Abstract
Many physiological processes, including most kidney-related functions, follow specific rhythms tied to a 24-hour cycle. This is largely because circadian genes operate in virtually every cell type in the body. In addition, many non-canonical genes have intrinsic circadian rhythms, especially within the liver and kidney. This new level of complexity applies to the control of renal electrolyte excretion. Furthermore, there is growing evidence that paracrine and autocrine factors, especially the endothelin system, are regulated by clock genes. We have known for decades that excretion of electrolytes is dependent on time-of-day, which could play an important role in fluid volume balance and blood pressure control. Here, we review what is known about the interplay between paracrine and circadian control of electrolyte excretion. The hope is that recognition of paracrine and circadian factors can be considered more deeply in the future when integrating with well-established neuro-endocrine control of excretion.
Keywords: circadian rhythms, clock genes, endothelin, purinergic receptors, sodium, potassium
INTRODUCTION
The control of renal fluid and electrolyte handling is an extremely complex effort involving a wide range of endocrine, paracrine and autocrine factors that balance intake with output. In terms of endocrine factors, the amount of work dedicated to the renin-angiotensin-aldosterone system is immense and arguably the most studied aspect of kidney function. The physiological role of antidiuretic hormone is also very well established as is the role of atrial natriuretic peptide, which is now approaching its 40th anniversary of its discovery (1). Paracrine and autocrine control of renal excretory function, such as endothelin (ET) (2), ATP/purinergic receptors (3), kinins (4), arachidonic acid and P450 metabolites (5), and nitric oxide (NO) (6), has been studied and reviewed in isolation, but few studies have integrated the balance of actions. To make matters even more complicated, renal hemodynamics and neural inputs can directly and indirectly modulate the activity of these systems. Although most would agree that intrarenal hemodynamics can impact renal tubular function, the difficulty in measuring medullary blood flow has resulted in few laboratories considering this as impacting their system. The role of renal nerves has gained considerable new attention in the past 10+ years as the result of a series of clinical trials demonstrating promise for renal denervation for the treatment of resistant hypertension (7). Furthermore, only in very recent years have we recognized that these balances are greatly impacted by sex and time of day.
While we have known for over 100 years that kidney excretory function follows a diurnal pattern, we have only begun to explore how this system works. A handful of labs have generated some very convincing evidence that the cell autonomous molecular clock functions to regulate renal tubular and vascular function in a circadian fashion (8). Given the current state of the field and the many reviews of individual factors, here, we focus on a few of the major paracrine/autocrine factors known to impact renal excretory function, specifically endothelin-1 (ET-1) and purinergic receptors, with a specific focus on how the intrinsic kidney clock controls renal excretory function.
KIDNEY CLOCK AND CIRCADIAN PHYSIOLOGY
For a long time, we have known that several aspects of kidney physiology in humans follow specific rhythms within a single 24-hour day (summarized in Figure 1). In 1950, Sirota et al. recruited 18 healthy adults and conducted a thorough study examining diurnal variations of renal function (9). They showed that urine volume during the night was significantly lower compared with daytime. Tubular reabsorption was considerably higher at night (9). Shortly thereafter, Mills and Stanbury carried out a study where healthy adults were placed on a 12-hour routine in place of the normal 24-hour pattern. They found that diurnal urinary excretion of water and electrolytes, namely sodium and potassium, was not significantly affected by the 12-hour cycle of eating, drinking and sleeping patterns (Figure 2). These findings led to the original hypothesis for an intrinsic mechanism for regulating renal function (10). However, it wasn’t until the past decade or so that we started to gain a further mechanistic understanding with regard to how this kidney rhythm was regulated.
Figure 1.
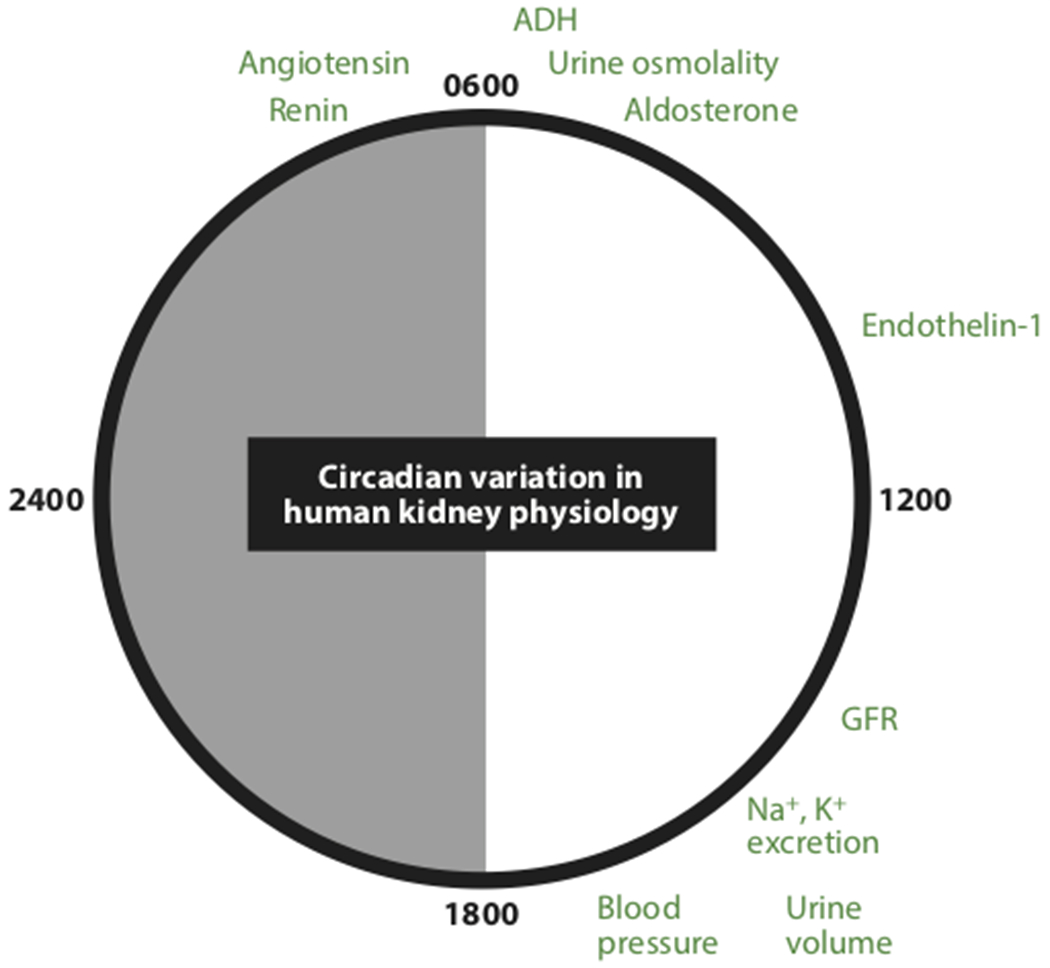
Approximate time of day when a variety of major renal excretory systems reach their maximum level. Minimums generally occur at the opposite side of this 24-h clock. Abbreviations: ADH, antidiuretic hormone; GFR, glomerular filtration rate.
Figure 2.
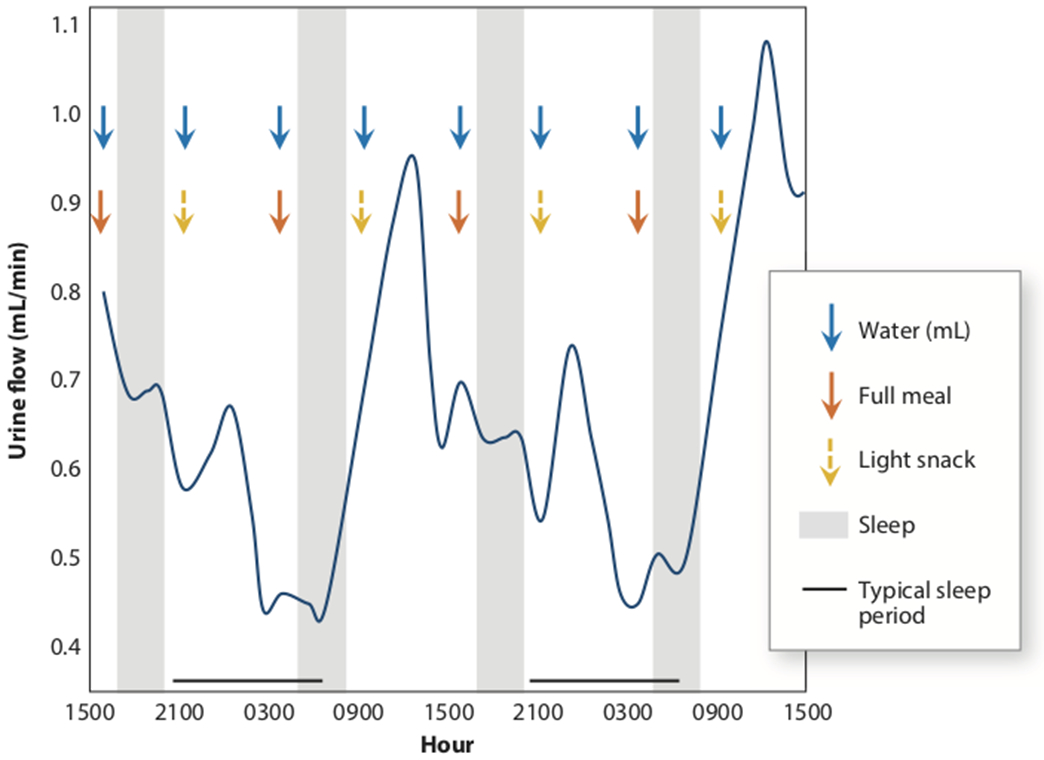
Persistence of circadian urine flow despite having a 12-h eating and sleeping pattern in healthy volunteers (10).
