Abstract
Efficient ciliary locomotion and transport require the coordination of motile cilia. Short-range coordination of ciliary beats can occur by biophysical mechanisms. Long-range coordination across large or disjointed ciliated fields often requires nervous system control and innervation of ciliated cells by ciliomotor neurons. The neuronal control of cilia is best understood in invertebrate ciliated microswimmers, but similar mechanisms may operate in the vertebrate body. Here, we review how the study of aquatic invertebrates contributed to our understanding of the neuronal control of cilia. We summarize the anatomy of ciliomotor systems and the physiological mechanisms that can alter ciliary activity. We also discuss the most well-characterized ciliomotor system, that of the larval annelid Platynereis. Here, pacemaker neurons drive the rhythmic activation of cholinergic and serotonergic ciliomotor neurons to induce ciliary arrests and beating. The Platynereis ciliomotor neurons form a distinct part of the larval nervous system. Similar ciliomotor systems likely operate in other ciliated larvae, such as mollusc veligers. We discuss the possible ancestry and conservation of ciliomotor circuits and highlight how comparative experimental approaches could contribute to a better understanding of the evolution and function of ciliary systems.
This article is part of the Theo Murphy meeting issue ‘Unity and diversity of cilia in locomotion and transport’.
Keywords: ciliary band, ciliary swimming, marine larva, calcium, Platynereis, evolution
1. Introduction
Ciliary locomotion occurs in the majority of unicellular eukaryotes [1,2] and is also widespread in animals. Animals can either swim or glide with cilia, both at larval stages and as adults. There is a great diversity in the mode of movement, the type of ciliation and the tissue-scale dynamics of cilia. Ciliary swimming is most common in the larval stages of marine invertebrates. The majority of bottom-dwelling marine invertebrate animals have a ciliated larval stage. These animals undergo a planktonic-to-benthic transition as part of their biphasic life cycle [3]. Ciliary gliding is often found in adult forms such as flatworms or placozoans where ciliary activity co-occurs with muscle-based or epithelial contractility [4,5]. Many animals also use cilia to generate feeding currents to capture food particles. Planktonic ciliary swimmers that also feed with cilia can display the most complex ciliary dynamics and have trade-offs between swimming and feeding [6].
In ciliary swimmers, gliders and feeders, the activity of cilia can change in response to environmental cues and is generally under nervous system control. For example, many ciliary swimmers can change their trajectory to move towards a light source by phototaxis [7]. Circadian or sensory-induced adjustments in ciliary beating allow planktonic organisms to regulate their depth in the water column [8]. There are several other contexts where ciliated fields and the flows they generate are important for animal physiology, including the establishment of symbiosis in squid [9], mixing the boundary layer in corals [10] or the movement of cerebrospinal fluid in the vertebrate brain [11]. All these activities require the coordination of multiple cilia across large ciliary fields, sometimes spanning the entire body. Here, we focus on the anatomical and functional organization of ciliary locomotor and feeding systems in invertebrates. We discuss different phenomena of ciliary coordination in ciliary bands and epithelia and the mechanisms of nervous system control. In some cases, large neurons known as ciliomotor neurons that innervate multiple ciliated cells are used to coordinate ciliary activity throughout an organism. The recently characterized whole-body ciliomotor circuit of the marine annelid Platynereis dumerilii [12] highlights the sophistication of a dedicated ciliomotor circuit. In Platynereis larvae, large biaxonal neurons form a morphologically and functionally distinct ciliomotor nervous system coordinating whole-body ciliary activity. We review the evidence suggesting that other ciliated larvae also have dedicated circuitry for the control of cilia. Future comparative studies could test the hypothesis that ciliomotor nervous systems have a unique evolutionary history with potentially deep origin in animal evolution [13,14].
2. Types of ciliary locomotor and feeding systems in invertebrates
Ciliary systems occur either as uniformly ciliated body surfaces or as ciliary bands with more densely concentrated cilia that run around the body or along appendages (figures 1 and 2). Cilia in ciliary bands often emanate from specialized multiciliated cells, distinct from monociliated epithelial cells. Ciliary bands often have a dual role, enabling the animal to both swim and feed.
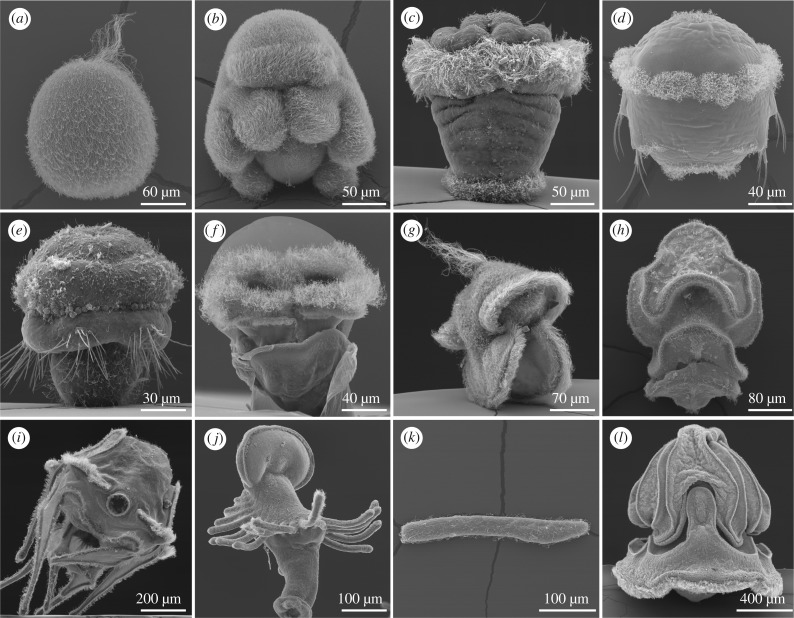
Figure 1.
The diversity of ciliated larvae. (a) Nematostella vectensis uniformly ciliated planula (cnidarian), (b) Mueller's larva of the flatworm Maritigrella crozieri, uniformly ciliated, (c) annelid trochophore with ciliary bands, (d) annelid trochophore with ciliary bands (P. dumerilii), (e) larva of the brachiopod Terebratalia transversa, (f) Aplysia californica, mollusc veliger with ciliary bands, (g) Lineus longissimus, nemertean pilidium larva, (h) starfish bipinnaria larva, (i) echinoderm 8-arm-larva (sea urchin), (j) phoronid actinotroch larva, (k) amphioxus chordate larva and (l) Schizocardium californicum hemichordate tornaria.
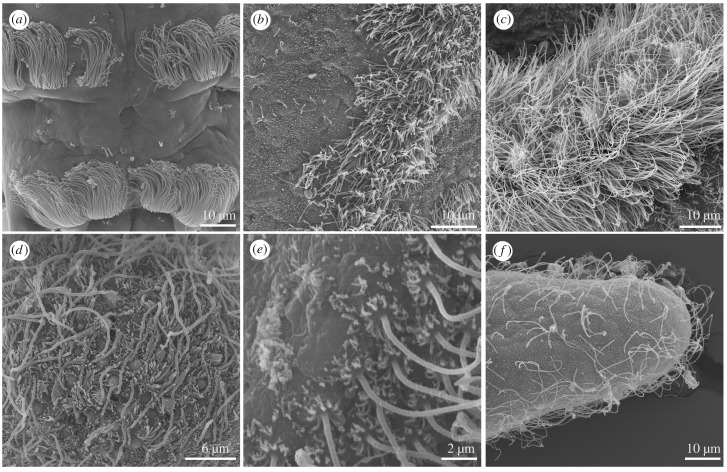
Figure 2.
Mono- and multiciliated surfaces. (a) Annelid multiciliated cells of the ciliary band (P. dumerilii). (b) Multiciliated cells on a hemichordate larva. (c) Multiciliated cells on a nemertean pilidium larva. (d) Monociliated epithelium in the planula of N. vectensis. (e) Monociliated cells on echinoderm larval arms. (f) Monociliated cells in an amphioxus larva.
(a). Locomotor cilia
Locomotor cilia occur in both larval and adult stages of invertebrates. Larval ciliary swimmers are present in many sponges and cnidarians, most spiralians, echinoderms, hemichordates and cephalochordates [15]. Ciliary swimming in adults is present in ctenophores, some flatworms and rotifers. Ciliary gliding is characteristic of placozoans and also occurs in some species of annelids, flatworms, nemerteans, gastrotrichs, gnathostomulids, gastropods and xenacoelomorphs [16–18].
There is a great diversity in the patterns of ciliation and the mode of ciliary beating across animals (figure 1). Locomotor cilia can occur either on ciliated epithelia (e.g. placozoans, flatworms, sponge, cnidarian and cephalochordate larvae) or organized in discrete ciliary bands (most lophotrochozoan and echinoderm larvae, ctenophore combs). The ciliated cells can either have one (sponges, cnidarians, the annelid Owenia, echinoderms) or multiple cilia (most lophotrochozoan larvae, sponge trichimella larvae) (figure 3). Both types have a broad phyletic distribution and it is currently unclear if multiciliation evolved multiple times independently. The molecular pathways driving centriole amplification in multiciliated cells are well understood, and it was experimentally demonstrated that changes in the levels of expression of genes involved in centriole amplification can induce multiciliation [20]. It may be that the fine-tuning of these pathways led to the repeated emergence of multiciliation during evolution.
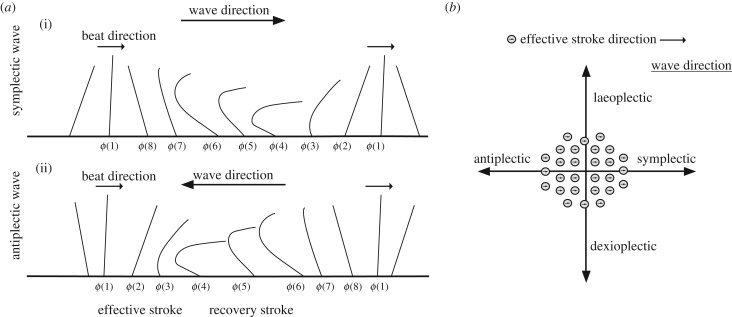
Figure 3.
