Abstract
Cardiovascular disease (CVD) is an important cause of morbidity and mortality after liver transplantation (LT). Although LT is associated with dyslipidemia, particularly atherogenic lipoprotein subparticles, the impact of these subparticles on CVD-related events is unknown. Therefore, the aim of the current study was to evaluate the impact of small dense (sdLDL-C) low-density lipoprotein (LDL) cholesterol (LDL-C) on CVD events. Prospectively enrolled patients (N = 130) had detailed lipid profile consisting of traditional lipid parameters and sdLDL-C and were followed for CVD events. The primary endpoint was a CVD composite consisting of myocardial infarction (MI), angina, need for coronary revascularization, and cardiac death. Mean age of the cohort was 58 ± 11 years, and the most common etiology of liver disease (LD) was hepatitis C virus (N = 48) and nonalcoholic steatohepatitis (N = 23). A total of 20 CVD events were noted after median follow-up of 45 months. The baseline traditional profile was similar in patients with and without CVD events. A serum LDL-C cutoff of 100 mg/dL was unable to identify individuals at risk of a CVD event (P = 0.86). In contrast, serum concentration of atherogenic sdLDL-C >25 mg/dL was predictive of CVD events with a hazard ratio of 6.376 (95% confidence interval, 2.65, 15.34; P < 0.001). This relationship was independent of diabetes, hypertension, sex, ethnicity, LD, obesity, and statin use. Conclusion: sdLDL-C independently predicted CVD events whereas LDL-C did not. Thus, sdLDL-C may provide a useful clinical tool in risk stratifying and managing patients after LT.
Cardiovascular disease (CVD) is an important cause of mortality in liver transplant recipients (LTRs) and is only expected to rise as more-effective therapies for recurrent liver disease (LD) are developed such as direct antiviral agonists.(1–3) CVD risk can be stratified into either nonmodifiable (age, sex, family history, etc.) or modifiable (lipid prof smoking cessation, weight loss, etc.) risk factors.(4,5) In clinical practice, risk of future CVD events is modified by positively impacting modifiable risk factors. Traditional lipid profile consisting of total cholesterol, low-density lipoprotein (LDL) cholesterol (LDL-C), high-density lipoprotein (HDL) cholesterol (HDL-C), and triglycerides are often used to risk stratify patients at increased risk for future CVD events and in need of lipid-lowering therapy.(6)
The recommendation to implement lipid-lowering therapy is based on perturbations of the traditional lipid profile, and the target levels have evolved over time.(7,8) This is partly attributed to the recognition of metabolic comorbidities as key contributors to future CVD risk, but also to suboptimal performance of traditional lipid profile to predict future CVD outcomes.(9,10) The traditional lipid profile fails to account for complex lipoprotein subparticles that have varying degrees of CVD risk, and measuring individual lipoproteins linked to atherosclerosis is a better predictor of future CVD events.(11–14) The key lipoproteins that are linked to CVD events include small dense LDL-C (sdLDL-C), LDL particle size, HDL-2 subtype, and very low-density lipoprotein (VLDL) cholesterol (VLDL-C).(11,15–17) Liver transplantation (LT) is associated with a propensity for proatherosclerotic lipoprotein milieu characterized by increase in sdLDL-C, VLDL size, and concentrations.(18) These findings are further exaggerated in patients with post-LT hepatic steatosis (HS) and by choice of immunosuppression with cyclosporine use favoring more atherogenic lipoproteins.(19) Despite the robust literature in the general population linking atherogenic sdLDL-C to CVD events, there are currently no such data in LTRs. Therefore, to overcome this significant knowledge gap, we conducted this prospective study to evaluate the performance of the traditional lipid profile and sdLDL-C to predict CVD events after LT.
Patients and Methods
The present study is an ancillary study of an ongoing study of cardiometabolic risk in LTRs. The study was reviewed and approved by the institutional review board of Virginia Commonwealth University (Richmond, VA). Data were analyzed in its entirety by the investigators who are fully responsible for the data and conclusion. The manuscript was reviewed and approved by all investigators before its submission.
PATIENT POPULATION
The study population consisted of LTRs followed at the author’s institution and were recruited to participate between January 1, 2012 and January 1, 2014. Informed consent was obtained for all patients prior to study enrollment. Patients with acute cellular rejection, graft cirrhosis, fibrosing cholestatic hepatitis C virus (HCV), multiorgan abdominal transplant, end-stage renal disease (ESRD) after LT on hemodialysis, and active malignancy were excluded. Impact of HCV therapy on lipoprotein subparticles is unknown. Also, HCV confers increased atherosclerotic risk, and therefore patients who were enrolled on active therapy may have a different atherogenic risk profile after sustained virological response.(20,21) Thus, in this proof-of-concept study, patients on active HCV therapy were excluded, but allowed to participate after they completed therapy. After an overnight fast, protocol-driven anthropometric measures and blood tests were collected.
Dyslipidemia was managed according to societal guidelines according to serum lipid levels and presence of cardiometabolic risk factors. The first choice of medication for management of dyslipidemia was β-hydroxy β-methylglutaryl-CoA reductase inhibitors or statin, which were titrated to desired lipid goal or patient tolerance. Patients intolerant to statin therapy were treated with alternative regiments, including fibrates, fish oil, and ezetimibe. All patients were followed regularly in the clinic at least every 6 months until either last follow-up or development of a cardiovascular event (CVE).
