Abstract
Females are relatively resistant to salt-sensitive hypertension than males, but the mechanisms are not completely elucidated. We recently demonstrated a decisive role of macula densa neuronal nitric oxide synthase β (NOS1β)-mediated tubuloglomerular feedback (TGF) in the long-term control of glomerular filtration rate (GFR), sodium excretion and blood pressure. In the present study, we hypothesized that the macula densa NOS1β-mediated TGF mechanism is different between male and female, thereby contributing to the sexual dimorphism of salt-sensitive hypertension. We used microperfusion, micropuncture, clearance of FITC–inulin and radio telemetry to examine the sex differences in the changes of macula densa NOS1β expression and activity, TGF response, natriuresis and blood pressure after salt loading in wild type and macula densa–specific NOS1 knockout (KO) mice. In wild type mice, a high salt diet induced greater increases in macula densa NOS1β expression and phosphorylation at Ser 1417, greater nitric oxide (NO) generation by the macula densa, and more inhibition in TGF response in vitro and in vivo in females than males. Additionally, the increases of GFR, urine flow rate and sodium excretion in response to an acute volume expansion were significantly greater in females than males. The blood pressure responses to Angiotensin II plus a high salt diet were significantly less in females than males. In contrast, these sex differences in TGF, natriuretic response and blood pressure were largely diminished in KO mice. In conclusion, macula densa NOS1β-mediated TGF is a novel and important mechanism for the sex differences in salt-sensitive hypertension.
Keywords: salt sensitivity hypertension, gender differences, macula densa, neuronal nitric oxide synthase, tubuloglomerular feedback
Graphical Abstract
INTRODUCTION
Hypertension affects about 30% of American adults and more than half of hypertensive patients are salt-sensitive.1-4 Before menopause, women have lower risk for most cardiovascular events, including salt-sensitive hypertension, than men.5-8 Moreover, various animal models of salt-sensitive hypertension also exhibit sex-related differences in blood pressure.9-13 However, the underlying mechanisms for the sexual dimorphism of salt-sensitive hypertension have not been fully clarified.5,14
Increases in glomerular filtration rate (GFR) in response to salt loading play a vital role in the rapid elimination of sodium to maintain salt-water balance and normal blood pressure. Impaired responses of GFR to salt loading have been observed in both humans15,16 and animal models17,18 with salt-sensitive hypertension. Tubuloglomerular feedback (TGF) is an important mechanism in control of GFR. It describes a negative feedback between tubule and afferent arteriole (Af-Art), in which an increase in NaCl delivery to the macula densa promotes the release of adenosine and/or ATP that constricts the Af-Art and thereby induces a tonic inhibition of single nephron GFR.19-21 Nitric oxide (NO) generated by neuronal nitric oxide synthase (NOS1) in the macula densa is a major modulator of TGF response, which buffers or attenuates the TGF response via a cGMP-dependent pathway.22-25 Recently, several studies from our laboratory have demonstrated that NOS1β is the primary and salt-sensitive splice variant of NOS1 in the macula densa, which contributes to most of the NO generation by the macula densa in response to salt loading;26-28 mice with the deletion of NOS1 from the macula densa exhibit augmented TGF response, impaired natriuresis and salt-sensitive hypertension.27 However, all of the above studies were performed in male mice.26-28 It is unknown whether the macula densa NOS1 β-mediated TGF in response to salt loading is different in females and if this difference in TGF responsiveness contributes to the sexual dimorphism of salt-sensitive hypertension.
In the present study, we hypothesized that a salt loading induces greater increases in macula densa NOS1β expression and activity in females than males; the higher expression and activity levels of macula densa NOS1β in females promote lower TGF response and higher GFR, thereby facilitating sodium excretion and protecting against salt-sensitive hypertension. Microperfusion, micropuncture, renal clearance of FITC–inulin and radio telemetry methods were utilized to examine the sex differences in the changes of macula densa NOS1β expression and phosphorylation, NO generation in the macula densa, TGF responsiveness, natriuretic response and mean arterial pressure (MAP) following a high salt diet in both wild type and macula densa–specific NOS1 knockout (KO) mice.
METHODS
Data, analytical methods, and study materials are available from the corresponding author on reasonable request. The detail methods are available in the online supplement.
Animals
C57BL/6 mice (male and female, 12 weeks old) were purchased from Jackson Laboratory. The KO mice (male and female, 12 weeks old) were generated by crossing NKCC2cre mice with NOS1flox/flox mice as we described previously.27 The number of animals used was indicated in the figure legends. Mice were fed with either high salt diet (4.0% NaCl; Envigo,) or normal salt diet (0.4% NaCl; Envigo) for 2 weeks. All protocols were approved by the Institutional Animal Care and Use Committee at the University of South Florida College of Medicine.
Statistical analysis
Statistical analysis was performed using Prism 8 (GraphPad Software; San Diego, CA). The effects of interest were tested using t-test, or two-way analysis of variance (ANOVA) followed by Sidak multiple comparisons test when appropriate. Data were presented as a mean ± SEM, and a p-value <0.05 was considered statistically significant.
RESULTS
A high salt diet induces greater increases of macula densa NOS1β expression and phosphorylation at Ser 1417 in female than male mice
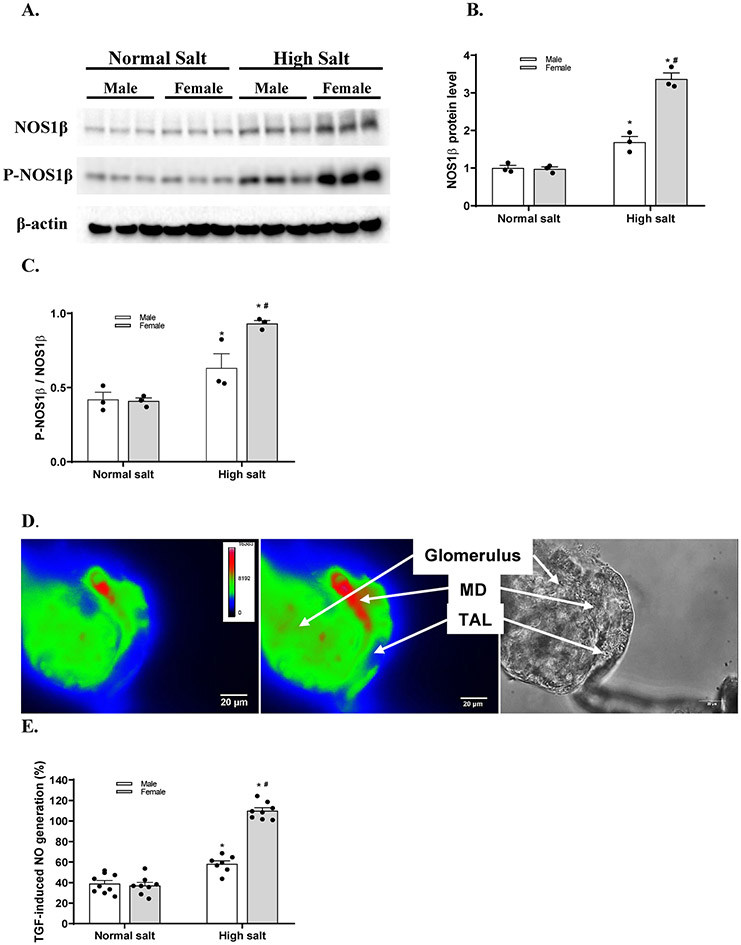
To determine the sex differences in the effect of salt loading on the expression and phosphorylation levels of NOS1 splice variants in the macula densa, we measured the protein levels of NOS1 and P-NOS1 in the renal cortex,27,29,30 where most of the NOS1 comes from macula densa cells, in male and female C57BL/6 mice fed either a normal salt diet or a high salt diet (Figure 1A). The NOS1β and P-NOS1β/NOS1β levels in the renal cortex were not significantly different between male and female mice on a normal salt diet. A 2 weeks of high salt diet increased the NOS1β and P-NOS1β/NOS1β levels by 68.7±10.9% and 49.3±12.8% respectively in male mice, and by 246.1±21.2% and 129.1±26.9% respectively in female mice (Figure 1B and 1C). These results demonstrated that the salt loading induced greater increases of macula densa NOS1β expression and phosphorylation at Ser 1417 in female than male mice.
