Abstract
AIM2 is a cytosolic innate immune receptor which recognizes double-stranded DNA (dsDNA) released during cellular perturbation and pathogenic assault. AIM2 recognition of dsDNA leads to the assembly of a large multiprotein oligomeric complex termed the inflammasome. This inflammasome assembly leads to the secretion of bioactive interleukin-1β (IL-1β) and IL-18 and induction of an inflammatory form of cell death called pyroptosis. Sensing of dsDNA by AIM2 in the cytosol is crucial to mediate protection against the invading pathogens including bacteria, virus, fungi and parasites. AIM2 also responds to dsDNA released from damaged host cells, resulting in the secretion of the effector cytokines thereby driving the progression of sterile inflammatory diseases such as skin disease, neuronal disease, chronic kidney disease, cardiovascular disease and diabetes. Additionally, the protection mediated by AIM2 in the development of colorectal cancer depends on its ability to regulate epithelial cell proliferation and gut microbiota in maintaining intestinal homeostasis independently of the effector cytokines. In this review, we will highlight the recent progress on the role of AIM2 inflammasome as a guardian of cellular integrity in modulating chronic inflammatory diseases, cancer and infection.
Keywords: AIM2, Gasdermin, Pyroptosis, Inflammasome, Cell death
Introduction
The innate immune system is made up of a set of germ-line–encoded pattern recognition receptors (PRRs) which act as a shield against pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) [1]. The PRRs are classified according to their cellular localization, ligand specificity and function. Based on their cellular localization, PRRs are classified as membrane-bound PRRs or cytoplasmic PRRs. The membrane-bound PRRs include Toll-like receptors (TLRs) and the C-type lectin receptors (CLRs), while the cytoplasmic PRRs include the NOD-like receptor (NLRs), the RIG-I–like receptors (retinoic acid-inducible gene-I–like receptors, or RLRs) and the absent in melanoma 2 (AIM2)-like receptors (ALRs) [2]. The ligand specificities of these receptors and their signaling cascades are relatively well characterized. The ability of intracellular DNA to cause immune responses has led to the discovery of several ALRs. The number of ALR genes varies widely among mammals, ranging from four in humans (AIM2, IFI16, PYHIN1 and MNDA) to 13 in mice [3]. Although ALRs have been characterized by the pyrin signaling domain (PYD) and the DNA-binding HIN domain, there is a significant lack of orthology among mammalian ALRs with the exception of AIM2 [4]. Unlike other DNA sensors that are involved in the interferon (IFN)-stimulatory DNA (ISD) pathway, AIM2 assembles a macromolecular complex termed the inflammasome in response to cytosolic double-stranded DNA (dsDNA) [5] [6] [7] [8]. AIM2 inflammasome activation drives pyroptosis and proteolytic maturation of the proinflammatory cytokines IL-18 and IL-1β [7]. dsDNA binds to the HIN domain of AIM2 regardless of its sequence. The sugar phosphate backbone of dsDNA interacts with the positively charged HIN-200 domain via electrostatic interactions that relieve PYD for self-oligomerization and its interaction with ASC for inflammasome activation [9]. A recent study has demonstrated that the PYD of AIM2 can also self-oligomerize to induce its activation [10]. AIM2-dependent effector cytokines and pyroptosis contribute to host protection against microbial infection [11]. Although the AIM2 inflammasome is protective in infectious diseases, it is often detrimental in several sterile inflammatory diseases including atherosclerosis [12] [13], chronic kidney disease (CKD) [14], skin disease [15], liver disease [16] and neuroinflammation [17]. In addition to these inflammasome-dependent functions, AIM2 also inhibits colorectal tumorigenesis, [18] [19] [20] which is independent of its inflammasome function. In this review, we will discuss the recent progress on the regulation of the AIM2 inflammasome and its biological functions in sterile inflammatory diseases, infectious diseases and cancer.
Regulation of AIM2 inflammation activation
Inappropriate inflammasome activation leads to the progression and development of several inflammatory diseases. Therefore, fine tuning of inflammasome activation in homeostasis and disease pathogenesis is crucial. In homeostatic conditions, sensing of self-DNA by AIM2 is regulated by conserved mechanisms that have been evolved to compartmentalize self-DNA to the nucleus or degrade mislocalized DNA [21]. Interestingly, DNA sequences, such as the TTAGGG repeat, which is mostly found in mammalian telomeric DNA, inhibit AIM2 inflammasome activation [22] by competing with dsDNA to maintain the inhibitory state of AIM2. Under homeostatic conditions, an intramolecular complex of the PYD and HIN domains of AIM2 maintains the inhibitory state; this is released upon binding of dsDNA to the HIN domain, thereby allowing PYD to be free for the subsequent interaction with ASC and polymerization [6] [7]. The molecules which hinder the binding of dsDNA to the HIN domain are likely to suppress AIM2 inflammasome activation. One such molecule is interferon-inducible protein (IFI) 16β, which is a newly identified human IFI16 isoform that has similar structure to mouse p202, which is a disease locus for lupus. IFI16β and p202 interact with the HIN domain to inhibit AIM2 oligomerization and subsequent polymerization of ASC, which are the essential steps of AIM2 inflammasome activation [8] [23]. Interestingly, IFN-inducible protein PYD-only protein 3 (POP3) also negatively regulates AIM2 inflammasome activation, but it does so uniquely by competing with ASC and inhibiting its recruitment to AIM2 [24] (Fig. 1). Besides this inhibition, the viral protein pUL83 released during human cytomegalovirus (HCMV) infection interacts with AIM2 to inhibit AIM2 inflammasome activation [25]. Similarly, herpes simplex virus-1 (HSV-1) tegument protein, VP22, inhibits oligomerization of AIM2, which is required for activation of the AIM2 inflammasome, thus promoting viral replication [26]. Hepatitis B e-antigen (HbeAG) was found to inhibit AIM2 inflammasome activation by interfering with the transcription and mature secretion of IL-1β in peripheral blood mononuclear cells (PBMCs) [27]. Similarly, HCMV IE86 protein was reported to inhibit the AIM2 inflammasome by obstructing pro–IL-1β mRNA synthesis and degradation of the immature protein [28].
Figure 1.

Regulation of AIM2 inflammasome activation. AIM2 is a dsDNA sensor and is composed of C-terminal HIN-200 domain and N-terminal pyrin domain. AIM2 forms an inflammasome when dsDNA from a number of microbial pathogens and host DNA from cellular damage binds to the HIN-200 domain of AIM2. The cytosolic bacterium Francisella novicida induces the production of type I IFNs via the c-GAS/STING pathway. Type I interferons activate IRF1. IRF1 then induces the upregulation of Gbp5 and Irgb10. These interferon-inducible proteins directly attack the bacterial membrane of F. novicida, releasing its DNA into the cytoplasm; its DNA binds to AIM2. The DNA virus MCMV, transfected poly(dA:dT), host DNA from cellular damage and fungal ligands from Aspergillus fumigatus activate AIM2 via a non-canonical pathway bypassing the requirement of type I IFNs. Ionizing radiation induces dsDNA breaks in host cells, leading to the redistribution of AIM2 and ASC into the DNA breakage area of nucleus. AIM2 and ASC then translocate to the cytoplasm to assemble an inflammasome complex. AIM2 activation can be inhibited by a mouse p202, a bipartite protein containing two HIN domains that interact with the HIN domain of AIM2. AIM2 can also be inhibited by human POP1 and POP3. During inflammasome activation, ASC brings caspase-1 to the inflammasome complex by CARD-CARD interactions. Activated caspase-1 then leads to the induction of pyroptosis via the proteolytic cleavage of the N-terminal domain of gasdermin-D that generates pores on the host cell membrane from which the proteolytically cleaved form of proinflammatory cytokines IL-1β and IL-18 are released.
Post-translational modification has already been demonstrated to play a crucial role in the activation of inflammasomes [29]. Such post-translational modification may regulate AIM2 inflammasome activation. Indeed, ubiquitinated TRIM11 has been reported to facilitate AIM2 inflammasome degradation upon DNA stimulation via p62-dependent selective autophagy [30]. Molecules which are also involved in interferon production and signaling are known to regulate AIM2 inflammasome activation during Francisella novicida (F. novicida) infection. However, transfection of dsDNA into the cytoplasm can directly activate AIM2 in the cells lacking IFN-associated molecules, ruling out a requirement for “priming” in AIM2 inflammasome activation [11]. Type I IFN signaling induced during F. novicida infection upregulates IFN-inducible proteins including guanylate-binding proteins (GBPs) and immunity-related GTPases (IRGs) by the transcription factor IRF1 [31]. Moreover, GBP family members GBP2 and GBP5 together with IRGB10 induce bacteriolysis, thereby releasing bacterial DNA into the host cytosol for sensing by the AIM2 [31] (Fig. 1). Although much progress has been made in understanding AIM2 inflammasome assembly, several questions remain unanswered. Carefully designed experiments are required to answer these questions.
Role of AIM2 in sterile inflammatory diseases
Skin disease
AIM2 expression is strongly upregulated in inflamed skin such as in psoriasis, atopic dermatitis, contact dermatitis and experimental wounds, and AIM2 is associated with the development of skin diseases in humans [15]. In the model of psoriasis, the mice lacking IL-1 receptor antagonist (IL-1Ra) develop severe skin inflammation, suggesting a pathogenic role for IL-1β in skin disease [32]. Importantly, the cytosolic DNA-induced IL-1β release in the keratinocytes indicates the involvement of the AIM2 inflammasome in the production of IL-1β. The abrogation of poly (dA:dT)-mediated IL-1β release in Aim2-silenced primary human keratinocytes further supports that AIM2 plays a pathogenic role in skin inflammatory diseases [33]. However, the source of cytosolic DNA in psoriatic keratinocytes remains unknown. It could be possible that the extracellular DNA from dying cells may be recognized by cytosolic AIM2. Further studies are warranted to fully understand the specific function of the AIM2 inflammasome in the skin and its mechanistic role in keratinocytes that promotes sterile inflammatory skin diseases.
