Abstract
Uptake of nutritional supplementation during pulmonary rehabilitation (PR) for people with chronic obstructive pulmonary disease (COPD) has been limited by an absence of rigorous evidence-based studies supporting use. The objective was to report and summarise the current evidence supporting the use of nutritional supplementation to improve outcomes during PR in stable COPD patients. A systematic search was conducted up to 7 August 2019 (registration number CRD42018089142). The preferred reporting items for systematic reviews and meta-analyses guidelines were used. Six databases were included: Medical Literature Analysis and Retrieval System Online or MEDLARS Online, Allied and Complementary Medicine Database, the Cochrane Database of Systematic Reviews, Excerpta Medica dataBASE, Cumulative Index of Nursing and Allied Health Literature and Web of Science. This systematic search generated 580 initial matches, of which 22 studies (917 COPD participants) met the pre-specified criteria and were included. Sixteen of 19 studies that used nutritional supplements in addition to PR did not show additional benefit compared to PR alone when measuring exercise capacity. Nutritional supplements significantly increased body weight in 7 of 11 studies. Body mass index increased significantly in two of six studies. Handgrip strength did not improve, while quadriceps muscle strength significantly improved in 3 of 11 studies. Four of eight studies showed a significant improvement in inspiratory muscle function. Only 2 of 14 studies demonstrated a significant improvement in quality of life with supplementation in addition to PR. There remains insufficient evidence on the effect of nutritional supplementation on improving outcomes during PR in patients with COPD due to heterogeneity in supplements, outcome measures and PR programmes. Therefore, controversy remains and further research is needed.
Keywords: Chronic obstructive pulmonary disease, pulmonary rehabilitation, nutrition, nutritional supplementation
Introduction
Patients with chronic obstructive pulmonary disease (COPD) experience daily symptoms, reduced exercise capacity and susceptibility to exacerbations, resulting in reduced health-related quality of life.1–3 The international Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy document summarises current approaches to COPD management.1 Cost-effective treatment approaches for COPD, described in the ‘value pyramid’4 include smoking cessation, influenza vaccination and pulmonary rehabilitation (PR). Multiple high-quality randomised controlled trials (RCTs) and meta-analyses have demonstrated that PR is an effective management strategy in COPD, since it improves exercise performance, reduces dyspnoea, reduces the risk of exacerbation and improves health-related quality of life.5–10
Exercise limitation is one of the most common problems for COPD patients and this may be compounded by reduced muscle mass and malnutrition. Some COPD patients may lose body weight and skeletal muscle mass, which leads to muscle weakness and dysfunction, impacting functional ability and quality of life.11 Muscle disuse, caused by a prolonged sedentary lifestyle and voluntary immobilisation, leads to further muscle deconditioning and thus reduced muscle strength and endurance.12 It has also been postulated that COPD is associated with a myopathy, which may be driven by systemic inflammation.12 Being underweight is associated with an increased risk of mortality in COPD and weight loss predicts mortality and morbidity in chronic lung disease patients.8,13 Therefore, patients with COPD are at risk of significant morbidity and mortality as a result of changes in body composition and nutritional and metabolic status.
It has been suggested that healthy older adults require additional nutrients compared with younger adults to preserve bone and lean mass. For instance, it is recommended that young adults require 0.7 g of protein/kg body weight per day while the recommendation for older adults is 1.2–1.5 g protein/kg body weight/day, especially for people with conditions that require higher levels of protein, such as COPD.14 Nutritional supplements have been used to overcome malnutrition in patients with COPD. It has been suggested that nutritional support integrated with exercise training may improve exercise activity, decrease mortality and improve muscle strength in undernourished COPD patients.15,16 A meta-analysis of nutritional supplementation for stable COPD by Ferreira et al. included 17 randomised clinical trials and concluded that nutritional supplements increased muscle mass and body weight and improved respiratory function and exercise tolerance in COPD patients who were poorly nourished.17,18 Additionally, Collins et al. demonstrated in a meta-analysis of nutritional support and functional capacity in COPD that nutritional supplements improved weight and handgrip strength in COPD patients.19 Both reviews only included randomised clinical trials and it was not necessary for participants to be engaged in PR. We hypothesised that an integrated approach of exercise training and nutritional support might be the best way to seek functional improvements. However, the uptake of nutritional supplementation during PR, where the potential benefit may be greatest, has been limited by the absence of rigorous evidence-based studies supporting use. The objective of this systematic review was to report and summarise the current evidence for using nutritional supplementation during PR in stable COPD patients to enhance PR outcomes.
Methods
Search strategy
The preferred reporting items for systematic reviews and meta-analyses guidelines were used for this systematic review, with Prospero registration number CRD42018089142.20 The search was conducted up to 7 August 2019 using Medical Literature Analysis and Retrieval System Online or MEDLARS Online, Excerpta Medica dataBASE, Allied and Complementary Medicine Database, the Cochrane Database of Systematic Reviews, Cumulative Index of Nursing and Allied Health Literature and Web of Science database (Supplemental Material Tables A1 to A5). The search strategy and terms used in this systematic review are described in Supplemental Material. The bibliography of eligible articles and existing systematic reviews in the field were also screened.
Inclusion criteria
The PICO (P: population, patient, problem; I: intervention; C: control, comparison or comparator; O: outcome) criteria for included studies appear in Table 1. Studies were included in the systematic review if they met all of the following criteria: studies of patients with a confirmed diagnosis of COPD; no evidence of recent exacerbation, as described in the individual studies; patients enrolled on a PR or other exercise training programme and patients receiving nutritional supplementation (caloric, non-caloric, powder, liquid, capsule or tablets) during PR.
Table 1.
PICO criteria used for the inclusion of studies.
| Criteria | Definition |
|---|---|
| Participants | Patients with a confirmed diagnosis of COPD, no evidence of recent exacerbation, enrolled on a pulmonary rehabilitation or other exercise training program |
| Intervention | Any nutritional supplement given during pulmonary rehabilitation |
| Comparator | Placebo, other nutritional supplement regime, no nutritional supplements |
| Outcome | Exercise function, body composition, peripheral muscle strength, respiratory muscle function and quality of life. |
| Study design | No restrictions |
COPD: chronic obstructive pulmonary disease; PICO: P—population, patient, problem; I—intervention; C—control, comparison or comparator; O—outcome.
Exclusion criteria
We excluded book chapters, systematic reviews (but screened the reference lists), non-English manuscripts, conference abstracts with no full-text and non-full text articles.
The main outcomes of interest were to investigate the impact of nutritional supplementation during PR programmes on exercise function, body composition, peripheral muscle strength, respiratory muscle function and quality of life.
Data collection
Three authors (AMA, JRH, and SM) screened the titles and abstracts to exclude irrelevant studies. Full texts of the relevant studies were read by the first author (AMA) to evaluate if they fulfilled the inclusion criteria. The reference lists of included studies and excluded systematic reviews were also screened; two additional studies were found, and the senior authors (JRH and SM) discussed eligibility. Disagreements between authors were resolved by discussion.
Quality assessment
The first and seventh authors (AMA and JRH) performed risk of bias assessment using the Cochrane risk of bias tool to assess randomised studies, which comprises seven questions, and the modified Newcastle–Ottawa scale to assess cohort studies, which is also made up of seven questions.21,22 For the randomised trials, we scored each of the seven domains as 0 (low risk of bias) or 1 (high risk of bias or bias unclear). There was, therefore, a total score between 0 and 7 in which a higher score equates to a higher risk of bias. For cohort studies, each of the seven domains was scored from 0 (high risk of bias) to 3 (low risk of bias) and we took a mean of the domains to result in a score between 0 and 3, where a higher score represents a lower risk of bias.
Synthesis of results
The main purpose of this systematic review was to report and summarise the current evidence of using nutritional supplementation during PR in stable COPD. A meta-analysis was not attempted due to methodological heterogeneity between studies. Our discussion focuses on the studies at lower risk of bias.
