Abstract
Deletion 5q or monosomy 5 (-5/5q-) in acute myeloid leukemia (AML) is a common high-risk feature that is referred to allogeneic stem cell transplantation. However, -5/5q- is frequently associated with other high-risk cytogenetic aberrations such as complex karyotype, monosomal karyotype, monosomy 7 (-7), or 17p abnormalities (abn (17p)), the significance of which is unknown. In order to address this question, we studied adult patients with AML harboring -5/5q- having their first allogeneic transplantation between 2000 and 2015. Five hundred and one patients with -5/5q- have been analyzed. Three hundred and thirty-eight patients (67%) were in first remission and 142 (28%) had an active disease at time of allogeneic transplantation. The 2-year probabilities of overall survival and leukemia-free survival were 27% and 20%, respectively. The 2-year probability of treatment-related mortality was 20%. We identified four different cytogenetic groups according to additional abnormalities with prognostic impact: -5/5q- without complex karyotype, monosomal karyotype or abn(17p), -5/5q- within a complex karyotype, -5/5q- within a monosomal karyotype and the combination of -5/5q- with abn(17p). In multivariate analysis, factors associated with worse overall survival and leukemia-free survival across the four groups were active disease, age, monosomal karyotype, and abn(17p). The presence of -5/5q- without monosomal karyotype or abn(17p) was associated with a significantly better survival rate while -5/5q- in conjunction with monosomal karyotype or abn(17p) translated into a worse outcome. The patients harboring the combination of -5/5q- with abn(17p) showed very limited benefit from allogeneic transplantation.
Introduction
Allogeneic stem cell transplantation (SCT) is a standard of care in patients with intermediate and high-risk acute myeloid leukemia (AML).1,2 High-risk AML is mainly defined by the presence of determined poor-risk cytogenetic abnormalities at diagnosis together with specific mutational events.3–6 In general, conventional post-remission high-dose chemotherapy is not capable of eradicating the leukemic-initiating stem-cell population of high-risk AML, harboring strong chemoresistance mechanisms,7 and only the potent graft-versus-leukemia (GvL) effect mediated by SCT may provide the capability to eradicate this cell population and overcome the poor prognosis of these high-risk AML subtypes, as previously demonstrated.2,8–10 Among the heterogeneous group of high-risk AML, prognosis can be further stratified based on specific genetic abnormalities, and the potential benefit of SCT differs among these diverse AML subtypes.11 Monosomy 5 or deletion of the long arm of chromosome 5 (-5/5q) has been part of the definition of high-risk AML for many years.12 Furthermore, monosomal karyotype (MK) described ten years ago referred to a cytogenetic risk category constantly associated with a very poor outcome.13,14 Within this subgroup, patients harboring a single monosomy, including monosomy 5, have a relatively better outcome than patients with two or more monosomies.15 We recently reported the outcome of SCT in 125 patients with AML and abnormalities of the short arm of chromosome 17 [abn(17p)] transplanted in first remission. The addition of -5/5q- to abn(17p) translated into a very bad outcome with a 2-year leukemia-free survival (LFS) of about 12%.16 The benefit of SCT in this subgroup appears very limited, which raises the question of the role of SCT in these patients. However, this observation was based on a limited number of patients and it was difficult to draw any conclusions as to whether the dismal outcomes were driven by -5/5q- itself or by the combination of -5/5q- with abn(17p) or TP53 mutations. In addition, the frequent association of -5/5q- with abn(17p) suggests co-operation between TP53 deletion/mutations and loss of putative tumor suppressor genes localized in the commonly deleted 5q region.17-19 However, -5/5q- is also well-represented in patients with MK and complex karyotype (CK) without abn(17p). The interaction observed between -5/5q- and abn(17p) in our previous dataset raised the question of the impact of other additional adverse cytogenetic abnormalities such as monosomy 7 or deletion 7q (-7/7q-), abn(17p), CK and MK on the outcomes of AML with -5/5q- after SCT, and this formed the rationale for our current retrospective study.
Methods
Patient selection and data collection
This is a retrospective registry-based analysis on behalf of the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT). The EBMT is a non-profit, scientific society representing more than 600 transplant centers, mainly in Europe, that are required to report all consecutive stem cell transplantations and their follow up once a year. Data are entered, managed and maintained in a central database with internet access; each EBMT center is represented in this database. Audits are routinely performed to determine the accuracy of the data. Patients or legal guardians provide informed consent authorizing the use of their personal information for research purposes. The study was approved by the ALWP review board.
Eligibility criteria for the study included all patients >18 years with de novo or secondary AML transplanted between 1st January 2000 and 31st December 2015 from an HLA-matched sibling or a fully-matched (10/10) unrelated donor. Patients undergoing second transplantation, as well as patients receiving a haploidentical or cord-blood transplantation, were excluded. We further selected patients harboring -5/5q- and having a full karyotype report within the database in order to study the prognostic effect of additional cytogenetic features. A total of 501 patients from 148 centers met the study inclusion criteria and have been selected for further analysis. Myeloablative conditioning (MAC) and reduced-intensity conditioning (RIC) have been defined elsewhere.20
The following variables were selected and included in the analysis: year of transplantation, age, gender, white blood cell count (WBC) at diagnosis, number of induction courses to achieve complete remission (CR), status at transplantation, time from diagnosis to SCT, type of conditioning regimen, use of total body irradiation (TBI), in vivo T-cell depletion (including both anti-thymocyte globulins and alemtuzumab), cytomegalovirus (CMV) status of donor and recipient, donor type, source of stem cells, Karnofsky performance status (KPS) at transplantation, engraftment, presence of acute and chronic graft-versus-host disease (GvHD), and grade of acute GvHD. For the analysis of additional cytogenetic abnormalities, we included in our analysis the presence of abn(17p), -7/7q-, MK and CK classified according to cytogenetic status according to Medical Research Council UK criteria.5 MK has been defined according to Breems et al.,13 and CK was defined by the presence of >3 chromosomal abnormalities.