Chronic kidney disease and diabetes
Chronic inflammation mediated by inflammasome activation is associated with the pathogenesis of CKD. The inflammasome effector cytokines IL-18 and IL-1β are shown to be detrimental during CKD in a mouse model of ischemia-reperfusion injury (IRI) [34] [35]. Mice deficient in IL-18 have shown better kidney function with reduced accumulation of neutrophils and macrophages and decreased expression of proinflammatory molecules when compared with IL-18–sufficient mice. Bone marrow chimeras in these mice have demonstrated that IL-18 produced from the hematopoietic compartment contributed to the renal damage [35]. The role of these cytokines in driving CKD in an NLRP3-dependent manner is well established [36], and the contribution of the AIM2 inflammasome in the production of these cytokines has only recently been suggested. This study demonstrated that Aim2−/− macrophages that have engulfed necrotic cell DNA showed attenuated IL-1β release [14]. In this study, Aim2−/− mice showed reduced renal injury, fibrosis and inflammation when compared with wild type littermates in a mouse model of unilateral urethral obstruction (UUO). This is correlated with the reduced levels of the inflammasome effector cytokines IL-β and IL-18 found in the kidney samples from Aim2−/− mice. A bone marrow chimera study suggests that AIM2 functions in the myeloid compartment in promoting the disease [14]. Overall, these findings suggest that the necrotic DNA in the kidney is recognized by the AIM2 inflammasome to drive macrophage IL-1β and IL-18 release in the pathogenesis of CKD (Fig. 2).
Figure 2.
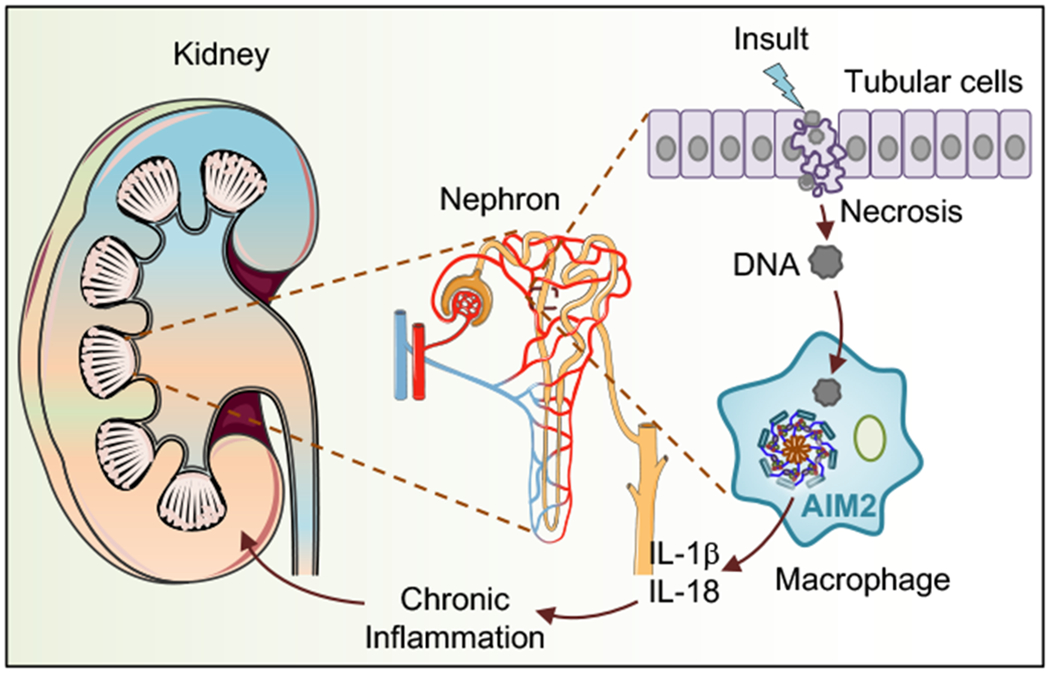
Role of AIM2 in chronic kidney disease. Acute kidney injury leads to the necrosis of tubular cells and releases DNA. The released DNA is taken up by neighboring macrophages, activating the AIM2 inflammasome. The activation of the AIM2 inflammasome leads to the formation of proinflammatory cytokines, such as IL-1β and IL-18, promoting chronic kidney inflammation.
Although one of the predisposing factors of chronic kidney failure is diabetes [37], the role of the AIM2 inflammasome in diabetes has not been well explored. A recent study has found increased expression of AIM2 in monocytes from patients with type 2 diabetes (T2D) compared with its expression in monocytes from healthy control patients. Likewise, the increased level of cell-free mitochondrial-DNA (mtDNA) in sera from patients with T2D compared with the level in sera from healthy control patients is positively correlated with the pathology. The increased content of mtDNA in peripheral blood or leukocytes is shown to be associated with T2D nephropathy or hyperglycemia, respectively [38]. The mtDNA potentially may engage AIM2 inflammasome activation for the release of effector cytokines. The mtDNA released during mitochondrial stress resulting from infection or potentially dietary lipid overload has been shown to assemble AIM2 inflammasome [39]. These findings suggest that the expression and activation of AIM2 and the increase in circulating mtDNA levels may contribute to the inflammatory process in patients with T2D [40].
Atherosclerosis
The dynamic interplay between macrophages, vascular smooth muscle cells (VSMCs) and myeloid cells dictates the development of cardiovascular diseases. Many clinical and experimental studies have reported that the innate immune responses mounted by inflammasome–dependent cytokines IL-18 and IL-1β facilitate the development of cardiovascular diseases such as atherosclerosis and abdominal aortic aneurysm (AAA) [41] [42]. The CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcome Study) has recently revealed that therapeutic IL-1β neutralization in patients with established atherosclerotic disease significantly reduces the incidence of a recurrent cardiovascular event [43], implicating that inflammasome–mediated cytokines can be atherogenic. Although most of the previous studies have suggested a role for the NLRP3 inflammasome in promoting atherosclerosis [44] [45], a later study has explored the pathogenic function of the AIM2 inflammasome in atherosclerosis [12]. Using the atherosclerosis prone ApoE−/− mouse model, the study demonstrated the improved histopathological features in high-fat diet–fed ApoE−/− mice lacking AIM2 when compared with littermate ApoE−/− mice. Similarly, the treatment of hypercholesterolemic mice with AIM2-antagonizing synthetic oligonucleotide A151 reduced the levels of atherogenic cytokines IL-1β and IL-18 within the atherosclerotic lesions [12]. Abundant Aim2 expression has correlated with heightened deposition of dsDNA at later stages of atherosclerosis. It is noteworthy that AIM2 has recently been identified in human atherosclerotic lesions in proximity to necrotic cores [13]. It is possible that dsDNA released from necrotic cells during advanced atherosclerosis activates the AIM2 inflammasome for the release of atherogenic cytokines. The cell death mediated by AIM2 may aid in the deposition of dsDNA in the necrotic cores. Oxidized low-density lipoprotein (ox-LDL), a major risk factor for atherosclerosis, has been shown to promote VSMCs death in an AIM2-dependent manner (Fig. 3). The observation that overexpression of AIM2 resulted in more gasdermin D (GSDMD) activity and vice versa in atherosclerotic plaques of ApoE−/− mice has further supported the role of AIM2 in mediating pyroptotic cell death [46]. In addition, recruitment of more macrophages in the plaque of ApoE−/− mice is associated with the ability of AIM2 to promote intracellular adhesion molecule-1 (ICAM-1) expression in VSMCs. AIM2 also promotes the migration of VSMCs by increasing TGF-β, SMAD2 and SMAD3 activity [47] (Fig. 3). Although the precise regulatory mechanism by which AIM2 induces these genes has not been reported, we can postulate that the development of atherosclerosis might be due to the indirect effect of cytokines released in response to AIM2 inflammasome activation. Furthermore, the detrimental role of AIM2 on cardiovascular health has been indicated by another study which showed increased AIM2 expression in the heart tissue of mice subjected to streptozotocin (STZ)-induced diabetes. Also, when H9C2 cardiac myoblasts are treated with higher concentration of glucose, the AIM2 expression and pyroptotic cell death increased; whereas, the silencing of AIM2 alleviated GSDMD activity and pyroptosis in these cells [48]. However, how AIM2 gets activated in the cardiac myoblasts during hyperglycemia is a question of discussion. One possibility is that STZ–induced reactive oxygen species may be releasing dsDNA into the cytosol to activate AIM2. Overall, these findings suggest a detrimental role for AIM2 in the progression of cardiovascular diseases and provide a new insight into the molecular mechanisms of vascular inflammation. Nevertheless, understanding the cellular sources of dsDNA and their mechanism to activate the AIM2 inflammasome in the context of these disease would be an interesting question to follow up.
Figure 3.
