Abstract
Genital warts, which are one of the most common sexually transmitted diseases (STDs), result from persistent infection with human papillomavirus (HPV), especially subtypes 6 or 11. Topical application of 5% imiquimod cream is currently recommended as a first-line treatment choice for genital warts, but the clearance and patient compliance rates remain less than sufficient. In the current study, we developed a temperature-sensitive gel that contains the host-defense peptides caerin 1.1 and 1.9, which were originally isolated from Australian tree frogs of the genus Litoria. Growth of HPV16 E6/E7-transformed TC-1 cells was inhibited in vitro and in vivo following injection of the tumor with the caerin gel in a TC-1 tumor mouse model. Furthermore, when the caerin gel was topically applied, the inhibitory effect remained, and T, NK cells were attracted to the tumor site. In addition, the gel maintained a similar level of bioactivity after incubation at room temperature for 30 days. Our results suggest that this caerin gel, following further optimization, may provide an alternative method for the management of genital warts.
Keywords: Caerin peptides, genital warts, gel
Introduction
Genital warts (condyloma acuminate, venereal warts, and ano-genital warts), which are one of most common sexually transmitted diseases (STDs), result from infection with low-risk human papillomavirus (HPV), especially subtypes 6 and 11 [1]. HPV6 and 11 account for approximately 90% of genital warts [1-3], and current estimates suggest that approximately 1% of sexually active individuals have symptomatic genital warts worldwide [4]. The clinical manifestation of genital warts varies significantly [5]. Although not a life-threating disease, genital warts cause discomfort and distress and may negatively affect the individual’s quality of life and relationship with a partner [6]. Genital warts may occur as a single lesion or as multiple lesions, usually 5 to 15 lesions with diameters of 1 to 10 mm [7]. Growth rates and spread of genital warts are also variable; however, accelerated growth and severe symptoms may occur in pregnant women or immunosuppressed individuals, such as in patients with HIV infection [8-10].
HPV vaccines based on papillomavirus-like particles (PV VLPs) were introduced a decade ago and effectively reduced the epidemiology of genital warts in countries where HPV vaccines were adopted [11,12]. The vast majority of genital warts, therefore, occur in developing countries, where HPV vaccines remain to be introduced or vaccine coverage needs to be increased [4]. Therefore, effective therapies against genital warts remain a priority.
Topical application of 5% imiquimod cream (Aldara, Loughborough, UK), podophyllotoxin, or sinecatechin/polyphenon E are recommended as a first-line treatment for genital warts, and subsequent physical or chemical ablation is recommended for larger warts [1,13]. Imiquimod, which is a Toll-like receptor (TLR) 7/8 ligand, acts as an immune modulator [1,14] and induces the secretion of proinflammatory cytokines [15], including interferon-alpha (IFN-α) [16]. Applied as a cream three times a week for 16 weeks, imiquimod achieves clearance rates between 16% and 50% [1]. The most common adverse effects include local erosions, erythema, and a burning sensation [1], and 2.5% or 3.75% imiquimod (Aldara) have been introduced as a means to reduce these side effects [17].
Peptides isolated from amphibians have been shown to kill bacteria via a unique mechanism, possibly involving cell membrane lysis. Some of these peptides are highly active against cancer cells. More than 200 host-defense peptides have been isolated and identified from skin secretions of Australian frogs and toads. Specifically, the caerin 1 peptides have previously been shown to be potent membrane-active peptides and to stop the formation of nitric oxide by neuronal nitric oxide synthase [18]. Caerin 1.1 peptide exhibits an anti-cancer effect against several human cancer cell lines. The caerin 1.9 peptide exhibits antimicrobial activity against a wide spectrum of gram-positive and gram-negative microbial strains and has recently been shown to inhibit cancer cell growth in vitro [19]. In addition, the caerin 1.1 and 1.9 peptides inhibit HIV-infected T cells within minutes of exposure at concentrations that are non-toxic to T cells and also inhibit the transfer of HIV from dendritic cells (DCs) to T cells [20]. Recently, caerin 1.1 and 1.9 peptides have been found to have cytotoxicity against HPV 16 early protein E6/E7-transformed TC-1 cells in vitro, and the anti-cancer efficacy improved when caerin 1.1 and 1.9 were used together [21]. Moreover, proteomics analysis revealed that the caerin 1.9 peptide stimulates multiple signaling pathways, including proinflammatory pathways [1,21].
Based on these previous studies, we have developed a temperature-sensitive gel that contains caerin 1.1 and 1.9 peptides and demonstrated that this caerin gel inhibits HPV16 E6/E7-transformed TC-1 cell growth in vitro and in vivo when injected locally to the tumor in a mouse model. The gel remains highly bioactive after incubation at room temperature for 30 days. Moreover, the temperature-sensitive gel inhibits subcutaneously transplanted TC-1 growth in mice when topically applied to the tumor and attracts T cells and NK cells to the tumor site.
Materials and methods
Mice
Six-to-eight-week-old, specific pathogen-free (SPF) female C57BL/6 (H-2b) mice were purchased from Guangdong Medical Laboratory Animal Center and maintained at the Animal Resource Centre (First Affiliated Hospital of Guangdong Pharmaceutical University, Guangdong Province, China). Experiments were approved and performed in compliance with the guidelines of the Animal Experimentation Ethics Committee (Ethics Approval Number: FAHGPU20160316). All mice were housed under SPF conditions on a 12-h light/12-h dark cycle at 22°C with a humidity of 75%. Five mice were kept in each cage, and animals were provided with sterilized standard mouse food and water. TC-1 tumor-bearing mice were administered 1% sodium pentobarbital via i.p. injection preceding treatment. Mice were sacrificed by CO2 inhalation at the end of each experiment, and death was confirmed by the absence of a heartbeat.
Cell lines and peptide synthesis
A murine TC-1 cell line transformed with HPV16 E6/E7 was obtained from Shanghai Institute for Cell Resources Centre (Chinese Academy of Sciences) and cultured according to the protocols provided by the supplier. Briefly, TC-1 cells were cultured at 37°C with 5% CO2 in complete RPMI 1640 media (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS, Gibco), 100 U of penicillin/mL and 100 mg of streptomycin/mL (Gibco), 0.2 mM nonessential amino acid solution, 1.0 mM sodium pyruvate, 2 mM L-glutamine, and 0.4 mg/mL G418 [22].