Types of metachrony. (a) Side view of a row of beating cilia. Symplectic metachronal waves (i) propagate in the same, while antiplectic waves (ii) propagate in the direction opposite to the direction of the effective stroke. (b) Top view of a bundle of cilia. Metachronal waves can propagate orthogonally to the beat plane. Laeoplectic waves propagate to the left, and dexioplectic to the right relative to the effective stroke of the cilia. Based on [19].
Cilia can be simple or compound, with compound cilia linked by filamentous bridges and able to support a larger body size and greater swimming speed [21]. Among animals, compound cilia occur in ctenophores, the largest ciliary swimmers [17]. The compound cilia in ctenophore comb plates are structurally complex, with multiple cilia grouped in bundles and adjacent cilia connected by a unique structure, the compartmenting lamella [22]. Compound cilia also occur in some single-celled ciliates like Stentor [23]. Table 1 summarizes the types of ciliation and ciliary movement across animals.
Table 1.
| animal group | Placozoa | sponges, cnidarians, flatworms, ectoprocts, cephalochordates | some bryozoan larvae | echinoderms, phoronids, brachiopods | molluscs, some bryozoans and rotifers, the annelid Chaetopterus | most spiralian phyla, some rotifers, tunicates | Ctenophores |
|---|---|---|---|---|---|---|---|
| ciliated surface | ciliated epithelia | ciliated epithelia | ciliated epithelia | ciliary bands | ciliary bands | ciliary bands | ciliated comb plates |
| ciliated cells | monociliated | monociliated | multiciliated | monociliated | multiciliated | multiciliated | multiciliated |
| type of cilia | separate | separate | separate | separate | separate | separate | compound |
| type of ciliary movement | uncoordinated | dexioplectic metachronal waves | dexioplectic metachronal waves | dexioplectic metachronal waves | laeoplectic metachronal waves | dexioplectic metachronal waves | antiplectic metachronal waves |
(b). Cilia in suspension feeding
Suspension feeding is widespread among larval and adult aquatic animals. Many animals have specialized ciliated structures like arms and tentacles to aid feeding, including the larvae of echinoderms, enteropneusts and lophophorates (brachiopods, phoronids, ectoprocts). Larval ascidians do not have ciliated feeding structures, but adults feed by filtering food particles through the branchial basket [25].
Feeding ciliary systems overlap with locomotory systems in some planktonic larvae with ciliary bands. There are two main suspension-feeding systems in these larvae: the upstream and downstream collecting systems. Larvae with one ciliary band use an upstream collecting system that concentrates food particles upstream of the ciliary band. Larvae with multiple ciliary bands rely on a downstream collecting system, also known as opposed-band feeding, where food particles are collected downstream of the main ciliary band [26]. Some planktotrophic pilidium larvae of nemerteans have ciliary bands, but they use muscular contractions of the lappet to induce local flexures of the ciliary band that efficiently funnel algae into the mouth [27].
(c). Swimming–feeding trade-off
It has been suggested that larval forms, behaviours and preferred habitats result in part from a trade-off that exists between swimming and feeding. Feeding and swimming efficiencies depend largely on the length of cilia and the size of the ciliary bands [28]. Echinoderms, hemichordates and lophophorates have long ciliated arms or lobes and an upstream collecting system. In the case of these groups, the decreased feeding efficiency of short (20–25 µm) cilia on monociliated cells is compensated for by an extension in the size of the ciliary band. On the other hand, cilia on multiciliated cells are longer, have faster effective strokes and permit their carriers to feed using opposing flow currents between the opposing ciliary bands [28].
In the bipinnaria larva of Patiria miniata, a starfish that uses only one ciliary band for both swimming and feeding (upstream collecting system), it was demonstrated that the cilia can change stroke direction, generating different complex patterns of vortices depending on whether the larva swims or feeds [6].
3. Ciliary coordination by biophysical and cellular mechanisms
For directional movement, changes in motion and efficient filter feeding, the activity of beating cilia needs to be coordinated and regulated. Ciliary coordination can occur at different scales, from local coordination of adjacent cilia to the coordination of cilia on distant parts of the body (e.g. segmental ciliary bands). The coordination is owing to biophysical, cellular and neuronal mechanisms.
(a). Metachronal waves
Most ciliated fields display metachronal waves, which are more efficient than non-metachronal beating in terms of energetics and flow generation [29,30]. Metachronal waves have an important contribution to swimming dynamics. The waves contribute to flow generation and could thus in principle exert a torque (turning force) on a swimming body. In addition, torque can also be generated by the azimuthal offset of the cilia [31]. The torque, together with the posterior-directed flow from effective ciliary strokes, generates the helical swimming trajectory characteristic of most larvae [32]. In helically swimming larvae, the direction of body rotation is usually opposite to the direction of wave propagation [24]. Understanding the generation of the different types of waves is an important future challenge for understanding ciliary coordination and swimming mechanics.
The direction of wave propagation relative to the effective ciliary stroke distinguishes four major forms of metachrony (figure 3). Symplectic waves propagate in the direction of the effective stroke and antiplectic waves in the opposite direction. Diaplectic waves are perpendicular to the effective stroke and can propagate either to the left (laeoplectic) or to the right (dexioplectic) [24]. In ciliary bands, the most common form of metachrony is dexioplectic, although some molluscs, bryozoans and larvae of the annelid Chaetopterus show laeoplectic waves [24]. Other exceptions include placozoans, where ciliary beating seems to be uncoordinated [15,16], and ctenophore comb cilia where the waves are antiplectic [15,16] (table 1).
Flow-based hydrodynamic coupling of adjacent cilia of the same ciliary band or the same ciliated epithelium contributes to the generation of metachronal waves. Mathematical models of ciliary beating and coordination are able to recapitulate metachronal synchronization [33,34]. In the unicellular green alga Chlamydomonas reinhardtii, basal-body coupling also contributes to ciliary coordination [35], but it is unclear whether this mechanism also occurs in ciliary bands in animals. In the comb plates of ctenophores, there is an additional level of short-range coordination, whereby adjacent cilia are directly coupled by filamentous bridges [17,22].
(b). Gap junctions
In some ciliated surfaces, there are gap junctions facilitating electrical coupling between ciliated cells. This may allow the fast propagation of signals leading to the coordination of ciliary activity across cells [36]. In the tunicates Oikopleura [37] and Corella [38], water flow into the adult animal is aided by the beating cilia of the branchial sac. Some, but not all, of the ciliated cells are innervated, and gap junctions between the ciliated cells ensure rapid signal propagation and coordinated beating [38,39]. Gap junctions have also been identified via electron microscopy between velar ciliated cells in mollusc larvae [36] and comb plate ciliated cells of ctenophores [40].
4. Neuronal and paracrine mechanisms of ciliary coordination
Long-range ciliary coordination has been observed between different ciliary bands in many organisms. The coordination can extend to three different aspects of ciliary activity that cannot be fully accounted for by hydrodynamic coupling and gap junctions: simultaneous ciliary reversals, arrests and frequency changes [41]. In several instances, it has been noted that these events are influenced by neurotransmitters and neuropeptides and accompanied by calcium-dependent action potentials. Ciliary bands are innervated in many animals, and the activity of ciliomotor neurons, where demonstrated, controls the phenomena of long-range ciliary coordination. Below we discuss the types of phenomena where long-range ciliary coordination has been observed. We also discuss the neuronal or paracrine mechanisms that have been suggested to ensure coordination.
(a). Coordination of ciliary closures
Coordinated closures have been observed in the ciliary bands of annelids [12,42], molluscs [36,43,44] and echinoderms [45], in the ciliated epithelia of placozoans [16], in the gill bar cilia of amphioxus [46] and in the branchial basket cilia of juvenile and adult tunicates [25,47]. The extent and duration of ciliary arrests can be varied and depend on the species and the developmental stage [48].
Alternating phases of spontaneous ciliary closures and beating control swimming depth in planktonic larvae [49]. Ciliary arrests also occur as part of startle and avoidance responses to mechanical stimuli [50,51] and in response to chemical stimuli, including settlement cues [43,52]. In hemichordates and echinoderms, mechanical stimulation leads to ciliary reversal or stoppage [45,51,53]. In the neuron-less placozoan Trichoplax adhaerens, the gliding movement halts when encountering food, likely owing to a pause in the activity of cilia [16].
The signalling mechanisms of ciliary closures have been studied in pharmacological, electrophysiological, calcium imaging and cell ablation experiments. Electrophysiological recordings revealed that ciliary closures are accompanied by bursts of membrane depolarization in the ciliated cells of larval annelids [8,49], molluscs [36,54], echinoderms [55] and the branchial baskets of adult tunicates [25]. The depolarizations lead to an increase in the concentration of intracellular calcium, as shown by calcium imaging in larval Platynereis [12].
Neurons that drive these ciliary depolarizations have been identified in larval Platynereis [12] and in the central ganglion of adult tunicates [25,38]. These two examples are also telling of the molecular mechanisms driving arrests.
Studies on Platynereis uncovered that the rate of change of intracellular calcium, and not absolute concentration, triggers closures. As long as the calcium concentration in the ciliated cells is increasing, the cilia remain arrested. Ciliary beating resumes when the calcium concentration starts decreasing [12]. The dependence of ciliary activity on the rate of calcium change was also shown to be important during sperm chemotaxis, suggesting a similar mechanism of adaptive signalling [56].
More information about the second messenger cascades involved in triggering ciliary closures came from pharmacological experiments in the tunicate Ciona intestinalis. In the Ciona branchial basket cilia, the calcium-dependent arrests are modulated by a pathway involving cAMP. It was shown that an increase in cAMP concentration reactivates the arrested cilia, which suggests there are antagonistic effects of calcium and cAMP [47].
While the details of signalling mechanisms driving ciliary closures remain largely unknown, some information is available about the neurotransmitters and neuromodulators that induce them. In larval Platynereis [8,12], cholinergic neurons were shown to induce closures. Pharmacological experiments in molluscs [44], the annelid Spirobranchus [42] and hemichordates [51] indicate that acetylcholine and probably also catecholamines may be responsible for inducing ciliary closure, while serotonin inhibits closures. In most of these experiments, it is difficult to distinguish direct neurotransmitter effects on the ciliated cells from potential indirect effects, for example, on presynaptic pacemaker systems.
