Supplemental Digital Content is Available in the Text.
Key Words: cornea, stromal, gene therapy, safety
Purpose:
Drug delivery directly to the corneal stroma currently relies on microscopic injections that demonstrate low reproducibility and clinician-dependent variability. With use of biological drugs such as adeno-associated viral (AAV) vectors, precise and consistent drug deposition is critical to reduce concerns related to off-target transduction and the host's immune response to the viral capsid and/or transgene-derived product. Therefore, a precise corneal injection (PCI) microneedle was designed to allow accurate depth-specific injections into the corneal stroma in a macroscopic setting.
Methods:
High-frequency ultrasound and confocal microscopy demonstrated the consistent ability to predetermine the precise injection depth using PCI needles of varying sizes. Next, a comparison between a standard 31-G needle and PCI needles was performed in vivo using AAV vector gene delivery.
Results:
Intrastromal corneal injections using the PCI microneedle resulted in less vector leakage at the site of injection and fewer anterior chamber penetrations compared with a standard 31-G needle. Although reporter gene expression appeared similar when the vector was administered with either needle type, a trend toward increased vector genomes was noted in the PCI-injected corneas at the experimental conclusion. As hypothesized, corneal perforation resulted in increased detection of AAV vector genomes in nontarget tissues, highlighting the importance of consistency for biological drug applications in the cornea.
Conclusions:
Further development of the PCI microneedle is warranted especially for AAV corneal gene therapy and offers the potential to enhance transduction while significantly reducing safety concerns and intraclinician and interclinician injection variability.
Corneal intrastromal injections are becoming increasingly routine for treatment of ocular diseases including infectious keratitis (ie, fungal and bacterial infections) and corneal neovascularization.1–5 Compared with topical application, injections are advantageous because they increase the local concentration and exposure time of the drug in the deep corneal stromal layers and require less frequent administration.5 Currently, intrastromal injections use 27- to 31-gauge needles directed obliquely into the cornea; however, these injections are technically difficult, result in variable corneal injection locations and depth, vary between clinicians, and require the need for magnification, such as an operating microscope, to perform the injections.6
Corneal gene therapy has been advocated for treatment of a diverse group of corneal stromal abnormalities, such as fibrosis, corneal dystrophy, transplant rejection, and corneal opacities associated with lysosomal storage diseases.6–9 Currently, the most popular gene delivery format relies on adeno-associated viral (AAV) vectors which remain primarily episomal and are capable of inducing gene expression for many years after a single administration. Regarding AAV corneal gene therapy, keratocytes of the normally avascular and compartmentalized stroma are an ideal target because they are largely quiescent and therefore will not dilute the nonintegrated nonreplicated AAV vector genome. Reports have demonstrated that AAV vectors administered by a single corneal intrastromal injection can result in widespread transduction throughout the entire cornea with impressive long-term transgene expression. In addition, eliminating exposure of the viral particles and transgene to the immune system will decrease development of neutralizing antibodies (NABs) and reduce immune response and inactivation of the gene therapy product.10 In the cornea, escaping immune detection of the intrastromal treatment may be achieved by restricting the gene therapy to the corneal stroma through eliminating exposure of the ocular surface and anterior chamber (AC) to the viral vector.
The purpose of this study was to validate a simple and consistent fixed-depth needle to reduce the variability of corneal intrastromal drug administration without the use of an operating microscope. Therefore, a purpose-designed precise corneal injection (PCI) microneedle was investigated for injection reproducibility, depth, and extent of corneal distribution after stromal injection of fluorescein or a biological gene therapy AAV vector. The results were compared with the current paradigm of corneal stroma drug administration, which relies on a standard beveled needle. The results demonstrate that the PCI needle provides consistent and precise macroscopic injections at predefined depths. Injections using either needle resolved quickly, were well tolerated, and contributed to similar levels of gene expression in a kinetic manner by live imaging. However, postmortem biodistribution experiments demonstrated that more AAV vector genomes were detected in the cornea with less exposure to nontarget tissues using the PCI microneedle. This technology will be especially important for increasing the safety of corneal gene therapy by reducing the overall intrastromal injection variability.
METHODS
Comparison and Feasibility of Corneal Intrastromal Injections (Ex Vivo)
To initially evaluate corneal intrastromal injections, fresh cadaver porcine eyes were used. Ocular tissues were placed on ice immediately after removal at a local United States Department of Agriculture--certified slaughter house, transported to the research laboratory on ice, and used for experiments within 8 hours after animal death. Only eyes with normal, clear corneas based on visual inspection were used. Injections were made on whole porcine cadaver eyes placed in a fixation device (Mastel Mandell Eye Mount; Mastel Precision Surgical Instruments, Rapid City, SD) with the vacuum adjusted to provide a normotensive intraocular pressure of 15 to 20 mm Hg, as measured using the Tonovet tonometer (Icare Finland).
