Abstract
Allergic asthma with high plasma IgE levels is a significant risk factor of human abdominal aortic aneurysm (AAA). This study tests a direct role of IgE in angiotensin-II (Ang-II) perfusion- and peri-aortic CaCl2 injury-induced AAA in mice. In both models, IgE-deficiency in Apoe−/−Ige−/− mice blunts AAA growth and reduces lesion accumulation of macrophages, CD4+ and CD8+ T cells, and lesion MHC class-II expression, CD31+ microvessel growth, and media smooth muscle cell loss, compared with those from Apoe−/− control mice. Real time-PCR reveals significant reductions in expression of neutrophil chemoattractants MIP-2α and CXCL5 in AAA lesions or macrophages from Apoe−/−Ige−/− mice, along with reduced lesion Ly6G+ neutrophil accumulation. Consistent with reduced lesion inflammatory cell accumulation, we find significant reductions of plasma and AAA lesion IL6 expression in Apoe−/−Ige−/− mice. Immunofluorescent staining and FACS analysis show that AAA lesion neutrophils express FcεR1. Mechanistic study demonstrates that IgE induces neutrophil FcεR1 expression, activates MAPK signaling, and promotes IL6 production. This study supports a direct role of IgE in AAA by promoting lesion chemokine expression, inflammatory cell accumulation, MAPK signaling, and cytokine expression. IgE inhibition may represent a novel therapeutic approach in AAA management.
Keywords: IgE, abdominal aortic aneurysm, neutrophil, IL6, FcεR1, MAPK
INTRODUCTION
Abdominal aortic aneurysm (AAA) remains a significant cause of death in adults as a result of aortic rupture.1,2 When invasive open or endovascular surgeries remain the only treatment for large (greater than 50 mm), symptomatic, or ruptured AAA, there is no effective medication to prevent or slow the growth of small or asymptomatic AAA.2,3 Accumulating studies demonstrate that AAA is an inflammatory disease, in which inflammatory cell activation plays essential roles. Therefore, targeting pro-inflammatory phenotypes holds a potential promise as an efficient therapeutic strategy.4–6 Inflammatory cell recruitment and expression of pro-inflammatory cytokines are key pathological features of human AAA. Among the inflammatory cells, neutrophils are among the most abundant cell types and the first cell population recruited to the site of injured aortic wall.7,8 After infiltration and activation, neutrophils release pro-inflammatory molecules such as interleukin-6 (IL6), neutrophil-associated proteases and extracellular traps (NETs), matrix metalloproteinases (MMP) such as MMP-9, cysteinyl cathepsins, and myeloperoxidase to induce aortic wall inflammation, vascular cell apoptosis, and matrix degradation,9–13 leading to aortic rupture. Depletion of circulating neutrophils by using anti-neutrophil antibody or targeting neutrophil recruitment required chemokines or adhesion molecules inhibited AAA development in aortic elastase perfusion or peri-aortic calcium phosphate injury-induced mouse AAA models,7,8,14,15 supporting an important role of neutrophils in AAA formation.
Plasma and tissue immunoglobulin E (IgE) is a signature molecule of asthma and allergic responses. Allergic airway inflammation expedited the progression of angiotensin-II (Ang-II) perfusion-induced AAA in mice.16 Our prior studies found that plasma IgE levels were much higher in patients or animals with both AAA and asthma than those who only suffered from AAA or asthma. A classic role of IgE is to bind to its high affinity receptor FcεR1 on mast cells as the most common mechanism of mast cell activation. Yet, we found that increased plasma and tissue IgE acts as an important pro-inflammatory stimulus to activate not only mast cells, but also CD4+ T cells, CD8+ T cells, and macrophages, as mechanisms of IgE in promoting AAA formation.16–18 These inflammatory cells all express FcεR1, although at lower levels than do mast cells. Neutrophils also express FcεR1. IgE-mediated neutrophil activation participates importantly in infectious and allergic diseases, such as cerebral malaria, allergy, and asthma among the others.19–23 Studies also show that IgE binding to its receptor FcεR1 affects neutrophil survival and cytokine secretion.24,25
Although a direct role of IgE in AAA remains untested, FcεR1-deficiency protected mice from Ang-II perfusion-induced AAA in mice.17,26 Accumulating evidences suggest that the pathobiological activities of FcεR1 include more than just mediating IgE activities. We showed that FcεR1 formed complexes with toll-like receptor-4 (TLR4) and Na+-H+ exchanger-1 (Nhe1). Deficiency of FcεR1, TLR4, or Nhe1 blocked IgE activity in inducing macrophage expressions of cytokines and chemokines and macrophage and vascular cell apoptosis.27,28 Fibrinogen ligand or disintegrin rhodostomin activates Nhe1 by forming complexes with integrin αIIbβ3, followed by activating the sodium-calcium exchanger NCX1.29 Activation of Nhe1 promotes the opening of integrin αvβ3 headpiece, thereby increasing cell adhesion and migration.30,31 Therefore, TLR4 ligand lipopolysaccharide (LPS) and integrins may indirectly affect FcεR1 activity. Here we test a direct role of IgE in Ang-II perfusion-induced and peri-aortic CaCl2 injury-induced AAA in mice using IgE-deficient mice. Mechanistic studies reveal a previously unrecognized role of IgE in promoting neutrophil cytokine expression and mitogen-activated protein kinase (MAPK) signaling activation.
MATERIALS AND METHODS
Mouse AAA models
Apolipoprotein E (ApoE)-deficient Apoe−/−Ige−/− mice, Apoe−/−Fcer1a−/− mice, and Apoe−/− control mice was generated by crossbreeding Ige–/– mice (C57BL/6) or FcεR1−/− mice (C57BL/6) with Apoe−/− mice (C57BL/6). Ang-II perfusion- and peri-aortic CaCl2 injury-induced experimental AAA was produced as described previously.16,17 Briefly, 8~10-week old male mice were either infused with 1000 ng/kg/min Ang-II (A9525, Sigma-Aldrich, St. Louis, MO) subcutaneously delivered by Alzet model 2004 osmotic minipumps (DURECT, Cupertino, CA) or peri-aortic application of 0.5 M CaCl2 for 28 days to generate AAA. All mice were kept on a 12 hrs/12 hrs light/dark cycle with high fat diet for Ang-II-induced AAA or standard chow diet for CaCl2-induced AAA. Mice were sacrificed with carbon dioxide narcosis, followed by cardiac puncture blood collection. Plasma IL6 (88-7064-88, Invitrogen, Carlsbad, CA), interferon-γ (IFN-γ) (88-7314-88, Invitrogen), and IL2 (88-7024-88, Invitrogen) levels were determined by ELISA according to the manufacturer’s protocols. The maximal suprarenal aortic diameter of each aneurysm was measured from post-mortem mice after the peri-aortic tissue was carefully removed from the aortic wall. Aortic diameters of mice were measured using a surgical microscope (Zeiss Stemi SV11) equipped with a micrometer eyepiece (14 mm/0.1, SG20.T0218c, Motic Instruments, Inc., Vancouver, British Columbia, Canada). All animal procedures conformed with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health and were approved by the Harvard Medical School Standing Committee on Animals (Protocol # 03759).
Mouse aortic tissue immunohistochemical analyses
Mouse aortic tissues were harvested and embedded in optimal cutting temperature (OCT) compound, and aorta segments at 6 μm were prepared as described previously.16,17 The serial sections were stained with antibodies for macrophages (Mac-3, 1:900, 553322, BD Biosciences, San Jose, CA), T cells (CD4, 1:90; 553043, and CD8, 1:100, 01041D, BD Biosciences), major histocompatibility complex class-II MHC-II) (1:250, 556999, BD Biosciences), smooth muscle cell (SMC) (α-actin, 1:750, F3777, Sigma-Aldrich), CD31 (1:1500, 553370, BD Biosciences) and neutrophils (Ly6G, 1:200, BE0075, BioXcell, West Lebanon, NH). CD4+ and CD8+ T cells, and CD31+ microvessels were counted blindly and presented as numbers of total cells per mm2 of lesion area. Ly6G+ neutrophils were counted and presented as number of cells per section. MHC-II-positive and Mac-3-positive areas were measured using computer-assisted image analysis software (Image-Pro Plus; Media Cybernetics, Bethesda, MD) and presented as positive area per mm2 of lesion area. Media SMC accumulation was graded according to the grading keys described previously.32
Immunofluorescent staining
For immunofluorescent staining, 6 μm frozen sections of aorta were fixed and permeabilized with cold-acetone for 5 min and blocked in phosphate-buffered saline (PBS) containing 5% fetal bovine serum (FBS) for 1 hr at room temperature. Sections were co-stained with FcεR1 (1:50, 06727, Sigma-Aldrich) and Ly6G (1:350, BE0075, BioXcell) in 1% FBS at 4 °C overnight. After incubation, the sections were further incubated with Alexa Flour 488-conjugated donkey anti-rat IgG (1:300, A21208, Invitrogen) and Alexa Flour 555-conjugated goat anti-rabbit IgG (1:300, A21428, Invitrogen) in PBS for 1 hr at room temperature. Nuclei were stained for 5 min at room temperature in PBS containing DAPI (0.5 μg/ml, 4083, Cell Signaling Technology, Danvers, MA). Coverslips were mounted with ProLong Gold antifade reagent (P36930, Invitrogen). Images were acquired using an Olympus confocal microscope.
