Abstract
The generation of ciliary waveforms requires the spatial and temporal regulation of dyneins. This review catalogues many of the asymmetric structures and proteins in the cilia of Chlamydomonas, a unicellular alga with two cilia that are used for motility in liquid medium. These asymmetries, which have been identified through mutant analysis, cryo-EM tomography and proteomics, provide a wealth of information to use for modelling how waveforms are generated and propagated.
This article is part of the Theo Murphy meeting issue ‘Unity and diversity of cilia in locomotion and transport’.
Keywords: Chlamydomonas, dynein arms, mutational analysis, cryo-EM tomography
1. Introduction
Cilia and flagella are often described as microtubule-based structures with ninefold symmetry. In this review, a discussion of the structural and biochemical asymmetries in Chlamydomonas cilia and their potential roles will be presented. It is likely that similar asymmetries are present in motile cilia in other organisms. Cilia have nine doublet microtubules (DMTs) that surround two singlet microtubules. These organelles and their associated structures are able to generate both asymmetric and symmetric waveforms that move single cells or move fluid in multi-ciliated tissues.
Motile cilia are highly conserved organelles composed of over 500 proteins that assemble into the 9 + 2 structure, which is the canonical structure associated with these organelles [1–3] (figure 1a). The nine outer DMTs each consist of one complete microtubule, the A-tubule, with 13 protofilaments, and an incomplete microtubule, the B-tubule, with 10 protofilaments and an inner junction filament composed of two proteins, FAP20/BUG22 and Parkin coregulated gene protein (PACRG) [4–6]. Axonemal complexes attach to the outer DMT to form the structural feature of the axoneme, termed the 96 nm repeat. The 96 nm repeat contains multiple structures; they are the four outer dynein arms, two complete radial spokes (S1 and S2) and a third vestigial spoke (S3), the Modifier of Inner Arms (MIA) complex, the nexin–dynein regulatory complex (N-DRC), the calmodulin- and spoke-associated complex (CSC) and multiple inner dynein arms [7–10]. A molecular ruler composed of CCDC39 and CCDC40 is important for the docking of several structures [11]. Mutations in CCDC39 or CCDC40 result in the loss of the N-DRC and misplacement of the radial spokes [11,12]. Additional filaments are observed on the surface of the A-tubule using the hybrid Tygress method, which achieves 12 Å resolution [13]. These filaments may provide additional rulers for other complexes. In the centre of the cylinder of nine outer doublets is the central pair complex (CPC), composed of singlet microtubules, C1 and C2, and their associated appendages. These microtubules are more similar in their structure and stability to the cytoplasmic microtubules.
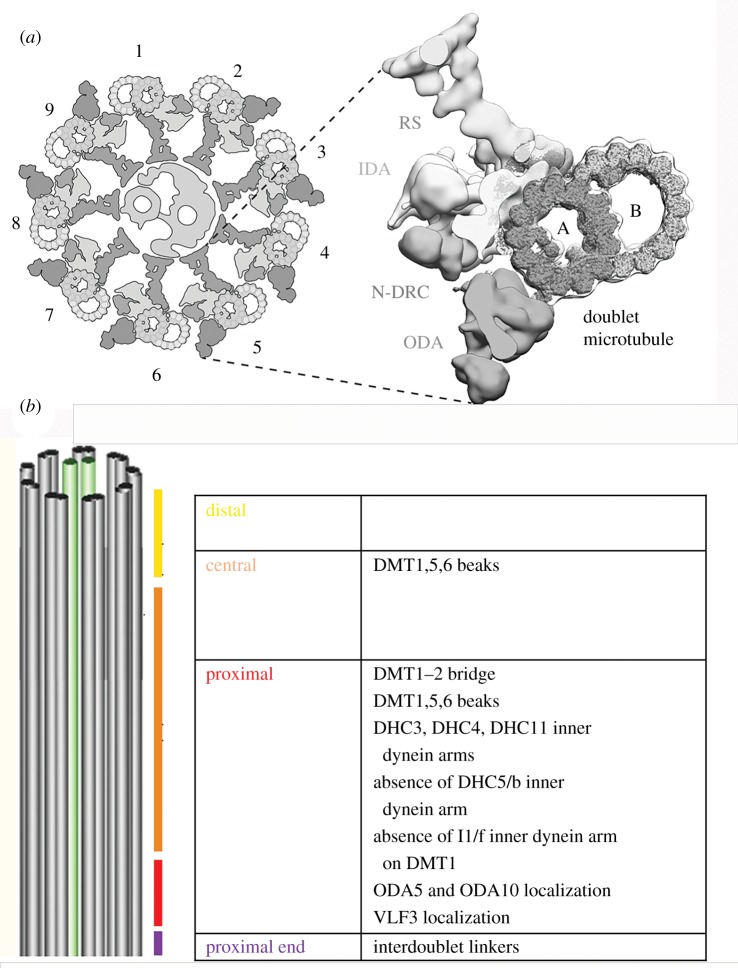
Figure 1.
Diagrams of the Chlamydomonas cilium. (a) Cross-sectional view of the cilium with one doublet microtubule enlarged to show a radial spoke (RS), an inner dynein arm (IDA), the N-DRC and an outer dynein arms (ODAs) as well as the A and B microtubules of the doublet microtubule. The DMTs are labelled 1–9. On doublet microtubule 1 (DMT1), there is no outer arm dynein. The central pair microtubules reside in the middle. (b) A longitudinal view to see the four regions and the unique features of these regions along the length the Chlamydomonas axoneme.
Chlamydomonas cilia exhibit two different waveforms. One is termed asymmetric and is characterized by a whip-like, or breaststroke, motion. The whip-like movement can be broken into two phases. The power stroke begins with the cilium fully extended. Then, the cilium moves through the liquid to lie next to the cell body. The recovery stroke involves an unfolding of the cilium to return to the fully extended starting position (figure 2a). This waveform is often referred to as the ciliary waveform as it is used by the lateral cilia of the clam gill or mammalian respiratory cilia. The waveform results in the Chlamydomonas cell moving forward with the cilia leading the way. In a wild-type Chlamydomonas cell, the beat frequency is generally about 60 Hz and the swimming velocity is about 150 µm s−1. A sinusoidal, or flagellar waveform as it is often called, is used by both vertebrate and invertebrate sperm tails. The waveform is initiated at the base of the cilium and propagated along the cilium to the tip, resulting in the backward motion of a Chlamydomonas cell with the cilia trailing (figure 2b).
Figure 2.
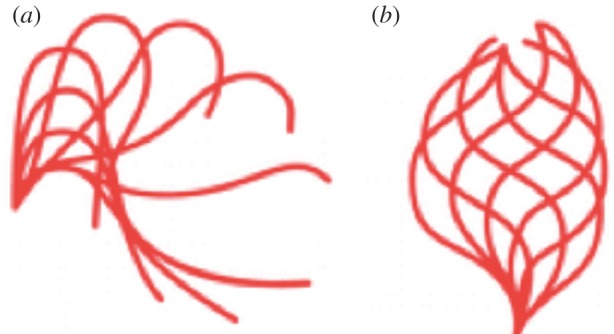
The two waveforms of Chlamydomonas. (a) The asymmetric waveform with the power and recovery stroke. (b) The symmetric waveform, which moves the cells backwards.
