Abstract
Dye-sensitized photoelectrochemical (DSPEC) cells are an emerging approach to producing solar fuels. The recent development of delafossite CuCrO2 as a p-type semiconductor has enabled H2 generation through the coassembly of catalyst and dye components. Here, we present a CuCrO2 electrode based on a high-surface-area inverse opal (IO) architecture with benchmark performance in DSPEC H2 generation. Coimmobilization of a phosphonated diketopyrrolopyrrole (DPP-P) or perylene monoimide (PMI-P) dye with a phosphonated molecular Ni catalyst (NiP) demonstrates the ability of IO-CuCrO2 to photogenerate H2. A positive photocurrent onset potential of approximately +0.8 V vs RHE was achieved with these photocathodes. The DPP-P-based photoelectrodes delivered photocurrents of −18 μA cm–2 and generated 160 ± 24 nmol of H2 cm–2, whereas the PMI-P-based photocathodes displayed higher photocurrents of −25 μA cm–2 and produced 215 ± 10 nmol of H2 cm–2 at 0.0 V vs RHE over the course of 2 h under visible light illumination (100 mW cm–2, AM 1.5G, λ > 420 nm, 25 °C). The high performance of the PMI-constructed system is attributed to the well-suited molecular structure and photophysical properties for p-type sensitization. These precious-metal-free photocathodes highlight the benefits of using bespoke IO-CuCrO2 electrodes as well as the important role of the molecular dye structure in DSPEC fuel synthesis.
Keywords: dye-sensitized, molecular catalyst, photocatalysis, solar fuels, p-type semiconductor, delafossite
Introduction
Solar conversion of water into chemical energy carriers offers a sustainable alternative to fossil fuels.1−3 Dye-sensitized photoelectrochemical (DSPEC) cells featuring molecular catalysts are a promising technology for solar water splitting due to their engineering flexibility, easy modification, and assembly.4−8 In these systems, semiconductor-immobilized molecular dyes harvest solar light and transfer charge to a catalytic site, facilitating the synthesis of solar fuels. Significant progress with dye-sensitized photocathodes (DSPCs) and photoanodes has been made possible through a deeper understanding of charge transfer processes and performance-limiting recombination routes.9−18 Although efficiency has improved in recent years, DSPCs still suffer from low photocurrents and poor catalytic activity, representing a bottleneck in state of the art DSPEC devices.
In DSPCs, light absorption by the dye is typically followed by hole injection into the semiconductor, creating a reduced dye that can transfer an electron to the coimmobilized catalyst. The steady-state photocurrent is representative of the kinetic interplay between productive charge transfer and detrimental recombination pathways. Therefore, higher photocurrents can be achieved by blocking the dominant recombination mechanisms between holes in the p-type semiconductor and (1) the reduced dye (geminate recombination) or (2) the reduced catalyst.19−25
Methods to minimize catalyst recombination in DSPCs through layer-by-layer assemblies26−29 and molecular dye–catalyst dyad complexes30−37 have shown some success, but the photocatalytic activity is still limited. Modular assembly through a coimmobilization route is generally preferred, as it provides flexibility in the construction of the electrode, avoiding complex synthetic modification and permitting the use of already established molecular species.38 The undesirable geminate recombination pathway can be rationally addressed through engineering of the dye structure. Charge transfer from an immobilized dye to a semiconductor is highly dependent on the spatial separation.39−43 Consequently, localizing charge density in the photoreduced dye (i.e., the ground state LUMO) away from the semiconductor surface reduces charge recombination kinetics. Such strategies have proven beneficial in p-type dye-sensitized solar cells (p-DSSCs) to effectively increase the photocurrent and photovoltage.16,44,45 In particular, organic dyes have proven to be proficient rivals to conventional precious-metal dyes, commonly displaying favorable qualities for p-type sensitization such as high extinction coefficients and internal acceptor units.16,46−48 The two dyes used in this study are based on diketopyrrolopyrrole (DPP) and perylene monoimide (PMI) chromophores, both of which exhibit beneficial characteristics for incorporation in DSPCs.
Nonetheless, the key component in both recombination routes is the semiconductor. NiO is currently the dominant wide-bandgap p-type semiconductor in DSPCs owing to simple preparation using solution-based techniques.26,49−52 However, problems associated with the high density of traps and low hole mobility have been identified,53−55 which account for the scarcity of reports of H2 generation with coimmobilized dye and catalyst components.20,21,38,56−59 Improved DSPCs can be realized through the development of alternative wide-bandgap p-type semiconductors. Delafossite structures are suitable candidates due to their solution processability, high hole mobility, and metal oxide character.60−63 CuGaO2 and CuCrO2 have been employed in DSPCs, both enabling an early photocurrent onset due to their highly anodic valence band positions.32,64−66 In particular, CuCrO2 formed using a sol–gel method was able to generate H2 in a coimmobilized molecular dye/catalyst assembly.64 The DSPC outperformed an analogous NiO-based photocathode, but low dye and catalyst loadings limited product generation. This issue can in principle be addressed through development of meso- and macroporous architectures such as inverse opal (IO) structures, which has previously proven effective for the incorporation of dye and catalyst components on metal oxides.27,28,67−75
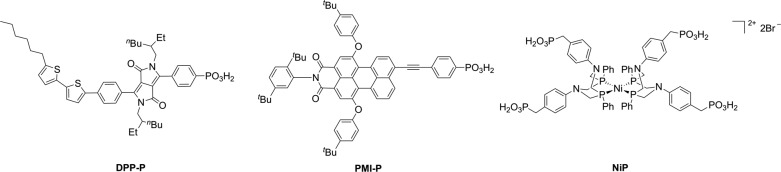
Here, IO-CuCrO2 electrodes are reported through a bottom-up templating method using organic microsphere templates and delafossite CuCrO2 particles. Two organic dyes (DPP-P76 and PMI-P,77Figure 1) were explored in this study and coimmobilized with a DuBois-type molecular Ni catalyst78−82 (NiP, Figure 1). Photoelectrochemical (PEC) H2 generation was used to demonstrate the influence of the novel CuCrO2 morphology and dye structure on performance.
Figure 1.
Chemical structures of molecular dyes DPP-P and PMI-P and molecular H2 evolution catalyst NiP used in this study.
Results and Discussion
Synthesis and Characterization of IO-CuCrO2 Electrodes
IO-CuCrO2 electrodes were constructed using a bottom-up templating method using mixtures of preformed CuCrO2 nanoparticles (NPs) with polystyrene (PS) beads (750 nm diameter). CuCrO2-NPs were synthesized using a modified hydrothermal synthesis procedure.83 In brief, Cu(NO3)2·3H2O (6.25 mmol) and Cr(NO3)3·9H2O (6.25 mmol) were stirred in Milli-Q H2O (70 mL) and NaOH (5 g) was added. The resulting alkaline solution was placed in a PTFE-lined autoclave and heated to 240 °C for 60 h. The NPs were washed consecutively with dilute HCl and EtOH three times and then dried in vacuo. Transmission electron microscopy (TEM) analysis showed that crystalline particles of approximately 15 nm in length and 5 nm in diameter were obtained (Figure S1) with a Brunauer–Emmett–Teller (BET) surface area of 86 m2 g–1. Powder X-ray diffraction (XRD) analysis confirmed the 3R delafossite structure (Figure S2).
The CuCrO2-NPs were dispersed in a 4/1 H2O/MeOH mixture through sonication. The PS beads were washed with MeOH, and a portion of the CuCrO2-NP mixture was added. The dispersion was sonicated and drop-cast on precleaned ITO-coated glass confined to a circular area with Parafilm and left to dry under ambient conditions. The Parafilm was removed, and the PS template was dissolved in toluene. The films were subsequently washed with acetone and dried before being sintered at 500 °C for 1 h under argon. Scanning electron microscopy (SEM) analysis showed that the IO-CuCrO2 films were approximately 2 μm thick with macropores roughly 600–700 nm in diameter (Figure 2a,b). XRD patterns of the IO-CuCrO2 films displayed peaks consistent with the 3R delafossite polytype (Figure S2).
Figure 2.
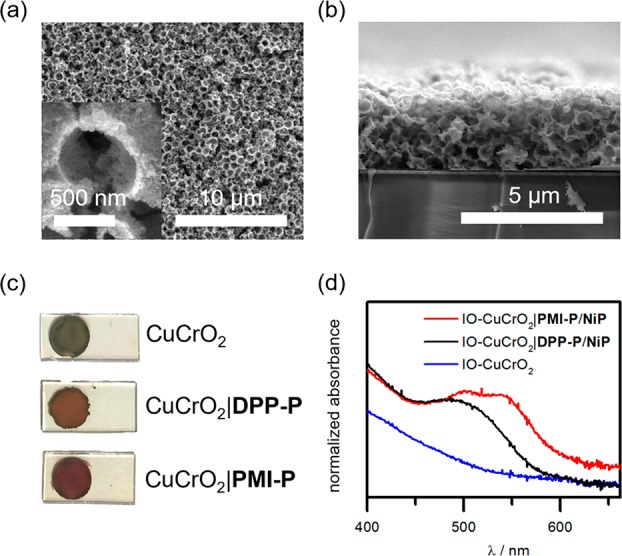
(a) Top-down and (b) cross-sectional SEM images of IO-CuCrO2 electrodes. (c) Photographs and (d) diffuse reflectance UV–vis spectra of the dye- and catalyst-loaded electrodes.
