Abstract
Aberrant glycosylation resulting from altered expression of sialyltransferases, such as ST3 β-galactoside α2-3-sialyltransferase 6, plays an important role in disease progression in multiple myeloma (MM). Hypersialylation can lead to increased immune evasion, drug resistance, tumor invasiveness, and disseminated disease. In this study, we explore the in vitro and in vivo effects of global sialyltransferase inhibition on myeloma cells using the pan-sialyltransferase inhibitor 3Fax-Neu5Ac delivered as a per-acetylated methyl ester pro-drug. Specifically, we show in vivo that 3Fax-Neu5Ac improves survival by enhancing bortezomib sensitivity in an aggressive mouse model of MM. However, 3Fax-Neu5Ac treatment of MM cells in vitro did not reverse bortezomib resistance conferred by bone marrow (BM) stromal cells. Instead, 3Fax-Neu5Ac significantly reduced interactions of myeloma cells with E-selectin, MADCAM1 and VCAM1, suggesting that reduced sialylation impairs extravasation and retention of myeloma cells in the BM. Finally, we showed that 3Fax-Neu5Ac alters the post-translational modification of the α4 integrin, which may explain the reduced affinity of α4β1/α4β7 integrins for their counter-receptors. We propose that inhibiting sialylation may represent a valuable strategy to restrict myeloma cells from entering the protective BM microenvironment, a niche in which they are normally protected from chemotherapeutic agents such as bortezomib. Thus, our work demonstrates that targeting sialylation to increase the ratio of circulating to BM-resident MM cells represents a new avenue that could increase the efficacy of other anti-myeloma therapies and holds great promise for future clinical applications.
Introduction
Multiple myeloma (MM) is characterized by clonal expansion of malignant plasma cells in the bone marrow (BM). Despite significant advances in treatment, MM remains incurable, with drug resistance mediated by the BM microenvironment being an important contributory factor.1,2 A related remarkable feature of MM is the ability for MM cells to spread from one BM site to another, which implies a persistent trafficking of circulating MM cells into and out of the BM microenvironment.3,4
Homing into the BM is physiologically governed by a diverse array of molecules such as Stromal cell-derived factor 1α (SDF1α), E-selectin, and various integrin co-receptors including Mucosal vascular addressin cell adhesion molecule 1 (MAD-CAM1).5 In the context of MM, SDF1α plays a major role in migration, adhesion, homing, and possibly retention of MM cells in the BM.6–9 Mediators of SDF1α activity in MM include matrix metalloproteinase and integrin α4β1-dependent adhesion on fibronectin and Vascular cell adhesion molecule 1 (VCAM1).10–12 Recently, E-selectin has also been shown to play a role in homing and retention of MM cells in the BM.13,14 In particular, we have shown that sialofucosylated structures recognized by E-selectin, such as Sialyl Lewisa/x (SLea/x), enable MM cells to escape the cytotoxic effects of bortezomib in vivo most likely by hiding in the BM.14 Indeed, MM cells enriched for E-selectin ligands recognized by the monoclonal antibody Heca452, were resistant to bortezomib treatment in vivo and this resistance was reversed by a small glycomimetic molecule GMI-1271, which inhibits the interaction between E-selectin and E-selectin ligands.14 Thus, SDF1α and E-selectin may act co-operatively to allow extravasation of MM cells into the BM niche where they can proliferate and evade drug treatments.
Post-translational glycosylation of proteins and lipids plays many physiological and pathophysiological roles. There is a growing appreciation that aberrant glycosylation is considered a hallmark of cancer,15,16 with one of the most prominent changes being a role for hypersialylation as a driver of tumor progression, metastasis and invasion.17,18 Hypersialylation is largely the result of overexpression of sialyltransferases (STs), which catalyze the attachment of sialic acids via different glycosidic linkages (α2-3, α2-6, or α2-8) to the underlying glycan chain.17,19 We have previously established an important role for aberrant sialylation in homing and survival in MM.20 Specifically, high expression of the ST3 β-galactoside α2-3-sialyltrans-ferase 6 (ST3GAL6) in MM cell lines and patient samples is associated with inferior outcomes. Knocking down ST3GAL6 reduces sialic acid expression on MM cells, decreasing their ability to home to the BM. Since ST3GAL6 participates in the generation of SLea/x structures, which forms the minimal E-selectin ligand, and may also be involved in sialylation of other structures important in MM homing and adhesion,21–23 we sought to investigate if we could therapeutically target sialylation on MM cells, and whether this would affect BM homing and survival in mice.
Here we show that pre-treatment of MM cells enriched for E-selectin ligands with 3Fax-Neu5Ac, a global inhibitor of the ST family,24 significantly reduces cell surface sialylation of these cells, prolongs survival in xenograft mice and enhances their in vivo sensitivity to bortezomib. In vitro, 3Fax-Neu5Ac impairs the interaction between MM cells and E-selectin under shear stress and, surprisingly, also greatly reduces their interaction with VCAM1 and MAD-CAM1 under similar conditions. In this respect, we show that 3Fax-Neu5Ac alters the post-translational modification of integrin α4 on MM cells. This implies a dual effect on homing, whereby blockade of selectin ligands and integrin-mediated interactions with BM endothelial cells prevents extravasation of MM cells in the BM. Our results suggest great potential for improved patient outcomes by targeting sialylation on MM cells, especially when used in combination with other active MM agents.
Methods
Selection of E-selectin ligand-enriched cells
The E-selectin ligand-enriched MM1S cell line (MM1SHeca452) was generated from GFP+/Luc+ MM1S and parental MM1S cell lines by two rounds of fluorescently-activated cell sorting (FACS) using the fluorescent Heca452 antibody (Biolegend; San Diego, CA, USA). Cells were maintained in RPMI-1640 (VWR; Radnor, PA, USA) containing L-glutamine, 10% heat inactivated fetal bovine serum (HI-FBS, VWR), and 1X antibiotic-antimycotic (Corning; Kennebunk, ME, USA).
Animal experiments
All experimental studies and procedures involving mice were performed in accordance with protocols approved by the governing Institutional Animal Care and Use Committee (IACUC) and all state and federal laws. In the toxicity study, 8-week old male and female C57BL/6J mice (n=8) received 0, 6.25, 12.5 or 25 mg/kg body weight doses of 3Fax-Neu5Ac (EMD Millipore; Burlington, USA) delivered intraperitoneally (i.p.) once daily for seven days. The drug was dissolved in dimethyl sulfoxide (DMSO) (VWR) and subsequently diluted 2-fold in PEG-300 (Sigma Aldrich; St. Louis, MO, USA). Mice were monitored daily for signs of discomfort, especially at the site of injection. In the homing study, 6-week old female Fox Chase SCID-Beige mice (Charles River Laboratory; Wilmington, MA, USA) (n=9 or 10) were inoculated via tail vein injections with 5x106 Heca452-enriched GFP+/Luc+ MM1S cells, which had been pre-treated with either vehicle or 300 mM 3Fax-Neu5Ac for seven days in culture before inoculation. Starting one day post inoculation, mice received either vehicle (PBS) or bortezomib (Selleck; Houston, TX, USA) injections intraperitoneally twice weekly. To monitor toxicity, mice were weighed twice weekly. Mice were frequently monitored for clinical signs of treatment-related side effects. Survival end points were mouse death or euthanasia as required by the IACUC (a single observation of >30% body weight loss, 3 consecutive measurements of >25% body weight loss, or impaired hind limb use). Survival differences were analyzed by the Kaplan-Meier method.
