Abstract
Ischemic stroke is caused by a thromboembolic occlusion of cerebral arteries. Treatment is focused on fast and efficient removal of the occluding thrombus, either via intravenous thrombolysis or via endovascular thrombectomy. Recanalization, however, is not always successful and factors contributing to failure are not completely understood. Although the occluding thrombus is the primary target of acute treatment, little is known about its internal organization and composition. The aim of this study, therefore, was to better understand the internal organization of ischemic stroke thrombi on a molecular and cellular level. A total of 188 thrombi were collected from endovascularly treated ischemic stroke patients and analyzed histologically for fibrin, red blood cells (RBC), von Willebrand factor (vWF), platelets, leukocytes and DNA, using bright field and fluorescence microscopy. Our results show that stroke thrombi are composed of two main types of areas: RBC-rich areas and platelet-rich areas. RBC-rich areas have limited complexity as they consist of RBC that are entangled in a meshwork of thin fibrin. In contrast, platelet-rich areas are characterized by dense fibrin structures aligned with vWF and abundant amounts of leukocytes and DNA that accumulate around and in these platelet-rich areas. These findings are important to better understand why platelet-rich thrombi are resistant to thrombolysis and difficult to retrieve via thrombectomy, and can guide further improvements of acute ischemic stroke therapy.
Introduction
Ischemic stroke is mainly caused by a thrombus that is occluding one or multiple arteries in the brain. As a consequence of the impaired cerebral blood flow, irreversible damage occurs in the associated brain tissue. Currently, only two US Food and Drug Administration (FDA)-approved treatment regimens are available to remove the thrombus and thus recanalize the occluded blood vessel in stroke patients: (i) pharmacological thrombolysis using recombinant tissue plasminogen activator (rt-PA), which promotes degradation of fibrin in the thrombus; and (ii) mechanical removal of the thrombus via endovascular thrombectomy.
Despite recent advances, efficient recanalization in ischemic stroke patients remains a challenge. rt-PA can only be administered within the first 4.5 hours after the onset of stroke symptoms due to the risk of cerebral bleeding when treatment is delayed. As a consequence, rt-PA treatment is available to less than 15% of patients in most European countries.1 Strikingly, even in patients who receive rt-PA, more than half fail to respond to the drug.2,3 Factors contributing to this so-called rt-PA resistance are not well understood, but size and characteristics of the thrombus itself are thought to play an important role. As of 2015, several positive trials have instigated large scale implementation of endovascular treatment, based on mechanical removal of the occluding thrombus.4–9 These positive trials have shown the benefits of this approach, but also revealed procedural challenges that can hamper efficient treatment. One of the most important obstacles in endovascular therapy is that thrombi tend to differ in consistency and removability. Indeed, mechanical thrombectomy is not successful in removing the thrombus in up to 20% of the patients.10 Beside vascular access, thrombus composition is considered an important factor responsible for thrombectomy failure.10,11
In spite of the fact that the occluding thrombus is the primary target in both pharmacological and mechanical recanalization therapy, very little is known about the general composition and structural organization of stroke thrombi or about the interplay between their cellular and molecular components. The main reason for this lack of knowledge was the unavailability of stroke thrombi in the past. However, endovascular thrombectomy procedures now provide patient thrombus material for detailed analysis.11
Good understanding of thrombus structure and composition will be crucial to meet the pressing demand for improved pharmacological or endovascular recanalization efficiency in acute stroke treatment. An increasing number of studies have now started to report first insights into stroke thrombus composition, mostly based on Hematoxylin & Eosin (H&E) staining and looking at fibrin and red blood cells (RBC) only. However, more specific staining can reveal novel molecular and cellular markers that could be extremely important for stroke pathophysiology. The aim of this study was to assess and define the internal organization and common structural features of stroke thrombi, using specific immunohistochemical and immunofluorescence histology procedures.
Methods
Patient thrombi
Thrombi (n=188) were collected from acute ischemic stroke patients after a thrombectomy procedure was performed at the AZ Groeninge Hospital in Kortrijk, Belgium, regardless of prior treatment with rt-PA. All patients or their legal representative gave written consent under the approval of the AZ Groeninge Hospital ethical committee (AZGS2015065). Thrombi were retrieved using a stent retriever and/or aspiration device according to the judgement of the treating neurointerventionalist. Thrombus material collected from multiple passes of one patient was pooled and further considered as one thrombus. Of the 188 collected thrombi, eleven thrombi were excluded because insufficient material was available to perform all analyses.
Thrombus histology
After retrieval, thrombi were gently removed from the device, washed in saline and immediately incubated in 4% paraformaldehyde for 24 hours at room temperature. Next, samples were embedded in paraffin and cut into 5 mm sections. To check for differences in content throughout the thrombus, sections were analyzed for fibrin, RBC, platelets, and von Willebrand Factor (vWF) every 75 mm in randomly selected thrombi. No substantial differences in the quantity and general organization of these components were found between different sections of a single thrombus.12 Thus, one section per thrombus, exposing a large thrombus surface, was deemed representative and was used to quantify the Martius Scarlet Blue (MSB) staining.