Dye and Catalyst Properties
DPP-P,76PMI-P,77 and NiP(78) were synthesized as previously reported. The phosphonic acid anchoring groups facilitate immobilization on metal oxide surfaces, and both dyes have previously been employed in colloidal dye-sensitized photocatalysis systems with TiO2-NPs and NiP using sacrificial reagents.76,77,84DPP-P was previously shown to be an effective dye in a CuCrO2 photocathode with NiP and is therefore a suitable choice for comparative purposes in this study.64 PMI dyes are renowned for their high stability and intense visible light absorption.45 PMI-based photocathodes have been established with heterogeneous catalysts85 and with molecular catalysts in solution,86 but no examples with an immobilized molecular catalyst currently exist.
The two dyes exhibit similar electrochemical properties in solution (Table S1). Importantly, both dye anions exhibit sufficient thermodynamic driving force (Edye/dye– ≈ −0.7 V vs RHE) to reduce NiP to a catalytically active state (ENiP,onset = −0.21 V vs RHE).78 Hole injection into the valence band of CuCrO2 (Efb ≈ +1.0 V vs RHE) is facilitated by the highly anodic excited state reduction potentials (EDPP-P*/DPP-P– = +1.57 V vs RHE and EPMI-P*/PMI-P– = +1.47 V vs RHE).64,87PMI-P displays a broad and intense absorption signal from 450 to 600 nm with a maximum at 536 nm (εmax = 3.8 × 104 M–1 cm–1, E00 = 2.20 eV) and a shoulder at 500 nm in solution-based UV–vis spectra. DPP-P also displays a broad absorption signal (425–550 nm) with a maximum centered at 496 nm (εmax = 2.6 × 104 M–1 cm–1, E00 = 2.27 eV) (Figure S3).
The intense visible light absorption and favorable electrochemical properties for both dyes are well-suited for application in DSPCs with NiP as the catalyst. To assess their ability to reduce the immobilized catalyst, photocathodes were assembled for PEC analysis.
Photocathode Assembly
Photocathodes were constructed through sequential anchoring of the dye followed by the catalyst. IO-CuCrO2 electrodes were soaked in a solution of PMI-P or DPP-P (0.2 mM, DMF, 15 h) before rinsing with DMF and then H2O. The resulting IO-CuCrO2|dye electrodes were dried under N2 and then immersed in a NiP solution (1 mM, MeOH, 3 h) under a N2 atmosphere to form IO-CuCrO2|dye/NiP electrodes (see the Experimental Section for details).
The color of the IO-CuCrO2 electrodes changed from light green to red or orange for the PMI-P- or DPP-P-sensitized electrodes, respectively (Figure 2c). UV–vis spectra of the films showed the characteristic visible light absorption profiles of the dyes. IO-CuCrO2|PMI-P electrodes displayed higher intensity for the peak at 500 nm than at 536 nm as opposed to the solution-based experiments—this could be attributed to the underlying absorption of CuCrO2 or to the presence of aggregates (Figure 2d).77,88
The loading of each dye was quantified following desorption in basic solution by electronic absorption spectrophotometry, and the amount of catalyst was determined using inductively coupled plasma-optical emission spectroscopy (ICP-OES) (Table S2). The high-surface-area IO-CuCrO2 electrodes loaded approximately 20 nmol cm–2 of the combined molecular species with a dye/catalyst ratio of approximately 3/1. The loading corresponds to a 5-fold increase over the previously reported sol–gel CuCrO2 electrodes (loading approximately 4 nmol cm–2).64
Photoelectrochemical H2 Generation
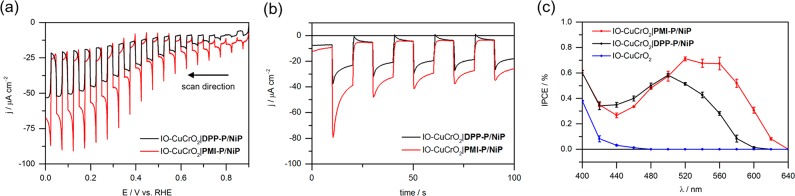
PEC analysis was conducted in aqueous Na2SO4 solution (0.1 M, pH 3) under chopped visible light illumination (100 mW cm–2, AM 1.5G, λ > 420 nm). Mildly acidic conditions are optimal for the catalytic H2 generation performance of NiP.78,89,90 Linear sweep voltammograms (LSVs) showed that cathodic photocurrents were obtained for IO-CuCrO2|DPP-P/NiP and IO-CuCrO2|PMI-P/NiP electrodes with an onset potential of approximately +0.8 V vs RHE (Figure 3a). Chronoamperometry analysis conducted at 0.0 V vs RHE displayed higher photocurrents for the PMI-P photocathodes (j ≈ −25 μA cm–2) than the corresponding DPP-P electrodes (j ≈ −18 μA cm–2) (Figure 3b). The enhancement can be assigned to the red-shifted, more intense, and broader absorption profile of the PMI dye.
Figure 3.
(a) LSVs and (b) chronoamperometry analysis of IO-CuCrO2 electrodes at 0.0 V vs RHE. (c) IPCE plots of IO-CuCrO2 electrodes at +0.3 V vs RHE with monochromatic light intensity maintained at 0.8 mW cm–2. Conditions (a–c): aqueous Na2SO4 (0.1 M, pH 3), chopped visible light illumination (100 mW cm–2, AM 1.5G, λ > 420 nm), scan rate 5 mV s–1 for voltammograms, 25 °C cell temperature maintained. A geometric electrode area of 0.25 cm2 was used for all experiments.
Transient photocurrent spikes were observed for both photocathodes, which are representative of charge accumulation at the semiconductor/electrolyte solution interface. Previous systems have also associated such spikes with recombination,36,91 but the exact origin is not easily identifiable because both dominant recombination routes can contribute to the effect. In this study, the PMI-P-based photocathodes generate the highest cathodic spikes, suggesting substantial charge accumulation at the interface. Kinetic modeling could provide further insight into the exact mechanism and has been successfully applied in similar systems92 but is beyond the scope of the current study.
All electrodes displayed a dark current previously assigned to a CuII/I redox couple and associated oxygen intercalation in LSVs.64,93 Interestingly, the dark current decreased upon immobilization of the individual dye and catalyst components (Figure S4) and the same effect was observed upon anchoring of an insulating organic molecule (phenylphosphonic acid) (Figure S5). The result agrees with a likely binding mechanism through M–O–P bonds,94 which shields the Cu sites from the electrolyte solution, thus lowering the observed redox signal.
Incident photon-to-current conversion efficiency (IPCE) measurements resembled the UV–vis spectra for each dye (Figure 3c). The inversion in peak maxima for the IO-CuCrO2|PMI-P/NiP photocathodes in comparison with the solution absorption suggests that aggregates contribute to photocurrent in this arrangement. Although often associated with adverse effects, aggregation due to π–π stacking of PMI units in related dye-sensitized systems has recently been shown to mediate charge separation on long time scales.95 A similar effect relating to charge accumulation on the PMI units could favor charge transfer and contribute to the photocurrent in this case. The higher values for PMI-P electrodes are representative of higher catalytic activity due to the greater degree of visible light absorption.
