Abstract
Background
Olfactory dysfunction (OD) refers to a reduced or absent ability to smell. OD negatively impacts health and quality of life and its prevalence increases with advancing age. Since OD may be an early marker of dementia and impending death, more knowledge regarding risk factors of OD in aging is warranted. The objective was therefore to explore longitudinally which demographic, genetic, clinical, lifestyle, and cognitive factors predict the development of OD.
Methods
The study included participants aged 60–90 years from the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K), who did not have OD at baseline and were reassessed with an odor identification task at a 6-year follow-up (n = 1,004). Risk factors of OD were assessed with multivariable logistic regression analyses.
Results
The percentage of incident OD cases was 14.2% over 6 years in the total sample and this number increased monotonically with age. Increasing age, carrying the ε4 allele of the APOE gene, atrial fibrillation, cerebrovascular disease, and current smoking were found to be risk factors for the development of OD, whereas better olfactory identification and verbal episodic memory proficiency at baseline were identified as protective factors.
Conclusions
In addition to nonmodifiable factors (age and genetic risk), several modifiable risk factors of OD were identified. This suggests that it might be possible to reduce OD incidence through the management of vascular risk factors and maintenance of a healthy lifestyle.
Keywords: Olfactory impairment, Epidemiology, Sensory, Longitudinal design
Olfactory dysfunction (OD), absence or reduced ability to smell, is a common condition in old age, negatively impacting health and quality of life (1). The prevalence of OD typically ranges between 20% and 30% in older adults, and becomes more prevalent with increasing age (2–4). To illustrate, prevalence rates above 60% have been reported for 90-year olds (2,3). Older individuals with OD commonly report perceiving food as flavorless, and having a reduced appetite, which may lead to underweight, poor health, and frailty (1). In addition, OD is associated with reduced safety (eg, food poisoning, inability to detect gas leaks) and increased mortality (5–7). The evidence further suggests that OD might be an indicator of accelerated brain aging, as a number of reports indicate that olfactory loss is associated with cognitive decline and impending dementia (8,9). For these reasons, it is of interest to gain further knowledge regarding the nature and development of OD in old age.
Current evidence on OD is almost exclusively based on cross-sectional studies conducted on different age cohorts. In order to assess how olfactory abilities change during aging, and to determine concomitant factors of OD development, longitudinal prospective measurements are needed. To date, only a few longitudinal studies have focused on predictors of OD (10–12). In one of these studies, using a sample of 1,556 individuals in the 53–91 age range, the overall percentage of incident OD cases over 5 years was 12.5%, and increased with age for both men and women (10). A history of nasal polyps, deviated septum, and heavy alcohol use were significant predictors of OD, whereas physical activity, and use of lipid-lowering agents and oral steroids were associated with reduced risk. Other longitudinal studies have found that subclinical atherosclerosis (12) and male sex (11) predict OD. Findings from cross-sectional studies indicate that a multitude of factors are associated with OD, even when cognitive status and neurodegenerative disorders are controlled for. In general, a large portion of the variance in OD is associated with demographic factors such as increasing age (13), male sex (2), and low education (14). Genetic factors also play a role in OD. For instance, the ε4 allele of the APOE gene has a negative effect on olfactory function (15,16), and the brain-derived neurotrophic factor (BDNF) val66met polymorphism moderates rate of olfactory decline in old age. Specifically, Val/Val carriers were found to decline faster on an odor identification task, suggesting that the BDNF Met allele may be protective against an accelerated olfactory decline in the later stages of life (17). Evidence from the human and animal literature has indicated that dopamine receptors are expressed in the olfactory bulb and that dopaminergic interneurons participate in olfactory processing (18). Dopaminergic transmission in the human brain is influenced by the metabolic breakdown of dopamine, a process catalyzed by the catechol O-methyltransferase (COMT) protein (19). The most common variation of the COMT gene, coding for this protein, is the Val158Met polymorphism, in which the variant containing Val increases dopamine catabolism and significantly lower synaptic dopamine levels (18).
Research also shows that a series of clinical and lifestyle factors are linked to OD. Murphy and colleagues (2) reported cardiovascular and cerebrovascular disease, nasal dysfunction, and a history of cancer to be associated with OD. Others report that conditions such as depression, head trauma, diabetes, and high cholesterol are more common in people with OD (20,21). Lifestyle factors, such as heavy alcohol consumption (22), current smoking (23), overweight (24), and underweight (4) have also been related to OD. Interestingly, lifestyle habits such as physical activity and social network size, both linked to successful brain aging, have been associated with a lower occurrence of olfactory impairment (25,26).
We have previously reported cross-sectional data from the same population-based study (Swedish National Study of Aging and Care in Kungsholmen or SNAC-K) as used in the present study. These revealed that advancing age, history of coronary heart disease, male sex, and fewer years of education were associated with concurrent OD (3). Here, we report the 6-year follow-up of participants without OD at the initial assessment (ie, the cases in the previous cross-sectional study (3) are not included). The use of a longitudinal prospective design, which is rare in previous studies on OD, enables measurement of the number of new cases, and evaluation of risk factors of OD (ie, whether factors that have been associated with OD in cross-sectional studies also constitute reliable predictors for the development of OD). Specifically, we were interested in which demographic, genetic, clinical, lifestyle, and cognitive factors predicted the development of OD across a 6-year follow-up interval.
