Abstract
Gout is a common inflammatory arthritis triggered by monosodium urate deposition after longstanding hyperuricemia. In the general community, the disease is largely polygenic in genetic architecture, with many polymorphisms having been identified in gout or urate-associated traits. In a small proportion of cases, rare high penetrant mutations associated with monogenic segregation of the disease in families have been demonstrated to be disease causative. In this study, we recruited a two-generation pedigree with early-onset gout. To elucidate the genetic predisposition, whole-exome sequencing (WES) was performed. After comprehensive variant analyses and cosegregation testing, we identified a missense variant (c.277C>A, p.L93M) in SLC16A9, an extremely rare variant in genetic databases. Moreover, in silico assessments showed strong pathogenicity. This variant cosegregated with the disease phenotype perfectly in the family and is located in a highly conserved functional domain. A few studies supported our results of the association between SLC16A9 and gout and serum urate levels. In conclusion, we provide the first evidence for the association of rare missense in SLC16A9 with early-onset gout. These findings not only expand our current understanding of gout but also may have further implications for the treatment and prevention of gout.
1. Introduction
Gout is a common inflammatory arthritis caused by the deposition of monosodium urate (MSU) crystals in and around the joints following longstanding hyperuricemia [1]. It affects 1-2% of adults in developed countries [1–3] and has a prevalence of 1.14% in eastern China [4]. Similar to other complex phenotypes, gout results from the interplay between inherited genetic risk variants and environmental factors [5]. Genome-wide association studies (GWAS) have confirmed the importance of genetic basis in gout. Several genetic loci have been associated with gout, such as ABCG2, ALDH16A1, BCAS3, RFX3, KCNQ1, ATXN2, CUX2, GCKR, PDZK1, CNTN5, and mitochondrial genetic variation [6–14]. For example, ABCG2 dysfunctional variants have a strong impact on the progression of hyperuricemia. The most common dysfunction variant rs2231142 (p.Q141K) increases the risk of gout and hyperuricemia, significantly influences the age of onset of gout, and is highly associated with a familial gout history [15]. Moreover, ABCG2 dysfunction was reported as a strong independent risk factor for pediatric-onset hyperuricemia/gout [16].
Notably, almost all these loci identified in gout GWAS were also associated with serum urate levels, indicating the shared genetic basis between gout and serum urate concentrations [5]. This is mainly because elevated serum urate levels are a critical risk factor for gout onset [17]. However, as GWAS for gout have been relatively limited in size and power compared with the GWAS of serum urate levels, less is known about the specific genetic contribution to gout as opposed to genetic associations of hyperuricemia. Association with gout at most urate-associated loci is still unclear.
SLC16A9 encodes monocarboxylate transporter 9 (MCT9), a member of solute carrier (SLC) superfamily that comprises more than 400 transporters [18]. SLC16A9 is ubiquitously expressed, including at particularly high levels in the kidney [19, 20]. Recent GWAS and meta-analysis revealed a significant association between polymorphisms of SLC16A9 and serum urate concentrations [7, 21, 22]. However, a role for variants of SLC16A9 and gout itself has not been demonstrated [7, 11]. To date, the transport substrate of MCT9 is still unknown and the function of SLC16A9 remains poorly understood, especially its potential association with gout.
Despite dozens of genetic loci identified in gout or urate-associated traits, little is known about the genetic aetiology of patients presenting with early-onset gout (EOG), which was defined as before the age of 40 years [23–26]. Previous studies have reported rs2231142 (Q141K) in ABCG2 as a genetic factor in early-onset gout [16, 27, 28]. In this study, we investigated a two-generation pedigree with early-onset gout. To elucidate the genetic predisposition, whole-exome sequencing (WES) was performed, and we identified a rare missense mutation (c.277C>A, p.L93M) in SLC16A9, providing new evidence for the association of SLC16A9 with gout.
2. Materials and Methods
2.1. Participant Recruitment
This study conformed to the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. Informed consent was obtained from the patient. Patients were clinically evaluated by rheumatologist according to the 2015 gout classification criteria by an American College of Rheumatology/European League against Rheumatism Collaborative Initiative [29, 30]. Peripheral blood samples were collected from patients and unaffected family members, from which genomic DNA was extracted.