The human cervical cancer HeLa cell line was purchased from the Shanghai Institutes for Biological Sciences (Chinese Academy of Sciences). The cell lines were cultured in complete DMEM media (Gibco) supplemented with 10% heat-inactivated FCS (Gibco), 100 U of penicillin/mL and 100 μg of streptomycin/mL (Gibco), 0.2 mM nonessential amino acid solution, 1.0 mM sodium pyruvate, and 2 mM L-glutamine at 37°C with 5% CO2.
Caerin 1.1 (GLLSVLGSVAKHVLPHVLPHVVPVIAEHL-NH2, simplified as F1), caerin 1.9 (GLFGVLGSIAKHVLPHVVPVIAEKL-NH2, simplified as F3), and control peptide (GTELPSPPSVWFEAEFK, simplified as P3) were synthesized by Mimotopes Proprietary Limited (Wuxi, China). The purity of the peptides was determined to be greater than 95% by reverse-phase HPLC at Mimotopes.
Temperature-sensitive gel preparation
Poloxamer 407 (relative molecular weight 12600, batch number WPAK592B) and poloxamer 188 (relative molecular weight 8400, batch number WPAK539B) were purchased from Badische Anilin-und-Soda-Fabrik (BASF; Ludwigshafen, Germany).
The empty gel matrix was first prepared. Briefly, 46 g of poloxamer 407 and 10 g of poloxamer 188 were mixed with 200 ml of distilled water and stored at 4°C until the poloxamers were completely dissolved. The preparation was stirred until a white condensation gum matrix was formed. The F peptide gel was prepared by mixing 10 mg of peptide F1 with 10 ml of the gel matrix. After the F1 was completely dissolved, the solution was filtered through a 0.22-μm microporous membrane filter to prepare a 1 mg/ml F1 gel. The same method was used to prepare 1 mg/ml F3, F1 and F3, and P3 gels, using the indicated peptides. The F1, F3, F1 and F3, and P3 gels were stored at 4°C until use.
To test the stability of the gels, all gels were incubated at room temperature for 30 days. The physical appearance of the F1 and F3 gel was characterized following incubation in a water bath at different temperatures ranging from 22°C to 37°C. The pH of the gels was measured using a pH meter (Mettler Toledo, Columbus, OH, USA; S220-K), and the viscosity of the gels was measured by a viscometer (Shanghai Changji Geological Instruments Co., Ltd., China; NDJ-8S). Briefly, 50 ml of each gel were separately added to the sample container, and the dynamic viscosity at 60 rpm was recorded at 25°C. The dynamic viscosity at 3 rpm was recorded at 37°C. In addition, the hygroscopicity of the gels was examined. For each of the prepared gels, 30 ml were weighed and then sealed in a sterile environment with tin foil paper (Glad, Oakland, CA, USA; F5M) at room temperature. After 7 days, the gel was weighed for comparison with the original weights. Gel sterility was observed using a microscope (Olympus Corporation, Japan; IX73). These assessments were determined following incubation of the gels at 37°C for 7 days.
3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
Cell proliferation was determined with an MTT assay (Sigma, St. Louis, MO, USA) following the manufacturer’s instructions as described previously [23]. Briefly, 5×103 TC-1 or HeLa cells were cultured in flat-bottomed 96-well plates. Approximately 0-15 µg of different peptides or gels were added to the cells and cultured overnight at 37°C with 5% CO2. Each treatment was performed in triplicate. Then 10 µl of MTT stock solution were added to the wells and incubated for an additional 4 hours. Finally, 150 µl DMSO was added to stop the reaction. Results were analyzed using an ELISA plate reader (Multiskan GO, Thermo Fisher Scientific) at 570 nm according to the manufacturer’s protocol. The survival percentage (S%) was calculated using the following equation: S% = (Treated-Control)/(Untreated-Control) × 100%.
Tumor challenge
TC-1 tumor challenge has been described elsewhere [24]. Briefly, TC-1 cells, at approximately 70% confluency, were harvested with 0.25% trypsin and washed repeatedly with phosphate-buffered saline (PBS). TC-1 tumor cells (5×105/mouse) were resuspended in 0.1 ml of PBS and injected subcutaneously into the left flank. TC-1 tumor-bearing mice (5/group) were gently held to expose the tumor, and the width and length of the tumor were measured by electronic digital caliper. The tumor volume size was calculated as 1/2× length × width × width.
Scoring of the body condition was performed by passing the finger over the mouse sacroiliac bones in order to monitor the health of tumor-bearing mice [23]. The following scores were defined: 5, obese mouse; 4, mouse well-fleshed, and bones barely felt; 3, mouse is in optimal condition, and bones are palpable; 2, mouse is thin, and bones are prominent; and 1, muscle wasting is advanced, fat deposits have disappeared, and bones are very prominent. Euthanasia was mandatory for scores of 1. Mice were sacrificed when the tumor diameter reached 20 mm, as approved by the ethics committee, or when ulcerated. Mice were also sacrificed if the tumor interfered with eating, drinking, urinating, defecating, or walking as described elsewhere [25]. In some experiments, TC-1 tumor-bearing mice were sacrificed, and tumors were dissected and weighed.
Treatment of TC-1 tumor-bearing mice with caerin peptides
On days 4 and 5 post-TC-1 challenge, when the tumors were 3 mm in diameter, the mice were injected intratumorally with 100 µl PBS containing 30 µg of F1/F3 peptides, F1/F3 gel, PBS, or gel only for 7 days consecutively.
In other experiments, subcutaneously transplanted TC-1 tumor-bearing mice were divided into five separate groups that received one of the following treatments: 5% imiquimod cream, F1/F3 gel, P3 gel, no treatment, or gel only. The different gels were applied to the shaved skin above the TC-1 tumor areas daily for 7 days consecutively, while 5% imiquimod cream was applied every 2 days. An additional group of TC-1 tumor-bearing mice were treated with 5% imiquimod every 2 days, alternating with F1/F3 gel on the days when imiquimod cream was not used. Then, 2 days after final treatment, mice were sacrificed, and the tumors were isolated and weighed. The tumor tissue was then dissected, dehydrated, embedded, and prepared for analysis.
