Abstract
Encountered in a number of clinical conditions, repeated hypoxia/reoxygenation during the neonatal period can pose both a threat to immediate survival as well as a diminished quality of living later in life. This review focuses on our current understanding of central respiratory rhythm generation and the role that hypoxia and reoxygenation play in influencing rhythmogenesis. Here, we examine the stereotypical response of the inspiratory rhythm from the preBötzinger complex (preBötC), basic neuronal mechanisms that support rhythm generation during the peri-hypoxic interval, and the physiological consequences of inspiratory network responsivity to hypoxia and reoxygenation, acute and chronic intermittent hypoxia, and oxidative stress. These topics are examined in the context of Sudden Infant Death Syndrome, apneas of prematurity, and neonatal abstinence syndrome.
1. Introduction
The challenge of ventilation begins with our first breath. Immediately following birth, the transition from the low oxygen intrauterine environment to the oxygen rich state of room air becomes a significant life-threatening challenge when stable breathing is not yet established (Alvaro and Rigatto, 2016; Hillman et al., 2012). Maternal issues, such as maternal hypertension, preeclampsia (Hubbard and Shingleton, 1985) and gestational diabetes (Gross et al., 1983) can lead to premature birth, which can result in the instability of neonatal breathing. When combined with immediate birthing complications, such as aspiration of meconium-stained amniotic fluids, caesarian-section delivery, and neonatal abstinence syndrome, the risk for respiratory instability increases substantially (“Neonatal drug withdrawal. American Academy of Pediatrics Committee on Drugs,” 1998; Reuter et al., 2014). Unstable breathing during the neonatal period can quickly transition from a couple of skipped breaths to a prolonged hypoxic insult, which can result in life-long problems that extend far beyond breathing, and in the most extreme cases, cause infant death (Bancalari and Claure, 2018). Even after the immediate perinatal period, during the first weeks of life, infants, that otherwise appear healthy, are at risk for Sudden Infant Death Syndrome (SIDS), an outcome where oscillations in blood oxygenation is a factor that can lead to SIDS (Waggener et al., 1990). The gravity of such outcomes due to respiratory instability are clinically well-recognized. Thus, when the potential for unstable neonatal breathing arises, practitioners place significant effort to monitor neonatal breathing and mitigate the occurrence of hypoxia. However, since our understanding into the basis and development of respiratory control in the neonate remains incomplete, there are few options to treat unstable breathing in the newborn. This review examines current advances made in understanding the development and involvement of brainstem networks in controlling respiration in response to and recovery from hypoxia. We will focus particularly on findings derived from the study of inspiratory rhythm generation from the preBötzinger complex (preBötC) during different forms of hypoxia and exposure to reactive oxygen species.
1.1. The neonatal ventilatory response to hypoxia and the perinatal respiratory networks of the brainstem
Respiratory control can be broadly categorized as sensory input, rhythm generation, and motor output (Barlow and Estep, 2006; Bellingham, 1998; Braman, 1995; Grillner, 2003). Balanced integration across these areas of control is necessary for maintaining stable and dynamically responsive breathing (Estelle B. Gauda and Martin, 2012). Yet in the neonate, many aspects of respiratory control are still developing. In rodents, the adult ventilatory response to hypercapnia appears to plateau following first week of life and into adulthood (Stunden et al., 2001) and similarly, carotid body discharge in response to hypoxia, hypercapnia, or hypoxic hypercapnia is stronger in adult (> 5 weeks of age) when compared to the neonate (P5 to P7 of age) (Pepper et al., 1995). Thus, it is during the neonatal period that susceptibility to experiencing a hypoxic event may be enhanced due to underdeveloped responses to deviations on blood gases.
Term and preterm (< 38 week) human infants exhibit a biphasic ventilatory response to drops in oxygen (Martin et al., 1998; Rigatto et al., 1975). It is characterized by an initial rapid, yet transient, increase in minute ventilation. This initial increase is later followed by a decline in ventilation that often falls well-below baseline breathing. This stereotypical ventilatory response to hypoxia is preserved also in neonatal rodents (Garcia et al., 2017), which have served as useful models for studying brainstem networks involved with the control of breathing and the mechanisms that support the responsiveness of neonatal breathing to hypoxia (Garcia et al., 2017; Viemari et al., 2003).
The rodent respiratory network is distributed throughout the brainstem and is composed of at least three groups of network oscillators: the preBötzinger complex (preBötC)(Anderson and Ramirez, 2017; Del Negro et al., 2018); the retrotrapezoid/parafacial respiratory group (RTN/pFRG)(Guyenet and Mulkey, 2010), and the recently discovered post-inspiratory complex (PiCo) (Anderson et al., 2016). While PiCo has been described in neonate (Anderson et al., 2016), it is unknown whether this network is active in the embryo. However, both the preBötC and RTN/pFRG appear to be active well before birth(Guyenet and Mulkey, 2010; Thoby-Brisson et al., 2005). Inspiratory activity from the brainstem can be recorded from the spinal cord respiratory motor pools as early as embryonic day 16 (E16) (Viemari et al., 2003). Respiratory activity from the embryonic brainstem emerges as a result of interactions between the preBötC and the RTN/pFRG (Champagnat et al., 2011; Tupal et al., 2014). While rhythmicity from the preBötC starts between E15 to E18 (Chevalier et al., 2016a,b; Pagliardini et al., 2003; Thoby-Brisson et al., 2005), activity from RTN/pFRG is present at E14.5 (Thoby-Brisson et al., 2009). During the early postnatal development, RTN/pFRG undergoes a shift in activity from a pre-inspiratory to an inspiratory pattern while preBötC activity remained unchanged (Oku et al., 2007). In juveniles (postnatal day 7 to 13) transection below the RTN/pFRG abolishes active expiration but does not affect inspiration (Janczewski and Feldman, 2006). Thus, while the preBötC is responsible for the inspiratory rhythm, the RTN/pFRG forms the basis of a functionally independent expiratory oscillator (Thoby-Brisson et al., 2009). The RTN/pFRG also contains several cellular constituents such as Phox2b expressing neurons that provide chemosensory drive in response to CO2 and acidification (Guyenet et al., 2005; Stornetta et al., 2006) and astrocytes that contribute to central chemoreception (Mulkey and Wenker, 2011; Wenker et al., 2010). Inspiratory activity at embryonic ages exhibits sensitivity to neuromodulation (Di Pasquale et al., 1994; Fujii and Arata, 2010; Viemari et al., 2003) and can respond to changes in oxygenation (Chevalier et al., 2016a,b; Viemari et al., 2003) indicating that the respiratory network has the potential to be dynamically responsive well-before birth. However, many cellular and physiological aspects of respiratory control continue to develop and change well-after birth (Prabhakar et al., 2007; Stunden et al., 2001; Wong-Riley and Liu, 2005) which leaves the neonate without the full complement of mechanisms to support the stability of adult breathing and may create vulnerabilities during the neonatal period where the responsiveness of breathing is underdeveloped (Rigatto and Brady, 1972).