Results
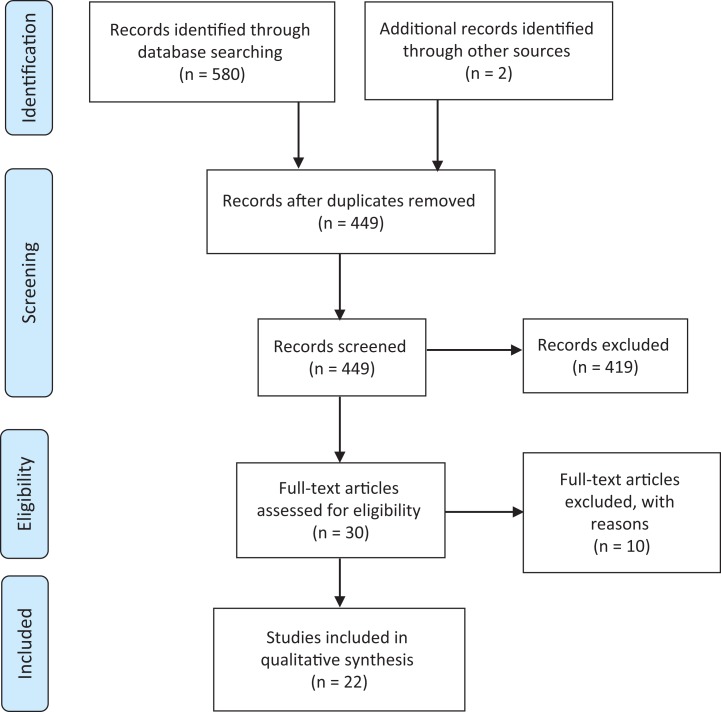
Initially, 580 studies were considered potentially eligible. However, after removing duplicates, 449 titles and abstracts were included. Screening the titles and abstracts resulted in 30 of 449 studies being considered for full-text reading. After reading the full text of 30 studies, 10 further studies were excluded (Supplemental Material Table A6). Screening the reference list of eligible studies revealed two further relevant studies. Thus, 22 studies in total met the inclusion criteria for the systematic review (see Figure 1).
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses flow diagram.
The 22 studies comprised 5 cohort studies and 17 RCTs. The sample size and study duration varied between 8 and 80 participants and 6 weeks to 4 months, respectively. A full description of the included RCTs and cohort studies appears in Tables 2 and 3, respectively. The risk of bias assessment for RCT and cohort studies appears in Tables A7 and A8 in Supplemental Material, respectively.
Table 2.
Detailed description of the included RCT studies.
| Author and risk of bias | Mean age, GOLD stage and BMI | Subject | Intervention | Pulmonary rehabilitation | Outcomes measures | Result |
|---|---|---|---|---|---|---|
| van de Bool et al.23
Bias: 1/7 |
Age: 62 years old. GOLD: 2. BMI: 22.7 kg/m2. |
N = 73 (‘low muscle mass’). |
Intervention: 125 mL of 9.4 g protein, 28.1 g carbohydrate and 4.1 g fat,
leucine, n-3 PUFA and vitamin D once per day. Placebo: Flavoured non-caloric aqueous solution. |
Duration: 4 months Location: outpatient. Session detail: 40 sessions, two to three times per week. 1. High intensity endurance exercise by cycle ergometry. 2. Treadmill walking. 3. Progressive resistance exercise of upper and lower body. 4. Education session. |
1. Body composition: Body mass, BMC, SMM and FM. 2. Muscle function: Quadriceps muscle strength, MIP. 3. Exercise performance: cycle endurance time (CET) and 6MWT. 4. Anxiety/depression: HADS. 5. Physical activity: 7 days. |
1. Body composition: Significant improvement in body mass (1.5 ± 0.6 kg,
p < 0.05) and FM (1.6 ± 0.5 kg, p <
0.01) in the intervention group. 2. Muscle function: No significant differences between the groups. 3. Exercise performance: No significant differences between the groups. 4. Anxiety/depression: No significant differences between the groups. 5. Physical activity: significant benefit in physical activity (929.5 ± 459.2 steps/day, p < 0.05). |
| Paulin et al.24
Bias: 1/7 |
Age: I versus P (56.5 vs. 65.2 years old). GOLD: 3 and 4. BMI: I versus P (24.5 vs. 25.1 kg/m2). |
N = 16 | Intervention (I): B12 500 mg/day for 8 weeks. Placebo (P): Maltodextrin. |
Duration: 8 weeks. Location: outpatient. Session detail: 3 days/week, 40 minutes of aerobic and resistance exercise. |
1. Cardiopulmonary exercise testing: Incremental or constant-load
protocols. |
1. Exercise performance: No significant differences between the groups. |
| Ahnfeldt et al.25
Bias: 4/7 |
Age: I versus P (67 vs. 70 years old). GOLD: 2 and 3. BMI: I versus P (24.3 vs. 23.4 kg/m2). |
N = 35 | Intervention (I): Protein bar (each 134.8 kcal of energy, 9.3 g protein, 14.6
carbohydrate, 4.2 fat) two times per day for 9 weeks. Placebo (P): No. |
Duration: 9 weeks. Location: outpatient Session detail: A—1 hour 2 times per week and home-based one time per week of: 1. Endurance. 2. Resistance. 3. Interval training. 4. Educational class. |
1. Muscle function: lower muscle strength. 2. Exercise performance: SWT. 3. Quality of life: SGRQ. |
1. Muscle function: No significant differences between the groups. 2. Exercise performance: No significant differences between the groups. 3. Quality of life: No significant differences between the groups. |
| Gurgun et al.26
Bias: 2/7 |
Age: I versus P (64 vs. 66 years old). GOLD: 3 and 4. BMI: I versus P (17.8 vs. 20 kg/m2). |
N = 30 (“wasted”) | Intervention (I): 250 mL 83.3% carbohydrate, 30% fat, 16.7% proteins, three
times per day. Placebo (P): No. |
Duration: 8 weeks. Location: outpatient. Session detail: Two times per week 60–80 minutes/day: A—Education. B—Exercise training include: 1. Warm-up and bicycle ergometer for 15 minutes. 2. Treadmill (15 minutes). 3. Upper and lower extremity strength (5–10 minutes). 4. Breathing and relaxation therapies (15–20 minutes each). |
1. Body composition: Body weight, BMI, FFMI. 2. Exercise performance: 6MWT, ISWT, and ESWT. 3. Quality of life: SGRQ. 4. Anxiety/depression: HADS. 5. Breathlessness scale: MRC and Borg. 6. Muscle size: QuadCSA. |
1. Body composition: Significant improvement in weight (1.1 ± 0.9 kg,
p < 0.05), BMI (0.2 ± 1.4 kg/m2,
p < 0.05) and FFMI (0.6 ± 0.5 kg/m2,
p < 0.05) in the intervention group. 2. Exercise performance: No significant differences between the groups. 3. Quality of life: No significant differences between the groups. 4. Anxiety/depression: No significant differences between the groups. 5. Breathlessness scale: No significant differences between the groups. 6. Muscle size: Significant increase in QuadCSA (2.5 ± 4.1 cm2, p < 0.05) in the intervention group. |
| Hornikx et al.27
Bias: 3/7 |
Age: I versus P (67 vs. 69 years old). GOLD: 2, 3 and 4. BMI: I versus P (25 vs. 24 kg/m2). |
N = 49 | Intervention (I): vitamin D monthly dosage (100.000 UI
cholecalciferol). Placebo (P): Arachidis oleum: 4 mL. |
Duration: 3 months. Location: outpatient. Session detail: Three times per week 90 minutes training of: 1. Cycling. 2. Walking on treadmill. 3. Stair climbing and arm cranking. 4. Strength exercises for extremities. |
1. Muscle function: quadriceps strength, MIP and MEP. 2. Exercise performance: incremental cycle ergometer and 6MWD. 3. Quality of life: CRDQ. |
1. Muscle function: Significant increase in MIP (11 ± 12 cmH2O,
p = 0.004) but no differences between groups in quadriceps
strength and MEP. 2. Exercise performance: No significant differences between the groups. 3. Quality of life: No significant differences between the groups. |
| Sugawara et al.28
Bias: 1/7 |
Age: 77 years old. GOLD: 3. BMI: not recorded. |
N = 31 |
Intervention: Mein (contains 200 kcal 20% protein, 25% lipid, 53.2% sugar, 1.8
fibre, Fisher is 3.7, antioxidant vitamins A, C and E) (two times per day 200 mL)
for 12 weeks + provided meal with dietary instruction. Placebo: No. |
Duration: 12 weeks. Location: Home-based. Session detail: A—Breathing retraining: 1. Pursed-lip breathing. 2. Diaphragmatic breathing. 3. Slow deep breathing. B—Exercise training: 1. Upper and lower limb exercises. 2. Respiratory muscle stretching calisthenics. 3. Level walking for least 15 minutes. 4. Inspiratory and expiratory muscle exercises. C—Education program. D—Physiotherapist supervision every 2 weeks in hospital. E—Periodic visits at home. |
1. Body composition: Body weight, FFM, FMI, (AC), (AMC), %IBW. 2. Muscle function: MIP and MEP, quadriceps strength. 3. Exercise performance: 6MWD. 4. Quality of life: CRQ. 5. Breathlessness scale: MRC. |
Data reported as change in ratio in interventional group versus placebo group,
