Abstract
Background
In the phase III MONARCH 2 study (NCT02107703), abemaciclib plus fulvestrant significantly improved progression‐free survival (PFS) versus placebo plus fulvestrant in patients with hormone receptor‐positive (HR+), HER2‐negative advanced breast cancer (ABC). This study assessed patient‐reported pain, global health‐related quality of life (HRQoL), functioning, and symptoms.
Materials and Methods
Abemaciclib or placebo (150 p.o. mg twice daily) plus fulvestrant (500 mg, per label) were randomly assigned (2:1). The modified Brief Pain Inventory, Short Form (mBPI‐sf); European Organization for Research and Treatment of Cancer (EORTC) QoL Core 30 (QLQ‐C30); and Breast Cancer Questionnaire (QLQ‐BR23) assessed outcomes. Data were collected at baseline, cycle 2, every two cycles 3–13, thereafter at every three cycles, and 30 days postdiscontinuation. Longitudinal mixed regression and Cox proportional hazards models assessed postbaseline change and time to sustained deterioration (TTSD) by study arm.
Results
On‐treatment HRQoL scores were consistently maintained from baseline and similar between arms. Patients in the abemaciclib arm (n = 446) experienced a 4.9‐month delay in pain deterioration (mBPI‐sf), compared with the control arm (n = 223), and significantly greater TTSD on the mBPI‐sf and analgesic use (hazard ratio, 0.76; 95% CI, 0.59–0.98) and QLQ‐C30 pain item (hazard ratio, 0.62; 95% CI, 0.48–0.79). TTSD for functioning and most symptoms significantly favored the abemaciclib arm, including fatigue, nausea and vomiting, and cognitive and social functioning. Only diarrhea significantly favored the control arm (hazard ratio, 1.60; 95% CI, 1.20–2.10).
Conclusion
HRQoL was maintained on abemaciclib plus fulvestrant. Alongside superior PFS and manageable safety profile, results support treatment with abemaciclib plus fulvestrant in a population of patients with endocrine‐resistant HR+, HER2‐negative ABC.
Implications for Practice
In MONARCH 2, abemaciclib plus fulvestrant demonstrated superior efficacy and a manageable safety profile for patients with in hormone receptor‐positive (HR+), HER2‐negative (−) advanced breast cancer (ABC). Impact on health‐related quality of life (HRQoL) is important to consider, given the palliative nature of ABC treatment. In this study, abemaciclib plus fulvestrant, compared with placebo plus fulvestrant, significantly delayed sustained deterioration of pain and other patient‐reported symptoms (including fatigue, nausea, vomiting), and social and cognitive functioning. Combined with demonstrated clinical benefit and tolerability, the stabilization of patient‐reported symptoms and HRQoL further supports abemaciclib plus fulvestrant as a desirable treatment option in endocrine resistant, HR+, HER2− ABC.
Keywords: Abemaciclib, Advanced breast cancer, Quality of life, Patient‐reported outcomes
Short abstract
This study assessed patient‐reported pain and global health‐related quality of life, functioning, and symptoms of patients participating in the phase III MONARCH 2 study.
Introduction
Abemaciclib is an orally administered, potent, small‐molecule, cyclin‐dependent kinase (CDK) 4 and 6 inhibitor administered on a twice‐daily continuous schedule 1, 2, 3, 4. Preclinical evidence indicates that continuous dosing promotes sustained cell cycle arrest, leading to cellular senescence or apoptosis 2, 4, 5. Compared with other CDK4 and 6 inhibitors, abemaciclib is 14 times more potent against cyclin D1‐CDK4 than cyclin D3‐CDK6 in enzymatic assays 1, 4, 6 and exhibits greater selectivity for CDK4 compared with CDK6 3. Additionally, abemaciclib has recently demonstrated advantageous activities compared with other CDK4 and 6 inhibitors, including inhibiting other kinases, CDK1‐cyclin B and CDK2‐cyclin A/E, implicated in CDK4 and 6‐inhibition resistance 7.
In MONARCH 1, as a single agent (200 mg twice daily on a continuous schedule), abemaciclib demonstrated an overall response rate (ORR) of 19.7%, with a clinical benefit rate of 42.4% in a heavily pretreated patient population with progressive advanced breast cancer (ABC) 1. In MONARCH 2, abemaciclib plus fulvestrant, compared with placebo plus fulvestrant, demonstrated significantly improved progression‐free survival (PFS; hazard ratio, 0.55; 95% confidence interval [CI], 0.45–0.68; p < .001), and an ORR of 48.1% versus 21.3%, in women with hormone receptor‐positive (HR+), HER2‐negative (−) ABC who progressed on or after (within 12 months) prior endocrine therapy (ET) 4. In MONARCH 3, abemaciclib plus a nonsteroidal aromatase inhibitor also demonstrated significantly improved PFS (hazard ratio, 0.54; 95% CI, 0.41–0.72; p < .001) 8.
CDK4 and 6 inhibitors in combination with ET have been recommended by the National Comprehensive Cancer Network 9 and the European Society for Medical Oncology (ESMO) for HR+, HER2− ABC 10. When combined with ET, CDK4 and 6 inhibitors have demonstrated superior PFS results when treating patients with HR+, HER2− ABC compared with ET alone 4, 11, 12. These combination therapies have also been associated with higher response rates, but additional treatment‐related toxicities could negatively impact health‐related quality of life (HRQoL) 13. Given the palliative nature of ABC treatment, consideration of treatment choices that minimize the impact to HRQoL is important in guiding treatment decisions 14, 15, 16, 17. Thus, it is now recommended that patient‐reported HRQoL be considered alongside efficacy and safety to better understand the impact of treatment 10, 14. The patient‐reported HRQoL measures collected in the MONARCH 2 trial were prespecified in the protocol as a secondary objective. The primary research aim of this report was to assess the impact of abemaciclib plus fulvestrant, compared with placebo plus fulvestrant, on the HRQoL symptom of pain. In addition, the broader impact of abemaciclib plus fulvestrant on global HRQoL, functioning, and other symptoms was assessed.
