Abstract
Background
National notifiable diseases surveillance system (NNDSS) data in developing countries are usually incomplete, yet the total number of fatal cases reported is commonly used in national priority-setting. Melioidosis, an infectious disease caused by Burkholderia pseudomallei, is largely underrecognized by policy-makers due to the underreporting of fatal cases via the NNDSS.
Methods
Collaborating with the Epidemiology Division (ED), Ministry of Public Health (MoPH), we conducted a retrospective study to determine the incidence and mortality of melioidosis cases already identified by clinical microbiology laboratories nationwide. A case of melioidosis was defined as a patient with any clinical specimen culture positive for B. pseudomallei. Routinely available microbiology and hospital databases of secondary care and tertiary care hospitals, the national death registry, and NNDSS data were obtained for analysis.
Results
A total of 7126 culture-confirmed melioidosis patients were identified from 2012 to 2015 in 60 hospitals countrywide. The total number of cases diagnosed in Northeast, Central, South, East, North, and West Thailand were 5475, 536, 374, 364, 358, and 19 cases, respectively. The overall 30-day mortality was 39% (2805/7126). Only 126 (4%) deaths were reported to the NNDSS. Age, presentation with bacteremia and pneumonia, prevalence of diabetes, and 30-day mortality differed by geographical region (all P < .001). The ED at MoPH has agreed to include the findings of our study in the next annual report of the NNDSS.
Conclusions
Melioidosis is an important cause of death in Thailand nationwide, and its clinical epidemiology may be different by region. In developing countries, NNDSS data can be supplemented by integrating information from readily available routine data sets.
Keywords: notifiable diseases, surveillance system, melioidosis, Burkholderia pseudomallei, epidemiology
Using readily available microbiology and hospital datasets from 60 secondary-care or tertiary-care hospitals from 2012 to 2015 in Thailand, we identified 2,805 fatal culture-confirmed melioidosis cases. In developing countries, integrated information from routine databases could supplement the National Notifiable Diseases Surveillance System data.
National notifiable diseases surveillance system (NNDSS) data are a key part of public health decision-making in all countries, including priority-setting, planning, resource mobilization and allocation, and monitoring and evaluation of disease prevention and control programs [1]. However, incomplete NNDSS data frequently affect priority-setting and actions by policy-makers, particularly with regards to bacterial diseases in low- and middle-income countries (LMICs) [2–4]. In high-income countries, the completeness of NNDSS data can range from 6% to 99%, and invasive bacterial infections are less likely to be reported compared with AIDS and tuberculosis [5, 6]. One of the solutions for high-income countries is the use of an automated computerized system to capture a combination of case data and laboratory data, create reports, and submit the reports to the responsible authority [7–10]. Affordable solutions to improve the completeness and accuracy of NNDSS data in LMICs are still needed [11, 12].
Melioidosis, an often fatal infectious disease caused by the Gram-negative bacterium Burkholderia pseudomallei, is endemic in tropical developing countries [13, 14]. Humans usually acquire melioidosis from B. pseudomallei in the environment via skin inoculation, ingestion, and inhalation. Diabetes is the most common clinical risk factor. The majority of patients present with sepsis with or without pneumonia or localized abscesses [15]. The mortality of melioidosis cases ranges from 10% to 63% [14, 16–18]. A modeling study estimated that there are about 165 000 melioidosis cases per year worldwide, of which 89 000 (54%) die [13]. Melioidosis is difficult to diagnose due to nonspecific clinical manifestations and a relative lack of microbiological laboratories in tropical developing countries [14]. The gold standard for the diagnosis of melioidosis is culture [19]. B. pseudomallei is not part of the normal human flora, and its isolation from any clinical sample is regarded as diagnostic of melioidosis. An indirect hemagglutination assay (IHA), which detects crude antibodies raised against B. pseudomallei, is neither sensitive nor specific, and it is not recommended for the diagnosis of melioidosis in disease-endemic regions [19].
Although the capacity and utilization of microbiological laboratories in public referral hospitals in Thailand are high [20], the national burden and epidemiology of melioidosis remain poorly understood. The NNDSS was established in Thailand in 1968, and melioidosis has been a notifiable disease since 2002 [2]. About 10 melioidosis deaths have been formally reported to the NNDSS each year [2]. However, a single hospital in Northeast Thailand continuously publishes scientific papers reporting about 100 fatal melioidosis cases each year [17, 21]. A modeling study estimated that there could be about 2800 fatal melioidosis cases annually in the country [13]. The low numbers of deaths from melioidosis reported to the NNDSS has meant that melioidosis has not been prioritized by the Ministry of Public Health (MoPH) in Thailand [2]. Here, we aim to determine the incidence, mortality, and clinical epidemiology of melioidosis cases already diagnosed by routine clinical microbiological laboratories in all secondary care and tertiary care hospitals in Thailand from 2012 to 2015, compare our findings with NNDSS data, and supplement the annual report of the NNDSS with our findings.
METHODS
Study Area and Population
In 2012, Thailand had a population of 64.4 million, consisted of 77 provinces, and covered 513 120 km2. The country can be divided into 6 geographical regions, comprising Northeast, North, East, West, South, and Central [22]. Thai health care services are delivered by multiple levels of health care facilities [23]. In each province, there are primary care units (PCUs) located in subdistricts, community hospitals (district level), and at least 1 general or regional hospital. Severely ill patients presenting to PCUs and community hospitals are often referred to general hospitals (acting as secondary care hospitals) or regional hospitals (acting as tertiary care hospitals). In 2012, there were 68 public general hospitals and 28 public regional hospitals in Thailand [24]. Unlike PCUs and community hospitals, these are equipped with a microbiology laboratory capable of performing bacterial culture using standard methodologies for bacterial identification and susceptibility testing provided by the Bureau of Laboratory Quality and Standards, MoPH, Thailand [25].
Study Design and Source of Data
Collaborating with the Epidemiology Division (ED) of the Department of Disease Control, MoPH, Thailand, we conducted a retrospective, multicenter surveillance study in all public general and regional hospitals in Thailand. From the hospitals that agreed to participate, data were collected from microbiology and hospital databases between January 2012 and December 2015. Hospital number (HN) and admission number (AN) were used as a record linkage between the 2 databases and to identify individuals who had repeat admissions. Diagnoses in the hospital data were recorded using 10th revision of the International Classification of Disease (ICD) codes. Date of death and ICD-10 codes was also extracted from these data.