Statistical analysis and end point definitions
The primary end point was LFS. Secondary end points included relapse incidence (RI), non-relapse mortality (NRM), overall survival (OS), acute and chronic GvHD, and refined GvHD-free/relapse-free survival (GRFS). All outcomes were measured from the time of transplant. LFS was defined as survival without relapse; patients alive without relapse were censored at the time of last contact. OS was based on death from any cause. NRM was defined as death without previous relapse. GRFS was defined as survival without grade 3-4 acute GvHD, extensive chronic GvHD, relapse or death.21 Surviving patients were censored at the time of last contact. The probabilities of OS, LFS, and GRFS were calculated by the Kaplan-Meier test, and those of acute and chronic GvHD, NRM, and relapse by the cumulative incidence estimator to accommodate competing risks. For NRM, relapse was the competing risk, and for relapse, the competing risk was NRM. For acute and chronic GvHD, death without the event and relapse were the competing risks.
For all univariate analyses, continuous variables were categorized and the median value was used as a cut-off point. A Cox proportional hazards model was used for multivariate regression including factors associated with LFS in univariate analysis and individual cytogenetic abnormalities. Finally, we defined four groups according to the presence of CK, MK and the presence or not of individual cytogenetic abnormalities significantly associated with the outcome. Patients’, disease and transplant-related characteristics for the four groups were compared by using χ2 statistics for categorical variables and the Kruskall-Wallis test for continuous variables. Factors differing in distribution between the groups or conceptually important were included in the final Cox model. We performed a first multivariate analysis including the following individual cytogenetics: abn(11q23), abn(17p) and -7/7q-. Then, MK and CK were added to the same model and thereafter we performed a stepwise selection for the cytogenetics variables (P in/out =0.10). Abn 17p, MK and CK remained in the Cox model for OS. Abn 17p and MK also remained in the Cox model for relapse, LFS and GRFS. The final Cox model contained all variables that were selected for at least one end point. Proportional hazards assumptions were checked systematically using the Grambsch-Therneau residual-based test. All interactions between cytogenetics groups and other co-variates were tested. Results were expressed as hazard ratio (HR) with 95% confidence interval (CI). Statistical analyses were performed with SPSS 24.0 (SPSS Inc., Chicago, IL, USA) and R 3.4.1 [R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/.]
Results
Patients’ characteristics
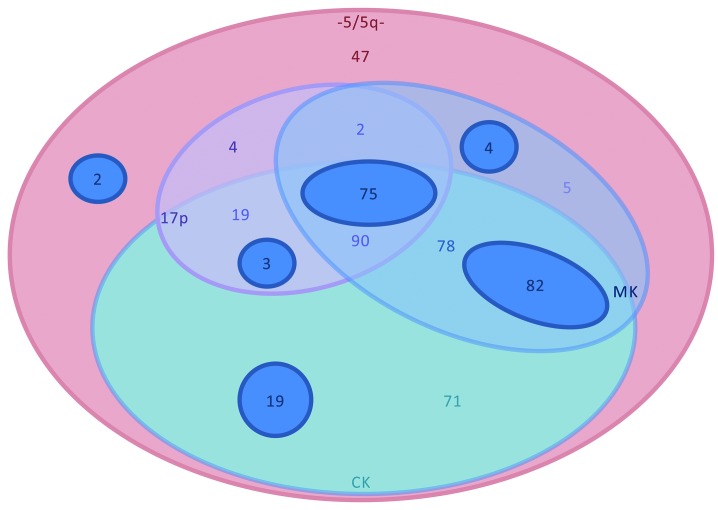
Patients’ characteristics are summarized in Table 1. Five hundred and one patients met the study inclusion criteria. Median follow up was 57 months [Interquartile Range (IQR): 27-116 months] and median age was 55-years old (range: 18-75 years). The main MAC regimen was the combination of cyclophosphamide with TBI followed by cyclophosphamide and busulfan, or fludarabine and busulfan. The main RIC regimen was the association of fludarabine and busulfan, followed by fludarabine and low-dose TBI, or fludarabine and melphalan. The most frequent GvHD prophylaxis was the association of cyclosporine and methotrexate (44%) followed by cyclosporine and mycophenolate mofetil (29%). Additional cytogenetic abnormalities besides -5/5q are illustrated in Figure 1. The vast majority of the patients showed a CK (87%) and/or MK (67%) in combination with -5/5q-. Patients also showed frequent association with abn(17p) (39%) and -7/7q- (37%), although the vast majority of these additional cytogenetic features were observed in the context of a complex or monosomal karyotype. Very few patients presented with abn(3q26) (n=22) or abn(11q23) (n=42). Most of those adverse cytogenetic features were not present as a single additional abnormality but rather existed in combination.
Table 1.
Patients’ characteristics from the entire cohort (n=501).
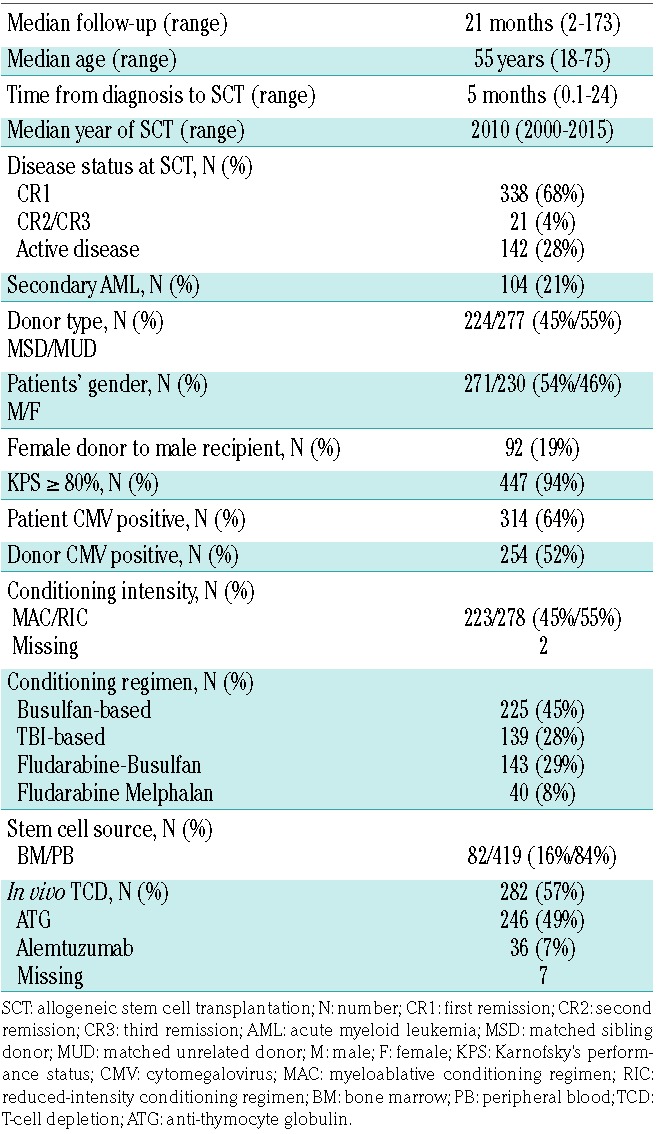
Figure 1.
