Abstract
Background:
Renal allograft rejection is more frequent under belatacept-based, compared to tacrolimus-based, immunosuppression. We studied kidney transplant recipients experiencing rejection under belatacept-based early corticosteroid withdrawal following T cell depleting induction in a recent randomized trial (BEST Trial, clinicaltrials.gov #NCT01729494) to determine mechanisms of rejection and treatment.
Methods:
Peripheral mononuclear cells, serum creatinine levels, and renal biopsies were collected from 8 patients undergoing belatacept-refractory rejection. We used flow cytometry, histology and immunofluorescence to characterize CD8+ effector memory T cell (TEM) populations in the periphery and graft before and after mammalian target of rapamycin (mTOR) inhibition.
Results:
Here, we found that patients with belatacept-refractory rejection (BRR) did not respond to standard antirejection therapy and had a substantial increase in alloreactive CD8+ T cells with a CD28low/DRhi/CD38hi/CD45RO+ TEM. These cells had increased activation of the mTOR pathway, as assessed by phosphorylated ribosomal protein S6 (p-RPS6) expression. Notably, everolimus (an mTOR inhibitor) treatment of patients with BRR halted the in vivo proliferation of TEM cells, their ex vivo alloreactivity, and resulted in their significant reduction in the peripheral blood. The frequency of circulating FoxP3+ regulatory T cells was not altered. Importantly, everolimus led to rapid resolution of rejection as confirmed by histology.
Conclusions:
Thus, while prior work has shown that concomitant belatacept + mTOR inhibitor therapy is effective for maintenance immunosuppression, our preliminary data suggest that everolimus may provide an available means for effecting “rescue” therapy for rejections occurring under belatacept that are refractory to traditional antirejection therapy with corticosteroids and polyclonal antilymphocyte globulin.
INTRODUCTION
While calcineurin inhibitor (CNI)-based therapies have remained the cornerstone of immunosuppression for kidney transplantation, their long-term usage leads to nephrotoxicity, cardiovascular and metabolic effects that directly contribute to allograft loss. A major advantage of the first biologic agent to receive FDA approval for maintenance immunosuppression, belatacept, is that it is devoid of nephrotoxicity. Belatacept was derived from CTLA4Ig (abatacept), a fusion protein consisting of the extracellular domain of human CTLA4 fused to a fragment of the Fc domain of a human IgG1 antibody1. A site-directed mutagenesis approach enables belatacept to bind to CD80/86 present on antigen presenting cells (APCs) with higher affinity than abatacept, preventing T cells from receiving signal two, through CD28, required for T cell activation1. Therefore, belatacept enables immunosuppression without the nephrotoxicity and adverse cardiovascular/metabolic effects of CNIs2,3.
Indeed, results from the belatacept Phase 3 registration trials (BENEFIT and BENEFIT EXT) have shown that (i) patient and graft survival, as well as the mean estimated glomerular filtration rate (eGFR) are significantly higher with belatacept than with CNI-based regimens, (ii) renal function benefits associated with belatacept were sustained, and (iii) blood pressure was consistently lower with belatacept than with CNIs, and increases in blood lipids were smaller in subjects treated with belatacept2–5. However, these initial clinical trials were conducted with concomitant corticosteroid (CCS) therapy and without T cell depleting induction4–7. Since almost 50% of kidney transplant recipients in the US are now conducted under early CCS cessation, it is important to determine whether such early CCS elimination can be achieved under belatacept. Indeed, in both a small scale pilot study and a large study (>300 patient trial clinicaltrials.gov #NCT01729494) simultaneous CNI- and steroid-free immunosuppression was achieved with rejection results similar to the BENEFIT trial5,8,9, where small increases in acute rejection incidence and increased acute rejection severity were observed.
The immunologic mechanisms and critical cellular components that mediate rejection under belatacept remain to be fully defined. One potential explanation is association of CD8+CD28− effector T cells that robustly produce cytokines when stimulated with allogeneic cells as shown by several studies in humans and non-human primates10–14. Regardless of mechanism, it is of considerable interest to develop new therapeutic approaches to target belatacept-refractory T cells and promote graft acceptance. In this regard, concomitant administration of mammalian target of rapamycin (mTOR) inhibitors (mTORi, either sirolimus or everolimus) with belatacept from the time of transplant appears to lessen acute rejection events8,15. However, it remains unclear if mTORi could be used to control rejection that has emerged under a belatacept/ mycophenolate mofetil (MMF) based protocol. This is particularly intriguing because mTOR plays a critical role in regulating CD8+ T cell effector and memory differentiation16.
Here, we describe our experience in a large randomized trial examining simultaneous CNI avoidance/early corticosteroid withdrawal under belatacept and T cell depleting induction therapy. We found that belatacept refractory rejections (BRRs) were: (i) refractory to traditional therapy with rabbit anti-thymocyte globulin (rATG)/MMF or high dose tacrolimus, and (ii) associated with expansion of CD28lowCD38hiCD8+ effector memory T cells (TEM) that possessed significant mTOR activity. Notably, we found that treatment with an mTORi, everolimus, significantly reduced the proliferation of these alloreactive CD8+ TEM cells and promoted the histologic resolution of rejection. Thus, our data, although preliminary and in a limited number of patients, show that mTORi may provide a novel means for addressing refractory rejection occurring under belatacept-based immunosuppression.
MATERIALS AND METHODS
Cohort and treatment
Patients who were enrolled in the BEST trial (BEST, NCT #01729494) at the University of Cincinnati Medical Center and The Christ Hospital undergoing a for-cause renal allograft biopsy were eligible for this study. All protocols were in compliance and approved by the University of Cincinnati Institutional Review Board, and patients were provided informed consent prior to study procedures (IRB# 2015–2934, 2017–4696).
The standard of care approach for treating rejection consisted of rATG for acute cellular rejection (ACR) of grade 2 or higher, or steroid pulsing for ACR 1A and 1B. For patients failing therapy with steroids, rATG was used as a second-line therapy. However, the patients described in this report did not respond to such treatments and were initially started on tacrolimus rescue therapy. Tacrolimus rescue therapy was implemented by initial dosing at 0.05–0.1 mg/kD given twice daily with target level of 10–12 ng/mL for at least 7–10 days followed by repeat biopsy if serum creatinine did not decrease17. Increased target levels up to 15 ng/mL were utilized in patients who failed to demonstrate histologic resolution of inflammatory infiltrates. The rATG rejection therapy protocol that was used in this study has been our standard of care for 20 years at the University of Cincinnati for Banff 2A and 2B rejections and has provided rejection reversal rates of 85% or greater. Dosing included FDA label rATG (Thymoglobulin) given at 1.5 mg/kg per dose. Dosing intervals were determined using monitoring of peripheral blood CD3+ T cells with repeat doses given only when peripheral CD3+ counts were greater than 25 cells/μL. Total duration of CD3+ suppression was 10–14 days.
Isolation and phenotypic analysis of PBMCs
Peripheral mononuclear cells (PBMCs) were isolated through Ficoll-Paque density gradient. 2×106 PBMCs were stained for phenotypic analysis with the following fluorochrome-conjugated
antibodies: CD3-V500 (BD Biosciences), CD4-APCeF780 (eBiosciences), CD8-BV605 (Biolegend), CD28-PE-Cy7 (Biolegend), CD38-APC (eBiosciences), CD45RO-FITC (eBiosciences), HLA-DR-AF700 (eBiosciences), CD45RA-PerCP-Cy5.5 (Biolegend). Viability was determined with Live/Dead fixable blue dead cell stain (Invitrogen). Intracellular stain was performed with FoxP3-Pacific Blue (eBiosciences) and Ki-67-BV786 (BD Biosciences) following the eBiosciences Fixation/Permeabilization protocol (Invitrogen). Samples were analyzed on a BD LSRFortessa analyzer (BD Biosciences). Flow cytometry data analysis was done with FlowJo V10, LLC Software.