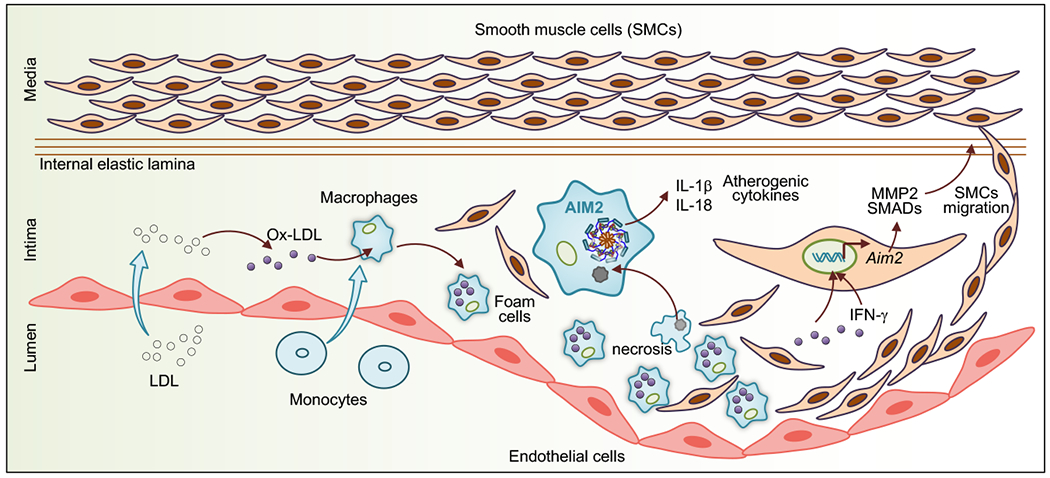
Role of AIM2 in atherosclerosis. dsDNA released from necrotic cells during advanced atherosclerosis activates the AIM2 inflammasome in macrophages and releases inflammasome effector cytokines, IL-1β and IL-18. These cytokines promote the formation of atherosclerotic plaque. Additionally, oxidized LDL promotes Aim2 gene expression in vascular smooth muscle cells. IFN-γ produced from activated macrophages and oxidized LDL activates MMP-2, SMAD-2 and SMAD-3, which are dependent on AIM2 and enhance the proliferation of smooth muscle cells, worsening atherosclerosis.
Neuronal disease
Neuroinflammation is an immune response that guards host neuronal cells from harmful stimuli mainly by regulating the production of proinflammatory cytokines [49]. The reduced brain injury in IL18−/− mice compared with that in wild type mice subjected to hypoxic ischemia suggests a detrimental role for the inflammasome in the pathophysiology of neuroinflammation [50]. Neuronal cell death contributes to many chronic neurodegenerative diseases [51]. AIM2 inflammasome activation in neurons by the cerebrospinal fluid (CSF) from patients with traumatic brain injury suggests a potential pathogenic role for AIM2 in neuronal disease [52]. In this study, the embryonic cortical neurons expressing a functional AIM2 underwent pyroptosis when exposed to the CSF from the patients with traumatic brain injury as compared with those exposed to the CSF from patients without traumatic brain injury. Further, the detrimental role of AIM2 on ischemic brain injury has been also shown in a rodent model of brain stroke [17]. The addition of dsDNA to cultured neurons induces IL-1β secretion in an AIM2-dependent manner and consequently enhances axon extension and downregulates dendritic growth, leading to the regulation of neuronal morphology. In addition, Aim2−/− mice have shown lower locomotor activity, increased anxious behavior and reduced memory of auditory fear, suggesting a pathogenic role for the AIM2 inflammasome in neuronal morphology and mouse behaviors [53]. Although the activation of the AIM2 inflammasome has been found to be pathogenic in the context of sterile neuronal diseases, AIM2 inflammasome-mediated pyroptosis is protective to limit EV-A71–induced viral replication in neuronal cells [54], suggesting a context-specific outcome for AIM2 inflammasome activation.
Colitis and cancer
Chronic inflammatory conditions predispose tissue for the development of cancer [55]. Remarkably, inflammasome signaling is implicated in virtually every aspect of tumor development, performing either tumor suppressive or pro-tumorigenic functions [20] [56]. Studies have indicated that the role of AIM2 in tumorigenesis is bidirectional and that the outcome varies depending on the type of cancer. AIM2 has tumor-suppressive function in colon cancer and hepatic cancer [20] but has tumor-promoting function in cutaneous squamous cell carcinoma (SCC) [57].
In the small and large intestine of patients with inflammatory bowel disease, AIM2 is predominantly expressed in macrophages and epithelial cells, suggesting a role for AIM2 in gut-related inflammatory diseases [58] [59]. The Aim2 gene contains a site for microsatellite instability that results in frequent gene mutation in colorectal cancer (CRC) and small bowel cancers [60] [61]. Two independent studies have suggested that AIM2 is required to inhibit the development of CRC [18] [62]. Studies have shown that in the colon of AIM2-deficient mice, there was a greater number of tumors compared with the number in wild type mice. However, these mice produced similar levels of the inflammasome effector cytokines, indicating an inflammasome-independent function of AIM2 in colorectal tumorigenesis. AIM2 inhibits overt proliferation of colonic stem cells and promotes cell death by suppressing PI3K/AKT pathways [18] [62] (Fig. 4). A similar negative regulatory role in limiting PI3K/AKT signaling and mTOR activation to suppress CRC has been described for the putative inflammasome sensor NLRC3 [63] [64]. Further, it has been shown that AIM2 reduces proliferation of colon cancer cells by inducing cell cycle arrest in the G2/M phase [65]. A recent study found that AIM2-deficient mice were highly susceptible to the dextran sulfate sodium-induced acute colitis model, which was linked to the dysbiosis of the gut microbiota. Mechanistically, the AIM2 inflammasome-mediated release of IL-18 triggers the upregulation of the IL-22–binding protein and antimicrobial peptides that modulate gut homeostasis [66] (Fig. 4).
Figure 4.
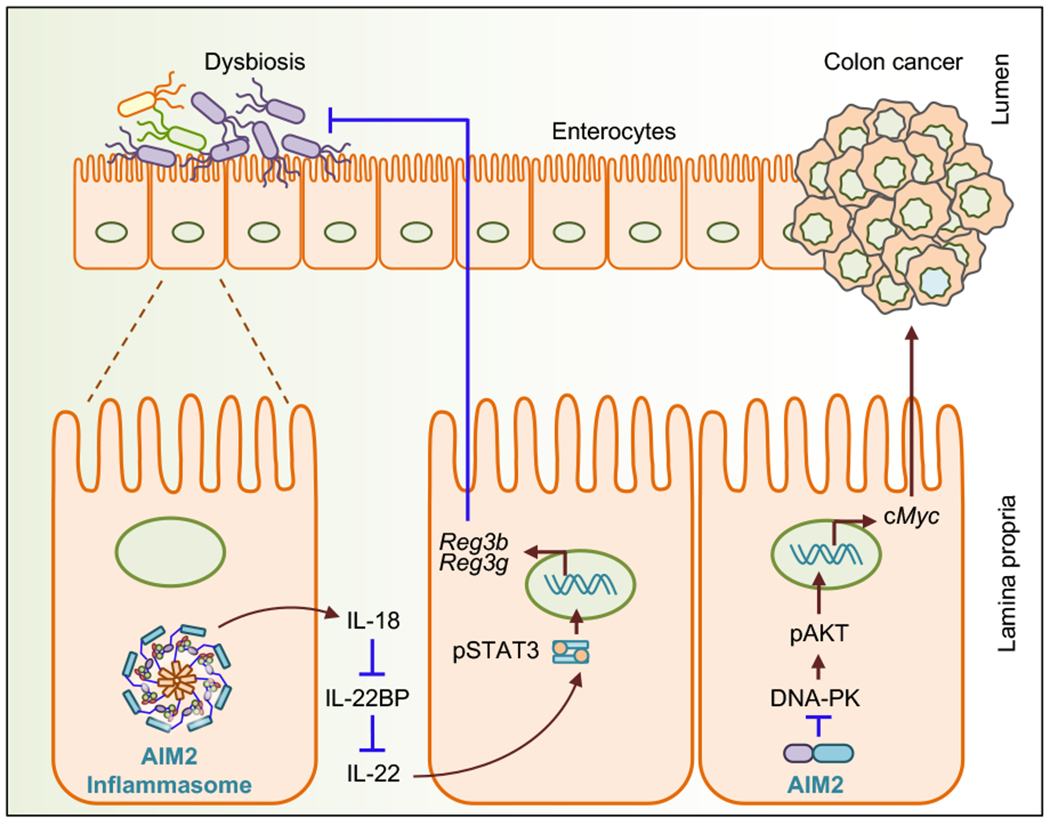
Role of AIM2 in colitis and colon cancer. AIM2-mediated IL-18 production from the intestinal epithelial cells downregulates the expression of IL-22BP and consequently enhances the availability of IL-22 in the colon. IL-22 then regulates the expression of antimicrobial peptides such as Reg3β and Reg3γ to regulate colitis. AIM2 interacts with DNA-PK in colonic epithelial cells and inhibits the phosphorylation of AKT and c-Myc, resulting in the prevention of colon tumor formation.
Host self-DNA enveloped in the nuclear or mitochondrial membrane is exposed following damage of the membrane envelope integrity via ionizing radiation and chemotherapeutic drugs [67]. Host-DNA released after intestinal injury or DNA derived from the gut microbiota may serve as a ligand to activate AIM2 in the colon [68]. Indeed, AIM2 senses DNA damage in the nucleus of intestinal epithelial cells and bone marrow cells in response to dsDNA breaks caused by ionizing radiation or chemotherapeutic agents [68]. This study revealed that, unlike canonical recognition of DNA in the cytosol, AIM2 can localize to the damaged nucleus and become activated, triggering cell death [68]. In the same study, AIM2 was also shown to contribute to the pathogenesis of chemotherapy-induced DNA damage, highlighting that targeting AIM2 by pharmaceuticals may have therapeutic benefits during radiation therapy [68]. Additionally, the susceptibility of Aim2−/− mice to the development of tumorigenesis can partly be rescued by cohousing them with wild type mice, suggesting the possible therapeutic use of healthy donor gut microbiota in patients suffering from intestinal inflammation and colon cancer [18].
