Abstract
Purpose of review
Cannabis (marijuana, weed, pot, ganja, Mary Jane) is the most commonly used federally illicit drug in the United States. The present review provides an overview of cannabis and cannabinoids with relevance to the practice of nephrology so that clinicians can best take care of patients.
Recent findings
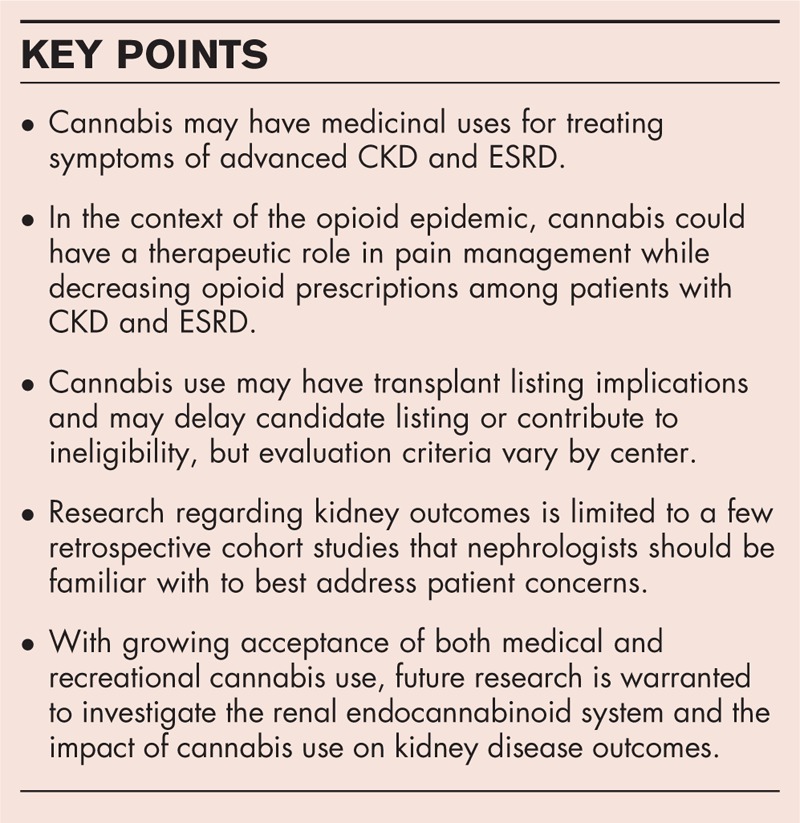
Cannabis may have medicinal benefits for treating symptoms of advanced chronic kidney disease (CKD) and end-stage renal disease including as a pain adjuvant potentially reducing the need for opioids. Cannabis does not seem to affect kidney function in healthy individuals. However, renal function should be closely monitored in those with CKD, the lowest effective dose should be used, and smoking should be avoided. Cannabis use may delay transplant candidate listing or contribute to ineligibility. Cannabidiol (CBD) has recently exploded in popularity. Although generally well tolerated, safe without significant side effects, and effective for a variety of neurological and psychiatric conditions, consumers have easy access to a wide range of unregulated CBD products, some with inaccurate labeling and false health claims. Importantly, CBD may raise tacrolimus levels.
Summary
Patients and healthcare professionals have little guidance or evidence regarding the impact of cannabis use on people with kidney disease. This knowledge gap will remain as long as federal regulations remain prohibitively restrictive towards prospective research.
Keywords: cannabidiol, kidney, marijuana, nephrology, renal
INTRODUCTION
Cannabis (marijuana, weed, pot, ganja, Mary Jane; Fig. 1) is the most commonly used federally illicit drug in the United States. As of December 2019, 33 states and the District of Columbia have medical cannabis programs. Eleven states and the District of Columbia have legalized recreational use. Several countries worldwide have legalized recreational use whereas many others have medical cannabis and decriminalization laws. The prevalence of cannabis use more than doubled between 2001 and 2013 in the United States [1] particularly among people over the age of 50 and even more so among those over 65 years [2▪,3,4▪,5▪]. These age groups are enriched with chronic illness including chronic kidney disease (CKD) that is associated with excess morbidity and mortality [6].
FIGURE 1.
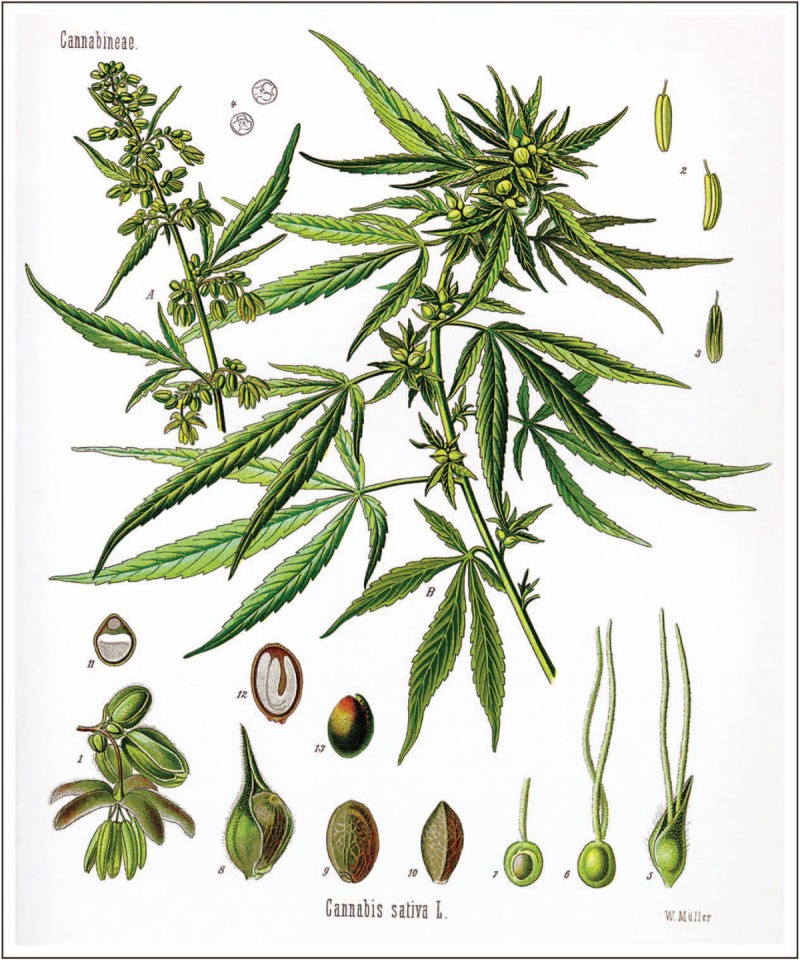
Cannabis sativa W.O.Müll. (A) flowering male and (B) seed-bearing female plant, actual size; (1) male flower, enlarged detail; (2) and (3) pollen sac of same from various angles; (4) pollen grain of same; (5) female flower with cover petal; (6) female flower, cover petal removed; (7) female fruit cluster, longitudinal section; (8) fruit with cover petal; (9) same without cover petal; (10) same; (11) same in cross-section; (12) same in longitudinal section; (13) seed without hull. From Franz Eugen Köhler's Medizinal-Pflantzen. Published and copyrighted by Gera-Untermhaus, FE Köhler in 1887 (1883–1914). Original figure is now in the public domain. https://commons.wikimedia.org/wiki/File:Cannabis_sativa_Koehler_drawing.jpg.