Mixed lymphocyte reactions
Donor and recipient PBMCs were thawed and rested for 1 hour in media with DNase I (Roche). For stimulators, APCs were isolated from donor PBMCs with CD3 MicroBeads (Miltenyi Biotec). For responders, recipient PBMCs were stained with CellTrace™ Violet Cell proliferation dye (ThermoFisher Scientific). 3×104 recipient responder PBMCs were cultured for 5 days in serum-free AIM V media with 8×104 isolated stimulator APCs from donor PBMCs. Cells were then washed with PBS, blocked with Fc Block, stained for surface markers CD3-V500 (BD Biosciences), CD4-APCeF780 (eBiosciences), CD8-BV605 (Biolegend), CD28-PE-Cy7 (Biolegend), CD38-APC (eBiosciences), CD45RO-FITC (eBiosciences), (eBiosciences), and CD45RA-PerCP-Cy5.5 (Biolegend). Viability was determined with Live/Dead fixable blue dead cell stain (Invitrogen). Samples were analyzed on a BD LSRFortessa analyzer (BD Biosciences). Flow cytometry data analysis was done with FlowJo V10, LLC Software.
Phospho-flow cytometry
Donor and recipient PBMCs were thawed and rested for 1 hour in media with DNase I (Roche). APCs were isolated from donor PBMCs with CD3 MicroBeads (Miltenyi Biotec). Recipient PBMCs were stained with CellTrace™ Violet Cell proliferation dye (ThermoFisher Scientific). 3×105 APCs were cultured with 106 recipient PBMCs for 2 hours in serum-free AIM V media. Upon stimulation, cells were fixed in Fixation/Permeabilization solution (BD Biosciences) at 4°C for 20 minutes. Cells were then permeabilized with 1X BD Perm/Wash at 4°C for 25 minutes. After being washed, cells were blocked with Fc Block, stained for surface markers and intracellular phospho-S6 ribosomal protein Ser235/236 (Cell Signaling Technology) with anti-rabbit IgG PE conjugate secondary antibody (Cell Signaling Technology). Samples were analyzed on a BD LSRFortessa analyzer (BD Biosciences). Flow cytometry data analysis was done with FlowJo V10, LLC Software.
Isolation of graft cells
For phenotyping analysis, the renal biopsy was cryopreserved in a solution containing DMSO18. For analysis, the biopsies were thawed, washed of any remaining cryopreservative with complete RPMI, and cut into pieces that were digested with 0.25 mg/mL Liberase LT (Roche) and 100 U/mL DNase I (Roche) at 37°C for 12 minutes on a shaker18. Dissociated cells and tissue were harvested through a 70 μm cell strainer (Fisher Scientific) into complete RPMI on ice. The remaining undigested tissue on top of the strainer was pushed through with the plastic end of a 3 mL syringe plunger (BD) and RPMI. Cell suspension was centrifuged at 400 x g at 4°C for 10 minutes and resuspended in media to determine 95% viability assessed by trypan blue exclusion dye (ThermoFisher Scientific).
Immunofluorescence staining
Formalin-fixed paraffin embedded sections of kidney biopsies were obtained and stained via immunofluorescence after de-paraffinization through overnight incubation in a 55° C oven. This was followed by three 5-minute washes in Histo-clear (National Diagnostics) and re-hydration of slides. Antigen retrieval was performed with 10 mM sodium citrate at pH 6 with a pressure cooker at high pressure for 15 minutes. Slides were cooled to room temperature and washed with 1X PBS/0.5% Triton-X 100 (Sigma-Aldrich) followed by a 20-minute wash in 3% hydrogen peroxide. After washing in 1X PBS, slides were blocked with 1X PBS/5%BSA/5%NDS for 1 hour at room temperature. Primary antibody cocktail containing CD8 (Bio-Rad), CD28 (Santa Cruz Biotechnology), CD38 (Abcam), and VEGF (R&D Systems) were added to the slides and incubated overnight at 4°C on a rotor. After washing in 1X PBS, the secondary antibody cocktail containing anti-mouse, anti-rat, anti-rabbit, and anti-goat Alexa Fluor 750, 647, 568, and 488 (Abcam) were incubated with the slides for 2 hours at room temperature. Slides were then washed in 1X PBS and incubated with DAPI (Invitrogen) for 2 minutes before mount and coverslip with ProLong Gold Anti-Fade Reagent (Invitrogen). All images were taken with a Nikon Eclipse Ti2 Widefield Microscope (Nikon), and all analysis was done with NIS Elements software (Nikon).
Statistical analysis
All graphs and statistical analysis were performed with GraphPad Prism software. Statistical analysis was performed using a Student’s t-test. A P value of < 0.05 was considered statistically significant.
RESULTS
Belatacept refractory rejections do not respond to standard of care treatment
Similar to the reported BENEFIT trial results5, the 1-year results from belatacept-treated group in the Belatacept-based Early Steroid Withdrawal Trial (BEST) shows a significant increase (~15%) in biopsy-proven acute rejection incidence when compared to the tacrolimus-treated control group, and also demonstrated greater severity when judged by Banff criteria (Table 1. BEST trial cohort)9. Based on these clinical observations, we analyzed BRRs in more detail. Of the 38 rejections identified in the BEST trial, 9 were removed from subsequent analysis as 4 had antibody mediated rejection (AMR), 3 had evidence of mixed acute rejection (MAR), and 2 had complicating thrombotic microangiopathy (TMA) (Table 1, supplemental figure 1). 29 patients presented with belatacept-refractory acute cellular rejection (ACR), of which, 17 were treated with standard of care rejection therapy. 2 of these patients resolved, 1 had BK viremia, and the other 14 failed to resolve (supplemental figure 1). These 14 patients were distinct from our prior experience defining the histologic and renal functional response to traditional antirejection therapy (CCS +/− rATG + tacrolimus rescue therapy with target troughs of 10–15 ng/mL followed by a 4-week taper)17. For this reason, we followed the serum creatinine, clinical course, and Banff classification of 4 representative patients with acute BRR in the BEST trial (Figure 1A–D; supplemental tables 1 and 2).
Table 1:
1-year results of the BEST trial shows higher rates of acute rejection and of increased histologic severity in BELA treated patients when compared to TAC treated patients but without adverse effects on graft function.
| Group A | Group B | Group C | p- value Group A vs Group C | p- value Group B vs Group C | |
|---|---|---|---|---|---|
| Patients analyzed | 105 | 102 | 105 | ||
| Induction | alemtuzumab | rATG | rATG | ||
| Immunosuppression | BELA | BELA | Tac | ||
| BPAR (%) | 18 (17.1) | 20 (19.6) | 5(4.08) | 0.014 | 0.003 |
| 1st BPACR Grade ≥ 2a | 5 (4.8) | 8 (7.8) | 0 | 0.12 | 0.009 |
rATG, rabbit anti-thymocyte globulin; BELA, belatacept; Tac, tacrolimus; BPAR, biopsy proven acute rejection; BPACR, biopsy proven acute cellular rejection.
