Abstract
The Systolic Blood Pressure Intervention Trial demonstrated reduced cardiovascular outcomes. We evaluated diabetes incidence in this randomized trial that compared intensive blood pressure strategy (systolic blood pressure <120 mmHg) versus standard strategy (<140 mm Hg). Participants were ≥50 years of age, with systolic 130-180 mm Hg and increased cardiovascular risk. Participants were excluded if they had diabetes mellitus, polycystic kidney disease, proteinuria >1 gm/day, heart failure, dementia, or stroke. Post randomization exclusions included participants missing blood glucose or ≥126 mg/dL (6.99 mmol/L), or on hypoglycemics. The outcome was incident diabetes: fasting blood glucose ≥126 mg/dL (6.99 mmol/L), diabetes self-report, or new use of hypoglycemics. The secondary outcome was impaired fasting glucose (100 to 125 mg/dL [5.55-6.94 mmol/L]) among those with normoglycemia (<100 mg/dL [5.55 mmol/L]). There were 9361 participants randomized and 981 excluded, yielding 4187 and 4193 participants assigned to intensive and standard strategies. There were 299 incident diabetes events (2.3% per year) for intensive and 251 events (1.9% per year) for standard, rates of 22.6 (20.2, 25.3) versus 19.0 (16.8, 21.5) events per 1000 person-years of treatment, respectively (adjusted hazard ratio 1.19, 95% confidence intervals [0.95, 1.49]). Impaired fasting glucose rates were 26.4 (24.9, 28.0) and 22.5 (21.1, 24.1) per 100 person-years for intensive and standard strategies (adjusted hazard ratio 1.17 [1.06, 1.30]). Intensive treatment strategy was not associated with increased diabetes but was associated with more impaired fasting glucose. The risks and benefits of intensive blood pressure targets should be factored into individualized patient treatment goals.
ClinicalTrials.gov number:
Keywords: Blood Pressure determination, Hypertension, Diabetes mellitus, Random Allocation, Cardiovascular disease
Graphical abstract
Hypertension, obesity and diabetes commonly co-exist, and all three conditions are associated with increased insulin resistance. Hyperinsulinemia can increase blood pressure via several mechanisms, including increased renal sodium reabsorption, activation of the sympathetic nervous system, alteration of transmembrane ion transport, and increased vascular resistance.1-4
Hypertension control has a complex relationship with diabetes. Control of hypertension can influence hyperinsulinemia and the subsequent risk for other insulin resistant states such as diabetes.5 In a large cohort study of low cardiovascular risk individuals in the United Kingdom, higher blood pressure was associated with an increased risk of incident diabetes.6 Antihypertensive medications, however, are associated with both increased (thiazide diuretics and beta blockers)7, 8 and decreased (angiotensin converting enzyme inhibitors and angiotensin II receptor blockers) risk for the development of diabetes.9 This relationship between blood pressure target goals and development of incident diabetes has not been explored in a higher cardiovascular risk population.
The Systolic Blood Pressure Intervention Trial (SPRINT) is a National Institutes of Health-sponsored trial of two differing blood pressure control strategies in persons free of diabetes mellitus who are at increased risk of developing cardiovascular disease (CVD). The relative hazard of the primary composite endpoint of SPRINT, which included myocardial infarction, acute coronary syndrome without myocardial infarction, stroke, acute decompensated heart failure, or death from cardiovascular causes, was significantly lower in the intensive arm (goal systolic blood pressure (SBP) <120 mm Hg) compared with the standard arm (goal SBP <140 mm Hg).10 National evaluations of hypertension control prior to the release of the SPRINT results demonstrated that attaining SBP goals of <140 mm Hg is difficult to achieve and often requires multiple medications.11-14
Understanding the risks and benefits associated with pursuing more aggressive blood pressure control is part of patient’s and provider’s ability to make an informed decision.15 The risks and benefits of developing or delaying diabetes are an integral part of this decision. Our aim was to test the hypothesis that more intensive blood pressure control is associated with a lower diabetes incidence among the SPRINT population and among certain high-risk subgroups, including those with underlying chronic kidney disease (CKD).
METHODS
Data Reproducibility Statement
All SPRINT anonymized data and materials are publicly available at the BioLINCC and can be accessed at https://biolincc.nhlbi.nih.gov/studies/sprint_pop/
Study Design and population
SPRINT was a randomized, controlled, open-label trial that was stopped in August 2015, because it demonstrated that treating to a target SBP of <120 mm Hg (Intensive) compared to <140 mm Hg (Standard) was more effective in preventing cardiovascular disease (CVD) outcomes and death. Participants were recruited from 102 clinical sites (organized into 5 clinical center networks) in the United States and Puerto Rico between November 2010 and March 2013. Additional details regarding randomization and the trial intervention have been reported previously.10, 16 Briefly, participants were eligible for inclusion if they were ≥50 years of age and had a screening SBP 130 to 180 mm Hg (on antihypertensive drug treatment or not), with an increased risk of cardiovascular (CV) events. Additional risk was defined by one or more of the following: clinical or subclinical CVD other than stroke, age ≥75 years, CKD, or a 10-year risk of CVD by Framingham risk score of 15% or greater.16 Participants were excluded if they had known diabetes mellitus, polycystic kidney disease, screening urine protein of >1 g/day or equivalent, symptomatic heart failure, ejection fraction <35% or stroke.
