Geographic and Racial Differences in Stroke (REGARDS) is an ongoing population-based study designed to investigate stroke and its mortality among 30,183 non-Hispanic black and non-Hispanic white adults from the continental U.S. aged ≥45 years. The study was designed to oversample black adults and participants from the stroke belt region (North Carolina, South Carolina, Georgia, Alabama, Mississippi, Arkansas, Louisiana, and Tennessee), including the stroke buckle region (coastal areas of North Carolina, South Carolina, and Georgia). The baseline assessment (2003–2007) included a computer-assisted telephone interview to record sociodemographics, health behaviors, and previous medical history followed by an in-home visit to collect anthropometrics, blood pressure, electrocardiogram, and blood and urine specimens (17). Participants or their proxies were contacted semiannually to identify any hospitalizations, emergency room visits, overnight stays in nursing homes or rehabilitation centers, and death during the preceding 6 months. Institutional review boards of all participating institutions reviewed and approved the study protocol. Informed consent was obtained from all participants.
For the current study, participants with prevalent stroke or missing stroke status at baseline (n = 2,036) or missing diabetes status at baseline (n = 984) were excluded. A complete case analysis was conducted after excluding participants with missing information on covariates (n = 3,710) or follow-up time (n = 451), resulting in a final sample size of 23,002 (Supplementary Fig. 1).
Diabetes
Prevalent diabetes was defined based on a single measurement of glucose (fasting glucose ≥126 mg/dL [≥7 mmol/L] or random glucose ≥200 mg/dL [≥11.1 mmol/L]) or use of any glucose-lowering medications at baseline (18).
Incident Stroke
Medical records were retrieved for participants with any suspected stroke events identified during the semiannual follow-up call (17). Incident stroke events were adjudicated by a physician committee based on World Health Organization definition (focal neurological deficits lasting >24 h) or supportive neuroimaging (for symptoms lasting <24 h) (19). Stroke events (fatal and nonfatal) that occurred through 30 September 2017 were included.
Covariates
Age, sex, race, education (high school or less vs. more than high school), annual household income in dollars (<20,000, 20,000–34,999, 35,000–74,999, and ≥75,000), health insurance coverage (yes or no), alcohol consumption (never, moderate, or heavy) and smoking status (never, past, or current smoker) were self-reported (18,20). Physical activity was assessed using validated question (“How many times per week do you engage in intense physical activity, enough to work up a sweat?”) and categorized as none, 1–3 times/week, or ≥4 times/week (21). Height and weight were measured following a standardized protocol and used to calculate BMI. History of myocardial infarction (MI) and history of atrial fibrillation were determined by electrocardiogram findings or self-report (18). Left ventricular hypertrophy was identified from baseline electrocardiogram using Sokolow-Lyon criteria (22). Blood pressure was determined by the average of two readings taken 30 s apart, after a 5-min rest. Triglycerides and HDL cholesterol were measured by colorimetric reflectance spectrophotometry, and LDL cholesterol was assessed by the Friedewald formula (23). Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (24). Albumin-to-creatinine ratio was calculated based on urine albumin measured by BN ProSpec Nephelometer and urine creatinine measured by Modular-P analyzer (25). A medication inventory was recorded during the in-home visit.
Statistical Analyses
Poisson regression was used to obtain age-adjusted incidence rates per 1,000 person-years and incidence rate ratios comparing those with diabetes to those without diabetes by age (<65 years or ≥65 years), race (black or white), and sex (men or women). Kaplan-Meier method was used to compute cumulative incidence of stroke by diabetes status, age, race, and sex. Cox proportional hazards regression was used to estimate hazard ratios (HRs) for the association of diabetes with stroke. Model 1 was unadjusted. Model 2 was adjusted for demographic factors (education, annual household income, geographic region, and health insurance coverage) and behavioral factors (alcohol intake, smoking status, and physical activity). Model 3 included additional adjustment for clinical factors (history of MI, history of atrial fibrillation, left ventricular hypertrophy, eGFR, albumin-to-creatinine ratio, triglyceride-to-HDL ratio, LDL cholesterol, statin use, systolic blood pressure, use of antihypertensive medications, and BMI). Proportionality of hazards was assessed with a product term between follow-up time and diabetes status. Effect-measure modification was assessed using a four-way product term (age, race, sex, and diabetes) and lower-order product terms. Because of the limited statistical power for tests of interaction (26,27), a higher α has been recommended (28) and is commonly used in epidemiological studies. Therefore, we used an a priori P value of 0.15 along with reviewing stratum-specific associations and background knowledge about hypothesized associations with age, race, and sex to assess effect-measure modification. A sensitivity analysis was also done to evaluate the diabetes–stroke association including ischemic strokes only. SAS version 9.4 (SAS Institute, Cary, NC) or R version 3.6.0 was used for all analyses.
