Significance
Using realistic simulation of dengue and plausible control programs in the state of Yucatán, Mexico, we show that vaccination programs can have amplifying or interfering dynamic effects when combined with expanded vector control effort. These results should inform planning in regions considering an integrated dengue control program, particularly if using CYD-TDV, which we found to underperform when combined with increasing vector control.
Keywords: dengue, vector control, dengue vaccines, mathematical modeling
Abstract
Viruses transmitted by Aedes mosquitoes, such as dengue, Zika, and chikungunya, have expanding ranges and seem unabated by current vector control programs. Effective control of these pathogens likely requires integrated approaches. We evaluated dengue management options in an endemic setting that combine novel vector control and vaccination using an agent-based model for Yucatán, Mexico, fit to 37 y of data. Our intervention models are informed by targeted indoor residual spraying (TIRS) experiments; trial outcomes and World Health Organization (WHO) testing guidance for the only licensed dengue vaccine, CYD-TDV; and preliminary results for in-development vaccines. We evaluated several implementation options, including varying coverage levels; staggered introductions; and a one-time, large-scale vaccination campaign. We found that CYD-TDV and TIRS interfere: while the combination outperforms either alone, performance is lower than estimated from their separate benefits. The conventional model hypothesized for in-development vaccines, however, performs synergistically with TIRS, amplifying effectiveness well beyond their independent impacts. If the preliminary performance by either of the in-development vaccines is upheld, a one-time, large-scale campaign followed by routine vaccination alongside aggressive new vector control could enable short-term elimination, with nearly all cases avoided for a decade despite continuous dengue reintroductions. If elimination is impracticable due to resource limitations, less ambitious implementations of this combination still produce amplified, longer-lasting effectiveness over single-approach interventions.
Dengue virus (DENV) is an Aedes mosquito-borne pathogen that threatens nearly half the global population, with estimated annual infections of 390 million people resulting in 96 million symptomatic cases (1–5). The expanding geographic range of Aedes spp., rapid urbanization, lack of sustained effective vector control strategies, and increased human mobility have primed the world for increased transmission of dengue and other Aedes-borne diseases, including chikungunya, Zika, and yellow fever (6–9).
The World Health Organization (WHO) considers mosquito control a necessary component of dengue management (10), but due to global trends in DENV transmission, the dengue prevention community has generally acknowledged that vector control is not sufficient for containment (6, 7, 11). Research and development of vaccines have expanded to address this gap (12–19), but the complex immune response to dengue has proven a major challenge (20). DENV has four serotypes, and infection induces long-lived immunity against the infecting serotype as well as temporary immunity against the other serotypes. While initially protective, this differential immune response can later increase the risk of symptomatic and severe disease (21). To be efficacious, a vaccine needs to evoke broad, durable immunity while avoiding this negative cross-reactive response.
Phase III trial results for a candidate vaccine, CYD-TDV (commercially known as Dengvaxia), were initially promising, indicating that it was effective against all serotypes (12, 13). Follow-up studies, however, concluded that it had similar long-term immune outcomes to natural dengue infections: temporary broad immunity followed by increased risk of disease for first natural infections (14, 22). Recipients with past natural infections showed 68 to 90% efficacy depending on serotype (23). The WHO now recommends only vaccinating people with a laboratory-confirmed prior dengue infection (24, 25). Preliminary results of human challenge and immunogenicity experiments for two other dengue vaccines developed by NIH/Butantan/Merck and Takeda indicate tetravalent responses in 90 to 100% (18, 19) and 60 to 100% (16, 17) of individuals, respectively. Both vaccines are currently in phase III trials that will soon produce additional results about efficacy.
Novel vector controls are also being developed. In addition to more experimental approaches, like Wolbachia and gene drives (11), insecticide-based methods with proven performance against malaria are now being considered for Aedes aegypti. Field experiments and mathematical modeling have shown that household-based targeted indoor residual spraying [TIRS; applying residual insecticides only to Ae. aegypti resting sites; e.g., exposed low walls (26, 27)] could be effective at preventing dengue cases in endemic areas (28, 29). TIRS has also been shown to be less expensive than classic indoor residual spraying (IRS) while maintaining a high level of efficacy (30).
Unfortunately, both vaccination and vector control approaches have operational downsides. Without large-scale, one-time campaigns in broad age groups (also known as “catch-up” campaigns), forecast models suggest that routine vaccination of narrow age groups with a conventional vaccine against dengue would take decades to achieve high effectiveness (31). Expanded vector control, on the other hand, initially shows remarkable effectiveness but dynamically fades over time as naturally acquired immunity in the population declines (29, 32). The timing of these trends is complementary, and simple ordinary differential equation models suggest that combining the interventions might address their separate weaknesses (33).
While it is important to understand general trends, simple models may not provide sufficiently accurate quantitative forecasts for real-world decision making. Based on preliminary estimates of intervention performance, we used our fitted model of dengue transmission in the Mexican state of Yucatán to quantitatively assess different deployment strategies. While future intervention decisions will need to be guided by empirical data not yet available, our results will be useful for narrowing the potential programs for detailed consideration. This work also demonstrates an intervention assessment framework, which can be rapidly adjusted to account for updated intervention performance.