Flow cytometry analysis of tumor-infiltrating T cells and NK cells
Flow cytometry was performed on mononuclear cells isolated from TC-1 tumors from mice. Tumors were excised and cut into small pieces after removal of connective tissues. Tumor tissue was then incubated for 1 h, with occasional shaking, in an enzyme mixture that consisted of 1 mg/ml collagenase D (Roche, Basel, Switzerland), 20 mg/ml of DNase I (Sigma), and 10% FCS in RPMI-1640 medium at 37°C. The digested tissue was filtered through a 70-μm nylon mesh filter, and the resultant cells were washed twice in flow cytometry buffer (PBS with 2% FCS; Gibco/Thermo Fisher Scientific). Cells were subsequently stained with conjugated monoclonal antibodies and their appropriate isotype controls for 15 minutes at 22°C. Directly conjugated fluorochrome antibodies included anti-mouse Alexa Fluor647 CD3 antibody (BD Pharmingen, San Diego, CA, USA), anti-mouse FITC CD45.2 antibody (BD Pharmingen), and anti-mouse BV421 NK1.1 antibody (BD Horizon, San Diego, CA, USA). Propidium iodide (eBioscience, San Diego, CA, USA) staining was used to exclude dead cells. After washing, cells were analyzed on a flow cytometer (FACS Aria II; BD Biosciences, San Jose, CA, USA) and analyzed using FlowJo software (TreeStar, Ashland, Oregon).
Statistical analysis
Tumor weights are expressed as the mean ± SD, and statistical significance of differences between groups was determined using the two-tailed Student’s t-test. Tumor volumes among different groups were analyzed by one-way ANOVA using Prism 5.0 (Graphpad Software, San Diego, CA, USA). MTT data were calculated as percentage of inhibition compared with untreated control group, and the paired two-tailed t-test was used to compare the results. Differences were deemed significant when the P-value was less than 0.05.
Results
Preparation and physical characteristics of temperature-sensitive F1/F3 gel
The physical appearance of the F1/F3 gel was characterized within temperatures ranging from 4°C to 37°C, as these values correspond to typical temperatures for storage and use of the gel, to evaluate its temperature sensitivity. F1/F3 gel maintained a transparent liquid phase from 4°C to 35°C, and when the temperature was increased to 37°C, the gel developed a homogenous gel-like consistency (Figure S1).
The pH of the gel matrix, P3 gel, and F1/F3 gel at 25°C and 37°C was determined to be 6.86 using a pH meter. The viscosities of the gel matrix, P3 gel, and F1/F3 gel at 25°C and 37°C were determined to be similar using a digital viscometer (Table S1). With respect to hygroscopicity, after 7 days of incubation at room temperature, the weights (85.04 g) of the gel matrix, P3 gel, and F1/F3 gel did not significantly differ from the original weights (85.5 g for all), indicating that none of these gels is hygroscopic. Observation of the gel matrix, P3 gel, F1/F3 gel using a microscope revealed no contamination even after incubation at 37°C for 7 days.
F1/F3 gel inhibits TC-1 and HeLa cell growth in vitro
F1/F3 peptides inhibit TC-1 cell growth but do not inhibit growth of primary human HMC cells, a primary human mesangial cell line, in vitro [21] (Figure S2); however, it is possible that these peptides may have lost their inhibitory activity during gel preparation. Thus, we investigated whether the F1/F3 gel has similar bioactivity against HPV+ cell lines compared to F1 or F3 peptides in PBS. TC-1 cells, derived from primary lung epithelial cells of C57BL/6 mice, were immortalized with retrovirus vector LXSN16E6E7 and subsequently transformed with the pVEJB plasmid expressing the activated human c-Ha-ras oncogene. HeLa cell is a HPV18+ human cervical cancer cell line. At 15 µg/ml, F1, F3, and F1/F3 gels, and F1, F3, and F1/F3 peptides in PBS were able to inhibit TC-1 and HeLa cell growth. For TC-1 cells, 44%, 53%, and 18% of cells remained viable after treatment with F1, F3, and F1/F3 peptides, respectively, while 28%, 38%, and 13% of cells remained viable after treatment with F1, F3, and F1/F3 gels, respectively. For HeLa cells, 31%, 63%, and 18% of cells were viable after treatment with F1, F3, and F1/F3 peptides, respectively, while 24%, 43%, and 11% of cells were viable after treatment with the F1, F3, and F1/F3 gel preparations, respectively. TC-1 cell survival following treatment with F1 and F3 gels was significantly less than following treatment with F1 and F3 peptides in PBS (P<0.05) or with gel only (P<0.05). Similar results were obtained after treatment with 5 µg of the different F peptides. F1 and F3 peptides had additive effects on inhibition of growth of both cell lines in MTT assay compared with F1 or F3 in PBS or in gel added individually (Figure 1), as observed in our previous studies [21]. The control P3 peptide at either 5 or 15 µg/ml did not inhibit TC-1 or HeLa cell growth when applied as free peptide or in gel preparation (Figure 1).
Figure 1.
