Abstract
Microorganisms can be found in virtually any environment. In humans, the largest collection of microorganisms is found in the gut ecosystem. The adult gut microbiome consists of more genes than its human host and typically spans more than 60 genera from across the taxonomic tree. In addition, the gut contains the largest number of neurons in the body, after the brain. In recent years, it has become clear that the gut microbiome is in communication with the brain, through the gut–brain axis. A growing body of literature shows that the gut microbiome plays a shaping role in a variety of psychiatric disorders, including major depressive disorder (MDD). In this review, the interplay between the microbiome and MDD is discussed in three facets. First, we discuss factors that affect the onset/development of MDD that also greatly impinge on the composition of the gut microbiota—especially diet and stressful life events. We then examine the interplay between the microbiota and MDD. We examine evidence suggesting that the microbiota is altered in MDD, and we discuss why the microbiota should be considered during MDD treatment. Finally, we look toward the future and examine how the microbiota might become a therapeutic target for MDD. This review is intended to introduce those familiar with the neurological and psychiatric aspects of MDD to the microbiome and its potential role in the disorder. Although research is in its very early days, with much yet to be the understood, the microbiome is offering new avenues for developing potentially novel strategies for managing MDD.
Keywords: antidepressant, gut–brain axis, major depressive disorder, microbiome, psychobiotic
With the exception of a few notable instances, the fields of microbiology and psychiatry have not gone hand in hand. It is worth noting, however, that the syphilis-causing microbe Treponema pallidum was responsible for filling large parts of Victorian mental asylums. Also of note, the 1908 Nobel prize winner in Physiology or Medicine, Élie Metchnikoff, adhered to the idea that bacteria in fermented milk were beneficial against autointoxication, a term historically used to describe a wide range of symptoms such as fatigue and melancholia.1 Building on Metchnikoff’s ideas the psychiatrist Henry Cotton, medical director of New Jersey State Hospital at Trenton, was convinced that the bacteria on the teeth of his patients were the source of their psychiatric conditions. Infamously, he would have their teeth pulled as part of their treatment.2 More recently, as the neurocognitive effects of HIV infection became evident, psychiatry once more saw a connection with microbiology. For the most part, however, in our heavily specialized medical and scientific training, the practitioners of these disciplines have rarely crossed paths.
This situation changed in tandem with the emergence of new technologies such as next-generation sequencing and the increase in processing power required to analyze large amounts of data. The microbes found in and on the human body have been mapped through projects like the Human Microbiome Project,3 LifeLines-DEEP,4 Flemish Gut,5 TwinsUK,6 MetaHIT,7 and ELDERMET.8 In humans, the greatest abundance of microbes is found in the gut. According to current estimates, the gut microbiome consists of around 3 × 1013 microbes from more than 60 genera and weighs approximately 200 grams.9 Increasing efforts and research studies are currently investigating the microbiome–gut–brain axis (MGBA).10–16 While the precise mechanisms involved in microbiome-to-brain dialogue are still an open question in the field, routes of communication include the immune system,13,17 synthesis and metabolism of metabolites and neurotransmitters,18 and activation of the vagus nerve.19,20
Over the years, it has become clear, especially from animal studies, that the gut microbiome can influence a broad range of factors, including the development of type 2 diabetes,21 Alzheimer’s disease,22 and major depressive disorder (MDD).18,23,24 Intriguingly, the gut microbiome has been linked to several physiological functions relevant to depression; these functions are described in Figure 1. From a clinical point of view, episodes of depression are associated with a dysregulated hypothalamic–pituitary–adrenal (HPA) axis,25 and conversely, improved depressive symptoms are associated with stabilization of the HPA axis.26,27 The gut microbiota plays a role in the programming and reactivity of the HPA axis. A direct link between the microbiota and HPA function was shown in a study from Sudo and colleagues,28 who showed exaggerated corticosterone (CORT) and adrenocorticotrophin (ACTH) levels in germ-free mice in response to restraint stress when compared to conventionally housed, specific pathogen–free (SPF) mice. In rats, administration of probiotics (Lactobacillus sp.) during the early stress period was able to normalize basal CORT levels, which are increased following maternal separation.29 In addition to affecting HPA-axis function, the microbiota could influence central nervous system function directly by means of neuronal activation of stress circuits. Studies involving the oral administration of the pathogenic bacteria Citrobacter rodentium and Campylobacter jejuni show that gut microbes can mediate the stress response by activating vagal pathways.30,31 Alterations in the microbiome can also lead to hyperactivation of the immune system, with production of inflammatory cytokines typically observed in depression.32 Finally, depression has been associated with impairment of the microbiome’s ability to produce neuroactive metabolites and with disrupted intestinal barrier function (see Figure 1).33
Figure 1.
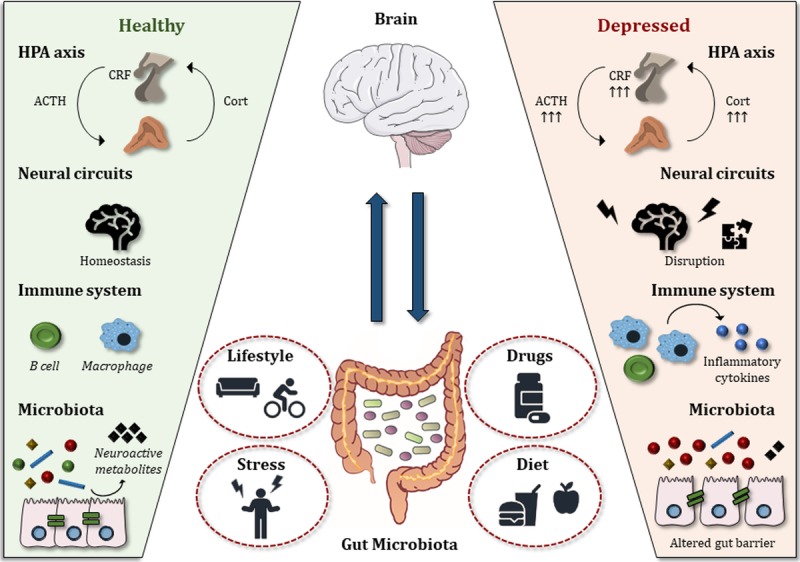
Impact of the gut microbiota on the gut–brain axis in health and depression. Left panel: A stable and balanced gut microbiota is essential for normal gut–brain axis signaling. Right panel: In major depressive disorder, alterations in the gut microbiota negatively affect the gut–brain axis at several levels. The HPA axis becomes hyperactivated; neural circuits and neurotransmitter levels are disrupted; the immune system produces excessive proinflammatory cytokines; and the intestinal barrier is disrupted. Middle panel: Factors that influence the microbiome and, in turn, the onset/development of depression include lifestyle, medications, stress, and dietary habits. ACTH, adrenocorticotropic hormone; Cort, corticosterone (rodents) or cortisol (humans); CRF, corticotropin-releasing factor; HPA axis, hypothalamus–pituitary–adrenal axis.
Animal research has played an integral part in the development of the microbiome field. Numerous important findings with high potential translational value have been made in rodent models (see Text Box 1).48,49 Because of the regular housing and feeding conditions for rodents, for example, the inter-individual variation in the microbiome is much lower than in humans, facilitating the detection of changes in the microbiome even in lower group sizes.50 Furthermore, experimental manipulations such as germ-free environments can be applied in animals but are not feasible in humans. The use of germ-free models has been crucial in linking the microbiome to many key brain processes and behaviors,51–53 despite the limitation of abnormal neurodevelopment present in germ-free animals.