Secreted peptides can also have an effect on ciliary closures. In Trichoplax, the coordinated ciliary pauses may be owing to diffusible neuropeptide-like molecules [57]. Treatment of Platynereis larvae with synthetic neuropeptides revealed that several peptides can induce or inhibit ciliary arrests [49,58]. The site of action of these neuropeptides is not known, but they may modulate the ciliomotor pacemaker circuit in these larvae [12]. Neuropeptides can modulate pacemaker systems as demonstrated, for example, in the crustacean somatogastric ganglia [59,60].
(b). Coordination of ciliary reversals
Ciliary reversals, or reversals of the direction of the effective stroke of ciliary beating, have been observed in ctenophores and some deuterostomes (echinoderms and tunicates).
In ctenophores, ciliary reversals occur during prey capture [61]. Upon contact with prey, the ctenophore comb cilia briefly stop beating (quiescence). Quiescence is followed by a unilateral ciliary reversal in the ctene rows that were catching the prey. Reversals can also be induced by electrical, mechanical or chemical stimulation of some larval ctenophores [62]. Reversals were demonstrated to be calcium-dependent and triggered by voltage-dependent calcium channels [17,62].
In echinoderm larvae, contact with food particles leads to brief local ciliary reversals in the ciliary band [37,63]. Larger-scale, coordinated reversals are observed as an avoidance response upon contact with obstacles and they lead to the animal swimming backwards [45]. The reversals are accompanied by action potentials [55] and involve cholinergic and catecholaminergic neurotransmission [45,64,65]. Pharmacological experiments implicate an ionotropic (nicotinic) acetylcholine receptor in stimulating the avoidance response-related reversals [45]. However, specific ciliomotor neurons mediating this behaviour have not yet been identified.
In the branchial basket of the tunicate Oikopleura, coordinated reversals of ciliary beat in two ciliated rings induce a reversal of the water current through the pharynx [37]. The reversals are accompanied by membrane depolarizations of the ciliated cells. This happens spontaneously, as well as in response to mechanical or electrical stimulation. It is presumed that reversals increase in instances of greater particle density in natural conditions. The ciliated cells of Oikopleura are innervated with peripheral nerves. As spontaneous reversals continue after the removal of the brain, it was suggested that a peripheral pacemaker system exists to induce them [37].
(c). Control of ciliary beat frequency
Similar to ciliary closures, ciliary beat frequency (CBF) can be modulated by neurotransmitters and neuropeptides to control swimming speed or feeding behaviour. Serotonin and dopamine are the two transmitters most commonly associated with a change in CBF. Serotonin generally increases CBF and inhibits closures. Dopamine most commonly decreases CBF, with a few exceptions.
Serotonin is the most common cilioexcitatory neurotransmitter in aquatic embryos and larvae. In encapsulated embryos of the gastropod Helisoma, specific serotonergic neurons mediate hypoxia-induced increases in CBF [66,67]. This induces rapid rotations of the embryos, and more efficient oxygen diffusion owing to increased stirring. This serotonin-mediated response acts through G-protein-coupled receptors. One receptor signals through the Gq pathway, leading to increases in intracellular Ca2+ [68]. The hypoxia response is also accompanied by increased cAMP levels in the ciliated cells, mediated by another, Gs-coupled serotonin receptor [69]. The different serotonin receptors may have a function during different phases of the behavioural response [69].
Similar cilioexcitatory effects of both serotonin and cAMP were demonstrated in pharmacological experiments in annelids [12,49] and echinoderms [70,71]. Serotonin treatments also lead to increased CBF in mollusc velligers [44] and echinoderm plutei [72]. In a rare example of surface ciliation in a vertebrate, the CBF of Xenopus laevis epidermal larval cilia is controlled by serotonin secreted from specialized epidermal cells binding to the ionotropic 5-HT3 receptor on ciliated cells [73]. Serotonin was found to have cilioexcitatory effects in other vertebrate tissues as well, including the mouse trachea [74] and rat ependymal cells, where the cilioexcitatory effects are calcium-dependent [75].
Dopamine was demonstrated to decrease CBF in pharmacological experiments on echinoderm plutei and bipinnariae [53,70], mollusc veligers [44] and annelid trochophores [42]. In all these species, dopamine treatment also induces more frequent ciliary closures. As an exception, in the embryos of the snail Lymnaea, dopaminergic neurons seem to induce CBF increases during the hypoxia response [67]. In sea urchin embryos, dopamine increases swimming speed likely through a cilioexcitatory effect [70,76]. Experiments in echinoderms suggest a role for acetylcholine, adrenaline and noradrenaline in decreasing CBF [53,70].
In addition to neurotransmitters, neuropeptides also exhibit stimulatory and inhibitory effects on CBF. In Platynereis, 9 of 11 neuropeptides tested were found to have a cilioexcitatory effect, while the remaining two neuropeptides reduced CBF [49]. Neuropeptide antibody stainings have revealed peptidergic nerves along ciliary bands in several larvae. RFamide-like neuropeptides are commonly detected along ciliary bands [44,67,77–80]. In Platynereis larvae, FMRFamide increases CBF and leads to higher positioning in the water column, while in the Crepidula fornicata veliger, it has the opposite effect [44]. In the nemertean Lineus longissimus, two neuropeptides (excitatory peptides 1 and 2) increase CBF [81]. While the influence of peptides on CBF has not been explored in vertebrates in great detail, it has been shown thus far that the melanin-concentrating hormone exhibits cilioexcitatory effects in the mouse ependymal cells [82].
The signalling cascades involved in coordinated changes in ciliary activity generally involve calcium as a second messenger. The diverse effects of calcium on ciliary activity may partly be owing to differences in calcium channels, signal location or dynamics, or interactions with other second messengers. For example, the fine-tuning of ciliary closure dynamics is achieved through an antagonism between calcium and cAMP signalling in Ciona [47]. CBF is generally regulated by cAMP (e.g. [49,71]) and may interact with calcium to fine-tune responses. In addition, the rate of change in calcium concentration can also be important [12,56]. Finally, different processes rely on different calcium channels. Ciliary reversals are mediated by voltage-dependent calcium channels [62]; CBF changes can be triggered through Gq signalling and the inositol trisphosphate receptor [68].
5. Innervation of ciliary bands
The phenomena of long-range ciliary coordination discussed above are commonly under neuronal control.
The most unambiguous data about the innervation of larval ciliary bands are available from electron microscopy studies. Electron microscopy enables the identification of neurons forming synapses on ciliated cells. Synapses from nerves running along ciliary bands or ciliated epithelia have been described in ctenophores [62], the larvae of platyhelminths [83], annelids [12,48,84], molluscs [54], nemerteans [85] and echinoderms [53].
The axons of neurons that synapse on ciliated cells run along the ciliary bands. In some cases, these nerves form a distinct ciliomotor nervous system that is clearly distinguishable from the central nervous system. The best example of a distinct ciliomotor nervous system can be found in the Mueller's larva of the polyclad flatworm Pseudoceros canadensis, which has a unique intraepithelial nervous system associated with the ciliary band [83]. The ciliomotor nervous system is separated from the central nervous system by the basement membrane and there are only two points of contact between the two systems. Many of the cells of the ciliomotor nervous system are bipolar sensory cells with sensory dendrites among the cells of the ciliary band. Pilidium larvae of nemerteans also have a distinct ciliomotor nervous system. In these larvae, the main ciliary band is innervated by the marginal nerve, the largest nerve in the body. Additional nerves connect the marginal nerve to the oral nerve that innervates the accessory oral ciliary bands [85,86].
Further knowledge about the innervation of ciliary bands comes from immunofluorescence or histological stainings. Serotonin immunoreactivity has been detected in the ciliary nerves in most groups of ciliated animals (table 2; [101]). Glyoxal-induced fluorescence imaging also shows catecholamine presence in the ciliary band nerves of nemerteans [86], annelids [108], phoronids [109], echinoderms [110,111] and enteropneusts [105]. Cholinergic innervation has been characterized in ciliary bands of echinoderms [112], enteropneusts [105], annelids [12] and molluscs [113].
Table 2.
Summary of ciliation and the neuronal control of cilia across metazoans. DA, dopamine; NPs, neuropeptides; PKC, protein kinase C.