Materials and Methods
Study Design and Treatment
A detailed study design has been previously reported 4. MONARCH 2 was a phase III, randomized, double‐blind, placebo‐controlled study of abemaciclib or placebo (150 mg twice daily) plus fulvestrant (500 mg per label) in women with HR+, HER2− ABC who progressed while on or after prior ET within 12 months 4. As previously reported, patients in the abemaciclib arm initially received 200 mg twice daily. After a review of dose modification, interruption, and discontinuation rate, the starting dose was reduced to 150 mg for new patients to improve tolerability; all patients receiving 200 mg underwent a mandatory dose reduction to 150 mg 4. Prior to joining the study, all patients provided informed consent. All appropriate ethical and institutional review boards approved the trial before commencing. This study was performed in compliance with the Declaration of Helsinki. A steering committee oversaw the conduct of the trial, and an independent data monitoring committee conducted quarterly safety data reviews.
Patient‐Reported Symptoms and Quality of Life Measures
Outcomes were assessed at baseline, cycle 2, every two cycles 3–13, thereafter every three cycles, and once 30 days postdiscontinuation.
Pain
Pain was assessed in three ways: by the modified Brief Pain Inventory, Short Form (mBPI‐sf) 18; the pain item from the European Organization for Research and Treatment of Cancer (EORTC) QoL Questionnaire Core 30 (QLQ‐C30) 19; and analgesic use. Pain intensity, assessed with the mBPI‐sf, included four pain items (worst, least, average, and now) and a composite interference score 18. Focused analysis for this outcome was on “worst pain.” The EORTC QLQ‐C30 pain items asked, “During the past week, have you had pain?” and “During the past week, did pain interfere with your daily activities?” Both items were scored on a scale of 1 (not at all) to 4 (very much) and summarized into a single pain‐symptom scale. Individual prescription and over‐the‐counter analgesic medication were recorded for each patient and at each study visit. Pain medication was classified into medication categories, using the World Health Organization analgesic ladder 20. A medication category was assigned based on the maximum analgesic therapy administered for that cycle on a routine basis.
Quality of Life
Global HRQoL, symptoms, and functioning were assessed with the EORTC QLQ‐C30 19 and Breast Cancer Questionnaire (QLQ‐BR23) 21 The EORTC QLQ‐C30 19 assess three dimensions: (a) global health status and/or HRQoL (2 items to create 1 scale); (b) functioning (15 items to create the 5 functioning scales): physical (5 items), role (2 items), emotional (4 items), cognitive (2 items), social (2 items); and (c) symptoms (13 items to create 9 symptom scales): fatigue (3 items), nausea and vomiting (2 items), pain (described above, 2 items), dyspnea (1 item), insomnia (1 item), appetite loss (1 item), constipation (1 item), diarrhea (1 item), and financial impact (1 item).
Symptom assessment focused on frequency as opposed to interference and impact on quality of life. Symptoms and functioning were scored on a 4‐point scale (not at all, a little, quite a bit, and very much; higher scores indicating greater symptom burden or higher or better functioning), whereas global HRQoL was scored on a scale of 1 to 7 (very poor to excellent; higher scores indicating better function).
The QLQ‐BR23 21 collected disease‐specific data. Functioning (body image, sexuality, and future perspective) and symptom scales (arm, breast, upset by hair loss, and systemic therapy side effects) were collected and scored on 4‐point scales (not at all to very much; higher scores indicating greater symptom burden or higher or better functioning).
Statistical Analyses
Questionnaire compliance per cycle was measured as the percentage of patients completing each instrument. Possible reasons for noncompliance included “missing” (i.e., patient data was missing and no reason recorded), “study site failed to administer questionnaire,” and “patient refusal.”
Change from baseline was assessed using mixed‐effects repeated‐measures models, by study arms and cycle, and followed intention‐to‐treat including all data and cycles for which at least 25% of patients completed questionnaires in both study arms. Time to worsening of pain (i.e., time to deterioration [TTD]) was created using combined data from the mBPI‐sf and analgesic use and defined as either a “worst pain” (increase of ≥2 points postbaseline on the mBPI‐sf, based on previously established minimally important differences [MID]) 22 or an analgesic drug class increase of ≥1 level. TTD was described using the Kaplan‐Meier method and was compared between study arms using a log‐rank test. Post hoc analyses investigated time to sustained deterioration (TTSD) using Cox proportional hazard models. For the mBPI‐sf, TTSD used the above definition of an increase of ≥2 points postbaseline increase in pain, followed by all subsequent scores meeting the MID criteria compared with baseline. For all EORTC outcomes, TTSD was defined as a ≥ 10‐point deterioration compared with a patient's baseline score, followed by all subsequent scores meeting the MID criteria compared with baseline. The ≥10‐point criteria was based on established thresholds for EORTC QLQ‐C30 MID 23, 24. For all analyses, there were no adjustments for multiple comparisons and p values were set at ≤ .05 statistical significance. Analyses were conducted using SAS Version 9.2 or later (SAS, Cary, NC).
Results
Baseline Patients and Disease Characteristics
As previously described 4, 669 patients were randomly assigned (2:1, stratified by visceral site and ET resistance) to receive abemaciclib plus fulvestrant (n = 446) or placebo plus fulvestrant (n = 223; Fig. 1). Baseline patient and disease characteristics were well balanced in the study arms and similar to those reported in the previously reported efficacy sample 4. Specifically, patients in the abemaciclib arm had a median age of 59 years (range, 32–91), whereaas those in the control arm had a median age of 62 years (range: 32–87). In the abemaciclib arm, 53.1% of patients were white, 33.4% were Asian, and 6.5% self‐reported as other race; in the control arm, 61.0% of patients were white, 29.1% were Asian, and 5.8% self‐reported as other race. In the abemaciclib arm, 54.9% of patients had visceral metastatic sites, 27.6% had bone only, and had 16.8% other. In the control arm, 57.4% of patients had visceral metastatic sites, 25.6% had bone only, and 17.0% had other 4.