Controlled-potential photoelectrolysis (CPPE) was required to confirm the origin of the photocurrent. CPPE experiments were conducted with each electrode at applied potentials of 0.0 and +0.3 V vs RHE in aqueous Na2SO4 (0.1 M, pH 3) over the course of 2 h under constant light illumination (100 mW cm–2, AM 1.5G, λ > 420 nm) (Table 1 and Figure S6). IO-CuCrO2|DPP-P/NiP and IO-CuCrO2|PMI-P/NiP photocathodes generated appreciable amounts of H2 at each applied potential, with the highest activity observed at 0.0 V vs RHE. IO-CuCrO2|PMI-P/NiP electrodes generated 215 ± 10 nmol of H2 cm–2 with a Faradaic efficiency (FE) of 41 ± 8%, giving a turnover number for NiP (TONNiP) of 48 ± 2. IO-CuCrO2|DPP-P/NiP showed slightly lower performance, generating 160 ± 24 nmol of H2 cm–2 with a FE of 40 ± 14% and a TONNiP of 36 ± 5. No H2 was detected for IO-CuCrO2, IO-CuCrO2|dye, or IO-CuCrO2|NiP electrodes, confirming the need for both dye and catalyst molecules for catalysis. These control experiments also rule out any contribution from electrode-derived heterogeneous catalysts as previously observed in other systems.96 The modest FE is at least partially attributed to the persistent dark current originating from the CuII/I redox couple.64,93
Table 1. PEC H2 Generation with Corresponding TONNiP and FE for IO-CuCrO2|PMI-P/NiP and IO-CuCrO2|DPP-P/NiP Photocathodes at Different Applied Potentials over the Course of 2 ha.
| H2 (nmol cm–2) | TONNiP | FE (%) | |
|---|---|---|---|
| IO-CuCrO2|PMI-P/NiP | |||
| +0.3 V vs RHE | 184 ± 22 | 41 ± 5 | 45 ± 6 |
| +0.0 V vs RHE | 215 ± 10 | 48 ± 2 | 41 ± 8 |
| IO-CuCrO2|DPP-P/NiP | |||
| +0.3 V vs RHE | 72 ± 9 | 16 ± 2 | 25 ± 1 |
| +0.0 V vs RHE | 160 ± 24 | 36 ± 5 | 40 ± 14 |
Conditions: aqueous Na2SO4 (0.1 M, pH 3), UV-filtered simulated solar light (100 mW cm–2, AM 1.5G, λ > 420 nm, 25 °C), geometrical surface area of 0.25 cm2. TONNiP was calculated using the catalyst loading determined from ICP-OES measurements.
Postcatalysis ICP-OES showed that approximately 85% and 75% of the initial NiP loaded was retained on the surface of IO-CuCrO2|PMI-P/NiP and IO-CuCrO2|DPP-P/NiP electrodes, respectively (Table S2). Corresponding sol–gel derived CuCrO2|DPP-P/NiP photocathodes preserved only 50% of the catalyst, suggesting that the immobilized NiP is stabilized against desorption by the IO-CuCrO2 structures.
Comparison with State of the Art
The photocathodes presented here display high activities for coimmobilized molecular DSPCs. Incorporation of a coumarin 343 dye coimmobilized with a bimetallic iron-based catalyst on NiO generated photocurrents of −10 μA cm–2 at +0.16 V vs RHE with a FE of 50% but was extremely short lived due to degradation of the catalyst with a final TON ≤ 3.57 A cobalt diimine-dioxime system featuring a coanchored push–pull dye on NiO generated photocurrents of −7.5 μA cm–2 at +0.14 V vs RHE, producing H2 with an FE of 10%.38 Immobilization of the same push–pull dye with a cobaloxime catalyst on CuGaO2 boosted H2 generation, increasing the FE to 74%; this further stresses the importance of altering the semiconductor unit in DSPCs.66 The high photocurrents, FEs, and TONs of the PMI-P (−25 μA cm–2, FE = 41%, TON = 48) and DPP-P (−18 μA cm–2, FE = 40%, TON = 36) photocathodes presented here highlight the benefits of using CuCrO2. Furthermore, the first example of a PMI-sensitized DSPC with an immobilized molecular catalyst is presented. The high performance of the PMI system directs research toward modification of similar chromophores that benefit from high extinction coefficients and favorable structural properties for sensitization of p-type semiconductors.
NiP has previously been incorporated as the catalyst in DSPCs. Layer-by-layer assembly of a phosphonated Ru-polypyridyl dye and NiP on NiO using a ZrIV linker facilitated H2 generation but with low activity.26 Incorporation of a donor–dye–catalyst assembly with a Ru-based dye reported photocurrents of −56 μA cm–2 at +0.05 V vs RHE with a TON of ∼16 and an FE of 53%.27 A subsequent trilayered NiO/ITO assembly without the donor assembly enhanced the FE to 90%.28 The PMI and DPP photocathodes in the current study avoid precious-metal components but retain high activity. Although not as efficient as the best Ru-dye NiP-based examples, they are competitive despite the simple coimmobilization strategy. Layer-by-layer assemblies using organic dyes with CuCrO2 could further reduce catalyst recombination to enhance activity in future studies.
The benefits of modifying NiO through doping and defect passivation/elimination has been outlined in previous reports.28,97,98 Similar improvements to CuCrO2 electrodes are expected to result in enhanced performance. Here, the development of our previous CuCrO2 system through altering the semiconductor morphology increased photocurrents and product yields. The amount of H2 generated is 70% higher with the IO-CuCrO2 electrodes, the enhanced performance being assigned to higher molecular loadings (500% increase) of dye and catalyst in the extended IO network. The sustained photocurrent of the IO-CuCrO2 photocathode is approximately twice that of the previous CuCrO2 system; however, the lower TON achieved is likely due to a significant amount of each species that is grafted on the electrode but does not contribute to catalysis. Significant light scattering/absorption of the IO films is another likely cause, where light harvesting from dye molecules buried within the film is highly limited. The issue could be addressed through pore size modification and thickness control to rationally tailor the IO network for a specific dye/catalyst combination without significantly reducing the molecular loading. Similar approaches to increase the surface area could prove effective with other p-type semiconductors in future studies.
The advantage of bypassing synthetically challenging complexes through coimmobilization increases the diversity of available photocathodes. Well-studied organic chromophores such as the two employed here can be integrated with the established range of Earth-abundant molecular catalysts,1 enabling inexpensive and efficient reduction reactions. Future developments of dye–catalyst assemblies based on IO-CuCrO2 could provide an understanding of the catalysis-limiting processes in DSPCs.
Conclusions
We report two inverse opal CuCrO2 photocathodes using different organic dyes with a coimmobilized molecular Ni bis(diphosphine) catalyst. The IO architecture enables high loadings of molecular species, resulting in a 70% enhancement in H2 generation over a previously reported analogous sol–gel CuCrO2 photocathode. This PMI-sensitized CuCrO2 electrode benefits from a broad and intense absorption profile, ensuring that a large portion of visible light can be effectively harvested. Both IO-CuCrO2|PMI-P/NiP and IO-CuCrO2|DPP-P/NiP photocathodes generate photocurrents of −25 and −18 μA cm–2, respectively, at 0.0 V vs. RHE under visible light illumination (100 mW cm–2, AM 1.5G, λ > 420 nm). The photocurrent onset potential was approximately +0.8 V vs RHE for both architectures. DPP-P- and PMI-P-sensitized photocathodes produce appreciable amounts of H2 over the course of 2 h CPPE. The IO-CuCrO2|PMI-P/NiP photoelectrodes perform best, producing 215 ± 10 nmol of H2 cm–2 at 0.0 V vs RHE with an FE of 41 ± 8% and a TONNiP of 48 ± 2. Under the same conditions, IO-CuCrO2|DPP-P/NiP generates 160 ± 24 nmol of H2 cm–2 with a FE of 40 ± 14% and a TONNiP of 36 ± 5. The high performance of PMI-P reflects the favorable molecular structure and absorption spectrum, which is well-suited for sensitization of p-type semiconductors.
The novel IO-CuCrO2 morphology emphasizes the importance of high-surface-area structures and more suitable p-type semiconductors in DSPCs. Coimmobilization of catalyst and dye components proves to be a viable method to form DSPCs with CuCrO2, avoiding the need for a complicated synthetic protocol. Furthermore, the procedure enables control over pore size through the use of different-sized template spheres, which could assist the development of DSPCs in future studies. Integration of CO2 reduction catalysts and development of dyes tailored for this CuCrO2 surface could provide vital advancements for delafossite-based photocathodes. The novel and transferable IO-CuCrO2 structure presented here extends the range of materials available for fuel-generating DSPCs.
Experimental Section
Materials and Methods
NiP,78DPP-P,76 and PMI-P(77) were synthesized as previously reported. Milli-Q H2O (R > 18.2 MΩ cm) was used for all electrochemical and analytical measurements. Cu(NO3)2·3H2O (Sigma-Aldrich, ≥99%), Cr(NO3)3·9H2O (Sigma-Aldrich, ≥99%), and anhydrous NaOH pellets (Sigma-Aldrich, ≥98%) were used for the CuCrO2-NP preparation.83 PS beads (Polysciences Inc., 750 nm, 2.6% w/v) were used for IO-CuCrO2 synthesis. ITO-coated glass sheets (Vision Tek Systems Ltd., R = 12 Ω cm–2, thickness of 1.1 mm) were cut into 1 × 3 cm2 slides prior to cleaning.
Synthesis of CuCrO2-NPs
Cu(NO3)2·3H2O (1.51 g, 6.25 mmol) and Cr(NO3)3·9H2O (2.50 g, 6.25 mmol) were stirred in Milli-Q H2O (70 mL), and NaOH (5.0 g) was added (final solution pH 13). The solution was stirred at room temperature for 2 h, and then 12 mL was decanted into a PTFE-lined autoclave (23 mL total volume). The autoclave was heated to 240 °C for 60 h before the CuCrO2-NPs were removed. The particles were washed with HCl (0.1 M, 15 mL) and centrifuged (8000 rpm, 5 min), and the supernatant was removed. They were then washed with EtOH (15 mL) and recentrifuged, and the supernatant was removed. The washing steps were repeated for a total of three washes before the CuCrO2-NPs were dried in vacuo. The dry NPs were ground using a pestle and mortar and stored under vacuum before use.
