Abstract
The mitochondrial unfolded protein response (UPRmt) is an evolutionarily conserved adaptive response that functions to maintain mitochondrial homeostasis following mitochondrial damage. In Caenorhabditis elegans, the nervous system plays a central role in responding to mitochondrial stress by releasing endocrine signals that act upon distal tissues to activate the UPRmt. The mechanisms by which mitochondrial stress is sensed by neurons and transmitted to distal tissues are not fully understood. Here, we identify a role for the conserved follicle-stimulating hormone G protein-coupled receptor, FSHR-1, in promoting UPRmt activation. Genetic deficiency of fshr-1 severely attenuates UPRmt activation and organism-wide survival in response to mitochondrial stress. FSHR-1 functions in a common genetic pathway with SPHK-1/sphingosine kinase to promote UPRmt activation, and FSHR-1 regulates the mitochondrial association of SPHK-1 in the intestine. Through tissue-specific rescue assays, we show that FSHR-1 functions in neurons to activate the UPRmt, to promote mitochondrial association of SPHK-1 in the intestine, and to promote organism-wide survival in response to mitochondrial stress. We propose that FSHR-1 functions cell nonautonomously in neurons to activate UPRmt upstream of SPHK-1 signaling in the intestine.
Keywords: FSHR-1, paraquat, sphingosine kinase, UPRmt
THE mitochondrial unfolded protein response (UPRmt) functions to maintain mitochondrial protein homeostasis in response to mitochondrial dysfunction caused by mitochondrial DNA damage, incorrect mitochondrial protein folding, or impaired oxidative phosphorylation. Failure to appropriately control and maintain protein homeostasis in the mitochondria is associated with the development of numerous diseases, neurodegeneration, and ageing (Durieux et al. 2011; Liu et al. 2014; Pellegrino et al. 2014; Nargund et al. 2015; Fiorese et al. 2016; Martinez et al. 2017). The UPRmt is initiated when mitochondrial proteostasis is disrupted, the detection of which by mitochondrial and cytosolic factors leads to epigenetic modifications and transcriptional responses in the nucleus that restore mitochondrial function. A critical sensor and activator of the UPRmt in Caenorhabditis elegans and in mammals is the leucine zipper transcription factor ATSF-1/ATF5 (Fiorese et al. 2016). ATFS-1 is normally targeted to mitochondria where it is degraded, but upon mitochondrial stress, mitochondrial import is disrupted and ATFS-1 is targeted instead to the nucleus where it regulates the expression of a cascade of genes including the conserved HSP70-like chaperone, hsp-6, which is targeted to the mitochondria to restore protein folding (Nargund et al. 2012). The intestinal UPRmt can be activated by mitochondrial stress originating either cell autonomously in the intestine or cell nonautonomously in the nervous system. Mitochondrial stress in neurons activates the UPRmt in the intestine through the release of neuropeptides, serotonin, and/or Wnt ligands (Berendzen et al. 2016; Shao et al. 2016; Zhang et al. 2018).
Genetic screens for additional factors that activate the UPRmt have revealed important roles for mitochondrial ceramide produced by SPTL-1/serine palmitoyltransferase and sphingosine-1-phosphate (S1P) produced by SPHK-1/sphingosine kinase in the activation of the UPRmt (Liu et al. 2014; Kim and Sieburth 2018a). SPHK-1 recruitment to mitochondria from cytoplasmic pools may serve as an early signal to activate UPRmt (Kim and Sieburth 2018a). SPHK-1 mitochondrial recruitment is positively regulated by mitochondrial stress originating either from the intestine or from the nervous system. Neuronal mitochondrial stress activates intestinal SPHK-1 by a mechanism that involves neuropeptide, but not serotonin, signaling (Kim and Sieburth 2018a). Neuropeptides exert their biological functions primarily through activating G protein-coupled receptors (GPCRs) on target cells to trigger downstream signaling events (Frooninckx et al. 2012). However, the specific neuropeptides and the GPCRs functioning in SPHK-1-mediated UPRmt activation have not been identified.
FSHR-1 is a GPCR-containing extracellular Leucine-Rich Repeats (LRRs) protein that is homologous to the follicle-stimulating hormone receptor family (Powell et al. 2009). FSHR-1 plays a critical role in activating innate immunity in response to infection by pathogenic bacteria, and functions in the intestine to promote protection against pathogenic infection and to regulate antimicrobial gene expression. (Cho et al. 2007; Powell et al. 2009; Miller et al. 2015). Interestingly, infection by pathogenic bacteria leads to multiple cellular responses in the intestine, including the activation of the UPRmt (Liu et al. 2014; Pellegrino et al. 2014).
In this study, through the analysis of fshr-1 null mutants, we show that FSHR-1 positively regulates UPRmt activation and promotes the mitochondrial association of SPHK-1 in the intestine. Through tissue-specific rescue experiments, we find that FSHR-1 functions in the nervous system to exert its function in UPRmt activation. We propose that FSHR-1 is part of a neuroendocrine signaling network that functions to regulate the UPRmt through intertissue signaling.
Materials and Methods
C. elegans strains
Strains used in this study were maintained at 22° following standard methods. Young adult hermaphrodites derived from the wild-type reference strain N2 Bristol were used for all experiments. The following mutant strains were used. SJ4100: zcIs13[Phsp-6::GFP], OJ4113: vjIs138[Pges-1::sphk-1::gfp], OJ4143: vjIs148[Pges-1::tomm-20::mCherry], ST66: ncIs17[Phsp-16.2::gfp], OJ2329: vjIs208[Pgst-4::gfp], uthIs375[unc-119p::cco-1 HP + rol-6(su1006)](Zhang et al. 2018), OJ997: sphk-1(ok1097), GR2250: mgIs73[cyp-14A4p::gfp], and KP3397: fshr-1(ok778). The sphk-1(ok1097) and fshr-1(ok778) strains were outcrossed at least six times with wild-type animals prior to analysis.
Molecular biology
fshr-1 complementary cDNA (cDNA) was cloned from C. elegans wild-type cDNA and then inserted into the pPD49.26 expression vector using standard molecular biology techniques. The following plasmids were generated: pSK55[Pges-1::fshr-1], pSK56[Prab-3::fshr-1], and pSK98[Prgef-1::fshr-1]. Sequence files of plasmids are available upon request.