We evaluated distinct mechanisms representing CYD-TDV and an extrapolation of the ongoing phase III trials for the NIH/Butantan/Merck and Takeda vaccines considering realistic deployment options: 80% attempted coverage via routine vaccination in children as well as adding a one-time catch-up campaign at the same coverage. Quantitative findings vary substantially depending on the vaccine mechanism assumed. When simulating the combination of CYD-TDV [given only to test-seropositive 9-y-old children, consistent with current WHO recommendations (25)] and TIRS, the two interventions interfere across a range of transmission settings; while their combination generally outperforms either alone, the result is lower than what might be naïvely expected given their individual benefits. However, if either of the NIH/Butantan/Merck or Takeda vaccine trials achieves the target efficacy outcomes—namely, demonstrating a tetravalent, durable, highly efficacious vaccine—then we predict that new vaccine will have an amplifying interaction with TIRS, suggesting a promising integration of control methods currently in development.
Results
Using an agent-based model of DENV transmission in the state of Yucatán, Mexico (Fig. 1), we forecast the impact of vaccination and vector control interventions independently and in combination. These interventions were introduced in a population with stable dengue dynamics, and we simulated 40 y of intervention to capture long-term trends.
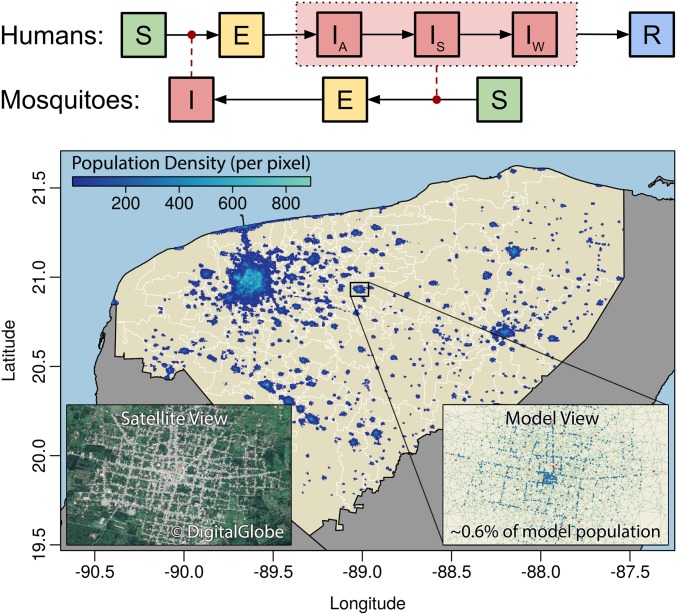
Fig. 1.
Agent-based model structure. (Upper) Our model’s state transition diagram for individual humans (E, Exposed; I, infectious [A, asymptomatic; S, symptomatic; and W, withdrawn from daily activity]; R, recovered; and S, susceptible) and mosquitoes (E, exposed; I, infectious; and S, susceptible). Solid arrows denote possible transitions; dashed arrows denote the influence of infectious mosquitoes and humans on DENV transmission rates. For humans, this series of transitions can occur for each serotype; each mosquito may only be infected by a single serotype. (Lower) Overall model spatial structure, zooming to Izamal (Insets) to illustrate detailed structure. Households, workplaces, and schools (Right Inset) are placed based on government data and are consistent with satellite imagery (Left Inset); SI Appendix, Fig. S1 shows enlarged versions of Insets. Pixel size in density map is 430 × 460 m.
To assess intervention performance, we focus on annual effectiveness, which is the fraction of cases (i.e., symptomatic infections) prevented across the entire population, aggregated at yearly intervals from the start of the intervention. The reduction is measured against a baseline scenario without interventions. We report cumulative effectiveness (i.e., the fraction of all cases prevented since the start of the intervention) as well as interquartile prediction intervals in SI Appendix.
Single-Intervention Scenarios.
We updated our previous single-intervention simulations (29, 31, 34) to reflect model improvements and to provide a consistent benchmark for comparison (Fig. 2). These updated results are broadly consistent with those previously published. Here, we consider two vaccine intervention models: 1) a conventional vaccine model with tetravalent, durable efficacy against infection of 70% (hereafter D70E, with hypothetical efficacy based on the results in refs. 16–19; see Fig. S13 for alternative efficacy values) and 2) a natural infection-like model of CYD-TDV, which is initially perfectly efficacious but linearly wanes to zero efficacy over 2 y and contributes to infection history, administered only to individuals who test seropositive, with 20% false seronegative and 5% false seropositive rates (SI Appendix, Figs. S8–S11 show effects of alternative test performance assumptions). For both vaccine models, we assume that the efficacy is the probability that each exposure is resisted, also known as a “leaky” vaccine mechanism. Materials and Methods has full vaccine model details.