Additional cytogenetic abnormalities. Distribution of additional cytogenetic abnormalities. Only 47 patients harbored -5/5q- without -7/7q-, CK, MK or abn(17p). The vast majority of the patients showed a CK (87%) and/or MK (67%) in combination with -5/5q-. The main groups were the combination of CK, MK and abn(17p), the association of MK and CK and the patients with CK. The dark blue circles illustrate patients with -7/7q- among the different cytogenetic groups.
Transplantation outcomes: relapse incidence, non-relapse mortality, leukemia-free survival, overall survival and graft-versus-host disease in the entire cohort
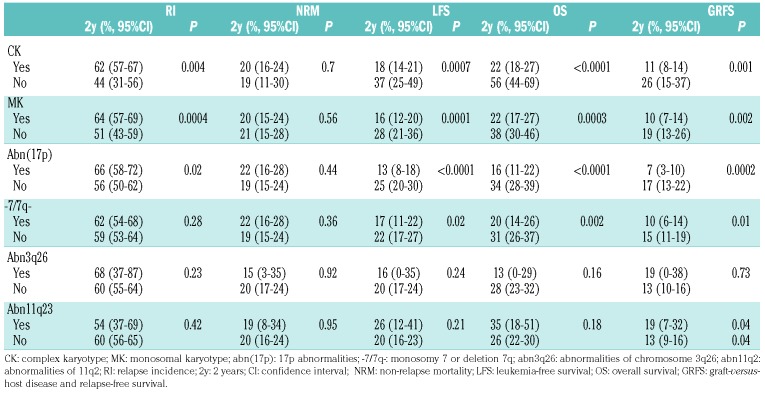
The 2-year cumulative incidence of relapse in the overall series was 59.9% (95%CI: 55.3-64.2) (Online Supplementary Figure S1A), and the median time to relapse was four months (IQR 0.2-130). In univariate analysis, a matched sibling donor (MSD), and the presence of additional cytogenetic abnormalities defined as CK, MK and abn(17p) were all associated with an increased RI (Table 2). The 2-year probability of NRM was 19.9% (95%CI: 16.4-23.7) (Online Supplementary Table S1B). NRM was strongly associated with donor type and disease status in univariate analysis. None of the additional cytogenetic events impacted NRM (Table 2).
Table 2.
Univariate analysis of additional cytogenetic abnormalities.
The 2-year probability of LFS in this entire cohort was 20.2% (95% CI: 16.4-23.9) (Online Supplementary Figure S1C). In univariate analysis, we found that younger age (< 55-year old), being in first complete remission (CR1), better KPS (>80%) and administration of MAC were all significantly associated with better LFS. CK, MK, abn(17p) and -7/7q- also impacted on LFS (Table 2). The 2-year OS was 27% (95%CI: 22.8-31.2) (Online Supplementary Figure S1D). Similarly to LFS, younger age (< 55-year old), being in CR1, better KPS (>80%) and administration of MAC led to better OS in univariate analysis. Notably, CK, MK, abn(17p) and -7/7q- impacted prognosis (Table 2).
The cumulative incidence of grade II-IV acute GvHD was 29.3% (95%CI: 25.2-33.4) and the 2-year cumulative incidence of chronic GvHD was 27.3% (95%CI: 23.2-31.5), leading to a 2-year probability of GRFS of 13.1% (95%CI: 10-16.3). In univariate analysis, the use of MUD and not being in remission at SCT were associated with a higher incidence of grade II-IV acute GvHD. In contrast, advanced disease status was associated with a lower risk of chronic GvHD, a fact probably due to the high risk of early relapse among patients not transplanted in remission. A female donor to a male recipient led to higher incidence of chronic GvHD in univariate analysis. The presence of additional cytogenetic abnormalities was not asso ciated with the risk of developing acute or chronic GvHD. The factors associated with GRFS were the same as those described for LFS and OS (see above and Table 2). The main cause of death was disease-related (61%), followed by infections (16%) and GvHD (13%).
The multivariate analysis performed in the entire cohort confirmed the strong impact of disease status at the time of transplantation on RI, NRM, LFS and OS (Online Supplementary Table S1). Increasing age was associated with higher NRM, which translated into significantly worse LFS and OS without impacting RI. The use of MUD was associated with higher NRM with no effect on OS. A good performance status at SCT was associated with less relapse and improved LFS and OS. Conditioning intensity did not impact any SCT outcome parameters in multivari ate analysis. While active disease at SCT and MUD were associated with higher incidence of grade II-IV GvHD, no factor was associated with chronic GvHD in multivariate analysis (Online Supplementary Table S2). In our stepwise selection of cytogenetic variables (as described in the methods), -7/7q- lost any significance on outcomes and we kept only CK, MK and abn(17p) in our final multivariate model. There was a significant correlation between Abn(17p) and decreased LFS, OS and GRFS.
