Abstract
It is vital to understand processes of microplastic ingestion and egestion by aquatic organisms in order to evaluate the potential effects and impacts of microplastics in aquatic ecosystems. In this study, goldfish (Carassius auratus) was used to investigate ingestion and egestion of polyethylene (PE) microplastics and how these processes were affected by size, color, and shape of microplastics. Results showed that goldfish ingested white PE microplastics only in the presence of fish feed and that microplastics larger than 2 mm were rejected even after being ingested. However, in the presence of food, more green and black microplastics were ingested compared with red, blue, and white microplastics while significantly higher amounts of microplastic films were ingested compared with fragments and filaments. Microplastics ingested by goldfish were egested within 72 h. However, the egestion rate of filaments was the lowest among all tested microplastic shapes. The presence of food appeared to reduce film and filament residues in fish after 72 h. Results of this study imply that different features of microplastics result in different exposure risks for fish. Thus, the specific features of microplastics (e.g. their shape, color, and size) should be considered in future ecotoxicological studies.
Keywords: Environmental chemistry, Environmental hazard, Environmental pollution, Environmental risk assessment, Environmental toxicology, Microplastics, Fish, Ingestion, Egestion, Environmental features
Environmental chemistry; Environmental hazard; Environmental pollution; Environmental risk assessment; Environmental toxicology; Microplastics; Fish; Ingestion; Egestion; Environmental features.
1. Introduction
Microplastics (plastic particles <5 mm) have become a worldwide environmental concern due to their wide distribution and their potential ecological risks (Machado et al., 2018; Rochman, 2018). Both marine and freshwater ecosystems are considered to be important sinks of microplastics (Cole et al., 2011; Zhang et al., 2018). Aquatic organisms including zooplanktons, benthic macroinvertebrates, fishes, and even marine mammals have been demonstrated to have microplastics detected in their digestive tracts (Cole et al., 2013; Jabeen et al., 2017; Li et al., 2018a; Lusher et al., 2017). Microplastic exposure can have physiological, behavioral, growth, reproductive, and genetic impacts on aquatic organisms (Chae and An, 2017; Green et al., 2017). Direct physical damage from microplastics and adverse effects from hazardous substances released from them might be responsible for the toxicity of microplastics (Chae and An, 2017; Koelmans et al., 2016; Lu et al., 2016). This implies that residence time in organisms as well as the features of microplastics are related to their toxic effects. However, factors affecting the ingestion and retention of microplastics in organisms are still poorly understood.
Ingestion of microplastics by fish can be affected by their feeding habits. Filter-feeding fish can ingest microplastics during filtration (Fossi et al., 2014) while trophic transfer and initiative predation are both potential pathways for microplastics to enter predators and opportunistic feeders (Ferreira et al., 2018; Grigorakis et al., 2017; Lu et al., 2016; Ory et al., 2018). In finless porpoise, unintentional ingestion of microplastics has been speculated as the avenue for microplastics found in neonatal porpoise (Xiong et al., 2018). Previous work has also considered the egestion of microplastics via feces by variety of organisms, which could affect not only the fate and distribution of microplastics but also their toxic effects on organisms (Cole et al., 2013; Dawson et al., 2018; Grigorakis et al., 2017; Hämer et al., 2014; Katija et al., 2017; Ory et al., 2018).
Ingestion and egestion of microplastics by aquatic organism have been investigated in many laboratory experiments (Cole et al., 2016; Lu et al., 2016). However, compared to the diversity of microplastics in the environment, microplastics used in these studies are often simple and even hardly found in the environment. Polystyrene (PS) microbeads are the most commonly used microplastics in ecotoxicological studies (Chae and An, 2017) but PS microbeads are rarely detected in the environment (Zhang et al., 2018). Microplastics in the environment are more diverse in shape, color, size, and type (Cole et al., 2011), and all these features might affect their ingestion and egestion by organisms (Grigorakis et al., 2017; Lehtiniemi et al., 2018; Ory et al., 2018). Selective feeding for different colors of microplastics has been observed previously in fish and other organisms (de Sa et al., 2015; Ory et al., 2017, 2018; Santos et al., 2016). However, more studies are needed to reveal the influence of microplastics with different shapes, sizes, and colors on their ingestion and egestion by fish.
In this study, goldfish (Carassius auratus) was used as model organism to assess the ingestion and egestion of microplastics with environmental features. Polyethylene (PE) microplastics of different sizes, colors, and shapes were used to evaluate their ingestion by goldfish. The effects of shapes and food for the egestion of microplastics were also evaluated to assess whether the egestion processes of microplastics are related to any of these features.