Synthesis of IO-CuCrO2 Electrodes
PS beads (750 nm, 2.6% w/v suspension in H2O, 0.5 mL) were centrifuged, and the supernatant was removed; the beads were then washed with MeOH and centrifuged again to give a PS pellet. A dispersion of CuCrO2-NPs (7.5 wt %, MeOH/H2O 1/4, 140 μL) was added to the PS pellet and sonicated (5 min at <10 °C). The solution was drop-cast (4 μL) on ITO-coated glass (0.5 cm2, confined with Parafilm) and dried in air for 3 h. The Parafilm was removed and the PS template dissolved in toluene for 15 h before being rinsed with acetone then H2O and dried in vacuo. Annealing under Ar (500 °C, 5 °C min–1 ramp rate, 1 h, 150 sccm flow rate) using a tube furnace fitted with a quartz tube, end seals, and insulation plugs (Carbolite Gero) was required to sinter the particles to form the final IO-CuCrO2 structures.
Physical Characterization
A Tescan MIRA3 FEG-SEM was used to obtain all SEM images. TEM analysis was conducted using a FEI Phillips Technai F20-G2 TEM, operating at an accelerating voltage of 200 kV (Electron Microscopy Suite, Cavendish Laboratory, University of Cambridge). XRD measurements were taken with a PANalytical BV X’Pert Pro X-ray diffractometer. UV–vis absorption spectra were acquired using a Varian Cary 50 spectrophotometer operated in transmission mode or with a diffuse reflectance accessory (Barrelino) for powder samples. Emission spectra were recorded on a Fluoromax-4 Horiba Jobin Yvon spectrofluorimeter (PMI) or with an Edinburgh Instruments FS5 spectrofluorimeter (DPP). N2 gas adsorption measurements were carried out using a Micromeritics 3 Flex instrument (Micromeritics, Norcross, GA, USA) with powder samples. Samples were degassed for 10 h at 100 °C, and measurements were carried out in liquid N2. BET specific surface area values were obtained from fitting N2 isotherms using the Microactive software.
Immobilization of Dyes and Catalyst
The dye species were immobilized through soaking in a bath containing DPP-P or PMI-P (0.2 mM) for 15 h in DMF, maintained at 25 °C. The IO-CuCrO2|dye electrodes were rinsed with DMF and water before being dried under N2. These electrodes were then soaked in NiP (1 mM, MeOH) for 3 h under a N2 atmosphere. The IO-CuCrO2|dye/NiP electrodes were rinsed with MeOH and then water and dried under N2 in the dark. All electrodes were used directly after immobilization. A bare IO-CuCrO2 electrode was soaked in a solution of phenylphosphonic acid (0.5 M, DMF) for 2 h and then rinsed with DMF and H2O for the comparative dark current study.
Quantification of Immobilized DPP-P and NiP
DPP-P and PMI-P loadings were quantified using UV–vis spectroscopy following desorption from IO-CuCrO2|dye/NiP electrodes by scraping the CuCrO2-NP powder from the surface (0.25 cm2) and sonicating in tetrabutylammonium hydroxide (TBAOH) 30-hydrate (0.1 M, DMF, 1 mL) for 30 min. Higher TBAOH concentrations and longer sonication times were avoided to prevent dye decomposition. The absorption maximum at 500 and 536 nm for DPP-P and PMI-P electrodes, respectively, was determined and fitted to a calibration curve (conducted in 0.1 M TBAOH in DMF) to determine the loading values. NiP loading was determined by ICP-OES following overnight digestion of the electrodes (0.25 cm2) in aqueous HNO3 (70%, 1 mL) and subsequent dilution to 10% v/v with Milli-Q H2O. Values for nitric acid solution, IO-CuCrO2, IO-CuCrO2|DPP-P, IO-CuCrO2|PMI-P, and pre- and postelectrolysis CuCrO2|DPP-P/NiP and CuCrO2|PMI-P/NiP electrodes were determined in triplicate.
Photoelectrochemical Measurements
PEC measurements were conducted using an Ivium CompactStat potentiostat in a custom two-compartment electrochemical cell featuring a flat quartz window and a Nafion membrane. A three-electrode setup was used with a Pt counter electrode, Ag/AgCl/KClsat reference electrode, and an IO-CuCrO2-based working electrode (0.25 cm2 active area). N2-purged (15 min) aqueous Na2SO4 electrolyte solution (0.1 M, pH 3) was used for all measurements. Electrodes were illuminated from the front using a calibrated Newport Oriel solar light simulator (150 W, 100 mW cm–2, AM 1.5G) with an IR water filter and a UQG Optics UV-Filter (λ > 420 nm).
CPPE experiments with each photocathode were conducted in triplicate at +0.3 and 0.0 V vs RHE in a custom two-compartment electrochemical cell featuring a flat quartz window and a Nafion membrane. Both compartments were purged with 2% CH4 in N2 for 30 min prior to electrolysis, and the amount of H2 was determined using a Shimadzu Tracera GC2010 Plus gas chromatograph using a barrier ionization discharge detector and a molsieve column (kept at 130 °C) with He as the carrier gas. All PEC cells were left for 2 h following electrolysis to allow solution-dissolved H2 to equilibrate with the gas headspace. The partial pressure of H2 was calculated to account for dissolved gas in the solution, and this was added to the amount of H2 in the headspace to determine the total H2 and Faradaic efficiency.
IPCE Measurements
A three-electrode setup with a Pt counter electrode, Ag/AgCl/KClsat reference electrode, and an IO-CuCrO2|dye/NiP or IO-CuCrO2 working electrode was used in a custom three-necked cell with a flat borosilicate glass window for IPCE measurements. The electrolyte solution was Na2SO4 (0.1 M, pH 3), and an applied potential of +0.3 V vs RHE was maintained for all measurements. Monochromatic light was supplied with a 300 W xenon lamp solar light simulator connected to a monochromator (MSH300, LOT Quantum design). The intensity was calibrated to 0.8 mW cm–2 for each individual wavelength, and experiments with each electrode were conducted in triplicate with different electrodes using an active area of 0.25 cm2. Photocurrents were determined at each recorded wavelength through light chopping using an Ivium CompactStat potentiostat. Error bars represent standard deviation from the mean.
Acknowledgments
The work presented was supported by the Christian Doppler Research Association (Austrian Federal Ministry for Digital and Economic Affairs and the National Foundation for Research, Technology and Development), the OMV Group (E.R., C.E.C., and J.W.), the EPSRC NanoDTC in Cambridge (EP/L015978/1; E.R. and C.E.C.), and an EPSRC PhD DTA studentship (EP/M508007/1; D.A.-G.). We thank Laura Healy (Department of Geography, University of Cambridge) for ICP-OES measurements. We are grateful to Agence Nationale de la Recherche (ANR) for financial support of this research through the program POSITIF (ANR-12-PRGE-0016-01) and Région des Pays de la Loire for the project LUMOMAT (F.O. and Y.F.). We appreciate suggestions and comments on the manuscript from Esther Edwardes Moore and Dr. Souvik Roy.
Glossary
Abbreviations
- ITO
indium tin oxide
- ICP-OES
inductively coupled plasma-optical emission spectrometry
- SEM
scanning electron microscopy
- Efb
flatband potential
- BET
Brunauer-Emmett-Teller
- XRD
X-ray diffraction
- LSV
linear sweep voltammetry
- RHE
reversible hydrogen electrode
- LUMO
lowest unoccupied molecular orbital
- TBAOH
tetrabutylammonium hydroxide
- DSPEC
dye-sensitized photoelectrochemical
- IO
inverse opal
- DSPC
dye-sensitized photocathode
- p-DSSC
p-type dye-sensitized solar cell
- DPP
diketopyrrolopyrrole
- PMI
perylene monoimide
- PEC
photoelectrochemical
- NP
nanoparticle
- PS
polystyrene
- IPCE
incident photon-to-current conversion efficiency
- CPPE
controlled potential photoelectrolysis
- FE
Faradaic efficiency
- TON
turnover number
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscatal.9b02984.
Additional figures and tables as described in the text (PDF)
Author Contributions
All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Notes
Data related to this publication are available at the University of Cambridge data repository: https://doi.org/10.17863/CAM.43804.