Outcomes by cytogenetic subgroups
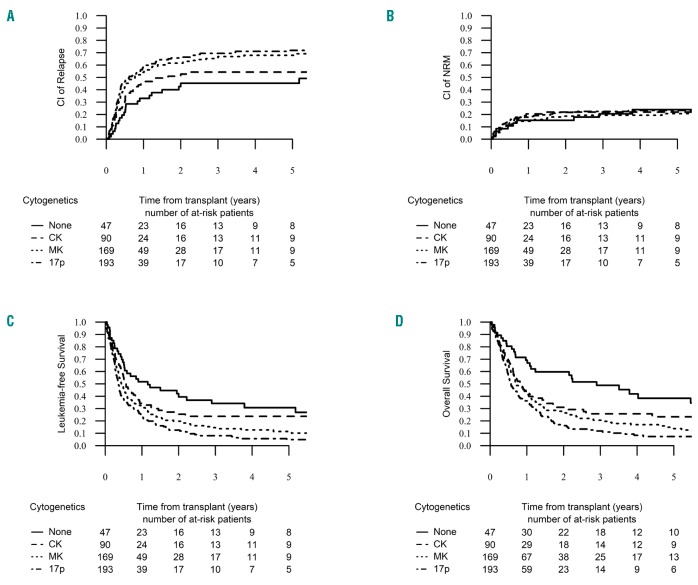
In order to elucidate the impact of additional cytogenetic abnormalities on outcomes of patients with AML and -5/5q-, we defined four different subgroups within our entire cohort in a hierarchical manner according to the presence of CK, MK and abn(17p), based on their prognostic impact shown in univariate and multivariate analysis and their capability to distinguish biologically and clinically meaningful cytogenetic categories. Our study contains 154 monosomy 5 and 347 deletion 5q. We decided to study -5 and 5q- together in order to analyze the impact of MK separately from -5. Indeed, all of our -5 patients except one fulfilled the definition of MK. Thus, the “5q sole group” contained 47 patients with 5q abnormalities but absence of additional -7/7q-, abn(17p), CK or MK. Notably, no case of -5 was included in this group. The “CK group” included 90 patients who fulfilled the definition of CK but without abn(17p) or MK. Only one patient with -5 was included in this group. The “MK group” was comprised of the group of patients with -5/5q- within a MK with the exception of abn(17p), and finally, the “abn(17p) group” encompassed the combination of -5/5q- with abn(17p) regardless of the presence of other cytogenetic features. Due to the lack of significance of -7/7q- in our multivariate analysis, this abnormality was not taken into account in our prognostic classification. Patients’ characteristics were well balanced between those four cytogenetic subgroups (Online Supplementary Table S3). The 2-year probability of NRM was similar across the four groups (P=0.86), but the 2-year cumulative incidence of relapse increased significantly from the “5q sole” up to the “abn(17p)” group, reaching 45.3% (95%CI: 29.9-59.5), 52.7% (95%CI: 40.9-63.1), 61.5% (95%CI: 53.5-68.6) and 65.7% (95%CI: 58.1-72.3) in the 5q sole, CK, MK and abn(17p) groups, respectively (P=0.006) (Figure 2A and B). Median time to relapse was 6.2 months (IQR: 3.5-16.3), for the “5q sole group”, 4.7 months (IQR: 2.3-8) for the “CK group”, 4.5 months (IQR: 2.3-9.1) for the “MK group” and 3.9 months (IQR: 2.2-8.7) for the “abn(17p) group” (P=0.12). This different RI across cytogenetic subgroups also determined other important outcomes. Thus, the 2-year probability of LFS was 39.4% (95%CI: 24.8-54) for the “5q sole group”, 25.4% (95%CI: 15.6-35.3) for the “CK group”, 19.8% (95%CI: 13.5-26.1) for the “MK group” and 12.6% (95%CI: 7.5-17.7) for the “abn(17p) group” (P<0.001) (Figure 2C). The 2-year probability of OS also decreased significantly from the “5q sole group” down to the “abn(17p) group”, reaching 59.7% (95% CI: 45.2-74.2, 31% [95% CI: 20.5-41.6], 6.5% [95% CI: 19.4-33.5] and 16.3% [95% CI: 10.5-22] in each group, respectively (P<0.001) (Figure 2D). The 2-year probability of GRFS followed the same trend, with 26.5% [95% CI: 13-39.9] for the “5q sole group”, 17% [95% CI: 8.5-25.4] for the “CK group”, 14.2% [95% CI: 8.7-19.7] for the “MK group” and only 6.6% [95% CI: 2.8-10.4] for the “abn(17p) group” (P<0.001) (Online Supplementary Figure S2). In contrast, the cumulative incidence of grade II-IV acute GvHD and the 2-year cumulative incidence of chronic GvHD were not different across the four groups (P=0.33 and P=0.8, respectively).
Figure 2.
Relapse incidence (RI), non-relapse mortality (NRM), leukemia-free survival (LFS) and overall survival (OS) by cytogenetic groups. The 2-year cumulative incidence of relapse increased significantly from the “none group” up to the “abn(17p) group”, reaching 45.3% [95% CI: 29.9-59.5], 52.7% [95% CI: 40.9-63.1], 61.5% [95% CI: 53.5-68.6] and 65.7% [95% CI: 58.1-72.3] in the none, CK, MK and abn(17p) groups, respectively (P=0.006) (A). The 2-year probability of NRM was similar across the four groups, reaching 19.9% [95% CI: 16.4-23.7] (P=0.86) (B). The 2-year probability of LFS was 39.4% [95% CI: 24.8-54] for the “none group”, 25.4% [95% CI: 15.6-35.3] for the “CK group”, 19.8% [95% CI: 13.5-26.1] for the “MK group” and 12.6% [95% CI: 7.5-17.7] for the “abn(17p) group” (P<0.001) (C). The 2-year probability of OS decreased significantly from the “none group” down to the “abn(17p) group”, reaching 59.7% [95% CI: 45.2-74.2], 31% [95% CI: 20.5-41.6], 26.5% [95% CI: 19.4-33.5] and 16.3% [95% CI: 10.5-22] in each group respectively (P<0.001) (D).