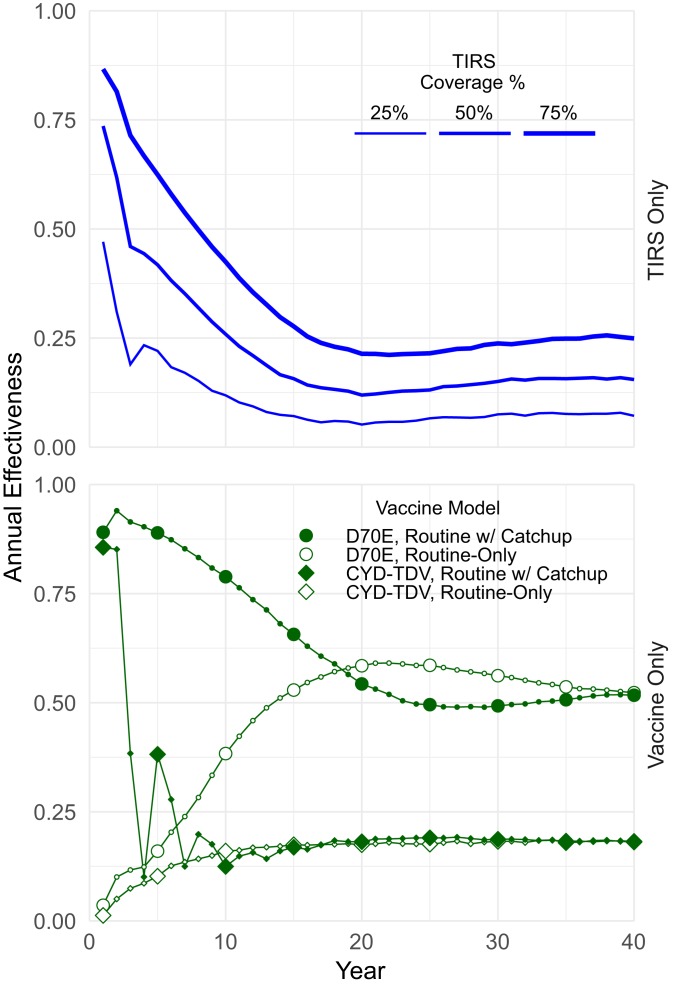
Fig. 2.
Simulated performance for single interventions. Overall annual effectiveness for TIRS-only (Upper; blue) and vaccine-only interventions (Lower; green). Results are aggregated annually, with some points enlarged for readability. We generally use the visual distinctions introduced in this figure throughout the manuscript.
At the population level, these distinct vaccine models initially perform indistinguishably, but CYD-TDV performance drops off after 2 y, coincident with waning protection at the individual level. As expected for both vaccine models, a catch-up campaign (i.e., a large-scale, one-time program covering individuals older than routine recipients) increases the population-level effect, but that effect wanes, either quickly with vaccine efficacy (CYD-TDV model) or more slowly with demographic turnover (D70E model), and performance eventually converges with conducting routine-only vaccination. TIRS has high early effectiveness but rapidly fades as naturally acquired population-level immunity wanes (29).
Combination Interventions.
We forecast performance of both vaccine mechanisms in combination with TIRS. One way to estimate population-level benefits of combined interventions is to assume that interventions prevent the same proportion of cases whether used separately or in combination. For example, if intervention prevents 50% of cases and intervention prevents 60%, we might estimate 20% [] to remain. However, interventions at the population scale may interact, which can be either amplifying (i.e., having greater effect than the naïve estimate) or interfering (i.e., having lesser effect). We illustrate this for our scenarios in Fig. 3; Fig. 3, Upper shows example independent interventions and corresponding naïve estimate benchmarks, and Fig. 3, Lower compares this benchmark with the actual simulated effectiveness. Because infectious disease systems are generally nonlinear (including our model) (SI Appendix, Fig. S7) and the individual interventions do not approach elimination, we expect amplification.
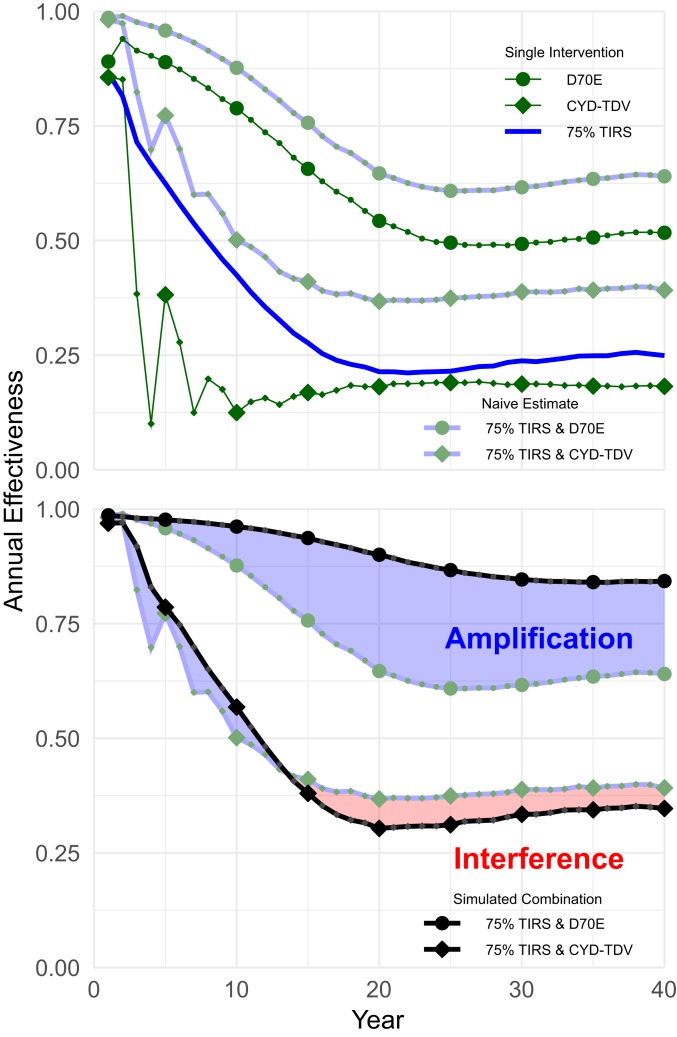
Fig. 3.
