Abstract
We previously demonstrated that 20 mM sucrose promotes the upper axillary bud outgrowth in two-node stems of Chrysanthemum morifolium. In this study, we aimed to screen for potential genes involved in this process. Quantitative reverse transcription (qRT)-PCR analysis of sugar-related genes in the upper axillary bud of plants treated with 20 mM sucrose revealed the specific expression of the gene CmSWEET17. Expression of this gene was increased in the bud, as well as the leaves of C. morifolium, following exogenous sucrose treatment. CmSWEET17 was isolated from C. morifolium and a subcellular localization assay confirmed that the protein product was localized in the cell membrane. Overexpression of CmSWEET17 promoted upper axillary bud growth in the two-node stems treatment as compared with the wild-type. In addition, the expression of auxin transporter genes CmAUX1, CmLAX2, CmPIN1, CmPIN2, and CmPIN4 was upregulated in the upper axillary bud of CmSWEET17 overexpression lines, while indole-3-acetic acid content decreased. The results suggest that CmSWEET17 could be involved in the process of sucrose-induced axillary bud outgrowth in C. morifolium, possibly via the auxin transport pathway.
Keywords: CmSWEET17, sucrose, bud outgrowth, Chrysanthemum morifolium, auxin
1. Introduction
The chrysanthemum, one of the most well-known flowers in the world, originated in China and possesses great ornamental and economic value. However, the production of single-headed cut chrysanthemum flowers requires a large amount of manpower and material resources to manually remove the axillary buds. This labor-intensive process limits the development of the ornamental chrysanthemum industry to a certain extent [1]. Currently, reports on the shoot branching of chrysanthemum focus mainly on the effects of hormones, including strigolactone [2], auxin [3], and cytokinin [4], as well as other factors such as temperature [5] and transcription factor BRC1 [6]. However, few studies have reported on the effect of sugar on the regulation of the shoot branching of chrysanthemum [7].
Sugar, as both an energy source and a signaling molecule, plays a crucial role in processes of plant growth and development, including flowering [8,9], meristematic proliferation [10], and anthocyanin biosynthesis [11]. Studies have shown that sugar also affects shoot branching and bud outgrowth [12,13]. In the rose plant, exogenous sugars (sucrose, palatinose, and psicose) promote axillary bud growth in vitro [14]. Furthermore, budbreak in the walnut plant is associated with the sucrose absorption [15]. Additionally, gentiobiose, a rare disaccharide, acts as a signal to promote bud growth in Gentiana, and exogenous gentiobiose facilitates bud germination [16].
Since 1934, when Thimann and Skoog demonstrated that apical dominance was associated with auxin [17], this hormone has been regarded as the key signal controlling bud outgrowth and apical dominance [18]. The growth of axillary buds is accompanied by auxin export from the buds and an increase in the expression of the auxin transport gene PINs [4,19]. In recent years, studies have shown that the initial signaling molecule regulating plant apical dominance could be sugar rather than auxin [20,21]; and that auxin acts on subsequent continuous growth stages [22]. However, the mechanism by which sugar regulates axillary bud outgrowth remains unclear.
In plants, sugar is produced mainly by photosynthesis in leaves, from where it is then transported to other parts of the plant to serve its function. This process requires specific sugar transporting proteins, such as sucrose transporters SUC/SUT and bidirectional sugar transporters SWEET [23]. Sugar transporters are indispensable for sugar function. Sugar transport protein 1 (STP1) regulates shoot branching by controlling the expression of genes involved in hormone biosynthesis and signaling in Arabidopsis thaliana [24].
SWEET proteins belong to the MtN3/saliva family, with the majority being composed of seven predicted α-helical transmembrane (TM) domains, and a few of only three TM domains [25]. The N- and C-terminals are located in the inner and outer cytoplasm, respectively [26]. In A. thaliana and rice, SWEETs participate in phloem transport of sucrose [27], nectar secretion [28], and regulating gibberellin-mediated physiological processes [29]. There are 17 SWEET members in A. thaliana [25,30]. AtSWEET17, which belongs to the fourth subfamily [31], participates in the regulation of fructose accumulation in leaves, as demonstrated by the decrease in fructose content of leaves in sweet17-1 and sweet17-2 mutants [32]. SWEET17 expression can be induced by fructose and darkness; the fructose content, in the leaves of SWEET17 overexpression lines, has been shown to decrease sharply under cold stress [33]. The expression of DsSWEET17 in Dianthus spiculifolius is affected by exogenous fructose, glucose, salt stress, and osmotic stress and following its transformation into A. thaliana, root growth was promoted [31]. Transgenic lines of PtSWEET17a overexpression in poplar result in a growth promoting phenotype, which is manifested as a thicker stem diameter and increased plant height [34]. However, to the best of our knowledge, the involvement of SWEET17 in shoot branching has not been reported to date.
A previous study found that treatment with 20 mM sucrose could promote axillary bud outgrowth in C. morifolium as compared with the control (Figure S1) [35]. In this study, a quantitative analysis was performed to screen for key genes involved in this process, revealing differential expression of the gene CmSWEET17. To further investigate the role of this gene, transgenic engineering, gene expression quantification, and auxin content determination were carried out.
2. Materials and Methods
2.1. Plants and Growth Conditions
Plantlets of chrysanthemum (Chrysanthemum morifolium ‘Jinba’) were propagated under sterile conditions with MS medium, followed by growth in a tissue culture room at 23 °C with a photoperiod of 16 h light and 8 h dark and a light intensity of 100 to approximately 120 µmol photons·m−2·s−1.
