Abstract
Endophytic bacteria (EB) are both a novel source of bioactive compounds that confer phytopathogen resistance and inducers of secondary metabolites in host plants. Twenty-seven EB isolated from various parts of Metasequoia glyptostroboides, Ginkgo biloba, Taxus brevifolia, Pinus densiflora, Salix babylonica, and S. chaenomeloides could produce salicylic acid (SA). The highest producers were isolates EB-44 and EB-47, identified as Pseudomonas tremae and Curtobacterium herbarum, respectively. Nicotiana benthamiana grown from EB-44-soaked seeds exhibited a 2.3-fold higher endogenous SA concentration and increased resistance against P. syringae pv. tabaci, the causative agent of tobacco wildfire disease, than plants grown from water-soaked seeds. N benthamiana and N. tabacum grown from EB-44-treated seeds developed 33% and 54% disease lesions, respectively, when infected with P. syringae pv. tabaci, and showed increased height and weight, in addition to 4.6 and 1.4-fold increases in nicotine accumulation, respectively. The results suggest that SA-producing EB-44 can successfully colonize Nicotiana spp., leading to increased endogenous SA production and resistance to tobacco wildfire disease. The newly isolated EB can offer an efficient and eco-friendly solution for controlling wildfire disease and nicotine accumulation in Nicotiana, with additional application for other important crops to increase both productivity and the generation of bioactive compounds.
Keywords: Pseudomonas tremae, endophytic bacteria, salicylic acid, disease resistance, wildfire disease, nicotine synthesis
1. Introduction
Endophytes comprise fungi or bacteria that colonize plant tissues without harming the host plant [1]. Bioactive compounds from endophytes, such as Bacillus, Pseudomonas, and Rhizobium, are known to promote host plant growth [2,3], whereas endophytic bacteria (EB) can confer resistance to pathogen-induced diseases in the host plant [4,5,6,7,8]. Bacterial species such as P. fluorescens (strain CHA0), P. aeruginosa (7NSK2), and Serrtia macrcescens (strain 90-166) produce salicylic acid (SA) and their colonization of host plants increases the endogenous SA levels, in addition to enhancing host defences [9,10,11]. The results of EB application from several studies have indicated that SA promotes plant growth and stimulates plant defence mechanisms by inducing systemic acquired resistance (SAR) [12,13,14].
Wildfire disease caused by Pseudomonas syringae pv. tabaci (Pst) is one of the most destructive tobacco diseases [15,16,17]. This disease is characterized by the emergence of small brown or black water-soaked lesions surrounded by a broad, chlorotic halo on leaves, resulting from exotoxin production by Pst [18]. An integrated approach for the management of wildfire disease consists of (i) using seeds treated with silver nitrate; (ii) using certified tobacco seeds, rotating seedbed sites, and properly fumigating seedbed areas; (iii) spraying with a suitable copper-based compound and acibenzolar-S-methyl; (iv) spraying with antibiotics; and (v) using wildfire-resistant cultivars [18,19]. Application of copper-based compounds in combination with acibenzolar-S-methyl and streptomycin is especially effective at controlling the disease [18]. Since 1955, streptomycin has been used as a major antibiotic to control various bacterial diseases, including fire blight, soft rot, bacterial spot, and wildfire diseases of different crops [20]. However, streptomycin-resistant bacterial strains have emerged because of indiscriminate and long-term use of this antibiotic [21,22]. A recent study showed that tobacco wildfire disease infection rates were significantly decreased by the foliar application of a bacterial mix of Bacillus (87.74%), Alcaligenes (7.69%), Pseudochrobactrum (2.86%), and Achromobacter (1.05%) [23]. Although development of wildfire-resistant cultivars may be an alternative and eco-friendly method of controlling the disease, this process is time-consuming and laborious and these cultivars are not widely available. Therefore, development of a practical and convenient alternative for controlling wildfire disease is urgently required.
Nicotine, a plant alkaloid, is the most important compound in tobacco [24]. Nicotine plays important roles in tobacco plants, including in protection against insects and herbivores and regulation of plant growth [25,26]. Inoculation with phytopathogenic Pst and P. syringae pv. tomato results in increased nicotine levels in tobacco plants [27,28,29], while multiple signal molecules, including jasmonic acid (JA) and auxin, can also stimulate the synthesis of nicotine [30]. Numerous studies have reported the isolation of nicotine-degrading bacteria, including Pseudomonas sp. [31,32], Ochrobactrum intermedium [33], Rhodococcus sp. [34], and Agrobacterium sp. S33 [35]. To the best of our knowledge, however, there are no reports indicating that EB treatment promotes nicotine accumulation in tobacco.