LIPOPROTEIN PROFILE
The tradition lipid profile, consisting of HDL-C, total cholesterol, LDL-C, and triglycerides, was collected for all patients. Additional lipoprotein subparticles, as outlined below, were collected. LDLs: LDL-C, LDL particle concentration (LDL-P) and size, sdLDL-C, sdLDL particle concentration, and % sdLDL-C.(11,15,22,23); HDLs: HDL-C, HDL particle concentration (HDL-P), HDL-C subclass 2 (HDL2-C), and apolipoprotein A-1 (apoA-1). HDL2 refers to medium-sized HDL particles(17,24); and VLDLs: serum apolipoprotein B (apoB), triglycerides, VLDL particle size, and VLDL particle concentration (VLDL-P).(25)
Lipoprotein cholesterol concentration (sdLDL-C, HDL-C, and LDL-C) was measured by qualitative assay kits as described.(26) Percent sdLDL was calculated from sdLDL-C and LDL-C. VLDL, LDL, and HDL particle number and size were measured by proton nuclear magnetic resonance spectroscopy at LipoScience (Raleigh, NC).
STATISTICAL ANALYSIS
The primary outcome of the current study was a composite endpoint consisting of angina, myocardial infraction (MI), need for revascularization, or cardiac death.(27–29) Patients with angina who subsequently had CVD on either abnormal cardiac stress test or cardiac catherization were included, whereas patients with chest pain and negative cardiac chest pain were excluded from the analysis. Patients were considered to be high risk if sdLDL-C >25 mg/dL or LDL-C >100 mg/dL.(11,12,30) Kaplan-Meier curves were constructed, and differences between curves were evaluated using the log-rank test. Impact of sdLDL-C, biochemical, and clinical parameters on risk of CVD event was evaluated by Cox regression analysis. Cox regression models were constructed with LD, ethnicity, sex, hypertension, diabetes, obesity (body mass index [BMI] >30 kg/m2), statin use, and sdLDL-C (>25 mg/dL) as covariates. Continuous variables are presented as a mean ± SD for normally distributed variables and as a median with interquartile range (IQR) for skewed data. Categorical variables are reported as numbers and percent. Comparisons between groups for continuous variables were made using the Student t test, whereas categorical variables were compared using the κ2 test. Baseline and follow-up measurements were compared using a paired t test. A nominal P value of <0.05 was considered statistically significant. Linear regression models were constructed with sdLDL-C as the outcome measure. Covariates included: immunosuppression use (tacrolimus vs. cyclosporine), age, sex, ethnicity, BMI, LD, comorbid conditions (diabetes, hypertension, or obesity), VLDL-related lipoproteins (VLDL size, concentration), LDL size, and HDL-related parameters. Those variables noted to have a P value ≤0.1 were subsequently included in multivariate models at baseline. Data analysis was performed using SPSS software (version 24.0; IBM, Chicago, IL).
Results
PATIENT CHARACTERISTICS
A total of 173 patients were screened, and, of these, 130 met entry criteria and were enrolled into the study and included in the final analysis (Table 1). The most common exclusion criterion was active HCV therapy (N = 41); however, 10 of these patients were subsequently enrolled after completing therapy. Other common exclusion criteria included dual solid organ transplants (N = 6 simultaneous liver kidney and N = 1 combined liver-heart transplants), graft cirrhosis (N = 3), and ESRD (N = 2). Leading etiologies of chronic LD (CLD) leading to cirrhosis requiring LT were HCV (N = 48), nonalcoholic steatohepatitis (NASH; N = 23), and alcohol-related cirrhosis (N = 15). At enrollment, the study cohort consisted largely of males (N = 81) and non-Hispanic whites (N = 99). Mean age of the cohort was 58 ± 11 years, and mean BMI was 29.2 ± 5.7 kg/m2. Prevalence of cardiometabolic conditions, including diabetes, hypertension, and dyslipidemia, was 45 (35%), 106 (82%), and 52 (40%), respectively, at baseline on enrollment.
TABLE 1.