| organism | species | developmental stage | ciliated cells | ciliary bands | innervation | signalling | neurotransmitters | neuropeptides | CBF (Hz) | arrests | sensory input |
|---|---|---|---|---|---|---|---|---|---|---|---|
| sponges | Amphimedon queenslandica [87,88] | parenchymella larva | all larvae have monociliated epithelial cells, except hexactinellid trichimella larvae (multiciliated) [89] | rows of cilia on larval surface except for pole | no neuropeptides found in the genome | negative phototaxis | |||||
| placozoans | Trichoplax adhaerens [16,57] | adult | monociliated | ciliated epithelium | none | none | FFNPa, ELPE, MFPF and WPPF cause cilia to pause and the animal to flatten, diverse effects | when feeding | food | ||
| ctenophores | various [17,62,90,91] | adult, cydippid larva | multiciliated, filamentous bridges between cilia facilitate mechanical coordination | 8 paired ciliated comb rows | beating usually initiated at the pacemaker balancer cilia in the aboral statocyst; synapses shown onto ciliated cells | elevated Mg levels abolish ciliary function, implying Ca-signalling | only Glu, no other classic neurotransmitters | several ctenophore-specific neuropeptides | 7 (Martensia ovumi), 14–15 (Beroe), 5–13 (Leucothea pulchra) | arrests upon stimulation; quiescence and ciliary reversals during prey capture | mechanical, chemical or electrical stimuli inhibit ciliary movement |
| cnidarians | Tripedalia cystophora [92] | planula | monociliated | ciliated epithelium | not known | ocelli (directional light signals) used for steering swimming | |||||
| molluscs | Calliostoma ligatum [36]; Phestilla sibogae [43,52]; Haliotis rufescens [93]; Crepidula fornicata [44] | veliger | multiciliated | velar cilia | Ca-dependent action potentials lead to arrest; settlement-induced arrests mediated through GABA; gap junctions between cilia | 5HT increases CBF, abolishes arrests; DA increases CBF | FMRFamide decreases CBF, larvae lower in water column | 5–7 Hz | spontaneous and induced | responds to dissolved settlement cues (prey extract) with arrests | |
| Helisoma trivolvis [66,94,95] | early embryo (no larval stage) | multiciliated | pedal and dorsolateral (prototrochal) ciliary bands | serotonergic sensory-motor ENC1 neurons; type 5 and 7 receptors in the foot ciliated cells | Ca-signalling through PKC | serotonin increases CBF | ENC1 sensory-motor neurons directly respond to hypoxia —acceleration in rotational swimming | ||||
| Lymnea stagnalis [67] | early embryo (no larval stage) | multiciliated | ciliated apical plate region, pedal and dorsolateral (prototrochal) ciliary bands | transient apical catecholaminergic (TAC) neurons | dopamine may act on D1 receptor | serotonin and dopamine increase CBF | FMRFamide in TAC neurons | ∼14 Hz in pedal cilia | dopaminergic and serotonergic neurons respond to hypoxia —acceleration in rotational swimming | ||
| annelids | P. dumerilii [12,49,50] | trochophore | multiciliated | prototroch and metatroch | full ciliomotor circuit reconstructed | Ca-dependent action potentials | 5HT increases CBF, catecholamines decrease it | RYa, FVMa, DLa, FMRFa, FVa, LYa, YFa, L11, and SPY increase CBF, FLa and WLD decrease CBF; RYa, FVMa, DLa, FMRFa and FVa reduce arrests, FLa, WLD and MIP increase arrest | ∼15 Hz | spontaneous and induced; 5HT decreases closure frequency | phototaxis, startle response, settlement-induced arrests |
| Capitella teleta [96] Spirobranchus giganteus [42]; Phyllodoce sp. [48] | trochophore | multiciliated | prototroch and metatroch | prototroch nerve | Ca-dependent | β-blockers (alprenolol) lead to arrest | DLamide, FVamide, RYamide immunoreactivity in apical organ neurons with projections to ciliary band | yes, partial in Phyllodoce | |||
| Owenia fusiformis, Owenia collaris [15] | mitraria larva | monociliated tentacle cells | primary ciliary band with 2 rows of cells, later also secondary ciliary band on posterior end | ||||||||
| nemerteans | Lineus albocinctus, Micrura purpurea [86]; L. longissimus [81]; Micrura alaskensis [27,97] | pilidium | multiciliated | marginal nerve (5HT); peptidergic (EP) nerves projecting from apical organ to the nerves underneath ciliary bands | 2 excitatory NPs (EP1, EP2) increase CBF | 9.6 Hz (apical) and 10.3 Hz (lateral ciliary band) | in response to feeding | arrests upon mechanosensory stimuli related to feeding | |||
| platyhelminths | P. canadensis [83]; M. crozieri [79] | Mueller's larva | multiciliated | ciliary band | ciliary nerve | 5HT immunoreactivity in ciliary band nerve | FMRFa immunoreactivity in ciliary band nerve | no | |||
| cycliophorans | Symbion pandora [98,99] | chordoid larva | multiciliated | 2 ventral anterior bands, ciliated body field, ciliated foot | no 5HT immunoreactivity in anterior ciliary bands | ||||||
| bryozoans (ectoprocts) | Fredericella sultana (phylactolaemate) [77] | larva | multiciliated | ciliated epidermis | 5HT and DA stimulate negative phototaxis | FMRFamide immunoreactivity in ciliated cells | phototaxis | ||||
| Flustrellidra hispida, Bugula fulva, Alcyonidium gelatinosum, and Bowerbankia gracilis (gymnolaemate) [78]; Cryptosula sp. [96]; Bugula neritina [100] | coronate larva | multiciliated | 1 ciliary band (corona) | FMRFamide immunoreactivity in ciliated cells, RYamide immunoreactivity in lateral cells projecting to ciliary band | |||||||
| entoprocts | various [101,102] | swimming-type larva | multiciliated | prototroch, metatroch, ciliated food groove and gastrotroch | prototroch nerve | no 5HT immunoreactivity along ciliary band | |||||
| phoronids | Phoronis muelleri [15,80] | actinotroch | multiciliated | preoral, postoral and tentacle ciliary bands; archaeotroch on posterior end; all monociliated | 5HT-like immunoreactivity in tentacles and archaeotroch | FMRFamide immunoreactivity in ciliated cells near neurophil | |||||
| rotifers | various [15,103] | adult | multiciliated | 3 ciliary bands: trochus, circumapical field and cingulum; pseudotrochus in other species | no known innervation | ||||||
| brachiopods | T. transversa [104] | larva | monociliated | ciliary bands | FMRFamide induces defence behaviour (sinking) | defence response to mechanical stimuli | |||||
| echinoderms | Psammechinus miliaris [70,71]; Pseudocentrotus depressus, Hemicentrorus pulcherrimus [70,72]; Lytechinus pictus [45] | pluteus | monociliated | 1, circumoral | excitatory role of cAMP, Ca involved in both excitation and inhibition; suggested that nicotinic AChR is involved | 5HT and beta-adrenergic agonists increase, DA decreases CBF, DA, adrenaline and cholinergic agents cause ciliary reversal and arrest | coordinated arrests and reversals | avoidance response | |||
| Pisaster ochraceus [53] | bipinnaria | monociliated | 1, circumoral | ciliary nerve; aminergic sensory cells | cholinergic agents, DA and adrenaline reduce beating | no, no reversals either | avoidance response (reduced ciliary beating) | ||||
| hemichordates | Balanoglossus biminiensis [51]; Balanoglossus proterogonius [105] | tornaria | monociliated cells in the two circumoral bands, multiciliated in teletroch | two circumoral bands, teletroch | innervated in part by fibres from the apical plate and adoral nerve centres; unknown teletroch innervation | cholinesterase activity in the epithelium along the length of the oral ciliary bands, but not in the telotroch; single catecholaminergic cells in postoral band and teletroch; cholinergic agents induce teletroch arrest | Yes (15–20 s). Some parts of the telotroch may stop beating while others continue | avoidance response | |||
| tunicates | Ciona intestinalis [47], Chelyosoma productum [25] | adult | multiciliated | around the stigmata of the branchial basket (stigmatal ciliary system) | ciliary arrest (CA) neurons (part of the visceral nerve of the central ganglia) directly controlling ciliary arrests | Ca-dependent action potentials lead to arrest; cAMP activates quiescent cilia | spontaneous or in response to mechanical, electrical, or chemical stim- ulation; 1–2 s in duration | ||||
| cephalochordates | Branchiostoma floridae | larva [106] | monociliated | ciliated epidermis; loss of cilia from 24 h post-fertilization | |||||||
| juvenile [46,107] | monociliated | gill bar lateral cilia | neuronal control confirmed; innervation by atrial nervous system | FMRFamide immunoreactivity in the atrial nervous system innervating the cilia |
It was shown through these tissue stainings that the ciliated velum of the mollusc veliger is innervated by bipolar and tripolar cholinergic neurons. Bipolar neurons were found at the base of the velum, connecting it with the cerebral ganglia [113].
6. The ciliomotor circuit in the Platynereis dumerilii larva
The most comprehensive characterization of ciliary band innervation comes from the reconstruction of the ciliomotor nervous system in the Platynereis nectochaete larva [12]. Here, all neurons that synapse on locomotor cilia have been reconstructed by serial electron microscopy (figure 4). The neurons form a distinct ciliomotor circuit with a function in the control of ciliary closures and beating. Most ciliomotor neurons are morphologically unique and have two axons emanating from the cell body. These neurons are the largest in the body, with very long axons, spanning the entire prototroch ciliary band or all segmental ciliary bands [12]. Through immunofluorescence, in situ hybridization and transgenesis, the Platynereis ciliary neurons have been classified into 11 cholinergic, five serotonergic and three mixed peptidergic–catecholaminergic neurons [12].
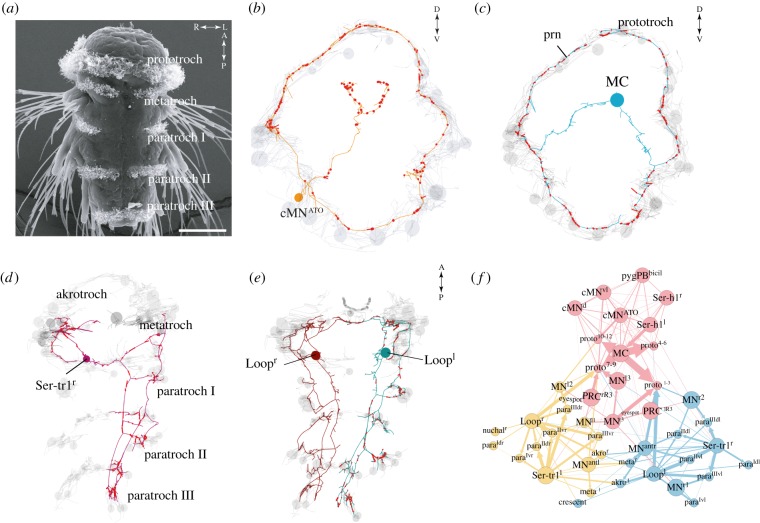
Figure 4.
The ciliomotor circuit of the Platynereis larva. (a) SEM of a Platynereis nectochaete (3 days old) larva with ciliary bands labelled. Scale bar 50 µm. (b) serial scanning transmission electron microscopy (ssTEM)-based reconstructions of one of three catecholaminergic neurons (anterior view) and (c) of the closure-inducing cholinergic MC neuron (anterior view) in the Platynereis ciliomotor circuit. Ciliated cells are shown in grey. (d) Reconstruction of the serotonergic Ser-tr1 and (e) cholinergic Loop ciliomotor neurons (ventral views). (f) Synaptic connectivity graph of all ciliomotor neurons and ciliary band cells.