Bioluminescent imaging
Starting on day 7, and biweekly until day 30, tumor burden was assessed with bioluminescence imaging (BLI) in an IVIS® Lumina LT (Perkin Elmer Inc.; Waltham, MA, USA) equipped with a CCD camera (cooled at −90°C), mounted on a light-tight specimen chamber. Mice were injected with 150 mg/kg i.p. filter-sterilized D-luciferin substrate (VivoGlo, Promega; Madison, WI, USA) and imaged after 10 minutes. Data were acquired and analyzed using LivingImage software 4.5.1. (PerkinElmer). BLI flux equaling the radiance (photons/s) in each pixel integrated over the region of interest (ROI) area (cm2), where the ROI was the whole mouse, was used to quantify tumor burden. BLI and mouse weight data were graphed and analyzed only for days in which all mice remained in the study to avoid artifacts due to mouse death.
Statistical analysis
All data are expressed as mean±standard error of the mean (SEM), unless otherwise noted. Student’s t-test, ordinary one-way or two-way ANOVA tests were used to determine significance, using P<0.05 as the cut-off, with Tukey’s multiple comparison post-hoc testing unless otherwise noted. **** P<0.0001; *** P<0.001; ** P<0.01; * P<0.05. GraphPad Prism 6.02 software (La Jolla, CA, USA) was used to compute all statistical calculations unless otherwise noted.
Additional information concerning materials and methods can be found in the Online Supplementary Appendix.
Results
Treatment of mice with 3Fax-Neu5Ac causes a dose dependent decrease in sialoside expression on multiple organs systemically
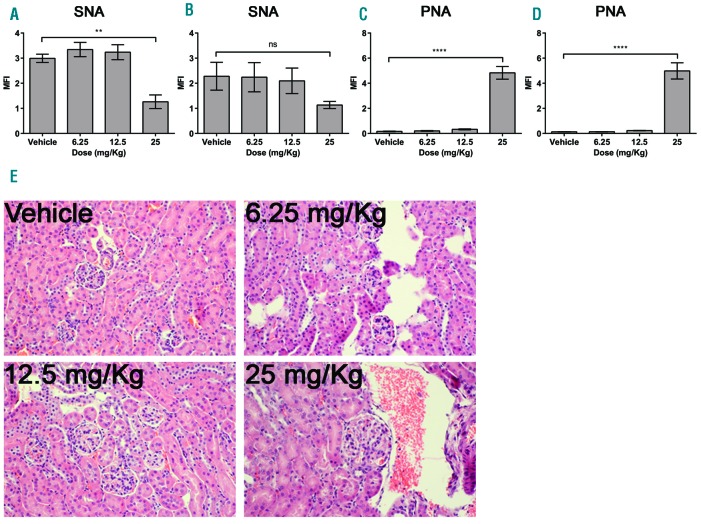
Building upon the previous 3Fax-Neu5Ac in vivo experience,25,26 we first studied the effects of global desialylation in vivo after systemic administration of 3Fax-Neu5Ac. Mice were treated with 6.25, 12.5, and 25 mg/kg of 3Fax-Neu5Ac daily for seven consecutive days. Sialylation was monitored using two different lectins after seven days of treatment: the Sambucus nigra lectin (SNA), which binds α2-6 linked sialic acids, and the Peanut agglutinin lectin (PNA), which binds to desialylated T antigen. In mice treated with 25 mg/kg 3Fax-Neu5Ac, there was a clear decrease in SNA staining in the kidney, spleen and liver (Online Supplementary Figure S1A-C), consistent with a reduction in α2-6 linked sialic acid expression. Moreover, at the same dose, there was a contemporary increase in PNA staining (Online Supplementary Figure S2A-C) consistent with decreased sialic acid expression leading to exposure of terminal galactose residues, such as the T antigen (Galβ1-3GalNAcαSer/Thr). To determine the effects of 3Fax-Neu5Ac treatment on sialylation of cells of the immune system, peripheral blood B cells were used as representative immune cells. The median fluorescent intensity (MFI) values for SNA and PNA positive staining were determined on the seventh day of treatment and the seventh day after the final treatment. Similar to what was observed in histology sections, 3Fax-Neu5Ac treatment induced a decrease in the SNA MFI with the highest dose (25 mg/kg) having a significant fold change compared to the control at the seventh day of dosing (Figure 1A). As expected, the PNA lectin MFI significantly increased with 3Fax-Neu5Ac treatment by the final day of treatment as well (Figure 1C). Sialic acid expression remained low after seven days of recovery after the 25 mg/kg dose, as particularly evident in cells stained with PNA, although a trend was also observed in the SNA-stained cells (Figure 1B and D), suggesting that recovery takes longer than seven days. Because sialylation is crucial to kidney filtration, we also determined the effects of the dose regimen in the kidneys. H&E staining showed no obvious histological changes in the kidney after seven days of recovery (Figure 1E). However, mice that received the highest dose, 25 mg/kg, did experience edema in the peritoneal cavity, as previously reported.27 These data demonstrate that 3Fax-Neu5Ac can successfully inhibit the expression of sialic acid systemically, but that local BM- or myeloma-specific delivery may be necessary to overcome effects on other organs in future studies.
Figure 1.
Decreased sialylation of B cells and kidney toxicity following systemic 3Fax-Neu5Ac treatment. Peripheral blood B cells were stained with SNA (A and B) or PNA (C and D) on the seventh day of dosing (A and C) and after seven days of recovery post last injection (B and D). Bars represent mean±Standard Error of Mean of three independent experiments. The one-way ANOVA was used to determine statistical significance with Dunnett’s multiple comparison post-hoc testing. **P<0.01; ****P<0.0001; ns: non-significant. MFI: median fluorescence intensity. (E) Representative images of Hematoxylin & Eosin stained kidneys taken after seven days of recovery. Images were taken at 20x magnification of four representative mice.