Transgenic lines
Transgenic strains were generated by injecting expression constructs (10–25 ng/μl) and the co-injection marker pJQ70 [Pofm-1::mCherry, 40 ng/μl], KP#708[Pttx-3::rfp, 40 ng/μl], or KP#1106 [Pmyo-2::gfp, 10 ng/μl] into N2 worms or the indicated mutants using standard techniques (Mello et al. 1991). At least three lines for each transgene were tested and a representative transgene was used for further experiments. The following transgenic lines were made: vjEx1448[Prab-3::fshr-1], vjEx1449[Pges-1::fshr-1], vjEx1000[Pges-1::gfp], and vjEx1831[Prgef-1::fshr-1].
Microscopy and analysis
Fluorescence microscopy experiments were performed following previous methods (Kim and Sieburth 2018a). Briefly, L4-stage or young adult worms were immobilized by using 2,3-butanedione monoxime (BDM, 30 mg/ml; Sigma [Sigma Chemical], St. Louis, MO) in M9 buffer then mounted on 2% agarose pads for imaging. To quantify the fluorescence intensity of Phsp-6::GFP or Pgst-4::GFP, Z stacks of the intestine posterior to the vulva were selected as a representative region because of low basal expression in the absence of stress. Images were captured with the Nikon (Garden City, NY) eclipse 90i microscope equipped with a Nikon PlanApo 40×, or 60× or 100× objective (NA = 1.4), and a PhotometricsCoolsnap ES2 or a Hamamatsu Orca Flash LT + CMOS camera. Metamorph 7.0 software (Universal Imaging/Molecular Devices) was used to capture serial image stacks, and the maximum intensity was measured (Kim and Sieburth 2018b). Intensity quantification analysis was performed on the same day to equalize the absolute fluorescence levels between samples within the same experimental set. For Figure 1A, each sample cluster of worms was captured using a 4× objective lens for the representative images as shown in Figure 1A, then captured by a 40× objective lens for fluorescence intensity quantification.
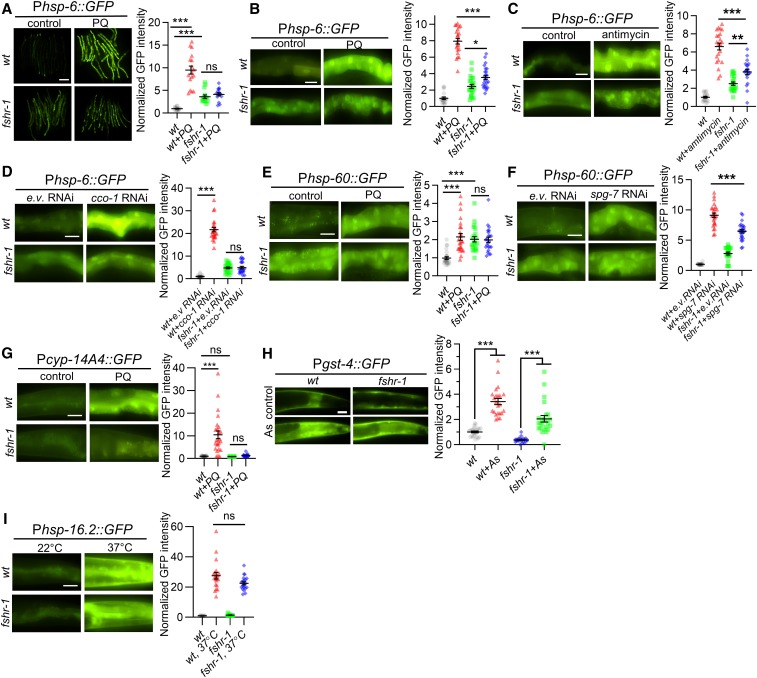
Figure 1.
FSHR-1 promotes UPRmt activation. (A) Representative low-magnification images of several wt adults or fshr-1 mutants expressing the zcIs13 [Phsp-6::GFP] transgene (GFP driven by the hsp-6 promoter), following treatment with M9 (control) or PQ for 24 hr. (B and C) Left: representative high-magnification images of intestines posterior to the vulva in young adult wt animals or fshr-1 mutants expressing Phsp-6::GFP following treatment with M9 (control) or PQ (B), or DMSO (control) or antimycin (C) for 24 hr. Right: average GFP fluorescence intensity is quantified. (D) Left: representative images of Phsp-6::GFP fluorescence in intestines of wt or fshr-1 mutants following e.v. control or cco-1/cox-5B RNAi treatment by feeding. Right: average GFP fluorescence intensity is quantified. (E and F) Left: representative intestine images of wt or fshr-1 mutants expressing the zcIs9 [Phsp-60::GFP] transgene following M9 and e.v. (G) (control), or PQ or spg-7/AFG3L2 RNAi treatment. Right: average GFP fluorescence intensity is quantified. (G) Left: representative images of wt or fshr-1 mutants expressing the mgIs73 [Pcyp-14A4::gfp] transgene in the absence or presence of PQ. Right: average GFP fluorescence intensity is quantified. (H) Left: representative images of posterior intestines from animals expressing the antioxidant reporter transgene [Pgst-4::GFP] in wt or fshr-1 mutants in the absence or presence of As. Right: average GFP fluorescence intensity is quantified. (I) Left: representative images of posterior intestines from animals expressing heat shock transgene ncIs17 [Phsp-16.2::GFP] in wt or fshr-1 mutants following 22° (control) or 37° exposure for 20 min. Right: average GFP fluorescence intensity is quantified. Bar represents 10 µm. Error bars indicate ± SEM. One-way ANOVA with Bonferroni post-test *P < 0.1; **P < 0.01; ***P < 0.001. As, arsenite; e.v., empty vector; ns, not statistically significant; PQ, paraquat; RNAi, RNA interference; UPRmt, mitochondrial unfolded protein response; wt, wild-type.
RNA interference
A feeding RNA interference (RNAi) knockdown assay was performed following established protocols (Kamath and Ahringer 2003). Briefly, gravid adult animals were placed on RNAi plates seeded with HT115(DE3) bacteria transformed with L4440 vector containing a genomic fragment of the gene to be knocked down (or empty L4440 vector as a control) to collect eggs, then removed after 4 hr to obtain an age-matched synchronized worm population. Young adult animals were used for subsequent assays.
Stress induction assays
For drug-induced stress, transgenic L4 animals were transferred to fresh NGM plates seeded with HB101 bacteria, then 80 μl of stock solutions dissolved in M9 buffer of paraquat were added to plates for a final concentration of 0.4 mM paraquat. After 24 hr, adults were selected for fluorescence microscopy analysis. For imaging of animals that had been subjected to RNAi-induced knockdown, L4 animals grown on RNAi plates were transferred to new RNAi plates to obtain synchronized animals and imaged 24 hr later. For Pgst-4::GFP imaging, young adult animals were incubated with 5 mM arsenite or 50 mM paraquat in liquid solution (in M9 buffer) for 1 hr, then drug was washed out and worms were transferred onto new NGM plates for 4 hr before imaging. M9 buffer was used as a control for paraquat and arsenite treatment.