2. Methods
2.1. Materials
Polyethylene (PE) plastic is one of the most frequently detected plastic types in the environment and can be found with different sizes, colors, and shapes (Zhang et al., 2018). So, PE raw plastic materials, including plastic sheet, cling film, and fishing line bought from local retail stores, were used in this study. The plastic sheets used in this work had different colors (green, blue, red, black, and white). Raw plastic materials were ground or cut into microplastic fragments, films, and filaments by an IKA (Staufen, Germany) A11 basic analytical mill with liquid nitrogen. Then, ground microplastics were sieved through stainless steel mesh of 3 mm, 2 mm, and 0.5 mm sizes to obtain microplastics of three different size categories (3–5 mm, 2–3 mm, and 0.5–2 mm). All these microplastics were stored in glass petri dishes before experiment.
Goldfish are a common, easily raised, and highly tolerant opportunist species. The experiments involving goldfish were approved by the Institutional Animal Care and Use Committee of the Institute of Hydrobiology, Chinses Academy of Sciences. Goldfish used in this study were bought from a local aquarium market and acclimated for two weeks prior to experiments. Commercial fish feed was also obtained from the aquarium market. The feed was brown and spherical with a diameter of about 1–3 mm. Goldfish were fed twice a day (morning and afternoon) with about 0.3 g of feed per fish.
2.2. Experimental design
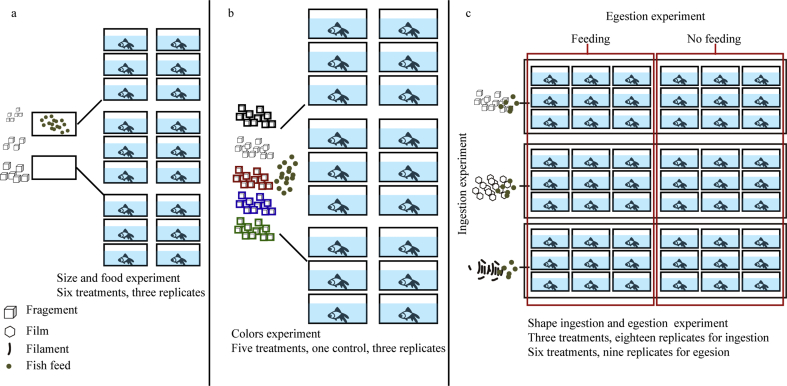
The experimental design of this study was presented in Figure 1 and the details of each experiment was provided below.
Figure 1.
The experimental design of this study: a) the ingestion of microplastics of different sizes with or without food; b) ingestion of microplastics of different colors; and c) Ingestion and egestion of microplastics of different shapes.
2.2.1. Ingestion of microplastics of different sizes
Semitransparent plastic tanks with dimensions of 25 × 14 × 6 cm and volume of 2 L were used as the test vessels. Goldfish fasted for 48 h were used in the experiments. One fish was added to each tank with 1.5 L water and three tanks were performed for each treatment as replicates. White microplastic fragments in three size categories (0.5~2mm, 2~3mm, and >3mm) were added at a concentration of 100 items/L with 0.3 g of feed or without food, respectively, which is close to the highest microplastic abundances in the environment reported in the effluents of wastewater treatment plants (Li et al., 2018b). After added, the microplastic and feed particles were stirred softly with a glass rod. However, all microplastics were floating on the surface of the water as the feed due to the low density of PE (0.88–0.96 g/cm3). After 1 h of exposure, fish were taken out from tanks, washed carefully with filtered deionized water, and narcotized with tricaine mesylate (MS-222) anesthetic to prevent egestion of microplastics caused by the stress reaction. The digestive tract of each fish was sampled to determine the quantity of the ingested microplastics following the method introduced in 2.3. The water in each tank was also filtered onto a Whatman GF/C filter to determine the concentration of microplastics remaining in the water. A blank control without microplastics was also included.
2.2.2. Ingestion of microplastics of different colors
This experiment was carried out following similar procedures as described above with one goldfish in each tank and three tanks for each treatment as replicates. Only 0.5–2 mm fragments were used and added at a concentration of 100 items/L with 0.3 g fish feed. Fragments of five different colors (white, black, blue, red, and green) were tested separately in this experiment.
2.2.3. Ingestion and egestion of microplastics of different shapes
This experiment was carried out following similar procedures as color experiment with one fish in each tank except that the experimental treatments involved microplastics of different shapes (fragment, film, and filament). Fragments, film, and filament used in this experiment were white, transparent, and cyan, respectively, and all 0.5–2 mm. In each of the three treatments (fragment, film, filament), eighteen replicates of each treatment were carried out to meet the demand of the egestion experiment (see below).
After 1 h of exposure, fish in each shape treatment were taken out from tanks, washed with filtered deionized water, and each fish was transferred into a new separate tank without microplastics. All eighteen fish in from each shape treatment were assigned equally to two groups. One group was fasted while the other group was fed every morning and afternoon for 72 h. During the 72h egestion experiment, Fish were transferred to new tanks at 1 h, 2 h, 3 h, 6 h, 10 h, 24 h, 48 h, and 72 h. The water in the former tanks were filtered through a Whatman GF/C filter. Filters were collected to analyze egested microplastics. After 72 h, the experimental fish were taken out from tanks and dissected as described above to determine the quantity of microplastic remaining in their digestive tracts. No fish died during experiment.