Estimated vs. simulated combined effectiveness. Naïve estimates of combination performance (Upper) calculated by assuming that single interventions act independently. Actual simulation (Lower) shows that performance of combined interventions may exceed (amplification) or fall short of (interference) naïve estimates.
As shown in Fig. 3, Lower, the most ambitious scenario—75% TIRS coverage combined with routine D70E vaccination and a one-time catch-up campaign—results in amplification that maintains population-level effectiveness above 95% for a decade. Under these circumstances, DENV circulation is only maintained in our model by external introductions, suggesting that elimination is plausible. In contrast, TIRS with CYD-TDV shows short-term, modest amplification and long-term interference.
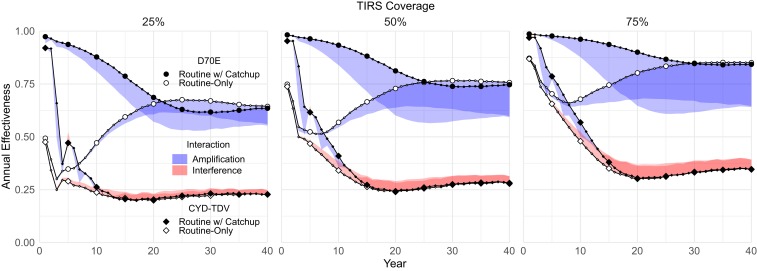
Fig. 4 shows similar trends for all of the vector control plus vaccine coverage scenarios. All combinations exceeded the performance of their individual components. In general, D70E has amplifying interactions with TIRS, while CYD-TDV had interfering ones. The interaction magnitude increases with increasing TIRS coverage. Vaccination programs with a catch-up campaign also increase the short-term interaction magnitude. This matches intuition: as interventions are scaled up, they have the potential to interact more strongly.
Fig. 4.
Combined intervention effectiveness. D70E combined with TIRS ([Left] 25%, [Center] 50%, and [Right] 75% coverage) quickly results in dengue control that equals or exceeds naïve estimates. Because CYD-TDV eligibility depends on past natural infection, reducing DENV transmission with TIRS leads to interference in our model.
CYD-TDV only shows amplification during the 10 to 15 y after a catch-up campaign. Catch-up vaccination candidates are older, and thus, they are more likely to be seropositive and eligible for the vaccine; after vaccination, this large group strictly benefits from future TIRS. For routine vaccination, however, each new cohort has reduced seroprevalence due to past TIRS and thus, reduced eligibility. This CYD-TDV model would still benefit seronegative recipients if they are ultimately likely to experience two or more infections (34), and therefore, declining eligibility reduces future benefits. This effect could be mediated by repeat testing (35), but we did not explore that in this work.
Staggered Starts for D70E Combination Interventions.
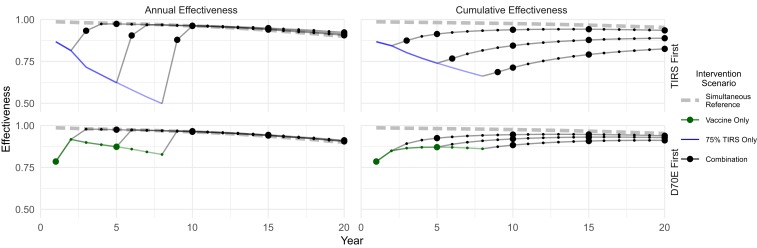
In some settings, beginning two ambitious interventions at the same time might be unrealistic, but a staggered introduction of the interventions might be feasible (e.g., one intervention is possible now, but the other is not ready to deploy, or both are available, but are too costly to start concurrently). Because both aggressive TIRS and routine D70E vaccination with a one-time catch-up campaign are individually highly effective in the first few years, concurrent starts might have little advantage. We evaluated combined interventions that do not begin at the same time (Fig. 5), instead delaying the second intervention by 2, 5, or 8 y. We also considered a less aggressive catch-up campaign for D70E: instead of all individuals older than age 2, we targeted only up to age 50 and spread the campaign over 2 y rather than completing it in 1 y. In all instances, we found that reducing the aggressiveness of the catch-up campaign had minimal consequences, and within 2 y of adding the second intervention, annual effectiveness was indistinguishable from starting interventions concurrently with the more intense catch-up campaign. The impact on cumulative effectiveness increased with the second intervention delay but was above 80% after 20 y in all scenarios.
Fig. 5.
Staggered starts for D70E combinations. Staggered starts (by 2, 5, and 8 y) for both TIRS followed by D70E (Upper) and D70E followed by TIRS (Lower) compared with concurrent start (dashed gray lines). Delaying either D70E or TIRS has limited impact: after the second intervention is introduced, annual effectiveness rapidly approaches that of simultaneous starts. All staggered scenarios have 75% TIRS coverage and routine vaccination with a smaller, slower, one-time catch-up campaign.
