In autoimmune hemolytic anemia (AIHA), auto-antibodies directed against red blood cells (RBC) lead to the breakdown of these cells through complement-dependent and -independent mechanisms. Multimeric IgG or IgM auto-antibodies bind to RBC and recruit C1, thereby triggering activation of the classical complement pathway. This results in the deposition of the opsonic fragment C3b on the RBC and may, upon increasing opsonization, lead to the formation of membrane attack complexes (MAC). Complement-opsonized RBC are cleared extravascularly via complement receptor-mediated phagocytosis mainly by liver macrophages, whereas IgG-opsonized RBC are phagocytosed via Fc-gamma receptors by splenic macrophages. Additionally, intravascular hemolysis may occur due to complement-induced MAC formation on the RBC membrane as a consequence of strong complement activation (by, for example, IgM auto-antibodies) causing direct hemolysis in the circulation. A significant proportion of patients with AIHA have IgM auto-antibodies that are not detectable using the most common diagnostic techniques and complement activation accompanied by intravascular hemolysis.1 Intravascular hemolysis is, in turn, directly related to the course and severity of the disease.2
Immunosuppressants are the first-line treatment in AIHA and are given with the aim of reducing auto-antibody production. However, they do not act immediately and some patients are unresponsive.3 In severe cases, symptomatic anemia in patients is corrected by RBC transfusion.4 However, the efficacy of RBC transfusion is reduced because the RBC auto-antibodies react with both recipient and donor RBC causing the destruction of transfused cells.5 Complement inhibition may be implemented to halt ongoing hemolysis in patients refractory to immunosuppressants and to improve the utility of RBC transfusions by preventing hemolysis of donor RBC. Currently, the only available therapeutic complement inhibitors are eculizumab, used for the treatment of paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome and generalized myasthenia gravis, and C1 esterase inhibitor (C1-INH), approved for the treatment of hereditary angioedema. Eculizumab inhibits complement activation at the level of C5 and blocks MAC formation, thereby preventing intravascular hemolysis; however, it does not halt opsonization or extravascular hemolysis, which renders this drug less suitable for AIHA patients.6 Although complement inhibition at the C1 level using C1-INH has been shown to prevent MAC formation and complement-mediated extravascular hemolysis in vitro and in vivo,7,8 treatment of AIHA with C1-INH has limitations. Due to the low-affinity interaction with its substrates, high doses of C1-INH are needed.8 Moreover, C1-INH shows broad protease specificity and regulates different plasma cascade systems, which might be suboptimal features in the perspective of therapeutic use. Sutimlimab, a humanized monoclonal antibody directed against human C1s, specifically blocks the classical pathway of complement. Although sutimlimab has been recently shown to be safe in a randomized first-in-human study in healthy volunteers, antidrug antibodies were detected in some of the treated volunteers, which could compromise the inhibitory capacity of this antibody.9 In the present study, we investigated the potential therapeutic effect of a small peptide of the Compstatin family (Cp40) in AIHA. Cp40 targets complement activation at the level of C3 and is therefore expected to block both extra- and intravascular hemolysis. Cp40 has been previously shown to inhibit C3 deposition on RBC in an in vitro malaria model10 and complement opsonization and hemolysis of RBC from patients with paroxysmal nocturnal hemoglobinuria.11 Furthermore, Cp40 has been tested for safety in nonhuman primates12 and is under clinical development for the treatment of age-related macular degeneration.13