Methods
Participants
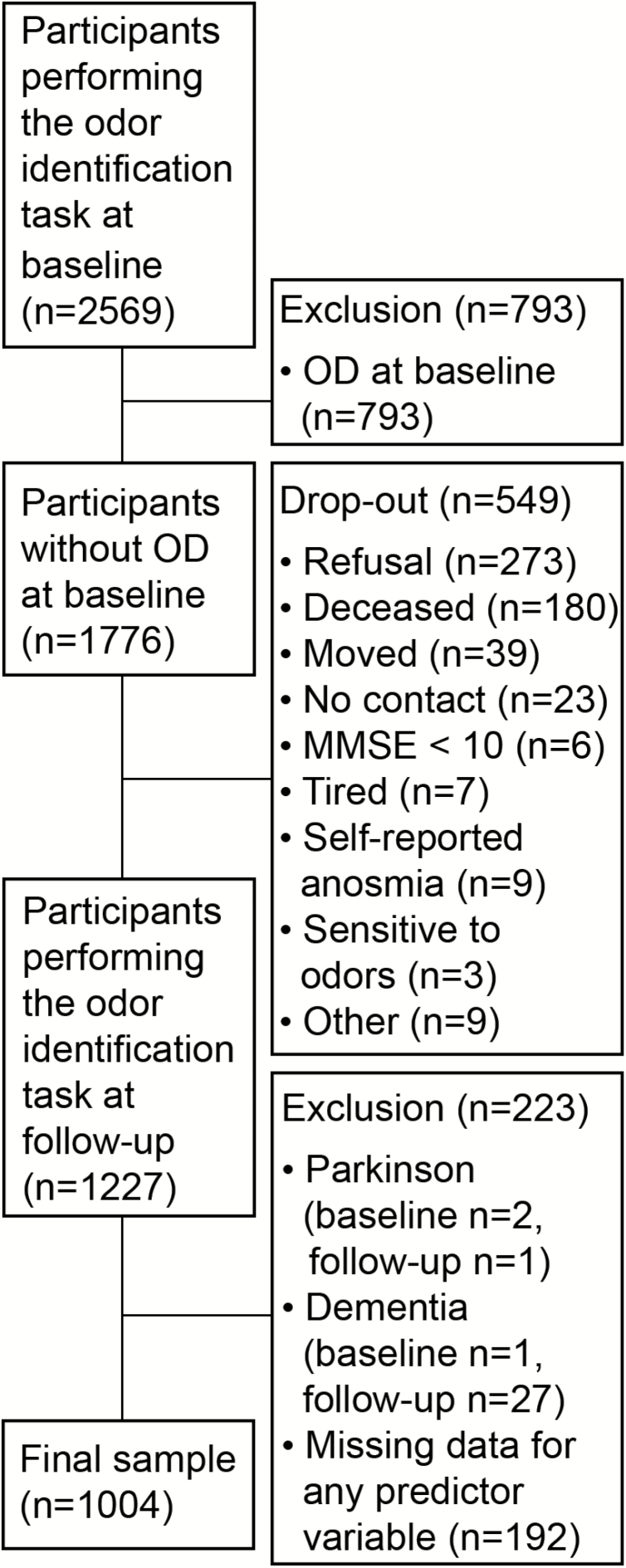
Data from the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K) were used. SNAC-K is a longitudinal study consisting of three parts: an interview conducted by a nurse, a medical examination, and neuropsychological testing. Between 2001 and 2004, 4,590 individuals aged 60 years and older from the island of Kungsholmen in central Stockholm were randomly selected from the population registry according to 11 predefined age cohorts (60, 66, 72, 78, 81, 84, 87, 90, 93, 96, and ≥99 years). Out of 3,363 individuals participating in the baseline assessment (participation rate: 73.3%), 2,569 individuals completed an odor identification task. The participants were re-examined by the time they reached the age of the next age cohort. Hence, the younger cohorts were re-examined every 6 years and older cohorts every 3 years. In the present study, data from the baseline assessment and the 6-year follow-up (to which all participants were re-invited) were used. Among those who participated in the odor identification task at baseline, 793 had OD and were excluded from the sample. Among the 1,776 remaining participants, 1,227 (69.1%) returned for the odor identification task at follow-up. Participants at risk for developing OD who did not return to the follow-up assessment were more likely to be older, have fewer years of education, lower odor identification ability and general cognitive performance (as assessed by the Mini-Mental State examination) at baseline. Among these, the following exclusion criteria were applied: Parkinson’s disease (CERAD criteria) at baseline (n = 2) or follow-up (n = 1), dementia or questionable dementia (DSM-IV criteria) at baseline (n = 1) or follow-up (n = 27), and missing data for any of the predictor variables (n = 192). When comparing logistic regression models it is important that the samples used to fit the smaller and larger models are the same, which becomes an issue when there is missing data (27). This motivated our exclusion of individuals with missing data for any of the predictors. This resulted in a final sample of 1,004 participants (Figure 1).
Figure 1.
Flowchart of the sample selection. OD = Olfactory dysfunction.
All parts of the SNAC-K project have been approved by the Ethics Committee at Karolinska Institutet and the Regional Ethics Review Board in Stockholm (Dnrs 01-114, 04-929/3, Ö 26–2007) and were conducted according to the Declaration of Helsinki. Informed consent was collected from all participants. In cases where the participant was severely cognitively impaired, informed consent was given by next-of-kin.
Assessment of Olfactory Function
Olfactory ability was assessed by the standardized “Sniffin’ Sticks” odor identification test consisting of 16 odors (apple, banana, clove, coffee, cinnamon, fish, garlic, lemon, leather, licorice, peppermint, pineapple, rose, turpentine, mushroom, and gasoline (28)). Each odor was presented during 5 seconds using felt tip-pens placed underneath the participants’ noses. After each presentation, the participants were instructed to freely identify the odor by naming. If they could not identify the odor or respond incorrectly, they were asked to select one out of four response alternatives, of which one was correct (cued identification). The total number of correctly identified odors by either free or cued identification (range 0–16) represented the number of correct answers in the present study. OD was defined as having a score of 10 or less, based on established cutoff scores for reduced olfactory acuity (28). The sample in the present study only included participants at risk of OD, that is, individuals without OD at baseline. These individuals were then divided into two groups according to whether or not they had developed OD at follow-up.