For the main analysis we further excluded participants who may have begun the trial with diabetes. Additional post randomization exclusions included those who were missing blood glucose at randomization, had a fasting glucose at randomization of 126 mg/dL (≥6.99 mmol/L) or higher or were on a glucose lowering medication. For the secondary analysis of impaired fasting glucose (IFG) incidence, we excluded participants with baseline blood glucose ≥100 mg/dL (≥ 5.55 mmol/L). The study was approved by the institutional review board of record for each clinical site and all participants provided informed consent.
Measurements
Sociodemographic data were collected at randomization and clinical data were obtained at randomization and every 3 months. Race and ethnicity information was obtained via self-report. Blood pressure was determined using the mean of 3 properly sized automated cuff readings, taken 1 minute apart after 5 minutes of quiet rest.17 Laboratory data, including fasting blood glucose, were collected at baseline and at the 2 and 4 year or close out visits. If a blood glucose sample was marked as “non-fasting,” the value was considered missing for this analysis. Samples were centrifuged and shipped on ice to the central laboratory. Glucose was measured in serum using the hexokinase method on a Roche analyzer. Medical history was collected annually and included any reported use of antidiabetic medications and diagnosed diabetes.
Study Outcomes
The primary outcome was incident diabetes, defined as time to reaching a fasting blood glucose ≥126 mg/dL(≥6.99 mmol/L) 18, participant self-report of diabetes at annual examination; or new use of hypoglycemic medications listed at an annual exam (Table S1). The secondary outcome was incidence of IFG, defined as time to first fasting blood glucose between 100 and 125 mg/dL (5.55- 6.94 mmol/L) among participants with normoglycemia (blood glucose < 100 mg/dL, <5.55 mmol/L) at baseline.
Covariates
Study covariates, collected at randomization, included age, gender, race, body mass index (BMI) in kilograms per meters squared and all antihypertensive medication categories used at baseline. Baseline estimated glomerular filtration rates (eGFR) were calculated from serum creatinine measurement and demographics using the Modification of Diet in Renal Disease (MDRD) four- component equation.19
Statistical analyses and Power
Statistical analyses were conducted at the coordinating center using SAS software, version 9.4 (SAS Institute, Cary N.C.). Baseline characteristics were compared among participants randomized to intensive versus standard blood pressure strategies. The primary analysis was not a pre-specified analysis of the main trial. The cumulative incidence of new onset diabetes was compared between the intensive versus standard blood pressure strategies using a competing risk proportional hazards regression model among the cohort of patients who were free of glucose lowering medications or had a blood glucose <126mg/dL (<6.99 mmol/L) at randomization. The p-value for the unadjusted hazard was calculated through maximum likelihood estimates. We also conducted multiple sensitivity and subgroup analyses with no correction for multiple testing. The first analysis utilized the randomized population and analyzed them as intention to treat regardless of their baseline glucose or medications. The second, evaluated and compared the highest measured blood glucose achieved by intervention arm. The third evaluated the odds of a fasting blood sugar increase of 10 mg/dL (0.55 mmol/L) or 20 mg/dL (1.11 mmol/L) between randomization and the highest fasting follow-up blood sugar between study arms. For these analyses we used a logistic regression model adjusted for the above covariates. For both the primary and randomized trial populations, Cox proportional hazards models were constructed to compare outcomes for intensive versus standard (referent) adjusted for baseline covariates (age, gender, race, BMI, eGFR, and baseline antihypertensive medications). Follow-up time was censored at the study stoppage date (August 20th, 2015), death, loss to follow-up, or reaching the primary outcome (diabetes incidence). The proportional hazards assumptions were verified through examination of log-log plots. We conducted subgroup analyses and tested for effect measure modification by creating interaction treatment terms (treatment arm* subgroup) using the following groups: age (≥65, <65 years), race (black, white), and the presence of CKD as defined by Kidney Disease Improving Global Outcomes (KDIGO) classification of CKD20 at randomization (eGFR ≥60 ml/min, eGFR 45-59 ml/min, eGFR <45 ml/min). Maximum likelihood estimates and p-values from the likelihood ratio statistics for each term were used to check tests of interaction.
For the primary composite outcome and the population of patients who were eligible for inclusion based on the post randomization exclusion criteria, power was 45% to detect a Hazard Ratio of 1.17. Power increased to 85% for the secondary outcome of IFG (blood glucose between 100 and 125 mg/dL [5.55- 6.94 mmol/L]).