CYD-TDV Sensitivity to Mosquito Population Density.
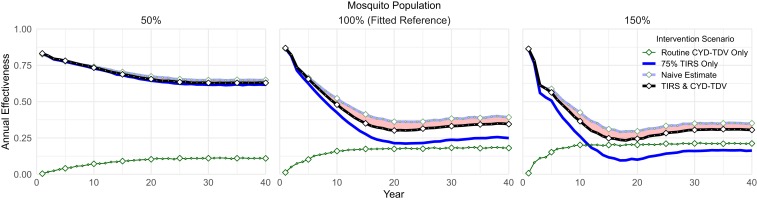
As shown previously (34), CYD-TDV vaccine effectiveness is sensitive to transmission intensity in the target population. To extend these results to combined interventions, we evaluated effectiveness of this vaccine mechanism for different transmission levels by varying mosquito population density (Fig. 6). CYD-TDV effectiveness still follows mosquito density and thus, transmission when serostatus testing is required. The interaction between CYD-TDV and TIRS remains interfering at all densities that we considered. At the lowest transmission level, however, this effect is greatly diminished, likely because 75% TIRS in a low-transmission setting causes individuals in the model to typically experience zero or one lifetime infection and thus, have no need for CYD-TDV. Shifting from the as fitted to the high-transmission setting, the long-term effectiveness of TIRS decreases by roughly half, implying reduced effect on seroprevalence in routine vaccination candidates and shrinking the interference deficit.
Fig. 6.
CYD-TDV sensitivity to mosquito population density. As transmission increases, long-term performance of routine CYD-TDV combinations approaches the naïve estimate. Combinations consistently outperform either intervention alone, although only marginally outperforming TIRS in the lowest transmission setting.
Discussion
Forecasts indicate that even ambitious vector control programs or historically high levels of durable, efficacious vaccine coverage would fail to reduce DENV transmission below the endemic threshold year round (29, 31, 34). Based on data for the state of Yucatán, DENV transmissibility is high enough that, given regular reintroductions, the long-term burden is only reduced by about half by even the most ambitious single-intervention strategies that we considered in our model. Reducing mosquito populations reduces dengue incidence for several years, but after population immunity declines as a result, even small numbers of mosquitoes can effectively transmit DENV (36). Similarly, for a leaky vaccine model, even the high coverage achieved by long running programs cannot prevent repeated exposures from producing cases. A higher force of infection, common in other dengue-endemic settings, would drive this benefit lower.
However, these interventions complement each other, contributing effectiveness when the other is lacking. Reasoning from a simple dynamics perspective suggests that combining them may yield consistent high effectiveness. We quantify this effect and confirm that the intuition holds for D70E and TIRS using a detailed model of DENV transmission in the state of Yucatán, Mexico. Our model clearly shows the advantages of integrating a new vector control intervention like TIRS in combination with an efficacious vaccine: an ambitious control scenario—75% TIRS coverage, 80% routine vaccination of 2-y-old children, and a large-scale, one-time catch-up campaign vaccinating older individuals—predicts a high, long-term impact: >83% reduction in cases every year for 40 y. Short-term effectiveness was even better, with >96% effectiveness for the first decade and >90% for the first 20 y. If ongoing phase III trials for new dengue vaccines show efficacy comparable with or better than the 70% that we assume, these results suggest that elimination could be possible with a more expansive deployment of this strategy as under these conditions, the model only sustains dengue by occasional external reintroductions. Dengue control organizations have long advocated for integrated interventions, and this model can aid in allocating a finite budget to components of an integrated program. The appropriate allocation would be determined by more conclusive empirical evidence for intervention efficacies as well as other logistical concerns. For example, a sufficiently high-efficacy vaccine would lessen the need for extensive new vector control for dengue, but vector control might reduce the burden of other Aedes-borne diseases. Limited supply and high demand for a new, highly efficacious vaccine might make additional vector control more practical than a one-time mass vaccination campaign in order to achieve early effectiveness, while a low-efficacy vaccine would suggest more emphasis on vector control over a long time horizon (SI Appendix, Fig. S13 has alternative vaccine efficacy results).
Even without prioritizing elimination, understanding the benefits of combining interventions may justify larger investments in control programs. One might naïvely assume that because vector control and vaccination are independent interventions with, for example, individual effectiveness rates of 25 and 50% after 20 y, respectively, the combination would jointly prevent 62.5% of cases. However, that treats the interventions as if they only prevented symptoms and not infections (i.e., a linear effect) and ignores reductions to onward transmission. For interventions that reduce transmission, amplification may be possible: infectious disease spread in general is exponential, saturating as the system approaches infection of the entire susceptible population. For a disease as transmissible as dengue, a single intervention might not be enough to reduce cases much below the saturating limit, while combined interventions could still reduce transmission close to or in principle, below the epidemic threshold. We see these enhanced returns for D70E and TIRS combinations: for the 25 and 50% effectiveness example above, we actually forecast an 83% reduction rather than 62.5%. This amplifying interaction generally fails for CYD-TDV, however, because the vaccine’s benefits vary with the rate of past infection.