In this study, we isolated EB from different tissues of six plant species and measured SA production. Endophytic bacteria that constitutively produce SA could provide a means to develop SAR against wildfire disease in tobacco through successful colonization of the host tobacco plants. To investigate the potential beneficial effects of SA-producing EB on host plants, we applied SA-producing EB to Nicotiana benthamiana and N. tabacum L. and evaluated resistance against wildfire disease, plant growth, and nicotine accumulation.
2. Materials and Methods
2.1. Collection of Plant Materials and Isolation of EB
Leaves, fruits, seeds, and cones of gymnosperm and angiosperm species, including Metasequoia glyptostroboides, Ginkgo biloba, Taxus brevifolia, Pinus densiflora, Salix babylonica, and S. chaenomeloides, were collected from Yeungnam University campus, Gyeongsan, Republic of Korea, in October 2014. The collected plant material was immediately taken to the laboratory in plastic bags for EB isolation. Plant tissues with visible superficial injury were excluded, and only healthy tissues were used for EB isolation.
Isolation of EB was carried out as per the standard procedure [36]. To isolate single EB colonies, the ground tissue extracts were diluted 1 × 10−1 and 10−2-fold with a sterilized NaCl solution (0.9%), spread onto plates containing four different types of media (25% yeast extract, nutrient broth, and agar (YNA), 25% nutrient agar (NA), de Man, Rogosa, and Sharpe (MRS), and Luria–Bertani (LB)), and incubated for up to 15 days at 28 °C. All the colonies were counted and the values were expressed as colony-forming units (CFUs) per gram of fresh tissue. This single colony isolation procedure was repeated three times for confirmation of each isolated EB, and morphological characteristics such as the form, margin, colour, and height of the colonies were documented. Isolated EB were cultured in the original isolation media and stored as 50% glycerol stocks at −80 °C until use.
The isolated EB were identified based on 16S rRNA gene sequencing. Sequencing data, alignment, and the constructed phylogenetic tree were analysed using Molecular Evolutionary Genetics Analysis (MEGA) software [37,38].
2.2. Measurement of SA Production in Isolated EB and Host Plants
In vitro screening for SA-producing EB was carried out following the standard protocol [9,10], with slight modifications. For inoculation, 20 µL aliquots of the glycerol stocks of each EB culture were diluted in 5 mL of casamino acid broth (CAB) in 50 mL Falcon tubes and incubated at 28 °C for 36 h at 180 rpm in the dark. Subsequently, 100 µL of this culture was transferred to 30 mL of CAB, incubated for 36 h, and centrifuged at 3500 rpm for 15 min at 4 °C, and the supernatant was collected. The pH of the supernatant was adjusted to ~1.5–2.0 with 1 M HCl, following which the supernatant was mixed with an equal volume of ethyl acetate and incubated overnight at room temperature. The ethyl acetate layer was collected the following day and evaporated at 50 °C on a rotary evaporator (A-1000S, EYELA, Tokyo, Japan). The dried extracts were collected in tubular glass vials, suspended in 1 mL of HPLC-grade methanol, and then filtered through a 0.45-µm syringe filter (SN-01345, Thermo Fisher Scientific, Waltham, MA, USA).
Endogenous SA was quantified according to the method described by Press et al. [10], with minor modifications. The filtrated 20 µL solution of each EB was injected into an HPLC system consisting of a pump equipped with an Eclipse XDB-C18 column (5 µm, 4.6 × 250 mm, Agilent Technologies, Santa Clara, CA, USA) and a UV detector (YL9100, Young-Lin, Korea). The isocratic mobile phase containing 0.2 M sodium acetate buffer (pH 5.5) in 10% methanol was applied at a flow rate of 0.8 mL/min. The wavelength and column temperature were set at 302 nm and 40 °C, respectively. The levels of SA were quantified by comparing the area of the corresponding peaks with the standard curve created using different concentrations (500, 250, 125, 62.5, and 31.25 µg/mL) of free SA (Duchefa Biochemie, Haarlem, The Netherlands). Quantification of the SA produced by the EB was repeated three times using HPLC.
The concentrations of free SA in the fresh leaves of M. glyptostroboides, G. biloba, T. brevifolia, Pinus densiflora, S. babylonica, S. chaenomeloides, and N. benthamiana were measured, as described by Dhakal et al. [39], using approximately 0.5 g of leaves.
2.3. Growth of Tobacco Species Inoculated with SA-Producing EB
Among the 27 EB isolates that produced SA, the highest SA-producing strains, EB-44 and EB-47, and a non-SA-producing strain, EB-52, were grown in culture broth, centrifugated at 3500 rpm for 15 min at 4 °C, and resuspended to OD600 0.5 in 10 mM MgCl2. As a positive control, 1 mM salicylic acid in 0.3% Tween 20 was sprayed on 7-week-old N. tabacum plants growing in a glasshouse.