1.2. Fundamental elements of inspiratory rhythm generation
The preBötC can be isolated in a single brainstem slice preparation and is where the principles and mechanisms underpinning inspiratory rhythmogenesis have been extensively studied (Butera et al., 1999; Koshiya and Smith, 1999; Smith et al., 1991). In the seminal work localizing the preBötC by Smith et al. (Smith et al., 1991), rhythm generation from the inspiratory network was shown to be attenuated in response to microinjection of the AMPA receptor antagonist, CNQX, suggesting that inspiratory rhythmogenesis from the preBötC is largely dependent on excitatory transmission. Subsequent studies examining the basis of rhythm generation in the preBötC have confirmed this observation (Lieske and Ramirez, 2006; Morgado-Valle and Feldman, 2007; Shao et al., 2003) and the necessity of glutamatergic transmission for rhythm generation is a well-accepted key principle. In isolation of fast synaptic transmission, individual preBötC neurons can be categorized as intrinsic bursting neurons, weak/tonic spiking neurons, or silent neurons (Ramirez et al., 2011). Intrinsic bursting neurons in the inspiratory network can be further divided by the underlying con-ductances that drive bursting into either persistent sodium current (INaP) bursters or non-specific cation current (ICAN) bursters (Ramirez et al., 2004). Additional intrinsic and synaptic properties contribute to stabilizing and shaping the phenotype of these general preBötC classes of preBötC neurons, and we refer the reader to the following reviews (Ramirez et al., 2012, 2011; Ramirez et al., 2004) for a more extensive understanding of the cellular and network properties that support rhythmogenesis from the preBötC beyond the peri-hypoxic interval.
1.3. Oxygenation and the preBötC
The isolated preBötC is capable of generating different neural rhythms reminiscent of eupnea, gasping, and sighing (Lieske et al., 2000) and can be revealed by changing the state of oxygenation surrounding the network (Garcia et al., 2017, 2013b; Hill et al., 2011; Lieske et al., 2000). When the inspiratory network becomes initially hypoxic, normal rhythmogenesis (Fig. 1A) increases in frequency and begins to generate augmented bursts (Fig. 1B) while later in hypoxia, augmented bursting ceases and rhythmogenesis slows (Fig. 1C). This initial augmentation followed by depression is remarkably similar to the neonatal ventilatory response to hypoxia (Garcia et al., 2016; Pena et al., 2008). Upon reoxygenation, a paradoxical depression in in vitro rhythmogenesis occurs (Fig. 1D) which is also strikingly similar to post hypoxic ventilatory depression (Coles et al., 1998; Garcia et al., 2013b). The similarities between the stereotypical in vitro response of the rhythm generating network to hypoxia and the stereotypical hypoxic response of neonatal breathing supports the notion that the preBötC is a significant contributor to all phases of breathing during the peri-hypoxic interval.
Fig. 1.
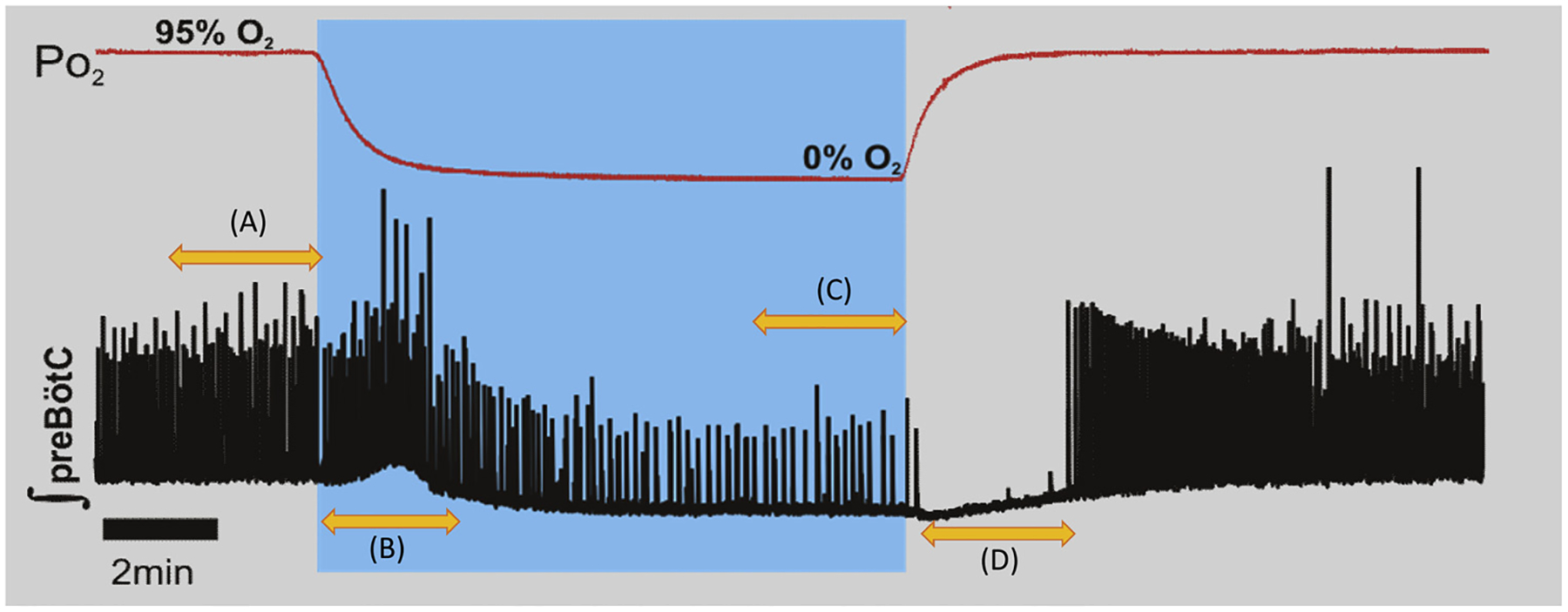
The stereotypical response of the isolated preBötC to hypoxia and reoxygenation. (A) The in vitro preBötC generates a spontaneous rhythm in well-oxygenated conditions (95% O2 5% CO2). (B) Switching from to 95% N2 5% CO2 produces hypoxic conditions as oxygen can still be measured in the circulating media (see Hill et al., 2011). During the initial period of hypoxic exposure, the in vitro rhythm increases in frequency and begins to generate augmented bursts. (C) Later during hypoxia augmented bursting ceases and rhythmogenesis slows. (D) Upon reoxygenation, the preBötC rhythm is initially suppressed delaying post-hypoxic recovery.
Although it is tempting to conclude that the preBötC is responsible for driving changes in the breathing rhythm during transitions in oxygenation, rhythmogenesis from the preBötC appears to stereotypically respond to relative changes in oxygenation as opposed to sensing absolute changes in the oxygen environment (Hill et al., 2011). This observation suggests that the contribution of the preBötC is only responsive to changes in oxygenation and sensory input from outside inspiratory network is still required for normal adult physiology. Indeed, targeted microinjections of colchicine to block axonal transport in the adult preBötC reduces, but does not eliminate, both hypercapnic and hypoxic ventilatory responses (Wu et al., 2005). During the neonatal period when central CO2/H+ chemoreception (Cerpa et al., 2017; Stunden et al., 2001), peripheral O2 (Pawar et al., 2008) and peripheral CO2/H+ (Pepper et al., 1995) chemoreception are still developing, the intrinsic responsiveness of the preBötC to changes in oxygenation may have a greater role in influencing neonatal breathing responses to deviations in blood gases.
1.4. Neuromodulation of rhythmogenesis during hypoxia
Intrinsic properties of individual preBötC neurons play an important role in maintaining hypoxic rhythmogenesis. In particular, INaP-dependent activity appears to be critical for driving the preBötC rhythm under low O2 tensions as riluzole and R56865, INaP blockers, prevent hypoxic rhythmogenesis, but has little effect on rhythmogenesis under well-oxygenated control conditions (Paton et al., 2006; Pena et al., 2004). Intracellular recordings from synaptically isolated preBötC neurons also demonstrate that INaP dependent bursters are intrinsically active during hypoxia while ICAN and weak/tonic spiking neurons are silenced by hypoxia (Pena et al., 2004).