Figure 1. High salt diet induces greater increases of macula densa NOS1β expression and activity in female mice than male mice.
(A) The immunoblots of NOS1, P-NOS1 and the loading control of β-actin. The renal cortical levels of NOS1β (B) and P-NOS1β/NOS1β (C) in male and female C57BL/6 mice with normal salt diet or high salt diet. n=3; *P<0.01 versus normal salt; #P<0.01 versus male mice. (D) The NO generation in the macula densa was measured in isolated perfused JGA with DAF-2 DA. The bright field image exhibited the anatomic structure of the perfused JGA. The florescent image of DAF-2 DA loaded JGA showed the NO generation in the macula densa. (E) The TGF-induced NO generation by the macula densa in male and female C57BL/6 mice with normal salt diet or high salt diet. n=7-9; *P<0.01 versus normal salt; #P<0.05 versus male mice. Statistical difference was calculated by two-way ANOVA followed by Sidak multiple comparisons test.
A high salt diet induces greater NO generation at the macula densa in female than male mice
To determine the sex differences in the effect of salt loading on the NO generation in the macula densa, we measured the TGF-induced NO generation in male and female C57BL/6 mice fed either a normal salt diet or a high salt diet.27,29 The TGF-induced NO generation by the macula densa was not significantly different between male and female mice on a normal salt diet. A 2 weeks of high salt diet increased the production of NO by 58.1±7.1% (from 117.1±10.7 to 184.8±15.2 units/min) in male mice and by 109.9±8.3% (from 115.1±7.1 to 241.3±9.7 units/min) in female mice when NaCl concentration in tubular perfusate was increased from 10 to 80 mM (Figure 1D). These results demonstrated that the salt loading induced greater NO generation by the macula densa in female than male mice.
A high salt diet induces more inhibitions of TGF response in vitro and in vivo in female than male mice
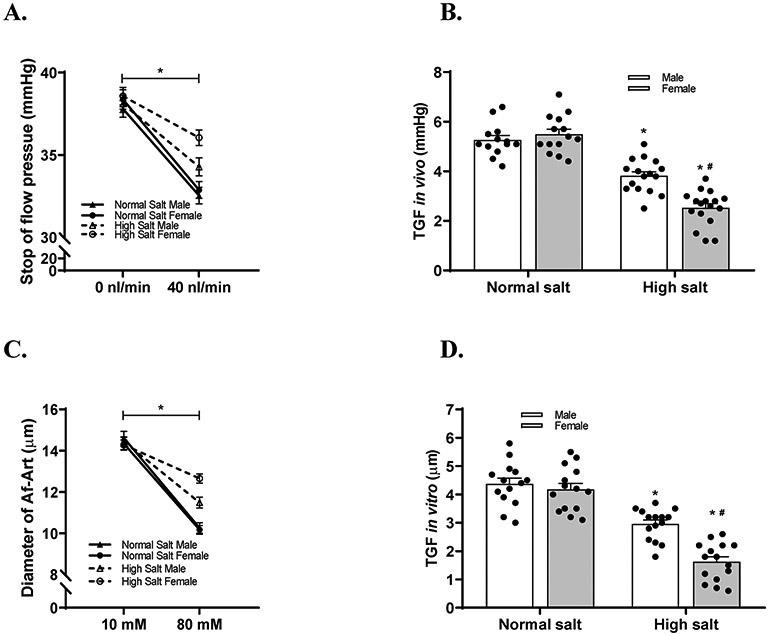
To determine the sex differences in the effect of salt loading on TGF responsiveness, we measured TGF response in vivo with micropuncture in male and female C57BL/6 mice fed either a normal salt diet or a high salt diet.27,29 The TGF response in vivo was not significantly different between male and female mice on a normal salt diet. Following a 2 weeks of high salt diet, the TGF response in vivo, indicated by ΔPsf,, was reduced by 27.4±12.8% (from 5.26±0.69 mmHg to 3.81±0.67 mmHg) in male mice and by 54.1±13.1% (from 5.49±0.78 mmHg to 2.52±0.72 mmHg) in female mice (Figure 2A and 2B).
Figure 2. High salt diet induces more inhibitions in TGF response in vitro and in vivo in female mice than male mice.
(A-B) TGF response in vivo was indicated by the change of stop flow pressure when the tubular perfusion rate was increased from 0 to 40 nl/min. TGF response in vivo was measured and compared in male and female C57BL/6 mice with normal salt diet or high salt diet. n=10-13 tubules/4-5 mice; *P<0.01 versus normal salt; #P<0.05 versus male mice. (C and D) TGF response in vitro was indicated by the change of Af-Art diameter while the macula densa perfusate was switched from 10 to 80 mM NaCl. TGF response in vitro was measured and compared in male and female C57BL/6 mice with normal salt diet or high salt diet. n=11-12; *P<0.01 versus normal salt; #P<0.05 versus male mice. Statistical difference was calculated by two-way ANOVA followed by Sidak multiple comparisons test.
To eliminate systemic confounding factors such as hormones and sympathetic activity, we also measured TGF response in vitro in isolated and double perfused JGAs. The TGF response in vitro was not significantly different between male and female mice on a normal salt diet. Following a 2 weeks of high salt diet, the TGF response in vitro, indicated by the change in luminal diameter of Af-Art, was decreased by 32.3±12.6% (from 4.3±0.7 μm to 2.9±0.5 μm) in male mice and by 61.1±15.9% (from 4.1±0.8 μm to 1.6±0.7 μm) in female mice (Figure 2C and 2D).
These results demonstrated that the salt loading induced more inhibition of TGF response in female than male mice.
The sex differences in the effect of salt loading on TGF response are dependent on macula densa NOS1β
To determine the significance of macula densa NOS1β in the sex differences in the effect of salt loading on TGF responsiveness, we measured the changes of TGF response in vivo and in vitro following a 2 weeks of high salt diet in male and female NOS1flox/flox, as well as KO mice.