AAA lesion and spleen single cell preparation and flow cytometry analysis
To determine FcεR1 expression on neutrophils in AAA lesions, Ang-II perfusion-induced AAA lesions from Apoe−/− or Apoe−/−Fcer1a−/− mice were minced into small pieces and digested into single cell suspension in an aorta dissociation enzyme stock solution (125 U/ml collagenase type-XI, 60 U/ml hyaluronidase type I-s, 60 U/ml DNase1, and 450 U/ml collagenase type-I; all enzymes were obtained from Sigma-Aldrich) at 37°C for 1 hr and filtered through a 70 μm cell strainer (322350, BD Bioscience). Single cell preparation was labeled with fixable viability dye eFluor™ 450 (1:200, 65-0863-14, Invitrogen), PerCP-cyanine5.5-conjugated CD45 (1:200, 45-0451-82, Invitrogen), APC-conjugated CD11b (1:200, 17–0112-82, Invitrogen), APC-eFluor™ 780-conjugated Ly6G (1:200, 47-5931-82, eBioscience, San Diego, CA), and FITC-conjugated FcεR1 (1:200, 11–5898-85, eBioscience) for subsequent FACS analysis.
To characterize the immune cell in spleen from Apoe−/− and Apoe−/−Ige−/− mice, spleen was removed, placed in cold PBS, and grinded into 5 ml PBS before filtering through a 70 µm cell strainer. Splenocytes were collected after depleting the red blood cells. Cells were then washed with PBS and stained with the fixable viability dye eFluor™ 450 or eFluor™ 660 and cell surface marker antibodies including PerCP-cyanine5.5-conjugated CD45, APC-conjugated CD11b, PE-conjugated Ly6G (1:200, 551461, BD PharMingen, Billerica, MA), FITC-conjugated Ly6C (1:200, 53-5932-82, Invitrogen), PE/Cy7-conjugated F4/80 (1:200, 123114, BioLegend, San Diego, CA), Pacific Blue™-conjugated CD3ε (1:200, 100334, BioLegend), PE-conjugated CD8a (1:200, 100708, BioLegend), FITC-conjugated CD4 (1:200, 11-0042-85, Invitrogen), APC-conjugated CD117 (c-Kit) (1:200, 17-1171-83, Invitrogen), and FITC-conjugated FcεR1 for subsequent FACS analysis.
Macrophage and neutrophil isolation and IgE treatment
Bone-marrow-derived macrophages (BMDMs) were prepared from 8 weeks old Apoe−/− and Apoe−/−Ige−/− mice. Fresh bone-marrow cells were isolated from the femurs and tibias bone and cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 20 ng/ml of macrophage-colony stimulating factor (M-CSF, PeproTech, Rocky Hill, NJ) for 9 days. Cells were then stimulated with LPS (Sigma-Aldrich) (100 ng/ml, 24 hrs) and collected for RNA extraction.
Bone-marrow-derived neutrophils were isolated from 8~10 weeks old Apoe−/−and Apoe−/−Fcer1a−/− mice according to established protocol.33 Briefly, femur and tibia bones were flushed with RPMI 1640, and cells were collected and treated with ammonium-chloride-potassium lysis buffer (A10492-01, Fisher Scientific, Hampton, NH) to lyse red blood cells. Cell pellet was suspended in HBSS with phenol red and neutrophils were separated and collected by Percoll density gradient centrifugation. Neutrophil purity was confirmed by FACS analysis using APC-conjugated CD11b (1:200, 17-0112-83, eBioscience) and APC-eFluor™ 780-conjugated Ly6G (1:200, 47-5931-82, eBioscience), and Giemsa staining according to manufacturer’s protocol (GS500, Sigma-Aldrich). After isolation, neutrophils were plated (200,000/well) on a 6-well plate in RPIM 1640 supplemented with 10% FBS and stimulated with 25 μg/ml aggregated and cytokinergic IgE SPE-7 (D8406, Sigma-Aldrich), monomeric and poorly cytokinergic IgE (H1-DNP-ε-206),27,34 or AAA lesion lysate for 6 hrs. After stimulation, neutrophil pellet and supernatant were separated by centrifuging at 1000 rpm for 5 min. Cell pellet was collected for RNA and protein extraction and the supernatant was used for measuring IL6 levels using a commercial ELISA kit (88-7064-88, Invitrogen). AAA lysates was prepared from Ang-II perfusion-induced Apoe−/−Apoe−/− mice. Briefly, AAA was carefully removed and homogenized into RPIM 1640 using Tissyelyser-II (Qiagen, Germantown, MD) for 5 min with a frequency of 30/s. The supernatant was separated and collected by centrifugation at 1,000 g for 15 min and the protein concentration was determined using Pierce BCA assay (PI23223, Fisher Scientific).
RNA isolation and real-time-PCR
Mouse aortic tissues were homogenized using the TissueLyser II (Qiagen) according to manufacturer’s instructions. Total RNA from mouse aortas and cultured neutrophils were extracted using the TRIzol reagent (15596018, Invitrogen) according to the manufacturer. Reverse transcriptions were performed by using high-capacity cDNA reverse transcription kit (4368813, Applied Biosystems, Foster City, CA). PCR was performed using the SyberGreen GoTaq qPCR master mix (A6001, Promega, Madison, WI) according to the manufacturer. mRNAs levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Changes in expression were calculated using the ΔΔCt method. Primers used for real-time-PCR include: C-X-C motif chemokine ligand 2 (CXCL2), forward: CCAACCACCAGGCTACAGG, reverse: GCGTCACACTCAAGCTCTG; C-X-C motif chemokine ligand 5 (CXCL5), forward: GTTCCATCTCGCCATTCATGC, reverse: GCGGCTATGACTGAGGAAGG; IL6, forward: CCTTCCTACCCCAATTTCCAA, reverse: AGATGAATTGGATGGTCTTGGTC; and GAPDH, forward: TGTCATACTTGGCAGGTTTCT, reverse: CGTGTTCCTACCCCCAATGT.
Protein extraction and immunoblot
Total protein from cultured neutrophils was extracted using the RIPA buffer (BP-115, Boston BioProdcuts, Ashland, MA) with protease inhibitor and phosphatase inhibitor. Protein concentrations were determined using Pierce BCA assay (PI23223, Fisher Scientific). Equal amount of proteins (20 μg per lane) was separated on a 4-20% Mini-PROTEAN TGX Gel (456-1096, Bio-Rad Laboratories, Hercules, CA). Separated proteins were transferred to PVDF membranes using the Transfer Turbo Blot system (Bio-Rad Laboratories) and the Trans-Blot Turbo RTA transfer kit (170-4272, Bio-Rad Laboratories). Membranes were blocked with 5% nonfat milk for 1 hr at room temperature. After blocking, the membranes were incubated overnight at 4 °C with antibodies against IL6 (1:1000, 12912), phosphor-c-Jun N-terminal kinase (p-JNK, 1:1000, 9255), total (t)-JNK (1:1000, 9252,), p-p38 (1:1000, 9211), t-p38 (1:1000, 9212), phosphor-extracellular-signal-regulated kinase (p-ERK1/2, 1:1000, 9101), t-ERK1/2 (1:1000, 9107), and β-actin (1:4000, 4970), all from Cell Signaling Technology. Proteins on the membranes were visualized by chemiluminescence detection kit (Super signal west femto maximum sensitivity substrate, Fisher Scientific). ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to analyze signal abundance.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 7.0 (GraphPad Software, Inc., CA). Unpaired 2-tailed Student t test was used to determine statistical significance between 2 groups for normally distributed continuous variables. For comparison of multiple groups, ANOVA followed by Tukey multiple comparison analysis or 2-way ANOVA followed by Bonferroni post hoc test were used. For data without normal distribution, nonparametric Mann-Whitney U test or Kruskal-Wallis test were applied. All data are presented as mean±SEM. P<0.05 was considered statistically significant for all tests.