2.2. Two-Node Stem Treatments
The two-node stem was established in order to study the development of axillary buds (Figure S1). When a seedling grew to ~10 cm, the stem was cut into a two-node stem containing two axillary buds. The two-node stems were treated with 0 or 20 mM sucrose, as described previously [35]. The treatments consisted of MS medium with 20 mM mannitol (control) or 20 mM sucrose. The treatment groups were grown under darkness to avoid photosynthesis-induced sucrose production. After 10 days of treatment, the phenotype of axillary bud length was changed and the axillary buds from each group were collected for subsequent RNA extraction. Due to the small size of the axillary buds, the sampling was repeated 4 to approximately 5 times, and each sample was taken at the same time of day.
2.3. Quantitative Reverse Transcription PCR (qRT-PCR) Analysis
Total RNA was extracted from the axillary buds using Trizol reagent according to the manufacturer’s protocol (Takara, Shiga, Japan). First-strand cDNA was synthesized using a PrimeScript™ RT Reagent kit (Takara). The qPCR reactions were run using the Mastercycler® EP Realplex Real-Time PCR system (Eppendorf, Hamburg, Germany), as described previously [36]. Using the existing transcriptome library (http://www.ncbi.nlm.nih.gov/bioproject/PRJNA329030), we screened sugar-related genes, including signal key signaling genes hexokinase (HXK), sucrose non-fermenting-related kinase 1 (SnRK1), and target of rapamycin (TOR), as well as sugar transporter SUC and SWEET genes. Gene-specific primers were designed using Primer Premier 5.0 (Premier Biosoft International, Palo Alto, CA, USA), and the sequences are listed in Table S1. The chrysanthemum CmEF1α gene (GenBank acc. no., AB679278.1) was used as an internal control.
2.4. Exogenous Sucrose Treatment
When the C. morifolium seedlings grew to 15 cm and produced 10 true leaves, they were treated with 50 mM sucrose, as previously described [9]. Following treatment, the second top leaf (fully unfolded) and axillary bud were collected at 0, 0.5, 1, 3, 6, and 24 h. The leaves and axillary buds were snap frozen in liquid nitrogen and stored at −80 °C, for subsequent RNA extraction and quantitative analysis of CmSWEET17.
2.5. Isolation of CmSWEET17 and Sequence Analysis
The sequence of the sugar transporter gene CmSWEET17 was obtained using ORFfinder (https://www.ncbi.nlm.nih.gov/orffinder/), and the full length was cloned using gene-specific primers (Table S1). The PCR reaction was composed of 10 μL 5x Phusion HF Buffer, 2 μL each primer (10 μM), 1 μL dNTP (10 μM), 0.5 μL dimethyl sulfoxide (DMSO), 34 μL H2O, 0.5 μL Phusion High-Fidelity DNA Polymerases (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 0.5 μL of cDNA. The PCR cycling regime comprised an initial denaturation step at 98 °C for 30 s, followed by 40 cycles at 98 °C/10 s, 55 °C/30 s, and 72 °C/30 s, with a final extension step at 72 °C for 8 min. The resulting amplicon (876 bp in length) was introduced into the pMD19-T vector (Takara) for sequencing. After successfully obtaining the CmSWEET17 sequence, prediction of the transmembrane domains of was performed using TMHMM (http://www.cbs.dtu.dk/services/TMHMM/). Amino acid sequence alignment and construction of a phylogenetic tree was carried out alongside AtSWEET family members from A. thaliana, using molecular evolutionary genetics analysis software DNAMAN version 6 (Lynnon Biosoft, San Ramon, CA, USA).
2.6. Subcellular Localization of the CmSWEET17 Protein
The open reading frame of the CmSWEET17 gene was amplified using forward and reverse primers incorporating SalI and NotI restriction sites, respectively (CmSWEET17-1A-F and CmSWEET17-1A-R, Table S1). The PCR reagents and conditions were the same as Section 2.5. Restriction enzymes, SalI and NotI, were used to digest the resulting PCR products and the pENTR™ 1A Dual Selection entry vector (Thermo Fisher Scientific, Inc.), and the double enzyme products were recovered by DNA Gel Extraction Kit (Axygen) and ligated with solution I (Takara). Subsequently, the resulting vector (pENTR1A-CmSWEET17) was used to construct a green fluorescent protein (GFP) fusion vector pMDC43-CmSWEET17 by means of the Gateway® LR Clonase® II enzyme (Thermo Fisher Scientific, Inc.). The construct was transiently introduced into onion epidermal cells using a PDS-1000/He helium-driven particle accelerator (Bio-Rad Laboratories, Inc., California, USA), which vacuum level, helium pressure, and target interval were 26 to 28 inches Hg (mercury), 1300 psi (pounds per square inch), and 6 cm, respectively. And the cells were then incubated at 25 °C for 16 h in the dark. The GFP fluorescence was monitored by laser scanning confocal microscopy (LSM780; Zeiss, Oberkochen, Germany) at 488 nm. To induce plasmolysis, a few drops of 20% mannitol solution were added to the onion epidermis cells, which were then incubated at room temperature for ~20 min under dark conditions.
2.7. Chrysanthemum Transformation
The pMDC43-CmSWEET17 plasmid was used as an overexpression vector in Agrobacterium tumefaciens strain EHA105 using the freeze-thaw method, and subsequently into C. morifolium, as described previously [37]. Hygromycin-resistant plants were obtained, and their DNA was extracted, as described previously [35], and verified by PCR analysis (primers pMDC43-F and CmSWEET17-R, Table S1). After the DNA verification, the potential overexpression lines (OX) of CmSWEET17 were validated using qRT-PCR (primers CmSWEET17-Q-F/R, Table S1). The overexpression lines were treated as per the two-node stem treatments described in Section 2.2, and the bud length was measured under a stereomicroscope, with buds from 10 stem segments measured for each treatment.