Results
Baseline participant characteristics by age and diabetes status are reported in Table 1. The prevalence of diabetes was 17.6% among those aged <65 years and 20.8% among those aged ≥65 years. Compared with those without diabetes, participants with diabetes were more likely to be black, have high school education or less, and have annual income <$20,000. Participants with diabetes had higher BMI, lower cholesterol levels, and higher systolic blood pressure and were more likely to have an eGFR <60 mL/min/1.73 m2, left ventricular hypertrophy, and history of atrial fibrillation (Table 1).
Table 1.
Baseline characteristics of REGARDS participants by diabetes status and age (2003–2007)
| Characteristic | Age <65 years |
Age ≥65 years |
||
|---|---|---|---|---|
| No diabetes (n = 9,810) | Diabetes (n = 2,093) | No diabetes (n = 8,793) | Diabetes (n = 2,306) | |
| Age (years) | 57.0 (5.0) | 58.0 (4.6) | 72.6 (5.9) | 72.1 (5.5) |
| Black (%) | 37.9 | 60.3 | 32.1 | 52.9 |
| Women (%) | 57.7 | 55.3 | 52.6 | 49.5 |
| High school graduate or less (%) | 30.0 | 42.9 | 38.9 | 49.8 |
| Annual household income (%) | ||||
| <$20,000 | 11.5 | 22.3 | 17.8 | 26.1 |
| $20,000–34,999 | 17.9 | 23.8 | 28.7 | 30.3 |
| $35,000–74,999 | 34.6 | 30.2 | 29.6 | 24.3 |
| ≥$75,000 | 26.4 | 14.4 | 10.4 | 6.5 |
| Refused | 9.6 | 9.4 | 13.5 | 12.9 |
| Has health insurance (%) | 89.1 | 86.5 | 98.9 | 99.0 |
| Geographic region (%) | ||||
| Buckle | 21.8 | 23.1 | 19.5 | 22.6 |
| Belt | 35.2 | 38.1 | 33.1 | 34.0 |
| Nonbelt | 43.0 | 38.8 | 47.5 | 43.5 |
| Alcohol consumption (%) | ||||
| Never | 54.9 | 72.2 | 62.1 | 74.9 |
| Moderate | 20.2 | 16.9 | 14.9 | 13.7 |
| Heavy | 24.9 | 10.9 | 23.0 | 11.5 |
| Smoking status (%) | ||||
| Never | 47.7 | 45.6 | 46.3 | 41.5 |
| Former | 34.2 | 37.1 | 44.6 | 48.4 |
| Current | 18.1 | 17.3 | 9.1 | 10.1 |
| Physical activity (%) | ||||
| None | 29.2 | 37.1 | 32.6 | 41.5 |
| 1–3 times/week | 40.4 | 38.3 | 34.5 | 31.4 |
| ≥4 times/week | 30.4 | 24.6 | 32.9 | 27.1 |
| BMI (kg/m2) | 29.2 (6.1) | 33.8 (6.8) | 27.7 (5.3) | 31.0 (5.9) |
| Total cholesterol (mg/dL) | 198.2 (38.0) | 182.9 (41.0) | 191.6 (38.4) | 175.7 (37.4) |
| LDL cholesterol (mg/dL) | 120.4 (34.1) | 107.1 (36.4) | 113.5 (33.5) | 100.8 (32.7) |
| HDL cholesterol (mg/dL) | 53.1 (16.2) | 47.2 (13.9) | 53.7 (16.6) | 47.6 (14.4) |
| Statin use (%) | 20.7 | 43.9 | 32.7 | 49.5 |
| Systolic blood pressure (mmHg) | 123.6 (15.5) | 130 (16.6) | 128.9 (16.3) | 132.8 (17.3) |
| Use of antihypertensive drugs (%) | 41.4 | 73.7 | 55.0 | 75.9 |
| eGFR <60 mL/min/1.73 m2 (%)† | 2.8 | 8.7 | 14.8 | 24.5 |
| Albumin-to-creatinine ratio ≥30 μg/mg (%) | 7.6 | 25.9 | 13.3 | 30.9 |
| History of MI (%) | 6.7 | 13.3 | 13.7 | 20.0 |
| History of atrial fibrillation (%) | 5.7 | 8.6 | 9.6 | 10.9 |
| Left ventricular hypertrophy (%) | 7.2 | 11.0 | 10.3 | 14.3 |
| Use of glucose-lowering medication | — | 81.6 | — | 85.2 |
Data are mean (SD) unless otherwise noted.