Treatment of MM1SHeca452 with 3Fax-Neu5Ac decreases sialylation in vitro
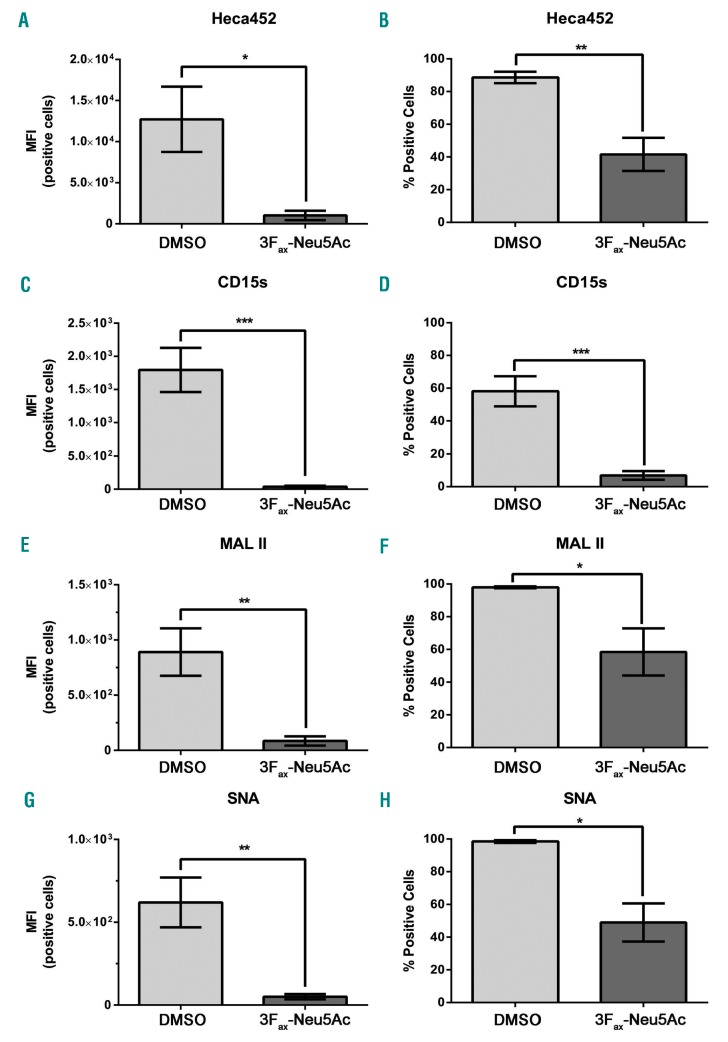
We next examined whether 3Fax-Neu5Ac could significantly reduce the expression of sialic acid on MM1SHeca452 cells. These cells are enriched for E-selectin ligand expression compared to the MM1S parental line and in vivo generate a very aggressive disease which displays resistance to bortezomib.14 The MM1SHeca452 cell line has been extensively characterized; their sensitivity to bortezomib, clonogenic potential, and proliferation in vitro are identical to the parental line and their aggressive phenotype becomes evident only in vivo.14 Sialylation was monitored using the Heca452 and CD15s antibodies, which recognize the sialofucosylated structure SLea/x, the Maackia Amurensis Lectin II (MAL II), which preferentially binds to α2-3 linked sialic acid, and SNA. Over a seven day span in culture, 300 mM of 3Fax-Neu5Ac significantly decreased the Heca452 staining in a time-dependent manner (Online Supplementary Figure S3). After seven days of treatment, 3Fax-Neu5Ac decreased the MFI and the total number of the Heca452, CD15s, MALII and SNA positive cells (Figure 2). Importantly, 3Fax-Neu5Ac treatment did not induce a significant change in the sensitivity to borte-zomib in vitro (Figure 4). Based on these data, we chose to pre-treat the MM1SHeca452 cells with 300 mM of 3Fax-Neu5Ac for seven days to significantly reduce sialylation on the cell surface.
Figure 2.
3Fax-Neu5Ac treatment decreases sialylation in the MM1SHeca452 cell line. MM1SHeca452 cells were treated with 300 mM 3Fax-Neu5Ac or dimethyl sulfoxide (DMSO) (vehicle control) for seven days. After treatment, cells were collected and stained with the Heca452 (A and B), CD15s (C and D), MALII (F and G), or SNA (G and H) antibodies or lectins. Bars represent mean±Standard Error of Mean of three independent experiments. Unpaired Student’s t-test was used to determine statistical significance. *P<0.05; ** P<0.01; ***P<0.001. MFI: median fluorescence intensity.
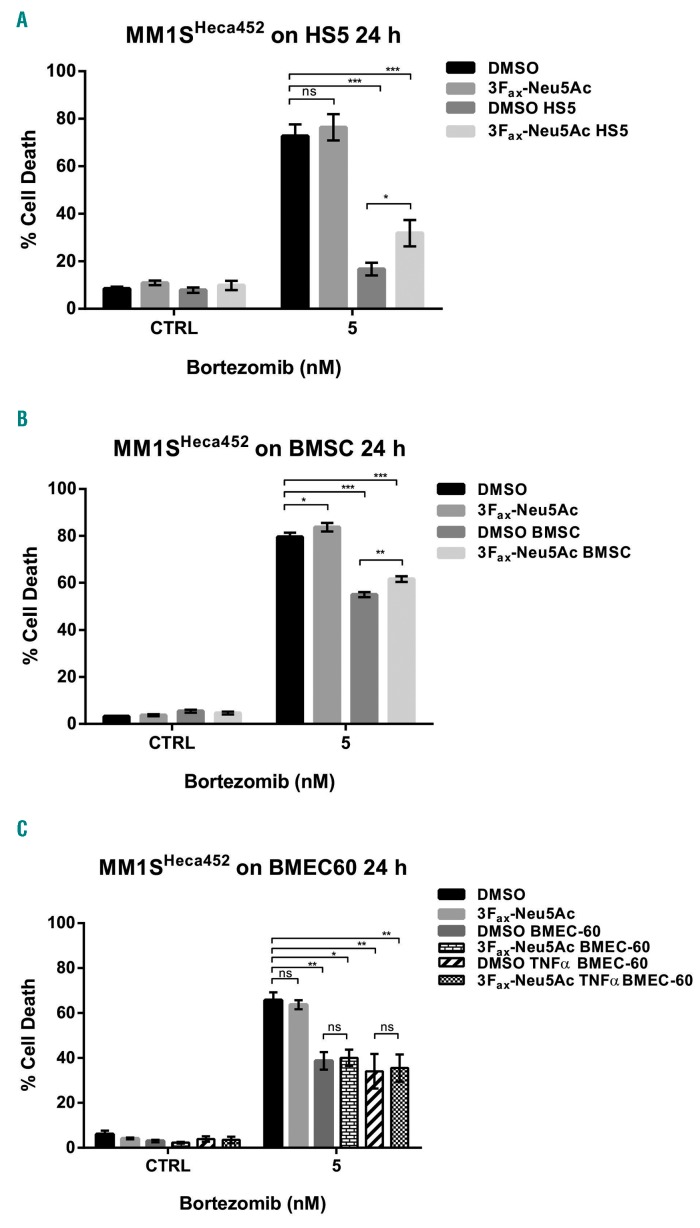
Figure 4.
3Fax-Neu5Ac treatment has a minimal impact on stroma-mediated bortezomib resistance in MM1SHeca452 cell line. MM1SHeca452 cells were treated with 300 mM 3Fax-Neu5Ac or dimethyl sulfoxide (DMSO) [vehicle control (CTRL)] for seven days. After treatment, cells were seeded onto 80% confluent layer of HS5 expressing GFP (A), patient-derived bone marrow stromal cells (BMSC) (B) and BMEC-60 stained with Tag-it Violet™ (C) or plastic. BMEC-60 were also stimulated with 10 ng/mL TNFα for 4 hours (h) to induce activation. Cells were co-cultured for 24 h and then treated with 5 nM bortezomib for a further 24 h. After incubation, cells were collected and cell death was examined by Annexin V-APC and PI staining. One-way ANOVA was used to determine statistical significance with Dunnett’s multiple comparison post-hoc testing. *P<0.05; **P<0.01; ***P<0.001; ns: non-significant.