Baseline Clinical Characteristics of the Study Cohort
| No CVD Event | CVD Event | P Value | ||
|---|---|---|---|---|
| Clinical characteristics | ||||
| N | 130 | 110 | 20 | |
| Age (years) | 58 ± 11 | 58 ± 11 | 58 ± 11 | 0.86 |
| Sex, male (%) | 81 (62) | 66 (60) | 15 (75) | 0.21 |
| Ethnicity | 0.85 | |||
| White (%) | 99 (76) | |||
| Black (%) | 25 (19) | 21 (19) | 4 (20) | |
| Etiology of LD | 0.02 | |||
| HCV (%) | 48 (37) | 45 (41) | 3 (15) | |
| Alcohol (%) | 15 (12) | 13 (12) | 2 (10) | |
| NASH (%) | 23 (18) | 16 (15) | 7 (35) | |
| Follow-up time from LT (months) | 66 ± 56 | 63 ± 54 | 87 ± 69 | 0.23 |
| Cardiometabolic conditions | ||||
| BMI (kg/m2) | 29.2 ± 5.7 | 28.4 ± 5.2 | 33.5 ± 6.3 | 0.002 |
| Diabetes (%) | 45 (35) | 34 (31) | 11 (55) | 0.04 |
| Dyslipidemia (%) | 52 (40) | 39 (35) | 13 (65) | 0.02 |
| Hypertension (%) | 106 (82) | 88 (75) | 18 (90) | 0.37 |
| Obesity (%) | 52 (40) | 39 (35) | 13 (65) | 0.02 |
| Laboratory paramaters | ||||
| ALT (IU/L) | 48 ± 38 | 50 ± 40 | 37 ± 21 | 0.04 |
| AST (IU/L) | 47 ± 30 | 49 ± 31 | 37 ± 17 | 0.02 |
| Alkaline phosphatase (IU/L) | 136 ± 98 | 139±103 | 114 ± 65 | 0.17 |
| Bilirubin, total (mg/dL) | 0.78 ± 0.77 | 0.82 ± 0.83 | 0.54 ± 0.16 | 0.001 |
| Bilirubin, direct (mg/dL) | 0.33 ± 0.33 | 0.35 ± 0.36 | 0.20 ± 0.07 | <0.001 |
| Immunosuppressants | ||||
| Cyclosporine (%) | 41 (32) | 36 (33) | 5 (25) | 0.61 |
| Rapamune (%) | 27 (21) | 20 (18) | 7 (35) | 0.13 |
| Tacrolimus (%) | 78 (60) | 68 (62) | 10 (50) | 0.33 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
BASELINE LIPID PROFILE
Mean values of the detailed lipid profile are reported in Table 2. At baseline, serum LDL-C, HDL-C, total cholesterol, and triglycerides levels were 97 ± 35, 54 ± 18, 177 ± 47, and 182 ± 31 mg/dL, respectively. Elevated serum LDL-C, as defined by LDL-C >100 mg/dL, was present in 55 patients (42%). Lipoprotein subparticles are also detailed in Table 2. Specifically, the concentration of major atherogenic liproprotein subparticles were as follows: sdLDL 27.0 ± 14.0 mg/dL, VLDL-P 5.65 ± 5.84 umol/L, apoB 84 ± 33 mg/dL, and LDL size of 20.9 ± 0.78 nm. Concentration of HDL-2, the major antiatherogenic HDL subparticle, was 19.6 ± 11.1 mg/dL.
TABLE 2.
Baseline Lipid Characteristics of the Entire Cohort
| Entire Cohort (N = 130) | No CVD Event (N = 110) | CVD Event (N = 20) | P Value | |
|---|---|---|---|---|
| HDL | ||||
| apoA-1 (mg/dL) | 136 ± 27 | 137 ± 28 | 131 ± 23 | 0.41 |
| HDL-C (md/dL) | 54 ± 18 | 55 ± 17 | 49 ± 16 | 0.14 |
| HDL-P (umol/L) | 29.5 ± 8.0 | 30 ± 8 | 29 ± 5 | 0.49 |
| HDL2-C (mg/dL) | 19.6 ± 11.1 | 20 ± 11 | 16 ± 9 | 0.13 |
| LDL | ||||
| LDL-C (mg/dL) | 97 ± 35 | 97 ± 34 | 96 ± 42 | 0.87 |
| LDL-P (umol/L) | 1,372 ± 556 | 1,352 ± 537 | 1,487 ± 657 | 0.41 |
| LDL particle size (nm) | 20.9 ± 0.78 | 20.9 ± 0.78 | 20.4 ±0.67 | 0.06 |
| sdLDL-C (mg/dL) | 27.0 ± 14.0 | 25.4 ± 13.5 | 36.0 ± 13.8 | 0.002 |
| % sdLDL-C | 26.9 ± 9.8 | 25.4 ± 9.3 | 35 ± 8.3 | <0.001 |
| VLDL | ||||
| apoB (mg/dL) | 84 ± 33 | 82 ± 27 | 89 ± 37 | 0.38 |
| Triglycerides (mg/dL) | 182 ± 131 | 161 ± 89 | 229 ± 98 | 0.008 |
| VLDL particle size (nm) | 49.6 ± 6.4 | 49.4 ± 6.7 | 50.9 ± 4.1 | 0.35 |
| VLDL-P (umol/L) | 5.65 ± 5.84 | 5.42 ± 6.15 | 7.19 ± 2.77 | 0.49 |
Serum sdLDL-C was strongly associated with VLDL-related parameters, but not HDL-C, in regression analysis. Serum sdLDL-C was positively associated with serum triglycerides (standardized β-coefficient, 0.631; P < 0.001), apoB (standardized β-coefficient, 0.795; P < 0.001), and VLDL-P (standardized β-coefficient, 0.624; P < 0.001). A positive trend between sdLDL-C and VLDL particle size was also noted. In contrast, an inverse relationship between sdLDL-C and LDL size (standardized β-coefficient, −0.392; P < 0.001) and tacrolimus, rather than cyclosporine, use (standardized β-coefficient, −0.201; P = 0.038) was demonstrated. sdLDL-C was also associated with BMI (standardized β-coefficient, 0.232; P = 0.008) and hypertension (standardized β-coefficient, 0.226; P = 0.01) at baseline. In the multivariate regression model, the association between sdLDL-C and apoB (β-coefficient, 0.691 ± 0.031; P < 0.001), VLDL-P (β-coefficient, 0.234 ± 0.142; P = 0.001), and LDL-size (β-coefficient, −0.170 ± 1.048; P = 0.006) was maintained.
CVEs
The primary composite CVD outcome occurred in 20 patients after median follow-up of 45 months (IQR, interquartile range, 35, 48).