Ciliary movement is generated by the controlled sliding of adjacent outer DMTs in the axoneme. The dynein motors use the energy derived from adenosine triphosphate hydrolysis to generate the sliding movement, which is converted into bending to create the waveform. If all of the ciliary dyneins were active at one time, the cilia would be in a rigor state or tug-of-war, which results in no net movement or bending. In order to generate an effective bend, dynein motor function must be controlled both along the length of the axoneme and around the circumference of the axoneme across a defined axis. In elegant cryo electron microscopy (cryo-EM) tomography studies, Lin & Nicastro unexpectedly found that most dyneins are in an active state conformation, with a smaller population of inactive dyneins [14]. The locations of the inactive dyneins are asymmetric and bend-dependent. They propose a switch-inhibition mechanism in which the bend is generated by inhibiting, rather than activating, dyneins on one side of the cilium. The data suggest that the initiation of a bend starts with the inhibition of the inner dynein arms (a, d and g) on specific DMTs. Events on DMT 2 to 4 are needed for initiating the asymmetric waveform and events on DMT 7–9 are needed for initiating the symmetrical waveform. Once enough dyneins are inhibited, the dyneins on the other site help to start a bend. This bend is propagated until an unknown signal reactivates these dyneins and the axoneme straightens. In paralysed mutants (pf), all the dyneins are active and in a tug-of-war with each other [14].
The asymmetrical structural features described in this review are likely to be key for generating the waveforms. This review discusses proximal/distal differences, radial/doublet specific differences and cis/trans differences. The Chlamydomonas cilium has been divided into four parts: the very proximal portion of the axoneme (0.3 µm), the proximal portion (2 µm), the central portion (7 µm) and the distal portion (2–3 µm) [10] (figure 1b). Part of the distal segment is marked by an extension of the A-tubule [10]. The DMTs are numbered DMT1–9. Two cilia are indicated as cis and trans based on their position relative to the single eyespot.
(a). The dynein arms
The outer dynein arms are positioned on the outer circumference of eight of the nine outer DMTs every 24 nm. The outer dynein arm attachment is stabilized by a complex of three proteins [15]. Multiple mutations fail to assemble all, or part of the outer dynein arms, as monitored by electron microscopy and by biochemical analysis of salt-extracted mutant axonemes [16–23]. These loci encode most of the 16 structural proteins of the outer dynein arm (figure 3). The role of the outer dynein arms in ciliary movement has been determined from analysis of the mutant phenotypes. While the ciliary waveform is normal, oda− mutants display significantly reduced ciliary beat frequencies. The outer dynein arms are needed for increasing the power of the ciliary stroke. In these mutant strains, the ciliary beat is reduced by more than one-half and results in a reduced forward swimming speed [24–26].
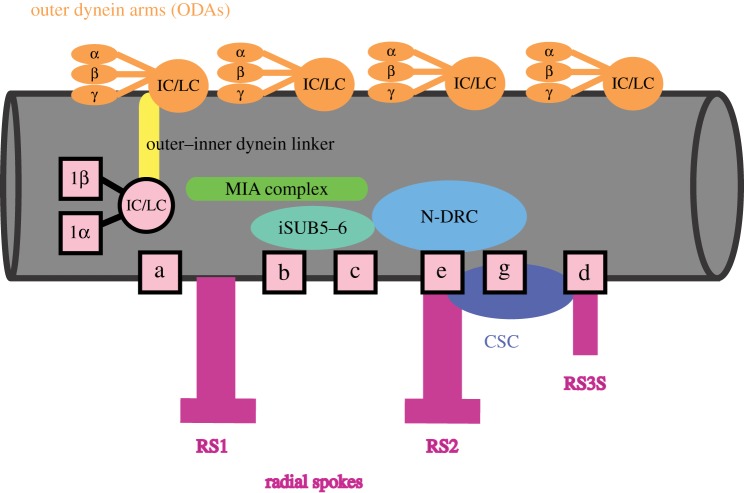
Figure 3.
The 96 nm repeat found along the central region of a doublet microtubule. The outer dynein arms are shown in orange and have the three heavy chains and the intermediate and light chains (IC/LC). The inner dynein arms are in pink. The I1/f dynein, which is two-headed, is indicated by its heavy chains (1α and 1β) as well as the IC/LC proteins. The six other inner dynein arm complexes are shown as boxes. The regulatory MIA complex is shown in green, the outer-inner dynein linker (OIDL) complex is in yellow, the iSUB5–6 complex is in teal, the N-DRC complex is shown in light blue, and the CSC complex is in dark blue. The radial spokes are in magenta.
Inner dynein arms are found along the inner circumference of the nine outer DMTs and function to generate the ciliary waveforms. Inner dynein arm (ida−) mutations result in a shallow ciliary waveform with reduced amplitude that produces reduced forward swimming speeds [27]. Unlike the outer dynein arms, there are at least 10 biochemically distinct inner arm structures in the 96 nm repeat [28,29]. One of the inner dynein arms (I1/f) is a two-headed structure with 10 subunits [30]. The remaining six inner dynein arms are single-headed motors with only one heavy chain; they are biochemically defined as dyneins a, b, c, d, e and g [28]. In addition, there are three minor dynein heavy chains. Inner arm mutants in nine genes have been isolated that affect their assembly [30–40], and these mutant strains show altered waveforms. Additional proteins associated with specific inner arms have been identified using biochemical analyses [41–44].
2. Proximal differences
(a). The inner dynein arms
Three (DHC3, DHC4 and DHC11) of 14 dynein heavy chain genes in Chlamydomonas produce proteins that are found at low abundance. Immunofluorescence microscopy revealed that DHC11 localizes exclusively to the proximal approximately 2 µm portion, and DHC3 and DHC4 are likely to be localized to this proximal portion [45]. This conclusion is supported by proteomic data of isolated axonemes. DHC3, DHC4 and DHC11 peptides are present at about 12% of the single-headed inner arm dynein proteins and at about 6% of the I1/f inner dynein arm. DHC11 may replace inner dynein arm a (IAa) in the proximal portion [10]. By contrast, inner dynein b (DHC5/IAb) shows an inverse distribution pattern in which it is missing from the proximal 2 µm, but is present along the rest of the axoneme.
(b). Doublet microtubule bridge
In the proximal axoneme, doublet microtubule 1 (DMT1) has a bridge with DMT2 [46–48]. This bridge is not unique to Chlamydomonas. In sea urchins, two of the doublets are attached by a bridge (DMT5–DMT6) with a 96 nm periodicity [48]. The bridge in sea urchins is made up of two parts called the inner and the outer parts. The inner part of the bridge, i-SUB5–6, is present along the entire length of a DMT in both Strongylocentrotus (DMT5) and Chlamydomonas (DMT1) cilia. It localizes between the I1/f dynein and the N-DRC (figure 3).
(c). Polyglutamylation of tubulin
Axonemal microtubules contain post-translational modifications on tubulin that include acetylation, phosphorylation, polyglycylation and polyglutamylation [49]. The functional importance for ciliary function is not clear for all of the modifications. Tubulin polyglutamylation adds multiple glutamates onto the gamma carboxyl group of any of several glutamine residues in the carboxyl terminus of either α- or β-tubulin. Different proteins are required for polyglutamylation (PolyE) along the length of the axoneme in Chlamydomonas. PolyE staining appears to be restricted to the B-tubule and is found along the length of the axoneme expect for the distal end [50]. In Chlamydomonas, mutations in the genes TTLL9 or FAP234, encoding either the tubulin tyrosine ligase-like enzyme or the subunit needed for its localization, [51] affect polyglutamylation of α-tubulin [12,52]. The proximal end of the axoneme (approx. 1.5 µm) remains polyglutamylated in these mutants [53]. Double mutant analysis suggests that loss of ttll9 activity regulates inner dynein arms [54]. In mutants of the molecular ruler (CCDC39/40), there is a reduction in the signal intensity of polyglutamylated α-tubulin relative to polyglutamylated β-tubulin. The PolyE signal in the proximal region requires CCDC39 [12]. CCDC39 appears to be required for the transport or docking of a different TTLL enzyme, which acts at the proximal end.