The 3-day-old Platynereis larva (nectochaete) has multiple segmentally arranged ciliary bands, where the beating and closures show a rhythmic pattern and cross-band synchronization. Imaging of neuronal activity reported by the calcium sensor GCaMP6 showed that the activity of the serotonergic ciliomotor neurons correlates with ciliary beating, whereas cholinergic neurons are active during closures. Laser ablation of a major head cholinergic neuron (MC neuron, figure 4) abolished the rhythmic closures of the main ciliary band innervated by this neuron.
The ciliomotor circuit is under the control of a central pattern generator (CPG), the ciliomotor pacemaker. The three peptidergic–catecholaminergic neurons of the ciliomotor circuit activate rhythmically and likely form the pacemaker. Two of them are active during ciliary closures and one during the phases of beating. This rhythmically active circuit driving alternating phases of swimming and sinking (during closures) may enable the larvae to maintain a constant depth in the water column [49].
The activity of this pacemaker seems to be under the influence of different neuropeptides and hormones released in response to sensory cues or following a circadian rhythm. Several neuropeptides expressed in sensory–neurosecretory neurons in the larval brain influence larval vertical distribution through changing the ciliomotor rhythm (inhibiting or stimulating ciliary closures) [96]. A reduction in closures moves the larvae upwards in the plankton, whereas more frequent closures lead to sinking. Sensory cues may trigger neuropeptide release and concomitant changes in ciliary closures. For example, during larval settlement, chemical cues likely lead to a release of myoinhibitory peptides from chemosensory–neurosecretory neurons [114]. Exposing larvae to these peptides increases ciliary closures, which causes the larva to sink.
The frequency and duration of ciliary closures also change in a diurnal cycle, with more frequent closures occurring during nighttime. This effect may be mediated by melatonin signalling acting on cholinergic ciliomotor neurons [8].
Platynereis larvae also respond to vibrational stimuli by ciliary arrests [50]. The stimuli are detected by ciliated mechanosensory neurons called the collar-receptor neurons (CRs). CRs synapse on different interneurons that in turn synapse on the cholinergic intersegmental ciliomotor neurons. This feed-forward circuit can explain how a vibrational stimulus leads to the coordinated arrest of all locomotor cilia in the larva.
7. The evolution of ciliomotor cell types and circuits
We can note several general principles and similarities in the regulation of ciliary locomotion across different groups of animals (figure 5). To achieve coordinated movement of cilia across longer distances, neuronal input is required and achieved through the release of neurotransmitters and neuropeptides. Even in placozoans—animals that lack a nervous system—a function for neuropeptides in stopping ciliary gliding has been confirmed. In different groups where their effects were studied, ciliary responses to neurotransmitters were shown to be similar. Serotonin application increases CBF and decreases the occurrence of ciliary closures, while by contrast, acetylcholine and catecholamines decrease CBF and increase closures [12,42,44,66,70,72].
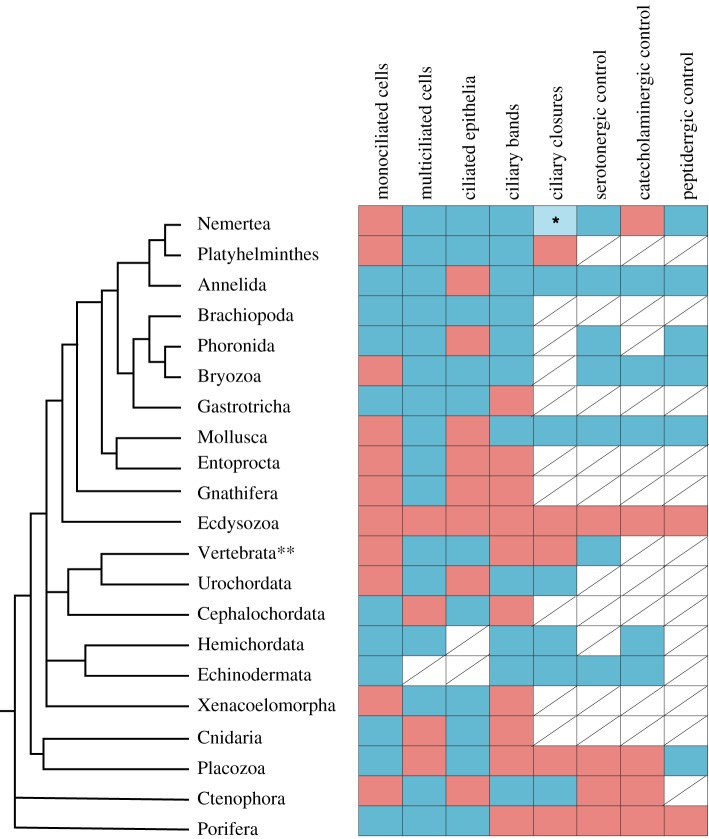
Figure 5.
Types of invertebrate ciliary systems and their control. Blue squares indicate presence, and red squares absence of a trait. Squares with no available data are crossed out. Phylogeny is based on [115–117]. *Nemerteans show brief arrests coupled with muscle contractions upon contact with food particles. **Only motile cilia on the body surface (anuran larvae) are considered.
The general involvement of serotoninergic and catecholaminergic neurons in ciliary control suggests that such ciliomotor neurons may trace back to the protostome–deuterostome common ancestor.
In animals where neuronal control of ciliary activity has been demonstrated, such as molluscs, annelids, nemerteans, echinoderms and chordates [12,25,36,42–45,47], spontaneous ciliary closures have been recorded, implying the existence of a pacemaker system (CPGs) involved in generating the rhythm of beating versus closure, similar to Platynereis larvae [12]. Changes in the pattern of closures were shown to be induced by chemical [8,52] and mechanical [45,51,53] stimuli, suggesting that there is sensory innervation modulating the presumptive pacemaker function.
Apart from the pacemaker neurons in Platynereis, we know very little about the generation of ciliary rhythms in other animals. Electron microscopic studies identified large and morphologically distinct neurons in other larvae that span the whole body to innervate ciliated cells [83,85,86].
We hypothesize that ciliomotor neurons are special and form a distinct part of the nervous system with a unique function and evolutionary history. We call this the ciliomotor nervous system. In the Platynereis larva, the comprehensive characterization of the ciliomotor nervous system revealed many unique characteristics. First, all ciliomotor neurons, with two exceptions, have a unique biaxonal morphology where two axons emanate directly from the neuronal soma and project in two directions. Second, the ciliomotor neurons show a distinct activity profile that drives ciliary activity. Third, the ciliomotor nervous system has a unique connectivity pattern and forms a distinct subnetwork in the larval nervous system. Fourth, the ciliomotor system must be specific to the larval stages as ciliation and ciliary swimming are lost in the juvenile worms and are absent from adults. The developmental fate of the ciliomotor neurons is not known, but they will either disappear or completely change function.
In agreement with what we have found in Platynereis, a morphological reconstruction of the ciliomotor system in the platyhelminth Muller's larva by Lacalli [83] revealed that the ciliomotor system in this larva is clearly distinguishable from the central nervous system. In the pilidium larva, the largest and most distinct neuron innervates the ciliary margin. Giant serotonergic neurons with bi- or multiaxonal morphology have also been described in the phoronid larva [118]. These studies suggest that ciliomotor nervous systems form a distinct part of larval nervous systems, with unique characteristics and potentially a unique evolutionary history.
From the perspective of comparative neurobiology, ciliomotor neural circuits represent an interesting model system as they can be unambiguously identified through cell tracing in electron microscopy datasets (tracing backwards from ciliated cells). Such connectomic reconstructions of the circuitry underlying ciliary coordination in different animals would be valuable to understanding the evolution of these systems. Unravelling the evolution of ciliomotor circuits will also require research into the function and molecular specification of the cell types composing these circuits. This would require a combination of behavioural experiments, functional imaging (e.g. using genetically encoded calcium indicators) and genetic approaches. For example, an exciting subject of cell-type and circuit evolution would be a comparison of the annelid larval circuit to circuits in mollusc ciliated larvae. Both larval types show spontaneous and mechanically induced coordinated synchronized arrests that extend to all cilia, which suggests that a similar pacemaker operates in these larvae. In addition, the main ciliary band (prototroch) of annelid and mollusc larvae are likely homologous as they derive from the same blastomeres during the spiral cleavage pattern [119–121]. More generally, it would be interesting to study how ciliomotor systems compare across lophotrochozoan larvae. What are the differences between larvae with distinct ciliary bands and uniformly ciliated larvae? How is the nervous system in larvae with ciliary bands made of multiciliated or monociliated cells? How do systems regulating locomotory ciliary bands and feeding ciliary bands compare to each other?
Ciliomotor cell types likely coevolved with ciliary bands and their comparative study across animal groups may also reveal which larval types are homologous and which evolved independently. These are exciting questions for future neuro-evo-devo studies.
Acknowledgements
We thank Elizabeth A. Williams and Daniel Thiel for their comments. We also thank Èlia Benito-Gutiérrez for providing the amphioxus samples and Chris Lowe for the Schizocardium sample.
Data accessibility
This article has no additional data.
Authors' contributions
M.M. and G.J. wrote the paper. G.J. collected and fixed the specimens and J.B. prepared and imaged them by SEM.
Competing interests
We declare we have no competing interests.
Funding
This project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 766053 (EvoCell).