Text Box 1
What Have We Learned from Animal Research?
Animal research offers some unique advantages compared to human studies
It is possible to maintain a highly controlled environment, spanning from diet to housing conditions (temperature, humidity, air flow) and genetics—all factors that reduce the inter-individual variation of microbiome composition.
Moreover, compared to a human life expectancy of approximately 70 years, a mouse has a lifespan of 2–3 years, enabling researchers to study the entire life cycle.
Particularly important in the microbiome field, germ-free rodents are raised in germ-free isolators and represent a crucial tool in determining whether the microbiome plays a causal role in a given host function. In addition to the germ-free model, the effects of perturbation or depletion of the microbiome with unbalanced diets or antibiotics in animals allow for experimental setups that can provide important mechanistic insights.
Through animal research we are starting to delineate the routes of communication involved in the gut–brain signaling, including the vagus nerve, the production of microbial metabolites, and the involvement of the immune system.
Finally, the access to the entire gastrointestinal tract in animal models has been instrumental in studying mechanisms of digestive pathophysiology and in characterizing the microbiome along different sections of the gastrointestinal tract.
Nonetheless, there are limitations and challenges
One limitation of animal research relates to the different anatomical structures in rodents versus humans, including brain and gastrointestinal anatomy, which are particularly relevant in gut–brain research. Moreover, rodent brains, in addition to anatomical differences, undergo more postnatal development than humans.
Moreover, the highly controlled conditions previously mentioned as an advantage can also be construed as a disadvantage in that such regulated environments can be poorly translatable into human conditions.
Particularly when studying a heterogeneous disorder such as major depressive disorder, interpreting changes in mood and behavior in animals is challenging. Robust batteries of behavioral tests and paradigms have been set up to minimize variance in this regard,34 but the specifics of these paradigms are outside of the scope of this review. Apart from changes in behavior, physiological readouts, such as blood levels of corticosterone, are often used.
Changes in animal studies linked to depression
It is worth mentioning the observed neurobiological changes that were reported in animals with an altered microbiome. For more than four decades, it has been known that stress can change the microbiome in mice, specifically decreasing Lactobacillus.35 Since then, more preclinical studies have reported many similar changes in the composition of the microbiome.36,37 Recently, chronic intermittent hypoxia was found to affect not only the physiology, including the autonomic nervous system, but also the microbiome in rodents.38,39 The gut microbiome is also known to modulate the physiology and behavior of the animal. For instance, mice without a microbiome show a heightened myelination in the prefrontal cortex,40 altered RNA-splicing in the amygdala in response to social interaction,41 and an altered immune system.42 Neurodevelopmental differences—including neurogenesis, a process that is dysregulated in depression—have been observed in mice with a humanized microbiome, where specific microbes seemed to be necessary for normal neurodevelopment.43 Interventions targeting the microbiome have been found to protect against physiological and neuroimmune changes due to aging44 and against the behavioral and cognitive effects of stress.45–47
In this review, we will first discuss factors that affect the onset/development of MDD that also affect the composition of the gut microbiome. Then we will examine the interplay between the microbiome and MDD. In particular, we will examine evidence suggesting that the microbiome is altered in MDD, and discuss why the microbiome should be considered during MDD treatment. Finally, we look toward the future and examine how the microbiome might become a therapeutic target in MDD, potentially affecting future clinical practice.
SCULPTING THE GUT MICROBIOME
The gut microbiome is a highly dynamic system, undergoing constant change over time. The degree and manner of change is thought to be determined by a vast combination of factors, ranging from stage of life to exercise. In the context of MDD and the microbiome, two such factors stand out especially—namely, diet and stress. Other factors, such as exercise and aging, that have been shown to affect microbiota composition will also be examined in the context of MDD.
Diet Alters the Gut Microbiome: Relevance for MDD
Though the precise mechanism is unknown at this point in time, diet is known to markedly shape the composition of the gut microbiome.54–56 Furthermore, quality of diet is known to influence the severity of MDD. For instance, intake of the biologically related compounds folate and vitamin B12 were inversely correlated with severity of depression in a large cross-sectional study.57 In a meta-analysis covering 16 studies, 15 of which were in non–clinically depressed populations, dietary intervention led to a significant improvement in mood but not in anxiety.58 Notably, the SMILES trial, a 12-week dietary intervention using a modified Mediterranean diet to target MDD in adults, has shown convincingly that diet can be effective in alleviating MDD symptoms.59,60 Recently, numerous researchers have called for further research to understand the interplay between diet, the microbiome, and MDD.61–64 Along the same lines, one of the outcomes of the European Union’s recent MyNewGut project—an initiative focused on understanding and promoting health by targeting the gut microbiome—was a dietary recommendation intended to improve MDD symptoms by targeting the gut microbiome through the increased consumption of fiber and fish.64 These food groups, which are an important part of the Mediteranean diet, are associated with an increased abundance of bacteria with anti-inflammatory properties. That well-studied diet is known not only to affect the gut microbiome by increasing the abundance of microbes that produce short-chain fatty acids (SCFAs), but also to shorten episodes of depression.65,66
Stress Alters the Gut Microbiome: Relevance for MDD
Similar to the link between diet and the microbiome, the link between stress and MDD is known and well described.67,68 Recently, it has become clear that stress influences the microbiome. Often, the reported changes are on the level of alpha diversity (defined as diversity within the ecosystem) or beta diversity (defined as difference between ecosystems, often in terms of composition), rather than involving the specific microbes being affected.69–73 While human studies are much needed in this area, the available evidence suggests that the human microbiome is similarly involved in maternal and early-life stress, with high-stress microbiomes featuring an increased diversity of Clostridium genera, which is generally associated with inflammation and disease.74 Many studies exist in animal models showing the impact of stress on the microbiome—including rodents,29,36,37,46,75,76 pigs,77 and primates.78,79 Generally, these studies report decreased alpha diversity in the stress group, reduced levels of Lactobacillus, a health-promoting genus that is abundant in early life, and reduced SCFA production. It is difficult, however, to identify specific trends between these studies. For one, the differing methods and databases used in the studies make it challenging to compare the specific microbes that have changed. Furthermore, functional analysis based on 16S sequencing (the most common method of sequencing) is limited by the quality of the tools and databases used, making it difficult to formulate mechanisms explaining the interactions between observed changes in the microbiome and the phenotype.
The gut microbiome also influences the response to stress. Relatively early in the microbiome field, Sudo and colleagues28 discovered that germ-free mice show an exaggerated stress response as measured by CORT and ACTH. In the same study, this exaggerated response was found to be normalized after introducing a probiotic but worsened after introducing a pathogenic strain. More recently, the field has moved toward the notion that the microbiome plays an important role in resilience to stress, especially during early development. The adverse effects of stress have been found, in rodents, to be ameliorated by fiber-rich or milk-associated oligosaccharides, which are preferentially metabolized by certain gut microbes but are difficult to metabolize for the host.45,71–73,80,81 This finding is itself supported by the finding that there is selectivity in favor of strains found in the mother’s microbiome in the microbiome of breast-fed infants.82
It must be noted here that several intervention studies targeting the microbiome and reporting improvements in MDD symptoms and mood have been published. The nature of those interventions will be discussed later in this review.
Other Factors That Influence the Gut Microbiome: Relevance for MDD
In addition to dietary habits and stress—which, as discussed above, have been linked to changes in mood and behavior through alterations of the gut microbiome—other factors known to affect the gut microbiota could indirectly influence the onset or development of MDD. These factors include circadian rhythm, exercise, and aging.