not as absolute values. 1. Body composition: Significant improvement in body weight (2.6 ± 3 kg vs -0.2 ±1.4 kg, p = 0.0010), FMI (8.6 ± 10.7 kg/m2 versus 0.6 ± 10.6 kg/m2, p = 0.048), %AC (2.4 ± 3.7% vs -0.7 ± 2.4%, p = 0.0134), and %IBW (2.7 ± 3% vs. −0.2 ± 1.3%, p = 0.0017) in the intervention group. 2. Muscle function: MIP (39.2 ± 38.9 cmH2O vs. 0.1 ± 24.1 cmH2O, p = 0.0030) and quadriceps strength (10.0 ± 13.3 kg/kg vs. −1.6 ± 9.5 kg/kg, p = 0.0079) increased significantly in the intervention group. 3. Exercise performance: 6MWD (19.7 ± 24.7 m vs. −7.1 ± 50.8 m, p = 0.0137) improved significantly in the intervention group. 4. Quality of life: total score (6.2 ± 7.5 vs. −2.7 ± 13.1, p = 0.0374) and emotional domain (8.9 ± 14.4 vs. −3.9 ± 12.2, P = 0.0097) increased significantly in the intervention group. 5. Breathlessness scale: MRC 22.6 ± 40.6 vs. −4.4 ± 17.2 (p = 0.0339) improved significantly in the intervention group. |
| Baldi et al.29
Bias: 3/7 |
Age: I versus P (73 vs. 70 years old). GOLD: 3. BMI: I versus P (19.9 vs. 21 kg/m2). |
N = 28 depleted | Intervention (I): Amino acids 4 g two times per day for 12 weeks. Placebo (P): No. |
Duration: 4 weeks. Location: inpatient Session detail: five days per week. 30 minutes submaximal cycle ergometry. 30 minutes walking and 1 arm exercise session. Then: Duration: 8 weeks Location: Home Session detail: Twice per day 30 minutes unloaded bicycle training. |
1. Body composition: weight and FFM. | Data reported as change in the interventional group versus change in the placebo
group. 1. Body composition: Significant increase in weight (3.8 ± 2.6 kg, p versus = 0.0002). (−0.1 ± 1.1 kg, p = 0.81) and FFM (1.5 ± 2.6 kg, p versus = 0.05). (−0.1 ± 2.3 kg, p = 0.94). |
| Laviolette et al.30
Bias: 2/7 |
Age: I versus P (63 vs. 68 years old). GOLD: 2 and 3. BMI: I versus P (29.7 vs. 26.7 kg/m2). |
N = 22 | Intervention (I): Whey protein 20 g in 120 mL/day for 16 weeks (8 without PR and
8 with PR). Placebo (P): casein 20 g in 120 mL/day for 16 weeks (8 without PR and 8 with PR). |
Duration: 8 weeks Location: not specified Session detail: Three times per week. 90 minutes of: 1. Endurance. 2. Resistance exercise. 3. Education and self-management strategies. |
Baseline, 8th, and 16th week: 1. Body composition: weight. 2. Muscle function: quadriceps muscle strength and fatigue. 3. Exercise performance: constant work rate cycle endurance. 4. Quality of life: CRQ. 5. Lung function: spirometry and lung volumes. |
1. Body composition: No significant differences between the groups. 2. Muscle function: No significant differences between the groups. Exercise performance: No significant differences between the groups. 4. Quality of life: No significant differences between the groups. 5. Lung function: No significant difference between groups. |
| Wetering et al.31
Bias: 3/7 |
Age: 64 years old. GOLD: 2. BMI: 21.7 kg/m2. |
N = 30 (“wasted”) | Intervention: respifor (high-carbohydrate supplement; 125 mL, 188 kcal) three
times per day for 4 months. Placebo: No. |
Duration: 4 months. Location: outpatient. Session detail: 1. Two times per week for 30 minutes of intensive exercise. 2. 1, 2 and 3 months dietician counselling for weight losing and muscle-wasted patients. 3. Education program. 4. Smoking cessation. |
1. Body composition: FFMI and BMI. 2. Muscle function: MIP and quadriceps average power. 3. Exercise performance: Peak exercise capacity (W max), cycle endurance test (CET) and 6MWD. 4. Quality of life: SGRQ. |
1. Body composition: Significant increase in BMI (mean difference 1
kg/m2, p < 0.05), and FFMI (mean difference
0.9kg/m2, p < 0.05). 2. Muscle function: Significant increase in MIP (mean difference 1.4 kPa, p < 0.05) and QAP (mean difference 13.1 W, p < 0.05). 3. Exercise performance: Significant increase in W max (mean difference 11.7 W, p < 0.05), CET (mean difference 525 second, p < 0.05), and 6MWD (mean difference 27.2 m, p < 0.05). 4. Quality of life: No statistically significant difference although absolute difference between groups at 6.1 units is greater than the MCID. |
| Deacon et al.32
Bias: 2/7 |
Age: I versus P (68 vs. 68 years old). GOLD: 3. BMI: I versus P (28.1 vs. 25.7 kg/m2). |
N = 80 | Intervention (I): Creatine Loading phase: 22 g daily, 4 divided doses for 5 days. Maintenance phase: (PR) 3.76 g daily. Placebo (P): lactose. |
Duration: 7 weeks. Location: outpatient Session detail: three times per week of: 1. Endurance training. 2. Individually prescribed resistance training using gym equipment and free weights. |
1. Body composition: weight, FFM and FM. 2. Muscle function: quadriceps, triceps and biceps. 3. Exercise performance: ISWT and ESWT. 4. Quality of life: CRQ-SR. |
1. Body composition: No significant differences between the groups. 2. Muscle function: No significant differences between the groups. 3. Exercise performance: No significant differences between the groups. 4. Quality of life: No significant differences between the groups. |
| Borghi-Silva et al.33
Bias: 1/7 |
Age: I versus P (69 vs. 65 years old). GOLD: 3. BMI: I versus P (22 vs. 23 kg/m2). |
N = 16 | Intervention (I): Oral l-carnitine 2 g, twice per day in 10 mL bottle
for 6 weeks. Placebo (P): Saline solution. |
Duration: 6 weeks. Location: outpatient. Session detail: 1 hour three times per week: 30 minutes treadmill, inspiratory muscle training. |
1. Body composition: Triceps skinfold, mid-arm circumference and BMI. 2. Muscle function: MIP and MEP. 3. Exercise performance: incremental exercise test (treadmill) and 6MWT. 4. Breathlessness scale: Borg. |
Data reported as change in interventional group vs change in the placebo
group. 1. Body composition: No significant differences between the groups. 2. Muscle function: MIP (40 ± 14 cmH2O vs. 14 ± 5 cmH2O, p < 0.05) but not MEP, increased significantly in the intervention group. 3. Exercise performance: No significant differences between the groups. 4. Breathlessness: No significant differences between the groups. |
| Faager et al.34
Bias: 1/7 |
Age: I versus P (67 vs. 64 years old). GOLD: 3. BMI: I versus P (25 vs. 22 kg/m2). |
N = 23 | Intervention (I): creatine 0.3 g/kg body weight/day, divided in four doses/day
for 7 days. Creatine 0.07 g/kg body weight/day one dose/day for remaining 7 weeks. Placebo (P): Glucose. |
Duration: 8 weeks. Location: outpatient. Session detail: Two times per week for 60–75 minutes of exercise training and education consisting of: 1. Ergometer cycling. 2. Arm muscle training with dumbbells. 3. Rising and getting up from a stool and getting up onto a low stool. 4. Thera band exercises for shoulder girdle. 5. Thigh muscle training with weight cuffs. 6. Abdominal muscle training. 7. Flexibility exercises for thorax and adjacent joints. |
1. Body composition: weight. 2. Muscle function: Grip strength, maximal right knee strength and fatigue. 3. Exercise performance: ESWT. 4. Quality of life: SGRQ. 5. Lung function: spirometry. |
1. Body composition: No significant differences between the groups. 2. Muscle function: No significant differences between the groups. 3. Exercise performance: No significant differences between the groups. 4. Quality of life: No significant differences between the groups. 5. Lung function: No significant differences in FEV1 between the groups. |
| Broekhuizen et al.35
Bias: 3/7 |
Age: I versus P (62 vs. 64 years old. GOLD: 3. BMI: I versus P (22.5 vs. 22.1 kg/m2). |
N = 80 | Intervention (I): PUFA 1 g 9 capsules/day. Placebo (P): 9 capsules/day of palm and sunflower oil, vitamin E. Depleted patients n = 48 respifor (see above) 3times per day. |