Cannabis is the dried flower bud of the Cannabis sativa and Cannabis indica plants, and naturally contains numerous phytocannabinoids. Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are the most abundant and well described phytocannabinoids, with differing activities and affinities for the ubiquitously expressed Gi/o-protein-coupled cannabinoid receptors CB1 and CB2. THC is the primary psychoactive component of cannabis and is a partial agonist to CB1 and CB2 receptors. In contrast, CBD is nonintoxicating and has little affinity for these receptors but acts as a negative allosteric modulator of CB1 with pharmacological effects on other receptor systems including GPR55, TRPV1, 5-HT1A, adenosine A2A, and nonreceptor mechanisms [7]. Plant breeding has created numerous genetically unique Cannabis chemovars, enhancing certain desired effects. For example, chemovars with a higher concentration of THC are selectively produced for recreational use, because THC activation of CB1 mediates the psychotropic effects of cannabis, whereas medical cannabis generally has higher CBD levels than recreational chemovars, often even exceeding the THC content. In fact, symptom relief may be obtained with THC doses lower than what is needed to induce psychotropic effects. Endogenous cannabinoids are eicosanoids derived from cell membrane phospholipids. The two primary endocannabinoids are anandamide/N-arachidonoylethanolamine and 2-arachidonoylglycerol, which are the natural ligands for the cannabinoid receptors. The endocannabinoid system is present in many tissues including the kidney where it has been shown to influence renal blood flow [8,9], glomerular filtration rate [10], fibrosis [11–13], proteinuria [14–21], and tubular function [22–27]. The endocannabinoid system has been comprehensively reviewed elsewhere [28,29▪,30] including specific interactions with the kidney [31,32,33▪,34▪,35–38]. Whole cannabis contains numerous cannabinoid compounds with different affinities, making the predicted cumulative effect on cannabis receptors, and potential renal effects difficult to predict.
Physicians remain poorly educated with respect to cannabis and the endocannabinoid system [39,40▪]. The federal stigma against cannabis in the United States, leading up to the Marihuana Tax Act of 1937 and the Controlled Substances Act of 1970, have strongly limited research and prevented teaching about the drug in medical education. State legalized consumption of cannabis is in conflict with federal law where it remains a Schedule I controlled substance without accepted medical use and a high potential for abuse. Despite this, the World Health Organization classifies CBD as having no potential for abuse [41] and several oral cannabinoid-based pharmaceuticals are U.S. Food and Drug Administration (FDA) approved, having demonstrated efficacy in treating certain medical conditions. Cannabis derived CBD (Epidiolex) is an FDA approved medication for pediatric epilepsy whereas synthetic THC is FDA approved as dronabinol (Marinol, Syndros), and a synthetic THC analogue as nabilone (Cesamet). The cannabis extract nabiximols (Sativex, THC/CBD 1:1) is approved for medical use outside of the United States.
Box 1.
no caption available
CANNABIS AND CANNABINOIDS
Cannabis can be home grown or purchased from retail and medical dispensaries, dependent on the jurisdiction. Cannabis contains over 200 phytocannabinoids, terpenoids, and flavonoids that may act in concert, described as the ‘entourage effect’, so that the combination of plant components act synergistically to be more efficacious than the individual isolated compounds [42,43]. Cannabis can be consumed as dried flower bud through smoking burned plant material or heated in a vaporizer to the vaporizing points for the various cannabinoids (311°F--428°F) without burning the plant and generating smoke. Recently, electronic cigarettes and vape pens have become a popular means to inhale heated aerosol from a concentrated oil containing cannabinoids and/or nicotine. Unfortunately, some THC oil concentrates from illicit manufacturers/underground vape-makers have been associated with fatal lung injury attributed to inhalation of chemical irritants [44▪,45], including vitamin E acetate, used as an oil diluent [46].
Cannabis and isolated cannabinoids can also be processed into foods or ‘edibles’. Over 90% of current users consume cannabis through inhalation whereas oral consumption accounts for less than 10% of cannabis use [47]. Inhalation provides an onset of action within minutes and allows for real-time dose titration. Peak effects are seen within 15–30 min with a half-life of 1–2 h. After oral consumption, the onset of action may be delayed up to 1–2 h, with peak effects at 2–3 h and half-life of 3–6 h. Inexperienced users who do not feel an effect right away may be tempted to overconsume innocuous appearing edible preparations that can lead to drug accumulation and prolonged adverse side effects. For this reason, edibles have been associated with higher rates of emergency room visits, primarily for acute psychiatric symptoms, intoxication, and cardiovascular symptoms [48▪]. Cannabinoids are highly lipophilic and bioavailability is increased with high fat intake compared to consumption on an empty stomach [49]. THC and CBD may interact with the metabolism of prescription medications [50]. CBD is metabolized by CYP3A4 and CYP2C19 with a growing body of evidence suggesting it is also a potent inhibitor of these pathways [51,52] including CYP2C9 [53] and CYP2D6 [54]. The clinical relevance of these interactions is largely unknown. THC has a large volume of distribution (Vd) with slow elimination from the body. Cannabinoids are primarily cleared by the liver and the minority of inactive metabolites are excreted in the urine, accounting for 20% of metabolite elimination. Terminal half-life varies based on frequency of use: several days in infrequent users to over 1 month in heavy chronic users. Cannabinoid pharmacokinetics have been comprehensively reviewed [55,56▪,57]. Evidence regarding the pharmacokinetics of cannabinoids in people with impaired kidney function is scarce. The only published study evaluated 200mg of oral CBD and found no statistically significant differences in maximum measured plasma concentration (Cmax), time to Cmax, area under the plasma concentration–time curve (AUC) from time zero to last measurable concentration, or AUC from time zero to infinity values between participants with severe renal impairment (mean eGFR ∼22 ml/min/1.73 m2) and normal renal function [58▪]. Given the primary hepatic metabolism, dose adjustments are unlikely to be needed. The large Vd for THC and high protein binding [57] suggest limited clearance with hemodialysis.
Dosing recommendations for cannabis and cannabinoid preparations have been previously published [59▪]. Almost 90% of current adult cannabis use is in part or entirely for recreational reasons and slightly less than half is in part or entirely for medicinal purposes [47]. CBD-infused oils, tinctures, creams, food items, and drinks, along with a host of other products, have exploded in popularity since the passage of the Agriculture Improvement Act of 2018 (2018 Farm Bill), which removed hemp (Cannabis sativa with <0.3% THC) from the Controlled Substances Act and legalized its domestic agricultural production as a commodity. CBD is sold in health food stores, retail shops, dispensaries, pharmacies, convenience stores, and on the internet. Already, 14% of Americans use CBD, primarily for pain, anxiety, insomnia, and arthritis [60]. CBD is well tolerated, safe, and effective for a variety of neurological and psychiatric conditions [61–63,64▪], although at high doses, CBD may increase liver enzymes and interact with some prescription medications [65▪]. Consumers have easy access to a wide range of unregulated CBD products with inaccurate labeling and false health claims. A clinical guide on the therapeutic actions and safety of CBD and hemp oils has been recently published [66▪]. In addition to CBD isolate, hemp extract may be marketed as ‘full spectrum’, which contains whole plant extract and a variety of compounds. Hemp seed oils are also sold but do not contain any phytocannabinoid compounds.
There is no evidence to suggest that CBD has any adverse effect on kidney function. In fact, CBD prevented cisplatin induced nephrotoxicity in a mouse model by reducing oxidative stress [67]. However, some products may contain toxic contaminants such as heavy metals, pesticides, and solvents. A study of 84 CBD products sold online found that 42% of products contained more CBD than stated on the label, 26% were overlabeled, whereas only 31% contained the stated amount [68]. Additionally, 20% of these products were contaminated with THC that could potentially be detected on a urine toxicology screen. Some products do not contain a sufficient quantity of CBD to achieve pharmacological activity.
Consumers should scrutinize labels, ensure that the product has been made with good manufacturing practice, ensure cannabinoid extraction using carbon dioxide, ensure organic certification by the U.S. Department of Agriculture, and purchase from a certified medical dispensary or company that has a certificate of analysis. If CBD is regularly consumed, careful monitoring of clinical parameters and drug interactions is warranted.