Supplementary Material
References
- Dalle K. E.; Warnan J.; Leung J. J.; Reuillard B.; Karmel I. S.; Reisner E. Electro- and Solar-Driven Fuel Synthesis with First Row Transition Metal Complexes. Chem. Rev. 2019, 119, 2752–2875. 10.1021/acs.chemrev.8b00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M. G.; Warren E. L.; McKone J. R.; Boettcher S. W.; Mi Q.; Santori E. A.; Lewis N. S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. 10.1021/cr1002326. [DOI] [PubMed] [Google Scholar]
- Tachibana Y.; Vayssieres L.; Durrant J. R. Artificial Photosynthesis for Solar Water-Splitting. Nat. Photonics 2012, 6, 511–518. 10.1038/nphoton.2012.175. [DOI] [Google Scholar]
- Brennaman M. K.; Dillon R. J.; Alibabaei L.; Gish M. K.; Dares C. J.; Ashford D. L.; House R. L.; Meyer G. J.; Papanikolas J. M.; Meyer T. J. Finding the Way to Solar Fuels with Dye-Sensitized Photoelectrosynthesis Cells. J. Am. Chem. Soc. 2016, 138, 13085–13102. 10.1021/jacs.6b06466. [DOI] [PubMed] [Google Scholar]
- Gibson E. A. Dye-Sensitized Photocathodes for H2 Evolution. Chem. Soc. Rev. 2017, 46, 6194–6209. 10.1039/C7CS00322F. [DOI] [PubMed] [Google Scholar]
- Zhang B.; Sun L. Artificial Photosynthesis: Opportunities and Challenges of Molecular Catalysts. Chem. Soc. Rev. 2019, 48, 2216–2264. 10.1039/C8CS00897C. [DOI] [PubMed] [Google Scholar]
- Xu P.; McCool N. S.; Mallouk T. E. Water Splitting Dye-Sensitized Solar Cells. Nano Today 2017, 14, 42–58. 10.1016/j.nantod.2017.04.009. [DOI] [Google Scholar]
- Hennessey S.; Farràs P. Production of Solar Chemicals: Gaining Selectivity with Hybrid Molecule/Semiconductor Assemblies. Chem. Commun. 2018, 54, 6662–6680. 10.1039/C8CC02487A. [DOI] [PubMed] [Google Scholar]
- Troian-Gautier L.; DiMarco B. N.; Sampaio R. N.; Marquard S. L.; Meyer G. J. Evidence That ΔS‡ Controls Interfacial Electron Transfer Dynamics from Anatase TiO2 to Molecular Acceptors. J. Am. Chem. Soc. 2018, 140, 3019–3029. 10.1021/jacs.7b13243. [DOI] [PubMed] [Google Scholar]
- Xu P.; Mallouk T. E. Charge Transfer Dynamics in Aqueous Dye-Sensitized Photoelectrochemical Cells: Implications for Water Splitting Efficiency. J. Phys. Chem. C 2019, 123, 299–305. 10.1021/acs.jpcc.8b11356. [DOI] [Google Scholar]
- Black F. A.; Clark C. A.; Summers G. H.; Clark I. P.; Towrie M.; Penfold T.; George M. W.; Gibson E. A. Investigating Interfacial Electron Transfer in Dye-Sensitized NiO Using Vibrational Spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 7877–7885. 10.1039/C6CP05712H. [DOI] [PubMed] [Google Scholar]
- D’Amario L.; Antila L. J.; Pettersson Rimgard B.; Boschloo G.; Hammarström L. Kinetic Evidence of Two Pathways for Charge Recombination in NiO-Based Dye-Sensitized Solar Cells. J. Phys. Chem. Lett. 2015, 6, 779–783. 10.1021/acs.jpclett.5b00048. [DOI] [PubMed] [Google Scholar]
- Smeigh A. L.; Le Pleux L.; Fortage J.; Pellegrin Y.; Blart E.; Odobel F.; Hammarström L. Ultrafast Recombination for NiO Sensitized with a Series of Perylene Imide Sensitizers Exhibiting Marcus Normal Behaviour. Chem. Commun. 2012, 48, 678–680. 10.1039/C1CC16144J. [DOI] [PubMed] [Google Scholar]
- Shan B.; Farnum B. H.; Wee K.-R.; Meyer T. J. Generation of Long-Lived Redox Equivalents in Self-Assembled Bilayer Structures on Metal Oxide Electrodes. J. Phys. Chem. C 2017, 121, 5882–5890. 10.1021/acs.jpcc.6b12416. [DOI] [Google Scholar]
- Föhlinger J.; Maji S.; Brown A.; Mijangos E.; Ott S.; Hammarström L. Self-Quenching and Slow Hole Injection May Limit the Efficiency in NiO-Based Dye-Sensitized Solar Cells. J. Phys. Chem. C 2018, 122, 13902–13910. 10.1021/acs.jpcc.8b01016. [DOI] [Google Scholar]
- Nikolaou V.; Charisiadis A.; Charalambidis G.; Coutsolelos A. G.; Odobel F. Recent Advances and Insights in Dye-Sensitized NiO Photocathodes for Photovoltaic Devices. J. Mater. Chem. A 2017, 5, 21077–21113. 10.1039/C7TA06500K. [DOI] [Google Scholar]
- Odobel F.; Pellegrin Y. Recent Advances in the Sensitization of Wide-Band-Gap Nanostructured p-Type Semiconductors. Photovoltaic and Photocatalytic Applications. J. Phys. Chem. Lett. 2013, 4, 2551–2564. 10.1021/jz400861v. [DOI] [Google Scholar]
- Moore G. F.; Blakemore J. D.; Milot R. L.; Hull J. F.; Song H.; Cai L.; Schmuttenmaer C. A.; Crabtree R. H.; Brudvig G. W. A Visible Light Water-Splitting Cell with a Photoanode Formed by Codeposition of a High-Potential Porphyrin and an Iridium Water-Oxidation Catalyst. Energy Environ. Sci. 2011, 4, 2389–2392. 10.1039/c1ee01037a. [DOI] [Google Scholar]
- Zhang L.; Favereau L.; Farré Y.; Mijangos E.; Pellegrin Y.; Blart E.; Odobel F.; Hammarström L. Ultrafast and Slow Charge Recombination Dynamics of Diketopyrrolopyrrole–NiO Dye Sensitized Solar Cells. Phys. Chem. Chem. Phys. 2016, 18, 18515–18527. 10.1039/C6CP01762B. [DOI] [PubMed] [Google Scholar]
- Brown A. M.; Antila L. J.; Mirmohades M.; Pullen S.; Ott S.; Hammarström L. Ultrafast Electron Transfer between Dye and Catalyst on a Mesoporous NiO Surface. J. Am. Chem. Soc. 2016, 138, 8060–8063. 10.1021/jacs.6b03889. [DOI] [PubMed] [Google Scholar]
- Gilbert Gatty M.; Pullen S.; Sheibani E.; Tian H.; Ott S.; Hammarström L. Direct Evidence of Catalyst Reduction on Dye and Catalyst Co-Sensitized NiO Photocathodes by Mid- Infrared Transient Absorption Spectroscopy. Chem. Sci. 2018, 9, 4983–4991. 10.1039/C8SC00990B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgström M.; Blart E.; Boschloo G.; Mukhtar E.; Hagfeldt A.; Hammarström L.; Odobel F. Sensitized Hole Injection of Phosphorus Porphyrin into NiO: Toward New Photovoltaic Devices. J. Phys. Chem. B 2005, 109, 22928–22934. 10.1021/jp054034a. [DOI] [PubMed] [Google Scholar]
- Morandeira A.; Boschloo G.; Hagfeldt A.; Hammarström L. Photoinduced Ultrafast Dynamics of Coumarin 343 Sensitized P-Type-Nanostructured NiO Films. J. Phys. Chem. B 2005, 109, 19403–19410. 10.1021/jp053230e. [DOI] [PubMed] [Google Scholar]
- Daeneke T.; Yu Z.; Lee G. P.; Fu D.; Duffy N. W.; Makuta S.; Tachibana Y.; Spiccia L.; Mishra A.; Bäuerle P.; Bach U. Dominating Energy Losses in NiO P-Type Dye-Sensitized Solar Cells. Adv. Energy Mater. 2015, 5, 1401387. 10.1002/aenm.201401387. [DOI] [Google Scholar]
- Gardner J. M.; Beyler M.; Karnahl M.; Tschierlei S.; Ott S.; Hammarström L. Light-Driven Electron Transfer between a Photosensitizer and a Proton-Reducing Catalyst Co-Adsorbed to NiO. J. Am. Chem. Soc. 2012, 134, 19322–19325. 10.1021/ja3082268. [DOI] [PubMed] [Google Scholar]
- Gross M. A.; Creissen C. E.; Orchard K. L.; Reisner E. Photoelectrochemical Hydrogen Production in Water Using a Layer-by-Layer Assembly of a Ru Dye and Ni Catalyst on NiO. Chem. Sci. 2016, 7, 5537–5546. 10.1039/C6SC00715E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan B.; Das A. K.; Marquard S. L.; Farnum B. H.; Wang D.; Bullock R. M.; Meyer T. J. Photogeneration of Hydrogen from Water by a Robust Dye-Sensitized Photocathode. Energy Environ. Sci. 2016, 9, 3693–3697. 10.1039/C6EE02903E. [DOI] [Google Scholar]
