Abstract
Atopic dermatitis (AD) and psoriasis are common skin diseases with a high negative impact on patients' quality of life. Both diseases are mediated by a pro‐inflammatory infiltrate consisting of several cell types, such as T‐cells, antigen‐presenting cells and granulocytes and display disturbed keratinocyte differentiation. Given the fact that histamine levels are also highly elevated in inflamed skin, it is likely that histamine plays a relevant role in disease pathology. However, antagonists blocking histamine H1 receptor or H2 receptors are largely ineffective in reducing chronic symptoms in AD and psoriasis. Over the last years, much research has been undertaken to shed light into the mode of action of the most recently discovered histamine H4 receptor. This research has shown that H4 receptor antagonists display antipruritic and anti‐inflammatory effects not only in mouse models but also in first human clinical trials, and therefore, H4 receptors might present a novel therapeutic target. In this review, we summarize the effects of the H4 receptors on different cell types, mouse models and clinical studies in regard to AD and psoriasis respectively.
Linked Articles
This article is part of a themed section on New Uses for 21st Century. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.3/issuetoc
Abbreviations
- AD
atopic dermatitis
- APCs
antigen‐presenting cells
- CD
cluster of differentiation
- DCs
dendritic cells
- DNCB
dinitrochlorobenzene
- FcεRI
high‐affinity IgE receptor
- LCs
Langerhans cells
- mDCs
myeloid dendritic cells
- 4MH
4‐methylhistamine
- OVA
ovalbumin
- PASI
Psoriasis Area Severity Index
- pDCs
plasmacytoid dendritic cells
- poly I : C
polyinosinic–polycytidylic acid
- SCORAD
score of atopic dermatitis
- slan DCs
6‐sulfo LacNAc‐expressing dendritic cells
- SNP
single nucleotide polymorphism
- TDI
toluene diisocyanate
- TNCB
trinitrochlorobenzene
- Treg
regulatory T‐cells
- TSLP
thymic stromal lymphopoietin
Introduction
Histamine, a pleiotropic mediator, is involved in a variety of physiological and pathological processes in both the CNS and the periphery (Panula et al., 2015). Over the last century, four GPCRs were identified binding histamine as a ligand. The H1 receptor was the first histamine receptor to be discovered, in 1937 by Bovet and Staub (1937) (Simons and Simons, 2011), followed by the H2 receptor in 1972 (Black et al., 1972) and the H3 receptor in 1983 (Arrang et al., 1983). Antagonists blocking the H1 receptor or the H2 receptor became successful drugs for the treatment of allergic diseases and gastric acid secretion, respectively, and they are still in use today (Thurmond, 2015). The H3 receptor modulates histamine actions in the brain, and an inverse agonist of this receptor has been approved for the treatment of narcolepsy since 2016 (Baumann, 2017). However, no antagonists of the H1, H2 or H3 receptors are able to reduce the inflammatory and pruritic symptoms in chronic inflammatory skin diseases like atopic dermatitis (AD) or psoriasis, although it is well known that histamine is elevated in inflamed skin and plays a relevant role in disease pathology (Gutzmer et al., 2011). In the early 2000s, the H4 receptor was described by several groups (Nakamura et al., 2000; Oda et al., 2000; Liu et al., 2001a). Various selective H4 receptor ligands and the use of H4 receptor −/− mice allowed the analysis of the expression and the function of these receptors (Thurmond, 2015; Ko et al., 2018). This revealed a relevant role of H4 receptors in mediating pruritus and in the modulation of cellular responses in several immune and epithelial cells, which are important in the pathogenesis of inflammatory skin diseases (Gutzmer et al., 2011; Thurmond, 2015). Moreover, in first clinical trials, H4 receptor antagonists reduced inflammation and scratching behaviour in patients with AD (Murata et al., 2015; Werfel et al., 2018).
In this review, we summarize the current knowledge regarding the expression and function of H4 receptors with respect to inflammatory skin diseases. Furthermore we focus on different human cell types, mouse models and clinical studies in the most frequently occurring skin diseases, AD and psoriasis.
The role of H4 receptors in AD
Disease pattern
AD is a chronic inflammatory skin disease and affects up to 20% of children and up to 3% of adults, and the prevalence is still increasing (Nutten, 2015). It is associated with a characteristic distribution of eczematous skin lesions and intensive itch as dominant clinical features, which has a high negative impact on patients' quality of life (Werfel et al., 2016b). The pathophysiology is complex with mainly a Th2‐driven systemic immune dysfunction accompanied by an interaction with keratinocytes. Although numerous risk and trigger factors have been identified, including a genetic predisposition, skin barrier disruption or environmental conditions, such as exposure to allergens or microbes, the underlying mechanism of the development of AD still remains unclear (Werfel, 2009; Nutten, 2015). One of the characteristics of AD is the presence of T‐cells in the affected skin with an initial Th2 polarization and a more Th1‐dominated milieu in chronic AD (Werfel et al., 2016a). However, other cell populations are also critical for the initiation and maintenance of the disease, such as dendritic cell (DC) types expressing high‐affinity IgE receptors (FcεRIs), which are able to take up and present antigens penetrating the epidermis. Moreover, mast cells, eosinophils, basophils and NK cells are elevated in the skin of AD patients and contribute to disease pathology, although their exact role still needs to be elucidated (Werfel et al., 2016a). Accompanied by the increased number of immune cells in the skin, there are also several inflammatory mediators which are up‐regulated such as IL‐4, IL‐5, IL‐13, IL‐31, the chemokine CCL17, thymic stromal lymphopoietin (TSLP) and histamine, which on one side amplify eczematous lesions and on the other side directly mediate pruritus (Werfel et al., 2016a). A schematic overview is illustrated in Figure 1.
Figure 1.
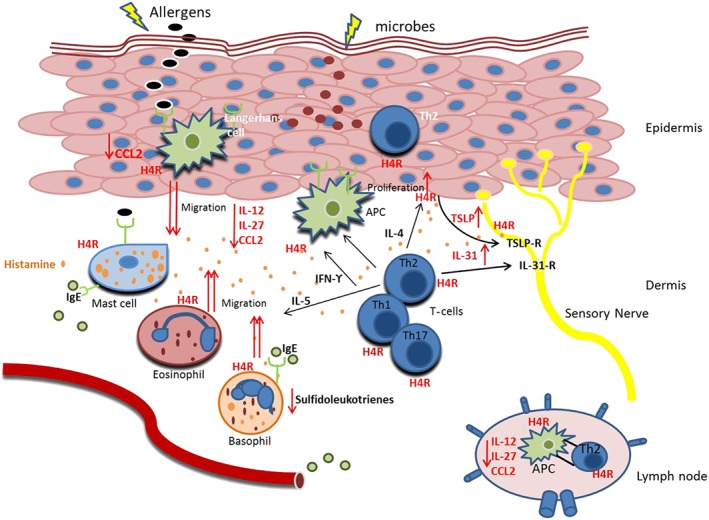
The role of histamine H4 receptors on human cells relevant in AD. These receptors (H4R) are expressed on keratinocytes and different immune cell populations, which play a role in inflamed skin of AD. In the Th2‐dominated phase of acute inflammation, various cell types such as mast cells, granulocytes, Th cells, macrophages and APCs are detectable in the skin. Engagement of the FcεRI triggers release of histamine from mast cells and basophils. Histamine in turn is able to mediate several pro‐inflammatory and anti‐inflammatory effects via its binding to H4 receptors. In basophils, stimulation of H4 receptors reduces the FcεRI cross‐linking‐mediated release of sulfidoleukotrienes. In APCs, various pro‐inflammatory cytokines like IL‐12, IL‐27 and CCL2 are down‐regulated. However, the decrease of these cytokines results to an impaired Th1 polarization and to a shift towards a Th2‐driven immune response, which further can lead to an exacerbation of the symptoms seen in acute AD. In line with this, histamine via H4 receptors increases the chemotaxis of APCs, eosinophils and basophils that is essential for the recruitment of the cells into the skin. Moreover, stimulation of H4 receptors up‐regulates the production of IL‐31 in Th2 cells and TSLP release from keratinocytes. IL‐31 and TSLP are able to trigger a Th2 polarization and to directly mediate pruritus. In addition, histamine through H4 receptors increases the proliferation rate in keratinocytes of AD patients. Conclusively, the pro‐inflammatory effects of H4 receptors seem to be more important in disease pathology. The effects mediated by H4 receptors are highlighted in red.
Strategies for the treatment of AD are limited. Systemic glucocorticoids and cyclosporine should not be used for long‐term treatment due to various side effects. Dupilumab, a human monoclonal antibody against the IL‐4 receptor α chain that is shared by the IL‐4 receptor and IL‐13 receptor, has been documented to significantly improve most of the clinical outcomes. Dupilumab showed long‐term efficacy and an acceptable safety profile: injection‐site reactions and conjunctivitis were more common in patients treated with dupilumab when compared with placebo. Therefore, dupilumab has been approved in the USA and in the European Union for the treatment of moderate‐to‐severe AD only (Werfel, 2018). Thus, there is still an urgent need to extend the spectrum of potential drugs for long‐term therapy in AD patients.