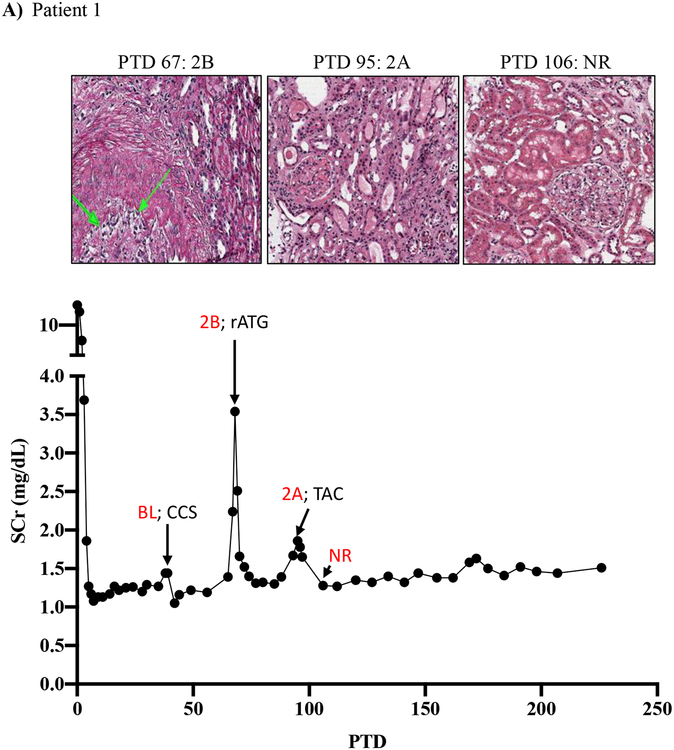
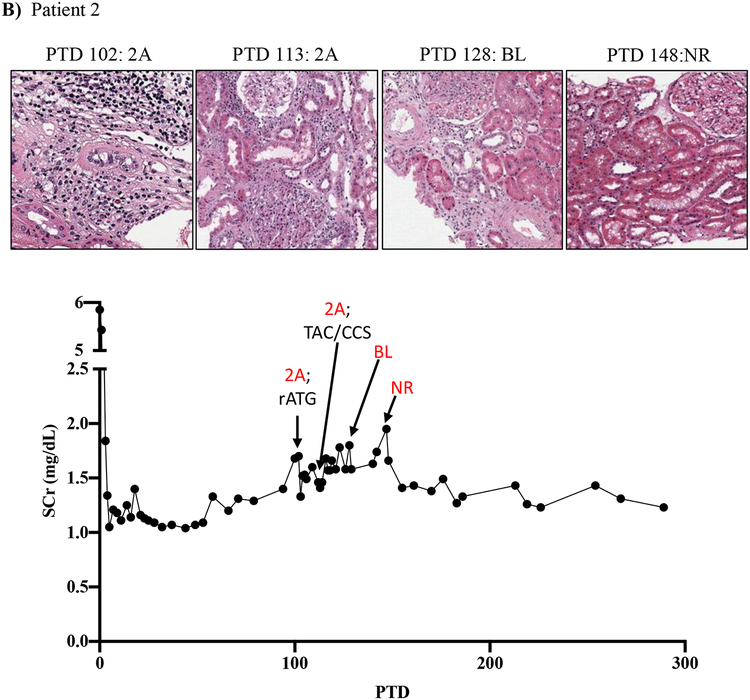
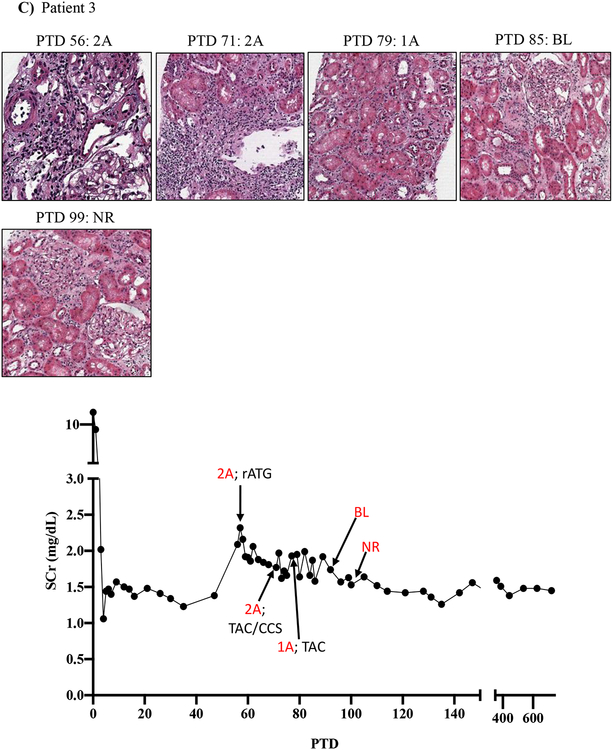
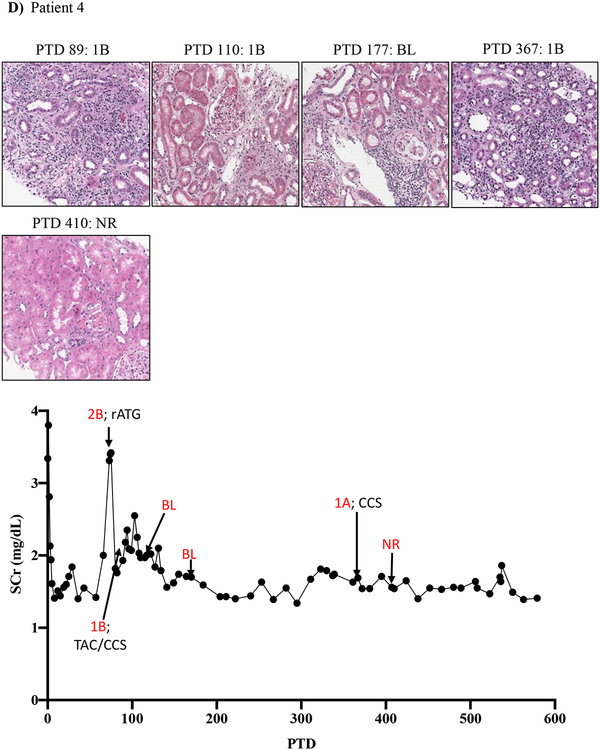
Figure 1: Resistance of belatacept-refractory rejection to standard of care immunosuppression.
A-D) Plots showing post-transplant serum creatinine, histology, Banff lesion score (red) and treatment course of four patients that underwent rejection under belatacept. Renal allograft pathology was determined by H&E staining (micrographs) at specified timepoints. A) Shows a 46-year-old male that received his first transplant from living donor after ESRD due to PCKD. Prior to transplant he had a cPRA of 0%. Initial immunosuppression included rATG induction, belatacept, MMF, and early corticosteroid withdrawal at 5 days post-transplant. B) Shows the history of a 56-year-old Caucasian male that received first kidney transplant after ESRD due to DM and had a 0% cPRA and non-prior HLA exposure history. Postoperatively, he received alemtuzumab induction with belatacept, MMF and early corticosteroid cessation at 5 days post-transplant. C) Shows a 53-year-old AA woman with ESRD due to necrotizing GN and the recipient of a first kidney transplant. She had a 0% cPRA with no prior history of HLA exposure. Immunosuppressive therapy included rATG induction, belatacept, MMF, and CCS cessation on PTD 5. D) Displays a 60-year-old male with ESRD due to DM that underwent kidney transplantation from a deceased donor and received alemtuzumab induction, belatacept, MMF, and CCS cessation at 5 days post-transplant.
BL, borderline; CCS, corticosteroids; DM, diabetes mellitus; ESRD, end stage renal disease; GN, glomerulonephritis; MMF, mycophenolate mofetil; NR, no rejection; PCKD, polycystic kidney disease; rATG, rabbit anti-thymocyte globulin; TAC, tacrolimus.
Patient one showed renal allograft dysfunction on post-transplant day (PTD) 39, and a biopsy revealed a borderline lesion with negative C4d staining, which was treated with high dose CCS. Repeat renal allograft biopsy performed on PTD 67 revealed Banff 2B ACR with negative C4d staining, which was treated with rATG therapy. Repeat biopsy on PTD 77 did not provide adequate tissue, and repeat biopsy on PTD 95 revealed Banff 2A rejection, which was treated with tacrolimus rescue therapy. It was not until PTD 106 that repeat biopsy revealed resolution of rejection (Figure 1A).
Patient two showed renal dysfunction on PTD 102, which upon biopsy revealed a Banff 2A rejection that was treated with four doses of rATG over a 12-day period. A repeat biopsy on PTD 113 revealed persistent Banff 2A rejection, which was then treated with pulse corticosteroids and tacrolimus rescue therapy in combination. An additional repeat biopsy on PTD 128 revealed persistent borderline rejection, and it wasn’t until PTD 148 that another repeat biopsy revealed no rejection (Figure 1B).
Patient three underwent renal allograft biopsy on PTD 56 for renal dysfunction evaluation which revealed a Banff 2A rejection that was treated with five rATG doses over 10 days. Repeat renal allograft biopsy on PTD 71 revealed persistent Banff 2A rejection, which was treated with CCS and tacrolimus rescue therapy. Repeat biopsy on PTD 79 revealed Banff 1A rejection, and tacrolimus therapy was continued. A persistent borderline lesion was found on PTD 85, and two weeks later on PTD 99 a repeat biopsy revealed resolution of rejection (Figure 1C).