†eGFR estimated using Chronic Kidney Disease Epidemiology Collaboration equation.
During a median follow-up of 9.8 years, 1,018 stroke events occurred (ischemic stroke, N = 914; hemorrhagic stroke, N = 104). The crude stroke incidence rate for those with diabetes was 7.55 per 1,000 person-years (95% CI 6.70–8.51) compared with 4.48 per 1,000 person-years (95% CI 4.17–4.82) for those without diabetes. The cumulative incidence of stroke was generally higher among those with diabetes compared with those without diabetes (Fig. 1). Additionally, the stroke incidence rates were higher for those aged ≥65 years than those aged <65 years regardless of diabetes status (Supplementary Table 1).
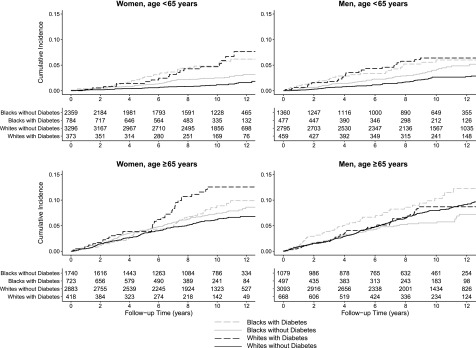
Figure 1.
Kaplan-Meier curves for cumulative incidence of stroke by age, race, sex, and diabetes status. Unadjusted cumulative incidence of stroke shown by age-sex strata comparing those with and without diabetes among black adults and white adults. The solid black line represents white adults with diabetes, dashed black line represents white adults without diabetes, solid gray line represents black adults with diabetes, and dashed gray line represents black adults without diabetes.
Results are presented by race-sex groups for those aged <65 and ≥65 years (age, race, sex, and diabetes four-way interaction, P = 0.1087). Among those aged <65 years, diabetes was associated with an increased hazard of stroke for each race-sex group, although the magnitude of the association was weaker and not statistically significant for black men (Table 2). In unadjusted analyses among those aged <65 years, white women with diabetes had over four times the risk of stroke compared with white women without diabetes (HR 4.53 [95% CI 2.58–7.93]). Moreover, diabetes was associated with double the risk of stroke among black women and white men. Adjustment for demographic, behavioral, and clinical factors attenuated these associations for white women (HR 3.72 [95% CI 2.10–6.57]), black women (HR 1.88 [95% CI 1.22–2.90]), and white men (HR 2.04 [95% CI 1.27–3.27]). Results were similar when only ischemic stroke events were analyzed (Supplementary Table 2).
Table 2.