RESULTS
IgE-deficiency attenuates Ang-II perfusion-induced suprarenal AAA in Apoe−/− mice
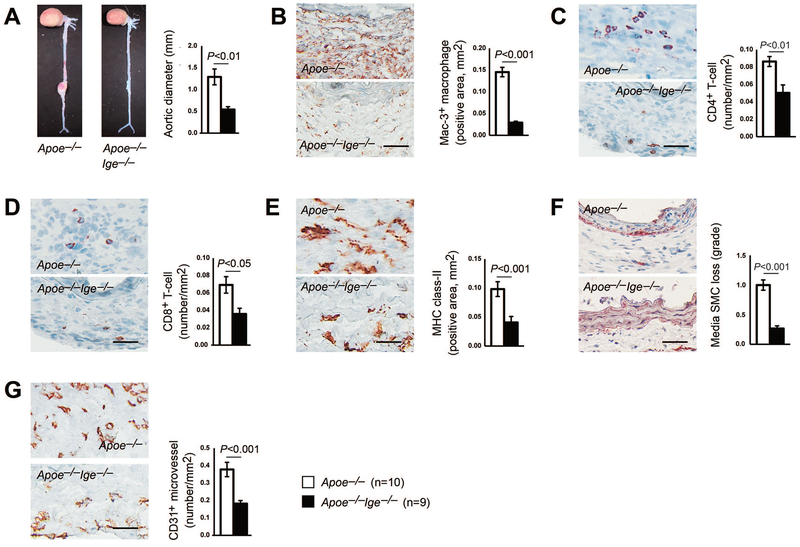
To examine a direct role of IgE in AAA formation, we generated compound Apoe−/−Ige−/− mutant mice by crossbreeding IgE-deficient (Ige–/–) mice with Apoe−/− mice and performed subcutaneous Ang-II (1000 ng·kg−1·min−1) perfusion to produce AAA. Although we did not test whether Apoe−/−Ige−/− mice differ from Apoe−/− mice in lifespan beyond 12 months of age, FACS analysis revealed more CD11b+Ly6G+ neutrophils, CD11b+F4/80+ macrophages, and CD11b+Ly6Cint and CD11b+Ly6Clo monocytes in spleens from Apoe−/−Ige−/− mice than those in age-matched Apoe−/− mice (Supplemental Figure S1A–S1C). In contrast, spleens from Apoe−/−Ige−/− mice had fewer CD8+ T cells and CD117+FcεR1+ mast cells than those from Apoe−/− mice (Supplemental Figure S1D/S1E). Splenic CD11b+Ly6Chi monocytes and CD4+ T cells did not differ between the two types of mice (Supplemental Figure S1C/S1D). Changes in these immune cells may affect AAA development from Apoe−/−Ige−/− mice. After 4 weeks of Ang-II perfusion, IgE-deficiency markedly blunted the expansion of suprarenal abdominal aortas in Apoe−/−Ige−/− mice, much smaller than those in IgE-sufficient Apoe−/− mice (1.29±0.18 mm vs. 0.54±0.07 mm, P<0.01, Figure 1A). In line with the reduction of maximal aortic diameters in Apoe−/−Ige−/− mice, histological assessment of AAA lesions revealed an 80% reduction of macrophage-positive areas by Mac-3 staining (Figure 1B), a 42% reduction of CD4+ T cells and 48% of CD8+ T cells by CD4 and CD8 staining (Figure 1C/1D), and a 58% reduction of MHC-II-positive areas (Figure 1E), compared with the lesions from Apoe−/− mice. Furthermore, as shown by immunohistochemistry in Figures 1F and 1G, Apoe−/−Ige−/− mice exhibited a reduction in media SMC loss and microvessel numbers (CD31 staining), compared with those from Apoe−/− mice, although we did not test whether IgE-deficiency affected systolic or diastolic blood pressures between Apoe−/−Ige−/− and Apoe−/− mice before and after Ang-II perfusion. These data indicate that IgE-deficiency alleviates inflammatory response and results in reduced SMC loss, angiogenesis, and AAA lesion progression in Ang-II-perfused Apoe−/−Ige−/− mice.
Figure 1.
IgE-deficiency reduces angiotensin-II (Ang-II)-induced AAA in mice. Aortic diameters (A), lesion Mac-3+ macrophage contents (B), CD4+ T-cell numbers (C), CD8+ T-cell numbers (D), MHC class-II levels (E), media SMC loss (F), and CD31+ microvessel numbers (G) in AAA lesions from both Apoe−/− and Apoe−/−Ige−/− mice. Data are mean±SEM. Representative images are shown to the left. Scale: 50 μm. The number of mice per group and mouse genotypes is indicated.
IgE-deficiency prevents infrarenal AAA formation in peri-aortic CaCl2-injured mice
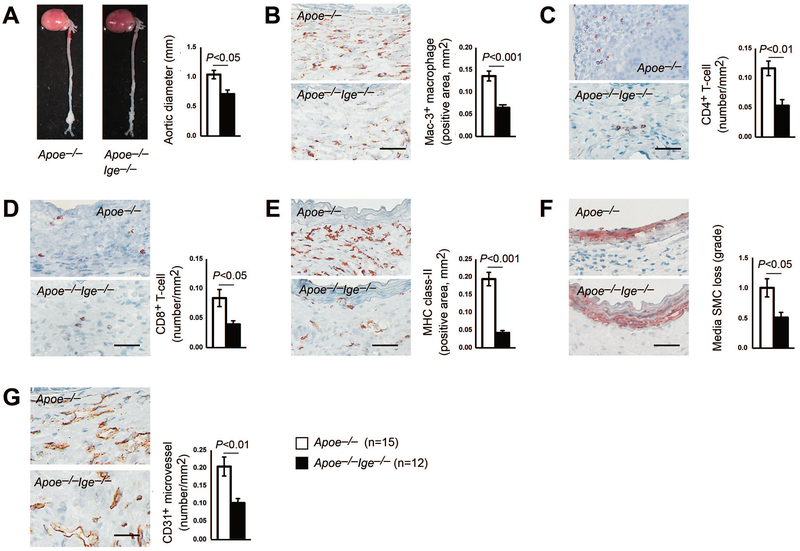
To further establish the protective role of IgE ablation in AAA formation, an independent peri-aortic CaCl2 injury-induced infrarenal AAA model was introduced in the current study. As we anticipated, IgE-deficient Apoe−/−Ige−/− mice exhibited a significant reduction of infrarenal maximal outer aortic diameters than those from IgE-sufficient Apoe−/− mice (1.04±0.07 mm vs. 0.71±0.07 mm, P<0.01, Figure 2A). Consistent with the protective effects found in Ang-II perfusion-induced suprarenal AAA, AAA lesion inflammation (increases in macrophage positive areas, CD4+ and CD8+ T-cell numbers, and MHC-II-positive areas) (Figures 2B–2E), aortic media SMC loss (Figure 2F), and CD31+ microvessel numbers (Figure 2G) were all significantly lower in lesions from Apoe−/−Ige−/− mice than in lesions from Apoe−/− mice. Because CaCl2 injury-induced AAA did not change systolic or diastolic blood pressure,35 we also did not monitor the blood pressure differences between the groups. Taken together, these observations demonstrate that IgE plays an important role in both chronic and acute injury-induced suprarenal and infrarenal AAA formation.
Figure 2.
IgE-deficiency reduces peri-aortic CaCl2 injury-induced AAA in mice. Aortic diameters (A), lesion Mac-3+ macrophage contents (B), CD4+ T-cell numbers (C), CD8+ T-cell numbers (D), MHC class-II levels (E), media SMC loss (F), and CD31+ microvessel numbers (G) in AAA lesions from both Apoe−/− and Apoe−/−Ige−/− mice. Data are mean±SEM. Representative images are shown to the left. Scale: 50 μm. The number of mice per group and mouse genotypes is indicated.