Under well-oxygenated conditions, the majority of preBötC neurons normally exist as weak/tonic spiking neurons while silent and intrinsic bursting neurons only constitute a minority (Carroll and Ramirez, 2013; Carroll et al., 2013; Nieto-Posadas et al., 2014). This composition provides sufficient stability in well-oxygenated states, yet in the presence of enhanced serotonergic (Tryba et al., 2006), substance P-ergic (Pena and Ramirez, 2004), or adrenergic (Viemari and Ramirez, 2006) neuromodulation, weak/tonic spiking neurons can assume a burster phenotype. These observations show that firing phenotype is not a fixed property among all preBötC neurons. Increasing intrinsically bursting neurons throughout the respiratory network may enhance the robustness of rhythm generation when the inspiratory network is burdened. Recent multielectrode studies examining network interaction during hypoxic rhythmogenesis indicate that during the hypoxia, functional connectivity appears to wane when compared to rhythmogenesis under normal conditions (Nieto-Posadas et al., 2014). However, when serotoninergic 2A (Pena and Ramirez, 2002) or alpha-2 adrenergic receptors (Viemari et al., 2011) are blocked during a transition to hypoxia, the loss of neuromodulatory signaling increases the likelihood for failed rhythmogenesis.
in vivo exposure to intermittent hypoxia leads to an increase in catecholaminergic neurons of the ventral lateral medulla and in gene expression of serotonin transporter (Slc6a4) 5-HTT and tryptophan hydroxylase 2, two critical components important to maintenance of serotoninergic neuromodulation (Givan and Cummings, 2016). Furthermore, loss of central serotonergic neuromodulation increases the chance of failed autoresuscitation (Chen et al., 2013), impairs hypoxia-triggered arousal (Young et al., 2017), and increases apneas (Hodges et al., 2009; Young et al., 2017). Combined with the observations made in the inspiratory network, these findings suggest that enhancing neuromodulation is a natural response to the recurrence of hypoxia and may serve as a potential mechanism that defends against unstable breathing by supporting reliable rhythmogenesis in response to changing blood gases.
1.5. SIDS, hypoxia, and post-hypoxic recovery
Sudden infant death syndrome (SIDS) is a diagnosis provided when the death of an infant less than one year of age cannot be explained by other means(Byard, 2018; Ferguson, 2015). The triple-risk model of SIDS first proposed by Filliano and Kinney (Filiano and Kinney, 1994) states that the SIDS emerges as the result of intersection among three factors: a vulnerable infant, a critical developmental period, and an exogenous stressor (i.e., environmental challenge). Biological sex and age are thought to be significant factors that contribute to the first two factors in the triple-risk model of SIDS. Males are more likely to succumb to SIDS (Mage and Donner, 2004, 2014) while the peak occurrence of SIDS is between three and six months (Mage and Donner, 2009). Furthermore, postmortem analysis suggests that deficits in serotonergic neuromodulation may further increase vulnerability to SIDS (Kinney et al., 2001). Thus, if a vulnerable infant encounters even the briefest hypoxic challenge, the threshold for SIDS may be much lower (Ramirez et al., 2018; Thach, 2008). In the sleeping infant, the prone position increases likelihood for unescapable hypoxic exposure and the susceptibility for suffocation due to the potential inability of the infant in repositioning his or her head to prevent rebreathing into the cushion, pillow, or bedding (Erck Lambert et al., 2019). Rebreathing into cushion, pillow, or bedding, will initially lead to hypercapnia and subsequently, if left unchecked hypoxia. Impaired central and peripheral chemoreception have been is largely recognized as significant contributing factors to SIDS occurrence (For review see: (E. B. Gauda et al., 2007; Porzionato et al., 2018)). For example, hyposensitivity of peripheral chemoreception may contribute not only to the blunted ventilatory response to hypoxic hypercapnia, but also may lead to a failure in arousal. Thus, deficits in the arousal response during these hypoxic challenges are thought to contribute to these outcomes (Garcia et al., 2013a). Together hypoxia and the failure to arouse to such challenge may be considered two significant factors contributing to SIDS.
Further supporting the perspective that hypoxic challenge is a significant factor that can lead to infant death, increased rates of both SIDS (Barkin et al., 1981) and unexplained neonatal hospital death (Yangzom et al., 2008) have been documented in high altitude populations. Hypoxia may alter neonatal cardiac maturation, leading to the impaired cardiac conduction, and decreasing the threshold for cardiac arrhythmias and the occurrence of sudden death (Neary et al., 2013). In cases of prematurity, hypoxia may result from underdevelopment of respiratory centers that cause hypoventilation and diaphragmatic malfunction. Other studies have also demonstrated that intrauterine growth restriction via prenatal hypoxia not only leads to a modification in inspiratory rhythm but also alterations in catecholaminergic modulation (Tree et al., 2016). However, recent findings suggest the period of post-hypoxic recovery of breathing and rhythmogenesis from the preBötC may also be a factor contributing to SIDS.
Following hypoxia, rhythmogenesis is initially suppressed but later frequency rebounds (Garcia et al., 2013b; Hill et al., 2011). This biphasic response of preBötC rhythm may contribute to the paradoxical occurrence of post-hypoxic ventilatory depression—a transition period that can increase the chance for unstable breathing and contribute to the risk of SIDS. Both postnatal age and sex appear to be factors that can influence inspiratory rhythmogenesis during reoxygenation (Garcia et al., 2013b). The latency of post-hypoxic recovery in rhythmogenesis from the preBötC increases with postnatal age (Garcia et al., 2013b). During the second week of life, NMDA receptor expression (Liu and Wong-Riley, 2005), GABAA receptor subunit expression (Liu and Wong-Riley, 2006) and chloride homeostasis (Liu and Wong-Riley, 2012) change and may contribute to the prolongation of post-hypoxic recovery. In addition to the prolongation of post-hypoxic recovery, a sex-based difference emerges in the post-hypoxic recovery of preBötC rhythms during the second week of life (Garcia et al., 2013b) whereby post hypoxic depression is prolonged in the preBötC rhythms from males when compared to females. This sex-based difference in post-hypoxic recovery was eliminated by either activating or blocking the ATP-sensitive potassium channel (KATP), suggesting that the dynamic activity of the KATP largely contributes to the post-hypoxic recovery of the rhythmogenesis form the preBötC as opposed to other potential differences such as expression of the channel. Thus, as the activity of KATP is regulated by the availability of ATP, sex-based differences in metabolic activity within the respiratory network may impact the breathing rhythm during peri-hypoxic intervals.