2.8. Indole-3-Acetic Acid (IAA) Content Measurement
The buds from the two-node stem treatment were harvested, flash frozen in liquid nitrogen, and stored at −80 °C (sampling as described in Section 2.2). Auxin (IAA) was extracted and measured using ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), as described previously [38].
2.9. Statistical Analysis
Statistical analyses were carried out using Excel 2017 (Microsoft Corporation), and data are expressed as the mean ± SE of at least three separate experiments and p ≤ 0.05 was considered to indicate a statistically significant difference. The images were processed using Adobe Photoshop software.
3. Results
3.1. Expression of CmSWEET17 in Response to Sucrose
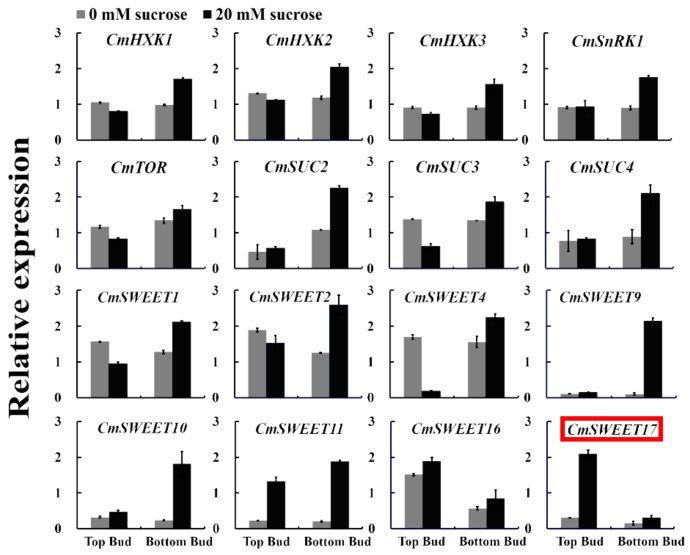
To screen for genes that potentially serve a role in sucrose-induced promotion of axillary bud outgrowth in the two-node stem system (Figure S1), a qRT-PCR analysis of sugar-related genes was performed. The only gene detected to be specifically expressed in the upper bud of plants treated with 20 mM sucrose was CmSWEET17 (Figure 1). This gene could, therefore, be involved in the process of sucrose-promoted outgrowth of the upper bud.
Figure 1.
Relative expression of sugar-related genes in the axillary bud following the two-node stems treatment.
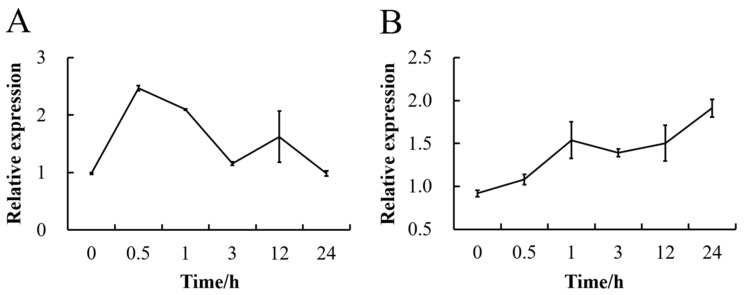
To verify whether sucrose affects the expression of CmSWEET17, we sprayed C. morifolium seedlings with 50 mM sucrose solution and analyzed the gene expression profile in the leaves using qRT-PCR. The data showed that the treatment resulted in a rapid increase in CmSWEET17 expression, peaking at 30 min, when it was 2.52× higher than the baseline level (Figure 2A). The expression level then decreased gradually over time, returning to baseline at 24 h (Figure 2A). In the axillary buds, the expression of CmSWEET17 increased gradually from 0 to 24 h, at 24 h, when it was 2.08× higher than the baseline level (Figure 2B). The results demonstrate that exogenous sucrose treatment can lead to an increase in the expression of CmSWEET17 in the leaves and lateral buds of C. morifolium.
Figure 2.
Change in expression level of the CmSWEET17 gene in response to exogenous sucrose treatment (A) in the leaf, and (B) in the axillary bud.
3.2. CmSWEET17 Sequence Analysis
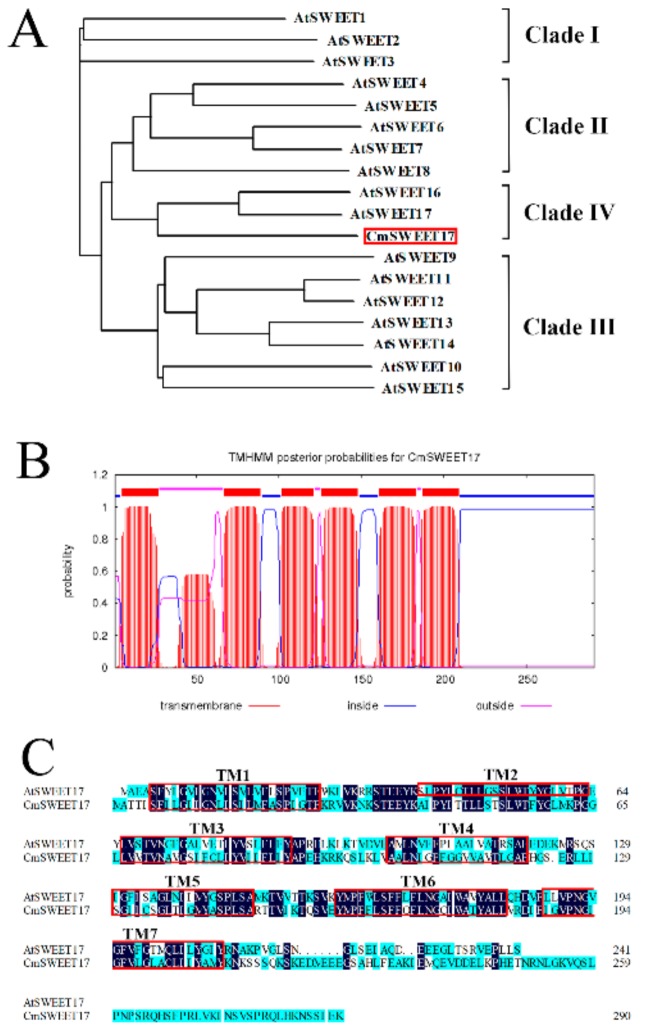
To clone the CmSWEET17 gene, we designed the specific primers (CmSWEET17-F and CmSWEET17-R, Supplementary Table S1). The open reading frame (ORF) of CmSWEET17 was found to be 876 bp, which encoded 291 amino acids. Phylogenetic analysis revealed that CmSWEET17 was most closely related to AtSWEET17, belonging to clade IV of the Arabidopsis AtSWEET family (Figure 3A). Using the TMHMM algorithm, CmSWEET17 was predicted to have six transmembrane domains (Figure 3B), SWEET family genes in other species generally contain seven transmembrane domains. CmSWEET17 lacks a second transmembrane domain (TM2) as compared with AtSWEET17 in Arabidopsis thaliana (Figure 3C).