The 2017 Nobel Prize in Physiology and Medicine was awarded to Jeffery Hall, Michael Rosbash and Michael Young for their discoveries of molecular mechanisms controlling circadian rhythms. The readers are referred to excellent review articles for molecular circadian clock regulation (11; 12). Briefly (Figure 3), circadian proteins Clock and Bmal1 act as master transcription factors and activate Period (Per), Cryptochrome (Cry), and many other target genes. Per and Cry translocate to the nucleus and act as the repressive part in the feedback loop. As shown in Figure 4, several critically important genes related to sodium transport in the kidney are expressed in a circadian pattern such as the sodium/hydrogen exchanger 3 (NHE3), the sodium-glucose cotransporter 2 (SGLT2), the epithelial sodium channel alpha subunit (αENaC)(13). The utilization of global and tissue specific knockout animal models has facilitated a more thorough understanding regarding specific roles of circadian components in different organs, including the kidney. The kidney has the second greatest number of genes that follow a circadian expression pattern after the liver (14), yet little is known about how these systems impact excretory function. Here, we list animal models deficient in clock components and summarize recent progress in the understanding of circadian regulation of renal physiology with a focus on blood pressure (summarized in Figure 5) and renal electrolyte excretion. Readers are directed to recent review articles in circadian renal function and its clinical implications (15; 16).
Figure 3.
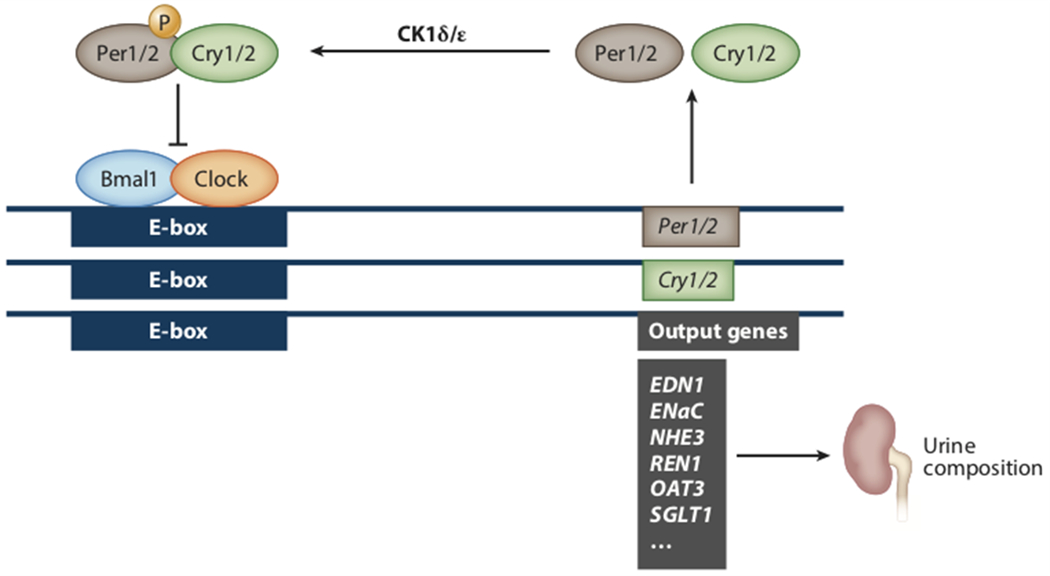
Simplified view of the core circadian clock. Bmal1 and Clock heterodimerize before binding to E-box elements on the promoters of a wide range of genes including Per and Cry. Per and Cry proteins then heterodimerize before then inhibiting Bmal1 and Clock binding to the E-box. However, this translocation is dependent upon phosphorylation by CK1δ/ε. Figure based on a variety of sources but primarily Reference 8. Abbreviations: EDN1, endothelin-1; ENaC, epithelial sodium channel alpha subunit or SCNN1A; NHE3, sodium/hydrogen exchanger 3 or SLC9A3; OAT3, organic anion transporter 3 or SLC22A8; REN1, renin 1; SGLT1, sodium-glucose cotransporter 1 or SLC5A1.
Figure 4.

Approximate rhythm of mRNA expression for major Na+ transporters in the mouse kidney. Figure based on the Circadian Expression Profiles Database (CircaDB) originally established by Panda et al. (13). Abbreviations: αENaC, alpha epithelial sodium channel alpha subunit; NHE3, sodium/hydrogen exchanger 3; NKCC, potassium chloride channel; SGLT2, sodium-glucose cotransporter 2.
Figure 5.

Approximation of blood pressures over the course of 24 h in mice with various clock gene knockouts (KO) compared to wild types (wt). All panels are based on 12:12 light:dark conditions while maintained on standard rodent diets. Red arrows depict significant differences in KO versus wt in light and dark conditions. Per1 KO strains are shown for two different genetic backgrounds, C57Bl/6 and 129/sv. For Clock, there is a mutant strain that has a non-functional protein expressed and a traditional KO strain. Kidney Bmal1 specific KO include the Pax8-Cre that is specific for the entire nephron and the Ren1-Cre that is specific for cells expressing renin, which includes juxtaglomerular cells as well as renal epithelium.
Bmal1
Bmal1 has been one of the most intensively studied circadian genes and is often referred to as a component of the core clock, not to be confused with the central clock that refers to the molecular clock within the central nervous system, specifically in the suprachiasmatic nucleus. Various mouse models with specific depletions of Bmal1 exhibited disrupted blood pressure phenotypes with or without impaired renal electrolyte excretion. The global Bmal1 knockout (Bmal1KO) mouse was first generated in 2000 by Bunger et al. and was known to have a complete loss of circadian rhythmicity in activity (17). Subsequently, Curtis et al. showed that Bmal1KO mice did not have a circadian blood pressure rhythm such that blood pressure did not rise during the active phase (18). Further efforts have been made to uncover the mechanisms underlying Bmal1 control of circadian blood pressure and renal electrolyte excretion using a variety of tissue specific knockout mice. Wang et al. showed that loss of peroxisome proliferator-activated receptor gamma, or PPARγ, in smooth muscle (SM22 Cre) led to decreased day/night variation of blood pressure in mice, which was further shown to be caused by impaired rhythmicity of Bmal1 (19). Xie et al. utilized the same SM22 Cre and observed that depletion of Bmal1 in smooth muscle reduced the night-day amplitude of circadian blood pressure rhythms in mice without affecting their locomotor activity (20). In addition, Chang et al. generated perivascular adipose tissue (PVAT) specific Bmal1 knockout mouse and showed that loss of Bmal1 in PVAT led to a super-dipper phenotype, where the inactive phase of blood pressure was low compared with control mice (21). Collectively, these studies were of great importance as they provided solid evidence that peripheral circadian components participate in circadian blood pressure regulation.
Regarding the kidney clock, Firsov and colleagues have generated a renal tubular Bmal1 deficient mouse model using Pax8 Cre (22). These mice lack Bmal1 expression in the entire nephron. They observed a slight decrease in systolic but not diastolic blood pressure and an intact circadian blood pressure rhythm. In addition, the rhythms of sodium and potassium excretion were preserved, although we do not know how the mice would respond to a challenge of low or high dietary electrolytes. In another kidney-specific Bmal1 knockout model generated by the same group, depletion of Bmal1 in renin-secreting cells (Ren1 Cre) increased GFR, attenuated diurnal rhythms of sodium and water excretion, and reduced plasma aldosterone concentrations (23). However, circadian blood pressure rhythms remained intact even though both systolic and diastolic blood pressure were significantly decreased compared to control mice. These studies suggested that kidney-specific Bmal1 may be important in maintaining blood pressure and/or electrolyte rhythms; however, further studies are needed to determine whether Bmal1 in the kidney controls the rhythmicity of blood pressure.
Period
The Gumz lab has published a line of work illustrating an important role for Period (Per1) in the regulation of blood pressure and renal electrolyte excretion as well as the renal effects of aldosterone. This lab generated mice where the clock gene Per1 was deleted in two strains of mice (the salt-sensitive 129/sv and salt-resistant C57BL/6). Blood pressure phenotypes were different between these two strains of mice. Depletion of Per1 in 129/sv mice produced a significantly lower blood pressure compared with control mice (24); however, loss of Per1 in C57BL/6 mice resulted in an increase in blood pressure observed only during the active phase (25). Following high salt plus mineralocorticoid treatment, male knockout mice developed a non-dipping blood pressure pattern, that is, blood pressure was not reduced during the inactive (lights on) phase (25). However, females did not display this non-dipping phenotype (26). Consistent with the blood pressure changes, Per1 also positively regulates sodium transporters in the kidney (27; 28). Male Per1 KO mice have a loss of diurnal sodium excretion and elevated ET-1 mRNA in the kidney; changes in ET-1 were not observed in female Per1 KO mice. Given the sex differences in ET-1 dependent natriuresis in the kidney (29), these findings suggest that there could be a sex difference in how the clock regulates ET-1 dependent sodium homeostasis. Importantly, a very recent study by Alli and colleagues reported that knockdown of Per1 with siRNA in Xenopus 2F3 distal nephron cells reduced ENaC activity as assessed by patch clamp techniques (30).
Mice lacking another isoform of the Per gene, Per2, were shown to have aortic endothelial dysfunction and significantly lower diastolic blood pressure during the active phase (31). Pati et al. treated a series of Period isoform knockout (Per2KO, Per2,3KO and Per1,2,3KO) mice with low salt diet and were able to show a loss of circadian blood pressure due to a high inactive phase blood pressure, which was restored by losartan (32). These findings suggest that angiotensin contributes to Period-mediated regulation of blood pressure, but whether this response has effects on kidney electrolyte handling is unknown.