1.6. Reactive oxygen species
As discussed in the previous section, the transition from hypoxia to a well-oxygenated state (i.e., reoxygenation) may be a period of vulnerability for breathing, as respiratory rhythmogenesis has a delayed recovery upon reoxygenation. Many biological systems exhibit an oxidative burst during reoxygenation(Granger and Kvietys, 2015; Spranger et al., 1998) that can cause cellular injury(Granger and Kvietys, 2015) and, in the context of the preBötC, affect stable rhythmogenesis. The oxidative burst results from one or more types of reactive oxygen species (ROS) being produced (Granger and Kvietys, 2015; Halliwell, 1991; Li and Jackson, 2002). During reoxygenation, one type of ROS, superoxide anion, may be produced from the mitochondria (Bao et al., 2009; Gruber et al., 2013) or other cellular processes (Bedard and Krause, 2007; Berry and Hare, 2004). Superoxide anion is rapidly converted, via superoxide dismutase, into hydrogen peroxide (H2O2)(Granger and Kvietys, 2015). H2O2 is another type of ROS and can be further metabolized by catalase or other peroxidases(Granger and Kvietys, 2015). Both superoxide anion and H2O2 have been implicated in cell signaling, but when they accumulate, can also be sources for oxidative stress (Granger and Kvietys, 2015). For example, in presence of a transition metal, like Fe2+, H2O2 is rapidly converted into the short-lived ROS, hydroxyl radical(Granger and Kvietys, 2015). Unlike superoxide anion and H2O2, hydroxyl radical does not act as a signaling molecule but rather, serves as a source of oxidative stress (Halliwell, 1991).
Acute H2O2 suppresses synaptic transmission in both the adult and neonatal hippocampus (Avshalumov and Rice, 2002; Garcia et al., 2011) yet H2O2 differentially affects rhythm generation from the preBötC (Garcia et al., 2011). Application of H2O2 to the in vitro slice triggers a biphasic change in the frequency of rhythmogenesis that is characterized by an initial depression followed by an augmentation phase. This response was remarkably similar to the stereotypical response of the isolated preBötC to reoxygenation from hypoxia and could be traced to induvial neuronal responses to H2O2. The initial depression of rhythmogenesis by H2O2 appears to be the result of oxidative stress, as hydroxyl radical, produced by co-application of Fe2+ and H2O2 (to produce hydroxyl radical) leading to monotonic depression in rhythmic frequency. By contrast, in the presence of the iron chelator, deferox-amine, H2O2 only causes augmentation of rhythmic frequency from the preBötC. The chelation of free iron minimized the conversion of H2O2 to hydroxyl radical, presumably unmasking the action that H2O2 has independent of hydroxyl radical. Indeed, optogenetic stimulation of microglia and hypoxia causes increases in extracellular H2O2 in ventrolateral medullary slices (Pardo-Pena et al., 2018). Thus, rhythmogenesis from the preBötC may be influenced by ROS in a species dependent fashion and such effects may have particular relevance to reoxygenation.
1.7. Intermittent hypoxia
While it may only take a single bout of hypoxia to destabilize breathing, intermittent oscillations in arterial O2 are common to several conditions during neonatal life. To determine how such oscillationsin-fluence neonatal respiratory control, exposure to intermittent hypoxia has been used to experimentally model unstable breathing (Garcia et al., 2017, 2016; Peng et al., 2004; Prabhakar et al., 2007; Zanella et al., 2014). Studies in the preBötC have used both acute intermittent hypoxia and chronic intermittent hypoxia to understand the consequences of intermittent hypoxia versus hypoxia on inspiratory rhythmogenesis relevant to conditions where neonatal apneas are evident(Garcia et al., 2016; Zanella et al., 2014).
1.8. Acute intermittent hypoxia
Neonatal abstinence syndrome (NAS) is a condition where the neonatal infant experiences drug withdrawal due to maternal narcotic use or abuse during pregnancy (Kocherlakota, 2014; “Neonatal drug withdrawal. American Academy of Pediatrics Committee on Drugs, 1998). As many drugs readily pass from the maternal blood stream through the placenta to the fetus(Ross et al., 2015), illicit drug abuse during pregnancy can cause the fetus to become addicted to the substance(s) used by the mother (Behnke et al., 2013). As a result, the sudden discontinuation of fetal exposure to substances that were used or abused by the mother during pregnancy commonly lead to withdrawal symptoms in the newborn and is associated with increased adrenergic tone (Oji-Mmuo et al., 2019) and disrupted serotonergic tone (Lunden and Kirby, 2013). Such departures from the normal neuromodulatory state could increase the risk of unstable breathing and oscillations in blood gases. Indeed, in premature infants suffering from NAS, intermittent hypoxemic episodes can worsen (Abu Jawdeh et al., 2017).
At the level of the preBötC, acute intermittent hypoxia (IH) produces the stereotypical responses of rhythmogenesis during each cycle of hypoxia and reoxygenation (Blitz and Ramirez, 2002; Zanella et al., 2014) and leads to long-lasting augmentation in the frequency of rhythmogenesis during the post-hypoxic period (Blitz and Ramirez, 2002; Camacho-Hernandez et al., 2018). Acute IH also has little impact on the transmission of the premotor rhythm originating from the preBötC and generated by the hypoglossal motor pool demonstrating the robustness of the network to repeated changes in oxygenation (Zanella et al., 2014). However, when combined with enhanced adrenergic tone, acute IH can cause long-lasting instabilities in inspiratory rhythmogenesis.
Enhanced adrenergic tone co-incidental with acute IH leads to the emergence of post-hypoxic subnetwork bursting in the preBötC (Zanella et al., 2014). This subnetwork bursting in the premotor network fails to produce the motor bursts in the hypoglossal motor nucleus and subsequently, enhances irregularities both in the preBötC rhythm and inspiratory motor rhythm. This appears to be the result of functional remodeling that involves changes in glycinergic inhibition and activation ofalpha 2 adrenergic receptors (Zanella et al., 2014). Thus, increased adrenergic tone and acute IH may serve to perpetuate perinatal respiratory instability causing the preBötC to enter into an unstable state where synaptic inhibition disrupts transmission from the premotor network to respiratory motor pools.
1.9. Chronic intermittent hypoxia
The occurrence of intermittent hypoxia during perinatal life is often experienced over the course of days and even weeks. For the majority of infants (< 30 weeks of gestation) suffering from apneas of prematurity, chronic intermittent hypoxemia associated with the condition does not diminish until after the 40th week of gestation (Eichenwald and Committee on, F., and Newborn, A. A. o. P., 2016). Perinatal exposure to chronic intermittent hypoxia (IH) enhances the neonatal ventilatory response to acute hypoxia (Garcia et al., 2016; Peng et al., 2004). The effects of chronic IH on neonatal ventilation involve both enhanced peripheral O2 chemosensitivity of the carotid bodies (Peng et al., 2004; Prabhakar et al., 2007) and changed inspiratory rhythmogenesis from the preBötC (Garcia et al., 2017, 2016). Chronic IH impacts inspiratory rhythmogenesis in a state dependent manner. Following exposure to chronic IH, excitability during a network burst is reduced among individual inspiratory neurons and is co-incidental with a loss of fidelity of individual neurons with the network rhythm (Garcia et al., 2016). These cellular effects on preBötC neurons result in increased short-term variability of both amplitude and period of the network rhythm and consequently increases transmission failure of the preBötC rhythm to the hypoglossal motor pool in local respiratory circuit. Biochemically, chronic IH increases oxidative stress in the preBötC and irregular rhythmogenesis and transmission failure can be mitigated by administration of the antioxidant, manganese(III) tetrakis(1-methyl-4-pyridyl) porphyrin (MnTMPyP), during neonatal chronic IH exposure.
Neonatal CIH causes ROS-dependent endothelin signaling to augment the hypoxic response of the carotid bodies (Pawar et al., 2009). Similarly, in the adrenal medulla, neonatal chronic IH leads to a ROS-mediated suppression of multiple nicotinic receptor subunits which thereby impairs nicotinic receptor mediated activation of the adrenal medulla (Souvannakitti et al., 2010) and can last into adulthood (Souvannakitti et al., 2009). Thus, the role of chronic IH-dependent ROS in remodeling cellular and physiological activity does not appear to be a unique feature at the level of the preBötC but rather, is evident throughout the neonatal nervous system.
