ABSTRACT
Background
The cerebellum's role in dystonia is increasingly recognized. Dystonia can be a disabling and refractory condition; deep brain stimulation can help many patients, but it is traditionally less effective in acquired dystonia. New surgical targets would be instrumental in providing treatment options and understanding dystonia further.
Objective
To evaluate the efficacy of deep brain stimulation of the cerebellum in acquired dystonia.
Methods
We report our management of a 37‐year‐old woman with severe left arm and leg dystonia, a complication of an ischemic stroke in childhood. She had already had 2 thalamotomies with only transient benefit. These procedures, in addition to her initial stroke that had damaged the basal ganglia, left traditional deep brain stimulation targets unavailable.
Results
After implantation of bilateral deep cerebellar nuclei, dystonia improved with a 40% reduction in severity on scales and subjective reports of improved posturing, gait, and pain. This improvement has been maintained for almost 2 years after implantation.
Conclusion
Cerebellar stimulation has potential for therapeutic benefit in acquired dystonia and should be further explored.
Keywords: Deep brain stimulation, dystonia, cerebellum
https://onlinelibrary.wiley.com/page/journal/23301619/homepage/mdc312876-sup-v001_1.htm
https://onlinelibrary.wiley.com/page/journal/23301619/homepage/mdc312876-sup-v002_2.htm
Although dystonia was classically thought to arise from basal ganglia pathology, increasing evidence supports a critical role of the cerebellum in its pathophysiology as well.1 Through pharmacologic or genetic alteration of cerebellar output pathways, irregular cerebellar activity leads to high‐frequency burst firing of the basal ganglia and is associated with dystonic posturing in mice.2, 3, 4 Subsequent inhibition of cerebellar outflow, either electrically or pharmacologically, reduces the abnormal basal ganglia activity and improves dystonia.3, 4 Whereas classically the cerebellum and basal ganglia communicate through rubro‐thalamo‐cortical connections, rapid modulation may occur through a more direct, disynaptic pathway, with 1 relay in the thalamus. This pathway has recently been identified in rodents and nonhuman primates5, 6, 7, 8 and, in animal models, conveys the aberrant activity that underlies dystonic posturing.3 In humans, structural abnormalities in the cerebellum or its afferent pathways have been implicated as the cause of dystonia in several cases.9, 10 Similarly, in a small autopsy study, a reduced density of cerebellar Purkinje cells was found in patients with cervical dystonia when compared with healthy controls.11
The role of the cerebellum in dystonia indicates its potential as a therapeutic target for deep brain stimulation (DBS). In mouse models of dystonia, DBS of cerebellar output nuclei improved dystonic posturing and general mobility.4 In humans, the invasive stimulation of the cerebellar hemispheres has been described previously for spasticity and dystonia with variable effect.12, 13 Recently, stimulation of the deep cerebellar nuclei has been employed for spasticity and dystonia as a result of cerebral palsy.14, 15 Identifying a new target would be clinically very useful; although DBS of the globus pallidus and subthalamic nucleus are already useful targets for the treatment for medically refractory‐isolated dystonia, stimulation may be less effective in acquired dystonia.16, 17, 18 Furthermore, DBS has limited utility when traditional targets are damaged from prior injury.19 A new target could expand management opportunities for many refractory patients and further the understanding of dystonia pathophysiology. Here we report clinical improvement after bilateral cerebellar stimulation in dystonia secondary to hypoxic ischemic injury.
Presentation
A 37‐year‐old woman presented to our center for treatment of left arm and leg dystonia. She had had a normal birth and early development with no delayed milestones. At 11 months old, she developed diabetic ketoacidosis and was diagnosed with type 1 diabetes. At 5 years of age, she developed a gastrointestinal illness, severe hyperglycemia, and suffered a brainstem stroke that caused respiratory arrest and hypoxic ischemic injury to the bilateral basal ganglia. (Fig. 1). She was comatose for 18 weeks although eventually recovered with residual left arm and leg weakness and cognitive impairment. At 7 years of age, she developed dystonic movements in her left arm and leg. Dystonia became severe enough to cause left arm dislocations from severe backward extension and hip problems from severe circumduction. Besides type 1 diabetes, she had also developed celiac disease. Her sister also had celiac disease, but otherwise her family medical history was unremarkable, with no one affected by movement disorders or other related conditions. Her dystonia was refractory to medical therapy, and she underwent 2 right thalamotomies at ages 16 and 19. Following each procedure, symptoms abated for a few years but then returned, a tendency previously reported after thalamotomy.20 By the age of 30, she was having significant functional impairment from dystonia, having had repeated falls, a left arm fracture, and hip dislocation. She also complained of severe pain in the left leg and chronic left arm cramping.