IgE-deficiency reduces AAA lesion chemokine expression and neutrophil accumulation
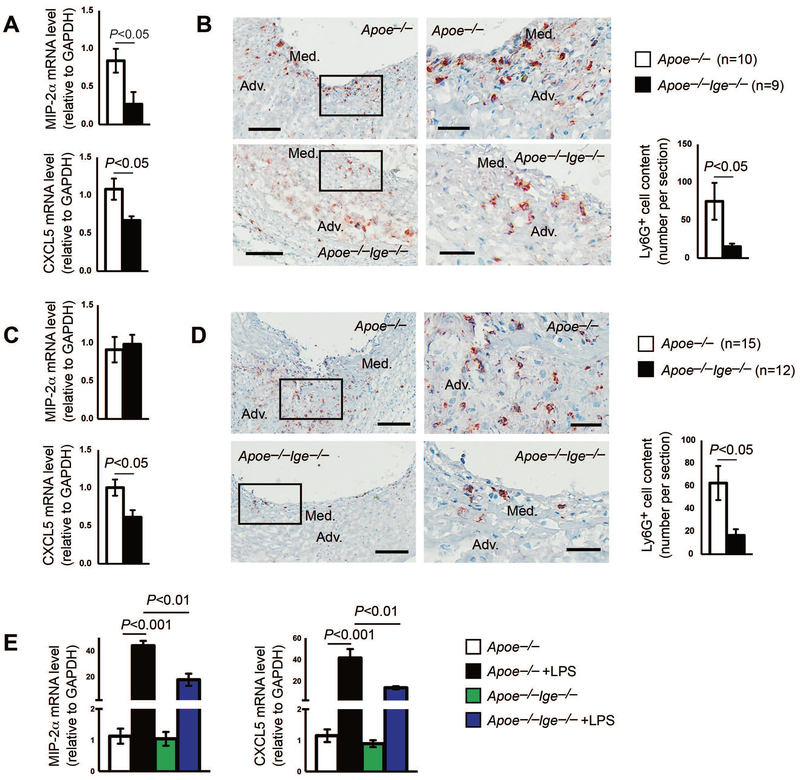
Neutrophils are the first cell population recruited to the injured aortic wall and play a critical role in AAA formation and progression.36,37 We next asked whether IgE-deficiency also affected neutrophil accumulation during AAA formation. We collected both Ang-II perfusion-induced suprarenal AAA lesion tissues and peri-aortic CaCl2 injury-induced infrarenal aortic AAA lesion tissues and measured the mRNA levels of macrophage inflammatory protein 2-alpha (MIP-2α) and chemokine CXC motif ligand 5 (CXCL5), both are major neutrophil chemotactic chemokines that are responsible for neutrophil recruitment to the site of inflammation from circulation.37,38 As shown in Figure 3A, the expression of MIP-2α repressed by 68% and the expression of CXCL5 reduced by 38% in Ang-II perfusion-induced AAA lesions from Apoe−/−Ige−/− mice, compared to those from Apoe−/− mice. Consistent with these RT-PCR data, immunohistochemical staining showed that the accumulation of Ly6G+ neutrophils in lesions from Apoe−/−Ige−/− mice was significantly blunted compared with those from Apoe−/− mice (Figure 3B), although we detected more splenic neutrophils in Apoe−/−Ige−/− mice than in Apoe−/− mice (Supplemental Figure S1A). Ly6G+ neutrophils appeared throughout the media and adventitia (Figure 3B).
Figure 3.
IgE-deficiency suppresses neutrophil accumulation in Ang-II perfusion-induced AAA lesions. MIP-2α and CXCL5 mRNA levels (A) and Ly6G+ neutrophil contents (B) in Ang-II perfusion-induced AAA lesions from Apoe−/− and Apoe−/−Ige−/− mice. MIP-2α and CXCL5 mRNA levels (C) and Ly6G+ neutrophil contents (D) in peri-aortic CaCl2 injury-induced AAA lesions from Apoe−/− and Apoe−/−Ige−/− mice. E. MIP-2α and CXCL5 mRNA levels in BMDMs from Apoe−/− and Apoe−/−Ige−/− mice treated with and without LPS. Data are mean±SEM from three independent experiments. Representative images for panels B and D are shown to the left. Scale: 200 μm; insert: 50 μm. The number of mice per group and mouse genotypes is indicated.
We obtained similar observations from peri-aortic CaCl2 injury-induced AAA. Although the expression of MIP-2α did not differ between AAA lesions from Apoe−/− and Apoe−/−Ige−/− mice, the expression of CXCL5 reduced by 39% in peri-aortic CaCl2 injury-induced AAA lesions from Apoe−/−Ige−/− mice (Figure 3C). Again, immunofluorescent staining showed that Ly6G+ neutrophils were many fewer in AAA lesion from Apoe−/−Ige−/− mice than those in lesions from Apoe−/− mice (Figure 3D). Ly6G+ neutrophils also appeared in the media and adventitia in these AAA lesions (Figure 3D). Macrophages are abundant inflammatory cells in AAA lesions. MIP-2α and CXCL5 expression in macrophages may affect neutrophil accumulation. In BMDMs from Apoe−/− mice, LPS greatly increased the expression of these neutrophil chemokines. Such induction was significantly blocked in cells from Apoe−/−Ige−/− mice (Figure 3E).
IgE-deficiency reduces IL6 expression in plasma and AAA lesions
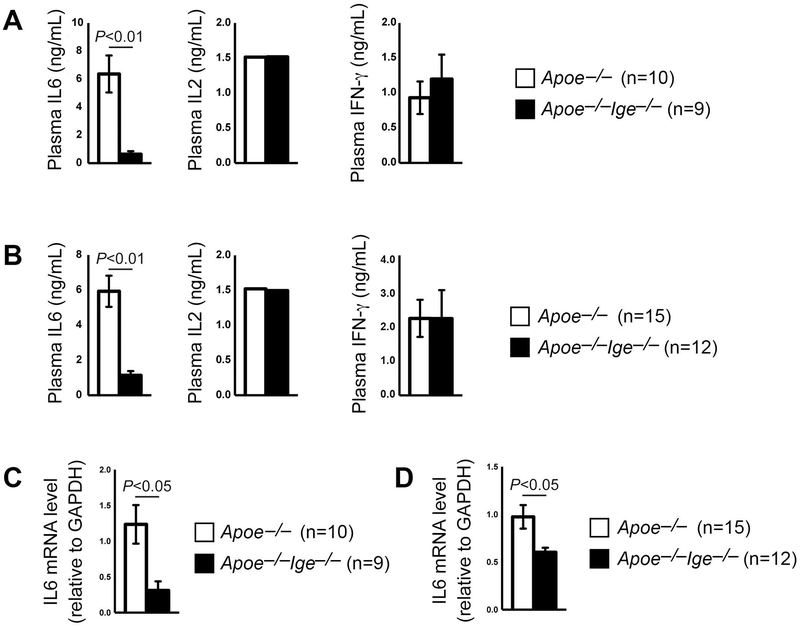
Increases of plasma and AAA lesion pro-inflammatory cytokines are signatures of AAA progression and contribute to aortic wall SMC apoptosis, elastin fragmentation, and aortic rupture.4,5 As we anticipated, along with reduced AAA formation in Apoe−/−Ige−/− mice, the increase of plasma IL6 levels in Apoe−/− mice was greatly blunted in Apoe−/−Ige−/− mice from both Ang-II- and CaCl2-induced AAA models, although plasma IL2 and IFN-γ levels did not differ between the two genotypes of mice from both models (Figure 4A/4B). IgE-deficiency also reduced AAA lesion IL6 expression. RT-PCR analysis of both Ang-II perfusion- and peri-aortic CaCl2 injury-induced AAA lesions from Apoe−/−Ige−/− mice demonstrated significant reduction of IL6 mRNA levels, compared with those from Apoe−/− mice (Figure 4C/4D).
Figure 4.
IgE-deficiency reduces plasma and AAA lesion IL6 levels. A. ELISA determined plasma IL6, IL2 and INF-γ levels in Apoe−/− and Apoe−/−Ige−/− mice after Ang-II perfusion-induced AAA. B. ELISA determined plasma IL6, IL2 and INF-γ levels in Apoe−/− and Apoe−/−Ige−/− mice after peri-aortic CaCl2 injury-induced AAA. RT-PCR determined the IL6 mRNA levels in AAA lesions from Apoe−/− and Apoe−/−Ige−/− mice after Ang-II perfusion-induced (C) and peri-aortic CaCl2 injury-induced AAA (D). Data are mean±SEM. The number of mice per group and mouse genotypes is indicated.