It been shown not only that the microbiota modulates circadian rhythm83,84 but that circadian disruptions can affect the intestinal microbiota.85 Dysregulation of the peripheral or central clocks can lead to microbiome changes, as one recent study has demonstrated utilizing transgenic mice containing deletions of circadian clock genes.85 These mice showed changes to the microbiome and a dampening or abolishment of microbiota compositional oscillations.84,86,87 In one study, the dysregulation of the microbiome was rescued by specifically timed feeding, either exclusively during light or dark.84 MDD can be associated with a dysregulation of the circadian clock;88,89 more work is needed to understand the relationship between the microbiome, circadian rhythms, and brain health, including MDD.
A growing body of literature examines the effect of exercise on the gut microbiota and the gut–brain axis. In particular, moderate levels of exercise have been found to have positive effects on stress, immunity, and energy homeostasis.90,91 Moreover, a case-control mouse study reports free access to exercise was significantly associated with an increase in the relative abundance of the genera Bifidobacterium and Lactobacillus and the species Blautia coccoides and Eubacterium rectale, as well as with an increase in microbiota diversity,92 having potential positive effects on brain and behavior. Several studies in humans and mice have reported changes in the microbiome subsequent to exercise;93–95 however, the magnitude and nature of microbiome-mediated positive effects of exercise on brain function and MDD remain to be investigated.96
During aging, the stability of the microbiome deteriorates.97 It is worth noting, however, that we still lack an exact characterization of the aging gut microbiome. In humans, aging and age-related impairments such as frailty have been linked to a decrease in microbiota diversity.8,98 Conversely, aged (24-month-old) mice exhibit increased microbial diversity than their younger counterparts.99 Intriguingly, the aged gut microbiota composition can also contribute to inflammaging,100 a term used to describe the heightened proinflammatory state and concordant decrease in adaptive immunity observed at older age.101 Given the high prevalence of MDD in aging,102,103 it is tempting to speculate that the microbiome might be at the intersection of aging and mood; this hypothesis needs to be further verified, however, in targeted and large population-based studies.104
CONSIDERING THE GUT MICROBIOME AS A PART OF THE DEPRESSED PATIENT
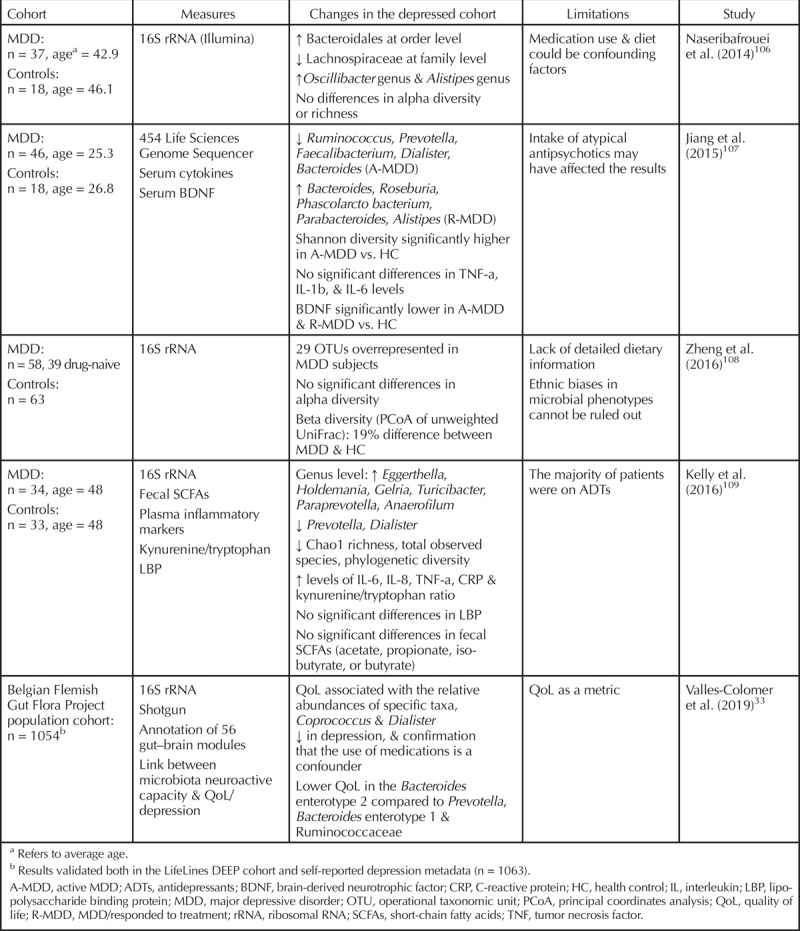
A wealth of studies, from different perspectives and experimental approaches, link the gut microbiome to MDD.18,61,105 Not only is it now apparent that the gut microbiome is altered in MDD, but some studies have also shown that transferring the microbiome of a depressed individual into a healthy rodent can induce depressive-like behavior in the recipient. Such data suggest a causal role for the microbiota in depression pathophysiology (Figure 1). In this section we will discuss the evidence supporting the role of the microbiome in MDD. A summary of the studies investigating the microbiome composition in depressed patients can be found in Table 1.
Table 1.
Human Studies Reporting Altered Microbial Composition in Depression
Gut Microbiome Is Altered in Depression
In recent years, more and more studies have reported that MDD patients have an altered gut microbiome composition when compared to healthy controls, although the specific nature of the alterations can differ from study to study.106–110 The alpha diversity of MDD patients tends to be lower overall, with a higher abundance of bacterial phyla generally associated with inflammation, like Bacteroidetes, and a reduction in phyla associated with a decrease in inflammation, like Firmicutes. The genera Lactobacillus and Bifidobacterium are sometimes reported as having a positive influence on mood. The variation in microbiome analysis methods, ranging from the use of different versions of reference databases or even entirely separate databases to differences in sampling methods, can account for discrepancies in findings between studies.111
An enterotype is, by definition, a classification of living organisms based on their bacteriological ecosystems in the gut microbiome. As part of the Flemish Gut Flora Project, a recently published study shows what might be the strongest available evidence for the existence of the gut–brain axis.33 In that study, the authors found that in their cohort of more than 1000 participants, depressed individuals were more likely than their healthy counterparts to fall into a certain enterotype. The enterotype in question was defined by having a lower bacterial load and a relatively low abundance of the bacterial genus Faecalibacterium. Furthermore, participants who reported a lower quality of life were also more likely to belong to this enterotype,33 which has been linked to inflammation in earlier research by the same group.112 In addition to their finding concerning general composition, the Belgian group identified several features of the microbiome, such as the abundance of butyrate-producing bacteria in the gut, that were associated with a higher quality-of-life score.33 In another important contribution to the field, this same article formulated what will likely become an invaluable tool for future research and understanding. The article identified 56 gut–brain modules, which are the pathways of metabolic function in the gut—for example, GABA metabolism—that potentially influence the brain. In looking at these modules, the authors found several to be altered in a subcohort of clinically depressed patients. A complication that the authors confronted in interpreting their data was that enterotype is a contested term in the microbiome field, and its definition and usefulness are subject to debate.113,114 To address these issues, the Flemish study utilized a Bayesian multinomial-mixture model in assessing enterotype classifications—as opposed to the classical, contested definition of enterotypes within the microbiome, which relies on microbiome profiles falling into distinct clusters.