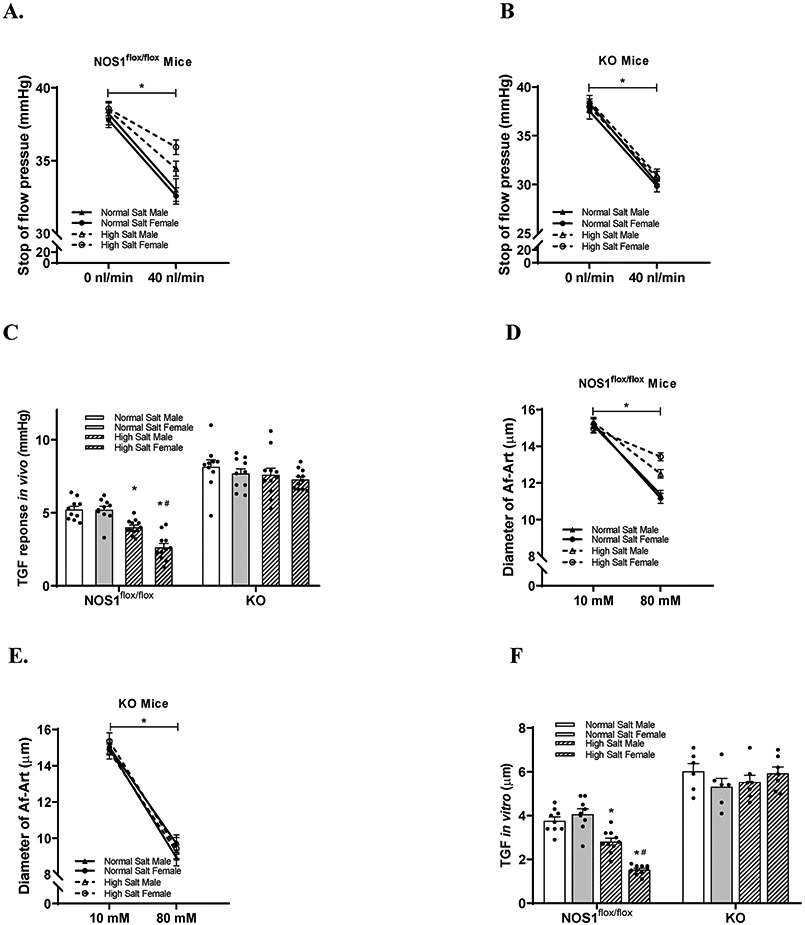
In NOS1flox/flox strain, TGF responses in vitro and in vivo were not significantly different between male and female mice on a normal salt diet. In male NOS1flox/flox mice on high salt diet, the TGF response in vivo and in vitro were reduced by 23.1±9.6% (from 5.22±0.72 to 4.01±0.50 mmHg) and 25.1±13.4% (from 3.75±0.53 to 2.81±0.50 μm) respectively compared with normal salt diet. In female NOS1flox/flox mice on high salt diet, the TGF response in vivo and in vitro were reduced by 49.3±17.3% (from 5.20±0.76 to 2.63±0.91 mmHg) and 62.4±5.3% (from 4.06±0.72 to 1.53±0.21 μm) respectively compared with normal salt diet. The high salt diet-induced inhibitions of TGF in vivo (Figure 3A and 3C) and in vitro (Figure 3D and 3F) were greater in female than male NOS1flox/flox mice by 1.6±0.4 μm and 1.36±0.62 mmHg respectively.
Figure 3. The sex differences in the effect of salt loading on TGF response are mediated by macula densa NOS1β.
(A-C) TGF response in vivo (n=10-12 tubules/3-5 mice) and (D-F) TGF response in vitro (n=6-10) in male and female NOS1flox/flox mice, as well as KO mice with normal salt diet or high salt diet. *P<0.01 versus normal salt; #P<0.05 versus male mice. Statistical difference was calculated by two-way ANOVA followed by Sidak multiple comparisons test.
In KO strain, TGF response in vivo and in vitro were not significantly different between male and female mice on a normal salt diet, but significantly enhanced compared with NOS1flox/flox mice. Following a 2 week of high salt diet, neither TGF response in vivo (Figure 3B and 3C) nor in vitro (Figure 3E and 3F) were significantly changed in KO mice or significantly different between males and females.
These results demonstrated that the sex differences in the effect of salt loading on TGF responsiveness were mediated by macula densa NOS1β in mice.
Macula densa NOS1β contributes to the sex differences in natriuretic responses to acute volume expansion
To determine the significance of macula densa NOS1β in the sex differences of natriuresis, we measured the changes in GFR, urine flow rate and sodium excretion after an acute volume expansion in male and female NOS1flox/flox, as well as KO mice.27
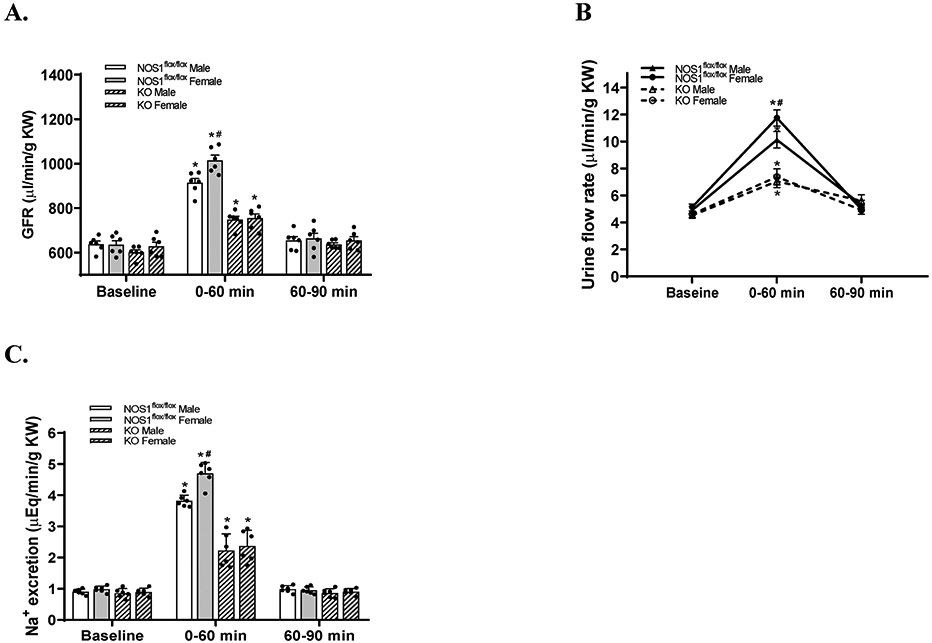
In NOS1flox/flox strain, the basal levels of GFR, urine flow rate and sodium excretion were not significantly different between male and female mice. In male NOS1flox/flox mice, an acute volume expansion increased GFR by 43.4±9.3% (from 637.8±33.3 to 913.2±47.8 μl/min/g KW), urine flow rate by 107.7±28.4% (from 4.9±0.6 to 10.1±1.4 μl/min/g KW), and sodium excretion by 324.7±32.9% (from 0.90±0.08 to 3.81±0.18 μEq/min/g KW) compared with baselines. In female NOS1flox/flox mice, an acute volume expansion increased GFR by 60.3±14.1% (from 635.4±44.6 to 1014.6±56.7 μl/min/g KW), urine flow rate by 128.3±18.7% (from 5.1±0.5 to 11.7±1.5 μl/min/g KW), and sodium excretion by 380.2±59.5% (from 0.98±0.09 to 4.69±0.35 μEq/min/g KW) compared with baselines. The acute volume expansion-induced increases in GFR (Figure 4A), urine flow rate (Figure 4B) and sodium excretion (Figure 4C) were greater in female than male NOS1flox/flox mice by 103.8±22.2 μl/min/g KW, 1.4±0.3 μl/min/g KW and 0.80±0.27 μEq/min/g KW, respectively.
Figure 4. The sex differences in natriuretic responses to acute volume expansion are dependent on macula densa NOS1β.