Thrombus sections were stained with H&E (HT110216, Sigma-Aldrich, St. Louis, MO, USA), Martius Scarlet Blue [fibrin (dark pink/red) and RBC (yellow) staining] or Feulgen’s reaction [DNA staining (pink), 1079070001, Merck Chemicals, MA, USA]. Alternatively, thrombus sections were examined via immunohisto-chemistry and immunofluorescence for the presence of vWF (A008202-2, Dako, Glostrup, Denmark, and ab11713, Abcam, Cambridge, UK, respectively), platelets (GPIbα, MA5-11642, Invitrogen, Waltham, MA, USA), fibrin(ogen) (A0080, Dako), and leukocytes (CD45, 304002, Biolegend, San Diego, CA, USA). For immunohistochemical stainings, nucleated cells were stained green using a Methyl Green solution (H-3402, Vector Laboratories). Images were acquired using a single slide scanner (Nanozoomer SQ, Hamamatsu Photonics, Japan). For immunofluorescent stainings, DNA was stained using 4,6-diamidino-2-phenylindole (DAPI, P36935, Invitrogen). RBC were visualized via their inherent autoflu-orescence at a wavelength of 555 nm. Images from immunofluores-cent stainings were acquired using an Axio Observer Z1 inverted fluorescent microscope (Zeiss, Carl Zeiss AG, Oberkochen, Germany) or a laser scanning confocal microscope (LSM710, Zeiss). Images were processed by Zen 2012 (blue edition, version 2.3, Zeiss) software. Negative controls of the immunohistochemical (Online Supplementary Figure S1A-D) and immunofluorescent (Online Supplementary Figure S1E and F) staining were achieved by omission of the primary antibody or by using isotype primary antibodies. A more detailed description of all histology procedures is provided in the Online Supplementary Methods.
Results
Red blood cell-rich and platelet-rich areas form distinct structural components of stroke thrombi
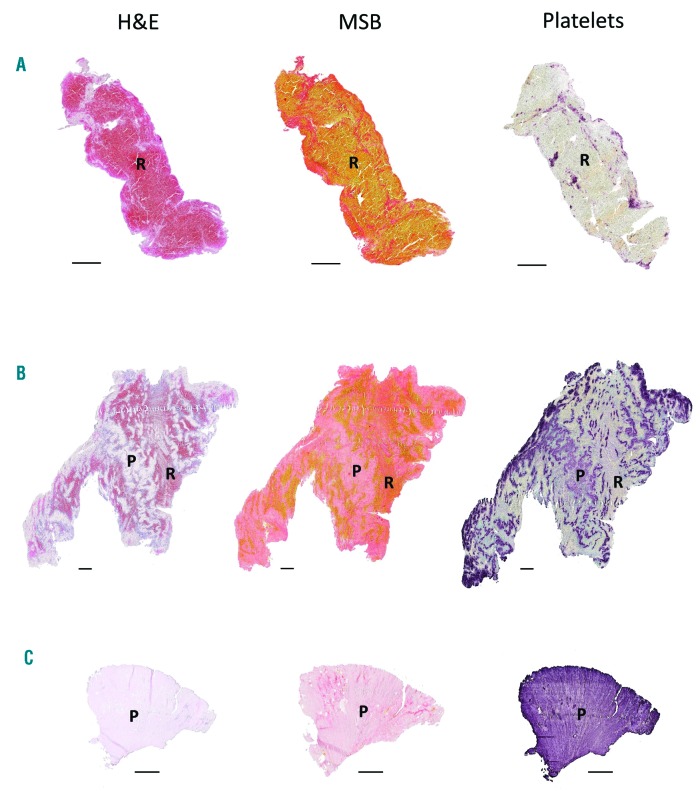
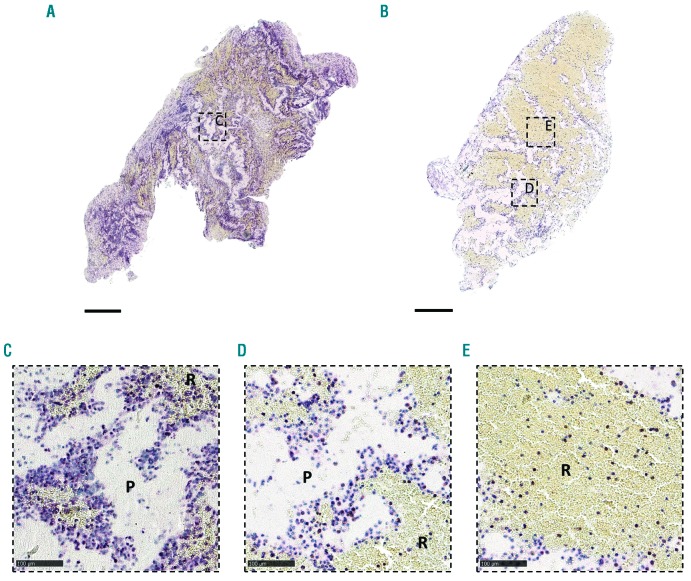
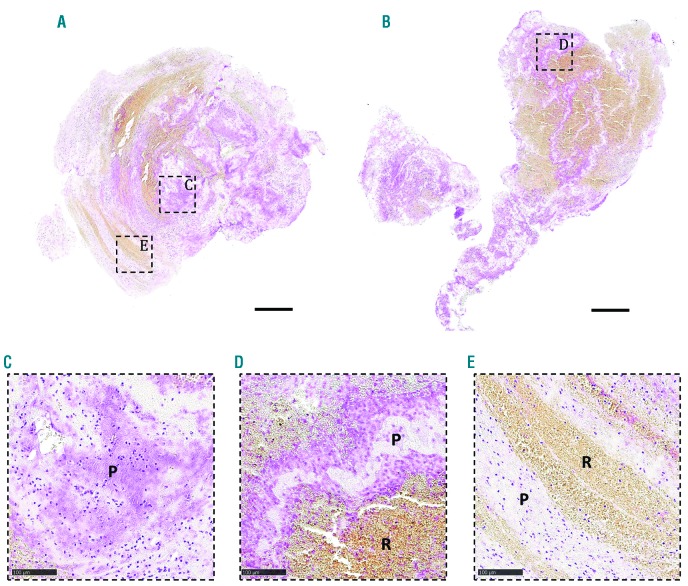
To better understand the structural features of acute ischemic stroke thrombi, we collected and histologically analyzed 177 thrombi retrieved by thrombectomy from patients with ischemic stroke. As shown in Online Supplementary Figure S2, the macroscopic appearance of retrieved thrombi was heterogenous in size, shape and color. All thrombi were sectioned and stained with H&E and MSB to visualize their general internal organization. H&E allows identification of fibrin/platelet aggregates (pink), RBC (red), and nucleated cells (dark blue) (Figure 1, left), whereas MSB staining selectively demonstrates the presence of fibrin (dark pink/red), RBC (yellow), and collagen (blue) (Figure 1, middle). H&E and MSB stainings revealed a typical common pattern in all thrombi, which is the presence of two distinct types of thrombus material: (i) RBC-rich/fibrin-poor material that appears red on H&E stainings and yellow on MSB stainings; and (ii) RBC-poor/fibrin-rich areas that appear as light pink areas on H&E staining and pink to red areas on MSB stainings. Interestingly, large amounts of blood platelets are only present in the RBC-poor/fibrin-rich areas and not in the RBC-rich areas, as shown via platelet-specific immunostaining (Figure 1, right). Consequently, thrombi can be RBC-rich/platelet-poor (Figure 1A), mixed (Figure 1B), or RBC-poor/platelet-rich (Figure 1C). Based on these clear and distinct differences, RBC-rich/fibrin-poor areas will be referred to as RBC-rich (R), whereas the term platelet-rich (P) will be used to indicate the RBC-poor/fibrin-rich/platelet-rich areas. Blue MSB staining, indicative for collagen, was not observed.