In vivo, pre-treatment of MM1SHeca452 cells with 3Fax-Neu5Ac reduces tumor burden and increases survival, and co-treatment with bortezomib further enhances these outcomes
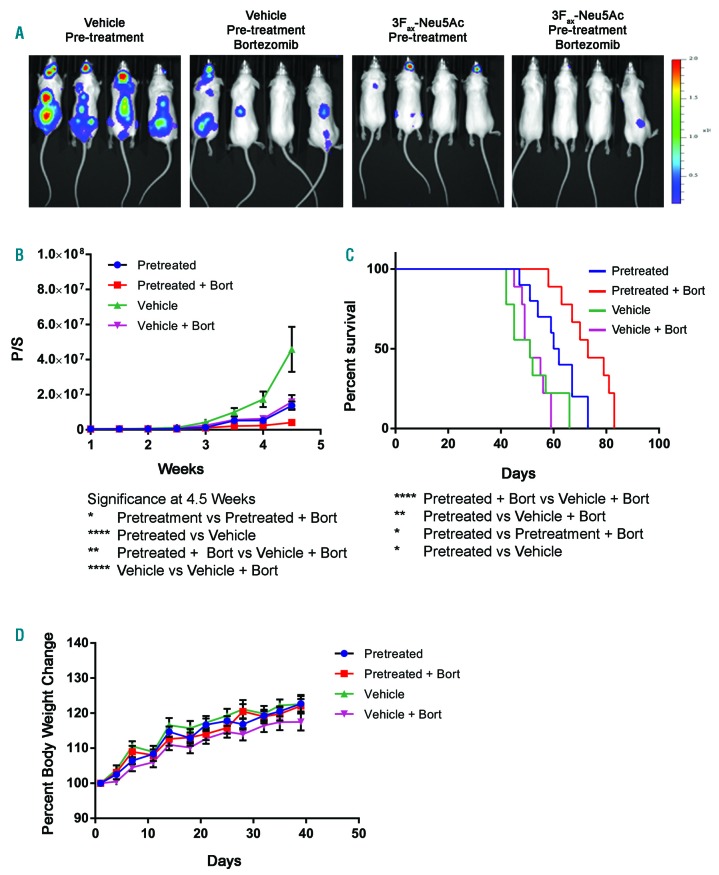
To study the impact of global sialylation inhibition specifically on MM cells and to avoid kidney toxicity related to 3Fax-Neu5Ac treatment, we pre-treated the MM1SHeca452 cells with 300 mM of 3Fax-Neu5Ac or vehicle for seven days, inoculated these cells into immunocom-promised mice, and followed tumor burden using bioluminescence imaging. Mice inoculated with 3Fax-Neu5Ac-pre-treated MM1SHeca452 (3Fax-Neu5Ac MM1SHeca452 mice) showed reduced tumor burden compared to mice inoculated with vehicle-pre-treated MM1SHeca452 (vehicle MM1SHeca452 mice) (Figure 3A and B). Two cohorts in this study in addition received bortezomib treatment, which decreased the tumor burden in the vehicle and 3Fax-Neu5Ac MM1SHeca452 mice (Figure 3A and B). The 3Fax-Neu5Ac MM1SHeca452 mice that received bortezomib treatment had the least tumor burden throughout the study compared to the other groups (Figure 3B). Importantly, even in the absence of bortezomib, pre-treatment of MM1SHeca452 cells with 3Fax-Neu5Ac reduced tumor burden compared to vehicle MM1SHeca452 mice, suggesting that 3Fax-Neu5Ac pre-treatment is beneficial even without the addition of chemotherapy. We also observed that the 3Fax-Neu5Ac MM1SHeca452 mice survived significantly longer than the vehicle MM1SHeca452 mice (Figure 3C). Notably, bortezomib treatment did not prolong survival of the vehicle MM1SHeca452 mice, confirming our previous observation that in this in vivo model, these MM cells are more refractory to bortezomib treatment (Figure 3C).14 Above all, pre-treatment of MM1SHeca452 cells with 3Fax-Neu5Ac in combination with bortezomib led to longer survival compared to the other groups, suggesting a synergistic therapeutic effect. No significant difference was observed in change in body weight between the treatment groups (Figure 3D). Overall, we demonstrate that 3Fax-Neu5Ac reduces tumor burden and increases survival, and that additional treatment with bortezomib has a synergistic effect with 3Fax-Neu5Ac.
Figure 3.
Effects of 3Fax-Neu5Ac pre-treatment on MM1SHeca452 xenograft mouse models. (A) Representative bioluminescence images taken at week 4.5 showing the differences in tumor burden between treatment groups. The colored scale represents luminescence in radiance unit (p/sec/cm2/sr) with maximum and minimum values of 2x106 and 1.5x105, respectively. Graph lines illustrate tumor burden (B), survival (C), and body weight (D) of treatment groups. Bars represent Standard Error of Mean. The two-way ANOVA was used to determine statistical significance with Tukey’s multiple comparison post-hoc testing. *P<0.05; **P<0.01; ****P<0.0001. Bort: borte-zomib
3Fax-Neu5Ac treatment partially reverts stroma but not endothelial-induced bortezomib resistance in vitro
To gain insight into the mechanism(s) of increased bortezomib sensitivity in response to 3Fax-Neu5Ac treat ment, we sought to investigate in vitro the effects of 3Fax-Neu5Ac pre-treatment on the sensitivity to bortezomib in MM1SHeca452 cells in co-culture conditions that partially recapitulate the BM environment. To this end, we used the well-established HS5 stromal cell line,28 primary BM stromal cells (BMSC) derived from MM patients, and the BM endothelial cell line BMEC-60. The latter was also treated with 10 ng/mL TNFα for four hours before co-culture to induce activation. BMEC-60, BMSC and, to an even greater extent, HS5 induced resistance to bortezomib (5 nM) in MM1SHeca452 cells (Figure 4A-C). 3Fax-Neu5Ac pretreatment caused only minor, although significant re-sen-sitization to bortezomib in the presence of HS5 and BMSC (Figure 4A and B) and did not reverse BMEC-60-induced bortezomib resistance (Figure 4C). Importantly, in the absence of BM-derived cells, the 3Fax-Neu5Ac pretreatment had only a minor effect on the MM1SHeca452 response to bortezomib. Together these data indicate that, in MM cells, bortezomib resistance that is BM stromal cell-driven, although maybe not endothelial cell-driven, can be partially reversed by inhibition of sialylation.