Predictors of OD
Candidate predictors were selected based on a review of the literature on variables that have been associated with OD in cross-sectional studies.
Demographic variables
Participants’ demographic information (ie, age, sex, and education) was collected through a nurse interview. Age was treated as a categorical variable consisting of five levels (corresponding to the age cohorts 60, 66, 72, 78, and ≥81 years), whereas sex and education were treated as dichotomous variables. The education variable involved a question asking for the highest level of education completed, which was recoded into (0) no university degree and (1) university degree.
Genetic factors
Matrix-Assisted Laser Desorption-Ionization-Time-of-Flight (MALDI-TOF) mass spectrometry-based single nucleotide polymorphism (SNP) genotyping using the Sequenom MassARRAY platform was performed on peripheral blood samples at the Mutation Analysis Facility (MAF) at Karolinska Institutet. For the present study, genotype information for Apolipoprotein E (APOE, rs429358), Brain-Derived Neurotrophic Factor (BDNF, rs6265) and Catechol-O-Methyltransferase (COMT, rs4680) was used. For APOE, participants were grouped as carriers or noncarriers of the ε4 allele (too few individuals carried two ε4 alleles to be included as a separate category). Dichotomization for BDNF and COMT was performed according to: BDNF—homozygous Val carriers versus carriers of any Met allele, and for COMT—carriers of any Val allele versus homozygous Met carriers.
Clinical and physical function factors
Clinical and functional assessments were carried out by trained physicians, nurses, and psychologists. Information was collected by physical examination, inpatient/outpatient registers, lab tests, self-reports, and/or proxy interviews. All diagnoses were coded in accordance with the International Classification of Diseases, 10th revision (ICD-10), and were treated as binary variables. In the present study, the following diagnoses were included: ischemic heart disease (ICD-10 I20-I25), atrial fibrillation (ICD-10 I48), heart failure (ICD-10 I50), epilepsy (ICD-10 G40), head trauma (ICD-10 S06), cerebrovascular disease (ICD-10 I60-I69), and depression (ICD-10 F33). Risk factors of vascular disease were also included as clinical factors due to previous reports linking vascular alterations to olfactory performance. These variables were: high cholesterol (total serum cholesterol level ≥6.22 mmol/L), hypertension (≥160/100 mmHg, or current use of antihypertensive medication), and diabetes (either of fasting glucose level ≥7.0 mmol/L, nonfasting glucose level ≥11.0 mmol/L, use of oral glucose-lowering agents or insulin injection).
Migraine and cancer have previously been associated with smell loss and were included as possible clinical predictors of OD in this study. Migraine was classified according to the International Headache Society (IHS) criteria (29). Cancer was defined as a former diagnosis of any type of cancer.
Given that associations between olfactory function and performance in motor function have been found (30), measures of physical function (ie, walking speed and grip strength) were included. Walking speed was assessed by timing (in seconds) the participants walking 6 or 2.4 m (if the participants reported walking slowly) at a self-selected speed (31). Grip strength was assessed with the “Grippit” (32). The participants squeezed a handle with maximum force, once with both hands. The force (in Newton) of the strongest hand was used in the analyses.
Behavioral and lifestyle factors
Behavioral and lifestyle factors (ie, physical inactivity, overweight, underweight, heavy alcohol consumption, current and past smoking, manufacturing occupation, social network, and leisure activities) were collected through standardized interviews performed by a nurse, and through a self-administrated questionnaire. Physical inactivity was defined as performing exercise less or equal to two to three times per month. Overweight and underweight were based on body mass index (BMI; weight in kilograms divided by the square of height in meters) and defined as BMI > 30 and BMI < 18, respectively. Heavy alcohol consumption was defined as drinking >14 drinks (with a drink defined as 12–14 g pure alcohol, corresponding to 10–15 cL wine/33 cL regular beer/4 cL spirits such as whiskey) per week for men and >7 drinks per week for women (33). Current smoking was defined in accordance with reporting to currently smoke regularly or sometimes. Past smoking was defined as having previously smoked, but not being a current smoker. The variable manufacturing occupation was derived from the longest-held occupation and dichotomized according to: (0) nonmanufacturing occupation (junior office workers, office workers, senior office workers, entrepreneurs, academic professions, and farm owners) and (1) manufacturing occupation (no trained skill/trained skill goods-producing and service-producing workers). A continuous social network index devised by Calderón-Larrañaga and colleagues (34) was used. This social network index is based on several items from the self-administrated questionnaire describing social connections and support. Leisure activities were assessed by four different factors (predominantly social, complex, predominantly physical, and low-level activities) obtained by a hierarchical cluster analysis performed by Köhncke and colleagues (35) on reported frequency of engagement in 21 activities listed in the self-administrated questionnaire.
Cognitive factors
Cognitive performance was assessed by an extensive cognitive test battery administered by trained psychologists in accordance with standardized protocols. For a detailed description of the cognitive tasks and their procedure, see Ref. (36). The cognitive variables included in the present study belonged to the following domains: episodic memory (free recall and recognition), semantic memory (vocabulary and general knowledge), letter fluency (F and A), category fluency (animals and professions), and perceptual speed (pattern comparison and digit cancelation). All cognitive variables were included in univariable, age-adjusted logistic regression analyses. For each cognitive domain, only the variable with the lowest p-value of its Wald statistic was included in the multivariable models. In addition to the cognitive battery, the Mini-Mental State examination (MMSE) (37) was administered as a measure of global cognition.
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics 25 (IBM Corporation; New York). Proportions of new cases with OD over the 6-year follow-up were expressed as percentages. The Wilson method was used to calculate 95% confidence intervals (38).