It is a common in Thailand for terminally ill patients to be discharged from hospital to be allowed to die at home [26, 27], so 30-day mortality was verified using the death registry data of the Ministry of Public Health, Thailand. NNDSS data were obtained from the ED, MoPH. The data variables included province, type of health care facilities, total number of cases, and total number of deaths.
Definitions
Culture-confirmed melioidosis was defined as a patient with a culture positive for B. pseudomallei from any clinical specimen. Comorbidities (diabetes mellitus, hypertension, chronic renal failure, chronic obstructive pulmonary disease [COPD], chronic liver disease, HIV, tuberculosis, thalassemia, and malignancy) were defined using ICD-10, Thai edition, codes (Supplementary Table 1) [28]. Bacteremia and bacteriuria were defined as blood and urine cultures positive for B. pseudomallei, respectively. Pneumonia was defined using ICD-10 codes or sputum culture positive for the organism. Hepatosplenic abscess, septic arthritis, and osteomyelitis were defined using ICD-10 codes.
Thirty-day mortality was determined on the basis of a record of death within 30 days of admission in the routine hospital database or by a record of death within that period in the national death registry. In-hospital mortality was determined using the discharge status recorded in the hospital admission data for that admission. In the event that a patient had more than 1 episode of admission due to culture-confirmed melioidosis, only the first episode was included in the study.
Statistical Analysis
The outcomes of interest were incidence and 30-day mortality, and their associations with regions, comorbidities, and clinical manifestations. The incidence rate (per 100 000 population per year) was calculated by dividing the cumulative incidence by the total population in the study province. The reporting completeness was calculated by dividing the total number of fatal cases reported by the total number of fatal cases observed. Interquartile ranges (IQRs) are presented in terms of 25th and 75th percentiles. Binary and continuous variables were compared using the chi-square test and Kruskal-Wallis test, respectively. The risk factors associated with 30-day mortality were evaluated using a univariable and multivariable logistic regression model stratified by hospital. The final multivariable logistic regression models were developed using a purposeful selection method [29]. Poisson regression models were used to assess changes in incidence rates over time and to compare incidence rates among regions. All models were stratified by hospital. A sensitivity analysis was done by evaluating factors associated with in-hospital mortality. All statistical analyses were performed using Stata, version 15.0 (StataCorp LP, College Station, TX, USA).
Ethical Considerations
Ethical permission for this study was obtained from the Institute for the Development of Human Research Protection, Ministry of Public Health (IHRP 2334/2556), the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University (MUTM 2014-017-01), and the Oxford Tropical Research Ethics Committee, University of Oxford (OXTREC 521-13). Written approval was given by the directors of the hospitals to use their routine hospital database for research. Individual consent was not sought from the patients as this was a retrospective study, and the Ethical and Scientific Review Committees approved the process.
RESULTS
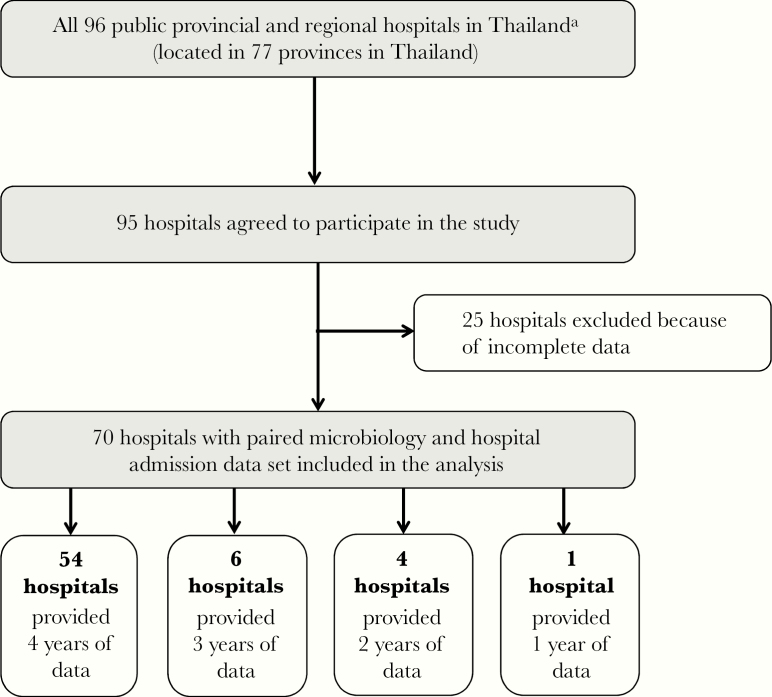
Of 96 public general and regional hospitals in Thailand, 95 (99%) agreed to participate in the study (Figure 1). Twenty-five hospitals (26%) were not included in the analysis because either the microbiology or hospital database was not obtained. Seventy hospitals included in the analysis were located in 61 provinces (Figure 2). A total of 54 hospitals (77%) provided data for 4 years (from 2012 to 2015), 6 hospitals (9%) for 3 years, 4 hospitals (6%) for 2 years, and 6 hospitals (8%) for 1 year (see supplementary Table 2).
Figure 1.
Flowchart of the study. aIn 2012, there were 68 public general hospitals (acting as secondary care hospitals) and 28 public regional hospitals (acting as tertiary care hospitals) in Thailand [24].
