Short abstract
This investigation was performed to verify whether lncRNA CRNDE sponging miR-181a-5p was involved with sepsis-relevant inflammatory dysfunctions. Aggregately 136 sepsis patients and 151 healthy people were recruited, and their fasting peripheral blood was gathered to detect expressions of CRNDE and miR-181a-5p. In addition, THP-1 cells were transfected with si-CRNDE, miR-181a-5p mimic, pcDNA3.1-TLR4 and si-TLR4, and then sepsis-specific inflammatory cytokines within the cells were quantified. The sponging relationships between CRNDE and miR-181a-5p, as well as between miR-181a-5p and TLR4, were ascertained by means of luciferase reporter gene assay. The experimental results revealed that over-expressed CRNDE and under-expressed miR-181a-5p were associated with shortened lifespan of sepsis patients. Mechanically, si-CRNDE-1 and miR-181a-5p mimic were able to reverse the promoting effects of LPS on production of NF-kB, TNF-α, IL-1β and IL-6 by THP-1 cells. Moreover, the expressional change of miR-181a-5p in THP-1 cells was in part owing to its being sponged by CRNDE. Lastly, TLR4, subjected to targeted modification of miR-181a-5p, was capable of disturbing the contribution of CRNDE and miR-181a-5p to THP-1 cells’ release of NF-kB, TNF-α, IL-1β and IL-6. Collectively, the CRNDE/miR-181a-5p/TLR4 axis seemed to have potential in modifying sepsis-related inflammatory pathogenesis, which offered a direction for sepsis diagnosis and treatment.
Keywords: Sepsis, LncRNA CRNDE, miR-181a-5p, TLR4, inflammation
Introduction
Sepsis, a systemic inflammatory response syndrome, usually occurs along with severe trauma, burns, shock and severe surgery, and it is clinically featured by massive release of inflammatory mediators into peripheral blood.1,2 The mortality caused by severe sepsis is expected to achieve 28–50%,3,4 and more unfortunately, patients with a dysfunctional cardiovascular system are more prone to death when they were complicated by sepsis.5–7 Hence, it is of utmost importance to clarify root causes that explain sepsis onset, and also biomarkers for the progression of sepsis. Acknowledging the fundamental role of pro-inflammatory cytokines in boosting sepsis development,8 exploring biomarkers that powerfully modify inflammatory responses might be beneficial to uncovering sepsis etiology.
To date, sepsis-focused research has been mostly related to protein-coding genes, rather than non-coding RNAs, such as long non-coding RNAs (lncRNAs), that fail to encode proteins.9,10 Nonetheless, the involvement of lncRNAs in immune reactions has been increasingly revealed,11–13 and the immune-modulatory effects of lncRNAs have also been confirmed in various immune cells, such as monocytes and dendritic cells.14 Furthermore, profiling of lncRNAs showed discrepant patterns during differentiation of Th1, Th2 and Th17 cells,15 which further highlighted the high-level involvement of lncRNAs in inflammation. Notable, lncRNA colorectal neoplasia differentially expressed (CRNDE), according to results derived from Kyoto Encyclopedia of Genes and Genomes (KEGG) database, was conjectured as an immunity-relevant gene, and a majority of its functions were centered on regulation of immune responses, such as TLR signaling, TNF-α signaling and the cytokine–cytokine receptor interaction pathway.16 The in vitro experiments also supported the role CRNDE played in activation of MyD88-independent TLR signaling and subsequent NF-κB signaling,16 whose normal functioning appeared fundamental for restraint of sepsis development.17–20 As a consequence, it is plausible that CRNDE could play a part in the acceleration of sepsis aggravation. In addition, miR-181a-5p was negatively modified by CRNDE, and the Wnt/β-catenin signaling regulated by it served as a powerful mediator underlying sepsis-correlated inflammation.21,22 In addition, the activity of NF-κB could be undermined after stimulation of over-expressed miR-181a,23 even though whether the inflammation-boosting capacity of NF-κB would be weakened by miR-181a remained uncertain. Furthermore, miR-181a-5p was additionally supposed as a modulator of immune responses in dendritic cells via negative modification of TNF-α.24 In summary, CRNDE and miR-181a-5p were tightly linked with inflammatory disorders inherent in sepsis etiology; nonetheless, whether CRNDE combined with miR-181a-5p indeed promoted or delayed sepsis development was still unknown.
In order to fill this knowledge gap, this investigation was designed to explore the inner linkage of CRNDE with miR-181a-5p in sepsis, which might guide fruitful diagnosis and treatment for sepsis in future.
Materials and methods
Inclusion of clinical samples
In total 136 sepsis patients aged between 18 and 76 yr, were recruited from the respiratory department of Chenzhou No.1 People’s Hospital, and their confirmed diagnoses were in accordance with the criteria formulated by American College of Chest Physicians and Critical Care Medicine in 2012.25 Sepsis cases were excluded when they were found with symptoms related with immune system disorders, hematological diseases, chronic organ dysfunctions, seriously impaired hepato-renal functions and cancers. In the meantime, 151 healthy people who took physical examinations in the hospital were incorporated into the control group. The fasting peripheral blood samples collected from patients with sepsis and healthy controls in the morning were centrifuged at 594 g for 10 min, after which the supernatants were stored at –80°C. All procedures required by this investigation have been approved by Chenzhou No.1 People’s Hospital and the ethics committee of Chenzhou No.1 People’s Hospital. All the participants and their relatives have signed informed consents after being fully notified.
Cell treatment and cell transfection
The THP-1 cell line, a human myeloid leukemia mononuclear cell line, was purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China). The cells were incubated in the atmosphere of 5% CO2 and 37°C, and their culture medium was managed as DMEM complete medium that contained 10% FBS.26
The THP-1 cells at the logarithmic phase were seeded at the concentration of 5.0 × 106/ml, and cell models of sepsis were built by stimulating the THP-1 cell line with 100 ng/ml LPS for 4 h.27 Also, the THP-1 cells that grew to 70%-80% confluence were transfected with siRNAs against CRNDE, si-NC, miR-181a-5p mimic, miR-NC and si-TLR4 (Genepharma, China) (Supplemental Table 1). After 48 h, the cells were used for the following experiments.