The purpose of having different enzymes for polyglutamylation at the proximal and central regions is not known. It is interesting to speculate that the length or spacing of the polyglutamate tails may be different and allow different proteins to bind and interact at the proximal and distal ends [55]. If B-tubules are highly modified by polyglutamylation, this modification might participate in multiple functions, which include stabilizing DMTs and regulating dynein motor activity.
(d). LF5 and length control
Recent work showed that length affects the motility properties of the cilium. Mechanical metrics (force, torque and power) all increased in proportion to the ciliary length. The mechanical efficiency of beating appeared to be maximal at the normal wild-type length of 10–12 µm [56]. In lf− (long flagella) mutants, the cilia are often two to three times longer than in wild-type cells and are unable to generate an effective waveform. Length control is regulated by the LF5 kinase, which is a homologue of CDKL5, and localizes to the proximal 1 µm of the cilia. This localization requires a complex of the LF1, LF2 and LF3 proteins [57]. The role of LF5 at the proximal end of the axoneme is not clear but could affect entry or exit of intraflagellar transport trains.
3. Proximal and radial asymmetries
(a). The outer–inner dynein linker
The outer–inner dynein linker (OIDL) connects the I1/f dynein arm to the outer dynein arms [10]. OIDLs in the central and distal regions are similar among all of the DMTs. However, at the proximal end, more OIDL densities are found on DMT2, DMT6, DMT7 and DMT8 than on the other DMTs. The OIDL contains the intermediate chain (IC2) of the outer dynein arm and DRC4 of the N-DRC [58]. It plays a role in regulating both inner and outer dynein arms.
(b). The ODA5/ODA10 protein complex
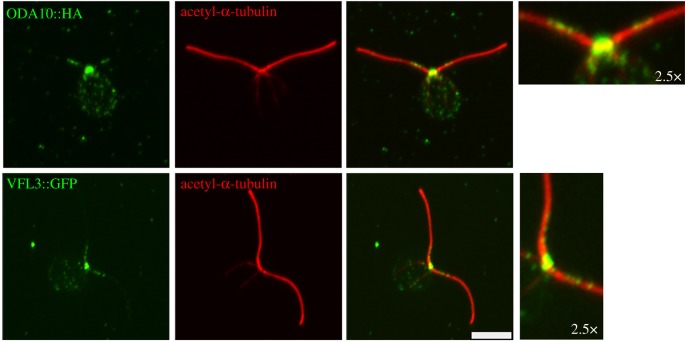
ODA5 and ODA10 play two roles. They are required for outer dynein arm assembly in the cytoplasm [59], and they are assembled into the cilia as well [60]. Using tagged transgenes, ODA10 and ODA5 were found to localize to the proximal 2–3 µm of the cilia (figure 4) and only to DMT1, with a 24 nm spacing, as assayed by immuno-EM [59]. Chlamydomonas has paralogues of ODA5 (ODA1/DC3) and of ODA10 (ODA3/DC1) which generate a separate docking complex (DC1/DC3) that assembles along most of the cilium and is associated with the stabilization of the outer dynein arms [15]. In humans, there are only two proteins; they are CCDC151 and CCDC114. They play a role in placement of the outer dynein arms in humans and are found along the entire length of the ciliary axoneme [61,62]. It is not known what restrains the ODA5/ODA10 complex to one microtubule in the proximal axoneme and what its role is in the proximal cilium.
Figure 4.
Localization of ODA10 and VFL3. Tagged transgenes (HA or GFP) for ODA10 and VFL3 show localization to the proximal region of the cilia. Antibodies to acetylated α-tubulin. The HA and GFP signals are shown in green and the acetylated α-tubulin is shown in red. (Online version in colour.)
Unlike in Chlamydomonas, the trypanosome waveform can start at the tip or the base of the axoneme. The initiation of the waveform is based on a proximal/distal asymmetry of the outer arm docking complexes. The trypanosomes have two paralogues of DC1 and two paralogues of DC3, which form a proximal docking complex and a distal docking complex. This asymmetry is set up by a gradient of proximal docking complexes using the intraflagellar transport machinery [63].
(c). VFL3/CCDC61 plays another role
The VFL3 protein has been suggested to play a role in segregation of the basal bodies in Chlamydomonas by assembling the distal striated fibres that hold the mature basal bodies together [64]. In addition, VLF3 has been postulated to have a role in the templating of new basal bodies [65]. Recently, we have found that the VFL3 protein also localizes to the proximal region of the cilia [66] (figure 4).
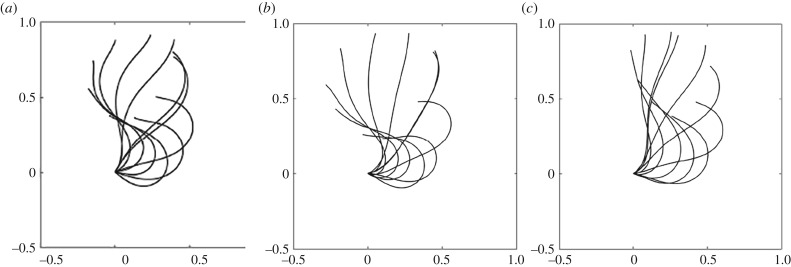
Analysis of the waveform of cilia in the vfl3 mutant using high-speed filming revealed that the flagellar waveform and frequency are similar to those of wild-type cells [67]. Wan & Goldstein showed that the two cilia in the vlf3 mutant display markedly different synchronization from the wild-type pattern. They used micromanipulation of flagella and concluded that a mechanism, internal to the cell, must provide additional flagellar coupling [68]. The waveform of biciliated cells in the vfl3-2 mutant is unchanged but the two cilia from the vfl3 mutant move independently (figure 5). We do not know if coupling offered by the striated fibres between the two basal bodies or the role of the VLF3 protein in the proximal cilia is responsible for the lack of synchrony between the two cilia [49]. As in wild-type cells, the cilia of vfl3 coordinately switch to a symmetrical, ciliary-type waveform during the shock response, which shows that the VFL3 protein is not needed for photoshock response.
Figure 5.
Waveforms of cilia from biciliated vfl3-2 cells are unchanged from those of uniciliated cells. (a) Uniciliated vfl3-2 cell, (b) biciliated vfl3-2 cell and (c) uniciliated uni1-2 cell.
(d). B-tubule projections or beaks show proximal and radial asymmetries
Structural analyses of axonemes revealed the presence of projections in the centre of the B-tubules. These projections are only in the proximal half of the axoneme and in only three outer doublet microtubules (DMT1, 5 and 6) [46]. They are referred to as beaks [46]. Mutant strains with defects in the MBO1 and MBO2 genes lack beaks in DMT5 and DMT6, but retain the beak in DMT1 [69]. The mbo− mutant cells fail to generate the asymmetric waveform required for normal forward swimming. As a result, the mutant cells move backwards only (mbo) using the symmetrical waveform. The MBO2 gene encodes a coiled-coil protein (CCDC146) [70] that localizes along the length of the ciliary axoneme. Proteomic analysis of mbo1 and mbo2 mutants revealed that the mutant axonemes are missing many more proteins than shown originally by two-dimensional electrophoresis ([69], M. Porter 2019, personal communication). The composition of the beaks remains unknown. The BUG22/FAP20 protein, which is part of the inner junction, plays a role in the placement of all the three beaks, and fap20/bug22 mutants show a symmetrical waveform that generates backwards swimming [4]. The role of MBO2 or FAP20 in beak assembly is not known. Loss of BUG22 in Drosophila sperm affects polyglycylation of tubulin [71], but polyglycylation has not been examined in the Chlamydomonas mutants.