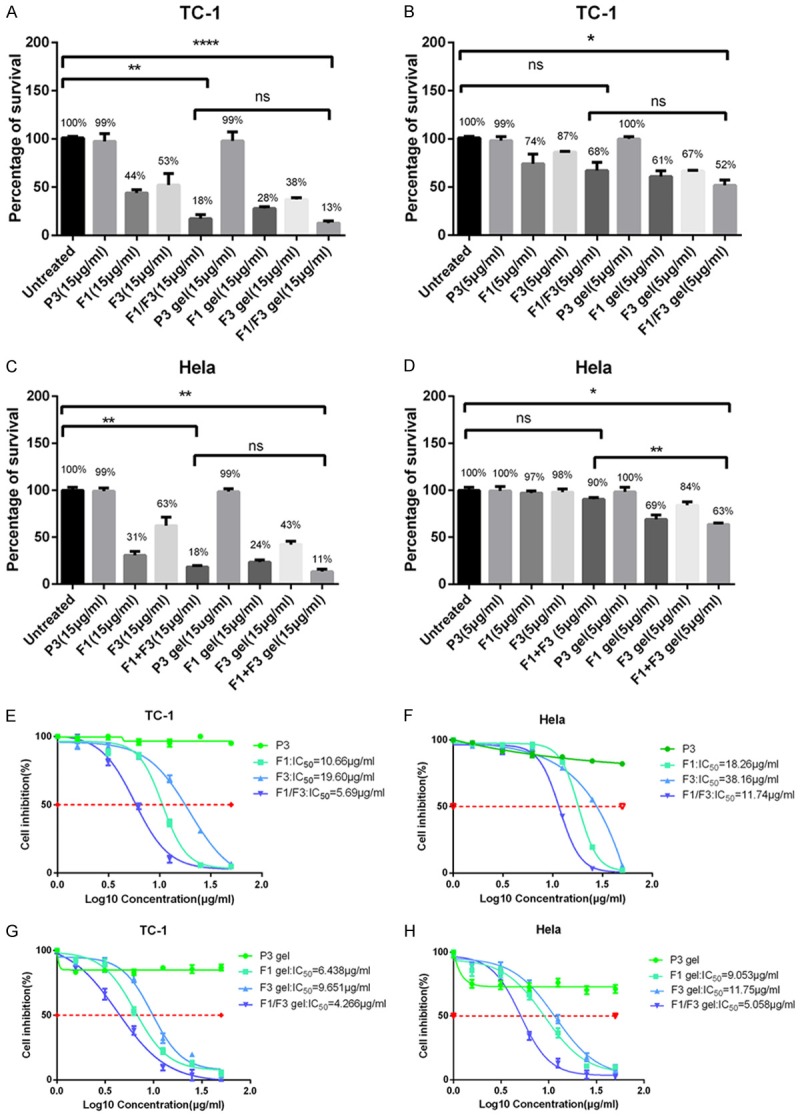
F1/F3 gel inhibits the proliferation of TC-1 and Hela cells. (A-D) Approximately 5×103 of TC-1 cells (A and B) or HeLa cells (C and D) were cultured either in media alone; with different concentrations (5 and 15 µg/ml) of F1 gel, F3 gel, F1/F3 gel, or P3 gel; with 15 µg/ml of F1, F3, or P3 peptides in PBS; or with gel only overnight before MTT assay was performed. Each bar represents the means of three replicates, and the error bars represent the standard deviations. The data are representative of two independent experiments. *P<0.05; **P<0.01; ****P<0.0001; ns, not significant. (E-H) IC50 values for F1, F3, and F1/F3 in free peptide in PBS or gel form were determined in cultures of 5×103 TC-1 cells (E and G) and HeLa cells (F and H). Cells were cultured in media alone or with different concentrations (1.5625, 3.125, 6.25, 12.5, 25, and 50 µg/ml) of the F1 gel, F3 gel, F1/F3 gel, P3 gel, F1 peptide, F3 peptide, or P3 peptide in PBS overnight before the MTT assay was performed. Each bar represents the means of three replicates, which were performed in triplicate, and the error bars represent the standard deviations. The result is the representative of three independent experiments.
Next, we determined the half maximal inhibitory concentration (IC50) values for F1, F3, and F1/F3 in PBS or in gels. For TC-1 cells, IC50 values for F1, F3, and F1/F3 in PBS were 10.66 μg/ml, 19.60 μg/ml, and 5.69 μg/ml, respectively, while the IC50 values for the F1, F3, and F1/F3 gels were 6.438 μg/ml, 9.651 μg/ml, and 4.266 μg/ml, respectively. For HeLa cells, the IC50 values for the F1, F3, and F1/F3 peptides in PBS were 18.26 μg/ml, 38.16 μg/ml, and 11.74 μg/ml, respectively, while the IC50 values for F1, F3, and F1/F3 gels were 9.053 μg/ml, 11.75 μg/ml, and 5.058 μg/ml, respectively (Figure 1).
Furthermore, the bioactivity of the F1/F3 gel stored at room temperature (22-24°C) was tested using the MTT assay. After storage at room temperature for 30 days, the F1/F3 gel was able to inhibit TC-1 cell growth at concentrations as low as 5 µg/ml (Figure 2).
Figure 2.
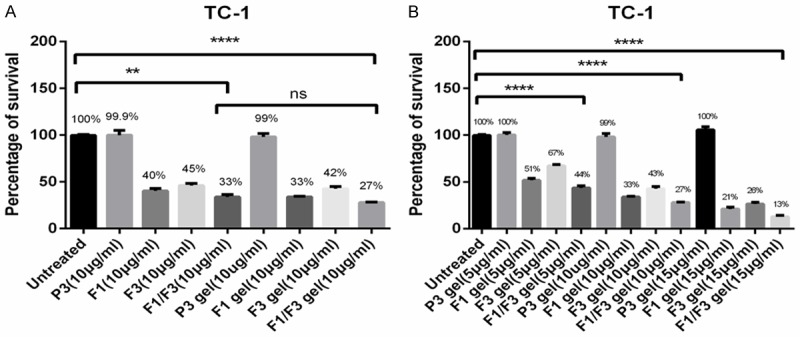
F1/F3 gel is stable at room temperature for 30 days. A and B. Approximately, 5×103 TC-1 cells were cultured either in media alone or with different concentrations (5, 10, and 15 µg/ml) of F1 gel, F3 gel, F1/F3 gel, or P3 gel or with 10 µg/ml of F1, F3, or P3 peptides in PBS at room temperature (22-24°C) for 30 days before the MTT assay was performed. Gel only treatment served as a control. Each bar represents the means of three replicates (performed in triplicate), and the error bars represent the standard deviations. These data are the representative of three independent experiments. **P<0.01; ****P<0.0001; ns, not significant.