RT-PCR
The total RNAs were extracted from blood or cells according to instructions of the Trizol kit (Invitrogen, USA), and they were then dissolved within diethylpyrocarbonate (DEPC)-treated distilled water. The concentration and quality of RNAs were measured using a spectrophotometer (model: Nano Drop 2000, Applied Biosystems, USA), and their quality was satisfied when the A260/A280 ratio ranged between 1.9 and 2.1. Afterward, the collected RNAs were reversely transcribed into cDNAs, aided by the PrimeScript™ RT reagent kit (Takara, Japan). Then with primers synthesized by Sangon (Shanghai, China) (Table 1), a PCR reaction was implemented on a PCR instrument (model: Applied Biosystems 7600, ABI, USA), as per the instructions of SYBR Premix ExTaq II Kit (Takara, Japan). The reaction steps were pre-denaturation at 95°C for 30 s, 40 cycles of denaturation at 95°C for 5 s, annealing at 60°C for 5 s and extension at 72°C for 30 s. Finally, expression of target genes was quantified on the basis of 2−ΔΔCt method, with U6 or GAPDH as the internal reference.
Table 1.
Primers used in RT-PCR analysis.
| Gene | Direction | Primer sequences (5'-3') |
|---|---|---|
| CRNDE | Forward | CGCGCCCGCGCGGCGGAGGA |
| Reverse | TATGAATTGCAGACTTTGCAGA | |
| miR-181a-5p | Forward | GCCGAACATTCAACGCTGTCG |
| Reverse | GTGCAGGGTCCGAGGT | |
| TLR4 | Forward | TGAGCAGTCGTGCTGGTATC |
| Reverse | CAGGGCTTTTCTGAGTCGTC | |
| NF-κB | Forward | CAGAGGGACAACAGCAATGA |
| Reverse | CCGTGTAAACCAAAGCCTA | |
| TNF-α | Forward | CCCTCCCCATGGAGCCAGCT |
| Reverse | GCACAGAGGCCAGGGGGCTA | |
| IL-1β | Forward | TCAGGCAGGCAGTATCACTC |
| Reverse | GCAAGGTCCACGGGAAAGAC | |
| IL-6 | Forward | TCGAGCCCACCGGGAACGAA |
| Reverse | GCAACTGGACCGAAGGCGCT | |
| GAPDH | Forward | GTCAACGGATTTGGTCTGTATT |
| Reverse | AGTCTTCTGGGTGGCAGTGAT | |
| U6 | Forward | GTGCTCGCTTCGGCAGCACAT |
| Reverse | ATGGAACGCTTCACGAATTTG |
Western blotting
The cells or blood were added with pre-cooled lysis buffer, and the supernatants were taken after 4°C and 9590 g centrifugation for 5 min. The concentration of total protein was detected using the Bradford method, and 40 μg of the protein was used for 10% SDS-PAGE. The samples were then transferred onto a polyvinylidene fluoride (PVDF) membrane via the wet method, and were blocked at 37°C within TBST that contained 5% skim milk powder for 1 h. Subsequently, the samples were incubated within primary Abs (rabbit anti-human, Abcam, USA) against NF-kB (1:1000, Cat. No.: ab32536), TNF-α (1:500, Cat. No.: ab6671), IL-1β (1:1000, Cat. No.: ab2105), IL-6 (1:500, Cat. No.: ab6672), TLR4 (1:500, Cat. No.: ab13556) and GAPDH (1:10000, Cat. No.: ab181602) at 4°C overnight. Then goat anti-rabbit secondary Abs marked with HRP (1:10000, Cat. No.: ab97051, Abcam, USA) were added to the samples for additional 1 h incubation at room temperature. The products were washed five times with TBST, each time for 5 min. After development and exposure in the dark, the Gene Tools software was applied to measure the expression level of target proteins, with GAPDH as the reference.
Luciferase reporter gene assay
The CRNDE and TLR4 sequences that contained specific target sites of miR-181a-5p were connected to psi-Check2 (Promega, USA), and were then amplified through PCR. The reclaimed products were called psi-Check2-CRNDE Wt and psi-Check2-TLR4 Wt. Meanwhile, psi-Check2-CRNDE Mut and psi-Check2-TLR4 Mut were constructed following identical procedures, except that the CRNDE and TLR4 sequences were mutated in their miR-181a-5p targeting sites. In line with the guidance of Lipofectamine TM2000 kit (Invitrogen, USA), miR-181a-5p mimic and miR-NC were, respectively, co-transfected into cells with psi-Check2-CRNDE Wt, psi-Check2-CRNDE Mut, psi-Check2-TLR4 Wt and psi-Check2-TLR4 Mut. Finally, the dual-luciferase reporter assay kit (Promega, USA) was used to determine the luciferase activity of cells.
Statistical analyses
The data were statistically analyzed using SPSS 13.0 software. The count data expressed as percentage (%) were compared through χ2 test, and the measurement data presented in the form of mean ± standard deviation (SD) were contrasted using student’s t-test. Survival curves were plotted utilizing the Kaplan–Meier method, with log-rank test adopted for between-group comparisons. Statistical significance was deemed at P values of < 0.05.
Results
Comparison of baseline clinical characteristics between sepsis patients and healthy controls
The patients with sepsis and the healthy controls were matched with respect to mean age (P = 0.130) and sex ratio (P = 0.204) (Table 2). However, significantly heightened levels of white blood cells (WBC), C-reactive protein (CRP) and procalcitonin (PCT) were detected among sepsis patients, when compared with healthy controls (P < 0.001). The average MAP, SOFA and APACHE II scores of sepsis patients were 0.34 ± 0.08, 7.64 ± 0.67 and 17.15 ± 1.22, respectively.