Gut Microbiome Can Transfer Depression
It must be noted that, even if the depressed microbiome is compositionally distinct from that of the general population, that in itself is not enough to conclude a causative role for the microbiome in the development of MDD. Two animal studies published in 2016 independently show in comparable, but distinct, manners that both mice and rats that received a fecal microbiome transplantation (FMT) from depressed humans displayed a heightened state of inflammation and increased anhedonia-like (as measured by the sucrose preference test) and anxiety-like behavior (as measured by the open field test and elevated plus maze test) compared to those who received FMT from healthy volunteers.108,109 The forced swim test led to conflicting outcomes; the study in mice found an increased immobility time, associated with depressive-like behavior, whereas the rat study found no difference. Potential confounders of these studies include the fact that depressed donors were prescribed medication and that a low number of donors was used for a higher amount of recipients. While only shown in rodents, the behavioral outputs of these studies and their respective translational implications support the notion that certain compositions of the microbiome can affect behavior and mood. A general trend among the microbiomes of depressed patients and animals with increased depressive-like behavior is a drop in alpha diversity, an increase in relative abundance of microbes associated with a proinflammatory state, and a heightened state of inflammation in the host.
CONSIDERING THE GUT MICROBIOME DURING MDD TREATMENT
As previously discussed, alterations of the microbiome can affect the onset/development of MDD at different levels (see Figure 1). Furthermore, several drugs, including psychotropic drugs, can influence the composition of the intestinal microbiota.115,116 Equally hard to confirm as to rule out, it has been speculated upon that the microbiome-targeted effects of psychotropic drugs might play a role in the mechanism of action or in the side effects of these medications. A recent study reports that the gut microbe Ruminococcus flavefaciens metabolizes fluoxetine and inhibits its mood-affecting effect.117
Psychotropic Drugs Influence the Gut Microbiome
In a large in vitro study, the growth of 40 microbes commonly found in the human gut was affected by supplementation of several commercial drugs, including psychotropic drugs.118 While this study used bacterial monocultures and therefore did not account for the vast complexity of the microbiome, it suggests that the composition of the microbiome will be affected by the intake of these common drugs. Interestingly, nearly all subclasses of the antipsychotics with different chemical structure targeted a more similar pattern of species than that presumed from their chemical structure, suggesting that the antimicrobial action may not only express as a side effect of antipsychotics but also be part of their mode of action.118 A hypothesized mechanism of action, for instance, would be that subpopulations of psychiatric patients (bearing microbiomes that are different from those of healthy individuals) might have beneficial treatment outcomes due to microbiome-targeting effects of such medications. In a recent study from our laboratory, chronic administration of psychotropic drugs was shown to influence the microbiome composition and diversity in rats.119 The authors point out that some drugs, including the selective serotonin reuptake inhibitor (SSRI) fluoxetine, specifically shape the microbiome in a distinct manner. What specific effects this compositional shift might have on the host mental well-being is still unknown. Many other studies have found antimicrobial activity in vitro with common SSRIs, reaffirming the idea that psychotropic drugs shape the gut microbiome.120,121 In perhaps a whim of history, isoniazid and iproniazid, two of the first antidepressants ever developed, were originally classified and marketed as antibiotics.122
The Gut Microbiome Influences Drug Metabolism
The whole new field of pharmacomicrobiomics focuses on the role played by the gut microbiome in xenobiotic (typically synthetic molecules that are foreign to a biological system) metabolism.123,124 Several drug classes, including cardiac glycosides, chemotherapeutics, and drugs used for immunotheraphy, are known to be metabolized by the gut microbiota,125–127 but no evidence is currently available on how microbial perturbations influence the metabolism of psychotropic drugs. More research is now warranted, especially considering that several psychotropics have been shown to alter the gut microbiota composition both in vitro and in vivo.
LOOKING TOWARD THE FUTURE: POSSIBLE APPROACHES
After acknowledging the concept that the microbiome and the brain are in a constant bidirectional relationship with each other, it is logical to consider the microbiome as a therapeutic target for MDD. MDD is a complex disorder, and many patients fail to respond to antidepressant treatment, while others respond but do not fully remit. In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, more than 40% of patients with MDD did not achieve remission, even after two optimally delivered trials of antidepressant medications.128 Moreover, adjunctive treatments are commonly employed to improve therapeutic outcomes. Any alternative approach should be considered for its therapeutic value, either in tandem with traditional antidepressant treatment or as a stand-alone treatment. Therefore, the microbiome represents a potential target in treating of MDD. It is easily modifiable, and its manipulations do not cause adverse effects when using safe microbes.
Supplementing the Gut Microbiome to Improve Depression
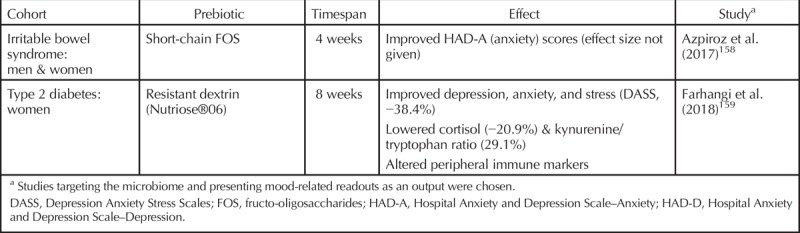
Apart from dietary interventions, treatments meant to alter the composition of the microbiome can be split into two categories: prebiotics and probiotics. Prebiotics are defined as foods that are not digestible by humans (such as fibers) and that have a beneficial effect on the host’s microbiome.129 Probiotics are live microbes that have a beneficial effect on the host (provided, of course, that they are ingested in adequate quantities).130–132 When both pre- and probiotics are coadministered, which happens increasingly, the term synbiotic is used. Microbiome interventions specifically designed to improve mental health are termed psychobiotics.133–135 It should be noted that psychobiotics do not necessarily have to target a clinical population but may also be intended for general use. Indeed, numerous studies are available reporting the beneficial effects of specific probiotics on mood.136–138 Human studies that involve probiotics or prebiotics and that have MDD symptoms or mood as an outcome measure are presented in Tables 2 and 3, respectively, with the reported effectiveness or lack thereof also reported. It must be stressed that, like any therapeutic, the type and quantity matter. Equally important, the beneficial effects observed in preclinical models need to be translated and confirmed in a human setting. In a relatively new field like the microbiome, a critical attitude and well-designed trials are not misplaced.
Table 2.