The expression of AIM2 is significantly decreased in cancerous liver tissue as compared with the distal non-cancerous liver tissues, and the expression of AIM2 is negatively correlated with the tumor burden, suggesting an association of AIM2 in liver cancer. The exogenous overexpression of AIM2 has shown to suppress the tumorigenicity in immunocompromised nude mice by suppressing mTOR-S6K1 pathways [69]. AIM2 silencing and overexpression studies in hepatocellular carcinoma (HCC) cells have revealed that the anti-cancerous effect of AIM2 is mediated by forming inflammasomes and inducing pyroptosis [69]. Hepatitis B virus x protein (HBx)-induced loss of AIM2 is associated with increased cell migration and invasion, leading to hepatic metastasis [70]. In this particular study, loss of AIM2 promotes epithelial-mesenchymal transition (EMT) by targeting fibronectin-1 (FN1) in the liver, suggesting the newly identified HBx/AIM2/FN1 axis as a novel potential therapeutic target for HCC metastasis. In contrast, a recent study has shown a detrimental role for AIM2 in the development of HCC [71]. Genetic deletion of AIM2 has resulted in reduced liver damage and tumor development in the diethylnitrosamine-induced mouse model of HCC. Could these contrasting roles of AIM2 in the tumorigenesis of HCC be explained by the use of different mouse models? If this is the case, AIM2 is beneficial in the transplantable model of HCC, but detrimental in the chemically induced model of HCC.
AIM2 is a potential oncogenic driver in cutaneous SCC and endometrial cancer [57] [72]. The decreased tumor growth of SCC in the absence of AIM2 is associated with its ability to suppress cell death and promote cellular proliferation by inducing cell cycle regulatory genes [57], which might be due to the indirect effect of cytokines released by SCC cells in response to AIM2 inflammasome activation. Furthermore, reduced invasion of cutaneous SCC in the absence of AIM2 is associated with decreased production of MMP-13 and MMP-1 [57]. Studies have suggested that AIM2–dependent release of alarmins promotes tumorigenesis [73]. Increased production of IL-1α from immunosuppressive tumor-associated plasmacytoid dendritic cells (pDCs) in lung cancer depends on the AIM2 inflammasome [73]. Disrupted mitochondrial iron metabolism in the absence of the mitophagy proteins PINK1 and PARK2 induces AIM2-dependent high mobility group box 1 release in pancreatic ductal adenocarcinoma, promoting the upregulation of immune checkpoint protein programmed cell death ligand 1 [74]. These two studies highlight the significance of understudied AIM2–mediated alarmins in tumorigenesis.
Role of AIM2 in infectious diseases
Bacterial infection
The activation of the AIM2 inflammasome is protective during bacterial infections. Two fundamental requirements for AIM2 inflammasome activation during bacterial infection are bacterial access to the cytoplasm and the release of bacterial DNA via bacteriolysis. AIM2 provides immunological surveillance to the host for pathogenic bacteria such as Francisella tularensis Live Vaccine Strain (LVS), Francisella subspecies novicida (F. novicida), Listeria monocytogenes, Streptococcus pneumonia, Mycobacterium species, Porphyromonas gingivalis, Staphylococcus aureus, Brucella abortus, Chlamydia muridarum and Legionella pneumophila [75] [76] [77] [78] [79] [80] [81] [82] [83] [84] [85] [86] [87]. A range of Gram-positive, Gram-negative and Gram-variable bacteria that actively engage AIM2 inflammasome activation during infection have been listed in Table 1, and the details of AIM2 recognition of these bacteria during infection has been discussed in our previously published review articles [19] [88]. The common mechanism for activation of the AIM2 inflammasome during all these bacterial infections can be explained by the ability of AIM2 to sense microbial dsDNA. For instance, L. pneumophila secretory effector protein SdhA is known to inhibit bacterial DNA release and thus interfere with AIM2 inflammasome recognition and activation [87]. AIM2 can further co-ordinate the activation of other inflammasome sensors like NLRP3 to mount an effective immune response to infection [89]. Furthermore, the requirement of type I interferon signaling for AIM2 inflammasome activation during F. novicida infection suggests that the regulatory mechanism for activating the AIM2 inflammasome is not limited to sensing dsDNA. The mutant strain of F. novicida which lacks the gene required for vacuole disruption fails to activate the AIM2 inflammasome, whereas the mutant that rapidly disrupts the vacuole hyperactivates AIM2. This indicates that cytosolic accessibility of the bacteria is a prerequisite for F. novicida–mediated AIM2 inflammasome activation.
Table 1:
List of microbial pathogens activating the AIM2 inflammasome
| Viruses | In vitro studies | In vivo studies |
|---|---|---|
| Mouse cytomegalovirus (DNA virus) | Reduced caspase-1 cleavage and IL-1β release in Aim2−/− mouse peritoneal macrophages, BMDMs and BMDCs [18] [76]. | Reduced serum IL-18 level 36 h p.i. [18]. |
| Human cytomegalovirus (DNA virus) | Reduced caspase-1 cleavage, IL-1β release and cell death in Aim2-silenced THP-1 cells [93]. | N/A |
| Vaccinia virus (DNA virus) | Reduced caspase-1 cleavage and IL-1β release in Aim2−/− mouse peritoneal macrophages and BMDCs [7] [76]. | N/A |
| Human papillomaviruses (DNA virus) | Reduced IL-1β and IL-18 release in Aim2-silenced human keratinocytes [95]. | N/A |
| Hepatitis B virus (DNA virus) | Reduced expression of IL-1β, IL-18 and caspase-1 in Aim2-silenced human glomerular mesangial cell line [96]. | N/A |
| Epstein-Barr virus (DNA virus) | Reduced caspase-1 activation in Aim2-silenced primary human monocytes [94]. | N/A |
| Modified vaccinia virus Ankara (DNA virus) | Reduced IL-1β and IL-18 secretion in Aim2-silenced human primary keratinocytes [108]. | N/A |
| Herpes-simplex virus type 1 (DNA virus) | Reduced IL-1β and IL-18 secretion in Aim2-silenced human primary keratinocytes [108]. | N/A |
| Enterovirus 71 (DNA virus) | Reduced caspase-1, cell death and IL-1β release in Aim2- silenced SK-N-SH neuronal cells [54]. | N/A |
| Chikungunya virus (RNA virus) | Reduced IL-1β release in Aim2-silenced human primary dermal fibroblasts [99]. | N/A |
| West Nile virus (RNA virus) | Reduced IL-1β release in Aim2-silenced primary human dermal fibroblasts [99]. | N/A |
| Influenza A virus (RNA virus) | Reduced IL-1β release from Aim2-silenced PR8-stimulated human alveolar macrophages [101]. | Reduced caspase-1 and IL-1β cleavage in lung homogenates of IAV-infected Aim2−/− mice [98]. |
| Zika virus (RNA virus) | Reduced IL-1β and Aim2 expression in Zika virus-infected human dermal fibroblasts [100]. | N/A |
| Bacteria | In-vitro studies | In-vivo studies |
| Francisella tularensis LVS or F. tularensis subspecies novicida (F. novicida) | Reduced caspase-1 activation, IL-1β and IL-18 release and pyroptosis in Aim2−/− mouse BMDMs or BMDCs [6] [18] [75] [76] [77] [90] [109]. | Increased overall susceptibility, reduced serum IL-18 levels 1 d p.i.[6] [18] [76]. |
| Reduced IL-1β in Aim2-silenced THP-1 cells [110]. | ||
| Listeria monocytogenes | Reduced caspase-1 cleavage, IL-1β release and pyroptosis in Aim2-silenced mouse BMDMs [78] [79] [80] [111] [112]. | N/A |
| Streptococcus pneumoniae | Reduced caspase-1 cleavage, IL-1β and IL-18 release and pyroptosis in Aim2-silenced peritoneal macrophages [113]. | N/A |
| Mycobacterium tuberculosis | Reduced IL-1β and IL-18 release in Aim2−/− and Aim2-silenced THP-1 human monocytic cell line [82] [114]. | Reduced IL-1β in BALF and IL-18 in serum at 3 w p.i. Reduced caspase-1 cleavage and increased infiltration of inflammatory cells in lungs [83]. |
| Mycobacterium bovis | Reduced caspase-1 cleavage, IL-1β release and pyroptosis in Aim2-silenced mouse macrophage J774A.1 [84]. | N/A |
| Porphyromonas gingivalis | Reduced caspase-1 cleavage, IL-1β and pyroptosis in Aim2-silenced THP-1 cells [84]. In contrast, a recent study found that this caspase-1 activation is AIM2 independent but NLRP3 dependent [115]. | N/A |
| Legionella pneumophila | Reduced caspase-1 cleavage and IL-1β release in Aim2−/− mouse BMDMs [87]. | N/A |
| Staphylococcus aureus | N/A | Increased susceptibility upon intracranial infection. Reduced IL-1β, IL-6, CCL2 and CXCL10 in wound abscess [86]. |
| Brucella abortus | Reduced caspase-1 cleavage, IL-1β and cell death in Aim2−/− mouse BMDMs [116] [117]. | Increased bacterial burden 4 w p.i. [117]. |
| Chlamydia muridarum | Reduced IL-1β and IL-18 in Aim2−/− mouse BMDMs [118]. | N/A |
| Chlamydia trachomatis | Reduced IL-1β and IL-18 in Aim2−/− mouse BMDMs [118]. | N/A |
| Fungi | In-vitro studies | In-vivo studies |
| Aspergillus fumigatus | IL-1β and IL-18 is modestly reduced in Aim2−/− mouse BMDCs due to redundant roles with NLRP3 [103]. | Similar susceptibility of Aim2−/− mice with wild type mice because of redundant roles of NLRP3 [103]. |
| Protozoa | In-vitro studies | In-vivo studies |
| Plasmodium berghei | IL-1β and pyroptosis were modestly decreased in Aim2−/− mouse BMDMs infected with iRBCs, synthetic and natural hemozoin because of its redundant roles with NLRP3 [105]. | Decreased neutrophils recruitment in peritoneal cavity of Aim2−/− mice due to redundant roles with NLRP3 [105]. |
The importance of type I IFNs for the activation of the AIM2 inflammasome during bacterial infections has been well established [11] [81] [90] [91]. Bacterial DNA sensing by other cytosolic sensors such as cyclic-GMP-AMP synthase (cGAS) and STING drive the production of type I IFNs [11] [77]. Type I IFNs bind to IFNα/β receptors (IFNARs) and form the IFN-stimulated gene factor 3 complex [92]. This complex then induces the expression of IFN-regulatory factor (IRF) 1, which in turn regulates the expression of GBPs and IRGs [11]. Bone marrow-derived macrophages (BMDMs) lacking type I IFN signaling components such as IFNAR1, IFNAR2, IRF9 and STAT1 have impaired AIM2 inflammasome activation during F. novicida infection [11]. GBPs and IRGs function in cell-autonomous immunity and have been shown to target both vacuolar and cytosolic pathogens. Although Gbp2−/− or Gbp5−/− BMDMs have reduced AIM2 inflammasome activation during F. novicida infection, reconstitution of GBP2 and GBP5 is unable to rescue the inflammasome activation in Ifnar1−/− macrophages, suggesting that type I IFN signaling induces other IFN-inducible proteins which may cooperate with GBPs for efficient bacteriolysis [11] [90]. Indeed, GBP targeting requires the recruitment of IRGB10 to cytosolic bacteria, which ultimately results in bacteriolysis, thereby releasing bacterial DNA into the cytoplasm for activation of the AIM2 inflammasome (Fig. 1). Consistent with all these in vitro findings, mice lacking AIM2, caspase-1, IRF1, GBP chromosome 3 and IRGB10 are all hypersusceptible to F. novicida infection and have reduced production of AIM2-dependent effector cytokines in sera [11] [31]. Overall, these findings suggest that AIM2 inflammasome activation is an effective antibacterial strategy to prevent the dissemination of bacteria into the cytosol.