Figure 3.
CmSWEET17 sequence analysis. (A) Phylogenetic tree of sequences including CmSWEET17 and AtSWEET family members from Arabidopsis thaliana. (B) Putative transmembrane TM domains within the CmSWEET17 sequence. (C) Amino acid sequence alignment of CmSWEET17 against AtSWEET17. Red boxes indicate TM domains and TM, transmembrane.
3.3. Subcellular Localization of CmSWEET17
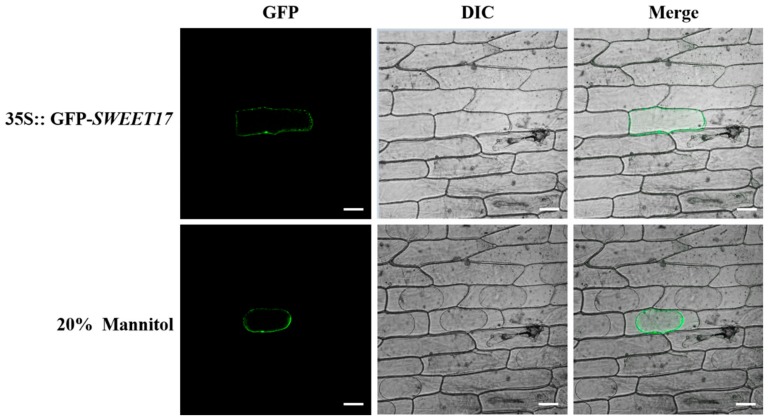
In order to determine the localization of the CmSWEET17 protein in living cells, we constructed the pMDC43-CmSWEET17 vector, delivered it into onion inner epidermis cells, and observed GFP signals. These signals were observed only in the cell membrane (Figure 4). To further verify whether CmSWEET17 was located in the cell membrane, 20% mannitol solution was used for plasmolysis. With the separation of the plasma membrane from the cell wall, the GFP signals also moved along with the cell membrane (Figure 4). These data indicate that CmSWEET17 is located in the cell membrane.
Figure 4.
Subcellular localization of CmSWEET17 protein in onion epidermis cells. GFP, green fluorescence channel; DIC, bright light channel; and MERGE, overlay plots. Scale bars, 50 μm.
3.4. Effect of CmSWEET17 Overexpression on Bud Outgrowth
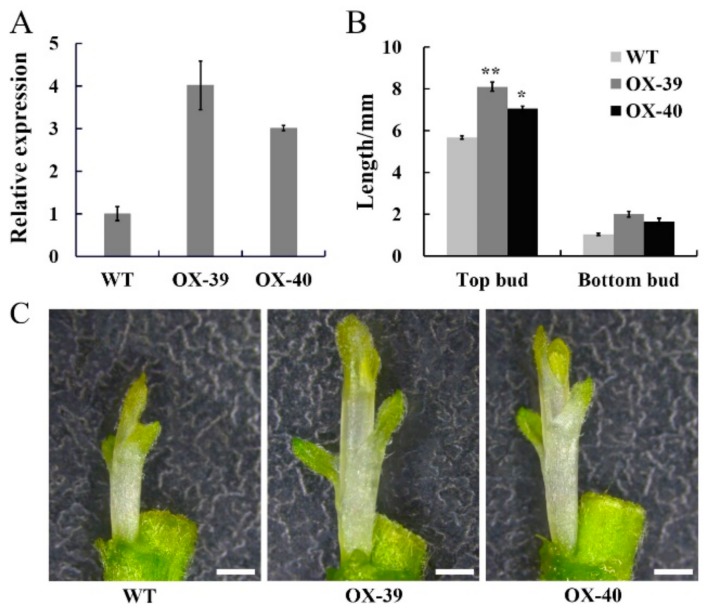
To verify the function of CmSWEET17, the overexpression vector pMDC43-CmSWEET17 was constructed and transformed into C. morifolium via the Agrobacterium-mediated leaf disc method, and overexpression lines were obtained. PCR analysis of the genomic DNA identified the OX-39 and OX-40 lines of CmSWEET17 (Figure S2). Subsequently, quantitative analysis revealed that CmSWEET17 expression in OX-39 and OX-40 was increased by 4.01 and 3.01 times, respectively, as compared with the control (Figure 5A).
Figure 5.