Duration: 8 weeks. Location: inpatient. Session detail: A—General physical training of: 1. Exercise in relation to daily activities. 2. Cycle ergometry. 3. Treadmill walking. 4. Swimming. B—Sports and games. C—Educational program. D—Regular meals. |
1. Body composition: BMI, weight, FFM, FM and FFMI. 2. Muscle function: quadriceps strength, handgrip and MIP. 3. Exercise performance: endurance time incremental bicycle ergometry and submaximal bicycle ergometry. 4. Lung function: spirometry. |
1. Body composition: No significant differences between the groups. 2. Muscle function: No significant differences between the groups. 3. Exercise performance: Maximal exercise capacity (peak workload (9.7 W difference, P = 0.009) and bicycle ergometry duration (4.3 minutes difference, p = 0.023) improved significantly in the intervention group. 4. Lung function: No significant differences between the groups. |
| Fuld et al.36
Bias: 3/7 |
Age: I versus P (64 vs. 62 years old). GOLD: 3. BMI: I versus P (23.2 vs. 24.3 kg/m2). |
N = 25 | Intervention (I): Creatine + glucose polymer (5 g creatine and 35 g
glucose/dose). A—Loading phase: three times daily for 14 days. B—Maintenance phase: one time daily for 10 weeks (PR). Placebo (P): Glucose polymer (40.7 g/dose). |
Duration: 8 weeks Location: outpatient Session detail: Two times per week each 1 hour consisting of: 1. A warm-up. 2. Mobility training. 3. Dynamic strength training of all extremities. 4. Whole body endurance training. 5. Education and behavioural interventions. |
1. Body composition: Body mass, FFM and FM. 2. Muscle function: MIP, lower limb muscle performance, handgrip. 3. Exercise performance: ISWT, ESWT, cycle ergometry. 4. Quality of life: SGRQ. 5. Lung function: Spirometry. |
Data reported as change in interventional group versus change in the placebo
group. 1. Body composition: FFM increased significantly by (2 kg vs. 0.4 kg, p < 0.05) in the creatine group. FM and BM: No significant differences between the groups. 2. Muscle function: Significant increase in lower limb strength (19.5 N.m vs. 12.2 N.m, p < 0.05), endurance (1216 J vs. 362 J, p < 0.05), handgrip strength (2.9 N vs. 0.6 N, p < 0.05) and endurance (15.6 repetitions vs. 8.4 repetitions, p < 0.05) in the creatine group. No significant change in MIP. 3. Exercise performance: No significant differences between the groups. 4. Quality of life: Total score decreased (5.9, p < 0.05) and activity domain deceased (5.3, p < 0.01) in the creatine group. 5. Lung function: No significant improvement in FEV1. |
| Steiner et al.37
Bias: 3/7 |
Age: I versus P (66 vs. 68 years old). GOLD: 3. BMI: I vs. P (23.9 vs. 23.5 kg/m2). |
N = 60. | Intervention (I): respifor (high-carbohydrate supplement; 125 mL, 188 kcal)
three times per day for 7 weeks Placebo (P): non-nutritive. |
Duration: 7 weeks. Location: outpatient. Session detail: two times per week of: 1. Endurance training (walking exercise + home walking program). 2. Circuit of low impact conditioning exercise. 3. Educational sessions. |
1. Body composition: weight, BMI, BM, lean mass, fat mass. 2. Muscle function: quadriceps and handgrip strength. 3. Exercise performance: ISWT and ESWT. 4. Quality of life: CRQ-SR. |
1. Body composition: Significant improvement in weight (0.63 kg,
p = 0.004), BMI (0.24 kg/m2, p =
0.002), and fat mass (0.67 kg, p = 0.001) in the intervention
group. 2. Muscle intervention: No significant differences between the groups. 3. Exercise performance: No significant differences between the groups. 4. Quality of life: No significant differences between the groups. |
| Vermeeren et al.38
Bias: 3/7 |
Age: part I 65 versus part II 62 years old. GOLD: 3. BMI: part I 20.6 versus part II 22.6 kg/m2. |
Part 1: N = 14 Part II: N = 11 |
Part I: Intervention 1: 1046 kJ, 21% protein, 34% fat, 45%
carbohydrate. Intervention 2: 2092 kJ, 21% protein, 36% fat, 43% carbohydrate. Placebo: 209 kJ coffee creamer and lemon syrup. Part II: respifor (see above) versus pulmocare (high fat supplement) 200 mL. |
Duration: not specified. Location: inpatient. Session detail: Not specified. |
1. Exercise performance: cycle ergometer. 2. Lung function: spirometry. 3. Self-reported: A—Change in breathlessness during meals. B—Leg pain. |
1. Exercise performance: Part I: No significant differences between the
groups. 2. Lung function: Part I: No significant differences between the groups. Part II: PEF (pre 3.1 L/second ± 1.0, post 3.3 L/second ± 1.2) increased significantly after the respifor supplement versus pulmocare (pre 3.1 L/second ± 0.9, post 3.1 L/second ± 0.9) (p < 0.05). 3. Self-reported symptoms: Part I: Satiety changed significantly after the supplements for the 2092-kJ supplement (p < 0.05). Part II: Significant increase in breathlessness at 30 and 60 minutes following a meal with pulmocare versus respifor (raw data not provided, p < 0.05). |
| Schols et al.39
Bias: 4/7 |
Age: not recorded. GOLD: 3. BMI: not recorded. |
N = 71 per protocol group. | Complex, three group study: p group: placebo steroid. N group: placebo steroid + nutritional supplement. N + A: 4 IM injections of nandrolone + nutritional supplement (not considered further). Nutrition: one time per day 200 mL for 57 days mixture of nutridrink (high energy), protifar (high protein) and fantomalt (high energy carbohydrate and oil). |
Duration: 57 days. Location: inpatient. Session detail: 1. General physical training related to daily activates. 2. Cycle ergometry. 3. Treadmill walking. 4. Walking circuits. 5. Swimming. |
Measurements were made at entry, 29 and 57 days: 1. Body composition: weight, arm circumference, skinfolds, FFM. 2. Muscle function: MIP. 3. Exercise performance: 12MWT. |
Comparing group p with group N. Patients were
stratified to depleted group versus non-depleted group: Depleted group: 1. Body composition: No significant difference in FFM or arm circumference between N and P but significant increase in skinfold and weight in the N groups (raw data not provided, p < 0.03). Non-depleted group: Only reported in per protocol analysis 2. Muscle function: No significant differences between the groups. 3. Exercise performance: No significant differences between the groups. |
I: intervention group; P: placebo or control group; 12MWT: 12-minute walk Test; 6MWD: 6-minute walk distance; BM: body mass; BMC: bone mineral content; BMI: body mass index; CRQ, Chronic Respiratory Disease Questionnaire; CWT: constant work rate test; ESWT: endurance shuttle walk test; FEV1: forced expiratory volume in 1 second; FFM: fat-free mass; FM: fat mass; FMI: fat mass index; IBW: ideal body weight; ISWT: incremental shuttle walking test; MEP: maximum expiratory pressure; FFMI: fat-free mass index; MIP: maximum inspiratory pressure; MMC: mid-arm muscle circumference; PEF: peak expiratory flow; QAP: quadriceps average power; QuadCSA: quadriceps cross-sectional area; SGRQ: St. George’s Respiratory Questionnaire; SMM: skeletal muscle mass; UI: International unit; LBM: lean body mass; LBMI: lean body mass index; SMI: skeletal muscle mass index; BCM: body cell mass; BMC: bone mineral content; ASM: appendicular skeletal muscle mass; EQ-5D-3 L: EuroQoL five-dimensions Questionnaire; W, Watt.