Figure 1.
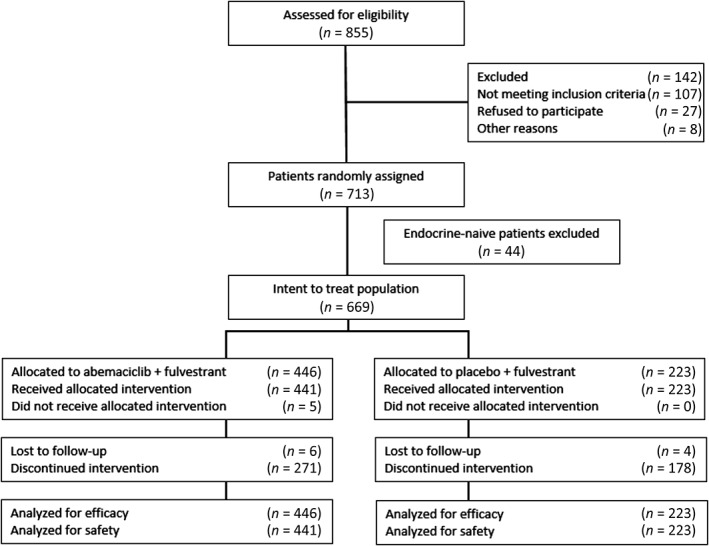
CONSORT diagram.
Patient‐Reported Symptoms and Quality of Life Measures
Compliance
Patient compliance rates for all questionnaires were ≥95% at baseline, ≥85% on therapy, and ≥77% at follow‐up (supplemental online Table 1). Nonresponse was balanced between study arms across the questionnaires. The most common reason for noncompliance across all study visits was “study site failed to administer questionnaire” (supplemental online Table 1).
Pain
At baseline, pain scores based on the mBPI‐sf and the EORTC QLQ‐C30 pain item were similar between study arms (Table 1). In mixed models, the mean change from baseline over the entire treatment course were similar between study arms (Table 1).
Table 1.
Baseline mean (SD) scores by study arm and within‐treatment group change from baseline: mBPI‐sf, EORTC, QLQ‐C30, EORTC QLQ‐BR23, and normative scores
| Baseline score, mean (SD) | Change from baseline,a LS mean (SE) | Reference valuesb | |||
|---|---|---|---|---|---|
| Abemaciclibc | Placeboc | Abemaciclibc | Placeboc | ||
| Summary of mBPI‐sfd | |||||
| Worst paine | 2.9 (2.7) | 2.6 (2.5) | −0.4 (0.1) | −0.2 (0.1) | N/A |
| Least paine | 1.4 (1.8) | 1.2 (1.6) | 0.01 (0.1) | 0.1 (0.1) | N/A |
| Pain on average | 2.3 (2.1) | 2.1 (2.1) | −0.2 (0.1) | −0.1 (0.1) | N/A |
| Pain right now | 1.7 (2.2) | 1.7 (2.1) | −0.1 (0.1) | −0.04 (0.1) | N/A |
| Mean interference score | 2.1 (2.4) | 1.8 (2.2) | −0.1 (0.1) | 0.00 (0.1) | N/A |
| Summary of EORTC QLQ‐C30f | |||||
| Global health | 64.0 (22.4) | 63.5 (22.8) | −1.4 (0.7) | 0.1 (1.0) | 60.2 (25.5) |
| Functional scalesf | |||||
| Physical | 77.4 (20.4) | 78.9 (19.6) | −1.2 (0.6) | −1.7 (0.9) | 81.6 (18.7) |
| Role | 75.9 (28.9) | 78.5 (26.9) | −2.2 (0.9) | −1.4 (1.2) | 67.4 (31.1) |
| Emotional | 72.2 (22.0) | 71.3 (23.6) | 3.9 (0.7) | 4.1 (1.0) | 65.9 (24.6) |
| Cognitive | 83.3 (19.8) | 83.6 (21.4) | −1.9 (0.7) | −0.8 (1.0) | 80.5 (23.2) |
| Social | 79.2 (26.3) | 82.9 (25.1) | −1.6 (0.8) | 0.4 (1.2) | 74.2 (28.4) |
| Symptom scalesd | |||||
| Fatigue | 32.0 (24.2) | 29.7 (22.8) | 3.7 (0.7) | 1.8 (1.1) | 36.3 (27.0) |
| Nausea and vomiting | 7.0 (16.7) | 4.7 (10.7) | 4.1 (0.5) | 0.7 (0.7) | 10.3 (19.7) |
| Pain | 30.7 (28.8) | 27.2 (26.0) | −3.7 (0.9) | −1.1 (1.2) | 30.9 (29.6) |
| Dyspnea | 17.5 (24.6) | 15.6 (23.1) | 3.9 (0.9) | 1.3 (1.2) | 20.4 (28.2) |
| Insomnia | 28.0 (30.3) | 27.4 (27.6) | −2.2 (0.9) | 0.1 (1.3) | 33.1 (32.6) |
| Appetite loss | 16.8 (25.6) | 16.2 (27.6) | 3.6 (0.8) | −1.7 (1.2) | 21.7 (31.0) |
| Constipation | 14.1 (24.4) | 12.8 (22.5) | −1.5 (0.7) | −0.7 (1.0) | 19.2 (28.8) |
| Diarrhea | 8.7 (18.9) | 7.3 (17.5) | 24.1 (0.9) | −0.5 (1.3) | 5.8 (15.2) |
| Financial difficulties | 20.1 (29.1) | 14.5 (24.1) | −2.2 (0.8) | −2.2 (1.1) | 18.6 (28.6) |
| Summary of EORTC QLQ‐BR23 | |||||
| Functional scalesf , g | |||||
| Body image | 76.7 (25.0) | 76.9 (26.5) | −1.0 (0.8) | 0.6 (1.1) | 81.9 (22.6) |