2.3. Microplastic analysis
To determine microplastic ingestion, each fish was killed and dissected, and its digestive tract was transferred to a 100-mL glass beaker covered with aluminum foil. The digestive tracts were digested with 30 % hydrogen peroxide for 72 h at 60 °C。After digestion, the residue in each beaker was filtered onto a Whatman GF/C filter.
To determine microplastic egestion, the residues on the filter collected at each sampling time were rinsed with 30% hydrogen peroxide into a 100-mL glass beaker to digest the faeces and food. After 72 h of digestion, the residues in each beaker were filtered onto a new Whatman GF/C filter. The filters were transferred to glass petri dishes and air-dried. Microplastics on the filters were examined and counted using a Nikon stereomicroscope (Tokyo, Japan) under 20–40 × magnification.
2.4. Data analysis
Data for ingestion and egestion of microplastics by goldfish were obtained directly from visual observation of microplastics on the filter. However, for comparison of egestion of microplastics, egestion rates were calculated because the goldfish used in the egestion group may have ingested different numbers of microplastics in the ingestion experiment, which would make the direct comparisons of quantity meaningless. Egestion rate for the first egestion event (vf) and for the whole egestion process (v) were calculated according to Eqs. (1) and (2), respectively:
| (1) |
| (2) |
where nf was the number of microplastics found in the first time, tf was the time of the sampling found firstly found microplastics egestion, n was the number of microplastics egested by each fish during the whole experiment time, and te was the time for all microplastics egested from the fish (if not all microplastics egested during the whole experiment, te = 72).
SPSS 20.0 was used for the statistical analyses. One-way analysis of variance (ANOVA) was used to test the differences among the ingestion of microplastics of different colors. The Least Significant Difference (LSD) method was used for the post hoc tests of different experimental groups. Nonparametric test, namely Kruskal-Wallis and Mann-Whitney post hoc test were applied to compare the differences of ingestion and egestion rates of microplastics in different shapes, since the data of these experiments did not follow the normal distribution. Finally, we conducted two-way ANOVA to test the effects of feeding condition and microplastic shape on egestion rates (vf and v) of goldfish. The LSD method was also used for the post hoc tests of main effects for each treatment. A significance level of 0.05 was used in all tests.
3. Results
3.1. Microplastic ingestion preferences of goldfish
In the size experiment, microplastic ingestion was only observed in the experimental group having microplastic fragments that were 0.5–2 mm and in the presence of food. No microplastics were found in the digestive tracts of fish in other treatments. We observed that microplastic fragments larger than 2 mm were ingested by the goldfish with food but then rejected (Video S1). Goldfish completely ignored white microplastic fragments in the absence of food (Video S2). The numbers of microplastics fragment ingested in this experiment were provided in Table S1. As a result, all subsequent ingestion experiments were carried out using 0.5–2mm microplastics with food present.
Supplementary content related to this article has been published online at https://doi.org/10.1016/j.heliyon.2019.e03063.
The following are the supplementary data related to this article:
Videos1Microplastics ingestion process of goldfish when food was present.
Videos2Microplastics ingestion process of goldfish in the absence of food.
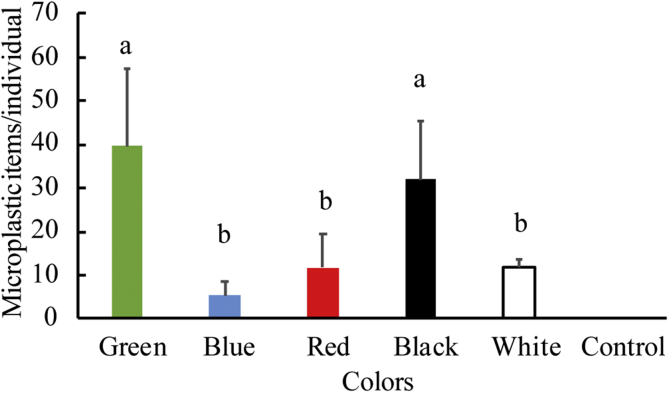
Color preferences for ingested microplastics were also observed (Figure 2). As indicated by the result of one-way ANOVA, significant differences were detected for ingestion of microplastics in different color treatments (F (4, 10) = 5.974, p = 0.010). Abundances of green and black microplastics in the digestive tracts of goldfish were significantly higher than that of other colors (p < 0.05). The detailed results of the One-way ANOVA and the LSD test are presented in Supplementary Information.
Figure 2.