Seeds of N. benthamiana and N. tabacum were soaked for 3 h in a suspension containing SA-producing EB at OD600 = 0.5 or 10 mM MgCl2. The seeds were then sown in plastic pots (12 cm diameter × 10 cm height) containing 17% peat moss, 70% coco peat, 5% zeolite, and 8% perlite and grown in a controlled walk-in chamber. Seedlings of N. tabacum were transferred to the glasshouse for further experiments and N. benthamiana seedlings were grown under fluorescent light at 120 μE/(m2·s) under a 16/8 h light/dark photoperiod at 23 °C in a walk-in chamber.
2.4. Disease Assay of Pst-Infected Tobacco Species
Disease assays were performed by infecting N. benthamiana and N. tabacum with the wildfire disease pathogen Pst. The bacteria were grown at 28 °C in King B medium supplemented with 50 mg/mL rifampicin. Overnight bacterial cultures were harvested by centrifugation at 3500 rpm for 15 min at 4 °C and then resuspended at OD600 0.1 in 10 mM MgCl2. Leaves of 4-week-old N. benthamiana and 6-week-old N. tabacum plants were infiltrated with 0.1 mL of bacterial suspensions using a 1 mL needleless syringe. Lesion areas were measured at 6 dpi using a digital Vernier calliper.
2.5. Pathogenicity Test of the Isolated SA-Producing EB
To test whether the SA-producing EB-44 and EB-47 isolates were pathogenic to N. benthamiana plants, the isolates were infiltrated into the leaves of N. benthamiana at a concentration of OD600 0.1. Pst and 10 mM MgCl2 were used as positive and negative controls, respectively. The incidence of wildfire disease symptoms was assessed up to 5 dpi.
2.6. Reisolation and Confirmation of SA-Producing EB
Reisolation of SA-producing EB isolates was performed to determine whether the EB could colonize the interior of N. benthamiana plants and affect their growth. Nicotiana benthamiana seeds were soaked in a suspension containing the SA-producing EB-44 at OD600 = 0.5 for 3 h and then grown for 4 weeks; the EB were subsequently reisolated from the leaves as described in the previous section. Nicotiana benthamiana seeds soaked in 10 mM MgCl2 were used as a control for comparison of plant growth. To confirm that the reisolated EB were the same as the original SA-producing isolate, the 16S rRNA gene sequences of the original and reisolated EB were compared. The experiment was repeated twice, with four replicates.
2.7. Quantification of Nicotine production in Tobacco Species Co-Cultivated with EB
Approximately 0.5 g of leaves collected from the upper parts of 4-week-old N. benthamiana and 8-week-old N. tabacum plants were ground in liquid nitrogen using a mortar and pestle and mixed with 3.5 mL of 90% methanol. The ground sample was sonicated in a sonicating water bath for 10 min to lyse the cells, and was then incubated for 3 h at room temperature at 100 rpm. The supernatant was collected in Eppendorf tubes, centrifuged at 14,000 × g for 10 min at 4 °C, and filtered using a 0.22 µm nylon filter for LC–MS analysis.
The filtered samples were transferred to autosampler vials and 5 µL aliquots were analysed by LC–MS. Nicotine levels were quantified using a LC–MS system composed of an HPLC apparatus (Model 2695; Waters, Milford, MA, USA) equipped with a reversed phase column (Luna C18, 4.6 × 150 mm, 5 μm; Phenomenex, Torrance, CA, USA) and a mass spectrometer (Model 3100; Waters). For HPLC, the conditions were as follows: the injection volume was 5 µL; the mobile phase consisted of 20 mM ammonium acetate buffer (solvent A, pH 7.2) and acetonitrile (solvent B); the gradient elution program was 50:50 (A/B) from 0 to 5 min, and a linear increase of B to 100% to 7 min, before returning to 50:50 (A/B) at 10 min at a flow rate of 0.5 mL/min. For the MS, the desolvation gas (N2) flow rate was 4 L/h, desolvation temperature was 350 °C, capillary voltage was 4 kV, cone voltage was 30 V, ionization mode was set to electrospray positive, and single ion recording was set at m/z = 163.