IgE promotes neutrophil IL6 expression and MAPK activation
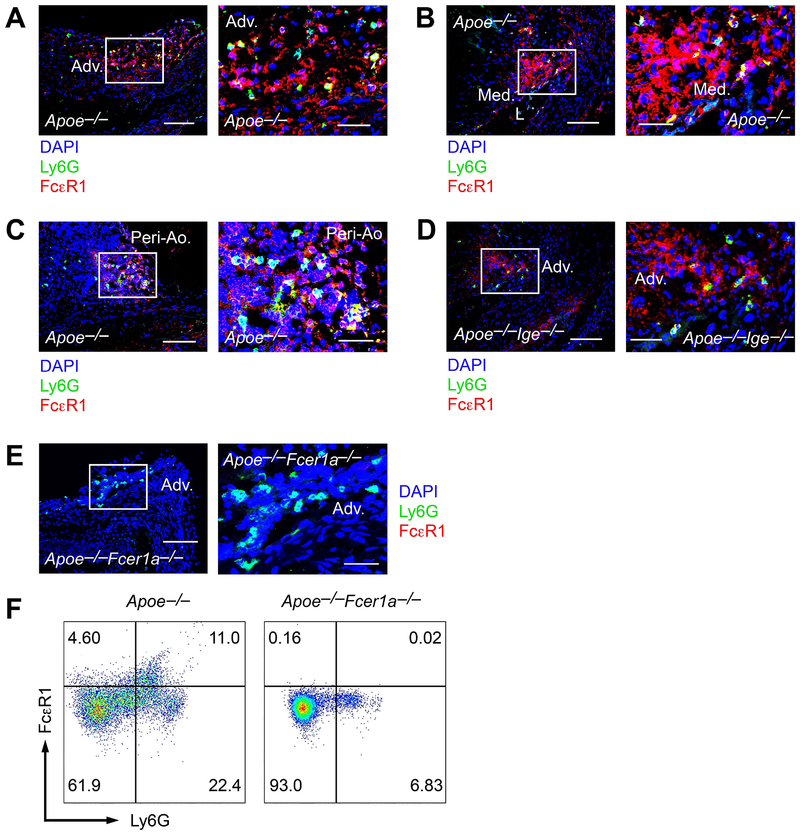
IgE-deficiency reduced neutrophil accumulation and IL6 expression in AAA lesions from Apoe−/−Ige−/− mice (Figures 3 and 4). During AAA development, aortic wall neutrophils are a major source of lesion IL6.39 Based on these prior studies and our own observations, we hypothesized that IgE controls neutrophil IL6 expression and secretion. Using Ang-II perfusion-induced AAA lesion frozen sections prepared from Apoe−/− and Apoe−/−Ige−/− mice, we performed immunofluorescent double staining with FcεR1 and Ly6G antibodies. Many Ly6G+ neutrophils in the adventitia (Figure 5A), media (Figure 5B), and peri-aortic inflammatory cell clusters (Figure 5C) in AAA lesions from Apoe−/− mice also expressed FcεR1. Although there were many fewer Ly6G+ neutrophils in AAA lesions from Apoe−/−Ige−/− mice than those from Apoe−/− mice (Figure 3B), we also detected Ly6G+FcεR1+ double positive neutrophils in AAA lesions from Apoe−/−Ige−/− mice (Figure 5D). AAA lesions from age-matched FcεR1-deficient Apoe−/−Fcer1a−/− mice tested the FcεR1 antibody specificity. Immunofluorescent staining showed that the Ly6G+ neutrophils in these lesions did not express FcεR1 (Figure 5E). To confirm further that neutrophils in AAA lesions expressed FcεR1, we prepared AAA lesion single cells from Ang-II perfusion-treated Apoe−/− and Apoe−/−Fcer1a−/− mice and performed FACS analysis. We detected Ly6G+FcεR1+ neutrophils in AAA lesions from Apoe−/− mice, but Ly6G+ neutrophils in AAA lesions from Apoe−/−Fcer1a−/− mice did not express FcεR1 (Figure 5F). Therefore, if not all, some neutrophils in mouse AAA lesions express IgE receptor FcεR1.
Figure 5.
FcεR1 expression on Ly6G+ neutrophils in Ang-II perfusion-induced AAA lesions. Immunofluorescent staining of Ly6G (green) and FcεR1 (red) double positive neutrophils in the adventitia (Adv.) (A), media (Med.) next to the lumen (L) (B), and peri-aorta (Peri-Ao.) inflammatory cell clusters (C) in AAA lesions from Apoe−/− mice. D. Ly6G (green) and FcεR1 (red) double positive neutrophils the adventitia (Adv.) in AAA lesions from the Apoe−/−Ige−/− mice. E. AAA lesions from Apoe−/−Fcer1a−/− mice were used as negative control to detect FcεR1 expression (red) on Ly6G neutrophils (green). Scale: 200 μm; insert: 70 μm. F. FACS analysis of Ly6G+FcεR1+ neutrophils in AAA lesions from Apoe−/− and Apoe−/−Fcer1a−/− mice (negative control).
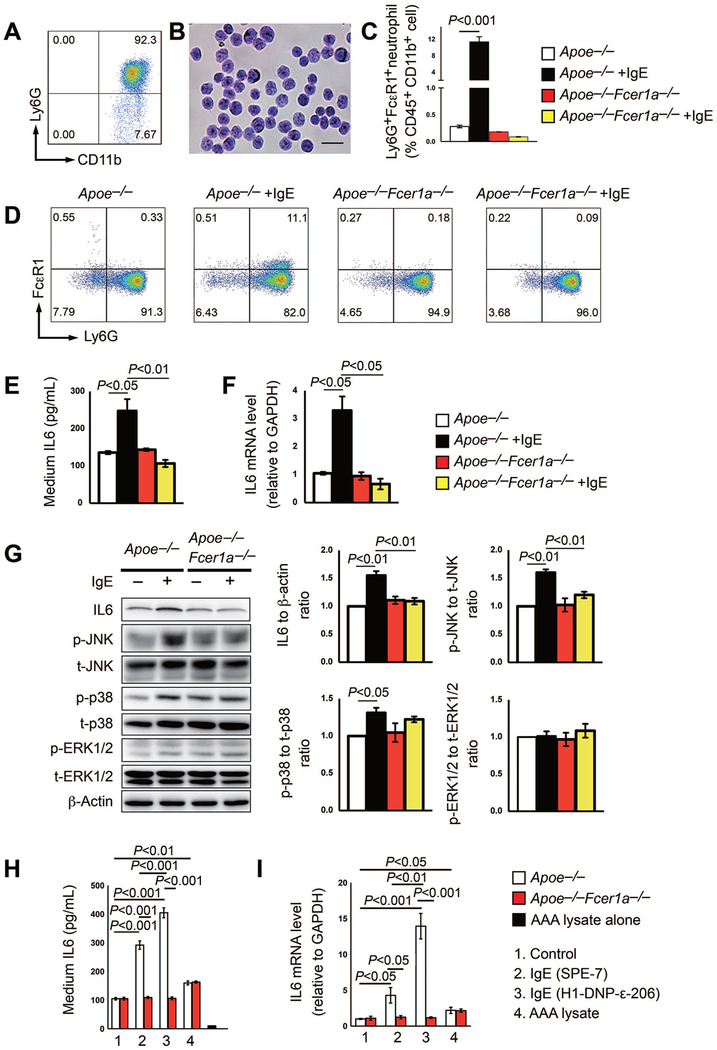
To examine the underlying activity and associated mechanism by which IgE activates neutrophils, we prepared bone-marrow-derived neutrophils from both Apoe−/− and IgE receptor FcεR1-deficient Apoe−/−Fcer1a−/− mice. FACS analysis verified the purity of our purified CD11b+Ly6G+ neutrophils (Figure 6A) and further confirmed by Giemsa staining (Figure 6B). When neutrophils from Apoe−/− mice were treated with 25 µg/ml of aggregated and cytokinergic IgE (SPE7) for 6 hrs, we detected increased expression of FcεR1 as determined by FACS analysis. Neutrophils from Apoe−/−Fcer1a−/− mice were used as negative control and failed to express FcεR1 after IgE stimulation (Figure 6C/6D). Under the same IgE treatment condition, neutrophils from Apoe−/− mice showed 2-fold increase of IL6 secretion to the culture media as determined by ELISA (Figure 6E) and 3-fold increase of IL6 mRNA levels as determined by RT-PCR (Figure 6F). In contrast, such IgE activities were muted when neutrophils from Apoe−/−Fcer1a−/− mice were used (Figures 6E/6F), suggesting that IgE activates neutrophil IL6 production via cell surface FcεR1.
Figure 6.
IgE activity in inducing neutrophil IL6 expression and MAPK activation. A/B. The purity of neutrophils isolated from Apoe−/− and Apoe−/−Fcer1a−/− mice bone marrow was verified by FACS and Giemsa staining. Scale: 50 μm. C/D. FACS analysis to detect FcεR1 expression on Ly6G+ neutrophils treated with and without 25 μg/ml of IgE for 6 hrs. Representative images are show in panel D. E/F. Neutrophil culture media supernatant IL6 levels and IL6 mRNA levels in neutrophils from Apoe−/− and Apoe−/−Fcer1a−/− mice treated with and without IgE as in C/D. G. Representative Western blot images and quantification of IL6, phosphor (p)-JNK, total (t)-JNK, p-p38, t-p38, p-ERK1/2, and t-ERK1/2 in cultured neutrophils from Apoe−/− and Apoe−/−Fcer1a−/− mice treated with or without 25 μg/ml IgE for 6 hrs. H/I. Neutrophil culture media supernatant IL6 levels and IL6 mRNA levels in neutrophils from Apoe−/− and Apoe−/−Fcer1a−/− mice treated with and without 25 μg/ml aggregated and cytokinergic IgE SPE-7, monomeric and poorly cytokinergic IgE H1-DNP-ε−206, and AAA lysate. AAA lysate alone without neutrophils was used as ELISA negative control. Data are mean±SEM from three independent experiments.
In response to pro-inflammatory stimuli, MAPK plays an essential role in neutrophil adhesion, migration, and activation.40–43 Therefore, we examined whether IgE also activated the MAPK signaling pathway as a mechanism to explain increased IL6 expression. When bone-marrow-derived neutrophils from Apoe−/− mice were treated with IgE, we detected significant increase of IL6, as determined by immunoblot analysis. From the same samples, we detected concurrent activation of both the phosphor (p)-JNK and p-p38, but not total (t)-JNK or t-p38. Yet, such activities of IgE were muted in neutrophils from Apoe−/−Fcer1a−/− mice (Figure 6G). In contrast, the activity of IgE in activating p-ERK1/2 remained moderate, regardless whether neutrophils were from Apoe−/− or Apoe−/−Fcer1a−/− mice (Figure 6G).