The interference that we predict between CYD-TDV and TIRS should be expected for CYD-TDV combined with any intervention that reduces the rate of natural infection. For the scenarios that we considered, the CYD-TDV and TIRS combination still outperformed either single approach, but the modest benefit of combining interventions implies lower cost-effectiveness per incremental investment compared with either intervention alone. In some low-transmission scenarios that we evaluated (SI Appendix), we observed actual net negatives, indicating that adding CYD-TDV to TIRS could perform worse than TIRS alone.
Beyond the interventions considered here, we would expect control programs that are truly mechanistically independent to show amplification when combined. If one of the interventions depends on a dynamic process that the other prevents, however, interference becomes possible.
Although there can be substantial benefits to combining interventions, simultaneously starting both could be logistically challenging in regions with a high dengue burden. Additionally, although candidate vaccines with similar performance may be on the horizon, D70E is still hypothetical, while TIRS (and other novel vector control approaches) could be deployed relatively soon. To address these potential challenges, we investigated staggering the start dates of the two interventions. These more gradual approaches to a combined intervention strategy are promising: the lower initial annual effectiveness associated with delaying either of the interventions recovers to the level of the concurrent start within a year of adding the second intervention. We also considered catch-up campaigns with a more restricted demographic group in this analysis, which had no discernible impact on intervention performance.
While the reasons for staggering campaigns might also suggest stopping vector control after some period, we did not investigate that option. Vector control might cease unintentionally due to other circumstances (for example, civil unrest), but a deliberate decision to cease vector control seems unlikely: vaccination might be sufficient for dengue, but Aedes control reduces transmission of multiple diseases. Furthermore, past results indicate that ceasing vector control, whether intentional or incidental, can lead to extreme outbreaks (29).
We also did not consider alternative timings for CYD-TDV combinations as we found that effectiveness was consistently below 40% after 15 y, regardless of whether it was coupled with TIRS. The accepted model of CYD-TDV is essentially one of protection against disease and only for vaccinees with some level of prior dengue immunity (22, 24). Although interventions involving CYD-TDV generally reduced the dengue disease burden in our model, CYD-TDV is likely not compatible with a strategy to eliminate or even substantially reduce dengue transmission for more than a few years. As transmission decreased, the vaccine became less useful, whether due to TIRS (in combination strategies) or CYD-TDV itself (in catch-up campaigns). This outcome should be carefully considered in locations contemplating using the CYD-TDV vaccine in combination with new vector control efforts.
Building a combined intervention program by deploying its components in different years is just one way to address logistical constraints. More generally, deciding how best to allocate finite resources among multiple interventions remains an open question to be regularly revisited as new data become available and additional products enter the market. While single interventions might, in principle, be able to achieve the predicted level of effectiveness of combined interventions, they would require very high individual efficacy and coverage. Those high-performance requirements are likely to be more difficult to achieve than introducing a combined intervention built with components that have good, but not astonishing, performance. Combining interventions may also have impacts beyond what we have explored here; for example, reduction of Aedes populations will have benefits with respect to other diseases spread by the vector.
As our understanding of the mode of action, performance, and costs of available interventions improves, the question of which combinations are most effective will need to be revisited. We show here, however, that interventions can be strongly synergistic, offering public health officials a more powerful control strategy than might be naïvely anticipated.
Materials and Methods
Using an agent-based model of dengue transmission in Yucatán, Mexico, we simulated the impact of a range of single and combined interventions; SI Appendix has full details. The transmission model was fit to dengue case, serotype, and seroprevalence data using AbcSmc (37) and then, run until dengue dynamics were stable. Each intervention scenario was simulated 10,000 times (1,000 parameter combinations from the fitted posterior each repeated with 10 different random seeds).
TIRS Vector Control Model.
TIRS campaigns occur annually. During a 90-d rollout period starting in late May (specifically Julian day 147, the optimal date identified in ref. 29), 75% of houses, randomly drawn each year, are treated with an insecticide that remains active for 90 d. Treatment date for individual houses is chosen randomly within the rollout period. In actively protected households, the insecticide causes 13% excess daily vector mortality, which results in an 80% reduction in the hypothetical mosquito population size at that location as well as shorter lifespans for infectious mosquitoes.
Vaccine Models.
We consider two vaccine efficacy models, which we refer to as D70E (representing a performance estimate for the vaccines currently in trials) and CYD-TDV (intended to explicitly model that vaccine).
The simple efficacy vaccine, D70E, is similar to but simpler than the vaccine used in ref. 31: durable and 70% efficacious against all serotypes conferred in a single dose. Vaccine performance is not affected by serostatus of the recipient. When breakthrough infections occur, we assume that the vaccine prevents 80.3% of severe cases, which instead manifest as nonsevere symptomatic cases (12). This vaccine model is plausible for some of the vaccines that are in phase II and phase III trials (17, 38) and serves a working estimate until successful licensure of one or more of those products. We also provide results for 50 and 90% efficacy vaccines for this mechanism in SI Appendix, which can provide guidance on how intermediate efficacy values that we did not simulate would perform.
