Abstract
Aim:
Comparison of casein phosphopeptides (CPPs) and amorphous calcium phosphate (ACP), tricalcium phosphate, and hydroxyapatite on assessment of dentine tubule occlusion on primary enamel using scanning electron microscope (SEM).
Materials and Methods:
A total of 20 freshly extracted noncarious primary molars were randomly divided into four groups (A to D) with five sections in each group; group A: negative control, group B: CPP-ACP, group C: tricalcium phosphate, and group D: Hydroxyapatite (HA). To assess tubule occlusion, 20 dentin sections of 2 mm thickness were obtained from cervical third of sound primary molars. Each section were processed to simulate the hypersensitive dentin and the test agents were brushed over the sections with an electric toothbrush and observed under SEM for calculation of the percentage of occluded tubules.
Results:
Group B and D showed greater percentage of tubule occlusion than group C. Intergroup comparison of tubule occlusion potential of Group B and D was not significant.
Conclusion:
HA showed significantly higher dentinal tubule occlusion when compared to CPP-ACP and tricalcium phosphate.
Keywords: CPP-ACP, dentinal hypersensitivity, hydroxyapatite, scanning electron microscope, tricalcium phosphate
Introduction
Dentine hypersensitivity refers to the transient and severe pain arising from stimulation of exposed dentine with cold, heat, and mechanical pressure. Many diseases, including physiological wear, dental erosion, enamel hypoplasia, wedge-shaped defects, bruxism, and deep carious lesions can lead to exposed dentine.[1] This condition is described clinically as an exaggerated response to a nonnoxious stimulus and is the result of dentin tubules exposure due to loss of enamel.[2] Its prevalence greatly varies between 3% and 98% depending on the population, study setting, and study design.[3,4]
Dental erosion and early childhood caries (ECC) is being recognized as a common condition in pediatric dentistry with complications of tooth sensitivity, altered aesthetics, and loss of occlusal vertical dimension. Recently, the prevalence of erosion in children has been reported to range from 10% to over 80%,[5] whereas for ECC it has been reported to range from 5% to 85% in developed countries.[6] Despite the increase in prevalence of these conditions, limited literature is available in support with regard to primary dentition. Bearing this in mind, we have undertaken this study to find substantial evidence for treatment of the same.
Dentin tubules occlusion is the most current therapeutic approach.[7] Recently, good clinical results were reported with products containing arginine/calcium carbonate, calcium sodium phosphosilicate (NovaMin), or strontium acetate.[8,9] Fluoride varnish was the first Food and Drug Administration-approved agent for treatment of hypersensitivity, and it protects the dentin surface by forming a protective layer of calcium fluoride.[10]
Recently, introduction of newer materials like casein phosphopeptides (CPPs) and amorphous calcium phosphate (ACP) (that is derived from bovine milk protein, casein, calcium, and phosphate),[11] tricalcium phosphates (calcium phosphate system, stable in aqueous environment, and does not affect the fluoride activity when added in the dentifrices)[12] and Remin pro (hydroxyapatite, HA that fills eroded enamel, fluoride seals dentinal tubule, and xylitol acts as an antibacterial agent) and has been recommended for the management of dentinal hypersensitivity.[13] Therefore, considering all the factors, aim of the present study is to evaluate and compare CPP-ACP, tricalcium phosphate, and HA on assessment of dentine tubule occlusion on primary enamel using SEM.
Materials and Method
Ethical Approval was obtained from the institutional review board (CDS/2017/1787) after which the study was undertaken. A total of 20 primary molars extracted for therapeutic reasons were selected and were thoroughly cleaned free of debris and calculus using hand scalers and stored in 10% formalin till the time of commencement of study.
Assessment of dentine tubule occlusion
The 20 primary molars were used to prepare dentin discs of 2 mm thickness from the coronal portion of the tooth just below the level of the cementoenamel junction using double-sided diamond disk with a micromotor hand piece. These dentin discs were then polished with silicon carbide paper of 320, 600, and 800 grit and were ultrasonicated in distilled water for 10 min to remove the residual smear layer and then etched by immersing the specimens in a tray containing 6% citric acid for 2 min to simulate dentin hypersensitivity.