4. Radial asymmetries
(a). The central pair complex
Cilia in all studied metazoans have a fixed CPC orientation in which the plane through the two CPC microtubules is perpendicular to the bend plane. By contrast, the CPC has a variable orientation in Chlamydomonas. This is related to an overall helical twist of the CPC structure and a change in the orientation of the CPC during bending. The plane through the two CPC microtubules is parallel to the bend plane [72].
The central pair microtubules are asymmetric. The two microtubules, called C1 and C2, have differential stability [73]. The two central microtubules have different appendages based on cryo-EM and analysis of mutants [74–82]. Recent cryo-EM tomography shows that the C1 microtubule has six projections while the C2 microtubule has five projections [83]. The two central pair microtubules are connected by a bridge as well that has at least three appendages. The known mutants affect specific appendages. CPC1 and hydin mutations affect the appendages on the C2 microtubule (2b-2d), PF16 (SPAG6) mutations affect the C1 tubule, and PF6 mutations affect the appendage 1a. The PF20 mutation affects the bridge between the two microtubules [83]. Recent proteomics on the pf18 mutant, which lacks a CPC, suggests that it is composed of at least 44 proteins [84]. The CPC together with the radial spokes are needed for the ciliary waveform. Mutants affecting different appendages show a range of phenotypes. The fap47 mutant is intriguing in that it is defective in phototaxis [84].
(b). DMT1 and DMT9
As mentioned previously, DMT1 shows proximal differences. In addition, DMT1 and DMT9 are missing several single-headed IDAs (IAb, IAc and IAe); these are missing from the entire length of these two DMTs [48].
(c). The MIA complex and outer dynein arms are needed for assembly of I1/f dynein
Chlamydomonas has two responses to light: phototaxis and photoshock. Chlamyrhodopsin is the photoreceptor [85] and the signal transduction process involves transmembrane Ca2+ fluxes. The cell responds by changes in ciliary behaviour. The MIA1 and MIA2 genes were identified among mutants that showed reduced positive phototaxis and resulted in increased phosphorylation of the IC138 subunit of the I1/f dynein [86]. These genes encode FAP73 and FAP100, and they localize near I1/f dynein and may connect with the N-DRC. The assembly of the I1/f dynein arm requires both the MIA1 and ODA complexes [87]. Consequently, in the mia1 or mia2 mutants, the I1/f dynein arms are missing from DMT1, which lacks outer dynein arms. In a mia1; oda6 double mutant, the I1/f dynein is completely missing from the axoneme [87].
(d). Mutations that affect dynein docking on a subset of doublet microtubules
The ODA2 gene encodes the gamma-dynein heavy chain of the outer dynein arms. Some alleles cause loss of the entire outer arm complex while others show a partial reduction. Surprisingly, the alleles isolated as suppressors of central pair mutations show a partial reduction of outer dynein arms on a subset of doublets (DMT3,6,7,8,9) [88]. The bop2 mutation was identified as a suppressor that affects the assembly of inner dynein arms on only a subset of the DMTs [89,90]. By electron microscopy tomography, doublets 5, 6 and 8 show the most severe deficiency, doublet 9 has an intermediate phenotype, and doublets 2, 3, 4 and 7 show the least severe phenotype. A more recent examination of bop2 mutants using cryo-EM tomography shows that a subset of inner dynein arms is missing on DMT5–9 [91]. BOP2 encodes a WDR protein (FAP57), and a patient with a mutation in CFAP57 show primary ciliary dyskinesia [92].
5. cis and trans asymmetries
The two cilia in Chlamydomonas are called the cis and the trans cilia. The cis cilium is nearest to the eyespot, which lies on the equator of the cell. The cis cilium is templated by the daughter basal body and the trans cilium is templated by the older or mother basal body [93]. Differences in the behaviour of the cis and trans cilia are thought to play a key role in orientation of the cell to light and calcium signals [94].
Differences between the cis and trans cilia have been studied using detergent-extracted and demembranated cells. At lower calcium concentrations, the trans axoneme is inactivated but at higher calcium concentrations, the cis axoneme is inactivated. These intrinsic differences between the two cilia are thought to be important for phototaxis [95]. In living cells with one cilium, the ciliary beat frequency of the trans cilium is about 30% higher than that of the cis cilium. But the cilia generally beat with the same frequency when both are present [96]. More recently, high-speed, high-resolution imaging of pipette-held cells with two cilia again has shown synchronized breaststrokes. At a low rate, there is a ‘slip’ and the trans cilium shows a faster beat frequency by about 30% while the cis cilium retains the original beat frequency [97].
This difference between the cis and trans cilia beat frequencies is not observed in mutants lacking the outer arm docking complex (oda1 and oda3) or in the oda11 mutant, which lacks the α heavy chain, but remains in the other outer dynein arm mutants [98,99]. How the docking complex and the α heavy chain but not the loss of the entire outer dynein arm influences these intrinsic differences is not known. A protein with four EF-hand domains (ODA14/DC3) requires DC1 and DC3 for its assembly [100]. DC3 plays a role in sensing calcium and redox poise that could be involved in these intrinsic differences between the two cilia [21]. In the non-phototactic mutant, ptx1, both cilia show the trans beat frequency [101], which suggests that PTX1 may define the trans cilium behaviour. The PTX1 gene has not been identified as of yet. In another non-phototactic mutant, lsp1, both cilia show the cis beat frequency [102].
Mutants that assemble primarily the trans cilium are available [103]. These uniciliated cells facilitate recording of the waveform, although cilia behaviour may differ somewhat in uniciliated compared with biciliated cells [104]. Estimates of the ‘equivalent’ swimming speeds of uniciliated cells are much lower than the known swimming speeds of wild-type biciliated cells [56]. The reasons for these differences are not yet resolved. Biciliated Chlamydomonas may synchronize the two cilia by ‘cell body rocking’ with minimal direct hydrodynamic interactions between the two cilia [104], by hydrodynamic coupling [105] or by coupling of the basal bodies via the striated fibres [68]. Proteomics of cilia from the uni1 mutant [103] will help to determine the protein composition of the cis and trans cilia, and will help us to understand the intrinsic differences.
6. Why have asymmetries in the cilium?
Regulation of ciliary and/or flagellar motility requires the coordinated spatial control of dynein-driven microtubule sliding [106]. However, the mechanisms for regulating the location and symmetry of dynein activity are still not understood completely. It seems very likely that the asymmetries observed in the cilium of Chlamydomonas are critically important for the initiation and regulation of the waveform. This regulation will require proximal asymmetries as the initiation of the waveform occurs in the proximal region as well as radial asymmetries as proposed by Lin & Nicastro [14]. The presence of different dynein arms as well as the alterations in DMT1 and DMT2 in the proximal axoneme could have a role in initiating the waveform. DMT1–2 in Chlamydomonas are located in a plane almost perpendicular to the bending plane and show little or no interdoublet sliding [107,108].
The role of the central pair and radial spokes in regulating the dyneins is also demonstrated by the isolation of suppressor mutations that restore some motility to paralysed mutants that arise from defects in the CPC/RS and radial spokes [2,19,33,88,109,110]. These mutations affect both outer and inner dynein arms as well as the N-DRC, which modulates dynein activity. The loss of the N-DRC is able to turn-on dynein activity in the absence of CPC/RS signals. An alternative model suggests that interactions between dynein and the passive components of the axoneme can produce coordinated, propulsive oscillations like the fluttering of a flag. Steady, distributed axial forces, acting in opposite directions on coupled beams in viscous fluid, lead to dynamic structural instability and oscillatory, wave-like motion [111].