| Sexual functioning | 10.0 (18.3) | 10.0 (16.9) | 0.2 (0.5) | −0.6 (0.8) | 19.2 (23.2) |
| Future perspective | 40.2 (32.6) | 39.7 (31.3) | 12.0 (1.1) | 15.6 (1.5) | 47.6 (34.1) |
| Symptom scalesd , g | |||||
| Systemic therapy side effects | 16.4 (13.6) | 16.1 (13.0) | 7.7 (0.5) | 2.4 (0.7) | 15.8 (14.3) |
| Breast | 13.3 (17.0) | 12.9 (17.3) | −2.4 (0.5) | −2.7 (0.7) | 17.6 (16.7) |
| Arm | 18.6 (21.4) | 18.0 (20.7) | −1.7 (0.7) | −0.8 (1.0) | 21.0 (21.1) |
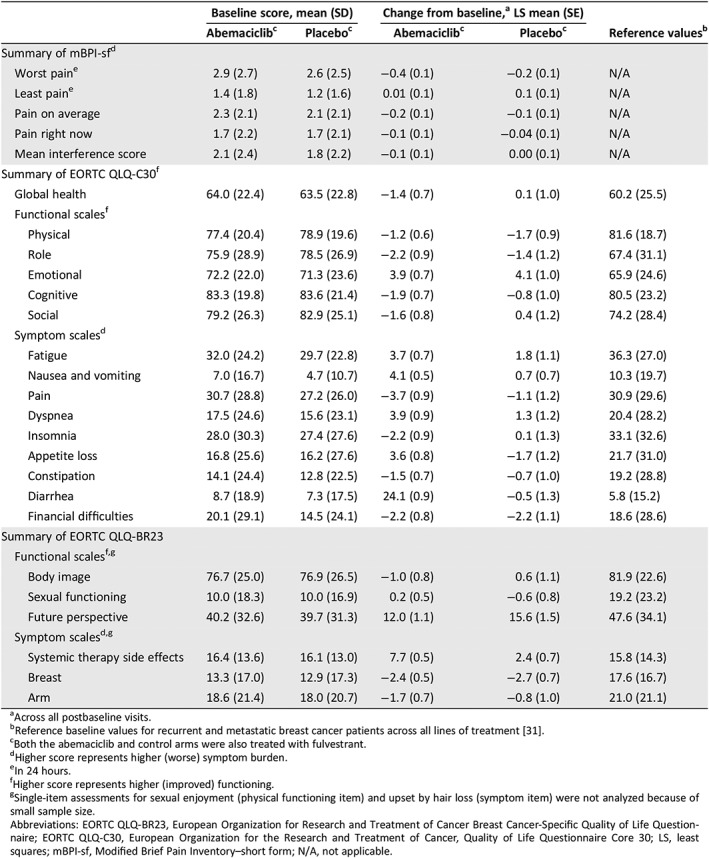
Across all postbaseline visits.
Reference baseline values for recurrent and metastatic breast cancer patients across all lines of treatment 31.
Both the abemaciclib and control arms were also treated with fulvestrant.
Higher score represents higher (worse) symptom burden.
In 24 hours.
Higher score represents higher (improved) functioning.
Single‐item assessments for sexual enjoyment (physical functioning item) and upset by hair loss (symptom item) were not analyzed because of small sample size.
Abbreviations: EORTC QLQ‐BR23, European Organization for Research and Treatment of Cancer Breast Cancer‐Specific Quality of Life Questionnaire; EORTC QLQ‐C30, European Organization for the Research and Treatment of Cancer, Quality of Life Questionnaire Core 30; LS, least squares; mBPI‐sf, Modified Brief Pain Inventory–short form; N/A, not applicable.
Analgesic use at any point in the study was similar in abemaciclib and control arms, with 159 (36.1%) and 74 (33.2%) patients reporting any opioid use, respectively. Specific strong opioid use included oxycodone (9.5% abemaciclib arm and 9.4% control arm) and morphine (5.7% abemaciclib arm and 7.2% control arm). Similarly, 246 (55.8%) abemaciclib plus fulvestrant patients and 119 (53.4%) placebo plus fulvestrant patients reported using any nonopioid analgesic.
When investigating TTD with the mBPI‐sf and analgesic use, patients in the abemaciclib plus fulvestrant arm experienced a 4.9‐month greater delay in median TTD of pain compared with those in the placebo plus fulvestrant arm; however, this was not statistically significant (16.8 vs 11.9 months; hazard ratio, 0.900; p = .400; Fig. 2). When investigating TTSD with the mBPI‐sf and analgesic use, sustained deterioration was significantly delayed in the abemaciclib arm compared with the control arm (hazard ratio, 0.76; 95% CI, 0.59;–0.98; Fig. 3). These results were similar when investigating mBPI‐sf scores alone without analgesic use (hazard ratio, 0.62; 95% CI, 0.47–0.82; Fig. 3). TTSD of pain, as measured by the EORTC‐QLQ‐C30, also significantly favored the abemaciclib arm compared with the control arm (hazard ratio, 0.62; 95% CI, 0.48–0.79; Fig. 3).
Figure 2.
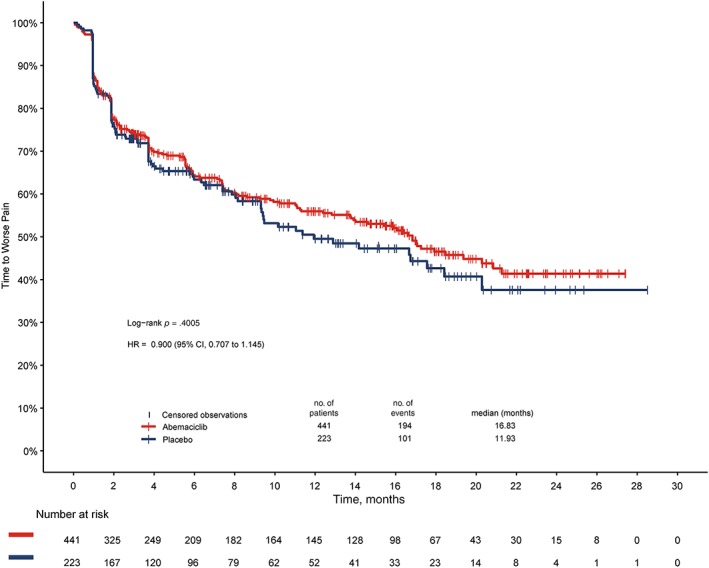
Time to deterioration of modified Brief Pain Inventory, Short Form “worst pain” and increased analgesic use.Abbreviations: CI, confidence interval; HR, hazard ratio.