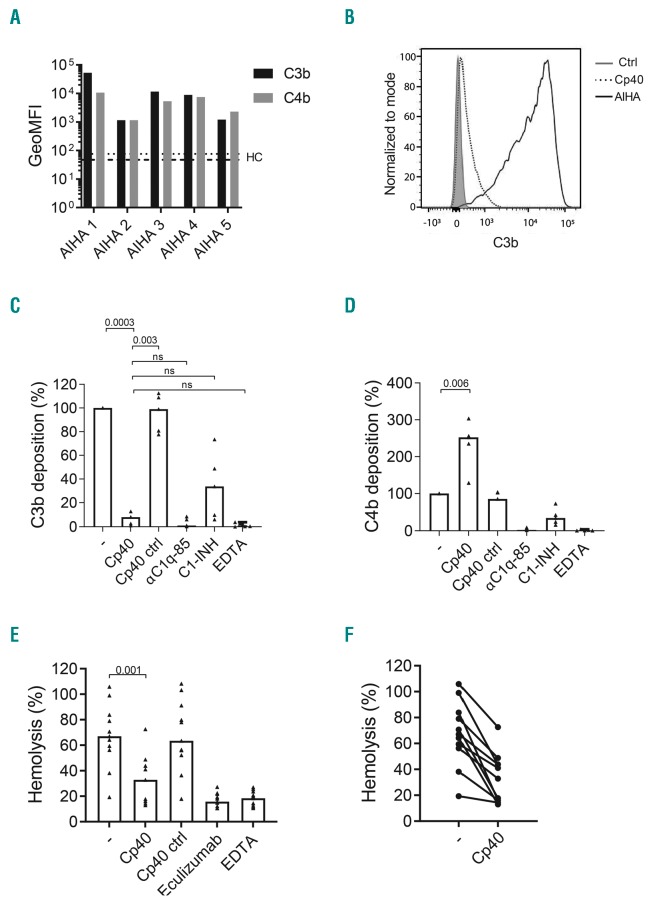
First, to examine the effect of Cp40 on complement deposition, donor RBC were incubated with sera from AIHA patients with a positive direct antiglobulin test (DAT) score for C3 deposition (details given in the Online Supplementary Material) and C4b and C3b deposition on the RBC was analyzed using flow cytometry. Although complement deposition was observed with all tested sera, opsonization levels differed among patients, presumably due to variability in titer and the subclass of the opsonizing auto-antibodies in the sera from the different patients (Figure 1A). Addition of Cp40 resulted in nearly complete reduction in C3b deposition on RBC sensitized with AIHA sera (Figure 1B, C). This reduction was stronger than that achieved with C1-INH and similar to the levels observed when a monoclonal antibody against C1q was used and in the ethylenediaminetetraacetic acid (EDTA) control (Figure 1C), the latter of which blocks all complement activity. No inhibition was observed with a sequence-scrambled Cp40 control peptide. As reported previously, higher levels of C4b were detected on the RBC membrane in the presence of Cp40 (Figure 1D), probably due to enhanced detection of C4b in the absence of surrounding C3b.14 Since Cp40 inhibits C3b deposition on RBC incubated with AIHA sera, we next examined the effect of Cp40 on MAC formation, which results in intravascular hemolysis. RBC were incubated with patients’ sera in the presence or absence of Cp40. As expected, Cp40 inhibited lysis of RBC opsonized with all the tested sera (Figure 1E, F). This inhibition was comparable to that observed in sera treated with eculizumab or EDTA, whereas the scrambled Cp40 control did not inhibit MAC formation. In conclusion, we found that Cp40 effectively prevents C3b deposition and MAC formation on RBC opsonized with AIHA sera from patients with a DAT score positive for C3. Previous studies have shown that Cp40 blocks C3 deposition and hemolysis of RBC in the context of malaria and paroxysmal nocturnal hemoglobinuria, which are both antibody-independent diseases.10,11 Our results confirm these findings using AIHA sera to opsonize RBC and suggest that Cp40 is a potential candidate for complement inhibition to prevent intravascular hemolysis, which has been associated with thrombosis and an unfavorable prognosis,3 in complement-driven AIHA.
Figure 1.
Inhibition of complement deposition and lysis of red blood cells opsonized with autoimmune hemolytic anemia sera by Cp40. Red blood cells (RBC) were opsonized with sera from patients with autoimmune hemolytic anemia (AIHA) in the presence of recalcified human plasma from an AB blood group donor as a source of complement factors and C3b and C4b deposition on the RBC was determined using flow cytometry. (A) Levels of C3b and C4b deposition on RBC after incubation with sera from five individual AIHA patients. Dotted and dashed lines represent the C3b and C4b deposition levels obtained with RBC opsonized with serum from a healthy control (HC). (B) Representative flow cytometry histogram showing the effect