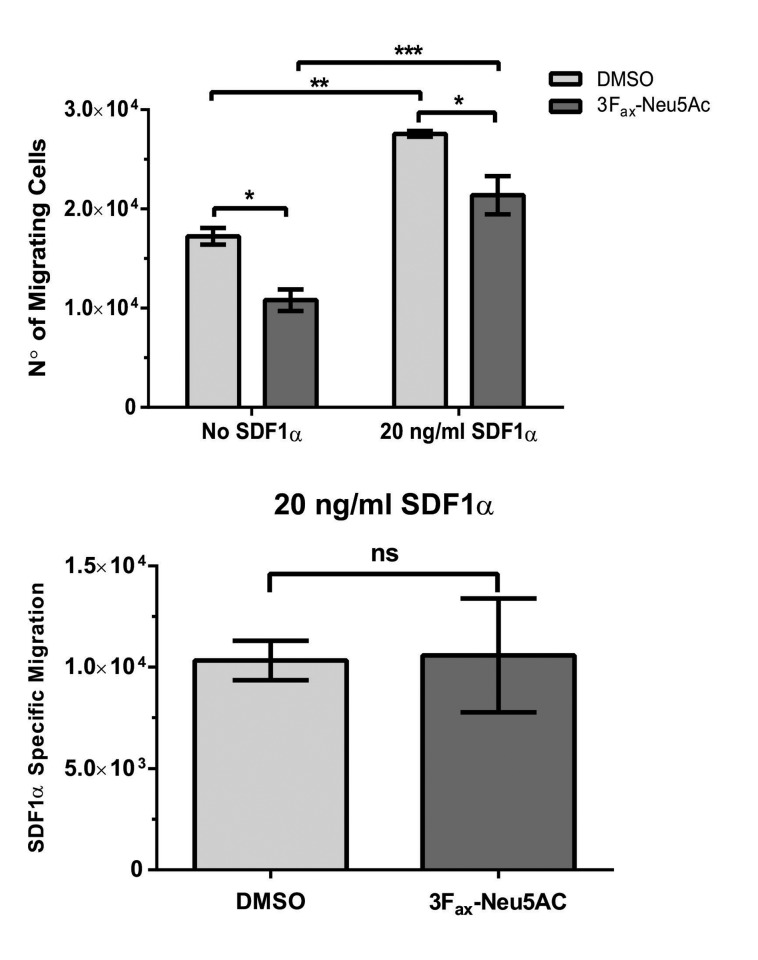
3Fax-Neu5Ac treatment does not affect migration in response to SDF1α
Since 3Fax-Neu5Ac did not completely reverse bortezomib resistance induced by BM cell lines and patient-derived-BMSC in vitro, we reasoned that the mechanism(s) of bortezomib re-sensitization in vivo induced by 3Fax-Neu5Ac may also involve a defect in the ability of the MM1SHeca452 cells to home into the protective BM microenvironment. To explore this possibility, we first examined the effects of 3Fax-Neu5Ac pre-treatment on migration in response to SDF1α in a transwell assay. DMSO and 3Fax-Neu5Ac pre-treated MM1SHeca452 cells showed enhanced migration in response to SDF1α (Figure 5A). However, spontaneous as well as SDF1α-induced migration were similarly inhibited by 3Fax-Neu5Ac pre-treatment (Figure 5A). Indeed, when we specifically examined migration in response to SDF1α by subtracting spontaneous migration (no SDF1α) to SDF1α-containing samples, we observed that 3Fax-Neu5Ac pre-treatment did not affect SDF1α-driven migration (Figure 5B). These data suggest that 3Fax-Neu5Ac pre-treatment has an impact on the motility of the cells but not specifically on SDF1α-induced migration.
Figure 5.
3Fax-Neu5Ac treatment reduces motility of MM1SHeca452 independently of SDF1α. MM1SHeca452 cells were treated with 300 mM 3Fax-Neu5Ac or dimethyl sulfoxide (DMSO) (vehicle control) for seven days. After treatment, cells were starved for 1 hour (h) and then seeded on the upper chamber of transwells. Lower chamber was filled with either serum-free media (No SDF1α) or serum-free media supplemented with SDF1α (20 ng/mL). Cells were allowed to migrate for 4 h at 37°C. After incubation, cells in the lower chambers were collected and counted using a BD Accuri flow cytometer. Data are presented as (A) raw data or (B) as the difference between migrating cells in SDF1α-containing media and control media. Bars represents mean±Standard Error of Mean of three independent experiments. One-way ANOVA test was used to determine statistical significance with Sidak’s multiple comparison post-hoc testing. *P<0.05; **P<0.01; ***P<0.001; ns: non-significant.
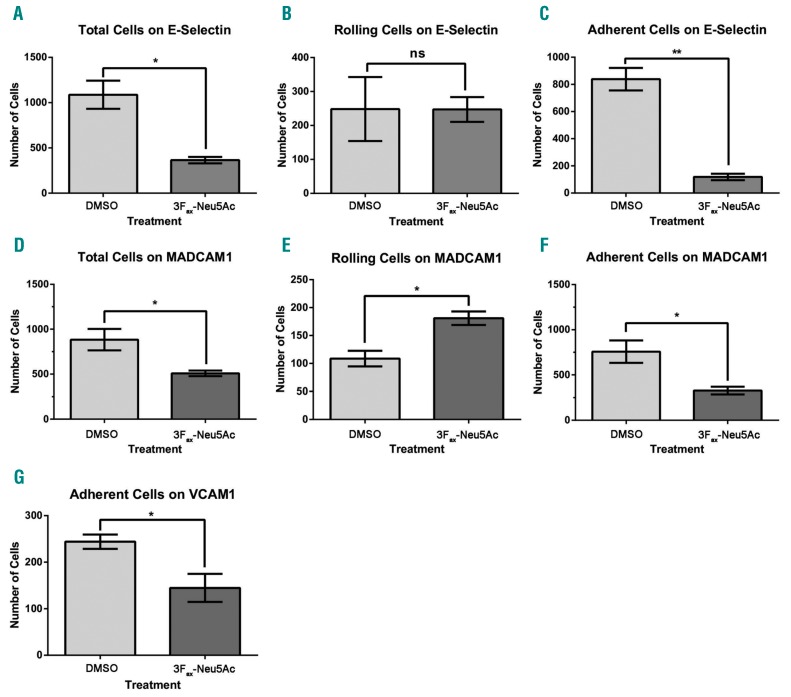
3Fax-Neu5Ac treatment impairs adhesion and rolling of MM1SHeca452 cells on E-selectin, MADCAM1 and VCAM1
We next examined whether 3Fax-Neu5Ac could influence adhesion and rolling on selectins and integrin co-receptors important in BM homing.3–5 To this end, we performed adhesion and rolling assays under shear stress on E-selectin, MADCAM1 and VCAM1-coated substrates. MM1SHeca452 cells showed robust interactions with E-selectin which could be subcategorized into firm adhesion and rolling (Figure 6A-C). 3Fax-Neu5Ac pre-treatment dramatically impaired this interaction by decreasing the number of adherent cells (Figure 6A and C). The number of rolling cells was not affected (Figure 6B). However, when we looked at the rolling velocity, we observed an increase in the velocity of the MM1SHeca452 cells pre-treated with 3Fax-Neu5Ac versus DMSO controls, indicating a decrease in the affinity of the E-selectin ligands for E-selectin (Online Supplementary Figure S4A-C). These data are consistent with a requirement of sialic acid for E-selectin binding. The MM1SHeca452 also showed robust adhesion and rolling on MADCAM1 under shear stress (Figure 6D-F). 3Fax-Neu5Ac pre-treatment induced a decrease in the total number of cells interacting with MADCAM1 and, in particular, decreased the number of adherent cells while increasing the number of rolling cells, suggesting a decrease in the affinity of integrin α4β7 for MADCAM1. Again, the velocity of the rolling cells was increased by 3Fax-Neu5Ac, indicating a weaker attachment (Online Supplementary Figure S4D-F). Finally, the 3Fax-Neu5Ac pre-treatment induced a reduction in the number of MM1SHeca452 cells interacting with VCAM1, which was exclusively adhesion (Figure 6G). Indeed, rolling on VCAM1 was not observed indicating strong interactions between integrin α4β1 and VCAM1. Altogether, these data indicate that desialylation of the MM1SHeca452 cells impairs interaction (a summation of adhesion and rolling) with E-selectin, MADCAM1 and VCAM1.
Figure 6.