Binary logistic regression analyses were conducted according to the recommendations of Hosmer, Lemeshow, and Sturdivant (27) to predict OD at follow-up using the predictors specified above, all of which were assessed at baseline. First, univariable logistic regression analyses for all predictors were conducted, from which candidates for multivariable models were chosen based on p < .20. The reason for choosing a liberal significance level of .20 in this screening procedure was that the more traditional significance level of .05 often fails to identify variables that are important at this stage (27). In the screening procedure, categorical predictors with zero frequency cells in cross-tabulation across the two levels of the outcome variable were excluded, as logistic regression models might fail to converge under this circumstance. Multicollinearity (r > 0.90) among predictors was not allowed (39). The variables that passed the screening procedure were included in a multivariable logistic regression model. The importance of each predictor in this model was assessed using the p-value of its Wald statistic. If a predictor did not contribute at the significance level of .05, it was eliminated and the smaller model obtained was compared to the original, larger model using the −2 log-likelihood ratio test. After this first part, each excluded predictor was again added to the model one at a time, and its significance was checked by the Wald statistic. If the predictor now contributed significantly to the model, it was kept. The purpose of this procedure is to identify variables that make an important contribution in the presence of other variables. Once the main effects model was decided on, interactions among pairs of the variables in the model were investigated. Only interactions that contributed significantly (p < .05) to the model were included. Overall goodness-of-fit (how accurately the predictions of the model reflect the observed data) was assessed by the Hosmer–Lemeshow goodness of fit test.
Results
The sample consisted of 1,004 individuals (643 women, 361 men) without OD at baseline. Their mean baseline age was 67.5 years (range, 60.1–90.8 years). The proportion of new cases with OD was 14.2% over 6 years (Table 1). Within sexes, the proportion increased monotonically with age for women. For men, the proportion of new cases with OD was selectively higher in the 66-year cohort than in the 72-year cohort, but otherwise it increased with age. Between sexes, the proportion of new cases with OD was similar for men and women in all age cohorts except for the 66-year cohort, in which the proportion of new cases with OD was significantly higher for men than women (Χ 2 (1, N = 258) = 5.58, p = .018).
Table 1.
Percent of Incident OD by Sex and Age at the 6-Year Follow-Up
| Age Cohort | Women | Men | All | OR (CI) by Cohort* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | OD % (n) | 95% CI | N | OD % (n) | 95% CI | N | OD % (n) | 95% CI | ||
| 60 | 238 | 8.4 (20) | 5.5–12.6 | 161 | 7.5 (12) | 4.3–12.6 | 399 | 8.0 (32) | 5.7–11.1 | |
| 66 | 161 | 9.3 (15) | 5.7–14.8 | 97 | 19.6 (19) | 12.9–28.6 | 258 | 13.2 (34) | 9.6–17.9 | 1.74 (1.05–2.90) |
| 72 | 119 | 12.6 (15) | 7.8–19.8 | 60 | 15.0 (9) | 8.1–26.1 | 179 | 13.4 (24) | 9.2–19.2 | 1.78 (1.01–3.11) |
| 78 | 78 | 29.5 (23) | 20.5–40.4 | 24 | 25.0 (6) | 12.0–44.9 | 102 | 28.4 (29) | 20.6–37.8 | 4.56 (2.60–7.99) |
| >80 | 47 | 36.2 (17) | 24.0–50.5 | 19 | 36.8 (7) | 19.2–59.0 | 66 | 36.2 (24) | 25.8–48.4 | 6.55 (3.53–12.16) |
| All | 643 | 14.0 (90) | 11.5–16.9 | 361 | 14.7 (53) | 11.4–18.7 | 1004 | 14.2 (143) | 12.2–16.5 |
Note: CI = Confidence interval; OD = Olfactory dysfunction; OR = Odds ratio.
*Reference category: 60 years..
Age-adjusted baseline risk factors, below the predefined alpha level of 0.20 to be considered for further multivariable analyses, are presented in Table 2. For a complete list of potential predictors tested in univariable models, see (Supplementary Table S1).
Table 2.
Results from Logistic Regression Analyses of Selected Predictors of OD Included in Multivariable Logistic Regression Models
| Categorical Variables | OD (n = 143) % (n) | No OD (n = 861) % (n) | Age Adjusted OR | 95% CI | p |
|---|---|---|---|---|---|
| APOE (ε4 carrier) | 33.6 (48) | 26.7 (230) | 1.51 | 1.02–2.22 | .040 |
| Current smoking | 17.5 (25) | 13.4 (115) | 1.75 | 1.07–2.87 | .026 |
| Physical inactivity | 23.8 (34) | 17.3 (149) | 1.57 | 1.01–2.43 | .043 |
| Atrial fibrillation | 15.4 (22) | 7.9 (68) | 1.69 | 0.99–2.90 | .055 |
| Head trauma | 16.8 (24) | 12.9 (111) | 1.62 | 0.98–2.66 | .058 |
| Cerebrovascular disease | 7.7 (11) | 3.1 (27) | 2.02 | 0.95–4.27 | .067 |
| Hypertension | 44.1 (63) | 43.2 (372) | 0.75 | 0.51–1.09 | .132 |
| Continuous variables | M (SD) | M (SD) | |||
| Odor identification at baseline | 12.5 (1.4) | 13.6 (1.4) | 0.60 | 0.52–0.69 | <.001 |
| MMSE | 29.2 (1.0) | 29.4 (0.8) | 0.81 | 0.66–0.99 | .044 |
| Perceptual speed | 14.3 (2.8) | 15.8 (3.1) | 0.91 | 0.85–0.97 | .004 |
| Episodic memory | 11.6 (2.7) | 12.1 (2.5) | 0.94 | 0.88–1.01 | .069 |
| Complex leisure activity | 3.5 (1.6) | 3.8 (1.8) | 0.93 | 0.84–1.02 | .133 |
| Social network index | 0.06 (0.4) | 0.14 (0.5) | 0.77 | 0.52–1.14 | .194 |
Note: CI = Confidence interval; MMSE = Mini-Mental State examination; OD = Olfactory dysfunction; OR = Odds ratio.