Figure 1.
Stroke thrombi typically consist of distinct red blood cell (RBC)-rich and platelet-rich areas. Consecutive thrombus sections were stained with Hematoxylin & Eosin (H&E), Martius Scarlet Blue (MSB) and an anti-platelet GPIbα antibody. Classical H&E staining (left) was used to visualize overall thrombus composition and organization. On H&E staining, RBC-rich areas appear red whereas RBC-poor areas appear light pink. On MSB staining (middle), red areas show the presence of fibrin, whereas RBC appear yellow. Platelets were stained purple using an anti-GPIbα antibody (right). Overall, stroke thrombi consist of two distinct areas: RBC-rich areas (R), and platelet-rich areas (P). Examples of representative thrombi are shown, which are RBC-rich/platelet-poor (A), mixed (B), and RBC-poor/platelet-rich (C). Scale = 500 mm.
Relative contribution of red blood cell-rich and platelet-rich regions
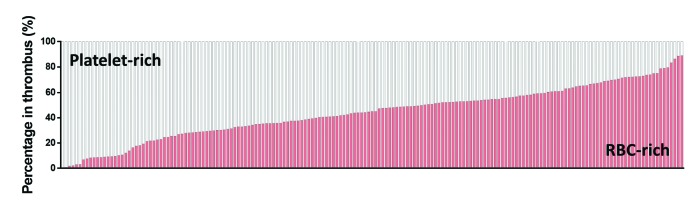
To assess the relative contribution of each type of thrombus material, we quantified the total amount of RBC-rich and platelet-rich areas for all thrombi (Figure 2). The amount of RBC-rich and platelet-rich areas varied between thrombi. Whereas some thrombi were platelet-dominant, others were RBC-dominant (Figure 2). Overall, the amount of RBC-rich material ranged from 0.4% to 89.0% (mean 43.9±20.4%) , which corresponds to a range from 11% to 99.6% (mean 56.1±20.4%) for platelet-rich material. Typically, both regions are interspersed through each other within a thrombus. However, some thrombi typically consisted of an RBC-rich core that was surrounded by platelet-rich material (Online Supplementary Figure S3).
Figure 2.
General stroke thrombus composition. Stroke thrombi (n=177, vertical bars) were quantitatively analyzed and the percentage of red blood cell (RBC)-rich areas (red) and platelet-rich areas (white) were determined. Thrombus composition ranges from very platelet-rich to very RBC-rich areas, with almost all thrombi containing significant amounts of both areas.
The relative amount of RBC-rich or platelet-rich material most likely affects the way stroke thrombi react to pharmacological or endovascular intervention. To better understand the specific characteristics of both regions, we performed a more detailed microscopic analysis.