In adults, chronic IH causes oxidative stress that also potentiates the activity from the carotid bodies (Del Rio et al., 2010; Peng et al., 2006) and may be an upstream contributor to the pro-inflammatory state caused by chronic IH that promotes the potentiation of the carotid body (Del Rio et al., 2012) and the hypoxic central chemoreflex pathway (Oyarce & Iturriaga, 2018). Increased hypoxia inducible factor 1a signaling (HIF1a) in the nervous system is upstream of oxidative stress produced by chronic IH (Peng et al., 2006). Although it is unknown whether chronic IH causes increased HIF1a signaling in the preBötC, recent transcriptional analysis of neonatal preBötC neuronshas revealed that HIF1a is expressed approximately three times higher in glutamatergic Dbx1 preBötC neurons than in non-Dbx1 neurons (Hayes et al., 2017). These observations suggest that chronic IH may indeed increase HIF1a signaling in the preBötC but this remains to be demonstrated. Should chronic IH increase HIF1a signaling in the preBötC, the role of such signaling remains to be determined. On one hand, HIF1a signaling may serve to be protective promoting survival during hypoxia, while on the other, increased HIF1a signaling due to oxidative stress may serve to promote a pro-oxidant state (Semenza and Prabhakar, 2007) to destabilize function in inspiratory brain circuit. However, the protective role and the pro-oxidant role for IH-dependent HIF1a signaling may not be mutually exclusive given the potential for reactive oxygen species to modulate rhythmogenesis (Garcia et al., 2011).
2. Conclusion
Neonatal breathing possesses the capability to respond to hypoxia but is often still vulnerable to disturbances that cause repeated oscillations in blood oxygen. Such destabilization can arise from a host of conditions, yet hypoxic challenge and unstable breathing must be overcome by the neonate in order to survive. While several parts of the nervous system contribute the control over breathing, inspiratory rhythm generation from the preBötC appears to emerge well-before full gestational age. The cellular and network properties of the inspiratory network normally permit a robust response to hypoxic challenge yet the transitions from well-oxygenated states to hypoxia and back reveal vulnerabilities in rhythm generation relevant to understanding conditions such as SIDS. Moreover, the response to recurrent hypoxia and reoxygenation, whether acute orchronic, reveals a hysteresis in rhythmogenesis from preBötC which may serve to perpetuate unstable breathing when respiratory control is underdeveloped. Even if hypoxic insult during neonatal life does not result in sudden death, exposure to hypoxia early in life may have consequences that are not yet fully understood. This underscores the importance for continued the study of respiratory control and the premotor circuits that drive breathing. Pinpointing critical vulnerabilities in the control of breathing, whether to under-development or to pathophysiology may facilitate novel therapies to better mitigate or even prevent neonatal hypoxic insult and its negative consequences.
Funding
This work was supported by a grant from The BSD Office of Diversity & Inclusion at The University of Chicago awarded to AJG, by a NIH R01 NS10742101 awarded to AJG and by NIH P01 HL144454-01A1.
References
- Abu Jawdeh EG, Westgate PM, Pant A, Stacy AL, Mamilla D, Gabrani A,Giannone P, et al. , 2017. Prenatal Opioid Exposure and Intermittent Hypoxemiain Preterm Infants: ARetrospective Assessment. Front. Pediatr 5, 253 10.3389/fped.2017.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro RE, Rigatto H, 2016. Control of breathing in newborns In: Buonocore G, Bracci R, Weindling M (Eds.), Neonatology: A Practical Approach to Neonatal Diseases. Springer International Publishing, Cham, pp. 1–16. [Google Scholar]
- Anderson TM, Garcia AJ 3rd, Baertsch NA, Pollak J, Bloom JC, Wei AD, Ramirez JM, et al. , 2016. A novel excitatory network for the control of breathing. Nature 536 (7614), 76–80. 10.1038/nature18944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TM, Ramirez JM, 2017. Respiratory rhythm generation: triple oscillator hypothesis. F1000Res 6, 139 10.12688/f1000research.10193.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Rice ME, 2002. NMDA receptor activation mediates hydrogen peroxide-induced pathophysiology in rat hippocampal slices. J. Neurophysiol 87 (6), 2896–2903. 10.1152/jn.2002.87.6.2896. [DOI] [PubMed] [Google Scholar]
- Bancalari E, Claure N, 2018. Respiratory instability and hypoxemia episodes in preterm infants. Am. J. Perinatol 35 (6), 534–536. 10.1055/s-0038-1637760. [DOI] [PubMed] [Google Scholar]
- Bao L, Avshalumov MV, Patel JC, Lee CR, Miller EW, Chang CJ, Rice ME, 2009. Mitochondria are the source of hydrogen peroxide for dynamic brain-cell signaling. J. Neurosci 29 (28), 9002–9010. 10.1523/JNEUROSCI.1706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkin RM, Hartley MR, Brooks JG, 1981. Influence of high altitude on sudden infant death syndrome. Pediatrics 68 (6), 891–892. [PubMed] [Google Scholar]
- Barlow SM, Estep M, 2006. Central pattern generation and the motor infrastructure for suck, respiration, and speech. J. Commun. Disord 39 (5), 366–380. 10.1016/j.jcomdis.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH, 2007. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev 87 (1), 245–313. 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Behnke M, Smith VC, Committee on Substance, A, Committee on, F, Newborn, 2013. Prenatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics 131 (3), e1009–e1024. 10.1542/peds.2012-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC, 1998. Driving respiration: the respiratory central pattern generator. Clin. Exp. Pharmacol. Physiol 25 (10), 847–856. [DOI] [PubMed] [Google Scholar]
- Berry CE, Hare JM, 2004. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J. Physiol. (Paris) 555 (Pt 3), 589–606. 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Ramirez JM, 2002. Long-term modulation of respiratory network activity following anoxia in vitro. J. Neurophysiol 87 (6), 2964–2971. 10.1152/jn.2002.87.6.2964. [DOI] [PubMed] [Google Scholar]
- Braman SS, 1995. The regulation of normal lung function. Allergy Proc. 16 (5), 223–226. [DOI] [PubMed] [Google Scholar]
- Butera RJ Jr., Rinzel J, Smith JC, 1999. Models of respiratory rhythm generation in the pre-Botzinger complex. I. Bursting pacemaker neurons. J. Neurophysiol 82 (1), 382–397. 10.1152/jn.1999.82.1.382. [DOI] [PubMed] [Google Scholar]
- Byard RW, 2018. The autopsy and pathology of sudden infant death syndrome In: Duncan JR, Byard RW (Eds.), SIDS Sudden Infant and Early Childhood Death: The Past, the Present and the Future. Adelaide (AU). [PubMed] [Google Scholar]
- Camacho-Hernandez NP, Lorea-Hernandez JJ, Pena-Ortega F, 2018. Microglial modulators reduce respiratory rhythm long-term facilitation in vitro. Respir. Physiol. Neurobiol 10.1016/j.resp.2018.07.012. [DOI] [PubMed] [Google Scholar]