The 20 dentine specimens were divided into the four above-mentioned groups (n = 5). The test agents were brushed over the specimens using an electric toothbrush (Colgate 360° sonic power®) at 20,000 strokes per minute for 2 min twice a day for 7 days. The specimens were rinsed in distilled water after each brushing session and stored in a closed container containing distilled water. After the last brushing session, specimens were washed with distilled water and coated (MED 010- Jeol, Japan) with a thin gold layer following which the specimens were analyzed in a scanning electron microscope (DSM 840 A-Geol. Japan) operating at 10 kv in 2000 × magnification. The area in the center of each specimen was scanned so as to obtain tubules in a circular cross section. Photomicrographs were taken to analyze the following study parameters [Figure 1].
Figure 1.
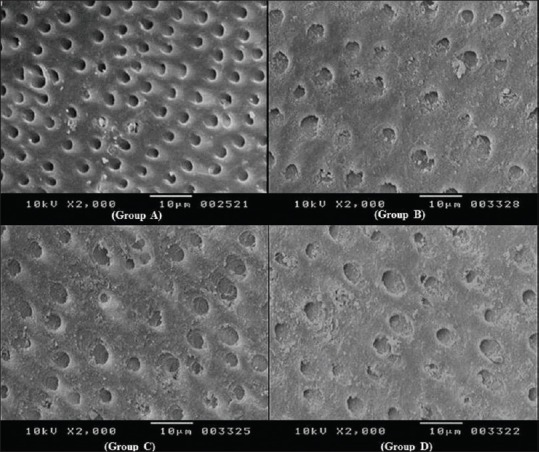
Dentinal tubule occlusion seen in all four groups (A to D)
Number of tubules occluded per unit area.
Number of tubules patent per unit area.
Quantitative analysis was performed by counting the numbers of dentinal tubules at 2000 × magnification considering a total area of 1600 μm2, grids were applied on the photomicrograph using CorelDraw CS3 software.
Results
The results were subjected for appropriate statistical analysis; Kruskal–Wallis test revealed that intergroup comparison of the mean percentage of tubule occlusion was statistically highly significant (P value < 0.001) [Table 1] and Mann–Whitney U test showed that the tubule occlusion potential was statistically significant among all groups except for Group B and D which was not significant [Table 2 and Figure 1].
Table 1.
Comparison of difference in tubules closed
| Study groups | Mean (%) | S.D | P (Kruskal-Wallis test) |
|---|---|---|---|
| Group A | 3.34 | 3.38 | <0.001** HS |
| Group B | 65.33 | 5.95 | |
| Group C | 48.18 | 7.33 | |
| Group D | 71.81 | 5.51 |
**P<0.001, HS (highly significant)
Table 2.
Multiple comparisons of all four groups
| Study groups | Multiple comparisons (Mann Whitney Test) |
|---|---|
| A & B | (P=0.008*) S |
| A & C | (P=0.008*) S |
| A & D | (P=0.009*) S |
| B & C | (P=0.008*) S |
| B & D | (P=0.151) NS |
| C & D | (P=0.009*) S |
**P<0.001, HS (highly significant). *P<0.05, S (significant). P>0.05, NS (not significant)
Discussion
Dentin hypersensitivity that can be a potential threat to the individual's oral health is closely related to the exposure and patency of dentinal tubules. Many factors may contribute to the exposure of dentinal tubules, such as occlusal wear, deep carious lesion, abrasion due to brushing, dietary erosion, parafunctional habit, abnormal tooth positioning, and abfractive lesions. The condition has been treated by a number of agents, which have been claimed to reduce pain by occluding dentinal tubules.[14] Desensitizing dentifrice is preferred treatment for hypersensitivities because tooth brushing is one of the easiest methods in a home care system.
Various in vivo studies have shown considerable decrease in hypersensitivity when teeth were brushed with desensitizing dentifrices.[15] The in vitro dentine disc dentinal tubule blockage experiment has become the gold standard for assessment of dentine hypersensitivity.[16,17] Utilizing safe and effective biological materials through their physical or chemical properties to block exposed dentinal tubules, to reduce, or inhibit the flow of tubular fluid and to avoid stimulating pulp nerve endings is thus an effective means for controlling dentin sensitivities.[18]
Human cervical dentine has reported to have 19,000 tubules per mm2 in superficial dentine. As the half-way point between the superficial dentine and the pulp is reached, the number of tubules reached to 30,000 tubules per mm[2,19] Dentinal tubule area was evaluated by SEM at 2,000 times magnification (total area of 16,00 μm2). Quantitative analysis of dentinal tubule number could be prejudice due to changes in direction of dentinal tubules and in the position of SEM samples. The presence of smear layer and small analyzed area could also introduce some bias. Hirayama et al.[20] reported that the tubules of primary dentin had smaller diameters because the peritubular dentin matrix was wider than that of permanent dentin.