References
- 1.Brumley DR, Polin M, Pedley TJ, Goldstein RE. 2015. Metachronal waves in the flagellar beating of Volvox and their hydrodynamic origin. J. R. Soc. Interface. 12, 20141358 ( 10.1098/rsif.2014.1358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamm SL. 1972. Ciliary motion in Paramecium. A scanning electron microscope study. J. Cell Biol. 55, 250–255. ( 10.1083/jcb.55.1.250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staver JM, Strathmann RR. 2002. Evolution of fast development of planktonic embryos to early swimming. Biol. Bull. 203, 58–69. ( 10.2307/1543458) [DOI] [PubMed] [Google Scholar]
- 4.Paskin TR, Jellies J, Bacher J, Beane WS. 2014. Planarian phototactic assay reveals differential behavioral responses based on wavelength. PLoS ONE 9, e114708 ( 10.1371/journal.pone.0114708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armon S, Bull MS, Aranda-Diaz A, Prakash M. 2018. Ultrafast epithelial contractions provide insights into contraction speed limits and tissue integrity. Proc. Natl Acad. Sci. USA 115, E10333–E10341. ( 10.1073/pnas.1802934115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilpin W, Prakash VN, Prakash M. 2017. Vortex arrays and ciliary tangles underlie the feeding–swimming trade-off in starfish larvae. Nat. Phys. 13, 380–386. ( 10.1038/nphys3981) [DOI] [Google Scholar]
- 7.Jékely G. 2009. Evolution of phototaxis. Phil. Trans. R. Soc. B 364, 2795–2808. ( 10.1098/rstb.2009.0072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tosches MA, Bucher D, Vopalensky P, Arendt D. 2014. Melatonin signaling controls circadian swimming behavior in marine zooplankton. Cell 159, 46–57. ( 10.1016/j.cell.2014.07.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nawroth JC, Guo H, Koch E, Heath-Heckman EAC, Hermanson JC, Ruby EG, Dabiri JO, Kanso E, McFall-Ngai M. 2017. Motile cilia create fluid-mechanical microhabitats for the active recruitment of the host microbiome. Proc. Natl Acad. Sci. USA 114, 9510–9516. ( 10.1073/pnas.1706926114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro OH, Fernandez VI, Garren M, Guasto JS, Debaillon-Vesque FP, Kramarsky-Winter E, Vardi A, Stocker R. 2014. Vortical ciliary flows actively enhance mass transport in reef corals. Proc. Natl Acad. Sci. USA 111, 13 391–13 396. ( 10.1073/pnas.1323094111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faubel R, Westendorf C, Bodenschatz E, Eichele G. 2016. Cilia-based flow network in the brain ventricles. Science 353, 176–178. ( 10.1126/science.aae0450) [DOI] [PubMed] [Google Scholar]
- 12.Verasztó C, Ueda N, Bezares-Calderón LA, Panzera A, Williams EA, Shahidi R, Jékely G. 2017. Ciliomotor circuitry underlying whole-body coordination of ciliary activity in the larva. eLife 6, e26000 ( 10.7554/eLife.26000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jekely G. 2011. Origin and early evolution of neural circuits for the control of ciliary locomotion. Proc. R. Soc. B 278, 914–922. ( 10.1098/rspb.2010.2027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jékely G, Keijzer F, Godfrey-Smith P. 2015. An option space for early neural evolution. Phil. Trans. R. Soc. B 370, 20150181 ( 10.1098/rstb.2015.0181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen C. 1987. Structure and function of metazoan ciliary bands and their phylogenetic significance. Acta Zool. 68, 205–262. ( 10.1111/j.1463-6395.1987.tb00892.x) [DOI] [Google Scholar]
- 16.Smith CL, Pivovarova N, Reese TS. 2015. Coordinated feeding behavior in Trichoplax, an animal without synapses. PLoS ONE 10, e0136098 ( 10.1371/journal.pone.0136098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamm SL. 2014. Cilia and the life of ctenophores. Invertebr. Biol. 133, 1–46. ( 10.1111/ivb.12042) [DOI] [Google Scholar]
- 18.Martin GG. 1978. Ciliary gliding in lower invertebrates. Zoomorphologie 91, 249–261. ( 10.1007/BF00999814) [DOI] [Google Scholar]
- 19.Childress S. 1981. Mechanics of swimming and flying. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 20.Klos DDA, Vladar EK, Werner ME, Mitchell JW, Hwang P, Mitchell BJ. 2013. Deuterosome-mediated centriole biogenesis. Dev. Cell 27, 103–112. ( 10.1016/j.devcel.2013.08.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chia F-S, Buckland-Nicks J, Young CM. 1984. Locomotion of marine invertebrate larvae: a review. Can. J. Zool. 62, 1205–1222. ( 10.1139/z84-176) [DOI] [Google Scholar]
- 22.Jokura K, Shibata D, Yamaguchi K, Shiba K, Makino Y, Shigenobu S, Inaba K. 2019. CTENO64 is required for coordinated paddling of ciliary comb plate in ctenophores. Curr. Biol. 29, 3510–3516. ( 10.1016/j.cub.2019.08.059) [DOI] [PubMed] [Google Scholar]
- 23.Wan KY, Hürlimann SK, Fenix AM, McGillivary RM, Makushok T, Burns E, Sheung JY, Marshall WF. 2019. Reorganization of complex ciliary flows around regenerating Stentor coeruleus. Phil. Trans. R. Soc. B 375, 20190167 ( 10.1098/rstb.2019.0167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knight-Jones EW. 1954. Relations between metachronism and the direction of ciliary beat in metazoa. J. Cell Sci. s3–95, 503–521. [Google Scholar]
- 25.Arkett SA. 1987. Ciliary arrest controlled by identified central neurons in a urochordate (ascidiacea). J. Comp. Physiol. A 161, 837–847. ( 10.1007/bf00610225) [DOI] [Google Scholar]
- 26.Strathmann RR, Jahn TL, Fonseca JRC. 1972. Suspension feeding by marine invertebrate larvae: clearance of particles by ciliated bands of rotifers, pluteus, and trochophore. Biol. Bull. 142, 505–519. ( 10.2307/1540326) [DOI] [Google Scholar]
- 27.von Dassow G, Emlet RB, Maslakova SA. 2013. How the pilidium larva feeds. Front. Zool. 10, 47 ( 10.1186/1742-9994-10-47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strathmann RR, Grünbaum D. 2006. Good eaters, poor swimmers: compromises in larval form. Integr. Comp. Biol. 46, 312–322. ( 10.1093/icb/icj031) [DOI] [PubMed] [Google Scholar]
- 29.Osterman N, Vilfan A. 2011. Finding the ciliary beating pattern with optimal efficiency. Proc. Natl Acad. Sci. USA 108, 15 727–15 732. ( 10.1073/pnas.1107889108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gueron S, Levit-Gurevich K. 1999. Energetic considerations of ciliary beating and the advantage of metachronal coordination. Proc. Natl Acad. Sci. USA 96, 12 240–12 245. ( 10.1073/pnas.96.22.12240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedley TJ, Brumley DR, Goldstein RE. 2016. Squirmers with swirl: a model for Volvox swimming. J. Fluid Mech. 798, 165–186. ( 10.1017/jfm.2016.306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jékely G, Colombelli J, Hausen H, Guy K, Stelzer E, Nédélec F, Arendt D. 2008. Mechanism of phototaxis in marine zooplankton. Nature 456, 395–399. ( 10.1038/nature07590) [DOI] [PubMed] [Google Scholar]
- 33.Elgeti J, Gompper G. 2013. Emergence of metachronal waves in cilia arrays. Proc. Natl Acad. Sci. USA 110, 4470–4475. ( 10.1073/pnas.1218869110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guirao B, Joanny J-F. 2007. Spontaneous creation of macroscopic flow and metachronal waves in an array of cilia. Biophys. J. 92, 1900–1917. ( 10.1529/biophysj.106.084897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan KY, Goldstein RE. 2016. Coordinated beating of algal flagella is mediated by basal coupling. Proc. Natl Acad. Sci. USA 113, E2784–E2793. ( 10.1073/pnas.1518527113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arkett SA, Mackie GO, Singla CL. 1987. Neuronal control of ciliary locomotion in a gastropod veliger (Calliostoma). Biol. Bull. 173, 513–526. ( 10.2307/1541697) [DOI] [PubMed] [Google Scholar]
- 37.Galt CP, Mackie GO. 1971. Electrical correlates of ciliary reversal in oikopleura. J. Exp. Biol. 55, 205–212. [Google Scholar]
- 38.Mackie GO, Paul DH, Singla CM, Sleigh MA, Williams DE. 1974. Branchial innervation and ciliary control in the ascidian Corella. Proc. R. Soc. Lond. B 187, 1–35. ( 10.1098/rspb.1974.0058) [DOI] [PubMed] [Google Scholar]
- 39.Spencer AN. 1974. Non-nervous conduction in invertebrates and embryos. Integr. Comp. Biol. 14, 917–929. ( 10.1093/icb/14.3.917) [DOI] [Google Scholar]
- 40.Satterlie RA, Case JF. 1978. Gap junctions suggest epithelial conduction within the comb plates of the ctenophore Pleurobrachia bachei. Cell Tissue Res. 193, 87–91. ( 10.1007/BF00221603) [DOI] [PubMed] [Google Scholar]
- 41.Murakami A. 1989. The control of cilia in metazoa: ciliary functions and Ca-dependent responses. Comp. Biochem. Physiol. A Physiol. 94, 375–382. ( 10.1016/0300-9629(89)90557-4) [DOI] [Google Scholar]
- 42.Marsden JR, Hassessian H. 1986. Effects of Ca2+ and catecholamines on swimming cilia of the trochophore larva of the polychaete Spirobranchus giganteus (Pallas). J. Exp. Mar. Biol. Ecol. 95, 245–255. ( 10.1016/0022-0981(86)90257-1) [DOI] [Google Scholar]
