Abstract
Background
The incidence of oral mucositis (any grade) after everolimus treatment is 58% in the general population and 81% in Asian patients. This study hypothesized that professional oral care (POC) before everolimus treatment could reduce the incidence of everolimus‐induced oral mucositis.
Materials and Methods
This randomized, multicenter, open‐label, phase III study evaluated the efficacy of POC in preventing everolimus‐induced mucositis. Patients were randomized into POC and control groups (1:1 ratio) and received everolimus with exemestane. Patients in the POC group underwent teeth surface cleaning, scaling, and tongue cleaning before everolimus initiation and continued to receive weekly POC throughout the 8‐week treatment period. Patients in the control group brushed their own teeth and gargled with 0.9% sodium chloride solution or water. The primary endpoint was the incidence of all grades of oral mucositis. We targeted acquisition of 200 patients with a 2‐sided type I error rate of 5% and 80% power to detect 25% risk reduction.
Results
Between March 2015 and December 2017, we enrolled 175 women from 31 institutions, of which five did not receive the protocol treatment and were excluded. Over the 8 weeks, the incidence of grade 1 oral mucositis was significantly different between the POC group (76.5%, 62 of 82 patients) and control group (89.7%, 78 of 87 patients; p = .034). The incidence of grade 2 (severe) oral mucositis was also significantly different between the POC group (34.6%, 28 of 82 patients) and control group (54%, 47 of 87 patients; p = .015). As a result of oral mucositis, 18 (22.0%) patients in the POC group and 28 (32.2%) in the control group had to undergo everolimus dose reduction.
Conclusion
POC reduced the incidence and severity of oral mucositis in patients receiving everolimus and exemestane. This might be considered as a treatment option of oral care for patients undergoing this treatment. Clinical trial identification number: NCT 02069093.
Implications for Practice
The Oral Care‐BC trial that prophylactically used professional oral care (POC), available worldwide, did not show a greater than 25% difference in mucositis. The 12% difference in grade 1 or higher mucositis and especially the ∼20% difference in grade 2 mucositis are likely clinically meaningful to patients. POC before treatment should be considered as a treatment option of oral care for postmenopausal patients who are receiving everolimus and exemestane for treatment of hormone receptor‐positive, HER2‐negative advanced breast cancer and metastatic breast cancer. However, POC was not adequate for prophylactic oral mucositis in these patients, and dexamethasone mouthwash prophylaxis is standard treatment before everolimus.
Keywords: Oral care, Oral mucositis, Everolimus, Breast cancer
Short abstract
Oral mucositis is a clinically significant complication of mucotoxic cancer therapy. This article focuses on the effect of dental intervention, before everolimus treatment, on oral mucositis.
Introduction
Oral mucositis is a clinically significant complication of mucotoxic cancer therapy that affects 5%–40% of patients receiving standard‐dose chemotherapy and more than 75% of patients receiving either high‐dose chemotherapy with stem cell transplantation or radiation therapy for head and neck cancer 1, 2, 3. Molecularly targeted therapeutic drugs and chemotherapy drugs used for cancer treatment can cause oral mucositis, resulting in pain and other symptoms. The pain makes it difficult for the patient to ingest orally and can result in malnutrition. Difficulties with oral ingestion and poor oral cleaning can also result in an increase of bacteria inside the mouth, which is associated with systemic risks due to an increased risk of aspiration pneumonitis. Oral mucositis can also result in postponement of changes in treatment, discontinuation of treatment, and prolongation of hospitalization 4, 5.
Although guidelines on oral mucositis 2, 3 state that dental intervention is preferred when implementing cancer treatment, such interventions are not strongly recommended. A retrospective study of 495 patients with nonhead and non‐neck cancers showed that commencement of oral care reduced the incidence of complicating oral mucositis from 38.5% to 10.5% 6, but this is the only such study, and there is insufficient evidence for a strong recommendation. Saito et al. demonstrated that prophylactic professional oral health care reduced the risk of oral mucositis in a small prospective study to assess the usefulness of such care in preventing mucositis in patients undergoing adjuvant chemotherapy 7.
In a randomized phase III study of the treatment of hormone receptor‐positive, hormone therapy‐resistant refractory breast cancer, the progression‐free survival was significantly longer in a concomitant everolimus group compared with an exemestane monotherapy group 8. The incidence of oral mucositis of any grade as an adverse drug reaction of everolimus was 58%, and an analysis of Asian patients showed that the corresponding value in these patients was 81% 9, with an incidence of 91% in Japanese patients 10. However, the mechanism underlying the occurrence of everolimus‐induced oral mucositis has not yet been elucidated.
Oral care is recommended with an evidence level of 3 by The Mucositis Study Group of Multinational Association for Supportive Care in Cancer and International Society of Oral Oncology 2, 3. However, there is a dearth of evidence for recommending oral care, with no data from large‐scale randomized studies. Thus, it is unknown whether the occurrence of oral mucositis can be reduced by implementation of professional oral care (POC) in the form of tooth surface cleaning, scaling, and oral hygiene instruction from a dental and oral surgeon or a dental hygienist before everolimus treatment. Therefore, this study hypothesized that the occurrence of oral mucositis will be reduced by the implementation of dental intervention in the form of tooth surface cleaning, scaling, and oral hygiene instruction from a dentist and oral surgeon and use of Neostelin Green mouthwash before everolimus treatment.