The role of H4 receptors in human cells relevant in AD
T‐cells
T‐cells are considered to be the driving force of the inflammatory response in AD (Gutzmer et al., 2011; Werfel et al., 2016a). More specifically, Th2 cells expressing cytokines such as IL‐4, IL‐5 and IL‐13 have been found to be elevated in skin biopsies from AD patients, compared with normal control skin from healthy subjects or to non‐lesional skin from AD patients (Hamid et al., 1994). It has been proposed that this Th2‐dominated micromilieu observed in AD and especially the cytokine IL‐4 lead to the up‐regulation of H4 receptors in CD4+ T‐cells. In line with this, H4 receptors are predominantly expressed in in vitro‐differentiated Th2 cells compared with Th1‐differentiated cells (Sugata et al., 2007; Gutzmer et al., 2009). In contrast, the stimulation of naïve CD4+ T‐cells with IL‐4 results in the down‐regulation of the H1 receptor mRNA expression levels (Jutel et al., 2001).
Stimulation of the H4 receptors in human peripheral blood mononuclear cells and Th2 cells leads to up‐regulation of IL‐31 mRNA expression levels (Gutzmer et al., 2009), a cytokine that is strongly related to the induction of pruritus.
Antigen‐presenting cells
A resident population of Langerhans cells (LCs) is located in the epidermis, and populations of monocytes, DCs and macrophages infiltrate the dermis. This heterogeneous group of antigen‐presenting leukocytes is important for the activation of both the innate and adaptive arms of the immune system mainly by their capacity for potent antigen presentation and T‐cell activation.
Monocytes/DCs
The mRNAs for H1, H2 and H4 receptors are expressed in human monocytes (Gutzmer et al., 2005; Glatzer et al., 2014; Capelo et al., 2016; Mommert et al., 2018c). The H4 receptor has a functional role on these cells, as induction of calcium influx, down‐regulation of the chemokine CCL2 and down‐regulation of polyinosinic–polycytidylic acid (poly I : C)‐induced expression of IL‐12 in response to stimulation, were observed. These effects were reversed by pretreatment of the cells with H4 receptor antagonists (Gutzmer et al., 2005; Dijkstra et al., 2007). In addition, IL‐27 expression is down‐regulated via H2 and H4 receptors in human monocytes. Stimulating human keratinocytes with supernatants from histamine stimulated monocytes resulted in less production of the Th1‐associated chemokine CXCL10, when compared with supernatants from unstimulated samples. This experiment demonstrates that the functional effect of histamine in monocytes resulted in decreased activation of keratinocytes (Gschwandtner et al., 2012).
Similar to the effects on monocytes, down‐regulation of poly I : C‐induced expression of IL‐12 and spontaneous down‐regulation of CCL2 via H4 receptors were also described in inflammatory dendritic epidermal cells (Dijkstra et al., 2008).
LCs, as immature antigen‐presenting cells (APCs), resides in the supra‐basal layers of the epidermis in close contact with keratinocytes. Expression of H4 receptors was detected in in vitro‐generated monocyte‐derived LCs at mRNA level. In naturally occurring CD207+ LCs, H4 receptors were also detected by flow cytometry or by immunofluorescence. Stimulation with different histamine receptor agonists showed a down‐regulation of CCL2 expression and induced migration via H4 receptors. These effects were blocked by H4 receptor antagonists (Gschwandtner et al., 2010). On one hand, increased migration may foster antigen presentation by LCs, and on the other hand, down‐regulation of CCL2 may restrain accumulation of immune cells in the dermis.
Beyond expression on their immature precursors, expression of mRNAs for H1, H2 and H4 receptors have been detected in different subtypes of mature APCs such as in 6‐sulfo LacNAc‐expressing DCs (slan DCs) and in myeloid DCs (mDCs) (Glatzer et al., 2014). Agonists of H2 and H4 receptors down‐regulated LPS‐induced expression of TNF‐α and IL‐12 in slan DCs (Gschwandtner et al., 2011b).
Macrophages
As a result of the pathogenic and tissue changes during AD, monocytes may differentiate into macrophages and respond to environmental cues with polarization into distinct functional phenotypes. In the presence of the Th2 cytokines IL‐4 or IL‐13, the M2a macrophage phenotype is induced. In human monocytes, levels of H1, H2 and H4 receptors are low but are up‐regulated during the differentiation process from monocytes to DCs or macrophages (Gutzmer et al., 2005; Glatzer et al., 2014; Capelo et al., 2016; Mommert et al., 2018b). Targeting the H1, H2 and H4 receptors on M2 macrophages affected the M2 phenotype by differentially regulating the expression of the macrophage differentiation marker CD68 or of the scavenger receptor CD163. In addition, the expression of the anaphylatoxin C3a receptor was down‐regulated via H4 receptors (Mommert et al., 2018c). The M2 phenotype is characterized by secreting specific chemokines such as CCL17 and CCL22, which are abundant in AD. In particular, CCL17 represents a sensitive biomarker for disease activity in AD (Morita et al., 2010).
The role of histamine in CCL17 and CCL22 expression in human monocytes and M2 macrophages was investigated in a recent study by Mommert et al., (2018b). Activation of monocytes or fully differentiated M2 macrophages by Th2 cytokines led to pronounced secretion of CCL17 and CCL22. Remarkably, in contrast to CCL22 expression, the IL‐4‐induced or IL‐13‐induced CCL17 mRNA and protein production was selectively potentiated by stimulating the H2 receptors but not by agonists specific for H1 or H4 receptors (Mommert et al., 2018b). By applying the H4 receptor antagonist JNJ‐7777120, Miyano et al. (2016) demonstrated that these receptors were responsible for enhanced spontaneously or peptidoglycan‐induced production of CCL17 and as opposed to our study also of CCL22 production in monocyte‐derived LCs in patients with AD.
Summing up, APCs and macrophages regularly express the mRNA for H1, H2 and H4 receptors. Histamine may have a potent function in the physiopathology of inflammatory skin diseases through a tight control of Th1/Th2 cytokine and chemokine production. On one hand, mainly Th1‐related cytokines or chemokines such as IL‐12, IL‐27 or CXCL10 (Gutzmer et al., 2005; Gschwandtner et al., 2012; Glatzer et al., 2014) are down‐regulated via H4 receptors leading to a shift into a more Th2‐dominated milieu, which may antagonize a Th1 cell‐mediated inflammation. On the other hand, histamine acts as chemoattractant via H4 receptors which may lead to an accumulation of immune cells building up the dermal infiltrate. In addition, up‐regulation of CCL17 production in M2 macrophages or CCL17 and CCL22 in LCs via H2 or H4 receptors, respectively, provides inflammatory effects.
Granulocytes
Eosinophils and basophils infiltrate the skin under pathophysiological conditions, and an increased cell number in the blood correlates with disease severity in AD (Werfel et al., 2016a). Although the specific role of both cell types remains unclear for the pathogenesis of AD, it is possible that eosinophils and basophils trigger the inflammatory response by secreting a broad spectrum of pro‐inflammatory cytokines.
It has been shown that human eosinophils express functional H4 receptors and that histamine mediates chemotaxis, calcium influx and shape change (Buckland et al., 2003; Ling, 2004; Reher et al., 2012; Thurmond, 2015). Thus, it seems that H4 receptors have a pro‐inflammatory role in regard to the activation of eosinophils. However, studies of immunological functions are still lacking to elucidate the exact contribution of H4 receptors on eosinophils in disease pathology.
Human basophils are characterized by expression of the FcεRI on their surface. We showed in a recent study that highly purified basophils express mRNAs for H1, H2 and H4 receptors but not for H3 receptors (Mommert et al., 2016a). Interestingly, H4 receptors were highly expressed and showed even higher mRNA expression levels when compared with the expression levels of the H1 and H2 receptors. Migration of basophils was induced by histamine and by a H4 receptor agonist.
Peripheral blood samples from healthy donors, patients with a history of allergic diseases and hymenoptera venom‐sensitized patients were stimulated with different histamine receptor specific agonists. We observed a significant reduction in FcεRI cross‐linking‐mediated surface expression of CD63 and CD203c on basophils and a decreased release of sulfido‐leukotrienes. Both effects were mainly mediated via H4 receptors. Although basophils migrate in the direction of histamine or H4 receptor agonists and may accumulate at the site of allergic inflammation, our data indicate a substantial role of H4 receptors in fostering an intrinsic self‐termination mechanism for IgE‐dependent basophil activation to prevent excessive activation of these cells (Mommert et al., 2016a).