2.8. Statistical Analysis
All data are expressed as means ± standard deviations (SDs) of three or four independent replications from each experiment. Statistical analysis of the results was conducted using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test at p < 0.05 using the Statistical Analysis Software (SAS) (Version: SAS 9.4; SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Isolation of EB and Measurement of SA in EB and the Host Plants
In total, 134 EB were isolated from various tissues of the six selected plant species; namely: Metasequoia glyptostroboides, Ginkgo biloba, Taxus brevifolia, Pinus densiflora, Salix babylonica, and S. chaenomeloides. Ginkgo biloba and M. glyptostroboides were selected because both species are considered living fossil plants. Ginkgos are an ancient plant line, with the earliest representatives having been found in approximately 280-million-year-old rocks from the Permian age. Metasequoia glyptostroboides was first identified as a living fossil species in 1941, and its unmineralized stumps and leaves from the warm Eocene Epoch approximately 45 million years ago can still be observed. Taxus brevifolia and P. densiflora were selected as gymnosperms with medicinal uses, while Salix babylonica and S. chaenomeloides were selected as angiosperms and based on their accumulation of SA in their bark.
The SA concentrations in the leaves of M. glyptostroboides, G. biloba, T. brevifolia, P. densiflora, S. babylonica, S. chaenomeloides, and N. benthamiana were analysed. The leaves of S. babylonica and N. benthamiana presented SA levels of 966.50 µg/g and 833.67 µg/g of fresh weight (FW), respectively. The SA concentrations in the leaves of M. glyptostroboides, G. biloba, T. brevifolia, P. densiflora, and S. chaenomeloides were below the detectable limit. Endophytic bacteria were isolated from leaves (73 isolates, 54.48%), fruits (49 isolates, 36.57%), seeds (eight isolates, 5.97%), and cones (four isolates, 2.98%) (Table 1). Tissue-specific EB colony-forming units (CFUs) per gram of fresh tissue were in the range of 1.8 × 102 to 6.0 × 104 CFUs/g in fruits and 1.8 × 103 to 1.5 × 105 CFUs/g in leaves, and presented values of 7.2 × 103 CFUs/g in seeds and 1.2 × 103 CFUs/g in cones (Table 1).
Table 1.
Isolation of salicylic acid-producing endophytic bacteria from six plant species.
| Plant Species | Total no. of Isolates | Tissue | No. of Isolates | CFUs/g of Fresh Tissue |
No. of EB Isolates | |
|---|---|---|---|---|---|---|
| SA | No SA | |||||
|
Metasequoia
glyptostroboides |
8 | Leaves | 4 | 3.3 × 104 | 0 | 4 |
| Cones | 4 | 1.2 × 103 | 2 | 2 | ||
| Ginkgo biloba | 50 | Leaves | 11 | 1.8 × 103 | 1 | 10 |
| Fruits | 39 | 6.0 × 104 | 1 | 38 | ||
| Taxus brevifolia | Leaves | 23 | 8.6 × 104 | 2 | 21 | |
| 41 | Seeds | 8 | 7.2 × 103 | 0 | 8 | |
| Fruits | 10 | 1.8 × 102 | 1 | 9 | ||
| Pinus densiflora | 2 | Leaves | 2 | 9.6 × 103 | 0 | 2 |
| Salix babylonica | 24 | Leaves | 24 | 1.5 × 105 | 20 | 4 |
| Salix chaenomeloides | 9 | Leaves | 9 | 5.9 × 104 | 0 | 9 |
| Total | 134 | 134 | - | 27 | 107 | |
EB: endophytic bacteria; SA: salicylic acid; CFUs = colony forming units.
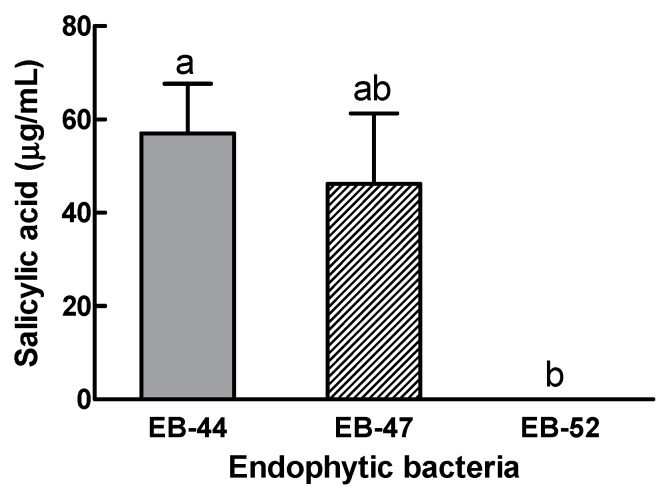
When EB were identified by HPLC as producing SA, the peaks of the EB extracts exactly matched the retention time of the SA standard at 6.9 min. Of the 134 EB isolates, 27 could produce SA, and the highest percentage of SA-producing EB was obtained from S. babylonica (74.07%) (Table 1). When the SA-producing EB were identified by 16S rRNA gene sequencing (Supplementary Table S1), the two EB producing the greatest quantities of SA were Pseudomonas tremae (EB-44, 57.05 µg/mL) and Curtobacterium herbarum (EB-47, 46.22 µg/mL) (Figure 1 and Supplementary Table S1). The constructed phylogenetic tree indicated that the 16S rRNA sequences of EB-44 and EB-47 showed the greatest similarities to those of P. tremae TO1 and C. herbarum P420/07, respectively. These two SA-producing isolates (EB-44 and EB-47), along with one non-SA-producing isolate (EB-52; C. plantarum) as a control, were selected for further experiments. EB-44, EB-47, and EB-52 were isolated from the leaves of S. babylonica.