Results from the multivariable logistic regressions are presented in Table 3. The findings from the Hosmer–Lemeshow tests indicate that the observed probabilities did not deviate from the expected in any of the models, indicating a good fit for all models. In model 1, the predictors: odor identification at baseline, age, current smoking, APOE (ε4 carrier), atrial fibrillation and cerebrovascular disease, contributed significantly to the model. These predictors were then included in model 2. According to the −2 log-likelihood ratio test, there was a significant difference between the performance of the reduced model (model 2) in comparison with the full model (model 1) in predicting the development of OD: Χ 2 (8) = 15.59, p = .048. After again adding (one at a time) the previously excluded predictors to model 2, only episodic memory significantly contributed to the model and was thus reinstated (model 3). There was no significant difference between model 3 in comparison with model 1 in predicting the development of OD: Χ 2 (7) = 11.61, p = .114; hence, model 3 was chosen as the final model. The interaction terms did not contribute significantly to the final model, and were consequently not included.
Table 3.
Multivariable Logistic Regression Models for Predicting OD
| Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wald | Wald | Wald | ||||||||||
| Predictors | OR | 95% CI | Χ 2 | p | OR | 95% CI | Χ 2 | p | OR | 95% CI | Χ 2 | p |
| Odor identification | 0.59 | 0.51–0.69 | 48.58 | <.001 | 0.59 | 0.51–0.68 | 53.12 | <.001 | 0.58 | 0.51–0.68 | 53.64 | <.001 |
| Age* | 20.79 | <001 | 31.06 | <.001 | 28.74 | <.001 | ||||||
| 66 | 1.82 | 1.04–3.19 | 4.34 | .037 | 1.69 | 0.99–2.88 | 3.64 | .057 | 1.66 | 0.97–2.85 | 3.64 | .057 |
| 72 | 1.72 | 0.92–3.22 | 2.68 | .101 | 1.55 | 0.86–2.79 | 2.12 | .146 | 1.51 | 0.83–2.72 | 2.22 | .136 |
| 78 | 3.95 | 1.99–7.84 | 14.45 | <.001 | 3.79 | 2.06–6.97 | 18.24 | <.001 | 3.56 | 1.92–6.58 | 17.47 | <.001 |
| >80 | 4.72 | 2.20-0.11 | 15.21 | <.001 | 5.05 | 2.55–9.97 | 21.71 | <.001 | 4.56 | 2.29–9.07 | 19.98 | <.001 |
| Current smoking | 1.92 | 1.12–3.29 | 5.44 | .020 | 1.89 | 1.12–3.20 | 5.60 | .018 | 1.92 | 1.13–3.24 | 5.87 | .015 |
| Atrial fibrillation | 2.07 | 1.15–3.75 | 5.93 | .015 | 1.89 | 1.06–3.37 | 4.72 | .030 | 1.95 | 1.09–3.47 | 5.07 | .024 |
| Cerebrovascular disease | 2.35 | 1.02–5.39 | 3.94 | .047 | 2.42 | 1.06–5.55 | 4.37 | .037 | 2.39 | 1.05–5.44 | 4.26 | .039 |
| APOE (ε4 carrier) | 1.58 | 1.04–2.39 | 4.25 | .039 | 1.53 | 1.01–2.30 | 4.05 | .044 | 1.54 | 1.02–2.33 | 4.23 | .040 |
| Episodic memory | 0.96 | 0.91–1.02 | 2.36 | .124 | 0.93 | 0.86–1.00 | 4.03 | .045 | ||||
| Perceptual speed | 0.99 | 0.95–1.04 | 0.80 | .371 | ||||||||
| MMSE | 0.83 | 0.66–1.04 | 2.57 | .109 | ||||||||
| Physical inactivity | 1.38 | 0.86–2.23 | 1.84 | .175 | ||||||||
| Head trauma | 1.40 | 0.82–2.38 | 1.49 | .222 | ||||||||
| Hypertension | 0.66 | 0.44–1.00 | 3.81 | .051 | ||||||||
| Complex leisure activity | 0.96 | 0.86–1.08 | 0.33 | .569 | ||||||||
| Social network index | 1.00 | 0.63–1.57 | 0.00 | .973 | ||||||||
Note: CI = Confidence interval; MMSE = Mini-Mental State examination; OD = Olfactory dysfunction; OR = Odds ratio.
*Reference category: 60 years. The −2 log likelihood was 682.3 for model 1, 697.9 for model 2 and 693.94 for model 3. Nagelkerke’s R2 was 0.23 for model 1, 0.21 for model 2 and 0.21 for model 3. Results from the Hosmer–Lemeshow goodness of fit: Χ 2 = 2.37, p = .967 for model 1, Χ 2 = 7.10, p = .526 for model 2 and Χ 2 = 6.08, p = .638 for model 3.
Discussion
OD is common among older adults but its predictors are not well known as the number of longitudinal studies conducted so far is few. We conducted a longitudinal study to address the development of incident OD in the older population, and the demographic, genetic, clinical, lifestyle, and cognitive risk factors that predict OD over a 6-year interval. Consistent with previous cross-sectional observations, age, carrying an APOE ε4 allele, cerebrovascular disease, and current smoking were reliable risk factors for the development of incident OD (2,13,16,23). A novel finding was that atrial fibrillation increased the risk, whereas episodic memory proficiency, along with higher baseline olfactory function, were protective factors against OD 6 years after the initial assessment.
The percentage of new cases of OD was 14.2% over 6 years. This corresponds closely to the study by Schubert and colleagues (10), who reported that 12.5% of their sample developed OD over 5 years. Age was a prominent risk factor for the development of OD, which is in accordance with a previous longitudinal finding (10), and with a wealth of evidence from cross-sectional studies reporting positive associations between age and occurrence of OD (13). The odds ratio of OD in the oldest cohort (>80 years) was about five times higher than that observed in the youngest cohort (60 years). The monotonic olfactory decline clearly shows the pervasiveness of smell loss also in dementia-free older populations. It is worth noting that, given that the studied sample represents individuals free of neurodegenerative disorders, the observed percentage of new OD cases across the 6-year measurement interval (14.2%) likely underestimates the incidence proportion relative to the general aging population (13).