Keratinocytes
mRNA expression analysis as well as immunohistochemical staining revealed that human keratinocytes express H4 receptors (Yamaura et al., 2009; Glatzer et al., 2013). We further showed that expression of mRNA for H4 receptors was more abundant in keratinocytes derived from patients with AD. Moreover, stimulation of keratinocytes with histamine via the H4 receptor induced proliferation, which was even more pronounced in keratinocytes derived from AD patients (Glatzer et al., 2013). Thus, stimulation of H4 receptors might contribute to the epidermal hyperplasia observed in AD patients. Regarding skin barrier function, the H4 receptor does not seem to play a role in the histamine‐induced inhibition of epidermal differentiation, which is more related to H1 receptors (Gschwandtner et al., 2013).
Although keratinocytes represent the outer barrier of the body, they also function as important regulatory and effector cells. Keratinocytes have the potential to secrete specific chemokines and cytokines, which play a relevant role in the initiation and perturbation of AD by attracting or stimulating different T‐cell subtypes (Werfel, 2009). One important cytokine in the pathogenesis of AD is TSLP, which triggers a type 2 inflammatory response and additionally acts on sensory neurons and thereby triggers itch (Ziegler, 2012). In a recently published study, we found that pre‐incubation with histamine prior to challenge with poly I : C resulted in a significant increase of TSLP production compared with stimulation with poly I : C alone in normal human epidermal keratinocytes. This effect was mainly mediated via H4 receptors (Schaper et al., 2016). Thus, decreasing TSLP production via blockade of H4 receptors could be one pathway for reducing a Th2 response and inhibiting scratching symptoms in AD patients. In another study, it has been shown that stimulation of H4 receptors increases IL‐8 mRNA expression in HaCaT cells, which also indicates a pro‐inflammatory effect of H4 receptors on human keratinocytes (Suwa et al., 2014).
Neurons
Pruritus that arises in the skin is mediated via exogenous and endogenous factors released by immune cells or keratinocytes. These factors induce activation of different receptors and signal cascades from periphery via dorsal root ganglia and spinal cord to the CNS (Steinhoff et al., 2006). Some studies showed that the H4 receptor was expressed in the CNS and in dorsal root ganglia of mice, rats and dogs, where this receptor also mediates functional effects (Strakhova et al., 2009; Rossbach et al., 2011; Galeotti et al., 2013; Rossbach and Baumer, 2014). This aspect is further discussed below. However, in humans, the data are limited, probably due to the difficulty in obtaining suitable material for research purposes. Strakhova et al. (2009) showed that transcripts of the H4 receptor are present in regions of the CNS, including spinal cord, hippocampus, cortex, thalamus and amygdala, with the highest levels of H4 receptor mRNA detected in the spinal cord. Connelly et al. (2009) reported that H4 receptors were prominently expressed in distinct deep laminae, particularly layer VI in the human cortex. However, the mechanism by which H4 receptors possibly mediate chronic itch in humans is still elusive and remains to be identified.
The role of H4 receptors in animal models of AD
Antagonists of H4 receptors have been studied in several mouse models of allergic dermatitis (Hirasawa et al., 2009; Rossbach et al., 2009; Cowden et al., 2010; Seike et al., 2010; Suwa et al., 2011; Kamo et al., 2014) and in a canine model of AD (Baumer et al., 2011). The H4 receptor antagonists JNJ‐7777120, JNJ‐28307474 and JNJ‐39758979 showed anti‐inflammatory properties in a mouse model of allergic dermatitis induced by the hapten FITC (Cowden et al., 2010), via reducing inflammation by down‐regulating the number of skin eosinophils and mast cells as well as the expression of several cytokines. In contrast, JNJ‐7777120 did not reduce the allergic inflammation induced by the haptens dinitrochlorobenzene (DNCB) and toluene diisocyanate (TDI) (Rossbach et al., 2009). It has to be taken into consideration that the models represent either an acute or a chronic inflammatory response with differences in the cell types involved. One characteristic of the FITC model is a Th2‐dominated response and a distinct eosinophilia, which is less pronounced in other models of hapten‐induced contact dermatitis such as the DNCB or TDI model. One possible explanation for the differing results could be that H4 receptors are more involved in Th2‐polarized inflammation. In line with this, in another hapten‐induced allergic dermatitis model, the trinitrochlorobenzene (TNCB) model, JNJ‐7777120 was only effective in reducing the extent of chronic lesions provoked by repeated application of the allergen to the skin of the back but failed to inhibit ear‐swelling induced by single epicutaneous challenge of TNCB to the ear (Seike et al., 2010). The attenuation of the chronic lesions was accompanied by a diminished mast cell and eosinophilic infiltration, which again suggests a relevant role for these cell populations in disease pathology (Seike et al., 2010). In a picryl chloride‐induced model of chronic allergic dermatitis established in NC/Nga mice, JNJ‐7777120 reduced skin lesions and inhibited the production of Th2 cytokines at lesional skin sites (Ohsawa and Hirasawa, 2012).
Antagonists at H4 receptors have also been tested in mouse models that mimic more aspects of human AD than the hapten‐induced models such as the Dermatophagoides farinae body allergen‐induced model of chronic allergic dermatitis in NC/Nga mice and in the ovalbumin (OVA) model (Kamo et al., 2014; Rossbach et al., 2016 ; Kochling et al., 2017). Dermatitis induced by Dermatophagoides farinae body ointment in NC/Nga mice was clearly not ameliorated either by JNJ‐7777120 or by JNJ‐28307474 (Kamo et al., 2014). In line with this, in the OVA model, neither the H4 receptor antagonist JNJ‐39758979 nor JNJ‐28307474 improved dermatitis severity (Rossbach et al., 2016; Kochling et al., 2017). In contrast, in H4 receptor−/− mice, OVA‐induced skin lesions were clearly diminished (Rossbach et al., 2016). One reason for the inconsistent results might be an insufficient concentration of the drug in the skin. Thus, it has to be examined whether additional topical treatment could improve skin symptoms. Notably, the anti‐inflammatory effect could at least partially be mimicked by JNJ‐28307474, only when this H4 receptor antagonist was given during sensitization and provocation phase of the allergic reaction (Rossbach et al., 2016). This finding indicates that it is necessary to block H4 receptors during initiation of the allergic inflammation, which is an important point to clarify, because pharmacological interventions usually occur after the establishment of the disease.
Interestingly, the combination of H1 receptor antagonists with H4 receptor antagonists provided synergistic anti‐inflammatory action in the OVA model as well as in NC/Nga mice (Ohsawa and Hirasawa, 2012; Kochling et al., 2017). In addition, Mahapatra et al. (2014) revealed that local cytokine responses in skin‐draining lymph nodes were only reduced by the combined application of H1 and H4 receptor antagonists. Such combinations of H1 and H4 receptor antagonists may provide a better option for the treatment of AD than H4 or H1 receptor antagonists alone. Corresponding with the results in allergen‐induced inflammation, inhibition of allergen‐induced pruritus was greater when both H1 and H4 receptors were blocked (Rossbach et al., 2009; Ohsawa and Hirasawa, 2012).
In a dog model of AD, preventive administration of JNJ‐7777120 and JNJ‐28307474 did not affect the development of acute skin lesions (Baumer et al., 2011). In this context, it has to be considered that H4 receptor homology among different species is the lowest among the histamine receptor family, and also, binding and functional activity of numerous H4 receptor agonists and antagonists clearly vary between different species (Liu et al., 2001b). Consequently, animal studies with the focus on H4 receptor expression and function must be interpreted with care.
In contrast to the heterogeneous results regarding the anti‐inflammatory effects of H4 receptor antagonists in the different mouse models, the antipruritic effects are very homogenous between studies. Several studies in rodents reported a reduction in scratching symptoms via H4 receptors in either histamine‐induced or allergen‐induced itch (Dunford et al., 2007; Rossbach et al., 2009; Cowden et al., 2010; Rossbach et al., 2011; Ohsawa and Hirasawa, 2012).
H4 receptors and SNPs mutations in AD
Genetic variations in disease‐specific target genes and associations between these genetic factors and skin disorders such as AD, psoriasis or lupus erythematosus had been postulated and described in the past.
Based on the genetic background of inflammatory skin diseases and the complex picture of immunomodulatory activities of H4 receptors, single nucleotide polymorphisms (SNPs) or copy number variations had been genotyped in a Chinese population suffering from AD or lupus erythematosus respectively (Yu et al., 2010a,b; Chen et al., 2013). These investigations gave first hints that genetic variations within the H4 receptor gene may play a role in the pathophysiology of skin diseases. Polymorphisms and copy number variations within the H4 receptor gene were found to be associated with AD (Yu et al., 2010b; Chen et al., 2013). Amplifications of copy number variations of this gene were also found to increase the risk of lupus erythematosus (Yu et al., 2010a). Micallef et al. (2013) has nicely reviewed the data of genetic variations and polymorphisms within the genes of the four histamine receptors and their associations to inflammatory diseases of the CNS and cancer.