In wild-type cells, the symmetrical waveform (backwards swimming) is initiated when cells are exposed to intense light signals, which cause an influx of calcium ions into the cells [112]. Several lines of evidence suggest that the outer dynein arms are potential targets of the central pair–radial spoke control system for generating the symmetrical waveform [113,114].
The continued analysis of these asymmetries in Chlamydomonas and other organisms will be key to understanding the generation of the asymmetric and symmetric waveforms. Further biophysical, microscopic and modelling studies of the many mutants affecting these asymmetrically localized proteins will be key to understanding the waveforms and the large molecular ciliary machine.
Acknowledgements
I thank Dr Huawen Lin for images of ODA10 and VFL3 localization and Dr Mathieu Bottier for the movie of vfl3 cells. Dr David Mitchell kindly provided the ODA10 strains and useful discussion.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
This work was supported by a grant from the National Institutes of Health (grant no. R01 GM032843) to S.K.D.
References
- 1.Pazour GJ, Agrin N, Leszyk J, Witman GB. 2005. Proteomic analysis of a eukaryotic cilium. J. Cell Biol. 170, 103–113. ( 10.1083/jcb.200504008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bower R, Tritschler D, Vanderwaal K, Perrone CA, Mueller J, Fox L, Sale WS, Holzbaur E. 2013. The N-DRC forms a conserved biochemical complex that maintains outer doublet alignment and limits microtubule sliding in motile axonemes. Mol. Biol. Cell 24, 1134–1152. ( 10.1091/mbc.e12-11-0801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin H, Guo S, Dutcher SK. 2018. RPGRIP1 L helps to establish the ciliary gate for entry of proteins. J. Cell Sci. 131, jcs220905 ( 10.1242/jcs.220905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng D, Cao M, Oda T, Pan J. 2014. The conserved ciliary protein Bug22 controls planar beating of Chlamydomonas flagella. J. Cell Sci. 127, 281–287. ( 10.1242/jcs.140723) [DOI] [PubMed] [Google Scholar]
- 5.Yanagisawa HA, Mathis G, Oda T, Hirono M, Richey EA, Ishikawa H, Marshall WF, Kikkawa M, Holzbaur E. 2014. FAP20 is an inner junction protein of doublet microtubules essential for both the planar asymmetrical waveform and stability of flagella in Chlamydomonas. Mol. Biol. Cell 25, 1472–1483. ( 10.1091/mbc.e13-08-0464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dymek EE, Lin J, Fu G, Porter ME, Nicastro D, Smith EF. 2019. PACRG and FAP20 form the inner junction of axonemal doublet microtubules and regulate ciliary motility. Mol. Biol. Cell, 30, 1805–1816. ( 10.1091/mbc.E19-01-0063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heuser T, Raytchev M, Krell J, Porter ME, Nicastro D. 2009. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J. Cell Biol. 187, 921–933. ( 10.1083/jcb.200908067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heuser T, Barber CF, Lin J, Krell J, Rebesco M, Porter ME, Nicastro D. 2012. Cryoelectron tomography reveals doublet-specific structures and unique interactions in the I1 dynein. Proc. Natl Acad. Sci. USA 109, E2067–E2076. ( 10.1073/pnas.1120690109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heuser T, Dymek EE, Lin J, Smith EF, Nicastro D. 2012. The CSC connects three major axonemal complexes involved in dynein regulation. Mol. Biol. Cell 23, 3143–3155. ( 10.1091/mbc.e12-05-0357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bui KH, Yagi T, Yamamoto R, Kamiya R, Ishikawa T. 2012. Polarity and asymmetry in the arrangement of dynein and related structures in the Chlamydomonas axoneme. J. Cell Biol. 198, 913–925. ( 10.1083/jcb.201201120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oda T, Yanagisawa H, Kamiya R, Kikkawa M. 2014. Cilia and flagella. A molecular ruler determines the repeat length in eukaryotic cilia and flagella. Science 346, 857–860. ( 10.1126/science.1260214) [DOI] [PubMed] [Google Scholar]
- 12.Lin H, Zhang Z, Guo S, Chen F, Kessler JM, Wang YM, Dutcher SK. 2015. A NIMA-related kinase suppresses the flagellar instability associated with the loss of multiple axonemal structures. PLoS Genet. 11, e1005508 ( 10.1371/journal.pgen.1005508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song K, Shang Z, Fu X, Lou X, Grigorieff N, Nicastro D. 2018. Structure of the ciliary axoneme at nanometer resolution reconstructed by TYGRESS. bioRχiv 363317 ( 10.1101/363317) [DOI] [Google Scholar]
- 14.Lin J, Nicastro D. 2018. Asymmetric distribution and spatial switching of dynein activity generates ciliary motility. Science 360, eaar1968 ( 10.1126/science.aar1968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oda T, Abe T, Yanagisawa H, Kikkawa M. 2016. Docking-complex-independent alignment of Chlamydomonas outer dynein arms with 24-nm periodicity in vitro. J. Cell Sci. 129, 1547–1551. ( 10.1242/jcs.184598) [DOI] [PubMed] [Google Scholar]
- 16.Kamiya R. 1988. Mutations at twelve independent loci result in absence of outer dynein arms in Chylamydomonas reinhardtii. J. Cell Biol. 107, 2253–2258. ( 10.1083/jcb.107.6.2253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell DR, Rosenbaum JL. 1985. A motile Chlamydomonas flagellar mutant that lacks outer dynein arms. J. Cell Biol. 100, 1228–1234. ( 10.1083/jcb.100.4.1228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell DR, Kang Y. 1991. Identification of oda6 as a Chlamydomonas dynein mutant by rescue with the wild-type gene. J. Cell Biol. 113, 835–842. ( 10.1083/jcb.113.4.835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter ME, Knott JA, Gardner LC, Mitchell DR, Dutcher SK. 1994. Mutations in the SUP-PF-1 locus of Chlamydomonas reinhardtii identify a regulatory domain in the beta-dynein heavy chain. J. Cell Biol. 126, 1495–1507. ( 10.1083/jcb.126.6.1495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takada S, Wilkerson CG, Wakabayashi K, Kamiya R, Witman GB. 2002. The outer dynein arm-docking complex: composition and characterization of a subunit (Oda1) necessary for outer arm assembly. Mol. Biol. Cell 13, 1015–1029. ( 10.1091/mbc.01-04-0201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casey DM, Yagi T, Kamiya R, Witman GB. 2003. DC3, the smallest subunit of the Chlamydomonas flagellar outer dynein arm-docking complex, is a redox-sensitive calcium-binding protein. J. Biol. Chem. 278, 42 652–42 659. ( 10.1074/jbc.M303064200) [DOI] [PubMed] [Google Scholar]
- 22.Ahmed NT, Mitchell DR. 2005. ODA16p, a Chlamydomonas flagellar protein needed for dynein assembly. Mol. Biol. Cell 16, 5004–5012. ( 10.1091/mbc.e05-07-0627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiBella LM, Sakato M, Patel-King RS, Pazour GJ, King SM. 2004. The LC7 light chains of Chlamydomonas flagellar dyneins interact with components required for both motor assembly and regulation. Mol. Biol. Cell 15, 4633–4646. ( 10.1091/mbc.e04-06-0461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brokaw CJ, Kamiya R. 1987. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil. Cytoskeleton 8, 68–75. ( 10.1002/cm.970080110) [DOI] [PubMed] [Google Scholar]