Figure 1.
Salicylic acid concentrations in EB-44 (Pseudomonas tremae), EB-47 (Curtobacterium herbarum), and EB-52 (C. plantarum) isolated from the leaves of Salix babylonica. Data represent means ± standard deviations of four independent replications for each treatment. Means with different letters are significantly different at p < 0.05 by Duncan’s multiple range test.
Pathogenicity tests were conducted to confirm the non-toxicity and non-pathogenicity of EB-44, EB-47, and EB-52 on N. benthamiana. In contrast to the brown lesions induced by Pst infiltration, no visible toxicity or disease symptoms were elicited in N. benthamiana leaves in response to EB infiltration.
3.2. Disease Resistance in Nicotiana plants Grown from Seeds Treated with SA-Producing EB
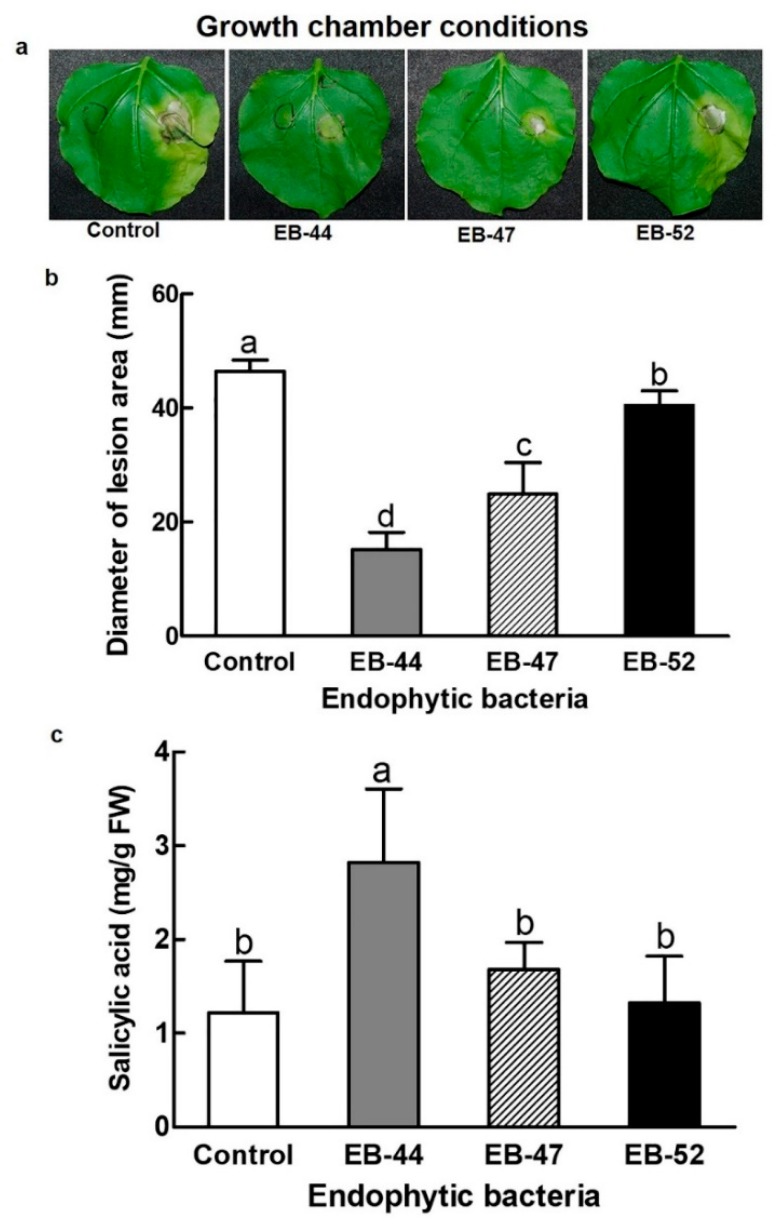
Resistance against Pst-induced disease in N. benthamiana was investigated in plants grown in a walk-in chamber from seeds soaked in an SA-producing EB suspension. Seeds of N. benthamiana were treated for 3 h with EB-44, EB-47, EB-52, or 10 mM MgCl2, and then grown for four weeks. With Pst infiltration into the leaves, the disease lesion area was considerably smaller in N. benthamiana plants grown from seeds treated with SA-producing EB than in those soaked in 10 mM MgCl2 or non-SA-producing EB (Figure 2). Control plants showed the largest lesion areas at 6 days post inoculation (dpi) (46.49 ± 1.93 mm), followed by plants treated with EB-52 (40.48 ± 5.54 mm), EB-47 (24.89 ± 2.99 mm), and EB-44 (15.23 ± 2.57 mm) (Figure 2a,b). The leaves of N. benthamiana grown from seeds treated with SA-producing EB-44 accumulated considerably higher SA levels (2823.17 µg/g FW) than those treated with 10 mM MgCl2 (1218.17 µg/g FW) (Figure 2c).