Table 2.
Comparison of baseline clinical features between sepsis patients and healthy controls.
| Clinical features | Sepsis patients | Healthy controls | t/χ2 Value | P Value |
|---|---|---|---|---|
| Gender | ||||
| Female | 66 | 62 | 1.62 | 0.204 |
| Male | 70 | 89 | ||
| Age (yr) | 56.04 ± 2.03 | 55.61 ± 2.68 | 1.52 | 0.130 |
| MAP score | 0.34 ± 0.08 | – | – | – |
| SOFA score | 7.64 ± 0.67 | – | – | – |
| APACHE II score | 17.15 ± 1.22 | – | – | – |
| WBC (×109/l) | 14.37 ± 5.61 | 7.42 ± 1.31 | 14.79 | < 0.001 |
| CRP (mg/l) | 125 ± 39 | 4.69 ± 1.53 | 37.88 | < 0.001 |
| PCT (μg/l) | 9.51 ± 4.52 | 0.22 ± 0.10 | 25.25 | < 0.001 |
MAP: mean arterial pressure; SOFA: sequential organ failure assessment; APACHE II: acute physiology and chronic health evaluation II; WBC: white blood cell count; CRP: C-reactive protein; PCT: procalcitonin.
Association of CRNDE and miR-181a-5p expressions with clinical traits of sepsis patients
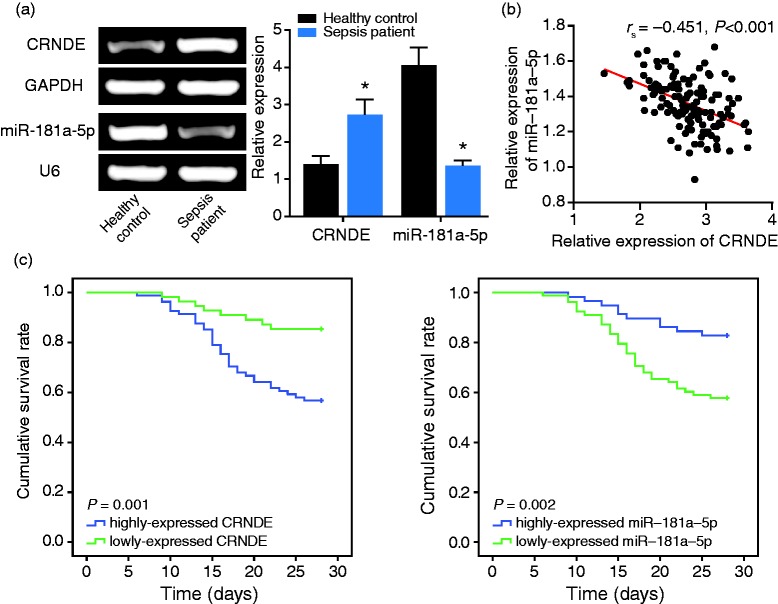
The sepsis patients displayed higher CRNDE expression and lower miR-181a-5p expression than healthy controls (P < 0.05) (Figure 1a). In addition, the sepsis patients were divided into over-expressed CRNDE (> median expression) and under-expressed (≤ median expression) CRNDE groups (Table 3). Similarly, the same sepsis patients were categorized into over-expressed miR-181a-5p group and under-expressed miR-181a-5p group, with the median expression of miR-181a-5p as the dividing point. The results revealed that sepsis patients carrying over-expressed CRNDE and under-expressed miR-181a-5p expression were tracked with higher MAP (≥ 0.34), SOFA (≥ 7.64) and APACHE II (≥ 17.15) scores, along with larger amounts of WBC (≥ 14.37 × 109/l), CRP (≥ 125 mg/l) and PCT (≥ 5.63 μg/l), than those categorized into the under-expressed CRNDE group and over-expressed miR-181a-5p group (P < 0.05). Interestingly, there emerged a negative correlation between CRNDE expression and miR-181a-5p expression among the recruited sepsis subjects (rs = -0.451, 95% CI: -0.575 to -0.305, P < 0.001) (Figure 1b).
Figure 1.
Association of CRNDE and miR-181a-5p expressions with the prognosis of sepsis patients. (a) The CRNDE and miR-181a-5p expressions were compared between sepsis patients and healthy controls. *: P < 0.05 when compared with healthy controls. (b) The CRNDE expression was negatively correlated with miR-181a-5p expression among the sepsis patients. (c) The low-expressed CRNDE and high-expressed miR-181a-5p were associated with favorable survival of sepsis patients, with high-expressed CRNDE and low-expressed miR-181a-5p, respectively, as the reference.
Table 3.
Association of CRNDE/miR-181a-5p expressions with the baseline clinical features of sepsis patients.
| Clinical features |
LncRNA CRNDE expression |
miR-181a-5p expression |
||||||
|---|---|---|---|---|---|---|---|---|
| High | Low | χ2 Value | P Value | High | Low | χ2 Value | P Value | |
| Gender | ||||||||
| Female | 35 | 31 | 31 | 35 | ||||
| Male | 46 | 24 | 2.27 | 0.132 | 27 | 43 | 0.98 | 0.322 |
| Age | ||||||||
| ≥ 56.04 | 39 | 32 | 35 | 36 | ||||
| < 56.04 | 42 | 23 | 1.32 | 0.250 | 23 | 42 | 2.69 | 0.101 |
| MAP score | ||||||||
| ≥ 0.34 | 62 | 30 | 31 | 61 | ||||
| < 0.34 | 19 | 25 | 7.24 | 0.007 | 27 | 17 | 9.32 | 0.002 |
| SOFA score | ||||||||
| ≥ 7.64 | 52 | 24 | 23 | 53 | ||||
| < 7.64 | 29 | 31 | 5.62 | 0.018 | 35 | 25 | 10.80 | 0.001 |
| APACHE II score | ||||||||
| ≥ 17.15 | 60 | 25 | 30 | 55 | ||||
| < 17.15 | 21 | 30 | 11.45 | 0.001 | 28 | 23 | 5.01 | 0.025 |
| WBC (×109/l) | ||||||||
| ≥ 14.37 | 52 | 27 | 28 | 51 | ||||
| < 14.37 | 29 | 28 | 3.07 | 0.080 | 30 | 27 | 4.00 | 0.046 |
| CRP (mg/l) | ||||||||
| ≥ 125 | 70 | 27 | 34 | 63 | ||||
| < 125 | 11 | 28 | 22.32 | < 0.001 | 24 | 15 | 7.98 | 0.005 |
| PCT (μg/l) | ||||||||
| ≥ 9.51 | 56 | 24 | 28 | 52 | ||||
| < 9.51 | 25 | 31 | 8.79 | 0.003 | 30 | 26 | 4.65 | 0.031 |
MAP: mean arterial pressure; SOFA: sequential organ failure assessment; APACHE II: acute physiology and chronic health evaluation II; WBC: white blood cell count; CRP: C-reactive protein; PCT: procalcitonin.