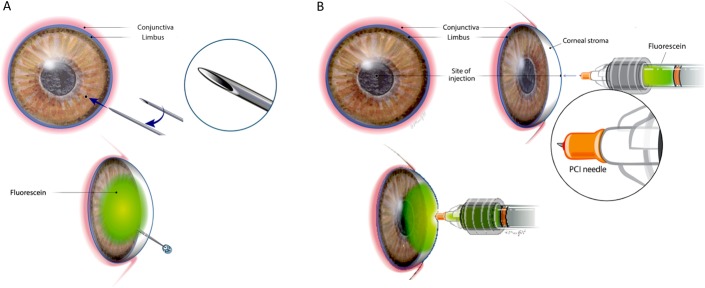
Corneal injections were made with either a commercially available 31-gauge insulin syringe (BD Ultra-Fine II Short Needle Insulin Syringe 31-Gauge 1cc 5/16"; Becton, Dickinson and Company, Franklin Lakes, NJ), as previously described,6 or a purpose-designed PCI microneedle (Theia Medical, Raleigh, NC), a 34-gauge needle with a defined fixed depth and bevel configuration optimized for corneal intrastromal injections (Fig. 1). For intrastromal injections made with the 31-gauge needle, the needle was directed obliquely and horizontally from the temporal limbus and extended to the central cornea with the bevel pointed down, followed by a slow injection of 50 μL of 0.01% sodium fluorescein (AK-Fluor fluorescein injection; USP, Lake Forest, IL) in balanced salt solution (BSS) (Fig. 1). In a separate set of eyes, PCI microneedles were used to inject directly into the axial corneal stroma from an anterior, perpendicular approach (Fig. 1). For these injections, a 650-μm length PCI microneedle was used to inject 10, 25, or 50 μL of 0.01% fluorescein into the central cornea. For the 10 μL injections, a 50-μL glass syringe (Microliter 700 Series Syringe; Hamilton Company, Reno, NV) was used. For the 25 and 50 μL injections, a 0.25-mL Sword Handle Fixed Male Medallion syringe was used (Merit Medical, Inc, South Jordan, UT). Injections were performed in triplicate, with 3 additional eyes serving as uninjected controls. To evaluate the location and depth of injection and resulting corneal thickness, high-frequency ultrasound (HFU) was performed with a 50-MHz linear probe in B mode (Aviso, Quantel Medical, Bozeman, MT). Sagittal images were obtained of the central cornea before injection and immediately after injection. Corneal thickness was measured using the ultrasound instrument caliper function by measuring the central portion of each image from the surface epithelium to endothelium.
FIGURE 1.
Intrastromal injection techniques and approach to the cornea when using a small-gauge needle (A) or a depth-defined PCI microneedle (B). Injections using the small-gauge needle, such as a 31-G needle (A), generally require the use of magnification such as an operating microscope by the user. The PCI microneedle (B) can be used for intrastromal injection without user magnification.
To determine the depth and consistency of injection using PCI microneedles, a separate set of eyes (n = 3/group) were injected using either a 330-μm (short), a 460-μm (medium), or a 600-μm (long) PCI microneedle. A 50-μL glass syringe (Microliter 700 Series Syringe; Hamilton Company, Reno, NV) was used to inject each eye with 10 μL of 0.01% sodium fluorescein. Digital photography and HFU images were obtained for each eye before, and immediately after, injection. For comparison and assessment of the injection location, on the ultrasound image, the distance from the corneal epithelium to the center of the injection site was measured using the ultrasound calipers for each eye.
To determine the tissue effect and extent of the PCI needle penetration into the cornea as a function of needle length, porcine cadaver eyes were fixed in a Mastel corneal vacuum mount, and either a 600- or 700-μm-long PCI needle was inserted into the cornea, but without fluid injection. After removal of the PCI needle, imaging of the corneal epithelium to endothelium was performed consecutively using confocal microscopy (Heidelberg Retina Tomograph 3 with Rostock Corneal Module; Heidelberg Engineering, GmBH, Dossenheim, Germany). Confocal imaging was also performed on a noninjected normal cornea.