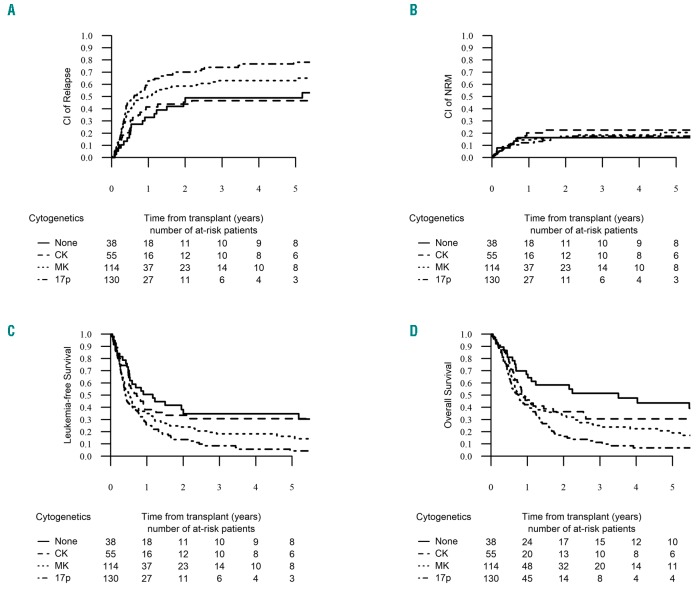
In multivariate analysis, taking the “5q sole group” as a reference, the “CK group” did not show any significant difference in RI, NRM, LFS, OS, LFS and GRFS. In contrast, patients in the “MK group” and “abn(17p) group” experienced higher incidence of RI and lower LFS, OS and GRFS compared to the “5q sole group” (Table 3). To minimize the strong impact of disease status on outcome, we decided to run the univariate by cytogenetic subgroups focusing on the 338 patients transplanted in CR1. The “MK group” and “abn(17p) group” had the same negative impact on RI, LFS and OS (Figure 3). As detailed above, the presence of -7/7q- was excluded from the “5q sole group”, but was present in 21% of the “CK group”, 51% of the “MK group”, and 40% of the “abn(17p) group”. Given the high overlap between the -7/7q and the “MK group”, we then performed a univariate analysis within the “MK group” comparing the outcome between patients with presence or absence of additional -7/7q- and did not find any significant impact on RI, NRM, LFS, OS and GRFS. We also looked at the impact of MK within the “abn(17p)”; MK lost its negative impact in this very high-risk subgroup, even though the group of abn(17p) patients without MK was rather small (n=26) (data not shown).
Table 3.
Multivariate analysis using a Cox proportional hazard model by cytogenetic subgroups. Only variables with a P<0.05 in univariate analysis.
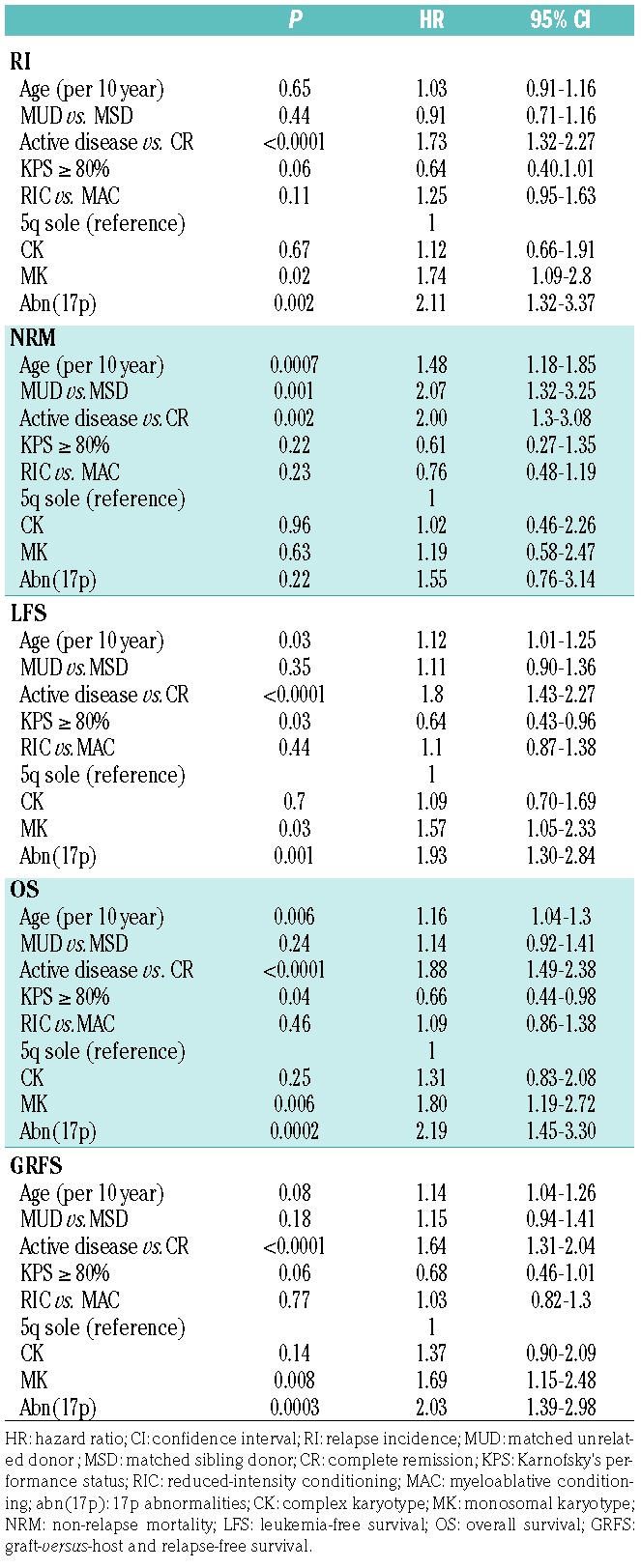
Figure 3.