Distribution of CVD outcomes included MI (N = 9), need for coronary revascularization (N = 8), and angina (N = 3). No CVD-associated death was noted within the study cohort. Using Cox proportional hazard analysis, only BMI was associated with risk of CVD event BMI (hazard ratio [HR], 1.103; 95% confidence interval [CI], 1.031; 1.179; P = 0.004). The traditional lipid profile, consisting of HDL-C, LDL-C, and total cholesterol, was not significantly different between patients that developed CVD events and those that did not (Fig. 1A–C; Tables 2 and 3). Serum triglycerides level was significantly higher at baseline in patients who had a CVD event (Fig. 1D). Of the lipoprotein subparticles evaluated, only baseline serum levels of sdLDL-C were significantly different in patients who had primary CVD-related outcomes (Fig. 2A–F). Presence of LDL-C >100 mg/dL was not predictive of CVD events (Fig. 3A). In contrast, baseline serum sdLDL-C was predictive of CVD events (Fig. 3B). At baseline, patients with sdLDL-C >25.0 mg/dL were more likely to have cardiometabolic diseases (Supporting Table S1). There were 17 CVD events observed in patients with sdLDL-C >25.0 mg/dL, whereas three CVD events occurred in those with sdLDL-C <25.0 mg/dL. A sdLDL-C level >25.0 mg/dL had an HR of 6.376 (95% CI, 2.65, 15.34; P < 0.001) for predicting CVEs. Although accounting for LD, ethnicity, sex, hypertension, obesity, and statin use, sdLDL-C remained significantly associated with the risk of a CVD event with an HR of 4.657 (95% CI, 1.335, 16.239; P = 0.016).
FIG. 1.
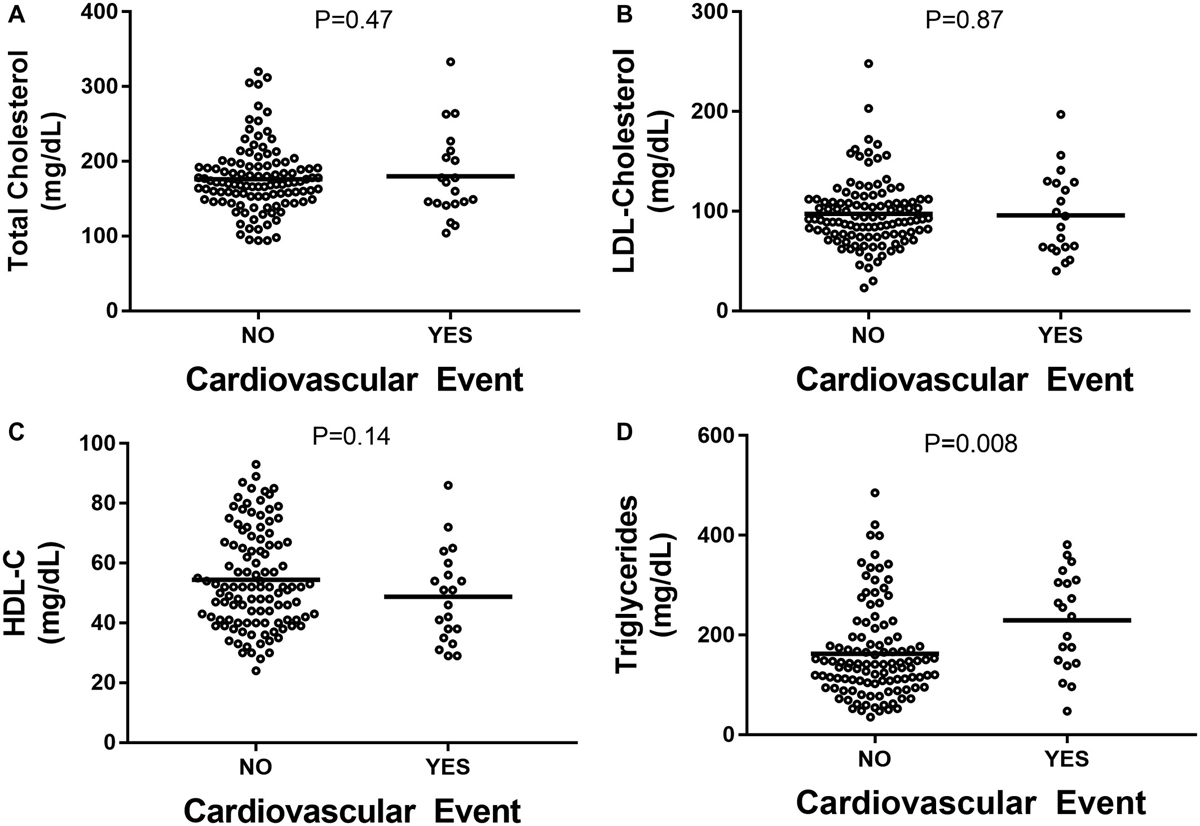
(A) Total serum cholesterol. (B) LDL-C. (C) HDL-C. (D) Serum triglycerides.
TABLE 3.