Intra-tumor injection of F1/F3 gel inhibits TC-1 cell growth in vivo
Next, we determined whether the F1/F3 gel inhibited TC-1 growth in vivo to confirm that F1/F3 peptides maintained their bioactivity after gel preparation. Five days after TC-1 cells were transplanted subcutaneously into C57BL/6 mice, F1/F3 gels dissolved in PBS at concentration equal to free F1/F3 were injected into the TC-1 tumors daily for 7 days. Then, 3 days later, mice were sacrificed, and tumors were isolated and weighed. Tumor sizes and weights were significantly lower in mice that received an intra-tumor injection with either F1/F3 gel or free F1/F3 peptide compared to those from mice that received localized injection of only gel dissolved in PBS (P<0.01). No differences were noted between the effects of the F1/F3 peptides and the F1/F3 gel (Figure 3A and 3B).
Figure 3.
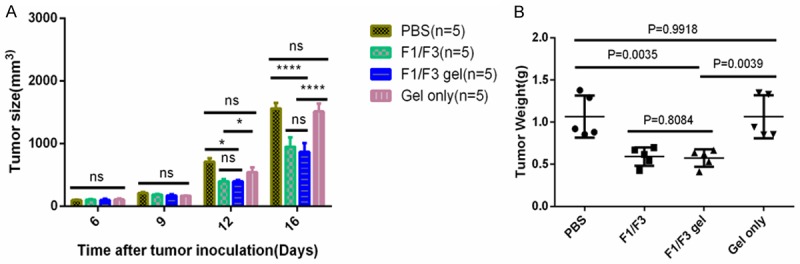
F1/F3 gel inhibits TC-1 tumor growth in vivo. Groups of five C57/BL6 mice were subcutaneously transplanted with 5×105 TC-1 tumor cells. When the tumors reached 3 mm in diameter, the mice were injected intratumorally with 100 µl PBS containing 30 µg of F1 and F3 peptide or F1/F3 gel for 7 days consecutively. Control groups received PBS or gel only. Then, 3 days after final injection, tumors were isolated from individual mice and analyzed for (A) volume and (B) weight. Each bar represents the means of measurements of tumors from the five mice, and the error bars represent the standard deviations. *P<0.05; ****P<0.0001; ns, not significant.
Topical application of F1/F3 gel inhibits TC-1 tumor growth in vivo
To investigate whether topical application of F1/F3 gel was able to inhibit TC-1 growth in vivo, TC-1 cells were subcutaneously transplanted into C57BL/6 mice. After 3 days, TC-1 tumor-bearing mice were divided into seven groups to receive either no treatment, gel only, P3 gel, F1/F3 gel, 5% imiquimod cream, 5% imiquimod plus gel only, or 5% imiquimod alternating with F1/F3 gel. These treatments were applied topically to the skin above the TC-1 tumor. The gels were applied daily for 7 days consecutively, except for 5% imiquimod, which was applied every other day as recommended by the manufacturer. Mice were sacrificed 2 days after the final treatment, and isolated tumors were weighed. Topical application of both F1 and F3 gel and imiquimod inhibited TC-1 growth compared with the untreated control group. The tumor weights from mice in the F1/F3 gel group were significantly less than those from mice that received gel only or P3 control gel (P<0.05). Interestingly, TC-1 tumor-bearing mice treated with F1/F3 gel alternating with 5% imiquimod had lower tumor weights than those treated with F1/F3 gel or 5% imiquimod cream alone, although these differences were not statistically significant (Figure 4).
Figure 4.
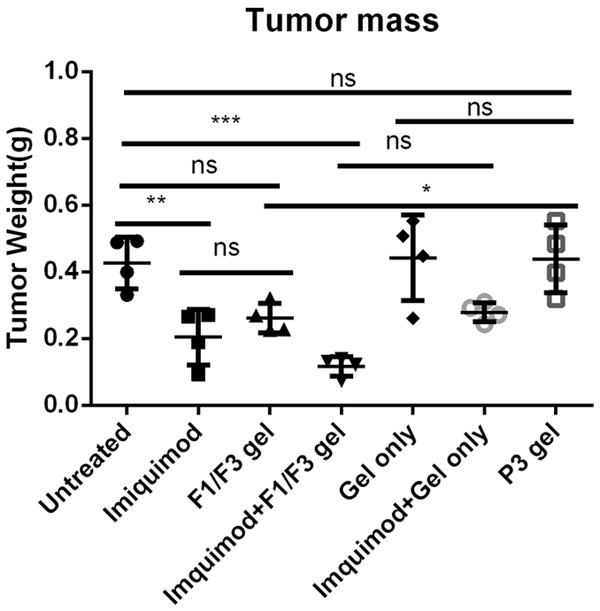
Topical application of F1/F3 gel inhibited TC-1 tumor growth, and this inhibition was enhanced by imiquimod. C57/BL6 mice were subcutaneously transplanted with 5×105 TC-1 tumor cells. Then, 3 days after TC-1 transplantation, the tumor areas of five mice per group were treated topically with either gel only, P3 gel, F1/F3 gel, or 5% imiquimod cream daily for 7 days, except 5% imiquimod, which was applied every 2 days. In another group, TC-1 tumor-bearing mice were treated with 5% imiquimod every other day and with F1/F3 gel on the alternate days. Mice were sacrificed 2 days after the final treatment for analysis of tumor weights. Data represent the tumor weights of individual mice, and the mean is shown. Error bars represent the standard deviations. These data reflect one representative result of three identical experiments. *P<0.05; **P<0.01; ***P<0.001; ns, not significant.