Cryptochrome, Clock, and Dec
Doi et al. generated double Cryptochrome (Cry1 and Cry2) null mice and showed that these mice exhibited a loss of circadian blood pressure rhythm, although the 24-hour average blood pressures were similar to wildtype mice. Interestingly, these mice developed significant hypertension relative to wildtype controls when placed on a high salt diet due to extremely high levels of circulating aldosterone (33); this effect was mitigated by administration of a mineralocorticoid receptor antagonist, eplerenone.
In contrast to the Cry1/2 knockouts, Clock mutant mice that generate a non-functional protein have intact circadian blood pressure rhythms; however, the blood pressure dipping was reduced such that the night-day amplitude was significantly attenuated compared to wildtype controls (34). This abnormality in circadian blood pressure and heart rate rhythms were entirely abolished by adrenalectomy (34). Furthermore, Zuber et al. generated Clock deficient mice that have a lower expression of the epithelium sodium channel (ENaC) at the beginning of inactive phase that is consistent with an observed increase in urinary sodium excretion (35). In addition, Clock mutant mice had an arrhythmic plasma aldosterone concentration and disrupted plasma potassium level compared to wild type controls. It is important to note that these two Clock mutant/deficient mouse strains (34; 36) were generated on different genetic backgrounds, which we know can lead to distinct differences in phenotype as reported by the Gumz lab for Per1 where mice on the 129/sv background had normal blood pressure rhythms, but at a lower level than wild types (24), but Per1 KO on the C57Bl/6 background were hypertensive but only during the active period (25).
Dec1 has been considered a fifth member of the clock-gene family and functions to repress the transcription of Per1 induced by the Clock/Bmal1 heterodimer (37). Nakashima et al. showed that mice lacking Dec1 gene had significantly lower blood pressure but an intact circadian rhythm (38). Evidence that Dec1 directly regulates Atp1b1, gene that encodes the beta subunit of sodium/potassium ATPase, was also reported in this study.
PARACRINE CONTROL AND CIRCADIAN EXCRETION
Endothelin-1
In this section, we summarize our current understanding of the connections between circadian system and ET-1 system and their synergistic impact on renal electrolyte excretion. ET-1 is a 21 amino acid peptide with very potent effects on many cell types throughout the body (39). Over the past several decades, considerable progress has been made characterizing the effects of ET-1 in the kidney and the renal microcirculation which serves, in large measure, to facilitate the excretion of high dietary salt (40). The primary sources of ET-1 production in the kidney are the collecting duct (CD) and particularly the inner medullary collecting duct (IMCD), but considerable ET-1 is also produced by the vascular endothelium and most likely a few other sources. Production of ET-1 is largely stimulated by high dietary salt. This occurs in both the CD and vascular endothelium. In an autocrine fashion, ET-1 in the CD primarily stimulates the ETB receptor, which in turn, activates nitric oxide synthase 1β (NOS1β) to produce NO, cGMP and subsequent inhibition of ENaC activity to facilitate diuresis. However, the role of ET-1 in response to high salt diet is evident in the renal microcirculation as well. Fellner et al. showed that impaired autoregulation by the renal afferent arteriole resulting from a chronic high salt diet is restored following ETB receptor blockade suggesting that ET-1 can contribute to increased GFR during high salt conditions (41). In recent years, considerable attention has been paid to circadian aspects of the ET-1 system following the important discovery from the Gumz lab showing that the largest change in gene expression produced by aldosterone in CD cells were Per1 and ET-1 (EDN1) (42).
In 1998, Hwang et al. recruited 11 normotensive subjects and 23 hypertensive patients and conducted an elegant study to determine the relation between hypertension and the diurnal excretion rate of ET-1 (43). Subjects with hypertension, but not end organ damage, exhibited regular circadian blood pressure rhythm. However, there was a one-hour phase delay in the hypertensive group compared with normotensive controls, that is, the peak in blood pressure consistently occurred at a later time. In general, the asynchrony of functional rhythms is thought to increase disease risk. Urine samples from these patients were collected 4 times per day for 3 consecutive days. Under a control diet (7g NaCl every 24h), urinary sodium and ET-1 excretion showed a similar diurnal rhythm between normotensive and hypertensive subjects. In addition, the diurnal pattern of ET-1 excretion was correlated with sodium excretion. Following saline infusion, nearly half of the hypertensive group showed a delayed natriuretic response where urinary sodium excretion was not increased compared with baseline during the first 4 hours. Interestingly, less urinary ET-1 excretion was also observed in these patients. This delayed response in sodium and ET-1 excretion happened in the morning hours (8am-12pm), which is roughly the beginning of the active period for humans. In contrast, the other half of hypertensive subjects showed an exaggerated natriuretic response as well as increased ET-1 excretion following saline infusion. This early study in humans demonstrated that in a specific population of hypertensive subjects, the timing of urinary sodium excretion can be delayed, which could be related to circadian excretion of ET-1. Consistent with these results, Johnston et al. showed that rats with dysfunctional ETB receptors (ETB-def) failed to excrete sodium in a timely manner (44). When challenged with an acute salt load at the beginning of the inactive period, both male and female ETB-def rats had a delayed natriuretic response compared with control rats. However, when the salt load was given at the beginning of the active period, female ETB-def rats were able to more promptly excrete the sodium whereas the natriuretic response in males was significantly delayed. This delay could be due, at least in part, to increased ETA receptor activity when ETB activity is reduced because administration of an ETA antagonist, atrasentan, was able to improve the delayed natriuretic response. These two studies from humans and rats suggest that diurnal/circadian excretion of sodium requires the ET-1 system in which the natriuretic effect mediated by the ETB receptor may be opposed by the ETA receptor. Furthermore, males and females have differential regulatory mechanisms of the ET-1 system that are time-dependent and may explain why females have less salt-dependent hypertension.
Dhaun et al. conducted a clinical trial examining nocturnal blood pressure dipping and arterial stiffness in non-diabetic chronic kidney disease (CKD) patients and healthy controls (45). They found that elevated plasma ET-1 levels were associated not only with hypertension but also non-dipping blood pressure that is characteristic in CKD patients. In addition, 6-week treatment with an ETA receptor antagonist, sitaxentan, significantly improved the nocturnal dipping, suggesting the involvement of ET system in the diurnal variation of blood pressure in the setting of CKD. The authors were not able to detect circadian variations in plasma ET-1 or urinary ET-1 which could be due to the relatively small sample size. It is also possible that the lack of observation for circadian activity of the ET-1 system may be related to difficulties in measuring paracrine activity in vivo. In rats, plasma ET-1 exhibits a discernable rhythm within 24 hours (46). Urinary excretion of ET-1 is higher in the active period compared with inactive period in normal rats but not in ETB-def rats, suggesting an impaired diurnal renal production of ET-1 when ETB receptors are not functional, which occurs in many forms of salt-sensitive animals.
High salt intake causes a highly significant phase shift (5.5-hour phase delay) of Bmal1 mRNA expression in the inner medulla, but not cortex of control rats (46). However, this high salt-induced alteration in Bmal1 expression was absent in ETB-def rats. In addition, Bmal1 mRNA expression in IMCD3 cells decreased following exogenous ET-1 treatment (47). In addition, Bmal1 knockdown reduced the expression of ENaC. These results provide strong evidence for involvement of Bmal1 in ET-1 system-mediated regulation of renal sodium excretion. Of note, high salt diet causes an increase in skin sodium content during the active phase compared with inactive phase in ETB-def rats, which may contribute to the increased blood pressure amplitude observed in these rats following high salt diet (48).
As noted earlier, Per1 also appears to be heavily involved in regulating the ET-1 system in the collecting duct. Gumz et al. first discovered in 2009 that Per1 positively regulates ENaC (27). Subsequently, the same lab showed that knockdown of Per1 in collecting duct cells leads to an induction of ET-1 and several other negative regulators of ENaC (24). In addition, mice lacking Per1 on a 129/sv background have lower blood pressures overall despite maintaining a normal amplitude (24). This was associated with increased renal production of ET-1 from both the inner medulla and the cortex. In another study, this lab also reported that ETA and ETB receptor expression in the kidney is time-dependent but not regulated by Per1 (49); mRNA levels of ETA and ETB receptors were assessed in wildtype and Per1 heterozygous mice at the middle of the inactive and active phases. In both wildtype and Per1 heterozygous mice, ETA expression was lower at active phase compared to inactive phase in both the renal cortex and inner medulla. In both wildtype and Per1 het strains, ETB expression was higher at active phase compared to inactive phase in the inner medulla, but cortical expression was lower at active phase compared to inactive phase. In contrast to Bmal1, Per1 acts as an upstream regulator of ET-1 production, which likely serves as a mediator for ET-1 regulation of sodium excretion.
Nitric Oxide
NO signaling is also key to promoting natriuresis and diuresis. NO inhibits sodium reabsorption in different segments along the nephron, primarily the thick ascending limb and the collecting duct. Work primarily from the Garvin lab has established that NO decreases chloride absorption in the think ascending limb of the loop of Henle and reduces the activity of the sodium, potassium 2 chloride channel (NKCC2) (50–52). In the CD, direct evidence has established that NO inhibits the amiloride sensitive ENaC (53). Additionally, NO can independently inhibit water reabsorption in the CD (54). All three isoforms of NO synthase (NOS 1, 2, and 3) are constitutively expressed along the nephron, but the distribution of these isoforms is quite specific. Most of the effects of NO on sodium and water handling have been attributed to NOS3 in the thick ascending limb and NOS1 in the CD. However, actual NOS activity has been shown to be at least 10 fold higher in the CD than any other segment.