Traditional Lipid Profile in Patients Stratified Based on CVD Events
| No CVD Events | CVD Events | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | P Value | Baseline | Follow-up | P Value | |
| LDL-C (mg/dL) | 97 ± 34 | 96 ± 29 | 0.88 | 96 ± 42 | 86 ± 40 | 0.88 |
| HDL-C (mg/dL) | 55 ± 17 | 48 ± 20 | 0.15 | 49 ± 15 | 41 ± 15 | 0.11 |
| Triglycerides (mg/dL) | 161 ± 89 | 169±228 | 0.002 | 229 ± 98 | 226±135 | 0.008 |
| Cholesterol (mg/dL) | 176 ± 45 | 186±122 | 0.74 | 180 ± 58 | 167 ± 45 | 0.50 |
FIG. 2.
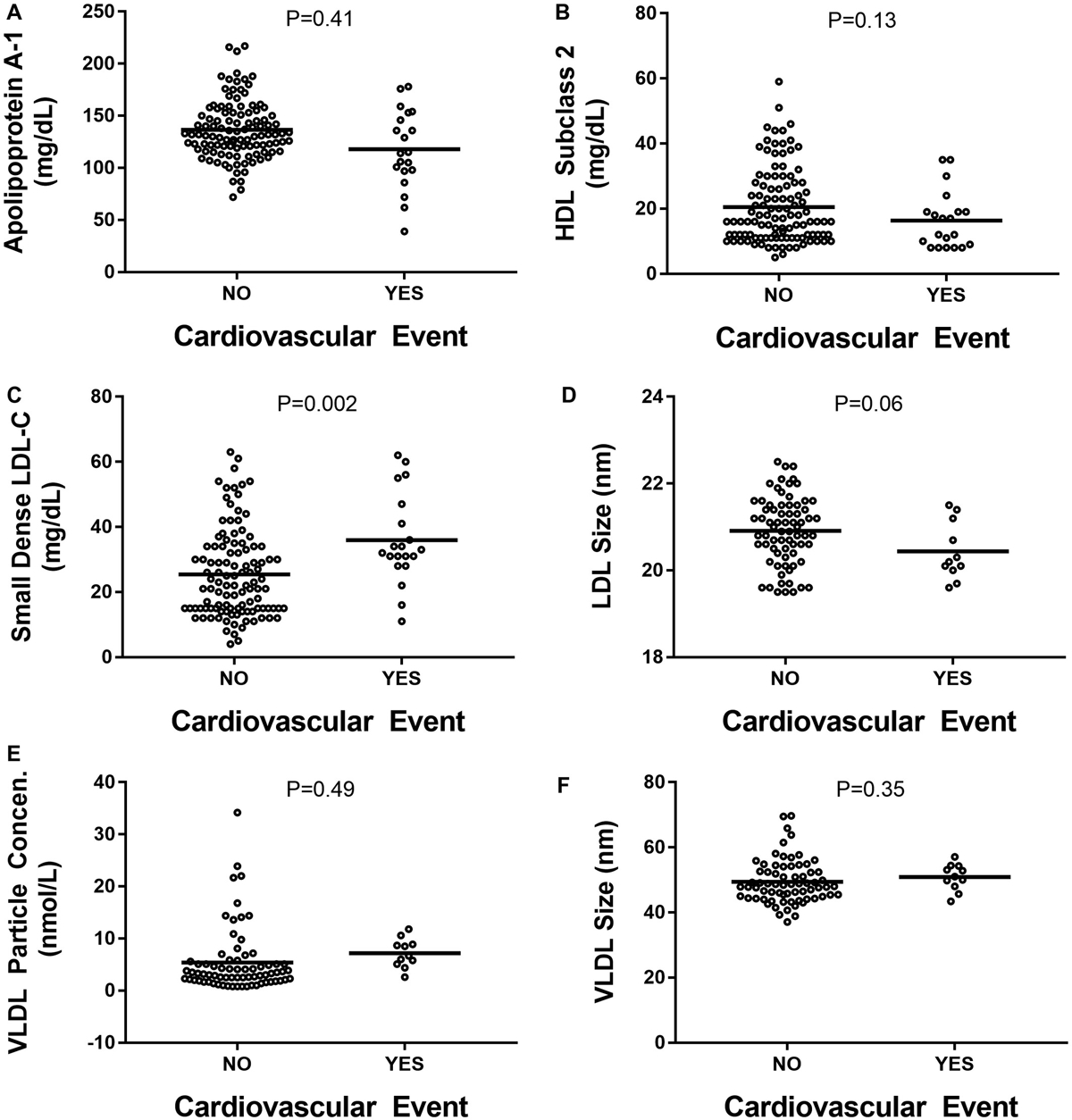
(A) apoA-1. (B) HDL-2. (C) sdLDL-C. (D) LDL particle size. (E) VLDL-P. (F) VLDL particle size.
FIG. 3.
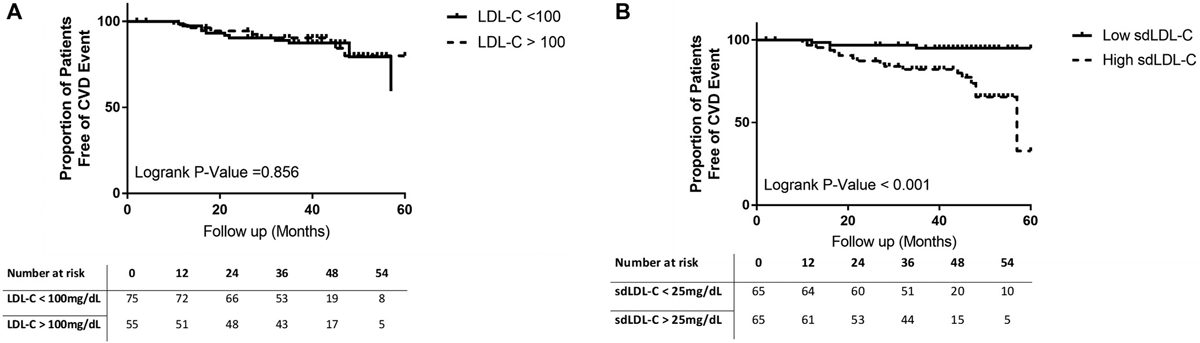
Kaplan-Meier plot showing cumulative probability of remaining free CVEs according to (A) LDL-C and (B) sdLDL-C.