Red blood cell-rich areas are composed of packed red blood cells within a meshwork of fibrin
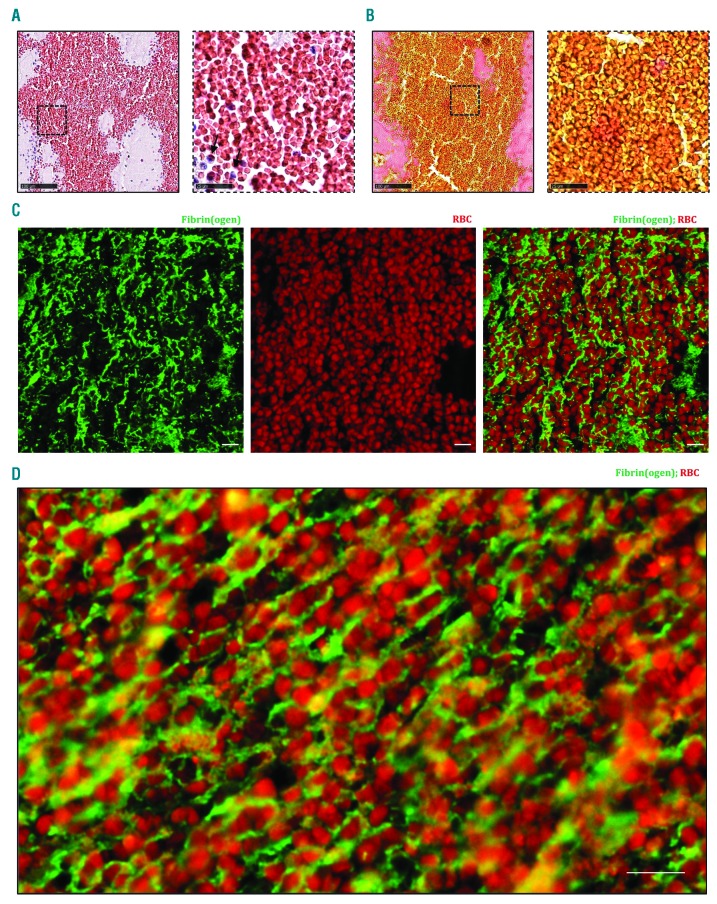
Higher magnifications of RBC-rich areas stained by H&E and MSB showed the presence of RBC that were packed together with little or no nucleated cells (Figure 3A and B). To further characterize these RBC-rich zones, immunofluorescent stainings were performed for fibrin(ogen), platelets and vWF. RBC were identified based on their inherent autofluorescence. Interestingly, fluorescent co-stainings showed densely-packed RBC within a meshwork of thin fibrin strands (Figure 3C and D). vWF (sometimes together with platelets) was only rarely detected and did not form a main constituent of these RBC-rich areas (Online Supplementary Figure S4). Taken together, the RBC-rich portion of ischemic stroke thrombi is composed of rather homogeneously distributed RBC that are densely packed and embedded in thin fibrin strands, with no other main structural components.
Figure 3.
Red blood cell (RBC)-rich areas are composed of densely packed RBC in a fibrin network. Hematoxylin & Eosin (H&E) staining (A) and Martius Scarlet Blue (MSB) staining (B) show the abundance of RBC with little or no nucleated cells (black arrows), appearing red in H&E staining and yellow in MSB staining. Fibrin is stained red in MSB staining. (A and B, right panels) Magnification of the area indicated in the left panel. Occasional presence of nucleated cells in RBC-rich areas (blue on H&E) is observed. (C and D) Immunofluorescent staining was performed to specifically visualize fibrin(ogen) (green) and RBC (autofluorescence, red). RBC are found within a network of fibrin(ogen). Scale bars are: (A and B, left panels) 100 mm; (A and B, right panels) 25 mm; (C and D) 10 mm.
Platelet-rich areas consist of dense structures of fibrin, von Willebrand Factor and platelets
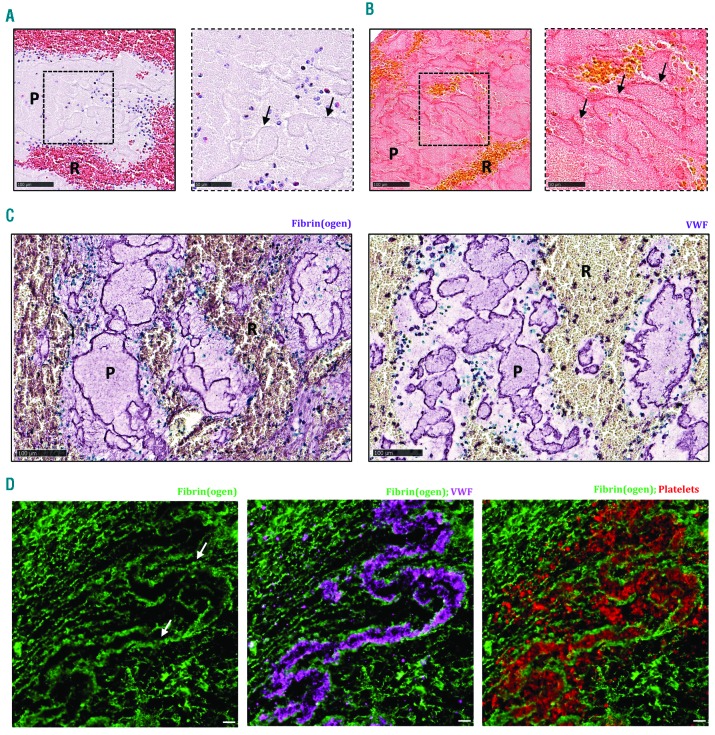
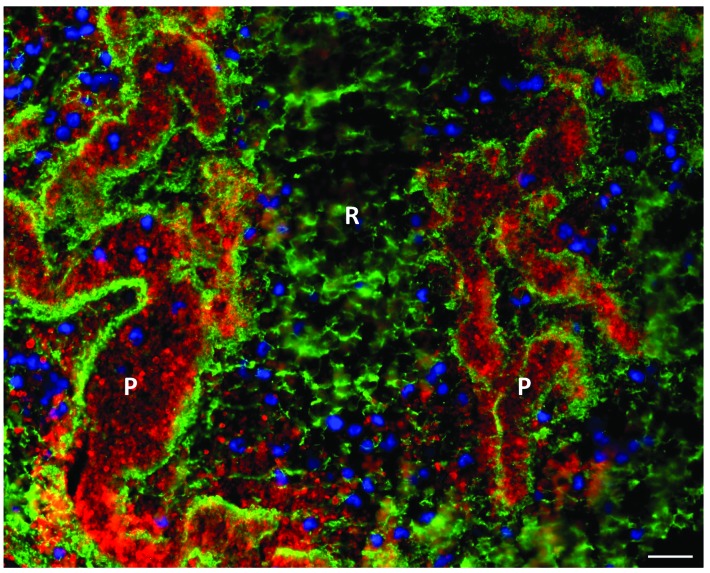
Platelet-rich thrombus material appeared light pink on H&E staining, without clear cellular elements (Figure 4A). MSB staining showed pink, dense fibrin structures that were clearly visible throughout the platelet-rich regions (Figure 4B). To gain more detailed insight into the microstructural organization of these platelet-rich areas, both immunohistochemical and immunofluorescent co-stainings were performed combining fibrin(ogen) with vWF and/or platelets. In accordance with the MSB staining, fluorescence microscopy for fibrin(ogen) confirmed the dense fibrin structures that demarcate platelet-rich zones (Figure 4C and D). Remarkably, co-staining with vWF showed that these dense fibrin structures are also positive for vWF (Figure 4C and D), suggesting an interaction between vWF and fibrin. Together, fibrin and vWF delineate substructures that are filled with platelets. Indeed, co-staining of platelets and fibrin(ogen) or vWF shows the presence of such platelet islands within these structures of fibrin and vWF. Hence, in contrast to the RBC-rich zones, the platelet-rich zones contain no RBC, but are mainly composed of dense formations of fibrin and vWF, packed with platelets. Figure 5 shows a typical overview picture of both zones in one thrombus, illustrating on the one hand platelet-rich areas surrounded by dense fibrin with little fibrin between platelets and on the other hand RBC-rich areas with densely packed RBC (not stained) entangled in a thin fibrin network.