HRs for the association of diabetes with risk of stroke by age, race, and sex groups: the REGARDS Study*
| Model 1 |
Model 2 |
Model 3 |
|||||
|---|---|---|---|---|---|---|---|
| n/N† | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Age <65 years at baseline | |||||||
| White women with diabetes | 18/373 | 4.53 | 2.58–7.93 | 3.92 | 2.23–6.89 | 3.72 | 2.10–6.57 |
| White women without diabetes | 38/3,296 | 1 | — | 1 | — | 1 | — |
| Black women with diabetes | 34/784 | 2.04 | 1.33–3.14 | 1.96 | 1.27–3.01 | 1.88 | 1.22–2.90 |
| Black women without diabetes | 53/2,359 | 1 | — | 1 | — | 1 | — |
| White men with diabetes | 25/459 | 2.59 | 1.63–4.11 | 2.31 | 1.45–3.68 | 2.01 | 1.27–3.27 |
| White men without diabetes | 63/2,795 | 1 | — | 1 | — | 1 | — |
| Black men with diabetes | 23/477 | 1.38 | 0.84–2.28 | 1.37 | 0.83–2.25 | 1.27 | 0.77–2.10 |
| Black men without diabetes | 48/1,360 | 1 | — | 1 | — | 1 | — |
| Age ≥65 years at baseline | |||||||
| White women with diabetes | 38/418 | 1.92 | 1.35–2.73 | 1.82 | 1.27–2.59 | 1.79 | 1.25–2.58 |
| White women without diabetes | 158/2,882 | 1 | — | 1 | — | 1 | — |
| Black women with diabetes | 47/723 | 1.10 | 0.78–1.55 | 1.06 | 0.75–1.49 | 1.05 | 0.74–1.48 |
| Black women without diabetes | 112/1,740 | 1 | — | 1 | — | 1 | — |
| White men with diabetes | 42/668 | 1.01 | 0.72–1.40 | 0.94 | 0.68–1.31 | 0.86 | 0.62–1.21 |
| White men without diabetes | 221/3,092 | 1 | — | 1 | — | 1 | — |
| Black men with diabetes | 42/497 | 1.79 | 1.20–2.67 | 1.78 | 1.19–2.65 | 1.68 | 1.13–2.52 |
| Black men without diabetes | 56/1,079 | 1 | — | 1 | — | 1 | — |
Model 1: unadjusted. Model 2: adjusted for age (centered), education, annual household income, health insurance coverage, geographic region of residence, alcohol consumption, smoking status, and physical activity. Model 3: model 2 adjustments plus adjustments for BMI, triglyceride-to-HDL ratio, LDL cholesterol, statin use, systolic blood pressure, use of antihypertensive medications, eGFR <60 mL/min/1.73 m2, albumin-to-creatinine ratio, MI, atrial fibrillation, and left ventricular hypertrophy.
*Interaction term age-race-sex-diabetes, P = 0.1087.
†n in the numerator is total stroke events in each group, and N in the denominator is total individuals in the corresponding group.
Among those aged ≥65 years, diabetes increased the risk of stroke for white women (HR 1.92 [95% CI 1.35–2.73]) and black men (HR 1.79 [95% CI 1.20–2.67]), but not for black women (HR 1.10 [95% CI 0.78–1.55]) or white men (HR 1.01 [95% CI 0.72–1.40]) in unadjusted analyses. These associations were attenuated but remained significant for white women and black men after multivariable adjustment (Table 2). In a sensitivity analysis assessing ischemic stroke events only among those aged ≥65 years, results were similar (Supplementary Table 2).
Conclusions
In this prospective cohort study, the magnitude of the association of diabetes with stroke varied by age, race, and sex jointly. While stroke incidence was higher among older adults (≥65 years), the magnitude of the diabetes–stroke association was generally more pronounced among those aged <65 years. Diabetes was associated with an increased risk of stroke for each race-sex group among those aged <65 years, except black men. However, among those aged ≥65 years, the higher risk of stroke associated with diabetes was observed only among black men and white women.
Our findings are similar to prior reports that showed the diabetes-stroke association was stronger among middle-aged adults than older adults (3,8,11,13). Findings from a retrospective cohort study reported that the stroke risk due to diabetes approximates the stroke risk associated with aging 15 years (29). Moreover, it has been postulated that the onset of arterial stiffness at an early age due to diabetes may be an underlying mechanism for increased stroke risk at younger ages (30).
In contrast to no sex differences reported in the diabetes–stroke association in multiple cohort studies (4–6,9), two meta-analyses of 102 studies (21 from the U.S.) and 64 studies (5 from the U.S.), respectively, reported a stronger association among women compared with men (7,8). A previous analysis of REGARDS participants reported sex differences in the diabetes–stroke association among white adults but not black adults (31). In contrast, the ARIC study reported a stronger association among women than men in the diabetes–cardiovascular disease association (a composite outcome of stroke, coronary heart disease, peripheral arterial disease, and heart failure) that was similar for black and white adults (32). However, the current analysis showed that sex together with age and race jointly influenced the diabetes–stroke association specifically. It has been reported that women with diabetes were less likely to attain control of stroke risk factors compared with men with diabetes (33). However, in our study, the higher risk for stroke among women with diabetes remained after adjustment for stroke risk factors. Women also have higher levels of atherogenic markers (e.g., E-selectin and soluble intracellular adhesion molecule) than men prior to the diagnosis of diabetes (34), which may contribute to some of the observed sex differences in the diabetes–stroke association.