Viral infection
AIM2 recognizes a cytosolic dsDNA virus and forms an inflammasome complex to provide host protection by driving pyroptosis (Fig. 1). Mouse cytomegalovirus (MCMV) and vaccinia virus are considered classical dsDNA-containing viruses which activate the AIM2 inflammasome in both the mouse BMDMs and bone marrow-derived dendritic cells (BMDCs) (Table 1). Additionally, mice deficient in AIM2 have an impaired ability to secrete IL-18 and have higher viral titer during MCMV infection [76]. The HCMV, which is a counterpart of MCMV, has also been found to activate the AIM2 inflammasome in human THP-1 monocytic cells [93]. Similarly, Epstein-Barr virus engages the AIM2 inflammasome in THP-1 cells [94]. Human papilloma viruses, which are dsDNA viruses, selectively infect human keratinocytes and drive the production of inflammasome effector cytokines in an AIM2-dependent manner [95]. The siRNA-mediated knockdown of the gene encoding AIM2 leads to reduced expression of IL-1β, IL-18 and caspase-1 in human glomerular mesangial cells infected with hepatitis B virus [96]. The expression of the gene encoding AIM2 is increased in the renal biopsies of patients with hepatitis B virus glomerulonephritis when compared with the renal biopsies from the patients with chronic glomerulonephritis. But, how AIM2 senses viral DNA derived from hepatitis B virus in renal tissue remains unknown [96]. It is possible that DNA from hepatitis B virus may be recognized by AIM2 to trigger the release of inflammasome effector cytokines inducing renal damage. Alternatively, some of the DNA viruses such as hepatitis B virus and other hepadnaviruses could be transcribed into an RNA template, which might activate NLRP3 [97] [98] rather than activating AIM2.
AIM2 also contributes to the secretion of IL-β during infection with RNA viruses. AIM2-silenced dermal fibroblasts infected with the RNA viruses chikungunya virus or West Nile virus showed reduced proteolytic cleavage and release of IL-1β [99]. Zika virus, a ssRNA virus, is able to stimulate AIM2 expression and the secretion of IL-1β in primary human skin fibroblasts [100]. Similarly, a human RNA virus Enterovirus A71 (EV-A71) induces pyroptosis in neuronal cells in an AIM2-dependent manner [54] (Table 1). The host DNA released from damaged cells in lungs during influenza A virus infection induces early AIM2-dependent IL-1β production, and AIM2 deficiency ameliorated symptoms and lung pathology [101]. In contrast to these findings, another study reported that AIM2 deficiency led to an elevated inflammatory response to influenza A virus infection [102]. These contrasting results could be due to difference in animal facilities, different composition of microbiota or the dose and source of H1N1 virus used for the infection. Therefore, further studies are required to understand the mechanism by which AIM2 recognizes RNA viruses and induces inflammasome activation and the subsequent maturation and secretion of IL-18 and IL-1β.
Other infections
Compared to bacterial and viral infection, the role of the AIM2 inflammasome in response to other pathogens such as fungi and parasites is less clear (Table 1). AIM2 and NLRP3 co-operatively induce a single cytoplasmic inflammasome platform in A. fumigatus–infected mouse BMDCs. Mice lacking both AIM2 and NLRP3 are highly susceptible to aspergillosis [103], suggesting that concerted activation of both the AIM2 and NLRP3 inflammasome is crucial for the host protection. In addition, IRGB10 induced by A. fumigatus in an IRF1-dependent manner targets Aspergillus hypha for the release of fungal ligands, which subsequently causes both AIM2 and NLRP3 inflammasome activation [104]. Such concerted activation of both the AIM2 and NLRP3 inflammasomes has also been reported in macrophages stimulated with Plasmodium berghei-infected red blood cells or synthetic or natural hemozoins [105].
Conclusion
In homeostatic conditions, sensing of self-DNA by AIM2 is tightly kept in check by conserved mechanisms that tag self-DNA to the nucleus or degrade mislocalized DNA [21]. Cellular perturbation during inflammatory diseases or microbial infection results in release of host or microbial dsDNA, which is then sensed by AIM2 to assemble the inflammasome complex and thereby allow the secretion of effector cytokines and induce pyroptosis. Although protective in microbial infection, AIM2 is pathogenic in sterile inflammatory diseases. The AIM2 inflammasome is crucial for the host defense against various pathogens such as bacteria, virus, fungi and parasites. On the other hand, AIM2 inflammasome activation drives pathogenesis of numerous sterile inflammatory diseases such as cardiovascular disease, skin disease, CKD and neuroinflammatory disease. Inflammasome-dependent cytokines IL-18 and IL-1β are shown to be detrimental during CKD in a mouse model of IRI [34]. DNA from necrotic cells in the kidney is recognized by AIM2, leading to assembly of an inflammasome complex to produce IL-1β and IL-18 from macrophages during the pathogenesis of CKD [14]. Likewise, AIM2 provides an inflammatory feedback loop during the progression of cardiovascular and neuroinflammatory diseases [12] [106]. In addition to these inflammasome-dependent functions, AIM2 operates in an inflammasome-independent mode to inhibit colitis-associated CRC by regulating the proliferation of intestinal stem cells and the composition of the gut microbiota [18]. These novel functions of AIM2 provide new insights into the molecular mechanisms of sterile inflammatory disease which could be useful to develop immune-targeted therapies. Although therapeutic targeting of AIM2 may be beneficial for sterile inflammatory diseases, inhibition of AIM2 may predispose individuals to infection. Therefore, understanding the nature of dsDNA sequence and its cellular source under these pathological conditions is an emerging area of study to explore AIM2 biology. Besides AIM2, the cGAS-STING pathway activates the inflammasome in response to dsDNA. Unlike in the murine system that exclusively engages the AIM2 inflammasome during bacterial and viral infections, primary human myeloid cells also activate the cGAS-STING-NLRP3 pathway [107] [88]. Although the exact mechanism underlying this disparity in the activation and utilization of different sensors between murine and human systems is not clearly understood, distinct hypotheses have posited that AIM2 might play a subordinate role in DNA-inflammasome responses and an inflammasome-independent role in tumorigenesis [18] [107]. Given that AIM2 is activated by certain microbial DNA but not by all the microbial DNA, understanding how DNA is released and detected by AIM2 will answer many unresolved questions. The question of whether AIM2 is involved equally in different sterile inflammatory disease awaits more investigations. Besides AIM2, multiple cytosolic dsDNA sensors have been identified. Is there any crosstalk between these DNA sensors and the AIM2 inflammasome that influence the pathogenesis of disease? Additionally, some physiological inhibitors that balance the activity of AIM2 have been reported [23] [8]. Further research is warranted to understand how these physiological inhibitors operate in both the infectious and inflammatory diseases. Overall, AIM2 functions as a guardian of cellular integrity in both infectious and sterile inflammatory diseases.
Acknowledgements
Research studies from our laboratory are supported by the U.S. National Institutes of Health (AR056296, CA163507, AI124346, and AI101935 to T.-D.K.), the American Lebanese Syrian Associated Charities (to T.-D.K.). We apologize to our colleagues whose work was not cited due to space limitations.
Footnotes
Conflicts of Interest
The authors declare no commercial or financial conflict of interest to disclose.