For Phsp-16.2::GFP imaging, young adult animals were incubated at 22° or 37° for 20 min, and then allowed to recover at 22° for 24 hr prior to imaging. For paraquat survival assays, young adult animals were placed onto NGM plates containing 10 mM paraquat for 17 hr. After 17 hr, the percentage of surviving animals was counted every 3 hr over the course of 15 hr. Survival assays were done in experimental duplicate for each biological duplicate.
Statistical analysis
We utilized one-way ANOVA with a Bonferroni post-test to evaluate the statistical significance for multiple sample comparisons including Figure 3B (26 hr), while Student’s t-tests were used for two-sample comparison using Graphpad Prizm 8. P-values < 0.01 or 0.001 are indicated with asterisks (**P < 0.01 and ***P < 0.001, respectively). Error bars in the figures indicate the SE of the mean (± SEM). At least 15 animals per sample were analyzed for fluorescence quantification and data points, and averages are presented as shaped box plots in the figures.
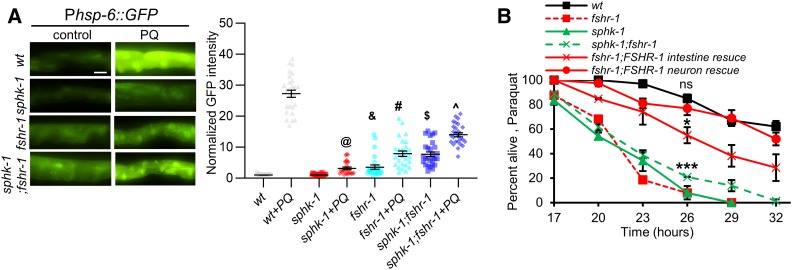
Figure 3.
FSHR-1 functions in a common pathway with SPHK-1 to activate the UPRmt. (A) Left: representative images of Phsp-6::GFP expression in the intestines of wild-type, sphk-1 mutants, fshr-1 mutants, and sphk-1;fshr-1 double mutants in the absence or the presence of PQ for 24 hr. Right: average GFP fluorescence intensity is quantified. (B) Survival rate curves of the indicated strains on plates containing 10 mM PQ. Bar represents 10 µm. One-way ANOVA with Bonferroni post-test: @P < 0.001 compared to sphk-1; &P < 0.001 compared to sphk-1; #P < 0.001 compared to fshr-1; $P < 0.001 compared to fshr-1; and ^P < 0.001 compared to sphk-1;fshr-1; ns: P > 0.05 compared to fshr-1;FSHR-1 neuron rescue; *P < 0.05 compared to wt; and ***P < 0.001 compared to wt. ns, not statistically significant; PQ, paraquat; UPRmt, mitochondrial unfolded protein response; wt, wild-type.
Data availability
Strains and plasmids used in this study are available upon request. The authors confirm that all data necessary for confirming the conclusions of the findings are present within the article and figures.
Results
FSHR-1/GPCR signaling regulates the UPRmt
Prior studies found that fshr-1 mutants have normal life spans (Powell et al. 2009), but exhibit enhanced sensitivity to lethality caused by the mitochondrial toxin and UPRmt activator paraquat (Miller et al. 2015). Because impairing UPRmt activation causes sensitivity to paraquat-induced lethality (Nargund et al. 2012; Gatsi et al. 2014; Liu et al. 2014; Kim and Sieburth 2018a), we speculated that FSHR-1 may positively regulate the UPRmt. To monitor UPRmt activation, we quantified the intestinal fluorescence of the UPRmt transcriptional reporter zcIs13, in which GFP is expressed under control of the hsp-6 promoter (Phsp-6::GFP). Paraquat is an oxidant that interferes with electron transport at the inner mitochondrial membrane, and has been used widely to acutely activate the UPRmt in both C. elegans and in mammalian cells (Nargund et al. 2012; Runkel et al. 2013; Fiorese et al. 2016; Kim and Sieburth 2018a). Wild-type animals treated with paraquat for 24 hr exhibited a roughly 10-fold increase in Phsp-6::GFP expression in the intestine compared to nontreated controls (Figure 1, A and B). In contrast, fshr-1 mutants showed a slight increase in baseline Phsp-6::GFP expression, and either no significant change or a small increase in Phsp-6::GFP expression following paraquat treatment (Figure 1, A and B). Antimycin is a mitochondrial electron transport chain (ETC) stressor, and antimycin treatment for 24 hr robustly induces the UPRmt (Liu et al. 2014). fshr-1 mutations attenuated the antimycin-induced increase in Phsp-6::GFP expression (Figure 1C). cco-1 (aka cox-5B) encodes the cytochrome c oxidase subunit in complex IV, which is the terminal electron acceptor of the ETC, and RNAi-mediated cco-1 knockdown is a potent activator of the UPRmt (Nargund et al. 2012; Berendzen et al. 2016; Merkwirth et al. 2016; Tian et al. 2016; Kim and Sieburth 2018a). cco-1 RNAi by feeding, which induces mitochondrial stress in the intestine (Zhang et al. 2018), significantly increased Phsp-6::GFP expression in wild-type animals but failed to increase Phsp-6::GFP expression in fshr-1 mutants (Figure 1D). fshr-1 mutations also blocked or attenuated the stress-induced increase in expression of two additional UPRmt reporters: Phsp-60::GFP and Pcyp-14A4::GFP [Figure 1, E–G, Nargund et al. (2012), and Mao et al. (2019)].
fshr-1 mutants were not defective in the induction of the antioxidant reporter Pgst-4::GFP in response to treatment with the mitochondrial reactive oxygen species (ROS) generator arsenite [Figure 1H; 1.6-fold increase in wild-type and 1.9-fold increase in fshr-1 mutants by arsenite (Inoue et al. 2005; Choe et al. 2009; Ruiz-Ramos et al. 2009; Prakash et al. 2015; Wu et al. 2016)]. In addition, fshr-1 mutants exhibited normal induction of the heat-shock reporter Phsp-16.2::GFP in response to heat shock at 37° for 20 min (Figure 1I). Together, these results suggest that FSHR-1 plays a specific role in activating the UPRmt in response to mitochondrial stress.