The changes in GFR (A), urine flow rate (B) and sodium excretion (C) during the 0-60 minute period and 60-90 minute period after acute volume expansion in male and female NOS1flox/flox mice, as well as KO mice. n=6; *P<0.01 versus baseline; #P<0.05 versus male mice. Statistical difference was calculated by two-way ANOVA followed by Sidak multiple comparisons test.
In KO strain, the basal levels of GFR, urine flow rate and sodium excretion were not significantly different between male and female mice and similar to NOS1flox/flox strain. Following an acute volume expansion, the changes in GFR, urinary flow rate and sodium excretion were not significantly different between males and females. The acute volume expansion increased GFR by 24.4±1.9% (from 601.4±28.3 to 748.3±37.1 μl/min/g KW) and 20.5±7.8% (from 627.9±45.7 to 755.1±46.5 μl/min/g KW) (Figure 4A), urine flow rate by 55.4±20.3% (from 4.5±0.4 to 7.1±1.2 μl/min/g KW) and 60.2±27.7% (from 4.5±0.6 to 7.3±1.4 μl/min/g KW) (Figure 4B), and sodium excretion by 162.9±63.3% (from 0.85±0.14 to 2.22±0.53 μEq/min/g KW) and 189.3±80.4% (from 0.89±0.12 μm to 2.37±0.50 μEq/min/g KW) (Figure 4C) compared with baselines in males and females, respectively.
These results demonstrated that the sex differences in natriuretic responses to the acute volume expansion were dependent on macula densa NOS1β in mice.
Macula densa NOS1β contributes to the sex differences in salt-sensitive hypertension
To determine the significance of macula densa NOS1β in the sex differences of salt-sensitive hypertension, we measured blood pressure responses to a subpressor dose of Ang II plus salt loading in male and female NOS1flox/flox, as well as KO mice.27,31,32
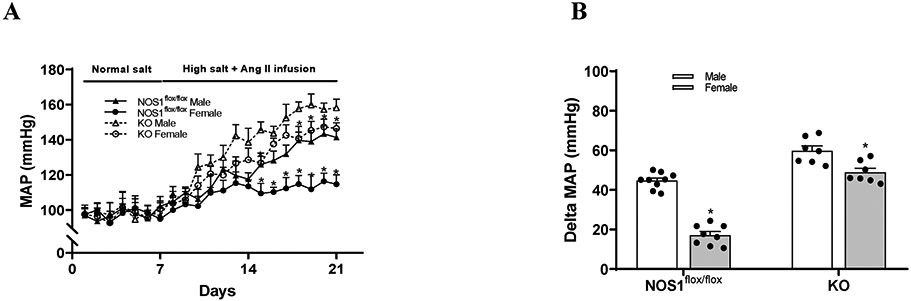
In NOS1flox/flox strain, the basal levels of MAP were not significantly different between male and female mice on a normal salt diet. Following a 2 weeks of high salt diet along with Ang II infusion, MAP raised by 46.2±5.3% (from 96.9±3.9 mmHg to 141.3±4.7 mmHg) in males and by 17.6±4.8% (from 97.6±3.8 mmHg to 114.7±5.3 mmHg) in females (Figure 5A and 5B) compared with baselines. The increases of blood pressure were significantly less in female than male NOS1flox/flox mice by 27.5±4.4 mmHg.
Figure 5. The sex differences in salt-sensitive hypertension are dependent on macula densa NOS1β.
(A and B) The MAP responses to sub-pressor Ang II infusion plus high salt diet in male and female NOS1flox/flox mice, as well as NOS1KO mice. n=7-9; *P<0.01 versus normal salt; #P<0.05 versus female mice. Statistical difference was calculated by two-way ANOVA followed by Sidak multiple comparisons test.
In KO strain, the basal levels of MAP were not significantly different between male and female mice on a normal salt diet and similar to NOS1flox/flox strain. Following a 2 weeks of high salt diet along with Ang II infusion, MAP raised by 60.9±8.9% (from 98.5±4.2 mmHg to 158.2±4.9 mmHg) in males and by 50.2±7.3% (from 97.6±3.1 mmHg to 146.5±3.2 mmHg) in females (Figure 5A and 5B) compared with baselines. The sex differences in the increases of blood pressure were 10.8±5.5 mmHg in KO mice, which was significantly diminished compared with that in NOS1flox/flox mice (27.5±4.4 mmHg).
These results demonstrated that macula densa NOS1β played a significant role in the sex differences of salt-sensitive hypertension in mice.
DISCUSSION
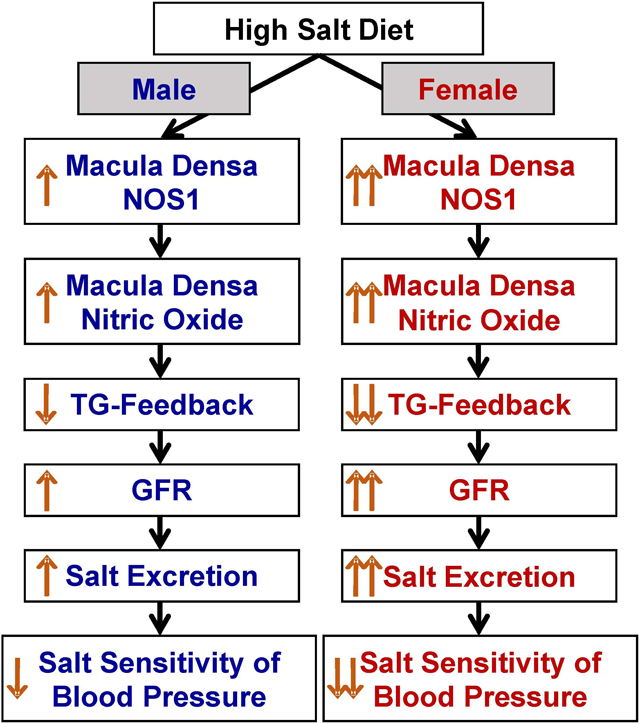
The present study demonstrated the significance of macula densa NOS1β-mediated TGF mechanism in the sexual dimorphism of salt-sensitive hypertension. We found that a high salt diet induced higher levels of macula densa NOS1β expression and phosphorylation at Ser 1417, greater NO generation at the macula densa and lower TGF response in female than male wild type mice. Moreover, female mice exhibited a greater natriuretic response to acute volume expansion and lower MAP in response to Ang II infusion plus a high salt diet. In contrast, these sex differences in TGF response, natriuresis and blood pressure were largely diminished in KO mice.
It is well-known that the sexual dimorphism of salt-sensitive hypertension exists in both humans5-8 and experimental animals.9-13 However, the underlying mechanisms for the sex differences in salt-sensitive hypertension have not been fully clarified.5,14 Our recent studies have demonstrated the significance of macula densa NOS1β-mediated TGF mechanism in the long-term control of sodium excretion and blood pressure.26-28 However, all of these previous studies were undertaken on male mice only without a comparison to females. The role of macula densa NOS1β-mediated TGF mechanism in the sexual dimorphism of salt-sensitive hypertension remains to be determined.