SYMPTOMS ASSOCIATED WITH CHRONIC KIDNEY DISEASE AND END-STAGE RENAL DISEASE
Over 1.5 million people with advanced CKD and about 750 000 people with end-stage renal disease (ESRD) live in the United States [6]. One-quarter to one-half of patients with CKD experience chronic symptoms such as pain, nausea, anorexia, sleep disturbance, anxiety, and depression [69], several of which are approved indications for medical cannabis. In addition, anxiety, depression, and insomnia are the most common psychiatric conditions that people self-treat with cannabis [70]. Evidence supports the use of cannabis in patient populations without CKD for treating several of these symptoms including chronic pain, nausea, and loss of appetite. The rationale for its use in patients with CKD and ESRD has been previously reviewed by myself and others [71,72,73▪].
Pain prevalence among patients with CKD and ESRD is as high as 50% [74]. Pain attributed to kidney disease occurs from polycystic kidney disease, renal colic from nephrolithiasis, renal osteodystrophy, or uremic neuropathy. Historically, cannabis has been recommended for a wide range of ailments including as a spasmolytic for cases of renal colic and to facilitate the excretion of small kidney stones [75].
Over 60% of dialysis patients receive at least one opioid prescription annually and approximately 20% of them take prescription opioids chronically [76]. Both short-term and chronic use of prescription opioids are associated with increased morbidity and mortality [76,77]. Cannabis could have a therapeutic role in pain management that deserves clinical consideration and further clinical trial investigation. Access to medical cannabis has been associated with decreased opioid prescriptions and dose reductions [78–82,83▪]. The National Academies concluded that substantial evidence exists for the use of cannabis and cannabinoids to treat chronic pain [84] while meta-analyses and systematic reviews of cannabis use, including prescription cannabinoids, have given mixed results for treating chronic pain [85–87].
IMPAIRED KIDNEY FUNCTION
Recreational cannabis is most often smoked (>90%) [47,88] and generally contains higher THC content whereas medical cannabis is vaporized or consumed orally and often has a higher CBD content. Medical cannabis programs in several states only allow for edibles and vaporizers and do not allow smoking. Existing research regarding cannabis is biased towards recreational cannabis consumed by smoking prior to or early into state legalization programs.
Among participants with an estimated glomerular filtration rate (eGFR) less than 60 ml/min/1.73 m2) in the multicenter Assessment, Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury (ASSESS-AKI) study self-reported chronic cannabis usage was associated with more rapid eGFR decline compared to those with an eGFR more than 60 ml/min/1.73 m2 over a median 4.1 years [89]. Cannabis usage was not associated with changes in albuminuria over time. Conversely, sicker patients who may already have progressive CKD may be more inclined to use medical cannabis for symptom management. An analysis of the Chronic Renal Insufficiency Cohort (CRIC) Study from 2003 to 2008 among 3939 adults with baseline eGFR between 20 and 70 ml/min/1.73 m2 did not demonstrate an association between cannabis use and CKD progression over 5.5 years of follow-up [90▪].
Among healthy individuals, analysis of the Coronary Artery Risk Development in Young Adults (CARDIA) Study did not demonstrate a longitudinal association between cannabis use and eGFR change, rapid eGFR decline, or prevalent albuminuria after 15 years of follow-up in 3765 participants [91]. Past or current cannabis use was reported by 83% of participants. The CARDIA study began in 1988 and at that time, cannabis had lower THC content and a lower THC/CBD ratio than current levels. Similar findings were revealed in a cross-sectional analysis of 13 995 adults aged 18–59 years in the nationally representative National Health and Nutrition Examination Survey (NHANES) from 2007 to 2014 that did not find a clinically significant effect of self-reported past or current cannabis use on serum creatinine, eGFR, microalbuminuria, or stage 2 or higher CKD [92].
Renal function in cannabis users with CKD should be closely monitored, the lowest effective dose should be used, and smoking should be avoided. It is currently unknown if other routes of administration attenuate kidney risk but they at least avoid potential pulmonary complications.
ACUTE KIDNEY INJURY
Synthetic cannabinoids are potent CB1 agonists originally developed as research compounds but have emerged on the marketplace as popular and potentially dangerous recreational drugs referred to as ‘spice’ or ‘K2’. Numerous cases during 2012 have linked synthetic cannabinoids to acute kidney injury (AKI) [93–95]. Specifically, the synthetic cannabinoid XLR-11 has been identified as a nephrotoxic compound [95] possibly related to effects on proximal tubule mitochondrial function [96]. Synthetic cannabinoids may be nephrotoxic, but a noncannabinoid contaminant has been proposed as an alternative explanation [97,98]. Nausea, vomiting, and flank pain are common in the majority of cases. Kidney biopsy most often demonstrates acute tubular necrosis with some cases of acute interstitial nephritis [99]. Synthetic cannabinoids are not detected on standard blood and urine toxicology screens. Therefore, nephrologists should have a high index of suspicion when diagnosing unexplained AKI.
Cannabinoid hyperemesis syndrome (CHS) [100] is a rare complication of heavy and frequent cannabis use over many years characterized by intractable vomiting that is relieved with hot showers. CHS is occasionally associated with prerenal AKI [101–106], treated with intravenous fluids and antiemetics. Interestingly, hypophosphatemia was observed in a case series of 6 men with CHS [107].
KIDNEY TRANSPLANTATION
CB2 is widely expressed on immune cells and cannabinoids have immunomodulatory effects in animal models of allogeneic transplantation and autoimmune diseases [108–111]. As such, cannabis and cannabinoids may have immunomodulatory effects among kidney transplant recipients. Interestingly, a study of routine kidney transplant biopsies revealed significant upregulation of glomerular and tubular CB1 expression in those with chronic allograft dysfunction compared to low levels in normal kidney allografts, suggesting a role for CB1 in allograft fibrosis [112].
Cannabis use in potential transplant recipients may have implications for pretransplant screening, such as delayed candidate listing or contributing to ineligibility [113▪], with implications for posttransplant outcomes. There is concern regarding adherence to immunosuppressive medications, the ability to follow instructions, and attendance of follow-up appointments. An American Society of Transplantation survey revealed that about half of transplant centers vary in their policy according to the organ, whereas slightly more than one-quarter of centers rejected all candidates regardless of organ [114▪]. Drug screening of potential transplant donors and recipients should consider the prolonged excretion of THC metabolites which may range from a few days in casual users to several weeks to over 1 month with chronic heavy use.
A retrospective single center study of kidney transplant candidates revealed that cannabis abuse and dependence were associated with a high prevalence of other substance use disorders, psychiatric comorbidities, and strong family histories of addictions, resembling other substance use populations that generally adversely affect kidney graft outcomes [115]. A study of a national kidney transplant database demonstrated that cannabis dependence or abuse (CDOA) in the year before transplant was not associated with death or graft failure in the year after transplant, but was associated with posttransplant psychosocial problems such as alcohol abuse, other drug abuse, noncompliance, schizophrenia, and depression [116▪]. CDOA after kidney transplantation was associated with cardiovascular, pulmonary, psychosocial complications, accidents, and fractures. Accordingly, CDOA was associated with an approximately two-fold increased risk of death-censored graft failure, all-cause graft loss, and death in the subsequent 2 years. Although associations likely, in part, reflect comorbid conditions or behaviors, CDOA after kidney transplantation appears to have consequences for allograft and patient outcomes. Additionally, patients who carry a formal diagnosis of CDOA likely reflect ‘extreme’ users who were not able to hide their use and raised suspicion. Data from patients with CDOA cannot be generalized to all as cannabis does not have adverse effects on life in everyone [117▪]. A single center study of 56 cannabis users out of 1225 kidney recipients from 2008 to 2013 demonstrated that recreational cannabis use, defined by positive urine toxicology screen and/or self-reported recent use, did not affect mortality, graft loss, or graft function 1-year posttransplant [118]. Finally, a single center study of 919 kidney transplant recipients from 2001 to 2015 revealed that smoking status was not significantly associated with acute rejection, eGFR, or pneumonia within 1-year posttransplant. Patients with isolated cannabis use had similar overall graft survival compared to nonusers [119▪].