Studies of Microbial Modulation of Mood by Probiotics
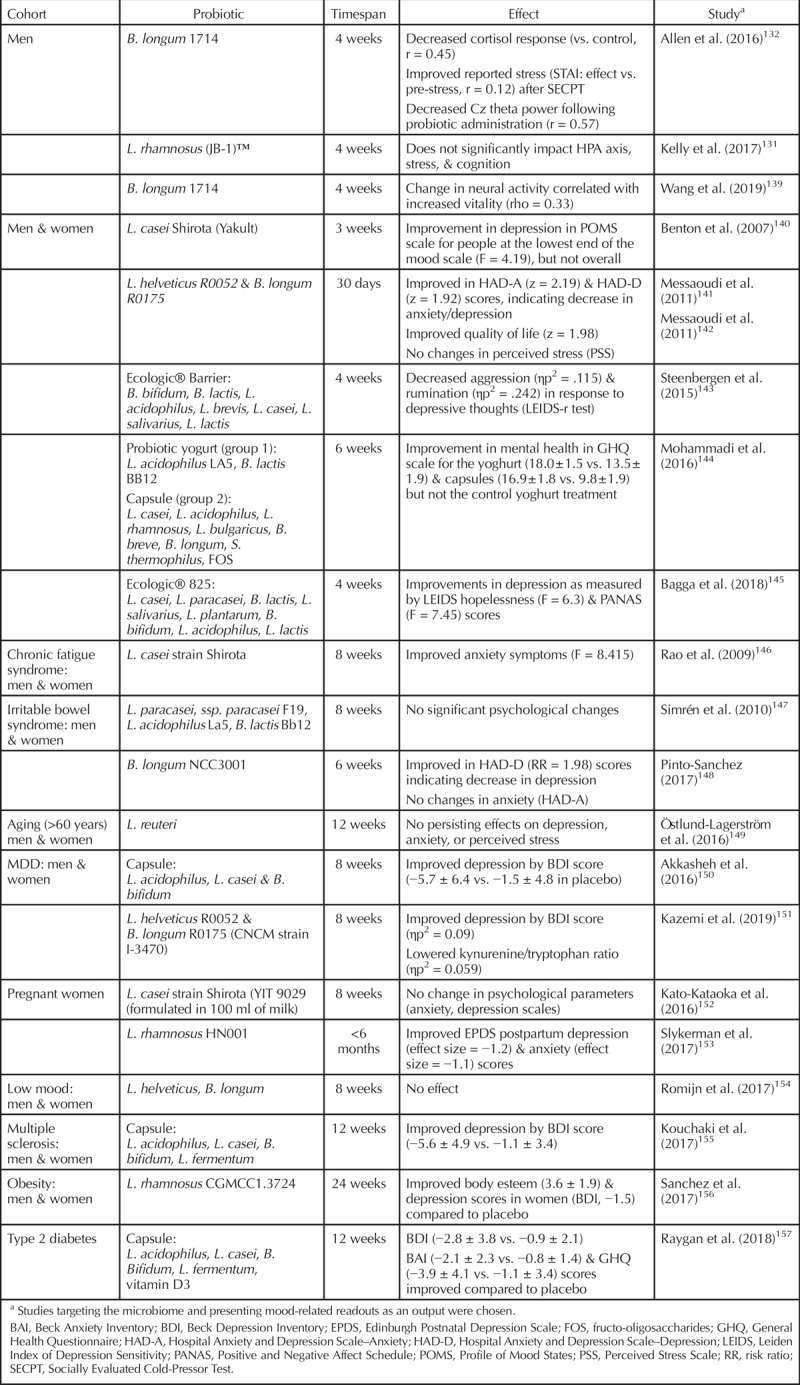
Table 3.
Studies of Microbial Modulation of Mood by Prebiotics
Fecal Microbiome Transplantation as a Therapy for MDD
Earlier in this review, studies were discussed where the microbiome was found to be a potential carrier of depressive mood.108,109 When looking at potential therapies, a fecal microbiome transplantation from a healthy donor represents a feasible approach. As discussed earlier in the review, microbiome features such as an altered microbial composition, or alpha diversity, are often associated with MDD symptoms. FMT has been shown to transfer these features to the recipient.160 While dedicated studies are not yet available, a recent study on the effect of FMT on inflammatory bowel disease has reported improved mood in recipients of a healthy microbiome (Table 4),161 suggesting potential clinical implications for this technique. More research is also warranted to investigate whether it is the microbes or their metabolites that have a beneficial effect through the FMT technique. For example, FMT with sterile fecal filtrate rather than fecal microbiota was sufficient to induce a therapeutic effect in patients with Clostridium difficile infection.162 This finding indicates that bacterial components, metabolites, or bacteriophages mediate some of the effects of FMT—which might represent an alternative approach to whole-microbiome FMT.163
Table 4.
Studies of Microbial Modulation of Mood by Fecal Microbiome Transplantation

CONCLUSIONS
In this review we have highlighted the role of the microbiome in MDD. Although this field of research is just emerging, accumulating evidence suggests that it deserves increasing attention in the biological psychiatry of MDD. The microbiome is altered in depressed patients, and common therapeutic drugs targeting MDD, such as SSRIs, affect the microbiome. The reverse is likely also true. Looking toward the future, the microbiome might be the place to probe when developing new treatments for MDD. An important future step in this field will be to translate the growing body of preclinical work into clinical practice, where the microbiome could be used as a tool to improve the patients’ response to psychiatric drugs such as antidepressants. The addition of microbiota profiling to MDD biomarkers already in place may provide further diagnostic precision and potentially improve personalized treatment. It is important to remember that MDD is a highly heterogeneous disorder and bears a complex pathophysiology. Genetic predisposition, environmental factors, such as significant psychosocial stress, and biological systems all play a role in the onset of this disorder. We support the concept that the gut microbiota be added to this model.
Throughout the review, we have stressed the need for more dedicated human studies on depression and the microbiome. Many of the existing studies in humans feature low sample sizes, likely confounding factors such as irritable bowel syndrome, or both. Mechanistic studies in clinically relevant populations are urgently needed. In this regard, randomized, controlled trials with multiple timepoints of microbiome collection are essential to tease out cause and effect. Moreover, longitudinal studies with probiotics, prebiotics, and other microbiota-targeted interventions are required to validate the psychobiotic approach. Interestingly, it is over 100 years ago since George Porter Philips put forward the concept of treating melancholia with lactobacillus;164 with more clinical research, we may be able to validate how much he was ahead of his time.
Declaration of interest
APC Microbiome Ireland has conducted research funded by many pharmaceutical and food companies. Drs. Dinan and Cryan have received research funding from 4D Pharma, Cremo, DuPont, Mead Johnson, Nutricia, and Suntory Wellness.
Footnotes
*Drs. Bastiaanssen and Cussotto contributed equally and have agreed to share first authorship.
Supported by Science Foundation Ireland Centre grant no. SFI/12/RC/2273 to the APC Microbiome Institute.
Original manuscript received 9 May 2019; revised manuscript received 27 July 2019, accepted for publication subject to revision 27 August 2019; revised manuscripts received 23 September 2019 and 26 October 2019.