Figure 1.
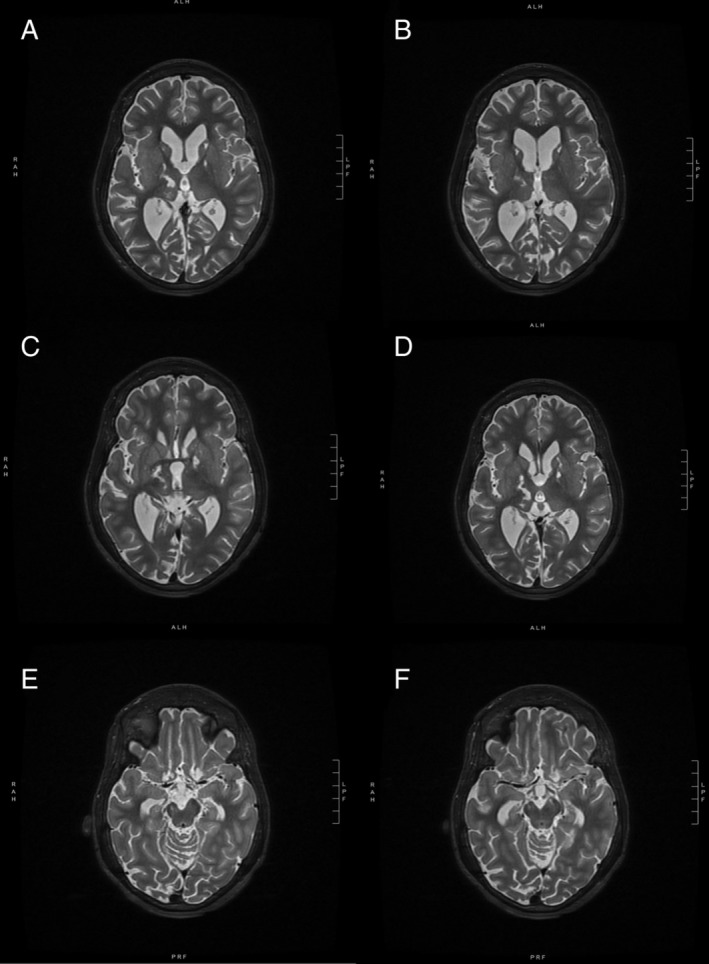
Preoperative T2 magnetic resonance imaging of the brain showed damage to multiple subcortical structures. Bilateral globus pallidus interna and caudate heads showed increased T2 signal and cystic damage thought to be from prior intervention or prior ischemic damage (A,B). Increased T2 signal is also seen in the right thalamus as a sequela of prior thalamotomies (C,D). Either because of prior intervention or ischemic damage, the right cerebellar peduncle shows atrophy, and the bilateral subthalamic nuclei are difficult to identify (E,F). Also notable is the thinning of the corpus callosum, prominent ventricles, and cerebellar and cerebral volume loss, all of which were thought to be related to either subsequent degeneration from or directly related to her initial injury.