Predictive role of CRNDE and miR-181a-5p for prognosis of patients with sepsis
Over-expressed CRNDE was associated with poorer overall survival than under-expressed CRNDE (P = 0.001), while on the contrary, under-expressed miR-181a-5p might prolong the lifespan of patients with sepsis in comparison to over-expressed miR-181a-5p (P = 0.002) (Figure 1c). In addition, both CRNDE and miR-181a-5p were identified as significant predictors for the prognosis of sepsis patients, according to results of univariate and multivariate analyses (P < 0.05) (Table 4). Concurrently, higher SOFA score and APACHE II score were also reflective of poor prognosis of patients with sepsis than lower scores (P < 0.05).
Table 4.
Identification of potentially significant predictors for the prognosis of sepsis patients.
| Clinical features |
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P Value | Hazard ratio | 95% CI | P Value | |
| LncRNA CRNDE expression | ||||||
| High vs. low | 4.47 | 1.88–10.66 | 0.001 | 3.43 | 1.15–10.26 | 0.027 |
| miR-181a-5p expression | ||||||
| High vs. low | 0.28 | 0.13–0.64 | 0.003 | 0.41 | 0.15-0.95 | 0.042 |
| Gender | ||||||
| Female vs. male | 1.02 | 0.49–2.10 | 0.961 | 1.86 | 0.77–4.52 | 0.171 |
| Age (yr) | ||||||
| ≥ 56.04 vs. < 56.04 | 1.08 | 0.52–2.22 | 0.839 | 1.26 | 0.53–3.02 | 0.603 |
| MAP score | ||||||
| ≥ 0.34 vs. < 0.34 | 1.60 | 0.72–3.58 | 0.253 | 1.01 | 0.39–2.64 | 0.981 |
| SOFA score | ||||||
| ≥ 7.64 vs. < 7.64 | 3.84 | 1.70–8.68 | 0.001 | 2.84 | 1.09-7.36 | 0.032 |
| APACHE II score | ||||||
| ≥ 17.15 vs. < 17.15 | 5.78 | 2.23–15.00 | < 0.001 | 3.73 | 1.28–10.88 | 0.016 |
| WBC (×109/l) | ||||||
| ≥ 14.37 vs. < 14.37 | 1.33 | 0.63–2.79 | 0.45 | 1.13 | 0.46–2.75 | 0.788 |
| CRP (mg/l) | ||||||
| ≥ 125 vs. < 125 | 1.80 | 0.77–4.23 | 0.178 | 0.59 | 0.19–1.82 | 0.356 |
| PCT (μg/l) | ||||||
| ≥ 9.51 vs. < 9.51 | 1.47 | 0.70–3.11 | 0.312 | 1.22 | 0.50–3.00 | 0.659 |
MAP: mean arterial pressure; SOFA: sequential organ failure assessment; APACHE II: acute physiology and chronic health evaluation II; WBC: white blood cell count; CRP: C-reactive protein; PCT: procalcitonin.
Contribution of CRNDE and miR-181a-5p to macrophages’ release of cytokines
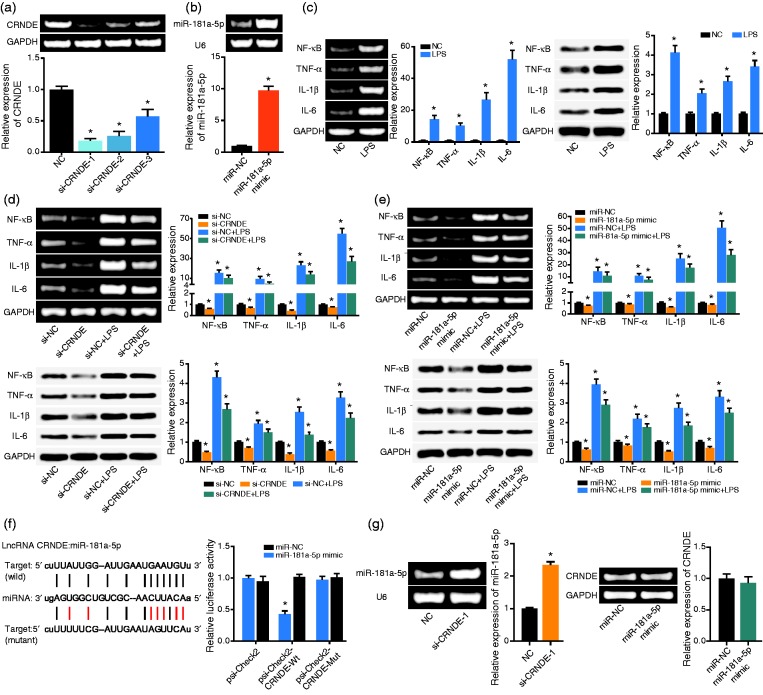
The expression of CRNDE was down-regulated evidently after transfection of si-CRNDE-1, si-CRNDE-2 and si-CRNDE-3 (P < 0.05) (Figure 2a). The si-CRNDE-1 was adopted as the silencing strategy for its superior efficacy in lowering CRNDE expression. Regarding miR-181a-5p, transfection of miR-181a-5p mimic resulted in a higher expression of miR-181a-5p than no treatment (P < 0.05) (Figure 2b). In addition, LPS-treated cells generated higher levels of NF-kB (P < 0.001), TNF-α (P < 0.001), IL-1β (P < 0.001) and IL-6 (P < 0.001) than NC group (all P < 0.05) (Figure 2c). Nevertheless, si-CRNDE-1 (Figure 2d) and miR-181a-5p mimic (Figure 2e) seemed to partly block the effects of LPS on cells’ secretion of NF-kB, TNF-α, IL-1β and IL-6 (P < 0.05), though the action effect of miR-181a-5p mimic was less pronounced than that of si-CRNDE-1 (P < 0.05).