Patient four underwent renal allograft biopsy on PTD 73 for evaluation of renal dysfunction, which revealed a Banff 2B ACR that was treated with rATG. A repeat biopsy on PTD 89 revealed Banff 1B ACR, which was treated with high dose CCS and tacrolimus rescue therapy for 12 weeks. The patient had a persistent borderline lesion as evidenced by biopsies on PTD 110 and PTD 177. Another repeat biopsy on PTD 367 revealed Banff 1A ACR, which was treated with high dose CCS, and it was not until yet another repeat biopsy on PTD 410 revealed resolution of rejection (Figure 1D).
The lack of any (or minimal) histologic and clinical improvement following treatment with rATG has been rarely encountered in over 30 years of experience in immunosuppressive drug development by our senior author (ESW unpublished observations)19. Indeed, despite aggressive treatment with rATG and/or high dose CNI/CCS, rejections persisted for more than 30 days in several patients (Figure 1A, C, D, Table 2). Notably, these persisting rejections were not due to medication non-compliance as patients were closely monitored for adherence and their belatacept infusions were documented. Our impressions from this experience were consistent with those in the BENEFIT trial and indicated an urgent need for more effective antirejection therapies tailored to underlying BRR biology.
Table 2:
Demographics and treatment data of four patients undergoing resistance of BRR to standard of care in the BEST trial.
| Patient | 1 | 2 | 3 | 4 | Mean | SD |
|---|---|---|---|---|---|---|
| Induction | rATG | alemtuzumab | rATG | alemtuzumab | ||
| Immunosuppression | BELA | BELA | BELA | BELA | ||
| Age at transplant | 48 | 56 | 60 | 54 | 54.25 | 5.05799 |
| Cause of ESRD | PCKD | DM | Necrotizing GN | DM | ||
| EBV status | POS | POS | POS | POS | 100% POS | |
| CMV status | NEG | NEG | POS | NEG | 25% POS | |
| rATG dose for rejection (mg/kg) | 6 | 6 | 9 | 4.5 | 6.4 | 1.9 |
| Days of CD3 suppression (CD3 <25 cell/μL) | 14 | 14 | 14 | 10 | 13.0 | 2.0 |
SD, standard deviation; rATG, rabbit anti-thymocyte globulin; BELA, belatacept; ESRD, end stage renal disease; PCKD, polycystic kidney disease; DM, diabetes mellitus; GN, glomerulonephritis; EBV, Epstein-Barr virus; POS, positive; CMV, cytomegalovirus; NEG, negative.
Belatacept refractory rejections correlate with the emergence of CD8+ effector memory T cells
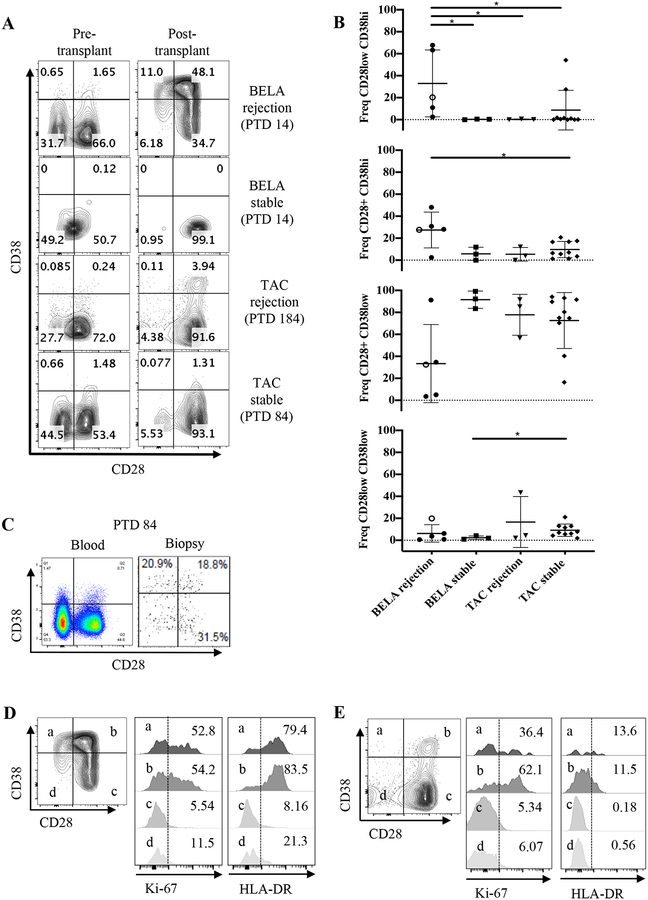
To understand the nature of acute BRR, we characterized PBMCs from stable patients and patients undergoing rejection under either belatacept- or tacrolimus-based immunosuppression from a cohort of patients enrolled in the BEST trial (Figure 2). PBMCs were isolated and analyzed by flow cytometry for lymphocyte activation (CD38, HLA-DR) and proliferation (Ki-67) markers pre- and post-transplant in stable patients and at the time of rejection. Consistent with prior studies12, our data show significant expansion of CD8+CD28−CD38hi (17 fold) and CD8+CD28+CD38hi (28 fold) T cells in the blood of a belatacept-treated patient that presented symptoms of rejection 14 days post-transplant (Figure 2A, B, gating strategy in supplemental figure 2). Notably, we also observed these cells in the graft at PTD 84 (Figure 2C). These CD8+CD38+ cells had a memory phenotype (based on CD45RO expression), expressed the activation marker HLA-DR and were highly proliferative (Figure 2D). In contrast, rejection under tacrolimus was associated with CD38+CD28+HLA-DR+ cells and a relative paucity of CD38hiCD28low cells (Figure 2E).
Figure 2: Emergence of CD8+CD28−CD38hi and CD8+CD28+CD38hi effector memory T cells in patients with belatacept-resistant allo-rejection.
A) CD8+ T cells gated from CD3+CD45RO+ were analyzed for the expression of CD28 and CD38 in stable and rejection patients under belatacept or tacrolimus. BELA rejection on patient 7 is PTD 14, which is the time of diagnosed rejection and prior to any SOC rejection therapy. TAC rejection depicts the timepoint of rejection diagnosis at PTD 184. Stable patients’ samples were chosen to match rejectors PTD. B) Frequencies of CD8+ CD45RO+ CD28+/− CD38+/− TEM populations in the rejector and stable patients. Open circle marks the CD8+ profile for patient 7 at PTD 185, after SOC rejection therapy. Results are presented as means ± standard deviation. C) At moment of confirmed rejection PTD 84 for patient 7, CD8+ TEM cells are present in the graft as shown by flow cytometric analysis of cells extracted from a previously frozen biopsy. D) Belatacept rejection CD8+ effector memory T cells and E) tacrolimus rejection CD8+ effector memory T cells were analyzed with Ki-67 for proliferation and HLA-DR for activation. BELA rejection n=4, BELA stable n=3, TAC rejection n=4, TAC stable n=10. *p<0.05.
mTOR inhibition can rescue belatacept refractory rejections
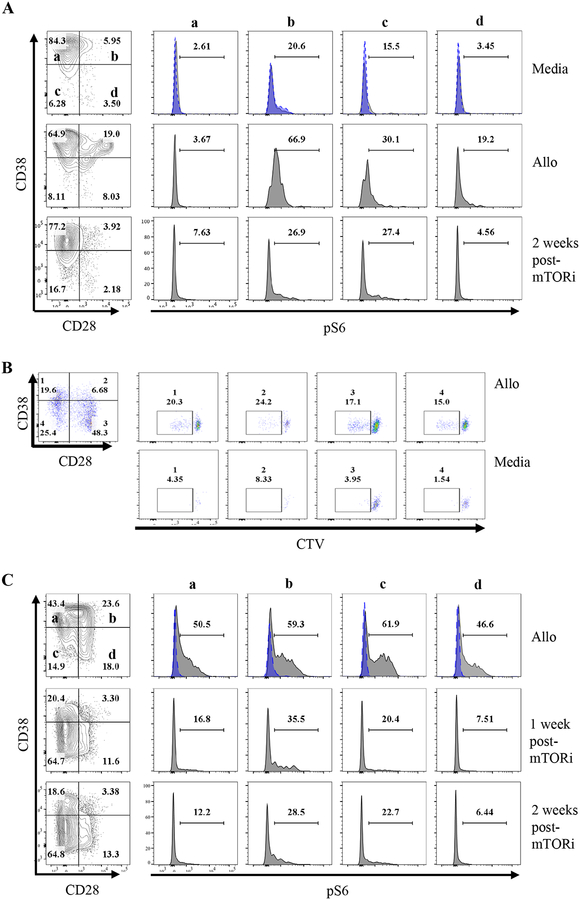
Prior work showed that inhibition of mTOR can halt the proliferation of human alloreactive CD8+ TEM20 as well as prevent most BRR in nonhuman primates when administered concomitantly with belatacept21. We inquired whether belatacept-refractory alloreactive CD8+ T cells show upregulation of phosphorylated ribosomal protein S6 (p-RPS6), thereby indicating the presence of an active mTOR/AKT signaling pathway within alloreactive T cells. Interestingly, we found that CD8+CD28−CD38+ cells and CD8+CD28+CD38+ cells emerging at time of rejection under belatacept have increased p-RPS6 at time of rejection (PTD 14) in response to allogeneic stimulator cells (Figure 3A, B). Notably, even after rejection and tacrolimus/rATG therapy, these BRR patients also had a persisting mixed-lymphocyte reaction response in the peripheral blood whether measured by proliferation or pRS6 (Figure 3A–C). Importantly, administration of everolimus reduced p-RPS6 expression in these patients, showing their decreased alloreactivity (Figure 3A, C).