Comparison of Intrastromal Injection Using a 31-Gauge Versus a PCI Microneedle In Vivo
All animals in this study were used in accordance with the NIH Guide for the Care and Use of Laboratory Animals; use adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research; and this study was approved and monitored by the North Carolina State University Institutional Animal Use and Care committee. Normal outbred male New Zealand White rabbits (Oryctolagus cuniculus) were used. Although the rabbits were under anesthesia and after the application of 5% betadine solution, sterile saline irrigation, and a topical anesthetic (0.5% proparacaine HCL; [Alcaine, Alcon Laboratories, Fort Worth, TX]), a 31-gauge (BD Ultra-Fine II Short Needle Insulin Syringe 31-Gauge 1cc 5/16"; Becton, Dickinson and Company) or a 318-μm length PCI microneedle was used for intrastromal injections under an operating microscope (Carl Zeiss OPMI VISU 200 Operating Microscope; Carl Zeiss Meditec, Inc Dublin, CA). With either needle, the right eye received 25 μL of self-complementary AAV8-CMV-green fluorescent protein (GFP) (Lot #AV7289 rAAV8.Hpa-TRS-sk, Gene Therapy Center, University of North Carolina, Chapel Hill, NC) (1e10 viral genomes [vg]), whereas the left eye received 25 μL of saline (n = 6 rabbits for each injection). Assessment of injection leakage (mild, moderate, severe) and the presence of intracameral penetration were recorded at the time of injection for each eye. Before injection and on days 1 to 6, 9, 14, and 16, complete ophthalmic examinations using slit-lamp biomicroscopy (Kowa SL-17 slit lamp; Kowa USA, Torrance, CA), pachymetry (Pachpen; Keeler, Malvern, PA), and intraocular pressure (Tonovet tonometer; Icare, Vantaa, Finland) were performed. Results of examination of ocular surface morphology and anterior and posterior segment inflammation were recorded using the modified Hackett–McDonald (ie, without application of fluorescein) ocular scoring system.11 Images of all corneas were collected immediately after injection (time 0) and repeated at each examination time (days 1–6, 9, 14, and 16 after injection) using digital ocular photography (Nikon D200, AF-S DX Micro NIKKOR 85 mm f/3.5 G Lens; Nikon Corporation, Tokyo, Japan) with a fixed magnification. Digital images were analyzed to determine distribution of the corneal injection by measuring the area (pixel counts) of the visible corneal stromal opacity when visible (ImageJ 1.52k; National Institutes of Health, Bethesda, MD).
Rabbits were also imaged before injection, immediately after injection, and then at days 6 and 16 after injection using a noncontact spectral domain optical coherence tomography (SD-OCT) instrument (Envisu R-class SD-OCT; Bioptigen, Inc, Morrisville, NC), which contains a super-luminescent light emitting diode delivering light at a wavelength of 840 nm. Imaging was performed using the hand held probe of the SD-OCT device fitted with a noncontact 12-mm telecentric lens for image acquisition. After adjusting the arm reference length on the SD-OCT device by manufacturer recommendations, SD-OCT was set to 1000 A scans per B scan, and 100 B scans in total, to generate a radial volume of 8 mm in diameter. B scans and en face reconstructed images were reviewed.
In Vivo Expression of GFP
In addition, in vivo corneal GFP expression was imaged on days 6, 9, and 16 after injection using a scanning laser ophthalmoscope (SLO) with a 482-nm laser source (Infrared cSLO; RetiMap Roland Consult, Wiesbaden, Germany). The intensity of GFP fluorescence expression was then quantitated from the in vivo images using a previously described method for calculating corrected total cell fluorescence (CTCF), where CTCF = Integrated density—(Area of selected cell/fluorescence × Mean fluorescence of background readings).6
Corneal Histology
On day 18 after injection, the rabbits were euthanized and eyes were collected and analyzed histologically or by probe-based quantitative qPCR analysis (3 eyes of each group for histology and 3 from each group for qPCR). Corneas were excised, fixed, embedded in paraffin, sectioned at a thickness of 5 μm, and stained with hematoxylin and eosin before examination by light microscopy.
Viral Genome Distribution
Viral genome (vg) biodistribution was assessed by qPCR in the cornea, liver, submandibular and mesenteric lymph node (LN), and heart muscle. To detect the viral genome (vg) in these tissues, gDNA were isolated using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA). The vector genome was quantitatively analyzed by qPCR using the probe technology as described above. In short, the amount of vector-specific GFP genome copies was standardized against an amplicon from a single copy housekeeping gene β-actin. qPCR was carried out with an initial denaturation step at 95°C for 10 minutes, followed by 45 cycles of denaturation at 95°C for 10 seconds and annealing/extension at 56°C for 45 seconds for GFP probe detection. The results are presented as the relative fold change calculated using the 2−ΔΔCt method.