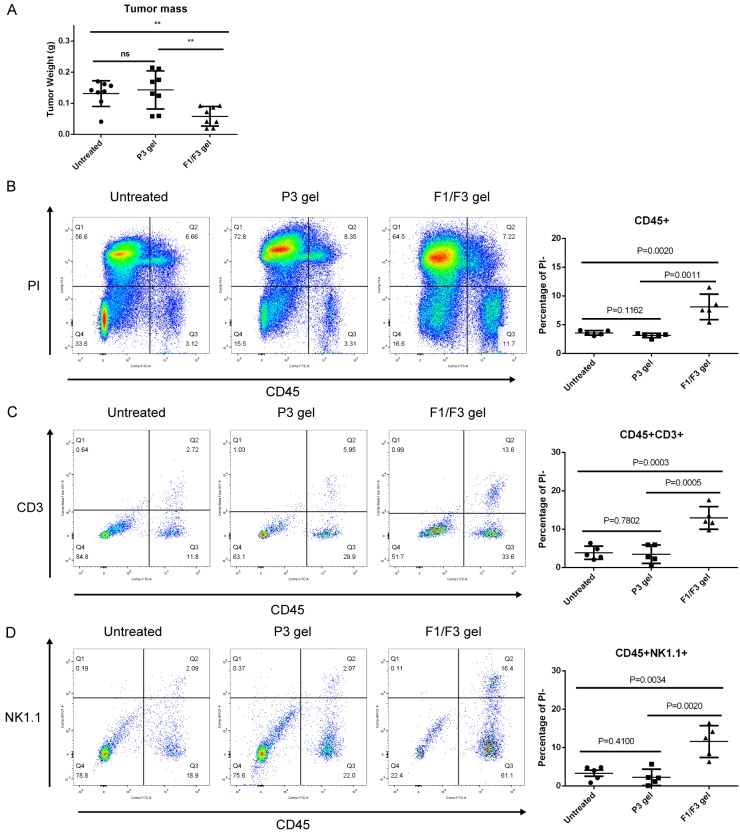
Topical application of F1/F3 gel attracts T cells and NK cells to TC-1 tumors
Previously, we demonstrated that intra-tumor injection of F1/F3 peptides to TC-1 tumor-bearing mice inhibits TC-1 tumor growth in C57BL/6 mice but not in nude mice [36]. These results suggested that adaptive immune cells were involved in TC-1 tumor growth inhibition by these caerin 1 peptides. We therefore examined the numbers of tumor-infiltrating T cells in TC-1 tumors in mice treated with F1/F3 gel using flow cytometry. The numbers of both CD45+CD3+ T cells and NK1.1+ cells infiltrating to TC-1 tumors were significantly increased in mice treated with F1/F3 gel compared to those in mice that remained untreated or treated with P3 control gel (Figure 5).
Figure 5.
Topically applied F1/F3 gel promotes infiltration of T cells and NK cells to the TC-1 tumor site. C57/BL6 mice were subcutaneously transplanted with 5×105 TC-1 tumor cells. After 3 days, P3 gel or F1/F3 gel (n=8/group) was applied daily to the shaved skin area above the tumor for 7 days consecutively. Mice were sacrificed 2 days after the final treatment, and (A) isolated tumors were weighed using an electronic balance. Five tumors from each group were randomly selected for analysis. Single cell suspensions were stained with anti-CD45.2, anti-CD3, and anti-NK1.1 antibodies and subjected to flow cytometry. (B) Infiltration of CD45+ cells in tumor tissues. (C) Infiltration of CD45+CD3+ T cells in tumor tissues. (D) Infiltration of CD45+NK1.1+ cells in tumor tissues. PI was used to exclude dead cells. One representative result from three identical experiments is shown. Data are shown for each individual mouse, and means and standard deviations are indicated by bars. *P<0.05; **P<0.01; ns, not significant.
Discussion
The overall prevalence of genital warts based on retrospective administrative databases, medical chart reviews, and prospectively collected physician reports ranges from 0.13% to 0.56%, whereas prevalence ranges from 0.2% to 5.1% based on genital examinations [26]. Introduction of a prophylactic vaccine against HPV infection has greatly reduced the incidence of genital warts in countries, such as Australia, where the vaccine is well distributed [11,12,27]. Although recent studies reported a decline in the prevalence of genital warts in the United States after dissemination of the HPV vaccine in 2006 [28], the prevalence of ano-genital warts has risen steadily for the past 35 years, according to a study published in 2012 [14]. To date, approximately 47 million women worldwide (95% confidence intervals [CI], 39-55 million) have received the full course of HPV vaccine, indicating a total population coverage of 1.4% (95% CI 1.1-1.6). Additionally, 59 million women (95% CI, 48-71 million) have received at least one dose, representing a total population coverage of 1.7% (95% CI, 1.4-2.1), suggesting that the global disease burden of genital warts remains high [29].
Although genital warts are a non-life-threatening disease, they cannot be cured; however, removal of warts by chemical or physical ablation or alternatively by topical application of 5% imiquimod cream (Aldara) or sinecatechin (polyphenon E) ointment to the warts [1] are the commonly employed methods for the treatment of ano-genital warts. Imiquimod is a TLR7/8 ligand [30], which induces a non-specific innate immune response via activation of TLR7/8 receptors [15]. Aldara is applied as a 5% imiquimod cream three times a week for up to 16 weeks, and the most common adverse effects include local erosions, erythema, and a burning sensation [1]. More recently, Aldara creams containing 2.5% or 3.75% imiquimod have been introduced to reduce the side effects [17,31]. The lengthy treatment course, obvious side effects, and low patient compliance rates indicates a need for development of better therapies for genital warts.
Caerin peptides isolated from an Australian tree frog inhibit tumor growth both in vitro and in vivo [19,21]. A temperature-sensitive gel containing caerin 1.1 and caerin 1.9 (F1/F3 gel; Figure S1), inhibited the proliferation of TC-1 and HeLa cells in vitro (Figure 1) and inhibited subcutaneous transplanted TC-1 tumor cell growth in vivo (Figure 3). Topical application of F1/F3 gel over the subcutaneous transplanted TC-1 (Figure 4) also inhibited TC-1 tumor growth. Moreover, the F1/F3 gel remained bioactive when stored at room temperature for one month (Figure 2). Finally, topical application of F1/F3 gel attracted T cells and NK cell to the tumor site (Figure 5). These results suggest that the F1/F3 gel has potential as an alternative therapeutic for the treatment of ano-genital warts.