References
- 1.Newton K and Dixit VM, Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol 2012. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeuchi O and Akira S, Pattern recognition receptors and inflammation. Cell 2010. 140: 805–820. [DOI] [PubMed] [Google Scholar]
- 3.Cridland JA, Curley EZ, Wykes MN, Schroder K, Sweet MJ, Roberts TL, Ragan MA, Kassahn KS and Stacey KJ, The mammalian PYHIN gene family: phylogeny, evolution and expression. BMC Evol Biol 2012. 12: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunette RL, Young JM, Whitley DG, Brodsky IE, Malik HS and Stetson DB, Extensive evolutionary and functional diversity among mammalian AIM2-like receptors. J Exp Med 2012. 209: 1969–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL and Superti-Furga G, An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol 2009. 10: 266–272. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, Eisenlohr L, Landel CP and Alnemri ES, The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol 2010. 11: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E and Fitzgerald KA, AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009. 458: 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, Hume DA and Stacey KJ, HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 2009. 323: 1057–1060. [DOI] [PubMed] [Google Scholar]
- 9.Jin T, Perry A, Jiang J, Smith P, Curry JA, Unterholzner L, Jiang Z, Horvath G, Rathinam VA, Johnstone RW, Hornung V, Latz E, Bowie AG, Fitzgerald KA and Xiao TS, Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 2012. 36: 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu A, Kabaleeswaran V, Fu T, Magupalli VG and Wu H, Crystal structure of the F27G AIM2 PYD mutant and similarities of its self-association to DED/DED interactions. J Mol Biol 2014. 426: 1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Man SM, Karki R, Malireddi RK, Neale G, Vogel P, Yamamoto M, Lamkanfi M and Kanneganti TD, The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol 2015. 16: 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulin N, Viola JR, Maas SL, de Jong R, Fernandes-Alnemri T, Weber C, Drechsler M, Doring Y and Soehnlein O, Double-Strand DNA Sensing Aim2 Inflammasome Regulates Atherosclerotic Plaque Vulnerability. Circulation 2018. 138: 321–323. [DOI] [PubMed] [Google Scholar]
- 13.Hakimi M, Peters A, Becker A, Bockler D and Dihlmann S, Inflammation-related induction of absent in melanoma 2 (AIM2) in vascular cells and atherosclerotic lesions suggests a role in vascular pathogenesis. J Vasc Surg 2014. 59: 794–803. [DOI] [PubMed] [Google Scholar]
- 14.Komada T, Chung H, Lau A, Platnich JM, Beck PL, Benediktsson H, Duff HJ, Jenne CN and Muruve DA, Macrophage Uptake of Necrotic Cell DNA Activates the AIM2 Inflammasome to Regulate a Proinflammatory Phenotype in CKD. J Am Soc Nephrol 2018. 29: 1165–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Koning HD, Bergboer JG, van den Bogaard EH, van Vlijmen-Willems IM, Rodijk-Olthuis D, Simon A, Zeeuwen PL and Schalkwijk J, Strong induction of AIM2 expression in human epidermis in acute and chronic inflammatory skin conditions. Exp Dermatol 2012. 21: 961–964. [DOI] [PubMed] [Google Scholar]
- 16.Lozano-Ruiz B, Bachiller V, Garcia-Martinez I, Zapater P, Gomez-Hurtado I, Moratalla A, Gimenez P, Bellot P, Frances R, Such J and Gonzalez-Navajas JM, Absent in melanoma 2 triggers a heightened inflammasome response in ascitic fluid macrophages of patients with cirrhosis. J Hepatol 2015. 62: 64–71. [DOI] [PubMed] [Google Scholar]
- 17.Denes A, Coutts G, Lenart N, Cruickshank SM, Pelegrin P, Skinner J, Rothwell N, Allan SM and Brough D, AIM2 and NLRC4 inflammasomes contribute with ASC to acute brain injury independently of NLRP3. Proc Natl Acad Sci U S A 2015. 112: 4050–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Man SM, Zhu Q, Zhu L, Liu Z, Karki R, Malik A, Sharma D, Li L, Malireddi RK, Gurung P, Neale G, Olsen SR, Carter RA, McGoldrick DJ, Wu G, Finkelstein D, Vogel P, Gilbertson RJ and Kanneganti TD, Critical Role for the DNA Sensor AIM2 in Stem Cell Proliferation and Cancer. Cell 2015. 162: 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Man SM, Karki R and Kanneganti TD, AIM2 inflammasome in infection, cancer, and autoimmunity: Role in DNA sensing, inflammation, and innate immunity. Eur J Immunol 2016. 46: 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karki R and Kanneganti TD, Diverging inflammasome signals in tumorigenesis and potential targeting. Nat Rev Cancer 2019. 19: 197–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawane K, Motani K and Nagata S, DNA Degradation and Its Defects. Cold Spring Harbor Perspectives in Biology 2014. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaminski JJ, Schattgen SA, Tzeng TC, Bode C, Klinman DM and Fitzgerald KA, Synthetic oligodeoxynucleotides containing suppressive TTAGGG motifs inhibit AIM2 inflammasome activation. J Immunol 2013. 191: 3876–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang PH, Ye ZW, Deng JJ, Siu KL, Gao WW, Chaudhary V, Cheng Y, Fung SY, Yuen KS, Ho TH, Chan CP, Zhang Y, Kok KH, Yang W, Chan CP and Jin DY, Inhibition of AIM2 inflammasome activation by a novel transcript isoform of IFI16. EMBO Rep 2018. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khare S, Ratsimandresy RA, de Almeida L, Cuda CM, Rellick SL, Misharin AV, Wallin MC, Gangopadhyay A, Forte E, Gottwein E, Perlman H, Reed JC, Greaves DR, Dorfleutner A and Stehlik C, The PYRIN domain-only protein POP3 inhibits ALR inflammasomes and regulates responses to infection with DNA viruses. Nat Immunol 2014. 15: 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Ma D, Huang H, Lu Y, Liao Y, Liu L, Liu X and Fang F, Interaction between HCMV pUL83 and human AIM2 disrupts the activation of the AIM2 inflammasome. Virol J 2017. 14: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maruzuru Y, Ichinohe T, Sato R, Miyake K, Okano T, Suzuki T, Koshiba T, Koyanagi N, Tsuda S, Watanabe M, Arii J, Kato A and Kawaguchi Y, Herpes Simplex Virus 1 VP22 Inhibits AIM2-Dependent Inflammasome Activation to Enable Efficient Viral Replication. Cell Host Microbe 2018. 23: 254–265 e257. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, He G, Chen Y, Zhang X and Wu S, Differential Activation of NLRP3, AIM2, and IFI16 Inflammasomes in Humans with Acute and Chronic Hepatitis B. Viral Immunol 2018. 31: 639–645. [DOI] [PubMed] [Google Scholar]
- 28.Botto S, Abraham J, Mizuno N, Pryke K, Gall B, Landais I, Streblow DN, Fruh KJ and DeFilippis VR, Human Cytomegalovirus Immediate Early 86-kDa Protein Blocks Transcription and Induces Degradation of the Immature Interleukin-1beta Protein during Virion-Mediated Activation of the AIM2 Inflammasome. MBio 2019. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Liu Z and Xiao TS, Post-translational regulation of inflammasomes. Cell Mol Immunol 2017. 14: 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu T, Tang Q, Liu K, Xie W, Liu X, Wang H, Wang RF and Cui J, TRIM11 Suppresses AIM2 Inflammasome by Degrading AIM2 via p62-Dependent Selective Autophagy. Cell Rep 2016. 16: 1988–2002. [DOI] [PubMed] [Google Scholar]
- 31.Man SM, Karki R, Sasai M, Place DE, Kesavardhana S, Temirov J, Frase S, Zhu Q, Malireddi RKS, Kuriakose T, Peters JL, Neale G, Brown SA, Yamamoto M and Kanneganti TD, IRGB10 Liberates Bacterial Ligands for Sensing by the AIM2 and Caspase-11-NLRP3 Inflammasomes. Cell 2016. 167: 382–396 e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shepherd J, Little MC and Nicklin MJ, Psoriasis-like cutaneous inflammation in mice lacking interleukin-1 receptor antagonist. J Invest Dermatol 2004. 122: 665–669. [DOI] [PubMed] [Google Scholar]
- 33.Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Goss C, Anz D, Simanski M, Glaser R, Harder J, Hornung V, Gallo RL, Ruzicka T, Besch R and Schauber J, Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med 2011. 3: 82ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anders HJ, Of Inflammasomes and Alarmins: IL-1beta and IL-1alpha in Kidney Disease. J Am Soc Nephrol 2016. 27: 2564–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu H, Craft ML, Wang P, Wyburn KR, Chen G, Ma J, Hambly B and Chadban SJ, IL-18 contributes to renal damage after ischemia-reperfusion. J Am Soc Nephrol 2008. 19: 2331–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hutton HL, Ooi JD, Holdsworth SR and Kitching AR, The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology (Carlton) 2016. 21: 736–744. [DOI] [PubMed] [Google Scholar]
- 37.Anders HJ, Huber TB, Isermann B and Schiffer M, CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol 2018. 14: 361–377. [DOI] [PubMed] [Google Scholar]
- 38.Al-Kafaji G, Aljadaan A, Kamal A and Bakhiet M, Peripheral blood mitochondrial DNA copy number as a novel potential biomarker for diabetic nephropathy in type 2 diabetes patients. Exp Ther Med 2018. 16: 1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dang EV, McDonald JG, Russell DW and Cyster JG, Oxysterol Restraint of Cholesterol Synthesis Prevents AIM2 Inflammasome Activation. Cell 2017. 171: 1057–1071 e1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catano Canizales YG, Uresti Rivera EE, Garcia Jacobo RE, Portales Perez DP, Yadira B, Rodriguez Rivera JG, Amaro RG, Enciso Moreno JA and Garcia Hernandez MH, Increased Levels of AIM2 and Circulating Mitochondrial DNA in Type 2 Diabetes. Iran J Immunol 2018. 15: 142–155. [PubMed] [Google Scholar]