In the present study, we found that the expression level of NOS1β in the renal cortex was similar between male and female C57BL/6 mice on a normal salt diet, while females had a greater increase in NOS1β expression than males on a high salt diet. In addition, it has been reported that the phosphorylation of NOS1 at Ser1417 by cAMP-dependent protein kinase (PKA) increased the NOS activity,33-35 and the phosphorylation at Ser847 by calmodulin-dependent protein kinase (CaM-K) decreased its activity.36,37 In this study, we found that the phosphorylation level of NOS1β at Ser1417 in the renal cortex was not significantly different between male and female C57BL/6 mice on a normal salt diet, while females had a greater upregulation in NOS1β phosphorylation at Ser1417 than males on a high salt diet. Furthermore, the TGF-induced NO production in the macula densa was similar between male and female C57BL/6 mice on a normal salt diet, but significantly higher in females than males on a high salt diet. These results demonstrated that the salt loading induced greater increases in both expression and activity of macula densa NOS1 β in female than male mice. Consistent with our findings in C57BL/6 mice, the renal cortical NOS1 expression was shown to be similar between male and female Sprague Dawley rats,38,39 as well as spontaneously hypertensive rats40 on a normal salt diet. However, the sex differences in NOS1 expression on a high salt diet were not examined in these studies. It was also reported that manipulation of sex hormones did not significantly alter the renal cortical NOS1 expression or activity in spontaneously hypertensive rats41 and Fischer-344 rats,42 suggesting that the sex hormones may not play an essential role in regulating the expression or phosphorylation of NOS1 in the macula densa. Since the PI3K/Akt43-46 and cAMP/PKA35,47,48 pathways have been recognized to participate in the regulation of NOS1 expression and phosphorylation, we speculate that the sex differences existing in these signaling pathways49-54 might be associated with the sexual dimorphism of the salt loading-induced changes in macula densa NOS1β, which will be examined in the future studies.
NO generated by NOS1β at the macula densa is a key modulator of TGF response, which buffers or attenuates the TGF response via a cGMP-dependent pathway.22,23,54 The TGF mechanism describes a negative feedback between tubule and Af-Art, where an increase in NaCl delivery to the macula densa promotes the release and formation of ATP and/or adenosine, which then constricts the Af-Art, thereby resulting in a tonic inhibition of single nephron GFR.55-58 It was reported that TGF response was similar between the age-matched male and female FVB/N mice with a sodium-controlled diet (0.25% NaCl).59 Nevertheless, the sex differences in the effect of salt loading on TGF response remain unknown. In the present study, we found that the TGF response was not significantly different between male and female C57BL/6 mice on a normal salt diet, which is consistent with the previous finding in FVB/N mice. However, female mice had a greater inhibition in TGF response than male mice on a high salt diet. Recently, our laboratory generated a macula densa-specific NOS1 knockout mouse strain by crossing an NKCC2cre line with a NOS1flox/flox line. This floxed mouse line targets the exon 6 of the NOS1 gene and excision of this exon by Cre recombinase inactivates all splice variants of NOS1.27,60 Furthermore, because the expression of NOS1 is negligible in the thick ascending limb compared with that in the macula densa,61,62 this NKCC2cre/NOS1flox/flox line is considered as a macula densa–selective NOS1 knockout model. Thus, in the present study, this KO model was utilized to determine the significance of macula densa NOS1 in the sexual dimorphism of TGF response on a high salt diet. We found that the salt loading-induced inhibition of TGF was significantly greater in female than male NOS1flox/flox mice, but the sex differences were almost eliminated in the KO mice, indicating that the sex differences in the effect of salt loading on TGF responsiveness are mediated by macula densa NOS1β.
The increase of GFR plays a critical role in sodium excretion following salt loading to restore salt-water balance and protect against salt-sensitivity of blood pressure. An acute salt loading via saline infusion or chronic salt loading via high salt diet was reported to increase GFR in healthy normotensive subjects without significant changes in blood pressure.63-67 In contrast, the salt loading-induced rises of GFR were attenuated or absent in salt-sensitive subjects with significant elevations in blood pressure.15,16 Similar phenotypes were also observed in experimental animal models. A saline infusion or high salt diet increased GFR in Dahl salt-resistant rats without significant changes in blood pressure. On the contrary, the salt loading-induced rises of GFR were largely attenuated in Dahl salt-sensitive rats with significant increases in blood pressure.17,18,68,69 Moreover, sexual dimorphism of pressure-natriuresis has been shown in Sprague-Dawley rats, wherein females exhibited a greater sodium excretion than males at a defined renal perfusion pressure.70,71 However, the mechanisms underlying these sex differences in natriuresis remain unclear. In the present study, to determine the significance of macula densa NOS1β in the sex differences of natriuretic responses to salt loading, we measured the changes in GFR, urine flow rate and sodium excretion in response to an acute volume expansion in male and female KO mice and compared with the NOS1flox/flox mice. We found that the increases of GFR, urine flow rate and sodium excretion in response to the acute volume expansion were significantly greater in female than male NOS1flox/flox mice, whereas these sex differences were almost eliminated in the KO mice, indicating that the sex differences in natriuretic responses to salt loading are dependent on macula densa NOS1β.
Chronic administration of subpressor dose of Ang II is a well-established and widely-used animal model that mimics many characteristics of salt-sensitive hypertension in humans.72-75 Many previous studies have demonstrated that females are relatively resistant to subpressor Ang II-induced hypertension compared with males and the sex differences in blood pressure responses are further exaggerated with salt loading.10,11,59,76 However, the mechanisms for these sex differences in subpressor Ang II-induced salt-sensitive hypertension have not been fully clarified. In the present study, to determine the significance of macula densa NOS1β in the sexual dimorphism of salt-sensitive hypertension, we measured the blood pressure responses to subpressor Ang II along with a high salt diet in male and female NOS1flox/flox mice, as well as KO mice. We found that the increases of blood pressure were nearly 30 mmHg greater in male than female NOS1flox/flox mice, whereas the sex differences in the increases of blood pressure were less than 10 mmHg in the KO mice, which indicates that macula densa NOS1β plays an essential role in the sexual dimorphism of subpressor Ang II-induced salt-sensitive hypertension. In addition, our recent study27 has demonstrated that high salt diet significantly increased the MAP by about 10 mmHg in the KO mice, but not in the NOS1flox/flox mice. Therefore, it is the true salt-sensitivity of blood pressure. The reason why we added Ang II is to exaggerate the blood pressure response to high salt diet.
Although the macula densa NOS1β-mediated TGF is an important mechanism for the sex differences in salt-sensitive hypertension, we are aware that the knockout of macula densa NOS1 does not completely eliminate the differences in blood pressure responses to subpressor Ang II plus salt loading between male and female mice, and the other factors also contribute to the sexual dimorphism of salt-sensitive hypertension. For example, macula densa angiotensin type 2 receptor (AT2R)-mediated TGF response was reported to be critical in the sex differences in Ang II-induced hypertension59. Whereas several other studies demonstrated that Ang II enhanced TGF response and stimulated cytosolic calcium increases in the macula densa cells through only AT1 receptors but not AT2 receptors.77,78 Many previous studies have also indicated the involvement of sex hormones in the sexual dimorphism of salt-sensitive hypertension. It was reported that the sex differences in the DOC-salt hypertension were completely abolished by gonadectomy.79,80 Ovariectomy was shown to exacerbate the development of hypertension in females to a comparable level of males in Dahl salt-sensitive rats.81-83 The pressor responses to Ang II were found to be attenuated in males with castration and augmented in females with ovariectomy.10 Moreover, a previous study from our laboratory demonstrated that testosterone enhanced TGF response by stimulating superoxide production in macula densa cells via androgen receptors.84 However, whether the effects of these factors are dependent on macula densa NOS1-mediated TGF response is still elusive and needs to be determined in future studies.