of Cp40 on the C3b deposition on RBC opsonized with one tested AIHA serum. (C, D) Detection of C3b and C4b on RBC incubated with sera from the same five patients as shown in (A) in the presence of Cp40, scrambled Cp40 control peptide, αC1q-85, C1 esterase inhibitor (C1-INH) or ethylenediaminetetraacetic acid (EDTA). (E) Hemolysis of RBC after incubation with patients’ sera (n=10) in the presence of recalcified human plasma as a source of complement factors with either Cp40, Cp40 scrambled peptide, αC1q-85, C1-INH or EDTA. Median and data points are shown. (F) Hemolysis induced by opsonization of RBC with all tested patients’ sera with or without Cp40. Percentage lysis is normalized to a 100% lysis control that was obtained by incubating the RBC with distilled water. Statistical differences were calculated using one-way analysis of variance; ns=not significant. GeoMFI: geometric mean fluorescence intensity; Ctrl: control;
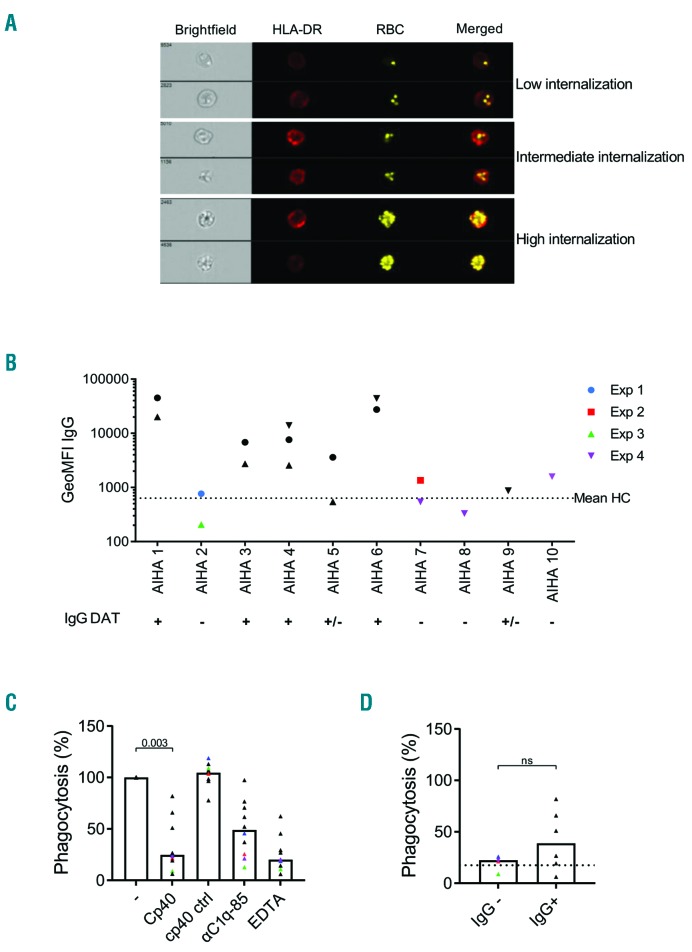
In addition to complement-mediated intravascular hemolysis, extravascular hemolysis by phagocytosis of opsonized RBC is a major cause of RBC breakdown in AIHA. We studied the effect of Cp40 on extravascular hemolysis by measuring the phagocytic uptake of opsonized RBC by macrophages. We found that opsonization of the RBC with AIHA serum resulted in different levels of internalization of the RBC by macrophages (Figure 2A). Patients’ sera were then divided into two groups based on results obtained in the DAT, either positive for complement deposition only or positive for complement deposition and IgG opsonization, which was confirmed using flow cytometry (Figure 2B). As expected, a clear reduction in phagocytosis of the RBC by macrophages was observed for all tested sera when Cp40 was added during opsonization (Figure 2C) while IgG opsonization levels remained unchanged (Online Supplementary Figure S1) indicating that inhibition of complement deposition on the RBC by Cp40 diminishes internalization. Overall, the same degree of internalization inhibition was achieved using Cp40 and EDTA but Cp40 was more effective than αC1q-85 at preventing RBC phagocytosis (Figure 2C). The same samples were divided according to their DAT score for IgG (Figure 2D). For samples with complement-activating capacity only, RBC internalization by Cp40 was reduced to the same extent as that by EDTA (dotted line; median, 22.45; range, 25.8-8.97). As expected, phagocytosis of RBC opsonized with complement and IgG-positive AIHA sera (Figure 2D) was less well inhibited by Cp40 (median, 38.9; range, 81.91-7.3) probably due to IgG binding to Fc-gamma receptors. This suggests that complement inhibition at the level of C3 with Cp40 is potentially beneficial in AIHA patients with chronic hemolysis caused by complement activation. Complement and Fc-gamma receptors are the two major classes of receptors involved in extravascular RBC breakdown in AIHA. Our results show that Cp40 can inhibit complement-mediated extravascular hemolysis of RBC opsonized with AIHA sera. Previous studies showed that complement inhibition at the C5 level has positive effects in AIHA patients with cold agglutinin disease.15 Our findings are in line with these results and additionally show that complement regulation by Cp40 can be even more beneficial since both intra- and extravascular hemolysis are prevented. Overall, we conclude that Cp40 is a promising new treatment for complement-mediated AIHA. Future research should confirm these findings in vivo.
Figure 2.