3Fax-Neu5Ac treatment impairs adhesion and rolling of MM1SHeca452 on E-selectin, VCAM1 and MADCAM1 under shear stress. MM1SHeca452 cells were treated with 300 mM 3Fax-Neu5Ac or dimethyl sulfox-ide (DMSO) (vehicle control) for seven days. After treatment, cells were collected, washed and resuspended at 2x106/mL. Eighty μL of cell suspension were loaded onto E-selectin- (A-C), MADCAM1- (D-F) and VCAM1-(G) coated microfluidic channels and adhesion/rolling assay was performed at 0.5 dyne/cm2 at RT using the Mirus Evo NanoPump. Rolling cells were imaged using an A-Plan 10X/0.25 objective of an A10 Vert.A1 microscope equipped with a QIClick F-M-12 Mono camera. Images were acquired using the Vena Flux Assay software and analyzed using the Image-Pro Premiere. Bars represent the mean±Standard Error of Mean of three independent experiments. Unpaired Student’s t-test was used to determine statistical significance. *P<0.05; **P<0.01; ns: non-significant.
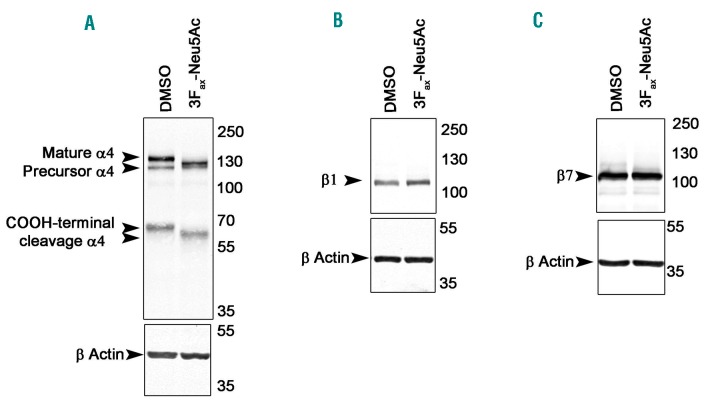
3Fax-Neu5Ac treatment alters the post-translational modifications on integrin α4
Next, we examined whether 3Fax-Neu5Ac treatment decreased protein expression of integrins α4β7 or α4β1, which bind VCAM1 and MADCAM1 respectively, on MM1SHeca452 cells.29–31 Flow cytometry analysis revealed that 3Fax-Neu5Ac did not decrease α4, β1 or β7 expression (Online Supplementary Figure S5D-F). We then investigated whether 3Fax-Neu5Ac affected post-translational modifications on these integrins, which in turn would result in an altered mobility on SDS-PAGE. Indeed, Western blot analysis of integrin α4 revealed a marked shift of the mature as well as the C-terminal cleavage form in response to 3Fax-Neu5Ac, suggesting an alteration of α4 post-translational modifications probably due to its desialylation (Figure 7A). To our knowledge, this is the first evidence that integrin α4 is post-translationally sialylated. The integrins β1 and β7 were not heavily affected by 3Fax-Neu5Ac pre-treatment (Figure 7B and C). Similar results were obtained in the parental MM1S cell line (Online Supplementary Figure S6A-C). Altogether, these data indicate that 3Fax-Neu5Ac primarily alters integrin α4 post-translationally, which most likely results in the observed weaker interaction of the MM cells with MAD-CAM1 and VCAM1.
Figure 7.
Post-translational modification of integrin α4 is altered by 3Fax-Neu5Ac treatment. Whole cell extracts from MM1SHeca452 cells treated for seven days with 300 mM 3Fax-Neu5Ac or dimethyl sul-foxide (DMSO) (vehicle control) were subjected to SDS PAGE, transferred to nitrocellulose membrane and blotted for integrin α4 (A), β1 (B), and β7 (C).
Discussion
In this study, we examined whether global inhibition of sialylation could increase bortezomib sensitivity in an aggressive MM mouse model, which employs xenotrans-plantation of MM cells that have been enriched for E-selectin ligands.14 To this end, we used the pan-sialyltransferase inhibitor 3Fax-Neu5Ac, which had been previously shown to efficiently block sialylation in leukemic cells.24
In a preliminary dose-finding in vivo study, we observed that 3Fax-Neu5Ac decreased sialylation in various tissues, including cells of the immune system. At its effective dose, 25 mg/kg, 3Fax-Neu5Ac induced edema in the peritoneal cavity of mice suggesting that desialylation of the glomerulus could lead to dose-limiting toxicity, as previously reported.27 To overcome 3Fax-Neu5Ac-induced kidney toxicity and to examine the role of sialylation specifically in MM, we treated the E-selectin enriched MM1S cell line, MM1SHeca452, with 3Fax-Neu5Ac before inoculation, an approach that has been successfully used to uncover a critical role of sialylation in melanoma metastasis and growth in vivo.32
The vehicle-pre-treated MM1SHeca452 cells showed an initial response to bortezomib in vivo. Indeed, bortezomib was able to reduce tumor burden, however, despite this initial response, bortezomib alone was not able to improve survival. These data would suggest that the surviving MM1SHeca452 cells were so aggressive that they still induced death in mice at a similar rate to the non-bortezomib-treated mice. 3Fax-Neu5Ac pre-treatment of MM1SHeca452 cells effectively blocked α2-3 and α2-6 sialylation as well as expression of SLea/x. More importantly, pretreatment of MM1SHeca452 cells with 3Fax-Neu5Ac blunted the aggressive nature of these cells. Indeed, 3Fax-Neu5Ac treatment reduced tumor burden, increased bortezomib sensitivity and, most importantly, improved survival, suggesting that sialylation contributes to the aggressive phenotype of the MM1SHeca452 cells and inhibiting it could represent a valuable treatment in MM. Currently, systemic administration of 3Fax-Neu5Ac is not feasible due to irreversible nephrotoxicity. While it is possible that a different dose and schedule could reveal a therapeutic window, clearly the potential for off-target toxicity is a major obstacle to clinical development. This is particularly relevant in MM where the kidney is one of the organs whose function is greatly impaired by the disease. However, alternative approaches could be explored to address this issue, including the development of more selective sialyltransferase inhibitors or the use of a targeted delivery system, which would release 3Fax-Neu5Ac selectively into the BM microenvironment or the MM cells. Indeed, Bull et al. have previously reported that targeted delivery of antibody-labeled nanoparticles containing 3Fax-Neu5Ac into melanoma cells facilitates long-term sialic acid blockade and, importantly, reduces lung metastasis in vivo.33 A similar strategy could be employed using antibodies specific to MM antigens (such as CD38 and BCMA) or by incorporating bisphosphonates into the nanoparticles to target the BM.34 Achievement of sufficiently high local BM concentrations of 3Fax-Neu5Ac should result in sialylation inhibition on MM cells, without off target toxicity. Inhibiting sialylation using these approaches could also target the tumor microenvironment including the immune environment. For instance, it has been recently reported that sialic acid blockade via intra-tumoral injection of 3Fax-Neu5Ac could suppress tumor growth by enhancing T-cell-mediated tumor immunity.35 In addition to a reduction in sialic acid expression by tumor cells, sialyltransferase inhibition converted the immune suppressive tumor microenvironment to an immune promoting one with significantly higher numbers of activated effector immune cells, including CD8+ T cells and natural killer (NK) cells, along with a reduction in regulatory T cells (Tregs).35 Sialyltransferase inhibition also led to anti-tumor effects, which were mediated by CD8+ effector cells as well as potential activation of stimulated dendritic cells (DC).35
A number of different mechanisms could account for the 3Fax-Neu5Ac-mediated increased-sensitization of the MM1SHeca452 cells to bortezomib in vivo. First, we explored the possibility that 3Fax-Neu5Ac could directly inhibit BM-mediated bortezomib resistance in vitro. HS5, patient-derived BMSC and the BM endothelial cell line BMEC-60 showed significant inhibition of bortezomib-induced cell death in MM1SHeca452. However, we observed that 3Fax-Neu5Ac induced only a partial re-sensitization to borte-zomib on HS5 and patient-derived BMSC, suggesting that desialylation plays a minor role in blocking BM-mediated drug resistance. The BM microenvironment can induce drug resistance through cell adhesion-mediated drug resistance (CAM-DR) and soluble factors.1,36–39 It is possible that in our in vitro model system, inhibition of sialylation is not enough to inactivate all the pathways responsible for the BM-mediated bortezomib resistance.1,39,40 Therefore, it is conceivable that the increased-sensitization to bortezomib observed in vivo may be predominantly due to mechanisms other than blockade of BM-mediated drug resistance. Nonetheless, an important future direction will be to test 3Fax-Neu5Ac in in vitro models that more faithfully reproduce the tumor microenvironment to better understand the effects of 3Fax-Neu5Ac on the microenvironment-mediated drug resistance.