REFERENCES
- 1.Metchnikoff II. The prolongation of life: optimistic studies. New York: Springer, 2004. [Google Scholar]
- 2.Scull A. Madhouse: a tragic tale of megalomania and modern medicine. New Haven: Yale University Press, 2005. [Google Scholar]
- 3.Methé BA, Nelson KE, Pop M, et al. A framework for human microbiome research. Nature 2012;486:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tigchelaar EF, Zhernakova A, Dekens JAM, et al. Cohort profile: LifeLines DEEP, a prospective, general population cohort study in the northern Netherlands: study design and baseline characteristics. BMJ Open 2015;5:e006772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science 2016;352:560–4. [DOI] [PubMed] [Google Scholar]
- 6.Goodrich JK, Davenport ER, Beaumont M, et al. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe 2016;19:731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012;488:178–84. [DOI] [PubMed] [Google Scholar]
- 9.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 2016;14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–12. [DOI] [PubMed] [Google Scholar]
- 11.Lyte M. Microbial endocrinology: host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes 2014;5:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schirmer M, Smeekens SP, Vlamakis H, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 2016;167:1125–36.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017;112:399–412. [DOI] [PubMed] [Google Scholar]
- 15.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009;6:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev 2019;99:1877–2013. [DOI] [PubMed] [Google Scholar]
- 17.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 2009;9:313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster JA, McVey Neufeld K-A. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 2013;36:305–12. [DOI] [PubMed] [Google Scholar]
- 19.Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. In: Lyte M, Cryan JF, eds. Microbial endocrinology: the microbiota-gut-brain axis in health and disease. New York: Springer, 2014:115–33. [DOI] [PubMed] [Google Scholar]
- 20.Fülling C, Dinan TG, Cryan JF. Gut microbe to brain signaling: what happens in vagus…. Neuron 2019;101:998–1002. [DOI] [PubMed] [Google Scholar]
- 21.Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012;490:55. [DOI] [PubMed] [Google Scholar]
- 22.Hu X, Wang T, Jin F. Alzheimer’s disease and gut microbiota. Sci China Life Sci 2016;59:1006–23. [DOI] [PubMed] [Google Scholar]
- 23.Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil 2013;25:713–9. [DOI] [PubMed] [Google Scholar]
- 24.Pereira J, Rea K, Nolan Y, O’Leary O, Dinan T, Cryan J. Depression’s unholy trinity: dysregulated stress, immunity, and the microbiome. Annu Rev Psychol 2019; Sep 30 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25.Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J Psychiatry Neurosci 2004;29:185. [PMC free article] [PubMed] [Google Scholar]
- 26.Heuser IJ, Schweiger U, Gotthardt U, et al. Pituitary-adrenal-system regulation and psychopathology during amitriptyline treatment in elderly depressed patients and normal comparison subjects. Am J Psychiatry 1996;153:93–9. [DOI] [PubMed] [Google Scholar]
- 27.Nickel T, Sonntag A, Schill J, et al. Clinical and neurobiological effects of tianeptine and paroxetine in major depression. J Clin Psychopharmacol 2003;23:155–68. [DOI] [PubMed] [Google Scholar]
- 28.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 2004;558:263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 2007;56:1522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goehler LE, Park SM, Opitz N, Lyte M, Gaykema RP. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav Immun 2008;22:354–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia citrobacter rodentium. Physiol Behav 2006;89:350–7. [DOI] [PubMed] [Google Scholar]
- 32.Wong ML, Inserra A, Lewis MD, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry 2016;21:797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valles-Colomer M, Falony G, Darzi Y, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol 2019;4:623–32. [DOI] [PubMed] [Google Scholar]
- 34.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 2005;4:775–90. [DOI] [PubMed] [Google Scholar]
- 35.Tannock GW, Savage DC. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun 1974;9:591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bharwani A, Mian MF, Foster JA, Surette MG, Bienenstock J, Forsythe P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology 2016;63:217–27. [DOI] [PubMed] [Google Scholar]
- 37.Partrick KA, Chassaing B, Beach LQ, McCann KE, Gewirtz AT, Huhman KL. Acute and repeated exposure to social stress reduces gut microbiota diversity in Syrian hamsters. Behav Brain Res 2018;345:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucking EF, O’Connor KM, Strain CR, et al. Chronic intermittent hypoxia disrupts cardiorespiratory homeostasis and gut microbiota composition in adult male guinea-pigs. EBioMedicine 2018;38:191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Connor KM, Lucking EF, Golubeva AV, et al. Manipulation of gut microbiota blunts the ventilatory response to hypercapnia in adult rats. EBioMedicine 2019;44:618–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoban AE, Stilling RM, Ryan FJ, et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 2016;6:e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stilling RM, Moloney GM, Ryan FJ, et al. Social interaction-induced activation of RNA splicing in the amygdala of microbiome-deficient mice. eLife 2018;7:e33070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thion MS, Low D, Silvin A, et al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 2018;172:500–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu J, Lu L, Yu Y, Cluette-Brown J, Martin CR, Claud EC. Effects of intestinal microbiota on brain development in humanized gnotobiotic mice. Sci Rep 2018;8:5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boehme M, van de Wouw M, Bastiaanssen TF, et al. Mid-life microbiota crises: middle age is associated with pervasive neuroimmune alterations that are reversed by targeting the gut microbiome. Mol Psychiatry 2019 May 16 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 45.Provensi G, Schmidt SD, Boehme M, et al. Preventing adolescent stress-induced cognitive and microbiome changes by diet. Proc Natl Acad Sci U S A 2019:116:9644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burokas A, Arboleya S, Moloney RD, et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry 2017;82:472–87. [DOI] [PubMed] [Google Scholar]
- 47.O’Mahony SM, McVey Neufeld K-A, Waworuntu RV, et al. The enduring effects of early life stress on the microbiota-gut-brain axis are buffered by dietary supplementation with milk fat globule membrane and a prebiotic blend. Eur J Neurosci 2019 Jul 24 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 48.Franklin CL, Ericsson AC. Microbiota and reproducibility of rodent models. Lab Anim (NY) 2017;46:114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen TLA, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech 2015;8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hugenholtz F, de Vos WM. Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci 2018;75:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 2011;108:3047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luczynski P, McVey Neufeld KA, Oriach CS, Clarke G, Dinan TG, Cryan JF. Growing up in a bubble: using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol 2016;19(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luczynski P, Whelan SO, O’Sullivan C, et al. Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur J Neurosci 2016;44:2654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 2009;1:6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang C, Yin A, Li H, et al. Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children. EBioMedicine 2015;2:968–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanchez-Villegas A, Henriquez P, Bes-Rastrollo M, Doreste J. Mediterranean diet and depression. Public Health Nutr 2006;9:1104–9. [DOI] [PubMed] [Google Scholar]
- 58.Firth J, Marx W, Dash S, et al. The effects of dietary improvement on symptoms of depression and anxiety: a meta-analysis of randomized controlled trials. Psychosom Med 2019;81:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacka FN, O’Neil A, Opie R, et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med 2017;15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Opie RS, O’Neil A, Jacka FN, Pizzinga J, Itsiopoulos C. A modified Mediterranean dietary intervention for adults with major depression: dietary protocol and feasibility data from the SMILES trial. Nutr Neurosci 2018;21:487–501. [DOI] [PubMed] [Google Scholar]