PERSPECTIVES
This study demonstrated a novel mechanism for the sex differences in salt-sensitive hypertension, wherein a high salt diet induces higher levels of macula densa NOS1β expression and phosphorylation at Ser 1417 in females than males, which results in a greater inhibition in TGF response, thereby facilitating sodium excretion and protecting against salt-sensitivity in blood pressure. These findings establish a critical role of macula densa NOS1β-mediated TGF mechanism in the sexual dimorphism of salt-sensitive hypertension.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is New?
Using a variety of sophisticated techniques and a novel macula densa-specific NOS1 knockout model, we identified a new mechanism for the sex differences in salt-sensitive hypertension, wherein salt loading induces more upregulation in the expression and activity of macula densa NOS1β in females than males, which results in greater inhibition in tubuloglomerular feedback response, thereby facilitating sodium excretion and protecting against salt-sensitivity in blood pressure.
What Is Relevant?
Females are relatively resistant to salt-sensitive hypertension compared with males. However, the underlying mechanism for the sexual dimorphism of salt-sensitive hypertension has not been fully elucidated.
Summary.
Macula densa NOS1β-mediated tubuloglomerular feedback is a novel mechanism for the sex differences in salt-sensitive hypertension.
Acknowledgments
SOURCES OF FUNDING
This work was supported by American Heart Association Predoctoral Fellowship and American Society of Nephrology Ben J. Lipps Research Fellowship Awards (to J. Zhang.), American Society of Nephrology Ben J. Lipps Research Fellowship Award (to J. Wei.), American Physiological Society STRIDE Summer Research Fellowship Award (to L. Qu.), American Heart Association Career Development Award 18CDA34110441 (to L. Wang.), the National Institutes of Health grants DK099276, HL142814, and HL137987 (to R. Liu.), and National Natural Science Foundation of China 81900347 (to J. Zhu.).
Footnotes
DISCLOSURES
None.
Reference List
- 1.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension 2004; 44(4):398–404. [DOI] [PubMed] [Google Scholar]
- 2.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA 2002; 287(8):1003–1010. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension 1996; 27(3 Pt 2):481–490. [DOI] [PubMed] [Google Scholar]
- 4.Luft FC. Salt and hypertension: recent advances and perspectives. J Lab Clin Med 1989; 114(3):215–221. [PubMed] [Google Scholar]
- 5.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension 2001; 37(5):1199–1208. [DOI] [PubMed] [Google Scholar]
- 6.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension 1995; 25(3):305–313. [DOI] [PubMed] [Google Scholar]
- 7.Wiinberg N, Hoegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens 1995; 8(10 Pt 1):978–986. [DOI] [PubMed] [Google Scholar]
- 8.Khoury S, Yarows SA, O’Brien TK, Sowers JR. Ambulatory blood pressure monitoring in a nonacademic setting. Effects of age and sex. Am J Hypertens 1992; 5(9):616–623. [DOI] [PubMed] [Google Scholar]
- 9.Dahl LK, Knudsen KD, Ohanian EV, Muirhead M, Tuthill R. Role of the gonads in hypertension-prone rats. J Exp Med 1975; 142(3):748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol 2005; 288(5):H2177–H2184. [DOI] [PubMed] [Google Scholar]
- 11.Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hypertension 2008; 51(4):1170–1176. [DOI] [PubMed] [Google Scholar]
- 12.Bayorh MA, Socci RR, Eatman D, Wang M, Thierry-Palmer M. The role of gender in salt-induced hypertension. Clin Exp Hypertens 2001; 23(3):241–255. [DOI] [PubMed] [Google Scholar]
- 13.Ouchi Y, Share L, Crofton JT, Iitake K, Brooks DP. Sex difference in the development of deoxycorticosterone-salt hypertension in the rat. Hypertension 1987; 9(2):172–177. [DOI] [PubMed] [Google Scholar]
- 14.Maranon R, Reckelhoff JF. Sex and gender differences in control of blood pressure. Clin Sci (Lond) 2013; 125(7):311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campese VM, Parise M, Karubian F, Bigazzi R. Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension 1991; 18(6):805–812. [DOI] [PubMed] [Google Scholar]
- 16.Stadler P, Pusterla C, Beretta-Piccoli C. Renal tubular handling of sodium and familial predisposition to essential hypertension. J Hypertens 1987; 5(6):727–732. [DOI] [PubMed] [Google Scholar]
- 17.Hua JL, Kaskel FJ, Juno CJ, Moore LC, McCaughran JA Jr. Salt intake and renal hemodynamics in immature and mature Dahl salt-sensitive (DS/JR) and salt-resistant (DR/JR) rats. Am J Hypertens 1990; 3(4):268–273. [DOI] [PubMed] [Google Scholar]
- 18.Simchon S, Manger WM, Carlin RD, Peeters LL, Rodriguez J, Batista D, Brown T, Merchant NB, Jan KM, Chien S. Salt-induced hypertension in Dahl salt-sensitive rats. Hemodynamics and renal responses. Hypertension 1989; 13(6 Pt 1):612–621. [DOI] [PubMed] [Google Scholar]
- 19.Vallon V Tubuloglomerular feedback and the control of glomerular filtration rate. News Physiol Sci 2003; 18:169–174. [DOI] [PubMed] [Google Scholar]
- 20.Schnermann J, Traynor T, Yang T, Arend L, Huang YG, Smart A, Briggs JP. Tubuloglomerular feedback: new concepts and developments. Kidney Int Suppl 1998; 67:S40–S45. [DOI] [PubMed] [Google Scholar]
- 21.Briggs JP, Schnermann J. The tubuloglomerular feedback mechanism: functional and biochemical aspects. Annu Rev Physiol 1987; 49:251–273. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs G, Komlosi P, Fuson A, Peti-Peterdi J, Rosivall L, Bell PD. Neuronal nitric oxide synthase: its role and regulation in macula densa cells. J Am Soc Nephrol 2003; 14(10):2475–2483. [DOI] [PubMed] [Google Scholar]
- 23.Ren YL, Garvin JL, Carretero OA. Role of macula densa nitric oxide and cGMP in the regulation of tubuloglomerular feedback. Kidney Int 2000; 58(5):2053–2060. [DOI] [PubMed] [Google Scholar]
- 24.Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, Schmidt HH. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci U S A 1992; 89(24):11993–11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mundel P, Bachmann S, Bader M, Fischer A, Kummer W, Mayer B, Kriz W. Expression of nitric oxide synthase in kidney macula densa cells. Kidney Int 1992; 42(4):1017–1019. [DOI] [PubMed] [Google Scholar]