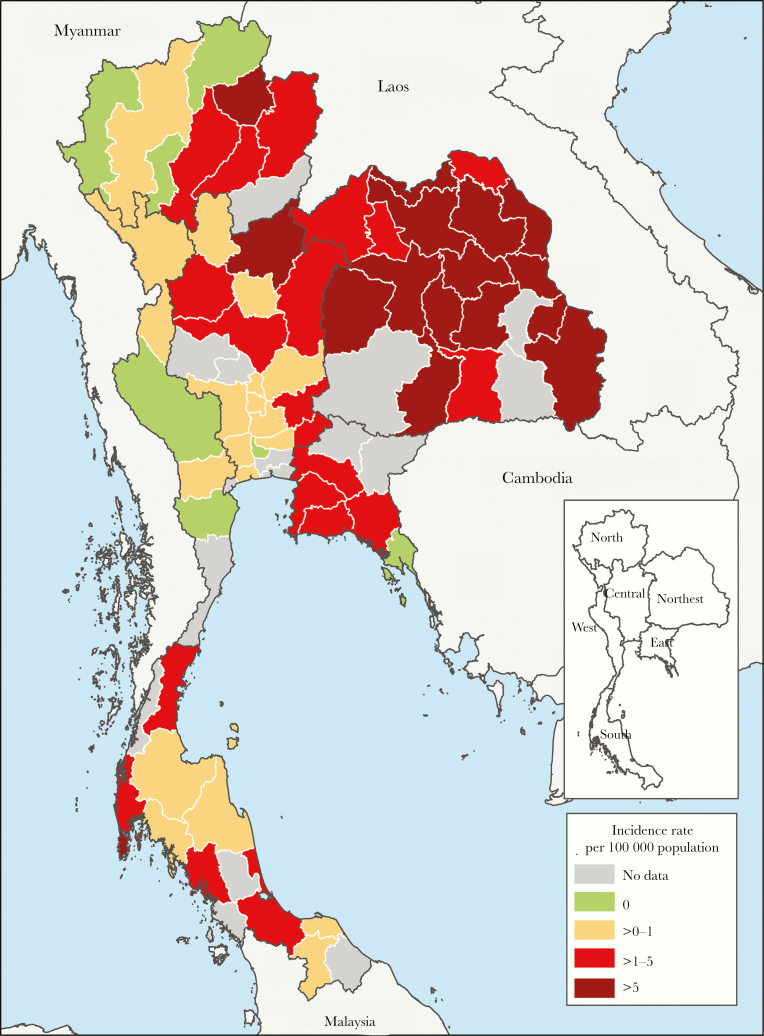
Figure 2.
Incidence rates of culture-confirmed melioidosis in Thailand from 2012 to 2015. Provinces are categorized based on incidence rates of culture-confirmed melioidosis observed (dark red, >5 cases per 100 000 population per year; red, >1–5 cases per 100 000 population per year; yellow, >0–1 cases per 100 000 population per year; green, no cases observed; and gray, no data)
A total of 8 476 596 admission records from 6 228 644 patients were evaluated, and 7626 admission records had at least 1 clinical sample culture positive for B. pseudomallei. Multiple admissions during which B. pseudomallei was grown from clinical specimens were noted in 421 patients. Only the first episode of culture-confirmed melioidosis in 7126 patients was included in further analysis.
Incidence of Melioidosis
The total numbers of culture-confirmed melioidosis cases identified in 2012, 2013, 2014, and 2015 were 1735, 1757, 1932, and 1702, respectively (Table 1). Overall, melioidosis cases were already diagnosed in 60 hospitals located in 52 provinces (Figure 2). The average incidence rate of melioidosis during the 4-year study period was 3.95 per 100 000 population per year and was significantly different by region (P < .001). There was no clear trend over the study period. The total number of cases diagnosed in Northeast, Central, South, East, North, and West Thailand were 5475, 536, 374, 364, 358, and 19 cases, respectively. The incidence rate was highest in Northeast Thailand (8.73 per 100 000 population per year) and lowest in West Thailand (0.23 per 100 000 population per year; P < .001) (Supplementary Table 3).
Table 1.
Total Number of Culture-Confirmed Melioidosis Cases Diagnosed by Routine Clinical Microbiology Laboratories in Public Secondary Care and Tertiary Care Hospitals in Thailand in 2012 to 2015
| No. of Culture-Confirmed Melioidosis Casesb | |||||||
|---|---|---|---|---|---|---|---|
| Regions | No. of Participating Hospitals | No. of Provincesa | 2012 | 2013 | 2014 | 2015 | Total |
| Northeast | 17 | 17 | 1332 | 1359 | 1481 | 1303 | 5475 |
| Central | 21 | 17 | 112 | 142 | 155 | 127 | 536 |
| South | 12 | 10 | 97 | 75 | 95 | 107 | 374 |
| East | 5 | 5 | 85 | 84 | 113 | 82 | 364 |
| North | 9 | 8 | 99 | 93 | 85 | 81 | 358 |
| West | 6 | 4 | 10 | 4 | 3 | 2 | 19 |
| Total | 70 | 61 | 1735 | 1757 | 1932 | 1702 | 7126 |
aEight provinces had the data obtained from more than 1 hospital, including Lopburi (2), Phang Nga (2), Phayao (2), Ratchaburi (3), Saraburi (2), Singburi (2), Songkhla (2), and Suphanburi (2).
bOf 70 provincial or regional hospitals included in the study, 65, 58, 64, and 62 provided data for years 2012, 2013, 2014, and 2015, respectively.
Clinical Epidemiology of Melioidosis
Of the 7126 patients, 4839 (68%) were male, and the median age (IQR; range) was 54 (44.5–63; <1–100) years (Supplementary Table 4). Using ICD-10 codes, we found that the most common comorbidities reported were diabetes mellitus (43%), followed by hypertension (15%) and chronic kidney disease (11%). The most common clinical specimens that were culture positive for B. pseudomallei were blood (n = 4910, 69%), sputum (n = 1555, 22%), urine (n = 341, 5%), pleural fluid (n = 92, 1%), cerebrospinal fluid (n = 13, 0.2%), and unidentified pus or fluid (n = 1143, 16%). Using the combination of ICD-10 codes and the microbiology laboratory database, we found that the most common clinical presentation was bacteremia (69%), followed by pneumonia (38%), hepatosplenic abscesses (8%), and bacteriuria (5%).
Age, comorbidities, and clinical presentations of melioidosis in Thailand differed by geographical region (Supplementary Table 4). The median age of patients was highest in North Thailand (57 years) and lowest in West Thailand (48 years; P < .001). The prevalence of diabetes mellitus was highest in South Thailand (48%) and lowest in North Thailand (21%; P < .001). Presentation with bacteremia was highest in East Thailand (78%) and lowest in West Thailand (63%). Presentation with pneumonia was also highest in East Thailand (46%) and lowest in West Thailand (16%).