- Carroll MS, Ramirez JM, 2013. Cycle-by-cycle assembly of respiratory network activity is dynamic and stochastic. J. Neurophysiol 109 (2), 296–305. 10.1152/jn.00830.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MS, Viemari JC, Ramirez JM, 2013. Patterns of inspiratory phase-dependent activity in the in vitro respiratory network. J. Neurophysiol 109 (2), 285–295. 10.1152/jn.00619.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerpa VJ, Wu Y, Bravo E, Teran FA, Flynn RS, Richerson GB, 2017. Medullary 5-HT neurons: switch from tonic respiratory drive to chemoreception during postnatal development. Neuroscience 344, 1–14. 10.1016/j.neuroscience.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagnat J, Morin-Surun MP, Bouvier J, Thoby-Brisson M, Fortin G, 2011. Prenatal development of central rhythm generation. Respir. Physiol. Neurobiol 178 (1), 146–155. 10.1016/j.resp.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Chen J, Magnusson J, Karsenty G, Cummings KJ, 2013. Time- and age-dependent effects of serotonin on gasping and autoresuscitation in neonatal mice. J Appl Physiol (1985) 114 (12), 1668–1676. 10.1152/japplphysiol.00003.2013. [DOI] [PubMed] [Google Scholar]
- Chevalier M, De Sa R, Cardoit L, Thoby-Brisson M, 2016a. Mechanisms underlying adaptation of respiratory network activity to modulatory stimuli in the mouse embryo. Neural Plast, 2016, 3905257 10.1155/2016/3905257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier M, Toporikova N, Simmers J, Thoby-Brisson M, 2016b. Development of pacemaker properties and rhythmogenic mechanisms in the mouse embryonic respiratory network. Elife 5 10.7554/eLife.16125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles SK, Ernsberger P, Dick TE, 1998. Post-hypoxic frequency decline does not depend on alpha2-adrenergic receptors in the adult rat. Brain Res. 794 (2), 267–273. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Funk GD, Feldman JL, 2018. Breathing matters. Nat. Rev. Neurosci 19 (6), 351–367. 10.1038/s41583-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio R, Moya EA, Iturriaga R, 2010. Carotid body and cardiorespiratory alterations in intermittent hypoxia: the oxidative link. Eur. Respir. J 36 (1), 143–150. 10.1183/09031936.00158109. [DOI] [PubMed] [Google Scholar]
- Del Rio R, Moya EA, Parga MJ, Madrid C, Iturriaga R, 2012. Carotid body inflammation and cardiorespiratory alterations in intermittent hypoxia. Eur. Respir. J 39 (6), 1492–1500. 10.1183/09031936.00141511. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Monteau R, Hilaire G, 1994. Involvement of the rostral ventro-lateral medulla in respiratory rhythm genesis during the peri-natal period: an in vitro study in newborn and fetal rats. Brain Res. Dev. Brain Res 78 (2), 243–252. [DOI] [PubMed] [Google Scholar]
- Eichenwald EC, Committee on F, & Newborn A. A. o. P, 2016. Apnea of prematurity. Pediatrics 137 (1). 10.1542/peds.2015-3757. [DOI] [PubMed] [Google Scholar]
- Lambert Erck, Parks AB, Cottengim SE,C, Faulkner M, Hauck FR, ShapiroMendoza CK, 2019. Sleep-related infant suffocation deaths attributable to Soft bedding, overlay, and wedging. Pediatrics 143 (5). 10.1542/peds.2018-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AH, 2015. Ignored disease or diagnostic dustbin? Sudden infant death syndrome in the british context. Soc. Hist. Med 28 (3), 487–508. 10.1093/shm/hkv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano JJ, Kinney HC, 1994. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol. Neonate 65 (3–4), 194–197. 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- Fujii M, Arata A, 2010. Adrenaline modulates on the respiratory network development. Adv. Exp. Med. Biol 669, 25–28. 10.1007/978-1-4419-5692-7_5. [DOI] [PubMed] [Google Scholar]
- Garcia AJ 3rd, Dashevskiy T, Khuu MA, Ramirez JM, 2017. Chronic intermittent hypoxia differentially impacts different states of inspiratory activity at the level of the preBotzinger complex. Front. Physiol 8, 571 10.3389/fphys.2017.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AJ 3rd, Khan SA, Kumar GK, Prabhakar NR, Ramirez JM, 2011. Hydrogen peroxide differentially affects activity in the pre-Botzinger complex and hippocampus. J. Neurophysiol 106 (6), 3045–3055. 10.1152/jn.00550.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AJ 3rd, Koschnitzky JE, Ramirez JM, 2013a. The physiological determinants of sudden infant death syndrome. Respir. Physiol. Neurobiol 189 (2), 288–300. 10.1016/j.resp.2013.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AJ 3rd, Rotem-Kohavi N, Doi A, Ramirez JM, 2013b. Post-hypoxic recovery of respiratory rhythm generation is gender dependent. PLoS One 8 (4), e60695 10.1371/journal.pone.0060695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AJ 3rd, Zanella S, Dashevskiy T, Khan SA, Khuu MA, Prabhakar NR, Ramirez JM, 2016. Chronic intermittent hypoxia alters local respiratory circuit function at the level of the preBotzinger complex. Front. Neurosci 10, 4 10.3389/fnins.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauda EB, Cristofalo E, Nunez J, 2007. Peripheral arterial chemoreceptors and sudden infant death syndrome. Respir. Physiol. Neurobiol 157 (1), 162–170. 10.1016/j.resp.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Gauda EB, Martin RJ, 2012. Control of Breathing. Elsevier, pp. 584–597. [Google Scholar]
- Givan SA, Cummings KJ, 2016. Intermittent severe hypoxia induces plasticity within serotonergic and catecholaminergic neurons in the neonatal rat ventrolateral medulla. J Appl Physiol (1985) 120 (11), 1277–1287. 10.1152/japplphysiol.00048.2016. [DOI] [PubMed] [Google Scholar]
- Granger DN, Kvietys PR, 2015. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 6, 524–551. 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, 2003. The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci 4 (7), 573–586. 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Gross TL, Sokol RJ, Kwong MS, Wilson M, Kuhnert PM, 1983. Transient tachypnea of the newborn: the relationship to preterm delivery and significant neonatal morbidity. Am. J. Obstet. Gynecol 146 (3), 236–241. 10.1016/00029378(83)90742-1. [DOI] [PubMed] [Google Scholar]
- Gruber J, Fong S, Chen CB, Yoong S, Pastorin G, Schaffer S, Halliwell B, et al. , 2013. Mitochondria-targeted antioxidants and metabolic modulators as pharmacological interventions to slow ageing. Biotechnol. Adv 31 (5), 563–592. 10.1016/j.biotechadv.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, 2010. Retrotrapezoid nucleus and parafacial respiratory group. Respir. Physiol. Neurobiol 173 (3), 244–255. 10.1016/j.resp.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA, 2005. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J. Neurosci 25 (39), 8938–8947. 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, 1991. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am. J. Med 91 (3C), 14S–22S. [DOI] [PubMed] [Google Scholar]
- Hayes JA, Kottick A, Picardo MCD, Halleran AD, Smith RD, Smith GD, Del Negro CA, et al. , 2017. Transcriptome of neonatal preBotzinger complex neurones in Dbx1 reporter mice. Sci. Rep 7 (1), 8669 10.1038/s41598-01709418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AA, Garcia AJ 3rd, Zanella S, Upadhyaya R, Ramirez JM, 2011. Graded reductions in oxygenation evoke graded reconfiguration of the isolated respiratory network. J. Neurophysiol 105 (2), 625–639. 10.1152/jn.00237.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman NH, Kallapur SG, Jobe AH, 2012. Physiology of transition from intrauterine to extrauterine life. Clin. Perinatol 39 (4), 769–783. 10.1016/j.clp.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB, 2009. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J. Neurosci 29 (33), 10341–10349. 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard JL, Shingleton HM, 1985. Sexual function of patients after cancer of the cervix treatment. Clin. Obstet. Gynaecol 12 (1), 247–264. [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL, 2006. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J. Physiol. (Paris) 570 (Pt 2), 407–420. 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Filiano JJ, White WF, 2001. Medullary serotonergic network deficiency in the sudden infant death syndrome: review of a 15-year study of a single dataset. J. Neuropathol. Exp. Neurol 60 (3), 228–247. [DOI] [PubMed] [Google Scholar]