2.2. Whole-Exome Sequencing
Whole-exome sequencing (WES) was performed on the proband. Briefly, genomic DNA was sheared into 200- to 250-base pair (bp) fragments using a Covaris S220 ultrasonicator. Then the fragments were ligated with adapters to both ends, amplified by ligation-mediated polymerase chain reaction, purified, and hybridized. Nonhybridized fragments were washed out. Enrichment of the DNA libraries was performed using the Exome Enrichment V5 Kit (Agilent Technologies, Palo Alto, CA, USA) according to the manufacturers' protocol. Subsequently, enriched DNA libraries were sequenced on a HiSeq X Ten sequencer (Illumina, San Diego, CA, USA). All raw sequencing data were processed according to a customized bioinformatics pipeline described previously [31]. After the quality control test, the reads were mapped to the reference human genome (hg19) using SOAPaligner software and further visualized using the SplicingViewer software [32]. SNV and Indel calls as well as annotation were performed using GATK tool and mirTrios with integrated ANNOVAR tool [33].
2.3. Variant Analyses and Identification
We used the following databases for selecting rare variants as an initial filtration: Genome Aggregation Database (http://gnomad.broadinstitute.org/), Exome Aggregation Consortium (ExAC, http://exac.broadinstitute.org/), NHLBI Exome Sequencing Project (ESP, http://evs.gs.washington.edu/EVS/), and 1000 Genome (http://www.1000genomes.org). Variants with a minor allele frequency of over 0.01 in any of these databases were discarded. The effects of the candidate variants were assessed using in silico prediction programs. Missense variants were analyzed by M-CAP (http://bejerano.stanford.edu/mcap/), Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/), and MutationTaster (http://mutationtaster.org/). Direct Sanger sequencing was then used to confirm the segregated variants in the present family, using an ABI 3500 Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA).
2.4. SLC16A9 Amplification and Genotyping
An additional cohort of unrelated cases (n = 30) with gout was recruited, and their DNA was submitted for Sanger sequencing. Primers were designed to amplify all coding regions and the intron-exon boundaries of the SLC16A9 gene. The PCR products were purified and sequenced on an ABI 3500 Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA).
3. Results
3.1. Clinical Observations
The proband was a 25-year-old male from a Han Chinese family, having suffered his first gout flare at the age of 20. His father was also diagnosed with gout having the first gout flare at the age of 25, whereas all the other family members were unaffected (Table 1 and Figure 1(a)).
Table 1.
Summary of clinical observations of the participants in this study.
| ID | Gender | Age (y) | SUA | HUA | SCr | BUN | FEUa | UPH | Onset age (y) | Arthritis | Tophi | TG | TC | Obesity | HBP | HG | Obesity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| II:1 | F | 63 | 257 | − | 51 | 6.8 | 6.5 | − | − | − | − | − | − | + | − | − | |
| II:4 | M | 48 | 581 | + | 69 | 4.2 | 3.77 | 6.0 | 25 | + | − | − | + | + | + | + | + |
| II:5 | F | 46 | 305 | − | 44 | 6.0 | 5.5 | − | − | − | + | − | + | − | − | + | |
| III:1 | M | 45 | 321 | − | 50 | 5.3 | 6.0 | − | − | − | − | − | − | − | − | − | |
| III:2 | M | 25 | 517 | + | 71 | 5.1 | 4.63 | 5.0 | 20 | + | − | − | − | − | − | − | − |
SUA, serum uric acid, μmol/l; HUA, hyperuricemia; SCr, serum creatine, μmol/l; BUN, blood urea nitrogen, mmol/l; FEUa, fractional excretion of uric acid,%; (hyperuricemia: male > 420 μmol/l; female > 360 μmol/l); UPH, urine PH; TG, triglyceride; TC, total cholesterol; HBP, high blood pressure; HG, hyperglycemia.
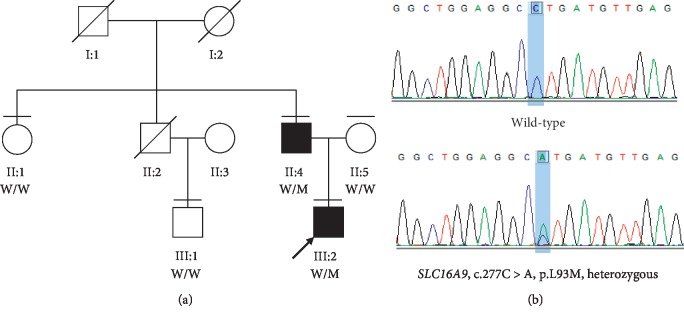
Figure 1.