The CYD-TDV model is based on the mechanism identified by ref. 22 as providing the best explanation of the CYD-TDV trial data and is the same model used by the UF modeling group in ref. 34. CYD-TDV is modeled as a three-dose vaccine delivered at 6-mo intervals only administered to individuals who test seropositive. The vaccine mechanism includes three effects: 1) immunity against infection (supported by ref. 39) that wanes linearly following each dose, from perfect to nothing over 2 y, resulting in a complete loss of immunity against infection after 3 y from the initial dose; 2) initial immunity against severe disease of 80.3% in the case of breakthrough infections, which also wanes linearly to nothing over 2 y from the most recent dose; and 3) the vaccine increments infection history like a natural infection, meaning that individuals who falsely test seropositive are at enhanced disease risk on their first natural infection. Seropositive recipients thus unambiguously benefit from the vaccine, if only temporarily, while seronegative recipients eventually have an increased risk of disease from their next natural infection. Consistent with current WHO guidance (25), the vaccine is only administered to seropositive individuals, which we determine using a serological test. In the results, we assume a test with 5% false seropositive rate and 20% false seronegative rate; SI Appendix has additional details and consequences of alternate test characteristics.
Vaccination with either mechanism probabilistically prevents infections when an exposure occurs based on the vaccines efficacy at that time. Over each simulated day, infectious mosquitoes may bite individuals who are at the same locations (i.e., house, workplace, or school). Those individuals who do not have naturally acquired immunity (i.e., no previous infection with the exposure serotype and no infection with any serotype within the last 2 y) are considered exposed due to such bites. If exposed individuals have received a vaccine, they can resist infection with probability equal to the vaccine efficacy. For durable vaccines, efficacy is constant; for waning vaccines, efficacy depends on the time since the individual was vaccinated. For both models, every exposure is an independent chance to break through the vaccine protection (i.e., the vaccine efficacy model is leaky).
The two vaccine models have different target age eligibility. For CYD-TDV, we consider routine-only vaccination of 9-y-old children and one-time catch-up campaigns for 10- to 50-y-old individuals. Because CYD-TDV is only recommended for use in seropositive candidates, the size of the eligible population is affected by recent dengue epidemics. In our fitted model population, seroprevalence of 9-y-old children has a median of 56% (interquartile range: [51, 63%]) across replicates at steady state (SI Appendix has additional characterization of the synthetic population). After interventions are introduced, dengue incidence generally decreases, and thus, so does seroprevalence among 9-y-old children, decreasing the eligible population for CYD-TDV.
For D70E, for all but the staggered start date combinations shown in Fig. 5, we consider routine-only vaccination of 2-y-old children and one-time catch-up campaigns for 3 y and older. For the results in Fig. 5, we limit the D70E catch-up campaign to 3- to 50-y-old individuals spread over 2 y. For both vaccine models and both routine administration and the one-time catch-up campaign, we consider 80% of the target group for vaccine coverage. Since CYD-TDV is only administered to test-seropositive individuals, the realized coverage may be lower.
Effectiveness and Interaction Calculations.
We calculate effectiveness (annually or cumulatively) for a single or combination intervention strategy as
The effectiveness difference between simulated and estimated combined interventions of a vaccination strategy and a mosquito control strategy is
To calculate these values, we match baseline and intervention runs by parameter combination and report medians in the text; interquartile prediction intervals are in SI Appendix.
Data Availability
All code necessary to reproduce the simulation results is available from GitHub, https://github.com/tjhladish/dengue. SI Appendix documents the detailed data sources. IPUMS-International microcensus data may be obtained by request from https://international.ipums.org/international/; all other data are publicly available from the sources indicated.
Supplementary Material
Acknowledgments
This project was funded in part by NIH/National Institute of General Medical Sciences Grant U54 GM111274 and NSF Grant Division of Environmental Biology/Ecology and Evolution of Infectious Diseases (DEB/EEID) 1640698. We also thank Juliet R. C. Pulliam and Stefan Flasche for feedback on the draft manuscript.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1903496117/-/DCSupplemental.