H4 receptors and clinical studies in AD
Due to the dual function of H4 receptor blockade (direct reduction of itch and inhibition of inflammatory response), H4 receptors may represent a promising candidate for the treatment of AD in humans. Until now, only few reports of clinical data exist regarding H4 receptor antagonists. The first compound used in clinical studies was JNJ‐39758979, which could reduce histamine‐induced scratching after 2 and 6 h. These results confirmed data from mice studies and demonstrated that H4 receptors are involved in mediating pruritic symptoms in neurons (Thurmond, 2015). In a phase 2 clinical trial, this compound was tested in adult Japanese patients with moderate AD over a period of 6 weeks. Because of two cases of agranulocytosis, most likely related to reactive metabolites and not to H4 receptor antagonism, the study had to be discontinued prematurely (Murata et al., 2015). However, despite low patient numbers, analysis of the data revealed a significant reduction in pruritus in patients treated with JNJ‐39758979 compared with placebo group, as well as an improvement of eczema (Murata et al., 2015). In another randomized, placebo‐controlled phase 2a study, the selective H4 receptor antagonist ZPL‐3893787 was tested in males and females with moderate‐to‐severe AD and administered p.o. over a period of 8 weeks (Werfel et al., 2018). Overall, the compound was well tolerated, and adverse events reported on ZPL‐3893787 and placebo groups were comparable. After treatment with ZPL‐3893787, patients displayed a 50% reduction in Eczema Area and Severity Index score (vs. placebo group 27%) and a reduction of 41% in the score of AD (SCORAD) (vs. placebo group reduction of 26%). The effect on pruritus was similar between ZPL‐3893787 and placebo and not statistically significant. However, the pruritus score obtained from the SCORAD revealed clear improvement for the ZPL‐3893787 group compared with the placebo group (Werfel et al., 2018). Based on the two latter studies, H4 receptor antagonists showed clinically significant antipruritic and anti‐inflammatory effects in AD patients. Further clinical studies with larger sample sizes are needed to clearly characterize the risk/benefit ratio and to additionally characterize groups of AD patients, who may particularly benefit from H4 receptor antagonist and who may not. In a recently published phase 2a study with JNJ‐39758979 in adults with uncontrolled asthma, the H4 receptor antagonist did not meet the primary endpoint. However, significant improvements in pre‐bronchodilator FEV1 were observed with JNJ‐39758979 versus placebo at week 12 in pre‐specified subgroups, which displayed elevated sputum eosinophils or blood eosinophils at baseline (Kollmeier et al., 2018). Thus, it seems that the H4 receptor antagonist was specifically effective in asthma patients with an eosinophilic inflammation. Whether there are similar subgroups in AD patients warrants further investigations.
The role of H4 receptors in psoriasis
Disease pattern
Psoriasis is a multifactorial chronic skin disease, affecting approximately 3% of the Western population. Genetic, environmental and behavioural factors are supposed to play a role in the pathogenesis and course of the disease. However, the exact aetiology of psoriasis remains unclear (Lowes et al., 2008, 2014).
Plaque psoriasis (psoriasis vulgaris) is the most common form of the disease and usually presents as symmetrical erythematous papules or plaques covered with thick silvery scales. The histological characteristics of psoriasis are the marked thickening of the epidermis due to hyper‐proliferative keratinocytes and the elongated rete ridges. Other important histological features of psoriasis include a collection of neutrophils termed Munro microabscess, which is located in the stratum granulosum (Nograles et al., 2009; Lowes et al., 2014). In normal healthy human skin, relevant amounts of T‐cells are present. These skin‐resident T‐cells composed primarily of T effector memory cells are mainly Th1 biased and are able to respond to stimulation (Clark, 2010). Besides T‐cells, a population of dermal DCs such as mDCs, macrophages and in small numbers plasmacytoid DCs (pDCs) reside in the dermis and have the capacity to take up antigens. Respective cells mature during migration to draining lymph nodes and present these antigens to T‐cells and B‐cells (Zaba et al., 2009). In psoriasis, IL‐23 is overproduced mainly by DCs and stimulates survival and proliferation of Th17 cells. A critical function of Th17 cells in psoriasis rather than of Th1 cells had been accepted and led to reclassify psoriasis as a more Th17‐driven disease. Th17 cells are implicated in the pathogenesis of psoriasis by producing high amounts of IL‐17A and IL‐22 (Boutet et al., 2018).
Importantly, mast cells also reside, preferentially located in the upper dermis of psoriatic lesions, and levels of the anaphylatoxins C3a and C5a were detected in scales of psoriatic lesions (el‐Lati et al., 1994; Mashiko et al., 2015). Histamine could be released by anaphylatoxins acting via their cognate receptors, which are expressed on mast cells (Giang et al., 2018). The study of Krogstad et al. detected increased histamine levels in lesional skin when compared with uninvolved skin of psoriasis patients by microdialysis technique and demonstrated a detailed and direct proof that histamine is present in enhanced concentrations in psoriatic skin and may contribute to the inflammation (Krogstad et al., 1997). Later studies gave more indirect hints that histamine is present in psoriatic skin. For instance, it has been shown in a recently published study that antihistamines (clemastine and levocetirizine) targeting H1 receptors had a moderate effect in reducing itch in patients with psoriasis (Domagała et al., 2017). In the following sections, we will discuss the expression levels of the histamine receptors, in particular of H4 receptors, on cell populations that are present in human skin under both steady‐state and inflammatory conditions. A schematic overview is presented in Figure 2. The function of histamine, with an emphasis on H4 receptors, will be discussed regarding its potential role in contributing to the dramatic increase of the individual cell numbers in the dermal infiltrate by inducing migration and phenotypic changes of these cells in regard to the expression of characteristic markers or release of cytokines and chemokines that occurs in the initiation phase of psoriasis.
Figure 2.
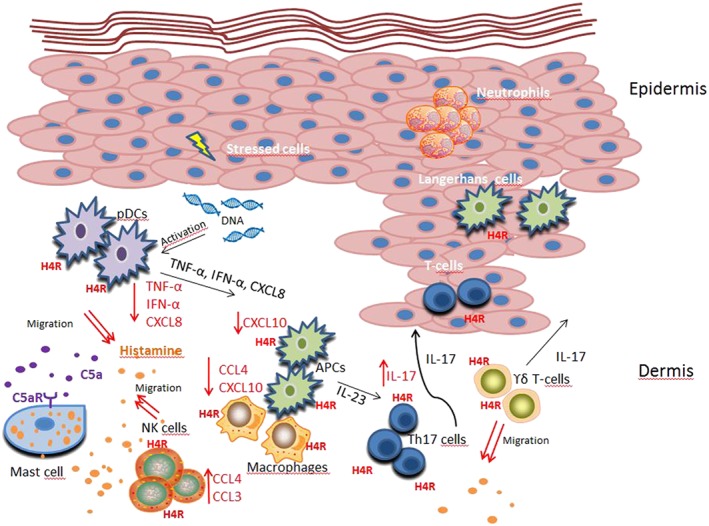
The role of histamine H4 receptors on human cells relevant to psoriasis. H4 receptors (H4R) are expressed on keratinocytes and different immune cells, which play a role in psoriasis. Triggering the complement component 5a receptor (C5aR) on mast cells leads to release of histamine. Histamine acts via H4 receptors on Th17 cells to release IL‐17 and promotes NK cells to produce CCL3 and CCL4. Stressed keratinocytes release self‐DNA, which in turn activates pDCs to produce IFN‐α. The stimulation of H4 receptors on pDCs suppresses the secretion of the TNF‐α, IFN‐α and CXCL8. Histamine reduces, via H4 receptors, the release of the chemokines CXCL10 or CXCL10 and CCL4 in APCs and macrophages respectively. Histamine itself induces chemotaxis via H4 receptors of γδ T‐cells, NK cells and of pDCs. The anti‐inflammatory effects of the H4 receptors seem to be more pronounced when compared with the inflammatory effects of the receptor in the pathophysiology of psoriasis. Thus, H4 receptor agonists, rather than H4 receptor antagonists, may prevent amplification of the symptoms. The effects mediated by H4 receptors are highlighted in red.
The role of H4 receptors on human cells relevant in psoriasis
T‐cells
Besides Th1 cells and IFN‐γ that have been historically described as main drivers of psoriasis, the IL‐23/IL‐17 axis and IL‐17 are now known to play a pivotal role in psoriasis and psoriasis arthritis. The main producers of IL‐17, in response to different combinations of cytokines, are Th17 cells. Beyond Th17 cells, other immune cells such as γδ T‐cells or NK cells are able to synthesize IL‐17. Th17 cells are detected in psoriatic skin lesions (Al‐Mossawi et al., 2013). IL‐17 levels are elevated in the serum of psoriasis patients. The results of clinical trials targeting IL‐17 and its receptor with biological agents reinforce the proposition that the IL‐17 pathway is an essential target in the pathogenesis of psoriasis (Lowes et al., 2008; Kirkham et al., 2014; Boutet et al., 2018).