CmSWEET17 overexpression lines promote bud growth in Chrysanthemum morifolium. (A) Relative CmSWEET17 expression in WT (wild-type) and overexpression lines OX-39 and OX-40 and (B) the length of the bud following treatment of two-node stem. Data are represented as the mean ± SE of n = 10. (C) Phenotype of axillary bud growth in WT, OX-39, and OX-40. Scale bars, 1 mm. * p ≤ 0.05 and ** p ≤ 0.01 vs. WT.
In order to test whether CmSWEET17 regulates the outgrowth of the axillary bud, the two-node stem treatment was applied to the OX-39, OX-40, and wild type (WT) lines. After 10 days, the length of upper and lower buds of the WT plant were 5.67 and 1.04 mm, respectively, whereas the upper buds of OX-39 and OX-40 were 8.11 and 7.06 mm, respectively, and their lower buds were 2.01 and 1.67 mm, respectively (Figure 5B,C). These results indicate that CmSWEET17 served a role in the promotion of the axillary bud outgrowth in C. morifolium.
3.5. Effect of CmSWEET17 Overexpression on Auxin Export from Buds
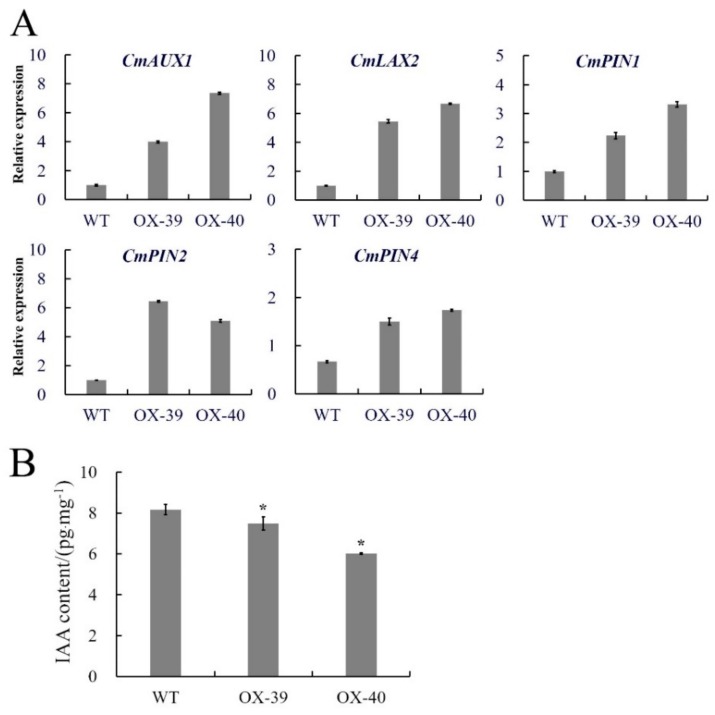
In order to explore the possible pathway by which CmSWEET17 promotes axillary bud outgrowth, we performed quantitative analyses on the axillary buds from overexpression lines following two-node stem treatment. The results showed that the expression levels of auxin transporter genes CmAUX1, CmLAX2, CmPIN1, CmPIN2, and CmPIN4 in the upper buds of the OX-39 and OX-40 lines were increased as compared with those in the WT plants (Figure 6A). Further determination of IAA content revealed that the levels in the WT, OX-39, and OX-40 lines were 8.16, 7.49, and 6.02 pg.mg−1, respectively, indicating a significant decrease of auxin content in the overexpression lines (Figure 6B). These data imply that CmSWEET17 overexpression may induce auxin export from buds by upregulating the expression of auxin transporter protein, thus, promoting axillary bud outgrowth.
Figure 6.
Differences in transgenic CmSWEET17 overexpression lines. (A) Relative gene expression and (B) IAA content analysis of WT and overexpression lines (OX-39 and OX-40). * p ≤ 0.05 vs. WT. WT, wild-type and IAA, indole-3-acetic acid.
4. Discussion
Sucrose is a critical energy and signaling molecule in plants, playing an important role in a number of processes related to growth and development [39]. Previous studies of our group have demonstrated that 20 mM sucrose promotes the upper bud outgrowth in the two-node stem system in C. morifolium [35]; therefore, we speculated that sugar-related genes are involved in this process. Using the existing transcriptome library, the expression levels of sugar-related genes HXK, SnRK1, TOR, SUCs, and SWEETs were measured, revealing that CmSWEET17 was highly expressed in the growing apical buds (Figure 1). Furthermore, exogenous sucrose treatment led to an increase in the expression of CmSWEET17 in both the leaves and axillary buds of C. morifolium (Figure 2). In A. thaliana, SWEET17, a fructose transport protein, was found to respond to the absence of light and fructose [33]. Likewise, in C. morifolium, the expression of CmSWEET17 responded to sucrose. This suggests that CmSWEET17 could be involved in the regulation of sucrose-induced promotion of axillary bud outgrowth in C. morifolium, which is positively correlated with the axillary bud growth.
Two types of mechanisms are known to be involved in the unloading of sucrose in sink tissues, the symplasmic and apoplasmic pathways. In the symplasmic pathway, sucrose is transported directly to the sink cells through plasmodesmata, without requiring transporters and energy consumption. Conversely, in the apoplasmic pathway, sucrose is first degraded into monosaccharides (fructose and glucose), then transported to parenchyma cells by monosaccharide transport proteins, and finally into the sink cells through plasmodesma. CmSWEET17, a fructose transport protein, therefore, could be involved in the apoplasmic pathway of sucrose unloading in sink tissues, a hypothesis which requires further study.