Identification of SLC16A9 missense in the family with early-onset gout. (a) Pedigree and cosegregation results. Affected individual is represented as a filled square. Normal individuals are shown as empty symbols. (b) Sanger sequencing confirmed the segregation of the rare missense variant, c.277C>A (p.L93M).
Both proband and his affected father experienced recurrent acute monoarticular arthritis affecting the first metatarsophalangeal joint (MTP1) and/or knee starting at 20 and 25 years of age, respectively. The symptoms generally started at night and peaked within 24 hours, preventing walking and could not bear touch. The symptomatic course lasted no more than one week. The symptoms typically completely resolved within one or two days after taking nonsteroidal anti-inflammatory drug and colchicine. Patients have normal intelligence and are competent of the job. The muscle tension and renal function are normal, and no urate nephrolithiasis has been found. In both cases, serum uric acid was increased but not achieved to an extremely high level (see in Table 1), and the fractional excretion of uric acid (FEUa) was decreased (the normal range for FEUa is 7%–12%) [30, 34], consistent with renal underexcretion (RUE) gout. So the purine overproduction gout (HGPRT deficiency, PRPS1 superactivity) was excluded in the family. The detailed clinical information is summarized in Table 1.
3.2. Genetic Assessments
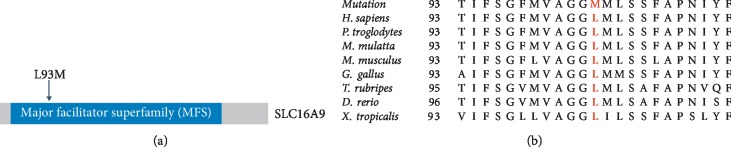
To reveal the genetic predisposition, WES was performed on the proband (III:2). The mean read depth for the WES was >100X and the coverage of the targeted regions (>1X) reached >99%. Variant analyses and a step-by-step filtering strategy by combination of minor allele frequency, in silico assessments, gene function, and cosegregation analysis were carried out [35–37]. A total of six candidate variants were submitted for cosegregation analysis and only one survived, a rare missense mutation (c.277C>A, p.L93M) in SLC16A9. This is an extremely rare variant (rs550527563) in all of the databases (Table 2). For example, the allele frequency is 0.0032% (8 in 251050) and 0.0033% (4 in 120986) in gnomAD and ExAC, respectively, while it is absent in ESP (Table 2). All these alleles are from Asian, while it is absent in Caucasians. Moreover, in silico assessments showed strong pathogenicity for this variant including the M-CAP, a newly developed tool for variants with uncertain significance in clinical exomes at high sensitivity [38]. Importantly, segregation testing in all available family members indicated that L93M cosegregated with the disease phenotype in this pedigree (Figures 1(a) and 1(b)). Both patients harbor a heterozygous variant while the healthy individuals do not have the nucleotide change. The variant c.277C>A results in a switch from leucine to methionine in the major facilitator superfamily (MFS) domain (Figure 2(a)). Multiple orthologous sequence alignment revealed that leucine at position 93 is in a highly conserved region across different species (Figure 2(b)).
Table 2.
Variant identified in patients with early-onset gout.
| ID | Variant | Type | Frequency (allele count) | In silico assessments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| gnomAD | ExAC | ESP | 1K | Polyphen-2 | MutationTaster | LRT | M-CAP | |||
| II:4 | c.277C>A, p.L93M | Hetero | 0.0032% (8) | 0.0033% (4) | 0 | 0.04% (2) | Damaging | Damaging | Damaging | Possibly pathogenic |
| III:2 | c.277C>A, p.L93M | Hetero | 0.0032% (8) | 0.0033% (4) | 0 | 0.04% (2) | Damaging | Damaging | Damaging | Possibly pathogenic |
Figure 2.
(a) Domain structure of SLC16A9 and location of L93M variant. (b) Conservation analyses of the mutated residues 93 in SLC16A9 across different species.
The probability of being loss-of-function (LoF) intolerant (pLI) is 0.64, and the expected number of LoF is 11.7 while the observed number is only 2, suggesting the probability of being a functionally important variant [39]. Expanded screening of SLC16A9 in a cohort of 30 patients with gout failed to identify any additional rare variants in this gene. Taken together, WES revealed a putative causal variant in SLC16A9 in a family with early-onset gout.