Mortality Involving Melioidosis
A total of 2805 cases died within 30 days of hospital admission, giving a 30-day mortality of 39% (2805/7126). Death in melioidosis patients often occurred rapidly, with 1076 deaths (39%) occurring within the first 2 days of admission, 894 (32%) from day 3 to day 7, and the remaining 835 (30%) after 7 days of admission.
In the univariable logistic regression models, 30-day mortality was associated with older age, comorbidities, clinical presentation, and region (Supplementary Table 5). In the final multivariable model, 30-day mortality was associated with the comorbidities of chronic kidney disease and liver disease, and presentation with bacteremia, pneumonia, and bacteriuria (Table 2). Male gender, comorbidities of diabetes and thalassemia, and presentation with hepatosplenic abscesses, septic arthritis, and osteomyelitis were associated with survival. Sensitivity analysis showed that factors associated with in-hospital mortality were similar to factors associated with 30-day mortality, except that some P values were slightly higher (Supplementary Tables 6 and 7).
Table 2.
Factors Associated With 30-Day Mortality in 7126 Culture-Confirmed Melioidosis Cases in 2012–2015, by Multivariable Logistic Regression Model, Stratified by Hospital
| Baseline Characteristics | Died (n = 2805) | Survived (n = 4321) | Adjusted Odds Ratio(95% CI) | P |
|---|---|---|---|---|
| Gender (male), No. (%) | 1908 (68.0) | 2931 (67.8) | 0.84 (0.74–0.94) | .004 |
| Age, median (IQR), y | 56 (46–65) | 53 (43–61) | 1.01 (1.01–1.02) | <.001 |
| Comorbidities,a No. (%) | ||||
| Liver disease | 371 (13.2) | 290 (6.7) | 1.89 (1.57–2.28) | <.001 |
| Chronic kidney disease | 410 (14.6) | 405 (9.4) | 1.54 (1.30–1.83) | <.001 |
| Thalassemia | 36 (1.3) | 115 (2.7) | 0.60 (0.38–0.92) | .02 |
| Diabetes mellitus | 1061 (37.8) | 1984 (45.9) | 0.57 (0.50–0.64) | <.001 |
| Clinical manifestations, No. (%) | ||||
| Bacteremiab | 2391 (85.2) | 2519 (58.3) | 5.66 (4.93–6.51) | <.001 |
| Pneumoniac | 1574 (56.1) | 1131 (26.2) | 4.44 (3.94–4.99) | <.001 |
| Bacteriuriad | 209 (7.5) | 132 (3.1) | 3.14 (2.41–4.09) | <.001 |
| Hepatosplenic abscesse | 100 (3.6) | 480 (11.1) | 0.35 (0.28–0.45) | <.001 |
| Septic arthritise | 85 (3.0) | 300 (6.9) | 0.61 (0.46–0.81) | .001 |
| Ostemomyelitise | 6 (0.2) | 57 (1.3) | 0.36 (0.14–0.91) | .03 |
| Regions, No. (%) | ||||
| Northeast | 2190 (78.1) | 3285 (76.0) | 1 | .05 |
| Central | 215 (7.7) | 321 (7.4) | 0.95 (0.72–1.25) | |
| East | 158 (5.6) | 206 (4.8) | 0.91 (0.66–1.25) | |
| North | 111 (4.0) | 247 (5.7) | 0.60 (0.43–0.84) | |
| South | 129 (4.6) | 245 (5.7) | 0.87 (0.64–1.17) | |
| West | 2 (0.1) | 17 (0.4) | 0.26 (0.06–1.24) |
Abbreviations: CI, confidence interval; IQR, interquartile range.
aComorbidities identified by using the ICD-10 codes listed in Supplementary Table 1.
bBlood culture positive for B. pseudomallei.
cUsing ICD-10 codes or sputum culture positive for B. pseudomallei.
dUrine culture positive for B. pseudomallei.
eUsing ICD-10 codes.
Comparison Between Hospital Data and NNDSS Data
The total number of melioidosis cases reported to the NNDSS during the study period was 12 305, of which 141 were reported as fatal cases (Table 3). Of 2805 fatal melioidosis cases identified by the microbiology and hospital databases of the participating hospitals during the study period, 126 were reported as fatal cases to Report 506, giving a completeness of 4% (126/2805).
Table 3.
Comparison Between Incidences and Mortalities of Melioidosis Diagnosed by Microbiology Laboratories and Those Officially Reported to the National Notifiable Disease Surveillance System (Report 506) in Thailand From 2012 to 2015
| Culture-Confirmed Melioidosisa | Report 506 Datab | ||||
|---|---|---|---|---|---|
| Year | Type of Hospital | No. of Cases Diagnosed | No. of Mortality Outcome | No. of Cases Reported | No. of Mortality Outcome Reported |
| 2012 | PCUs or community hospitals | NA | NA | 2426 | 2 |
| Regional or general hospitals not included in the study | NA | NA | 259 | 7 | |
| Regional or general hospital included in the study | 1735 | 683 | 1018 | 4 | |
| 2013 | PCUs or community hospitals | NA | NA | 1821 | 0 |
| Regional or general hospitals not included in the study | NA | NA | 210 | 1 | |
| Regional or general hospital included in the study | 1757 | 737 | 799 | 3 | |
| 2014 | PCUs or community hospitals | NA | NA | 1677 | 3 |
| Regional or general hospitals not included in the study | NA | NA | 174 | 1 | |
| Regional or general hospital included in the study | 1932 | 750 | 695 | 8 | |
| 2015 | PCUs or community hospitals | NA | NA | 2042 | 1 |
| Regional or general hospitals not included in the study | NA | NA | 217 | 0 | |
| Regional or general hospital included in the study | 1702 | 635 | 967 | 111 c | |
Abbreviations: IHA, indirect hemagglutination assay; PCU, primary care unit.
aSeventy of 96 public general and regional hospitals in Thailand participated in the study.
bBased on the national notifiable disease surveillance system in Thailand. Both probable and confirmed melioidosis cases are reported. Probable cases are defined as clinically compatible illness with IHA titer ≥1:160 or IFA >1:400. Confirmed melioidosis cases are defined as clinically compatible illness with any clinical specimen culture positive for B. pseudomallei or a 4-fold rise in IHA or IFA.
cIn 2015, 107 of 111 fatal cases (96%) were reported by a single regional hospital, Sunpasitthiprasong Hospital, Ubon Ratchathani, in Northeast Thailand.