Relapse incidence (RI), non-relapse mortality (NRM), leukemia-free survival (LFS) and overall survival (OS) by cytogenetic groups in patients in first remission. The 2-year cumulative incidence of relapse increased significantly from the “none group” up to the “abn(17p) group”, reaching 48.9% [95% CI: 30.9-64.7], 43.9% [95% CI: 29.6-57.4], 58.7% [95% CI: 48.7-67.4] and 70.1% [95% CI: 60.6-77.8] in the none, CK, MK and abn(17p) groups, respectively (P=0.006) (A). The 2-year probability of NRM was similar across the four groups, reaching 19.9% [95% CI: 16.4-23.7] (P=0.87) (B). The two-year probability of LFS was 34.7% [95% CI: 18.6-50.9] for the “none group”, 33.5% [95% CI: 19.9-47] for the “CK group”, 23.9% [95% CI: 15.7-32] for the “MK group” and 13.6% [95% CI: 7-20.2] for the “abn(17p) group” (P<0.001) (C). The 2-year probability of OS decreased significantly from the “none group” down to the “abn(17p) group”, reaching 58.5% [95% CI: 42.3-74.6], 36.5% [95% CI: 22.6-50.3], 33% [95% CI: 23.9-42] and 16.1% [95% CI: 8.9-23.3] in each group, respectively (P<0.001) (D).
Discussion
-5/5q- is a common finding in AML, consistently associated with poor outcomes after standard chemotherapy with long-term overall survival of about 5%.5,22 SCT has been shown to significantly improve the outcome of high-risk AML subsets, with a probability of disease cure in the range of 40%.1,8,9,23 Nonetheless, in our large cohort of 501 AML patients harboring -5/5q undergoing first SCT, the 2-year probability of OS and LFS was only 27% and 20%, respectively, outcomes which clearly appear inferior to those reported for other high-risk cytogenetic AML,8 suggesting an independent deleterious effect of -5/5q- on transplant outcome. Indeed, in the EBMT registry, we found 3,021 patients with adverse cytogenetics according to the MRC classification with the exception of -5/5q-, and we found a 2-year OS and LFS of 43% and 37%, respectively. In contrast, our results resemble those of patients with MK AML.24–26 Indeed, most of the patients in our cohort harbored additional adverse cytogenetic features, such as MK (67%), which may have been confounded with the true impact of -5/5q-. Moreover, inferior outcomes of this cohort may also be explained by the fact that about 30% of our patients had active disease at the time of SCT, which appears, as expected, to be a strong predictor for worse outcomes in multivariate analysis.8 Nevertheless, even when focusing on patients in CR1, the observed outcomes in the current cohort are still in the range of 25% at two years, suggesting that our population represents a higher-risk group. Not surprisingly, younger age and a better performance status were both associated with better OS and LFS in line with previously published data,1,27,28 but this observation should be weighed against the underlying selection bias inherent in such a registry-based study. Conditioning intensity lost all impact on outcomes in multivariate analysis. This observation has been confirmed in other studies where the benefit of conditioning intensity was lost in chemorefractory disease, such as MK AML and those involving TP53 deregulation.26,29
The main objective of our study was to evaluate the impact of additional cytogenetic abnormalities in a cohort of AML patients with -5/5q-. The presence of -5/5q- is rarely an isolated event in AML as it is frequently associated with other adverse cytogenetic features, such as CK, MK, -7/7q- or abn(17p).5,22,30 The independent impact of -5/5q- was questioned by Breems et al. in the first report on MK, in which any single monosomy carried a better outcome than the full definition of MK,13,15,31 with no specific effect for -5/5q-. More recently, Middeke et al. described 236 high-risk AML patients after SCT, and found that -5/5q- was associated with worse outcomes compared to CK and/or MK AML, and that abn(17p) translated into the worst survival after SCT.32 Those data suggested that the bad prognosis of MK AML after SCT was mainly related to the presence of -5/5q- and/or abn(17p), but these observations have not been completely confirmed by others.15,33 In our multivariate Cox model, we found that either the presence of MK or abn(17p) were both significantly associated with worse OS and LFS, while CK and -7/7q- had no impact on any outcome parameter. Most of those additional cytogenetic abnormalities and/or characteristics are typically not present as a single additional event to -5/5q- (Figure 1) making it difficult to weigh the impact of each individual additional event. To avoid the confounding effect of largely overlapping cytogenetic categories, we decided to define four well-delimited groups based on a hierarchical prognostic effect of MK and additional abn(17p) in -5/5q- AML: the “5q sole group”, “CK group”, “MK group”, and “abn(17p) group”. These cytogenetic categorizations allowed us to confirm the strong deleterious prognostic effect of additional MK and abn(17p) in this entity in multivariate analysis. In contrast, we did not observe differences in any outcome parameters between the “5q sole group” and the “CK group” with a relatively better 2-year OS (close to 40% for patients transplanted in CR1). The additional cytogenetic abnormalities found in both of those groups could only be numerical abnormalities and some structural abnormalities. The weaker prognostic impact of numerical abnormalities such as trisomy has already been suggested in other studies.13,34 On the contrary, the presence of -5/5q- within MK is translated into worse LFS and OS, which is in agreement with most published data,24,33 but different from the report from Middeke et al.32 Finally, we confirmed the deleterious effect on outcomes of the combination of -5/5q- with any abn(17p), which has been suggested from our previous dataset.16 The impact of abn(17p) clearly appears stronger than MK, as MK did not impact outcomes within the “abn(17p)” group.