Figure 2.
The regulatory effects of CRNDE and miR-181a-5p on the release of cytokines. (a) The expression of CRNDE was determined after respective transfection of si-CRNDE-1, si-CRNDE-2 and si-CRNDE-3. *: P < 0.05 when compared with NC group. (b) The expression of miR-181a-5p was measured after transfection of miR-181a-5p mimic. *: P < 0.05 when compared with miR-NC group. (c) The mRNA and protein levels of NF-kB, TNF-α, IL-1β and IL-6 within THP-1 cell line were monitored after treatment of LPS. *: P < 0.05 when compared with NC group. (d) The mRNA and protein levels of NF-kB, TNF-α, IL-1β and IL-6 within THP-1 cell line were determined among si-NC, si-CRNDE, si-NC + LPS and si-CRNDE + LPS groups. *: P < 0.05 when compared with si-NC group. (e) The mRNA and protein levels of NF-kB, TNF-α, IL-1β and IL-6 within THP-1 cell line were compared among miR-NC, miR-181a-5p mimic, miR-NC + LPS and miR-181a-5p mimic + LPS groups. *: P < 0.05 when compared with miR-NC group. (f) The CRNDE sponged miR-181a-5p in certain sites, and the luciferase activity was compared among psi-Check2-CRNDE-Wt + miR-181a-5p mimic group, psi-Check2-CRNDE-Mut + miR-181a-5p mimic group and psi-Check2-CRNDE-Wt + miR-NC group. *: P < 0.05 when compared with psi-Check2-CRNDE-Wt + miR-NC group. (g) The miR-181a-5p expression was determined after transfection of si-CRNDE-1, and the expression of CRNDE was drawn after treatment of miR-181a-5p. *: P < 0.05 when compared with miR-NC group.
The sponging relationship between CRNDE and miR-181a-5p in macrophages
The luciferase activity of psi-Check2-CRNDE Wt + miR-181a-5p mimic group was significantly reduced when compared with psi-Check2-CRNDE Mut + miR-181a-5p mimic group (P < 0.05), which showed no significant distinction from psi-Check2 + miR-181a-5p group in terms of luciferase activity (P > 0.05) (Figure 2f). Moreover, depression of CRNDE expression could induce up-regulation of miR-181a-5p expression (P < 0.05), yet the expression of CRNDE was unaffected after transfection of miR-181a-5p mimic (P > 0.05) (Figure 2g).
Modulation of TLR4 expression by CRNDE and miR-181a-5p
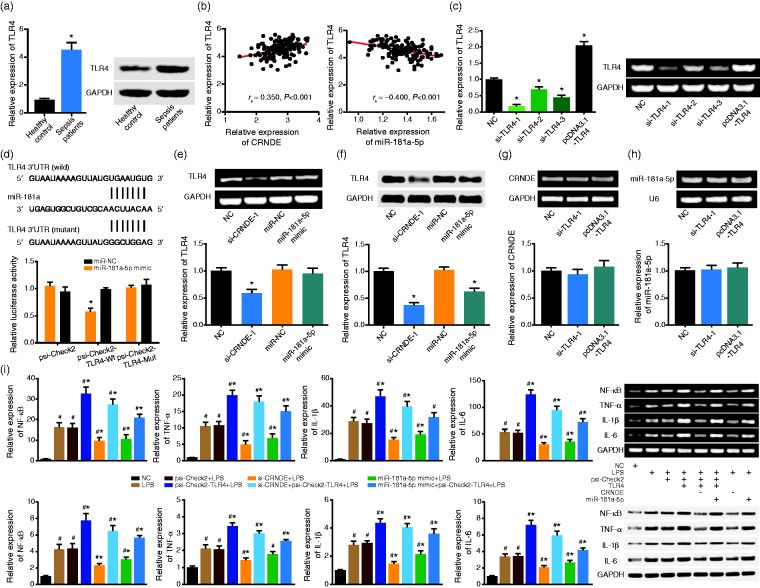
Among the patients included, expression of TLR4 in sepsis patients was about 4.5 fold of that in the control population (P < 0.05) (Figure 3a), and TLR4 expression was up-regulated with rise of CRNDE expression (rs = 0.350, 95% CI: 0.193–0.489, P < 0.001) and decline of miR-181a-5p expression (rs = –0.400, 95% CI: –0.532 to –0.248, P < 0.001) (Figure 3b). In addition, transfection of si-TLR4-1 brought about the lowest expression of TLR4 in comparison to si-TLR4-1, si-TLR4-2 and si-TLR4-3 groups (P < 0.05), while expression of TLR4 was promoted significantly within cells treated by psi-Check2-TLR4 (P < 0.05) (Figure 3c). Furthermore, co-transfection of psi-Check2-TLR4 Wt and miR-181a-5p mimic observably lowered the luciferase activity of macrophages in comparison to psi-Check2-TLR4 Mut + miR-181a-5p mimic group and psi-Check2-TLR4 Wt + miR-NC group (P < 0.05) (Figure 3d). Transfection of si-CRNDE-1 and miR-181a-5p mimic could both decrease the protein level of TLR4 within macrophages (P < 0.05) (Figure 3e and 3f), while si-TLR4-1 and psi-Check2-TLR4 could barely alter the expression of CRNDE and miR-181a-5p within cells (P > 0.05) (Figure 3g and 3h).