Serum Neutralizing Antibody Analysis
Serum was collected from each rabbit before corneal injection (time 0) and at euthanasia (day 18 after injection). Serum neutralizing antibody to viral capsids was evaluated as previously described.12,13 Briefly, HEK cells were seeded in a 48-well plate at 50,000 cells/well in duplicate 24 hours before vector transduction. Preinjection serum and 18-day postinjection serum were used 1:1 and then serially diluted 1:2 to 1:2048 in PBS to a final volume of 13 μL and incubated with AAV-CMV Firefly Luciferase of the same capsid as that injected in 13 μL PBS for 2 hours at 4°C. Serum/vector mixture was then added to the cells, and a luciferase assay was performed 48 hours posttransduction using the Promega Luciferase Assay System (Bright-Glo; Promega, Madison, WI) using a PerkinElmer Victor 3 1420 Multilabel Counter Luminometer. Results were plotted to determine the point at which serum dilution suppressed transduction to less than 50% of preinjection serum levels.12,13
Statistical and Data Analysis
Wilcoxon tests (nonparametric inflammatory scores) or t tests/analysis of variance (parametric data, corneal thickness, intraocular pressure, vg copy numbers) were used to determine significance among the treatment groups. Differences were considered significant at P ≤ 0.05, and all probabilities and results were calculated using computerized statistical software (JMP Pro, v. 13.2; SAS Inc, Cary, NC).
RESULTS
Comparison and Feasibility of Corneal Intrastromal Injections (Ex Vivo)
Corneal intrastromal injections (50 μL of 0.01% sodium fluorescein) (Fig. 1) were successfully performed using the standard 31-gauge needle in the cadaver porcine corneas; however, these injections resulted in variable locations (ie, not consistently axial), corneal area distribution, and stromal depths of the injectate. In addition, there was endothelial perforation in 1 of 4 intrastromal injections with injection into the AC (Supplemental Digital Content 1, Supplemental Figure 1, http://links.lww.com/ICO/A917). Corneal intrastromal injection into approximately 1000-μm-thick cadaver porcine corneas using a 650-μm PCI microneedle resulted in the injectate radiating concentrically from the injection site evenly and no intracameral penetrations (Supplemental Digital Content 2, Supplemental Figure 2, http://links.lww.com/ICO/A918). The injection using the PCI microneedle repeatedly placed the injectate in the central stroma with a direct injection volume/area/corneal thickness correlation that significantly increased with volume (P < 0.0001), as measured by the fluorescein area, percent of corneal coverage, and corneal thickness (Supplemental Digital Content 2, Supplemental Figure 2, http://links.lww.com/ICO/A918).
Evaluation of Depth of Injection and Needle Penetration
Evaluation of HFU images immediately after a 10-μL injection of 0.01% fluorescein in ex vivo porcine corneas demonstrated the depth of injections was significantly (P < 0.004) and directly related to the length of the microneedle (330–600 μM) (Fig. 2 and Supplemental Figure 4, Supplemental Digital Content 4, http://links.lww.com/ICO/A951). Therefore, it is conceivable that by using appropriate-length microneedles, specific areas of the cornea, for example, the deep cornea to target corneal endothelial cells for transduction, could be targeted with low volumes of the injectate (ie, 10 μL or less).
FIGURE 2.
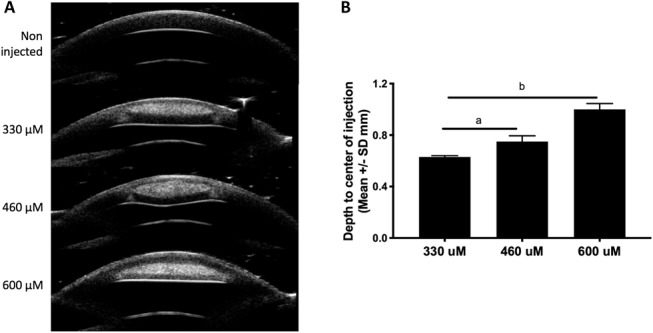
Depth of corneal stromal injection using different lengths of the injection device. A, HFU images in a noninjected eye and after injection of 10 μL of 0.01% fluorescein solution using a 330-, 460-, or 600-μM length needle. B, Depth to the center of the injection location was significantly different when using a short, medium, or long needle. N = 3 per injection. A, Depth of injection using a medium needle was significantly greater than that of a short needle (P = 0.02). B, Depth of injection with a long needle was significantly greater than that using a medium or short needle (P < 0.004).