On neurologic exam, she had normal cranial nerves and full strength throughout her right side and left leg. She had mild proximal and distal weakness in her left arm and could not extend her fingers fully, although testing was limited by dystonia and difficulty with voluntary movement; she had full passive range of motion throughout (Video S1). Her sensation was intact throughout and reflexes were 2+, although absent in her left leg. She had no clonus, plantar flexor responses in both feet, and when her dystonia was not active, normal tone throughout. She had no cerebellar abnormalities on exam. Finger and foot taps were slow on her right side although without clear decrement; she had no definite bradykinesia or tremor, although again assessment of the left arm was limited. Her right leg had occasional involuntary movements with gait, although these appeared compensatory for her imbalance. In her left arm, she had dystonia consisting of left arm abduction and pronation, backward extension, left wrist flexion, and finger curling. She had knee and hip flexion while seated and leg circumduction, knee extension, and plantar flexion with walking. Walking was also limited by frequent painful calf muscle cramping. The Burke‐Fahn‐Marsden Dystonia Rating Scale Movement Subscore (BFMDRS) was 44, and the Disability Subscore was 11. Brain imaging revealed traditional DBS lead targets for dystonia absent or too damaged to implant (Fig. 1).
Methods
Surgical Procedure
Given the limited treatment options and the potential role of the cerebellum in dystonia, we proceeded with cerebellar DBS. We implanted Medtronic 3387 leads (Medtronic, Minneapolis, MN, USA) with frame‐based stereotaxy in the prone position under general endotracheal anesthesia. Intraoperative 3D imaging (Medtronic O‐arm) was used for anatomic target confirmation. To modulate cerebellar activity, we targeted the dentate nuclei. The dentate are the major source of outflow projections from cerebellum to motor cortex,21 but also may more rapidly modulate the basal ganglia through the more direct disynaptic pathway.3, 22 Furthermore, improvement in dystonia after lesioning of the dentate has been reported in several cases.23, 24 For the contacts of the leads to traverse the dentate, the tip of each lead was targeted to the origin of the superior cerebellar peduncle. Given the location of the target and the suboccipital approach, the stereotactic frame needed to be placed much lower than usual, requiring taping of the shoulders to prevent contact with the frame. We targeted bilateral hemispheres as diffusion tensor imaging showed both decussating and nondecussating cerebello‐thalamic pathways, consistent with prior literature.25 Postoperative computerized tomography scan computationally fused with preoperative magnetic resonance imaging (Stealth Cranial 8 software, Medtronic, Minneapolis, MN, USA) confirmed the expected lead placement (Fig. 2). For further confirmation, the dentate nucleus was autosegmented (Brainlab Elements software, Brainlab, Munich, Germany) on the preoperative magnetic resonance imaging, and the lead trajectory derived from postoperative computerized tomography was superimposed on the nuclear outline (Fig. 3). Internal pulse generators (Activa SC, Medtronic, Minneapolis, MN, USA) were then placed in the pectoral area bilaterally. The patient had transient worsening of balance postoperatively but otherwise tolerated the procedure without complication.
Figure 2.
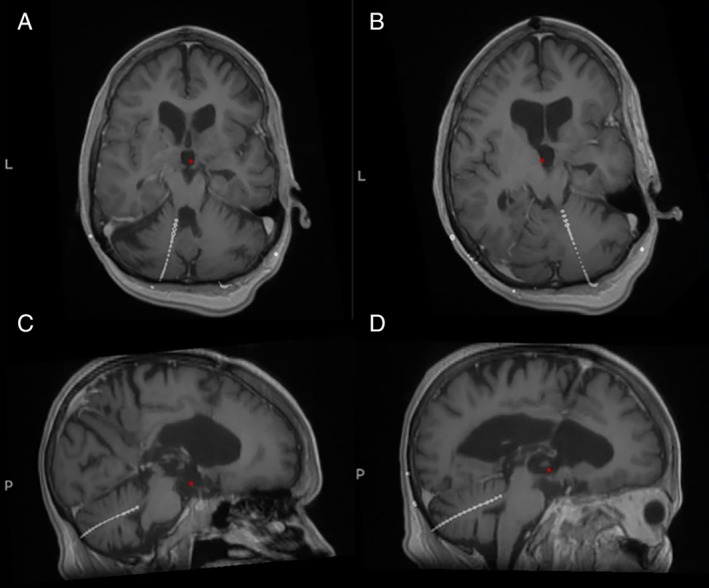
Intraoperative computerized tomography scan fused with preoperative magnetic resonance imaging show the left (A,C) and right (B,D) lead tips in the expected locations.
Figure 3.