Digest of the Study Protocol
Oral Care‐BC was a Japan‐based, phase III, multicenter randomized clinical trial that assessed the effectiveness of POC in preventing oral mucositis in patients treated with everolimus and exemestane for hormone receptor‐positive, human epidermal growth factor receptor type 2 (HER2)‐negative metastatic breast cancer. This trial concept was already showed in Japanese journal of clinical oncology 11. This study was a multicenter phase III trial, and patients were randomly assigned in a 1:1 ratio (stratified according to center, use of bone‐modifying agent, age, and history of receiving chemotherapy within 3 months). Eligible patients were enrolled at 31 investigation sites from academic and community settings in Japan on the basis of the following key inclusion criteria: women aged 20 years or older who were postmenopausal and had metastatic histologically or cytologically confirmed hormone receptor‐positive, HER2‐negative breast cancer; were newly prescribed everolimus 10 mg and exemestane 25 mg; had Eastern Cooperative Oncology Group (ECOG) performance status of 0–1; and showed adequate renal function (serum creatinine level ≤1.5 × upper limit of normal). Patients with an edentulous jaw, oral mucositis within 1 month, chemotherapy administered within 1 mouth prior to randomization (except bisphosphonates or denosumab), and severe or uncontrolled medical conditions were excluded. The institutional review boards at each of the 31 study sites approved the study protocol; all patients gave written informed consent before all study‐related procedures.
Procedures
Using a dynamic allocation method that minimizes the effects of the allocation adjustment factors discussed below, the Comprehensive Support Project for Oncology Research (CSPOR) Data Center randomly allocated treatment protocols to participants who were then categorized into the POC and control (C) groups in a ratio of approximately 1:1. The allocation algorithm was employed by the person responsible for biostatistical analysis and considered the following factors: age at enrollment (<65 years vs. ≥65 years), use of bone‐modifying agents (yes vs. no), chemotherapy within the last 3 months (yes vs. no), and facility (facility name).
Patients were randomly allocated to the two groups at enrollment, and the protocol treatment (everolimus 10 mg once a day and exemestane 25 mg once a day) was initiated within 3 weeks from the date of enrollment. “Protocol treatment completion” was defined as oral management for a period of 8 weeks in the C and POC groups.
In the POC group, patients received scaling, crown polishing, brushing instruction, tongue plaque removal instruction, gargling instruction (with Neostelin Green 0.2% mouthwash solution, which is an antiseptic mouthwash containing benzethonium chloride as an active ingredient), and dexaltin ointment that is steroidal ointment for the oral cavity containing 0.1% desamethasone use when grade 1 oral mucositis occurred. In the C group (brushing instruction only group), patients received instructions for gargling (with physiological saline) and brushing and were prohibited from using ointments for oral mucositis up to the appearance of grade 2 oral mucositis. The duration of the oral management protocols was 8 weeks in both groups. Dose modification or interruption of everolimus was allowed for management of adverse events. Everolimus use was paused in patients developing grade 2 or 3 stomatitis, and the treatment was resumed after recovery to a maximum severity of grade 1 stomatitis. Patients were discontinued from the study or from everolimus and exemestane treatment for the following reasons: diagnosis of grade 4 stomatitis and other nonhematologic toxicities, diagnosis of grade 3 noninfectious pneumonitis, disease progression requiring a treatment change, or withdrawal of consent.
At each center, the oncologist and dentist or an oral surgeon assessed and documented oral mucositis separately. Each patient was assessed weekly for oral mucositis until 8 weeks. Safety assessments were performed according to the Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE 4.0). Adverse events were monitored throughout the study duration, for 28 days after the last treatment dose (the last dose of everolimus and exemestane), or until study discontinuation. Laboratory assessments of complete blood count, biochemical profile, and liver function profile for adverse event monitoring were performed at screening or baseline and then at every week until 8 weeks.
Interventions were provided by a licensed dentist or a dental hygienist under the supervision of a dentist at 31 sites. Training manuals were prepared to standardize POC. The oncologist and dentist evaluated oral mucositis every week until 8 weeks by using CTCAE v3.0 for a comparison with BOLERO‐2.
Outcomes
The primary endpoint was the incidence of grade 1 or worse oral mucositis evaluated by the oncologist over 8 weeks for comparisons between the POC and C groups. For better assessment of the clinical effect of Everolimus‐associated stomatitis on patients, we evaluated the primary endpoint using a composite assessment. Oral mucositis was graded by the oncologist with the composite score of CTCAE 3.0.
The following secondary endpoints were assessed throughout the 8‐week period. Other endpoints included the incidence of oral mucositis grade greater than or equal to 2 evaluated by an oncologist, incidence of oral mucositis grade greater than or equal to 3 evaluated by an oncologist, incidence of oral mucositis grade greater than or equal to 1 evaluated by a dental and oral surgeon, incidence of oral mucositis grade greater than or equal to 2 evaluated by a dental and oral surgeon, incidence of oral mucositis grade greater than or equal to 3 evaluated by a dental and oral surgeon and time to the onset of oral mucositis, and the duration of each grade of oral mucositis, incidence of patients in suspension or dose reduction of everolimus treatment because of oral mucositis, Oral Assessment Guide (Revised) 12, health‐related quality of life, and time to treatment failure (TTF) were assessed.
Statistical Analysis
Main Analysis And Assessment Criteria
The incidence of oral mucositis of grade greater than or equal to 1 was calculated in the POC and C groups, and their difference was tested. The occurrence of oral mucositis was included if it occurred within 8 weeks from the initiation of protocol treatment, regardless of the completion of dental intervention or protocol treatment (everolimus and exemestane). Although Fisher's exact test was originally planned as the primary analysis, to overcome censoring prior to 8 weeks follow‐up without incidence of oral mucositis, weighted chi‐squared test was performed, in which participants were weighted by the inverse of censoring probability 13, which was nonparametrically estimated by the Kaplan‐Meier method in each of the strata defined by treatment and stratification factors used for randomization. If the result of this test was statistically significant with a two‐sided α‐level of 5% and the incidence of oral mucositis was lower in the POC group than in the C group, the dental intervention was considered to have demonstrated some efficacy. The two‐sided 95% confidence interval of the difference in incidence was also calculated for the purpose of analysis and interpretation.