Figure 3: Effector memory CD8+ T cells emerging under belatacept have increased p-RPS6 expression.
PBMCs isolated from patients 6 (A) and 7 (B, C) (n=2) undergoing rejection were analyzed for p-RPS6 before and after (1 and 2 weeks) mTORi. PBMCs were cultured alone, or with their respective donor APCs (allo) for 2 hours. Cells were then fixed and stained for CD45, CD3, CD8, CD38, CD28 and p-RPS6 phospho-flow analysis. Blue dashed-line histograms show isotype control. B) Allo reactive cells were detected by cell trace violet dilution in a mixed lymphocyte reaction assay in PBMCs of patient 7 at PTD 56 following rejection standard of care treatment were mixed with donor cells for 5 days and analyzed by flow cytometry.
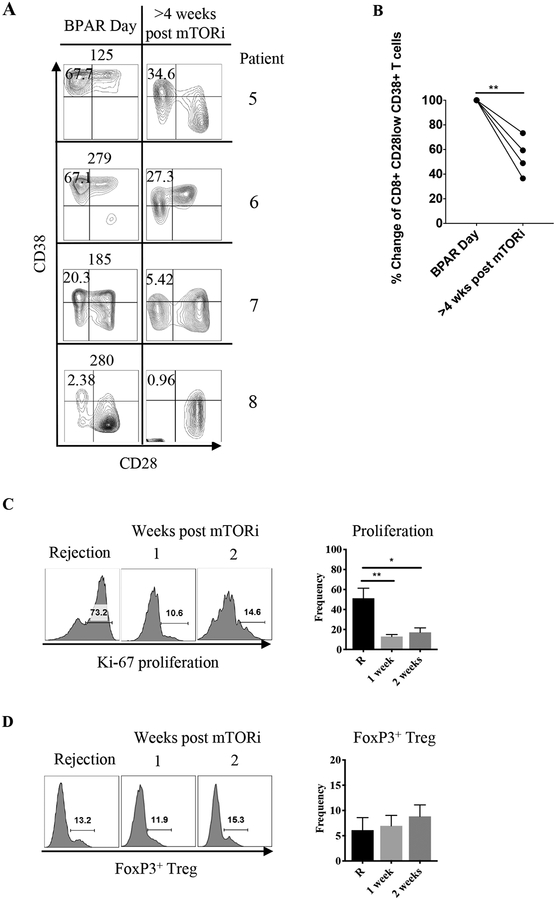
Given the increased mTOR activity, persisting allograft inflammation, and alloreactivity that was culled by everolimus, we next inquired whether mTORi could limit such alloreactivity and reverse ongoing allograft rejection. Thus, we decided to use mTORi as a “rescue” therapy for patients demonstrating BRR with additional resistance to standard of care rATG/tacrolimus rescue therapy. Four patients undergoing BRR from the BEST trial cohort, which had an expansion of CD8+CD28lowCD38hi TEM cells present in their peripheral blood to varying degrees, were treated with everolimus (with dosing to achieve target levels of 6–9 ng/mL) (Figure 4A). Weekly monitoring of peripheral blood CD8+CD28lowCD38hi TEM cells by flow cytometry demonstrated a significant decrease in the frequency of CD8+ TEM cells that were CD28lowCD38hi in each patient (Figure 4A, B). Consistent with these data, everolimus also drove a dramatic loss of cellular proliferation as assessed by Ki-67 staining within CD8+ CD28lowCD38hi TEM, similar to prior work in non-transplant models22 (Figure 4C). Interestingly, everolimus did not alter the percentage of FoxP3+ CD4+ Treg (Figure 4D). Moreover, everolimus also decreased the abundance of CD38hiCD8+ T cells in the kidney allograft of two different patients (supplemental figure 3A, B).
Figure 4: Everolimus diminishes activated CD8+CD28−CD38hi effector memory T cells.
A) Four patients (patients 5, 6, 7 and 8) presenting with belatacept–refractory rejection were treated with everolimus (n=4). PBMCs were analyzed for presence of CD38hi TEM cells pre- and post-everolimus therapy. Quadrants were drawn based on internal positive controls. B) Percent change at 4 weeks or more after everolimus treatment. Percent change was determined by calculating the decrease difference between pre and post, dividing the decrease by the original number and multiplying it by 100. C) Proliferation assessment by Ki-67 at rejection and 1 and 2 weeks post-everolimus. D) Effect of everolimus on FoxP3+ Tregs 1 and 2 weeks after treatment.
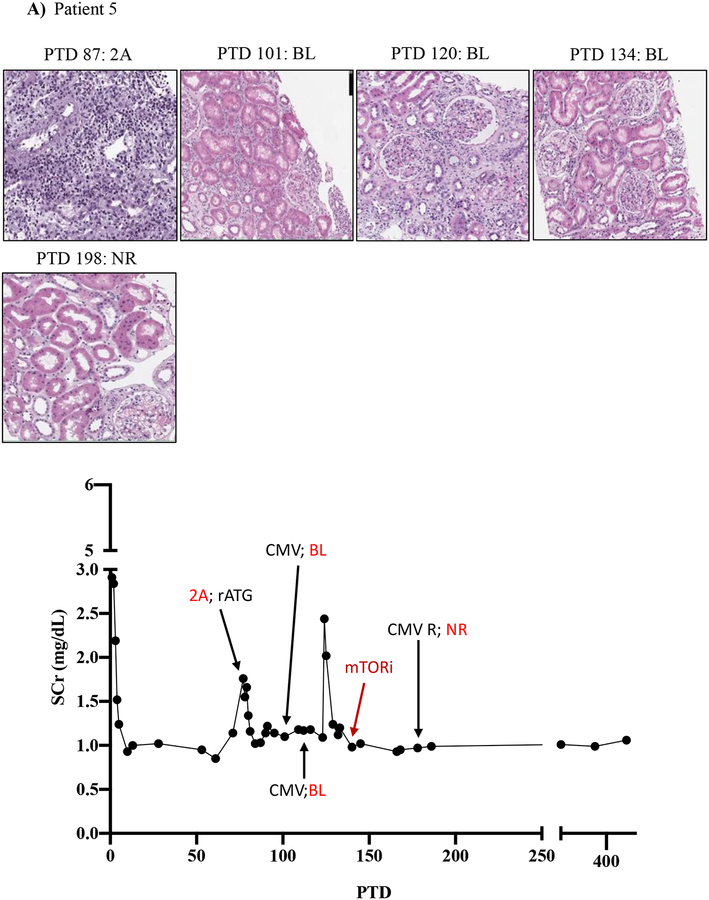
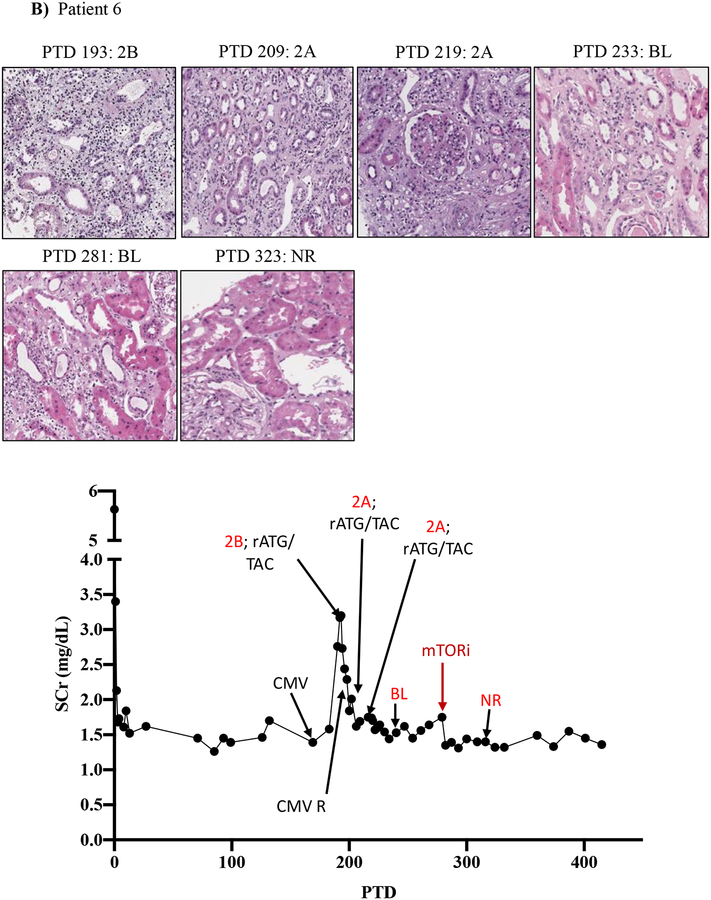
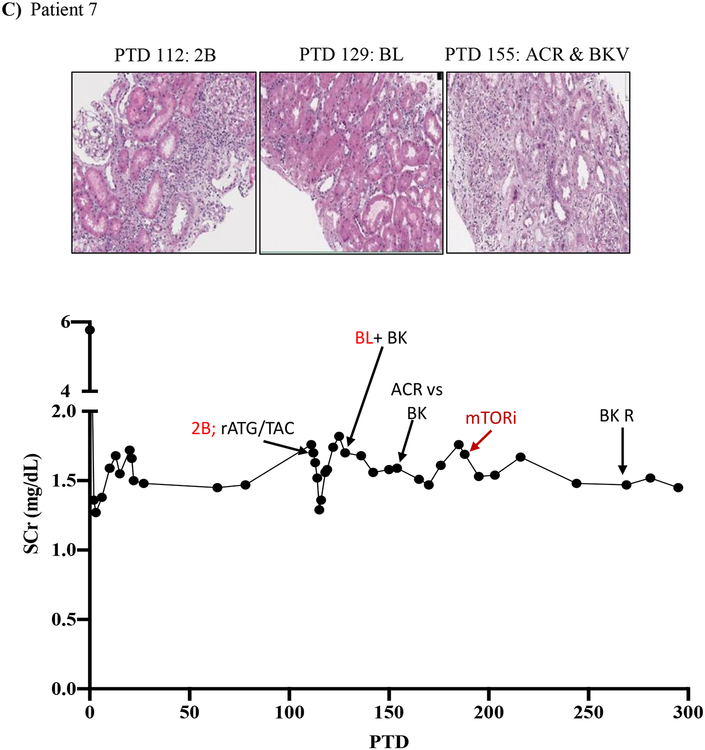