None of the other demographic factors were associated with the risk of developing OD between the baseline and the 6-year follow-up. Although more men than women exhibited OD at baseline (3), the OD incidence proportion was similar for men and women. A possible exception to this pattern is that the number of new cases of OD was higher among men in the second youngest (66 years) age cohort, suggesting that smell impairment may start earlier in life in men than women. This finding in agreement with findings from previous research assessing cognitive functions, where women often have a somewhat higher level of overall cognitive performance, although the rate of age-related cognitive decline is similar between the sexes (40). Likewise, the present findings showed no influence of education on the rate of OD development, despite cross-sectional evidence, indicating that lower education is related to a higher prevalence of smell impairment (3). Again, parallels can be drawn to cognitive research in which cross-sectional evidence of associations between level of cognitive function and educational attainment has been found, whereas longitudinal studies have found that education does not alter the rate of age-related cognitive decline (41,42). These results highlight the importance of conducting longitudinal research, as factors related to the level of olfactory functioning are not necessarily related to change therein.
Of the genetic factors included, only the ɛ4 allele of the APOE gene constituted a risk factor for the development of OD. This observation corroborates previous research on the associations between the ɛ4 allele and olfactory impairment and olfactory memory decline (15,16). Given that the ε4 allele is a risk factor for developing dementia and Alzheimer’s disease (43), older individuals carrying the ε4 allele may be more likely to be in a preclinical phase of dementia. They may thus be affected by early brain changes that have negative consequences for both general memory abilities and olfactory abilities.
In accordance with previous studies that have found vascular risk factors (ie, subclinical atherosclerosis; 12 and stroke (44) to be predictors of OD, the present study also found vascular risk factors (ie, atrial fibrillation and cerebrovascular disease) to predict OD. Atrial fibrillation (ie, rapid or irregular heartbeat) alters the pulsatile dynamics of the cerebrovascular system resulting in local hypoperfusion and hypertensive events. The hemodynamic cerebral effect of atrial fibrillation has been suggested as a mechanism underlying cognitive impairment (45) and might also be relevant for the genesis of OD. This is so because brain structures relevant for olfaction might undergo ischemic damage, due to transient hypoperfusion or transient hypertensive events. In a similar vein, a cerebrovascular disease might cause structural damage in brain areas involved in olfaction. Toward this end, poor olfaction has been associated with increased mortality from neurodegenerative and cardiovascular diseases in a recent study (6).
Among the life-style factors examined, only current smoking was a significant predictor of OD. Cross-sectional observations have shown that current smokers, but not past smokers, have a higher prevalence of olfactory impairment (23). We extend these findings by demonstrating that current smoking is associated with future OD risk, whereas past smoking is not. These results are in line with findings from a previous longitudinal study showing a stronger trend toward increased risk of olfactory decline (defined as a two-step decrease of the San Diego Odor Identification Test) over 5 years in current when compared with former smokers (12). A possible explanation is the reversibility of metaplastic changes (ie, squamous metaplasia) or sinonasal inflammation caused by smoking (23). Taken together, these findings suggest that the repercussions of smoking on olfactory function are reversible, making smoking another modifiable risk factor of OD. A recent study found that olfactory impairment persists 15 years after smoking cessation (46). However, individuals who had quit smoking more than 15 years ago had the same odor identification performance as those who never smoked. Additional prospective studies on smoking cessation are warranted in order to assess the reversibility of OD.
Some protective factors for OD were also identified. Scoring higher on the odor identification task at baseline was, unsurprisingly, strongly associated with a lower risk of OD 6 years later. Higher episodic memory performance was found to be protective for OD as well. Positive associations between episodic memory and odor identification have previously been reported (47). This probably reflects that high olfactory memory performance requires intact memory abilities in general. It may also reflect that preserved brain integrity in old age is linked to both episodic memory and olfactory proficiency. Prior studies with a prospective design have found odor identification deficits to predict episodic memory and global cognitive decline (48). In contrast to the present study, in which the outcome is development of OD, the outcomes of interest in prior studies have been cognitive decline and/or conversion to dementia. An important goal in future studies is therefore to investigate the temporal relationship between episodic memory decline and impaired odor identification.
Limitations of the present study include that individuals with severe olfactory impairment could not be tested. We also excluded participants with dementia due to difficulties in reliably assessing their olfactory abilities. These exclusions are likely to lead to an underestimation of the observed effects. In addition, we did not have access to clinical diagnoses specific to nasal diseases, which prevented us from addressing such associations.
In summary, several modifiable prospective risk factors of OD were identified, indicating that there are possibilities for reducing olfactory impairment in aging. An apparent lifestyle modification that could reduce olfactory impairment is smoking cessation. Other lifestyle and behavioral modifications that have been shown to decrease the risk of cerebrovascular disease and atrial fibrillation, such as dietary changes and a more physically active life (49,50), might also reduce the risk of OD. Thus, the results from the present study suggest that there are possibilities to prevent, or at least reduce, olfactory impairment in old age. Future intervention studies might further elucidate these issues, as well as investigate whether prevention of OD reduces mortality or dementia risk. The results of the present study highlight the importance of conducting longitudinal research, as factors related to the level of olfactory functioning in cross-sectional studies (eg, sex and education) are not necessarily related to change in olfactory functioning.
Funding
SNAC-K is financially supported by the Swedish Ministry of Health and Social Affairs, the participating County Councils and Municipalities, and the Swedish Research Council. This work was further funded by a program grant entitled “Our unique sense of smell” awarded by the Swedish Foundation for Humanities and Social Sciences (M14-0375:1) to ML and a research grant from the Swedish Research Council awarded to EL (2017-01759).