Previously, we showed that in the same model system, the small molecule glycomimetic GMI-1271, which inhibits interactions between E-selectin and E-selectin ligands, could increase the number of MM cell in circulation, where they are more susceptible to bortezomib.14 In a similar way, we hypothesized that inhibition of sialylation by 3Fax-Neu5Ac could also inhibit homing and retention of MM cells in the BM. To this end, we examined the interaction of vehicle or 3Fax-Neu5A-treated MM1SHeca452 with E-selectin under shear stress. We found that 3Fax-Neu5Ac treatment, by reducing SLea/x, effectively inhibited the interaction between the MM1SHeca452 cells and E-selectin, confirming previous observations.24 However, we reasoned that global suppression of sialylation could have effects beyond E-selectin. Indeed, 3Fax-Neu5Ac induced a general reduction in the motility of treated cells. This prompted us to investigate whether desialylation would alter adhesion and rolling mediated by α4β7 and α4β1 integrins, which are highly expressed on MM cells.41–44 In a shear stress adhesion assay, we observed that 3Fax-Neu5Ac reduced the number of adherent cells on VCAM1 and, surprisingly, adhesion on MADCAM1. MADCAM1 is an immunoglobulin superfamily adhesion molecule expressed by mucosal venules that helps direct lymphocyte trafficking into Peyer’s patches and the intestinal lamina propria.29,45 There is also evidence that interaction between HSC and endothelial MADCAM1 in the BM promotes the homing and engraftment of HSC in mice.46–48 In a similar way, MADCAM1 could co-operate with SDF1α and E-selectin to facilitate homing of MM cell in the BM.
Indeed, MADCAM1 ligand α4/β7 has been shown to play a critical role in MM-cell adhesion, migration, invasion, BM homing, and adhesion-mediated drug resistance.43,49 Moreover, it was shown that the expression levels of β7 integrin on MM cells correlates with poor survival in MM patients.43 Our results suggest the possibility of reduced interactions between endothelial MADCAM1 and α4/β7 on MM cells as a result of desialylation. Indeed, we showed that 3Fax-Neu5Ac altered the SDS-PAGE mobility of the α4 chain and in particular of its mature forms, suggesting that desialylation interferes with α4 maturation. The interaction between MM cells and MADCAM1 becomes apparent only under shear stress as we failed to detect adhesion on MADCAM1 under static conditions (data not shown). This is highly reminiscent of L-selectin on leukocytes that requires a threshold shear stress to establish rolling and adhesion, below which no interactions are observed.50 Thus, it is possible that MAD-CAM1 mediates or facilitates homing but not retention of the MM cells in the BM.
In conclusion, targeting sialylation in MM cells has the potential to block the ability of MM cells to home to the BM, which, in turn, could reduce the severity of the disease, because most existing therapies against MM, like bortezomib, are maximally effective on circulating MM cells.
Supplementary Material
Acknowledgments
The authors would also like to acknowledge the Flow Cytometry Core Facility at NUI Galway. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/2/457
Funding
The authors would like to acknowledge the Health Research Board (CSA 2012/10) and NIH’s National Institute of General Medical Sciences (NIH P30 GM106391, P30GM103392, P20GM121301, and U54GM115516) for funding this work.
The authors’ work is also supported by start-up funds from the Maine Medical Center Research Institute, a pilot awarded to Dr. Reagan and core facilities from the Massachusetts General Hospital Center for Skeletal Research (NIH/NIAMS P30AR066261), and a pilot grant from the American Cancer Society (Research Grant #IRG-16-191-33; Reagan PI).
References
- 1.Di Marzo L, Desantis V, Solimando AG, et al. Microenvironment drug resistance in multiple myeloma: emerging new players. Oncotarget. 2016;7(37):60698–60711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawano Y, Moschetta M, Manier S, et al. Targeting the b one marrow microenvironment in multiple myeloma. Immunol Rev. 2015;263(1):160–172. [DOI] [PubMed] [Google Scholar]
- 3.Moschetta M, Kawano Y, Sacco A, et al. Bone Marrow Stroma and Vascular Contributions to Myeloma Bone Homing. Curr Osteoporos Rep. 2017;15(5):499–506. [DOI] [PubMed] [Google Scholar]
- 4.Natoni A, Macauley MS, O’Dwyer ME. Targeting Selectins and Their Ligands in Cancer. Front Oncol. 2016;6:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahin AO, Buitenhuis M. Molecular mechanisms underlying adhesion and migration of hematopoietic stem cells. Cell Adh Migr. 2012;6(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alsayed Y, Ngo H, Runnels J, et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007;109(7):2708–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azab AK, Runnels JM, Pitsillides C, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113(18):4341–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouyssou JM, Ghobrial IM, Roccaro AM. Targeting SDF-1 in multiple myeloma tumor microenvironment. Cancer Lett. 2016;380(1):315–318. [DOI] [PubMed] [Google Scholar]
- 9.Waldschmidt JM, Simon A, Wider D, et al. CXCL12 and CXCR7 are relevant targets to reverse cell adhesion-mediated drug resistance in multiple myeloma. Br J Haematol. 2017;179(1):36–49. [DOI] [PubMed] [Google Scholar]
- 10.Gazitt Y, Akay C. Mobilization of myeloma cells involves SDF-1/CXCR4 signaling and downregulation of VLA-4. Stem Cells. 2004;22(1):65–73. [DOI] [PubMed] [Google Scholar]
- 11.Menu E, Asosingh K, Indraccolo S, et al. The involvement of stromal derived factor 1alpha in homing and progression of multiple myeloma in the 5TMM model. Haematologica. 2006;91(5):605–612. [PubMed] [Google Scholar]
- 12.Parmo-Cabanas M, Bartolome RA, Wright N, Hidalgo A, Drager AM, Teixido J. Integrin alpha4beta1 involvement in stromal cell-derived factor-1alpha-promoted myeloma cell transendothelial migration and adhesion: role of cAMP and the actin cytoskeleton in adhesion. Exp Cell Res. 2004;294(2):571–580. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Moreno M, Leiva M, Aguilera-Montilla N, et al. In vivo adhesion of malignant B cells to bone marrow microvascula-ture is regulated by alpha4beta1 cytoplasmic-binding proteins. Leukemia. 2016; 30(4):861–872. [DOI] [PubMed] [Google Scholar]
- 14.Natoni A, Smith TAG, Keane N, et al. E-selectin ligands recognised by HECA452 induce drug resistance in myeloma, which is overcome by the E-selectin antagonist, GMI-1271. Leukemia. 2017;31(12):2642–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glavey SV, Huynh D, Reagan MR, et al. The cancer glycome: carbohydrates as mediators of metastasis. Blood Rev. 2015;29(4):269–279. [DOI] [PubMed] [Google Scholar]