Figure 3.
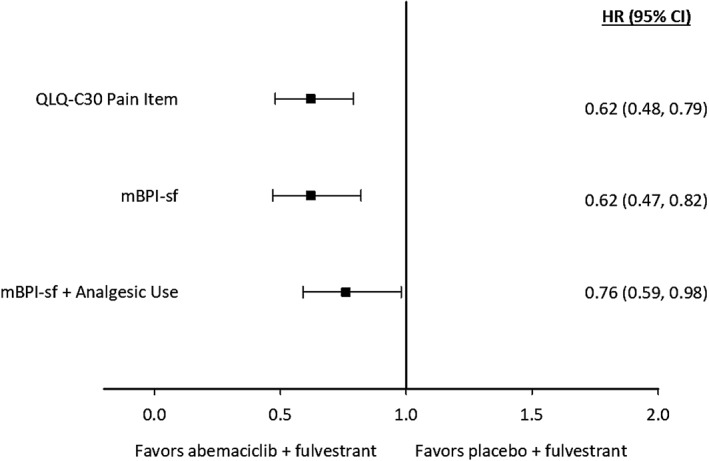
Forest plot of time to sustained deterioration for pain, as measured by the modified Brief Pain Inventory, Short Form (mBPI‐sf) with analgesic use, mBPI‐sf alone, and the European Organization for Research and Treatment of Cancer Quality of Life Core 30 (QLQ‐C30).Abbreviations: CI, confidence interval; HR, hazard ratio.
Quality of Life
At baseline, the EORTC QLQ‐C30 global health status score and the QLQ‐C30 and QLQ‐BR23 functional and symptom scores were similar between study arms and comparable to established reference values for patients with recurrent or metastatic breast cancer (Table 1) 21. Change from baseline for most functional and symptom scores were similar between study arms, but four scores statistically favored the control arm over time: appetite loss (between treatment group difference ± SE, 5.31 ± 1.43; p < .001), nausea and/or vomiting (3.42 ± 0.88; p < .001), diarrhea (24.64 ± 1.56; p < .001), and systemic therapy side effects (5.21 ± 0.87; p < .001, supplemental online Figs. 1 and 2). The symptom upset by hair loss was not analyzed because of small sample size. The diarrhea score was the only item with a clinically meaningful (≥10 points) difference (supplemental online Fig. 1). Furthermore, this higher symptom burden of nausea, vomiting, appetite loss, diarrhea, and systemic therapy side effects was more likely to be reported by patients in early visits while on treatment 25. Dose reductions or omissions and symptom‐specific treatment (i.e., antidiarrheal medication) were effective at reducing these symptoms to baseline or near‐baseline levels at later study visits and at follow‐up for diarrhea 25.
TTSD favored the abemaciclib arm, compared with the control arm, in HRQoL (reflected in the global health status score) and all functional and most symptom scales on the QLQ‐C30 (Fig. 4) and BR‐23 (Fig. 5). Specifically, QLQ‐C30 symptoms favoring the abemaciclib arm at statistically significant levels were fatigue, nausea and vomiting, insomnia, constipation, and financial difficulties (Fig. 4). Arm and breast symptoms measured by the BR‐23 also significantly favored the abemaciclib arm (Fig. 5). Functioning scales favoring the abemaciclib arm at statistically significant levels were physical, role, emotional, cognitive, and social (Fig. 4). The EORTC QLQ‐C30 global health status score, dyspnea, and appetite loss favored the abemaciclib arm but did not reach statistical significance (Fig. 4). Assessed by the BR‐23, systemic therapy side effects, future perspective, sexual functioning, and body image all favored the abemaciclib arm but did not reach statistical significance (Fig. 5). Diarrhea is the only symptom that favored the placebo plus fulvestrant arm (hazard ratio, 1.60; 95% CI, 1.20–2.10; Fig. 4).
Figure 4.
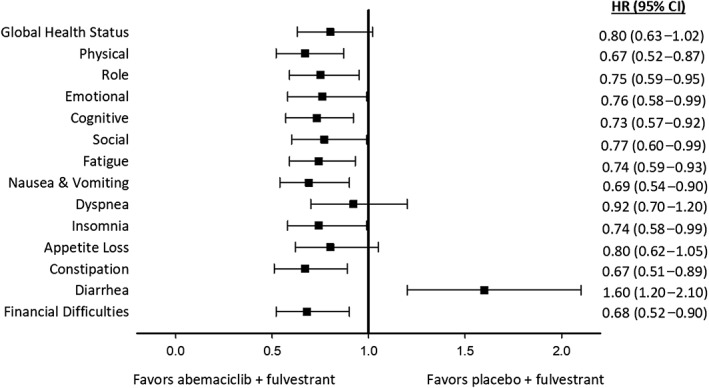
Forest plot of time to sustained deterioration of European Organization for Research and Treatment of Cancer Quality of Life Core 30 symptom and functioning items.Abbreviations: CI, confidence interval; HR, hazard ratio.
Figure 5.