- Kocherlakota P, 2014. Neonatal abstinence syndrome. Pediatrics 134 (2), e547–561. 10.1542/peds.2013-3524. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Smith JC, 1999. Neuronal pacemaker for breathing visualized in vitro. Nature 400 (6742), 360–363. 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- Li C, Jackson RM, 2002. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am. J. Physiol., Cell Physiol 282 (2), C227–C241. 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Ramirez JM, 2006. Pattern-specific synaptic mechanisms in a multifunctional network. I. Effects of alterations in synapse strength. J. Neurophysiol 95 (3), 1323–1333. 10.1152/jn.00505.2004. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM, 2000. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps [see comment]. Nat. Neurosci 3 (6), 600–607. 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT, 2005. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol (1985) 98 (4), 1442–1457. 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT, 2006. Developmental changes in the expression of GABAA receptor subunits alpha1, alpha2, and alpha3 in brain stem nuclei of rats. Brain Res. 1098 (1), 129–138. 10.1016/j.brainres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT, 2012. Postnatal development of Na(+)-K(+)-2Cl(−) co-transporter 1 and K(+)-Cl(−) co-transporter 2 immunoreactivity in multiple brain stem respiratory nuclei of the rat. Neuroscience 210, 1–20. 10.1016/j.neuroscience.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunden JW, Kirby LG, 2013. Opiate exposure and withdrawal dynamically regulate mRNA expression in the serotonergic dorsal raphe nucleus. Neuroscience 254, 160–172. 10.1016/j.neuroscience.2013.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mage DT, Donner EM, 2004. The fifty percent male excess of infant respiratory mortality. Acta Paediatr. 93 (9), 1210–1215. [PubMed] [Google Scholar]
- Mage DT, Donner EM, 2014. Is excess male infant mortality from sudden infant death syndrome and other respiratory diseases X-linked? Acta Paediatr. 103 (2), 188–193. 10.1111/apa.12482. [DOI] [PubMed] [Google Scholar]
- Mage DT, Donner M, 2009. A unifying theory for SIDS. Int J Pediatr, 2009, 368270 10.1155/2009/368270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RJ, DiFiore JM, Jana L, Davis RL, Miller MJ, Coles SK, Dick TE, 1998. Persistence of the biphasic ventilatory response to hypoxia in preterm infants. J. Pediatr 132 (6), 960–964. [DOI] [PubMed] [Google Scholar]
- Morgado-Valle C, Feldman JL, 2007. NMDA receptors in preBotzinger complex neurons can drive respiratory rhythm independent of AMPA receptors. J. Physiol. (Paris) 582 (Pt 1), 359–368. 10.1113/jphysiol.2007.130617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Wenker IC, 2011. Astrocyte chemoreceptors: mechanisms of H+ sensing by astrocytes in the retrotrapezoid nucleus and their possible contribution to respiratory drive. Exp. Physiol 96 (4), 400–406. 10.1113/expphysiol.2010.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary MT, Mohun TJ, Breckenridge RA, 2013. A mouse model to study the link between hypoxia, long QT interval and sudden infant death syndrome. Dis. Model. Mech 6 (2), 503–507. 10.1242/dmm.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neonatal drug withdrawal. American Academy of Pediatrics Committee on Drugs, 1998. Pediatrics 101 (6), 1079–1088. [PubMed] [Google Scholar]
- Nieto-Posadas A, Flores-Martinez E, Lorea-Hernandez JJ, Rivera-Angulo AJ, PerezOrtega JE, Bargas J, Pena-Ortega F, 2014. Change in network connectivity during fictive-gasping generation in hypoxia: prevention by a metabolic intermediate. Front. Physiol 5, 265 10.3389/fphys.2014.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oji-Mmuo CN, Speer RR, Gardner FC, Marvin MM, Hozella AC, Doheny KK, 2019. Prenatal opioid exposure heightens sympathetic arousal and facial expressions of pain/distress in term neonates at 24–48 hours post birth. J. Matern. Fetal. Neonatal. Med 1–181. 10.1080/14767058.2019.1588876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku Y, Masumiya H, Okada Y, 2007. Postnatal developmental changes in activation profiles of the respiratory neuronal network in the rat ventral medulla. J. Physiol. (Paris) 585 (Pt 1), 175–186. 10.1113/jphysiol.2007.138180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarce MP, Iturriaga R, 2018. Proinflammatory cytokines in the nucleus of the solitary tract of hypertensive rats exposed to chronic intermittent hypoxia. Adv. Exp. Med. Biol 1071, 69–74. 10.1007/978-3-319-91137-3_8. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Ren J, Greer JJ, 2003. Ontogeny of the pre-Botzinger complex in perinatal rats. J. Neurosci 23 (29), 9575–9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Pena K, Lorea-Hernandez JJ, Camacho-Hernandez NP, Ordaz B, VillasanaSalazar B, Morales-Villagran A, Pena-Ortega F, 2018. Hydrogen peroxide extracellular concentration in the ventrolateral medulla and its increase in response to hypoxia in vitro: possible role of microglia. Brain Res. 1692, 87–99. 10.1016/j.brainres.2018.04.032. [DOI] [PubMed] [Google Scholar]
- Paton JF, Abdala AP, Koizumi H, Smith JC, St-John WM, 2006. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat. Neurosci 9 (3), 311–313. 10.1038/nn1650. [DOI] [PubMed] [Google Scholar]
- Pawar A, Nanduri J, Yuan G, Khan SA, Wang N, Kumar GK, Prabhakar NR, 2009. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol 296 (3), R735–R742. 10.1152/ajpregu.90490.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar A, Peng YJ, Jacono FJ, Prabhakar NR, 2008. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol (1985) 104 (5), 1287–1294. 10.1152/japplphysiol.00644.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Meza-Andrade R, Paez-Zayas V, Gonzalez-Marin MC, 2008. Gasping generation in developing Swiss-Webster mice in vitro and in vivo. Neurochem. Res 33 (8), 1492–1500. 10.1007/s11064-008-9616-x. [DOI] [PubMed] [Google Scholar]
- Pena F, Parkis MA, Tryba AK, Ramirez JM, 2004. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron 43 (1), 105–117. 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM, 2002. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J. Neurosci 22 (24), 11055–11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Ramirez JM, 2004. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J. Neurosci 24 (34), 7549–7556. 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Rennison J, Prabhakar NR, 2004. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J Appl Physiol (1985) 97 (5), 2020–2025. 10.1152/japplphysiol.00876.2003. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Prabhakar NR, et al. , 2006. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J. Physiol. (Paris) 577 (Pt 2), 705–716. 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper DR, Landauer RC, Kumar P, 1995. Postnatal development of CO2-O2 interaction in the rat carotid body in vitro. J. Physiol. (Paris) 485 (Pt 2), 531–541. 10.1113/jphysiol.1995.sp020749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzionato A, Macchi V, De Caro R, 2018. Central and peripheral chemoreceptors in sudden infant death syndrome. J. Physiol. (Paris) 596 (15), 3007–3019. 10.1113/JP274355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Peng YJ, Kumar GK, Pawar A, 2007. Altered carotid body function by intermittent hypoxia in neonates and adults: relevance to recurrent apneas. Respir. Physiol. Neurobiol 157 (1), 148–153. 10.1016/j.resp.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Doi A, Garcia AJ 3rd, Elsen FP, Koch H, Wei AD, 2012. The cellular building blocks of breathing. Compr. Physiol 2 (4), 2683–2731. 10.1002/cphy.c110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Koch H, Garcia AJ 3rd, Doi A, Zanella S, 2011. The role of spiking and bursting pacemakers in the neuronal control of breathing. J. Biol. Phys 37 (3), 241–261. 10.1007/s10867-011-9214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Ramirez SC, Anderson TM, 2018. Sudden infant death syndrome, sleep, and the physiology and pathophysiology of the respiratory network In: Duncan JR, Byard RW (Eds.), SIDS Sudden Infant and Early Childhood Death: The Past, the Present and the Future. Adelaide (AU).. [PubMed] [Google Scholar]