FSHR-1/GPCR functions cell nonautonomously in neurons to regulate UPRmt
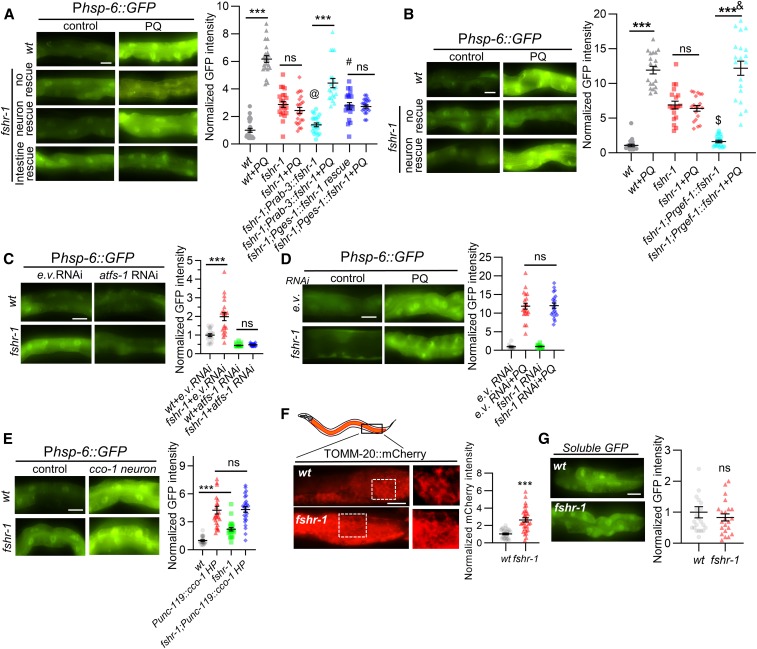
FSHR-1 is expressed primarily in the intestine and in a subset of neurons (Sieburth et al. 2005; Cho et al. 2007; Cao et al. 2017). To determine in which tissue FSHR-1 functions to regulate the UPRmt, we examined Phsp-6::GFP induction in transgenic fshr-1 mutants expressing fshr-1 cDNA in either the nervous system (using the rab-3 or the rgef-1 promoter) or the intestine (using the ges-1 promoter). Pan-neuronal fshr-1 either partially (rab-3 promoter) or fully (rgef-1 promoter) rescued the paraquat-induced hsp-6 expression defects of fshr-1 mutants (Figure 2, A and B). In contrast, intestinal fshr-1 cDNA expression failed to rescue the paraquat-induced hsp-6 expression defects of fshr-1 mutants (Figure 2A). RNAi-mediated knockdown of fshr-1 by bacterial feeding, which efficiently knocks down genes in the intestine but not in the nervous system (Kamath et al. 2001; Timmons et al. 2001; Asikainen et al. 2005), did not attenuate paraquat-induced hsp-6 expression, confirming that fshr-1 does not function in the intestine to regulate the UPRmt (Figure 2D). Taken together, these results suggest that FSHR-1 primarily functions cell nonautonomously in the nervous system to positively regulate UPRmt activation.
Figure 2.
FSHR-1 functions in the nervous system to regulate the UPRmt. (A and B) Left: representative images of posterior intestines of young adult animals or fshr-1 mutants expressing the Phsp-6::GFP transgene following treatment with M9 (control) or PQ for 24 hr. “fshr-1 neuronal rescue” denotes fshr-1 mutants expressing fshr-1 cDNA under the neuron-specific promoter rab-3 (A) or rgef-1 (B). “fshr-1 intestine rescue” denotes fshr-1 mutants expressing fshr-1 cDNA under the intestine-specific promoter ges-1. Right: average GFP fluorescence intensity is quantified. (C) Left: representative images of intestines of wt or fshr-1 mutants expressing Phsp-6::GFP following e.v. control or atfs-1 RNAi treatment by feeding. Right: average GFP fluorescence intensity is quantified. (D) Left: representative images of intestines of wt worms expressing Phsp-6::GFP following e.v. control or fshr-1 RNAi treatment by feeding in the absence or presence of PQ. Right: average GFP fluorescence intensity is quantified. (E) Left: representative images of Phsp-6::GFP fluorescence in intestines of wt or fshr-1 mutants in the absence or presence of transgene uthIs375[unc-119p::cco-1 HP + rol-6(su1006)]). Right: average GFP fluorescence intensity is quantified. (F) Left: representative images of intestinal cells of wt or fshr-1 mutants expressing the mitochondrial marker TOMM-20::mCherry under the intestinal-specific promoter ges-1. Right: average mCherry fluorescence intensities are quantified. (G) Left: representative images of animals expressing soluble GFP in the intestine (Pges-1::GFP) in wt or fshr-1 mutants. Right: average GFP fluorescence intensities are quantified. Bar in the panel represents 10 µm. Error bars indicate ± SEM. One-way ANOVA with Bonferroni post-test ***P < 0.001; #P < 0.001 compared to fshr-1; Prab-3::fshr-1; @P > 0.05 compared to wt; $P > 0.05 compared to wt; and &P > 0.05 compared to wt + PQ. cDNA, complementary DNA; e.v., empty vector; ns, not statistically significant; PQ, paraquat; RNAi, RNA interference; UPRmt, mitochondrial unfolded protein response; wt, wild-type.
The intestinal UPRmt can be activated by cell-autonomous stress originating in the intestine or by cell-nonautonomous stress originating in the nervous system (Nargund et al. 2012; Berendzen et al. 2016; Merkwirth et al. 2016; Zhang et al. 2018). fshr-1 is required for proper UPRmt activation elicited by intestinal stress (Figure 1, D and F). To determine whether fshr-1 promotes UPRmt activation elicited by neuronal stress, we examined Phsp-6::GFP expression in animals in which cco-1 was selectively silenced in the nervous system. Neuronal cco-1 knockdown by hairpin-mediated RNAi is a potent activator of the intestinal UPRmt (Zhang et al. 2018). Neuronal cco-1 knockdown elicited similar intestinal Phsp-6::GFP expression in fshr-1 mutants as in wild-type controls (Figure 2E). The fold induction was slightly reduced in fshr-1 mutants [4.3-fold for fshr-1(+) vs. 2.2 fold for fshr-1(-)] due to increased baseline hsp-6 expression in fshr-1 mutants (Figure 2E). Thus, fshr-1 appears to be largely dispensable for UPRmt activation when stress originates from the nervous system.
Neuronal FSHR-1 regulates baseline hsp-6 and hsp-60 expression
Under nonstressed conditions, fshr-1 mutants exhibited small but significant increases in Phsp-6::GFP expression in the intestine compared to wild-type controls (Figure 1, A–D), and larger increases in Phsp-60::GFP expression (Figure 1, E and F) that could reach levels similar to those seen under stressed conditions (Figure 1E). The increase in Phsp-6::GFP expression was blocked by RNAi-mediated knockdown of atfs-1 (Figure 2C) and was restored to wild-type levels by pan-neuronal, but not by intestinal, fshr-1 cDNA expression (Figure 2, A and B), suggesting that the UPRmt is activated even in the absence of stress in fshr-1 mutants. Together, these results indicate that neuronal FSHR-1 functions cell nonautonomously to keep UPRmt activity low in the absence of stress.