Neutrophil expression of IL6 in response to IgE stimulation was not limited to autoreactive aggregated and cytokinergic IgE SPE7 (Figure 6C–6G), but also monomeric and poorly cytokinergic IgE H1-DNP-ε-206. IgE H1-DNP-ε−206 remained active in stimulating neutrophil secretion of IL6 to the culture media and cellular IL6 mRNA levels, as determined by ELISA and RT-PCR (Figure 6H/6I). Same concentration (25 μg/ml) of AAA lysate served as experimental positive control. Although at much lower levels, AAA lysate increased neutrophil IL6 secretion and mRNA levels (Figure 6H/6I). AAA lysate alone without neutrophils was used as ELISA negative control (Figure 6H).
DISCUSSION
Earlier studies demonstrated an essential role of IgE in AAA by activating mast cells, macrophages, and T cells using the IgE receptor FcεR1-deficient Fcεr1a−/− mice.17,26 IgE induces the expression of IL6 and IFN-γ in macrophages, CD4+ and CD8+ T cells. Such IgE activities were muted when cells from Fcεr1a−/− mice were used.17,27 Yet, studies also showed that IgE induced FcεR1 complex formation with TLR4 and Nhe1 in macrophages,27,28 and possibly other inflammatory and vascular cells. It is possible that IgE actions involve not only FcεR1 but also TLR4 or Nhe1. Consistent with this hypothesis, both TLR4 and Nhe1 contribute to AAA growth. Deficiency of TLR4 protected mice from Ang-II perfusion-induced AAA.31,44 We recently showed that Nhe1-deficiency also reduced diet-induced atherosclerosis28 and Ang-II perfusion-induced AAA formation in mice (Shi, unpublished observation). We showed that IgE activities in activating foam cell formation and associated signaling transduction (p-AKT, p-PI3K, and p-mTOR), and in inducing protease expression and apoptosis were blocked in macrophages from Apoe−/−Nhe1+/– mice.28 IgE-induced macrophage expression of IL6 and monocyte chemoattractant protein-1 (MCP-1) and macrophage apoptosis were also muted in cells from TLR4-deficient Tlr4–/– mice.27 Therefore, the activities of TLR4 and Nhe1 may also be affected in Fcεr1a−/− mice, thereby contributing to reduced AAA in these mice.17 This hypothesis is supported by the observations that both the autoreactive aggregated and cytokinergic IgE SPE7 and the monomeric and poorly cytokinergic IgE H1-DNP-ε-206 promoted neutrophil IL6 expression (Figure 6H/6I) and macrophage extracellular pH reduction, death, and IL6 expression in the absence of antigen dinitrophenyl-human serum albumin (DNP-HSA).27 FcεR1 alone may not explain these IgE activities. IgE-induced FcεR1 complex formation with TLR427 and Nhe128 may mediate these IgE activities in the absence of IgE antigens, a possibility that merits further investigation. Nevertheless, the current study tests a direct role of IgE in AAA using IgE-deficient Ige–/– mice in both Ang-II perfusion-induced and peri-aortic CaCl2 injury-induced AAA models. Results demonstrate that IgE-deficiency blunted AAA formation and reduced lesion chemokine expression, inflammatory cell accumulation, and cytokine expression. FcεR1, Nhe1, and TLR4 may all contribute to these IgE activities.
While IgE-mediated activations of mast cells, macrophages, and T cells are equally important in AAA development,17,27 this study focuses on a role of IgE in promoting neutrophil accumulation in AAA lesions, and neutrophil IL6 expression and MAPK activation. Accumulating evidences suggest that neutrophils play pathogenic role in AAA. Patients with AAA demonstrate increased blood neutrophil counts and neutrophil activation marker NGAL (neutrophil gelatinase-associated lipocalin).45 Targeting neutrophils with antibodies or blocking chemokine expression necessary for neutrophil recruitment ameliorates experimental AAA formation.7,8,14 Here we revealed a role of IgE in regulating neutrophil expression of CXC chemokines CXCL5 and MIP-2α that have been shown essential to neutrophil chemoattraction.46–49 That is probably why we detected many fewer neutrophils in AAA lesions from the Apoe−/−Ige−/− mice than those in Apoe−/− mice (Figure 4B/4D). As a major IL6-producing cell type in AAA lesions,39 neutrophils in AAA lesions may produce IL6 to contribute to lesion inflammation and tissue remodeling. Low numbers of lesion neutrophils in Apoe−/−Ige−/− mice after Ang-II- and CaCl2-induced AAA may have contributed to reduced AAA in these mice. Yet, our study did not test a direct role of IgE-mediated neutrophil activation in AAA. One way to test a role of neutrophil-derive IL6 in AAA is to adoptively transfer in vitro prepared neutrophils from wild-type and IL6-deficent mice into mice subjected to AAA formation. However, such technique may be limited to studies of acute inflammatory disease.50–52 Because of the short lifespan of donor neutrophils (within 24 hrs),53 daily adoptive transfer of neutrophils to study chronic diseases can be technically challenging in addition to causing unnecessary stress to the subjects that may artificially complicate data interpretation. Therefore, neutrophil-selective IL6-deficiency may be required to test a role of neutrophil IL6 in AAA, a hypothesis that merits further investigation.
MAPK signaling is common in inflamed aortic wall but also contributes to neutrophil activation.37,54 Inhibition of the JNK and p38 signaling pathways attenuated AAA growth.54,55 FcεR1 expression on neutrophils is required for IgE-induced MAPK activation. Although not tested, IgE may activate the MAPK signaling pathways in other aortic wall inflammatory and vascular cells that express FcεR1.17,27,28 This mechanism may involve not only IL6, but also other MAPK signaling-associated pro-inflammatory cytokines, chemokines, and proteases from inflammatory and vascular cells, thereby damaging the aortic wall. For example, IL6 and MAPK signaling stimulate MMP and cysteinyl cathepsin expression in macrophages and fibroblasts.56,57 Both MMPs and cysteinyl cathepsins play essential roles in AAA development.58,59 Although not tested in this study, reduced numbers of CD31+ microvessel contents in AAA lesions from Apoe−/−Ige−/− mice (Figure 1G/2G) suggest impaired protease expression and extracellular matrix degradation.
Together, results from this study suggest that in additional to mast cells, macrophages, and T cells, IgE also contribute to neutrophil accumulation and activation in experimental suprarenal and infrarenal AAA lesions. Like what have been tested in the IgE targeting molecules FcεR1, TLR4, and Nhe1 from prior studies, IgE-deficiency protects mice from AAA development. Strategies aiming at decreasing IgE expression or blocking IgE actions may provide novel therapeutic approaches to mitigate AAA formation and progression.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Ms. Eugenia Shvartz for her technical assistance. The authors also thank Dr. Hans Oettgen from the Division of Immunology, Boston Children’s Hospital, Harvard Medical School for providing the IgE-deficient mice. This work was supported by awards from the National Natural Science Foundation of China (81300176 to CZY), the Fundamental Research Funds for the Central Universities, SCUT (D2180650 to CZY), the Science Foundation of Guangzhou First People’s Hospital (2019 to JL), and the National Institute of Health (HL123568, HL60942, and AG058670 to GPS).