No racial differences in the diabetes–stroke association were reported in the National Health and Nutrition Examination Survey I study (1971–1975) (35), an early analysis of the ARIC study (1987–1995) (9), or the Northern Manhattan Study with follow-up into the late 2000s (6). However, a more recent analysis from the ARIC study reported a stronger diabetes–stroke association among black adults than white adults (10,11). Different results were also reported in the GCNKSS over time. In an initial analysis (1993–1994), the incidence rate ratios for stroke for those with diabetes compared with those without diabetes were generally higher for black adults than white adults at younger ages (12). However, more recent data from the GCNKSS (1993–2011) reported that the association of diabetes with stroke was greater among white adults than black adults (13). Although diabetes is a known risk factor for stroke, we observed that age, race, and sex together influenced the diabetes–stroke association such that stroke risk among those aged <65 years was increased for white women, black women, and white men, whereas stroke risk among those aged ≥65 years was increased for white women and black men. The potential explanatory factors underlying these differences are unclear. These associations could be reflective of broader changes in cardiovascular complications of diabetes. A recent study from Centers for Disease Control and Prevention described a resurgence of diabetes complications, including stroke incidence, since 2010 (16). The observed increase in stroke incidence was most apparent among adults with diabetes age <65 years, while stroke incidence has plateaued among adults with diabetes age ≥65 years (16). However, this study did not investigate race and sex differences in these trends, so it is unclear whether these changes are similar across race-sex groups. In addition, the ARIC study recently reported that the association of diabetes with a composite cardiovascular event end point (that included incident stroke) was stronger among black women than black men, highlighting the importance of considering both race and sex concurrently (32,36). In light of the changing trends in stroke incidence and the higher diabetes prevalence among black adults compared with white adults in the U.S., it is important to investigate whether the diabetes–stroke association varied within subgroups by race, sex, and age.
This study has several potential limitations. Our study used a single measure of blood glucose at baseline to assess diabetes status in addition to medication use. We did not have a second measure available to confirm diabetes classification; however, 83.4% of adults with diabetes were using glucose-lowering medications. Additionally, survival bias is a possibility because black adults have a higher mortality rate than white adults (37), and women have greater life expectancy than men (38). A previous analysis of REGARDS participants showed that racial disparities in stroke risk are attenuated at older ages (20), and it has been suggested that stroke risk would be greater among older black adults than older white adults if survival was similar in both races (39). However, a recent methodological analysis showed that racial differences in attrition among REGARDS participants did not appreciably affect observed associations in incident hypertension, a stroke risk factor (40). Our study did not have information available on diabetes duration or glycemic control, which are independently associated with stroke risk. However, when we adjusted for insulin use as a proxy indicator of diabetes severity, our results were similar (data not shown). We were also not able to differentiate between type 1 and type 2 diabetes in our study population, but the majority of our participants most likely had type 2 diabetes given the older age of our participants (mean age 64 years at baseline) and that ∼95% of diabetes cases in the U.S. are type 2 diabetes (14). While the study was designed to investigate contributing factors to stroke risk by oversampling black adults and adults from the stroke belt and stroke buckle region of the U.S., there were fewer stroke events among those aged <65 years, resulting in less precise estimates, and hemorrhagic strokes could not be evaluated separately due to the low number of events. Additionally, we included product terms in our regression models to allow the diabetes–stroke association to vary across subgroups. We used a higher α to assess effect-measure modification given the lower power to detect statistical interaction in epidemiological studies (27,28); however, the tradeoff with this approach is an increase in type I error (26). Although age, race, and sex appeared to modify the diabetes–stroke association in our study, additional investigation of these associations is needed. Lastly, this study included non-Hispanic white and non-Hispanic black participants only, so we were unable to investigate the diabetes–stroke association among other racial and ethnic groups. This study also has several strengths, including its prospective cohort design, rigorous adjudication of events using published guidelines, and contemporaneous collection of extensive data using standardized protocols with rigorous quality control measures.