Figure 4.
Platelet-rich areas consist of dense fibrin structures lined with von Willebrand Factor (vWF) and filled with platelets. Hematoxylin & Eosin (H&E) staining (A) and Martius Scarlet Blue (MSB) staining (B) show the presence of dense fibrin structures, indicated by the black arrows, within platelet-rich areas. (C) Immunohistochemical staining was used to specifically visualize fibrin(ogen) (left panel, purple) and vWF (right panel, purple). (D) Immunofluorescence analysis allowed to visualize fibrin (green), vWF (purple), and platelets (red). Dense fibrin structures (white arrows) are lined with vWF and filled with platelets. Scale bars are: (A and B, left panels, and C) 100 μm; (A and B, right panels) 25 mm; (D) 10 mm. P: platelet-rich area; R: RBC-rich area.
Figure 5.
Immunofluorescent overview picture of red blood cell (RBC)-rich and platelet-rich areas. Immunofluorescent analysis was used to visualize fibrin(ogen) (green), platelets (red), and nuclei (blue). RBC-rich areas consist of thin fibrin strands and RBC (not stained), whereas platelet-rich areas consist of dense fibrin structures packed with platelets. Nucleated cells were mainly found near platelet-rich areas. Scale bar = 20 mm. P: platelet-rich area; R: RBC-rich area.
Leukocytes and DNA are mainly present on the interface between red blood cell-rich and platelet-rich areas
We and others have previously shown the abundant presence of leukocytes in stroke thrombi, but apart from their presence, not much is known about their specific cellular or molecular distribution.11,13 Remarkably, when staining for RBC-rich and platelet-rich areas, we observed that leukocytes are primarily found at the interface between RBC-rich and platelet-rich areas (Figure 6A-D and Online Supplementary Figure S5). Besides their specific presence in these boundary zones, leukocytes are also abundantly present within the platelet-rich zones. In contrast, leukocytes are not commonly found in RBC-rich areas, where, if present, they are homogenously distributed throughout the RBC (Figure 6E).
Figure 6.
Leukocytes accumulate in platelet-rich areas and at the interface between platelet-rich and red blood cell (RBC)-rich areas. Stroke thrombi were immunohistochemically analyzed for leukocytes (purple). (A and B) Two representative images of stroke thrombi stained for leukocytes. (C-E) Magnifications show that leukocytes tend to accumulate in platelet-rich areas (C) or at the boundary between platelet-rich and RBC-rich areas (D), whereas leukocytes are homogenously spread within RBC-rich areas (E). Scale bars are: (A and B) 500 mm; (C-E) 100 mm. P: platelet-rich area; R: RBC-rich area.
Interestingly, leukocytes have been shown to promote thrombus formation by the formation of extracellular DNA traps. We and others recently described the presence of neutrophil extracellular DNA traps (NET) in acute ischemic stroke thrombi.14,15 To further examine the presence and internal organization of DNA networks in stroke thrombi, we performed a highly sensitive Feulgen’s DNA staining on a subset of 100 stroke thrombi. Strikingly, large extracellular DNA networks, appearing as extracellular smears, were seen throughout the majority of thrombi. Again, abundant amounts of extracellular DNA were observed particularly in the platelet-rich areas and in the boundary areas between platelet-rich and RBC-rich regions (Figure 7). No extracellular DNA was found within the RBC-rich regions (Figure 7E). In conclusion, leukocytes and networks of extracellular DNA were found specifically on the interface of the platelet-rich and RBC-rich regions, and in the platelet-rich regions.
Figure 7.
Extracellular DNA accumulates in platelet-rich areas and on the interface between platelet-rich and red blood cell (RBC)-rich areas. Stroke thrombi were stained using a Feulgen’s reaction to visualize intra (nuclei) and extracellular (smears) DNA (pink). (A and B) Two representative images of stroke thrombi stained for DNA. (C-E) Magnifications show that extracellular DNA tends to accumulate within platelet-rich areas (C) or at the boundary between platelet-rich and RBC-rich areas (D). No extracellular DNA is observed in RBC-rich areas (E). Scale bar: (A and B) 500 μm; (C-E) 100 mm. P: platelet-rich area; R: RBC-rich area.