Mitochondria marked by the mitochondrial localization signal of TOMM-20 fused to mCherry (TOMM-20::mCherry) are distributed in a highly reticulated pattern within the cytoplasm of intestinal cells [expressed under the ges-1 promoter; Figure 2E, Palikaras et al. (2015), and Kim and Sieburth (2018a)]. fshr-1 mutants exhibited a normal overall pattern of TOMM-20:mCherry fluorescence in intestinal cells (Figure 2E), but the average fluorescence intensity of TOMM-20:mCherry associated with mitochondria was significantly increased by about threefold in fshr-1 mutants compared to wild-type controls (Figure 2F). In control experiments, we found that fshr-1 mutants exhibited no change in the fluorescence intensity of soluble GFP expressed under the ges-1 promoter (Figure 2G), suggesting that transgene expression was not altered in fshr-1 mutants. The increase in TOMM-20 fluorescence intensity in fshr-1 mutants could reflect either an increase in mitochondrial mass, a decrease in TOMM-20 turnover, or an increase in TOMM-20 targeting to the outer mitochondrial membrane. Alterations in mitochondrial mass, morphology, or mitochondrial protein import have been linked to UPRmt activation (Ungvari et al. 2011; Houtkooper et al. 2013; Lerner et al. 2013; Mouchiroud et al. 2013; Mao et al. 2019).
FSHR-1 and SPHK-1 function in a common pathway to activate the UPRmt
SPHK-1 functions in the intestine to activate the UPRmt in response to a variety of mitochondrial stressors, including paraquat (Kim and Sieburth 2018a). To test whether FSHR-1 functions in a common pathway with SPHK-1 to activate the UPRmt, we examined genetic interactions between sphk-1 and fshr-1 mutants. sphk-1 mutants exhibited a significant reduction in paraquat-induced expression of Phsp-6::GFP (89% reduction), which is similar to that exhibited by fshr-1 mutants [92% reduction; Figure 3A and Kim and Sieburth (2018a)]. Double mutants lacking both sphk-1 and fshr-1 exhibited increased baseline expression of hsp-6, which was similar to that of fshr-1 mutants, and had defects in paraquat-induced hsp-6 induction that were no more severe than those seen in single mutants (93% reduction, Figure 3A).
sphk-1 mutants exhibit reduced survival rates when exposed to toxic levels of paraquat (Kim and Sieburth 2018a), which are similar to those of fshr-1 mutants (Figure 3B). The survival rates of double mutants lacking both sphk-1 and fshr-1 were no more severe than those of either single mutant (Figure 3B). Expression of sphk-1 cDNA in the intestine fully rescued the increased paraquat-induced lethality of sphk-1 mutants (Kim and Sieburth 2018a). Expression of fshr-1 cDNA in the nervous system fully rescued the increased paraquat-induced lethality of fshr-1 mutants, whereas expression of fshr-1 cDNA in the intestine partially restored paraquat sensitivity to fshr-1 mutants [Figure 3B and Miller et al. (2015). Taken together, FSHR-1 and SPHK-1 may function in a common pathway to activate the UPRmt, and to promote organism-wide protection from mitochondrial stress-induced lethality. Moreover, FSHR-1 functions in the nervous system to activate the UPRmt and promote survival, whereas SPHK-1 functions exclusively in the intestine.
FSHR-1/GPCR regulates mitochondrial association of SPHK-1 in the intestine
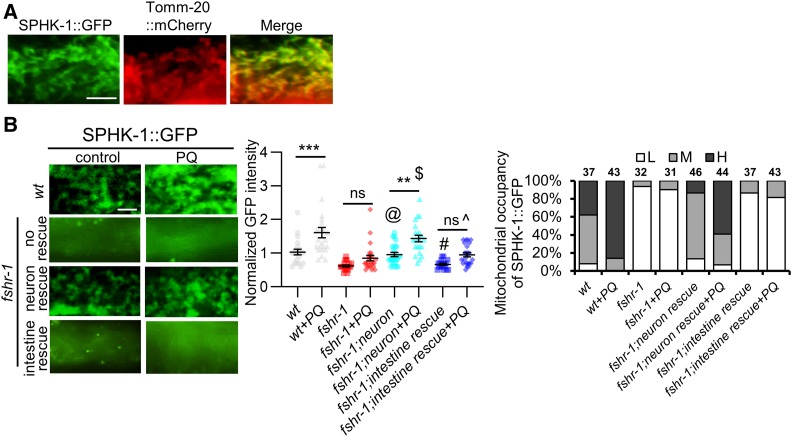
SPHK-1 localizes to intestinal mitochondria and is recruited to mitochondria from cytosolic pools by mitochondrial stress (Kim and Sieburth 2018a). SPHK-1::GFP fusion proteins, which are fully functional in rescuing the UPRmt defects of sphk-1 mutants, colocalize with TOMM-20::mCherry (Figure 4A). Mitochondrial stress induces a significant increase in mitochondrial SPHK-1::GFP fluorescence, as well as an increase in the number of intestinal cells in which SPHK-1::GFP adopts a mitochondrial distribution pattern [referred to as mitochondrial occupancy; Figure 4B and Kim and Sieburth (2018a)]. To determine whether FSHR-1 regulates SPHK-1 mitochondrial association, we examined the mitochondrial abundance of SPHK-1::GFP fusion proteins before and after paraquat exposure. fshr-1 mutants exhibited two defects in SPHK-1::GFP mitochondrial association. First, fshr-1 mutants exhibited a nearly twofold reduction in SPHK-1::GFP mitochondrial association compared to wild-type controls in the absence of paraquat (Figure 4B). Second, the paraquat-induced increase of mitochondrial SPHK-1::GFP association was completely abolished in fshr-1 mutants (Figure 4B). Thus, FSHR-1 positively regulates SPHK-1 association with mitochondria under nonstressed conditions and FSHR-1 is required for stress-induced SPHK-1 mitochondrial targeting.
Figure 4.