Supplementary Material
Acknowledgments
We thank the participants as well as all staff involved in the data collection and management of the SNAC-K study.
Conflict of Interest
None reported.
References
- 1. Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life—an updated review. Chem Senses. 2014;39:185–194. doi: 10.1093/chemse/bjt072 [DOI] [PubMed] [Google Scholar]
- 2. Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288:2307–2312. doi: 10.1001/jama.288.18.2307 [DOI] [PubMed] [Google Scholar]
- 3. Seubert J, Laukka EJ, Rizzuto D, et al. . Prevalence and correlates of olfactory dysfunction in old age: a population-based study. J Gerontol A Biol Sci Med Sci. 2017;72:1072–1079. doi: 10.1093/gerona/glx054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karpa MJ, Gopinath B, Rochtchina E, et al. . Prevalence and neurodegenerative or other associations with olfactory impairment in an older community. J Aging Health. 2010;22:154–168. doi: 10.1177/0898264309353066 [DOI] [PubMed] [Google Scholar]
- 5. Ekström I, Sjölund S, Nordin S, et al. . Smell loss predicts mortality risk regardless of dementia conversion. J Am Geriatr Soc. 2017;65:1238–1243. doi: 10.1111/jgs.14770 [DOI] [PubMed] [Google Scholar]
- 6. Liu B, Luo Z, Pinto JM, et al. . Relationship between poor olfaction and mortality among community-dwelling older adults: a cohort study. Ann Intern Med. 2019;170:673–681. doi: 10.7326/M18-0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One. 2014;9:e107541. doi: 10.1371/journal.pone.0107541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Behrman S, Chouliaras L, Ebmeier KP. Considering the senses in the diagnosis and management of dementia. Maturitas. 2014;77:305–310. doi: 10.1016/j.maturitas.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 9. Stanciu I, Larsson M, Nordin S, Adolfsson R, Nilsson LG, Olofsson JK. Olfactory impairment and subjective olfactory complaints independently predict conversion to dementia: a longitudinal, population-based study. J Int Neuropsychol Soc. 2014;20:209–217. doi: 10.1017/S1355617713001409 [DOI] [PubMed] [Google Scholar]
- 10. Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Olfactory impairment in older adults: five-year incidence and risk factors. Laryngoscope. 2011;121:873–878. doi: 10.1002/lary.21416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. The rate of age-related olfactory decline among the general population of older U.S. Adults. J Gerontol A Biol Sci Med Sci. 2015;70:1435–1441. doi: 10.1093/gerona/glv072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schubert CR, Cruickshanks KJ, Fischer ME, et al. . Carotid intima media thickness, atherosclerosis, and 5-year decline in odor identification: the beaver dam offspring study. J Gerontol A Biol Sci Med Sci. 2015;70:879–884. doi: 10.1093/gerona/glu158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doty RL, Kamath V. The influences of age on olfaction: a review. Front Psychol. 2014;5:20. doi: 10.3389/fpsyg.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larsson M, Nilsson LG, Olofsson JK, Nordin S. Demographic and cognitive predictors of cued odor identification: evidence from a population-based study. Chem Senses. 2004;29:547–554. doi: 10.1093/chemse/bjh059 [DOI] [PubMed] [Google Scholar]
- 15. Olofsson JK, Nordin S, Wiens S, Hedner M, Nilsson LG, Larsson M. Odor identification impairment in carriers of ApoE-varepsilon4 is independent of clinical dementia. Neurobiol Aging. 2010;31:567–577. doi: 10.1016/j.neurobiolaging.2008.05.019 [DOI] [PubMed] [Google Scholar]
- 16. Josefsson M, Larsson M, Nordin S, Adolfsson R, Olofsson J. APOE-ɛ4 effects on longitudinal decline in olfactory and non-olfactory cognitive abilities in middle-aged and old adults. Sci Rep. 2017;7:1286. doi: 10.1038/s41598-017-01508-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hedner M, Nilsson LG, Olofsson JK, et al. . Age-related olfactory decline is associated with the BDNF Val66met polymorphism: evidence from a population-based study. Front Aging Neurosci. 2010;2:24. doi: 10.3389/fnagi.2010.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marin C, Vilas D, Langdon C, et al. . Olfactory dysfunction in neurodegenerative diseases. Curr Allergy Asthma Rep. 2018;18:42. doi: 10.1007/s11882-018-0796-4 [DOI] [PubMed] [Google Scholar]
- 19. Hemby SE, Trojanowski JQ, Ginsberg SD. Neuron-specific age-related decreases in dopamine receptor subtype mRNAs. J Comp Neurol. 2003;456:176–183. doi: 10.1002/cne.10525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lombion-Pouthier S, Vandel P, Nezelof S, Haffen E, Millot JL. Odor perception in patients with mood disorders. J Affect Disord. 2006;90:187–191. doi: 10.1016/j.jad.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 21. Boyce JM, Shone GR. Effects of ageing on smell and taste. Postgrad Med J. 2006;82:239–241. doi: 10.1136/pgmj.2005.039453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rupp CI, Kurz M, Kemmler G, et al. . Reduced olfactory sensitivity, discrimination, and identification in patients with alcohol dependence. Alcohol Clin Exp Res. 2003;27:432–439. doi: 10.1097/01.ALC.0000057945.57330.2C [DOI] [PubMed] [Google Scholar]