Microplastic abundances in the digestive tracts of goldfish in different color groups (the bars and error bars are mean values and standard deviations, respectively. n = 3). The bars with different letters are significantly different (p < 0.05).
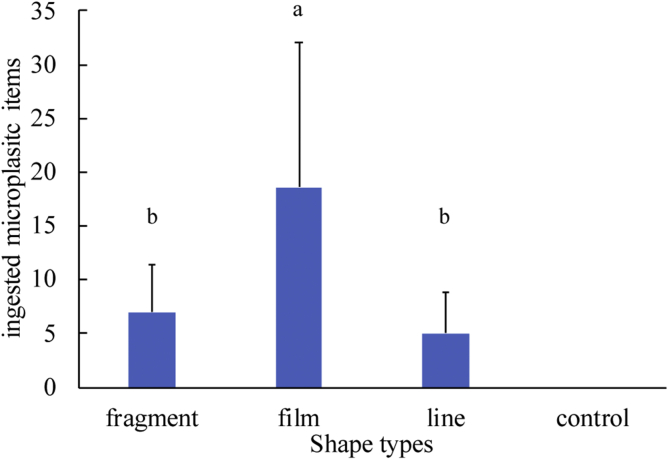
The result of independent samples nonparametric test also indicated that there were also significant different for the ingestion of microplastics of different shapes (H = 18.586, p < 0.001). Microplastic film was the most readily ingested shape as indicated by the highest abundances in the digestive tract of goldfish (Figure 3). Meanwhile, there was no significant difference in ingestion between fragments and filaments. The detailed results of the independent samples nonparametric test are presented in Supplementary Information.
Figure 3.
Microplastic abundances in the digestive tracts of goldfish in different shape groups (the bars and error bars are mean values and standard deviations, respectively. n = 18). The bars with different letters are significant different (p < 0.05).
3.2. Egestion of microplastics by goldfish
All shapes of microplastics ingested by the goldfish could be egested. Microplastic fragments were thoroughly egested from all fish after 72 h, while microplastics remained in the digestive tracts for 2, 4, and 3 out of 9 fish in the no-feeding film group, no-feeding filament group, and feeding filament group, respectively (Table S2 – S4). Food availability appeared to reduce film and filament residues in fish after 72 h.
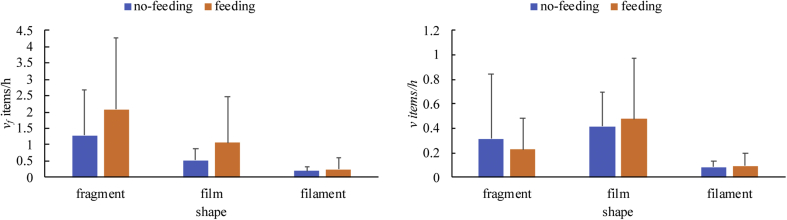
Two-way ANOVA showed that shape had a significant main effect on both v and vf, while the main effect of food presence and its interaction with shape were not significant (Table 1, Figure 4). Pairwise tests indicated that the vf was significantly higher for fragments than for film and filament (p < 0.05, Table 1, Table S10), while the v was significantly higher for film relative to filament (p < 0.05, Table 1, Table S12).
Table 1.
Summary of tests of the Two-way ANOVA of the effects of feeding × shape on egestion rates of microplastics (vf and v). Values which do not differ at the 0.05 level in LSD tests are joined by an underline. P-values < 0.05 are bolded.
| Partial η2 | F | p-value | Treatment (post hoc test) | |
|---|---|---|---|---|
| vf | ||||
| Food | 0.044 | 2.052 | 0.159 (NS) | |
| Shape | 0.223 | 6.446 | 0.003 | Fragmenta; filmb; filamentb |
| Food * shape | 0.018 | 0.419 | 0.660 (NS) | |
| v | ||||
| Food | <0.001 | 0.005 | 0.942 (NS) | |
| Shape | 0.171 | 4.654 | 0.015 | Filma; fragment ab; filamentb |
| Food * shape | 0.009 | 0.201 | 0.819 (NS) | |
NS, not significant.
a and b in the table present the significant differences (p < 0.05) between different groups.
Figure 4.
The egestion rates of different shapes of microplastics for goldfish in different treatments. a) vf; b) v. The bars and error bars are mean values and standard deviations, respectively. n = 9. The detail of statistics was presented in Supplementary Information.
4. Discussion
4.1. Influence of microplastic features on ingestion
In this study, the presence of food affected the ingestion of white microplastics by goldfish. This result implies ingestion of microplastics by goldfish involves co-capture with food, which has also been found in experiments with the medusa fish Seriolella violacea (Ory et al., 2018). Co-capture is also considered to be involved in microplastic ingestion by some large aquatic mammals (Lusher et al., 2017). Raw white microplastics may not attract the fish to catch them without flavor components and color that are of interest to goldfish. However, in the presence of food, microplastics might enter the mouth of fish if they are close to food pellets in the water. Study of sea birds indicates that plastic particles with algae living on the surface can release infochemicals to entice the sea birds to eat them directly (Cózar et al., 2017). Odor has also been established for goldfish as involved in finding food (Hara, 2006; Rolen et al., 2003; Stacey and Kyle, 1983). However, the short duration of the experiment prevents the formation of biofilms on the microplastics in this study. The effect of attached biofilms on the ingestion of microplastics by fish requires further studies.