Results
Study Cohort and Participant Characteristics
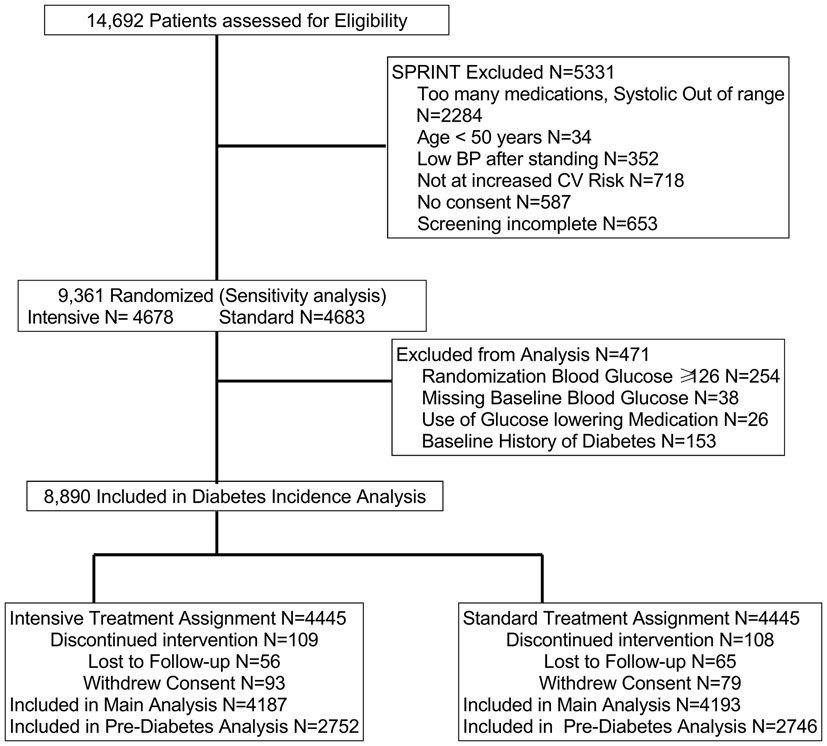
There were 14,692 persons assessed for eligibility and 9,361 participants enrolled from November 2010 through March 2013 and randomized (Intensive N=4678 and Standard N= 4683, sensitivity analysis population). For the main analysis of diabetes incidence, we further excluded 471 participants: baseline blood glucose ≥126 mg/dL (≥6.99 mmol/L) (N=254); missing blood glucose (N=38); or use of a glucose lowering medication or report of diabetes at baseline (N=179). The secondary analysis of IFG excluded an additional 2882 participants with blood glucose between 100-125 mg/dL (5.55- 6.94 mmol/L) (Figure 1). The median baseline blood glucose was 97 mg/dL (5.38 mmol/L), and there was no difference between groups. Other descriptive characteristics of the participants are presented in Table 1.
Figure 1:
Flow of participants included in Analyses
Table 1:
Characteristics of Patients in the primary analysis
| Participant Characteristics | Standard Blood Pressure N=4193 |
Intensive Blood Pressure N=4187 |
|---|---|---|
| Gender, Male (%) | 2742 (65.4) | 2696 (64.4) |
| Age, years, median interquartile range (IQR) | 67 (61, 76) | 67 (61, 76) |
| Race/Ethnicity (%) | ||
| Black | 1296 (30.9) | 1253 (29.9) |
| White | 2773 (66.1) | 2787 (66.6) |
| Ethnicity (%) Hispanic | 435 (10.4) | 455 (10.9) |
| Fasting Glucose mg/dL, median (IQR) | 97 (91, 104) | 97 (90,104) |
| Fasting Glucose mmol/L, median (IQR) | 5.38 (5.05, 5.77) | 5.38 (5.00, 5.77) |
| Systolic Blood pressure mm/Hg, median (IQR) | 139 (130, 149) | 138 (129, 149) |
| Diastolic Blood pressure mm/Hg, median (IQR) | 78 (70, 86) | 78 (70, 86) |
| Body Mass Index* (kg/meter2), median (IQR) | 28.9 (25.8, 32.6) | 28.9 (25.8, 32.9) |
| Low Density Lipoprotein mg/dL, median (IQR) | 109 (87, 134) | 110 (87, 133) |
| Low Density Lipoprotein mmol/L, median (IQR) | 2.82 (2.25, 3.47) | 2.85 (2.25, 3.44) |
| Triglycerides mg/dl, median (IQR) | 106 (77, 150) | 106 (76, 147) |
| Triglycerides mmol/L, median (IQR) | 1.20 (0.87, 1.69) | 1.20 (0.86, 1.66) |
| Creatinine mg/dL, median (IQR) | 1.00 (0.86, 1.21) | 1.00 (0.86, 1.21) |
| Creatinine mmol/L, median (IQR) | 0.09 (0.08, 0.11) | 0.09 (0.08, 0.11) |
| Glomerular filtration rate* ml/min, median (IQR) | 70.9 (58.3, 84.3) | 71.4 (58.0, 84.5) |
| No Proteinuria, Albumin to Creatinine ratio < 30 mg/gm (%) | 3277 (79.1) | 3278 (79.0) |
| Smoking status (%) | ||
| Never | 1850 (44.2) | 1853 (44.3) |
| Former | 1797 (42.9) | 1785 (42.6) |
| Current | 541(12.9) | 547 (13.1) |
| Missing | 5 (0.1) | 2 (0.04) |
| Framingham 10-yr cardiovascular disease risk score (%) | ||
| Cardiovascular risk < 10% | 301 (7.2) | 280 (6.7) |
| Cardiovascular risk 10- 20% | 1456 (34.8) | 1486 (35.5) |
| Cardiovascular risk ≥20% | 2431 (58.0) | 2418 (57.8) |
| Medications at Randomization (%) | ||