Patients with -5/5q- AML in CR1 without MK and/or abn(17p) appear to benefit from allogeneic SCT, with long-term survival achieved in more than 40% of the patients. In contrast, patients harboring the combination of -5/5q- with abn(17p) represent a very poor subgroup due to an intrinsic and well-known chemoresistance and to a potential lack of sensitivity to a GvL effect.16 If SCT remains the only option for those high-risk patients in CR1, it should be integrated into a post-transplant intervention program including low-dose decitabine,35 prophylactic donor leukocyte infusions,36,37 a combination of both or other P53-independent therapeutic agents. Lenalidomide has been shown to have a specific effect on myelodysplastic syndrome (MDS) with isolated 5q-through inhibition of the 5q- clone, leading to 60% hematologic response and 40% cytogenetic response.38–40 However, responses have been much lower in patients with higher-risk MDS and AML, especially if harboring CK or MK.38 Combinations with standard chemotherapy or hypomethylating agents are associated with objective responses even in patients harboring high-risk features38,41 with the exception of TP53 mutated clones.42,43 Another option might be to integrate lenalidomide as maintenance therapy after SCT, but previous experiences raised serious concerns about an increased risk of acute GvHD.44,45 However, interesting results from the combination of lenalidomide and azacytidine have been recently published.46
In conclusion, our study, based on a large cohort of patients with AML and -5/5q- undergoing SCT, showed that this strategy led to long-term survival in about 20% of the patients, which seems inferior to other high-risk AML subsets. One of the largest limitations in this study might be the lack of centralized cytogenetic analysis and the selection of patients with an available full cytogenetic report; an essential requirement for the proposed analysis. Active disease at the time of SCT remains the strongest prognostic factor of worse survival and precautions have to be taken when bridging these patients to SCT. Novel therapeutic pre-transplant strategies must be developed to increase the proportion of patients in remission before SCT. Finally, we found that the benefit from SCT in this cytogenetic entity is highly dependent on the presence of particular additional adverse cytogenetic features. Indeed, patients without MK or abn(17p) benefit the most from SCT, whereas the additional presence of MK and/or abn(17p) leads to a very poor outcome. SCT is therefore questionable in this subgroup of patients with the current standard approach, especially if they are not in CR1 at the time of SCT. Development of pre-transplant and post-transplant pharmacological and immunological interventions to sustain a response in a larger proportion of patients is urgently needed in these patients.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/2/414
References
- 1.Cornelissen JJ, van Putten WL, Verdonck LF, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007;109(9): 3658–3666. [DOI] [PubMed] [Google Scholar]
- 2.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. [DOI] [PubMed] [Google Scholar]
- 4.Dohner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015;373(12):1136–1152. [DOI] [PubMed] [Google Scholar]
- 5.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. [DOI] [PubMed] [Google Scholar]
- 6.Grimwade D, Ivey A, Huntly BJ. Molecular landscape of acute myeloid leukemia in younger adults and its clinical relevance. Blood. 2016;127(1):29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoch C, Kern W, Kohlmann A, et al. Acute myeloid leukemia with a complex aberrant karyotype is a distinct biological entity characterized by genomic imbalances and a specific gene expression profile. Genes Chromosomes Cancer. 2005;43(3):227–238. [DOI] [PubMed] [Google Scholar]
- 8.Schlenk RF, Dohner K, Mack S, et al. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German-Austrian trial AMLHD98A. J Clin Oncol. 2010;28(30):4642–4648. [DOI] [PubMed] [Google Scholar]
- 9.Stelljes M, Beelen DW, Braess J, et al. Allogeneic transplantation as post-remission therapy for cytogenetically high-risk acute myeloid leukemia: landmark analysis from a single prospective multicenter trial. Haematologica. 2011;96(7):972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Versluis J, Hazenberg CL, Passweg JR, et al. Post-remission treatment with allogeneic stem cell transplantation in patients aged 60 years and older with acute myeloid leukaemia: a time-dependent analysis. Lancet Haematol. 2015;2(10):e427–e436. [DOI] [PubMed] [Google Scholar]
- 11.Ferrant A, Labopin M, Frassoni F, et al. Karyotype in acute myeloblastic leukemia: prognostic significance for bone marrow transplantation in first remission: a European Group for Blood and Marrow Transplantation study. Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Blood. 1997;90(8):2931–2938. [PubMed] [Google Scholar]
- 12.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92(7):2322–2333. [PubMed] [Google Scholar]
- 13.Breems DA, Van Putten WL, De Greef GE, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26(29):4791–4797. [DOI] [PubMed] [Google Scholar]
- 14.Haferlach C, Alpermann T, Schnittger S, et al. Prognostic value of monosomal karyotype in comparison to complex aberrant karyotype in acute myeloid leukemia: a study on 824 cases with aberrant karyotype. Blood. 2012;119(9):2122–2125. [DOI] [PubMed] [Google Scholar]
- 15.Jang JE, Min YH, Yoon J, et al. Single monosomy as a relatively better survival factor in acute myeloid leukemia patients with monosomal karyotype. Blood Cancer J. 2015;5:e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poire X, Labopin M, Maertens J, et al. Allogeneic stem cell transplantation in adult patients with acute myeloid leukaemia and 17p abnormalities in first complete remission: a study from the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT). J Hematol Oncol. 2017;10(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castro PD, Liang JC, Nagarajan L. Deletions of chromosome 5q13.3 and 17p loci cooperate in myeloid neoplasms. Blood. 2000;95(6):2138–2143. [PubMed] [Google Scholar]
- 18.Kulasekararaj AG, Smith AE, Mian SA, et al. TP53 mutations in myelodysplastic syndrome are strongly correlated with aberrations of chromosome 5, and correlate with adverse prognosis. Br J Haematol. 2013;160(5):660–672. [DOI] [PubMed] [Google Scholar]