Discussion
This study provides a detailed description of compositional features of ischemic stroke thrombi. We found that stroke thrombi consist of RBC-rich and platelet-rich areas. RBC-rich areas have a limited complexity and are composed of densely packed RBC that are contained in a mesh-work of thin fibrin, with a small number of leukocytes that are spread homogeneously throughout the RBC. In contrast, platelet-rich areas are more complex and contain various scaffolds that include fibrin, vWF and DNA. Dense fibrin structures are aligned with vWF and are packed with platelets. In addition, leukocytes and DNA tend to accumulate within the platelet-rich areas and on the interface between platelet- and RBC-rich areas. Why different parts of the same thrombus have such distinct underlying architecture is an intriguing point. Local hemodynamic forces are known to regulate the thrombotic pathways and thus the biochemical make-up of thrombi. Interestingly, also thrombus contraction, which is mediated by contractile forces of platelets on fibrin, has been suggested to mediate spatial separation of RBC and platelet aggregates.16 Thrombus contraction leads to the compression of RBC to so-called polyhedrocytes, forming large clusters of densely packed RBC, and to redistribution of platelets to the exterior.16 Although our histology does not allow us to unequivocally identify the typical convex, irregular polyhedral structure of compressed RBC, some confocal images (e.g. Figure 3C) are reminiscent of polyhedrocytes observed in contracted thrombi.16,17 Thrombus contraction was reported to be reduced in patients with ischemic stroke, but could have profound effects on thrombus organization, thrombus volume (and thus blood flow past thrombo-embolic occlusions), and thrombus density.18 More studies are needed to fully understand the potential effect of thrombus contraction in ischemic stroke patients.
In general, our findings are important to advance our understanding of ischemic stroke pathophysiology and, more importantly, to guide the development of better recanalization strategies in stroke patients via thrombolysis or thrombectomy.
As far as thrombolysis is concerned, recombinant tissue plasminogen activator is currently the only FDA-approved drug for pharmacological thrombolysis of ischemic stroke thrombi. However, it is only effective in less than half of the patients that receive rt-PA.2,3 The mechanisms underlying this so-called "rt-PA-resistance" are not completely understood, but previous reports indicated that RBC-dominant thrombi respond better to rt-PA than platelet-dominant thrombi.19–27 Our histological findings provide new molecular insights on the composition of RBC-rich and platelet-rich thrombus material. It seems plausible that rt- PA, which promotes the degradation of fibrin, can have a direct and efficient thrombolytic effect on the RBC-rich areas in which thin fibrin is the main extracellular scaffold. In contrast, however, our histological analyses reveal that platelet-rich thrombus material contains denser fibrin structures that also include vWF. In addition, we show that platelet-rich areas comprise substantial amounts of extracellular DNA. Therefore it is tempting to speculate that vWF and DNA, together with fibrin, form the structural basis of platelet-rich thrombi, and that vWF and DNA, at least partially, could be responsible for the observed rt-PA-resistance of platelet-rich thrombi in patients.
Extracellular DNA and histones have indeed been shown to modify the structure of fibrin, making it more resistant to enzymatic degradation via rt-PA.28 The exact source of DNA observed in our study remains to be investigated, but neutrophils support thrombosis via the formation of neutrophil extracellular DNA structures that can act as a thrombogenic scaffold.29–31 Importantly, ex vivo thrombolysis experiments have shown that rt-PA in combination with DNAse-1 is more effective than rt-PA alone, further underlining the potential importance of extracellular DNA for rt-PA-resistance.14,15
Our observation that vWF is closely associated with fibrin in platelet-rich regions further supports a potentially novel link between vWF and fibrin in thrombus formation. It has been shown in vitro that fibrin and vWF can interact with each other via covalent crosslinking by FXIII or via thrombin-dependent incorporation, enhancing thrombus formation.32–34 Our histological data on vWF and fibrin indicate that fibrin degradation via plasmin alone might not be sufficient to achieve effective thrombolysis of platelet-rich thrombus material. Indeed, we and others have recently shown that targeting vWF, for example using the vWF-cleaving enzyme ADAMTS13, improves thrombolysis of rt-PA-resistant thrombi, reducing ischemic stroke brain injury in mice.19,35 Of note, plasmin can also cleave vWF, and plasmin activity was shown to regulate ADAMTS13 activity.36–38 It will be interesting to further elucidate the interplay between plasmin and ADAMTS13 in the degradation of fibrin/vWF structures that are present in ischemic stroke thrombi.
Taken together, our detailed histological analysis reveals different structural features in ischemic stroke thrombi that could be highly relevant for developing efficient pharmacological thrombolysis strategies. The presence of fibrin, vWF and DNA could explain why the current ‘one size fits all’ therapy aiming only at fibrinolysis via rt-PA is not effective in all patients. Furthermore, we found that some thrombi consist of an RBC-rich core surrounded by a dense platelet-rich shell, which could further hamper rt-PA-mediated thrombolysis.
As far as thrombectomy is concerned, emerging evidence indicates that RBC-rich stroke thrombi are more easily retrieved via endovascular procedures in comparison to more complex fibrin/platelet-rich thrombi.15,39 Even though it may seem intuitive that the retriever devices and techniques available today favor softer thrombi, the mechanisms that render platelet-rich thrombi more resistant to thrombectomy are not completely understood. Weafer et al. have recently shown that the degree of clot integration into the thrombectomy device is decreased in fibrin-rich thrombi, making these thrombi more resistant to mechanical removal.40 Our study reveals that platelet-rich thrombus material from ischemic stroke patients has particular features that include dense fibrin/vWF structures, leukocytes and DNA. Thick fibrin strands have been shown to increase clot rigidity and fibrin was shown to influence the thrombus coefficient of friction and level of physical compression.10,41,42 Interestingly, whether or not the association of vWF with fibrin influences the mechanical properties of thrombi needs to be further investigated. Of note, DNA is able to modify the structure of fibrin, rendering it more resistant to mechanical forces.28,42 In fact, Ducroux et al. found a positive correlation between the amount of neutrophil extracellular DNA traps and the number of device passes needed to achieve successful recanalization.15 Further studies are now needed to better understand how fibrin, DNA, vWF and platelets influence not only the mechanical properties of the occluding thrombus, but also its interaction with the thrombectomy device and the vessel wall.