| Aspirin | 2119 (50.6) | 2193 (52.5) |
| Angiotensin Converting Enzyme Inhibitors | 1506 (35.9) | 1591 (38.0) |
| Angiotensin II Receptor Blockers | 901 (21.5) | 877 (21.0) |
| Beta Blockers | 1254 (29.9) | 1346 (32.2) |
| Calcium Channel Blockers | 1471 (35.1) | 1425 (34.0) |
| Thiazide and potassium sparing diuretics | 1603 (38.3) | 1682 (40.1) |
| Non- Selective alpha Blockers | 189 (4.5) | 200 (4.8) |
| Loop Diuretics | 180 (4.3) | 188 (4.5) |
| Other Antihypertensive medications | 283 (6.8) | 336 (8.0) |
| Statin Lipid Lowering Drugs | 1871 (44.9) | 1793 (43.1) |
| Corticosteroid | 226 (5.4) | 229 (5.5) |
| Hormones | 133 (3.2) | 126 (3.0) |
Estimated glomerular filtration rate calculated using MDRD equation (N=17 participants missing baseline value) Body mass index (N=51 participants missing randomization BMI)
In the primary analysis, the median [Interquartile range (IQR)] follow-up prior to censoring or reaching an outcome was 3.3 (2.8, 3.8) years among intensive and 3.2 (2.8, 3.8) years among standard participants. Six percent of blood glucose values after randomization were noted to be non-fasting and considered missing. Most participants had one follow up blood sugar measure (6634 participants had one follow-up blood glucose and 1529 participants had two fasting blood glucose measures). A total of 7.1% of participants reached the outcome of new onset diabetes in the intensive treatment arm and 6% in the standard arm. Reasons for censoring were death (3.4% versus 4.6%) or study closeout visit (89.5% versus 89.5%).
Time to diabetes incidence
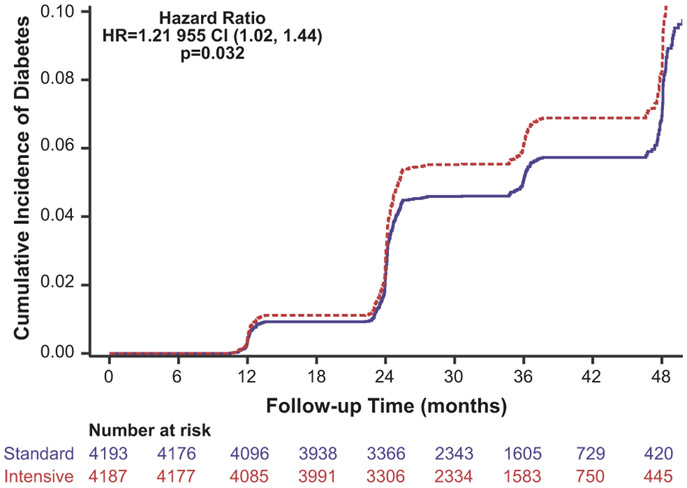
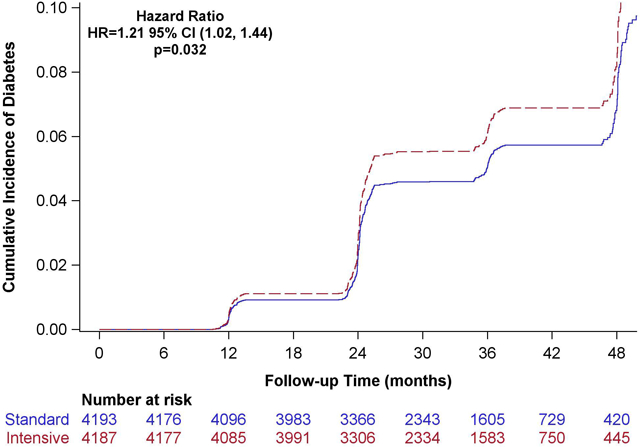
There were 299 composite events among intensive participants and 251 composite events among standard participants, resulting in 22.6 (20.2, 25.3) versus 19.0 (16.8, 21.5) events per 1000 person-years of treatment respectively (unadjusted hazard ratio 1.21 [95% confidence interval [95% CI] 1.02, 1.44, p=0.032 Figure 2). Once adjusted for baseline covariates the adjusted hazard ratio [aHR] was 1.17, (95% CI 0.99, 1.39) (Table 2). Elevated fasting glucose event rates were 12.7 (10.9, 14.7) and 10.4 (8.8, 12.3) per 1000 person-years, among intensive and standard treatment participants, respectively (aHR 1.19, 95% CI 0.95, 1.49). New self-report of diabetes rates was 11.9 (10.2, 13.9) versus 8.8 (7.4, 10.6) per 1000 person-years, respectively (aHR 1.33, 95% CI 1.05,1.70). New self-reported use of hypoglycemic medications was not statistically different for the intensive (8.8 [7.3, 10.5 per 1000 person-years] versus standard treatment strategy (7.1 [5.8, 8.7] per 1000 person-years) with an aHR of 1.22 (95% CI 0.92, 1.62).
Figure 2:
Cumulative Incidence of development of Diabetes by Standard or Intensive Blood pressure control, unadjusted Hazard Ratio and 95% confidence intervals presented.