FSHR-1 regulates mitochondrial association of SPHK-1. (A) High-magnification images of SPHK-1::GFP (left), TOMM-20::GFP (middle), and merge (right) in wt animals coexpressing Pges-1::sphk-1::gfp and Pges-1::tomm-20::mCherry transgenes. (B) Left: representative images of the indicated mutants expressing intestinal SPHK-1::GFP in the absence or presence of PQ for 24 hr. fshr-1 neuron and intestine rescue denote transgenic fshr-1 mutants expressing fshr-1 cDNA under the pan-neural promoter rab-3 or the intestinal promoter ges-1, respectively. Middle: average fluorescence intensities in the indicated strains expressing SPHK-1::GFP are quantified. Right: mitochondrial occupancy of indicated transgenes is quantified in the absence or presence of PQ. H, M, or L indicates the percentage of animals showing mitochondrially localized SPHK-1::GFP in more than two-thirds of the intestine (H), one-half of the intestine (M), or only in a few intestinal cells (L). Bar represents 2 µm. One-way ANOVA with Bonferroni post-test **P < 0.01; ***P < 0.001; @P > 0.05 compared to wt; #P > 0.05 compared to fshr-1 mutants; $P > 0.05 compared to wt + PQ; and ^P > 0.05 compared to fshr-1 + PQ. cDNA, complementary DNA; ns, not statistically significant; PQ, paraquat; wt, wild-type.
Neuronal fshr-1 cell-nonautonomously regulates mitochondrial association of SPHK-1 in the intestine
To determine in which tissue FSHR-1 functions to regulate the mitochondrial association of SPHK-1, we quantified SPHK-1::GFP mitochondrial fluorescence in fshr-1 mutants expressing fshr-1 cDNA in either the nervous system or in the intestine. We found that pan-neuronal expression of fshr-1 cDNA in fshr-1 mutants restored both the mitochondrial occupancy and fluorescence intensity of SPHK-1::GFP to wild-type levels in the absence of stress. Similarly, neuronal fshr-1 cDNA also restored normal paraquat-induced SPHK-1::GFP mitochondrial recruitment to fshr-1 mutants. In contrast, intestinal fshr-1 cDNA expression failed to rescue the SPHK-1::GFP fluorescence defects of fshr-1 mutants (Figure 4B). These results suggest that FSHR-1 functions exclusively in the nervous system to positively regulate baseline SPHK-1 abundance in the intestine in the absence of stress, and to promote paraquat-induced SPHK-1 mitochondrial association.
Discussion
The nervous system coordinates various stress responses by releasing diffusible factors that act upon distal tissues to activate cellular defense programs (Nussbaum-Krammer and Morimoto 2014; Berendzen et al. 2016; Shao et al. 2016). Here, we show that the conserved GPCR FSHR-1 is part of an integrated organism-wide response to mitochondrial stress that functions to activate the UPRmt in the intestine. fshr-1 promotes UPRmt activation in response to either acute exposure to mitochondrial toxins or to chronic mitochondrial dysfunction, and functions in a common pathway with sphk-1 to promote survival in response to toxic mitochondrial stress and UPRmt activation. fshr-1 positively regulates the mitochondrial association of SPHK-1 in the intestine in the absence of stress, as well as stress-induced SPHK-1 mitochondrial recruitment. fshr-1 functions in the nervous system, but not in the intestine, to promote UPRmt activation and stress-induced SPHK-1 mitochondrial recruitment. Finally, neuronal fshr-1 regulates intestinal mitochondrial homeostasis in the absence of stress by a mechanism that may involve regulating mitochondrial mass and/or mitochondrial protein abundance. We propose a model whereby neuronal fshr-1 signaling keeps UPRmt activity low in the absence of stress and positively regulates the UPRmt in response to intestinal stress. The underlying mechanism by which fshr-1 regulates the UPRmt may be by establishing or maintaining proper mitochondrial homeostasis in the intestine.
fshr-1 functions cell nonautonomously in the nervous system to activate the UPRmt; however, our genetic evidence suggests that it is not likely to function in one of the previously identified “mitokine” pathways that signal neuronal stress to the intestine. Disruption of these pathways (e.g., egl-20/Wnt or flp-2/neuropeptide) blocks the ability of stress originating in the nervous system to promote intestinal UPRmt activation (Shao et al. 2016; Kim and Ewbank 2018; Zhang et al. 2018). We found that fshr-1 is required for UPRmt activation in response to intestinal stress but not neuronal stress. Thus, fshr-1 is not likely to be a component of a neuronal mitokine pathway but instead may promote UPRmt activation by a mechanism that does not involve the direct response to neuronal stress.
Previous studies have established an important role for FSHR-1 in activating the innate immune response following infection with pathogenic bacteria. FSHR-1 promotes both survival following infection and the behavioral avoidance response to pathogenic bacteria, and FSHR-1 activates a number of antimicrobial and antioxidant genes in response to pathogens (Powell et al. 2009; Miller et al. 2015). FSHR-1 is proposed to not participate in the detection of the pathogenic bacteria themselves, but instead to participate in the detection of cellular damage (e.g., ROS) resulting from bacterial infection (Miller et al. 2015). Pathogenic bacterial infection is also a potent activator of the UPRmt, and UPRmt activation by pathogens promotes survival by inducing the expression of innate immune genes (Pellegrino et al. 2014). We speculate that fshr-1 may activate the innate immune response by contributing to activation of the UPRmt in response to a broad array of insults (e.g., pathogenic infection, ROS, and mitochondrial dysfunction). Interestingly, we found that intestinal fshr-1 does not regulate the UPRmt, yet intestinal fshr-1 expression protects fshr-1 mutants from paraquat toxicity, suggesting that activation of the UPRmt may not be necessary to elicit resistance to paraquat. Paraquat is an ROS generator that also activates the intestinal antioxidant response, and fshr-1 promotes the expression of antioxidant genes (Powell et al. 2009; Miller et al. 2015). Thus, the paraquat sensitivity of fshr-1 mutants may arise from defects in the antioxidant response in the intestine.
FSHR-1 also regulates intestinal mitochondrial homeostasis in the absence of stress. Whether FSHR-1 does so by regulating mitochondrial mass or mitochondrial protein import, or both, is not clear based on our quantitative imaging of mitochondrial markers. Interestingly, FSHR signaling negatively regulates mitochondrial biogenesis in mammals (Liu et al. 2017). Enhanced mitochondrial biogenesis is correlated with UPRmt activation. Inducers of mitochondrial biogenesis, such as nicotinamide riboside and poly(ADP-ribose) polymerase inhibitors (PARPi), disrupt mito–nuclear protein homeostasis resulting in UPRmt activation via the sirtuin 1 (SIRT1) pathway in mammals and C. elegans. (Mouchiroud et al. 2013). In addition, rapamycin and resveratrol, which increase mitochondrial content, also induce UPRmt activation in C. elegans (Ungvari et al. 2011; Houtkooper et al. 2013; Lerner et al. 2013). In worms, mutants with defects in the import of mitochondrial proteins into the matrix exhibit increased hsp-60 expression that is dependent upon atfs-1 (Nargund et al. 2012). We speculate that the defects in paraquat-induced UPRmt activation in fshr-1 mutants may be due to an underlying defect in the mitochondrial association of SPHK-1 under normal conditions.