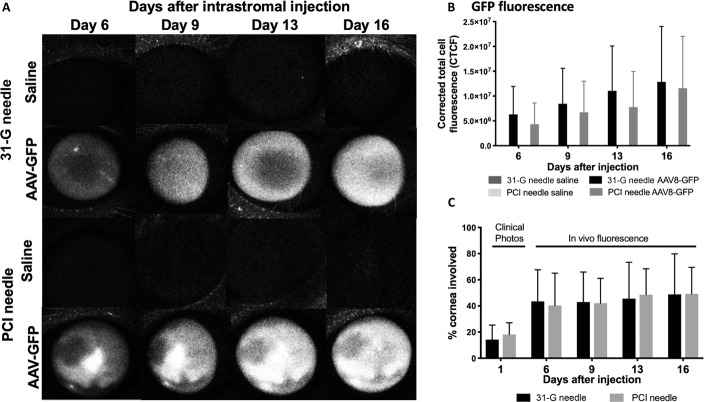
FIGURE 4.
In vivo expression of GFP. A, Using a SLO, GFP expression was detected on days 6, 9, 13, and 16 after intrastromal injection of 25 μL of saline (balanced salt solution) or AAV8-GFP using either a 31-G or a PCI needle. Fluorescence was not visible in the saline-dosed eyes; however, corneal expression was noted in increasing density in the right eyes (representative images, n = 6/injection). B, Mean fluorescence CTCF using the 31-G or PCI needle in the right corneas increased at each time point after injection but was not significantly different from each other at any day. C, Area of the cornea with GFP expression in vivo. The area of GFP fluorescence was higher at 6, 9, 13, and 16 days compared with the visible injection site immediately after injection (measured on clinical photographs), suggesting that diffusion of the virus beyond the injection site occurred. There was no significant difference with type of injection.
Confocal microscopy showed that the PCI needle created a linear incision into the corneal epithelium with minimal changes to the surrounding tissue. The depth of penetration of a 600-μM and 700-μM PCI needle was 604 and 689 μM, respectively. Slit-like incisions were visible through the stromal layers. At the edge of the incisions and at the maximum depth of the needle penetration, there was a denser, brighter appearance of the corneal lamellae, suggesting a slight compression of the tissue (Fig. 2 and Supplemental Figure 4, Supplemental Digital Content 4, http://links.lww.com/ICO/A951).
Comparison of Intrastromal Injection of Gene Therapy Using a 31-Gauge versus a PCI Microneedle In Vivo
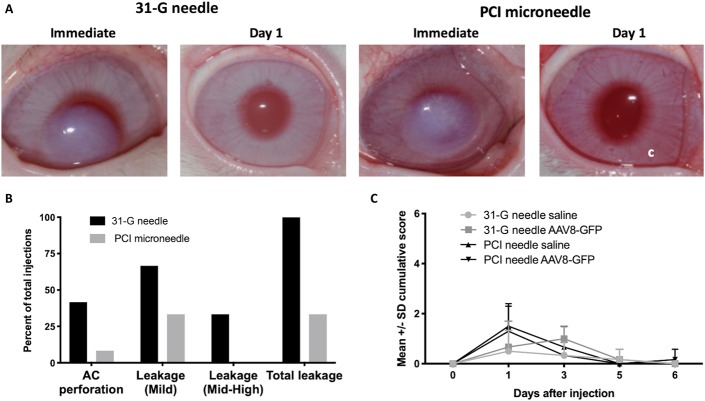
Intrastromal injections into normal, anesthetized rabbits were performed under an operating microscope by an experienced surgeon with an intent to provide axial intrastromal injections into normal rabbit corneas with a mean thickness of 367 ± SD 30 μM. Of the 12 injections using the 31-gauge needle, 5 resulted in AC perforations, 4 had moderate or high injection site drug leakage, and 8 had mild leakage, either during or on removal of the needle from the cornea (Fig. 3). Of the 12 injections made with the PCI microneedle, 1 injection was observed to penetrate into the AC, 1 had moderate surface injectate leakage, and 4 had mild leakage; all surface leakage occurred after removal of the needle from the cornea (Fig. 3). Using either the 31-gauge or PCI microneedle, no adverse effects (other than localized corneal opacity as described below) were observed after injection, and mean ocular inflammatory scores, intraocular pressure, and corneal thickness returned to near baseline by 24 hours and normalized in all eyes by 5 days after injection (Fig. 3 and Supplemental Digital Content 3, Supplemental Figure 3, http://links.lww.com/ICO/A919).