Table 2:
Diabetes Incidence rate by Intensive versus Standard blood pressure strategy
| Primary and Secondary outcomes | Standard Blood Pressure N= 4193 |
Intensive Blood Pressure N= 4187 |
|---|---|---|
| Composite diabetes outcome | ||
| N events | 251 | 299 |
| Person time years | 3217.2 | 13225.3 |
| Incidence rate per 1000 person-years of treatment strategy (95% Confidence Intervals) | 19.0 (16.8, 21.5) | 22.6 (20.2, 25.3) |
| Unadjusted Hazard Ratio (95% Confidence Intervals) | Reference | 1.21 (1.02, 1.44) |
| Adjusted Hazard Ratio (95% Confidence Intervals) * | Reference | 1.17 (0.99, 1.39) |
| Fasting glucose ≥ 126mg/ dL (≥6.99 mmol/L) | ||
| N events | 139 | 168 |
| Person time years | 13320.2 | 13235.2 |
| Incidence rate per 1000 person-years of treatment strategy (95% Confidence Intervals) | 10.4 (8.8, 12.3) | 12.7 (10.9, 14.7) |
| Adjusted Hazard Ratio (95% Confidence Intervals) * | Reference | 1.19 (0.95, 1.49) |
| Self-Report of Diabetes | ||
| N events | 117 | 158 |
| Person time years | 13227.2 | 13252.3 |
| Incidence rate per 1000 person-years of treatment strategy (95% Confidence Intervals) | 8.8 (7.4, 10.6) | 11.9 (10.2, 13.9) |
| Adjusted Hazard Ratio (95% Confidence Intervals) * | Reference | 1.34 (1.05, 1.70) |
| New use of hypoglycemic meds | ||
| N events | 94 | 116 |
| Person time years | 13236.2 | 13253.2 |
| Incidence rate per 1000 person-years of treatment strategy (95% Confidence Intervals) | 7.1 (5.8, 8.7) | 8.8 (7.3, 8.5) |
| Adjusted Hazard Ratio (95% Confidence Intervals) * | Reference | 1.22 (0.92, 1.62) |
| Secondary Outcome | N= 2746 | N= 2752 |
| Impaired Fasting glucose: 100- 125 mg/ dL [5.55- 6.94 mmol/L] | ||
| N events | 679 | 793 |
| Person time years | 3013.9 | 2998.7 |
| Incidence rate per 100 person-years of treatment strategy (95% Confidence Intervals) | 22.5 (21.1, 24.1) | 26.4 (24.9, 28.0) |
| Adjusted Hazard Ratio (95% Confidence Intervals) | Reference | 1.17 (1.06, 1.30) |
| Sensitivity Analysis: Randomized Population | N= 4683 | N=4678 |
| N events | 251 | 299 |
| Person time years | 13670 | 13717 |
| Incidence rate per 1000 person-years of treatment strategy (95% Confidence Intervals) | 18.4 (16.2, 20.8) | 21.8 (19.5, 24.4) |
| Adjusted Hazard Ratio (95% Confidence Intervals) † | Reference | 1.18 (0.99, 1.40) |
Confidence intervals for incidence rates calculated using Wilsons distribution
Adjusted model includes age, race, gender, body mass index, antihypertensive medication classes at randomization and estimated glomerular filtration rate. Adjusted model includes 8312 participants (N=68 participants missing baseline estimated glomerular filtration rate or body mass index)
Adjusted model includes 9246 participants (N=115 participants missing estimated glomerular filtration rate or body mass index)
Among the 2752 and 2746 participants with normal blood glucose in the intensive and standard arms at baseline, the IFG incidence rates were 26.4 (24.9, 28.0) and 22.5 (21.1, 24.1) per 100 person-years (aHR 1.17, 95% CI 1.06, 1.30). In the sensitivity analysis of the randomized trial (intention to treat) population there were 21.8 (16.2, 20.8) vs 18.4 (19.5, 24.4) events per 1000 person-years for intensive versus standard treatment strategies (aHR 1.18, 95% CI 0.99, 1.40).
We assessed median [IQR] fasting blood glucose at 24 months and 48 months by study arm. Median blood glucose did not differ over follow-up time. At 24 months median fasting blood glucose was 98 (91,106) [5.43 (5.05, 5.88)] and 97 (90, 105) mg/dL [5.38 (4.99, 5.83) mmol/L]; at 48 months median fasting blood glucose was 97 (90, 105) [5.38 (4.99, 5.83) mmol/L] and 98 (90, 105) mg/dL [5.43 (4.99, 5.83) mmol/L] among intensive and standard participants.
For each participant, we determined the highest blood sugar during follow-up and compared those values between study arms and stratified by baseline blood glucose (Table S2). Among intensive therapy the maximum mean blood glucose was 100.7 [5.59 mmol/L] (standard deviation [SD]16.7; 0.93) compared to standard therapy mean 99.4 [5.52 mmol/L] (SD=17.1; 0.95), p=0.0008. In the final sensitivity analyses we evaluated the odds of a 10 mg/dL (0.55 mmol/L) or 20 mg/dL (1.1 mmol/L) change in fasting blood sugar between study arms. There were 1524 patients who had a 10 mg/dL (0.55 mmol/L) increase in blood sugar (adjusted Odds Ratio [aOR] 1.26, 95% CI 1.12, 1.45 for intensive compared to standard). There were 496 participants who had a 20 mg/dL (1.1 mmol/L) increase in fasting blood sugar (aOR 1.23, 95% CI 1.03, 1.48) for intensive compared to standard.