Bioinformatics analysis of the CmSWEET17 protein revealed that it contains six TM domains (Figure 3B), while the equivalent SWEET17 protein in other species contains seven [31,33]. Moreover, studies in other species have found that SWEET17 is localized mainly on the vacuole membrane [31,32], whereas the results of this study revealed that, in C. morifolium, CmSWEET17 is localized on the cell membrane (Figure 4). Whether this difference in localization is related to the absence of a TM domain in the structure of CmSWEET17 remains unknown.
Transgenic experiments were conducted to verify whether CmSWEET17 is involved in the regulation of axillary bud outgrowth. After obtaining CmSWEET17 overexpression lines (OX-39 and OX-40), the two-node stem system was treated with 20 mM sucrose. The results showed that axillary bud outgrowth was promoted in the OX-39 and OX-40 lines (Figure 5). In the rose, the growth of axillary buds was found to be positively correlated with sugar metabolism [40], and the expression of sucrose transporter RhSUC2 was shown to be increased during the progress of axillary bud outgrowth [41]. In A. thaliana, the regulation of shoot branching by sugar transporter protein 1 (AtSTP1) depended on extracellular sugar content [24]. A quantitative analysis in this study showed that the expression of auxin transport genes CmAUX1, CmLAX2, CmPIN1, CmPIN2, and CmPIN4 was increased in the axillary buds of the OX-39 and OX-40 lines (Figure 6A), suggesting in increase in auxin transport. Further determination of auxin content revealed that the levels decreased in the CmSWEET17 overexpression lines (Figure 6B). Export of auxin from axillary buds is key to their growth [42] and requires the participation of auxin transport proteins [18]. During the growth of plant axillary buds, expression of auxin transport genes, PIN1 and AUX1, was found to be increased [43,44]. Sugar has been regarded as the initial regulator to promote axillary bud outgrowth in recent years, and auxin is known to be involved in subsequent continuous growth stages [20,22]. In the rose and pea, sucrose could be the upstream signal of the key hormonal mechanisms controlling bud outgrowth. Sucrose has been shown to induce the expression of RhPIN1, leading to an increase in auxin export flow, promoting the growth of axillary buds [21]. The results of this study showed that, in C. morifolium, CmSWEET17 could promote the axillary bud outgrowth by the regulating auxin transport pathway. However, the exact mechanism by which CmSWEET17 regulates auxin transport to promote the axillary bud outgrowth requires further investigation.
5. Conclusions
In this study, CmSWEET17 was found to be upregulated in the upper axillary buds of the two-node stem system following treatment with 20 mM sucrose. The data provide a preliminary confirmation of a novel function of CmSWEET17 in promoting axillary bud outgrowth. Furthermore, this function could occur via the auxin transporter pathway, increasing the expression of auxin transporters, as well as auxin export from the axillary bud.
Acknowledgments
The authors thank Hengfu Yin (Research Institute of Subtropical Forestry, Chinese Academy of Forestry, Hangzhou, China) for revision of the manuscript, and Jijun Yan and Jinfang Chu (National Centre for Plant Gene Research, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China) for their expertise in determining the IAA contents of chrysanthemum.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/1/26/s1, Supplementary Figure S1: Sucrose promotes axillary bud growth in Chrysanthemum morifolium, Supplementary Figure S2: Gel electrophoresis with the products of PCR analysis of genomic DNA (primers pMDC43-F and CmSWEET17-R). Supplementary Table S1: Sequences of primers used in the present study.
Author Contributions
W.L., F.C., and J.J. conceived the study; W.L. and B.P. wrote the manuscript with contributions from W.L., B.P., and A.S.; F.C. and J.J. revised the manuscript. Culture and treatment of plant materials were conducted by B.P. and W.L.; bioinformatics analysis by A.S.; qRT-PCR and subcellular localization by B.P.; and the auxin measurements and chrysanthemum transformation by W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 31872149 and 31870649), Jiangsu Agricultural Industry Technology System (JATS[2018]278), Natural Science Fund of Jiangsu (BK2019076), Fundamental Research Funds for the Central Universities (KYZ201832) and the Priority Academic Program Development of Jiangsu Higher Education Institution.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Liang L., Zhao L., Challis R., Leyser O. Strigolactone regulation of shoot branching in chrysanthemum (Dendranthema grandiflorum) J. Exp. Bot. 2010;61:3069–3078. doi: 10.1093/jxb/erq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xi L., Wen C., Fang S., Chen X., Nie J., Chu J., Yuan C., Yan C., Ma N., Zhao L. Impacts of strigolactone on shoot branching under phosphate starvation in chrysanthemum (Dendranthema grandiflorum cv. Jinba) Front. Plant Sci. 2015;6:694. doi: 10.3389/fpls.2015.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dierck R., Leus L., Dhooghe E., Van Huylenbroeck J., De Riek J., Van Der Straeten D., De Keyser E. Branching gene expression during chrysanthemum axillary bud outgrowth regulated by strigolactone and auxin transport. Plant Growth Regul. 2018;86:23–36. doi: 10.1007/s10725-018-0408-2. [DOI] [Google Scholar]