NONSTANDARD ABBREVIATIONS
- AAA
abdominal aortic aneurysm
- Ang-II
angiotensin-II
- IgE
immunoglobulin E
- IL6
interleukin-6
- NETs
neutrophil extracellular traps
- MMP
matrix metalloproteinases
- MAPK
mitogen-activated protein kinase
- ApoE
apolipoprotein E
- OCT
optimal cutting temperature
- MHC-II
major histocompatibility complex class-II
- SMC
smooth muscle cell
- BMDM
bone-marrow-derived macrophage
- PBS
phosphate-buffered saline
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- CXCL
C-X-C motif chemokine ligand
- p-JNK
phosphor-c-Jun N-terminal kinase
- p-ERK1/2
phosphor-extracellular-signal-regulated kinase
- MIP-2α
macrophage inflammatory protein 2-alpha
- DNP-HSA
dinitrophenyl-human serum albumin
- TLR4
Toll-like receptor 4
- Nhe1
Na+-H+ exchanger
- NGAL
neutrophil gelatinase-associated lipocalin
- MCP-1
monocyte chemoattractant protein-1
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Sampson UK, Norman PE, Fowkes FG, Aboyans V, Yanna S, Harrell FE Jr., Forouzanfar MH, Naghavi M, Denenberg JO, McDermott MM, Criqui MH, Mensah GA, Ezzati M, and Murray C (2014) Global and regional burden of aortic dissection and aneurysms: mortality trends in 21 world regions, 1990 to 2010. Glob Heart 9, 171–180 e110. [DOI] [PubMed] [Google Scholar]
- 2.Sakalihasan N, Limet R, and Defawe OD (2005) Abdominal aortic aneurysm. Lancet 365, 1577–1589. [DOI] [PubMed] [Google Scholar]
- 3.Moxon JV, Parr A, Emeto TI, Walker P, Norman PE, and Golledge J (2010) Diagnosis and monitoring of abdominal aortic aneurysm: current status and future prospects. Curr Probl Cardiol 35, 512–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golledge J (2019) Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol 16, 225–242. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu K, Mitchell RN, and Libby P (2006) Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 26, 987–994. [DOI] [PubMed] [Google Scholar]
- 6.Dale MA, Ruhlman MK, and Baxter BT (2015) Inflammatory cell phenotypes in AAAs: their role and potential as targets for therapy. Arterioscler Thromb Vasc Biol 35, 1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannawa KK, Eliason JL, Woodrum DT, Pearce CG, Roelofs KJ, Grigoryants V, Eagleton MJ, Henke PK, Wakefield TW, Myers DD, Stanley JC, and Upchurch GR Jr. (2005) L-selectin-mediated neutrophil recruitment in experimental rodent aneurysm formation. Circulation 112, 241–247. [DOI] [PubMed] [Google Scholar]
- 8.Eliason JL, Hannawa KK, Ailawadi G, Sinha I, Ford JW, Deogracias MP, Roelofs KJ, Woodrum DT, Ennis TL, Henke PK, Stanley JC, Thompson RW, and Upchurch GR Jr. (2005) Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation 112, 232–240. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe K, Fujioka D, Saito Y, Nakamura T, Obata JE, Kawabata K, Watanabe Y, Mishina H, Tamaru S, Hanasaki K, and Kugiyama K (2012) Group X secretory PLA2 in neutrophils plays a pathogenic role in abdominal aortic aneurysms in mice. Am J Physiol Heart Circ Physiol 302, H95–104. [DOI] [PubMed] [Google Scholar]
- 10.Kim HW, Blomkalns AL, Ogbi M, Thomas M, Gavrila D, Neltner BS, Cassis LA, Thompson RW, Weiss RM, Lindower PD, Blanco VM, McCormick ML, Daugherty A, Fu X, Hazen SL, Stansfield BK, Huo Y, Fulton DJ, Chatterjee T, and Weintraub NL (2017) Role of myeloperoxidase in abdominal aortic aneurysm formation: mitigation by taurine. Am J Physiol Heart Circ Physiol 313, H1168–H1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spinosa M, Su G, Salmon MD, Lu G, Cullen JM, Fashandi AZ, Hawkins RB, Montgomery W, Meher AK, Conte MS, Sharma AK, Ailawadi G, and Upchurch GR Jr. (2018) Resolvin D1 decreases abdominal aortic aneurysm formation by inhibiting NETosis in a mouse model. J Vasc Surg 68, 93S–103S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan H, Zhou HF, Akk A, Hu Y, Springer LE, Ennis TL, and Pham CTN (2016) Neutrophil Proteases Promote Experimental Abdominal Aortic Aneurysm via Extracellular Trap Release and Plasmacytoid Dendritic Cell Activation. Arterioscler Thromb Vasc Biol 36, 1660–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ajibade AA, Wang Q, Cui J, Zou J, Xia X, Wang M, Tong Y, Hui W, Liu D, Su B, Wang HY, and Wang RF (2012) TAK1 negatively regulates NF-kappaB and p38 MAP kinase activation in Gr-1+CD11b+ neutrophils. Immunity 36, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He L, Fu Y, Deng J, Shen Y, Wang Y, Yu F, Xie N, Chen Z, Hong T, Peng X, Li Q, Zhou J, Han J, Wang Y, Xi J, and Kong W (2018) Deficiency of FAM3D (Family With Sequence Similarity 3, Member D), A Novel Chemokine, Attenuates Neutrophil Recruitment and Ameliorates Abdominal Aortic Aneurysm Development. Arterioscler Thromb Vasc Biol 38, 1616–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagano MB, Bartoli MA, Ennis TL, Mao D, Simmons PM, Thompson RW, and Pham CT (2007) Critical role of dipeptidyl peptidase I in neutrophil recruitment during the development of experimental abdominal aortic aneurysms. Proc Natl Acad Sci U S A 104, 2855–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu CL, Wang Y, Liao M, Wemmelund H, Ren J, Fernandes C, Zhou Y, Sukhova GK, Lindholt JS, Johnsen SP, Zhang JY, Cheng X, Huang X, Daugherty A, Levy BD, Libby P, and Shi GP (2016) Allergic Lung Inflammation Aggravates Angiotensin II-Induced Abdominal Aortic Aneurysms in Mice. Arterioscler Thromb Vasc Biol 36, 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Lindholt JS, Sukhova GK, Shi MA, Xia M, Chen H, Xiang M, He A, Wang Y, Xiong N, Libby P, Wang JA, and Shi GP (2014) IgE actions on CD4+ T cells, mast cells, and macrophages participate in the pathogenesis of experimental abdominal aortic aneurysms. EMBO Mol Med 6, 952–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly BT, and Grayson MH (2016) Immunoglobulin E, what is it good for? Ann Allergy Asthma Immunol 116, 183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Futosi K, Fodor S, and Mocsai A (2013) Reprint of Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol 17, 1185–1197. [DOI] [PubMed] [Google Scholar]
- 20.Porcherie A, Mathieu C, Peronet R, Schneider E, Claver J, Commere PH, Kiefer-Biasizzo H, Karasuyama H, Milon G, Dy M, Kinet JP, Louis J, Blank U, and Mecheri S (2011) Critical role of the neutrophil-associated high-affinity receptor for IgE in the pathogenesis of experimental cerebral malaria. J Exp Med 208, 2225–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteseirin J, Bonilla I, Camacho MJ, Conde J, and Sobrino F (2001) IgE-dependent release of myeloperoxidase by neutrophils from allergic patients. Clin Exp Allergy 31, 889–892. [DOI] [PubMed] [Google Scholar]
- 22.Gounni AS, Lamkhioued B, Koussih L, Ra C, Renzi PM, and Hamid Q (2001) Human neutrophils express the high-affinity receptor for immunoglobulin E (Fc epsilon RI): role in asthma. FASEB J 15, 940–949. [DOI] [PubMed] [Google Scholar]
- 23.Alphonse MP, Saffar AS, Shan L, HayGlass KT, Simons FE, and Gounni AS (2008) Regulation of the high affinity IgE receptor (Fc epsilonRI) in human neutrophils: role of seasonal allergen exposure and Th-2 cytokines. PLoS One 3, e1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saffar AS, Alphonse MP, Shan L, Hayglass KT, Simons FE, and Gounni AS (2007) IgE modulates neutrophil survival in asthma: role of mitochondrial pathway. J Immunol 178, 2535–2541. [DOI] [PubMed] [Google Scholar]
- 25.Monteseirin J, Vega A, Chacon P, Camacho MJ, El Bekay R, Asturias JA, Martinez A, Guardia P, Perez-Cano R, and Conde J (2007) Neutrophils as a novel source of eosinophil cationic protein in IgE-mediated processes. J Immunol 179, 2634–2641. [DOI] [PubMed] [Google Scholar]
- 26.Guo W, Gao R, Zhang W, Ge W, Ren M, Li B, Zhao H, and Wang J (2019) IgE Aggravates the Senescence of Smooth Muscle Cells in Abdominal Aortic Aneurysm by Upregulating LincRNA-p21. Aging Dis 10, 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Cheng X, Xiang MX, Alanne-Kinnunen M, Wang JA, Chen H, He A, Sun X, Lin Y, Tang TT, Tu X, Sjoberg S, Sukhova GK, Liao YH, Conrad DH, Yu L, Kawakami T, Kovanen PT, Libby P, and Shi GP (2011) IgE stimulates human and mouse arterial cell apoptosis and cytokine expression and promotes atherogenesis in Apoe−/− mice. J Clin Invest 121, 3564–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu CL, Zhang X, Liu J, Wang Y, Sukhova GK, Wojtkiewicz GR, Liu T, Tang R, Achilefu S, Nahrendorf M, Libby P, Guo J, Zhang JY, and Shi GP (2019) Na(+)-H(+) exchanger 1 determines atherosclerotic lesion acidification and promotes atherogenesis. Nat Commun 10, 3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi YH, Ho PY, Chen TW, Lin WJ, Gukassyan V, Tsai TH, Wang DW, Lew TS, Tang CY, Lo SJ, Chen TY, Kao FJ, and Lin CH (2009) Membrane targeting and coupling of NHE1-integrinalphaIIbbeta3-NCX1 by lipid rafts following integrin-ligand interactions trigger Ca2+ oscillations. J Biol Chem 284, 3855–3864. [DOI] [PubMed] [Google Scholar]