Occluding dentinal tubule agents can create a barrier by precipitating proteins and calcium/phosphate ions on surface or within the tubule orifices. The mechanism of action of various chemical desensitizing agents is still not well understood.[21] Most of the previous measurements of dentin permeability have been carried out on the coronal[22,23] or radicular dentin of human permanent teeth. However, no studies have been performed on primary teeth. Permeability is defined as the ability of a membrane to permit solutes or solvents to pass through it.[24]
The agents used in the present study used are HA (Remin pro), CPP-ACP (GC tooth mousse), and tricalcium phosphate (Clinpro tooth crème) which significantly shown occlusion of the dentine tubules by above-mentioned topical desensitizing agents.
CPP-ACP is the acronym for a complex of CPPs and ACP, which is used as a desensitizing agent for the present study. Dentin surface treated with CPP-ACP showed substantial crystal-like deposits within the tubule lumen. Nevertheless, in few zones, the layer of amorphous calcium phosphate present on the dentin covered the orifices of dentinal tubules [Figure 1]. When the peptide complex binds to plaque or the tooth surface, it is said to deliver bioavailable calcium and phosphate for remineralization, resulting in occlusion of dentin tubules.[25]
In the present study, CPP-ACP showed 65.33% of tubule occlusion when compared to tricalcium phosphate (TCP) group with 48.18% tubule occlusion. Contradictory to our results, a study done by Prabhakar et al.[14] stated that there was 29.51% tubule occlusion seen with GC MI paste plus as it had shown weak ability to occlude dentinal tubule when compared to NaF and Clinpro tooth creame. GC MI paste had almost similar composition with that of GC tooth mousse, which was used for the present study.
TCP has been considered as one possible means for enhancing the levels of calcium in plaque and saliva. Combining calcium phosphate and fluoride ions in oral care products is problematic and can lead to loss of bioavailable fluoride ion due to a reaction between the calcium phosphate phase and the fluoride ion. In an approach to overcome this incompatibility of calcium phosphates and fluoride ions, new technologies have been developed. This technology is functionalized tricalcium phosphate TCP where tricalcium phosphate particles have been ball milled with sodium lauryl sulfate and has been included in a tooth creame with sodium fluoride marketed as Clinpro tooth crème (3 M ESPE).[26]
In the present study, TCP showed 48.18% of tubule occlusion when compared to CPP-ACP (65.33%) and HA (71.81%). This SEM observation was supported by Prabhakar et al.[14] in which Clinpro tooth creame showed 45.74% of tubule occlusion and similar findings were noted in the previous study conducted by Mackey.[27] Presence of fluoride concentration of 950 ppm may contribute to the occlusion of tubules apart from calcium phosphate system.
HA is one of the most biocompatible and bioactive materials and is widely applied to coat artificial joints and tooth roots. In the present study, hydroxyapatite (Remin pro) was used as desensitizing agent, which is a water-based cream, containing HA, fluoride, and xylitol. HA which is the main constituent of Remin Pro fills the superficial enamel lesions and the tiniest irregularities that arise from erosion. Fluoride which is also one of the content of Remin pro gets converted to fluorapatite when it comes in contact with saliva, thus strengthens the tooth and renders it more resistant to acid attacks and also it seals dentinal tubule. Xylitol reduces the harmful effects of bacteria and their metabolic product to lactic acid. Since HA has a crystal structure similar to human teeth, preliminary researches exploring the effects of HA in easing dentine hypersensitivity, remineralization of early enamel lesion by adding HA to dentifrice have been reported in recent years.[28]
In 2012, a study by Yuan et al.[18] concluded that HA added to ordinary dentifrice showed a significantly increased effect of dentinal tubule occlusion which was similar to commercially available antidentin sensitive dentrifices as reported by commercial research of such products. Similarly, in the present study HA (Remin pro) showed highly significant dentinal tubule occlusion when compare with CPP-ACP and tricalcium phosphate. A recent study evaluated the effectiveness of five different remineralizing agents on dentine permeability for treatment of dental hypersensitivity. It was seen that all five remineralizing agents occlude dentine tubules and reduce dentin permeability which is similar to our study.[29] Sharda et al.[30] evaluated the effectiveness of desensitizing dentifrice and mouthwash on dentin hypersensitivity and tooth remineralization. It was seen that desensitizing mouthwash was as effective as desentizing toothpaste. George et al. evaluated dentinal tubule occlusion using a desensitizing toothpaste and mouthwash for a period of 60 days using SEM. They found dentinal tubule occlusion was more with desentizing toothpaste which was similar to our study.[31]
Dentine tubule occlusion in an in vitro may be quite different when compared with dynamic, complex biological system, which usually occurs in the oral cavity in vivo. Thus, direct extrapolations to clinical conditions must be exercised with caution because of the obvious limitations of in vitro studies. The present study is significant as dentine tubule occlusion was seen in all groups indicating that remineralizing agents can be used for the treatment of dentine hypersensitivity in primary teeth allowing practitioners to provide best treatment for their patients, bringing a paradigm shift in treatment options. However, there is a need for the further long-term research under clinical conditions to prove the efficacy of these agents.