More green and black microplastics ingested implies that co-capture may not be the only factor involved in microplastic ingestion and suggests that the goldfish visual system also plays a role. Goldfish have an acute visual system (Yager and Thorpe, 1970). Despite many previous research on color perception by goldfish (Neumeyer, 1992; Ohnishi, 1993), there are no relevant studies of goldfish evaluating its color preference for food. However, study of rainbow trout indicates that fish often prefer food in particular colors while study of zebrafish indicates that this kind of color preference can be found and trained when plastic strips are used as simulative food (Ginetz and Larkin, 1973; Spence and Smith, 2008). The food used in this study was dark brown and, under natural conditions, the food of goldfish includes plants (green) and insects (dark in color) (Kottelat and Freyhof, 2007). This suggests that goldfish may mistake microplastics with similar colors as food. In laboratory experiments, Ory et al. (2018) found a preference for black microplastics in Seriolella violacea, a marine planktivore. A study on Pomatoschistus microps also found a preference for microplastics with colors similar to its prey (de Sa et al., 2015). In a field study, preferences for colored microplastics similar to prey of Decapterus muroadsi have also been documented (Ory et al., 2017). (Santos et al., 2016) have also found that sea turtles which perceive floating plastic from below preferentially ingest dark plastic fragments.
The developed gustatory abilities of fish could help them segregate edible from inedible items after oral intake (Kasumyan and Doving, 2003). However, given the size and shape selectivity shown in this study, sensation of foreign materials might also play a role during the ingestion of microplastics by goldfish. Compared to large plastic debris, small microplastics are more easily ingested (Wright et al., 2013). The goldfish used in this study could eat food pellets with diameter from 1 to 3 mm. This means that most microplastics used in this work could easily pass the mouth of goldfish and indeed co-capture was observed for larger microplastics during the experiment. However, after being captured, the goldfish expelled large microplastic particles after chewing (Video S1). This implies that microplastic size could affect ability of the fish to sense them during ingestion and chewing, making smaller microplastics more readily swallowed. Indeed, fish are sensitive to hard materials during swallowing (Houlihan et al., 2008). Previous study of juvenile striped killifish (Fundulus majalis), tomcods (Microgadus tomcod), and juvenile centrolophidae (Seriolella violacea) have all shown expulsion of millimeter-sized foreign materials including microplastics (Colton et al., 1974; Ory et al., 2018). Our results indicate that particle size is one factor affecting the likelihood of rejection of plastics that have been taken into the mouth.
The shape of microplastics could also affect swallowing. Although all microplastics used in this study are made from PE, their different shapes may make them be sensed by goldfish differently. Films are the thinnest among the shapes and easy to deform, which may make them difficult to be sensed when goldfish swallow. Microplastics in natural waters are diverse in not only polymer type but also shapes (Jabeen et al., 2017; Zhang et al., 2018). Our results suggest that soft and easily deformed types of microplastics such as films might be more likely to be swallowed by fish.
4.2. Egestion of microplastics
The egestion times of microplastics in this study are comparable to clearance times of plastic particles for fish reported in previous studies (from 33 h to 10 days) (Gassel et al., 2013; Grigorakis et al., 2017; Hoss and Settle, 1990; Ory et al., 2018) and are substantially longer than the time required by fish to digest and egest food pellets (2 d maximum). This implies that microplastic particles may be retained longer in the digestive tract than food and thus have more time to interact with the digestive system. This is important because microplastics can transport organic pollutants to the organisms after being ingested (Koelmans et al., 2016; Tanaka et al., 2015; Wardrop et al., 2016). Longer retention time implies a higher risk of such release. However, microplastics in natural waters are diverse and their capacity for adsorption and release of organic pollutants can vary widely (Wu et al., 2016). Thus, the differences of ingestion and egestion for the diversity of microplastics used in our study may lead to different ecological risks.
The higher vf of microplastic fragments than those of films and filaments and higher v of microplastic films than that of filaments in this study implies that, among these three tested shapes, filament microplastics move most slowly in the intestinal tract of goldfish. The microplastic filaments used in this study are slenderer than the other two shapes, which may make them more easily trapped in the convoluted intestinal tract. This implies that some shapes of microplastics may be more likely to be retained in the intestinal tract of fish. Aggregations of organic particles of slender and thin microplastics such as microfibers are common in the environment and affect the fate and bioavailability of microfibers (Porter et al., 2018; Zhao et al., 2018). The aggregation process may also happen in the intestinal tract of the goldfish with abundant organic particles, which may in turn affect the egestion of microplastic filaments.