We detected expression of mRNA for H1, H2 and H4 receptors in CD4+ T‐cells, which were polarized to Th17 cells in the presence of IL‐1β and IL‐23. Importantly, H4 receptors could be detected in situ on IL‐17‐positive cells in psoriatic skin. Stimulation of the histamine receptors on these polarized Th17 cells with histamine and specific agonists revealed an up‐regulation of IL‐17 mRNA expression and secretion of IL‐17 protein from respective cells mediated via H4 receptors. Elevated histamine levels in psoriatic skin may target H4 receptors on T‐cells leading to an increase of IL‐17, which may exacerbate the inflammatory process (Mommert et al., 2012). This scenario is further supported by a study from Wakahara et al. (2012) who showed that in the presence of IL‐3, basophil‐derived histamine augments the IL‐17 production in human memory T‐cells. Treatment of the basophil memory T‐cell cocultures with different histamine receptor antagonists revealed that blockers of H2 and H4 receptors significantly suppressed IL‐17 production (Wakahara et al., 2012). Future research will figure out whether the promising H4 receptor antagonists, which have been shown to improve the symptoms of AD (Werfel et al., 2018), would be beneficial to support the efficacy of biological agents targeting IL‐17 in psoriasis.
γδ T‐cells are unconventional T‐cells comprising a relatively small subset of T‐cells in peripheral blood. They are defined by expression of heterodimeric T‐cell receptors composed of γ and δ chains. Expression of H1, H2 and H4 receptors in γδ T‐cells isolated from human peripheral blood were detected at mRNA level by PCR and at protein level by Western blot analysis (Truta‐Feles et al., 2010). In these cells, histamine induced actin polymerization, intracellular Ca2+ mobilization and chemotaxis, through Pertussis toxin‐sensitive Gi proteins via H4 receptors (Truta‐Feles et al., 2010).
Antigen‐presenting cells
Plasmacytoid dendritic cells
pDCs that are characterized by plasma cell‐like shape and specific surface markers play an essential role in psoriasis, as increased serum levels of pDC‐derived cytokines correlate with disease severity (Gschwandtner et al., 2011a). pDCs obtained from blood of psoriasis patients expressed higher levels of H4 receptors when compared with pDCs from AD patients or to pDCs from healthy controls. Agonists at H4 receptors induced migration of pDCs (Gschwandtner et al., 2011a).
Stimulation of H2 and H4 receptors resulted in a down‐regulation of CpG dinucleotide‐induced production of TNF‐α, IFN‐α and CXCL8 but not of the chemokine CXCL10 in human pDCs. Importantly, the histamine‐induced down‐regulation of these cytokine or chemokine productions was more pronounced in pDCs derived from psoriasis patients, compared with cells from healthy controls (Gschwandtner et al., 2011a).
Notably, CXCL10 mRNA and protein expressions were affected by H2 or H4 receptors in human monocytes and mDCs. Agonists of these two receptors caused a significant decrease in poly I : C‐induced expression of CXCL10 in monocytes and mDCs (Glatzer et al., 2014).
Macrophages
Macrophages accumulate in the dermis in acute or chronic inflammatory skin diseases, such as AD or psoriasis, and play a central role in regulating local inflammation by secreting many subtype specific mediators and cytokines (Biswas and Mantovani, 2010).
Expression of mRNA for H1, H2 and H4 receptors was up‐regulated in the presence of GM‐CSF during the differentiation process of monocyte‐derived human M1 macrophages. During the differentiation process of M1 macrophages, CXCL10 expression was down‐regulated in response to histamine or to a H4 receptor agonist. In fully differentiated M1 macrophages, the IFN‐γ‐ and LPS‐induced mRNA and protein expression of the Th1‐related chemokine CCL4 was decreased via H4 receptors (Mommert et al., 2018c).
NK cells
NK cells are a specialized subset of CD56+CD16+ cells with the ability to kill cancer and virally infected cells in a non‐MHC‐dependent manner. NK cells are divided into two groups depending on the relative expression levels of the NK cell marker CD56 in low (CD56 dim) or high (CD56 bright) density. In psoriatic skin, CD56 bright NK cells, which represent the more immune‐regulatory cells of both subtypes, were detected in the mid‐dermis as part of the cellular infiltrate (Ottaviani et al., 2006). NK cells may be involved in psoriasis by releasing cytokines such as IFN‐γ, TNF‐α or IL‐22 (Jacobs et al., 2001).
The expression of H1 and H4 receptors was detected in permeabilized IL‐2‐activated NK cells by flow cytometry using rabbit antihistamine receptor antibodies. Histamine induced chemotaxis of NK cells that was blocked by pre‐incubation with a H3/H4 receptor antagonist (Damaj et al., 2007). In a more recently published study, we detected the expression of H1 and H4 receptor mRNA in purified NK cells and, in contrast to results from Damaj et al., the expression of mRNA for H2 receptors was also detectable. The expression of mRNA for H3 receptors was not detected (Mommert et al., 2015).
A comprehensive microarray‐based mRNA expression profiling revealed only few genes to be differentially regulated comparing H4 receptor‐stimulated versus non‐stimulated human NK cells. Among them, the mRNA of TNF‐α, CCL3, CCL4 and CCL3L3 showed slightly increased expression levels upon stimulation via H4 receptors. Follow‐up studies confirmed a significant up‐regulation of CCL3 and CCL4 (Mommert et al., 2015). The enhanced production of these chemokines via H4 receptors may contribute to migration and accumulation of various immune cells into the dermal infiltrate.
The role of H4 receptors in murine models of psoriasis
Apart from several human in vitro studies, which focused on the effect of H4 receptors in various cell types relevant for the pathogenesis of psoriasis, only one in vivo mouse study exists until now. In this study, the imiquimod‐induced skin inflammation model, first described by van der Fits et al. (2009), was applied. In this widely used murine model of preclinical studies of psoriasis, daily application of Aldara‐creme® onto the skin leads to an inflammation, which mimics several aspects of human psoriasis. Kim et al. (2016) demonstrated that the H4 receptor agonist 4‐methylhistamine (4MH) (20 to 40 mg·kg−1) significantly attenuated the psoriatic characteristics, including epidermal, hyperplasia, hyperkeratosis and lymphocyte infiltration. Furthermore, the number of CD4+CD25+Foxp3+ regulatory T‐cells (Treg) was significantly increased by treatment with 4MH (40 mg·kg−1). However, when interpreting the data, it should be taken into account that 4MH shows considerable in vivo agonist activity at H2 receptors, at doses >3 mg·kg−1 i.v. in rodents (Lim et al., 2009). Thus, the high doses of at least 20 mg·kg−1·day−1 4MH for 10 consecutive days applied by Kim et al. may have had agonist action at H2, rather than at H4 receptors.
More studies with specific histamine receptor ligands are needed to really distinguish between effects mediated via H2 or H4 receptors.
H4 receptors and SNPs mutations in psoriasis
Here, we summarize our recently published data about genetic variations within the promotor region of the human H4 receptor gene in psoriasis patients. Three SNPs in the promotor region and one SNP located in an intron of the H4 receptor gene were analysed by PCR and pyrophosphate DNA sequencing in patients diagnosed with chronic psoriasis and healthy controls (Mommert et al., 2016b).
The genotype distributions and allele frequencies of the four SNPs in the H4 receptor gene did not show obvious differences between the whole group of psoriasis patients and healthy controls. However, we found differences by trend in subgroup analysis: we detected that mutant genotypes of two SNPs located within the promoter region, rs17203314 and rs615283, were more frequent in patients with severe psoriasis according to the Psoriasis Area Severity Index when compared with the control groups. A significant association of rs615283 with psoriasis palmoplantaris, a severe form of psoriasis, was detected. To sum up, our study revealed possible associations of variations in the H4 receptor gene between severe psoriasis, subtypes of psoriasis and special clinical features of psoriasis in relationship to the control groups. Further studies are needed to confirm these results with larger sample sizes (Mommert et al., 2016b).
H4 receptors and clinical studies in psoriasis
In contrast to the H4 receptor antagonists JNJ‐39758979 and ZPL‐3893787, which have been used in clinical trials in the field of AD, another H4 receptor antagonist, toreforant (JNJ‐38518168), has completed efficacy studies in psoriasis and rheumatoid arthritis. The phase 2 testing in the rheumatoid arthritis patients was terminated prematurely because of patient fatality and secondary haemophagocytic lymphohistiocytosis (NCT00941707). However, post hoc analysis showed no significant improvement with toreforant. In the phase 2 study for the treatment of subjects with moderate‐to‐severe psoriasis (NCT02295865), toreforant was generally safe and well tolerated, but the study did not meet predefined success criteria (Frankel et al., 2018).
Summary
A range of in vitro studies in human cells as well as in mouse models, have pointed to a relevant role of H4 receptors in the pathogenesis of inflammatory skin diseases. However, it is worth noting that there are contradictory results regarding the anti‐inflammatory and pro‐inflammatory function of H4 receptors in different cell types.