Advances in understanding NO function in salt-dependent hypertension were once hindered largely due to the normotensive blood pressure phenotype in global NOS1KO mice (55). It was later found that the original global NOS1KO was in fact a strain that only lacks the alpha subunit of NOS1, while the splice variant, NOS1ß, remains active in the so-called NOS1 KO mouse. Hyndman et al. demonstrated that depletion of NOS1ß in principal cells of the collecting duct in mice leads to impaired sodium excretion and salt-sensitive hypertension, which alters the salt-resistant pressure-natriuresis relation observed in C57BL/6 mice (56). The synergistic effect of ET-1 and NO system in regulating sodium excretion has been confirmed by several labs. ET-1 stimulates NOS3 expression in the thick ascending limb and thereby increases the production of NO, through activating ETB receptor (57). The signaling mechanism for the rapid (seconds to minutes) paracrine/autocrine effects of ET-1 on NO production are not clear. In primary cultures of rat IMCD cells, Ye et al. provided evidence that endogenous ET-1 stimulates NOS3 gene expression although they did not measure NOS1 or its splice variants (58). Dr. Jennifer Pollock’s lab has shown that NOS1 and NOS3 activities are significantly lower in the inner medulla of ETB-def rats compared with normal rats (59). In addition, both ETA and ETB receptors were shown to regulate NOS activity. Gao et al. showed that mice lacking NOS3 in the nephron (Pax8 Cre) displayed delayed sodium excretion and salt-sensitive hypertension (60). In addition to the thick ascending limb and collecting duct, NOS activation in the macula densa has been shown to be important in regulating blood pressure and sodium excretion. Lu et al. showed that loss of NOS1ß in mice leads to salt-sensitive hypertension and impaired sodium and potassium excretion following acute volume expansion (61).
Although recent evidence suggests a strong capacity for NO regulation of renal sodium and water excretion, whether these NO-mediated effects depend on time-of-day remains largely unknown. An early study conducted by Tunctan et al. measured NOS activity over 24 hours in the kidney, brain, testis, lung, and aorta from male BALB/C mice (62). NOS activity measured in kidney homogenates exhibited circadian rhythmicity when mice were placed in a light/dark condition (14hr light, 10hr dark). However, under a constant lighting condition, circadian NOS activity in the kidney was absent, which was different from the brain and lung where NOS activities displayed circadian rhythms under both light/dark and constant lighting conditions. This suggests that NOS activity in the kidney could be entrained by light.
Functional evidence that NO signaling is heavily regulated by the circadian clock come from studies in the vasculature. Anea and colleagues showed that expression of phosphorylated NOS3 (or eNOS), an indirect measure of activity, was decreased in the carotid arteries of Bmal1KO mice, which was consistent with the reduced endothelial dependent vascular function observed in both Bmal1 KO and Clock mutant mice (63). Subsequently, this group showed that loss of Bmal1 was associated with increased production of endothelial superoxide by uncoupling NOS3 in the aorta (64). Westgate et al. found that plasma nitrate and nitrite, indirect measures of NO production, displayed diurnal variations in control mice as well as endothelial specific Bmal1 KO mice (65). In mesenteric resistance arteries from male rats, maximal contraction to phenylephrine was greater early in the inactive period compared with responses in the active period, while the acetylcholine relaxation was the opposite being greater in the active period (66). NOS3 protein expression also exhibited a time-of-day variation with higher levels being observed during the active period corresponding to the greater acetylcholine response. Chronic blockade of NOS with L-nitroarginine methyl ester (L-NAME) resulted in hypertension with a maintained circadian rhythm although at high doses, the amplitude of the blood pressure rhythm was exaggerated (67). NOS3 KO mice are hypertensive compared to genetic controls, but the amplitude of blood pressure variation is greater due to the increase in blood pressure during the active period (68). Although this clearly indicates that NOS3 is not required for a blood pressure rhythm, it does indicate that NOS3 functions in a circadian pattern to impact blood pressure. Unfortunately, none of these studies examined the kidney or kidney NOS in circadian blood pressure control, but given the critical role of the kidney in blood pressure control, studies are clearly justified to explore the mechanism responsible for diurnal NOS activity and whether this occurs in the kidney.
In aged mice, Kunieda and co-workers provided evidence that NO increases Per1 promoter activity and also helps to maintain circadian expression of Per2 in aortic smooth muscle cells (69). They also found that protein levels of phosphorylated NOS3 in aged mice exhibited an impaired circadian rhythm with lower levels during the inactive period compared with young mice. Once again, work in this area is underdeveloped and has not been addressed as to whether these mechanisms occur in the kidney. However, it has been reported that urinary nitrate excretion and cyclic GMP excretion, which are indicators for NO production, exhibited circadian rhythms in healthy subjects but not hypertensive patients (70).
Purinergic receptors
ATP-binding purinoceptors, P2 receptors, are expressed throughout renal tubular cells and renal vasculature and have been shown to be highly involved in paracrine regulation of renal tubular transport. Using microperfusion techniques, Bailey demonstrated that administration of an P2Y1 receptor agonist, 2meSADP, inhibited NHE3 activity and led to decreased sodium transport, which could be abolished by giving an P2Y1 antagonist, MRS2179 (71). Pochynyuk et al. provided direct evidence that purinergic signaling regulates ENaC in the CD (72). This group showed that ATP could decease ENaC activity through P2Y2 receptors on the apical side of the principal cells. Moreover, ENaC activity measured directly using patch-clamp methods was increased in CDs isolated from P2Y2 knockout mice compared to genetic controls. In addition to reduced expression of ENaC, P2Y2 knockout mice have lower plasma aldosterone and renin levels, increased expression of NKCC2, and increased water reabsorption (73). Additionally, these mice exhibit salt-resistant hypertension. Wildman et al. showed that P2X4 may also be involved in local regulation of ENaC in the CD (74), which is consistent with the hypertensive phenotype of the P2X4 knockout mice (75). Of note, P2X4 knockout mice have impaired endothelial dependent relaxation. Knockdown of P2X4 receptors in cultured endothelial cells inhibited NO production response to increases in shear stress. These studies demonstrate that P2 signaling is important in regulating sodium transport and most likely blood flow that may impact renal excretion of salt and water.
More recent evidence has shown that renal purinergic signaling and the ET-1 system may have some interaction within the CD. Pandit et al. showed that flow induced ET-1 expression in cultured IMCD3 cells could be prevented by inhibiting P2Y2 and P2X7 receptors (76). Gohar et al. observed that infusion of the P2 receptor agonist, UTP, into the renal medulla produced ET-1 dependent natriuresis in male but not female rats (77; 78). Further studies are needed to determine the specific mechanisms, including the more specific subtypes of P2 receptors that are responsible for the purinoceptor dependent ET-1 production.
Although direct evidence for circadian involvement in renal purinergic signaling has yet to be investigated, it is reasonable to speculate that the paracrine regulation of renal electrolyte excretion mediated by purinoceptors is, to some extent, time-of-day dependent, largely due to the intrinsic circadian characteristics of most, if not all, sodium transporters and the mounting evidence for circadian control of the ET-1 system. Of note, Palomino-Doza et al. conducted a study that involved over 1400 subjects from nearly 250 families and showed that nighttime diastolic blood pressure was strongly associated with P2X receptor polymorphism (79). However, work in this area has yet to determine whether renal purinergic signaling works in a circadian-dependent manner.
Intrarenal RAS
In 1990, Ingelfinger et al. utilized in situ hybridization to detect the existence of angiotensinogen in the kidney proximal tubule (80). This led to a tremendous amount of work in this area as recently reviewed in detail elsewhere (81; 82). In general, work in this area has provided evidence that intrarenal renin-angiotensin system (RAS) may also play a pivotal role in regulating blood pressure and electrolyte homeostasis in addition to the classic hormonal RAS. Gonzalez-Villalobos et al. showed that angiotensin II (Ang II) generated by the angiotensin converting enzyme (ACE) paradoxically led to increases in intrarenal Ang II (83). Moreover, in the presence of ACE inhibitors, the hypertensive response to chronic Ang II infusion was suppressed (84). Subsequently, this group conducted another study where they generated mice lacking kidney ACE and determined the role of renal ACE in hypertension induced by either Ang II infusion or NOS inhibition (85). Mice lacking renal ACE were hypertensive, and following Ang II infusion, the increases in intrarenal Ang II accumulation and activation of sodium transporters were inhibited.
In humans, Isobe et al. recruited 36 CKD patients and 14 non-CKD subjects and conducted a study to determine the relation between circadian blood pressure and circadian rhythms of intrarenal RAS (86). As expected, they observed that in non-CKD subjects, daytime BP is significantly higher compared with nighttime. In addition, levels of urinary angiotensinogen excretion, an indicator of intrarenal RAS activation, are similar between daytime and nighttime. Based on nighttime blood pressure data, CKD subjects were divided into riser (nighttime systolic blood pressure higher than 24-hour average) and non-riser categories (nighttime systolic blood pressure lower than 24-hour average). In non-riser CKD patients, urinary angiotensinogen excretion significantly decreased at night, which was similar to the blood pressure pattern. In risers, the diurnal pattern of urinary angiotensinogen excretion was not evident. Importantly, the nighttime/daytime ratio of urinary angiotensinogen positively correlated with nighttime/daytime ratio of urinary sodium excretion. Of note, nighttime activation of intrarenal RAS in CKD patients was associated with impaired melatonin secretion, which is often observed in CKD subjects (87). In addition, in a rat model of nephritis, an increase in the amplitude for circadian fluctuation of intrarenal RAS components were found (88). Given the role of melatonin in regulating a variety of circadian behaviors, these findings suggest a possible relation between melatonin and intrarenal RAS activity.
Figure 6 summarizes some of the known factors that are linked to clock genes and/or circadian control of renal tubular function. Several other elements such as 20-hydroxyeicosatetraenoic acid (20-HETE), bradykinin, and prostaglandin E2 (PGE2) have also been shown to participate in tubular function (89–92). However, evidence for their interactions with circadian control of excretory function is relatively lacking, although Nikolaeva et al. observed a circadian oscillation of 20-HETE in the kidney (36). It is important to note that this list is relatively sparse compared to the many cellular mechanisms that control what appears in the final urine. In that sense, this field is only in its infancy. Given the link between endocrine and paracrine factors such as aldosterone and ET-1 and the molecular clock, it is clear that this is an area that will be of great interest to hypertension investigators in the near future.