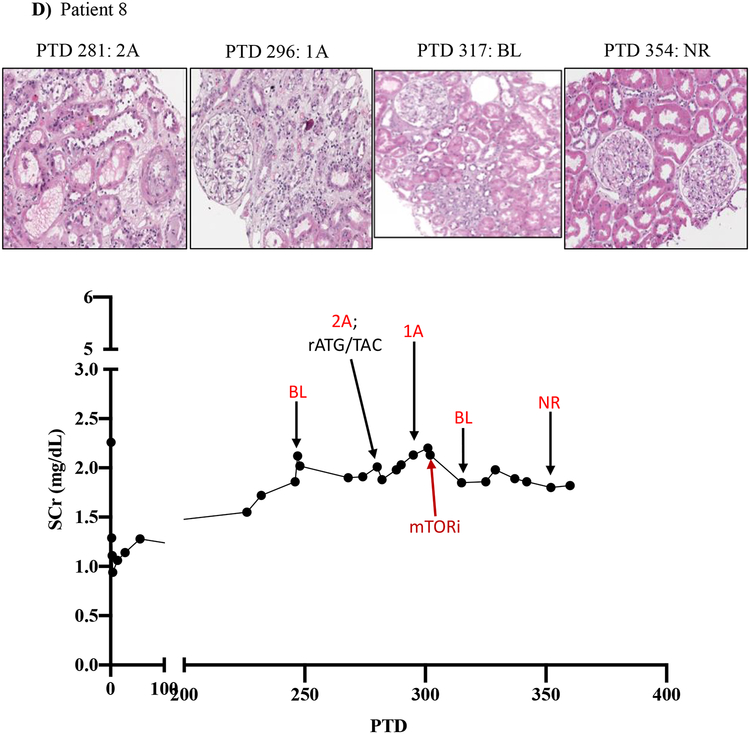
We then tracked serum creatinine and rejection histology of these four patients treated with everolimus during BRR (Figure 5; Table 3; supplemental tables 1 and 2). Patient five underwent renal allograft biopsy on PTD 87 to evaluate an increased serum creatinine (to 1.76 mg/dL from baseline of 0.97 mg/dL) that revealed a Banff grade 2A ACR. Rejection treatment included rATG (3 doses from PTD 88 to PTD 95). A repeat renal allograft biopsy on PTD 101 revealed borderline rejection, which was not treated due to development of CMV viremia. A repeat biopsy on PTD 120 and PTD 134 showed ongoing allograft inflammation graded as borderline rejection, and CMV viremia persisted despite valganciclovir therapy. On PTD 140, the patient was switched from MMF to everolimus therapy (target level 6–9 ng/mL) due to ongoing renal allograft inflammation/borderline rejection and persistent CMV viremia. CMV viremia resolved within 7 days, and follow-up renal allograft biopsy on PTD 198 demonstrated complete resolution with return of renal function back to pre-treatment baseline serum creatinine level of 0.99 mg/dL (Figure 5A).
Figure 5. mTOR inhibition can rescue belatacept resistant rejections.
A-D) Diagrams show serum creatinine, histology, Banff lesion score (red), and treatment course of four patients that underwent rejection under belatacept and were treated with mTORi. Renal allograft pathology was determined by H&E stain analysis (micrographs) at specified timepoints. A) Shows a 50-year-old Caucasian female with ESRD secondary to hypertension that received a first kidney transplant from a deceased donor. She had a low cPRA (13%) and no evidence of pre-transplant DSA. The patient received rATG induction, belatacept, MMF, and early steroid withdrawal (5 days). B) Shows a 53-year-old unsensitized (cPRA 0%) Caucasian male with ESRD due to DM that received a first renal transplant from a living donor. Immunosuppression included alemtuzumab, belatacept, MMF, and steroid withdrawal at 5 days. CMV infection occurred on PTD 170 and required cessation of MMF therapy for resolution. C) Shows a 46-year-old unsensitized Caucasian male with focal segmental glomerulosclerosis that underwent a first kidney transplant from a living related one haplotype mismatched donor. Immunosuppression included rATG induction, belatacept, MMF, and early steroid cessation at 5 days. D) Shows a 44-year-old unsensitized Caucasian male with ESRD due to obstructive uropathy that underwent living related donor kidney transplant. Immunosuppression consistent of rATG induction, belatacept and MMF maintenance therapy with early steroid cessation on PTD 5.
ACR, acute cellular rejection; BL, borderline; CCS, corticosteroids; DM, diabetes mellitus; ESRD, end stage renal disease; GN, glomerulonephritis; MMF, mycophenolate mofetil; NR, no rejection; rATG, rabbit Anti-thymocyte globulin; TAC, tacrolimus.
Table 3.