Conclusion
It was concluded that all the groups showed significant dentinal tubule occlusion; however, SEM observation revealed that dentinal tubule occlusion was seen significantly higher in HA when compared to CPP-ACP and TCP group.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Parolia A, Kundabala M, Mohan M. Management of dentinal hypersensitivity: A review. J Calif Dent Assoc. 2011;39:167–79. [PubMed] [Google Scholar]
- 2.Addy M, Hunter ML. Can tooth brushing damage your health? Effects on oral and dental tissues. Int Dent J. 2003;53:177–86. doi: 10.1111/j.1875-595x.2003.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 3.Splieth CH, Tachou A. Epidemiology of dentin hypersensitivity. Clin Oral Invest. 2013;17:S3–8. doi: 10.1007/s00784-012-0889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunha-Cruz J, Wataha JC, Heaton LJ, Rothen M, Sobieraj M, Scott J, et al. Northwest Practice-based Research Collaborative in Evidence-based dentistry. The prevalence of dentin hypersensitivity in general dental practices in the northwest United States. J Am Dent Assoc. 2013;144:288–96. doi: 10.14219/jada.archive.2013.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taji S, Seow WK. A literature review of dental erosion in children. Aust Dent J. 2010;55:358–67. doi: 10.1111/j.1834-7819.2010.01255.x. [DOI] [PubMed] [Google Scholar]
- 6.Çolak H, Dülgergil CT, Dalli M, Hamidi MM. Early childhood caries update: A review of causes, diagnoses, and treatments. J Nat Sci Biol Med. 2013;4:29–38. doi: 10.4103/0976-9668.107257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidlin PR, Sahrmann P. Current management of dentin hypersensitivity. Clin Oral Invest. 2013;17:S55–9. doi: 10.1007/s00784-012-0912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummins D. Recent advances in dentin hypersensitivity: Clinically proven treatments for instant and lasting sensitivity relief. Am J Dent. 2010;23(Spec No A):3A–13A. [PubMed] [Google Scholar]
- 9.Gendreau L, Barlow AP, Mason SC. Overview of the clinical evidence for the use of NovaMin in providing relief from the pain of dentin hypersensitivity. J Clin Dent. 2011;22:90–5. [PubMed] [Google Scholar]
- 10.Ritter AV, de La Dias W, Miguez P, Caplan DJ, Swift EJJ. Treating cervical dentin hypersensitivity with fluoride varnish: A randomized clinical study. J Am Dent Assoc. 2006;137:1013–20. doi: 10.14219/jada.archive.2006.0324. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds EC. Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res. 1997;76:1587–95. doi: 10.1177/00220345970760091101. [DOI] [PubMed] [Google Scholar]
- 12.Balakrishnan A, Jonathan R, Benin P, Kuumar A. Evaluation to determine the caries remineralization potential of three dentifrices: An in vitro study. J Conserv Dent. 2013;16:375–9. doi: 10.4103/0972-0707.114347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamin S, Roshni, Pradhan S, Nainan TM. Seal that heals. World J Dent. 2012;3:243–6. [Google Scholar]
- 14.Prabhakar AR, Manojkumar AJ, Basappa N. In vitro remineralization of enamel subsurface lesions and assessment of dentine tubule occlusion from NaF dentifrices with and without calcium. J Indian Soc Pedod Prev Dent. 2013;31:29–35. doi: 10.4103/0970-4388.112403. [DOI] [PubMed] [Google Scholar]
- 15.Arrais GC, Micheloni CD, Giannini M, Chan DC. Occluding effect of dentifrices on dentinal tubules. J Dent. 2003;31:577–84. doi: 10.1016/s0300-5712(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 16.Gillam DG, Mordan NJ, Sinodinou AD, Tang JY, Knowles JC, Gibson IR. The effects of oxalate-containing products on the exposed dentine surface: An SEM investigation. J Oral Rehabil. 2001;28:1037–44. doi: 10.1046/j.1365-2842.2001.00775.x. [DOI] [PubMed] [Google Scholar]