With regards to kidney donation, a retrospective single center study of 294 living kidney donors and 230 recipients between 2000 and 2016 showed that donor cannabis use did not demonstrate any deleterious effects on donor or recipient posttransplantation eGFR over a mean follow up of 2.1 years for donors and 5.2 years for recipients [120▪]. Among recipients of a kidney from a cannabis user, the rates of acute rejection, graft, and patient survival of the kidney allografts were similar to those from nonusers.
Although rare, fungal contamination of cannabis and pulmonary complications have been reported among kidney transplant recipients, including pulmonary aspergillosis associated with smoking cannabis [121,122] and exogenous lipid pneumonia secondary to smoking weed oil [123]. These cases have occurred prior to current cannabis legalization where microbial testing has become a regulatory requirement in the medical and recreational cannabis markets. Although sterilization of cannabis buds can eliminate fungi and may eliminate the risk of fatal opportunistic infections among immunosuppressed individuals [121,124], several toxigenic fungi and bacteria have been detected in cannabis samples [125,126]. Based on existing evidence, cannabis usage alone should not be the sole deciding factor for declining a patient for kidney transplant listing.
Several case reports demonstrate increased tacrolimus levels associated with CBD in a patient with interstitial nephritis and in non-kidney transplant recipients [127▪,128,129], whereas one small case series of low dose CBD for chronic pain among kidney transplant recipients did not reveal any change in tacrolimus levels [130]. Inaccurate product labeling and batch to batch variability of CBD products [68] may lead to unpredictable CNI levels, potential toxicity, or underdosing, especially with intermittent use of different cannabinoid products. Furthermore, CBD inhibits hepatic cyclosporine metabolism in vitro and in mice [131].
MEDICAL RISKS AND COMPLICATIONS
The public health impact of state legalization of cannabis remains unclear and these policies may have contributed to the increasing perception that cannabis is harmless. In fact, more than one-third of U.S. adults strongly or somewhat strongly agree that edible cannabis prevents health problems and more than a quarter strongly or somewhat strongly agree that smoking or vaping cannabis prevents health problems [132▪▪]. Medical cannabis as an option for those with CKD or ESRD, will include people who are older, frailer, and have more comorbid conditions and co-medications, potentially increasing susceptibility to adverse effects. The most common side effects are dizziness and dry mouth.
Smoking is associated with increased mortality among people with CKD and ESRD [133]. Although smoked cannabis is a source of oxidative stress to the respiratory tract [134] and associated with bronchial irritation and chronic bronchitis [135], regular heavy use is associated with lower risk for pulmonary complications compared to tobacco use [136,137]. A systematic review and meta-analysis demonstrated low-strength evidence that smoking cannabis more than once per week for at least 1 year was associated with cough, sputum production, and wheezing while evidence regarding cannabis use and obstructive lung disease and pulmonary function was insufficient [138▪▪].
CKD and ESRD are associated with increased cardiovascular morbidity and mortality [139]. Endocannabinoids are involved in various functions of the cardiovascular system including blood pressure regulation [140–142]. Some observational studies suggest a higher incidence of cardiovascular events with cannabis exposure, [143–145] while other studies do not [146,147]. Low strength evidence suggests that cannabis use is associated with tachycardia [148▪]. Acutely, cannabis may cause orthostatic hypotension whereas long-term cannabis use may be associated with a modest increase in systolic blood pressure [149]. Systematic reviews do not reveal any hemodynamic effects of either CBD [150] or THC [151].
Cognitive impairment is common among people with CKD and ESRD [152–154]. Acute cannabis usage may cause sedation and impair spatial-visual distortion while acute and long-term cannabis use can impair verbal learning, memory, and attention [155]. However, a comprehensive review of recreational cannabis and cognitive function revealed inconsistent findings across studies [156▪].
CONCLUSION
Given the rapidly expanding market for cannabis, large-scale longitudinal studies are needed to explore the long-term effects of chronic and frequent cannabis use. Consistent with recommendations regarding the use of tobacco and other smoked substances among patients with CKD and ESRD, smoked cannabis should be avoided among people with cardiovascular or pulmonary disease. Other routes of administration such as oral consumption may avoid these risks.
With growing acceptance of both medical and recreational cannabis and cannabinoids, further research is required to determine the efficacy, safety, and acceptability of medical and recreational cannabis use among people with CKD and ESRD. As clinicians, we should be informed and able to provide guidance with the most up to date information for our patients.
Acknowledgements
The author thanks Joseph Vassalotti and David Goldfarb for their review of the manuscript.
Financial support and sponsorship
This work was supported by a NIH T32DK007757 grant.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry 2015; 72:1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2▪.Han BH, Palamar JJ. Marijuana use by middle-aged and older adults in the United States, 2015–2016. Drug Alcohol Depend 2018; 191:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]; Prevalence of past-year cannabis use is increasing among middle-aged adults between 50 and 64 years and older adults ≥65 years.
- 3.Lloyd SL, Striley CW. Marijuana use among adults 50 years or older in the 21st century. Gerontol Geriatr Med 2018; 4:2333721418781668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪.Lum HD, Arora K, Croker JA, et al. Patterns of marijuana use and health impact: a survey among older Coloradans. Gerontol Geriatr Med 2019; 5:2333721419843707. [DOI] [PMC free article] [PubMed] [Google Scholar]; Descriptive account of legal medical and recreational cannabis use among people at least 60 years. Identified overall positive health impacts.
- 5▪.Reynolds IR, Fixen DR, Parnes BL, et al. Characteristics and patterns of marijuana use in community-dwelling older adults. J Am Geriatr Soc 2018; 66:2167–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]; Descriptive account of legal medical and recreational cannabis use among patients at a geriatric primary care clinic.