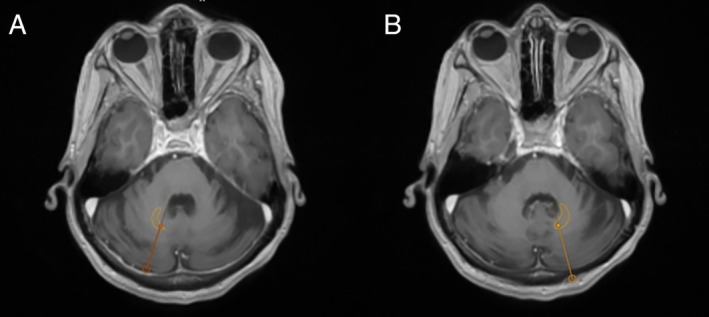
Preoperative magnetic resonance imaging of the brain with superimposed trajectories, derived from postoperative computerized tomography scan for the right (A) and left (B) leads. The dentate nucleus was autosegmented using Brainlab Elements software (Brainlab, Munich, Germany) and overlaid on the above image, showing lead projection through the nuclei.
Results
Postoperative Course
Subsequent monopolar review revealed simulation‐induced adverse effects localizable to cerebellum that occurred immediately in clinic. Distal contacts (0 and 1) on the left improved symptoms but caused titubation, appendicular ataxia, and ipsilateral leaning, whereas distal contacts on the right caused right‐sided leaning and head bobbing. Contact 3 on the right caused a language impairment consisting of reduced verbal fluency and word‐finding difficulty. The side effect threshold was highest in contacts 2 and 3 on the left, which she tolerated up to 3.5 V, beyond which stimulation was not attempted. We then lowered the stimulation, and she slowly increased the voltage at home, with some infrequent side effects (usually imbalance) that improved with decreasing stimulation. After repeated programming visits and adjustment at home, she was able to avoid stimulation‐related side effects all together. The optimal settings bilaterally were 1−, 2−, 3+, with a pulse width of 60 μs and a frequency of 130 Hz.
For several months, she increased left cerebellar stimulation at home to 2.8 V, and left leg pain and cramping gradually improved, as did dystonic posture and gait. After finding a stable setting in the left hemisphere, we turned on the right‐sided stimulation. She again slowly increased right‐sided stimulation to 1.2 V, at which point left arm posturing improved slightly (Video S2). Notably, no changes were seen on her right side, potentially because stimulation was kept relatively low. After the optimal programming settings were established, her dystonia was overall reduced in severity and was not as readily provoked. Her tone remained overall normal although at times she was slightly hypotonic, and the rest of her exam was similar to prior to surgery. She did have some residual focal areas of dystonia that were amenable to botulinum toxin injection, including left leg inversion and left toe curling. She showed improvement during the course of slow increases in stimulation over 6 months, with significantly less pain and cramping and no hip dislocation or falling. Her BFMDRS movement scale was 27.
To reevaluate benefit, we examined her OFF and ON stimulation approximately 2 years postoperatively and more than 6 months after botulinum toxin injection. With stimulation OFF for 24 hours, she developed cramping in her left leg and more circumduction with walking. These differences were supported by exam, with cramping and dystonia improving again in clinic after turning stimulation ON for 1 hour. The BFMDRS movement subscore, performed with the same rater, improved from 31 OFF stimulation to 18.5 after stimulation was ON for 1 hour, with improvement in left arm and leg dystonia and a slight improvement in neck dystonia. Her disability scale was 10.
Discussion
Previous experience in modulation of cerebellar activity in movement disorders has been mixed. Noninvasive stimulation with transcranial direct current stimulation and transcranial magnetic stimulation led to slight short‐term benefit in some studies.26, 27, 28 Long‐term improvement in dystonia has been reported after lesioning of the dentate nucleus.23, 24 Stimulation of the anterior cerebellar cortex has sometimes improved dystonic or athetoid movements in cerebral palsy, although typically a greater benefit reported in spasticity.12, 13, 29, 30, 31 Recently, implantation of electrodes targeting deep cerebellar nuclei was shown to improve dystonia and spasticity in cerebral palsy.14, 15 One study followed patients between 2 and 11 years and found improvement in dystonia by 13% to 35% according to the Unified Dystonia Rating Scale, and by >25% in half of the patients.15 Spasticity also improved from 10% to 30% in 8 patients, although the related pain did not significantly change.15
Although useful in understanding the potential role of the cerebellum in dystonia, interpretation from these cases is limited for several reasons. First, the movement disorders treated were largely mixed, involving spasticity and sometimes other hyperkinetic movement disorders besides dystonia. Second, the experience comes largely from cerebral palsy, where early perinatal injury to the brain may lead to changes in underlying brain connectivity and may limit the generalizability of stimulation. Our case demonstrates the potential efficacy of stimulation of the deep cerebellar nuclei in a predominantly dystonic phenotype compared with many previously reported cases from an injury acquired in childhood.