- 26.Lu D, Fu Y, Lopez-Ruiz A, Zhang R, Juncos R, Liu H, Manning RD Jr., Juncos LA, Liu R. Salt-sensitive splice variant of nNOS expressed in the macula densa cells. Am J Physiol Renal Physiol 2010; 298(6):F1465–F1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Y, Wei J, Stec DE, Roman RJ, Ge Y, Cheng L, Liu EY, Zhang J, Hansen PB, Fan F, Juncos LA, Wang L, Pollock J, Huang PL, Fu Y, Wang S, Liu R. Macula Densa Nitric Oxide Synthase 1beta Protects against Salt-Sensitive Hypertension. J Am Soc Nephrol 2016; 27(8):2346–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Chandrashekar K, Wang L, Lai EY, Wei J, Zhang G, Wang S, Zhang J, Juncos LA, Liu R. Inhibition of Nitric Oxide Synthase 1 Induces Salt-Sensitive Hypertension in Nitric Oxide Synthase 1alpha Knockout and Wild-Type Mice. Hypertension 2016; 67(4):792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Wei J, Jiang S, Xu L, Wang L, Cheng F, Buggs J, Koepsell H, Vallon V, Liu R. Macula Densa SGLT1-NOS1-Tubuloglomerular Feedback Pathway, a New Mechanism for Glomerular Hyperfiltration during Hyperglycemia. J Am Soc Nephrol 2019; 30(4):578–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei J, Zhang J, Wang L, Cha BJ, Jiang S, Liu R. A new low-nephron CKD model with hypertension, progressive decline of renal function, and enhanced inflammation in C57BL/6 mice. Am J Physiol Renal Physiol 2018; 314(5):F1008–F1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Chandrashekar K, Lu Y, Duan Y, Qu P, Wei J, Juncos LA, Liu R. Enhanced expression and activity of Nox2 and Nox4 in the macula densa in ANG II-induced hypertensive mice. Am J Physiol Renal Physiol 2014; 306(3):F344–F350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Qu HY, Song J, Wei J, Jiang S, Wang L, Wang L, Buggs J, Liu R. Enhanced hemodynamic responses to angiotensin II in diabetes are associated with increased expression and activity of AT1 receptors in the afferent arteriole. Physiol Genomics 2017; 49(10):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bredt DS, Ferris CD, Snyder SH. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem 1992; 267(16):10976–10981. [PubMed] [Google Scholar]
- 34.Adak S, Santolini J, Tikunova S, Wang Q, Johnson JD, Stuehr DJ. Neuronal nitric-oxide synthase mutant (Ser-1412 --> Asp) demonstrates surprising connections between heme reduction, NO complex formation, and catalysis. J Biol Chem 2001; 276(2):1244–1252. [DOI] [PubMed] [Google Scholar]
- 35.Hurt KJ, Sezen SF, Lagoda GF, Musicki B, Rameau GA, Snyder SH, Burnett AL. Cyclic AMP-dependent phosphorylation of neuronal nitric oxide synthase mediates penile erection. Proc Natl Acad Sci U S A 2012; 109(41):16624–16629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi Y, Nishio M, Naito Y, Yokokura H, Nimura Y, Hidaka H, Watanabe Y. Regulation of neuronal nitric-oxide synthase by calmodulin kinases. J Biol Chem 1999; 274(29):20597–20602. [DOI] [PubMed] [Google Scholar]
- 37.Komeima K, Hayashi Y, Naito Y, Watanabe Y. Inhibition of neuronal nitric-oxide synthase by calcium/ calmodulin-dependent protein kinase IIalpha through Ser847 phosphorylation in NG108-15 neuronal cells. J Biol Chem 2000; 275(36):28139–28143. [DOI] [PubMed] [Google Scholar]
- 38.Erdely A, Greenfeld Z, Wagner L, Baylis C. Sexual dimorphism in the aging kidney: Effects on injury and nitric oxide system. Kidney Int 2003; 63(3):1021–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji H, Pesce C, Zheng W, Kim J, Zhang Y, Menini S, Haywood JR, Sandberg K. Sex differences in renal injury and nitric oxide production in renal wrap hypertension. Am J Physiol Heart Circ Physiol 2005; 288(1):H43–H47. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan JC, Pardieck JL, Hyndman KA, Pollock JS. Renal NOS activity, expression, and localization in male and female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 2010; 298(1):R61–R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sullivan JC, Pardieck JL, Brinson K, Kang KT. Effects of estradiol on renal cyclic guanosine monophosphate and oxidative stress in spontaneously hypertensive rats. Gend Med 2009; 6(3):498–510. [DOI] [PubMed] [Google Scholar]
- 42.Sasser JM, Akinsiku O, Moningka NC, Jerzewski K, Baylis C, LeBlanc AJ, Kang LS, Sindler AL, Muller-Delp JM. Sexual dimorphism in development of kidney damage in aging Fischer-344 rats. Gend Med 2012; 9(4):219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinchee-Rodriguez K, Garg N, Venkatakrishnan P, Roman MG, Adamo ML, Masters BS, Roman LJ. Neuronal nitric oxide synthase is phosphorylated in response to insulin stimulation in skeletal muscle. Biochem Biophys Res Commun 2013; 435(3):501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka S, Hosogi S, Sawabe Y, Shimamoto C, Matsumura H, Inui T, Marunaka Y, Nakahari T. PPARalpha induced NOS1 phosphorylation via PI3K/Akt in guinea pig antral mucous cells: NO-enhancement in Ca(2+)-regulated exocytosis. Biomed Res 2016; 37(3):167–178. [DOI] [PubMed] [Google Scholar]
- 45.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron 2002; 35(4):605–623. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki M, Gonzalez-Zulueta M, Huang H, Herring WJ, Ahn S, Ginty DD, Dawson VL, Dawson TM. Dynamic regulation of neuronal NO synthase transcription by calcium influx through a CREB family transcription factor-dependent mechanism. Proc Natl Acad Sci U S A 2000; 97(15):8617–8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boissel JP, Bros M, Schrock A, Godtel-Armbrust U, Forstermann U. Cyclic AMP-mediated upregulation of the expression of neuronal NO synthase in human A673 neuroepithelioma cells results in a decrease in the level of bioactive NO production: analysis of the signaling mechanisms that are involved. Biochemistry 2004; 43(22):7197–7206. [DOI] [PubMed] [Google Scholar]
- 48.Yen DH, Chen LC, Shen YC, Chiu YC, Ho IC, Lou YJ, Chen IC, Yen JC. Protein kinase A-dependent neuronal nitric oxide synthase activation mediates the enhancement of baroreflex response by adrenomedullin in the nucleus tractus solitarii of rats. J Biomed Sci 2011; 18:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parks RJ, Howlett SE. Sex differences in mechanisms of cardiac excitation-contraction coupling. Pflugers Arch 2013; 465(5):747–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao Z, Liu L, Packwood W, Merkel M, Hurn PD, Van Winkle DM. Sex differences in the mechanism of Met5-enkephalin-induced cardioprotection: role of PI3K/Akt. Am J Physiol Heart Circ Physiol 2008; 294(1):H302–H310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng Z, Yang M, Zhang F, Yu J, Wang J, Ma L, Zhong Y, Qian L, Chen G, Yu L, Yan M. Gender-related difference of sevoflurane postconditioning in isolated rat hearts: focus on phosphatidylinositol-3-kinase/Akt signaling. J Surg Res 2011; 170(1):e3–e9. [DOI] [PubMed] [Google Scholar]
- 52.Camper-Kirby D, Welch S, Walker A, Shiraishi I, Setchell KD, Schaefer E, Kajstura J, Anversa P, Sussman MA. Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ Res 2001; 88(10):1020–1027. [DOI] [PubMed] [Google Scholar]