This study has several limitations that are worth considering. First, only thrombi from those patients in whom the thrombus did not dissolve spontaneously or during prior rt-PA treatment, and in whom the thrombus could be successfully retrieved, were available for study. This impedes the assessment of thrombi that were rt-PA susceptible or thrombectomy resistant. Second, the scope of this study was focused on the description of common structural features of patient stroke thrombi, and did not include clinical and procedural parameters. Ongoing research on larger sets of stroke thrombi are needed to further elucidate the clinical impact of the described structural features. Our hypothesis is that the relative contribution of platelet-rich and RBC-rich areas will most likely determine the success rate of any given pharmacological and endovascular recanalization strategy. Interestingly, non-contrast computed tomography (NCCT) and magnetic resonance imaging are able to identify the presence of RBC-dominant thrombi via the presence of a hyperdense artery sign or a blooming artefact.43,44 Such information could guide treatment selection in the future. Better understanding of thrombus composition and its link with stroke etiology could also help to assess the likely thrombus origin in patients with embolic stroke of undetermined source, and to guide the development of targeted strategies for secondary stroke prevention.
As a final note, we emphasize that we describe typical features that are generally found in the majority of ischemic stroke thrombi, and that the heterogeneity between thrombi (Figure 2) does not allow for a single typical thrombus model.
In conclusion, we show that stroke thrombi consist of RBC-rich areas and platelet-rich areas. We found that RBC-rich areas have a limited complexity, while platelet-rich areas are characterized by dense fibrin structures aligned with vWF and abundant amounts of leukocytes and extracellular DNA. These findings are important to further improve acute ischemic stroke therapy, especially concerning platelet-rich thrombi that are rt-PA resistant and difficult to retrieve via thrombectomy.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/2/498
Funding
This work was supported by research grants to SFDM from the Fonds voor Wetenschappelijk Onderzoek – Vlaanderen (FWO) (research grants G.0A86.13, G.0785.17 and 1509216N), the KU Leuven (OT/14/099 and ISP/14/02L2), the Queen Elisabeth Medical Foundation and by the European Union’s Horizon 2020 Research and Innovation Program INSIST under grant agreement No 777072. FD is a postdoctoral fellow of the FWO (12U7818N).
References
- 1.Aguiar de Sousa D, von Martial R, Abilleira S, et al. Access to and delivery of acute ischaemic stroke treatments: A survey of national scientific societies and stroke experts in 44 European countries. Eur Stroke J. 2019;4(1):13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prabhakaran S, Ruff I, Bernstein RA. Acute Stroke Intervention: A Systematic Review. JAMA. 2015;313(14):1451–1462. [DOI] [PubMed] [Google Scholar]
- 3.Vanacker P, Lambrou D, Eskandari A, et al. Improving the Prediction of Spontaneous and Post-thrombolytic Recanalization in Ischemic Stroke Patients. J Stroke Cerebrovasc Dis. 2015;24(8):1781–1786. [DOI] [PubMed] [Google Scholar]
- 4.Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treat ment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. [DOI] [PubMed] [Google Scholar]
- 5.Riedel CH, Zimmermann P, Ulf J-K, Stingele R, Deuschl G, Jansen O. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011;42(6):1775–1777. [DOI] [PubMed] [Google Scholar]
- 6.Saver JL, Goyal M, Bonafé A, et al. Stent- retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–2295. [DOI] [PubMed] [Google Scholar]
- 7.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–1018. [DOI] [PubMed] [Google Scholar]
- 8.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. N Engl J Med. 2015;372(24):2296–2306. [DOI] [PubMed] [Google Scholar]
- 9.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–1030. [DOI] [PubMed] [Google Scholar]
- 10.Yoo AJ, Andersson T. Thrombectomy in Acute Ischemic Stroke: Challenges to Procedural Success. J Stroke. 2017; 19(2):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Meyer SF, Andersson T, Baxter B, et al. Analyses of thrombi in acute ischemic stroke: A consensus statement on current knowledge and future directions. Int J Stroke. 2017;12(6):606–614. [DOI] [PubMed] [Google Scholar]
- 12.Staessens S, Fitzgerald S, Andersson T, et al. Histological stroke clot analysis after thrombectomy: technical aspects and recommendations. Int J Stroke. 2019. 10.1177/1747493019884527 [DOI] [PubMed] [Google Scholar]
- 13.Brinjikji W, Duffy S, Burrows A, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J Neurointerv Surg. 2017;9(6):529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laridan E, Denorme F, Desender L, et al. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;82(2):223–232. [DOI] [PubMed] [Google Scholar]
- 15.Ducroux C, Di Meglio L, Loyau S, et al. Thrombus Neutrophil Extracellular Traps Content Impair tPA-Induced Thrombolysis in Acute Ischemic Stroke. Stroke. 2018;49(3):754–757. [DOI] [PubMed] [Google Scholar]