- 4.Dierck R., De Keyser E., De Riek J., Dhooghe E., Van Huylenbroeck J., Prinsen E., Van Der Straeten D. Change in Auxin and Cytokinin Levels Coincides with altered expression of branching genes during axillary bud outgrowth in Chrysanthemum. PLoS ONE. 2016;11:e0161732. doi: 10.1371/journal.pone.0161732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoellhorn R.K., Barrett J.E., Bartusk C.A., Nell T. Elevated temperature affects axillary meristem development in Dendranthema × grandiflorum ‘Improved Mefo’. Hortscience. 2001;36:1049–1052. doi: 10.21273/HORTSCI.36.6.1049. [DOI] [Google Scholar]
- 6.Chen X., Zhou X., Xi L., Li J., Zhao R., Ma N., Zhao L. Roles of DgBRC1 in regulation of lateral branching in Chrysanthemum (Dendranthema ×grandiflora cv. Jinba) PLoS ONE. 2013;8:e61717. doi: 10.1371/journal.pone.0061717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan C., Ahmad S., Cheng T., Wang J., Pan H., Zhao L., Zhang Q. Red to far-red light ratio modulates hormonal and genetic control of axillary bud outgrowth in Chrysanthemum (Dendranthema grandiflorum ‘Jinba’) Int. J. Mol. Sci. 2018;19:1590. doi: 10.3390/ijms19061590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wahl V., Ponnu J., Schlereth A., Arrivault S., Langenecker T., Franke A., Feil R., Lunn J.E., Stitt M., Schmid M. Regulation of flowering Regulation of Flowering by Trehalose-6-Phosphate Signaling in Arabidopsis thaliana. Science. 2013;339:704–707. doi: 10.1126/science.1230406. [DOI] [PubMed] [Google Scholar]
- 9.Sun J., Wang H., Ren L., Chen S., Chen F., Jiang J. CmFTL2 is involved in the photoperiod- and sucrose-mediated control of flowering time in chrysanthemum. Hortic. Res. 2017;4:17001. doi: 10.1038/hortres.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skylar A., Sung F., Hong F., Chory J., Wu X. Metabolic sugar signal promotes Arabidopsis meristematic proliferation via G2. Dev. Biol. 2011;351:82–89. doi: 10.1016/j.ydbio.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y., Van den Ende W., Rolland F. Sucrose induction of anthocyanin biosynthesis is mediated by DELLA. Mol. Plant. 2014;7:570–572. doi: 10.1093/mp/sst161. [DOI] [PubMed] [Google Scholar]
- 12.Fichtner F., Barbier F.F., Feil R., Watanabe M., Annunziata M.G., Chabikwa T.G., Hofgen R., Stitt M., Beveridge C.A., Lunn J.E. Trehalose 6-phosphate is involved in triggering axillary bud outgrowth in garden pea (Pisum sativum L.) Plant J. 2017;92:611–623. doi: 10.1111/tpj.13705. [DOI] [PubMed] [Google Scholar]
- 13.Evers J.B. Sugar as a key component of the shoot branching regulation network. Plant Cell Environ. 2015;38:1455–1456. doi: 10.1111/pce.12519. [DOI] [PubMed] [Google Scholar]
- 14.Rabot A., Henry C., Baaziz K.B., Mortreau E., Azri W., Lothier J., Hamama L., Boummaza R., Leduc N., Pelleschi-Travier S., et al. Insight into the role of sugars in bud burst under light in the rose. Plant Cell Physiol. 2012;53:1068–1082. doi: 10.1093/pcp/pcs051. [DOI] [PubMed] [Google Scholar]
- 15.Decourteix M., Alves G., Bonhomme M., Peuch M., Baaziz K.B., Brunel N., Guilliot A., Rageau R., Améglio T., Pétel G. Sucrose (JrSUT1) and hexose (JrHT1 and JrHT2) transporters in walnut xylem parenchyma cells-their potential role in early events of growth resumption. Tree Physiol. 2008;28:215–224. doi: 10.1093/treephys/28.2.215. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi H., Imamura T., Konno N., Takeda T., Fujita K., Konishi T., Nishihara M., Uchimiya H. The gentio-oligosaccharide gentiobiose functions in the modulation of bud dormancy in the herbaceous perennial Gentiana. Plant Cell. 2014;26:3949–3963. doi: 10.1105/tpc.114.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thimann K.V., Skoog B.F., Kerckhoff W.G. On the Inhibition of Bud Development and other Functions of Growth Substance in Vicia Faba. Proc. R. Soc. Lond. Ser. B Contain. Pap. A Biol. Character. 1934;114:317–319. doi: 10.1098/rspb.1934.0010. [DOI] [Google Scholar]
- 18.Domagalska M.A., Leyser O. Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 2011;12:211–221. doi: 10.1038/nrm3088. [DOI] [PubMed] [Google Scholar]
- 19.Balla J., Kalousek P., Reinöhl V., Friml J., Procházka S. Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. Plant J. 2011;65:571–577. doi: 10.1111/j.1365-313X.2010.04443.x. [DOI] [PubMed] [Google Scholar]
- 20.Mason M.G., Ross J.J., Babst B.A., Wienclaw B.N., Beveridge C.A. Sugar demand, not auxin, is the initial regulator of apical dominance. PNAS. 2014;111:6092–6097. doi: 10.1073/pnas.1322045111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbier F., Péron T., Lecerf M., Perez-Garcia M.D., Barrière Q., Rolčík J., Boutet-Mercey S., Citerne S., Lemoine R., Porcheron B. Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J. Exp. Bot. 2015;66:2569–2582. doi: 10.1093/jxb/erv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chabikwa T.G., Brewer P.B., Beveridge C.A. Initial Bud Outgrowth Occurs Independent of Auxin Flow from Out of Buds. Plant Physiol. 2019;179:55–65. doi: 10.1104/pp.18.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruan Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014;65:33–67. doi: 10.1146/annurev-arplant-050213-040251. [DOI] [PubMed] [Google Scholar]