- 6.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2019; 73:A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and delta(9)-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol 2015; 172:737–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deutsch DG, Goligorsky MS, Schmid PC, et al. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest 1997; 100:1538–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pressly JD, Soni H, Jiang S, et al. Activation of the cannabinoid receptor 2 increases renal perfusion. Physiol Genomics 2019; 51:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koura Y, Ichihara A, Tada Y, et al. Anandamide decreases glomerular filtration rate through predominant vasodilation of efferent arterioles in rat kidneys. J Am Soc Nephrol 2004; 15:1488–1494. [DOI] [PubMed] [Google Scholar]
- 11.Lecru L, Desterke C, Grassin-Delyle S, et al. Cannabinoid receptor 1 is a major mediator of renal fibrosis. Kidney Int 2015; 88:72–84. [DOI] [PubMed] [Google Scholar]
- 12.Udi S, Hinden L, Ahmad M, et al. Dual inhibition of cannabinoid-1 receptor and iNOS attenuates obesity-induced chronic kidney disease. Br J Pharmacol 2019; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moradi H, Oveisi F, Khanifar E, et al. Increased renal 2-arachidonoylglycerol level is associated with improved renal function in a mouse model of acute kidney injury. Cannabis Cannabinoid Res 2016; 1:218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu YC, Lei CC, Shih YH, et al. Induction of proteinuria by cannabinoid receptors 1 signaling activation in CB1 transgenic mice. Am J Med Sci 2015; 349:162–168. [DOI] [PubMed] [Google Scholar]
- 15.Jourdan T, Szanda G, Rosenberg AZ, et al. Overactive cannabinoid 1 receptor in podocytes drives type 2 diabetic nephropathy. Proc Natl Acad Sci U S A 2014; 111:E5420–E5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barutta F, Corbelli A, Mastrocola R, et al. Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes 2010; 59:1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barutta F, Grimaldi S, Franco I, et al. Deficiency of cannabinoid receptor of type 2 worsens renal functional and structural abnormalities in streptozotocin-induced diabetic mice. Kidney Int 2014; 86:979–990. [DOI] [PubMed] [Google Scholar]
- 18.Barutta F, Grimaldi S, Gambino R, et al. Dual therapy targeting the endocannabinoid system prevents experimental diabetic nephropathy. Nephrol Dial Transplant 2017; 32:1655–1665. [DOI] [PubMed] [Google Scholar]
- 19.Barutta F, Piscitelli F, Pinach S, et al. Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes 2011; 60:2386–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jourdan T, Park JK, Varga ZV, et al. Cannabinoid-1 receptor deletion in podocytes mitigates both glomerular and tubular dysfunction in a mouse model of diabetic nephropathy. Diabetes Obes Metab 2018; 20:698–708. [DOI] [PubMed] [Google Scholar]
- 21.Zoja C, Locatelli M, Corna D, et al. Therapy with a selective cannabinoid receptor type 2 agonist limits albuminuria and renal injury in mice with type 2 diabetic nephropathy. Nephron 2016; 132:59–69. [DOI] [PubMed] [Google Scholar]
- 22.Hinden L, Udi S, Drori A, et al. Modulation of renal GLUT2 by the cannabinoid-1 receptor: implications for the treatment of diabetic nephropathy. J Am Soc Nephrol 2018; 29:434–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva GB, Atchison DK, Juncos LI, Garcia NH. Anandamide inhibits transport-related oxygen consumption in the loop of Henle by activating CB1 receptors. Am J Physiol Renal Physiol 2013; 304:F376–F381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chopda GR, Parge V, Thakur GA, et al. Tolerance to the diuretic effects of cannabinoids and cross-tolerance to a kappa-opioid agonist in THC-treated mice. J Pharmacol Exp Ther 2016; 358:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chopda GR, Vemuri VK, Sharma R, et al. Diuretic effects of cannabinoid agonists in mice. Eur J Pharmacol 2013; 721 (1–3):64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paronis CA, Thakur GA, Bajaj S, et al. Diuretic effects of cannabinoids. J Pharmacol Exp Ther 2013; 344:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkin KA, McAinch AJ, Briffa JF, et al. Cannabinoid receptor 2 expression in human proximal tubule cells is regulated by albumin independent of ERK1/2 signaling. Cell Physiol Biochem 2013; 32:1309–1319. [DOI] [PubMed] [Google Scholar]
- 28.Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 2018; 43:155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪.Di Marzo V. New approaches and challenges to targeting the endocannabinoid system. Nat Rev Drug Discov 2018; 17:623–639. [DOI] [PubMed] [Google Scholar]; Review of drug development targeting the endocannabinoid system.
- 30.Mechoulam R, Hanus LO, Pertwee R, Howlett AC. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev Neurosci 2014; 15:757–764. [DOI] [PubMed] [Google Scholar]
- 31.Tam J. The emerging role of the endocannabinoid system in the pathogenesis and treatment of kidney diseases. J Basic Clin Physiol Pharmacol 2016; 27:267–276. [DOI] [PubMed] [Google Scholar]
- 32.Park F, Potukuchi PK, Moradi H, Kovesdy CP. Cannabinoids and the kidney: effects in health and disease. Am J Physiol Renal Physiol 2017; 313:F1124–F1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪.Barutta F, Bruno G, Mastrocola R, et al. The role of cannabinoid signaling in acute and chronic kidney diseases. Kidney Int 2018; 94:252–258. [DOI] [PubMed] [Google Scholar]; Review of preclinical evidence supporting the role of the endocannabinoid system in acute and chronic kidney diseases.
- 34▪.Chua JT, Argueta DA, DiPatrizio NV, et al. Endocannabinoid system and the kidneys: from renal physiology to injury and disease. Cannabis Cannabinoid Res 2019; 4:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review of preclinical evidence supporting the role of the endocannabinoid system in acute and chronic kidney diseases.
- 35.Francois H, Lecru L. The role of cannabinoid receptors in renal diseases. Curr Med Chem 2018; 25:793–801. [DOI] [PubMed] [Google Scholar]
- 36.Hryciw DH, McAinch AJ. Cannabinoid receptors in the kidney. Curr Opin Nephrol Hypertens 2016; 25:459–464. [DOI] [PubMed] [Google Scholar]
- 37.Ritter JK, Li G, Xia M, Boini K. Anandamide and its metabolites: what are their roles in the kidney? Front Biosci (Schol Ed) 2016; 8:264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tam J, Hinden L, Drori A, et al. The therapeutic potential of targeting the peripheral endocannabinoid/CB1 receptor system. Eur J Intern Med 2018; 49:23–29. [DOI] [PubMed] [Google Scholar]
- 39.Evanoff AB, Quan T, Dufault C, et al. Physicians-in-training are not prepared to prescribe medical marijuana. Drug Alcohol Depend 2017; 180:151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40▪.Philpot LM, Ebbert JO, Hurt RT. A survey of the attitudes, beliefs and knowledge about medical cannabis among primary care providers. BMC Fam Pract 2019; 20:17. [DOI] [PMC free article] [PubMed] [Google Scholar]; Single center survey demonstrating knowledge gaps about the effectiveness of medical cannabis among primary care providers.
- 41.World Health Organization. Cannabidiol (CBD) Critical Review Report, Expert Committee on Drug Dependence, Fortieth Meeting, Geneva, 4-7 June 2018 [Internet]. 2018. Available from: https://www.who.int/medicines/access/controlled-substances/CannabidiolCriticalReview.pdf [Accessed 20 April 2019]. [Google Scholar]
- 42.Ben-Shabat S, Fride E, Sheskin T, et al. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol 1998; 353:23–31. [DOI] [PubMed] [Google Scholar]
- 43.Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011; 163:1344–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44▪.Chatham-Stephens K, Roguski K, Jang Y, et al. Characteristics of hospitalized and nonhospitalized patients in a nationwide outbreak of e-cigarette, or vaping, product use-associated lung injury - United States, November 2019. MMWR Morb Mortal Wkly Rep 2019; 68:1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]; Update from the CDC regarding the nationwide outbreak of e-cigarette, or vaping, product use-associated lung injury (EVALI)
- 45.Butt YM, Smith ML, Tazelaar HD, et al. Pathology of vaping-associated lung injury. N Engl J Med 2019; 381:1780–1781. [DOI] [PubMed] [Google Scholar]
- 46.Blount BC, Karwowski MP, Morel-Espinosa M, et al. Evaluation of bronchoalveolar lavage fluid from patients in an outbreak of e-cigarette, or vaping, product use-associated lung injury - 10 states, August-October 2019. MMWR Morb Mortal Wkly Rep 2019; 68:1040–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schauer GL, King BA, Bunnell RE, et al. Toking, vaping, and eating for health or fun: marijuana use patterns in adults, U.S., 2014. Am J Prev Med 2016; 50:1–8. [DOI] [PubMed] [Google Scholar]
- 48▪.Monte AA, Shelton SK, Mills E, et al. Acute illness associated with cannabis use, by route of exposure: an observational study. Ann Intern Med 2019; 170:531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]; Determined that edible cannabis is associated with a disproportionate increase in emergency department visits at a single institution.