Figure 2.
Disease resistance against Pseudomonas syringae pv. tabaci (Pst) and salicylic acid (SA) concentrations in the leaves of Nicotiana benthamiana grown from seeds treated with 10 mM MgCl2 (control), EB-44, EB-47, or EB-52 for 3 h. (a,b) The leaves of 4-week-old plants were infiltrated with 0.1 mL of Pst and the diameters of the infected areas were measured 6 days post inoculation. Left: 10 mM MgCl2 as control; right: 0.1 OD600 Pst. (c) The concentrations of SA in the leaves were measured before Pst infiltration. Data represent means ± standard deviations of four independent replications for each treatment. Means with different letters are significantly different at p < 0.05 by Duncan’s multiple range test. FW = fresh weight.
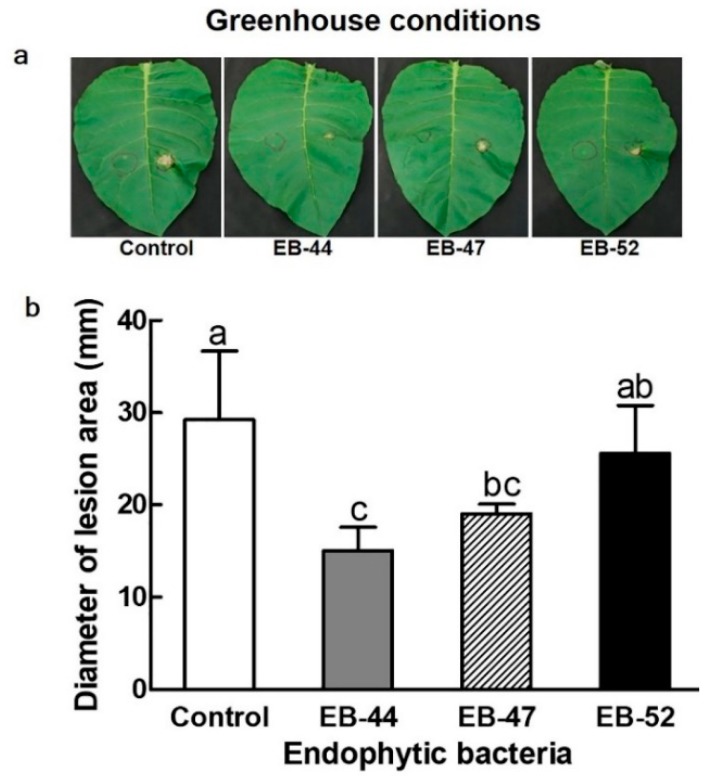
Resistance to Pst-induced disease in N. tabacum was further investigated in plants grown in a glasshouse from seeds treated for 3 h with SA-producing EB. Similar to that observed for the walk-in chamber, N. tabacum plants infiltrated with Pst and grown under glasshouse conditions presented markedly smaller lesion areas when treated with SA-producing EB than when treated with 10 mM MgCl2 or non-SA-producing EB (Figure 3). The greatest lesion area diameter at 6 dpi was observed in the control plants (29.24 ± 7.45 mm), followed by those treated with EB-52 (25.57 ± 5.21 mm), EB-47 (19.04 ± 1.06 mm), and EB-44 (15.06 ± 2.52 mm) (Figure 3a,b).
Figure 3.
The effect of salicylic acid-producing endophytic bacteria on the resistance of Nicotiana tabacum against Pseudomonas syringae pv. tabaci (Pst). Nicotiana tabacum plants grown from seeds treated with 10 mM MgCl2 (control), EB-44, EB-47, or EB-52 for 3 h were evaluated for disease incidence in 4-week-old leaves infiltrated with 0.1 mL of Pst; infection diameter was measured 6 days after inoculation. (a) Left: 10 mM MgCl2 treatment as control; right: 0.1 OD600 Pst. (b) Lesion diameter. Data represent means ± standard deviations of four independent replications for each treatment. Means with different letters are significantly different at p < 0.05 by Duncan’s multiple range test.