4. Discussion
In the present study, we recruited an unusual pedigree with early-onset gout. It is reasonable to speculate that it is possibly caused by rare monogenic variants because of two reasons, the family history and early-onset age. Firstly, this gout pedigree exhibited an autosomal dominant-like trait, consistent with a monogenic aetiology. More importantly, both proband and his affected father suffered first gout flare at early age. Epidemiological studies show that gout incidence increases with age until the age of 70 years and that onset before the age of 40 years is unusual [40, 41]. A few studies have demonstrated that complex disease with early-onset age could be caused by monogenic inheritance of mutated genes [42–44]. Therefore, aiming to investigate potential causal gene in this pedigree, we used WES which has proven to be highly robust and efficient in the identification of disease-causing genes in monogenic conditions or complex disorders [45]. Using this approach, we identified a rare missense in SLC16A9 gene by the comprehensive analyses including allele frequency, in silico assessments, gene function, and cosegregation analysis.
A few studies supported our results of the association between SLC16A9 and gout. The first evidence reported by Kolz et al. observed a SNP in SLC16A9, rs12356193, was significantly associated with serum uric acid levels by a meta-analysis of 28,141 individuals of European descent (P=1.1 × 10−8) [21]. Then the locus was successfully replicated in a cohort of 7,795 individuals [22]. Nakayama et al. investigated the relationship between another common variant (rs2242206) and gout. They found that the P value was significant in renal overload gout (ROL), but not with all gout susceptibility [46]. Subsequently, Köttgen et al. confirmed the SLC16A9 locus was associated with serum urate concentrations (rs1171614, P=2.3 × 10−28), but showed only nominal association with gout (rs1171614, P=1.7 × 10−2) [7]. Phipps-Green et al. tested 28 loci for association with gout in 1536 cases with gout and 2645 controls. At SLC16A9, the observed association with gout was restricted to the lower Polynesian ancestry group (rs12356193, P=0.006) [11]. Of note, the relationship between GWAS signals and genes underlying Mendelian phenotypes has been observed [47, 48]. Thus, it is reasonable to find rare pathogenic variants in GWAS signals. In addition to these genetic association studies, several studies also provided functional evidence. SLC16A9 is ubiquitously expressed and is especially expressed at a high level in the kidney [19, 20]. ALDH16A1 gene is associated with serum uric acid levels and gout, and RNA sequencing in the kidney of wild-type (WT) and Aldh16a1 knockout (KO) mice revealed changes in Slc16a9 are localized to the apical membrane of the proximal convoluted tubule cells and influence uric acid homeostasis [49]. These findings suggested the potential role of SLC16A9 in the aetiology of gout.
However, there are two main limitations in this study. Firstly, no functional genomics studies were performed in the present study. Experimental validations are essential to determine if interesting variants are indeed responsible for clinical symptoms [50, 51]. For example, a recent study demonstrates the rare variants of ABCG2 at both the clinical level and the functional level by complex approach [52]. Second is the lack of independent replication family. The genetic screening of SLC16A9 in gout pedigrees is required in the future studies. The copy number variations (CNVs) are not considered in this study [53].
In conclusion, we provide the first evidence for the association of rare missense in SLC16A9 with early-onset gout. These findings not only expand our current understanding of gout, but also may have further implications for the treatment and prevention of gout.
Acknowledgments
This study was supported by the Zhejiang Provincial Natural Science Foundation (LY20H100001), National Natural Science Foundation of China (31771390), and Wenzhou Science and Technology Bureau (Y20180129).
Data Availability
Summary data are available from the corresponding author on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
X.X. and X.F.H. conceived and designed the experiments; X.X. and L.S. recruited patients and collected samples; X.F.H., L.S., and C.Z. performed the experiments, analyzed data, and contributed equally to this work; Z.Z., H.C., and L.Z. contributed reagents/materials/analysis tools; X.X., X.F.H., and M.A.B. wrote and revised the manuscript. M.A.B. and X.X. also contributed equally to this work. X.F.H., L.S., and C.Z. contributed equally to this work.