The proportion of participants who were prescribed a thiazide-type diuretic was similar for intensive and standard arms at baseline (40.1% versus 38.3%, p=0.08). Over time the proportion prescribed a thiazide was higher in the intensive versus standard group (Figure S1).
Sub-group analyses intensive versus standard BP strategy by age, race and eGFR,
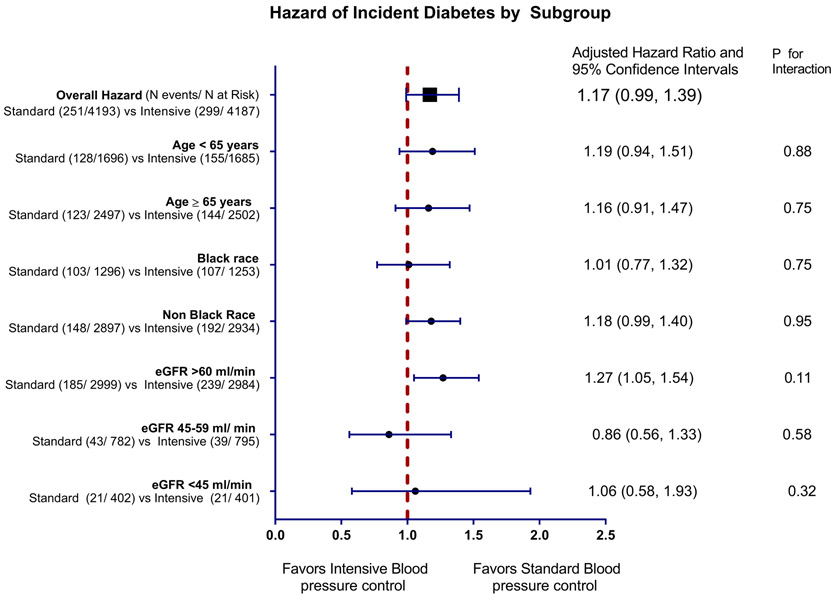
We tested for the interaction between randomized trial arm and subgroups defined by age, race and eGFR at baseline and none were statistically significant (Table S3 and Figure 3). An increased risk of diabetes with intensive treatment strategy was observed only among participants with eGFR ≥ 60 ml/min. Among participants with an eGFR 45 to 59 ml/min and eGFR 30 to 44 ml/min there was no increased hazard of incident diabetes aHR 0.86 (95% CI 0.56, 1.33) and 1.06 (95% CI 0.58, 1.93) with intensive treatment strategy.
Figure 3:
Hazard of Incident diabetes by subgroups stratified by age, race and estimated glomerular filtration rate at time of randomization
DISCUSSION
Among high cardiovascular risk participants randomized in the SPRINT study, the intensive treatment strategy compared with standard blood pressure treatment strategy was associated with small increased risk of new onset diabetes over the median follow-up of about 3 years, but confidence intervals were wide. In the sensitivity analyses which considered the full clinical trial population, the risk of incident diabetes was consistent with the main results. Overall, there was a increase in risk of IFG in the intensive treatment group and an increased odds of fasting glucose increase of > 10 or 20 mg/dL (0.55 or 1.1 mmol/L). This increased risk should be considered along with the main SPRINT study findings of reductions in CVD outcomes and death.
This study and the results add to the complex evidence for the interaction and association of lower blood pressure strategy with incident diabetes and the impact of antihypertensive medications. There are two existing observational studies nested within high cardiovascular risk trial populations, but neither examined specific SBP targets as they relate to incident diabetes. The first by Gupta et al. 21 evaluated 14,120 participants in the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) and found 1366 new onset diabetes events, with an incident diabetes rate of 18.6 per 1000 patient years over a median follow-up of 5.5 years. They reported that for each increase of 10 mmHg SBP, there is a 6% increased risk of incident diabetes. Patients in ASCOT were randomized to either Atenolol or Amlodipine with no interaction effect between risk of diabetes and medication. The second observational study was embedded within the Candesartan Antihypertensive Survival Evaluation in Japan (CASE-J) trial22. Among the 2685 high cardiovascular risk patients free of diabetes, 97 developed incident diabetes over the average of 3.3 years of follow-up. Pulse pressure was associated with incident diabetes (aHR per 1 SD increase 1.44 [1.15, 1.79]); while there was no association between SBP and diabetes (aHR per 1 SD increase 1.13 [0.90, 1.41]. Furthermore, they describe an interaction effect where the incidence of diabetes was lowest among candesartan users and appeared to be most beneficial at the highest pulse pressure.