- 23. Ajmani GS, Suh HH, Wroblewski KE, Pinto JM. Smoking and olfactory dysfunction: a systematic literature review and meta-analysis. Laryngoscope. 2017;127:1753–1761. doi: 10.1002/lary.26558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel ZM, DelGaudio JM, Wise SK. Higher body mass index is associated with subjective olfactory dysfunction. Behav Neurol. 2015;2015:675635. doi: 10.1155/2015/675635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schubert CR, Cruickshanks KJ, Nondahl DM, Klein BE, Klein R, Fischer ME. Exercise is associated with lower long-term risk of olfactory impairment in older adults. JAMA Otolaryngol Head Neck Surg. 2013;139:1061–1066. doi: 10.1001/jamaoto.2013.4759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zou LQ, Yang ZY, Wang Y, et al. . What does the nose know? Olfactory function predicts social network size in human. Sci Rep. 2016;6:25026. doi: 10.1038/srep25026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hosmer DW Jr, Lemeshow S, Sturdivant RX.. Applied Logistic Regression. Hoboken, NJ: John Wiley & Sons, Inc.; 2013. doi: 10.1002/9781118548387. [DOI] [Google Scholar]
- 28. Hummel T, Konnerth CG, Rosenheim K, Kobal G. Screening of olfactory function with a four-minute odor identification test: reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol. 2001;110:976–981. doi: 10.1177/000348940111001015 [DOI] [PubMed] [Google Scholar]
- 29. Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8(Suppl. 7):1–93. [PubMed] [Google Scholar]
- 30. Tian Q, Resnick SM, Studenski SA. Olfaction is related to motor function in older adults. J Gerontol A Biol Sci Med Sci. 2017;72:1067–1071. doi: 10.1093/gerona/glw222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Welmer AK, Rizzuto D, Laukka EJ, Johnell K, Fratiglioni L. Cognitive and physical function in relation to the risk of injurious falls in older adults: a population-based study. J Gerontol A Biol Sci Med Sci. 2017;72:669–675. doi: 10.1093/gerona/glw141 [DOI] [PubMed] [Google Scholar]
- 32. Nordenskiöld UM, Grimby G. Grip force in patients with rheumatoid arthritis and fibromyalgia and in healthy subjects. A study with the Grippit instrument. Scand J Rheumatol. 1993;22:14–19. doi: 10.3109/03009749309095105 [DOI] [PubMed] [Google Scholar]
- 33. Järvenpää T, Rinne JO, Koskenvuo M, Räihä I, Kaprio J. Binge drinking in midlife and dementia risk. Epidemiology. 2005;16:766–771. doi: 10.1097/01.ede.0000181307.30826.6c [DOI] [PubMed] [Google Scholar]
- 34. Calderón-Larrañaga A, Santoni G, Wang HX, et al. . Rapidly developing multimorbidity and disability in older adults: does social background matter? J Intern Med. 2018;283:489–499. doi: 10.1111/joim.12739 [DOI] [PubMed] [Google Scholar]
- 35. Köhncke Y, Laukka EJ, Brehmer Y, et al. . Three-year changes in leisure activities are associated with concurrent changes in white matter microstructure and perceptual speed in individuals aged 80 years and older. Neurobiol Aging. 2016;41:173–186. doi: 10.1016/j.neurobiolaging.2016.02.013 [DOI] [PubMed] [Google Scholar]
- 36. Laukka EJ, Lövdén M, Herlitz A, et al. . Genetic effects on old-age cognitive functioning: a population-based study. Psychol Aging. 2013;28:262–274. doi: 10.1037/a0030829 [DOI] [PubMed] [Google Scholar]
- 37. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 38. Altman DG, Machin D, Bryant TN, Gardner MJ, eds. Statistics with Confidence. 2nd ed Bristol, England: BMJ Books; 2000. ISBN:9780727913753. [Google Scholar]
- 39. Tabachnick BG, Fidell LS.. Using Multivariate Statistics. Boston, MA: Pearson Education, Limited; 2013. ISBN:0-205-89081-4. [Google Scholar]
- 40. Ferreira L, Ferreira Santos-Galduróz R, Ferri CP, Fernandes Galduróz JC. Rate of cognitive decline in relation to sex after 60 years-of-age: a systematic review. Geriatr Gerontol Int. 2014;14:23–31. doi: 10.1111/ggi.12093 [DOI] [PubMed] [Google Scholar]
- 41. Nyberg L, Pudas S. Successful memory aging. Annu Rev Psychol. 2019;70:219–243. doi: 10.1146/annurev-psych-010418-103052 [DOI] [PubMed] [Google Scholar]
- 42. Berggren R, Nilsson J, Lövdén M. Education does not affect cognitive decline in aging: a Bayesian assessment of the association between education and change in cognitive performance. Front Psychol. 2018;9:1138. doi: 10.3389/fpsyg.2018.01138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wehling E, Naess H, Wollschlaeger D, et al. . Olfactory dysfunction in chronic stroke patients. BMC Neurol. 2015;15(199):1–7. doi: 10.1186/s12883-015-0463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anselmino M, Scarsoglio S, Saglietto A, Gaita F, Ridolfi L. Transient cerebral hypoperfusion and hypertensive events during atrial fibrillation: a plausible mechanism for cognitive impairment. Sci Rep. 2016;6:28635. doi: 10.1038/srep28635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Siegel JK, Wroblewski KE, McClintock MK, Pinto JM. Olfactory dysfunction persists after smoking cessation and signals increased cardiovascular risk. Int Forum Allergy Rhinol. 2019;9:977–985. doi: 10.1002/alr.22357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilson RS, Arnold SE, Tang Y, Bennett DA. Odor identification and decline in different cognitive domains in old age. Neuroepidemiology. 2006;26:61–67. doi: 10.1159/000090250 [DOI] [PubMed] [Google Scholar]
- 48. Dintica CS, Marseglia A, Rizzuto D, et al. . Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology. 2019;92:e700–e709. doi: 10.1212/WNL.0000000000006919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120:472–495. doi: 10.1161/CIRCRESAHA.116.308398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. 2017;136:583–596. doi: 10.1161/CIRCULATIONAHA.116.023163 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.