Policy-Maker Engagement
The findings of our study were reported to the ED, MoPH, which agreed to include the findings in the next annual report of the NNDSS.
Discussion
Using routine microbiology and hospital databases, we show that in Thailand each year, about 1700 culture-confirmed melioidosis cases are diagnosed, of whom approximately 700 die. Only about 4% of the deaths were reported to the NNDSS Thailand. Integrating information from readily available microbiology and hospital data can reveal the burden of underreported notifiable diseases. This information could support priority-setting by policy-makers in LMICs. We propose, therefore, that integrating information from readily available data sets to improve national statistics and NNDSS data in LMICs should be considered and implemented.
We expect that including the findings of our study in the next annual report of the NNDSS could support initiatives toward a National Programme for Melioidosis to improve awareness, surveillance, diagnosis, treatment, and prevention of melioidosis by MoPH Thailand. Our finding of deaths involving melioidosis (about 700 fatal cases per year) is much higher than the mortalities involving dengue infection (about 100 fatal cases per year) shown in the annual report of the NNDSS Thailand [2]. In Thailand, dengue infection is considered high priority by policy-makers. There is a National Programme for Dengue Prevention Control focused on empowering individuals and communities for source reduction, health promotion, medical services, multisectoral networking, and enhancing capacity-building [30]. These activities improve dengue diagnosis and reporting, and most, if not all, diagnosed fatal cases are reported via the NNDSS.
Underreporting to the NNDSS could occur for a range of reasons. First, persons who are responsible for reporting notifiable diseases in many hospitals with microbiology laboratories (including epidemiologists, nurses, and doctors [31]) do not know that they should confirm a fatal outcome of all culture-confirmed melioidosis cases and report the death of any patients with culture-confirmed melioidosis to the NNDSS [2]. Second, laboratory isolation and identification of B. pseudomallei can take from 2 to 7 days, and many melioidosis cases could die before the culture results. In such cases, nurses and physicians would not be aware of the causative pathogen, and epidemiologists in the hospitals would not be informed and would not report the cases to the NNDSS [31]. Third, the definition of melioidosis used for the NNDSS Thailand is broad. The NNDSS Thailand recommends that both probable and confirmed melioidosis cases be reported [2]. Probable cases are defined as clinically compatible illness with an IHA titer ≥1:160 or immunofluorescence antibody test (IFA) >1:400. Confirmed melioidosis cases are defined as clinically compatible illness with any clinical specimen culture positive for B. pseudomallei or a 4-fold rise in IHA or IFA. However, IFA and IHA are not recommended for diagnosing melioidosis in disease-endemic areas; these tests are neither sensitive nor specific [19]. More than 60% of melioidosis cases reported to Report 506 are from PCUs or community hospitals that do not have microbiology laboratories, and a proportion of these reported cases are likely to be false-positive cases (ie, cases who do not have melioidosis but tested IFA or IHA positive due to previous exposure to environmental B. pseudomallei [19]).
Evaluating the incidence, mortality, and clinical epidemiology of a notifiable disease among provinces could give new information about diseases and highlight areas where diagnosis or reporting systems may need additional investigation. For example, culture-confirmed melioidosis cases were not identified in 9 provinces (10 hospitals) participating in our study, but a high incidence of culture-confirmed melioidosis cases was observed in neighboring provinces in our study. It is possible that B. pseudomallei may be misidentified as Pseudomonas spp. or that there may be contaminants in the laboratories in those provinces. This suggests that evaluation of protocols and operating procedures in microbiological laboratories in these provinces may be warranted.
The differences in clinical presentations and mortality of melioidosis across geographical regions could be due to several reasons, including differing characteristics of the baseline population, differences in the distribution of environmental B. pseudomallei [32, 33], virulence characteristics of B. pseudomallei [34], variation in risk of exposure and route of infection related to occupational activities [14, 35], and disparity among practices of physicians and clinical microbiological laboratories in the region. Selective culture media for B. pseudomallei, which can increase the sensitivity of bacterial isolation from nonsterile specimens such as sputum and urine, is only used in a limited number of hospitals in Northeast Thailand [2]. Variation in clinical presentations and comorbidities could also be due to different practices in recording ICD-10 codes by trained ICD coders or attending physicians in each region. This suggests that training for laboratory personnel and clinicians, informing clinicians of possible variation in clinical presentation of melioidosis cases, and workshops to improve communication between laboratory personnel, clinicians, ICD-10 coders, and persons responsible for NNDSS reporting should be provided countrywide. Further studies on differences in clinical presentations and mortality of melioidosis across regions are also required.
The lower overall mortality of patients with diabetes could be due to the use of glibenclamide [14, 36]. This has an anti-inflammatory effect, and patients taking glibenclamide before hospital admission have attenuated inflammatory responses [14, 36]. The lower overall mortality of patients with thalassemia could be due to early diagnosis of melioidosis in patients with a major underlying disease, unknown reasons (eg, increasing utilization of iron chelation therapy in Thailand), or residual confounding factors. Patients with thalassemia were reported to have a high mortality (59%, 16/28) in Sabah, Malaysia, and the incidence of melioidosis has decreased considerably since the universal availability of iron chelation therapy [37].
We propose a set of low-cost actions to improve NNDSS data in LMICs, including (1) routinely utilizing all laboratory databases (including microbiology, serology, and rapid diagnostic test databases) and hospital databases from all public and private hospitals to supplement NNDSS data of all notifiable diseases, (2) providing training to laboratory personnel countrywide to improve the sensitivity and accuracy of laboratory-diagnosed cases, (3) raising awareness among health care providers about diagnostic criteria and requirements for reporting the final outcome of all cases with notifiable diseases, and (4) updating criteria for diagnosing and reporting notifiable diseases. For example, patients culture positive for B. pseuodmallei should be defined as “culture-confirmed melioidosis cases” and reported to the NNDSS, similar to systems in Singapore, Australia, and Taiwan [38–40]. Melioidosis cases diagnosed based on the IHA or IFA without culture confirmation should be defined as “possible melioidosis cases” when reported to the NNDSS Thailand. These actions are being implemented or discussed with the ED, MoPH Thailand, in conjunction with the current NNDSS.