Although overall stroke incidence rates have declined in the U.S., rates among those aged <65 years were unchanged from 1990 to 2005 (11) and have begun to increase among younger adults with diabetes since 2010 (16). Given the increase in the burden of diabetes in the U.S., particularly among those aged <65 years, these findings suggest that targeted efforts are necessary earlier in life for stroke prevention, particularly among adults with diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank the other investigators, the staff, and the participants of the REGARDS Study for the valuable contributions. A complete list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Funding. This research project is supported by a National Institute of Neurological Disorders and Stroke, National Institutes of Health, and Department of Health and Human Services cooperative agreement U01-NS-041588. Additional support was provided by Centers for Disease Control and Prevention cooperative agreement U01-DP-006302.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke, the National Institutes of Health, the Centers for Disease Control and Prevention, or the Department of Health and Human Services.
Duality of Interest. M.M.S. and A.P.C. receive investigator-initiated research support from Amgen Inc. D.A.L. has been a consultant for the University of California, San Francisco (event adjudicator for Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke trial). No other potential conflicts of interest relevant to this article were reported.
Author Contributions. G.M. conceptualized the study, performed statistical analysis, interpreted the data, and drafted the manuscript. D.L.L. performed statistical analysis, interpreted the data, and critically revised the manuscript. S.E.J., B.M.K., and A.P.C. conceptualized the study, interpreted the data, and critically revised the manuscript. M.R.I., D.T.L., M.M.S., D.A.L., V.J.H., G.H., J.D.R., J.H.V., D.O.K., A.A., and J.F.M. interpreted the data and critically revised the manuscript. A.P.C. is the guarantor of this work and, as such, had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in poster form at the American Public Health Association 2018 Annual Meeting and Expo, San Diego, CA, 10–14 November 2018.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0442/-/DC1.
References
- 1.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation 2014;129:S74–S75]. Circulation 2014;129(Suppl. 2):S49–S73 [DOI] [PubMed] [Google Scholar]
- 2.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–1847 [DOI] [PubMed] [Google Scholar]
- 3.Dufouil C, Beiser A, McLure LA, et al. . Revised Framingham Stroke Risk Profile to reflect temporal trends. Circulation 2017;135:1145–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najarian RM, Sullivan LM, Kannel WB, Wilson PW, D’Agostino RB, Wolf PA. Metabolic syndrome compared with type 2 diabetes mellitus as a risk factor for stroke: the Framingham Offspring Study. Arch Intern Med 2006;166:106–111 [DOI] [PubMed] [Google Scholar]
- 5.Woodward M, Zhang X, Barzi F, et al.; Asia Pacific Cohort Studies Collaboration . The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia-Pacific region. Diabetes Care 2003;26:360–366 [DOI] [PubMed] [Google Scholar]
- 6.Banerjee C, Moon YP, Paik MC, et al. . Duration of diabetes and risk of ischemic stroke: the Northern Manhattan Study. Stroke 2012;43:1212–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014;383:1973–1980 [DOI] [PubMed] [Google Scholar]
- 8.Sarwar N, Gao P, Seshasai SR, et al.; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies [published correction appears in Lancet 2010;376:958]. Lancet 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folsom AR, Rasmussen ML, Chambless LE, et al. . Prospective associations of fasting insulin, body fat distribution, and diabetes with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Diabetes Care 1999;22:1077–1083 [DOI] [PubMed] [Google Scholar]
- 10.Huxley RR, Bell EJ, Lutsey PL, et al. . A comparative analysis of risk factors for stroke in blacks and whites: the Atherosclerosis Risk in Communities study. Ethn Health 2014;19:601–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koton S, Schneider AL, Rosamond WD, et al. . Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA 2014;312:259–268 [DOI] [PubMed] [Google Scholar]
- 12.Kissela BM, Khoury J, Kleindorfer D, et al. . Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care 2005;28:355–359 [DOI] [PubMed] [Google Scholar]
- 13.Khoury JC, Kleindorfer D, Alwell K, et al. . Diabetes mellitus: a risk factor for ischemic stroke in a large biracial population. Stroke 2013;44:1500–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention National Diabetes Statistics Report. Atlanta, GA, U.S. Department of Health and Human Services, 2017 [Google Scholar]
- 15.Geiss LS, Wang J, Cheng YJ, et al. . Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA 2014;312:1218–1226 [DOI] [PubMed] [Google Scholar]