FIGURE 3.
Clinical findings after intrastromal injection of 25 μL of BSS or AAV-GFP using either the 31-G or PCI needle in vivo. A, Clinical color images of the injection site. The injection resulted in corneal opacity immediately after injection, which resolved by day 1 after injection (n = 12 injections/group). B, Adverse events during the injection procedure. During the injection, AC perforation (46% vs. 8%) and injection site leakage (100% vs. 46%) were more frequent using the 31-G versus the PCI microneedle. C, Mean cumulative ocular inflammatory scores demonstrate only a mild inflammatory response at 1 day after injection, which resolved completely by day 5.
Intrastromal injection of 25 μL of BSS or AAV-GFP using either the 31-gauge or the PCI needle resulted in corneal opacity immediately after injection, which resolved by day 1 after injection (Fig. 3). On OCT images, immediately after injection, the corneas in both groups were diffusely thickened, which prevented evaluation of the entire depth of the cornea by OCT. In some corneas, a tangential incision site from the 31-gauge needle was visible, whereas with the PCI needle, a slight indentation of the corneal epithelium at the site of the PCI needle injection was observed. Corneal OCT anatomy returned to normal by 24 hours after injection (Fig. 3).
In Vivo Expression of Intrastromal GFP
On in vivo imaging using a SLO, GFP fluorescence was visible in all AAV8-GFP-injected eyes, but negative on all saline-injected eyes on days 6, 9, 13, and 16 after injection (Fig. 4). The fluorescence increased in density through day 16, but there was no significant difference in the area of corneal GFP fluorescence in eyes injected with 31-gauge or PCI needles (Fig. 4). The % area of the infiltrated cornea using the 31-gauge and PCI needles was 14.3% and 18.2%, respectively, immediately after injection (based on color images of visible corneal opacity), but the % area of corneal GFP expression was nearly 50% by day 16 after injection in both groups (Fig. 4), suggesting that diffusion of the virus beyond the visible injection site had occurred.
Viral Genome (vg) Distribution
In corneal tissues, viral genome copies (per μg of host genome cDNA) were on average higher in the PCI-injected right corneas compared with the 31-gauge needle injections although the difference was not significant (P = 0.17) (Fig. 5). In the opposite (left eye) saline-injected corneas, with both injection needle types, viral genome copies were not detected (Fig. 5). In peripheral tissues, there was a significantly higher number of vg copies measured in animals that had intracameral penetration, regardless of needle type used for injection (P = 0.0396) (Fig. 5). Furthermore, in rabbits injected with the 31-gauge needle, those that had intracameral penetration had significantly higher vg copies in the submandibular LNs compared with those rabbits without intracameral penetration (P = 0.028). Interestingly, the only rabbits that had elevated vg copies in the submandibular LNs (n = 3) and liver (n = 1) were those that had intracameral penetration with the 31-gauge needle during injection. The single intracameral penetration using the PCI needle did not have elevated vg copies in the peripheral tissues evaluated (Fig. 5). Surface ocular leakage at the time of injection did not correlate with elevated vg copies in peripheral tissues.
FIGURE 5.
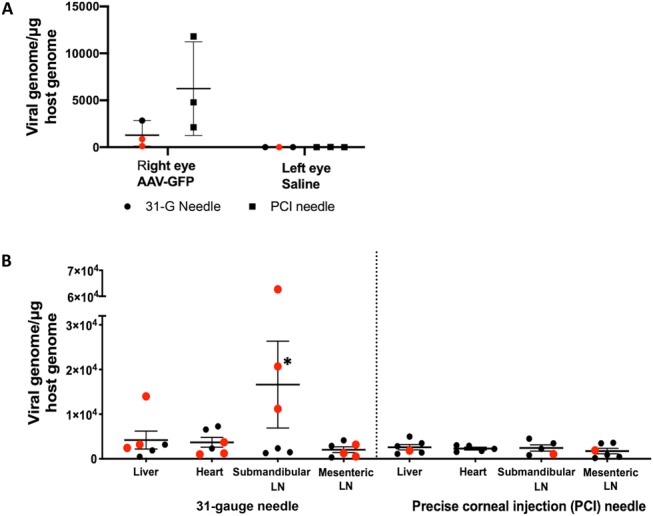
Expression of viral genomes. A, Expression of viral genomes in the cornea after intrastromal injection of AAV8-GFP (1 × 1010 vg) using either a 31-G or a PCI needle. Viral genomes per ug/host genome (scatterplot, with mean ± SD). The viral genome copies in the right eye using the PCI microneedle was on average higher than that measured in the left eye (injected with the 31-G needle), but the difference was not significant. Red data points associated with eyes with intracameral injection penetration. B, Extraocular viral genome (vg) distribution. More variance was observed among individual samples in all 31-G groups; however, no significant difference was observed between 31-G and PCI groups. Red data points associated with eyes with intracameral injection penetration. *Rabbit with a 1:4 viral serum titer.