The difference between the observed 701 fatal culture-confirmed melioidosis cases pear year in this study and the predicted 2838 fatal melioidosis cases per year in the previous modeling study [13] could be due to multiple reasons. First, only 74% of public general and regional hospitals in Thailand were included in the study, and only 77% of those hospitals provided data for all 4 years. Therefore, our data represented only about two-thirds of the already diagnosed melioidosis patients in Thailand. Second, it is possible that the participating hospitals may still misidentify a proportion of the B. pseudomallei isolates as contaminants or other bacteria [19]. Although it is possible that other bacteria could be misidentified as B. pseudomallei, we believe that it is rare based on the increasing clinical information and bacterial confirmation of melioidosis cases in all regions [2]. Third, the modeling study was based on data from large hospitals with research facilities, where the blood culture utilization rate is already high [20] and selective culture media for B. pseudomallei are used for nonsterile specimens collected from melioidosis-suspected cases [13, 19]. It is likely that some public general and regional hospitals will find more melioidosis cases if culturing practices change and selective culture media are used for melioidosis-suspected cases [16, 17]. Therefore, the number of cases and deaths from melioidosis reported here could represent a minimum estimate. Fourth, the previous model was imprecise, as shown by the wide 95% credible interval of the predicted mortality (1259 to 6678) due to limited data availability at that time [13]. The model could be revised and improved by using increasingly available data in the future.
The limitations of this study are that private hospitals, specialized hospitals such as military hospitals and psychiatric hospitals, hospitals in Bangkok, and university hospitals were not included in the study. The lack of culture-confirmed melioidosis identified in certain provinces should be interpreted with caution; an absence of risk for melioidosis acquisition in these areas should not be implied.
In conclusion, the high number of deaths from melioidosis reported in our study provides policy-makers with the evidence they need to accord a high priority to melioidosis as a major health problem in Thailand. Integrating information from readily available microbiology and hospital databases could be used to generate such information, supplement NNDSS data, and support priority-setting for policy-makers in LMICs.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We gratefully acknowledge the directors and epidemiological and laboratory teams of general and regional hospitals, as well as their administrative support staff, for providing microbiological and hospital admission data. The general and regional hospital network is comprised of Chorkaew Yangyuen (Samutprakarn Hospital), Aphinya Singkhongsin, Chanchira Chaichaem (Pranangklao Hospital), Phkaiwan Kropsanit (Pathumthani Hospital), Winai Suphapphot, Chaiwat Khaokaeo (Sena Hospital), Sasi Sichot (Phra Na Khon Sri Ayutthaya Hospital), Ratri Chalaemphak, Benchawan Khaisongkhram (Angthong Hospital), Praphon Chinthanu, Phutthakhun Wongsuwan (Banmi Hospital), Pritsana Wongnoi (Pranarai Maharaj Hospital), Witthaya Yotngoen (Singburi Hospital), Khongsak Sueachoi (Inburi Hospital), Pranom Chantharat (Jainad Narendra Hospital), Sangsan Sinbamrung, Duangkamon Chiratrachu (Phra Phutthabat Hospital), Waranya Sichanta (Saraburi Hospital), Panatda Thipruecha (Chonburi Hospital), Piyaphatcha Phongprasoet, Panatda Inphrom (Rayong Hospital), Pakkawi Siphueak, Ratchani Thamchamrat (Prapokklao Hospital), Phuangphikun PhonPrasit (Trat Hospital), Bunga Chanlee (BuddhaSothorn Hospital), Wiphawadi Dongchan (Chao Phya Abhaibhubejhr Hospital), Haruethai Khunothai (Nakhonnayok Hospital), Atchara Ampere (Somdet Phrayupharacha SaKaeo), Saifon Sutchai, Prayut Kaeomalang, Nonglak Prayunsoet (Maharat Nakhon Ratchasima Hospital), Ratana Chiracharuporn (Buriram Hospital), Suriya Senthong (Surin Hospital), Sunthon Romniyaphet (Sisaket Hospital), Jintana Kanchanabat, Praweennuch Watanachaiprasert, Thanasith Sananmuang (Sunpasitthiprasong Hospital), Somphon Chankaeo (Yasothon Hospital), Wiraphon Khwamman (Chaiyaphum Hospital), Kraison Bunsam, Phonnatcha Katiwong (Amnat Charoen Hospital), Kanyaphak Phanchampa (Buengkan Hospital), Sutthiphong Phonbun (Nongbualamphu Hospital), Marisa Uton, Thitiphan Khunphu (Sirindhorn Hospital of Khon Kaen Province), Kriangkri Kongsuk, Ritthikorn DongLuang (Khon Kaen Hospital), Suthep Thipsawang, Kochnipa Kwawong, Nida Thanaphatphairot (Udon Thani Hospital), Suphattra Likrachang, Kirana Phakdiburikun (Loei Hospital), Suphakon Saenthamphon (Nongkhai Hospital), Suchitra Nasingkhan, Kanchanaphon Taratai (Mahasarakham Hospital), Witthaya Ratmaet (Roiet Hospital), Natthasorn Chawaninthawisut (Kalasin Hospital), Phuwanat PhothiChai (Sakonnakhon Hospital), Phinthip