Stimulation in contacts 1 and 2, where we would expect the dentate nuclei to be located, had the best therapeutic response. Stimulation may act by modulating the aforementioned disynaptic pathway, proposed to involve a purely subcortical connection from cerebellum to thalamus to basal ganglia (although its presence has not been definitively demonstrated in humans). In nonhuman primate and human studies, the dentate nuclei contribute most of the cerebellum's direct output to this pathway.6, 7
On the other hand, modulation of well‐recognized cerebello‐thalamo‐cortical loops may be responsible for the therapeutic effect. The benefit of bilateral stimulation favors this anatomy: although the patient's left‐sided symptoms improved with stimulation of the ipsilateral cerebellum, her symptoms continued to improve with contralateral cerebellar stimulation, suggesting the presence of both a decussating and nondecussating pathway. The disynaptic pathway from the cerebellum to thalamus to basal ganglia has only been shown to project contralaterally,6, 7 but dentato‐rubro‐thalamic pathways project both ipsilaterally and contralaterally, with the nondecussating pathway contributing approximately 20% of the thalamic input.25 This bilateral projection was suggested by diffusion tensor imaging in our case. Bilateral stimulation of the cerebellum should therefore be considered for future procedures, even if symptoms are unilateral.
Although our case shows the potential for improving acquired dystonia with cerebellar stimulation, these results should be interpreted with some caution. Our patient did have altered anatomy as a result of her prior brain injury, although the cerebellum appeared spared from damage on magnetic resonance imaging. Importantly, the generalizability to idiopathic dystonia is unclear. For example, the time course of the stimulation's benefit in her case (with some improvement in symptoms after even 1 hour) was shorter than the benefit often seen in idiopathic dystonia, where clinical improvement occurs over weeks to months.32 The relatively rapid onset of improvement in this case may indicate a different underlying mechanism in acquired dystonia as opposed to idiopathic dystonia or utilization of a different pathway from the cerebellar target. On the other hand, the rapid benefit in this case may be indicative of an incomplete washout from her stimulation being off for 24 hours.
Finally, this is a single case, and the phenomenology, severity, and cause of dystonia is variable between patients. Even within 1 patient, dystonia can fluctuate, and neither the patient nor the raters were blinded to treatment in this case. On the other hand, the subjective improvement was sustained, and the ratings, if anything, may have underestimated her improvement: her OFF stimulation score at 2 years was not as high as preoperatively, and the timeline of stimulation washout in cerebellar stimulation is unknown. Furthermore, the BFMDRS does not capture pain, which improved significantly in her case. Overall, given how disabling and refractory to treatment dystonia can be, further exploration of the therapeutic benefit of cerebellar stimulation is warranted.
Author Roles
(1) Surgical Intervention: A. Conception and Planning of the Procedure, B. Execution of the Procedure; (2) Clinical Evaluation: A. Analysis of Clinical Response; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
E.G.B.: 2A, 3A, 3B
I.O.B.: 2A, 3B
N.S.L.: 1A, 2A, 3B
S.M.: 1A, 2A, 3B
P.A.S.: 1A, 1B, 2A, 3B
J.L.O.: 1A, 2A, 3B
Disclosures
Ethical Compliance Statement
The authors confirm that the approval of an institutional review board was not required for this work. Patient informed consent was obtained. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest
The authors have no financial disclosures relevant to this study.