- Shan B.; Sherman B. D.; Klug C. M.; Nayak A.; Marquard S. L.; Liu Q.; Bullock R. M.; Meyer T. J. Modulating Hole Transport in Multilayered Photocathodes with Derivatized P-Type Nickel Oxide and Molecular Assemblies for Solar-Driven Water Splitting. J. Phys. Chem. Lett. 2017, 8, 4374–4379. 10.1021/acs.jpclett.7b01911. [DOI] [PubMed] [Google Scholar]
- Orchard K. L.; Hojo D.; Sokol K. P.; Chan M.-J.; Asao N.; Adschiri T.; Reisner E. Catechol–TiO2 Hybrids for Photocatalytic H2 Production and Photocathode Assembly. Chem. Commun. 2017, 53, 12638–12641. 10.1039/C7CC05094A. [DOI] [PubMed] [Google Scholar]
- Põldme N.; O’Reilly L.; Fletcher I.; Portoles J.; Sazanovich I. V.; Towrie M.; Long C.; Vos J. G.; Pryce M. T.; Gibson E. A. Photoelectrocatalytic H2 Evolution from Integrated Photocatalysts Adsorbed on NiO. Chem. Sci. 2019, 10, 99–112. 10.1039/C8SC02575D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeffer N.; Massin J.; Lebrun C.; Renault O.; Chavarot-Kerlidou M.; Artero V. Covalent Design for Dye-Sensitized H2-Evolving Photocathodes Based on a Cobalt Diimine-Dioxime Catalyst. J. Am. Chem. Soc. 2016, 138, 12308–12311. 10.1021/jacs.6b05865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai H.; Sahara G.; Maeda K.; Higashi M.; Abe R.; Ishitani O. Hybrid Photocathode Consisting of a CuGaO2 p-Type Semiconductor and a Ru(II)–Re(I) Supramolecular Photocatalyst: Non-Biased Visible-Light-Driven CO2 Reduction with Water Oxidation. Chem. Sci. 2017, 8, 4242–4249. 10.1039/C7SC00940B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara G.; Kumagai H.; Maeda K.; Kaeffer N.; Artero V.; Higashi M.; Abe R.; Ishitani O. Photoelectrochemical Reduction of CO2 Coupled to Water Oxidation Using a Photocathode with a Ru(II)-Re(I) Complex Photocatalyst and a CoOx/TaON Photoanode. J. Am. Chem. Soc. 2016, 138, 14152–14158. 10.1021/jacs.6b09212. [DOI] [PubMed] [Google Scholar]
- Kamata R.; Kumagai H.; Yamazaki Y.; Sahara G.; Ishitani O. Photoelectrochemical CO2 Reduction Using a Ru(II)–Re(I) Supramolecular Photocatalyst Connected to a Vinyl Polymer on a NiO Electrode. ACS Appl. Mater. Interfaces 2019, 11, 5632–5641. 10.1021/acsami.8b05495. [DOI] [PubMed] [Google Scholar]
- Li T.-T.; Shan B.; Meyer T. J. Stable Molecular Photocathode for Solar-Driven CO2 Reduction in Aqueous Solutions. ACS Energy Lett. 2019, 4, 629–636. 10.1021/acsenergylett.8b02512. [DOI] [Google Scholar]
- Pati P. B.; Zhang L.; Philippe B.; Fernández-Terán R.; Ahmadi S.; Tian L.; Rensmo H.; Hammarström L.; Tian H. Insights into the Mechanism of a Covalently Linked Organic Dye–Cobaloxime Catalyst System for Dye-Sensitized Solar Fuel Devices. ChemSusChem 2017, 10, 2480–2495. 10.1002/cssc.201700285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bold S.; Zedler L.; Zhang Y.; Massin J.; Artero V.; Chavarot-Kerlidou M.; Dietzek B. Electron Transfer in a Covalent Dye-Cobalt Catalyst Assembly - a Transient Absorption Spectroelectrochemistry Perspective. Chem. Commun. 2018, 54, 10594–10597. 10.1039/C8CC05556D. [DOI] [PubMed] [Google Scholar]
- Kaeffer N.; Windle C. D.; Brisse R.; Gablin C.; Leonard D.; Jousselme B.; Chavarot-Kerlidou M.; Artero V. Insights into the Mechanism and Aging of a Noble-Metal Free H2-Evolving Dye-Sensitized Photocathode. Chem. Sci. 2018, 9, 6721–6738. 10.1039/C8SC00899J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque S. A.; Handa S.; Peter K.; Palomares E.; Thelakkat M.; Durrant J. R. Supermolecular Control of Charge Transfer in Dye-Sensitized Nanocrystalline TiO2 Films: Towards a Quantitative Structure-Function Relationship. Angew. Chem., Int. Ed. 2005, 44, 5740–5744. 10.1002/anie.200500363. [DOI] [PubMed] [Google Scholar]
- Hu K.; Blair A. D.; Piechota E. J.; Schauer P. A.; Sampaio R. N.; Parlane F. G. L.; Meyer G. J.; Berlinguette C. P. Kinetic Pathway for Interfacial Electron Transfer from a Semiconductor to a Molecule. Nat. Chem. 2016, 8, 853–859. 10.1038/nchem.2549. [DOI] [PubMed] [Google Scholar]
- Clifford J. N.; Palomares E.; Nazeeruddin M. K.; Grätzel M.; Nelson J.; Li X.; Long N. J.; Durrant J. R. Molecular Control of Recombination Dynamics in Dye-Sensitized Nanocrystalline TiO2 Films: Free Energy vs Distance Dependence. J. Am. Chem. Soc. 2004, 126, 5225–5233. 10.1021/ja039924n. [DOI] [PubMed] [Google Scholar]
- Ardo S.; Meyer G. J. Photodriven Heterogeneous Charge Transfer with Transition-Metal Compounds Anchored to TiO2 Semiconductor Surfaces. Chem. Soc. Rev. 2009, 38, 115–164. 10.1039/B804321N. [DOI] [PubMed] [Google Scholar]
- Han Y.; Dillon R. J.; Flynn C. J.; Rountree E. S.; Alibabaei L.; Cahoon J. F.; Papanikolas J. M.; Dempsey J. L. Interfacial Electron Transfer Yields in Dye-Sensitized NiO Photocathodes Correlated to Excited-State Dipole Orientation of Ruthenium Chromophores. Can. J. Chem. 2018, 96, 865–874. 10.1139/cjc-2017-0359. [DOI] [Google Scholar]
- Odobel F.; Pellegrin Y.; Gibson E. A.; Hagfeldt A.; Smeigh A. L.; Hammarström L. Recent Advances and Future Directions to Optimize the Performances of P-Type Dye-Sensitized Solar Cells. Coord. Chem. Rev. 2012, 256, 2414–2423. 10.1016/j.ccr.2012.04.017. [DOI] [Google Scholar]
- Morandeira A.; Fortage J.; Edvinsson T.; Le Pleux L.; Blart E.; Boschloo G.; Hagfeldt A.; Hammarström L.; Odobel F. Improved Photon-to-Current Conversion Efficiency with a Nanoporous p-Type NiO Electrode by the Use of a Sensitizer-Acceptor Dyad. J. Phys. Chem. C 2008, 112, 1721–1728. 10.1021/jp077446n. [DOI] [Google Scholar]
- Swierk J. R.; Méndez-Hernández D. D.; McCool N. S.; Liddell P.; Terazono Y.; Pahk I.; Tomlin J. J.; Oster N. V.; Moore T. A.; Moore A. L.; Gust D.; Mallouk T. E. Metal-Free Organic Sensitizers for Use in Water-Splitting Dye-Sensitized Photoelectrochemical Cells. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 1681–1686. 10.1073/pnas.1414901112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooyama Y.; Harima Y. Photophysical and Electrochemical Properties, and Molecular Structures of Organic Dyes for Dye-Sensitized Solar Cells. ChemPhysChem 2012, 13, 4032–4080. 10.1002/cphc.201200218. [DOI] [PubMed] [Google Scholar]
- Kirner J. T.; Finke R. G. Water-Oxidation Photoanodes Using Organic Light-Harvesting Materials: A Review. J. Mater. Chem. A 2017, 5, 19560–19592. 10.1039/C7TA05709A. [DOI] [Google Scholar]