- 24.Otori K., Tanabe N., Tamoi M., Shigeoka S. Sugar Transporter Protein 1 (STP1) contributes to regulation of the genes involved in shoot branching via carbon partitioning in Arabidopsis. Biosci. Biotechnol. Biochem. 2019;83:472–481. doi: 10.1080/09168451.2018.1550355. [DOI] [PubMed] [Google Scholar]
- 25.Xuan Y.H., Hu Y.B., Chen L.Q., Sosso D., Ducat D.C., Hou B.H., Frommer W.B. Functional role of oligomerization for bacterial and plant SWEET sugar transporter family. PNAS. 2013;110:E3685–E3694. doi: 10.1073/pnas.1311244110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L.Q., Hou B.H., Lalonde S., Takanaga H., Hartung M.L., Qu X.Q., Guo W.J., Kim J.G., Underwood W., Chaudhuri B. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature. 2010;468:527–532. doi: 10.1038/nature09606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L.Q., Qu X.Q., Hou B.H., Sosso D., Osorio S., Fernie A.R., Frommer W.B. Sucrose Efflux Mediated by SWEET Proteins as a Key Step for Phloem Transport. Science. 2012;335:207–211. doi: 10.1126/science.1213351. [DOI] [PubMed] [Google Scholar]
- 28.Lin I.W., Sosso D., Chen L.Q., Gase K., Kim S.G., Kessler D., Klinkenberg P.M., Gorder M.K., Hou B.H., Qu X.Q. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature. 2014;508:546–559. doi: 10.1038/nature13082. [DOI] [PubMed] [Google Scholar]
- 29.Kanno Y., Oikawa T., Chiba Y., Ishimaru Y., Shimizu T., Sano N., Koshiba T., Kamiya Y., Ueda M., Seo M. AtSWEET13 and AtSWEET14 regulate gibberellin-mediated physiological processes. Nat. Commun. 2016;7:13245. doi: 10.1038/ncomms13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han L., Zhu Y., Liu M., Zhou Y., Lu G., Lan L., Wang X., Zhao Y., Zhang X.C. Molecular mechanism of substrate recognition and transport by the AtSWEET13 sugar transporter. PNAS. 2017;114:10089–10094. doi: 10.1073/pnas.1709241114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou A., Ma H., Feng S., Gong S., Wang J. DsSWEET17, a tonoplast-localized sugar transporter from Dianthus spiculifolius, affects sugar metabolism and confers multiple stress tolerance in Arabidopsis. Int. J. Mol. Sci. 2018;19:1564. doi: 10.3390/ijms19061564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chardon F., Bedu M., Calenge F., Klemens P.A.W., Spinner L., Clement G., Chietera G., Léran S., Ferrand M., Lacombe B. Leaf fructose content is controlled by the vacuolar transporter SWEET17 in Arabidopsis. Curr. Biol. 2013;23:697–702. doi: 10.1016/j.cub.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Guo W.J., Nagy R., Chen H.Y., Pfrunder S., Yu Y.C., Santelia D., Frommer W.B., Martinoia E. SWEET17, a facilitative transporter, mediates fructose transport across the tonoplast of Arabidopsis roots and leaves. Plant Physiol. 2014;164:777–789. doi: 10.1104/pp.113.232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L. Ph.D. Thesis. Chinese Academy of Forestry; Beijing, China: 2016. Functional analysis of the sugar transporters SWEETs and SUT4 in Populus. [Google Scholar]
- 35.Liu W. Ph.D. Thesis. Nanjing Agricultural University; Nanjing, China: 2019. Molecular Mechanism of sucrose regulates axillary bud growth in Chrysanthemum. [Google Scholar]
- 36.Qi Y., Liu Y., Zhang Z., Gao J., Guan Z., Fang W., Chen S., Chen F., Jiang J. The over-expression of a chrysanthemum gene encoding an RNA polymerase II CTD phosphatase-like 1 enzyme enhances tolerance to heat stress. Hortic. Res. 2018;5:37. doi: 10.1038/s41438-018-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao Y., Sun J., Cao P., Zhang R., Fu Q., Chen S., Chen F., Jiang J. Functional analysis of alternative splicing of the FLOWERING LOCUS T orthologous gene in Chrysanthemum morifolium. Hortic. Res. 2016;3:16058. doi: 10.1038/hortres.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang B., Chu J., Yu T., Xu Q., Sun X., Yuan J., Xiong G., Wang G., Wang Y., Li J. Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis. PNAS. 2015;112:4821–4826. doi: 10.1073/pnas.1503998112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wind J., Smeekens S., Hanson J. Sucrose: Metabolite and signaling molecule. Phytochemistry. 2010;71:1610–1614. doi: 10.1016/j.phytochem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Girault T., Abidi F., Sigogne M., Pelleschi-Travier S., Boumaza R., Sakr S., Leduc N. Sugars are under light control during bud burst in Rosa Sp. Plant Cell Environ. 2010;33:1339–1350. doi: 10.1111/j.1365-3040.2010.02152.x. [DOI] [PubMed] [Google Scholar]
- 41.Henry C., Rabot A., Laloi M., Mortreau E., Sigogne M., Leduc N., Lemoine R., Sakr S., Vian A., Pelleschi-Travier S. Regulation of RhSUC2, a sucrose transporter, is correlated with the light control of bud burst in Rosa Sp. Plant Cell Environ. 2011;34:1776–1789. doi: 10.1111/j.1365-3040.2011.02374.x. [DOI] [PubMed] [Google Scholar]
- 42.Barbier F.F., Lunn J.E., Beveridge C.A. Ready, steady, go! A sugar hit starts the race to shoot branching. Curr. Opin. Plant Biol. 2015;25:39–45. doi: 10.1016/j.pbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Bennett T., Sieberer T., Willett B., Booker J., Luschnig C., Leyser O. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr. Biol. 2006;16:553–563. doi: 10.1016/j.cub.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 44.Kalousek P., Buchtová D., Balla J., Reinöhl V., Procházka S. Cytokinins and polartransport of auxin in axillary pea buds. Acta Univ. Agric. Et Silvic. Mendel. Brun. 2010;58:79–88. doi: 10.11118/actaun201058040079. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.