Though the presence of food dose not appear to influence the egestion rates of microplastics by goldfish, it still appears to reduce the residues of some shapes of microplastics in fish after 72 h. This implies that the presence of food may influence the egestion of microplastics to a certain extent. Grigorakis et al. (2017) found a similar retention of microplastics and food in goldfish, which implied that microplastics could be egested with food. However, they fed the fish with microplastics packed within the food. This likely made the microplastics more easily co-egested with the food than in our study, in which food and microplastics are separate. Egestion of microplastics is thought to have an important influence on the fate of microplastics in the water column, since they are easy to deposit to the sediment if packed with the organism's feces (Cole et al., 2016; Katija et al., 2017). It is also important to know if the absence of food will result in a longer exposure of microplastics in the digestive tract, which might enhance the effect of exposure. The incomplete agreement between our study and previous work suggests that more studies about food-related effects on microplastic egestion are needed, especially for different food and microplastic exposure patterns.
5. Conclusions
Our results indicate that the ingestion of microplastics by goldfish involves co-capture with food, especially for white microplastics, which are only ingested in the presence of food in this study. Furthermore, microplastics with food-like colors could be ingested more than microplastics with other colors by goldfish. The size of microplastic particles also affects their ingestion as they must be small enough to pass through the mouth and to not be recognized during swallowing. Microplastics films were more likely to be ingested than fragments and filaments. Shape also affected egestion rate of microplastics. Our results indicate that the diverse types of microplastics in the environment, which differ in shape, color, and size, have different likelihood of ingestion by fish and have different retention in the digestive tract of fish. This implies that different types of microplastics have different ecological risks. Future research on the ecological and physiological impacts of microplastics should consider the physical characteristics of the microplastics themselves as these appear to significantly modulate how they are encountered and processed by biota.
Declarations
Author contribution statement
Xiong Xiong: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yenan Tu: Performed the experiments.
Xianchuan Chen: Contributed reagents, materials, analysis tools or data.
Xiaoming Jiang, Huahong Shi: Analyzed and interpreted the data.
Chenxi Wu: Conceived and designed the experiments.
James J. Elser: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Natural Science Foundation of China (41501536), State Key Laboratory of Freshwater Ecology and Biotechnology (2016FBZ11), and Young Researcher Support Funding of Institute of Hydrobiology, Chinese Academy of Sciences (Y85E01).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Chae Y., An Y.J. Effects of micro- and nanoplastics on aquatic ecosystems: current research trends and perspectives. Mar. Pollut. Bull. 2017;124:624–632. doi: 10.1016/j.marpolbul.2017.01.070. [DOI] [PubMed] [Google Scholar]
- Cole M., Lindeque P., Fileman E., Halsband C., Goodhead R., Moger J., Galloway T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013;47:6646–6655. doi: 10.1021/es400663f. [DOI] [PubMed] [Google Scholar]
- Cole M., Lindeque P., Halsband C., Galloway T.S. Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 2011;62:2588–2597. doi: 10.1016/j.marpolbul.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Cole M., Lindeque P.K., Fileman E., Clark J., Lewis C., Halsband C., Galloway T.S. Microplastics alter the properties and sinking rates of zooplankton faecal pellets. Environ. Sci. Technol. 2016;50:3239–3246. doi: 10.1021/acs.est.5b05905. [DOI] [PubMed] [Google Scholar]
- Colton J.B., Burns B.R., Knapp Frederick D. Plastic particles in surface waters of the northwestern atlantic. Science. 1974;185:491–497. doi: 10.1126/science.185.4150.491. [DOI] [PubMed] [Google Scholar]