Discussion
Dysregulated lipid metabolism is at the core of CVD risk; however, the traditional lipid profile may fail to accurately risk stratify patients at high risk for CVD events.(11,15,22–24,31) This is in part attributed to the complex nature of lipoproteins which consist of several subparticles, not all of which have atherogenic potential.(16,26) Thus, it is the ratio of atherogenic to nonatherogenic lipoprotein subparticles that predict future risk of CVD events.(11,23,24) Previously, in cross-sectional cohorts of patients without diabetes or HS, we demonstrated that LT favors the shift toward proatherogenic lipoprotein subparticles that is independent of traditional cardiometabolic risk factors such as diabetes, obesity, age, and sex.(18,19) In the current study, we reaffirm these findings with an expanded cohort and build on these findings by demonstrating that sdLDL-C was a predictor of CVD events, whereas the traditional lipid profile was not. Normally, triglycerides are produced within the liver and packaged with apoB and secreted in the form of VLDL.(32) In circulation, VLDL is hydro-lyzed by lipoprotein lipase, resulting in production of large buoyant LDL particles, which have a high affinity of LDL receptor and are readily taken out from circulation.(33) However, as patients develop CLD, VLDL metabolism within the liver is perturbed, leading to increased production of large, triglyceride-laden VLDL particles, which are secreted from the liver.(34) These large VLDL particles are slowly metabolized intravascularly (5-day residence time), which are subject to exchange processes that remove cholesteryl ester from the particle core and replace it with triacylglycerol.(35,36) These abnormally altered LDL particles serve as substrate for hepatic lipase, and if the activity of the enzyme is high enough, lipolysis results in small denser LDL particles or small dense LDL (sdLDL).(37) These sdLDL particles have lower affinity for LDL receptor, thus spending extended time in circulation. Furthermore, sdLDL particles can more readily migrate through the arterial endothelium and into the intima of the arterial wall, leading to greater propensity for intravascular plaque formation.(38,39) Within the intima, sdLDL undergoes oxidative modification and promotes inflammatory cascade, leading to migration and differentiation of monocytes into macrophages, which take up lipoprotein particles by scavenger receptors, leading to formation of lipid-laden foam cells.(40,41) These lipid-laden macrophages transition to a maladaptive proinflammatory state, which leads to unstable plaque and eventually plaque rupture and CVD-related event.(42) In the present study, we demonstrate a strong and independent association between sdLDL and serum triglycerides, VLDL-P, and LDL size, reaffirming the role of hypertriglyceridemia and large VLDL as key precursors for formation of sdLDL-C.
Calcineurin inhibitors are the backbone of the vast majority of immunosuppressive regiments in LTRs, but can promote dyslipidemia.(43,44) Previous studies have shown that cyclosporine has a higher propensity of dyslipidemia through a number of mechanisms, including increased triglyceride secretion, reduction in bile acid synthesis, and reduced lipolysis in circulation.(45) Additionally, cyclosporine is associated with more atherogenic lipoprotein subparticles, and these findings were confirmed in the current study.(18,19) The putative pathogenic mechanism necessary for production of sdLDL-C is likely exacerbated by cyclosporine use.
The present study utilized detailed lipoprotein subparticles to link specific proteins with CVD outcomes using a prospective cohort and provides valuable information that can be readily incorporated into clinical practice to hopefully improve clinical outcomes. However, the study should be evaluated in the context of its limitations. First, because of a lack of published data evaluating sdLDL-C and CVD events in LTRs, we could not adequately determine the power and sample size a priori. As such, in the present exploratory study, we provide robust data with sdLDL-C and CVD events to help adequately power future studies. Second, the current study was not designed to compare the diagnostic performance of sdLDL-C and the traditional lipid profile, and adequately powered prospective studies are necessary to evaluate this further. Third, the study did not evaluate the impact of lipid-lowering therapy on lipoprotein subparticles, which may vary with the choice and strength of lipid-lowering therapy. Thus, to truly evaluate the impact of lipid-lowering therapy on lipoprotein would require a well-designed study with a large sample size treated with a single agent. The etiology of CLD may also impact the lipoprotein profile, and the present study did not account for these differences. Whereas this is true, a large number of patients matched for etiology and severity of Ld would be required and can only be conducted in large, multicenter, collaborative efforts. Within these limitations, the current study provides proof of concept showing the limited utility of the traditional lipid profile for risk stratifying patients.
In conclusion, in the current study, sdLDL-C was an independent predictor of CVD outcomes in LTRs. Generation of atherogenic sdLDL-C likely results from insulin resistance, exposure to chronic immunosuppression, and fatty liver after LT. Future studies aimed at lowering serum sdLDL-C and linking to improvement in clinical outcomes are necessary to improve CVD risk management in LTRs.