Financial Disclosures for the Previous 12 Months
E.G.B. receives research support from the Michael J. Fox Foundation, Biogen Inc, and the Gateway Institute for Brain Research Inc and has received consulting fees from NEJM Knowledge+, Oscar Health, and Rune Labs Inc. I.O.B. has received consulting fees from Bagatto Inc and Biogen Inc and honoraria from the American Academy of Neurology. P.A.S. receives research support from Medtronic Inc and Boston Scientific Inc. J.L.O. receives research support from the Michael J. Fox Foundation, Boston Scientific Inc, training grant support from Boston Scientific Inc and Medtronic Inc and grant support for a clinical trial from Biogen Inc and Cala Health Inc and has received consulting fees from Medtronic Inc and Acadia Pharmaceuticals Inc. N.S.L. and S.M. have nothing to disclose.
Supporting information
Video S1. Demonstration of dystonia prior to deep brain stimulation implantation and postimplantation but prestimulation (see video labels). Dystonic posturing of the left arm and leg is evident with sitting, and interference with gait is also apparent.
Video S2. Demonstration of poststimulation changes, with video segments 7 months and 23 months after surgery. See video labels for programming settings. Dystonia is still present but reduced in severity; arm appears more loose and cramping with walking is less frequent.
Acknowledgments
The authors are grateful for the assistance of Coralie De Hemptinne and Andrew Conner with figure preparation.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Tewari A, Fremont R, Khodakhah K. It's not just the basal ganglia: cerebellum as a target for dystonia therapeutics. Mov Disord 2017;32(11):1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calderon DP, Fremont R, Kraenzlin F, Khodakhah K. The neural substrates of rapid‐onset Dystonia‐Parkinsonism. Nat Neurosci 2011;14(3):357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen CH, Fremont R, Arteaga‐Bracho EE, Khodakhah K. Short latency cerebellar modulation of the basal ganglia. Nat Neurosci 2014;17(12):1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. White JJ, Sillitoe RV. Genetic silencing of olivocerebellar synapses causes dystonia‐like behaviour in mice. Nat Commun 2017;8:14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ichinohe N, Mori F, Shoumura K. A di‐synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res 2000;880(1–2):191–197. [DOI] [PubMed] [Google Scholar]
- 6. Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci 2005;8(11):1491–1493. [DOI] [PubMed] [Google Scholar]
- 7. Pelzer EA, Hintzen A, Goldau M, et al. Cerebellar networks with basal ganglia: feasibility for tracking cerebello‐pallidal and subthalamo‐cerebellar projections in the human brain. Eur J Neurosci 2013;38(8):3106–3114. [DOI] [PubMed] [Google Scholar]
- 8. Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A 2010;107(18):8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. LeDoux MS, Brady KA. Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord 2003;18(1):60–69. [DOI] [PubMed] [Google Scholar]
- 10. Batla A, Sanchez MC, Erro R, et al. The role of cerebellum in patients with late onset cervical/segmental dystonia?—Evidence from the clinic. Parkinsonism Relat Disord 2015;21(11):1317–1322. [DOI] [PubMed] [Google Scholar]
- 11. Prudente CN, Pardo CA, Xiao J, et al. Neuropathology of cervical dystonia. Exp Neurol 2013;241:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cooper IS, Upton ARM. Use of chronic cerebellar stimulation for disorders of inhibition. The Lancet 1978;1(8064):595–600. [DOI] [PubMed] [Google Scholar]
- 13. Fraioli B, Baldassarre L, Refice GM. Chronic paleocerebellar stimulation in dystonia and athetosis. Report of two cases. J Neurosurg Sci 1980;24(2):99–103. [PubMed] [Google Scholar]
- 14. Galanda M, Horvath S. Stereotactic stimulation of the anterior lobe of the cerebellum in cerebral palsy from a suboccipital approach. Acta Neurochirurgica Suppl 2007;97(2):239–243. [DOI] [PubMed] [Google Scholar]