- 61.Dash S, Clarke G, Berk M, Jacka FN. The gut microbiome and diet in psychiatry: focus on depression. Curr Opin Psychiatry 2015;28:1–6. [DOI] [PubMed] [Google Scholar]
- 62.Hamaker BR, Tuncil YE. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J Mol Biol 2014;426:3838–50. [DOI] [PubMed] [Google Scholar]
- 63.Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res 2017;179:223–44. [DOI] [PubMed] [Google Scholar]
- 64.Dinan TG, Stanton C, Long-Smith C, et al. Feeding melancholic microbes: MyNewGut recommendations on diet and mood. Clin Nutr 2019;38:1995–2001. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC. Shifts on gut microbiota associated to Mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol 2018;9:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lassale C, Batty GD, Baghdadli A, et al. Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry 2019;24:965–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology 2005;30:846–56. [DOI] [PubMed] [Google Scholar]
- 68.Breslau N, Davis GC. Chronic stress and major depression. Arch Gen Psychiatry 1986;43:309–14. [DOI] [PubMed] [Google Scholar]
- 69.Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF. The microbiome: stress, health and disease. Mamm Genome 2014;25:49–74. [DOI] [PubMed] [Google Scholar]
- 70.Gur TL, Worly BL, Bailey MT. Stress and the commensal microbiota: importance in parturition and infant neurodevelopment. Front Psychiatry 2015;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Weerth C. Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci Biobehav Rev 2017;83:458–71. [DOI] [PubMed] [Google Scholar]
- 72.Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress 2017;7:124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kentner AC, Cryan JF, Brummelte S. Resilience priming: translational models for understanding resiliency and adaptation to early life adversity. Dev Psychobiol 2019;61:350–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zijlmans MA, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015;53:233–45. [DOI] [PubMed] [Google Scholar]
- 75.O’Mahony SM, Hyland NP, Dinan TG, Cryan JF. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology (Berl) 2011;214:71–88. [DOI] [PubMed] [Google Scholar]
- 76.Golubeva AV, Crampton S, Desbonnet L, et al. Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology 2015;60:58–74. [DOI] [PubMed] [Google Scholar]
- 77.Mudd AT, Berding K, Wang M, Donovan SM, Dilger RN. Serum cortisol mediates the relationship between fecal ruminococcus and brain N-acetylaspartate in the young pig. Gut Microbes 2017;8:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol 1999;35:146–55. [PubMed] [Google Scholar]
- 79.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 2011;25:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tarr AJ, Galley JD, Fisher SE, Chichlowski M, Berg BM, Bailey MT. The prebiotics 3'Sialyllactose and 6'Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: evidence for effects on the gut-brain axis. Brain Behav Immun 2015;50:166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thompson RS, Roller R, Mika A, et al. Dietary prebiotics and bioactive milk fractions improve NREM sleep, enhance REM sleep rebound and attenuate the stress-induced decrease in diurnal temperature and gut microbial alpha diversity. Front Behav Neurosci 2017;10:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferretti P, Pasolli E, Tett A, et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 2018;24:133–5.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liang X, FitzGerald GA. Timing the microbes: the circadian rhythm of the gut microbiome. J Biol Rhythms 2017;32:505–15. [DOI] [PubMed] [Google Scholar]
- 84.Thaiss CA, Zeevi D, Levy M, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014;159:514–29. [DOI] [PubMed] [Google Scholar]
- 85.Voigt RM, Forsyth CB, Green SJ, et al. Circadian disorganization alters intestinal microbiota. PLoS One 2014;9:e97500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Voigt RM, Summa KC, Forsyth CB, et al. The circadian clock mutation promotes intestinal dysbiosis. Alcohol Clin Exp Res 2016;40:335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liang X, Bushman FD, FitzGerald GA. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci U S A 2015;112:10479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wittmann M, Schreiber W, Landgrebe M, Hajak G. Circadian rhythms and depression. Fortschr Neurol Psychiatr 2018;86:308–18. [DOI] [PubMed] [Google Scholar]
- 89.Robillard R, Carpenter JS, Rogers NL, et al. Circadian rhythms and psychiatric profiles in young adults with unipolar depressive disorders. Transl Psychiatry 2018;8:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bermon S, Petriz B, Kajeniene A, Prestes J, Castell L, Franco OL. The microbiota: an exercise immunology perspective. Exerc Immunol Rev 2015;21:70–9. [PubMed] [Google Scholar]
- 91.Mika A, Van Treuren W, Gonzalez A, Herrera JJ, Knight R, Fleshner M. Exercise is more effective at altering gut microbial composition and producing stable changes in lean mass in juvenile versus adult male F344 rats. PLoS One 2015;10:e0125889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Queipo-Ortuno MI, Seoane LM, Murri M, et al. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One 2013;8:e65465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Evans CC, LePard KJ, Kwak JW, et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS One 2014;9:e92193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petriz BA, Castro AP, Almeida JA, et al. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics 2014;15:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013;500:541–6. [DOI] [PubMed] [Google Scholar]
- 96.Cronin O, Barton W, Moran C, et al. Moderate-intensity aerobic and resistance exercise is safe and favorably influences body composition in patients with quiescent inflammatory bowel disease: a randomized controlled cross-over trial. BMC Gastroenterol 2019;19:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Claesson MJ, Cusack S, O’Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A 2011;108 suppl 1:4586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jackson MA, Jeffery IB, Beaumont M, et al. Signatures of early frailty in the gut microbiota. Genome Med 2016;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scott KA, Ida M, Peterson VL, et al. Revisiting Metchnikoff: age-related alterations in microbiota-gut-brain axis in the mouse. Brain Behav Immun 2017;65:20–32. [DOI] [PubMed] [Google Scholar]
- 100.Thevaranjan N, Puchta A, Schulz C, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2017;21:455–6.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000;908:244–54. [DOI] [PubMed] [Google Scholar]
- 102.Beutel ME, Brähler E, Wiltink J, et al. New onset of depression in aging women and men: contributions of social, psychological, behavioral, and somatic predictors in the community. Psychol Med 2019;49:1148–55. [DOI] [PubMed] [Google Scholar]
- 103.Charlton RA, Lamar M, Zhang A, et al. Associations between pro-inflammatory cytokines, learning, and memory in late-life depression and healthy aging. Int J Geriatr Psychiatry 2018;33:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Prenderville JA, Kennedy PJ, Dinan TG, Cryan JF. Adding fuel to the fire: the impact of stress on the ageing brain. Trends Neurosci 2015;38:13–25. [DOI] [PubMed] [Google Scholar]
- 105.Bastiaanssen TFS, Cowan CSM, Claesson MJ, Dinan TG, Cryan JF. Making sense of ... the microbiome in psychiatry. Int J Neuropsychopharmacol 2019;22:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil 2014;26:1155–62. [DOI] [PubMed] [Google Scholar]
- 107.Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 2015;48:186–94. [DOI] [PubMed] [Google Scholar]
- 108.Zheng P, Zeng B, Zhou C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 2016;21:786–96. [DOI] [PubMed] [Google Scholar]
- 109.Kelly JR, Borre Y, O’Brien C, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 2016;82:109–18. [DOI] [PubMed] [Google Scholar]
- 110.Aizawa E, Tsuji H, Asahara T, et al. Possible association of bifidobacterium and lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord 2016;202:254–7. [DOI] [PubMed] [Google Scholar]
- 111.Clooney AG, Fouhy F, Sleator RD, et al. Comparing apples and oranges?: Next generation sequencing and its impact on microbiome analysis. PLoS One 2016;11:e0148028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vandeputte D, Kathagen G, D’hoe K, et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature 2017;551:507. [DOI] [PubMed] [Google Scholar]
- 113.Jeffery IB, Claesson MJ, O’toole PW, Shanahan F. Categorization of the gut microbiota: enterotypes or gradients? Nat Rev Microbiol 2012;10:591. [DOI] [PubMed] [Google Scholar]