3.3. Growth Promotion of Nicotiana Plants Cultivated from Seeds Treated with SA-Producing EB
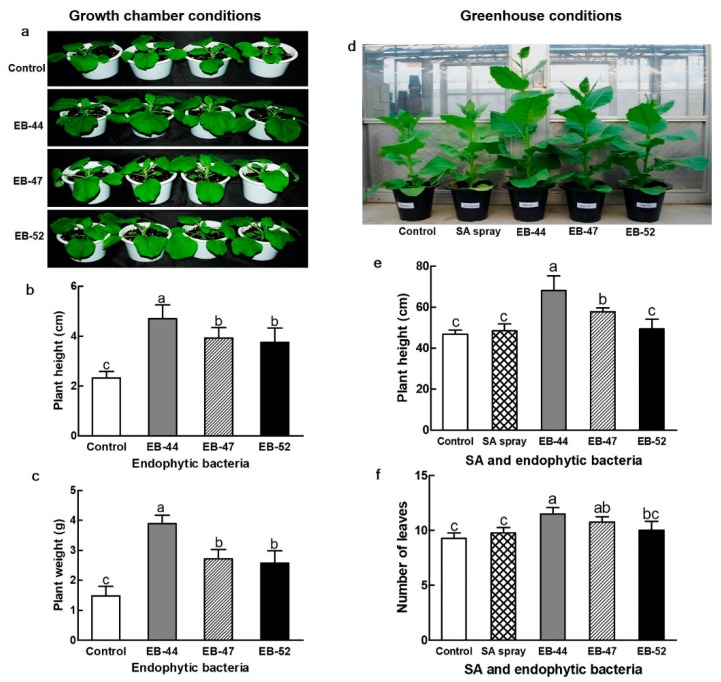
We evaluated the effect of SA-producing EB on the growth of N. benthamiana and N. tabacum grown from seeds treated with EB-44, EB-47, or EB-52 for 3 h. Compared to treatment with distilled water, seed EB inoculation increased both plant height and fresh weight. Under the growth chamber conditions used, N. benthamiana seed inoculation with EB-44, EB-47, or EB-52 increased plant height by 201.7%, 168.7%, and 160.9%, and increased fresh weight by 262.8%, 183.1%, and 173.6%, respectively, compared to distilled water treatment (Figure 4a–c). Under the greenhouse conditions, inoculation of N. tabacum seeds with EB-44, EB-47, or EB-52 increased plant height by 145.42%, 123.27%, and 105.34% and number of leaves by 124.32%, 116.22%, and 108.11%, respectively, compared to the control treatment (Figure 4d–f). For the two Nicotiana species and under both growth chamber and greenhouse conditions, two SA-producing isolates, EB-44 and EB-47, exerted greater plant growth-promoting effects than the non-SA-producing EB-52 isolate. As a positive control for verification of the effect of SA, 1 mM SA was sprayed on 7-week-old N. tabacum plants, which also resulted in increased plant height and leaf number by 103.31% and 105.41%, respectively (Figure 4d–f).
Figure 4.
Growth-promoting effect of salicylic acid (SA)-producing endophytic bacteria (EB) in Nicotiana. (a–c) Growth comparison of 3-week-old Nicotiana benthamiana plants in response to seed treatment with SA-producing EB or distilled water (Control). (d–f) Growth comparison of 8-week-old N. tabacum plants in response to seed treatment with SA-producing EB or water (Control). Data represent means ± standard deviations of four independent replications for each treatment. Means with different letters are significantly different at p < 0.05 by Duncan’s multiple range test.
We used 16S rRNA sequencing to confirm that the EB isolated from N. benthamiana were the same SA-producing EB used in the original seed inoculation. Bacteria were isolated from the leaves of 4-week-old N. benthamiana plants co-cultured with EB-44, and their population densities were counted (1.64 × 105 CFUs/g of fresh tissue). The bacteria reisolated from the leaves of plants grown from the EB-44-inoculated seeds were identified as EB-44 based on 16S rRNA gene sequences, and the 16S rRNA sequences of the isolates exactly matched that of the EB-44 used for the original seed inoculation.