The mechanisms underlying caerin peptide inhibition of tumor growth remain unclear. Caerin peptides activate HPV+ tumor cells to undergo apoptosis and secrete proinflammatory cytokines [19,21]. Furthermore, these peptides attract T cells to infiltrate the TC-1 tumor site, suggesting that adaptive immune responses elicited after F1/F3 gel application contribute to the regression of TC-1 tumors. Previously, we demonstrated that intra-tumor injection of F1/F3 peptide cannot inhibit TC-1 tumor growth in nude mice [36]. Caerin peptides also increase the efficacy of a HPV16 E7-specific therapeutic vaccine against TC-1 tumors via the recruitment of more T cells to the tumor site [36]. Together, these findings support the argument that the adaptive immune response is involved in the F1/F3-mediated inhibition of tumor growth in vivo.
Poloxamers are non-ionic poly ethylene oxide (PEO)-poly propylene oxide (PPO) copolymers [32] that are frequently used in pharmaceutical formulations as surfactants, emulsifying agents, solubilizing or dispersing agents, and in vivo absorbance enhancers [32,33]. A thermally reversible gel form of Pluronic F127 (poloxamer 407) was evaluated as a vehicle for the percutaneous administration of indomethacin [34], and in vivo studies suggested that 20% w/w aqueous gel may be of practical use as a base for topical administration of the drug. Moreover, poloxamer 407 gel was found suitable for transdermal delivery of insulin [35]. Thus, such poloxamers may promote the transdermal delivery of F1 and F3 peptides to the tumor site.
Aldara and F1/F3 gel exhibited synergistic effects when topically applied sequentially to TC-1 tumors (Figure 4), although the differences identified in our study were not statistically significant. Further studies are required to confirm whether Aldara and F1/F3 gel in combination will improve the inhibition of tumor growth, and we are currently investigating whether imiquimod promotes F1 and F3 peptides transdermal movement to the TC-1 tumor using a FITC-labelled F1 and F3 peptide gel.
In summary, a caerin 1.1 and 1.9 peptide gel formulation, called F1/F3 gel, inhibited TC-1 tumor growth following topical application to the tumor. Attempts are now being made to investigate whether this F1/F3 gel is suitable for use for the treatment of genital warts in the clinic, which remains a prominent public health problem worldwide.
Acknowledgements
This project was partly supported by the first affiliated hospital of Guangdong Pharmaceutical University; Foshan City Council Research Platform Fund (No. 2015AG1003, No. FS0AA-KJ218-1301-0039) and Science and Technology Research program of Guangdong province (No. 2016A020213001). We thank Dr. Jin-He Zhang for helping with some experiments performed at the department of nuclear medicine, southern theater general hospital of the PLA, Guangzhou 510000, China.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Yuan J, Ni G, Wang T, Mounsey K, Cavezza S, Pan X, Liu X. Genital warts treatment: beyond imiquimod. Hum Vaccin Immunother. 2018;14:1815–1819. doi: 10.1080/21645515.2018.1445947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leslie SW, Kumar S. StatPearls. Treasure Island (FL): 2018. Genital Warts. [Google Scholar]
- 3.Stanley M. Preventing cervical cancer and genital warts-how much protection is enough for HPV vaccines? J Infect. 2016;72(Suppl):S23–28. doi: 10.1016/j.jinf.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Gall SA. Female genital warts: global trends and treatments. Infect Dis Obstet Gynecol. 2001;9:149–154. doi: 10.1155/S1064744901000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynde C, Vender R, Bourcier M, Bhatia N. Clinical features of external genital warts. J Cutan Med Surg. 2013;17(Suppl 2):S55–60. [PubMed] [Google Scholar]
- 6.Bhatia N, Lynde C, Vender R, Bourcier M. Understanding genital warts: epidemiology, pathogenesis, and burden of disease of human papillomavirus. J Cutan Med Surg. 2013;17(Suppl 2):S47–54. [PubMed] [Google Scholar]
- 7.Banerjee S, Kaunelis D. Imiquimod for the treatment of genital warts: a review of clinical effectiveness and cost-effectiveness. Ottawa (ON): 2017. [PubMed] [Google Scholar]
- 8.Hamouda T, Freij MA, Saleh M. Management of genital warts in pregnancy. Clin Exp Obstet Gynecol. 2012;39:242–244. [PubMed] [Google Scholar]
- 9.Silverberg MJ, Ahdieh L, Munoz A, Anastos K, Burk RD, Cu-Uvin S, Duerr A, Greenblatt RM, Klein RS, Massad S, Minkoff H, Muderspach L, Palefsky J, Piessens E, Schuman P, Watts H, Shah KV. The impact of HIV infection and immunodeficiency on human papillomavirus type 6 or 11 infection and on genital warts. Sex Transm Dis. 2002;29:427–435. doi: 10.1097/00007435-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Bere A, Tayib S, Kriek JM, Masson L, Jaumdally SZ, Barnabas SL, Carr WH, Allan B, Williamson AL, Denny L, Passmore JA. Altered phenotype and function of NK cells infiltrating human papillomavirus (HPV)-associated genital warts during HIV infection. Clin Immunol. 2014;150:210–219. doi: 10.1016/j.clim.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Smith MA, Liu B, McIntyre P, Menzies R, Dey A, Canfell K. Trends in genital warts by socioeconomic status after the introduction of the national HPV vaccination program in Australia: analysis of national hospital data. BMC Infect Dis. 2016;16:52. doi: 10.1186/s12879-016-1347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith MA, Liu B, McIntyre P, Menzies R, Dey A, Canfell K. Fall in genital warts diagnoses in the general and indigenous Australian population following implementation of a national human papillomavirus vaccination program: analysis of routinely collected national hospital data. J Infect Dis. 2015;211:91–99. doi: 10.1093/infdis/jiu370. [DOI] [PubMed] [Google Scholar]