In regard to the disease pathology of AD, studies in T‐cells (up‐regulation of IL‐31), keratinocytes (up‐regulation of TSLP and increased proliferation) and AD mouse models (ameliorated symptoms in H4 receptor−/− mice and via blocking H4 receptors in Th2‐related models) clearly show a more pro‐inflammatory role of H4 receptors. In contrast, the results in APCs (down‐regulation of IL‐12, IL‐27 and CCL2) and basophils (down‐regulation of mediator release) point to a more anti‐inflammatory profile. However, the decrease of the latter cytokines results in an impaired Th1‐polarization and consequently leads to a shift towards a Th2‐driven immune response. This shift towards a Th2‐dominated response in conjunction with the up‐regulation of Th2 cytokines in turn can lead to an exacerbation of the symptoms seen in AD. Thus, even if H4 receptors are able to mediate the down‐regulation of some pro‐inflammatory cytokines, the Th2‐driven effects mediated via these receptors seem to dominate the immune response in AD. Consequently, blocking H4 receptors represents a promising treatment option in AD, and first positive results in clinical trials with H4 receptor antagonists in patients with AD strengthen this hypothesis.
As opposed to AD, in vitro studies on human immune cells, which play a role in psoriasis, provide more conflicting results to predict a successful treatment of the disease with H4 receptor antagonists. On one side, the enhanced production of IL‐17 and histamine‐induced migration of immune cells may foster the inflammation, and on the other side, down‐regulation of pro‐inflammatory cytokines or chemokines most pronounced in pDCs from psoriasis patients may control the inflammation. Psoriasis is mainly a Th1‐/Th17‐mediated disease. Because cytokines or chemokines such as IL‐12, IL‐27, TNF‐α, IFN‐α and CXCL10 or CXCL8 provide the local environment for polarizing Th1 or Th17 cells, down‐regulation of these mediators via H4 receptors is more in focus in psoriasis when compared with AD. Thus, in psoriasis, H4 receptor agonists may prevent the amplification of the symptoms, rather than H4 receptor antagonists. In line with this, an initial clinical study of an H4 receptor antagonist in psoriasis did not meet its endpoints.
Apart from AD and psoriasis, also other skin diseases accompanied by inflammation and pruritus may potentially be regulated by blockers of H4 receptors. For example, urticaria and prurigo are skin diseases characterized by intense itch. As H4 receptor antagonists display constant results in inhibiting pruritus in murine models, these histamine receptors may also be interesting therapeutic targets in patients suffering from urticaria or prurigo.
However, while further clinical trials are planned, more research is still needed to better understand H4 receptor regulation and immunomodulatory functions.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a, 2017b, 2017c).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgement
This study was supported by the Deutsche Forschungsgemeinschaft (DFG; Gu 434/6‐2).
Schaper‐Gerhardt K., Rossbach K., Nikolouli E., Werfel T., Gutzmer R., and Mommert S. (2020) The role of the histamine H4 receptor in atopic dermatitis and psoriasis, British Journal of Pharmacology, 177, 490–502, doi: 10.1111/bph.14550.
References
- Al‐Mossawi MH, Ridley A, Kiedel S, Bowness P (2013). The role of natural killer cells, gamma delta T‐cells and other innate immune cells in spondyloarthritis. Curr Opin Rheumatol 25: 434–439. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–s129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017b). The Concise Guide To PHARMACOLOGY 2017/18: Other proteins. Br J Pharmacol 174: S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrang JM, Garbarg M, Schwartz JC (1983). Auto‐inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature 302: 832–837. [DOI] [PubMed] [Google Scholar]
- Baumann CR (2017). Wide implications of a trial on pitolisant for cataplexy. Lancet Neurol 16: 173–174. [DOI] [PubMed] [Google Scholar]
- Baumer W, Stahl J, Sander K, Petersen LJ, Paps J, Stark H et al (2011). Lack of preventing effect of systemically and topically administered histamine H1 or H4 receptor antagonists in a dog model of acute atopic dermatitis. Exp Dermatol 20: 577–581. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Mantovani A (2010). Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11: 889–896. [DOI] [PubMed] [Google Scholar]
- Black JW, Duncan WA, Durant CJ, Ganellin CR, Parsons EM (1972). Definition and antagonism of histamine H2‐receptors. Nature 236: 385–390. [DOI] [PubMed] [Google Scholar]
- Boutet MA, Nerviani A, Gallo Afflitto G, Pitzalis C (2018). Role of the IL‐23/IL‐17 axis in psoriasis and psoriatic arthritis: the clinical importance of its divergence in skin and joints. Int J Mol Sci 19: 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovet D, Staub AM (1937). Action protectrice des éthers phénoliques au cours de l'intoxication histaminique. Comptes rendus des séances de la Société de Biologie et de ses Filiales (Paris) 124: 547–549. [Google Scholar]
- Buckland KF, Williams TJ, Conroy DM (2003). Histamine induces cytoskeletal changes in human eosinophils via the H4 receptor. Br J Pharmacol 140: 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelo R, Lehmann C, Ahmad K, Snodgrass R, Diehl O, Ringleb J et al (2016). Cellular analysis of the histamine H4 receptor in human myeloid cells. Biochem Pharmacol 103: 74–84. [DOI] [PubMed] [Google Scholar]
- Chen B, Ye T, Shao Y, Zhang J, Zhong Q, Hu X et al (2013). Association between copy‐number variations of the human histamine H4 receptor gene and atopic dermatitis in a Chinese population. Clin Exp Dermatol 38: 295–300 quiz 300–1. [DOI] [PubMed] [Google Scholar]
- Clark RA (2010). Skin‐resident T cells: the ups and downs of on site immunity. J Invest Dermatol 130: 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly WM, Shenton FC, Lethbridge N, Leurs R, Waldvogel HJ, Faull RL et al (2009). The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. Br J Pharmacol 157: 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowden JM, Zhang M, Dunford PJ, Thurmond RL (2010). The histamine H4 receptor mediates inflammation and pruritus in Th2‐dependent dermal inflammation. J Invest Dermatol 130: 1023–1033. [DOI] [PubMed] [Google Scholar]
- Damaj BB, Becerra CB, Esber HJ, Wen Y, Maghazachi AA (2007). Functional expression of H4 histamine receptor in human natural killer cells, monocytes, and dendritic cells. J Immunol 179: 7907–7915. [DOI] [PubMed] [Google Scholar]
- Dijkstra D, Leurs R, Chazot P, Shenton FC, Stark H, Werfel T et al (2007). Histamine downregulates monocyte CCL2 production through the histamine H4 receptor. J Allergy Clin Immunol 120: 300–307. [DOI] [PubMed] [Google Scholar]
- Dijkstra D, Stark H, Chazot PL, Shenton FC, Leurs R, Werfel T et al (2008). Human inflammatory dendritic epidermal cells express a functional histamine H4 receptor. J Invest Dermatol 128: 1696–1703. [DOI] [PubMed] [Google Scholar]
- Domagała A, Szepietowski J, Reich A (2017). Antihistamines in the treatment of pruritus in psoriasis. Postepy Dermatol Alergol 34: 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunford PJ, Williams KN, Desai PJ, Karlsson L, McQueen D, Thurmond RL (2007). Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J Allergy Clin Immunol 119: 176–183. [DOI] [PubMed] [Google Scholar]
- Frankel E, Song M, Li S, Jiang J, Thurmond RL, Randazzo B (2018). Efficacy and safety of toreforant, a selective histamine H4 receptor antagonist, for the treatment of moderate‐to‐severe plaque psoriasis: results from a phase 2 multicenter, randomized, double‐blind, placebo‐controlled trial. J Drugs Dermatol 17: 873–879. [PubMed] [Google Scholar]
- el‐Lati SG, Dahinden CA, Church MK (1994). Complement peptides C3a‐ and C5a‐induced mediator release from dissociated human skin mast cells. J Invest Dermatol 102: 803–806. [DOI] [PubMed] [Google Scholar]
- Galeotti N, Sanna MD, Ghelardini C (2013). Pleiotropic effect of histamine H4 receptor modulation in the central nervous system. Neuropharmacology 71: 141–147. [DOI] [PubMed] [Google Scholar]