References
- 1.Richette P., Bardin T. Gout. The Lancet. 2010;375(9711):318–328. doi: 10.1016/s0140-6736(09)60883-7. [DOI] [PubMed] [Google Scholar]
- 2.Wallace K. L., Riedel A. A., Joseph-Ridge N., Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. The Journal of Rheumatology. 2004;31(8):1582–1587. [PubMed] [Google Scholar]
- 3.Annemans L., Spaepen E., Gaskin M., et al. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000-2005. Annals of the Rheumatic Diseases. 2008;67(7):960–966. doi: 10.1136/ard.2007.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miao Z., Li C., Chen Y., et al. Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China. The Journal of Rheumatology. 2008;35(9):1859–1864. [PubMed] [Google Scholar]
- 5.Major T. J., Dalbeth N., Stahl E. A., Merriman T. R. An update on the genetics of hyperuricaemia and gout. Nature Reviews Rheumatology. 2018;14(6):341–353. doi: 10.1038/s41584-018-0004-x. [DOI] [PubMed] [Google Scholar]
- 6.Sulem P., Gudbjartsson D. F., Walters G. B., et al. Identification of low-frequency variants associated with gout and serum uric acid levels. Nature Genetics. 2011;43(11):1127–1130. doi: 10.1038/ng.972. [DOI] [PubMed] [Google Scholar]
- 7.Köttgen A., Albrecht E., Teumer A., et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nature Genetics. 2013;45(2):145–154. doi: 10.1038/ng.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C., Li Z., Liu S., et al. Genome-wide association analysis identifies three new risk loci for gout arthritis in Han Chinese. Nature Communications. 2015;6(1):p. 7041. doi: 10.1038/ncomms8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuo H., Yamamoto K., Nakaoka H., et al. Genome-wide association study of clinically defined gout identifies multiple risk loci and its association with clinical subtypes. Annals of the Rheumatic Diseases. 2016;75(4):652–659. doi: 10.1136/annrheumdis-2014-206191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama A., Nakaoka H., Yamamoto K., et al. GWAS of clinically defined gout and subtypes identifies multiple susceptibility loci that include urate transporter genes. Annals of the Rheumatic Diseases. 2017;76(5):869–877. doi: 10.1136/annrheumdis-2016-209632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phipps-Green A. J., Merriman M. E., Topless R., et al. Twenty-eight loci that influence serum urate levels: analysis of association with gout. Annals of the Rheumatic Diseases. 2016;75(1):124–130. doi: 10.1136/annrheumdis-2014-205877. [DOI] [PubMed] [Google Scholar]
- 12.Kawamura Y., Nakaoka H., Nakayama A., et al. Genome-wide association study revealed novel loci which aggravate asymptomatic hyperuricaemia into gout. Annals of the Rheumatic Diseases. 2019;78(10):1430–1437. doi: 10.1136/annrheumdis-2019-215521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gosling A. L., Boocock J., Dalbeth N., et al. Mitochondrial genetic variation and gout in Maori and Pacific people living in Aotearoa New Zealand. Annals of the Rheumatic Diseases. 2018;77(4):571–578. doi: 10.1136/annrheumdis-2017-212416. [DOI] [PubMed] [Google Scholar]
- 14.Tin A., Marten J., Halperin Kuhns V. L., et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nature Genetics. 2019;51(10):1459–1474. doi: 10.1038/s41588-019-0504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stiburkova B., Pavelcova K., Zavada J., et al. Functional non-synonymous variants of ABCG2 and gout risk. Rheumatology. 2017;56(11):1982–1992. doi: 10.1093/rheumatology/kex295. [DOI] [PubMed] [Google Scholar]
- 16.Stiburkova B., Pavelcova K., Pavlikova M., Ješina P., Pavelka K. The impact of dysfunctional variants of ABCG2 on hyperuricemia and gout in pediatric-onset patients. Arthritis Research & Therapy. 2019;21(1):p. 77. doi: 10.1186/s13075-019-1860-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riches P. L., Wright A. F., Ralston S. H. Recent insights into the pathogenesis of hyperuricaemia and gout. Human Molecular Genetics. 2009;18(R2):R177–R184. doi: 10.1093/hmg/ddp369. [DOI] [PubMed] [Google Scholar]