- 13.Karnes JB, Usatine RP. Management of external genital warts. Am Fam Physician. 2014;90:312–318. [PubMed] [Google Scholar]
- 14.Yanofsky VR, Patel RV, Goldenberg G. Genital warts: a comprehensive review. J Clin Aesthet Dermatol. 2012;5:25–36. [PMC free article] [PubMed] [Google Scholar]
- 15.Perry CM, Lamb HM. Topical imiquimod: a review of its use in genital warts. Drugs. 1999;58:375–390. doi: 10.2165/00003495-199958020-00017. [DOI] [PubMed] [Google Scholar]
- 16.Beutner KR, Spruance SL, Hougham AJ, Fox TL, Owens ML, Douglas JM Jr. Treatment of genital warts with an immune-response modifier (imiquimod) J Am Acad Dermatol. 1998;38:230–239. doi: 10.1016/s0190-9622(98)70243-9. [DOI] [PubMed] [Google Scholar]
- 17.Rosen T, Nelson A, Ault K. Imiquimod cream 2.5% and 3.75% applied once daily to treat external genital warts in men. Cutis. 2015;96:277–282. [PubMed] [Google Scholar]
- 18.Calabrese AN, Bowie JH, Pukala TL. Structural analysis of calmodulin binding by nNOS inhibitory amphibian peptides. Biochemistry. 2015;54:567–576. doi: 10.1021/bi5004124. [DOI] [PubMed] [Google Scholar]
- 19.Yuan J, You X, Ni G, Wang T, Cavezza S, Pan X, Liu X. Iodine-125 labeled Australian frog tree host-defense peptides caerin 1.1 and 1.9 better inhibit human breast cancer cells growth than the unlabeled peptides. (125)I-caerin 1.9 may better be used for the treatment of breast cancer. Hell J Nucl Med. 2018;21:115–120. doi: 10.1967/s002449910803. [DOI] [PubMed] [Google Scholar]
- 20.VanCompernolle S, Smith PB, Bowie JH, Tyler MJ, Unutmaz D, Rollins-Smith LA. Inhibition of HIV infection by caerin 1 antimicrobial peptides. Peptides. 2015;71:296–303. doi: 10.1016/j.peptides.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Ni G, Liang D, Cummins SF, Walton SF, Chen S, Wang Y, Mounsey K, Wei MQ, Yuan J, Pan X, Liu X, Wang T. Comparative proteomic study of the antiproliferative activity of frog host-defence peptide caerin 1.9 and its additive effect with caerin 1.1 on TC-1 cells transformed with HPV16 E6 and E7. Biomed Res Int. 2018;2018:7382351. doi: 10.1155/2018/7382351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Wang X, Wu X, Wei MQ, Zhang B, Liu X, Wang Y. IL-10 signalling blockade at the time of immunization inhibits Human papillomavirus 16 E7 transformed TC-1 tumour cells growth in mice. Cell Immunol. 2014;290:145–151. doi: 10.1016/j.cellimm.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Ni G, Chen S, Yang Y, Cummins SF, Zhan J, Li Z, Zhu B, Mounsey K, Walton S, Wei MQ, Wang Y, Zhou Y, Wang T, Liu X. Investigation the possibility of using peptides with a helical repeating pattern of hydro-phobic and hydrophilic residues to inhibit IL-10. PLoS One. 2016;11:e0153939. doi: 10.1371/journal.pone.0153939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S, Ni G, Wu X, Zhu B, Liao Z, Wang Y, Liu X. Blocking IL-10 signalling at the time of immunization renders the tumour more accessible to T cell infiltration in mice. Cell Immunol. 2016;300:9–17. doi: 10.1016/j.cellimm.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Ullman-Cullere MH, Foltz CJ. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci. 1999;49:319–323. [PubMed] [Google Scholar]
- 26.Patel H, Wagner M, Singhal P, Kothari S. Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis. 2013;13:39. doi: 10.1186/1471-2334-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali H, Guy RJ, Wand H, Read TR, Regan DG, Grulich AE, Fairley CK, Donovan B. Decline in in-patient treatments of genital warts among young Australians following the national HPV vaccination program. BMC Infect Dis. 2013;13:140. doi: 10.1186/1471-2334-13-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mann LM, Llata E, Flagg EW, Hong J, Asbel L, Carlos-Henderson J, Kerani RP, Kohn R, Pathela P, Schumacher C, Torrone EA. Trends of anogenital warts among sexually transmitted disease clinic patients-sexually transmitted disease surveillance network, united states, 2010-2016. J Infect Dis. 2019;219:1389–1397. doi: 10.1093/infdis/jiy684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, de Sanjose S, Castellsague X. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4:e453–463. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 30.Schon MP, Schon M. Imiquimod: mode of action. Br J Dermatol. 2007;157(Suppl 2):8–13. doi: 10.1111/j.1365-2133.2007.08265.x. [DOI] [PubMed] [Google Scholar]
- 31.Berman B, Wolf J. The role of imiquimod 3.75% cream in the treatment of external genital warts. Skin Therapy Lett. 2012;17:5–7. [PubMed] [Google Scholar]
- 32.Bodratti AM, Alexandridis P. Formulation of poloxamers for drug delivery. J Funct Biomater. 2018;9 doi: 10.3390/jfb9010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao Y, Thompson MJ, Wang Q, Tsai EW. Quantitation of poloxamers in pharmaceutical formulations using size exclusion chromatography and colorimetric methods. J Pharm Biomed Anal. 2004;35:1127–1142. doi: 10.1016/j.jpba.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki S, Tobiyama T, Takada M, Attwood D. Percutaneous absorption of indomethacin from pluronic F127 gels in rats. J Pharm Pharmacol. 1995;47:455–457. doi: 10.1111/j.2042-7158.1995.tb05829.x. [DOI] [PubMed] [Google Scholar]
- 35.Pillai O, Panchagnula R. Transdermal delivery of insulin from poloxamer gel: ex vivo and in vivo skin permeation studies in rat using iontophoresis and chemical enhancers. J Control Release. 2003;89:127–140. doi: 10.1016/s0168-3659(03)00094-4. [DOI] [PubMed] [Google Scholar]
- 36.Pan X, Ma BW, You XC, Chen S, Wu JL, Wang TF, Walton SF, Yuan JW, Wu XL, Chen GQ, Wang YJ, Ni GY, Liu XS. Synthesized natural peptides from amphibian skin secretions increase the efficacy of a therapeutic vaccine by recruiting more T cells to the tumour site. BMC Complement Altern Med. 2019;19:163. doi: 10.1186/s12906-019-2571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.