- 49.Birnbaum AK, Karanam A, Marino SE, et al. Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia 2019; 60:1586–1592. [DOI] [PubMed] [Google Scholar]
- 50.Rong C, Carmona NE, Lee YL, et al. Drug-drug interactions as a result of co-administering Delta(9)-THC and CBD with other psychotropic agents. Expert Opin Drug Saf 2018; 17:51–54. [DOI] [PubMed] [Google Scholar]
- 51.Yamaori S, Ebisawa J, Okushima Y, et al. Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci 2011; 88 (15–16):730–736. [DOI] [PubMed] [Google Scholar]
- 52.Jiang R, Yamaori S, Okamoto Y, et al. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab Pharmacokinet 2013; 28:332–338. [DOI] [PubMed] [Google Scholar]
- 53.Yamaori S, Koeda K, Kushihara M, et al. Comparison in the in vitro inhibitory effects of major phytocannabinoids and polycyclic aromatic hydrocarbons contained in marijuana smoke on cytochrome P450 2C9 activity. Drug Metab Pharmacokinet 2012; 27:294–300. [DOI] [PubMed] [Google Scholar]
- 54.Yamaori S, Okamoto Y, Yamamoto I, Watanabe K. Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metab Dispos 2011; 39:2049–2056. [DOI] [PubMed] [Google Scholar]
- 55.Taylor L, Gidal B, Blakey G, et al. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs 2018; 32:1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56▪.Millar SA, Stone NL, Yates AS, O'Sullivan SE. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol 2018; 9:1365. [DOI] [PMC free article] [PubMed] [Google Scholar]; Systematic review of cannabidiol pharmacokinetics in humans that highlights the paucity and discrepancies in data.
- 57.Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers 2007; 4:1770–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58▪.Tayo B, Taylor L, Sahebkar F, et al. A phase I, open-label, parallel-group, single-dose trial of the pharmacokinetics, safety, and tolerability of cannabidiol in subjects with mild to severe renal impairment. Clin Pharmacokinet 2019; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; First published cannabinoid pharmacokinetic study among people with renal impairment.
- 59▪.MacCallum CA, Russo EB, Sahebkar F, et al. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med 2018; 49:12–19. [DOI] [PubMed] [Google Scholar]
- 60.Brenan M. 14% of Americans Say They Use CBD Products. Gallup [Internet]. 2019 August 7, 2019. Available from: https://news.gallup.com/poll/263147/americans-say-cbd-products.aspx. [Accessed 7 Aug 2019] [Google Scholar]
- 61.Kenyon J, Liu W, Dalgleish A. Report of objective clinical responses of cancer patients to pharmaceutical-grade synthetic cannabidiol. Anticancer Res 2018; 38:5831–5835. [DOI] [PubMed] [Google Scholar]
- 62.Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med 2017; 376:2011–2020. [DOI] [PubMed] [Google Scholar]
- 63.Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med 2018; 378:1888–1897. [DOI] [PubMed] [Google Scholar]
- 64▪.Millar SA, Stone NL, Bellman ZD, et al. A systematic review of cannabidiol dosing in clinical populations. Br J Clin Pharmacol 2019; 85:1888–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review of cannabidiol dosing that highlights the potential wide range of activity in several pathologies but that pharmacokinetic studies and phase III trials are lacking.
- 65▪.Huestis MA, Solimini R, Pichini S, et al. Cannabidiol adverse effects and toxicity. Curr Neuropharmacol 2019; 17:974–989. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive systematic review of adverse effects and toxicity associated with cannabidiol, highlighting that adverse effects and potential drug--drug interactions must be taken into consideration.
- 66▪.VanDolah HJ, Bauer BA, Mauck KF. Clinicians’ guide to cannabidiol and hemp oils. Mayo Clin Proc 2019; 94:1840–1851. [DOI] [PubMed] [Google Scholar]; A clinical guide to cannabidiol and hemp oils to help clinicians advise their patients on the safest and most evidence-based formulations.
- 67.Pan H, Mukhopadhyay P, Rajesh M, et al. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther 2009; 328:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA 2017; 318:1708–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis 2007; 14:82–99. [DOI] [PubMed] [Google Scholar]
- 70.Osborn LA, Lauritsen KJ, Cross N, et al. Self-medication of somatic and psychiatric conditions using botanical marijuana. J Psychoactive Drugs 2015; 47:345–350. [DOI] [PubMed] [Google Scholar]
- 71.Rein JL, Wyatt CM. Marijuana and cannabinoids in ESRD and earlier stages of CKD. Am J Kidney Dis 2018; 71:267–274. [DOI] [PubMed] [Google Scholar]
- 72.Davison SN, Davison JS. Is there a legitimate role for the therapeutic use of cannabinoids for symptom management in chronic kidney disease? J Pain Symptom Manage 2011; 41:768–778. [DOI] [PubMed] [Google Scholar]
- 73▪.Ho C, Martinusen D, Lo C. A review of cannabis in chronic kidney disease symptom management. Can J Kidney Health Dis 2019; 6:2054358119828391. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review of key clinical studies and graded levels of evidence for nonsynthetic cannabinoids in the treatment of common symptoms encountered in advanced stages of CKD, including chronic pain, nausea and vomiting, anorexia, pruritus, and insomnia.
- 74.Koncicki HM, Unruh M, Schell JO. Pain management in CKD: a guide for nephrology providers. Am J Kidney Dis 2017; 69:451–460. [DOI] [PubMed] [Google Scholar]
- 75.Touw M. The religious and medicinal uses of cannabis in China, India and Tibet. J Psychoactive Drugs 1981; 13:23–34. [DOI] [PubMed] [Google Scholar]
- 76.Kimmel PL, Fwu CW, Abbott KC, et al. Opioid prescription, morbidity, and mortality in united states dialysis patients. J Am Soc Nephrol 2017; 28:3658–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Novick TK, Surapaneni A, Shin JI, et al. Associations of opioid prescriptions with death and hospitalization across the spectrum of estimated GFR. Clin J Am Soc Nephrol 2019; 14:1581–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wen H, Hockenberry JM. Association of medical and adult-use marijuana laws with opioid prescribing for medicaid enrollees. JAMA Intern Med 2018; 178:673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chihuri S, Li G. State marijuana laws and opioid overdose mortality. Inj Epidemiol 2019; 6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Powell D, Pacula RL, Jacobson M. Do medical marijuana laws reduce addictions and deaths related to pain killers? J Health Econ 2018; 58:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med 2014; 174:1668–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bradford AC, Bradford WD, Abraham A, Bagwell Adams G. Association between US State medical cannabis laws and opioid prescribing in the medicare part D population. JAMA Intern Med 2018; 178:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83▪.Ishida JH, Wong PO, Cohen BE, et al. Substitution of marijuana for opioids in a national survey of US adults. PLoS One 2019; 14:e0222577. [DOI] [PMC free article] [PubMed] [Google Scholar]; Survey of a nationally representative sample that found a substantial number of US adults substitute cannabis for opioids.
- 84.National Academies of Sciences E, Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. 2017; Washington, DC: The National Academies Press, 486 p. [PubMed] [Google Scholar]
- 85.Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 2015; 313:2456–2473. [DOI] [PubMed] [Google Scholar]
- 86.Nugent SM, Morasco BJ, O’Neil ME, et al. The effects of cannabis among adults with chronic pain and an overview of general harms: a systematic review. Ann Intern Med 2017; 167:319–331. [DOI] [PubMed] [Google Scholar]
- 87.Mücke M, Phillips T, Radbruch L, et al. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018; 3:CD012182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Russell C, Rueda S, Room R, et al. Routes of administration for cannabis use - basic prevalence and related health outcomes: a scoping review and synthesis. Int J Drug Policy 2018; 52:87–96. [DOI] [PubMed] [Google Scholar]
- 89.Rein JL, Texter LJ, Wurfel MM, et al. Marijuana use and kidney outcomes in the ASSESS-AKI Cohort [abstract]. J Am Soc Nephrol 2018; 29:479. [Google Scholar]
- 90▪.Bundy JD, Bazzano LA, Xie D, et al. Self-reported tobacco, alcohol, and illicit drug use and progression of chronic kidney disease. Clin J Am Soc Nephrol 2018; 13:993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Posthoc analysis of the CRIC cohort that found cannabis use to not be associated with progression of CKD.