- Giang J, Seelen MAJ, van Doorn MBA, Rissmann R, Prens EP, Damman J (2018). Complement activation in inflammatory skin diseases. Front Immunol 16: 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzer F, Gschwandtner M, Ehling S, Rossbach K, Janik K, Klos A et al (2013). Histamine induces proliferation in keratinocytes from patients with atopic dermatitis through the histamine 4 receptor. J Allergy Clin Immunol 132: 1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzer F, Mommert S, Kother B, Gschwandtner M, Stark H, Werfel T et al (2014). Histamine downregulates the Th1‐associated chemokine IP‐10 in monocytes and myeloid dendritic cells. Int Arch Allergy Immunol 163: 11–19. [DOI] [PubMed] [Google Scholar]
- Gschwandtner M, Bunk H, Kother B, Thurmond RL, Kietzmann M, Werfel T et al (2012). Histamine down‐regulates IL‐27 production in antigen‐presenting cells. J Leukoc Biol 92: 21–29. [DOI] [PubMed] [Google Scholar]
- Gschwandtner M, Mildner M, Mlitz V, Gruber F, Eckhart L, Werfel T et al (2013). Histamine suppresses epidermal keratinocyte differentiation and impairs skin barrier function in a human skin model. Allergy 68: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwandtner M, Mommert S, Kother B, Werfel T, Gutzmer R (2011a). The histamine H4 receptor is highly expressed on plasmacytoid dendritic cells in psoriasis and histamine regulates their cytokine production and migration. J Invest Dermatol 131: 1668–1676. [DOI] [PubMed] [Google Scholar]
- Gschwandtner M, Rossbach K, Dijkstra D, Baumer W, Kietzmann M, Stark H et al (2010). Murine and human Langerhans cells express a functional histamine H4 receptor: modulation of cell migration and function. Allergy 65: 840–849. [DOI] [PubMed] [Google Scholar]
- Gschwandtner M, Schakel K, Werfel T, Gutzmer R (2011b). Histamine H4 receptor activation on human slan‐dendritic cells down‐regulates their pro‐inflammatory capacity. Immunology 132: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzmer R, Diestel C, Mommert S, Kother B, Stark H, Wittmann M et al (2005). Histamine H4 receptor stimulation suppresses IL‐12p70 production and mediates chemotaxis in human monocyte‐derived dendritic cells. J Immunol 174: 5224–5232. [DOI] [PubMed] [Google Scholar]
- Gutzmer R, Gschwandtner M, Rossbach K, Mommert S, Werfel T, Kietzmann M et al (2011). Pathogenetic and therapeutic implications of the histamine H4 receptor in inflammatory skin diseases and pruritus. Front Biosci (Schol Ed) 3: 985–994. [DOI] [PubMed] [Google Scholar]
- Gutzmer R, Mommert S, Gschwandtner M, Zwingmann K, Stark H, Werfel T (2009). The histamine H4 receptor is functionally expressed on TH2 cells. J Allergy Clin Immunol 123: 619–625. [DOI] [PubMed] [Google Scholar]
- Hamid Q, Boguniewicz M, Leung DY (1994). Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest 94: 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa N, Ohsawa Y, Katoh G, Shibata K, Ishihara K, Seyama T et al (2009). Modification of the picryl chloride‐induced allergic dermatitis model in mouse ear lobes by 12‐O‐tetradecanoylphorbol 13‐acetate, and analysis of the role of histamine in the modified model. Int Arch Allergy Immunol 148: 279–288. [DOI] [PubMed] [Google Scholar]
- Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G et al (2001). CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol 31: 3121–3127. [DOI] [PubMed] [Google Scholar]
- Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J et al (2001). Histamine regulates T‐cell and antibody responses by differential expression of H1 and H2 receptors. Nature 413: 420–425. [DOI] [PubMed] [Google Scholar]
- Kamo A, Negi O, Tengara S, Kamata Y, Noguchi A, Ogawa H et al (2014). Histamine H4 receptor antagonists ineffective against itch and skin inflammation in atopic dermatitis mouse model. J Invest Dermatol 134: 546–548. [DOI] [PubMed] [Google Scholar]
- Kim CH, Lee JM, Yoo JK, Kim JS, Kim SU, Chang KT et al (2016). Inhibitory effect of imiquimod‐induced psoriasis‐like skin inflammation in mice by histamine H4 receptor agonist 4‐methylhistamine. Scand J Immunol 83: 409–417. [DOI] [PubMed] [Google Scholar]
- Kirkham BW, Kavanaugh A, Reich K (2014). Interleukin‐17A: a unique pathway in immune‐mediated diseases: psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology 141: 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K, Kim HJ, Ho PS, Lee SO, Lee JE, Min CR et al (2018). Discovery of a novel highly selective histamine H4 receptor antagonist for the treatment of atopic dermatitis. J Med Chem 61: 2949–2961. [DOI] [PubMed] [Google Scholar]
- Kochling H, Schaper K, Wilzopolski J, Gutzmer R, Werfel T, Baumer W et al (2017). Combined treatment with H1 and H4 receptor antagonists reduces inflammation in a mouse model of atopic dermatitis. J Dermatol Sci 87: 130–137. [DOI] [PubMed] [Google Scholar]
- Kollmeier AP, Greenspan A, Xu XL, Silkoff PE, Barnathan E, Loza MJ et al (2018). Phase 2a, randomized, double‐blind, placebo‐controlled, multicenter, parallel‐group study of an H4R‐antagonist (JNJ‐39758979) in adults with uncontrolled asthma. Clin Exp Allergy 48: 957–969. 10.1111/cea.13154. [DOI] [PubMed] [Google Scholar]
- Krogstad AL, Lonnroth P, Larson G, Wallin BG (1997). Increased interstitial histamine concentration in the psoriatic plaque. J Invest Dermatol 109: 632–635. [DOI] [PubMed] [Google Scholar]
- Lim HD, Adami M, Guaita E, Werfel T, Smits RA, de Esch IJ et al (2009). Pharmacological characterization of the new histamine H4 receptor agonist VUF 8430. Br J Pharmacol 157: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling P (2004). Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation. Br. J. Pharmacol. 142: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Ma X, Jiang X, Wilson SJ, Hofstra CL, Blevitt J et al (2001a). Cloning and pharmacological characterization of a fourth histamine receptor (H4) expressed in bone marrow. Mol Pharmacol 59: 420–426. [DOI] [PubMed] [Google Scholar]
- Liu C, Wilson SJ, Kuei C, Lovenberg TW (2001b). Comparison of human, mouse, rat, and guinea pig histamine H4 receptors reveals substantial pharmacological species variation. J Pharmacol Exp Ther 299: 121–130. [PubMed] [Google Scholar]
- Lowes MA, Kikuchi T, Fuentes‐Duculan J, Cardinale I, Zaba LC, Haider AS et al (2008). Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol 128: 1207–1211. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Suarez‐Farinas M, Krueger JG (2014). Immunology of psoriasis. Annu Rev Immunol 32: 227–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra S, Albrecht M, Behrens B, Jirmo A, Behrens G, Hartwig C et al (2014). Delineating the role of histamine‐1‐ and ‐4‐receptors in a mouse model of Th2‐dependent antigen‐specific skin inflammation. PLoS One 9: e87296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiko S, Bouguermouh S, Rubio M, Baba N, Bissonnette R, Sarfati M (2015). Human mast cells are major IL‐22 producers in patients with psoriasis and atopic dermatitis. J Allergy Clin Immunol 136: 351–9.e1. [DOI] [PubMed] [Google Scholar]
- Micallef S, Stark H, Sasse A (2013). Polymorphisms and genetic linkage of histamine receptors. Life Sci 93: 487–494. [DOI] [PubMed] [Google Scholar]
- Miyano K, Matsushita S, Tsuchida T, Nakamura K (2016). Inhibitory effect of a histamine 4 receptor antagonist on CCL17 and CCL22 production by monocyte‐derived Langerhans cells in patients with atopic dermatitis. J Dermatol 43: 1023–1029. [DOI] [PubMed] [Google Scholar]
- Mommert S, Aslan D, Ratz L, Stark H, Gutzmer R, Werfel T (2018a). The anaphylatoxin C3a receptor expression on human M2 macrophages is down‐regulated by stimulating the histamine H4 receptor and the IL‐4 receptor. J Innate Immun 10: 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommert S, Dittrich‐Breiholz O, Stark H, Gutzmer R, Werfel T (2015). The histamine H4 receptor regulates chemokine production in human natural killer cells. Int Arch Allergy Immunol 166: 225–230. [DOI] [PubMed] [Google Scholar]
- Mommert S, Gregor K, Rossbach K, Schaper K, Witte T, Gutzmer R et al (2018b). Histamine H2 receptor stimulation upregulates TH2 chemokine CCL17 production in human M2a macrophages. J Allergy Clin Immunol 141: 782–785.e5. [DOI] [PubMed] [Google Scholar]
- Mommert S, Gschwandtner M, Koether B, Gutzmer R, Werfel T (2012). Human memory Th17 cells express a functional histamine H4 receptor. Am J Pathol 180: 177–185. [DOI] [PubMed] [Google Scholar]
- Mommert S, Kleiner S, Gehring M, Eiz‐Vesper B, Stark H, Gutzmer R et al (2016a). Human basophil chemotaxis and activation are regulated via the histamine H4 receptor. Allergy 71: 1264–1273. [DOI] [PubMed] [Google Scholar]