Figure 3.
The role of TLR4 in mediating the impacts of CRNDE and miR-181a-5p on the release of cytokines. (a) The TLR4 expression was determined with sepsis patients and healthy controls. *: P < 0.05 when compared with healthy controls. (b) The expression of TLR4 assumed a positive correlation with the expression of CRNDE, yet a negative correlation with the expression of miR-181a-5p. (c) The expression of TLR4 was detected after respective treatments of THP-1 cells with si-TLR4-1, si-TLR4-2, si-TLR4-3 and pcDNA3.1-TLR4. *: P < 0.05 when compared with NC group. (d) The TLR4 was targeted by miR-181a in certain sites, and the luciferase activity of THP-1 cells was measured among psi-Check2-TLR4-Wt + miR-181a-5p mimic group, psi-Check2-TLR4-Mut + miR-181a-5p mimic group and psi-Check2-TLR4-Wt + miR-NC group. *: P < 0.05 when compared with psi-Check2-TLR4-Wt + miR-NC group. (e, f) The expression of TLR4 was assessed under the influence of si-CRNDE-1 and miR-181a-5p mimic. *: P < 0.05 when, respectively, compared with NC and miR-NC groups. (g, h) The CRNDE and miR-181a-5p expressions were detected after transfections of si-TLR4-1 and pcDNA3.1-TLR4. *: P < 0.05 when compared with NC group. (i) The mRNA and protein levels of NF-kB, TNF-α, IL-1β and IL-6 within THP-1 cell line were determined after respective treatment of NC, LPS, psi-Check2 + LPS, psi-Check2-TLR4 + LPS, si-CRNDE + psi-Check2-TLR4 + LPS, si-CRNDE + LPS, miR-181a-5p mimic + psi-Check2-TLR4 + LPS and miR-181a-5p mimic + LPS groups. #: P < 0.05 when compared with NC group. *: P < 0.05 when compared with LPS group.
Mediation of TLR4 in regulating the impacts of CRNDE and miR-181a-5p on macrophages’ release of cytokines
The mRNA and protein levels of NF-kB, TNF-α, IL-1β and IL-6 were boosted significantly under treatments of LPS and psi-Check2+LPS, when compared with NC group (P < 0.05) (Figure 3i). Moreover, dual treatments of psi-Check2-TLR4 and LPS further improved the expression levels of NF-kB, TNF-α, IL-1β and IL-6 when compared with single treatment of LPS (P < 0.05). Of note, additional transfection of psi-Check2-TLR4 (i.e. psi-Check2-TLR4 + si-CRNDE + LPS group) was associated with higher mRNA and protein levels of NF-kB, TNF-α, IL-1β and IL-6 than si-CRNDE + LPS group (P < 0.05). And treatment of psi-Check2-TLR4 (i.e. psi-Check2-TLR4 + miR-181a-5p mimic + LPS group) could reverse the effects of miR-181a-5p mimic (i.e. miR-181a-5p mimic + LPS group) on macrophages’ secretion of NF-kB, TNF-α, IL-1β and IL-6 (P < 0.05).
Discussion
Sepsis is a principal trigger of septic shock and multiple organ dysfunction syndrome, with an alarming growth rate of 1.5–9% annually.28,29 Despite conspicuous advances in anti-infective treatments, the death toll caused by severe sepsis stands high at 30–50%.29 Since a superfluous expression of pro-inflammatory cytokines, such as TNF-α, IL-1 and IFN-γ, are direct contributors to runaway inflammation and thereby sepsis onset,30 identification of biomarkers that prohibit inflammatory responses seems particularly vital to curb sepsis progression.
A number of pathways have been suggested to be sepsis related, including MAPK signaling,31 tyrosine kinase-signaling and transcriptional activator signaling (JAK-STAT),32 phosphatidylinositol 3-kinase/serine threonine protein kinase (PI3K/AKT) signaling33 and glycogen synthase-3 (GSK-3) signaling.33 Nevertheless, there has been scant evidence that verified the role of lncRNAs in sepsis, although over-expression of lncRNAs HOTAIR and MALAT1 were discovered in septic mouse models.34,35 The present study investigated lncRNA CRNDE as a potential modulator of inflammatory responses underlying sepsis pathogenesis. Specifically, the clinical analyses demonstrated that CRNDE was differentially expressed within sepsis patients of distinct prognosis (Figure 1c), and in vitro tests also uncovered that CRNDE could propel expression of sepsis-specific biomarkers, including TNF-α,36 IL-1β,37 NF-kB38 and IL-634 (Figure 2). CRNDE had been previously reported as a modulator of sepsis-initiated inflammatory pathways as mentioned above. For instance, the TLR signaling was disordered when CRNDE was over-expressed,16 and knock-out of CRNDE could dramatically alter the functions of NF-κB signaling, JAK/STAT signaling and PI3K/AKT signaling.39,40 Consequently, it was reasonable to speculate CRNDE as a potent biomarker that could greatly influence the inflammatory pathogenesis inherent in sepsis.