Serum Neutralizing Antibody Analysis
Serum NABs to the viral capsid were not detected in any rabbit before injection (time 0) and were detected in 1 rabbit (a titer level of 1:4) that had been injected with a 31-gauge needle. All the remaining rabbits injected with either needle type had no measurable serum titer to the capsid. The rabbit with the serum titer of 1:4 also had intracameral penetration during injection with the 31-gauge needle and also had elevated vg copies detected in the draining submandibular LN (Fig. 5).
Corneal Histology (H&E)
No abnormalities (inflammation, scarring, or toxicity) were noted on corneal histology examined by light microscopy in all corneas, including those injected with both needle types, AAV-GFP, or saline.
DISCUSSION
PCI microneedles were developed to improve injection accuracy and allow safe intracorneal injections in the clinic for a variety of targeted diseases and drug payloads. In cadaver corneas, compared with a standard 31-gauge needle, PCI microneedles allowed repeatable, axial corneal intrastromal injection without leakage to the ocular surface or intracameral penetration. Furthermore, by selecting appropriate PCI microneedle lengths, injection to the superficial, midstromal, or deep stromal layers of the cornea was specifically targeted in porcine corneas and allows the clinician to target specific corneal sites by selecting an appropriate-length PCI needle to reach the diseased tissue location and depth after use of high-resolution imaging, such as HFU or optical coherence tomography. Site-specific targeting of disease would be beneficial to reduce toxic side effects, immune complications, and systemic exposure of drugs including gene therapy vectors and their transgenic products. Targeted treatment would also reduce associated costs by reducing the required dose, the frequency of use, and secondary treatment of adverse events or side effects.
Intrastromal corneal injection to deliver AAV gene therapy is feasible using a commercially available 31-gauge needle; however, injections with the PCI microneedle resulted in diffuse corneal transgene expression (ie, >50% of corneal area expressing GFP by in vivo imaging) without production of a serum capsid antibody titer and low exposure of vg in peripheral organs. This is in contrast to the use of the 31-gauge needle to deliver the same dose of viral vector to the cornea, which resulted in an overall higher exposure of vg to peripheral tissues, especially the draining submandibular LNs, liver, and heart, and development of a serum titer in 1 rabbit to the viral capsid (Fig. 5). Although not all rabbits with inadvertent intracameral injection had peripheral vg copies detected or capsid-specific antibodies, the fact that a significant elevation in peripheral tissues of vg and development of serum titers were limited to those with AC penetration strongly suggests a correlation (Fig. 5).
Prevention of the development of NABs to the AAV capsid is a key parameter for success of gene therapy. In fact, in many clinical trials, preexisting titers to AAV capsids are common exclusion criteria,10 and in hemophilia, even low levels of NABs to the AAV vector capsid or transgene may result in a lack of efficacy of the treatment.14 For ocular therapy, in which a relatively low AAV titer is injected into an immune-privileged site, such as the subretinal space, very low humoral immune response develops, even with repeated injections.15,16 However, if the same dose of AAV vector is injected intravitreally or subconjunctivally, as studied in nonhuman primates, mice, and clinical patients, a high humoral neutralizing antibody response developed likely through breakdown of the blood ocular barrier and/or exposure to systemic blood circulation.10,12,15 Therefore, the cornea, like the subretinal space, is an immune-privileged site. Injection of a gene therapy vector intrastromally should not expose it to the peripheral immune system, and thus, development of neutralizing should not occur, especially if the viral vector and transgene are contained within the corneal stroma. In this study, not all rabbits with AC penetration had peripheral vg detected in peripheral tissues or developed NABs, possibly because they received variable doses of AAV intracamerally (ie, most was delivered intrastromally and an unknown volume into the AC during the injection). However, the results of this study support that limiting viral vector exposure to the corneal stroma (an immune-privileged site) and reduction of vector leakage will help prevent exposure of peripheral tissues to viral genomes and development of serum titers to viral capsids (and possibly transgene), thus increasing the safety of corneal gene therapy.