Figure 6.
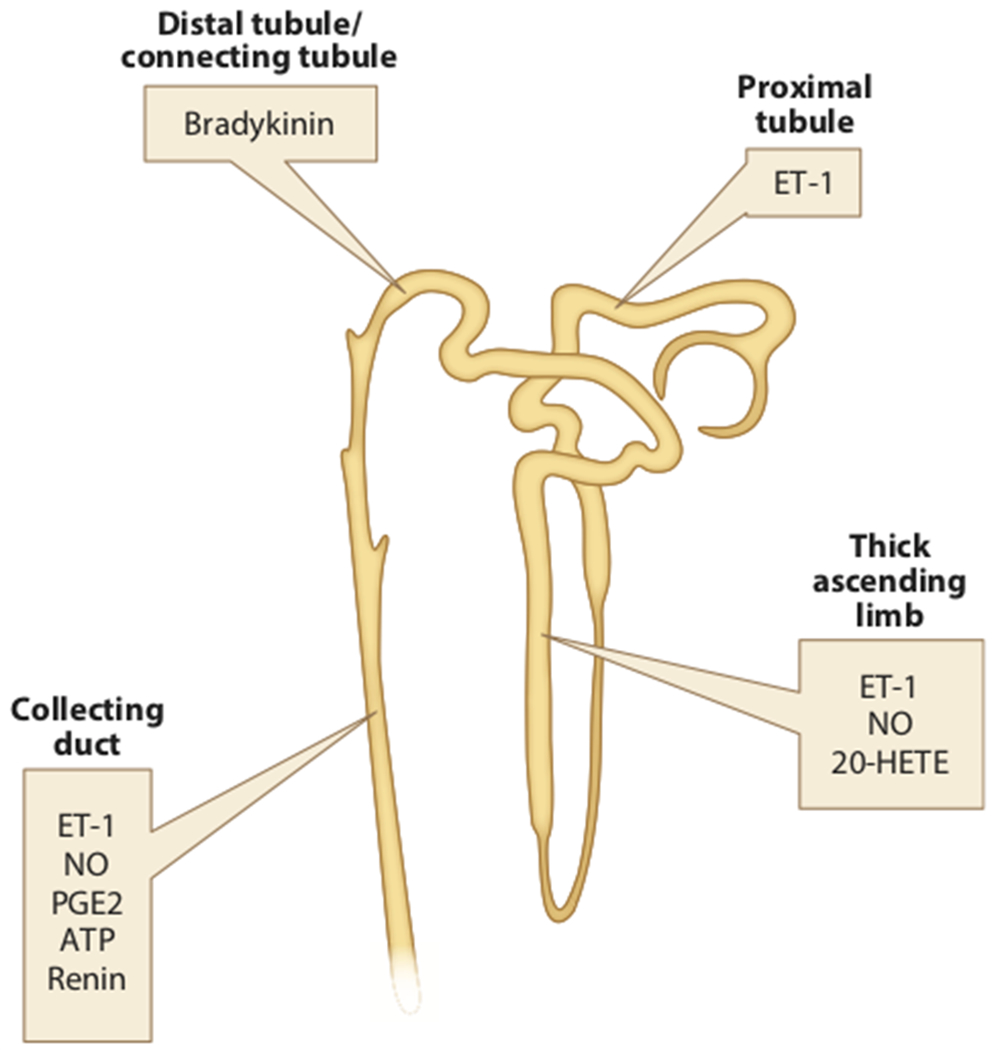
Primary site of action of major paracrine/autocrine factors in the nephron. Although these and other paracrine factors may have other sites of action, these have been established in vivo. Abbreviations: ET-1, endothelin 1; NO, nitric oxide; PGE2, prostaglandin E2.
POTASSIUM HOMEOSTASIS AND CIRCADIAN RHYTHMS
The role for circadian regulation of potassium balance was reviewed a few years ago (93). Thus, we will summarize recent progress with a focus on clinical implications of circadian misalignment of potassium homeostasis along with some novel insights from rodent studies. In 1999, Wilson et al. determined the effects of dietary potassium supplement on nocturnal blood pressure in African American adolescents (94). Participants that presented with normal blood pressures at baseline, but salt-sensitive, were given potassium supplements following a high sodium diet. High sodium diet caused non-dipping blood pressure in nearly half of the participants but was reversed to dipper status after potassium intervention. Similar results were achieved in salt-sensitive subjects in China (95). In CKD patients, Agarwal showed that urinary excretion of potassium was significantly higher at night compared with daytime (96), whereas the majority of potassium is normally excreted during daytime in healthy individuals (97). Bankir et al. also reported a similar relation between diurnal potassium excretion and circadian blood pressure in a larger population (98). It is possible that the beneficial effect of potassium supplementation on blood pressure may be due to a resetting of the diurnal pattern of potassium excretion and possibly aldosterone. Microarray analyses on mouse kidneys taken every 4 hours over 24 hours and found that the majority of known potassium-regulatory genes were expressed in a circadian-dependent manner, including genes that encode Na/K ATPase, H/K ATPase, Kir1.3, ROMK and the BK channel (35). In two distinct tissue-specific Bmal1 knockout models using Ren1(d) cre and Pax8 cre, the diurnal variation in potassium excretion was not consistently different compared with control mice (22; 23). However, the Clock knockout mouse has an increased inactive period potassium excretion under both normal light/dark and constant dark conditions (36). Future efforts should be directed to determine the mechanisms underlying circadian potassium regulation, and in particular, its relation with circadian regulation of sodium balance.
IMPLICATIONS FOR HYPERTENSION
In 2017, American Heart Association and American College of Cardiology released an updated guideline for the prevention, diagnosis, and treatment of high blood pressure in adults, in which the definition for stage 1 hypertension is systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 80 mmHg (99). This new guideline places an additional 14% of the US population into the category of having hypertension. With nearly half of the US population considered hypertensive, it is imperative that more accurate and reliable diagnostic methods are established including the use of ambulatory blood pressure monitoring (ABPM). ABPM provides enhanced abilities for diagnosing complicated yet not uncommon types of hypertension, including masked hypertension, white-coat hypertension, and nocturnal hypertension. Specifically, neither office blood pressure measurement or regular home blood pressure monitoring is able to accurately determine the existence of nocturnal hypertension, which has been shown to be prevalent in hypertensive patients (100).
The common definition for nocturnal high blood pressure is that the night-time blood pressure decreases less than 10% of day-time level. Based on the night-time “dipping” level, blood pressure can be recognized as in several categories: extreme dipper (larger than 20% dipping), dipper (10%-20% dipping), non-dipper (less than 10% dipping), and riser (higher than day-time blood pressure) (100; 101). The prevalence of nocturnal hypertension varies depending on ethnicity, gender, age, body mass index (BMI), sleeping disorder and many other factors. The Ambulatory Blood Pressure Collaboration in patients with Hypertension (ABC-H) Meta-analysis contains multiple studies from 10 cohorts with 17,312 hypertensive patients (102). The proportion of combined non-dippers and risers ranges from 37% to 65%. In another study by Harshfield et al., African American youth showed significantly higher night-time blood pressure compared to European Americans despite comparable day-time blood pressure (103). It was also noted in this study that sex was an important predictor of nocturnal blood pressure, where males were more prone to exhibit lower night-time blood pressure dipping. Li et al. showed higher prevalence of isolated nocturnal hypertension in East Asians compared to Africans and Europeans, which was associated with higher sodium consumption and lower potassium intake (104; 105). BMI has been shown to be related to nocturnal blood pressure dipping. Cuspidi et al. showed that overweight (BMI>25kg/m2) hypertensive patients had reduced nocturnal blood pressure dipping compared to lean patients (106). Obstructive sleep apnea has been shown to increase night-time blood pressure and that the severity of hypertension is correlated with apnea hypopnea index (107; 108). However, the extent of renal involvement in the day versus night blood pressures has not been established.
The night-time dipping of blood pressure is often attributed to endogenous circadian control of autonomic systems as well as exogenous factors such as activity and stress (109; 110). Night-time blood pressure may also serve as an independent target for blood pressure control (111). The absence of appropriate nocturnal dipping of blood pressure is associated with various cardiovascular events and end organ complications. In the ABC-H study, the nocturnal blood pressure decline was an independent predictor of various adverse outcomes, including myocardial infarction and stroke (102). In addition, among the various categories of blood pressure dipping, risers were shown to have the worst prognoses for events mentioned above.
Although nocturnal hypertension is often seen in patients with essential hypertension, it is not uncommon in several other pathological conditions. In fact, patients with CKD often exhibit an attenuated nocturnal blood pressure decline (112; 113). In a large cohort of CKD patients of Chinese ethnicity, isolated nocturnal hypertension was observed in more than 20% of participants. In addition, it was shown that CKD patients with isolated nocturnal hypertension had worse renal function and more prominent left ventricular hypertrophy (114). Moreover, non-dipping blood pressure is considered a risk factor for the progression of CKD. Davidson et al. conducted a retrospective study among hypertensive patients with normal baseline renal function and showed that non-dipping blood pressure preceded the decline of GFR and associated with an increase in serum creatinine level during a follow-up of 3.6 years (115). Garcia-Ortiz et al. showed an inverse relationship between nocturnal blood pressure dipping and albuminuria and that nocturnal dipping was a predictor of renal damage in hypertensive patients (116). In a prospective study conducted by Agarwal et al., nighttime blood pressure was associated with end stage renal disease and was a stronger predictor of total mortality compared with pressures during the day (117). In addition to CKD, nocturnal hypertension is a common comorbidity of both type 1 and type 2 diabetes and a major cardiovascular risk factor in diabetic patients (118). The mechanisms underlying this non-dipping phenomenon in diabetic patients remain to be elucidated although renal dysfunction, autonomic neuropathy and poor glycemic control have all been suggested (119; 120).