Table 3.
Detailed description of the included cohort studies.
| Author and risk of bias | Mean age, GOLD stage and BMI | Subject | Intervention | Pulmonary rehabilitation | Outcomes measures | Result |
|---|---|---|---|---|---|---|
| Kubo et al.40
Bias: 2.4 |
Age: I vs. P (70 vs. 71 years old). GOLD: 3. BMI: I vs. P (18.8 vs. 18.3 kg/m2). |
N = 8 | Intervention (I): 400 kcal and 8 g protein and abundance of branched chain amino
acids in 200 mL. Placebo (P): No. |
Duration: 8 weeks. Location: outpatient. Session detail: one time per week for 8 weeks: 1–90 minutes lecture and physical therapy: A: Breathing instruction B: Muscle strengthening exercise for lower limb. |
1. Exercise performance: 6MWD. 2. Quality of life: CRQ. |
1. Exercise performance: No significant differences between the
groups. 2. Quality of life: No significant differences between the groups. |
| Broekhuizen et al.41
Bias: 1.1 |
Age: A vs. B (62 vs. 63 years old). GOLD: 3. BMI A vs. B (20 vs. 19.7 kg/m2). |
N = 19 Historical controls: = 20 |
Group A: respifor (as above) 125 mL three times per day. Group B: historical One ensini (high carbohydrate supplement), one fortimel (high carbohydrate supplement), one nutridrink (high carbohydrate supplement), 200 mL three times per day for 8 weeks. |
Duration: 8 weeks. Location: inpatient. Session detail: Daily: 1. 2 times 20 minutes submaximal cycle ergometry. 2. 1 time 20 minutes treadmill exercise. 3. 1 time 30 minutes gymnastics. 4. One session of unsupported arm endurance and strength exercise training. 5. Educational programme. |
1. Body Composition: weight, FFM, FFMI and FM. 2. Exercise performance: incremental bicycle ergometry. 3. Quality of life: SGRQ. 4. Lung function: FEV1. |
1. Body composition: Group A: 1. Significant weight gain (1.9 kg, p = 0.019) vs. group B (1.2 kg). Both groups: Post-PR, significant gain in weight (A: 1.9 kg, p < 0.001; B: 1.2 kg, p < 0.001), FM (only group A 1.3 kg, p < 0.05), and FFM (A: 2 kg, p <0.001; B: 1.9 kg, p < 0.05). 2. Exercise performance: Both groups: Peak workload increased significantly during the incremental bicycle ergometry test (group A: 8.3 ± 17.1 W, p = 0.062; group B: 9 ± 9.4 W, p = 0.002). 3. Quality of life: SGRQ Group A: A: No significant differences (although numerical change in SGRQ was greater than the MCID). Group B: A: Worse score on the impact dimension. Both groups: No significant differences between the groups. 4. Lung function: No significant differences between the groups. |
| Creutzberg et al.42
Bias: 1.7 |
Age: I vs. P (65 vs. 65 years old). GOLD: 3. BMI: I vs. P (20.2 vs. 19.8 kg/m2). |
N = 64 (“depleted”) Historical controls = 28 |
Intervention (I): fortimel (as above), ensini (as above), fortipudding (high
carbohydrate supplement) three times per day for 8 weeks. Placebo (P): No. |
Duration: 8 weeks. Location: inpatient. Session detail: A—General physical training: 1. Swimming. 2. Sports. 3. Exercise in relation to daily activities. 4. Cycle ergometry. 5. Treadmill walking. B—Games. C—Educational program. |
1. Body composition: weight and FFM. 2. Muscle function: MIP. 3. Quality of life: SGRQ. |
1. Body composition: Significant increase in body weight (2.1 kg,
p < 0.05) and FFM (1.1 kg, p < 0.05) in
the intervention group. 2. Muscle function: No significant differences between the groups. 3. Quality of life: No significant differences between the groups. |
| Menier et al.43
Bias: 2.4 |
Age: 63 years old. GOLD: not recorded. BMI: not recorded. |
N = 60 | Intervention (I): amino acids 1 capsule/7 kg body weight/day, 6
weeks. Placebo (P): No. |
Duration: 5 weeks. Location: not specified Session detail: 5 days/week. (40 minutes). Intensity training endurance until exhaustion |
1. Exercise performance: Reached max power (W max) |
1. Exercise performance: No significant differences between the groups. |
| Creutzberg et al.44
Bias: 2.2 |
Age: group 1, 2, and 3 (69, 65, vs. 59 years old). GOLD: 3 BMI: group 1, 2, and 3 (39.9, 42.9, and 39.6 kg/m2) |
N = 24 (depleted group) |
Intervention: fortimel (as above), ensini (as above), fortipudding (as above) three times per day for 8 weeks. | Duration: 8 weeks Location: inpatient Session detail: not specified. Intensity depending on the tolerance of the patient. |
1. Body composition: Weight and FFM. |
Patients divided into (1) no weight gain <2%. (2) expected weight gain
>5%. (3) medium weight gain 2–5%: 1. Body composition: Weight significantly increased for group 3 (5.8 ± 1.2 kg, p < 0.001) vs. 1 and 2. FFM significantly increased for group 2 (FFM 1.5 ± 1.2 kg, p < 0.05) and group 3 (FFM 3.1 ± 1.8, p < 0.001) vs. group 1. |
I: intervention group; P: placebo or control group; 12MWT: 12-minute walk test; 6MWD: 6-minute walk distance; BMI: body mass index; CRQ: Chronic Respiratory Disease Questionnaire; FEV1: forced expiratory volume in 1 second, FFM: fat-free mass; FFMI: fat-free mass index; FM, fat mass; MIP: maximum inspiratory pressure; PR: pulmonary rehabilitation; SGRQ: St. George’s Respiratory Questionnaire.
Exercise capacity
Data on exercise function, performance, capacity or endurance were reported in 19 studies using the endurance shuttle walking test (ESWT), incremental shuttle walking test, 6-minute walk test (6MWT), 12-minute walk test, treadmill and incremental or constant work-load cycle ergometry. Seventeen studies found that using nutritional supplements such as high carbohydrates, vitamin D, creatine, or l-carnitine in addition to PR programs had no statistical benefit compared to PR alone.23–27,30,32–34,36–41,43 Three studies found that using nutritional supplements, such as, polyunsaturated fatty acids (PUFAs) and respifor, which are high in carbohydrates, had a statistically significant benefit on top of PR.28,31,35
There was only one study with positive findings at the lowest risk of bias (1/7), in which Sugawara et al. reported increases in 6-minute walk distance by 19.7 ± 24.7 m with this addition of supplement (less than the minimum clinically important difference). In this RCT, the intervention group received ready-to-drink oral nutritional supplement (ONS) twice a day composed of 200 kilocalories, 60% carbohydrates, 15% protein, 25% fat, 248 μg of omega-3 PUFAs 0.6 with vitamins A, C and E and a 12-week exercise programme while the control group underwent a 12-week exercise programme only.28 There were four RCTs with a similar low risk of bias, which demonstrated no benefit of supplementation. van de Bool et al.23 reported that using a high carbohydrate supplementation once a day (125 mL of 9.4 g protein, 28.1 g carbohydrate and 4.1 g fat, leucine, n-3 PUFA and vitamin D) over a period of 4 months within an outpatient PR did not show any significant improvement in exercise performance measured by cycle endurance time or 6MWT compared to the control PR group, who received flavoured non-caloric aqueous solution as a placebo. Similarly, the study by Paulin et al. found that using vitamin B12 for 8 weeks during outpatient, PR did not show any significant improvement in exercise performance or endurance compared to PR alone.24 Borghi-Silva et al. reported that using l-carnitine twice a day for 6 weeks did not demonstrate a significant improvement in exercise performance measured by treadmill performance and 6MWT when compared to the placebo group, who received saline solution for the same duration.33 Finally, Faager et al. concluded that using creatine for 8 weeks during PR did not improve exercise performance when measured by ESWT compared to the placebo (glucose) group who underwent the same PR.34
Body composition
Seventeen trials measured body composition including body weight, fat-free mass (FFM), fat-free mass index (FFMI) and body mass index (BMI).