Saiklang (Nakhonphanom Hospital), Yutphon Mankhong, Yothin Tairayawong (Mukdahan Hospital), Warawan Inthip (Nakornping Hospital), Dr. Thiraphong Tatiyaphonkunthiraphong (Lamphun Hospital), Thirin Ketwichit (Lampang Hospital), Yaowalak ChanDaeng (Uttaradit Hospital), Phana Thatsanawaythit, Itsareeya Boonrat (Phrae Hospital), Sopha Itsaranarongphan (Nan Hospital), Sanong Chaisue, Phanarat Phuangmali (ChiangKham Hospital), Chirawan Sithongphim (Phayao Hospital), Satorn Charatdamrongwat (Chiangrai Prachanukroh Hospital), Jintana Phothip, Duangdi Chomphu (Srisangwan Hospital), Ladda Raden (Sawanpracharak Hospital), Phitya Hema, Kanthika Ocharot, Mongkhon Uising (Uthaithani Hospital), Narong Mahayot (Kampangpetch Hospital), Onraphin Thiwai (Maesot Hospital), Preeyada Triprawat (Somdejprajaotaksin Maharaj Hospital), Kreangkrai Chatsut, Yuppharet Kaewprasern (Srisangworn Sukhothai Hospital), Meena Nakhon (Sukhothai Hospital), Aphinya Innoi, Thoranin Rakthanabodee (Buddhachinaraj Hospital), Siwaphorn Phongchin (Phichit Hospital), Jintana Phonphraram (Phetchabun Hospital), Somphon Niamlang (Damnoensaduak Hospital), Thanya Surakhamsang (Banpong Hospital), Thanyalak Borirak (Photharam Hospital), Nopphon Siangchin (Ratchaburi Hospital), Ratchani Watthanayaem, Suwan Manutchan (Pahonpol Payuha Sena Hospital), Ekachai Photnanthawong (Makarak Hospital), Dr. Pornsak Thirathonbun (SomdejPrasangkharach17 Hospital), Narong Wongkanha (Chaophraya Yommarat Hospital), Pitchayakhanid Yaemsoun, Pongphon Roeknaowarat (Nakhonpathom Hospital), Witthaya Sithong (Samutsakorn Hospital), Nonsi Sonthiyat (Kratumban Hospital), Narongchai SiamPhairi (Somdej Prabuddha Lertla Hospital), Chanthana Kalanuwat (Prajomklao Hospital), Saran Songsaeng (HuaHin Hospital), Chirawan Bunchusi (Maharaj Nakhonsithammarat Hospital), Chitchanin Niyomthai, Chutima Phayayam (Krabi Hospital), Boribun Chensamut (Takuapa Hospital), Aphisit Suwannarat, Suwanni Khwankhao, Phityaporn Chunchu (Phang Nga Hospital), Phatcharin Yatraksa (Vachira Phuket Hospital), Jittima Thongnak (Koh Samui Hospital), Ratchanok Withunphan (Suratthani Hospital), Chuenkhwan Kaeowichit, Phimnisa Phet (Ranong Hospital), Kritsanee Wichitakun (Chumporn Ketudomsak Hospital), Sakda Khaophong (Songkla Hospital), Wichian Patangkaro, Nattawan Chanmueang (Hat Yai Hospital), Nittaya Sakunsanti (Satun Hospital), Suriwan Phaksuphara, Umaporn Sina (Trang Hospital), Witthaya Wunchum (Phatthalung Hospital), Sirinthon Wongyoksuriya (Pattani Hospital), Mawin Deae, Ananni Sama (Betong Hospital), Wichai Wanmueang, Suphattra Mahachot (Yala Hospital), Arun Phutkaeo, Yukalipli Kaseng, Khodiyo Yamai (Narathiwat Ratchanakarin Hospital), Praphai Krirat, Naruemon Bunsiri (Sungai Kolok Hospital). We thank Saman Sayumphuruchinan (ED, MoPH), Wanwisa Khammak (Strategy and Planning Division, MoPH), and Prapass Wanapinij (MORU) for administrative and data management supports. We thank Prof. Brian J. Angus (University of Oxford, UK) and Prof. Joann L. Prior (Defence Science and Technology Laboratory, UK) for their scientific comments and advice.
Financial support. This work was supported by the Epidemiology Division, Department of Disease Control, Ministry of Public Health Thailand, and the Wellcome Trust (grant number 101103/Z/13/Z; intermediate fellowship to D.L.).
Potential conflicts of interest. We declare no competing interests; no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years, no other relationships or activities that could appear to have influenced the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. V.H. is the first author. D.L. and S.H. conceived and supervised the study. V.H. and D.L. designed the study and wrote the protocol. V.H. developed the data management plan and data collection and validation tools. P.K. and S.R. performed data collection with the supervision of S.K. and V.H. P.K., S.R., and V.H. performed data cleaning. V.H. and D.L. had full access to all materials and data, performed analyses, and drafted the first report with input from N.D., S.P., and S.H. All authors have reviewed the article, provided important input and revisions, and approved the final manuscript. D.L. is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Role of the funding source. All study procedures, data collection, data analyses, data interpretation, and writing of the report were performed without the sponsors’ involvement. Full access to data was granted to the corresponding author. All authors participated in the study design or analysis and approved the submission of the manuscript.
Patient and public involvement. No patients were involved in determining the research question or outcome measures, or in the interpretation and writing up of results. We plan to disseminate the results of the research to the public and to all public hospitals in Thailand through media outreach (eg, press release, communication media, and workshops) in the collaboration with the Epidemiology Division, Department of Disease Control, MoPH, Thailand.