- 16.Cines DB, Lebedeva T, Nagaswami C, et al. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood. 2014;123(10):1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tutwiler V, Mukhitov AR, Peshkova AD, et al. Shape changes of erythrocytes during blood clot contraction and the structure of polyhedrocytes. Sci Rep. 2018;8(1):17907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tutwiler V, Peshkova AD, Andrianova IA, Khasanova DR, Weisel JW, Litvinov RI. Contraction of Blood Clots Is Impaired in Acute Ischemic Stroke. Arter Thromb Vasc Biol. 2017;37(2):271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denorme F, Langhauser F, Desender L, et al. ADAMTS13-mediated thrombolysis of t-PA-resistant occlusions in ischemic stroke in mice. Blood. 2016;127(19):2337–2345. [DOI] [PubMed] [Google Scholar]
- 20.Niesten JM, van der Schaaf IC, van der Graaf Y, et al. Predictive value of thrombus attenuation on thin-slice non-contrast CT for persistent occlusion after intravenous thrombolysis. Cerebrovasc Dis. 2014; 37(2):116–122. [DOI] [PubMed] [Google Scholar]
- 21.Moftakhar P, English JD, Cooke DL, et al. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke. 2013;44(1):243–245. [DOI] [PubMed] [Google Scholar]
- 22.Nam HS, Kim EY, Kim SH, et al. Prediction of thrombus resolution after intravenous thrombolysis assessed by CT-based thrombus imaging. Thromb Haemost. 2012; 107(04):786–794. [DOI] [PubMed] [Google Scholar]
- 23.Puig J, Pedraza S, Demchuk A, et al. Quantification of thrombus Hounsfield units on noncontrast CT predicts stroke subtype and early recanalization after intravenous recombinant tissue plasminogen activator. Am J Neuroradiol. 2012;33(1):90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin JW, Jeong HS, Kwon H-JJ, Song KS, Kim J. High red blood cell composition in clots is associated with successful recanalization during intra-arterial thrombectomy. PLoS One. 2018;13(5):e0197492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi M, Park G, Lee J, et al. Erythrocyte Fraction Within Retrieved Thrombi Contributes to Thrombolytic Response in Acute Ischemic Stroke. Stroke. 2018; 49(3):652–659. [DOI] [PubMed] [Google Scholar]
- 26.Jang IK, Gold HK, Ziskind AA, et al. Differential sensitivity of erythrocyte-rich and platelet-rich arterial thrombi to lysis with recombinant tissue-type plasminogen activator. A possible explanation for resistance to coronary thrombolysis. Circulation. 1989;79(4):920–928. [DOI] [PubMed] [Google Scholar]
- 27.Tomkins AJ, Schleicher N, Murtha L, et al. Platelet rich clots are resistant to lysis by thrombolytic therapy in a rat model of embolic stroke. Exp Transl Stroke Med. 2015;7(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longstaff C, Varjú I, Sótonyi P, et al. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem. 2013;288(10):6946–6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010; 107(36):15880–15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arter Thromb Vasc Biol. 2012;32(8):1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grässle S, Huck V, Pappelbaum KI, et al. von Willebrand factor directly interacts with DNA from neutrophil extracellular traps. Arter Thromb Vasc Biol. 2014; 34(7):1382–1389. [DOI] [PubMed] [Google Scholar]
- 32.Miszta A, Pelkmans L, Lindhout T, et al. Thrombin-dependent Incorporation of von Willebrand Factor into a Fibrin Network. J Biol Chem. 2014;289(52):35979–35986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hada M, Kaminski M, Bockenstedt P, et al. Covalent crosslinking of von Willebrand factor to fibrin. Blood. 1986;68(1):95–101. [PubMed] [Google Scholar]
- 34.Keuren JFW, Baruch D, Legendre P, et al. Von Willebrand factor C1C2 domain is involved in platelet adhesion to polymerized fibrin at high shear rate. Blood. 2004;103(5):1741–1746. [DOI] [PubMed] [Google Scholar]
- 35.de Lizarrondo S, Gakuba C, Herbig BA, et al. Potent Thrombolytic Effect of N-Acetylcysteine on Arterial Thrombi. Circulation. 2017;136(7):646–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tersteeg C, de Maat S, De Meyer SF, et al. Plasmin cleavage of von Willebrand Factor as an emergency bypass for ADAMTS13 deficiency in thrombotic microangiopathy. Circulation. 2014; 129(12):1320–1331. [DOI] [PubMed] [Google Scholar]
- 37.Clark CC, Mebius MM, de Maat S, et al. Truncation of ADAMTS13 by Plasmin Enhances Its Activity in Plasma. Thromb Haemost. 2018;118(3):471–479. [DOI] [PubMed] [Google Scholar]
- 38.Crawley JTB, Lam JK, Rance JB, Mollica LR, O’Donnell JS, Lane DA. Proteolytic inactivation of ADAMTS13 by thrombin and plasmin. Blood. 2005;105(3):1085–93. [DOI] [PubMed] [Google Scholar]
- 39.Maekawa K, Shibata M, Nakajima H, et al. Erythrocyte-rich thrombus is associated with reduced number of maneuvers and procedure time in patients with acute ischemic stroke undergoing mechanical thrombectomy. Cerebrovasc Dis Extra. 2018;8(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weafer FM, Duffy S, Machado I, et al. Characterization of strut indentation during mechanical thrombectomy in acute ischemic stroke clot analogs. J Neurointerv Surg. 2019. January 19 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Gunning GM, Kevin M, Mirza M, Duffy S, Gilvarry M, Brouwer PA. Clot friction variation with fibrin content; implications for resistance to thrombectomy. J Neurointerv Surg. 2018;10(1):34–38. [DOI] [PubMed] [Google Scholar]
- 42.Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophys J. 1999;77(5):2813–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liebeskind DS, Sanossian N, Yong WH, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42(5):1237–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SK, Yoon W, Kim TS, Kim HS, Heo TW, Park MS. Histologic analysis of retrieved clots in acute ischemic stroke: correlation with stroke etiology and gradient-echo MRI. Am J Neuroradiol. 2015; 36(9):1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.