- Cózar A., Martí E., Duarte C.M., García-de-Lomas J., van Sebille E., Ballatore T.J., Eguíluz V.M., González-Gordillo J.I., Pedrotti M.L., Echevarría F., Troublè R., Irigoien X. The arctic ocean as a dead end for floating plastics in the north atlantic branch of the thermohaline circulation. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1600582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A.L., Kawaguchi S., King C.K., Townsend K.A., King R., Huston W.M., Nash S.M.B. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-03465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sa L.C., Luis L.G., Guilhermino L. Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions. Environ. Pollut. 2015;196:359–362. doi: 10.1016/j.envpol.2014.10.026. [DOI] [PubMed] [Google Scholar]
- Ferreira G.V.B., Barletta M., Lima A.R.A., Morley S.A., Justino A.K.S., Costa M.F. High intake rates of microplastics in a Western Atlantic predatory fish, and insights of a direct fishery effect. Environ. Pollut. 2018;236:706–717. doi: 10.1016/j.envpol.2018.01.095. [DOI] [PubMed] [Google Scholar]
- Fossi M.C., Coppola D., Baini M., Giannetti M., Guerranti C., Marsili L., Panti C., de Sabata E., Clò S. Large filter feeding marine organisms as indicators of microplastic in the pelagic environment: the case studies of the Mediterranean basking shark (Cetorhinus maximus) and fin whale (Balaenoptera physalus) Mar. Environ. Res. 2014;100:17–24. doi: 10.1016/j.marenvres.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Gassel M., Harwani S., Park J.S., Jahn A. Detection of nonylphenol and persistent organic pollutants in fish from the North Pacific Central Gyre. Mar. Pollut. Bull. 2013;73:231–242. doi: 10.1016/j.marpolbul.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Ginetz R.M., Larkin P.A. Choice of colors of food items by rainbow-trout (Salmo-Gairdneri) J. Fish. Res. Board Can. 1973;30:229–234. [Google Scholar]
- Green D.S., Boots B., O'Connor N.E., Thompson R. Microplastics affect the ecological functioning of an important biogenic habitat. Environ. Sci. Technol. 2017;51:68–77. doi: 10.1021/acs.est.6b04496. [DOI] [PubMed] [Google Scholar]
- Grigorakis S., Mason S.A., Drouillard K.G. Determination of the gut retention of plastic microbeads and microfibers in goldfish (Carassius auratus) Chemosphere. 2017;169:233–238. doi: 10.1016/j.chemosphere.2016.11.055. [DOI] [PubMed] [Google Scholar]
- Hämer J., Gutow L., Köhler A., Saborowski R. Fate of microplastics in the marine Isopod Idotea emarginata. Environ. Sci. Technol. 2014;48:13451–13458. doi: 10.1021/es501385y. [DOI] [PubMed] [Google Scholar]
- Hara T.J. Feeding behaviour in some teleosts is triggered by single amino acids primarily through olfaction. J. Fish Biol. 2006;68:810–825. [Google Scholar]
- Hoss D.E., Settle L.R. Proceedings of the Second International Conference on Marine Debris. NOAA Technical Memorandum. NOAA-TM-NMFS-SWFSC-154; Miami, FL: 1990. Ingestion of plastics by teleost fishes; pp. 693–709. [Google Scholar]
- Houlihan D., Boujard T., Jobling M. John Wiley & Sons; 2008. Food Intake in Fish. [Google Scholar]
- Jabeen K., Su L., Li J.N., Yang D.Q., Tong C.F., Mu J.L., Shi H.H. Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environ. Pollut. 2017;221:141–149. doi: 10.1016/j.envpol.2016.11.055. [DOI] [PubMed] [Google Scholar]
- Kasumyan A.O., Doving K.B. Taste preferences in fishes. Fish Fish. 2003;4:289–347. [Google Scholar]
- Katija K., Choy C.A., Sherlock R.E., Sherman A.D., Robison B.H. From the surface to the seafloor: how giant larvaceans transport microplastics into the deep sea. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1700715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelmans A.A., Bakir A., Burton G.A., Janssen C.R. Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016;50:3315–3326. doi: 10.1021/acs.est.5b06069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottelat M., Freyhof J. Publications Kottelat; 2007. Handbook of European Freshwater Fishes. [Google Scholar]
- Lehtiniemi M., Hartikainen S., Näkki P., Engström-Öst J., Koistinen A., Setälä O. Size matters more than shape: ingestion of primary and secondary microplastics by small predators. Food Webs. 2018;17 [Google Scholar]
- Li J., Green C., Reynolds A., Shi H., Rotchell J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018;241:35–44. doi: 10.1016/j.envpol.2018.05.038. [DOI] [PubMed] [Google Scholar]
- Li J.Y., Liu H.H., Chen J.P. Microplastics in freshwater systems: a review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018;137:362–374. doi: 10.1016/j.watres.2017.12.056. [DOI] [PubMed] [Google Scholar]
- Lu Y.F., Zhang Y., Deng Y.F., Jiang W., Zhao Y.P., Geng J.J., Ding L.L., Ren H.Q. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016;50:4054–4060. doi: 10.1021/acs.est.6b00183. [DOI] [PubMed] [Google Scholar]
- Lusher A.L., Hernandez-Milian G., Berrow S., Rogan E., O'Connor I. Incidence of marine debris in cetaceans stranded and bycaught in Ireland: recent findings and a review of historical knowledge ☆. Environ. Pollut. 2017 doi: 10.1016/j.envpol.2017.09.070. [DOI] [PubMed] [Google Scholar]