Transparency declaration. The manuscript’s guarantor (D.L.) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1. World Health Organization. WHO recommended surveillance standards, 2nd ed. Available at: https://apps.who.int/iris/handle/10665/65517. Accessed 13 August 2019. [Google Scholar]
- 2. Hinjoy S, Hantrakun V, Kongyu S, et al. . Melioidosis in Thailand: present and Future. Trop Med Infect Dis 2018; 3:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saxena AK, Azad CS. Neglected tropical bacterial diseases. In: Saxena AK, ed. Communicable Diseases of the Developing World. Switzerland: Springer International Publishing; 2018:169–244. [Google Scholar]
- 4. Bechtle M, Chen S, Efferth T. Neglected diseases caused by bacterial infections. Curr Med Chem 2010; 17:42–60. [DOI] [PubMed] [Google Scholar]
- 5. Doyle TJ, Glynn MK, Groseclose SL. Completeness of notifiable infectious disease reporting in the United States: an analytical literature review. Am J Epidemiol 2002; 155:866–74. [DOI] [PubMed] [Google Scholar]
- 6. Keramarou M, Evans MR. Completeness of infectious disease notification in the United Kingdom: a systematic review. J Infect 2012; 64:555–64. [DOI] [PubMed] [Google Scholar]
- 7. Vlieg WL, Fanoy EB, van Asten L, et al. . Comparing national infectious disease surveillance systems: China and the Netherlands. BMC Public Health 2017; 17:415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ward J, Hildebrandt C, Patel A. NEDSS Base System (NBS): electronic data exchange and workflow decision support. Online J Public Health Inform 2017; 9(1):e047. [Google Scholar]
- 9. Marc-Alain W, Arnold B, Edward van S, et al. . Automated, laboratory-based system using the Internet for disease outbreak detection, the Netherlands. Emerg Inf Dis 2003; 9(9):1046–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salmon M, Schumacher D, Burmann H, Frank C, Claus H, Höhle M. A system for automated outbreak detection of communicable diseases in Germany. Euro Surveill 2016; 21(13):30180–7. [DOI] [PubMed] [Google Scholar]
- 11. Toda M, Njeru I, Zurovac D, et al. . The impact of a SMS-based disease outbreak alert system (mSOS) in Kenya. Int J Infect Dis 2016; 45:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toda M, Njeru I, Zurovac D, et al. . Effectiveness of a mobile short-message-service-based disease outbreak alert system in Kenya. Emerg Infect Dis 2016; 22:711–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Limmathurotsakul D, Golding N, Dance DA, et al. . Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol 2016; 1:15008. [DOI] [PubMed] [Google Scholar]
- 14. Wiersinga WJ, Virk HS, Torres AG, et al. . Melioidosis. Nat Rev Dis Primers 2018; 4:17107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis 2010; 4:e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhengsri S, Baggett HC, Jorakate P, et al. . Incidence of bacteremic melioidosis in Eastern and Northeastern Thailand. Am J Trop Med Hyg 2011; 85:117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, et al. . Increasing incidence of human melioidosis in Northeast Thailand. Am J Trop Med Hyg 2010; 82:1113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zakuan Zainy D, Habsah H, Mohd Noor Siti S. Clinical characteristics and outcomes of bacteraemic melioidosis in a teaching hospital in a northeastern state of Malaysia: a five-year review. J Infect Dev Countr 2010; 4:430–5. [DOI] [PubMed] [Google Scholar]
- 19. Hoffmaster AR, AuCoin D, Baccam P, et al. . Melioidosis diagnostic workshop, 2013. Emerg Infect Dis 2015; 21(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teerawattanasook N, Tauran PM, Teparrukkul P, et al. . Capacity and utilization of blood culture in two referral hospitals in Indonesia and Thailand. Am J Trop Med Hyg 2017; 97:1257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ong CEL, Wongsuvan G, Chew JSW, et al. . Presence of Burkholderia pseudomallei in soil and paddy rice water in a rice field in Northeast Thailand, but not in air and rainwater. Am J Trop Med Hyg 2017; 97:1702–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kashino W, Piyaphanee W, Kittitrakul C, et al. . Incidence of potential rabies exposure among Japanese expatriates and travelers in Thailand. J Travel Med 2014; 21:240–7. [DOI] [PubMed] [Google Scholar]
- 23. Tangcharoensathien V, Jongudomsuk P.. From Policy to Implementation: Historical Events During 2001–2004 of Universal Converage in Thailand. Thailand: National Health Security Office; 2012. [Google Scholar]
- 24. Strategy and Planning Division, Ministry of Public Health, Thailand. Health resources 2012 Available at: http://thcc.or.th/download/gishealth/report-gis55.pdf. Accessed 16 May 2018.
- 25. Opartkiattikul N, Bejrachandra S. The external quality assessment schemes in Thailand. Rinsho Byori 2002; 50:121–5. [PubMed] [Google Scholar]
- 26. Kanoksil M, Jatapai A, Peacock SJ, Limmathurotsakul D. Epidemiology, microbiology and mortality associated with community-acquired bacteremia in Northeast Thailand: a multicenter surveillance study. PLoS One 2013; 8:e54714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hongsuwan M, Srisamang P, Kanoksil M, et al. . Increasing incidence of hospital-acquired and healthcare-associated bacteremia in Northeast Thailand: a multicenter surveillance study. PLoS One 2014; 9:e109324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thai Health Coding Center, Strategy and Planning Division, Ministry of Public Health, Thailand. International statistical classicfication of diseases and related health problems, tenth revision, Thai modification.2012. Available at: http://thcc.or.th/ebook1/2012/2012.html. Accessed 26 October 2018.
- 29. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008; 3:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization. Dengue/dengue haemorrhagic fever prevention and control 2011. Available at http://apps.searo.who.int/pds_docs/B3360.pdf. Accessed 26 October 2019.
- 31. Department of Epidemiology, Department of Disease Control, Ministry of Public Health, Thailand. Reporting of priority diseases guideline, Thailand 2012. Available at: http://www.boe.moph.go.th/files/report/20121008_18818829.pdf. Accessed 24 October 2019.
- 32. Vuddhakul V, Tharavichitkul P, Na-Ngam N, et al. . Epidemiology of Burkholderia pseudomallei in Thailand. Am J Trop Med Hyg 1999; 60:458–61. [DOI] [PubMed] [Google Scholar]
- 33. Hantrakun V, Rongkard P, Oyuchua M, et al. . Soil nutrient depletion is associated with the presence of Burkholderia pseudomallei. Appl Environ Microbiol 2016; 82:7086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sarovich DS, Price EP, Webb JR, et al. . Variable virulence factors in Burkholderia pseudomallei (melioidosis) associated with human disease. PLoS One 2014; 9:e91682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lim C, Peacock SJ, Limmathurotsakul D. Association between activities related to routes of infection and clinical manifestations of melioidosis. Clin Microbiol Infect 2016; 22(1):79 e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koh GC, Maude RR, Schreiber MF, et al. . Glyburide is anti-inflammatory and associated with reduced mortality in melioidosis. Clin Infect Dis 2011; 52:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fong SM, Wong KJ, Fukushima M, Yeo TW. Thalassemia major is a major risk factor for pediatric melioidosis in Kota Kinabalu, Sabah, Malaysia. Clin Infect Dis 2015; 60:1802–7. [DOI] [PubMed] [Google Scholar]
- 38. Sim SH, Ong CEL, Gan YH, et al. . Melioidosis in Singapore: clinical, veterinary, and environmental perspectives. Trop Med Infect Dis 2018; 3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith S, Hanson J, Currie BJ. Melioidosis: an Australian perspective. Trop Med Infect Dis 2018; 3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hsueh P-T, Huang W-T, Hsueh H-K, Chen Y-L, Chen Y-S. Transmission modes of melioidosis in Taiwan. Trop Med Infect Dis 2018; 3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.