- 41.Ramji DP and Davies TS, Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev 2015. 26: 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dihlmann S, Erhart P, Mehrabi A, Nickkholgh A, Lasitschka F, Bockler D and Hakimi M, Increased expression and activation of absent in melanoma 2 inflammasome components in lymphocytic infiltrates of abdominal aortic aneurysms. Mol Med 2014. 20: 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ and CANTOS Trial Group, Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017. 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 44.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V and Latz E, NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010. 464: 1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lukens JR, Dixit VD and Kanneganti TD, Inflammasome activation in obesity-related inflammatory diseases and autoimmunity. Discov Med 2011. 12: 65–74. [PMC free article] [PubMed] [Google Scholar]
- 46.Pan J, Han L, Guo J, Wang X, Liu D, Tian J, Zhang M and An F, AIM2 accelerates the atherosclerotic plaque progressions in ApoE−/− mice. Biochem Biophys Res Commun 2018. 498: 487–494. [DOI] [PubMed] [Google Scholar]
- 47.Pan J, Lu L, Wang X, Liu D, Tian J, Liu H, Zhang M, Xu F and An F, AIM2 regulates vascular smooth muscle cell migration in atherosclerosis. Biochem Biophys Res Commun 2018. 497: 401–409. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Pan J, Liu H, Zhang M, Liu D, Lu L, Tian J, Liu M, Jin T and An F, AIM2 gene silencing attenuates diabetic cardiomyopathy in type 2 diabetic rat model. Life Sci 2019. 221: 249–258. [DOI] [PubMed] [Google Scholar]
- 49.Shastri A, Bonifati DM and Kishore U, Innate immunity and neuroinflammation. Mediators Inflamm 2013. 2013: 342931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hedtjarn M, Leverin AL, Eriksson K, Blomgren K, Mallard C and Hagberg H, Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci 2002. 22: 5910–5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gorman AM, Neuronal cell death in neurodegenerative diseases: recurring themes around protein handling. J Cell Mol Med 2008. 12: 2263–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adamczak SE, de Rivero Vaccari JP, Dale G, Brand FJ 3rd, Nonner D, Bullock MR, Dahl GP, Dietrich WD and Keane RW, Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cereb Blood Flow Metab 2014. 34: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu PJ, Liu HY, Huang TN and Hsueh YP, AIM 2 inflammasomes regulate neuronal morphology and influence anxiety and memory in mice. Sci Rep 2016. 6: 32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yogarajah T, Ong KC, Perera D and Wong KT, AIM2 Inflammasome-Mediated Pyroptosis in Enterovirus A71-Infected Neuronal Cells Restricts Viral Replication. Sci Rep 2017. 7: 5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coussens LM and Werb Z, Inflammation and cancer. Nature 2002. 420: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karki R, Man SM and Kanneganti TD , Inflammasomes and Cancer. Cancer Immunol Res 2017. 5: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farshchian M, Nissinen L, Siljamaki E, Riihila P, Piipponen M, Kivisaari A, Kallajoki M, Grenman R, Peltonen J, Peltonen S, Quint KD, Bavinck JNB and Kahari VM, Tumor cell-specific AIM2 regulates growth and invasion of cutaneous squamous cell carcinoma. Oncotarget 2017. 8: 45825–45836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aguilera M, Darby T and Melgar S, The complex role of inflammasomes in the pathogenesis of Inflammatory Bowel Diseases - lessons learned from experimental models. Cytokine Growth Factor Rev 2014. 25: 715–730. [DOI] [PubMed] [Google Scholar]
- 59.Vanhove W, Peeters PM, Staelens D, Schraenen A, Van der Goten J, Cleynen I, De Schepper S, Van Lommel L, Reynaert NL, Schuit F, Van Assche G, Ferrante M, De Hertogh G, Wouters EF, Rutgeerts P, Vermeire S, Nys K and Arijs I, Strong Upregulation of AIM2 and IFI16 Inflammasomes in the Mucosa of Patients with Active Inflammatory Bowel Disease. Inflamm Bowel Dis 2015. 21: 2673–2682. [DOI] [PubMed] [Google Scholar]
- 60.Mori Y, Yin J, Rashid A, Leggett BA, Young J, Simms L, Kuehl PM, Langenberg P, Meltzer SJ and Stine OC, Instabilotyping: comprehensive identification of frameshift mutations caused by coding region microsatellite instability. Cancer Res 2001. 61: 6046–6049. [PubMed] [Google Scholar]
- 61.Schulmann K, Brasch FE, Kunstmann E, Engel C, Pagenstecher C, Vogelsang H, Kruger S, Vogel T, Knaebel HP, Ruschoff J, Hahn SA, Knebel-Doeberitz MV, Moeslein G, Meltzer SJ, Schackert HK, Tympner C, Mangold E, Schmiegel W and German HC, HNPCC-associated small bowel cancer: clinical and molecular characteristics. Gastroenterology 2005. 128: 590–599. [DOI] [PubMed] [Google Scholar]
- 62.Wilson JE, Petrucelli AS, Chen L, Koblansky AA, Truax AD, Oyama Y, Rogers AB, Brickey WJ, Wang Y, Schneider M, Muhlbauer M, Chou WC, Barker BR, Jobin C, Allbritton NL, Ramsden DA, Davis BK and Ting JP, Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med 2015. 21: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karki R, Man SM, Malireddi RKS, Kesavardhana S, Zhu Q, Burton AR, Sharma BR, Qi X, Pelletier S, Vogel P, Rosenstiel P and Kanneganti TD, NLRC3 is an inhibitory sensor of PI3K-mTOR pathways in cancer. Nature 2016. 540: 583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karki R, Malireddi RKS, Zhu Q and Kanneganti TD, NLRC3 regulates cellular proliferation and apoptosis to attenuate the development of colorectal cancer. Cell Cycle 2017. 16: 1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patsos G, Germann A, Gebert J and Dihlmann S, Restoration of absent in melanoma 2 (AIM2) induces G2/M cell cycle arrest and promotes invasion of colorectal cancer cells. Int J Cancer 2010. 126: 1838–1849. [DOI] [PubMed] [Google Scholar]
- 66.Ratsimandresy RA, Indramohan M, Dorfleutner A and Stehlik C, The AIM2 inflammasome is a central regulator of intestinal homeostasis through the IL-18/IL-22/STAT3 pathway. Cell Mol Immunol 2017. 14: 127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Di Micco A, Frera G, Lugrin J, Jamilloux Y, Hsu ET, Tardivel A, De Gassart A, Zaffalon L, Bujisic B, Siegert S, Quadroni M, Broz P, Henry T, Hrycyna CA and Martinon F, AIM2 inflammasome is activated by pharmacological disruption of nuclear envelope integrity. Proc Natl Acad Sci U S A 2016. 113: E4671–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu B, Jin C, Li HB, Tong J, Ouyang X, Cetinbas NM, Zhu S, Strowig T, Lam FC, Zhao C, Henao-Mejia J, Yilmaz O, Fitzgerald KA, Eisenbarth SC, Elinav E and Flavell RA, The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science 2016. 354: 765–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma X, Guo P, Qiu Y, Mu K, Zhu L, Zhao W, Li T and Han L, Loss of AIM2 expression promotes hepatocarcinoma progression through activation of mTOR-S6K1 pathway. Oncotarget 2016. 7: 36185–36197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen SL, Liu LL, Lu SX, Luo RZ, Wang CH, Wang H, Cai SH, Yang X, Xie D, Zhang CZ and Yun JP, HBx-mediated decrease of AIM2 contributes to hepatocellular carcinoma metastasis. Mol Oncol 2017. 11: 1225–1240. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Martinez-Cardona C, Lozano-Ruiz B, Bachiller V, Peiro G, Algaba-Chueca F, Gomez-Hurtado I, Such J, Zapater P, Frances R and Gonzalez-Navajas JM, AIM2 deficiency reduces the development of hepatocellular carcinoma in mice. Int J Cancer 2018. 143: 2997–3007. [DOI] [PubMed] [Google Scholar]
- 72.Woerner SM, Kloor M, Schwitalle Y, Youmans H, Doeberitz M, Gebert J and Dihlmann S, The putative tumor suppressor AIM2 is frequently affected by different genetic alterations in microsatellite unstable colon cancers. Genes Chromosomes Cancer 2007. 46: 1080–1089. [DOI] [PubMed] [Google Scholar]
- 73.Sorrentino R, Terlizzi M, Di Crescenzo VG, Popolo A, Pecoraro M, Perillo G, Galderisi A and Pinto A, Human lung cancer-derived immunosuppressive plasmacytoid dendritic cells release IL-1alpha in an AIM2 inflammasome-dependent manner. Am J Pathol 2015. 185: 3115–3124. [DOI] [PubMed] [Google Scholar]
- 74.Li C, Zhang Y, Cheng X, Yuan H, Zhu S, Liu J, Wen Q, Xie Y, Liu J, Kroemer G, Klionsky DJ, Lotze MT, Zeh HJ, Kang R and Tang D, PINK1 and PARK2 Suppress Pancreatic Tumorigenesis through Control of Mitochondrial Iron-Mediated Immunometabolism. Dev Cell 2018. 46: 441–455 e448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Belhocine K and Monack DM, Francisella infection triggers activation of the AIM2 inflammasome in murine dendritic cells. Cell Microbiol 2012. 14: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E and Fitzgerald KA, The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 2010. 11: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O’Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, Dixit VM and Monack DM, Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A 2010. 107: 9771–9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim S, Bauernfeind F, Ablasser A, Hartmann G, Fitzgerald KA, Latz E and Hornung V, Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur J Immunol 2010. 40: 1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sauer JD, Witte CE, Zemansky J, Hanson B, Lauer P and Portnoy DA, Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe 2010. 7: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu J, Fernandes-Alnemri T and Alnemri ES, Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J Clin Immunol 2010. 30: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fang R, Hara H, Sakai S, Hernandez-Cuellar E, Mitsuyama M, Kawamura I and Tsuchiya K, Type I interferon signaling regulates activation of the absent in melanoma 2 inflammasome during Streptococcus pneumoniae infection. Infect Immun 2014. 82: 2310–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saiga H, Nieuwenhuizen N, Gengenbacher M, Koehler AB, Schuerer S, Moura-Alves P, Wagner I, Mollenkopf HJ, Dorhoi A and Kaufmann SH, The Recombinant BCG DeltaureC::hly Vaccine Targets the AIM2 Inflammasome to Induce Autophagy and Inflammation. J Infect Dis 2015. 211: 1831–1841. [DOI] [PubMed] [Google Scholar]