Since FSHR-1 functions to protect animals from diverse stressors, the ligand for FSHR-1 is likely to originate from the host rather than from an exogenous source (Powell et al. 2009). FSHR-1 shares homology with three human GPCRs—FSHR, TSHR, and LHCGR—by virtue of nine LRRs found in its extracellular domain (Dolan et al. 2007). These GPCRs are activated by the heterodimeric glycopeptide hormones FSHα/β, TSHα/β, and LHα/β, respectively. In humans, FSH induces the generation of S1P by stimulating SphK1 activity for granulosa cell proliferation (Hernández-Coronado et al. 2016), suggesting potential functional conservation of ligand-activated FSHR-1 signaling. An additional mammalian glycopeptide hormone family member, thyrostimulin, is a heterodimer composed of the GPB5 α-subunit and the GPA2 β-subunit, which share homology with worm T23B12.8 and FLR-2, respectively (Rocco and Paluzzi 2016). Interestingly, genetic analysis implicates flr-2 in the neuronal control of intestinal functions (Oishi et al. 2009), but whether flr-2 regulates the UPRmt or innate immunity has not been investigated. Identifying the ligand(s) for FSHR-1 will help to clarify the cell-nonautonomous mechanisms by which FSHR-1 regulates the UPRmt pathway.
Acknowledgments
We thank the Ruvkun laboratory for providing the mgIs73 reporter strain and the Dillin laboratory for providing the uthIs375 strain. This work was supported by grants from the National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke to D.S. (NS-071085 and NS-099414). Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD-010440).
Footnotes
Communicating editor: B. Grant
Literature Cited
- Asikainen S., Vartiainen S., Lakso M., Nass R., and Wong G., 2005. Selective sensitivity of Caenorhabditis elegans neurons to RNA interference. Neuroreport 16: 1995–1999 [corrigenda: Neuroreport 17: 230–231 (2006)]. 10.1097/00001756-200512190-00005 [DOI] [PubMed] [Google Scholar]
- Berendzen K. M., Durieux J., Shao L. W., Tian Y., Kim H. E. et al. , 2016. Neuroendocrine coordination of mitochondrial stress signaling and proteostasis. Cell 166: 1553–1563.e10. 10.1016/j.cell.2016.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Packer J. S., Ramani V., Cusanovich D. A., Huynh C. et al. , 2017. Comprehensive single-cell transcriptional profiling of a multicellular organism. Science 357: 661–667. 10.1126/science.aam8940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S., Rogers K. W., and Fay D. S., 2007. The C. elegans glycopeptide hormone receptor ortholog, FSHR-1, regulates germline differentiation and survival. Curr. Biol. 17: 203–212. 10.1016/j.cub.2006.12.027 [DOI] [PubMed] [Google Scholar]
- Choe K. P., Przybysz A. J., and Strange K., 2009. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol. Cell. Biol. 29: 2704–2715. 10.1128/MCB.01811-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan J., Walshe K., Alsbury S., Hokamp K., O’Keeffe S. et al. , 2007. The extracellular leucine-rich repeat superfamily; a comparative survey and analysis of evolutionary relationships and expression patterns. BMC Genomics 8: 320 [corrigenda: BMC Genomics 10: 230 (2009)]. 10.1186/1471-2164-8-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux J., Wolff S., and Dillin A., 2011. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144: 79–91. 10.1016/j.cell.2010.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorese C. J., Schulz A. M., Lin Y. F., Rosin N., Pellegrino M. W. et al. , 2016. The transcription factor ATF5 mediates a mammalian mitochondrial UPR. Curr. Biol. 26: 2037–2043. 10.1016/j.cub.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frooninckx L., Van Rompay L., Temmerman L., Van Sinay E., Beets I. et al. , 2012. Neuropeptide GPCRs in C. elegans. Front. Endocrinol. (Lausanne) 3: 167 10.3389/fendo.2012.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatsi R., Schulze B., Rodriguez-Palero M. J., Hernando-Rodriguez B., Baumeister R. et al. , 2014. Prohibitin-mediated lifespan and mitochondrial stress implicate SGK-1, insulin/IGF and mTORC2 in C. elegans. PLoS One 9: e107671 10.1371/journal.pone.0107671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Coronado C. G., Guzmán A., Rodríguez A., Mondragón J. A., Romano M. C. et al. , 2016. Sphingosine-1-phosphate, regulated by FSH and VEGF, stimulates granulosa cell proliferation. Gen. Comp. Endocrinol. 236: 1–8. 10.1016/j.ygcen.2016.06.029 [DOI] [PubMed] [Google Scholar]
- Houtkooper R. H., Mouchiroud L., Ryu D., Moullan N., Katsyuba E. et al. , 2013. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497: 451–457. 10.1038/nature12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Hisamoto N., An J. H., Oliveira R. P., Nishida E. et al. , 2005. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 19: 2278–2283. 10.1101/gad.1324805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R. S., and Ahringer J., 2003. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30: 313–321. 10.1016/S1046-2023(03)00050-1 [DOI] [PubMed] [Google Scholar]
- Kamath R. S., Martinez-Campos M., Zipperlen P., Fraser A. G. and Ahringer J., 2001. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2: RESEARCH0002 10.1186/gb-2000-2-1-research0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. H., and Ewbank J. J., 2018. Signaling in the innate immune response (August 14, 2018), WormBook, ed. The C. elegans Research Community WormBook, doi/10.1895/wormbook.1.83.2, http://www.wormbook.org. 10.1895/wormbook.1.83.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., and Sieburth D., 2018a Sphingosine kinase activates the mitochondrial unfolded protein response and is targeted to mitochondria by stress. Cell Rep. 24: 2932–2945.e4. 10.1016/j.celrep.2018.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., and Sieburth D., 2018b Sphingosine kinase regulates neuropeptide secretion during the oxidative stress-response through intertissue signaling. J. Neurosci. 38: 8160–8176. 10.1523/JNEUROSCI.0536-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner C., Bitto A., Pulliam D., Nacarelli T., Konigsberg M. et al. , 2013. Reduced mammalian target of rapamycin activity facilitates mitochondrial retrograde signaling and increases life span in normal human fibroblasts. Aging Cell 12: 966–977. 10.1111/acel.12122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Samuel B. S., Breen P. C., and Ruvkun G., 2014. Caenorhabditis elegans pathways that surveil and defend mitochondria. Nature 508: 406–410. 