- 91.Ishida JH, Auer R, Vittinghoff E, et al. Marijuana use and estimated glomerular filtration rate in young adults. Clin J Am Soc Nephrol 2017; 12:1578–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu C, Papatheodorou SI, Danziger J, Mittleman MA. Marijuana use and renal function among US adults. Am J Med 2018; 131:408–414. [DOI] [PubMed] [Google Scholar]
- 93.Bhanushali GK, Jain G, Fatima H, et al. AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol 2013; 8:523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Buser GL, Gerona RR, Horowitz BZ, et al. Acute kidney injury associated with smoking synthetic cannabinoid. Clin Toxicol (Phila) 2014; 52:664–673. [DOI] [PubMed] [Google Scholar]
- 95.Centers for Disease Prevention. Acute kidney injury associated with synthetic cannabinoid use---multiple states, 2012. MMWR Morb Mortal Wkly Rep 2013; 62:93–98. [PMC free article] [PubMed] [Google Scholar]
- 96.Silva JP, Carmo H, Carvalho F. The synthetic cannabinoid XLR-11 induces in vitro nephrotoxicity by impairment of endocannabinoid-mediated regulation of mitochondrial function homeostasis and triggering of apoptosis. Toxicol Lett 2018; 287:59–69. [DOI] [PubMed] [Google Scholar]
- 97.Luciano RL, Perazella MA. Nephrotoxic effects of designer drugs: synthetic is not better!. Nat Rev Nephrol 2014; 10:314–324. [DOI] [PubMed] [Google Scholar]
- 98.Nanavati A, Herlitz LC. Tubulointerstitial injury and drugs of abuse. Adv Chronic Kidney Dis 2017; 24:80–85. [DOI] [PubMed] [Google Scholar]
- 99.Pendergraft WF, 3rd, Herlitz LC, Thornley-Brown D, et al. Nephrotoxic effects of common and emerging drugs of abuse. Clin J Am Soc Nephrol 2014; 9:1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut 2004; 53:1566–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Habboushe J, Sedor J. Cannabinoid hyperemesis acute renal failure: a common sequela of cannabinoid hyperemesis syndrome. Am J Emerg Med 2014; 32:690.e1-2. [DOI] [PubMed] [Google Scholar]
- 102.Bramstedt J, Dissmann R. Cannabinoid hyperemesis syndrome inducing acute prerenal failure and electrolyte disturbance. Dtsch Med Wochenschr 2011; 136:1720–1722. [DOI] [PubMed] [Google Scholar]
- 103.Baron M, Haymann JP, Wolfromm A, et al. The Case: The smoker and the nephrologist. Kidney Int 2011; 79:1385–1386. [DOI] [PubMed] [Google Scholar]
- 104.Price SL, Fisher C, Kumar R, Hilgerson A. Cannabinoid hyperemesis syndrome as the underlying cause of intractable nausea and vomiting. J Am Osteopath Assoc 2011; 111:166–169. [DOI] [PubMed] [Google Scholar]
- 105.Srihari P, Liu M, Punzell S, et al. Cannabinoid hyperemesis syndrome associated with compulsive showering and acute kidney injury. Prim Care Companion CNS Disord 2016; 18: doi: 10.4088/PCC.15l01847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klassen J, Wilson G. Cannabinoid hyperemesis syndrome masquerading as uremia: an educational case report. Can J Kidney Health Dis 2018; 5:2054358118791146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cadman PE. Hypophosphatemia in users of cannabis. Am J Kidney Dis 2017; 69:152–155. [DOI] [PubMed] [Google Scholar]
- 108.Lee WS, Erdelyi K, Matyas C, et al. Cannabidiol limits T cell-mediated chronic autoimmune myocarditis: implications to autoimmune disorders and organ transplantation. Mol Med 2016; 22:136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kozela E, Juknat A, Kaushansky N, et al. Cannabinoids decrease the th17 inflammatory autoimmune phenotype. J Neuroimmune Pharmacol 2013; 8:1265–1276. [DOI] [PubMed] [Google Scholar]
- 110.Yeshurun M, Shpilberg O, Herscovici C, et al. Cannabidiol for the prevention of graft-versus-host-disease after allogeneic hematopoietic cell transplantation: results of a phase II study. Biol Blood Marrow Transplant 2015; 21:1770–1775. [DOI] [PubMed] [Google Scholar]
- 111.Sido JM, Nagarkatti PS, Nagarkatti M. Delta(9)-Tetrahydrocannabinol attenuates allogeneic host-versus-graft response and delays skin graft rejection through activation of cannabinoid receptor 1 and induction of myeloid-derived suppressor cells. J Leukoc Biol 2015; 98:435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dao M, Lecru L, Vandermeersch S, et al. The cannabinoid receptor 1 is involved in renal fibrosis during chronic allograft dysfunction: Proof of concept. J Cell Mol Med 2019; 23:7279–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113▪.Ryan JE, Noeder M, Burke C, et al. Denying renal transplantation to an adolescent medical cannabis user: an ethical case study. Pediatr Transplant 2019; 23:e13467. [DOI] [PMC free article] [PubMed] [Google Scholar]; An enlightening and important ethical case study of a young kidney transplant candidate who used medical cannabis.
- 114▪.Levi ME, Montague BT, Thurstone C, et al. Marijuana use in transplantation: a call for clarity. Clin Transplant 2019; 33:e13456. [DOI] [PubMed] [Google Scholar]; Survey from the American Society of Transplantation comparing policies and concerns of cannabis use to actual observed complications. This report highlights a discordance among transplant centers in their perceived risks of cannabis compared to observed complications.
- 115.Stark AL, Hickson LJ, Larrabee BR, et al. Cannabis abuse and dependence in kidney transplant candidates. J Psychosom Res 2019; 121:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116▪.Alhamad T, Koraishy FM, Lam NN, et al. Cannabis dependence or abuse in kidney transplantation: implications for posttransplant outcomes. Transplantation 2019; 103:2373–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study of cannabis dependence or abuse on the impact of several clinically important post-kidney transplant outcomes.
- 117▪.Niederhaus SV. Cannabis Dependence or Abuse in Kidney Transplantation: One Perspective. Transplantation 2019; 103:2223–2224. [DOI] [PubMed] [Google Scholar]; Editorial to ref. 115 that offers a fair and important perspective to cannabis use among potential kidney transplant candidates and kidney transplant recipients.
- 118.Greenan G, Ahmad SB, Anders MG, et al. Recreational marijuana use is not associated with worse outcomes after renal transplantation. Clin Transplant 2016; 30:1340–1346. [DOI] [PubMed] [Google Scholar]
- 119▪.Fabbri KR, Anderson-Haag TL, Spenningsby AM, et al. Marijuana use should not preclude consideration for kidney transplantation. Clin Transplant 2019; 33:e13706.[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; Determined that isolated cannabis use was associated with similar first year outcomes and overall graft survival over a decade compared to nonusers at a single center.
- 120▪.Ruckle D, Keheila M, West B, et al. Should donors who have used marijuana be considered candidates for living kidney donation? Clin Kidney J 2019; 12:437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]; Determined that transplanted kidneys from cannabis using living donors had similar long term outcomes compared to kidneys from nonusers from a single center.