Theoretically, impaired sodium handling by the kidney may play an important role in elevated nocturnal blood pressure. Many have hypothesized that the loss of dipping in nighttime blood pressure is a pressure-natriuresis mechanism meant to compensate for an impaired ability to excrete sodium during day (98; 121; 122). Bankir et al. recruited 325 individuals of African descent and investigated the relationship between nighttime blood pressure and daytime urinary sodium excretion (98). They found that daytime urinary sodium concentration was inversely related to nocturnal systolic blood pressure and suggested that daytime sodium excretion was a determinant factor for nocturnal blood pressure. Similarly, another study carried out by Uzu et al. showed that, in Japanese patients who have metabolic syndrome, non-dipping blood pressure was associated with higher sodium sensitivity (123). Furthermore, it has been shown that patients with CKD have decreased day versus night ratio of natriuresis and require longer time at night till BP starts to fall, which may very well be the mechanism underlying the non-dipping blood pressure phenotype observed in subjects with impaired renal function (124).
TABLE.
Known mechanisms of molecular clock function in the nephron.
| Regulator | Target gene (mRNA) | nephron segment | reference(s) |
|---|---|---|---|
| Bmal1-Clock | NHE3 | proximal tubule | (125) |
| Per2, Cry1 | Bmal1-Clock | proximal tubule | (125) |
| Per1 | NHE3 | proximal tubule | (126) |
| Per1 | SGLT1 | proximal tubule | (126) |
| Bmal1 | OAT3 | proximal tubule | (22) |
| renin | Bmal1 | thick ascending limb | (23) |
| Per1 | pNCC | distal tubule | (28) |
| aldosterone | Per1 | collecting duct | (24; 26) |
| Per1 | αENaC | collecting duct | (27) |
| CK1δ/ε | Per1 | collecting duct | (127) |
FUTURE ISSUES.
The gap in knowledge regarding mechanisms that control renal electrolyte handling is enormous and only a handful of labs have focused on this significant area of research. Furthermore, in vivo validation of the impact of specific circadian genes on electrolyte excretion and blood pressure are needed. Such work related to time-of-day physiological variability will facilitate our understanding of renal and cardiovascular disease risk.
When performing clinical, whole animal physiology, and even cell culture studies, the time-of-day fluctuation of potential targets, caused by either their intrinsic circadian rhythms or as a result of the central clock, must be taken into consideration. This has been an overlooked aspect related to scientific rigor and reproducibility.
More studies are needed to determine the effect of diurnal potassium homeostasis on circadian blood pressure rhythm and its interaction with sodium transport as many investigators often overlook the role of the renin-angiotensin-aldosterone system in control of potassium homeostasis, and instead, focus solely on sodium balance. This is important for not only different types of hypertension, but also kidney diseases, especially IgA nephropathy, diabetic nephropathy and chronic kidney disease.
ACKNOWLEDGMENTS
The authors acknowledge grant support from an American Heart Association pre-doctoral fellowship to D.Z. (18PRE33990345) and the National Institutes of Health to D.M.P. (P01 HL95499 and P01 HL069999). Partial support was also provided by a UAB School of Medicine AMC21 Reload Multi-Investigator Grant. The authors also express their sincere appreciation to Drs. Bryan Becker, Megan Rhoads, and Paramita Pati for their helpful advice during the preparation of this manuscript.
Footnotes
DISCLOSURE STATEMENT
The authors have nothing to disclose.
LITERATURE CITED
- 1.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. 1981. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci 28:89–94 [DOI] [PubMed] [Google Scholar]
- 2.Kohan DE, Inscho EW, Wesson D, Pollock DM. 2011. Physiology of endothelin and the kidney. Compr Physiol 1:883–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Praetorius HA, Leipziger J. 2010. Intrarenal purinergic signaling in the control of renal tubular transport. Annu Rev Physiol 72:377–93 [DOI] [PubMed] [Google Scholar]
- 4.Rhaleb NE, Yang XP, Carretero OA. 2011. The kallikrein-kinin system as a regulator of cardiovascular and renal function. Compr Physiol 1:971–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imig JD, Khan MA. 2015. Cytochrome P450 and Lipoxygenase Metabolites on Renal Function. Compr Physiol 6:423–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baylis C 2012. Nitric oxide synthase derangements and hypertension in kidney disease. Curr Opin Nephrol Hypertens 21:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Townsend RR, Sobotka PA. 2018. Catheter-Based Renal Denervation for Hypertension. Curr Hypertens Rep 20:93. [DOI] [PubMed] [Google Scholar]
- 8.Richards J, Gumz ML. 2013. Mechanism of the circadian clock in physiology. Am J Physiol Regul Integr Comp Physiol 304:R1053–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sirota JH, Baldwin DS, Villarreal H. 1950. Diurnal variations of renal function in man. J Clin Invest 29:187–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills JN, Stanbury SW. 1952. Persistent 24-hour renal excretory rhythm on a 12-hour cycle of activity. J Physiol 117:22–37 [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi JS. 2015. Molecular components of the circadian clock in mammals. Diabetes Obes Metab 17 Suppl 1:6–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turek FW. 2016. Circadian clocks: Not your grandfather’s clock. Science 354:992–3 [DOI] [PubMed] [Google Scholar]
- 13.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, et al. 2002. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109:307–20 [DOI] [PubMed] [Google Scholar]
- 14.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. 2014. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 111:16219–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston JG, Pollock DM. 2018. Circadian regulation of renal function. Free Radic Biol Med 119:93–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Firsov D, Bonny O. 2018. Circadian rhythms and the kidney. Nat Rev Nephrol 14:626–35 [DOI] [PubMed] [Google Scholar]
- 17.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, et al. 2000. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103:1009–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. 2007. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A 104:3450–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, et al. 2008. Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab 8:482–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Z, Su W, Liu S, Zhao G, Esser K, et al. 2015. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Invest 125:324–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang L, Xiong W, Zhao X, Fan Y, Guo Y, et al. 2018. Bmal1 in Perivascular Adipose Tissue Regulates Resting-Phase Blood Pressure Through Transcriptional Regulation of Angiotensinogen. Circulation 138:67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolaeva S, Ansermet C, Centeno G, Pradervand S, Bize V, et al. 2016. Nephron-Specific Deletion of Circadian Clock Gene Bmal1 Alters the Plasma and Renal Metabolome and Impairs Drug Disposition. J Am Soc Nephrol 27:2997–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tokonami N, Mordasini D, Pradervand S, Centeno G, Jouffe C, et al. 2014. Local renal circadian clocks control fluid-electrolyte homeostasis and BP. J Am Soc Nephrol 25:1430–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stow LR, Richards J, Cheng KY, Lynch IJ, Jeffers LA, et al. 2012. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension 59:1151–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solocinski K, Holzworth M, Wen X, Cheng KY, Lynch IJ, et al. 2017. Desoxycorticosterone pivalate-salt treatment leads to non-dipping hypertension in Per1 knockout mice. Acta Physiol (Oxf) 220:72–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douma LG, Solocinski K, Holzworth MR, Crislip GR, Masten SH, et al. 2019. Female C57BL/6J mice lacking the circadian clock protein PER1 are protected from nondipping hypertension. Am J Physiol Regu Integr Compar Physiol 316:R50–R8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, et al. 2009. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest 119:2423–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards J, Ko B, All S, Cheng KY, Hoover RS, Gumz ML. 2014. A role for the circadian clock protein Per1 in the regulation of the NaCl co-transporter (NCC) and the with-no-lysine kinase (WNK) cascade in mouse distal convoluted tubule cells. J Biol Chem 289:11791–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gohar EY, Pollock DM. 2018. Sex-Specific Contributions of Endothelin to Hypertension. Curr Hypertens Rep 20:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alli A, Yu L, Holzworth M, Richards J, Cheng KY, et al. 2019. Direct and indirect inhibition of the circadian clock protein Per1: effects on ENaC and blood pressure. Am J Physiol Renal Physiol 316:F807–F13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vukolic A, Antic V, Van Vliet BN, Yang Z, Albrecht U, Montani JP. 2010. Role of mutation of the circadian clock gene Per2 in cardiovascular circadian rhythms. Am J Physiol Regu Integr Compar Physiol 298:R627–34 [DOI] [PubMed] [Google Scholar]
- 32.Pati P, Fulton DJ, Bagi Z, Chen F, Wang Y, et al. 2016. Low-Salt Diet and Circadian Dysfunction Synergize to Induce Angiotensin II-Dependent Hypertension in Mice. Hypertension 67:661–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, et al. 2010. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med 16:67–74 [DOI] [PubMed] [Google Scholar]