10.1038/nature13204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Ji Y., Yuen T., Rendina-Ruedy E., DeMambro V. E. et al. , 2017. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 546: 107–112. 10.1038/nature22342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao K., Ji F., Breen P., Sewell A., Han M. et al. , 2019. Mitochondrial dysfunction in C. elegans activates mitochondrial relocalization and nuclear hormone receptor-dependent detoxification genes. Cell Metab. 29: 1182–1191.e4. 10.1016/j.cmet.2019.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez B. A., Petersen D. A., Gaeta A. L., Stanley S. P., Caldwell G. A. et al. , 2017. Dysregulation of the mitochondrial unfolded protein response induces non-apoptotic dopaminergic neurodegeneration in C. elegans models of Parkinson’s disease. J. Neurosci. 37: 11085–11100. 10.1523/JNEUROSCI.1294-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., and Ambros V., 1991. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. 10.1002/j.1460-2075.1991.tb04966.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkwirth C., Jovaisaite V., Durieux J., Matilainen O., Jordan S. D. et al. , 2016. Two conserved histone demethylases regulate mitochondrial stress-induced longevity. Cell 165: 1209–1223. 10.1016/j.cell.2016.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. V., Grandi L. N., Giannini J. A., Robinson J. D., and Powell J. R., 2015. The conserved G-protein coupled receptor FSHR-1 regulates protective host responses to infection and oxidative stress. PLoS One 10: e0137403 10.1371/journal.pone.0137403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud L., Houtkooper R. H., Moullan N., Katsyuba E., Ryu D. et al. , 2013. The NAD(+)/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154: 430–441. 10.1016/j.cell.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargund A. M., Pellegrino M. W., Fiorese C. J., Baker B. M., and Haynes C. M., 2012. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 337: 587–590. 10.1126/science.1223560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargund A. M., Fiorese C. J., Pellegrino M. W., Deng P., and Haynes C. M., 2015. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPR(mt). Mol. Cell 58: 123–133. 10.1016/j.molcel.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum-Krammer C. I., and Morimoto R. I., 2014. Caenorhabditis elegans as a model system for studying non-cell-autonomous mechanisms in protein-misfolding diseases. Dis. Model. Mech. 7: 31–39. 10.1242/dmm.013011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi A., Gengyo-Ando K., Mitani S., Mohri-Shiomi A., Kimura K. D. et al. , 2009. FLR-2, the glycoprotein hormone alpha subunit, is involved in the neural control of intestinal functions in Caenorhabditis elegans. Genes Cells 14: 1141–1154. 10.1111/j.1365-2443.2009.01341.x [DOI] [PubMed] [Google Scholar]
- Palikaras K., Lionaki E., and Tavernarakis N., 2015. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 521: 525–528. 10.1038/nature14300 [DOI] [PubMed] [Google Scholar]
- Pellegrino M. W., Nargund A. M., Kirienko N. V., Gillis R., Fiorese C. J. et al. , 2014. Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature 516: 414–417. 10.1038/nature13818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. R., Kim D. H., and Ausubel F. M., 2009. The G protein-coupled receptor FSHR-1 is required for the Caenorhabditis elegans innate immune response. Proc. Natl. Acad. Sci. USA 106: 2782–2787. 10.1073/pnas.0813048106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash C., Soni M., and Kumar V., 2015. Biochemical and molecular alterations following arsenic-induced oxidative stress and mitochondrial dysfunction in rat brain. Biol. Trace Elem. Res. 167: 121–129. 10.1007/s12011-015-0284-9 [DOI] [PubMed] [Google Scholar]
- Rocco D. A., and Paluzzi J. P., 2016. Functional role of the heterodimeric glycoprotein hormone, GPA2/GPB5, and its receptor, LGR1: an invertebrate perspective. Gen. Comp. Endocrinol. 234: 20–27. 10.1016/j.ygcen.2015.12.011 [DOI] [PubMed] [Google Scholar]
- Ruiz-Ramos R., Lopez-Carrillo L., Rios-Perez A. D., De Vizcaya-Ruiz A., and Cebrian M. E., 2009. Sodium arsenite induces ROS generation, DNA oxidative damage, HO-1 and c-Myc proteins, NF-kappaB activation and cell proliferation in human breast cancer MCF-7 cells. Mutat. Res. 674: 109–115. 10.1016/j.mrgentox.2008.09.021 [DOI] [PubMed] [Google Scholar]
- Runkel E. D., Liu S., Baumeister R., and Schulze E., 2013. Surveillance-activated defenses block the ROS-induced mitochondrial unfolded protein response. PLoS Genet. 9: e1003346 [corrigenda: PLoS Genet. 12: e1006377 (2016)]. 10.1371/journal.pgen.1003346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L. W., Niu R., and Liu Y., 2016. Neuropeptide signals cell non-autonomous mitochondrial unfolded protein response. Cell Res. 26: 1182–1196. 10.1038/cr.2016.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth D., Ch’ng Q., Dybbs M., Tavazoie M., Kennedy S. et al. , 2005. Systematic analysis of genes required for synapse structure and function. Nature 436: 510–517. 10.1038/nature03809 [DOI] [PubMed] [Google Scholar]
- Tian Y., Garcia G., Bian Q., Steffen K. K., Joe L. et al. , 2016. Mitochondrial stress induces chromatin reorganization to promote longevity and UPR(mt). Cell 165: 1197–1208. 10.1016/j.cell.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L., Court D. L., and Fire A., 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263: 103–112. 10.1016/S0378-1119(00)00579-5 [DOI] [PubMed] [Google Scholar]
- Ungvari Z., Sonntag W. E., de Cabo R., Baur J. A., and Csiszar A., 2011. Mitochondrial protection by resveratrol. Exerc. Sport Sci. Rev. 39: 128–132. 10.1097/JES.0b013e3182141f80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. W., Deonarine A., Przybysz A., Strange K., and Choe K. P., 2016. The Skp1 homologs SKR-1/2 are required for the Caenorhabditis elegans SKN-1 antioxidant/detoxification response independently of p38 MAPK. PLoS Genet. 12: e1006361 10.1371/journal.pgen.1006361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Wu X., Chen P., Liu L., Xin N. et al. , 2018. The mitochondrial unfolded protein response is mediated cell-non-autonomously by retromer-dependent Wnt signaling. Cell 174: 870–883 e7. 10.1016/j.cell.2018.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids used in this study are available upon request. The authors confirm that all data necessary for confirming the conclusions of the findings are present within the article and figures.