- 25.Bayly PV, Lewis BL, Kemp PS, Pless RB, Dutcher SK. 2010. Efficient spatiotemporal analysis of the flagellar waveform of Chlamydomonas reinhardtii. Cytoskeleton 67, 56–69. ( 10.1002/cm.20424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim M, Huff E, Bottier M, Dutcher SK, Bayly PV, Meacham JM. 2019. Acoustic trap-and-release for rapid assessment of cell motility. Soft Matter 15, 4266–4275. ( 10.1039/c9sm00184k) [DOI] [PubMed] [Google Scholar]
- 27.King SM, Sale WS. 2018. Fifty years of microtubule sliding in cilia. Mol. Biol. Cell 29, 698–701. ( 10.1091/mbc.E17-07-0483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kagami O, Kamiya R. 1995. Separation of dynein species by high-pressure liquid chromatography. Methods Cell Biol. 47, 487–489. ( 10.1016/S0091-679X(08)60849-3) [DOI] [PubMed] [Google Scholar]
- 29.Porter ME, Sale WS. 2000. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J. Cell Biol. 151, F37–F42. ( 10.1083/jcb.151.5.F37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrone CA, Yang P, O'Toole E, Sale WS, Porter ME. 1998. The Chlamydomonas IDA7 locus encodes a 140-kDa dynein intermediate chain required to assemble the I1 inner arm complex. Mol. Biol. Cell 9, 3351–3365. ( 10.1091/mbc.9.12.3351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamiya R, Kurimoto E, Muto E. 1991. Two types of Chlamydomonas flagellar mutants missing different components of inner-arm dynein. J. Cell Biol. 112, 441–447. ( 10.1083/jcb.112.3.441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeDizet M, Piperno G. 1995. ida4-1, ida4-2, and ida4-3 are intron splicing mutations affecting the locus encoding p28, a light chain of Chlamydomonas axonemal inner dynein arms. Mol. Biol. Cell 6, 713–723. ( 10.1091/mbc.6.6.713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porter ME, Power J, Dutcher SK. 1992. Extragenic suppressors of paralyzed flagellar mutations in Chlamydomonas reinhardtii identify loci that alter the inner dynein arms. J. Cell Biol. 118, 1163–1176. ( 10.1083/jcb.118.5.1163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato-Minoura T, Hirono M, Kamiya R. 1997. Chlamydomonas inner-arm dynein mutant, ida5, has a mutation in an actin-encoding gene. J. Cell Biol. 137, 649–656. ( 10.1083/jcb.137.3.649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myster SH, Knott JA, Wysocki KM, O'Toole E, Porter ME. 1999. Domains in the 1α dynein heavy chain required for inner arm assembly and flagellar motility in Chlamydomonas. J. Cell Biol. 146, 801–818. ( 10.1083/jcb.146.4.801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perrone CA, Myster SH, Bower R, O'Toole ET, Porter ME. 2000. Insights into the structural organization of the I1 inner arm dynein from a domain analysis of the 1β dynein heavy chain. Mol. Biol. Cell 11, 2297–2313. ( 10.1091/mbc.11.7.2297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanagisawa HA, Kamiya R. 2004. A tektin homologue is decreased in Chlamydomonas mutants lacking an axonemal inner-arm dynein. Mol. Biol. Cell 15, 2105–2115. ( 10.1091/mbc.e03-11-0854) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter EL, et al. 2018. The IDA3 adapter, required for intraflagellar transport of I1 dynein, is regulated by ciliary length. Mol. Biol. Cell 29, 886–896. ( 10.1091/mbc.E17-12-0729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.VanderWaal KE, et al. 2011. bop5 mutations reveal new roles for the IC138 phosphoprotein in the regulation of flagellar motility and asymmetric waveforms. Mol. Biol. Cell 22, 2862–2874. ( 10.1091/mbc.e11-03-0270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yagi T, Minoura I, Fujiwara A, Saito R, Yasunaga T, Hirono M, Kamiya R. 2005. An axonemal dynein particularly important for flagellar movement at high viscosity. Implications from a new Chlamydomonas mutant deficient in the dynein heavy chain gene DHC9. J. Biol. Chem. 280, 41 412–41 420. ( 10.1074/jbc.M509072200) [DOI] [PubMed] [Google Scholar]
- 41.Wirschell M, Yang C, Yang P, Fox L, Yanagisawa HA, Kamiya R, Witman GB, Porter ME, Holzbaur E. 2009. IC97 is a novel intermediate chain of I1 dynein that interacts with tubulin and regulates interdoublet sliding. Mol. Biol. Cell 20, 3044–3054. ( 10.1091/mbc.e09-04-0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikeda K, Yamamoto R, Wirschell M, Yagi T, Bower R, Porter ME, Sale WS, Kamiya R. 2009. A novel ankyrin-repeat protein interacts with the regulatory proteins of inner arm dynein f (I1) of Chlamydomonas reinhardtii. Cell Motil. Cytoskeleton 66, 448–456. ( 10.1002/cm.20324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto R, Yanagisawa HA, Yagi T, Kamiya R. 2008. Novel 44-kilodalton subunit of axonemal Dynein conserved from Chlamydomonas to mammals. Eukaryot. Cell 7, 154–161. ( 10.1128/EC.00341-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto R, Yanagisawa HA, Yagi T, Kamiya R. 2006. A novel subunit of axonemal dynein conserved among lower and higher eukaryotes. FEBS Lett. 580, 6357–6360. ( 10.1016/j.febslet.2006.10.047) [DOI] [PubMed] [Google Scholar]
- 45.Yagi T, Uematsu K, Liu Z, Kamiya R. 2009. Identification of dyneins that localize exclusively to the proximal portion of Chlamydomonas flagella. J. Cell Sci. 122, 1306–1314. ( 10.1242/jcs.045096) [DOI] [PubMed] [Google Scholar]
- 46.Hoops HJ, Witman GB. 1983. Outer doublet heterogeneity reveals structural polarity related to beat direction in Chlamydomonas flagella. J. Cell Biol. 97, 902–908. ( 10.1083/jcb.97.3.902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bui KH, Sakakibara H, Movassagh T, Oiwa K, Ishikawa T. 2009. Asymmetry of inner dynein arms and inter-doublet links in Chlamydomonas flagella. J. Cell Biol. 186, 437–446. ( 10.1083/jcb.200903082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin J, Heuser T, Song K, Fu X, Nicastro D. 2012. One of the nine doublet microtubules of eukaryotic flagella exhibits unique and partially conserved structures. PLoS ONE 7, e46494 ( 10.1371/journal.pone.0046494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magiera MM, Singh P, Gadadhar S, Janke C. 2018. Tubulin posttranslational modifications and emerging links to human disease. Cell 173, 1323–1327. ( 10.1016/j.cell.2018.05.018) [DOI] [PubMed] [Google Scholar]
- 50.Lechtreck KF, Geimer S. 2000. Distribution of polyglutamylated tubulin in the flagellar apparatus of green flagellates. Cell Motil. Cytoskeleton 47, 219–235. () [DOI] [PubMed] [Google Scholar]
- 51.Kubo T, Yanagisawa HA, Liu Z, Shibuya R, Hirono M, Kamiya R. 2014. A conserved flagella-associated protein in Chlamydomonas, FAP234, is essential for axonemal localization of tubulin polyglutamylase TTLL9. Mol. Biol. Cell 25, 107–117. ( 10.1091/mbc.e13-07-0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kubo T, Yagi T, Kamiya R. 2012. Tubulin polyglutamylation regulates flagellar motility by controlling a specific inner-arm dynein that interacts with the dynein regulatory complex. Cytoskeleton 69, 1059–1068. ( 10.1002/cm.21075) [DOI] [PubMed] [Google Scholar]