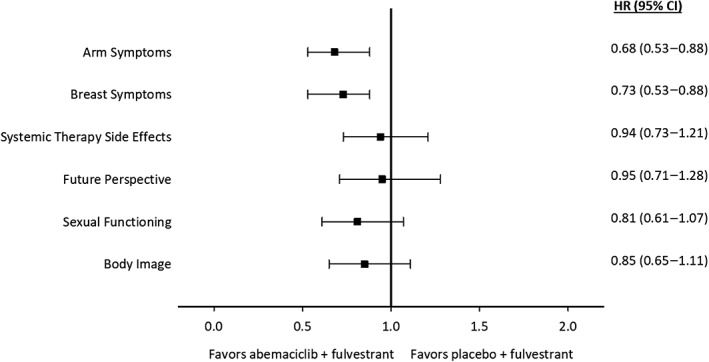
Forest plot of time to sustained deterioration of the European Organization for Research and Treatment of Cancer Breast Cancer Questionnaire symptom and functioning items.Abbreviations: CI, confidence interval; HR, hazard ratio.
Discussion
Given the palliative nature of care in the ABC setting, preserving and maintaining HRQoL and relieving symptoms are primary treatment goals 14, 15, 17. Combination treatment regimens may be associated with additional treatment‐related toxicities that may worsen HRQoL. Thus, ESMO guidelines have been recently revised to recommend that patient‐reported HRQoL should be considered alongside efficacy and safety to improve understanding of the impact on treatment to patients 10. This study found that global HRQoL, the symptom of pain, and additional symptoms and functioning were consistently maintained (i.e., did not deteriorate) from baseline across treatment in women with HR+, HER2− ABC receiving abemaciclib plus fulvestrant compared with those receiving placebo plus fulvestrant. These results demonstrate that, in spite of inherent increased toxicity associated with adding abemaciclib to fulvestrant treatment, there was tolerability across the treatment period.
The symptoms and functions captured in the outcomes assessed in this report are important from a patient perspective. In particular, pain is a symptom commonly reported as most significant among patients with ABC 16 and has been shown to be a strong predictor of overall HRQoL 26. In a study of 1,072 patients with breast cancer, pain and fatigue were commonly ranked as very important symptoms when receiving treatment, whereas maintaining overall HRQoL was the most important issue 27. Another recent study, investigating patient preference related to potential characteristics of cancer treatments, reported the most important item was physical function 28. Other HRQoL characteristics patients with ABC have reported as important include social functioning and nausea and/or vomiting 28. In addition to specific symptoms, TTSD analyses are an important addition to patient‐reported HRQoL analyses over time, as they capture the idea that, past the initial toxicity, patients will feel better and experience a longer period of time before sustained symptom and/or function deterioration if their symptoms are well managed and they are experiencing treatment response.
Importantly, the results reported here demonstrate maintained HRQoL, as measured by symptoms and functions that are important to patients. Specifically, patients in the abemaciclib arm, compared with the control arm, experienced a significant delayed TTSD for the symptoms of pain, fatigue, nausea and vomiting, and physical and social functioning. Although the prespecified TTD analyses of the mBPI‐sf and analgesic use outcome favored the abemaciclib arm, results were not statistically significant. However, this did not reflect the sustained element of deterioration but rather first occurrence, often well managed, and patients improved after the initial worsening. Thus, when we consider the sustained deterioration analyses, there was a significant TTSD for the abemaciclib arm in multiple pain outcomes including the mBPI‐sf alone, the mBPI‐sf with analgesic use, and the QLQ‐C30 pain scale.
Patients in the abemaciclib plus fulvestrant arm also experienced a significantly longer time prior to sustained deterioration for the symptoms of fatigue, nausea and vomiting, insomnia, constipation, and financial difficulties and for physical, role, social, emotional, and cognitive functioning. Diarrhea is the only symptom that significantly favored the control arm in TTSD analyses. Considered with prior results, which demonstrated improved PFS and ORR 4, these results indicate that patients taking abemaciclib plus fulvestrant experienced a consistency of HRQoL alongside treatment response and disease stabilization.
In MONARCH 2, diarrhea was the most commonly reported adverse event (abemaciclib arm, 86.4% vs placebo arm, 24.7%) 4. The previously disclosed safety profile is consistent with the HRQoL results reported herein, where diarrhea was the only patient‐reported symptom for which there was a statistically significant and clinically meaningful difference in favor of the placebo plus fulvestrant arm. However, diarrhea typically occurred in the first treatment cycle and in most cases was effectively managed with antidiarrheal medication and dose adjustments 4. Diarrhea did not frequently (29.9%) require dose interruption or reduction and returned to baseline levels for most patients by the postdiscontinuation follow‐up visit 4, 25. Only 2.9% of patients discontinued abemaciclib treatment because of diarrhea 4. Patient‐reported diarrhea may have been affected by the protocol amendment reducing the abemaciclib dose from 200 mg to 150 mg, but we did not examine that in this report. Although this study found a higher symptom burden over time in the abemaciclib arm compared with the control arm, as measured in change from baseline for nausea and vomiting, appetite loss, and systemic side effects, these symptoms did not meet the criteria for clinically meaningful differences. Furthermore, for all symptoms, this burden was reported during early study visits and returned to baseline or near‐baseline levels for most patients 25.
The significant results for TTSD of financial difficulties in the abemaciclib arm may be explained by patients in the abemaciclib arm are experiencing less deterioration and a longer period of PFS and are therefore missing less work. However, this hypothesis was not investigated in this study. Study drugs were provided in the same manner to patients in both study arms; given the out‐of‐pocket cost of drugs outside of the clinical trial setting, these results may not be generalizable to other patient populations.