- 17.Kolker JL, Vargas MA, Armstrong SR, Dawson DV. Effect of desensitizing agents on dentin permeability and dentin tubule occlusion. J Adhes Dent. 2002;4:211–21. [PubMed] [Google Scholar]
- 18.Yuan P, Shen X, Liu J, Hou Y, Zhu M, Huang J, et al. Effects of dentifrice containing hydroxyapatite on dentinal tubule occlusion and aqueous hexavalent chromium cations sorption: A preliminary study. PLoS One. 2012;7:e45283. doi: 10.1371/journal.pone.0045283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garberoglio R, Brannstrom M. Scanning electron microscope investigation of human dentinal tubules. Arch Oral Biol. 1976;21:355–62. doi: 10.1016/s0003-9969(76)80003-9. [DOI] [PubMed] [Google Scholar]
- 20.Hirayama A, Yamada M, Miake K. Analytical electron microscopic studies on the dentinal tubules of human deciduous teeth. J Dent Res. 1985;64:743. [Google Scholar]
- 21.West NX, Hughes JA, Addy M. The effect of pH on erosion of dentine and enamel by dietary acids in vitro. J Oral Rehabil. 2001;28:860–4. doi: 10.1046/j.1365-2842.2001.00778.x. [DOI] [PubMed] [Google Scholar]
- 22.Pashley EL, Talman R, Horner JA, Pashley DH. Permeability of normal versus carious dentin. Endod Dent Traumatol. 1991;7:207–11. doi: 10.1111/j.1600-9657.1991.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 23.Tagami J, Hosoda H, Burrow MF, Nakajima M. Effect of aging and caries on dentin permeability. Proc Finn Dent Soc. 1992;88(Suppl 1):149–54. [PubMed] [Google Scholar]
- 24.Pashley DH. Dentin-predentin complex and its permeability: Physiologic overview. J Dent Res. 1985;64:613–20. doi: 10.1177/002203458506400419. [DOI] [PubMed] [Google Scholar]
- 25.Kowalczyk A, Botulinski B, Jaworska M, Kierklo A, Pawinska M, Dabrowska E. Evaluation of the product based on Recaldent technology in the treatment of dentin hypersensitivity. Adv Med Sci. 2006;51:40–2. [PubMed] [Google Scholar]
- 26.Shen P, Manton DJ, Cochrane NJ, Walker GD, Yuan Y, Reynolds C, et al. Effect of added calcium phosphate on enamel remineralization by fluoride in a randomized controlled in situ trial. J Dent. 2011;39:518–25. doi: 10.1016/j.jdent.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Mackey AC. In Vitro Assessment of Dentin Tubule Occlusion by Hypersensitivity Dentifrices 2009 April 1-4. Available from: http://iadrconfexcom/iadr/2009miami/webprogram/Paper 120922 Html .
- 28.Kamath U, Sheth H, Mullur D, Soubhagya M. The effect of Remin Pro ® on bleached enamel hardness: An in-vitro study. Indian J Dent Res. 2013;24:690–3. doi: 10.4103/0970-9290.127612. [DOI] [PubMed] [Google Scholar]
- 29.Berkathullah M, Farook MS, Mahmoud O. The effectiveness of remineralizing agents on dentinal permeability? BioMed Res Int. 2018;2018:4072815. doi: 10.1155/2018/4072815. doi: 10.1155/2018/4072815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharda S, Prasad KV, Shetty PJ, Nikhil K. Effectiveness of desensitizing dentifrice and mouthwash on dentin hypersensitivity and tooth remineralization. Contemp Clin Dent. 2018;9:415–20. doi: 10.4103/ccd.ccd_167_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George G, Ranjini M A, Pai VS, Darsan J, Nadig RR. Evaluation of dentinal tubule occlusion using a desensitizing toothpaste and mouthwash for a period of 60 days: Scanning electron microscopy analysis. J Interdiscip Dent. 2018;8:96–101. [Google Scholar]