- Ramirez JM, Tryba AK, Pena F, 2004. Pacemaker neurons and neuronal networks: an integrative view. Curr. Opin. Neurobiol 14 (6), 665–674. 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Reuter S, Moser C, Baack M, 2014. Respiratory distress in the newborn. Pediatr. Rev 35 (10), 417–428. 10.1542/pir.35-10-417. quiz 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigatto H, Brady JP, 1972. Periodic breathing and apnea in preterm infants. II. Hypoxia as a primary event. Pediatrics 50 (2), 219–228. [PubMed] [Google Scholar]
- Rigatto H, Brady JP, de la Torre Verduzco R, 1975. Chemoreceptor reflexes in preterm infants: I. The effect of gestational and postnatal age on the ventilatory response to inhalation of 100% and 15% oxygen. Pediatrics 55 (5), 604–613. [PubMed] [Google Scholar]
- Ross EJ, Graham DL, Money KM, Stanwood GD, 2015. Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacology 40 (1), 61–87. 10.1038/npp.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Prabhakar NR, 2007. HIF-1-dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid. Redox Signal 9 (9), 1391–1396. 10.1089/ars.2007.1691. [DOI] [PubMed] [Google Scholar]
- Shao XM, Ge Q, Feldman JL, 2003. Modulation of AMPA receptors by cAMP-dependent protein kinase in preBotzinger complex inspiratory neurons regulates respiratory rhythm in the rat. J. Physiol. (Paris) 547 (Pt 2), 543–553. 10.1113/jphysiol.2002.031005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL, 1991. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254 (5032), 726–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souvannakitti D, Kumar GK, Fox A, Prabhakar NR, 2009. Neonatal intermittent hypoxia leads to long-lasting facilitation of acute hypoxia-evoked catecholamine secretion from rat chromaffin cells. J. Neurophysiol 101 (6), 2837–2846. 10.1152/jn.00036.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souvannakitti D, Kuri B, Yuan G, Pawar A, Kumar GK, Smith C, Prabhakar NR, et al. , 2010. Neonatal intermittent hypoxia impairs neuronal nicotinic receptor expression and function in adrenal chromaffin cells. Am. J. Physiol., Cell Physiol 299 (2), C381–C388. 10.1152/ajpcell.00159.201010.1152/ajpcell.00530.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger M, Kiprianova I, Krempien S, Schwab S, 1998. Reoxygenation increases the release of reactive oxygen intermediates in murine microglia. J. Cereb. Blood Flow Metab 18 (6), 670–674. 10.1097/00004647-199806000-00009. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Guyenet PG, et al. , 2006. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J. Neurosci 26 (40), 10305–10314. 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW, 2001. Development of in vivo ventilatory and single chemosensitive neuron responses to hypercapnia in rats. Respir. Physiol 127 (2–3), 135–155. [DOI] [PubMed] [Google Scholar]
- Thach B, 2008. Tragic and sudden death. Potential and proven mechanisms causing sudden infant death syndrome. EMBO Rep. 9 (2), 114–118. 10.1038/sj.embor.7401163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G, 2009. Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex. Nat. Neurosci 12 (8), 1028–1035. 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Trinh JB, Champagnat J, Fortin G, 2005. Emergence of the pre-Botzinger respiratory rhythm generator in the mouse embryo. J. Neurosci 25 (17), 4307–4318. 10.1523/JNEUROSCI.0551-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree K, Viemari JC, Cayetanot F, Peyronnet J, 2016. Growth restriction induced by chronic prenatal hypoxia affects breathing rhythm and its pontine catecholaminergic modulation. J. Neurophysiol 116 (4), 1654–1662. 10.1152/jn.00869.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM, 2006. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J. Neurosci 26 (10), 2623–2634. 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupal S, Huang WH, Picardo MC, Ling GY, Del Negro CA, Zoghbi HY, Gray PA, 2014. Atoh1-dependent rhombic lip neurons are required for temporal delay between independent respiratory oscillators in embryonic mice. Elife 3, e02265 10.7554/eLife.02265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Burnet H, Bevengut M, Hilaire G, 2003. Perinatal maturation of the mouse respiratory rhythm-generator: in vivo and in vitro studies. Eur. J. Neurosci 17 (6), 1233–1244. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Garcia AJ 3rd, Doi A, Ramirez JM, 2011. Activation of alpha-2 noradrenergic receptors is critical for the generation of fictive eupnea and fictive gasping inspiratory activities in mammals in vitro. Eur. J. Neurosci 33 (12), 2228–2237. 10.1111/j.1460-9568.2011.07706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Ramirez JM, 2006. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J. Neurophysiol 95 (4), 2070–2082. 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- Waggener TB, Southall DP, Scott LA, 1990. Analysis of breathing patterns in a prospective population of term infants does not predict susceptibility to sudden infant death syndrome. Pediatr. Res 27 (2), 113–117. 10.1203/00006450199002000-00002. [DOI] [PubMed] [Google Scholar]
- Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK, 2010. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J. Neurophysiol 104 (6), 3042–3052. 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MT, Liu Q, 2005. Neurochemical development of brain stem nuclei involved in the control of respiration. Respir. Physiol. Neurobiol 149 (1–3), 83–98. 10.1016/j.resp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wu M, Haxhiu MA, Johnson SM, 2005. Hypercapnic and hypoxic responses require intact neural transmission from the pre-Botzinger complex. Respir. Physiol. Neurobiol 146 (1), 33–46. 10.1016/j.resp.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Yangzom Y, Qian L, Shan M, La Y, Meiduo D, Hu X, Zetterstrom R, et al. , 2008. Outcome of hospital deliveries of women living at high altitude: a study from Lhasa in Tibet. Acta Paediatr. 97 (3), 317–321. 10.1111/j.1651-2227.2008.00628.x. [DOI] [PubMed] [Google Scholar]
- Young JO, Geurts A, Hodges MR, Cummings KJ, 2017. Active sleep unmasks apnea and delayed arousal in infant rat pups lacking central serotonin. J Appl Physiol (1985) 123 (4), 825–834. 10.1152/japplphysiol.00439.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella S, Doi A, Garcia AJ 3rd, Elsen F, Kirsch S, Wei AD, Ramirez JM, 2014. When norepinephrine becomes a driver of breathing irregularities: how intermittent hypoxia fundamentally alters the modulatory response of the respiratory network. J. Neurosci 34 (1), 36–50. 10.1523/JNEUROSCI.3644-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