Body weight was one of the most frequent outcomes measured before and after giving nutritional supplementation; 11 studies measured body weight in COPD patients with normal BMI. Seven studies reported that body weight increased significantly following nutritional supplementation compared to the placebo groups,26,37,39,28,29,42,44 and the study by Broekhuizen et al.41 compared two nutritional supplement regimes, respifor versus ensini, fortimel and nutridrink, which found that both interventions significantly increased body weight. Four studies reported that body weight did not significantly improve in the intervention groups when compared to the placebo groups.30,32,34,35 Of the RCTs in which body weight significantly increased, there was only one study, by Sugawara et al., that had a low risk of bias.28 This study reported a significant increase in body weight after 12 weeks of 2.6 ± 3 kg in those receiving the ready-to-drink (ONS, described above) with the mean baseline body weight of 50.8 kg, compared to those in the placebo group with the mean baseline body weight of 54.8 kg.28 In the study by Gurgun et al., there were significant improvements in body weight of 1.1 ± 0.9 kg, BMI 0.2 ± 1.4 kg/m2 and in FFMI (0.6 ±0.5 kg/m2) in those who received 250 mL of 83.3% carbohydrate, 30% fat and 16.7% protein three times a day as an intervention.26 Of the four studies with negative findings, one study was at low risk of bias.34 This study found no significant difference in body weight between the creatine intervention group and the placebo group after 8 weeks.
BMI was assessed before and after using supplementation in 5 of 24 studies.26,33,37,31,35 BMI significantly increased in the supplementation group when compared to the placebo group in three studies.26,37,31 Two studies reported no significant difference in BMI between participants who received nutritional supplementation with PR compared to PR only.33,35 One RCT at the lowest risk of bias showed no improvement in BMI with carnitine.33 In contrast, Gurgun et al. reported that BMI significantly increased after receiving nutritional supplement.26
FFM was evaluated in nine trials.32,36,39,41,28,35,29,42,44 Three studies demonstrated that FFM increased significantly in comparison with the placebo group but these studies all had some risk of bias.36,39,42 Two26,31 of four studies26,41,31,35 with some risk of bias reported that FFMI significantly increased in the supplemental group when compared to the placebo group. In contrast, the study by Broekhuizen et al. reported no significant difference in FFMI between the group who received PUFA as an intervention and the placebo group who received palm and sunflower oil with vitamin E capsule as a placebo.35
Peripheral muscle strength
Of the 24 studies included in this systematic review, 11 studies measured quadriceps muscle strength, handgrip strength or both23,25,27,30,32,34,36,37,28,31,35
Three studies reported that handgrip strength did not significantly improve in the intervention groups when compared to placebo.34,37,35 Faager et al. being at lowest risk of bias reported that using carnitine for 8 weeks during PR did not significantly improve handgrip strength when compared to the placebo group who received glucose.34 In contrast, the study by Fuld et al., which had a higher risk of bias, showed significant improvement in handgrip after using creatine three times a day for 2 weeks followed by once a day for 10 weeks.36
Quadriceps muscle strength was assessed in 11 studies.23,25,27,30,32,34,36,37,28,31,35 Of the 11 RCTs, only three studies with 86 participants in total demonstrated positive findings.36,28,31 The study by Sugawara et al., which had a low risk of bias, concluded that quadriceps muscle strength increased significantly after receiving a complex nutritional supplement when compared to the placebo group.36,28,31 However, nine studies reported that using nutritional supplementation during PR had no additional effect on quadriceps muscle strength.23,25,27,30,32,34,37,35 van de Bool et al. with a low risk of bias reported that using a high carbohydrate supplement showed no significant improvement in quadriceps strength when compared to the placebo group.23 Similarly, the study by Faager et al. showed that using creatine for 8 weeks in COPD patients enrolled in an 8-week PR programme did not reveal significant differences in quadriceps muscle strength compared with those who used placebo.34
Respiratory muscle function
Respiratory muscle function was assessed in 9 of the 24 included studies,23,27,33,36,39,28,31,35,42 of which 3 were at lowest risk of bias.23,33,28 Sugawara et al. reported that maximum inspiratory pressure (MIP) significantly improved in the interventional group (39.2 ± 38.9 cmH2O) after receiving nutritional supplement embedded in 12 weeks of PR compared with placebo (0.1 ± 24.1 cmH2O).28 A small study by Borghi-Silva et al. showed a significant improvement in MIP (40 ± 14 cmH2O) with carnitine compared to placebo (MIP; 14 ± 5 cmH2O).33 In contrast, a larger study by van de Bool et al. did not show a significant improvement in MIP when compared with placebo, who received glucose.23 None of the studies that measured maximal expiratory pressure (MEP) showed a significant difference between interventional and placebo groups.39,31,35
Quality of life
Quality of life was assessed in 14 of 24 studies.23,25–27,30,32,34,36,37,40,28,31,42 Eight studies used the St. George Respiratory Questionnaire (SGRQ),25,26,34,36,41,31,42 and six used the Chronic Respiratory Disease Questionnaire (CRQ).27,30,32,37,40,28 Overall, only two studies demonstrated a significant improvement in quality of life with supplementation in addition to PR.36,28 Sugawara et al., which was at lowest risk of bias, measured quality of life using the CRQ and showed a significant improvement in those receiving nutritional supplement compared with placebo, which was clinically significant (6.2 ± 7.5 vs. −2.7 ± 13.1).28 Thirteen studies showed negative findings including two RCTs at lowest risk of bias, including the study by Faager et al. using creatine supplementation and the study by van de Bool et al. using the high carbohydrate supplement. Faager et al. using creatine for 8 weeks during PR did not improve quality of life measured by SGRQ.34 Similarly, van de Bool et al. reported that 4 months of using oral nutritional intervention did not show symptoms of anxiety and depression.23
Discussion
This review is the first to summarise the potential effects of using nutritional supplementation during PR in patients with COPD. The studies varied in design, used differing supplements and measured different outcomes. In some, the primary purpose was to use the exercise component of PR to enhance the effect of nutrition, whereas others tested whether nutrition supplementation could enhance outcomes from PR. This results in considerable heterogeneity across studies, many of which were further limited by small sample size. It is, therefore, challenging to draw a single conclusion to address whether using a nutritional supplement has additional effects on exercise function, body composition, respiratory muscle function and quality of life during PR. We were also unable to perform meta-analysis due to this heterogeneity. Consequently, appropriately powered double-blinded RCT studies with suitable sample size using high energy/high protein nutritional supplement to investigate the effect of nutritional support in enhancing PR outcomes, and longer-term clinical outcomes, in COPD patients, are still needed. This would be particularly important in the high-risk group of COPD patients who are undernourished. This would support recommendations to incorporate nutritional support in PR management.19,45 High protein/high energy ONS is recommended by the British Association for Parenteral and Enteral Nutrition for patients with COPD due to high energy and protein requirements46 and PR services in different health contexts that need to consider how best to integrate nutritional assessment and, where successful, intervention into diverse methods of PR delivery.