- 83.Saiga H, Kitada S, Shimada Y, Kamiyama N, Okuyama M, Makino M, Yamamoto M and Takeda K, Critical role of AIM2 in Mycobacterium tuberculosis infection. Int Immunol 2012. 24: 637–644. [DOI] [PubMed] [Google Scholar]
- 84.Yang Y, Zhou X, Kouadir M, Shi F, Ding T, Liu C, Liu J, Wang M, Yang L, Yin X and Zhao D, the AIM2 inflammasome is involved in macrophage activation during infection with virulent Mycobacterium bovis strain. J Infect Dis 2013. 208: 1849–1858. [DOI] [PubMed] [Google Scholar]
- 85.Park E, Na HS, Song YR, Shin SY, Kim YM and Chung J, Activation of NLRP3 and AIM2 inflammasomes by Porphyromonas gingivalis infection. Infect Immun 2014. 82: 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hanamsagar R, Aldrich A and Kielian T, Critical role for the AIM2 inflammasome during acute CNS bacterial infection. J Neurochem 2014. 129: 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ge J, Gong YN, Xu Y and Shao F, Preventing bacterial DNA release and absent in melanoma 2 inflammasome activation by a Legionella effector functioning in membrane trafficking. Proc Natl Acad Sci U S A 2012. 109: 6193–6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Man SM, Karki R and Kanneganti TD, DNA-sensing inflammasomes: regulation of bacterial host defense and the gut microbiota. Pathog Dis 2016. 74: ftw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cunha LD, Silva ALN, Ribeiro JM, Mascarenhas DPA, Quirino GFS, Santos LL, Flavell RA and Zamboni DS, AIM2 Engages Active but Unprocessed Caspase-1 to Induce Noncanonical Activation of the NLRP3 Inflammasome. Cell Rep 2017. 20: 794–805. [DOI] [PubMed] [Google Scholar]
- 90.Meunier E, Wallet P, Dreier RF, Costanzo S, Anton L, Ruhl S, Dussurgey S, Dick MS, Kistner A, Rigard M, Degrandi D, Pfeffer K, Yamamoto M, Henry T and Broz P, Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat Immunol 2015. 16: 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Henry T, Brotcke A, Weiss DS, Thompson LJ and Monack DM, Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med 2007. 204: 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ivashkiv LB and Donlin LT, Regulation of type I interferon responses. Nat Rev Immunol 2014. 14: 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang Y, Liu L, Ma D, Liao Y, Lu Y, Huang H, Qin W, Liu X and Fang F, Human cytomegalovirus triggers the assembly of AIM2 inflammasome in THP-1-derived macrophages. J Med Virol 2017. 89: 2188–2195. [DOI] [PubMed] [Google Scholar]
- 94.Torii Y, Kawada JI, Murata T, Yoshiyama H, Kimura H and Ito Y, Epstein-Barr virus infection-induced inflammasome activation in human monocytes. PLoS One 2017. 12: e0175053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reinholz M, Kawakami Y, Salzer S, Kreuter A, Dombrowski Y, Koglin S, Kresse S, Ruzicka T and Schauber J, HPV16 activates the AIM2 inflammasome in keratinocytes. Arch Dermatol Res 2013. 305: 723–732. [DOI] [PubMed] [Google Scholar]
- 96.Zhen J, Zhang L, Pan J, Ma S, Yu X, Li X, Chen S and Du W, AIM2 mediates inflammation-associated renal damage in hepatitis B virus-associated glomerulonephritis by regulating caspase-1, IL-1beta, and IL-18. Mediators Inflamm 2014. 2014: 190860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S and Nunez G, Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 2006. 440: 233–236. [DOI] [PubMed] [Google Scholar]
- 98.Sander LE, Davis MJ, Boekschoten MV, Amsen D, Dascher CC, Ryffel B, Swanson JA, Muller M and Blander JM, Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature 2011. 474: 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ekchariyawat P, Hamel R, Bernard E, Wichit S, Surasombatpattana P, Talignani L, Thomas F, Choumet V, Yssel H, Despres P, Briant L and Misse D, Inflammasome signaling pathways exert antiviral effect against Chikungunya virus in human dermal fibroblasts. Infect Genet Evol 2015. 32: 401–408. [DOI] [PubMed] [Google Scholar]
- 100.Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Despres P, Amara A, Yssel H and Misse D, Biology of Zika Virus Infection in Human Skin Cells. J Virol 2015. 89: 8880–8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang H, Luo J, Alcorn JF, Chen K, Fan S, Pilewski J, Liu A, Chen W, Kolls JK and Wang J, AIM2 Inflammasome Is Critical for Influenza-Induced Lung Injury and Mortality. J Immunol 2017. 198: 4383–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schattgen SA, Gao G, Kurt-Jones EA and Fitzgerald KA, Cutting Edge: DNA in the Lung Microenvironment during Influenza Virus Infection Tempers Inflammation by Engaging the DNA Sensor AIM2. J Immunol 2016. 196: 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karki R, Man SM, Malireddi RKS, Gurung P, Vogel P, Lamkanfi M and Kanneganti TD, Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe 2015. 17: 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Briard B, Karki R, Malireddi RKS, Bhattacharya A, Place DE, Mavuluri J, Peters JL, Vogel P, Yamamoto M and Kanneganti TD, Fungal ligands released by innate immune effectors promote inflammasome activation during Aspergillus fumigatus infection. Nat Microbiol 2019. 4: 316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kalantari P, DeOliveira RB, Chan J, Corbett Y, Rathinam V, Stutz A, Latz E, Gazzinelli RT, Golenbock DT and Fitzgerald KA, Dual engagement of the NLRP3 and AIM2 inflammasomes by plasmodium-derived hemozoin and DNA during malaria. Cell Rep 2014. 6: 196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singhal G, Jaehne EJ, Corrigan F, Toben C and Baune BT, Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front Neurosci 2014. 8: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gaidt MM, Ebert TS, Chauhan D, Ramshorn K, Pinci F, Zuber S, O’Duill F, Schmid-Burgk JL, Hoss F, Buhmann R, Wittmann G, Latz E, Subklewe M and Hornung V, The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3. Cell 2017. 171: 1110–1124 e1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Strittmatter GE, Sand J, Sauter M, Seyffert M, Steigerwald R, Fraefel C, Smola S, French LE and Beer HD, IFN-gamma Primes Keratinocytes for HSV-1-Induced Inflammasome Activation. J Invest Dermatol 2016. 136: 610–620. [DOI] [PubMed] [Google Scholar]
- 109.Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, Weiss DS and Henry T, AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ 2012. 19: 1709–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Atianand MK, Duffy EB, Shah A, Kar S, Malik M and Harton JA, Francisella tularensis reveals a disparity between human and mouse NLRP3 inflammasome activation. J Biol Chem 2011. 286: 39033–39042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Warren SE, Armstrong A, Hamilton MK, Mao DP, Leaf IA, Miao EA and Aderem A, Cutting edge: Cytosolic bacterial DNA activates the inflammasome via Aim2. J Immunol 2010. 185: 818–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsuchiya K, Hara H, Kawamura I, Nomura T, Yamamoto T, Daim S, Dewamitta SR, Shen Y, Fang R and Mitsuyama M, Involvement of absent in melanoma 2 in inflammasome activation in macrophages infected with Listeria monocytogenes. J Immunol 2010. 185: 1186–1195. [DOI] [PubMed] [Google Scholar]
- 113.Fang R, Tsuchiya K, Kawamura I, Shen Y, Hara H, Sakai S, Yamamoto T, Fernandes-Alnemri T, Yang R, Hernandez-Cuellar E, Dewamitta SR, Xu Y, Qu H, Alnemri ES and Mitsuyama M, Critical roles of ASC inflammasomes in caspase-1 activation and host innate resistance to Streptococcus pneumoniae infection. J Immunol 2011. 187: 4890–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wassermann R, Gulen MF, Sala C, Perin SG, Lou Y, Rybniker J, Schmid-Burgk JL, Schmidt T, Hornung V, Cole ST and Ablasser A, Mycobacterium tuberculosis Differentially Activates cGAS- and Inflammasome-Dependent Intracellular Immune Responses through ESX-1. Cell Host Microbe 2015. 17: 799–810. [DOI] [PubMed] [Google Scholar]
- 115.Okano T, Ashida H, Suzuki S, Shoji M, Nakayama K and Suzuki T, Porphyromonas gingivalis triggers NLRP3-mediated inflammasome activation in macrophages in a bacterial gingipains-independent manner. Eur J Immunol 2018. 48: 1965–1974. [DOI] [PubMed] [Google Scholar]
- 116.Gomes MT, Campos PC, Oliveira FS, Corsetti PP, Bortoluci KR, Cunha LD, Zamboni DS and Oliveira SC, Critical role of ASC inflammasomes and bacterial type IV secretion system in caspase-1 activation and host innate resistance to Brucella abortus infection. J Immunol 2013. 190: 3629–3638. [DOI] [PubMed] [Google Scholar]
- 117.Costa Franco MM, Marim F, Guimaraes ES, Assis NRG, Cerqueira DM, Alves-Silva J, Harms J, Splitter G, Smith J, Kanneganti TD, de Queiroz N, Gutman D, Barber GN and Oliveira SC, Brucella abortus Triggers a cGAS-Independent STING Pathway To Induce Host Protection That Involves Guanylate-Binding Proteins and Inflammasome Activation. J Immunol 2018. 200: 607–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Finethy R, Jorgensen I, Haldar AK, de Zoete MR, Strowig T, Flavell RA, Yamamoto M, Nagarajan UM, Miao EA and Coers J, Guanylate binding proteins enable rapid activation of canonical and noncanonical inflammasomes in Chlamydia-infected macrophages. Infect Immun 2015. 83: 4740–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]