- Machado A.A.D., Kloas W., Zarfl C., Hempel S., Rillig M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018;24:1405–1416. doi: 10.1111/gcb.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeyer C. Tetrachromatic color-vision in goldfish - evidence from color mixture experiments. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 1992;171:639–649. [Google Scholar]
- Ohnishi K. Development of color-vision in goldfish - selective delayed maturation of blue vision. Vis. Res. 1993;33:1665–1672. doi: 10.1016/0042-6989(93)90032-r. [DOI] [PubMed] [Google Scholar]
- Ory N.C., Gallardo C., Lenz M., Thiel M. Capture, swallowing, and egestion of microplastics by a planktivorous juvenile fish. Environ. Pollut. 2018;240:566–573. doi: 10.1016/j.envpol.2018.04.093. [DOI] [PubMed] [Google Scholar]
- Ory N.C., Sobral P., Ferreira J.L., Thiel M. Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci. Total Environ. 2017;586:430–437. doi: 10.1016/j.scitotenv.2017.01.175. [DOI] [PubMed] [Google Scholar]
- Porter A., Lyons B.P., Galloway T.S., Lewis C. Role of marine snows in microplastic fate and bioavailability. Environ. Sci. Technol. 2018;52:7111–7119. doi: 10.1021/acs.est.8b01000. [DOI] [PubMed] [Google Scholar]
- Rochman C.M. Microplastics research - from sink to source. Science. 2018;360:28–29. doi: 10.1126/science.aar7734. [DOI] [PubMed] [Google Scholar]
- Rolen S.H., Sorensen P.W., Mattson D., Caprio J. Polyamines as olfactory stimuli in the goldfish Carassius auratus. J. Exp. Biol. 2003;206:1683–1696. doi: 10.1242/jeb.00338. [DOI] [PubMed] [Google Scholar]
- Santos R.G., Andrades R., Fardim L.M., Martins A.S. Marine debris ingestion and Thayer's law - the importance of plastic color. Environ. Pollut. 2016;214:585–588. doi: 10.1016/j.envpol.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Spence R., Smith C. Innate and learned colour preference in the zebrafish, Danio rerio. Ethology. 2008;114:582–588. [Google Scholar]
- Stacey N.E., Kyle A.L. Effects of olfactory tract lesions on sexual and feeding-behavior in the goldfish. Physiol. Behav. 1983;30:621–628. doi: 10.1016/0031-9384(83)90231-7. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Takada H., Yamashita R., Mizukawa K., Fukuwaka M., Watanuki Y. Facilitated leaching of additive-derived PBDEs from plastic by seabirds' stomach oil and accumulation in tissues. Environ. Sci. Technol. 2015;49:11799–11807. doi: 10.1021/acs.est.5b01376. [DOI] [PubMed] [Google Scholar]
- Wardrop P., Shimeta J., Nugegoda D., Morrison P.D., Miranda A., Tang M., Clarke B.O. Chemical pollutants sorbed to ingested microbeads from personal care products accumulate in fish. Environ. Sci. Technol. 2016;50:4037–4044. doi: 10.1021/acs.est.5b06280. [DOI] [PubMed] [Google Scholar]
- Wright S.L., Thompson R.C., Galloway T.S. The physical impacts of microplastics on marine organisms: a review. Environ. Pollut. 2013;178:483–492. doi: 10.1016/j.envpol.2013.02.031. [DOI] [PubMed] [Google Scholar]
- Wu C., Zhang K., Huang X., Liu J. Sorption of pharmaceuticals and personal care products to polyethylene debris. Environ. Sci. Pollut. Res. 2016;23:8819–8826. doi: 10.1007/s11356-016-6121-7. [DOI] [PubMed] [Google Scholar]
- Xiong X., Chen X., Zhang K., Mei Z., Hao Y., Zheng J., Wu C., Wang K., Ruan Y., Lam P.K.S., Wang D. Microplastics in the intestinal tracts of east asian finless porpoises (neophocaena asiaeorientalis sunameri) from yellow sea and Bohai sea of China. Mar. Pollut. Bull. 2018;136:55–60. doi: 10.1016/j.marpolbul.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Yager D., Thorpe S. Investigations of goldfish color vision. In: Stebbins W.C., editor. Animal Psychophysics: the Design and Conduct of Sensory Experiments. Springer US; Boston, MA: 1970. pp. 259–275. [Google Scholar]
- Zhang K., Shi H., Peng J., Wang Y., Xiong X., Wu C., Lam P.K.S. Microplastic pollution in China's inland water systems: a review of findings, methods, characteristics, effects, and management. Sci. Total Environ. 2018;630:1641–1653. doi: 10.1016/j.scitotenv.2018.02.300. [DOI] [PubMed] [Google Scholar]
- Zhao S.Y., Ward J.E., Danley M., Mincer T.J. Field-based evidence for microplastic in marine aggregates and mussels: implications for trophic transfer. Environ. Sci. Technol. 2018;52:11038–11048. doi: 10.1021/acs.est.8b03467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Videos1Microplastics ingestion process of goldfish when food was present.
Videos2Microplastics ingestion process of goldfish in the absence of food.