- 30.Babic AM, Chen CC, and Lau LF (1999) Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol 19, 2958–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin Z, Bagley J, Sukhova G, Baur WE, Park HJ, Beasley D, Libby P, Zhang Y, and Galper JB (2015) Angiotensin II-induced TLR4 mediated abdominal aortic aneurysm in apolipoprotein E knockout mice is dependent on STAT3. J Mol Cell Cardiol 87, 160–170. [DOI] [PubMed] [Google Scholar]
- 32.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, Knispel R, Xiong W, Thompson RW, Baxter BT, and Shi GP (2007) Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest 117, 3359–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swamydas M, Luo Y, Dorf ME, and Lionakis MS (2015) Isolation of Mouse Neutrophils. Curr Protoc Immunol 110, 3 20 21–23 20 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitaura J, Song J, Tsai M, Asai K, Maeda-Yamamoto M, Mocsai A, Kawakami Y, Liu FT, Lowell CA, Barisas BG, Galli SJ, and Kawakami T (2003) Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcepsilonRI. Proc Natl Acad Sci U S A 100, 12911–12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Sukhova GK, Liu J, Ozaki K, Lesner A, Libby P, Kovanen PT, and Shi GP (2015) Cathepsin G deficiency reduces periaortic calcium chloride injury-induced abdominal aortic aneurysms in mice. J Vasc Surg 62, 1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber C, Zernecke A, and Libby P (2008) The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol 8, 802–815. [DOI] [PubMed] [Google Scholar]
- 37.Sadik CD, Kim ND, and Luster AD (2011) Neutrophils cascading their way to inflammation. Trends Immunol 32, 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sokol CL, and Luster AD (2015) The chemokine system in innate immunity. Cold Spring Harb Perspect Biol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anzai A, Shimoda M, Endo J, Kohno T, Katsumata Y, Matsuhashi T, Yamamoto T, Ito K, Yan X, Shirakawa K, Shimizu-Hirota R, Yamada Y, Ueha S, Shinmura K, Okada Y, Fukuda K, and Sano M (2015) Adventitial CXCL1/G-CSF expression in response to acute aortic dissection triggers local neutrophil recruitment and activation leading to aortic rupture. Circ Res 116, 612–623. [DOI] [PubMed] [Google Scholar]
- 40.de Oliveira S, Boudinot P, Calado A, and Mulero V (2015) Duox1-derived H2O2 modulates Cxcl8 expression and neutrophil recruitment via JNK/c-JUN/AP-1 signaling and chromatin modifications. J Immunol 194, 1523–1533. [DOI] [PubMed] [Google Scholar]
- 41.Yang L, Zhou X, Huang W, Fang Q, Hu J, Yu L, Ma N, and Zhang W (2017) Protective Effect of Phillyrin on Lethal LPS-Induced Neutrophil Inflammation in Zebrafish. Cell Physiol Biochem 43, 2074–2087. [DOI] [PubMed] [Google Scholar]
- 42.Kasper B, Brandt E, Ernst M, and Petersen F (2006) Neutrophil adhesion to endothelial cells induced by platelet factor 4 requires sequential activation of Ras, Syk, and JNK MAP kinases. Blood 107, 1768–1775. [DOI] [PubMed] [Google Scholar]
- 43.Rosa SI, Rios-Santos F, Balogun SO, and Martins DT (2016) Vitexin reduces neutrophil migration to inflammatory focus by down-regulating pro-inflammatory mediators via inhibition of p38, ERK1/2 and JNK pathway. Phytomedicine 23, 9–17. [DOI] [PubMed] [Google Scholar]
- 44.Lai CH, Wang KC, Lee FT, Tsai HW, Ma CY, Cheng TL, Chang BI, Yang YJ, Shi GY, and Wu HL (2016) Toll-Like Receptor 4 Is Essential in the Development of Abdominal Aortic Aneurysm. PLoS One 11, e0146565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos-Mozo P, Madrigal-Matute J, Vega de Ceniga M, Blanco-Colio LM, Meilhac O, Feldman L, Michel JB, Clancy P, Golledge J, Norman PE, Egido J, and Martin-Ventura JL (2012) Increased plasma levels of NGAL, a marker of neutrophil activation, in patients with abdominal aortic aneurysm. Atherosclerosis 220, 552–556. [DOI] [PubMed] [Google Scholar]
- 46.Disteldorf EM, Krebs CF, Paust HJ, Turner JE, Nouailles G, Tittel A, Meyer-Schwesinger C, Stege G, Brix S, Velden J, Wiech T, Helmchen U, Steinmetz OM, Peters A, Bennstein SB, Kaffke A, Llanto C, Lira SA, Mittrucker HW, Stahl RA, Kurts C, Kaufmann SH, and Panzer U (2015) CXCL5 drives neutrophil recruitment in TH17-mediated GN. J Am Soc Nephrol 26, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mei J, Liu Y, Dai N, Favara M, Greene T, Jeyaseelan S, Poncz M, Lee JS, and Worthen GS (2010) CXCL5 regulates chemokine scavenging and pulmonary host defense to bacterial infection. Immunity 33, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Appelberg R (1992) Macrophage inflammatory proteins MIP-1 and MIP-2 are involved in T cell-mediated neutrophil recruitment. J Leukoc Biol 52, 303–306. [DOI] [PubMed] [Google Scholar]
- 49.Johnson EA, Dao TL, Guignet MA, Geddes CE, Koemeter-Cox AI, and Kan RK (2011) Increased expression of the chemokines CXCL1 and MIP-1alpha by resident brain cells precedes neutrophil infiltration in the brain following prolonged soman-induced status epilepticus in rats. J Neuroinflammation 8, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishi H, Furuhashi K, Cullere X, Saggu G, Miller MJ, Chen Y, Rosetti F, Hamilton SL, Yang L, Pittman SP, Liao J, Herter JM, Berry JC, DeAngelo DJ, Zhu C, Tsokos GC, and Mayadas TN (2017) Neutrophil FcgammaRIIA promotes IgG-mediated glomerular neutrophil capture via Abl/Src kinases. J Clin Invest 127, 3810–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen K, Nishi H, Travers R, Tsuboi N, Martinod K, Wagner DD, Stan R, Croce K, and Mayadas TN (2012) Endocytosis of soluble immune complexes leads to their clearance by FcgammaRIIIB but induces neutrophil extracellular traps via FcgammaRIIA in vivo. Blood 120, 4421–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirahashi J, Mekala D, Van Ziffle J, Xiao L, Saffaripour S, Wagner DD, Shapiro SD, Lowell C, and Mayadas TN (2006) Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity 25, 271–283. [DOI] [PubMed] [Google Scholar]
- 53.Gray RD, Hardisty G, Regan KH, Smith M, Robb CT, Duffin R, Mackellar A, Felton JM, Paemka L, McCullagh BN, Lucas CD, Dorward DA, McKone EF, Cooke G, Donnelly SC, Singh PK, Stoltz DA, Haslett C, McCray PB, Whyte MKB, Rossi AG, and Davidson DJ (2018) Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax 73, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang XJ, He C, Tian K, Li P, Su H, and Wan JB (2015) Ginsenoside Rb1 attenuates angiotensin II-induced abdominal aortic aneurysm through inactivation of the JNK and p38 signaling pathways. Vascul Pharmacol 73, 86–95. [DOI] [PubMed] [Google Scholar]
- 55.Tsai SH, Huang PH, Peng YJ, Chang WC, Tsai HY, Leu HB, Chen JW, and Lin SJ (2013) Zoledronate attenuates angiotensin II-induced abdominal aortic aneurysm through inactivation of Rho/ROCK-dependent JNK and NF-kappaB pathway. Cardiovasc Res 100, 501–510. [DOI] [PubMed] [Google Scholar]
- 56.Kothari P, Pestana R, Mesraoua R, Elchaki R, Khan KM, Dannenberg AJ, and Falcone DJ (2014) IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages. J Immunol 192, 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaguchi T, Naruishi K, Arai H, Nishimura F, and Takashiba S (2008) IL-6/sIL-6R enhances cathepsin B and L production via caveolin-1-mediated JNK-AP-1 pathway in human gingival fibroblasts. J Cell Physiol 217, 423–432. [DOI] [PubMed] [Google Scholar]
- 58.Keeling WB, Armstrong PA, Stone PA, Bandyk DF, and Shames ML (2005) An overview of matrix metalloproteinases in the pathogenesis and treatment of abdominal aortic aneurysms. Vasc Endovascular Surg 39, 457–464. [DOI] [PubMed] [Google Scholar]
- 59.Liu CL, Guo J, Zhang X, Sukhova GK, Libby P, and Shi GP (2018) Cysteine protease cathepsins in cardiovascular disease: from basic research to clinical trials. Nat Rev Cardiol 15, 351–370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.