In addition, we shed light on the role of miR-181a-5p in CRNDE-caused inflammatory responses, allowing that miR-181a-5p was subjected to targeted modulation of CRNDE (Figure 2f, 2g). In fact, the anti-inflammation function of miR-181a-5p has been documented earlier, such as suppression of NF-κB signaling,41 down-regulation of IL-8 expression42 and blockage of TNF-α expression.43 Within this study, miR-181a-5p was further demonstrated to impede over-expressed TNF-α, IL-1β, NF-κB and IL-6 that were characteristic for sepsis progression (Figure 2e). Building on these molecular mechanisms, under-expressed miR-181a-5p could serve as a pronounced indicator for poor prognosis of patients with sepsis (Table 3, Figure 1c). Beyond the above, TLR4, whose expression was suppressed under treatments of si-CRNDE and miR-181a-5p mimic (Figure 3d-3f), was discovered to influence the effects of miR-181a-5p on macrophages’ secretion of cytokines (Figure 3i). According to the cellular experiments, TLR4-induced inflammation in sepsis cell models was probably mediated by NF-Κb. In fact, there was clear evidence that TLR4 was capable of activating NF-κB,16 which then entered the nucleus to promote expression of inflammatory cytokines (e.g. TNF-α and IL-1).44 Intriguingly, the extent to which NF-κB was activated mirrored the prognostic condition of sepsis patients.45 The linkage of the CRNDE/miR-181a-5p/TLR4 axis and sepsis-related inflammation was thus formed.
Summing up the above, the CRNDE/miR-181a-5p/TLR4 axis was involved in dysfunctional inflammation underlying sepsis onset and development. Nonetheless, a comprehensive understanding of how CRNDE/miR-181a-5p/TLR4 axis plays a part in the underlying sepsis etiology would entail more proof from multiple angles. For instance, CRNDE could sponge more than miR-181a-5p to exert effects on sepsis-centered inflammation, and the miR-181a-5p/TLR4 axis might be influenced by other upstream lncRNAs. As for the clinical part, the included sepsis patients were Chinese-focused, and the derived study results might not be applicable for a sepsis population of other ethnicities. Above all, more convincing evidence is needed to overcome the deficits in experimental design mentioned above.
Supplemental Material
Supplemental material, INI880946 Supplemental Material for Linkage of lncRNA CRNDE sponging miR-181a-5p with aggravated inflammation underlying sepsis by Yijun Wang, Ziqiang Xu, Dongyou Yue, Zhenhua Zeng, Weijie Yuan and Ke Xu in Innate Immunity
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101: 1644–1655. [DOI] [PubMed] [Google Scholar]
- 2.Shukla P, Rao GM, Pandey G, et al. Therapeutic interventions in sepsis: Current and anticipated pharmacological agents. Br J Pharmacol 2014; 171: 5011–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moerer O, Schmid A, Hofmann M, et al. Direct costs of severe sepsis in three German intensive care units based on retrospective electronic patient record analysis of resource use. Intens Care Med 2002; 28: 1440–1446. [DOI] [PubMed] [Google Scholar]
- 4.Seymour CW, Cooke CR, Heckbert SR, et al. Prehospital intravenous access and fluid resuscitation in severe sepsis: An observational cohort study. Crit Care 2014; 18: 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco J, Muriel-Bombin A, Sagredo V, et al. Incidence, organ dysfunction and mortality in severe sepsis: A Spanish multicentre study. Crit Care 2008; 12: R158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parrillo JE, Parker MM, Natanson C, et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med 1990; 113: 227–242. [DOI] [PubMed] [Google Scholar]
- 7.Antonucci E, Fiaccadori E, Donadello K, et al. Myocardial depression in sepsis: From pathogenesis to clinical manifestations and treatment. J Crit Care 2014; 29: 500–511. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Wang X, Yan X, et al. LncRNA MALAT1 regulates sepsis-induced cardiac inflammation and dysfunction via interaction with miR-125b and p38 MAPK/NFkappaB. Int Immunopharmacol 2018; 55: 69–76. [DOI] [PubMed] [Google Scholar]
- 9.Batista PJ, Chang HY. Long noncoding RNAs: Cellular address codes in development and disease. Cell 2013; 152: 1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiStefano JK. The emerging role of long noncoding RNAs in human disease. Methods Mol Biol 2018; 1706: 91–110. [DOI] [PubMed] [Google Scholar]
- 11.Satpathy AT, Chang HY. Long noncoding RNA in hematopoiesis and immunity. Immunity 2015; 42: 792–804. [DOI] [PubMed] [Google Scholar]
- 12.Jiang C, Li X, Zhao H, et al. Long non-coding RNAs: Potential new biomarkers for predicting tumor invasion and metastasis. Mol Cancer 2016; 15: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters T, Hermans-Beijnsberger S, Beqqali A, et al. Long non-coding RNA Malat-1 is dispensable during pressure overload-induced cardiac remodeling and failure in mice. PloS One 2016; 11: e0150236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heward JA, Lindsay MA. Long non-coding RNAs in the regulation of the immune response. Trends Immunol 2014; 35: 408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu G, Tang Q, Sharma S, et al. Expression and regulation of intergenic long noncoding RNAs during T cell development and differentiation. Nat Immunol 2013; 14: 1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Li Q, Guo T, et al. LncRNA CRNDE triggers inflammation through the TLR3-NF-kappaB-Cytokine signaling pathway. Tumour Biol 2017; 39: 1010428317703821. [DOI] [PubMed] [Google Scholar]
- 17.Courtine E, Pene F, Cagnard N, et al. Critical role of cRel subunit of NF-kappaB in sepsis survival. Infect Immun 2011; 79: 1848–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poylin V, Fareed MU, O'Neal P, et al. The NF-kappaB inhibitor curcumin blocks sepsis-induced muscle proteolysis. Mediat Inflamm 2008; 2008: 317851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penner CG, Gang G, Wray C, et al. The transcription factors NF-kappab and AP-1 are differentially regulated in skeletal muscle during sepsis. Biochem Biophys Res Comm 2001; 281: 1331–1336. [DOI] [PubMed] [Google Scholar]