This study advances the research findings of the prior two by investigating if specific BP goals can influence diabetes risk; but the use of multiple antihypertensive regimens among both the intensive and the standard regimens adds further difficulty to understanding the blood pressure-diabetes relationship. Two meta-analyses of clinical trials evaluated the effects of antihypertensive medications on diabetes incidence and found protective associations with ACE inhibitor or ARB use. 9,23 In contrast, no association between incident diabetes and renin angiotensin system blockade was found in a large observational cohort of more than 130,000 patients 24. In this study, an increased risk of diabetes was found with the combination of a thiazide diuretic and a beta-blocker (OR 1.99; 95% CI 1.8, 2.2), and with a thiazide diuretic combined with a calcium channel blocker (OR 1.52; 95% CI 1.28, 1.82) compared to non-use of antihypertensives. The possible indirect (mediating) effects of medications on diabetes incidence was not the primary question under investigation in this study. Therefore, whether the use of certain medications alone or in combination influences the association between intensive treatment strategy and diabetes incidence remains uncertain. Further investigation is needed to understand the mediating relationship of medications as a contributor to IFG.
There are reports of insulin resistance in early stage CKD possibly due to shared diabetes risk factors including obesity and lifestyle which can influence glucose metabolism and insulin sensitivity.25-27 Although we did not measure insulin levels or insulin sensitivity, the subgroup analysis results suggest that there is no increased risk of diabetes in intensive strategy participants with reduced eGFR although confidence intervals were wide and power was limited for this group. This observation is consistent with the observation of Pham and colleagues 28 and does require further study to more clearly understand the interaction between renal dysfunction, blood pressure control and incident diabetes.
We note some study limitations. It is possible that there was reporting bias among participants randomized to intensive treatment. More participants randomized to intensive blood pressure treatment self-reported new onset diabetes, but the severity of their diabetes is uncertain since hypoglycemic medication use was not more common in the intensive arm and glycated hemoglobin level was not collected to confirm the diagnosis. Second, we accounted for certain covariates, including BMI and antihypertensive medications, at baseline, but did not account for time varying weight change or medication usage. The aim of our study was to evaluate if intensive treatment strategy was associated with diabetes incidence. Adjustment for covariates in the causal pathway of those treatment strategies is not appropriate. Third, our sample size and subgroups were determined a priori and were underpowered to determine risk of incident diabetes as demonstrated by the power calculation and wide confidence intervals for most groups. The sensitivity analysis evaluating the odds of a change in fasting blood glucose did demonstrate an association of intensive control with a 10 or 20 mg/dL (0.55 or 1.1 mmol/L) change in fasting glucose. Finally, the median duration of follow-up was only about 3 years. It remains unknown if longer follow-up would change this overall risk of incident diabetes.
Perspective
This study found that intensive blood pressure treatment in the SPRINT study was associated with a small non-significant increase in risk of incident diabetes. There was a statistically significant increased risk of IFG, with no difference in overall fasting glucose or diabetes medications used by randomization arm. If patients and providers were to pursue intensive goals a reduction in CVD risk would likely occur; however, these results reflect that this may come with an increased risk of IFG. A shared decision-making approach including the risks and benefits of pursuing intensive blood pressure targets should be factored into the individualized treatment goals and strategies for each patient.
Supplementary Material
Novelty and Significance.
What Is New
The risk of impaired fasting glucose is higher with intensive blood pressure control with no difference in overall fasting glucose or diabetes medications used by randomization arm.
What Is Relevant?
This small increased risk is of uncertain clinical significance but should be considered along with the main SPRINT study findings of reductions in CVD outcomes and death.
Summary
These results may strengthen the decision of patients and providers to pursue intensive blood pressure treatment as a means of cardiovascular risk reduction.
Acknowledgments
See Supplement for full SPRINT group
Dr. Roumie and Mr. Russell are responsible for the design, data analysis and drafting of the results in this manuscript. All authors are responsible for the critical review of the manuscript and the decision to publish.
Sources of Funding
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing, and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the additional acknowledgement list in Supplement.
We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS, Wake Forest University: UL1TR001420.
Footnotes
Disclosures: Roumie; Hung; Russell; Kreider; Nord; Ramsey; Kostis; Sweeny; Tamariz; Williams; Zias have no disclosures. Dr. Cushman reports institutional grant funding with Eli Lily and consulting with Sanofi and uncompensated consulting with Takeda. Dr. Basile reports grant funding with Eli Lily and consulting with Medtronic, ReCor and serves as a section editor for Up to Date. Dr. Rastogi reports research support from Bayer, AbbVie, and VPI.