The incidence of all grades of oral mucositis in Asian participants in the BOLERO‐2 study was 80%, and the incidence of all grades of oral mucositis upon everolimus administration in Japanese participants was assumed to be 80%. The implementation of dental oral management was expected to reduce the relative risk by 25%, and for a two‐sided α‐level of 5% and statistical power of 80% with Fisher's test, the necessary sample size in the two groups was 182. Taking subject exclusions into account, the target sample size was set at 200. The actual sample size was 175 in total, but the trial still had the power of 77%.
The study protocol was registered at the Web site of the University Hospital Medical Information Network, Japan (protocol identifier 000016109), on January 5, 2015, and at http://clinicaltrials.gov (NCT02376985).
Role of the Funding Source
This study was funded by CSPOR of Public Health Research Foundation. The research fund was provided to CSPOR by Novartis Pharma K. K (Nishiazabu Minato‐Ku, Japan). Novartis Pharma K. K. took no part in this study other than providing information relevant to proper use of the study drug. All decisions concerning the planning, implementation, and publication of this study were made by the steering committee
Results
Between March 2015 and December 2017, 175 patients were screened and 174 eligible patients from 31 centers were enrolled in the Oral Care‐BC trial (Fig. 1). Of the 174 women enrolled, 169 were evaluable for efficacy and safety; five women were unevaluable because they never received the protocol treatment. In the 169 evaluable patients, the median age was 64 years (range, 49–84) in the POC group and 64 years (range, 42–83) in the C group. Among these patients, 164 (97%) had an ECOG performance status of 0 or 1, and 19 (11%) received chemotherapy for metastatic disease before everolimus and exemestane treatment (Table 1).
Figure 1.
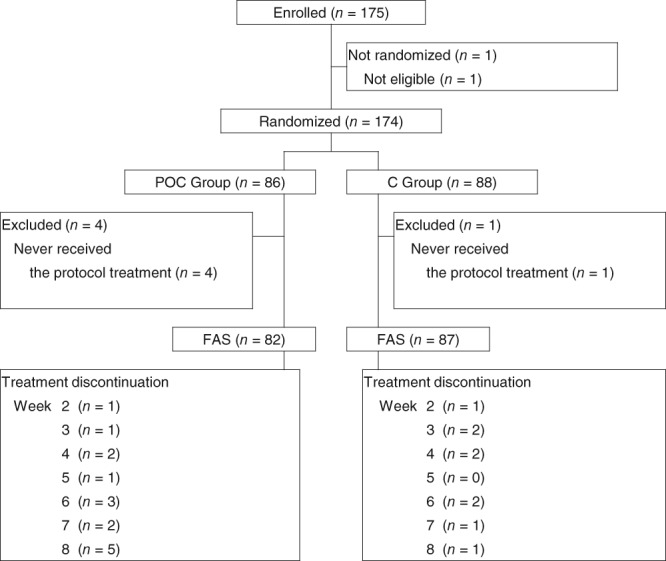
Consort diagram.
Abbreviations: C, control; FAS, full analysis set; POC, professional oral care.
Table 1.
Patient chracteristics
| POC Group (n = 82) | C Group (n = 87) | ||||
|---|---|---|---|---|---|
| Characteristics | n (%) | 95% CI | n (%) | 95% CI | p value |
| Age | .57† | ||||
| n, mean (SD) | 82, 63.7 (7.4) | 87, 62.9 (8.9) | |||
| median (min, max) | 64.0 (49, 84) | 64.0 (42, 83) | |||
| Age | .93* | ||||
| <65 yr | 42 (51.2) | 39.9–62.4 | 44 (50.6) | 39.6–61.5 | |
| ≥65 yr | 40 (48.8) | 37.6–60.1 | 43 (49.4) | 38.5–60.4 | |
| Bone‐modifying agent | .84* | ||||
| Not used | 39 (47.6) | 36.4–58.9 | 40 (46.0) | 35.2–57 | |
| Used | 43 (52.4) | 41.1–63.6 | 47 (54.0) | 43–64.8 | |
| Chemotherapy | .55* | ||||
| Not used | 74 (90.2) | 81.7–95.7 | 76 (87.4) | 78.5–93.5 | |
| Used | 8 (9.8) | 4.3–18.3 | 11 (12.6) | 6.5–21.5 | |
| PS | .14* | ||||
| 0 | 63, (76.8) | 66.2–85.4 | 72, (82.8) | 73.2–90 | |