- 20.Jiang M, Zhou M, Han Y, et al. Identification of NF-kappaB inhibitors in Xuebijing injection for sepsis treatment based on bioactivity-integrated UPLC-Q/TOF. J Ethnopharmacol 2013; 147: 426–433. [DOI] [PubMed] [Google Scholar]
- 21.Han P, Li JW, Zhang BM, et al. The lncRNA CRNDE promotes colorectal cancer cell proliferation and chemoresistance via miR-181a-5p-mediated regulation of Wnt/beta-catenin signaling. Mol Cancer 2017; 16: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma A, Yang WL, Ochani M, et al. Mitigation of sepsis-induced inflammatory responses and organ injury through targeting Wnt/beta-catenin signaling. Sci Rep 2017; 7: 9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozloski GA, Jiang X, Bhatt S, et al. miR-181a negatively regulates NF-kappaB signaling and affects activated B-cell-like diffuse large B-cell lymphoma pathogenesis. Blood 2016; 127: 2856–2866. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Wang FL, Wang HB, et al. TNF-alpha mRNA is negatively regulated by microRNA-181a-5p in maturation of dendritic cells induced by high mobility group box-1 protein. Sci Rep 2017; 7: 12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones AE, Puskarich MA. The Surviving Sepsis Campaign guidelines 2012: Update for emergency physicians. Ann Emerg Med 2014; 63: 35–47. [DOI] [PubMed] [Google Scholar]
- 26.Yu W, Zhang X, Wu H, et al. HO-1 is essential for tetrahydroxystilbene glucoside mediated mitochondrial biogenesis and anti-inflammation process in LPS-treated RAW264.7 macrophages . Oxidat Med Cell Longev 2017; 2017: 1818575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv Y, Hu S, Lu J, et al. Upregulating nonneuronal cholinergic activity decreases TNF release from lipopolysaccharide-stimulated RAW264.7 cells. Mediat Inflamm 2014; 2014: 873728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock 1998; 10: 79–89. [DOI] [PubMed] [Google Scholar]
- 29.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 30.Liu QY, Yao YM. Inflammatory response and immune regulation of high mobility group box-1 protein in treatment of sepsis. World J Emerg Med 2010; 1: 93–98. [PMC free article] [PubMed] [Google Scholar]
- 31.Zaki OS, Safar MM, Ain-Shoka AA, et al. Bone marrow mesenchymal stem cells combat lipopolysaccharide-induced sepsis in rats via amendment of P38-MAPK signaling cascade. Inflammation 2018; 41: 541–554. [DOI] [PubMed] [Google Scholar]
- 32.Cai B, Cai JP, Luo YL, et al. The specific roles of JAK/STAT signaling pathway in sepsis. Inflammation 2015; 38: 1599–1608. [DOI] [PubMed] [Google Scholar]
- 33.He H, Chang X, Gao J, et al. Salidroside mitigates sepsis-induced myocarditis in rats by regulating IGF-1/PI3K/Akt/GSK-3beta signaling. Inflammation 2015; 38: 2178–2184. [DOI] [PubMed] [Google Scholar]
- 34.Wu H, Liu J, Li W, et al. LncRNA-HOTAIR promotes TNF-alpha production in cardiomyocytes of LPS-induced sepsis mice by activating NF-kappaB pathway. Biochem Biophys Res Commun 2016; 471: 240–246. [DOI] [PubMed] [Google Scholar]
- 35.Yu Z, Rayile A, Zhang X, et al. Ulinastatin protects against lipopolysaccharide-induced cardiac microvascular endothelial cell dysfunction via downregulation of lncRNA MALAT1 and EZH2 in sepsis. Int J Mol Med 2017; 39: 1269–1276. [DOI] [PubMed] [Google Scholar]
- 36.Zhang M, Wang X, Bai B, et al. Oxymatrine protects against sepsis-induced myocardial injury via inhibition of the TNF-alpha/p38-MAPK/caspase-3 signaling pathway. Mol Med Rep 2016; 14: 551–559. [DOI] [PubMed] [Google Scholar]
- 37.Moraes CA, Santos G, de Sampaio e Spohr TC, et al. Activated microglia-induced deficits in excitatory synapses through IL-1beta: Implications for cognitive impairment in sepsis. Mol Neurobiol 2015; 52: 653–663. [DOI] [PubMed] [Google Scholar]
- 38.Selvaraj V, Nepal N, Rogers S, et al. Inhibition of MAP kinase/NF-kB mediated signaling and attenuation of lipopolysaccharide induced severe sepsis by cerium oxide nanoparticles. Biomaterials 2015; 59: 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu-Ge D, Yang YP, Jiang ZJ. Knockdown CRNDE alleviates LPS-induced inflammation injury via FOXM1 in WI-38 cells. Biomed Pharmacother 2018; 103: 1678–1687. [DOI] [PubMed] [Google Scholar]
- 40.Du DX, Lian DB, Amin BH, et al. Long non-coding RNA CRNDE is a novel tumor promoter by modulating PI3K/AKT signal pathways in human gastric cancer. Eur Rev Med Pharmacol Sci 2017; 21: 5392–5398. [DOI] [PubMed] [Google Scholar]
- 41.Sun X, Sit A, Feinberg MW. Role of miR-181 family in regulating vascular inflammation and immunity. Trends Cardiovasc Med 2014; 24: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galicia JC, Naqvi AR, Ko CC, et al. MiRNA-181a regulates Toll-like receptor agonist-induced inflammatory response in human fibroblasts. Genes Immun 2014; 15: 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia S, Tian H, Fan L, et al. Peripheral blood miR-181-5p serves as a marker for screening patients with osteoarthritis by targeting TNFalpha. Clin Lab 2017; 63: 1819–1825. [DOI] [PubMed] [Google Scholar]
- 44.Everhart MB, Han W, Sherrill TP, et al. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J Immunol 2006; 176: 4995–5005. [DOI] [PubMed] [Google Scholar]
- 45.Arnalich F, Garcia-Palomero E, Lopez J, et al. Predictive value of nuclear factor kappaB activity and plasma cytokine levels in patients with sepsis. Infect Immun 2000; 68: 1942–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, INI880946 Supplemental Material for Linkage of lncRNA CRNDE sponging miR-181a-5p with aggravated inflammation underlying sepsis by Yijun Wang, Ziqiang Xu, Dongyou Yue, Zhenhua Zeng, Weijie Yuan and Ke Xu in Innate Immunity