- Wood C. J.; Summers G. H.; Clark C. A.; Kaeffer N.; Braeutigam M.; Carbone L. R.; D’Amario L.; Fan K.; Farré Y.; Narbey S.; Oswald F.; Stevens L. A.; Parmenter C. D. J.; Fay M. W.; La Torre A.; Snape C. E.; Dietzek B.; Dini D.; Hammarström L.; Pellegrin Y.; Odobel F.; Sun L.; Artero V.; Gibson E. A. A Comprehensive Comparison of Dye-Sensitized NiO Photocathodes for Solar Energy Conversion. Phys. Chem. Chem. Phys. 2016, 18, 10727–10738. 10.1039/C5CP05326A. [DOI] [PubMed] [Google Scholar]
- Gibson E. A.; Awais M.; Dini D.; Dowling D. P.; Pryce M. T.; Vos J. G.; Boschloo G.; Hagfeldt A. Dye Sensitised Solar Cells with Nickel Oxide Photocathodes Prepared via Scalable Microwave Sintering. Phys. Chem. Chem. Phys. 2013, 15, 2411–2420. 10.1039/c2cp43592f. [DOI] [PubMed] [Google Scholar]
- Dini D.; Halpin Y.; Vos J. G.; Gibson E. A. The Influence of the Preparation Method of NiOx Photocathodes on the Efficiency of P-Type Dye-Sensitized Solar Cells. Coord. Chem. Rev. 2015, 304, 179–201. 10.1016/j.ccr.2015.03.020. [DOI] [Google Scholar]
- Lepleux L.; Chavillon B.; Pellegrin Y.; Blart E.; Cario L.; Jobic S.; Odobel F. Simple and Reproducible Procedure to Prepare Self-Nanostructured NiO Films for the Fabrication of P-Type Dye-Sensitized Solar Cells. Inorg. Chem. 2009, 48, 8245–8250. 10.1021/ic900866g. [DOI] [PubMed] [Google Scholar]
- D’Amario L.; Föhlinger J.; Boschloo G.; Hammarström L. Unveiling Hole Trapping and Surface Dynamics of NiO Nanoparticles. Chem. Sci. 2018, 9, 223–230. 10.1039/C7SC03442C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amario L.; Boschloo G.; Hagfeldt A.; Hammarström L. Tuning of Conductivity and Density of States of NiO Mesoporous Films Used in P-Type DSSCs. J. Phys. Chem. C 2014, 118, 19556–19564. 10.1021/jp504551v. [DOI] [Google Scholar]
- Mori S.; Fukuda S.; Sumikura S.; Takeda Y.; Tamaki Y.; Suzuki E.; Abe T. Charge-Transfer Processes in Dye-Sensitized NiO Solar Cells. J. Phys. Chem. C 2008, 112, 16134–16139. 10.1021/jp803919b. [DOI] [Google Scholar]
- Li F.; Fan K.; Xu B.; Gabrielsson E.; Daniel Q.; Li L.; Sun L. Organic Dye-Sensitized Tandem Photoelectrochemical Cell for Light Driven Total Water Splitting. J. Am. Chem. Soc. 2015, 137, 9153–9159. 10.1021/jacs.5b04856. [DOI] [PubMed] [Google Scholar]
- Antila L. J.; Ghamgosar P.; Maji S.; Tian H.; Ott S.; Hammarström L. Dynamics and Photochemical H2 Evolution of Dye–NiO Photocathodes with a Biomimetic FeFe-Catalyst. ACS Energy Lett. 2016, 1, 1106–1111. 10.1021/acsenergylett.6b00506. [DOI] [Google Scholar]
- van den Bosch B.; Rombouts J. A.; Orru R. V. A.; Reek J. N. H.; Detz R. J. Nickel-Based Dye-Sensitized Photocathode: Towards Proton Reduction Using a Molecular Nickel Catalyst and an Organic Dye. ChemCatChem 2016, 8, 1392–1398. 10.1002/cctc.201600025. [DOI] [Google Scholar]
- Fan K.; Li F.; Wang L.; Daniel Q.; Gabrielsson E.; Sun L. Pt-Free Tandem Molecular Photoelectrochemical Cells for Water Splitting Driven by Visible Light. Phys. Chem. Chem. Phys. 2014, 16, 25234–25240. 10.1039/C4CP04489D. [DOI] [PubMed] [Google Scholar]
- Bredar A. R. C.; Blanchet M. D.; Comes R. B.; Farnum B. H. Evidence and Influence of Copper Vacancies in P-Type CuGaO2 Mesoporous Films. ACS Appl. Energy Mater. 2019, 2, 19–28. 10.1021/acsaem.8b01558. [DOI] [Google Scholar]
- Renaud A.; Chavillon B.; Le Pleux L.; Pellegrin Y.; Blart E.; Boujtita M.; Pauporté T.; Cario L.; Jobic S.; Odobel F. CuGaO2: A Promising Alternative for NiO in p-Type Dye Solar Cells. J. Mater. Chem. 2012, 22, 14353–14356. 10.1039/c2jm31908j. [DOI] [Google Scholar]
- Powar S.; Xiong D.; Daeneke T.; Ma M. T.; Gupta A.; Lee G.; Makuta S.; Tachibana Y.; Chen W.; Spiccia L.; Cheng Y. B.; Götz G.; Bäuerle P.; Bach U. Improved Photovoltages for P-Type Dye-Sensitized Solar Cells Using CuCrO2 Nanoparticles. J. Phys. Chem. C 2014, 118, 16375–16379. 10.1021/jp409363u. [DOI] [Google Scholar]
- Yu M.; Draskovic T. I.; Wu Y. Cu(I)-Based Delafossite Compounds as Photocathodes in p-Type Dye-Sensitized Solar Cells. Phys. Chem. Chem. Phys. 2014, 16, 5026–5033. 10.1039/c3cp55457k. [DOI] [PubMed] [Google Scholar]
- Creissen C. E.; Warnan J.; Reisner E. Solar H2 Generation in Water with a CuCrO2 Photocathode Modified with an Organic Dye and Molecular Ni Catalyst. Chem. Sci. 2018, 9, 1439–1447. 10.1039/C7SC04476C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehnel M. F.; Creissen C. E.; Sahm C. D.; Wielend D.; Schlosser A.; Orchard K. L.; Reisner E. ZnSe Nanorods as Visible-Light Absorbers for Photocatalytic and Photoelectrochemical H2 Evolution in Water. Angew. Chem., Int. Ed. 2019, 58, 5059–5063. 10.1002/anie.201814265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle C.; Kumagai H.; Higashi M.; Brisse R.; Bold S.; Jousselme B.; Chavarot-Kerlidou M.; Maeda K.; Abe R.; Ishitani O.; Artero V. Earth-Abundant Molecular Z-Scheme Photoelectrochemical Cell for Overall Water-Splitting. J. Am. Chem. Soc. 2019, 141, 9593–9602. 10.1021/jacs.9b02521. [DOI] [PubMed] [Google Scholar]
- Mersch D.; Lee C. Y.; Zhang J. Z.; Brinkert K.; Fontecilla-Camps J. C.; Rutherford A. W.; Reisner E. Wiring of Photosystem II to Hydrogenase for Photoelectrochemical Water Splitting. J. Am. Chem. Soc. 2015, 137, 8541–8549. 10.1021/jacs.5b03737. [DOI] [PubMed] [Google Scholar]
- Sokol K. P.; Robinson W. E.; Warnan J.; Kornienko N.; Nowaczyk M. M.; Ruff A.; Zhang J. Z.; Reisner E. Bias-Free Photoelectrochemical Water Splitting with Photosystem II on a Dye-Sensitized Photoanode Wired to Hydrogenase. Nat. Energy 2018, 3, 944–951. 10.1038/s41560-018-0232-y. [DOI] [Google Scholar]
- Guldin S.; Hüttner S.; Kolle M.; Welland M. E.; Müller-Buschbaum P.; Friend R. H.; Steiner U.; Tetreault N. Dye-Sensitized Solar Cell Based on a Three-Dimensional Photonic Crystal. Nano Lett. 2010, 10, 2303–2309. 10.1021/nl904017t. [DOI] [PubMed] [Google Scholar]
- Shin J. H.; Kang J. H.; Jin W. M.; Park J. H.; Cho Y. S.; Moon J. H. Facile Synthesis of TiO2 Inverse Opal Electrodes for Dye-Sensitized Solar Cells. Langmuir 2011, 27, 856–860. 10.1021/la104512c. [DOI] [PubMed] [Google Scholar]
- Lee S. H. A.; Abrams N. M.; Hoertz P. G.; Barber G. D.; Halaoui L. I.; Mallouk T. E. Coupling of Titania Inverse Opals to Nanocrystalline Titania Layers in Dye-Sensitized Solar Cells. J. Phys. Chem. B 2008, 112, 14415–14421. 10.1021/jp802692u. [DOI] [PubMed] [Google Scholar]
- Lee J. W.; Lee J.; Kim C.; Cho C. Y.; Moon J. H. Facile Fabrication of Sub-100nm Mesoscale Inverse Opal Films and Their Application in Dye-Sensitized Solar Cell Electrodes. Sci. Rep. 2015, 4, 6804. 10.1038/srep06804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tétreault N.; Grätzel M. Novel Nanostructures for Next Generation Dye-Sensitized Solar Cells. Energy Environ. Sci. 2012, 5, 8506–8516. 10.1039/c2ee03242b. [DOI] [Google Scholar]
- Leung J. J.; Vigil J. A.; Warnan J.; Edwardes Moore E.; Reisner E. Rational Design of Polymers for Selective CO2 Reduction Catalysis. Angew. Chem., Int. Ed. 2019, 58, 7697–7701. 10.1002/anie.201902218. [DOI] [PubMed] [Google Scholar]