- 53.Kubo T, Hirono M, Aikawa T, Kamiya R, Witman GB. 2015. Reduced tubulin polyglutamylation suppresses flagellar shortness in Chlamydomonas. Mol. Biol. Cell 26, 2810–2822. ( 10.1091/mbc.E15-03-0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kubo T, Yanagisawa HA, Yagi T, Hirono M, Kamiya R. 2010. Tubulin polyglutamylation regulates axonemal motility by modulating activities of inner-arm dyneins. Curr. Biol. 20, 441–445. ( 10.1016/j.cub.2009.12.058) [DOI] [PubMed] [Google Scholar]
- 55.Valenstein ML, Roll-Mecak A. 2016. Graded control of microtubule severing by tubulin glutamylation. Cell 164, 911–921. ( 10.1016/j.cell.2016.01.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bottier M, Thomas KA, Dutcher SK, Bayly PV. 2019. How does cilium length affect beating? Biophys. J. 116, 1292–1304. ( 10.1016/j.bpj.2019.02.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tam LW, Ranum PT, Lefebvre PA. 2013. CDKL5 regulates flagellar length and localizes to the base of the flagella in Chlamydomonas. Mol. Biol. Cell 24, 588–600. ( 10.1091/mbc.e12-10-0718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oda T, Yagi T, Yanagisawa H, Kikkawa M. 2013. Identification of the outer-inner dynein linker as a hub controller for axonemal dynein activities. Curr. Biol. 23, 656–664. ( 10.1016/j.cub.2013.03.028) [DOI] [PubMed] [Google Scholar]
- 59.Dean AB, Mitchell DR. 2015. Late steps in cytoplasmic maturation of assembly-competent axonemal outer arm dynein in Chlamydomonas require interaction of ODA5 and ODA10 in a complex. Mol. Biol. Cell 26, 3596–3605. ( 10.1091/mbc.E15-05-0317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dean AB, Mitchell DR. 2013. Chlamydomonas ODA10 is a conserved axonemal protein that plays a unique role in outer dynein arm assembly. Mol. Biol. Cell 24, 3689–3696. ( 10.1091/mbc.E13-06-0310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hjeij R, et al. 2014. CCDC151 mutations cause primary ciliary dyskinesia by disruption of the outer dynein arm docking complex formation. Am. J. Hum. Genet. 95, 257–274. ( 10.1016/j.ajhg.2014.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alsaadi MM, et al. 2014. Nonsense mutation in coiled-coil domain containing 151 gene (CCDC151) causes primary ciliary dyskinesia. Hum. Mutat. 35, 1446–1448. ( 10.1002/humu.22698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edwards BFL, Wheeler RJ, Barker AR, Moreira-Leite FF, Gull K, Sunter JD. 2018. Direction of flagellum beat propagation is controlled by proximal/distal outer dynein arm asymmetry. Proc. Natl Acad. Sci. USA 115, E7341–E7350. ( 10.1073/pnas.1805827115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright RL, Chojnacki B, Jarvik JW. 1983. Abnormal basal-body number, location, and orientation in a striated fiber-defective mutant of Chlamydomonas reinhardtii. J. Cell Biol. 96, 1697–1707. ( 10.1083/jcb.96.6.1697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marshall W, Vucica Y, Rosenbaum J. 2001. Kinetics and regulation of de novo centriole assembly. Implications for the mechanism of centriole duplication. Curr. Biol. 11, 308–317. ( 10.1016/S0960-9822(01)00094-X) [DOI] [PubMed] [Google Scholar]
- 66.Ochil T, et al. Submitted. CCDC61/VFL3 is a paralog of SAS6 and promotes ciliary functions. [DOI] [PMC free article] [PubMed]
- 67.Hoops HJ, Wright RL, Jarvik JW, Witman GB. 1984. Flagellar waveform and rotational orientation in a Chlamydomonas mutant lacking normal striated fibers. J. Cell Biol. 98, 818–824. ( 10.1083/jcb.98.3.818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wan KY, Goldstein RE. 2016. Coordinated beating of algal flagella is mediated by basal coupling. Proc. Natl Acad. Sci. USA 113, E2784–E2793. ( 10.1073/pnas.1518527113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Segal RA, Huang B, Ramanis Z, Luck DJ. 1984. Mutant strains of Chlamydomonas reinhardtii that move backwards only. J. Cell Biol. 98, 2026–2034. ( 10.1083/jcb.98.6.2026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tam LW, Lefebvre PA. 2002. The Chlamydomonas MBO2 locus encodes a conserved coiled-coil protein important for flagellar waveform conversion. Cell Motil. Cytoskeleton 51, 197–212. ( 10.1002/cm.10023) [DOI] [PubMed] [Google Scholar]
- 71.Mendes MT, Gogendeau D, Pennetier C, Janke C, Basto R. 2014. Bug22 influences cilium morphology and the post-translational modification of ciliary microtubules. Biol. Open 3, 138–151. ( 10.1242/bio.20146577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mitchell DR, Nakatsugawa M. 2004. Bend propagation drives central pair rotation in Chlamydomonas reinhardtii flagella. J. Cell Biol. 166, 709–715. ( 10.1083/jcb.200406148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dutcher SK, Huang B, Luck DJ. 1984. Genetic dissection of the central pair microtubules of the flagella of Chlamydomonas reinhardtii. J. Cell Biol. 98, 229–236. ( 10.1083/jcb.98.1.229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Witman GB, Plummer J, Sander G. 1978. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. J. Cell Biol. 76, 729–747. ( 10.1083/jcb.76.3.729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adams GM, Huang B, Piperno G, Luck DJ. 1981. Central-pair microtubular complex of Chlamydomonas flagella: polypeptide composition as revealed by analysis of mutants. J. Cell Biol. 91, 69–76. ( 10.1083/jcb.91.1.69) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith EF, Lefebvre PA. 1996. PF16 encodes a protein with armadillo repeats and localizes to a single microtubule of the central apparatus in Chlamydomonas flagella. J. Cell Biol. 132, 359–370. ( 10.1083/jcb.132.3.359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith EF, Lefebvre PA. 1997. PF20 gene product contains WD repeats and localizes to the intermicrotubule bridges in Chlamydomonas flagella. Mol. Biol. Cell 8, 455–467. ( 10.1091/mbc.8.3.455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mitchell DR, Sale WS. 1999. Characterization of a Chlamydomonas insertional mutant that disrupts flagellar central pair microtubule-associated structures. J. Cell Biol. 144, 293–304. ( 10.1083/jcb.144.2.293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mitchell DR. 2003. Reconstruction of the projection periodicity and surface architecture of the flagellar central pair complex. Cell Motil. Cytoskeleton 55, 188–199. ( 10.1002/cm.10121) [DOI] [PubMed] [Google Scholar]