References
- 1.Bhatt S., et al. , The global distribution and burden of dengue. Nature 496, 504–507 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization , Dengue: Guidleines for Diagnosis, Treatment, Prevention and Control (WHO/TDR, Geneva, Switzerland, 2009). [PubMed] [Google Scholar]
- 3.San Martín J. L., et al. , The epidemiology of dengue in the Americas over the last three decades: A worrisome reality. Am. J. Trop. Med. Hyg. 82, 128–135 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzman M. G., et al. , Dengue: A continuing global threat. Nat. Rev. Microbiol. 8(suppl. 12), S7–S16 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady O. J., et al. , Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Neglected Trop. Dis. 6, e1760 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esu E., Lenhart A., Smith L., Horstick O., Effectiveness of peridomestic space spraying with insecticide on dengue transmission; systematic review. Trop. Med. Int. Health 15, 619–631 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Bowman L. R., Donegan S., McCall P. J., Is dengue vector control deficient in effectiveness or evidence?: Systematic review and meta-analysis. PLoS Neglected Trop. Dis. 10, 1–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiner R. C., Jr, et al. , Quantifying the epidemiological impact of vector control on dengue. PLoS Neglected Trop. Dis. 10, 1–11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett A. D. T., The reemergence of yellow fever. Science 361, 847–848 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Roiz D., et al. , Integrated Aedes management for the control of Aedes-borne diseases. PLoS Neglected Trop. Dis. 12, 1–21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achee N. L., et al. , A critical assessment of vector control for dengue prevention. PLoS Neglected Trop. Dis. 9, e0003655 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villar L., et al. , Efficacy of a tetravalent dengue vaccine in children in Latin America. N. Engl. J. Med. 372, 113–123 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Capeding M. R., et al. , Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 384, 1358–1365 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Sridhar S., et al. , Effect of dengue serostatus on dengue vaccine safety and efficacy. N. Engl. J. Med. 379, 327–340 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Schwartz L. M., Halloran M. E., Durbin A. P., Longini I. M. Jr, The dengue vaccine pipeline: Implications for the future of dengue control. Vaccine 33, 3293–3298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osorio J. E., et al. , Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: A randomised, placebo-controlled, phase 1 study. Lancet Infect. Dis. 14, 830–838 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sáez-Llorens X., et al. , Safety and immunogenicity of one versus two doses of Takeda’s tetravalent dengue vaccine in children in Asia and Latin America: Interim results from a phase 2, randomised, placebo-controlled study. Lancet Infect. Dis. 17, 615–625 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Kirkpatrick B. D., et al. Robust and balanced immune responses to all 4 dengue virus serotypes following administration of a single dose of a live attenuated tetravalent dengue vaccine to healthy, flavivirus-naive adults. J. Infect. Dis. 212, 702–710 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkpatrick B. D., et al. , The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci. Transl. Med. 8, 330ra36–330ra36 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Katzelnick L. C., et al. , Dynamics and determinants of the force of infection of dengue virus from 1994 to 2015 in Managua, Nicaragua. Proc. Natl. Acad. Sci. U.S.A. 42, 10762–10767 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katzelnick L. C., et al. , Antibody-dependent enhancement of severe dengue disease in humans. Science 358, 929–932 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson N. M., et al. , Benefits and risks of the Sanofi-Pasteur dengue vaccine: Modeling optimal deployment. Science 353, 1033–1036 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y., Meng Y., Halloran M. E., Longini I. M Jr, Dependency of vaccine efficacy on preexposure and age: A closer look at a tetravalent dengue vaccine. Clin. Infect. Dis. 66, 178–184 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilder-Smith A., et al. , Deliberations of the strategic advisory group of experts on immunization on the use of CYD-TDV dengue vaccine. Lancet Infect. Dis. 19, e31–e38 (2018). [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization , Dengue vaccine: WHO position paper, September 2018 - recommendations. Vaccine 37, 4848–4849 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Dzul-Manzanilla F., et al. , Indoor resting behavior of Aedes aegypti (diptera: Culicidae) in Acapulco, Mexico. J. Med. Entomol. 54, 501–504 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Chadee D. D., Resting behaviour of Aedes aegypti in Trinidad: With evidence for the re-introduction of indoor residual spraying (IRS) for dengue control. Parasites Vectors 6, 255 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vazquez-Prokopec G. M., Montgomery B. L., Horne P., Clennon J. A., Ritchie S. A., Combining contact tracing with targeted indoor residual spraying significantly reduces dengue transmission. Sci. Adv. 3, e1602024 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hladish T. J., et al. , Forecasting the effectiveness of indoor residual spraying for reducing dengue burden. PLoS Neglected Trop. Dis. 12, e0006570 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunbar M. W., et al. , Efficacy of novel indoor residual spraying methods targeting pyrethroid-resistant Aedes aegypti. PLoS Neglected Trop. Dis. 13, e0007203 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hladish T. J., et al. , Projected impact of dengue vaccination in Yucatán, Mexico. PLoS Neglected Trop. Dis. 10, e0004661 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamoto K. W., Gould F., Lloyd A. L., Integrating transgenic vector manipulation with clinical interventions to manage vector-borne diseases. PLoS Comput. Biol. 12, e1004695 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson M. A., Hombach J., Cummings D. A. T., Models of the impact of dengue vaccines: A review of current research and potential approaches. Vaccine 29, 5860–5868 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flasche S., et al. , The long-term safety, public health impact, and cost-effectiveness of routine vaccination with a recombinant, live-attenuated dengue vaccine (Dengvaxia): A model comparison study. PLoS Med. 13, e1002181 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson C. A. B., Abbas K. M., Clifford S., Flasche S., Hladish T. J., Serostatus testing & dengue vaccine cost-benefit thresholds. J. R. Soc. Interface 16, 20190234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ooi E.-E., Goh K.-T., Gubler D. J., Dengue prevention and 35 years of vector control in Singapore. Emerg. Infect. Dis. 12, 887–893 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hladish T. J., Sequential Monte Carlo approximate Bayesian computation with partial least squares parameter estimator. GitHub. https://github.com/tjhladish/abcsmc/ Accessed 4 December 2019.
- 38.Perkel J. M., NIH dengue vaccine leaps into phase 3 studies. Nat. Biotechnol. 34, 449 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Olivera-Botello G., et al. , Tetravalent dengue vaccine reduces symptomatic and asymptomatic dengue virus infections in healthy children and adolescents aged 2–16 years in Asia and Latin America. J. Infect. Dis. 214, 994–1000 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All code necessary to reproduce the simulation results is available from GitHub, https://github.com/tjhladish/dengue. SI Appendix documents the detailed data sources. IPUMS-International microcensus data may be obtained by request from https://international.ipums.org/international/; all other data are publicly available from the sources indicated.