- 16.Gregg EW, Hora I, Benoit SR. Resurgence in diabetes-related complications. JAMA 2019;321:1867–1868 [DOI] [PubMed] [Google Scholar]
- 17.Howard VJ, Cushman M, Pulley L, et al. . The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–143 [DOI] [PubMed] [Google Scholar]
- 18.Carson AP, Muntner P, Kissela BM, et al. . Association of prediabetes and diabetes with stroke symptoms: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Diabetes Care 2012;35:1845–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke 1989;20:1407–1431 [DOI] [PubMed] [Google Scholar]
- 20.Howard VJ, Kleindorfer DO, Judd SE, et al. . Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol 2011;69:619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kronish IM, Carson AP, Davidson KW, Muntner P, Safford MM. Depressive symptoms and cardiovascular health by the American Heart Association’s definition in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. PLoS One 2012;7:e52771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Neal WT, Howard VJ, Kleindorfer D, et al. . Interrelationship between electrocardiographic left ventricular hypertrophy, QT prolongation, and ischaemic stroke: the REasons for Geographic and Racial Differences in Stroke Study. Europace 2016;18:767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aaron KJ, Colantonio LD, Deng L, et al. . Cardiovascular health and healthcare utilization and expenditures among medicare beneficiaries: the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc 2017;6:e005106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warnock DG, Muntner P, McCullough PA, et al.; REGARDS Investigators . Kidney function, albuminuria, and all-cause mortality in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. Am J Kidney Dis 2010;56:861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufman JS, MacLehose RF. Which of these things is not like the others? Cancer 2013;119:4216–4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenland S. Interactions in epidemiology: relevance, identification, and estimation. Epidemiology 2009;20:14–17 [DOI] [PubMed] [Google Scholar]
- 28.Selvin S. Statistical Analysis of Epidemiologic Data. NewYork, Oxford University Press, 2004 [Google Scholar]
- 29.Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet 2006;368:29–36 [DOI] [PubMed] [Google Scholar]
- 30.Chen R, Ovbiagele B, Feng W. Diabetes and stroke: epidemiology, pathophysiology, pharmaceuticals and outcomes. Am J Med Sci 2016;351:380–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrett-Connor E, Wingard D, Wong N, Goldberg R. Chapter 18: Heart disease and diabetes. In Diabetes in America. 3rd edition. Bethesda, MD, National Institutes of Health, 2018, pp. 18-1–18-30 (NIH publ. no. 17-1468)
- 32.George KM, Selvin E, Pankow JS, Windham BG, Folsom AR. Sex differences in the association of diabetes with cardiovascular disease outcomes among African-American and white participants in the Atherosclerosis Risk in Communities Study. Am J Epidemiol 2018;187:403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winston GJ, Barr RG, Carrasquillo O, Bertoni AG, Shea S. Sex and racial/ethnic differences in cardiovascular disease risk factor treatment and control among individuals with diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009;32:1467–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donahue RP, Rejman K, Rafalson LB, Dmochowski J, Stranges S, Trevisan M. Sex differences in endothelial function markers before conversion to pre-diabetes: does the clock start ticking earlier among women? The Western New York Study. Diabetes Care 2007;30:354–359 [DOI] [PubMed] [Google Scholar]
- 35.Kittner SJ, White LR, Losonczy KG, Wolf PA, Hebel JR. Black-white differences in stroke incidence in a national sample. The contribution of hypertension and diabetes mellitus. JAMA 1990;264:1267–1270 [PubMed] [Google Scholar]
- 36.Moise N, Bertoni AG. Invited commentary: sex and race differences in diabetes and cardiovascular disease-achieving the promise of sex and race subgroup analyses in epidemiologic research. Am J Epidemiol 2018;187:411–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunningham TJ, Croft JB, Liu Y, Lu H, Eke PI, Giles WH. Vital signs: racial disparities in age-specific mortality among Blacks or African Americans - United States, 1999-2015. MMWR Morb Mortal Wkly Rep 2017;66:444–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arias E. United States life tables, 2011. Natl Vital Stat Rep 2015;64:1–63 [PubMed] [Google Scholar]
- 39.Mayeda ER, Banack HR, Bibbins-Domingo K, et al. . Can survival bias explain the age attenuation of racial inequalities in stroke incidence?: a simulation study. Epidemiology 2018;29:525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long DL, Howard G, Long DM, et al. . An investigation of selection bias in estimating racial disparity in stroke risk factors. Am J Epidemiol 2019;188:587– 597 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.