- Mommert S, Ratz L, Herwig K, Rost M, Gutzmer R, Werfel T (2016b). Genetic variations within the promotor region of the human histamine H4 receptor gene in psoriasis patients. Pharmacol Res 114: 121–127. [DOI] [PubMed] [Google Scholar]
- Mommert S, Ratz L, Stark H, Gutzmer R, Werfel T (2018c). The histamine H4 receptor modulates the differentiation process of human monocyte‐derived M1 macrophages and the release of CCL4/MIP‐1β from fully differentiated M1 macrophages. Inflamm Res 67: 503–513. [DOI] [PubMed] [Google Scholar]
- Morita E, Takahashi H, Niihara H, Dekio I, Sumikawa Y, Murakami Y et al (2010). Stratum corneum TARC level is a new indicator of lesional skin inflammation in atopic dermatitis. Allergy 65: 1166–1172. [DOI] [PubMed] [Google Scholar]
- Murata Y, Song M, Kikuchi H, Hisamichi K, Xu XL, Greenspan A et al (2015). Phase 2a, randomized, double‐blind, placebo‐controlled, multicenter, parallel‐group study of a H4R‐antagonist (JNJ‐39758979) in Japanese adults with moderate atopic dermatitis. J Dermatol 42: 129–139. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Itadani H, Hidaka Y, Ohta M, Tanaka K (2000). Molecular cloning and characterization of a new human histamine receptor, HHRH4. Biochem Biophys Res Commun 279: 615–620. [DOI] [PubMed] [Google Scholar]
- Nograles KE, Brasington RD, Bowcock AM (2009). New insights into the pathogenesis and genetics of psoriatic arthritis. Nat Clin Pract Rheumatol 5: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutten S (2015). Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab 66 (Suppl. 1): 8–16. [DOI] [PubMed] [Google Scholar]
- Oda T, Morikawa N, Saito Y, Masuho Y, Matsumoto S (2000). Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem 275: 36781–36786. [DOI] [PubMed] [Google Scholar]
- Ohsawa Y, Hirasawa N (2012). The antagonism of histamine H1 and H4 receptors ameliorates chronic allergic dermatitis via anti‐pruritic and anti‐inflammatory effects in NC/Nga mice. Allergy 67: 1014–1022. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Nasorri F, Bedini C, de Pita O, Girolomoni G, Cavani A (2006). CD56brightCD16− NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol 36: 118–128. [DOI] [PubMed] [Google Scholar]
- Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, Liu WL et al (2015). International Union of Basic and Clinical Pharmacology. XCVIII. Histamine receptors. Pharmacol Rev 67: 601–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reher TM, Neumann D, Buschauer A, Seifert R (2012). Incomplete activation of human eosinophils via the histamine H4‐receptor: evidence for ligand‐specific receptor conformations. Biochem Pharmacol 84 (2): 192–203. [DOI] [PubMed] [Google Scholar]
- Rossbach K, Baumer W (2014). PCR detects bands consistent with the expression of receptors associated with pruritus in canine dorsal root ganglia. Vet Dermatol 25: 9–e4. [DOI] [PubMed] [Google Scholar]
- Rossbach K, Nassenstein C, Gschwandtner M, Schnell D, Sander K, Seifert R et al (2011). Histamine H1, H3 and H4 receptors are involved in pruritus. Neuroscience 190: 89–102. [DOI] [PubMed] [Google Scholar]
- Rossbach K, Schaper K, Kloth C, Gutzmer R, Werfel T, Kietzmann M et al (2016). Histamine H4 receptor knockout mice display reduced inflammation in a chronic model of atopic dermatitis. Allergy 71: 189–197. [DOI] [PubMed] [Google Scholar]
- Rossbach K, Wendorff S, Sander K, Stark H, Gutzmer R, Werfel T et al (2009). Histamine H4 receptor antagonism reduces hapten‐induced scratching behaviour but not inflammation. Exp Dermatol 18: 57–63. [DOI] [PubMed] [Google Scholar]
- Schaper K, Rossbach K, Kother B, Stark H, Kietzmann M, Werfel T et al (2016). Stimulation of the histamine 4 receptor upregulates thymic stromal lymphopoietin (TSLP) in human and murine keratinocytes. Pharmacol Res 113 (Pt A): 209–215. [DOI] [PubMed] [Google Scholar]
- Seike M, Furuya K, Omura M, Hamada‐Watanabe K, Matsushita A, Ohtsu H (2010). Histamine H4 receptor antagonist ameliorates chronic allergic contact dermatitis induced by repeated challenge. Allergy 65: 319–326. [DOI] [PubMed] [Google Scholar]
- Simons FE, Simons KJ (2011). Histamine and H1‐antihistamines: celebrating a century of progress. J Allergy Clin Immunol 128: 1139–1150.e4. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Bienenstock J, Schmelz M, Maurer M, Wei E, Biro T (2006). Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J Invest Dermatol 126: 1705–1718. [DOI] [PubMed] [Google Scholar]
- Strakhova MI, Nikkel AL, Manelli AM, Hsieh GC, Esbenshade TA, Brioni JD et al (2009). Localization of histamine H4 receptors in the central nervous system of human and rat. Brain Res 1250: 41–48. [DOI] [PubMed] [Google Scholar]
- Sugata Y, Okano M, Fujiwara T, Matsumoto R, Hattori H, Yamamoto M et al (2007). Histamine H4 receptor agonists have more activities than H4 agonism in antigen‐specific human T‐cell responses. Immunology 121: 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa E, Yamaura K, Oda M, Namiki T, Ueno K (2011). Histamine H4 receptor antagonist reduces dermal inflammation and pruritus in a hapten‐induced experimental model. Eur J Pharmacol 667: 383–388. [DOI] [PubMed] [Google Scholar]
- Suwa E, Yamaura K, Sato S, Ueno K (2014). Increased expression of the histamine H4 receptor following differentiation and mediation of the H4 receptor on interleukin‐8 mRNA expression in HaCaT keratinocytes. Exp Dermatol 23: 138–140. [DOI] [PubMed] [Google Scholar]
- Thurmond RL (2015). The histamine H4 receptor: from orphan to the clinic. Front Pharmacol 6: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truta‐Feles K, Lagadari M, Lehmann K, Berod L, Cubillos S, Piehler S et al (2010). Histamine modulates γδ‐T lymphocyte migration and cytotoxicity, via Gi and Gs protein‐coupled signalling pathways. Br J Pharmacol 161: 1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD et al (2009). Imiquimod‐induced psoriasis‐like skin inflammation in mice is mediated via the IL‐23/IL‐17 axis. J Immunol 182: 5836–5845. [DOI] [PubMed] [Google Scholar]
- Wakahara K, Baba N, Van VQ, Begin P, Rubio M, Ferraro P et al (2012). Human basophils interact with memory T cells to augment Th17 responses. Blood 120: 4761–4771. [DOI] [PubMed] [Google Scholar]
- Werfel T (2009). The role of leukocytes, keratinocytes, and allergen‐specific IgE in the development of atopic dermatitis. J Invest Dermatol 129: 1878–1891. [DOI] [PubMed] [Google Scholar]
- Werfel T (2018). Novel systemic drugs in treatment of atopic dermatitis: results from phase II and phase III studies published in 2017/2018. Curr Opin Allergy Clin Immunol 18: 432–437. [DOI] [PubMed] [Google Scholar]
- Werfel T, Allam JP, Biedermann T, Eyerich K, Gilles S, Guttman‐Yassky E et al (2016a). Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol 138: 336–349. [DOI] [PubMed] [Google Scholar]
- Werfel T, Heratizadeh A, Aberer W, Ahrens F, Augustin M, Biedermann T et al (2016b). S2k guideline on diagnosis and treatment of atopic dermatitis – short version. J Dtsch Dermatol Ges 14: 92–106. [DOI] [PubMed] [Google Scholar]
- Werfel T, Layton G, Yeadon M, Whitlock L, Osterloh I, Jimenez P, et al (2018). Efficacy and safety of the histamine H4 receptor antagonist ZPL‐3893787 in patients with atopic dermatitis. J Allergy Clin Immunol; 10.1016/j.jaci.2018.07.047 [DOI] [PubMed] [Google Scholar]
- Yamaura K, Oda M, Suwa E, Suzuki M, Sato H, Ueno K (2009). Expression of histamine H4 receptor in human epidermal tissues and attenuation of experimental pruritus using H4 receptor antagonist. J Toxicol Sci 34: 427–431. [DOI] [PubMed] [Google Scholar]
- Yu B, Shao Y, Li P, Zhang J, Zhong Q, Yang H et al (2010a). Copy number variations of the human histamine H4 receptor gene are associated with systemic lupus erythematosus. Br J Dermatol 163: 935–940. [DOI] [PubMed] [Google Scholar]
- Yu B, Shao Y, Zhang J, Dong XL, Liu WL, Yang H et al (2010b). Polymorphisms in human histamine receptor H4 gene are associated with atopic dermatitis. Br J Dermatol 162: 1038–1043. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Krueger JG, Lowes MA (2009). Resident and “inflammatory” dendritic cells in human skin. J Invest Dermatol 129: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF (2012). Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol 130: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]