Exercise capacity has been used to quantify the direct effect of nutrition interventions and to predict mortality and morbidity in COPD patients and other diseases. In this systematic review, the majority of studies demonstrated no improvement in exercise outcomes with nutritional supplementation in addition to PR, compared to PR alone. There were four RCTs with negative findings at low risk of bias,23,24,33,34 which tested carbohydrate, B12, creatine and carnitine supplementation and just one small RCT with a positive finding, which used a ready-to-drink ONS twice a day composed of 200 kilocalories, 60% carbohydrates, 15% protein, 25% fat and 248 μg of omega-3 PUFAs 0.6 with vitamins A, C and E. These findings complement the meta-analysis of nutritional supplementation in stable COPD by Ferreira et al., which included 17 randomised clinical trials and concluded that nutritional supplements increased exercise tolerance in COPD patients who were poorly nourished when compared with baseline only, but which did not specifically consider use in the context of PR.17 A meta-analysis was not possible in our review due to considerable heterogeneity in studies, as described above.
Body composition is one of the outcome measures that might be expected to improve when using nutritional supplement in COPD. Being underweight is associated with an increased risk of mortality in COPD.13 Low body weight is observed in between 25% and 40% of COPD patients. Among these, 25% have moderate to severe weight loss and 35% have extremely low FFM.47 In this systematic review, we found that ready-to-drink ONS during PR may increase body weight in a population with normal body weight but not with carnitine or creatine. Importantly, improvements in body weight and FFM using nutritional supplementation during PR appear to occur especially in depleted, malnourished and muscle-wasted patients (who are at highest risk).23,26,31,29 In the meta-analysis by Ferreira et al, significant weight gain was noted compared to baseline in 11 RCTs and the meta-analysis of Collins et al. showed significant weight gain in favour of nutritional support when compared with control outside the context of a PR programme.17,18
In recent years, researchers have paid attention to the assessment of outcomes, such as quadriceps muscle strength and handgrip strength. Handgrip strength and quadriceps muscle strength are valid measurements of peripheral muscle strength and are associated with mortality, morbidity and increased length of hospital stay.19,48 In this systematic review, RCTs at low risk of bias did not support the concept that creatine, high carbohydrates, and l-carnitine increase peripheral muscle strength, and we found conflicting evidence for the benefits of a ready-to-drink ONS with one study having positive and another study having negative results. Collins et al. concluded that handgrip strength improved significantly in the intervention group when compared to usual care group with PR.19
Respiratory muscle weakness in COPD patients may be due to several factors, such as acute exacerbations, systemic inflammation and malnutrition.49 It has been suggested that nutritional supplements may improve respiratory muscle function. In this systematic review, we found two studies reporting that nutritional supplementation in addition to PR had an extra benefit in improving respiratory muscle function. This was demonstrated by measuring MIP and MEP. The effects were seen only on inspiratory measures, and the authors did not speculate on why they thought this was. Collins et al. concluded that MIP and MEP improved significantly in the intervention group when compared to usual care group. Ferreira et al. found that there was no significant difference between intervention control groups in MIP, but for malnourished patients with COPD, MIP and MEP improved significantly with nutritional support.17,19
Quality of life may be affected through multiple mechanisms in COPD. The available evidence from this review included one small study demonstrating an improvement in QOL measured by CRQ using ready-to-drink ONS, and two studies with negative results, one of which used creatine and one of which also used ONS. The meta-analysis by Ferreira et al reported significant improvement for quality of life measured using SGRQ for patients with COPD who were malnourished. Additionally, Naz and Sahin demonstrated that protein-rich nutritional supplement significantly improved the quality of life in patients with COPD who participated at PR when compared to PR alone.50
Strengths and limitations
To our knowledge, this is the only review that reports the effect of nutritional supplementation during PR in stable COPD. PR is an evidence-based and cost-effective intervention in COPD and thus maximising outcomes is of great interest to clinicians and patients alike. We have carefully searched the literature and registered our review in advance on PROSPERO. Three independent researchers examined the titles and abstracts for inclusion. Potential limitations include we only accessed studies in English, and the inherent variation, many of which had a risk of bias, for example, with inadequate sample size or absence of a power calculation, variation in outcomes measured, variety in study design or different PR protocols. Additionally, outcomes varied between studies, and we have not specifically considered the diversity of nutritional outcomes in this review, which focuses on clinical PR outcomes. There was significant diversity in the type, available substrate, energy imbalance or ingredients of the supplement either caloric or non-caloric and powder, liquid or tablets. We also observed a variation in the amount, contents and the duration of using supplements. Also, our review did not investigate the benefits of using nutritional supplements beyond the duration of PR, which could be important in clinical practice given that a major aim of PR programmes is to durably improve quality of life and reduce the risk of exacerbations and hospitalisations.
Conclusion
This is the first systematic review to report the value of nutritional supplementation during PR in patients with COPD. It is not possible to draw a definitive conclusion due to the heterogeneity of the supplements used, rehabilitation programmes and outcome measures. However, nutritional supplements may enhance the benefit of PR programmes, which would be of considerable benefit to those living with COPD. Not all studies showed positive results and there is a real need for further well-designed and rigorous research to address this area. This is particularly true in weight-losing and/or malnourished patients with COPD, who are at the highest risk of poor outcomes.
Supplemental material
supplemenary for Nutritional supplementation during pulmonary rehabilitation in COPD: A systematic review by Abdulelah M Aldhahir, Ahmed M Al Rajeh, Yousef S Aldabayan, Salifu Drammeh, Vanitha Subbu, Jaber S Alqahtani, John R Hurst and Swapna Mandal in Chronic Respiratory Disease
Acknowledgement
We thank Steven Bembridge Medical Librarian at Royal Free London NHS Foundation Trust, UK, for his assistance and support in refining the search strategy.
Authors’ note: John R Hurst and Swapna Mandal are joint senior authors.
Author contributions: AMA, JRH, and SM conceived and designed the study. AMA performed the initial search and data extraction, while JRH and SM checked the eligibility of the included articles. AMA and JRH performed the quality assessment for the included articles. AMA wrote the initial manuscript and YSA, JSA, SD, and AMR contributed to the writing of the manuscript. JRH, SM, and VS revised the manuscript. All authors read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JRH, SM, and AMA are running an RCT of protein supplementation to enhance PR outcomes in COPD. The product is being supplied by Nutricia. JRH received grants outside the submitted work from pharmaceutical companies that make medicines to treat COPD.
ORCID iD: Abdulelah M Aldhahir  https://orcid.org/0000-0002-4270-9494
https://orcid.org/0000-0002-4270-9494
Supplemental material: Supplemental material for this article is available online.
References
- 1. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive Summary. Am J Respir Crit Care Med 2017; 195: 557–582. Review. [DOI] [PubMed] [Google Scholar]
- 2. Janson C, Marks G, Buist S, et al. The impact of COPD on health status: findings from the BOLD study. Eur Resp J 2013; 42: 1472–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Remoortel H, Hornikx M, Demeyer H, et al. Daily physical activity in subjects with newly diagnosed COPD. Thorax 2013; 68: 962–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zoumot Z, Jordan S, Hopkinson NS. Emphysema: time to say farewell to therapeutic nihilism. Thorax 2014; 69: 973–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wust RCI, Degens H. Factors contributing to muscle wasting and dysfunction in COPD patients. Int J Chronic Obstr Pulm Dis 2007; 2: 289–300. Review. [PMC free article] [PubMed] [Google Scholar]
- 6. Lareau SC, Fahy B. Patient information series: pulmonary rehabilitation. Am J Respir Crit Care Med American Thoracic Society 2013; 198: 19–20. [Google Scholar]
- 7. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest 2007; 131: 4S–42S. Review. [DOI] [PubMed] [Google Scholar]
- 8. Nici L, Donner C, Wouters E, et al. American thoracic society/European respiratory society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006; 173: 1390–1413. Review. [DOI] [PubMed] [Google Scholar]
- 9. McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; (2): CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Evans R, Dunstan-Harvey T, Singh S. The minimum important difference of the incremental shuttle walk test distance in patients with COPD. Am J Respir Crit Care Med 2017; 195: C72 Conference Abstract. [DOI] [PubMed] [Google Scholar]
- 11. Engelen MP, Schols AM, Does JD, et al. Skeletal muscle weakness is associated with wasting of extremity fat-free mass but not with airflow obstruction in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 2000; 71: 733–738. [DOI] [PubMed] [Google Scholar]
- 12. Couillard A, Prefaut C. From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Resp J 2005; 26: 703–719. Review. [DOI] [PubMed] [Google Scholar]
- 13. Hallin R, Gudmundsson G, Suppli Ulrik C, et al. Nutritional status and long-term mortality in hospitalised patients with chronic obstructive pulmonary disease (COPD). Respir Med 2007; 101: 1954–1960. [DOI] [PubMed] [Google Scholar]
- 14. Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clinical Nutrition 2014; 33: 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schols A. Nutritional modulation as part of the integrated management of chronic obstructive pulmonary disease. Proc Nutr Soc 2003; 62: 783–791. Review. [DOI] [PubMed] [Google Scholar]
- 16. Efthimiou J, Fleming J, Gomes C, et al. The effect of supplementary oral nutrition in poorly nourished patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1988; 137: 1075–1082. [DOI] [PubMed] [Google Scholar]
- 17. Ferreira IM, Brooks D, White J, et al. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; 12: 1–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Collins PF, Stratton RJ, Elia M. Nutritional support in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Am J Clin Nutr 2012; 95: 1385–1395. 2012/04/20. [DOI] [PubMed] [Google Scholar]
- 19. Collins PF, Elia M, Stratton RJ. Nutritional support and functional capacity in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respirology 2013; 18: 616–629. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, et al. Reprint--preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther 2009; 89: 873–880. [PubMed] [Google Scholar]
- 21. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adapted Version of a Modified Newcastle-Ottawa Scale for Single Use in Specific Context. The Ottawa hospital research institution http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf (2016, accessed 31 January 2020).