Supplementary Material
Abbreviations:
- apoA-1
apolipoprotein A-1
- apoB
apolipoprotein B
- BMI
body mass index
- CI
confidence interval
- CLD
chronic LD
- CVD
cardiovascular disease
- CVE
cardiovascular event
- HCV
hepatitis C virus
- HDL
high-density lipoprotein
- HDL2-C
HDL-C subclass 2
- HDL-C
high-density lipoprotein cholesterol
- HR
hazard ratio
- LD
liver disease
- LDL
low-density lipoprotein
- LDL-C
low-density lipoprotein cholesterol
- LT
liver transplantation
- LTRs
liver transplant recipients
- MI
myocardial infarction
- NASH
nonalcoholic steatohepatitis
- sdLDL
small dense LDL
- sdLDL-C
small dense LDL-C
- VLDL
very low-density lipoprotein
- VLDL-C
very low-density lipoprotein cholesterol
- VLDL-P
VLDL particle concentration
Footnotes
Potential conflict of interest: Dr. Sterling received grants from AbbVie, Roche, and Gilead. Dr. Sanyal consults for and received grants from Echosens-Sandhill, Gilead, malinckrodt, Salix, Novartis, Galectin, and Sequana. He consults for and is employed by Sanyal Bio. He consults for and owns stock in Genfit. He consults for Pfizer, Nitto Denko, Nimbus, Boehringer Ingelheim, Hemoshear, Lilly, and Ardelyx. He received grants from Conatus, Bristol-Myers Squibb, and Merck. He received royalties from Elsevier and Uptodate. He owns stock in Akarna, NewCo LLC, Tiziana, and Natural Shield.
Supporting Information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.30518/suppinfo.
REFERENCES
- 1).Bhati C, Idowu MO, Sanyal AJ, Rivera M, Driscoll C, Stravitz RT, et al. Long Term Outcomes in Patients Undergoing Liver Transplantation for Nonalcoholic Steatohepatitis Related Cirrhosis. Transplantation 2017;101:1867–1874. [DOI] [PubMed] [Google Scholar]
- 2).Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant 2010;10:1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Agarwal K, Castells L, Müllhaupt B, Rosenberg WMC, McNabb B, Arterburn S, et al. Sofosbuvir/velpatasvir for 12 weeks in genotype 1–4 HCV-infected liver transplant recipients. J Hepatol 2018;69:603–607. [DOI] [PubMed] [Google Scholar]
- 4).Members WG, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016;133:e38–e60. [DOI] [PubMed] [Google Scholar]
- 5).Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 6).Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 7).Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. J Am Coll Cardiol 2014;63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 8).Nayor M, Vasan RS. Recent update to the US cholesterol treatment guidelines: a comparison with international guidelines. Circulation 2016;133:1795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Akosah KO, Schaper A, Cogbill C, Schoenfeld P. Preventing myocardial infarction in the young adult in the first place: how do the National Cholesterol Education Panel III guidelines perform? J Am Coll Cardiol 2003;41:1475–1479. [DOI] [PubMed] [Google Scholar]
- 10).Sachdeva A, Cannon CP, Deedwania PC, Labresh KA, Smith Jr SC, Dai D, et al. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J 2009;157:111–117.e2. [DOI] [PubMed] [Google Scholar]
- 11).Cromwell WC, Otvos JD, Keyes MJ, Pencina MJ, Sullivan L, Vasan RS, et al. LDL particle number and risk of future cardiovascular disease in the Framingham Offspring Study—implications for LDL management. J Clin Lipidol 2007;1:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol 2014;34:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Nishikura T, Koba S, Yokota Y, Hirano T, Tsunoda F, Shoji M, et al. Elevated small dense low-density lipoprotein cholesterol as a predictor for future cardiovascular events in patients with stable coronary artery disease. J Atheroscler Thromb 2014;21:755–767. [DOI] [PubMed] [Google Scholar]
- 14).Koba S, Hirano T, Ito Y, Tsunoda F, Yokota Y, Ban Y, et al. Significance of small dense low-density lipoprotein-cholesterol concentrations in relation to the severity of coronary heart diseases. Atherosclerosis 2006;189:206–214. [DOI] [PubMed] [Google Scholar]
- 15).Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. Am J Cardiol 2002;90:89–94. [DOI] [PubMed] [Google Scholar]
- 16).Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC Jr., et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2007;192:211–217. [DOI] [PubMed] [Google Scholar]
- 17).Assmann G, Schulte H, von Eckardstein A, Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis 1996;124(Suppl):S11–S20. [DOI] [PubMed] [Google Scholar]
- 18).Chhatrala R, Siddiqui MSBS, Stravitz RT, Driscoll C, Sanyal A, Sargeant C, et al. Evolution of the serum atherogenic risk in liver transplant recipients; role of lipoproteins, metabolic and inflammatory markers. Liver Transpl 2015;2015:623–630. [DOI] [PubMed] [Google Scholar]
- 19).Idowu MO, Chhatrala R, Siddiqui MSBSB, Driscoll C, Stravitz RT, Sanyal AJ, et al. De novo hepatic steatosis drives atherogenic risk in liver transplant recipients. Liver Transpl 2015;21:1395–1402. [DOI] [PubMed] [Google Scholar]
- 20).Alyan O, Kacmaz F, Ozdemir O, Deveci B, Astan R, Celebi AS, et al. Hepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon severity score system. Circ J 2008;72:1960–1965. [DOI] [PubMed] [Google Scholar]