| 1 | 14, (17.1) | 9.7–27 | 15 (17.2) | 10–26.8 | |
| 2 | 1 (1.2) | 0–6.6 | 0 (0.0) | 0–4.2 | |
| 3 | 0 (0.0) | 0–4.4 | 0 (0.0) | 0–4.2 | |
| 4 | 0 (0.0) | 0–4.4 | 0 (0.0) | 0–4.2 | |
| Missing | 4 (4.9) | 1.3–12 | 0 (0.0) | 0–4.2 | |
| BMI (kg/m2) | .76† | ||||
| n, mean (SD) | 78, 22.95 (3.84) | 87, 22.77 (3.55) | |||
| median (min, max) | 22.52 (14.9, 35.9) | 22.85 (16.4, 34.2) | |||
| BMI | .07† | ||||
| <25 | 54, (65.9) | 54.6–76 | 66 (75.9) | 65.5–84.4 | |
| ≥25 | 24 (29.3) | 19.7–40.4 | 21 (24.1) | 15.6–34.5 | |
| Missing | 4 (4.9) | 1.3–12 | 0 (0.0) | 0–4.2 | |
| Smoking | .50† | ||||
| Nonsmoker | 75 (91.5) | 83.2–96.5 | 83 (95.4) | 88.6–98.7 | |
| Smoker | 4 (4.9) | 1.3–12 | 3 (3.4) | 0.7–9.7 | |
| Missing | 3 (3.7) | 0.8–10.3 | 1 (1.1) | 0–6.2 | |
| Alcohol drinking | .90† | ||||
| Nondrinker | 64 (78.0) | 67.5–86.4 | 69 (79.3) | 69.3–87.3 | |
| Drinker | 14 (17.1) | 9.7–27 | 15 (17.2) | 10–26.8 | |
| Missing | 4 (4.9) | 1.3–12 | 3 (3.4) | 0.7–9.7 | |
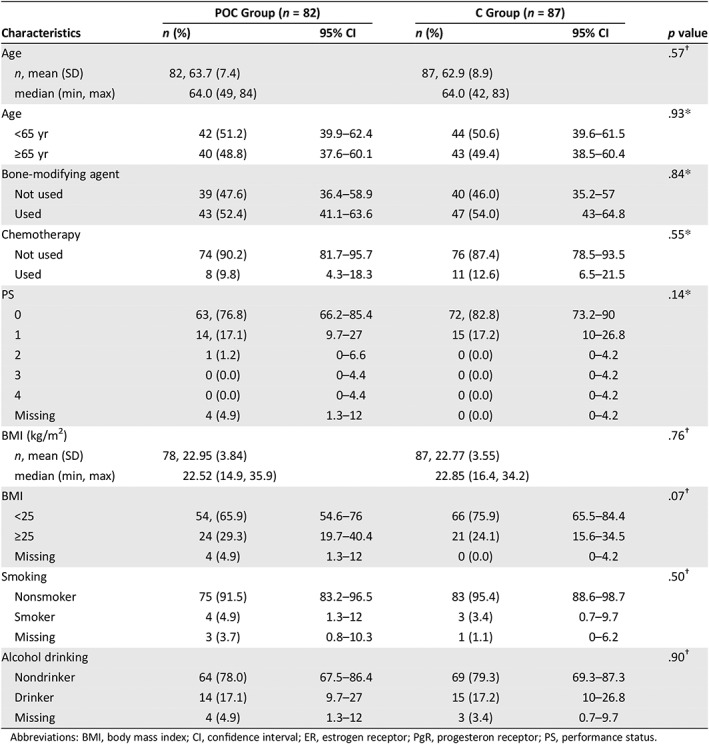
Abbreviations: BMI, body mass index; CI, confidence interval; ER, estrogen receptor; PgR, progesteron receptor; PS, performance status.
In the full analysis set of 169 patients treated with everolimus and exemestane, the primary endpoint of grade 1 or worse oral mucositis evaluated by an oncologist within 8 weeks occurred in 62 of 82 patients (75.6%) in the POC group and 78 of 87 patients (89.7%) in the C group (Table 1; p = .034). The risk difference was −11.88 (95% confidence interval [CI], −22.80 to −0.85; Table 2).
Table 2.
Primary analysis: Incidence probability of oral mucositis
| Oral mucositis grade 1 or worse (by oncologist) | POC group (n = 82), n (%) | C group (n = 87), n (%) | p valuea |
|---|---|---|---|
| Overall | .034 | ||
| Yes | 62 (75.6) | 78 (89.7) | |
| No | 20 (24.4) | 9 (10.3) | |
| Risk difference, % (95% CI)a | −11.88 (−22.82 to −0.95) | ||
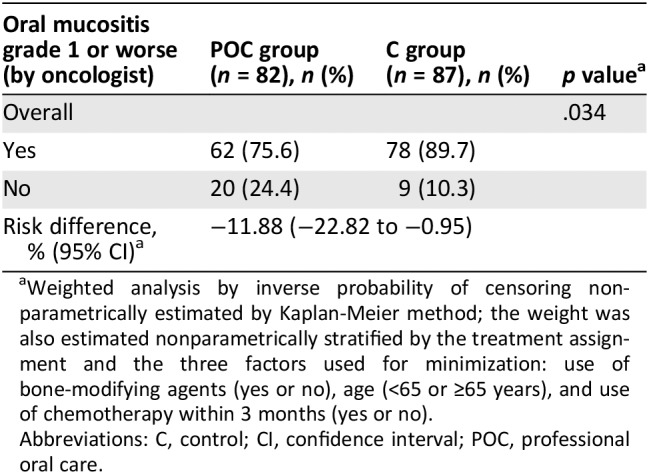
Weighted analysis by inverse probability of censoring nonparametrically estimated by Kaplan‐Meier method; the weight was also estimated nonparametrically stratified by the treatment assignment and the three factors used for minimization: use of bone‐modifying agents (yes or no), age (<65 or ≥65 years), and use of chemotherapy within 3 months (yes or no).
Abbreviations: C, control; CI, confidence interval; POC, professional oral care.
The secondary endpoint of grade 2 or worse oral mucositis evaluated by an oncologist occurred in 28 of 82 patients (34.1%) in the POC group and in 47 of 87 patients (54%) in the C group (Table 3; p = .013). The risk difference was −19.00 (95% CI, −33.71 to −4.28). Grade 3 or worse oral mucositis evaluated by the oncologist occurred in 5 of 82 patients (6.1%) in the POC group and 10 of 87 patients (11.5%) in the C group (Table 3; p = .281). The risk difference was −4.78 (95% CI, −13.40 to 3.83).
Table 3.