- Fang X.; Sokol K. P.; Heidary N.; Kandiel T. A.; Zhang J. Z.; Reisner E. Structure-Activity Relationships of Hierarchical Three-Dimensional Electrodes with Photosystem II for Semiartificial Photosynthesis. Nano Lett. 2019, 19, 1844–1850. 10.1021/acs.nanolett.8b04935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnan J.; Willkomm J.; Ng J. N.; Godin R.; Prantl S.; Durrant J. R.; Reisner E. Solar H2 Evolution in Water with Modified Diketopyrrolopyrrole Dyes Immobilised on Molecular Co and Ni Catalyst–TiO2 Hybrids. Chem. Sci. 2017, 8, 3070–3079. 10.1039/C6SC05219C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnan J.; Willkomm J.; Farré Y.; Pellegrin Y.; Boujtita M.; Odobel F.; Reisner E. Solar Electricity and Fuel Production with Perylene Monoimide Dye-Sensitised TiO2 in Water. Chem. Sci. 2019, 10, 2758–2766. 10.1039/C8SC05693E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M. A.; Reynal A.; Durrant J. R.; Reisner E. Versatile Photocatalytic Systems for H2 Generation in Water Based on an Efficient DuBois-Type Nickel Catalyst. J. Am. Chem. Soc. 2014, 136, 356–366. 10.1021/ja410592d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm M. L.; Stewart M. P.; Bullock R. M.; DuBois M. R.; DuBois D. L. A Synthetic Nickel Electrocatalyst with a Turnover Frequency Above 100,000 s–1 for H2 Production. Science 2011, 333, 863–866. 10.1126/science.1205864. [DOI] [PubMed] [Google Scholar]
- Shaw W. J.; Helm M. L.; DuBois D. L. A Modular, Energy-Based Approach to the Development of Nickel Containing Molecular Electrocatalysts for Hydrogen Production and Oxidation. Biochim. Biophys. Acta, Bioenerg. 2013, 1827, 1123–1139. 10.1016/j.bbabio.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Rosser T. E.; Hisatomi T.; Sun S.; Antón-García D.; Minegishi T.; Reisner E.; Domen K. La5Ti2Cu0.9Ag0.1S5O7 Modified with a Molecular Ni Catalyst for Photoelectrochemical H2 Generation. Chem. - Eur. J. 2018, 24, 18393–18397. 10.1002/chem.201801169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J. J.; Warnan J.; Nam D. H.; Zhang J. Z.; Willkomm J.; Reisner E. Photoelectrocatalytic H2 Evolution in Water with Molecular Catalysts Immobilised on p-Si via a Stabilising Mesoporous TiO2 Interlayer. Chem. Sci. 2017, 8, 5172–5180. 10.1039/C7SC01277B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong D.; Xu Z.; Zeng X.; Zhang W.; Chen W.; Xu X.; Wang M.; Cheng Y. B. Hydrothermal Synthesis of Ultrasmall CuCrO2 Nanocrystal Alternatives to NiO Nanoparticles in Efficient p-Type Dye-Sensitized Solar Cells. J. Mater. Chem. 2012, 22, 24760–24768. 10.1039/c2jm35101c. [DOI] [Google Scholar]
- Willkomm J.; Orchard K. L.; Reynal A.; Pastor E.; Durrant J. R.; Reisner E. Dye-Sensitised Semiconductors Modified with Molecular Catalysts for Light-Driven H2 Production. Chem. Soc. Rev. 2016, 45, 9–23. 10.1039/C5CS00733J. [DOI] [PubMed] [Google Scholar]
- Hoogeveen D. A.; Fournier M.; Bonke S. A.; Fang X. Y.; Mozer A. J.; Mishra A.; Bäuerle P.; Simonov A. N.; Spiccia L. Photo-Electrocatalytic Hydrogen Generation at Dye-Sensitised Electrodes Functionalised with a Heterogeneous Metal Catalyst. Electrochim. Acta 2016, 219, 773–780. 10.1016/j.electacta.2016.10.029. [DOI] [Google Scholar]
- Kamire R. J.; Majewski M. B.; Hoffeditz W. L.; Phelan B. T.; Farha O. K.; Hupp J. T.; Wasielewski M. R. Photodriven Hydrogen Evolution by Molecular Catalysts Using Al2O3-Protected Perylene-3,4-Dicarboximide on NiO Electrodes. Chem. Sci. 2017, 22, 32–57. 10.1039/C6SC02477G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré Y.; Maschietto F.; Föhlinger J.; Wykes M.; Planchat A.; Pellegrin Y.; Blart E.; Ciofini I.; Hammarström L.; Odobel F. Manuscript in preparation. [DOI] [PubMed]
- Würthner F.; Saha-Möller C. R.; Fimmel B.; Ogi S.; Leowanawat P.; Schmidt D. Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem. Rev. 2016, 116, 962–1052. 10.1021/acs.chemrev.5b00188. [DOI] [PubMed] [Google Scholar]
- Martindale B. C. M.; Hutton G. A. M.; Caputo C. A.; Reisner E. Solar Hydrogen Production Using Carbon Quantum Dots and a Molecular Nickel Catalyst. J. Am. Chem. Soc. 2015, 137, 6018–6025. 10.1021/jacs.5b01650. [DOI] [PubMed] [Google Scholar]
- Martindale B. C. M.; Joliat E.; Bachmann C.; Alberto R.; Reisner E. Clean Donor Oxidation Enhances the H2 Evolution Activity of a Carbon Quantum Dot-Molecular Catalyst Photosystem. Angew. Chem., Int. Ed. 2016, 55, 9402–9406. 10.1002/anie.201604355. [DOI] [PubMed] [Google Scholar]
- Ji Z.; He M.; Huang Z.; Ozkan U.; Wu Y. Photostable P-Type Dye-Sensitized Photoelectrochemical Cells for Water Reduction. J. Am. Chem. Soc. 2013, 135, 11696–11699. 10.1021/ja404525e. [DOI] [PubMed] [Google Scholar]
- Buesen D.; Hoefer T.; Zhang H.; Plumeré N. A Kinetic Model for Redox-Active Film Based Biophotoelectrodes. Faraday Discuss. 2019, 215, 39–53. 10.1039/C8FD00168E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketir W.; Saadi S.; Trari M. Physical and Photoelectrochemical Characterization of CuCrO2 Single Crystal. J. Solid State Electrochem. 2012, 16, 213–218. 10.1007/s10008-011-1307-x. [DOI] [Google Scholar]
- Queffélec C.; Petit M.; Janvier P.; Knight D. A.; Bujoli B. Surface Modification Using Phosphonic Acids and Esters. Chem. Rev. 2012, 112, 3777–3807. 10.1021/cr2004212. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Chien S.-C.; Sun J.; Hettiaratchy E. C.; Myers R. C.; Lin L.-C.; Wu Y. Excimer-Mediated Intermolecular Charge Transfer in Self-Assembled Donor-Acceptor Dyes on Metal Oxides. J. Am. Chem. Soc. 2019, 141, 8727–8731. 10.1021/jacs.9b03729. [DOI] [PubMed] [Google Scholar]
- Hoogeveen D. A.; Fournier M.; Bonke S. A.; Nattestad A.; Mishra A.; Bäuerle P.; Spiccia L.; Mozer A. J.; Simonov A. N. Origin of Photoelectrochemical Generation of Dihydrogen by a Dye-Sensitized Photocathode without an Intentionally Introduced Catalyst. J. Phys. Chem. C 2017, 121, 25836–25846. 10.1021/acs.jpcc.7b08067. [DOI] [Google Scholar]
- D’Amario L.; Jiang R.; Cappel U. B.; Gibson E. A.; Boschloo G.; Rensmo H.; Sun L.; Hammarström L.; Tian H. Chemical and Physical Reduction of High Valence Ni States in Mesoporous NiO Film for Solar Cell Application. ACS Appl. Mater. Interfaces 2017, 9, 33470–33477. 10.1021/acsami.7b01532. [DOI] [PubMed] [Google Scholar]
- Flynn C. J.; McCullough S. M.; Oh E.; Li L.; Mercado C. C.; Farnum B. H.; Li W.; Donley C. L.; You W.; Nozik A. J.; McBride J. R.; Meyer T. J.; Kanai Y.; Cahoon J. F. Site-Selective Passivation of Defects in NiO Solar Photocathodes by Targeted Atomic Deposition. ACS Appl. Mater. Interfaces 2016, 8, 4754–4761. 10.1021/acsami.6b01090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.