- 23. van de Bool C, Rutten EPA, van Helvoort A, et al. A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J Cachexia Sarcopenia Muscle 2017; 8: 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paulin FV, Zagatto AM, Chiappa GR, et al. Addition of vitamin B12 to exercise training improves cycle ergometer endurance in advanced COPD patients: a randomized and controlled study. Respir Med 2017; 122: 23–29. [DOI] [PubMed] [Google Scholar]
- 25. Ahnfeldt-Mollerup P, Hey H, Johansen C, et al. The effect of protein supplementation on quality of life, physical function, and muscle strength in patients with chronic obstructive pulmonary disease. Eur J Phys Rehabil Med 2015; 51: 447–456. [PubMed] [Google Scholar]
- 26. Gurgun A, Deniz S, Argin M, et al. Effects of nutritional supplementation combined with conventional pulmonary rehabilitation in muscle-wasted chronic obstructive pulmonary disease: a prospective, randomized and controlled study. Respirology 2013; 18: 495–500. [DOI] [PubMed] [Google Scholar]
- 27. Hornikx M, Van Remoortel H, Lehouck A, et al. Vitamin D supplementation during rehabilitation in COPD: a secondary analysis of a randomized trial. Respir Res 2012; 13: 84–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sugawara K, Takahashi H, Kashiwagura T, et al. Effect of anti-inflammatory supplementation with whey peptide and exercise therapy in patients with COPD. Respir Med 2012; 106: 1526–1534. [DOI] [PubMed] [Google Scholar]
- 29. Baldi S, Aquilani R, Pinna GD, et al. Fat-free mass change after nutritional rehabilitation in weight losing COPD: role of insulin, C-reactive protein and tissue hypoxia. Int J Chronic Obstr Pulm Dis 2010; 5: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laviolette L, Lands LC, Dauletbaev N, et al. Combined effect of dietary supplementation with pressurized whey and exercise training in chronic obstructive pulmonary disease: a randomized, controlled, double-blind pilot study. J Med Food 2010; 13: 589–598. [DOI] [PubMed] [Google Scholar]
- 31. van Wetering CR, Hoogendoorn M, Broekhuizen R, et al. Efficacy and costs of nutritional rehabilitation in muscle-wasted patients with chronic obstructive pulmonary disease in a community-based setting: a prespecified subgroup analysis of the intercom trial. J Am Med Dir Assoc 2010; 11: 179–187. [DOI] [PubMed] [Google Scholar]
- 32. Deacon SJ, Vincent EE, Greenhaff PL, et al. Randomized controlled trial of dietary creatine as an adjunct therapy to physical training in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 178: 233–239. [DOI] [PubMed] [Google Scholar]
- 33. Borghi-Silva A, Baldissera V, Sampaio LM, et al. L-carnitine as an ergogenic aid for patients with chronic obstructive pulmonary disease submitted to whole-body and respiratory muscle training programs. Braz J Med Biol Res 2006; 39: 465–474. [DOI] [PubMed] [Google Scholar]
- 34. Faager G, Soderlund K, Skold CM, et al. Creatine supplementation and physical training in patients with COPD: a double blind, placebo-controlled study. Int J Chronic Obstr Pulm Dis 2006; 1: 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Broekhuizen R, Wouters EF, Creutzberg EC, et al. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax 2005; 60: 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fuld JP, Kilduff LP, Neder JA, et al. Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax 2005; 60: 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steiner MC, Barton RL, Singh SJ, et al. Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: a randomised controlled trial. Thorax 2003; 58: 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vermeeren MA, Wouters EF, Nelissen LH, et al. Acute effects of different nutritional supplements on symptoms and functional capacity in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 2001; 73: 295–301. [DOI] [PubMed] [Google Scholar]
- 39. Schols AM, Soeters PB, Mostert R, et al. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo-controlled randomized trial. Am J Respir Crit Care Med 1995; 152: 1268–1274. [DOI] [PubMed] [Google Scholar]
- 40. Kubo H, Honda N, Tsuji F, et al. Effects of dietary supplements on the Fischer ratio before and after pulmonary rehabilitation. Asia Pac J Clin Nutr 2006; 15: 551–555. [PubMed] [Google Scholar]
- 41. Broekhuizen R, Creutzberg EC, Weling-Scheepers C, et al. Optimizing oral nutritional drink supplementation in patients with chronic obstructive pulmonary disease. Br J Nutr 2005; 93: 965–971. [DOI] [PubMed] [Google Scholar]
- 42. Creutzberg EC, Wouters EFM, Mostert R, et al. Efficacy of nutritional supplementation therapy in depleted patients with chronic obstructive pulmonary disease. Nutrition 2003; 19: 120–127. [DOI] [PubMed] [Google Scholar]
- 43. Menier R, Talmud J, Laplaud D, et al. Branched-chain amino acids and retraining of patients with chronic obstructive lung disease. J Sport Med Phys Fit 2001; 41: 500–504. [PubMed] [Google Scholar]
- 44. Creutzberg EC, Schols A, Weling-Scheepers C, et al. Characterization of nonresponse to high caloric oral nutritional therapy in depleted patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161: 745–752. [DOI] [PubMed] [Google Scholar]
- 45. Sugawara K, Takahashi H, Kasai C, et al. Effects of nutritional supplementation combined with low-intensity exercise in malnourished patients with COPD. Respir Med 2010; 104: 1883–1889. [DOI] [PubMed] [Google Scholar]
- 46. Managing Malnutrition in COPD, https://www.malnutritionpathway.co.uk/library/mm_copd.pdf (2016, accessed 07 November 2019).
- 47. Vermeeren MA, Creutzberg EC, Schols AM, et al. Prevalence of nutritional depletion in a large out-patient population of patients with COPD. Respir Med 2006; 100: 1349–1355. [DOI] [PubMed] [Google Scholar]
- 48. Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007; 62: 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gea J, Pascual S, Casadevall C, et al. Muscle dysfunction in chronic obstructive pulmonary disease: update on causes and biological findings. J Thorac Dis 2015; 7: E418–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Naz I, Sahin H. The effect of nutritional support on pulmonary rehabilitation outcomes in COPD patients with low body mass index. Eur Resp J 2018; 52 DOI: 10.1183/13993003.congress-2018.PA1497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplemenary for Nutritional supplementation during pulmonary rehabilitation in COPD: A systematic review by Abdulelah M Aldhahir, Ahmed M Al Rajeh, Yousef S Aldabayan, Salifu Drammeh, Vanitha Subbu, Jaber S Alqahtani, John R Hurst and Swapna Mandal in Chronic Respiratory Disease