Secondary analysis: Incidence probability of oral mucositis
| Oral mucositis grade by oncologist or dentist | POC Group (n = 82), n (%) | C Group (n = 87), n (%) | p valuea |
|---|---|---|---|
| Oral mucositis grade 2 or worse (by oncologist) | .013 | ||
| Yes | 28 (34.1) | 47 (54.0) | |
| No | 54 (65.9) | 40 (46.0) | |
| Risk difference, % (95% CI)a | −19.00 (−33.71 to −4.28) | ||
| Oral mucositis grade 3 or worse (by oncologist) | .281 | ||
| Yes | 5 (6.1) | 10 (11.5) | |
| No | 77 (93.9) | 77 (88.5) | |
| Risk difference, % (95% CI)a | −4.78 (−13.40 to 3.83) | ||
| Oral mucositis grade 1 or worse (by dental and oral surgeon) | .034 | ||
| Yes | 66 (80.5) | 81 (93.1) | |
| No | 16 (19.5) | 6 (6.9) | |
| Risk difference, % (95% CI)a | −10.25 (−19.72 to −0.79) | ||
| Oral mucositis grade 2 or worse (by dental and oral surgeon) | <.001 | ||
| Yes | 33 (40.2) | 61 (70.1) | |
| No | 49 (59.8) | 26 (29.9) | |
| Risk difference, % (95% CI)a | −29.00 − 43.33 to −14.66) | ||
| Oral mucositis grade 3 or worse (by dental and oral surgeon) | .117 | ||
| Yes | 1 (1.2) | 5 (5.7) | |
| No | 81 (98.8) | 82 (94.3) | |
| Risk difference, % (95% CI)a | −4.51 (−10.00 to 0.98) | ||
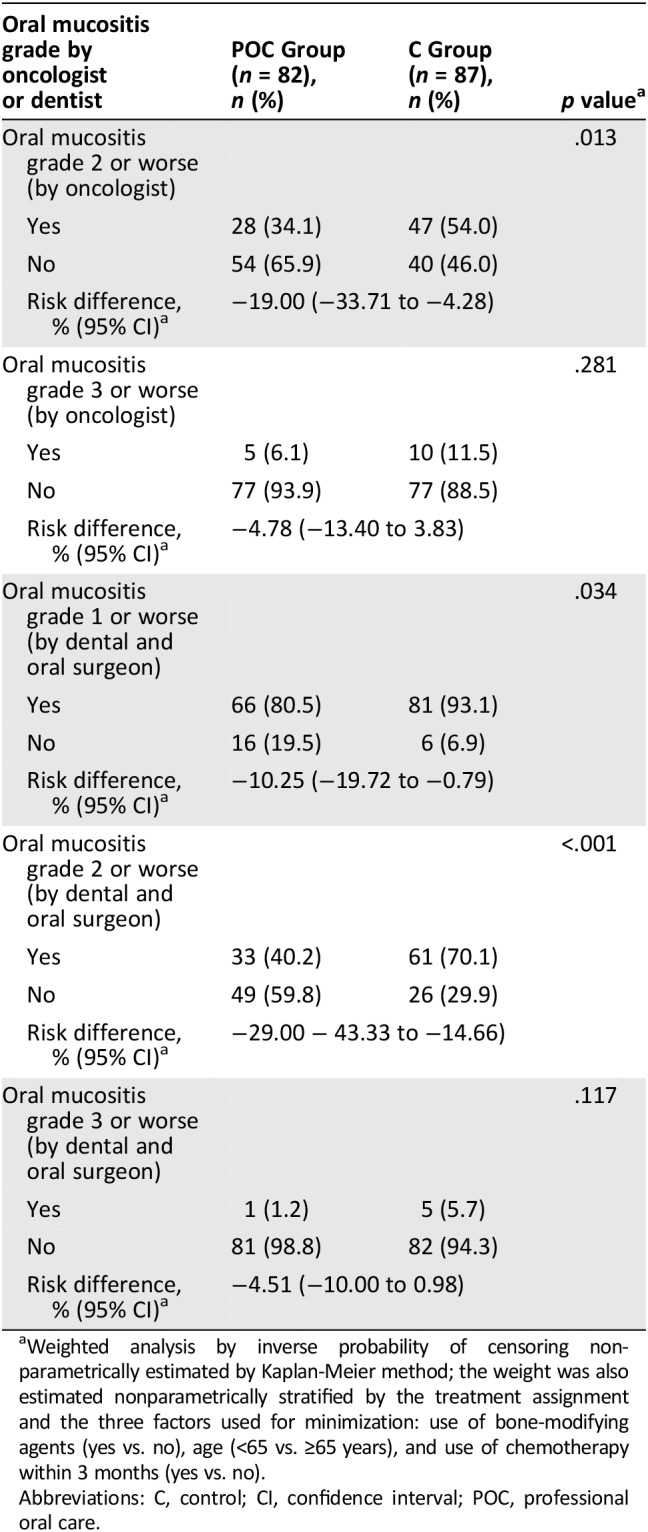
Weighted analysis by inverse probability of censoring nonparametrically estimated by Kaplan‐Meier method; the weight was also estimated nonparametrically stratified by the treatment assignment and the three factors used for minimization: use of bone‐modifying agents (yes vs. no), age (<65 vs. ≥65 years), and use of chemotherapy within 3 months (yes vs. no).
Abbreviations: C, control; CI, confidence interval; POC, professional oral care.
The other secondary endpoint of grade 1 or worse oral mucositis evaluated by a dentist or oral surgeon occurred in 66 of 81 patients (80.5%) in the POC group and in 82 of 87 patients (93.1%) in the C group (p = .034). The risk difference was −10.25 (95% CI, −19.72 to −0.79). Grade 2 or worse oral mucositis evaluated by a dentist or oral surgeon occurred in 33 of 82 patients (40.2%) in the POC group and 61 of 87 patients (69%) in the C group (Table 3) (p < .001). The risk difference was −29.00 (95% CI, −43.33 to −14.66). Grade 3 or worse oral mucositis evaluated by a dentist or oral surgeon occurred in 1 of 82 patients (1.2%) in the POC group and 5 of 87 patients (5.7%) in the C group (Table 3; p = .117). The risk difference was −4.51 (95% CI, −10 to 0.98; Fig. 2).