- 43.Leise EM, Hadfield MG. 2000. An inducer of molluscan metamorphosis transforms activity patterns in a larval nervous system. Biol. Bull. 199, 241–250. ( 10.2307/1543180) [DOI] [PubMed] [Google Scholar]
- 44.Penniman JR, Doll MK, Pires A. 2013. Neural correlates of settlement in veliger larvae of the gastropod, Crepidula fornicata. Invertebr. Biol. 132, 14–26. ( 10.1111/ivb.12014) [DOI] [Google Scholar]
- 45.Lacalli TC, Gilmour THJ. 1990. Ciliary reversal and locomotory control in the pluteus larva of Lytechinus pictus. Phil. Trans. R. Soc. Lond. B 330, 391–396. ( 10.1098/rstb.1990.0207) [DOI] [Google Scholar]
- 46.Bone Q. 1958. Nervous control of cilia in Amphioxus (Branchiostoma). Nature 181, 193–194. ( 10.1038/181193a0) [DOI] [Google Scholar]
- 47.Bergles D, Tamm S. 1992. Control of cilia in the branchial basket of Ciona intestinalis (Ascidacea). Biol. Bull. 182, 382–390. ( 10.2307/1542257) [DOI] [PubMed] [Google Scholar]
- 48.Lacalli TC. 1986. Prototroch structure and innervation in the trochophore larva of Phyllodoce (Polychaeta). Can. J. Zool. 64, 176–184. ( 10.1139/z86-028) [DOI] [Google Scholar]
- 49.Conzelmann M, Offenburger S-L, Asadulina A, Keller T, Munch TA, Jekely G. 2011. Neuropeptides regulate swimming depth of Platynereis larvae. Proc. Natl Acad. Sci. USA. 108, E1174–E1183. ( 10.1073/pnas.1109085108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bezares-Calderón LA, Berger J, Jasek S, Verasztó C, Mendes S, Gühmann M, Almeda R, Shahidi R, Jékely G. 2018. Neural circuitry of a polycystin-mediated hydrodynamic startle response for predator avoidance. eLife 7, e36262 ( 10.7554/eLife.36262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lacalli TC, Gilmour THJ. 2001. Locomotory and feeding effectors of the tornaria larva of Balanoglossus biminiensis. Acta Zool. 82, 117–126. ( 10.1046/j.1463-6395.2001.00075.x) [DOI] [Google Scholar]
- 52.Hadfield MG, Koehl MAR. 2004. Rapid behavioral responses of an invertebrate larva to dissolved settlement cue. Biol. Bull. 207, 28–43. ( 10.2307/1543626) [DOI] [PubMed] [Google Scholar]
- 53.Lacalli TC, Gilmour THJ, West JE. 1990. Ciliary band innervation in the bipinnaria larva of Pisaster ochraceus. Phil. Trans. R. Soc. Lond. B 330, 371–390. ( 10.1098/rstb.1990.0206) [DOI] [Google Scholar]
- 54.Mackie GO, Singla CL, Thiriot-Quievreux C. 1976. Nervous control of ciliary activity in gastropod larvae. Biol. Bull. 151, 182–199. ( 10.2307/1540713) [DOI] [PubMed] [Google Scholar]
- 55.Mackie GO, Spencer AN, Strathmann R. 1969. Electrical activity associated with ciliary reversal in an echinoderm larva. Nature 223, 1384–1385. ( 10.1038/2231384a0) [DOI] [Google Scholar]
- 56.Alvarez L, Dai L, Friedrich BM, Kashikar ND, Gregor I, Pascal R, Kaupp UB. 2012. The rate of change in Ca2+ concentration controls sperm chemotaxis. J. Cell Biol. 196, 653–663. ( 10.1083/jcb.201106096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Varoqueaux F, Williams EA, Grandemange S, Truscello L, Kamm K, Schierwater B, Jékely G, Fasshauer D. 2018. High cell diversity and complex peptidergic signaling underlie placozoan behavior. Curr. Biol. 28, 3495–3501. ( 10.1016/j.cub.2018.08.067) [DOI] [PubMed] [Google Scholar]
- 58.Senatore A, Reese TS, Smith CL. 2017. Neuropeptidergic integration of behavior in Trichoplax adhaerens, an animal without synapses. J. Exp. Biol. 220, 3381–3390. ( 10.1242/jeb.162396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weimann JM, Skiebe P, Heinzel HG, Soto C, Kopell N, Jorge-Rivera JC, Marder E. 1997. Modulation of oscillator interactions in the crab stomatogastric ganglion by crustacean cardioactive peptide. J. Neurosci. 17, 1748–1760. ( 10.1523/JNEUROSCI.17-05-01748.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thirumalai V, Marder E. 2002. Colocalized neuropeptides activate a central pattern generator by acting on different circuit targets. J. Neurosci. 22, 1874–1882. ( 10.1523/JNEUROSCI.22-05-01874.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tamm SL, Moss AG. 1985. Unilateral ciliary reversal and motor responses during prey capture by the ctenophore Pleurobrachia. J. Exp. Biol. 114, 443–461. [DOI] [PubMed] [Google Scholar]
- 62.Tamm SL, Tamm S. 1981. Ciliary reversal without rotation of axonemal structures in ctenophore comb plates. J. Cell Biol. 89, 495–509. ( 10.1083/jcb.89.3.495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strathmann RR. 1975. Larval feeding in echinoderms. Integr. Comp. Biol. 15, 717–730. ( 10.1093/icb/15.3.717) [DOI] [Google Scholar]
- 64.Ryberg E. 1974. The localization of cholinesterases and non-specific esterases in the echinopluteus. Zool. Scr. 2, 163–170. ( 10.1111/j.1463-6409.1974.tb00748.x) [DOI] [Google Scholar]
- 65.Wada Y, Mogami Y, Baba S. 1997. Modification of ciliary beating in sea urchin larvae induced by neurotransmitters: beat-plane rotation and control of frequency fluctuation. J. Exp. Biol. 200, 9–18. [DOI] [PubMed] [Google Scholar]
- 66.Kuang S, Doran SA, Wilson RJA, Goss GG, Goldberg JI. 2002. Serotonergic sensory-motor neurons mediate a behavioral response to hypoxia in pond snail embryos. J. Neurobiol. 52, 73–83. ( 10.1002/neu.10071) [DOI] [PubMed] [Google Scholar]
- 67.Goldberg JI, Rich DR, Muruganathan SP, Liu MB, Pon JR, Tam R, Diefenbach TJ, Kuang S. 2011. Identification and evolutionary implications of neurotransmitter–ciliary interactions underlying the behavioral response to hypoxia in Lymnaea stagnalis embryos. J. Exp. Biol. 214, 2660–2670. ( 10.1242/jeb.053009) [DOI] [PubMed] [Google Scholar]
- 68.Goldberg JI, Koehncke NK, Christopher KJ, Neumann C, Diefenbach TJ. 1994. Pharmacological characterization of a serotonin receptor involved in an early embryonic behavior of Helisoma trivolvis. J. Neurobiol. 25, 1545–1557. ( 10.1002/neu.480251207) [DOI] [PubMed] [Google Scholar]
- 69.Mapara S, Parries S, Quarrington C, Ahn K-C, Gallin WJ, Goldberg JI. 2008. Identification, molecular structure and expression of two cloned serotonin receptors from the pond snail, Helisoma trivolvis. J. Exp. Biol. 211, 900–910. ( 10.1242/jeb.013953) [DOI] [PubMed] [Google Scholar]
- 70.Soliman S. 1983. Pharmacological control of ciliary activity in the young sea urchin larva. Effects of monoaminergic agents. Comp. Biochem. Physiol. Part C: Comp. Pharmacol. 76, 181–191. ( 10.1016/0742-8413(83)90061-0) [DOI] [PubMed] [Google Scholar]
- 71.Soliman S. 1984. Pharmacological control of ciliary activity in the young sea urchin larva. Studies on the role of Ca2+ and cyclic nucleotides. Comp. Biochem. Physiol. C 78, 183–191. ( 10.1016/0742-8413(84)90067-7) [DOI] [PubMed] [Google Scholar]
- 72.Yoshihiro M, Keiko W, Chieko O, Akemi K, Baba SA. 1992. Regulation of ciliary movement in sea urchin embryos: Dopamine and 5-HT change the swimming behaviour. Comp. Biochem. Physiol. Part C: Comp. Pharmacol. 101, 251–254. ( 10.1016/0742-8413(92)90269-d) [DOI] [Google Scholar]
- 73.Walentek P, Bogusch S, Thumberger T, Vick P, Dubaissi E, Beyer T, Blum M, Schweickert A. 2014. A novel serotonin-secreting cell type regulates ciliary motility in the mucociliary epidermis of Xenopus tadpoles. Development 141, 1526–1533. ( 10.1242/dev.102343) [DOI] [PubMed] [Google Scholar]
- 74.König P, Krain B, Krasteva G, Kummer W. 2009. Serotonin increases cilia-driven particle transport via an acetylcholine-independent pathway in the mouse trachea. PLoS ONE 4, e4938 ( 10.1371/journal.pone.0004938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nguyen T, Chin WC, O'Brien JA, Verdugo P, Berger AJ. 2001. Intracellular pathways regulating ciliary beating of rat brain ependymal cells. J. Physiol. 531, 131–140. ( 10.1111/j.1469-7793.2001.0131j.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katow H, Suyemitsu T, Ooka S, Yaguchi J, Jin-Nai T, Kuwahara I, Katow T, Yaguchi S, Abe H. 2010. Development of a dopaminergic system in sea urchin embryos and larvae. J. Exp. Biol. 213, 2808–2819. ( 10.1242/jeb.042150) [DOI] [PubMed] [Google Scholar]
- 77.Gruhl A. 2010. Neuromuscular system of the larva of Fredericella sultana (Bryozoa: Phylactolaemata). Zool. Anz. 249, 139–149. ( 10.1016/j.jcz.2010.06.001) [DOI] [Google Scholar]
- 78.Gruhl A. 2009. Serotonergic and FMRFamidergic nervous systems in gymnolaemate bryozoan larvae. Zoomorphology 128, 135–156. ( 10.1007/s00435-009-0084-x) [DOI] [Google Scholar]
- 79.Rawlinson KA. 2010. Embryonic and post-embryonic development of the polyclad flatworm Maritigrella crozieri; implications for the evolution of spiralian life history traits. Front. Zool. 7, 12 ( 10.1186/1742-9994-7-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Temereva EN, Tsitrin EB. 2014. Development and organization of the larval nervous system in Phoronopsis harmeri: new insights into phoronid phylogeny. Front. Zool. 11, 3 ( 10.1186/1742-9994-11-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thiel D, Bauknecht P, Jékely G, Hejnol A. 2019. A nemertean excitatory peptide/CCHamide regulates ciliary swimming in the larvae of Lineus longissimus. Front. Zool. 16, 28 ( 10.1186/s12983-019-0326-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Conductier G, et al. 2013. Melanin-concentrating hormone regulates beat frequency of ependymal cilia and ventricular volume. Nat. Neurosci. 16, 845–847. ( 10.1038/nn.3401) [DOI] [PubMed] [Google Scholar]