- 18.César-Razquin A., Snijder B., Frappier-Brinton T., et al. A call for systematic research on solute carriers. Cell. 2015;162(3):478–487. doi: 10.1016/j.cell.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Halestrap A. P., Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Archiv European Journal of Physiology. 2004;447(5):619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 20.Halestrap A. P. The SLC16 gene family—structure, role and regulation in health and disease. Molecular Aspects of Medicine. 2013;34(2-3):337–349. doi: 10.1016/j.mam.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Kolz M., Johnson T., Sanna S., et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genetics. 2009;5(6) doi: 10.1371/journal.pgen.1000504.e1000504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Harst P., Bakker S. J. L., de Boer R. A., et al. Replication of the five novel loci for uric acid concentrations and potential mediating mechanisms. Human Molecular Genetics. 2010;19(2):387–395. doi: 10.1093/hmg/ddp489. [DOI] [PubMed] [Google Scholar]
- 23.Richette P., Doherty M., Pascual E., et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Annals of the Rheumatic Diseases. 2017;76(1):29–42. doi: 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 24.Yu K.-H., Luo S. F. Younger age of onset of gout in Taiwan. Rheumatology. 2003;42(1):166–170. doi: 10.1093/rheumatology/keg035. [DOI] [PubMed] [Google Scholar]
- 25.Zhang B., Fang W., Zeng X., et al. Clinical characteristics of early- and late-onset gout: a cross-sectional observational study from a Chinese gout clinic. Medicine (Baltimore) 2016;95(47):p. e5425. doi: 10.1097/md.0000000000005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascart T., Norberciak L., Ea H. K., Guggenbuhl P., Lioté F. GOSPEL 4—patients with early onset gout develop earlier severe joint involvement and metabolic comorbid conditions. Arthritis Care and Research (Hoboken) 2019;71(7):986–992. doi: 10.1002/acr.23706. [DOI] [PubMed] [Google Scholar]
- 27.Cleophas M., Joosten L., Stamp L., Dalbeth N., Woodward O., Merriman T. ABCG2 polymorphisms in gout: insights into disease susceptibility and treatment approaches. Pharmacogenomics and Personalized Medicine. 2017;10:129–142. doi: 10.2147/pgpm.s105854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuo H., Tomiyama H., Satake W., et al. ABCG2 variant has opposing effects on onset ages of Parkinson’s disease and gout. Annals of Clinical and Translational Neurology. 2015;2(3):302–306. doi: 10.1002/acn3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neogi T., Jansen T. L. T. A., Dalbeth N., et al. 2015 gout classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis & Rheumatology. 2015;67(10):2557–2568. doi: 10.1002/art.39254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neogi T., Jansen T. L. T. A., Dalbeth N., et al. 2015 gout classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Annals of the Rheumatic Diseases. 2015;74(10):1789–1798. doi: 10.1136/annrheumdis-2015-208237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang T., Liu Q., Li X., et al. RRBS-analyser: a comprehensive web server for reduced representation bisulfite sequencing data analysis. Human Mutation. 2013;34(12):1606–1610. doi: 10.1002/humu.22444. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q., Chen C., Shen E., Zhao F., Sun Z., Wu J. Detection, annotation and visualization of alternative splicing from RNA-Seq data with SplicingViewer. Genomics. 2012;99(3):178–182. doi: 10.1016/j.ygeno.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Li J., Jiang Y., Wang T., et al. mirTrios: an integrated pipeline for detection of de novo and rare inherited mutations from trios-based next-generation sequencing. Journal of Medical Genetics. 2015;52(4):275–281. doi: 10.1136/jmedgenet-2014-102656. [DOI] [PubMed] [Google Scholar]
- 34.Li Q. H., Liang J. J., Chen L. X., et al. Clinical characteristics and renal uric acid excretion in early-onset gout patients. Zhonghua Nei Ke Za Zhi. 2018;57(3):185–190. doi: 10.3760/cma.j.issn.0578-1426.2018.03.007. in Chinese. [DOI] [PubMed] [Google Scholar]
- 35.Huang X. F., Huang Z. Q., Lin D., et al. Unraveling the genetic cause of a consanguineous family with unilateral coloboma and retinoschisis: expanding the phenotypic variability of RAX mutations. Scientific Reports. 2017;7(1):p. 9064. doi: 10.1038/s41598-017-09276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang X.-F., Huang F., Wu K.-C., et al. Genotype-phenotype correlation and mutation spectrum in a large cohort of patients with inherited retinal dystrophy revealed by next-generation sequencing. Genetics in Medicine. 2015;17(4):271–278. doi: 10.1038/gim.2014.138. [DOI] [PubMed] [Google Scholar]