These results have several strengths and limitations for interpretation. First, one strength is the consistency of pain results across multiple instruments, namely the mBPI‐sf and the EORTC QLQ‐C30 pain item, combined with analgesic use. The EORTC QLQ‐C30 is broadly used in cancer trials as a well‐validated and reliable tool that has supported HRQoL claims in both U.S. Food and Drug Administration and European Medicines Evaluation Agency labels 19. Additionally, we report high compliance rates of the instruments used to assess pain, HRQoL, and other functional and symptom outcomes, ranging from the low end of 77% at follow‐up to 95% or greater at baseline. The self‐reported nature of the collected symptoms is a benefit, given there are often discrepancies between clinicians and patients in reporting of patient symptoms 29. TTSD analyses are another strength, given they allow for an initial toxicity and improvement; similar definitions have been previously used in similar studies of CDK 4 and 6 inhibitors 24, 30, but one study investigated deterioration stratified by PFS status 24. One limitation is that data cannot be assumed missing at random, but the relatively high compliance, balanced across study arms, minimizes this potential bias. There is also a limitation in not collecting assessments at every cycle, but data were collected at a relatively high frequency, as previously described. Analgesic use was collected and analyzed by category used on a regular basis, but more detailed analyses of analgesic use for pain was not completed. Finally, the assessments were not collected after the 30‐day follow‐up visit, so conclusions from this manuscript are limited to the treatment period.
Conclusion
Despite the long treatment course and increase of toxicities associated with the combination of therapies, HRQoL was not impaired. There were no differences over the course of treatment when comparing the abemaciclib plus fulvestrant and the placebo plus fulvestrant arms for pain, global HRQoL, and most symptoms and functioning. Furthermore, this study demonstrated favorability for the abemaciclib arm in TTSD for most symptoms and physical functioning. These results, combined with superior efficacy 4, indicate that abemaciclib combined with fulvestrant is a desirable treatment option for HR+, HER2− ABC in an endocrine‐resistant setting.
Author Contributions
Conception/design: Masakazu Toi, Clemens Stoffregen, Gregory L. Price
Provision of study material or patients: Peter A. Kaufman, Patrick Neven, Joohyuk Sohn, Clemens Stoffregen
Collection and/or assembly of data: Peter A. Kaufman, Masakazu Toi, Patrick Neven, Joohyuk Sohn, Clemens Stoffregen, Sarah Shekarriz
Data analysis and interpretation: Peter A. Kaufman, Masakazu Toi, Eva‐Marie Grischke, Valerie Andre, Clemens Stoffregen, Sarah Shekarriz, Gregory L. Price, Gebra Cuyun Carter, George W. Sledge, Jr.
Manuscript writing: Peter A. Kaufman, Masakazu Toi, Patrick Neven, Joohyuk Sohn, Eva‐Marie Grischke, Valerie Andre, Clemens Stoffregen, Sarah Shekarriz, Gregory L. Price, Gebra Cuyun Carter, George W. Sledge, Jr.
Final approval of manuscript: Peter A. Kaufman, Masakazu Toi, Patrick Neven, Joohyuk Sohn, Eva‐Marie Grischke, Valerie Andre, Clemens Stoffregen, Sarah Shekarriz, Gregory L. Price, Gebra Cuyun Carter, George W. Sledge, Jr.
Disclosures
Peter A. Kaufman: Eli Lilly and Company (RF, other), AstraZeneca (other), Novartis (RF); Masakazu Toi: Chugai, Takeda, Pfizer, Kyowa Hakko Kirin, C&C Research Laboratories, Taiho, Eisai, Daiichi‐Sankyo, AstraZeneca (RF, other), JBCRG Association, Astellas (RF), Eli Lilly and Company, Merck Sharpe & Dohme, Genomic Health, Novartis, Konica Minolta (other), JBCRG Association, Organisation for Oncology and Translational Research, Kyoto Breast Cancer Research Network (other‐Board of Directors); Patrick Neven: Pfizer, Novartis, Eli Lilly and Company, Roche (C/A), Kom op Tegen Kanker (RF), Eli Lilly Benelux (H); Joohyuk Sohn: Merck Sharpe & Dohme, Roche, Novartis, AstraZeneca, Eli Lilly and Company, Pfizer, Bayer, Glaxo Smith Kline, CONTESSA, Daiichi Sankyo (RF), Eli Lilly and Company (other); Valerie Andre: Eli Lilly & Company (E, OI); Clemens Stoffregen: Eli Lilly & Company (E, OI); Sarah Shekarriz: Eli Lilly & Company (E, OI); Gregory L. Price: Eli Lilly & Company (E, OI); Gebra Cuyun Carter: Eli Lilly & Company (E, OI); George W. Sledge: Eli Lilly & Company (E, other), Tessa Pharmaceuticals (other‐Board of Directors), Syndax (OI, other), Symphony Evolution, Radius Health, Symphony Evolution, Taiho Pharmaceuticals (H), Genentech/Roche (RF), Radius Health and Taiho Pharmaceuticals (other). Eva‐Marie Grischke indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table
Supplemental Figures
Acknowledgments
We thank all the patients, their families, the study sites, and the study personnel who participated in this study. We would also like to thank Jonathan Gable and Amy Lee Chong of Eli Lilly and Company for input and review. Euan MacPherson of EM Statistics and Kriss Harris of SAS Specialists Limited worked on time to sustained deterioration analyses. Eli Lilly and Company contracted with Syneos Health for writing and editing support from Andrea Metti, Ph.D., M.P.H. This study was sponsored by Eli Lilly and Company.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Dickler MN, Tolaney SM, Rugo HS et al. Monarch 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(−) metastatic breast cancer. Clin Cancer Res 2017;23:5218–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gelbert LM, Cai S, Lin X et al. Preclinical characterization of the CDK4/6 inhibitor ly2835219: In‐vivo cell cycle‐dependent/independent anti‐tumor activities alone/in combination with gemcitabine. Invest New Drugs 2014;32:825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patnaik A, Rosen LS, Tolaney SM et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non‐small cell lung cancer, and other solid tumors. Cancer Discov 2016;6:740–753. [DOI] [PubMed] [Google Scholar]