- 80.Dymek EE, Lefebvre PA, Smith EF. 2004. PF15p is the Chlamydomonas homologue of the katanin p80 subunit and is required for assembly of flagellar central microtubules. Eukaryot. Cell 3, 870–879. ( 10.1128/EC.3.4.870-879.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lechtreck KF, Witman GB. 2007. Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility. J. Cell Biol. 176, 473–482. ( 10.1083/jcb.200611115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dymek EE, Smith EF. 2012. PF19 encodes the p60 catalytic subunit of katanin and is required for assembly of the flagellar central apparatus in Chlamydomonas. J. Cell Sci. 125, 3357–3366. ( 10.1242/jcs.096941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carbajal-Gonzalez BI, Heuser T, Fu X, Lin J, Smith BW, Mitchell DR, Nicastro D. 2013. Conserved structural motifs in the central pair complex of eukaryotic flagella. Cytoskeleton 70, 101–120. ( 10.1002/cm.21094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao L, Hou Y, Picariello T, Craige B, Witman GB. 2019. Proteome of the central apparatus of a ciliary axoneme. J. Cell Biol. 218, 2051–2070. ( 10.1083/jcb.201902017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deininger W, Kroger P, Hegemann U, Lottspeich F, Hegemann P. 1995. Chlamyrhodopsin represents a new type of sensory photoreceptor. EMBO J. 14, 5849–5858. ( 10.1002/j.1460-2075.1995.tb00273.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.King SJ, Dutcher SK. 1997. Phosphoregulation of an inner dynein arm complex in Chlamydomonas reinhardtii is altered in phototactic mutant strains. J. Cell Biol. 136, 177–191. ( 10.1083/jcb.136.1.177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamamoto R, et al. 2013. The MIA complex is a conserved and novel dynein regulator essential for normal ciliary motility. J. Cell Biol. 201, 263–278. ( 10.1083/jcb.201211048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rupp G, O'Toole E, Gardner LC, Mitchell BF, Porter ME. 1996. The sup-pf-2 mutations of Chlamydomonas alter the activity of the outer dynein arms by modification of the gamma-dynein heavy chain. J. Cell Biol. 135, 1853–1865. ( 10.1083/jcb.135.6.1853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dutcher SK, Gibbons W, Inwood WB. 1988. A genetic analysis of suppressors of the PF10 mutation in Chlamydomonas reinhardtii. Genetics 120, 965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.King SJ, Inwood WB, O'Toole ET, Power J, Dutcher SK. 1994. The bop2-1 mutation reveals radial asymmetry in the inner dynein arm region of Chlamydomonas reinhardtii. J. Cell Biol. 126, 1255–1266. ( 10.1083/jcb.126.5.1255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin J, et al. 2019. FAP57/WDR65 targets assembly of a subset of inner arm dyneins and connects to regulatory hubs in cilia. Mol. Biol. Cell 30, 2659–2680. ( 10.1091/mbc.e13-08-0464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bustamante-Marin XM, et al. 2019. Mutation of CFAP57 causes primary ciliary dyskinesia by disrupting the asymmetric targeting of a subset of ciliary inner dynein arms. bioRxiv, 773028 ( 10.1101/773028) [DOI] [PMC free article] [PubMed]
- 93.Holmes J, Dutcher S. 1989. Cellular asymmetry in Chlamydomonas reinhardtii. J. Cell Sci. 94, 273–285. [DOI] [PubMed] [Google Scholar]
- 94.Dieckmann CL. 2003. Eyespot placement and assembly in the green alga Chlamydomonas. Bioessays 25, 410–416. ( 10.1002/bies.10259) [DOI] [PubMed] [Google Scholar]
- 95.Kamiya R, Witman GB. 1984. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J. Cell Biol. 98, 97–107. ( 10.1083/jcb.98.1.97) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kamiya R, Hasegawa E. 1987. Intrinsic difference in beat frequency between the two flagella of Chlamydomonas reinhardtii. Exp. Cell Res. 173, 299–304. ( 10.1016/0014-4827(87)90357-0) [DOI] [PubMed] [Google Scholar]
- 97.Wan KY, Leptos KC, Goldstein RE. 2014. Lag, lock, sync, slip: the many ‘phases’ of coupled flagella. R. Soc. Interface 11, 20131160 ( 10.1098/rsif.2013.1160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takada S, Kamiya R. 1997. Beat frequency difference between the two flagella of Chlamydomonas depends on the attachment site of outer dynein arms on the outer-doublet microtubules. Cell Motil. Cytoskeleton 36, 68–75. () [DOI] [PubMed] [Google Scholar]
- 99.Sakakibara H, Mitchell DR, Kamiya R. 1991. A Chlamydomonas outer arm dynein mutant missing the alpha heavy chain. J. Cell Biol. 113, 615–622. ( 10.1083/jcb.113.3.615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Casey DM, Inaba K, Pazour GJ, Takada S, Wakabayashi K, Wilkerson CG, Kamiya R, Witman GB. 2003. DC3, the 21-kDa subunit of the outer dynein arm-docking complex (ODA-DC), is a novel EF-hand protein important for assembly of both the outer arm and the ODA-DC. Mol. Biol. Cell 14, 3650–3663. ( 10.1091/mbc.e03-01-0057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Horst CJ, Witman GB. 1993. ptx1, a nonphototactic mutant of Chlamydomonas, lacks control of flagellar dominance. J. Cell Biol. 120, 733–741. ( 10.1083/jcb.120.3.733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Okita N, Isogai N, Hirono M, Kamiya R, Yoshimura K. 2005. Phototactic activity in Chlamydomonas ‘non-phototactic’ mutants deficient in Ca2+-dependent control of flagellar dominance or in inner-arm dynein. J. Cell Sci. 118, 529–537. ( 10.1242/jcs.01633) [DOI] [PubMed] [Google Scholar]
- 103.Huang B, Ramanis Z, Dutcher SK, Luck DJ. 1982. Uniflagellar mutants of Chlamydomonas: evidence for the role of basal bodies in transmission of positional information. Cell 29, 745–753. ( 10.1016/0092-8674(82)90436-6) [DOI] [PubMed] [Google Scholar]
- 104.Geyer VF, Julicher F, Howard J, Friedrich BM. 2013. Cell-body rocking is a dominant mechanism for flagellar synchronization in a swimming alga. Proc. Natl Acad. Sci. USA 110, 18 058–18 063. ( 10.1073/pnas.1300895110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brumley DR, Wan KY, Polin M, Goldstein RE. 2014. Flagellar synchronization through direct hydrodynamic interactions. eLife 3, e02750 ( 10.7554/eLife.02750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Viswanadha R, Sale WS, Porter ME. 2017. Ciliary motility: regulation of axonemal dynein motors. Cold Spring Harb. Perspect. Biol. 9, a018325 ( 10.1101/cshperspect.a018325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nakano I, Kobayashi T, Yoshimura M, Shingyoji C. 2003. Central-pair-linked regulation of microtubule sliding by calcium in flagellar axonemes. J. Cell Sci. 116, 1627–1636. ( 10.1242/jcs.00336) [DOI] [PubMed] [Google Scholar]
- 108.Lindemann CB, Lesich KA. 2010. Flagellar and ciliary beating: the proven and the possible. J. Cell Sci. 123, 519–528. ( 10.1242/jcs.051326) [DOI] [PubMed] [Google Scholar]
- 109.Huang B, Ramanis Z, Luck DJ. 1982. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for flagellar function. Cell 28, 115–124. ( 10.1016/0092-8674(82)90381-6) [DOI] [PubMed] [Google Scholar]
- 110.Rupp G, Porter ME. 2003. A subunit of the dynein regulatory complex in Chlamydomonas is a homologue of a growth arrest-specific gene product. J. Cell Biol. 162, 47–57. ( 10.1083/jcb.200303019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bayly PV, Dutcher SK. 2016. Steady dynein forces induce flutter instability and propagating waves in mathematical models of flagella. R. Soc. Interface 13, 20160523 ( 10.1098/rsif.2016.0523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holland EM, Harz H, Uhl R, Hegemann P. 1997. Control of phobic behavioral responses by rhodopsin-induced photocurrents in Chlamydomonas. Biophys. J. 73, 1395–1401. ( 10.1016/S0006-3495(97)78171-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wargo MJ, Smith EF. 2003. Asymmetry of the central apparatus defines the location of active microtubule sliding in Chlamydomonas flagella. Proc. Natl Acad. Sci. USA 100, 137–142. ( 10.1073/pnas.0135800100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.King SM. 2010. Sensing the mechanical state of the axoneme and integration of Ca2+ signaling by outer arm dynein. Cytoskeleton 67, 207–213. ( 10.1002/cm.20445) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.