- 19.Stoddart A, Fernald AA, Wang J, et al. Haploinsufficiency of del(5q) genes, Egr1 and Apc, cooperate with Tp53 loss to induce acute myeloid leukemia in mice. Blood. 2014;123(7):1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggeri A, Labopin M, Ciceri F, et al. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transplant. 2016;51(4):610–611. [DOI] [PubMed] [Google Scholar]
- 22.Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100(13):4325–4336. [DOI] [PubMed] [Google Scholar]
- 23.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–4083. [PubMed] [Google Scholar]
- 24.Brands-Nijenhuis AV, Labopin M, Schouten HC, et al. Monosomal karyotype as an adverse prognostic factor in patients with acute myeloid leukemia treated with allogeneic hematopoietic stem-cell transplantation in first complete remission: a retrospective survey on behalf of the ALWP of the EBMT. Haematologica. 2016;101(2):248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornelissen JJ, Breems D, van Putten WL, et al. Comparative analysis of the value of allo-geneic hematopoietic stem-cell transplantation in acute myeloid leukemia with monosomal karyotype versus other cytogenetic risk categories. J Clin Oncol. 2012;30(17): 2140–2146. [DOI] [PubMed] [Google Scholar]
- 26.Poire X, Labopin M, Cornelissen JJ, et al. Outcome of conditioning intensity in acute myeloid leukemia with monosomal karyotype in patients over 45 year-old: A study from the acute leukemia working party (ALWP) of the European group of blood and marrow transplantation (EBMT). Am J Hematol. 2015;90(8):719–724. [DOI] [PubMed] [Google Scholar]
- 27.Sorror M, Storer B, Sandmaier BM, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112(9):1992–2001. [DOI] [PubMed] [Google Scholar]
- 28.Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32(29):3249–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindsley RC, Saber W, Mar BG, et al. Prognostic Mutations in Myelodysplastic Syndrome after Stem-Cell Transplantation. N Engl J Med. 2017;376(6):536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strickland SA, Sun Z, Ketterling RP, et al. Independent Prognostic Significance of Monosomy 17 and Impact of Karyotype Complexity in Monosomal Karyotype/Complex Karyotype Acute Myeloid Leukemia: Results from Four ECOG-ACRIN Prospective Therapeutic Trials. Leuk Res. 2017;59:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raza S, TaherNazerHussain F, Patnaik M, et al. Autosomal monosomies among 24,262 consecutive cytogenetic studies: prevalence, chromosomal distribution and clinicopathologic correlates of sole abnormalities. Am J Hematol. 2011;86(4):353–356. [DOI] [PubMed] [Google Scholar]
- 32.Middeke JM, Beelen D, Stadler M, et al. Outcome of high-risk acute myeloid leukemia after allogeneic hematopoietic cell transplantation: negative impact of abnl(17p) and -5/5q. Blood. 2012;120(12): 2521–2528. [DOI] [PubMed] [Google Scholar]
- 33.Breems DA, Van Putten WL, Lowenberg B. The impact of abn(17p) and monosomy -5/del(5q) on the prognostic value of the monosomal karyotype in acute myeloid leukemia. Blood. 2013;121(15):3056–3057. [DOI] [PubMed] [Google Scholar]
- 34.Chilton L, Hills RK, Harrison CJ, et al. Hyperdiploidy with 49-65 chromosomes represents a heterogeneous cytogenetic subgroup of acute myeloid leukemia with differential outcome. Leukemia. 2014;28(2): 321–328. [DOI] [PubMed] [Google Scholar]
- 35.Pusic I, Choi J, Fiala MA, et al. Maintenance Therapy with Decitabine after Allogeneic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transplant. 2015;21(10):1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsirigotis P, Byrne M, Schmid C, et al. Relapse of AML after hematopoietic stem cell transplantation: methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant. 2016;51(11):1431–1438. [DOI] [PubMed] [Google Scholar]
- 37.Yafour N, Beckerich F, Bulabois CE, et al. How to prevent relapse after allogeneic hematopoietic stem cell transplantation in patients with acute leukemia and myelodysplastic syndrome. Curr Res Transl Med. 2017;65(2):65–69. [DOI] [PubMed] [Google Scholar]
- 38.Ades L, Prebet T, Stamatoullas A, et al. Lenalidomide combined with intensive chemotherapy in acute myeloid leukemia and higher-risk myelodysplastic syndrome with 5q deletion. Results of a phase II study by the Groupe Francophone Des Myelodysplasies. Haematologica. 2017; 102(4):728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Kantarjian H, Estrov Z, et al. A phase II study of lenalidomide alone in relapsed/refractory acute myeloid leukemia or high-risk myelodysplastic syndromes with chromosome 5 abnormalities. Clin Lymphoma Myeloma Leuk. 2012;12(5):341–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Germing U, Lauseker M, Hildebrandt B, et al. Survival, prognostic factors and rates of leukemic transformation in 381 untreated patients with MDS and del(5q): a multicenter study. Leukemia. 2012;26(6):1286–1292. [DOI] [PubMed] [Google Scholar]
- 41.Sekeres MA, O’Keefe C, List AF, et al. Demonstration of additional benefit in adding lenalidomide to azacitidine in patients with higher-risk myelodysplastic syndromes. Am J Hematol. 2011;86(1):102–103. [DOI] [PubMed] [Google Scholar]
- 42.Jadersten M, Saft L, Smith A, et al. TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression. J Clin Oncol. 2011;29(15):1971–1979. [DOI] [PubMed] [Google Scholar]
- 43.Sebaa A, Ades L, Baran-Marzack F, et al. Incidence of 17p deletions and TP53 mutation in myelodysplastic syndrome and acute myeloid leukemia with 5q deletion. Genes Chromosomes Cancer. 2012;51(12):1086–1092. [DOI] [PubMed] [Google Scholar]
- 44.Alsina M, Becker PS, Zhong X, et al. Lenalidomide maintenance for high-risk multiple myeloma after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(8):1183–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sockel K, Bornhaeuser M, Mischak-Weissinger E, et al. Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk myelodysplastic syndromes or acute myeloid leukemia and del(5q): results of the LENAMAINT trial. Haematologica. 2012;97(9):e34–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craddock C, Slade D, De Santo C, et al. Combination Lenalidomide and Azacitidine: A Novel Salvage Therapy in Patients Who Relapse After Allogeneic Stem-Cell Transplantation for Acute Myeloid Leukemia. J Clin Oncol. 2019:JCO1800889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.