- 37.Huang X.-F., Wu J., Lv J.-N., Zhang X., Jin Z.-B. Identification of false-negative mutations missed by next-generation sequencing in retinitis pigmentosa patients: a complementary approach to clinical genetic diagnostic testing. Genetics in Medicine. 2015;17(4):307–311. doi: 10.1038/gim.2014.193. [DOI] [PubMed] [Google Scholar]
- 38.Jagadeesh K. A., Wenger A. M., Berger M. J., et al. M-CAP eliminates a majority of variants of uncertain significance in clinical exomes at high sensitivity. Nature Genetics. 2016;48(12):1581–1586. doi: 10.1038/ng.3703. [DOI] [PubMed] [Google Scholar]
- 39.Lek M., Karczewski K. J., Minikel E. V., et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doherty M. New insights into the epidemiology of gout. Rheumatology. 2009;48(Suppl 2):ii2–ii8. doi: 10.1093/rheumatology/kep086. [DOI] [PubMed] [Google Scholar]
- 41.Kuo C.-F., Grainge M. J., Zhang W., Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nature Reviews Rheumatology. 2015;11(11):649–662. doi: 10.1038/nrrheum.2015.91. [DOI] [PubMed] [Google Scholar]
- 42.Barbier M., Wallon D., Le Ber I. Monogenic inheritance in early-onset dementia: illustration in Alzheimer’s disease and frontotemporal lobar dementia. Gériatrie et Psychologie Neuropsychiatrie du Viellissement. 2018;16(3):289–297. doi: 10.1684/pnv.2018.0744. [DOI] [PubMed] [Google Scholar]
- 43.Jin Z.-B., Wu J., Huang X.-F., et al. Trio-based exome sequencing arrests de novo mutations in early-onset high myopia. Proceedings of the National Academy of Sciences. 2017;114(16):4219–4224. doi: 10.1073/pnas.1615970114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bansal V., Gassenhuber J., Phillips T., et al. Spectrum of mutations in monogenic diabetes genes identified from high-throughput DNA sequencing of 6888 individuals. BMC Medicine. 2017;15(1):p. 213. doi: 10.1186/s12916-017-0977-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J., Shen E., Shi D., Sun Z., Cai T. Identification of a novel Cys146X mutation of SOD1 in familial amyotrophic lateral sclerosis by whole-exome sequencing. Genetics in Medicine. 2012;14(9):823–826. doi: 10.1038/gim.2012.50. [DOI] [PubMed] [Google Scholar]
- 46.Nakayama A., Matsuo H., Shimizu T., et al. Common missense variant of monocarboxylate transporter 9 (MCT9/SLC16A9) gene is associated with renal overload gout, but not with all gout susceptibility. Human Cell. 2013;26(4):133–136. doi: 10.1007/s13577-013-0073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chong J. X., Buckingham K. J., Jhangiani S. N., et al. The genetic basis of mendelian phenotypes: discoveries, challenges, and opportunities. The American Journal of Human Genetics. 2015;97(2):199–215. doi: 10.1016/j.ajhg.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freund M. K., Burch K. S., Shi H., et al. Phenotype-specific enrichment of mendelian disorder genes near GWAS regions across 62 complex traits. The American Journal of Human Genetics. 2018;103(4):535–552. doi: 10.1016/j.ajhg.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charkoftaki G., Chen Y., Han M., et al. Transcriptomic analysis and plasma metabolomics in Aldh16a1-null mice reveals a potential role of ALDH16A1 in renal function. Chemico-Biological Interactions. 2017;276:15–22. doi: 10.1016/j.cbi.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang X. F., Xiang L., Fang X. L., et al. Functional characterization of CEP250 variant identified in nonsyndromic retinitis pigmentosa. Human Mutation. 2019;40(8):1039–1045. doi: 10.1002/humu.23759. [DOI] [PubMed] [Google Scholar]
- 51.Huang X. F., Xiang L., Cheng W., et al. Mutation of IPO13 causes recessive ocular coloboma, microphthalmia, and cataract. Experimental & Molecular Medicine. 2018;50(4):p. 53. doi: 10.1038/s12276-018-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toyoda Y., Mančíková A., Krylov V., et al. Functional characterization of clinically-relevant rare variants in ABCG2 identified in a gout and hyperuricemia cohort. Cells. 2019;8(4):p. 363. doi: 10.3390/cells8040363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang X.-F., Mao J.-Y., Huang Z.-Q., et al. Genome-wide detection of copy number variations in unsolved inherited retinal disease. Investigative Opthalmology & Visual Science. 2017;58(1):424–429. doi: 10.1167/iovs.16-20705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Summary data are available from the corresponding author on request.