The benefits of the use of the fixed-depth PCI microneedle combined with the ability to use the device to make precise injections in a patient in the clinic with the use of only local anesthesia suggest that the PCI microneedle could be used for delivery of many corneal therapeutics and possibly used in the field by first responders or military personnel, as examples. Further development of the PCI microneedle for specific corneal disease indications, in addition to delivery of viral vectors, seems warranted.
ACKNOWLEDGMENTS
The authors thank UNC Vector Core for providing the gene therapy product, NC State laboratory animal resources and central procedures laboratory, and Darby Roberts and Laura Conatser for technical assistance.
Footnotes
Supported by North Carolina Biotechnology Center (B. C. Gilger), National MPS Society (B. C. Gilger and M. Hirsch).
B. C. Gilger, S. Patel, and V. Zarnitsyn are on U.S. Provisional Patent Application Serial No. 62/668,975, along with NC State University, regarding this technology. M. Hirsch is an inventor on unrelated technology licensed to AskBio. Self-complementary AAV was evaluated in this study, which is licensed to AskBio. B. C. Gilger, S. Patel, and V. Zarnitsyn are cofounders of Theia Medical, Inc. B. C. Gilger and M. Hirsch are cofounders of Bedrock Therapeutics, Inc.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.corneajrnl.com).
REFERENCES
- 1.Prakash G, Sharma N, Goel M, et al. Evaluation of intrastromal injection of voriconazole as a therapeutic adjunctive for the management of deep recalcitrant fungal keratitis. Am J Ophthalmol. 2008;146:56–59. [DOI] [PubMed] [Google Scholar]
- 2.Hu J, Zhang J, Li Y, et al. A combination of intrastromal and intracameral injections of Amphotericin B in the treatment of severe fungal keratitis. J Ophthalmol. 2016;2016:3436415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eguchi H, Hayashi Y, Miyamoto T, et al. Ineffectiveness of intrastromal voriconazole for filamentous fungal keratitis. Clin Ophthalmol. 2014;8:1075–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang SY, Lee GA. Intrastromal injection of antibiotic agent in the management of recalcitrant bacterial keratitis. J Cataract Refract Surg. 2011;37:960–962. [DOI] [PubMed] [Google Scholar]
- 5.Hashemian MN, Zare MA, Rahimi F, et al. Deep intrastromal bevacizumab injection for management of corneal stromal vascularization after deep anterior lamellar keratoplasty, a novel technique. Cornea. 2011;30:215–218. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch ML, Conatser LM, Smith SM, et al. AAV vector-meditated expression of HLA-G reduces injury-induced corneal vascularization, immune cell infiltration, and fibrosis. Sci Rep. 2017;7:17840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vance M, Llanga T, Bennett W, et al. AAV gene therapy for MPS1-associated corneal blindness. Sci Rep. 2016;6:22131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan RR, Tandon A, Sharma A, et al. Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy. Invest Ophthalmol Vis Sci. 2011;52:4833–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai ML, Chen SL, Chou PI, et al. Inducible adeno-associated virus vector-delivered transgene expression in corneal endothelium. Invest Ophthalmol Vis Sci. 2002;43:751–757. [PubMed] [Google Scholar]
- 10.Kotterman MA, Yin L, Strazzeri JM, et al. Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther. 2015;22:116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackett RB, McDonald TO. Ophthalmic toxicology and assessing ocular irritation. 1996;299–305 and 557–566. In: Maibach FNM, Maibach HI, eds. Dermatoxicology. 5th ed Washington, DC: Hemisphere Publishing Corporation. [Google Scholar]
- 12.Song L, Llanga T, Conatser LM, et al. Serotype survey of AAV gene delivery via subconjunctival injection in mice. Gene Ther. 2018;25:402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Wu S, Albright B, et al. Development of patient-specific AAV vectors after neutralizing antibody selection for enhanced muscle gene transfer. Mol Ther. 2016;24:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. [DOI] [PubMed] [Google Scholar]
- 15.Reichel FF, Peters T, Wilhelm B, et al. Humoral immune response after intravitreal but not after subretinal AAV8 in primates and patients. Invest Ophthalmol Vis Sci. 2018;59:1910–1915. [DOI] [PubMed] [Google Scholar]
- 16.Bennett J, Ashtari M, Wellman J, et al. Gene therapy: AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4:120ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]