Figure 2.
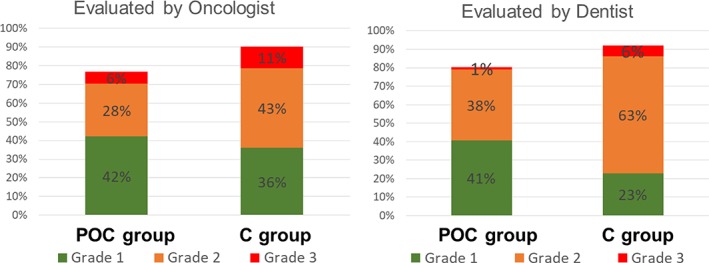
Incidence probability of oral mucositis. Incidence probability evaluated by oncologist (A) and evaluated by dentist (B).
Abbreviations: C, control; POC, professional oral care.
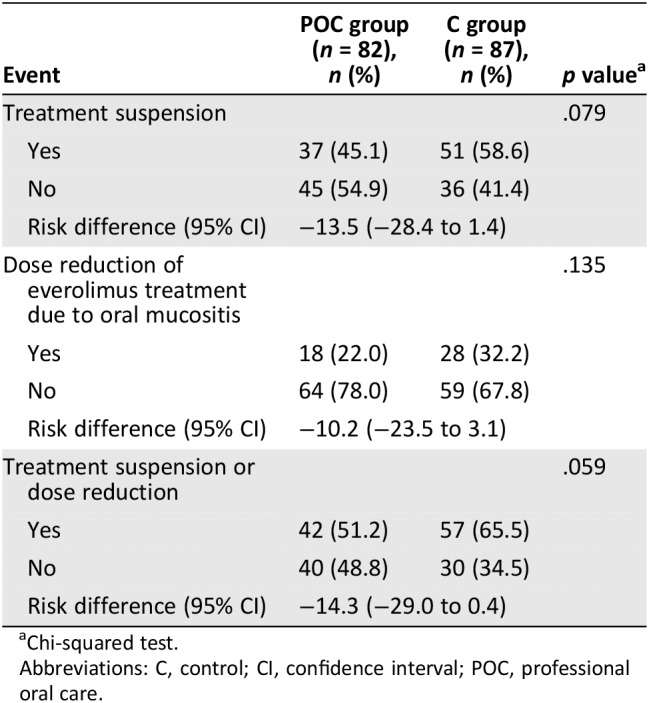
During the entire study duration, treatment delays caused by a suspected treatment‐related adverse event occurred in 37 patients (45.1%) in the POC group and 51 patients (58.6%) in the C group (p = .079) over the 8‐week period, with the most common adverse event being stomatitis (Table 4). Dose reduction of everolimus treatment because of oral mucositis occurred in 18 patients (22%) in the POC group and 28 patients (32.2%) in the C group (p = .135) because of a suspected treatment‐related adverse event, the most common of which was stomatitis.
Table 4.
Delay or dose‐reduction of everolimus
| Event | POC group (n = 82), n (%) | C group (n = 87), n (%) | p valuea |
|---|---|---|---|
| Treatment suspension | .079 | ||
| Yes | 37 (45.1) | 51 (58.6) | |
| No | 45 (54.9) | 36 (41.4) | |
| Risk difference (95% CI) | −13.5 (−28.4 to 1.4) | ||
| Dose reduction of everolimus treatment due to oral mucositis | .135 | ||
| Yes | 18 (22.0) | 28 (32.2) | |
| No | 64 (78.0) | 59 (67.8) | |
| Risk difference (95% CI) | −10.2 (−23.5 to 3.1) | ||
| Treatment suspension or dose reduction | .059 | ||
| Yes | 42 (51.2) | 57 (65.5) | |
| No | 40 (48.8) | 30 (34.5) | |
| Risk difference (95% CI) | −14.3 (−29.0 to 0.4) | ||
Chi‐squared test.
Abbreviations: C, control; CI, confidence interval; POC, professional oral care.
Sixty‐five (79%) of 82 patients in the POC group and 75 (86%) of 87 patients in the C group continued to receive treatment with protocol treatment after the 8‐week study period. The median TTF was 6.75 months in the POC group and 5.74 months in the C group. This difference was not statistically significant (p = .601). A total of 148 of the 169 patients stopped everolimus and exemestane; 100 patients showed disease progression (median, 13.9 week follow‐up; interquartile range, 6.9–23.9).
Overall, during the 8‐week period, adverse events occurred in 71 (86%) of the 82 patients in the POC group and 82 (94%) of the 87 patients in the C group (p = .059). The most frequently reported adverse events, regardless of causality, were oral mucositis, appetite loss, dysgeusia, fatigue, and constipation (Table 5). The incidence of oral mucositis and peripheral edema differed significantly between the POC and C groups. The incidence of other oral‐related events such as appetite loss and dysgeusia did not differ significantly between the two groups. No patient developed oral candidiasis during the study period.
Discussion
This study is the first randomized trial to compare POC with a control group to evaluate its effectiveness in reducing oral mucositis. The results of the Oral Care‐BC show that POC before everolimus treatment can prevent or reduce the incidence and severity of all‐grade oral mucositis evaluated by oncologists and dentists, especially grade 2 or worse oral mucositis at 8 weeks in comparison with the incidence in a control group. No significant difference in the suspension or dose reduction of everolimus was noted between the POC and C groups. POC for early‐onset disease and management of oral mucositis might have further improved tolerability and adherence.