- 53.Parks RJ, Ray G, Bienvenu LA, Rose RA, Howlett SE. Sex differences in SR Ca(2+) release in murine ventricular myocytes are regulated by the cAMP/PKA pathway. J Mol Cell Cardiol 2014; 75:162–173. [DOI] [PubMed] [Google Scholar]
- 54.Nazarian A, Sun WL, Zhou L, Kemen LM, Jenab S, Quinones-Jenab V. Sex differences in basal and cocaine-induced alterations in PKA and CREB proteins in the nucleus accumbens. Psychopharmacology (Berl) 2009; 203(3):641–650. [DOI] [PubMed] [Google Scholar]
- 55.Thomson S, Bao D, Deng A, Vallon V. Adenosine formed by 5’-nucleotidase mediates tubuloglomerular feedback. J Clin Invest 2000; 106(2):289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A 2001; 98(17):9983–9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren Y, Arima S, Carretero OA, Ito S. Possible role of adenosine in macula densa control of glomerular hemodynamics. Kidney Int 2002; 61(1):169–176. [DOI] [PubMed] [Google Scholar]
- 58.Ren Y, Garvin JL, Liu R, Carretero OA. Role of macula densa adenosine triphosphate (ATP) in tubuloglomerular feedback. Kidney Int 2004; 66(4):1479–1485. [DOI] [PubMed] [Google Scholar]
- 59.Brown RD, Hilliard LM, Head GA, Jones ES, Widdop RE, Denton KM. Sex differences in the pressor and tubuloglomerular feedback response to angiotensin II. Hypertension 2012; 59(1):129–135. [DOI] [PubMed] [Google Scholar]
- 60.Gyurko R, Leupen S, Huang PL. Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology 2002; 143(7):2767–2774. [DOI] [PubMed] [Google Scholar]
- 61.Tojo A, Gross SS, Zhang L, Tisher CC, Schmidt HH, Wilcox CS, Madsen KM. Immunocytochemical localization of distinct isoforms of nitric oxide synthase in the juxtaglomerular apparatus of normal rat kidney. J Am Soc Nephrol 1994; 4(7):1438–1447. [DOI] [PubMed] [Google Scholar]
- 62.Bachmann S, Bosse HM, Mundel P. Topography of nitric oxide synthesis by localizing constitutive NO synthases in mammalian kidney. Am J Physiol 1995; 268(5 Pt 2):F885–F898. [DOI] [PubMed] [Google Scholar]
- 63.Chiolero A, Maillard M, Nussberger J, Brunner HR, Burnier M. Proximal sodium reabsorption: An independent determinant of blood pressure response to salt. Hypertension 2000; 36(4):631–637. [DOI] [PubMed] [Google Scholar]
- 64.Visser FW, Krikken JA, Muntinga JH, Dierckx RA, Navis GJ. Rise in extracellular fluid volume during high sodium depends on BMI in healthy men. Obesity (Silver Spring) 2009; 17(9):1684–1688. [DOI] [PubMed] [Google Scholar]
- 65.Cannon PJ, Svahn DS, Demartini FE. The influence of hypertonic saline infusions upon the fractional reabsorption of urate and other ions in normal and hypertensive man. Circulation 1970; 41(1):97–108. [DOI] [PubMed] [Google Scholar]
- 66.Pechere-Bertschi A, Maillard M, Stalder H, Bischof P, Fathi M, Brunner HR, Burnier M. Renal hemodynamic and tubular responses to salt in women using oral contraceptives. Kidney Int 2003; 64(4):1374–1380. [DOI] [PubMed] [Google Scholar]
- 67.Rasmussen MS, Simonsen JA, Sandgaard NC, Hoilund-Carlsen PF, Bie P. Mechanisms of acute natriuresis in normal humans on low sodium diet. J Physiol 2003; 546(Pt 2):591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kassab S, Novak J, Miller T, Kirchner K, Granger J. Role of endothelin in mediating the attenuated renal hemodynamics in Dahl salt-sensitive hypertension. Hypertension 1997; 30(3 Pt 2):682–686. [DOI] [PubMed] [Google Scholar]
- 69.Zheng W, Ji H, Maric C, Wu X, Sandberg K. Effect of dietary sodium on estrogen regulation of blood pressure in Dahl salt-sensitive rats. Am J Physiol Heart Circ Physiol 2008; 294(4):H1508–H1513. [DOI] [PubMed] [Google Scholar]
- 70.Hilliard LM, Nematbakhsh M, Kett MM, Teichman E, Sampson AK, Widdop RE, Evans RG, Denton KM. Gender differences in pressure-natriuresis and renal autoregulation: role of the Angiotensin type 2 receptor. Hypertension 2011; 57(2):275–282. [DOI] [PubMed] [Google Scholar]
- 71.Khraibi AA, Liang M, Berndt TJ. Role of gender on renal interstitial hydrostatic pressure and sodium excretion in rats. Am J Hypertens 2001; 14(9 Pt 1):893–896. [DOI] [PubMed] [Google Scholar]
- 72.DICKINSON CJ, LAWRENCE JR. A slowly developing pressor response to small concentrations of angiotensin. Its bearing on the pathogenesis of chronic renal hypertension. Lancet 1963; 1(7295):1354–1356. [DOI] [PubMed] [Google Scholar]
- 73.Brown JJ, Lever AF, Robertson JI, Hodge RL, Lowe RD, Vane JR. Concurrent measurement of renin and angiotensin in the circulation of the dog. Nature 1967; 215(5103):853–855. [DOI] [PubMed] [Google Scholar]
- 74.Romero JC, Reckelhoff JF. State-of-the-Art lecture. Role of angiotensin and oxidative stress in essential hypertension. Hypertension 1999; 34(4 Pt 2):943–949. [DOI] [PubMed] [Google Scholar]
- 75.Granger JP, Schnackenberg CG. Renal mechanisms of angiotensin II-induced hypertension. Semin Nephrol 2000; 20(5):417–425. [PubMed] [Google Scholar]
- 76.Kittikulsuth W, Looney SW, Pollock DM. Endothelin ET(B) receptors contribute to sex differences in blood pressure elevation in angiotensin II hypertensive rats on a high-salt diet. Clin Exp Pharmacol Physiol 2013; 40(6):362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang H, Garvin JL, Carretero OA. Angiotensin II enhances tubuloglomerular feedback via luminal AT(1) receptors on the macula densa. Kidney Int 2001; 60(5):1851–1857. [DOI] [PubMed] [Google Scholar]
- 78.Liu R, Persson AE. Angiotensin II stimulates calcium and nitric oxide release from Macula densa cells through AT1 receptors. Hypertension 2004; 43(3):649–653. [DOI] [PubMed] [Google Scholar]
- 79.Crofton JT, Share L, Brooks DP. Gonadectomy abolishes the sexual dimorphism in DOC-salt hypertension in the rat. Clin Exp Hypertens A 1989; 11(7):1249–1261. [DOI] [PubMed] [Google Scholar]
- 80.Crofton JT, Share L. Gonadal hormones modulate deoxycorticosterone-salt hypertension in male and female rats. Hypertension 1997; 29(1 Pt 2):494–499. [DOI] [PubMed] [Google Scholar]
- 81.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension 2000; 35(1 Pt 2):484–489. [DOI] [PubMed] [Google Scholar]
- 82.Rowland NE, Fregly MJ. Role of gonadal hormones in hypertension in the Dahl salt-sensitive rat. Clin Exp Hypertens A 1992; 14(3):367–375. [DOI] [PubMed] [Google Scholar]
- 83.Otsuka K, Suzuki H, Sasaki T, Ishii N, Itoh H, Saruta T. Blunted pressure natriuresis in ovariectomized Dahl-Iwai salt-sensitive rats. Hypertension 1996; 27(1):119–124. [DOI] [PubMed] [Google Scholar]
- 84.Fu Y, Lu Y, Liu EY, Zhu X, Mahajan GJ, Lu D, Roman RJ, Liu R. Testosterone enhances tubuloglomerular feedback by increasing superoxide production in the macula densa. Am J Physiol Regul Integr Comp Physiol 2013; 304(9):R726–R733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.