REFERENCES
- 1.Reaven GM. Relationship between insulin resistance and hypertension. Diabetes Care. 1991;14 Suppl 4:33–8. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Insulin resistance, hyperinsulinemia, hypertriglyceridemia, and hypertension. Parallels between human disease and rodent models. Diabetes Care. 1991;14:195–202. [DOI] [PubMed] [Google Scholar]
- 3.Reaven GM and Hoffman BB. A role for insulin in the aetiology and course of hypertension? Lancet. 1987;2:435–7. [DOI] [PubMed] [Google Scholar]
- 4.Reaven GM, Lithell H and Landsberg L. Hypertension and associated metabolic abnormalities--the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–81. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA and Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–94. [DOI] [PubMed] [Google Scholar]
- 6.Emdin CA, Anderson SG, Woodward M and Rahimi K. Usual Blood Pressure and Risk of New-Onset Diabetes: Evidence From 4.1 Million Adults and a Meta-Analysis of Prospective Studies. J Am Coll Cardiol. 2015;66:1552–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bangalore S, Parkar S, Grossman E and Messerli FH. A meta-analysis of 94,492 patients with hypertension treated with beta blockers to determine the risk of new-onset diabetes mellitus. Am J Cardiol. 2007;100:1254–62. [DOI] [PubMed] [Google Scholar]
- 8.Gress TW, Nieto FJ, Shahar E, Wofford MR and Brancati FL. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Atherosclerosis Risk in Communities Study. N Engl J Med. 2000;342:905–12. [DOI] [PubMed] [Google Scholar]
- 9.Gillespie EL, White CM, Kardas M, Lindberg M and Coleman CI. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care. 2005;28:2261–6. [DOI] [PubMed] [Google Scholar]
- 10.Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK and Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan BM, Li J, Hutchison FN and Ferdinand KC. Hypertension in the United States, 1999 to 2012: progress toward Healthy People 2020 goals. Circulation. 2014;130:1692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fletcher RD, Amdur RL, Kolodner R, McManus C, Jones R, Faselis C, Kokkinos P, Singh S and Papademetriou V. Blood pressure control among US veterans: a large multiyear analysis of blood pressure data from the Veterans Administration health data repository. Circulation. 2012;125:2462–8. [DOI] [PubMed] [Google Scholar]
- 13.Gu Q, Burt VL, Dillon CF and Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health And Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126:2105–14. [DOI] [PubMed] [Google Scholar]
- 14.Booth JN 3rd, J Li, Zhang L, Chen L, Muntner P and Egan B. Trends in Prehypertension and Hypertension Risk Factors in US Adults: 1999-2012. Hypertension. 2017;70:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 16.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr., Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT Jr and Whelton PK. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson KC, Whelton PK, Cushman WC, Cutler JA, Evans GW, Snyder JK, Ambrosius WT, Beddhu S, Cheung AK, Fine LJ, Lewis CE, Rahman M, Reboussin DM, Rocco MV, Oparil S and Wright JT Jr. Blood Pressure Measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension. 2018;71:848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Standards of Medical Care in Diabetes-2017: Summary of Revisions. Diabetes Care. 2017;40:S4–S5. [DOI] [PubMed] [Google Scholar]
- 19.Huizinga MM, Roumie CL, Greevy RA, Liu X, Murff HJ, Hung AM, Grijalva CG and Griffin MR. Glycemic and weight changes after persistent use of incident oral diabetes therapy: a veterans administration retrospective cohort study. Pharmacoepidemiol Drug Saf. 2010;19:1108–12. [DOI] [PubMed] [Google Scholar]
- 20.Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, Moe SM, Shroff R, Tonelli MA, Toussaint ND, Vervloet MG and Leonard MB. Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder: Synopsis of the Kidney Disease: Improving Global Outcomes 2017 Clinical Practice Guideline Update. Ann Intern Med. 2018;168:422–430. [DOI] [PubMed] [Google Scholar]
- 21.Gupta AK, Dahlof B, Dobson J, Sever PS, Wedel H, Poulter NR and Anglo-Scandinavian Cardiac Outcomes Trial I. Determinants of new-onset diabetes among 19,257 hypertensive patients randomized in the Anglo-Scandinavian Cardiac Outcomes Trial--Blood Pressure Lowering Arm and the relative influence of antihypertensive medication. Diabetes Care. 2008;31:982–8. [DOI] [PubMed] [Google Scholar]
- 22.Yasuno S, Ueshima K, Oba K, Fujimoto A, Hirata M, Ogihara T, Saruta T and Nakao K. Is pulse pressure a predictor of new-onset diabetes in high-risk hypertensive patients?: a subanalysis of the Candesartan Antihypertensive Survival Evaluation in Japan (CASE-J) trial. Diabetes Care. 2010;33:1122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andraws R and Brown DL. Effect of inhibition of the renin-angiotensin system on development of type 2 diabetes mellitus (meta-analysis of randomized trials). Am J Cardiol. 2007;99:1006–12. [DOI] [PubMed] [Google Scholar]
- 24.Cooper--DeHoff RM, Bird ST, Nichols GA, Delaney JA and Winterstein AG. Antihypertensive drug class interactions and risk for incident diabetes: a nested case-control study. J Am Heart Assoc. 2013;2:e000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Muntner P, Hamm LL, Fonseca V, Batuman V, Whelton PK and He J. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol. 2003;14:469–77. [DOI] [PubMed] [Google Scholar]
- 26.Dave N, Wu J and Thomas S. Chronic Kidney Disease-Induced Insulin Resistance: Current State of the Field. Curr Diab Rep. 2018;18:44. [DOI] [PubMed] [Google Scholar]
- 27.Whaley-Connell A and Sowers JR. Insulin Resistance in Kidney Disease: Is There a Distinct Role Separate from That of Diabetes or Obesity? Cardiorenal Med. 2017;8:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pham H, Robinson-Cohen C, Biggs ML, Ix JH, Mukamal KJ, Fried LF, Kestenbaum B, Siscovick DS and de Boer IH. Chronic kidney disease, insulin resistance, and incident diabetes in older adults. Clin J Am Soc Nephrol. 2012;7:588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.