- 16.Vajaria BN, Patel PS. Glycosylation: a hall mark of cancer? Glycoconj J. 2017; 34(2):147–156. [DOI] [PubMed] [Google Scholar]
- 17.Bull C, Stoel MA, den Brok MH, Adema GJ. Sialic acids sweeten a tumor’s life. Cancer Res. 2014;74(12):3199–3204. [DOI] [PubMed] [Google Scholar]
- 18.Pearce OM, Laubli H. Sialic acids in cancer biology and immunity. Glycobiology. 2016; 26(2):111–128. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues E, Macauley MS. Hypersialylation in cancer: modulation of inflammation and therapeutic opportunities. Cancers (Basel). 2018;10(6):1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glavey SV, Manier S, Natoni A, et al. The sialyltransferase ST3GAL6 influences homing and survival in multiple myeloma. Blood. 2014;124(11):1765–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannagi R. Molecular mechanism for cancer-associated induction of sialyl Lewis X and sialyl Lewis A expression-The Warburg effect revisited. Glycoconj J. 2004; 20(5):353–364. [DOI] [PubMed] [Google Scholar]
- 22.Magnani JL. The discovery, biology, and drug development of sialyl Lea and sialyl Lex. Arch Biochem Biophys. 2004;426(2): 122–131. [DOI] [PubMed] [Google Scholar]
- 23.Varki A. Selectin ligands: will the real ones please stand up? J Clin Invest. 1997;100(11 Suppl):S31–35. [PubMed] [Google Scholar]
- 24.Rillahan CD, Antonopoulos A, Lefort CT, et al. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat Chem Biol. 2012;8(7):661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14(8):351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012;1253:16–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macauley MS, Arlian BM, Rillahan CD, et al. Systemic blockade of sialylation in mice with a global inhibitor of sialyltransferases. J Biol Chem. 2014;289(51):35149–35158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidmaier R, Baumann P, Meinhardt G. Cell-cell contact mediated signalling - no fear of contact. Exp Oncol. 2006;28(1):12–15. [PubMed] [Google Scholar]
- 29.Berlin C, Berg EL, Briskin MJ, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74(1):185–195. [DOI] [PubMed] [Google Scholar]
- 30.Elices MJ, Osborn L, Takada Y, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60(4):577–584. [DOI] [PubMed] [Google Scholar]
- 31.Osborn L, Hession C, Tizard R, et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59(6):1203–1211. [DOI] [PubMed] [Google Scholar]
- 32.Bull C, Boltje TJ, Wassink M, et al. Targeting aberrant sialylation in cancer cells using a fluorinated sialic acid analog impairs adhesion, migration, and in vivo tumor growth. Mol Cancer Ther. 2013; 12(10):1935–1946. [DOI] [PubMed] [Google Scholar]
- 33.Bull C, Boltje TJ, van Dinther EA, et al. Targeted delivery of a sialic acid-blocking glycomimetic to cancer cells inhibits metastatic spread. ACS Nano. 2015; 9(1):733–745. [DOI] [PubMed] [Google Scholar]
- 34.Swami A, Reagan MR, Basto P, et al. Engineered nanomedicine for myeloma and bone microenvironment targeting. Proc Natl Acad Sci U S A. 2014;111(28):10287–10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bull C, Boltje TJ, Balneger N, et al. Sialic acid blockade suppresses tumor growth by enhancing T-cell-mediated tumor immunity. Cancer Res. 2018;78(13):3574–3588. [DOI] [PubMed] [Google Scholar]
- 36.Roecklein BA, Torok-Storb B. Functionally distinct human marrow stromal cell lines immortalized by transduction with the human papilloma virus E6/E7 genes. Blood. 1995;85(4):997–1005. [PubMed] [Google Scholar]
- 37.Zhu D, Wang Z, Zhao JJ, et al. The Cyclophilin A-CD147 complex promotes the proliferation and homing of multiple myeloma cells. Nat Med. 2015;21(6):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanamandra N, Colaco NM, Parquet NA, et al. Tipifarnib and bortezomib are synergistic and overcome cell adhesion-mediated drug resistance in multiple myeloma and acute myeloid leukemia. Clin Cancer Res. 2006;12(2):591–599. [DOI] [PubMed] [Google Scholar]
- 39.Farrell ML, Reagan MR. Soluble and cell-cell-mediated drivers of proteasome inhibitor resistance in multiple myeloma. Front Endocrinol (Lausanne). 2018;9(218):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdi J, Chen G, Chang H. Drug resistance in multiple myeloma: latest findings and new concepts on molecular mechanisms. Oncotarget. 2013;4(12):2186–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim I, Uchiyama H, Chauhan D, Anderson KC. Cell surface expression and functional significance of adhesion molecules on human myeloma-derived cell lines. Br J Haematol. 1994;87(3):483–493. [DOI] [PubMed] [Google Scholar]
- 42.Luque R, Brieva JA, Moreno A, et al. Normal and clonal B lineage cells can be distinguished by their differential expression of B cell antigens and adhesion molecules in peripheral blood from multiple myeloma (MM) patients–diagnostic and clinical implications. Clin Exp Immunol. 1998;112(3):410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neri P, Ren L, Azab AK, et al. Integrin beta7- mediated regulation of multiple myeloma cell adhesion, migration, and invasion. Blood. 2011;117(23):6202–6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatsumi T, Shimazaki C, Goto H, et al. Expression of adhesion molecules on myelo-ma cells. Jpn J Cancer Res. 1996; 87(8):837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Streeter PR, Berg EL, Rouse BT, Bargatze RF, Butcher EC. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988;331(6151):41–46. [DOI] [PubMed] [Google Scholar]
- 46.Murakami JL, Xu B, Franco CB, et al. Evidence that beta7 integrin regulates hematopoietic stem cell homing and engraftment through interaction with MAdCAM-1. Stem Cells Dev. 2016; 25(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katayama Y, Hidalgo A, Peired A, Frenette PS. Integrin alpha4beta7 and its counter-receptor MAdCAM-1 contribute to hematopoietic progenitor recruitment into bone marrow following transplantation. Blood. 2004;104(7):2020–2026. [DOI] [PubMed] [Google Scholar]
- 48.Tada T, Inoue N, Widayati DT, Fukuta K. Role of MAdCAM-1 and its ligand on the homing of transplanted hematopoietic cells in irradiated mice. Exp Anim. 2008; 57(4):347–356. [DOI] [PubMed] [Google Scholar]
- 49.Masellis-Smith A, Belch AR, Mant MJ, Pilarski LM. Adhesion of multiple myeloma peripheral blood B cells to bone marrow fibroblasts: a requirement for CD44 and alpha4beta7. Cancer Res. 1997;57(5):930–936. [PubMed] [Google Scholar]
- 50.Finger EB, Puri KD, Alon R, Lawrence MB, von Andrian UH, Springer TA. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 1996; 379(6562):266–269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.