Demographics and treatment data of four patients undergoing BRR with resistance to standard of care in the BEST trial that were treated with mTORi rescue therapy.
| Patient | 5 | 6 | 7 | 8 | Mean | SD |
|---|---|---|---|---|---|---|
| Induction | rATG | alemtuzumab | rATG | rATG | ||
| Immunosuppression | BELA | BELA | BELA | BELA | ||
| Age at transplant | 50 | 53 | 46 | 44 | 48.25 | 4.0311 |
| Cause of ESRD | Hypertension | DM | MPGN | Hypertension | ||
| EBV status | POS | POS | POS | POS | 100% POS | |
| CMV status | POS | NEG | NEG | POS | 50% POS | |
| rATG dose for rejection (mg/kg) | 4.5 | 7.5 | 4.5 | 6 | 5.5 | 1.7 |
| Days of CD3 suppression (CD3 <25 cell/μL) | 10 | 16 | 10 | 14 | 12.5 | 3.0 |
SD, standard deviation; rATG, rabbit anti-thymocyte globulin; BELA, belatacept; ESRD, end stage renal disease; MPGN, membranoproliferative glomerulonephritis; DM, diabetes mellitus; EBV, Epstein-Barr virus; POS, positive; CMV, cytomegalovirus; NEG, negative.
Patient six had an increase in baseline serum creatine (1.42 mg/dL) to 3.17 mg/dL on PTD 193, and renal allograft biopsy showed Banff 2B ACR. Treatment was initiated with both rATG (6 doses over 10 days) and high dose tacrolimus (target level 12–15 ng/mL). Repeat biopsies on PTD 209 and 219 demonstrated a persistent Banff 2A ACR. Tacrolimus target levels were increased to 15–20 ng/mL and histologic improvement was noted by PTD 233. However, significant residual inflammation persisted as a borderline lesion on the biopsy on PTD 233 that continued to PTD 281. Due to the persisting borderline rejection, everolimus was initiated on PTD 323 and MMF was discontinued. Follow-up biopsy 5 weeks later showed clearance of inflammatory infiltrates/borderline rejection and renal function had returned to baseline (serum creatinine 1.32 mg/dL) (Figure 5B).
Patient seven experienced an elevation in serum creatinine to 1.76 mg/dL from a baseline of 1.45 mg/dL on PTD 112, and renal allograft biopsy revealed ACR Banff 2B. Treatment was initiated on PTD 113 with rATG (4 doses over 7 days) and tacrolimus. A follow-up biopsy on PTD 129 revealed borderline rejection and BK virus nephropathy, which persisted despite tacrolimus cessation. On PTD 155, repeat biopsy revealed ACR with BK virus nephropathy, and MMF dosing was reduced. However, BK viral loads continued to increase despite MMF reduction. On PTD 189 MMF was stopped and everolimus therapy was started. Over the next six weeks, BK viral loads became undetectable and serum creatinine improved to pretreatment baseline (1.45 mg/dL) (Figure 5C).
Patient eight experienced a progressive increase in baseline serum creatinine over a several month period, and on PTD 281 underwent renal allograft biopsy that demonstrated ACR Banff 2A. The patient was treated with rATG (3 doses over 8 days) and high dose tacrolimus. A repeat biopsy on PTD 296 showed persistent Banff 1A ACR, and he was treated with everolimus concomitant to MMF cessation. Four weeks later (PTD 317), a repeat biopsy showed improvement to borderline rejection. An additional repeat biopsy taken 37 days later (PTD 354) showed complete resolution of rejection (Figure 5D).
In patients five through eight, repeated renal allograft histologic assessment revealed a substantial reduction in inflammatory cell infiltrates following mTORi therapy (Figure 5A–D, Banff criteria scores in supplemental table 2). Notably, two patients who developed viremia (1 CMV, Figure 5A; 1 BK, Figure 5C) following repeated rejection therapies demonstrated concomitant resolution of rejection and clearance of viremia with everolimus therapy. Thus, in addition to its success reducing rejection rates when co-administered with belatacept as maintenance immunosuppression15, everolimus may allow BK and/or CMV viral clearance in addition to reversal of BRR.
DISCUSSION
Belatacept is a next generation immunosuppressive biologic developed for use in transplant to avoid the toxicities of CNIs23,24. However, its benefits are limited by a higher acute rejection incidence25. Several mechanisms have been proposed to explain BRR, including: (i) selection for CD8+CD28− TEM cells26,27; (ii) selection for CD4+CD57+CD28− T cells28; and (iii) high level of pre-existing CD28+ memory CD8+ T cells12. While these mechanisms are not mutually exclusive and may vary between patients, our data are consistent with an increase in CD8+CD28− and CD8+CD28+ TEM cells.
Our data show that alloreactive CD8+CD28lowCD38hi as well as CD8+CD28+CD38hi cells have substantial mTOR activity and that everolimus significantly diminishes CD8+CD28lowCD38hi abundance and proliferation. Prior work has shown that endothelial cells also possess significant mTOR activity during allograft rejection29,30. However, in those studies, mTOR activity is observed during AMR and promotes transplant vasculopathy downstream of anti-HLA class I antibodies30. While endothelial cell activation is also often associated with humoral or mixed acute rejection, these histologic rejection types are very infrequent under belatacept. Thus, during ACR, the direct effects of mTORi on CD8+ TEM cells likely plays a substantial role in the resolution of rejection.
Mechanistically, our data are consistent with the scenario in which mTORi prevents the expansion/function of alloreactive CD8+ T cells. Indeed, we observed a substantial loss of T cell proliferation and alloreactivity following mTORi therapy. Although mTORi has been reported to enhance Treg number and function, we did not observe measurable effects of everolimus on Treg frequency31,32. This raises the intriguing possibility that mTORi could promote active tolerance through functional enhancement of allo-specific Treg, although one study has argued against this possibility33. Thus, additional work is necessary to determine the effects of mTORi on maintenance of allograft tolerance.
Consistent with prior work showing that concomitant treatment with everolimus and belatacept is effective at preventing ACR with CCS-free regimens, we show that BRR themselves are responsive to everolimus. These data are important, as it is possible that the effects of mTORi could be different in preventing rejection (i.e. given concomitantly with belatacept from the time of transplant) versus given after rejection has developed (e.g. as a “rescue” therapy). It is formally possible that, even in the absence of mTORi, these rejections would have eventually responded to rATG or tacrolimus. However, our data suggest that mTORi had a substantial effect on hastening the resolution of BRR. First, without mTORi, lesions took months to resolve and required continual high-level tacrolimus/CCS therapy, while mTORi treatment generally resolved rejections within a few weeks. Second, mTORi drove a substantial reduction in CD8+ T cell proliferation, coincident with the resolution of rejection. Third, our data show persistent alloreactivity present before the onset of everolimus therapy that was significantly reduced after everolimus treatment (Figure 3). Together, our work showing that ACR occurring under belatacept is generally more severe and difficult to control with CNI/CCS/rATG based regimens, supports the implementation of larger scale studies to assess the broader efficacy of mTORi as maintenance immunosuppression or as a rescue therapy with belatacept. Although these data are preliminary and with a limited number of subjects, the strong scientific rationale and the mechanistic data provided argue for further exploration. Indeed, it is possible that mTOR conversion may best be instituted when acute rejection is first diagnosed rather than waiting for demonstration of treatment resistance. Therefore, future studies will be necessary to determine the appropriate role for mTOR inhibitors in the treatment of refractory rejection under belatacept therapy. If proven effective, mTOR therapy may provide an important solution to a major clinical limitation of belatacept therapy.
Supplementary Material
ACKNOWLEDGMENTS
We thank the CCHMC Confocal Imaging Core for technical guidance.
FUNDING
This research was supported by an Academic Research Center grant for Cincinnati Children’s Hospital, a collaborative pilot grant from the Institutional Clinical and Translational Science Award from the NIH (ULITR000077), and an NIH R21 AI142264 (D.A.H.).