- 15. Sokal P, Rudas M, Harat M, Szylberg L, Zielinski P. Deep anterior cerebellar stimulation reduces symptoms of secondary dystonia in patients with cerebral palsy treated due to spasticity. Clin Neurol Neurosurg 2015;135:62–68. [DOI] [PubMed] [Google Scholar]
- 16. Eltahawy HA, Saint‐Cyr J, Giladi N, Lang AE, Lozano AM. Primary dystonia is more responsive than secondary dystonia to pallidal interventions: outcome after pallidotomy or pallidal deep brain stimulation. Neurosurgery 2004;54(3):613–621. [DOI] [PubMed] [Google Scholar]
- 17. Badhiwala JH, Karmur B, Elkaim LM, et al. Clinical phenotypes associated with outcomes following deep brain stimulation for childhood dystonia. J Neurosurg Pediatr 2019;1–9. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18. Vidailhet M, Jutras MF, Grabli D, Roze E. Deep brain stimulation for dystonia. J Neurol Neurosurg Psychiatry 2013;84(9):1029–1042. [DOI] [PubMed] [Google Scholar]
- 19. Witt J, Starr PA, Ostrem JL. Use of pallidal deep brain stimulation in postinfarct hemidystonia. Stereotact Funct Neurosurg 2013;91(4):243–247. [DOI] [PubMed] [Google Scholar]
- 20. Yoshor D, Hamilton WJ, Ondo W, Jankovic J, Grossman RG. Comparison of thalamotomy and pallidotomy for the treatment of dystonia. Neurosurgery 2001;48:818–826. [DOI] [PubMed] [Google Scholar]
- 21. Dum RP, Li C, Strick PL. Motor and nonmotor domains in the monkey dentate. Ann N Y Acad Sci 2002;978:289–301. [DOI] [PubMed] [Google Scholar]
- 22. Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci 2018;19(6):338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zervas NT. Long‐term review of dentatectomy in dystonia musculorum deformans and cerebral palsy. Acta Neurochir 1977;S24:49–51. [DOI] [PubMed] [Google Scholar]
- 24. Teixeira M, França C, Andrade D, et al. Long‐term outcome of dentatotomy in a dystonic patient. Brazil Neurosurg 2016;35(4):307–309. [Google Scholar]
- 25. Meola A, Comert A, Yeh FC, Sivakanthan S, Fernandez‐Miranda JC. The nondecussating pathway of the dentatorubrothalamic tract in humans: human connectome‐based tractographic study and microdissection validation. J Neurosurg 2016;124(5):1406–1412. [DOI] [PubMed] [Google Scholar]
- 26. Franca C, de Andrade DC, Teixeira MJ, et al. Effects of cerebellar neuromodulation in movement disorders: a systematic review. Brain Stimul 2018;11(2):249–260. [DOI] [PubMed] [Google Scholar]
- 27. Bradnam LV, McDonnell MN, Ridding MC. Cerebellar intermittent theta‐burst stimulation and motor control training in individuals with cervical dystonia. Brain Sci 2016;6(4):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koch G, Porcacchia P, Ponzo V, et al. Effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stimul 2014;7(4):564–572. [DOI] [PubMed] [Google Scholar]
- 29. Cooper IS, Riklan M, Amin I, Waltz JM, Cullinan T. Chronic cerebellar stimulation in cerebral palsy. Neurology 1976;26:744–753. [DOI] [PubMed] [Google Scholar]
- 30. Cooper IS, Upton ARM, Amin I. Chronic cerebellar stimulation (CCS) and deep brain stimulation (DBS) in involuntary movement disorders. Appl Neurophysiol 1982;45(3):209–217. [DOI] [PubMed] [Google Scholar]
- 31. Davis R. Cerebellar stimulation for cerebral palsy spasticity, function, and seizures. Arch Med Res 2000;31(3):290–299. [DOI] [PubMed] [Google Scholar]
- 32. Tisch S, Limousin P, Rothwell JC, et al. Changes in forearm reciprocoal inhibition following pallidal stimulation for dystonia. Neurology 2006;66:1091–1093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Demonstration of dystonia prior to deep brain stimulation implantation and postimplantation but prestimulation (see video labels). Dystonic posturing of the left arm and leg is evident with sitting, and interference with gait is also apparent.
Video S2. Demonstration of poststimulation changes, with video segments 7 months and 23 months after surgery. See video labels for programming settings. Dystonia is still present but reduced in severity; arm appears more loose and cramping with walking is less frequent.