- 34.Sei H, Oishi K, Chikahisa S, Kitaoka K, Takeda E, Ishida N. 2008. Diurnal amplitudes of arterial pressure and heart rate are dampened in Clock mutant mice and adrenalectomized mice. Endocrinology 149:3576–80 [DOI] [PubMed] [Google Scholar]
- 35.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, et al. 2009. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A 106:16523–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikolaeva S, Pradervand S, Centeno G, Zavadova V, Tokonami N, et al. 2012. The circadian clock modulates renal sodium handling. J Am Soc Nephrol 23:1019–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honma S, Kawamoto T, Takagi Y, Fujimoto K, Sato F, et al. 2002. Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature 419:841–4 [DOI] [PubMed] [Google Scholar]
- 38.Nakashima A, Kawamoto T, Noshiro M, Ueno T, Doi S, et al. 2018. Dec1 and CLOCK Regulate Na+/K+-ATPase β1 Subunit Expression and Blood Pressure. Hypertension 72:746–54 [DOI] [PubMed] [Google Scholar]
- 39.Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, et al. 2016. Endothelin. Pharmacol Rev 68:357–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohan DE, Rossi NF, Inscho EW, Pollock DM. 2011. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91:1–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fellner RC, Guan Z, Cook AK, Pollock DM, Inscho EW. 2015. Endothelin contributes to blunted renal autoregulation observed with a high-salt diet. Am J Physiol Renal Physiol 309:F687–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gumz ML, Popp MP, Wingo CS, Cain BD. 2003. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol 285:F664–73. [DOI] [PubMed] [Google Scholar]
- 43.Hwang YS, Hsieh TJ, Lee YJ, Tsai JH. 1998. Circadian rhythm of urinary endothelin-1 excretion in mild hypertensive patients. Am J Hyper 11:1344–51 [DOI] [PubMed] [Google Scholar]
- 44.Johnston JG, Speed JS, Jin C, Pollock DM. 2016. Loss of endothelin B receptor function impairs sodium excretion in a time- and sex-dependent manner. Am J Physiol Renal Physiol 311:F991–F8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhaun N, Moorhouse R, MacIntyre IM, Melville V, Oosthuyzen W, et al. 2014. Diurnal variation in blood pressure and arterial stiffness in chronic kidney disease: the role of endothelin-1. Hypertension 64:296–304 [DOI] [PubMed] [Google Scholar]
- 46.Speed JS, Hyndman KA, Roth K, Heimlich JB, Kasztan M, et al. 2018. High dietary sodium causes dyssynchrony of the renal molecular clock in rats. Am J Physiol Renal Physiol 314:F89–F98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Speed JS, Hyndman KA, Kasztan M, Johnston JG, Roth KJ, et al. 2016. High salt intake alters renal medullary clock genes via ETB receptors. FASEB J 30:1216.9 [Google Scholar]
- 48.Speed JS, Hyndman KA, Kasztan M, Johnston JG, Roth KJ, et al. 2018. Diurnal pattern in skin Na+ and water content is associated with salt-sensitive hypertension in ETB receptor-deficient rats. Am J Physiol Regu Integr Compar Physiol 314:R544–R51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richards J, Welch AK, Barilovits SJ, All S, Cheng KY, et al. 2014. Tissue-specific and time-dependent regulation of the endothelin axis by the circadian clock protein Per1. Life Sci 118:255–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plato CF, Stoos BA, Wang D, Garvin JL. 1999. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol Renal Physiol 276:F159–63 [DOI] [PubMed] [Google Scholar]
- 51.Plato CF, Pollock DM, Garvin JL. 2000. Endothelin inhibits thick ascending limb chloride flux via ETB receptor-mediated NO release. Am J Physiol Renal Physiol 279:F326–33 [DOI] [PubMed] [Google Scholar]
- 52.Ortiz PA, Hong NJ, Garvin JL. 2001. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281:F819–25 [DOI] [PubMed] [Google Scholar]
- 53.Stoos BA, Garcia NH, Garvin JL. 1995. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol 6:89–94 [DOI] [PubMed] [Google Scholar]
- 54.Garcia NH, Stoos BA, Carretero OA, Garvin JL. 1996. Mechanism of the nitric oxide-induced blockade of collecting duct water permeability. Hypertension 27:679–83 [DOI] [PubMed] [Google Scholar]
- 55.Sallstrom J, Carlstrom M, Jensen BL, Skott O, Brown RD, Persson AE. 2008. Neuronal nitric oxide synthase-deficient mice have impaired renin release but normal blood pressure. Am J Hyper 21:111–6 [DOI] [PubMed] [Google Scholar]
- 56.Hyndman KA, Boesen EI, Elmarakby AA, Brands MW, Huang P, et al. 2013. Renal collecting duct NOS1 maintains fluid-electrolyte homeostasis and blood pressure. Hypertension 62:91–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herrera M, Garvin JL. 2004. Endothelin stimulates endothelial nitric oxide synthase expression in the thick ascending limb. Am J Physiol Renal Physiol 287:F231–5 [DOI] [PubMed] [Google Scholar]
- 58.Ye Q, Chen S, Gardner DG. 2003. Endothelin inhibits NPR-A and stimulates eNOS gene expression in rat IMCD cells. Hypertension 41:675–81 [DOI] [PubMed] [Google Scholar]
- 59.Sullivan JC, Goodchild TT, Cai Z, Pollock DM, Pollock JS. 2007. Endothelin(A) ETA and ETB receptor-mediated regulation of nitric oxide synthase 1 (NOS1) and NOS3 isoforms in the renal inner medulla. Acta Physiol (Oxf) 191:329–36 [DOI] [PubMed] [Google Scholar]
- 60.Gao Y, Stuart D, Takahishi T, Kohan DE. 2018. Nephron-Specific Disruption of Nitric Oxide Synthase 3 Causes Hypertension and Impaired Salt Excretion. J Am Heart Assoc 7(14). pii: e009236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu Y, Wei J, Stec DE, Roman RJ, Ge Y, et al. 2016. Macula Densa Nitric Oxide Synthase 1β Protects against Salt-Sensitive Hypertension. J Am Soc Nephrol 27:2346–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tunctan B, Weigl Y, Dotan A, Peleg L, Zengil H, et al. 2002. Circadian variation of nitric oxide synthase activity in mouse tissue. Chronobiol Int 19:393–404 [DOI] [PubMed] [Google Scholar]
- 63.Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, et al. 2009. Vascular disease in mice with a dysfunctional circadian clock. Circulation 119:1510–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anea CB, Cheng B, Sharma S, Kumar S, Caldwell RW, et al. 2012. Increased superoxide and endothelial NO synthase uncoupling in blood vessels of Bmal1-knockout mice. Circ Res 111:1157–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Westgate EJ, Cheng Y, Reilly DF, Price TS, Walisser JA, et al. 2008. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation 117:2087–95 [DOI] [PubMed] [Google Scholar]
- 66.Denniff M, Turrell HE, Vanezis A, Rodrigo GC. 2014. The time-of-day variation in vascular smooth muscle contractility depends on a nitric oxide signalling pathway. J Mol Cell Cardiol 66:133–40 [DOI] [PubMed] [Google Scholar]
- 67.Arraj M, Lemmer B. 2007. Endothelial nitric oxide is not involved in circadian rhythm generation of blood pressure: experiments in wild-type C57 and eNOS knock-out mice under light-dark and free-run conditions. Chronobiol Int 24:1231–40 [DOI] [PubMed] [Google Scholar]
- 68.Van Vliet BN, Chafe LL, Montani JP. 2003. Characteristics of 24 h telemetered blood pressure in eNOS-knockout and C57Bl/6J control mice. J Physiol 549:313–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kunieda T, Minamino T, Miura K, Katsuno T, Tateno K, et al. 2008. Reduced nitric oxide causes age-associated impairment of circadian rhythmicity. Circ Res 102:607–14 [DOI] [PubMed] [Google Scholar]
- 70.Bode-Boger SM, Boger RH, Kielstein JT, Loffler M, Schaffer J, Frolich JC. 2000. Role of endogenous nitric oxide in circadian blood pressure regulation in healthy humans and in patients with hypertension or atherosclerosis. J Investig Med 48:125–32 [PubMed] [Google Scholar]
- 71.Bailey MA. 2004. Inhibition of bicarbonate reabsorption in the rat proximal tubule by activation of luminal P2Y1 receptors. Am J Physiol Renal Physiol 287:F789–96 [DOI] [PubMed] [Google Scholar]
- 72.Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, et al. 2008. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem 283:36599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, et al. 2007. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21:3717–26 [DOI] [PubMed] [Google Scholar]
- 74.Wildman SS, Marks J, Turner CM, Yew-Booth L, Peppiatt-Wildman CM, et al. 2008. Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol 19:731–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, et al. 2006. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med 12:133–7 [DOI] [PubMed] [Google Scholar]
- 76.Pandit MM, Inscho EW, Zhang S, Seki T, Rohatgi R, et al. 2015. Flow regulation of endothelin-1 production in the inner medullary collecting duct. Am J Physiol Renal Physiol 308:F541–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gohar EY, Speed JS, Kasztan M, Jin C, Pollock DM. 2016. Activation of purinergic receptors (P2) in the renal medulla promotes endothelin-dependent natriuresis in male rats. Am J Physiol Renal Physiol 311:F260–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gohar EY, Kasztan M, Becker BK, Speed JS, Pollock DM. 2017. Ovariectomy uncovers purinergic receptor activation of endothelin-dependent natriuresis. Am J Physiol Renal Physiol 313:F361–F9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palomino-Doza J, Rahman TJ, Avery PJ, Mayosi BM, Farrall M, et al. 2008. Ambulatory blood pressure is associated with polymorphic variation in P2X receptor genes. Hypertension 52:980–5 [DOI] [PubMed] [Google Scholar]
- 80.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. 1990. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest 85:417–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Navar LG, Prieto MC, Satou R, Kobori H. 2011. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol 11:180–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carey RM. 2015. The intrarenal renin-angiotensin system in hypertension. Adv Chronic Kidney Dis 22:204–10 [DOI] [PubMed] [Google Scholar]