Oral care is based on the following principles: prevention of infections, pain control, maintenance of oral function, and the interplay between managing oral complications of cancer treatment and improving quality of life 14. The position paper for oral care described that oral and dental care must be recognized as a critical component of care prior to and during chemotherapy and hematopoietic stem cell transplantation and in survivorship. A multidisciplinary team approach is the key for success in the management of oral complications in hematology‐oncology patients, including those undergoing hematopoietic stem cell transplantation 14. In this context, POC can reduce early‐onset oral mucositis during treatment with everolimus.
Oral Care‐BC is a randomized control study because Asian patients show a higher incidence oral mucositis than western populations. Subgroup analyses in BOLERO‐2 study showed that oral mucositis occurred in 81% of the Asian population 9, with the corresponding number in the Japanese being as high as 91% 10. These findings could be attributable to the fact that the recommended dose of everolimus (10 mg) does not take weight and body mass index into account, and Asian individuals have a smaller body surface area.
The other study on prophylactic oral mucositis was the SWISH trial 15, which clearly showed that dexamethasone prophylaxis had a profound effect. In that trial, grade 2 or worse stomatitis was observed in only 2 (2%) of 85 patients by 8 weeks (95% CI, 0.29–8.24). The inclusion of video instructions about brushing the teeth at least twice daily with a soft‐bristled toothbrush, daily flossing, and maintenance of good oral hygiene in the SWISH trial may have improved adherence and outcomes compared with those observed in a real‐world situation.
In our study, POC had a profound effect on oral mucositis. However, 76.5% of the patients showed oral mucositis, indicating the need for other prophylactic approaches such as dexamethasone mouth wash. The POC might have prevented oral fungus infection, because dexamethasone mouth wash led to oral candidiasis in 2 of 85 patients.
These findings reinforce the importance of prophylaxis initiation concurrent with everolimus and exemestane treatment and the rationale for an early and short‐term intervention. As such, the most oral mucositis events from the safety set occurred within the first 8 weeks of treatment, which is consistent with the findings from larger datasets 16. In BOLERO‐2, 83% of grade 2 or worse oral mucositis events occurred within 8 weeks, further supporting the rationale that the 8‐week cutoff is sufficient to capture most events. However, whether continuous preventive usage of POC over a longer period could further decrease the incidence of oral mucositis remains unknown.
The concurrent use of POC and an mTOR inhibitor during the period of highest risk for oral mucositis is an effective strategy. POC may allow broader use of everolimus with less toxicity in other indications with a higher incidence of oral mucositis, such as advanced renal cell carcinoma (incidence of oral mucositis, 59%) 16 or advanced neuroendocrine tumors (70%).
We recognize that our study has some limitations. Dexamethasone mouthwash prophylaxis has been a standard treatment before everolimus administration, but we did not evaluate the POC effect in the presence of dexamethasone mouthwash usage in our research. In addition, the duration of the follow‐up was only 8 weeks. However, the patients from BOLERO‐2 served as the benchmark for the 8‐week incidence of oral mucositis, and this endpoint was considered to be valid because most events were observed within this time period 16. A phase III, randomized trial design for Oral Care‐BC was chosen because the Asian population shows a high incidence of oral mucositis. However, because our study was an open design with only a small difference between the POC and C groups observed, we cannot deny the possibility of bias in our assessment. Finally, our oral care protocol requires dental specialist consultation before everolimus treatment; however, oral health during cancer treatment is important to prevent not only stomatitis but also infection, osteonecrosis of the jaw.
Conclusion
The Oral Care‐BC trial that prophylactically used POC, available worldwide, did not show a greater than 25% difference in mucositis. The 12% difference in grade 1 or higher mucositis and especially the ∼20% difference in grade 2 mucositis are likely clinically meaningful to patients. POC before treatment should be considered a new treatment option of oral care for postmenopausal patients who are receiving everolimus and exemestane for treatment of hormone receptor‐positive, HER2‐negative advanced breast cancer and metastatic breast cancer, especially in the first 8 weeks of treatment and as needed thereafter. However, POC was not adequate for prophylactic oral mucositis in these patients, and steroid prophylaxis is standard treatment. In the future, POC may be considered as a treatment option in other diseases for which everolimus is indicated (advanced renal cell carcinoma, subependymal giant cell astrocytoma associated with tuberous sclerosis complex, and advanced neuroendocrine tumors).