- 114.Costea PI, Hildebrand F, Manimozhiyan A, et al. Enterotypes in the landscape of gut microbial community composition. Nat Microbiol 2018;3:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cussotto S, Clarke G, Dinan TG, Cryan JF. Psychotropics and the microbiome: a chamber of secrets. Psychopharmacology (Berl) 2019;236:1411–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Walsh J, Griffin BT, Clarke G, Hyland NP. Drug-gut microbiota interactions: implications for neuropharmacology. Br J Pharmacol 2018;175:4415–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lukić I, Getselter D, Ziv O, et al. Antidepressants affect gut microbiota and ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl Psychiatry 2019;9:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018;555:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cussotto S, Strain CR, Fouhy F, et al. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology (Berl) 2019;236:1671–85. [DOI] [PubMed] [Google Scholar]
- 120.Macedo D, Filho AJMC, Soares de Sousa CN, et al. Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J Affect Disord 2017;208:22–32. [DOI] [PubMed] [Google Scholar]
- 121.Munoz-Bellido JL, Munoz-Criado S, Garcia-Rodriguez JA. Antimicrobial activity of psychotropic drugs: selective serotonin reuptake inhibitors. Int J Antimicrob Agents 2000;14:177–80. [DOI] [PubMed] [Google Scholar]
- 122.Butler MI, Sandhu K, Cryan JF, Dinan TG. From isoniazid to psychobiotics: the gut microbiome as a new antidepressant target. Br J Hosp Med 2019;80:139–45. [DOI] [PubMed] [Google Scholar]
- 123.Rizkallah M, Saad R, Aziz R. The human microbiome project, personalized medicine and the birth of pharmacomicrobiomics. Curr Pharmacogenomics Pers Med 2010;8:182–93. [Google Scholar]
- 124.Clarke G, Sandhu KV, Griffin BT, Dinan TG, Cryan JF, Hyland NP. Gut reactions: breaking down xenobiotic-microbiome interactions. Pharmacol Rev 2019;71:198–224. [DOI] [PubMed] [Google Scholar]
- 125.Haiser HJ, Gootenberg DB, Chatman K, Sirasani G, Balskus EP, Turnbaugh PJ. Predicting and manipulating cardiac drug inactivation by the human gut bacterium eggerthella lenta. Science 2013;341:295–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol 2017;14:356–65. [DOI] [PubMed] [Google Scholar]
- 127.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359:91–7. [DOI] [PubMed] [Google Scholar]
- 128.Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry 2006;163:1519–30; quiz 665. [DOI] [PubMed] [Google Scholar]
- 129.Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 2017;14:491. [DOI] [PubMed] [Google Scholar]
- 130.Allen AP, Clarke G, Cryan JF, Quigley EMM, Dinan TG. Bifidobacterium infantis 35624 and other probiotics in the management of irritable bowel syndrome. Strain specificity, symptoms, and mechanisms. Curr Med Res Opin 2017;33:1349–51. [DOI] [PubMed] [Google Scholar]
- 131.Kelly JR, Allen AP, Temko A, et al. Lost in translation? The potential psychobiotic lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun 2017;61:50–9. [DOI] [PubMed] [Google Scholar]
- 132.Allen AP, Hutch W, Borre YE, et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry 2016;6:e939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anderson SC, Cryan JF, Dinan T. The psychobiotic revolution: mood, food, and the new science of the gut-brain connection. Washington, DC: National Geographic, 2017. [Google Scholar]
- 134.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry 2013;74:720–6. [DOI] [PubMed] [Google Scholar]
- 135.Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci 2016;39:763–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wallace CJK, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry 2017;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients 2016;8:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pirbaglou M, Katz J, de Souza RJ, Stearns JC, Motamed M, Ritvo P. Probiotic supplementation can positively affect anxiety and depressive symptoms: a systematic review of randomized controlled trials. Nutr Res 2016;36:889–98. [DOI] [PubMed] [Google Scholar]
- 139.Wang H, Braun C, Murphy EF, Enck P. Bifidobacterium longum 1714™ strain modulates brain activity of healthy volunteers during social stress. Am J Gastroenterol 2019;114:1152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr 2007;61:355–61. [DOI] [PubMed] [Google Scholar]
- 141.Messaoudi M, Lalonde R, Violle N, et al. Assessment of psychotropic-like properties of a probiotic formulation (lactobacillus helveticus R0052 and bifidobacterium longum R0175) in rats and human subjects. Br J Nutr 2011;105:755–64. [DOI] [PubMed] [Google Scholar]
- 142.Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (lactobacillus helveticus R0052 and bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011;2:256–61. [DOI] [PubMed] [Google Scholar]
- 143.Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun 2015;48:258–64. [DOI] [PubMed] [Google Scholar]
- 144.Mohammadi AA, Jazayeri S, Khosravi-Darani K, et al. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: a randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr Neurosci 2016;19:387–95. [DOI] [PubMed] [Google Scholar]
- 145.Bagga D, Reichert JL, Koschutnig K, et al. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes 2018;9:486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rao AV, Bested AC, Beaulne TM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog 2009;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Simrén M, Ohman L, Olsson J, et al. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome—a randomized, double-blind, controlled study. Aliment Pharmacol Ther 2010;31:218–27. [DOI] [PubMed] [Google Scholar]
- 148.Pinto-Sanchez MI, Hall GB, Ghajar K, et al. Probiotic bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 2017;153:448–59. [DOI] [PubMed] [Google Scholar]
- 149.Östlund-Lagerström L, Kihlgren A, Repsilber D, Björkstén B, Brummer RJ, Schoultz I. Probiotic administration among free-living older adults: a double blinded, randomized, placebo-controlled clinical trial. Nutr J 2016;15:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition 2016;32:315–20. [DOI] [PubMed] [Google Scholar]
- 151.Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin Nutr 2019;38:522–8. [DOI] [PubMed] [Google Scholar]
- 152.Kato-Kataoka A, Nishida K, Takada M, et al. Fermented milk containing lactobacillus casei strain shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef Microbes 2016;7:153–6. [DOI] [PubMed] [Google Scholar]
- 153.Slykerman RF, Hood F, Wickens K, et al. Effect of lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine 2017;24:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Romijn AR, Rucklidge JJ, Kuijer RG, Frampton C. A double-blind, randomized, placebo-controlled trial of lactobacillus helveticus and bifidobacterium longum for the symptoms of depression. Aust N Z J Psychiatry 2017;51:810–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kouchaki E, Tamtaji OR, Salami M, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr 2017;36:1245–9. [DOI] [PubMed] [Google Scholar]
- 156.Sanchez M, Darimont C, Panahi S, et al. Effects of a diet-based weight-reducing program with probiotic supplementation on satiety efficiency, eating behaviour traits, and psychosocial behaviours in obese individuals. Nutrients 2017;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Raygan F, Ostadmohammadi V, Bahmani F, Asemi Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry 2018;84:50–5. [DOI] [PubMed] [Google Scholar]
- 158.Azpiroz F, Dubray C, Bernalier-Donadille A, et al. Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: a randomized, double blind, placebo controlled study. Neurogastroenterol Motil 2017;29(2). [DOI] [PubMed] [Google Scholar]
- 159.Farhangi MA, Javid AZ, Sarmadi B, Karimi P, Dehghan P. A randomized controlled trial on the efficacy of resistant dextrin, as functional food, in women with type 2 diabetes: targeting the hypothalamic-pituitary-adrenal axis and immune system. Clin Nutr 2018;37:1216–23. [DOI] [PubMed] [Google Scholar]
- 160.Mazzawi T, Lied GA, Sangnes DA, et al. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS One 2018;13:e0194904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kurokawa S, Kishimoto T, Mizuno S, et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: an open-label observational study. J Affect Disord 2018;235:506–12. [DOI] [PubMed] [Google Scholar]
- 162.Ott SJ, Waetzig GH, Rehman A, et al. Efficacy of sterile fecal filtrate transfer for treating patients with clostridium difficile infection. Gastroenterology 2017;152:799–811.e7. [DOI] [PubMed] [Google Scholar]
- 163.Green J, Castle D, Berk M, et al. Faecal microbiota transplants for depression—Who gives a crapsule? Aust N Z J Psychiatry 2019;53:732–4. [DOI] [PubMed] [Google Scholar]
- 164.Phillips JGP. The treatment of melancholia by the lactic acid bacillus. J Ment Sci 1910;56:422–30. [Google Scholar]