Red blood cells opsonized with complement-activating serum from patients with autoimmune hemolytic anemia in the presence of Cp40 are not inter-nalized by macrophages. Red blood cells (RBC) labeled with PKH (yellow) were opsonized with serum from patients with autoimmune hemolytic anemia (AIHA) in the presence of normal human serum as a source of complement factors and incubated with M1 differentiated HLA-DR labeled (red) macrophages to induce antibody-and/or complement-mediated RBC internalization. (A) Representative flow cytometry image showing different levels of RBC internalization by macrophages. Panels 1, 2 and 3 show low, intermediate and high internalization of RBC by the M1 macrophages, respectively. (B) Sera from AIHA patients with a positive direct antiglobulin test (DAT) score for complement deposition (n=4 col-ored symbols) or complement deposition in combination with the presence of IgG auto-antibodies (n=6 black symbols) were incubated with healthy RBC and IgG deposition was determined using flow cytometry in different experiments (symbol code) to confirm DAT results. IgG deposition induced by incubating RBC with healthy control serum (HC) is depicted as a dotted line. (C) Internalization of RBC opsonized with the two different types of AIHA sera according to the DAT (C3 positive patients: colored symbols; C3 + IgG positive patients: black symbols) in the presence of Cp40, scrambled Cp40 control peptide, αC1q-85 or ethylene-diaminetetraacetic acid (EDTA) by M1 macrophages was determined using imaging flow cytometry. (D) Comparison of the levels of phagocytosis of RBC opsonized with IgG-positive or -negative sera from patients with AIHA (black vs. colored symbols) in the presence of Cp40. The dotted line represents the percentage of phagocytosis when EDTA was added. Median and data points are shown. Statistical differences in phagocytosis were calculated using one-way analysis of variance; ns=not significant. GeoMFI: geometric mean fluorescence intensity; HC: healthy control.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Meulenbroek E, de Haas M, Brouwer C, Folman C, Zeerleder SS, Wouters D. Complement deposition in autoimmune hemolytic anemia is a footprint for difficult-to-detect IgM autoantibodies. Haematologica. 2015;100(11):1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barcellini W, Fattizzo B, Zaninoni A, et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood. 2014;124(19):2930–2936. [DOI] [PubMed] [Google Scholar]
- 3.Faraci M, Zecca M, Pillon M, et al. Autoimmune hematological diseases after allogeneic hematopoietic stem cell transplantation in children: an Italian multicenter experience. Biol Blood Marrow Transplant. 2014;20(2):272–278. [DOI] [PubMed] [Google Scholar]
- 4.Petz LD. A physician’s guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol. 2004;124(6):712–716. [DOI] [PubMed] [Google Scholar]
- 5.Barros MM, Langhi DM, Jr, Bordin JO. Autoimmune hemolytic anemia: transfusion challenges and solutions. Int J Clin Transfus Med. 2017;5:9–18. [Google Scholar]
- 6.Hill A, Rother RP, Arnold L, et al. Eculizumab prevents intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria and unmasks low-level extravascular hemolysis occurring through C3 opsonization. Haematologica. 2010;95(4):567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi J, Rose EL, Singh A, et al. TNT003, an inhibitor of the serine protease C1s, prevents complement activation induced by cold agglutinins. Blood. 2014;123(26):4015–4022. [DOI] [PubMed] [Google Scholar]
- 8.Wouters D, Stephan F, Strengers P, et al. C1-esterase inhibitor con centrate rescues erythrocytes from complement-mediated destruction in autoimmune hemolytic anemia. Blood. 2013;121(7):1242–1244. [DOI] [PubMed] [Google Scholar]
- 9.Bartko J, Schoergenhofer C, Schwameis M, et al. A randomized, first-in-human, healthy volunteer trial of sutimlimab, a humanized antibody for the specific inhibition of the classical complement pathway. Clin Pharmacol Ther. 2018;104(4):655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindorfer MA, Cook EM, Reis ES, et al. Compstatin Cp40 blocks hematin-mediated deposition of C3b fragments on erythrocytes: Implications for treatment of malarial anemia. Clin Immunol. 2016;171:32–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Risitano AM, Ricklin D, Huang Y, et al. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014;123(13):2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reis ES, Berger N, Wang X, et al. Safety profile after prolonged C3 inhibition. Clin Immunol. 2018;197:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ClinicalTrials.gov. Safety of intravitreal POT-4 therapy for patients with neovascular age-related macular degeneration (AMD) (ASaP). 2010; https://clinicaltrials.gov/ct2/show/NCT00473928.
- 14.Wang J, Wang L, Xiang Y, Ricklin D, Lambris JD, Chen G. Using an in vitro xenoantibody-mediated complement-dependent cytoxicity model to evaluate the complement inhibitory activity of the peptidic C3 inhibitor Cp40. Clin Immunol. 2016;162:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth A, Huttmann A, Rother RP, Duhrsen U, Philipp T. Long-term efficacy of the complement inhibitor eculizumab in cold agglutinin disease. Blood. 2013;113(16):3885–3886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.