- 21).Patel SS, Nabi E, Guzman L, Abbate A, Bhati C, Stravitz RT, et al. Coronary artery disease in decompensated patients undergoing liver transplantation evaluation. Liver Transpl 2018;24: 333–342. [DOI] [PubMed] [Google Scholar]
- 22).Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, et al. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation 1997;95:69–75. [DOI] [PubMed] [Google Scholar]
- 23).Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation 2006;113:1556–1563. [DOI] [PubMed] [Google Scholar]
- 24).Williams PT, Feldman DE. Prospective study of coronary heart disease vs. HDL2, HDL3, and other lipoproteins in Gofman’s Livermore Cohort. Atherosclerosis 2011;214:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2001;104: 1108–1113. [DOI] [PubMed] [Google Scholar]
- 26).Siddiqui MS, Sterling RK, Luketic VA, Puri P, Stravitz RT, Bouneva I, et al. Association between high-normal levels of alanine aminotransferase and risk factors for atherogenesis. Gastroenterology 2013;145:1271–1279. e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Eisen A, Bhatt DL, Steg PG, Eagle KA, Goto S, Guo J, et al. Angina and future cardiovascular events in stable patients with coronary artery disease: insights from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. J Am Heart Assoc 2016;5:e004080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Berecki-Gisolf J, Humphreyes-Reid L, Wilson A, Dobson A. Angina symptoms are associated with mortality in older women with ischemic heart disease. Circulation 2009;120:2330–2336. [DOI] [PubMed] [Google Scholar]
- 29).Mozaffarian D, Bryson CL, Spertus JA, McDonell MB, Fihn SD. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J 2003;146:1015–1022. [DOI] [PubMed] [Google Scholar]
- 30).Sakai K, Koba S, Nakamura Y, Yokota Y, Tsunoda F, Shoji M, et al. Small dense low-density lipoprotein cholesterol is a promising biomarker for secondary prevention in older men with stable coronary artery disease. Geriatr Gerontol Int 2018;18:965–972. [DOI] [PubMed] [Google Scholar]
- 31).Herder C, Karakas M, Koenig W. Biomarkers for the prediction of type 2 diabetes and cardiovascular disease. Clin Pharmacol Ther 2011;90:52–66. [DOI] [PubMed] [Google Scholar]
- 32).Olofsson S-O, Wiklund O, Borén J. Apolipoproteins A-I and B: biosynthesis, role in the development of atherosclerosis and targets for intervention against cardiovascular disease. Vasc Health Risk Manag 2007;3:491–502. [PMC free article] [PubMed] [Google Scholar]
- 33).Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res 2002;43:1363–1379. [DOI] [PubMed] [Google Scholar]
- 34).Siddiqui MS, Fuchs M, Idowu MO, Luketic VA, Boyett S, Sargeant C, et al. Severity of nonalcoholic fatty liver disease and progression to cirrhosis associate with atherogenic lipoprotein profile. Clin Gastroenterol Hepatol 2014;13:1000–1008.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Yamada N, Shames DM, Takahashi K, Havel RJ. Metabolism of apolipoprotein B-100 in large very low density lipoproteins of blood plasma. Kinetic studies in normal and Watanabe heritable hyperlipidemic rabbits. J Clin Invest 1988;82:2106–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Packard CJ, Shepherd J. Lipoprotein heterogeneity and apolipoprotein B metabolism. Arterioscler Thromb Vasc Biol 1997; 17:3542–3556. [DOI] [PubMed] [Google Scholar]
- 37).Packard CJ. Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoprotein. Biochem Soc Trans 2003;31:1066–1069. [DOI] [PubMed] [Google Scholar]
- 38).Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 2007;116:1832–1844. [DOI] [PubMed] [Google Scholar]
- 39).Skålén K, Gustafsson M, Rydberg EK, Hultén LM, Wiklund O, Innerarity TL, et al. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 2002;417: 750–754. [DOI] [PubMed] [Google Scholar]
- 40).Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 1986;6:131–138. [DOI] [PubMed] [Google Scholar]
- 41).Haberland ME, Fogelman AM, Edwards PA. Specificity of receptor-mediated recognition of malondialdehyde-modified low density lipoproteins. Proc Natl Acad Sci U S A 1982;79: 1712–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).van der Vliet A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem 1997;272:7617–7625. [DOI] [PubMed] [Google Scholar]
- 43).Laryea M, Watt KD, Molinari M, Walsh MJ, McAlister VC, Marotta PJ, et al. Metabolic syndrome in liver transplant reciepients; prevalence and association with major vascular events. Liver Transpl 2007;13:1109–1114. [DOI] [PubMed] [Google Scholar]
- 44).Zimmermann A, Zobeley C, Weber MMM, Lang H, Galle PR, Zimmermann T. Changes in lipid and carbohydrate metabolism under mTOR- and calcineurin-based immunosuppressive regimen in adult patients after liver transplantation. Eur J Intern Med 2016;29:104–109. [DOI] [PubMed] [Google Scholar]
- 45).Hulzebos CV, Bijleveld CM, Stellaard F, Kuipers F, Fidler V, Slooff MJ, et al. Cyclosporine A-induced reduction of bile salt synthesis associated with increased plasma lipids in children after liver transplantation. Liver Transpl 2004;10:872–880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