- 4. Sledge GW Jr, Toi M, Neven P et al. MONARCH2: Abemaciclib in combination with fulvestrant in women with HR+/HER2‐ advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017;35:2875–2884. [DOI] [PubMed] [Google Scholar]
- 5. Lallena MJ, Boehnke K, Torres R et al. In‐vitro characterization of abemaciclib pharmacology in ER+ breast cancer cell lines. Cancer Res 2015;75(15 suppl):3101a. [Google Scholar]
- 6. Johnston S, Martin M, Di Leo A et al. Monarch 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019;17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hafner M, Mills CE, Subramanian K et al. Multiomics profiling establishes the polypharmacology of FDA‐approved CDK4/6 inhibitors and the potential for differential clinical activity. Cell Chem Biol 2019;26:1067–1080.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goetz MP, Toi M, Campone M et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer J Clin Oncol 2017;35:3638–3646. [DOI] [PubMed] [Google Scholar]
- 9. Gradishar W, Salemo KE. NCCN guidelines update: Breast cancer. J Natl Compr Canc Netw 2016;14(5 suppl):641–644. [DOI] [PubMed] [Google Scholar]
- 10. Cardoso F, Costa A, Senkus E et al. 3rd ESO‐ESMO International Consensus Guidelines for advanced breast cancer (ABC 3). Ann Oncol 2017;28:16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iwata H, Im SA, Masuda N et al. Paloma‐3: Phase III trial of fulvestrant with or without palbociclib in premenopausal and postmenopausal women with hormone receptor‐positive, human epidermal growth factor receptor 2‐negative metastatic breast cancer that progressed on prior endocrine therapy—Safety and efficacy in Asian patients. J Glob Oncol 2017;3:289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Shaughnessy J, Petrakova K, Sonke GS et al. Ribociclib plus letrozole versus letrozole alone in pateints with de novo HR+, HER2‐advanced breast cancer in the randomized monaleesa‐2 trial. Breast Cancer Res Treat 2018;168:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Modi S, Seidman A. Combination versus sequential single‐agent therapy for the treatment of metastatic breast cancer. EJC Suppl 2005;3:3–8. [Google Scholar]
- 14. Fallowfield LJ. Quality of life assessment using patient‐reported outcome (PRO) measures: Still a cinderella outcome? Ann Oncol 2018;29:2286–2287. [DOI] [PubMed] [Google Scholar]
- 15. Gerber B, Freund M, Reimer T. Recurrent breat cancer: Treatment strategies for maintaining and prolonging quality of life. Dtsc Arztebl Int 2010;107:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lemay K, Wilson KG, Buenger U et al. Fear of pain in patients with advanced cancer or in patients with chronic noncancer pain. Clin J Pain 2011;27:116–124. [DOI] [PubMed] [Google Scholar]
- 17. Marandino L, La Salvia A, Sonetto C et al. Deficiencies in health‐related quality‐of‐life assessment and reporting: A systematic review of oncology randomized phase III trials published between 2012 and 2016. Ann Oncol 2018;29:2288–2295. [DOI] [PubMed] [Google Scholar]
- 18. Cleeland CS. Pain Assessment in Cancer. Boca Raton, FL: CRC Press, 1991. [Google Scholar]
- 19. Aaronson NK, Ahmedzai S, Bergman B et al. The European Organisation for Research and Treatment of Cancer QLQ‐c30: A quality‐of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization . Cancer Pain Relief and Palliative Care: Report of a WHO Expert Committee. Geneva, Switzerland: World Health Organization, 1990. [PubMed] [Google Scholar]
- 21. Sprangers MAG, Groenvold M, Arraras JL et al. The European Organization for Research and Treatment of Cancer breast cancer‐specific quality‐of‐life questionnaire module: First results from a three‐country field study. J Clin Oncol 1996;14:2756–2768. [DOI] [PubMed] [Google Scholar]
- 22. Mathias SD, Crosby RD, Qian Y et al. Estimating minimally important differences for the worst pain rating of the brief pain inventory‐Short Form. J Support Oncol 2011;9:72–78. [DOI] [PubMed] [Google Scholar]
- 23. Osoba D, Rodrigues G, Myles J et al. Interpreting the significnace of changes in health‐related quality of life scores. J Clin Oncol 1998;16:139–144. [DOI] [PubMed] [Google Scholar]
- 24. Verma S, O'Shaughnessy J, Burris HA et al. Health‐related quality of life of postmenopausal women with hormone receptor‐positive, human epidermal growth factor receptor 2‐negative advanced breast cancer treated with ribociclib + letrozole: Results from MONALEESA‐2. Breast Cancer Res Treat 2018;170:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaufman PA, Toi M, Neven P et al. Health‐related quality of life (HRQOL) in MONARCH 2: Abemaciclib plus fulvestrant in women with HR+, HER2‐ advanced breast cancer (ABC) who progressed on endocrine therapy. J Clin Oncol 2018;36(15 suppl):1049a.29406801 [Google Scholar]
- 26. Cramarossa G, Chow E, Zhang L et al. Predictive factors for overall quality of life in patients with advanced cancer. Support Care Cancer 2013;21:1709–1716. [DOI] [PubMed] [Google Scholar]
- 27. Hollen PJ, Masaouel P, Gralla RJ. Determining issues of importance for the evaluation of quality of life and patient‐reported outcomes in breast cancer: Results of a survey of 1072 patients. Breast Cancer Res Treat 2015;151:679–686. [DOI] [PubMed] [Google Scholar]
- 28. Weidling E. Patient preferences for palliative treatment of locally advanced or metastatic breast cancer: An adaptive choice‐based conjoint analysis study from germany. Paper presented at: BMC Congress; January 2019; Berlin, Germany. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Atkinson TM, Li Y, Coffey CW et al. Reliability of adverse symptom event reporting by clinicians. Qual Lif Res 2012;21:1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harbeck N, Iyer S, Turner N et al. Quality of life with plabociclib plus fulvestrant in previously treated hormone receptor‐positive, HER2‐negative metastatic breast cancer: Patient‐reported outcomes from the PALOMA‐3 trial. Ann Oncol 2016;27:1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scott NW, Fayers PM, Aaronson NK et al. EORTC QLQ‐c30 Reference Values. Brussels, Belgium: EORTC Quality of Life Group, 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table
Supplemental Figures