ABBREVIATIONS
- ACR
acute cellular rejection
- AMR
antibody mediated rejection
- BELA
belatacept
- BENEFIT
Belatacept Evaluation of Neuroprotection and Efficacy as First-line Immunosuppression Trial
- BEST
Belatacept-based Early Steroid Withdrawal Trial
- BL
borderline
- BRR
belatacept-refractory rejections
- CCS
corticosteroids
- CNI
calcineurin inhibitor
- cPRA
calculated panel reactive antibodies
- CsA
cyclosporine
- DM
diabetes mellitus
- dnDSA
de novo donor specific antibodies
- DSA
donor specific antibodies
- eGFR
estimated glomerular filtration rate
- ESRD
end stage renal disease
- GN
glomerulonephritis
- MAR
mixed acute rejection
- MMF
mycophenolate mofetil
- mTOR
mammalian target of rapamycin
- mTORi
mammalian target of rapamycin inhibitors
- NR
no rejection
- PBMCs
peripheral blood mononuclear cells
- PCKD
polycystic kidney disease
- PTD
post-transplant day
- rATG
rabbit anti-thymocyte globulin
- SOC
Standard of care
- TAC
tacrolimus
- TEM
effector memory T cell
- TMA
thrombotic microangiopathy
Footnotes
DISCLOSURE
The authors of this manuscript have no conflict of interest to disclose.
REFERENCES
- 1.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(3):443–453. [DOI] [PubMed] [Google Scholar]
- 2.Talawila N, Pengel LH. Does belatacept improve outcomes for kidney transplant recipients? A systematic review. Transplant international : official journal of the European Society for Organ Transplantation. 2015;28(11):1251–1264. [DOI] [PubMed] [Google Scholar]
- 3.Heher E, Markmann JF. The Clearer BENEFITS of Belatacept. The New England journal of medicine. 2016;374(4):388–389. [DOI] [PubMed] [Google Scholar]
- 4.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(3):535–546. [DOI] [PubMed] [Google Scholar]
- 5.Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med. 2016;374(4):333–343. [DOI] [PubMed] [Google Scholar]
- 6.de Graav GN, Baan CC, Clahsen-van Groningen MC, et al. A Randomized Controlled Clinical Trial Comparing Belatacept With Tacrolimus After De Novo Kidney Transplantation. Transplantation. 2017;101(10):2571–2581. [DOI] [PubMed] [Google Scholar]
- 7.Newell KA, Mehta AK, Larsen CP, et al. Lessons Learned: Early Termination of a Randomized Trial of Calcineurin Inhibitor and Corticosteroid Avoidance Using Belatacept. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017;17(10):2712–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson R, Grinyo J, Vincenti F, et al. Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. Am J Transplant. 2011;11(1):66–76. [DOI] [PubMed] [Google Scholar]
- 9.Woodle EKD, Shields A, Leone J, Wiseman A, Matas A, West-Thielke P, King E, Alloway R. The BEST Trial: A Prospective Randomized Multicenter Trial of Belatacept-Based CNI- and Corticosteroid-Free Immunosuppression [abstract].. https://atcmeetingabstracts.com/abstract/the-best-trial-a-prospective-randomized-multicenter-trial-of-belatacept-based-cni-and-corticosteroid-free-immunosuppression/2018.
- 10.Xu H, Perez SD, Cheeseman J, Mehta AK, Kirk AD. The allo- and viral-specific immunosuppressive effect of belatacept, but not tacrolimus, attenuates with progressive T cell maturation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(2):319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Graav GN, Hesselink DA, Dieterich M, Kraaijeveld R, Weimar W, Baan CC. Down-Regulation of Surface CD28 under Belatacept Treatment: An Escape Mechanism for Antigen-Reactive T-Cells. PloS one. 2016;11(2):e0148604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathews DV, Wakwe WC, Kim SC, et al. Belatacept-Resistant Rejection Is Associated With CD28(+) Memory CD8 T Cells. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017;17(9):2285–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo DJ, Weaver TA, Stempora L, et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(1):22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver TA, Charafeddine AH, Agarwal A, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nature medicine. 2009;15(7):746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirk AD, Guasch A, Xu H, et al. Renal transplantation using belatacept without maintenance steroids or calcineurin inhibitors. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(5):1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollizzi KN, Patel CH, Sun IH, et al. mTORC1 and mTORC2 selectively regulate CD8(+) T cell differentiation. J Clin Invest. 2015;125(5):2090–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodle ES, Cronin D, Newell KA, et al. Tacrolimus therapy for refractory acute renal allograft rejection: definition of the histologic response by protocol biopsies. Transplantation. 1996;62(7):906–910. [DOI] [PubMed] [Google Scholar]
- 18.Rao Deepak A. B CC, Arazi Arnon, Davidson Anne, Liu Yanyan, Browne Edward P., Eisenhaure Thomas M., Chicoine Adam, Lieb David J., Smilek Dawn E., Tosta Patti, Lederer James A., Brenner Michael B., Hildeman David, Woodle E. Steve, Wofsy David, Anolik Jennifer H., Kretzler Matthias. A protocol for single-cell transcriptomics from cryopreserved renal tissue and urine for the Accelerating Medicine Partnership (AMP) RA/SLE network. bioRxiv Preprint Server, Cold Spring Harbor Laboratory 2018. [Google Scholar]
- 19.Gaber AO, First MR, Tesi RJ, et al. Results of the double-blind, randomized, multicenter, phase III clinical trial of Thymoglobulin versus Atgam in the treatment of acute graft rejection episodes after renal transplantation. Transplantation. 1998;66(1):29–37. [DOI] [PubMed] [Google Scholar]
- 20.Jones DL, Sacks SH, Wong W. Controlling the generation and function of human CD8+ memory T cells in vitro with immunosuppressants. Transplantation. 2006;82(10):1352–1361. [DOI] [PubMed] [Google Scholar]
- 21.Lo DJ, Anderson DJ, Weaver TA, et al. Belatacept and sirolimus prolong nonhuman primate renal allograft survival without a requirement for memory T cell depletion. Am J Transplant. 2013;13(2):320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merino D, San Segundo D, Medina JM, et al. Different in vitro proliferation and cytokine-production inhibition of memory T-cell subsets after calcineurin and mammalian target of rapamycin inhibitors treatment. Immunology. 2016;148(2):206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar D, LeCorchick S, Gupta G. Belatacept As an Alternative to Calcineurin Inhibitors in Patients with Solid Organ Transplants. Front Med (Lausanne). 2017;4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emamaullee J, Toso C, Merani S, Shapiro AM. Costimulatory blockade with belatacept in clinical and experimental transplantation - a review. Expert Opin Biol Ther. 2009;9(6):789–796. [DOI] [PubMed] [Google Scholar]
- 25.de Graav GN, Hesselink DA, Dieterich M, et al. An Acute Cellular Rejection With Detrimental Outcome Occurring Under Belatacept-Based Immunosuppressive Therapy: An Immunological Analysis. Transplantation. 2016;100(5):1111–1119. [DOI] [PubMed] [Google Scholar]
- 26.Mou D, Espinosa J, Lo DJ, Kirk AD. CD28 negative T cells: is their loss our gain? American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(11):2460–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trzonkowski P, Zilvetti M, Chapman S, et al. Homeostatic repopulation by CD28-CD8+ T cells in alemtuzumab-depleted kidney transplant recipients treated with reduced immunosuppression. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(2):338–347. [DOI] [PubMed] [Google Scholar]
- 28.Espinosa J, Herr F, Tharp G, et al. CD57(+) CD4 T Cells Underlie Belatacept-Resistant Allograft Rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(4):1102–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin YP, Valenzuela NM, Ziegler ME, Rozengurt E, Reed EF. Everolimus inhibits anti-HLA I antibody-mediated endothelial cell signaling, migration and proliferation more potently than sirolimus. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(4):806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jindra PT, Jin YP, Rozengurt E, Reed EF. HLA class I antibody-mediated endothelial cell proliferation via the mTOR pathway. J Immunol. 2008;180(4):2357–2366. [DOI] [PubMed] [Google Scholar]
- 31.Levitsky J, Miller J, Huang X, Gallon L, Leventhal JR, Mathew JM. Immunoregulatory Effects of Everolimus on In Vitro Alloimmune Responses. PloS one. 2016;11(6):e0156535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallon L, Traitanon O, Yu Y, et al. Differential Effects of Calcineurin and Mammalian Target of Rapamycin Inhibitors on Alloreactive Th1, Th17, and Regulatory T Cells. Transplantation. 2015;99(9):1774–1784. [DOI] [PubMed] [Google Scholar]
- 33.Tang Q, Vincenti F. Transplant trials with Tregs: perils and promises. J Clin Invest. 2017;127(7):2505–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.