Author Contributions
Conception/design: Naoki Niikura, Ken‐ichi Watanabe, Naoki Hayashi, Mariko Naito, Kosuke Kashiwabara, Toshinari Yamashita, Masahiro Umeda, Yoshihide Ota
Provision of study material or patients: Naoki Niikura, Katsuhiko Nakatukasa, Takeshi Amemiya, Ken‐ichi Watanabe, Hironobu Hata, Yuichiro Kikawa, Naoki Taniike, Takashi Yamanaka, Sachiyo Mitsunaga, Kazuhiko Nakagami, Moriyasu Adachi, Naoto Kondo, Yasuyuki Shibuya, Naoki Hayashi, Toshinari Yamashita, Masahiro Umeda, Hirofumi Mukai, Yoshihide Ota
Collection and/or assembly of data: Naoki Niikura, Katsuhiko Nakatukasa, Takeshi Amemiya, Ken‐ichi Watanabe, Hironobu Hata, Yuichiro Kikawa, Naoki Taniike, Takashi Yamanaka, Sachiyo Mitsunaga, Kazuhiko Nakagami, Moriyasu Adachi, Naoto Kondo, Yasuyuki Shibuya, Naoki Hayashi, Mariko Naito, Kosuke Kashiwabara, Toshinari Yamashita, Masahiro Umeda, Hirofumi Mukai, Yoshihide Ota
Data analysis and interpretation: Naoki Niikura, Ken‐ichi Watanabe, Naoki Hayashi, Mariko Naito, Kosuke Kashiwabara, Toshinari Yamashita, Masahiro Umeda, Hirofumi Mukai, Yoshihide Ota
Manuscript writing: Naoki Niikura, Yoshihide Ota
Final approval of manuscript: Naoki Niikura, Katsuhiko Nakatukasa, Takeshi Amemiya, Ken‐ichi Watanabe, Hironobu Hata, Yuichiro Kikawa, Naoki Taniike, Takashi Yamanaka, Sachiyo Mitsunaga, Kazuhiko Nakagami, Moriyasu Adachi, Naoto Kondo, Yasuyuki Shibuya, Naoki Hayashi, Mariko Naito, Kosuke Kashiwabara, Toshinari Yamashita, Masahiro Umeda, Hirofumi Mukai, Yoshihide Ota
Disclsoures
Naoki Niikura: Novartis, Bristol‐Myers Squibb, Chugai Pharmaceutical Co, Nihon Medi‐Physics Co. Ltd., Merck Sharp & Dohme, Daiichi‐Sankyo (RF‐to Tokai University), AstraZeneca, Novartis, Eisai and Pfizer (H); Yuichiro Kikawa: Eisai, Novartis, Taiho, Pfizer (H); Takashi Yamanaka: Chugai, Eisai, Novartis, Pfizer (H); Naoki Hayashi: Chugai‐Pharma, Novartis, AstraZeneca, Pfizer, Kirin Pharma, Genomic Health Inc, Devicor Japan, Allergan Japan (H); Toshinari Yamashita: Chugai, Nippon Kayaku, Kyowa Kirin (RF), Chugai, Nippon Kayaku, Kyowa Kirin, Eisai, Novartis Pharma, Taiho, AstraZeneca, Pfizer Japan (H); Hirofumi Mukai: Japanese Government, Eisai, Daiichi Sankyo, Nippon Kayaku, Pfizer (RF), AstraZeneca, Pfizer, Daiichi Sankyo, Taiho (ET). The other authors indicated no financial relationships.
Acknowledgments
We greatly appreciate all the women who participated in this trial. We also thank all investigators and their collaborators who were dedicated to this study.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Sonis ST, Elting LS, Keefe D et al. Perspectives on cancer therapy‐induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004;100:1995–2025. [DOI] [PubMed] [Google Scholar]
- 2. Lalla RV, Bowen J, Barasch A et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014;120:1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McGuire DB, Fulton JS, Park J et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients. Support Care Cancer 2013;21:3165–3177. [DOI] [PubMed] [Google Scholar]
- 4. Sonis ST, Oster G, Fuchs H et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem‐cell transplantation. J Clin Oncol 2001;19:2201–2205. [DOI] [PubMed] [Google Scholar]
- 5. Elting LS, Cooksley C, Chambers M et al. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy‐induced mucositis. Cancer 2003;98:1531–1539. [DOI] [PubMed] [Google Scholar]
- 6. Sonis S, Kunz A. Impact of improved dental services on the frequency of oral complications of cancer therapy for patients with non‐head‐and‐neck malignancies. Oral Surg Oral Med Oral Pathol 1988;65:19–22. [DOI] [PubMed] [Google Scholar]
- 7. Saito H, Watanabe Y, Sato K et al. Effects of professional oral health care on reducing the risk of chemotherapy‐induced oral mucositis. Support Care Cancer 2014;22:2935–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baselga J, Campone M, Piccart M et al. Everolimus in postmenopausal hormone‐receptor‐positive advanced breast cancer. N Engl J Med 2012;366:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noguchi S, Masuda N, Iwata H et al. Efficacy of everolimus with exemestane versus exemestane alone in Asian patients with HER2‐negative, hormone‐receptor‐positive breast cancer in BOLERO‐2. Breast Cancer 2014;21:703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ito Y, Masuda N, Iwata H et al. Everolimus plus exemestane in postmenopausal patients with estrogen‐receptor‐positive advanced breast cancer ‐ Japanese subgroup analysis of BOLERO ‐2 [in Japanese]. Gan To Kagaku Ryoho 2015;42:67–75. [PubMed] [Google Scholar]
- 11. Niikura N, Ota Y, Hayashi N et al. Evaluation of oral care to prevent oral mucositis in estrogen receptor‐positive metastatic breast cancer patients treated with everolimus (Oral Care‐BC): Randomized controlled phase III trial. Jpn J Clin Oncol 2016;46:879–882. [DOI] [PubMed] [Google Scholar]
- 12. Andersson P, Westergren A, Karlsson S et al. Oral health and nutritional status in a group of geriatric rehabilitation patients. Scand J Caring Sci 2002;16:311–318. [DOI] [PubMed] [Google Scholar]
- 13. Robins JM. An analytic method for randomized trials with informative censoring: Part II. Lifetime Data Anal 1995;1:417–434. [DOI] [PubMed] [Google Scholar]
- 14. Elad S, Raber‐Durlacher JE, Brennan MT et al. Basic oral care for hematology‐oncology patients and hematopoietic stem cell transplantation recipients: A position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT). Support Care Cancer 2015;23:223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rugo HS, Seneviratne L, Beck JT et al. Prevention of everolimus‐related stomatitis in women with hormone receptor‐positive, HER2‐negative metastatic breast cancer using dexamethasone mouthwash (SWISH): A single‐arm, phase 2 trial. Lancet Oncol 2017;18:654–662. [DOI] [PubMed] [Google Scholar]
- 16. Divers J, O'Shaughnessy J. Stomatitis associated with use of mTOR inhibitors: Implications for patients with invasive breast cancer. Clin J Oncol Nurs 2015;19:468–474. [DOI] [PubMed] [Google Scholar]