- 83.Lacalli TC. 1982. The nervous system and ciliary band of Müller's larva. Proc. R. Soc. Lond. B. 217, 37–58. ( 10.1098/rspb.1982.0093) [DOI] [PubMed] [Google Scholar]
- 84.Holborow PL, Laverack MS, Barber VC. 1969. Cilia and other surface structures of the trochophore of Harmothoë imbricata (Polychaeta). Zeitschrift für Zellforschung und Mikroskopische Anatomie 98, 246–261. ( 10.1007/BF00338328) [DOI] [PubMed] [Google Scholar]
- 85.Lacalli TC, West JE. 1985. The nervous system of a pilidium larva: evidence from electron microscope reconstructions. Can. J. Zool. 63, 1909–1916. ( 10.1139/z85-284) [DOI] [Google Scholar]
- 86.Hay-Schmidt A. 1990. Catecholamine-containing, serotonin-like and neuropeptide FMRFamide-like immunoreactive cells and processes in the nervous system of the pilidium larva (Nemertini). Zoomorphology. 109, 231–244. ( 10.1007/bf00312190) [DOI] [Google Scholar]
- 87.Leys SP, Degnan BM. 2001. Cytological basis of photoresponsive behavior in a sponge larva. Biol. Bull. 201, 323–338. ( 10.2307/1543611) [DOI] [PubMed] [Google Scholar]
- 88.Srivastava M, et al. 2010. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature. 466, 720–726. ( 10.1038/nature09201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maldonado M. 2006. The ecology of the sponge larva. Can. J. Zool. 84, 175–194. ( 10.1139/z05-177) [DOI] [Google Scholar]
- 90.Moroz LL, et al. 2014. The ctenophore genome and the evolutionary origins of neural systems. Nature 510, 109–114. ( 10.1038/nature13400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsumoto GI. 1991. Swimming movements of ctenophores, and the mechanics of propulsion by ctene rows. Hydrobiologia. 216–217, 319–325. ( 10.1007/bf00026481) [DOI] [Google Scholar]
- 92.Nordström K, Wallén R, Seymour J, Nilsson D. 2003. A simple visual system without neurons in jellyfish larvae. Proc. R. Soc. B 270, 2349–2354. ( 10.1098/rspb.2003.2504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barlow LA. 1990. Electrophysiological and behavioral responses of larvae of the red abalone (Haliotis rufescens) to settlement-inducing substances. Bull. Mar. Sci. 46, 537–554. [Google Scholar]
- 94.Kuang S, Goldberg JI. 2001. Laser ablation reveals regulation of ciliary activity by serotonergic neurons in molluscan embryos. J. Neurobiol. 47, 1–15. ( 10.1002/neu.1011) [DOI] [PubMed] [Google Scholar]
- 95.Christopher KJ, Young KG, Chang JP, Goldberg JI. 1999. Involvement of protein kinase C in 5-HT-stimulated ciliary activity in Helisoma trivolvis embryos. J. Physiol. 515, 511–522. ( 10.1111/j.1469-7793.1999.511ac.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Conzelmann M, Jékely G. 2012. Antibodies against conserved amidated neuropeptide epitopes enrich the comparative neurobiology toolbox. Evodevo 3, 23 ( 10.1186/2041-9139-3-23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maslakova SA. 2010. Development to metamorphosis of the nemertean pilidium larva. Front. Zool. 7, 30 ( 10.1186/1742-9994-7-30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Funch P. 1996. The chordoid larva of Symbion pandora (Cycliophora) is a modified trochophore. J. Morphol. 230, 231–263. () [DOI] [PubMed] [Google Scholar]
- 99.Wanninger A. 2005. Immunocytochemistry of the nervous system and the musculature of the chordoid larva of Symbion pandora (Cycliophora). J. Morphol. 265, 237–243. ( 10.1002/jmor.10354) [DOI] [PubMed] [Google Scholar]
- 100.Pires A, Woollacott RM. 1997. Serotonin and dopamine have opposite effects on phototaxis in larvae of the bryozoan Bugula neritina. Biol. Bull. 192, 399–409. ( 10.2307/1542749) [DOI] [PubMed] [Google Scholar]
- 101.Hay-Schmidt A. 2000. The evolution of the serotonergic nervous system. Proc. R. Soc. B 267, 1071–1079. ( 10.1098/rspb.2000.1111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fuchs J, Wanninger A. 2008. Reconstruction of the neuromuscular system of the swimming-type larva of Loxosomella atkinsae (Entoprocta) as inferred by fluorescence labelling and confocal microscopy. Org. Divers. Evol. 8, 325–335. ( 10.1016/j.ode.2008.05.002) [DOI] [Google Scholar]
- 103.Clément P. 1987. Movements in rotifers: correlations of ultrastructure and behavior. Hydrobiologia 1, 339–359. ( 10.1007/BF00025764) [DOI] [Google Scholar]
- 104.Thiel D, Bauknecht P, Jékely G, Hejnol A. 2017. An ancient FMRFamide-related peptide-receptor pair induces defense behavior in a brachiopod larva. Open Biol. 7, pii. 170136 ( 10.1098/rsob.170136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dautov SS, Nezlin LP. 1992. Nervous system of the tornaria larva (Hemichordata: Enteropneusta). A histochemical and ultrastructural study. Biol. Bull. 183, 463–475. ( 10.2307/1542023) [DOI] [PubMed] [Google Scholar]
- 106.Stokes MD, Dale Stokes M, Holland ND. 1995. Embryos and larvae of a lancelet, Branchiostoma floridae, from hatching through metamorphosis: growth in the laboratory and external morphology. Acta Zool. 76, 105–120. ( 10.1111/j.1463-6395.1995.tb00986.x) [DOI] [Google Scholar]
- 107.Baskin DG, Detmers PA. 1976. Electron microscopic study on the gill bars of amphioxus (Brachiostoma californiense) with special reference to neurociliary control. Cell Tissue Res. 166, 167–178. ( 10.1007/BF00227038) [DOI] [PubMed] [Google Scholar]
- 108.Starunov VV, Voronezhskaya EE, Nezlin LP. 2017. Development of the nervous system in Platynereis dumerilii (Nereididae, Annelida). Front. Zool. 14, 27 ( 10.1186/s12983-017-0211-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hay-Schmidt A. 1990. Distribution of catecholamine-containing, serotonin-like and neuropeptide FMRFamide-like immunoreactive neurons and processes in the nervous system of the actinotroch larva of Phoronis muelleri (Phoronida). Cell Tissue Res. 259, 105–118. ( 10.1007/BF00571435) [DOI] [Google Scholar]
- 110.Burke RD. 1983. The structure of the larval nervous system of Pisaster ochraceus (Echinodermata: Asteroidea). J. Morphol. 178, 23–35. ( 10.1002/jmor.1051780103) [DOI] [PubMed] [Google Scholar]
- 111.Burke RD, Brand DG, Bisgrove BW. 1986. Structure of the nervous system of the auricularia larva of Parasticopus californicus. Biol. Bull. 170, 450–460. ( 10.2307/1541854) [DOI] [Google Scholar]
- 112.Nezlin LP. 2000. Tornaria of hemichordates and other dipleurula-type larvae: a comparison. J. Zool. Syst. Evol. Res. 38, 149–156. ( 10.1046/j.1439-0469.2000.383144.x) [DOI] [Google Scholar]
- 113.Raineri M. 1995. Is a mollusc an evolved bent metatrochophore? A histochemical investigation of neurogenesis in Mytilus (Mollusca: Bivalvia). J. Mar. Biol. Assoc. UK 75, 571–592. ( 10.1017/S0025315400039023) [DOI] [Google Scholar]
- 114.Conzelmann M, Williams EA. 2013. Conserved MIP receptor–ligand pair regulates Platynereis larval settlement. Proc. Natl Acad. Sci. USA 110, 8224–8229. ( 10.1073/pnas.1220285110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marlétaz F, Peijnenburg KTCA, Goto T, Satoh N, Rokhsar DS. 2019. A new spiralian phylogeny places the enigmatic arrow worms among gnathiferans. Curr. Biol. 29, 312–318. ( 10.1016/j.cub.2018.11.042) [DOI] [PubMed] [Google Scholar]
- 116.Laumer CE, Gruber-Vodicka H, Hadfield MG, Pearse VB, Riesgo A, Marioni JC, Giribet G. 2018. Support for a clade of Placozoa and Cnidaria in genes with minimal compositional bias. eLife 7, e36278 ( 10.7554/eLife.36278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Philippe H, et al. 2019. Mitigating anticipated effects of systematic errors supports sister-group relationship between Xenacoelomorpha and Ambulacraria. Curr. Biol. 29, 1818–1826. ( 10.1016/j.cub.2019.04.009) [DOI] [PubMed] [Google Scholar]
- 118.Temereva E, Wanninger A. 2012. Development of the nervous system in Phoronopsis harmeri (Lophotrochozoa, Phoronida) reveals both deuterostome- and trochozoan-like features. BMC Evol. Biol. 12, 121 ( 10.1186/1471-2148-12-121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gharbiah M, Nakamoto A, Nagy LM. 2013. Analysis of ciliary band formation in the mollusc Ilyanassa obsoleta. Dev. Genes Evol. 223, 225–235. ( 10.1007/s00427-013-0440-1) [DOI] [PubMed] [Google Scholar]
- 120.Nielsen C. 2005. Trochophora larvae: cell-lineages, ciliary bands and body regions. 2. Other groups and general discussion. J. Exp. Zool. B Mol. Dev. Evol. 304, 401–447. ( 10.1002/jez.b.21050) [DOI] [PubMed] [Google Scholar]
- 121.Rouse GW. 1999. Trochophore concepts: ciliary bands and the evolution of larvae in spiralian Metazoa. Biol. J. Linn. Soc. Lond. 66, 411–464. ( 10.1111/j.1095-8312.1999.tb01920.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.