- 83.Gonzalez-Villalobos RA, Satou R, Seth DM, Semprun-Prieto LC, Katsurada A, et al. 2009. Angiotensin-converting enzyme-derived angiotensin II formation during angiotensin II-induced hypertension. Hypertension 53:351–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, et al. 2010. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol 298:F150–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, et al. 2013. The absence of intrarenal ACE protects against hypertension. J Clin Invest 123:2011–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Isobe S, Ohashi N, Fujikura T, Tsuji T, Sakao Y, et al. 2015. Disturbed circadian rhythm of the intrarenal renin-angiotensin system: relevant to nocturnal hypertension and renal damage. Clin Exp Nephrol 19:231–9 [DOI] [PubMed] [Google Scholar]
- 87.Ishigaki S, Ohashi N, Isobe S, Tsuji N, Iwakura T, et al. 2016. Impaired endogenous nighttime melatonin secretion relates to intrarenal renin-angiotensin system activation and renal damage in patients with chronic kidney disease. Clin Exp Nephrol 20:878–84 [DOI] [PubMed] [Google Scholar]
- 88.Isobe S, Ohashi N, Ishigaki S, Tsuji T, Sakao Y, et al. 2016. Augmented circadian rhythm of the intrarenal renin-angiotensin systems in anti-thymocyte serum nephritis rats. Hypertens Res 39:312–20 [DOI] [PubMed] [Google Scholar]
- 89.Williams JM, Murphy S, Burke M, Roman RJ. 2010. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol 56:336–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang DD, Gao ZX, Vio CP, Xiao Y, Wu P, et al. 2018. Bradykinin Stimulates Renal Na+ and K+ Excretion by Inhibiting the K+ Channel (Kir4.1) in the Distal Convoluted Tubule. Hypertension 72:361–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mamenko M, Zaika O, Pochynyuk O. 2014. Direct regulation of ENaC by bradykinin in the distal nephron. Implications for renal sodium handling. Cur Op Nephrol Hyper 23:122–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nasrallah R, Zimpelmann J, Eckert D, Ghossein J, Geddes S, et al. 2018. PGE2 EP1 receptor inhibits vasopressin-dependent water reabsorption and sodium transport in mouse collecting duct. Lab Invest 98:360–70 [DOI] [PubMed] [Google Scholar]
- 93.Gumz ML, Rabinowitz L. 2013. Role of circadian rhythms in potassium homeostasis. Seminars Nephrol 33:229–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilson DK, Sica DA, Miller SB. 1999. Effects of potassium on blood pressure in salt-sensitive and salt-resistant black adolescents. Hypertension 34:181–6 [DOI] [PubMed] [Google Scholar]
- 95.Guo TS, Dai Y, Ren KY, Mu JJ, Ren J, et al. 2017. Effects of salt loading and potassium supplement on the circadian blood pressure profile in salt-sensitive Chinese patients. Blood Press Monit 22:307–13 [DOI] [PubMed] [Google Scholar]
- 96.Agarwal R 2007. Relationship between circadian blood pressure variation and circadian protein excretion in CKD. Am J Physiol Renal Physiol 293:F655–9 [DOI] [PubMed] [Google Scholar]
- 97.Koopman MG, Koomen GC, Krediet RT, de Moor EA, Hoek FJ, Arisz L. 1989. Circadian rhythm of glomerular filtration rate in normal individuals. Clin Sci (Lond) 77:105–11 [DOI] [PubMed] [Google Scholar]
- 98.Bankir L, Bochud M, Maillard M, Bovet P, Gabriel A, Burnier M. 2008. Nighttime blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in African subjects. Hypertension 51:891–8 [DOI] [PubMed] [Google Scholar]
- 99.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, et al. 2018. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71:1269–324 [DOI] [PubMed] [Google Scholar]
- 100.Kario K 2018. Nocturnal Hypertension: New Technology and Evidence. Hypertension 71:997–1009 [DOI] [PubMed] [Google Scholar]
- 101.de la Sierra A, Gorostidi M, Banegas JR, Segura J, de la Cruz JJ, Ruilope LM. 2014. Nocturnal hypertension or nondipping: which is better associated with the cardiovascular risk profile? Am J Hyper 27:680–7 [DOI] [PubMed] [Google Scholar]
- 102.Salles GF, Reboldi G, Fagard RH, Cardoso CR, Pierdomenico SD, et al. 2016. Prognostic Effect of the Nocturnal Blood Pressure Fall in Hypertensive Patients: The Ambulatory Blood Pressure Collaboration in Patients With Hypertension (ABC-H) Meta-Analysis. Hypertension 67:693–700 [DOI] [PubMed] [Google Scholar]
- 103.Harshfield GA, Treiber FA, Wilson ME, Kapuku GK, Davis HC. 2002. A longitudinal study of ethnic differences in ambulatory blood pressure patterns in youth. Am J Hyper 15:525–30 [DOI] [PubMed] [Google Scholar]
- 104.Li Y, Staessen JA, Lu L, Li LH, Wang GL, Wang JG. 2007. Is isolated nocturnal hypertension a novel clinical entity? Findings from a Chinese population study. Hypertension 50:333–9 [DOI] [PubMed] [Google Scholar]
- 105.Li Y, Wang JG. 2013. Isolated nocturnal hypertension: a disease masked in the dark. Hypertension 61:278–83 [DOI] [PubMed] [Google Scholar]
- 106.Cuspidi C, Meani S, Valerio C, Negri F, Sala C, et al. 2008. Body mass index, nocturnal fall in blood pressure and organ damage in untreated essential hypertensive patients. Blood Press Monit 13:318–24 [DOI] [PubMed] [Google Scholar]
- 107.Nabe B, Lies A, Pankow W, Kohl FV, Lohmann FW. 1995. Determinants of circadian blood pressure rhythm and blood pressure variability in obstructive sleep apnoea. J Sleep Res 4:97–101 [DOI] [PubMed] [Google Scholar]
- 108.Suzuki M, Guilleminault C, Otsuka K, Shiomi T. 1996. Blood pressure “dipping” and “non-dipping” in obstructive sleep apnea syndrome patients. Sleep 19:382–7 [DOI] [PubMed] [Google Scholar]
- 109.Chau NP, Mallion JM, de Gaudemaris R, Ruche E, Siche JP, et al. 1989. Twenty-four-hour ambulatory blood pressure in shift workers. Circulation 80:341–7 [DOI] [PubMed] [Google Scholar]
- 110.Smolensky MH, Haus E. 2001. Circadian rhythms and clinical medicine with applications to hypertension. Am J Hyper 14:280S–90S [DOI] [PubMed] [Google Scholar]
- 111.Brook RD, Rajagopalan S. 2018. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Soc Hypertens 12:238. [DOI] [PubMed] [Google Scholar]
- 112.Fukuda M, Munemura M, Usami T, Nakao N, Takeuchi O, et al. 2004. Nocturnal blood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kidney Int 65:621–5 [DOI] [PubMed] [Google Scholar]
- 113.Tamura K, Kanaoka T, Ohsawa M, Haku S, Azushima K, et al. 2011. Emerging concept of anti-hypertensive therapy based on ambulatory blood pressure profile in chronic kidney disease. Am J Cardiovasc Dis 1:236–43 [PMC free article] [PubMed] [Google Scholar]
- 114.Wang C, Deng WJ, Gong WY, Zhang J, Tang H, et al. 2015. High prevalence of isolated nocturnal hypertension in Chinese patients with chronic kidney disease. J Am Heart Assoc 4:e002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Davidson MB, Hix JK, Vidt DG, Brotman DJ. 2006. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med 166:846–52 [DOI] [PubMed] [Google Scholar]
- 116.Garcia-Ortiz L, Gomez-Marcos MA, Martin-Moreiras J, Gonzalez-Elena LJ, Recio-Rodriguez JI, et al. 2009. Pulse pressure and nocturnal fall in blood pressure are predictors of vascular, cardiac and renal target organ damage in hypertensive patients (LOD-RISK study). Blood Press Monit 14:145–51 [DOI] [PubMed] [Google Scholar]
- 117.Agarwal R, Andersen MJ. 2006. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 69:1175–80 [DOI] [PubMed] [Google Scholar]
- 118.Kim YS, Davis S, Stok WJ, van Ittersum FJ, van Lieshout JJ. 2019. Impaired nocturnal blood pressure dipping in patients with type 2 diabetes mellitus. Hypertens Res 42:59–66 [DOI] [PubMed] [Google Scholar]
- 119.Sturrock ND, George E, Pound N, Stevenson J, Peck GM, Sowter H. 2000. Non-dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus. Diabet Med 17:360–4 [DOI] [PubMed] [Google Scholar]
- 120.Mulec H, Blohme G, Kullenberg K, Nyberg G, Bjorck S. 1995. Latent overhydration and nocturnal hypertension in diabetic nephropathy. Diabetologia 38:216–20 [DOI] [PubMed] [Google Scholar]
- 121.Uzu T, Kazembe FS, Ishikawa K, Nakamura S, Inenaga T, Kimura G. 1996. High sodium sensitivity implicates nocturnal hypertension in essential hypertension. Hypertension 28:139–42 [DOI] [PubMed] [Google Scholar]
- 122.Fujii T, Uzu T, Nishimura M, Takeji M, Kuroda S, et al. 1999. Circadian rhythm of natriuresis is disturbed in nondipper type of essential hypertension. Am J Kidney Dis 33:29–35 [DOI] [PubMed] [Google Scholar]
- 123.Uzu T, Kimura G, Yamauchi A, Kanasaki M, Isshiki K, et al. 2006. Enhanced sodium sensitivity and disturbed circadian rhythm of blood pressure in essential hypertension. J Hypertens 24:1627–32 [DOI] [PubMed] [Google Scholar]
- 124.Fukuda M, Mizuno M, Yamanaka T, Motokawa M, Shirasawa Y, et al. 2008. Patients with renal dysfunction require a longer duration until blood pressure dips during the night. Hypertension 52:1155–60 [DOI] [PubMed] [Google Scholar]
- 125.Wei N, Gumz ML, Layton AT. 2018. Predicted effect of circadian clock modulation of NHE3 of a proximal tubule cell on sodium transport. Am J Physiol Renal Physiol 315:F665–F76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Solocinski K, Richards J, All S, Cheng KY, Khundmiri SJ, Gumz ML. 2015. Transcriptional regulation of NHE3 and SGLT1 by the circadian clock protein Per1 in proximal tubule cells. Am J Physiol Renal Physiol 309:F933–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maywood ES, Chesham JE, Smyllie NJ, Hastings MH. 2014. The Tau mutation of casein kinase 1epsilon sets the period of the mammalian pacemaker via regulation of Period1 or Period2 clock proteins. J Biol Rhythms 29:110–8 [DOI] [PMC free article] [PubMed] [Google Scholar]