- 121.Ruchlemer R, Amit-Kohn M, Raveh D, Hanus L. Inhaled medicinal cannabis and the immunocompromised patient. Support Care Cancer 2015; 23:819–822. [DOI] [PubMed] [Google Scholar]
- 122.Marks WH, Florence L, Lieberman J, et al. Successfully treated invasive pulmonary aspergillosis associated with smoking marijuana in a renal transplant recipient. Transplantation 1996; 61:1771–1774. [DOI] [PubMed] [Google Scholar]
- 123.Vethanayagam D, Pugsley S, Dunn EJ, et al. Exogenous lipid pneumonia related to smoking weed oil following cadaveric renal transplantation. Can Respir J 2000; 7:338–342. [DOI] [PubMed] [Google Scholar]
- 124.McPartland JM, Pruitt PL. Medical marijuana and its use by the immunocompromised. Altern Ther Health Med 1997; 3:39–45. [PubMed] [Google Scholar]
- 125.McKernan K, Spangler J, Zhang L, et al. Cannabis microbiome sequencing reveals several mycotoxic fungi native to dispensary grade Cannabis flowers. F1000Res 2015; 4:1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McKernan K, Spangler J, Helbert Y, et al. Metagenomic analysis of medicinal Cannabis samples; pathogenic bacteria, toxigenic fungi, and beneficial microbes grow in culture-based yeast and mold tests. F1000Res 2016; 5:2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127▪.Leino AD, Emoto C, Fukuda T, et al. Evidence of a clinically significant drug-drug interaction between cannabidiol and tacrolimus. Am J Transplant 2019; 19:2944–2948. [DOI] [PubMed] [Google Scholar]; Case report describing an increase in serum tacrolimus levels associated with cannabidiol.
- 128.Moadel D, Chism K. Medical marijuana-induced tacrolimus toxicity. Psychosomatics 2019; 60:603–605. [DOI] [PubMed] [Google Scholar]
- 129.Hauser N, Sahai T, Richards R, Roberts T. High on cannabis and calcineurin inhibitors: a word of warning in an era of legalized marijuana. Case Rep Transplant 2016; 2016:4028492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cuñetti L, Manzo L, Peyraube R, et al. Chronic pain treatment with cannabidiol in kidney transplant patients in Uruguay. Transplant Proc 2018; 50:461–464. [DOI] [PubMed] [Google Scholar]
- 131.Jaeger W, Benet LZ, Bornheim LM. Inhibition of cyclosporine and tetrahydrocannabinol metabolism by cannabidiol in mouse and human microsomes. Xenobiotica 1996; 26:275–284. [DOI] [PubMed] [Google Scholar]
- 132▪▪.Keyhani S, Steigerwald S, Ishida J, et al. Risks and benefits of marijuana use: a National Survey of U.S. Adults. Ann Intern Med 2018; 169:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reports that U.S. adults view the risks and benefits of cannabis use more favorably than what existing evidence supports.
- 133.Liebman SE, Lamontagne SP, Huang LS, et al. Smoking in dialysis patients: a systematic review and meta-analysis of mortality and cardiovascular morbidity. Am J Kidney Dis 2011; 58:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sarafian TA, Magallanes JA, Shau H, et al. Oxidative stress produced by marijuana smoke. An adverse effect enhanced by cannabinoids. Am J Respir Cell Mol Biol 1999; 20:1286–1293. [DOI] [PubMed] [Google Scholar]
- 135.Tashkin DP, Baldwin GC, Sarafian T, et al. Respiratory and immunologic consequences of marijuana smoking. J Clin Pharmacol 2002; 42 (S1):71S–81S. [DOI] [PubMed] [Google Scholar]
- 136.Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc 2013; 10:239–247. [DOI] [PubMed] [Google Scholar]
- 137.Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years. JAMA 2012; 307:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138▪▪.Ghasemiesfe M, Ravi D, Vali M, et al. Marijuana use, respiratory symptoms, and pulmonary function: a systematic review and meta-analysis. Ann Intern Med 2018; 169:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]; Systematic review and meta-analysis of pulmonary complications associated with cannabis use.
- 139.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 140.Pacher P, Steffens S, Hasko G, et al. Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat Rev Cardiol 2018; 15:151–166. [DOI] [PubMed] [Google Scholar]
- 141.Alfulaij N, Meiners F, Michalek J, et al. Cannabinoids, the heart of the matter. J Am Heart Assoc 2018; 7: pii:e009099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Montecucco F, Di Marzo V. At the heart of the matter: the endocannabinoid system in cardiovascular function and dysfunction. Trends Pharmacol Sci 2012; 33:331–340. [DOI] [PubMed] [Google Scholar]
- 143.Jouanjus E, Lapeyre-Mestre M, Micallef J. French Association of the Regional Abuse and Dependence Monitoring Centres (CEIP-A) Working Group on Cannabis Complications. Cannabis use: signal of increasing risk of serious cardiovascular disorders. J Am Heart Assoc 2014; 3:e000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rumalla K, Reddy AY, Mittal MK. Recreational marijuana use and acute ischemic stroke: a population-based analysis of hospitalized patients in the United States. J Neurol Sci 2016; 364:191–196. [DOI] [PubMed] [Google Scholar]
- 145.Mittleman MA, Lewis RA, Maclure M, et al. Triggering myocardial infarction by marijuana. Circulation 2001; 103:2805–2809. [DOI] [PubMed] [Google Scholar]
- 146.Reis JP, Auer R, Bancks MP, et al. Cumulative lifetime marijuana use and incident cardiovascular disease in middle age: the coronary artery risk development in young adults (CARDIA) study. Am J Public Health 2017; 107:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meier MH, Caspi A, Cerda M, et al. Associations between cannabis use and physical health problems in early midlife: a longitudinal comparison of persistent cannabis vs tobacco users. JAMA Psychiatry 2016; 73:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148▪.Ghasemiesfe M, Ravi D, Casino T, et al. Acute cardiovascular effects of marijuana use. J Gen Intern Med 2019; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; Systematic review of cardiovascular complications associated with cannabis use.
- 149.Alshaarawy O, Elbaz HA. Cannabis use and blood pressure levels: United States National Health and Nutrition Examination Survey, 2005–2012. J Hypertens 2016; 34:1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sultan SR, Millar SA, England TJ, O'Sullivan SE. A systematic review and meta-analysis of the haemodynamic effects of cannabidiol. Front Pharmacol 2017; 8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sultan SR, Millar SA, O'Sullivan SE, England TJ. A systematic review and meta-analysis of the in vivo haemodynamic effects of delta(9)-tetrahydrocannabinol. Pharmaceuticals (Basel) 2018; 11: pii:E13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harhay MN, Xie D, Zhang X, et al. Cognitive impairment in non-dialysis-dependent CKD and the transition to dialysis: findings from the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis 2018; 72:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Drew DA, Weiner DE, Tighiouart H, et al. Cognitive decline and its risk factors in prevalent hemodialysis patients. Am J Kidney Dis 2017; 69:780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.O’Lone E, Connors M, Masson P, et al. Cognition in people with end-stage kidney disease treated with hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis 2016; 67:925–935. [DOI] [PubMed] [Google Scholar]
- 155.Broyd SJ, van Hell HH, Beale C, et al. Acute and chronic effects of cannabinoids on human cognition---a systematic review. Biol Psychiatry 2016; 79:557–567. [DOI] [PubMed] [Google Scholar]
- 156▪.Sagar KA, Gruber SA. Interactions between recreational cannabis use and cognitive function: lessons from functional magnetic resonance imaging. Ann N Y Acad Sci 2019; 1451:42–70. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive review focused on evidence from functional MRI studies that document acute and residual alterations in brain function during tasks spanning a variety of cognitive domains: executive function, attention and working memory, memory, motor skills, error monitoring, and reward and affective processing.