3.4. Nicotine Accumulation in Nicotiana Inoculated with SA-Producing EB
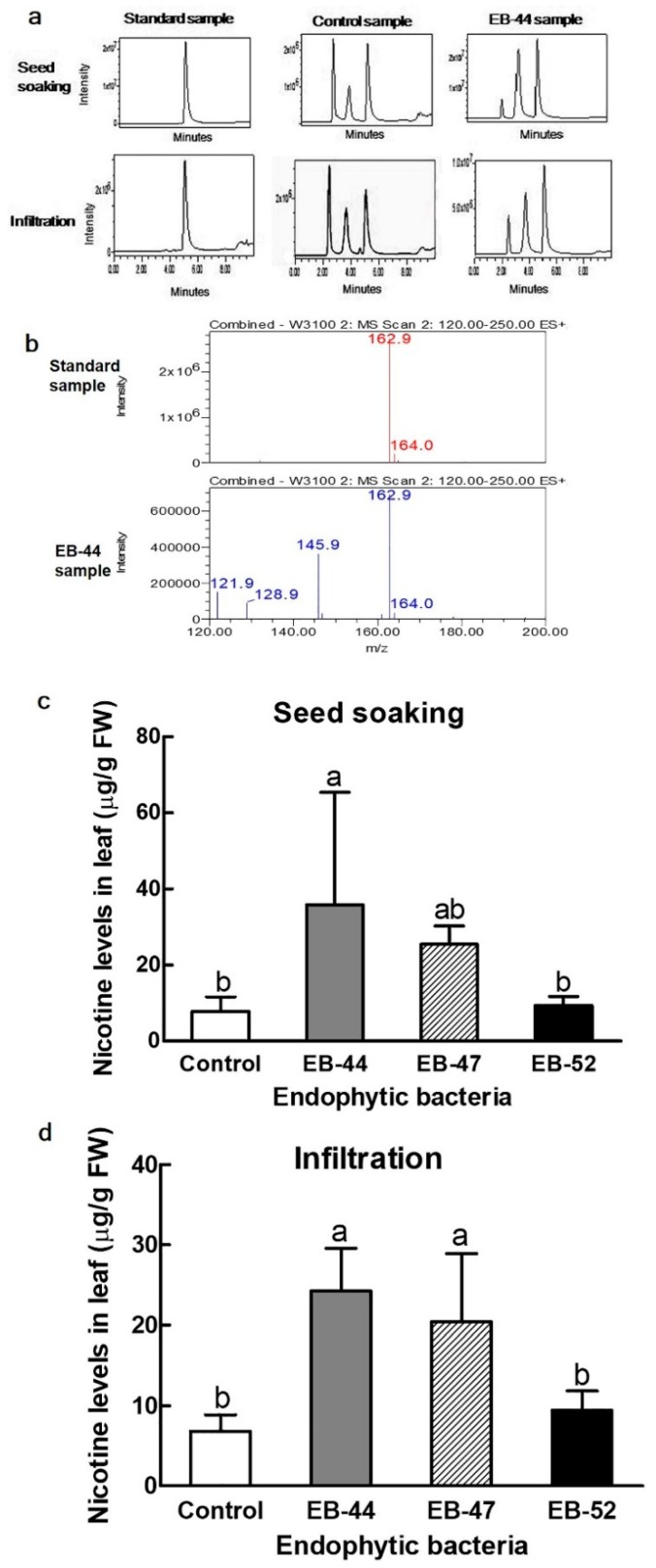
The nicotine content in the leaves of Nicotiana species was analysed by liquid chromatography–mass spectrometry (LC–MS). Compared to the chromatogram and mass spectra of a nicotine standard, the extract of N. benthamiana grown from seeds inoculated with SA-producing EB showed a peak at the same retention time (5.04 min) and mass spectrum profile of the standard at 162.9 m/z (Figure 5a,b). The leaves of N. benthamiana grown from seeds inoculated with EB-44, EB-47, or EB-52 accumulated 4.6, 3.2, and 1.2-fold higher nicotine levels than those imbibed with a 10 mM MgCl2 solution, respectively.
Figure 5.
Liquid chromatography–mass spectrometry (LC–MS) analysis for the identification and quantification of nicotine levels in the leaves of Nicotiana benthamiana treated with salicylic acid (SA)-producing endophytic bacteria (EB). (a) LC–MS chromatograms of the standard, control, and EB-44 samples with matching nicotine peaks at 5.04 min. (b) Mass spectra profiles of the matching (162.9 m/z) nicotine standard and EB-44. (c,d) Nicotine contents in the leaves of N. benthamiana grown from seeds treated with SA-producing EB or by leaf infiltration. Data represent means ± standard deviations of four independent replications for each treatment. Means with different letters are significantly different at p < 0.05 by Duncan’s multiple range test. FW = fresh weight.
Further experiments using SA-producing EB infiltration into leaves were performed to determine the direct infiltration of SA-producing EB into the leaves. Leaves infiltrated with EB-44, EB-47, or EB-52 contained 3.6, 2.9, and 1.4-fold higher nicotine concentrations, respectively, than those imbibed with a 10 mM MgCl2 solution (Figure 5c,d).
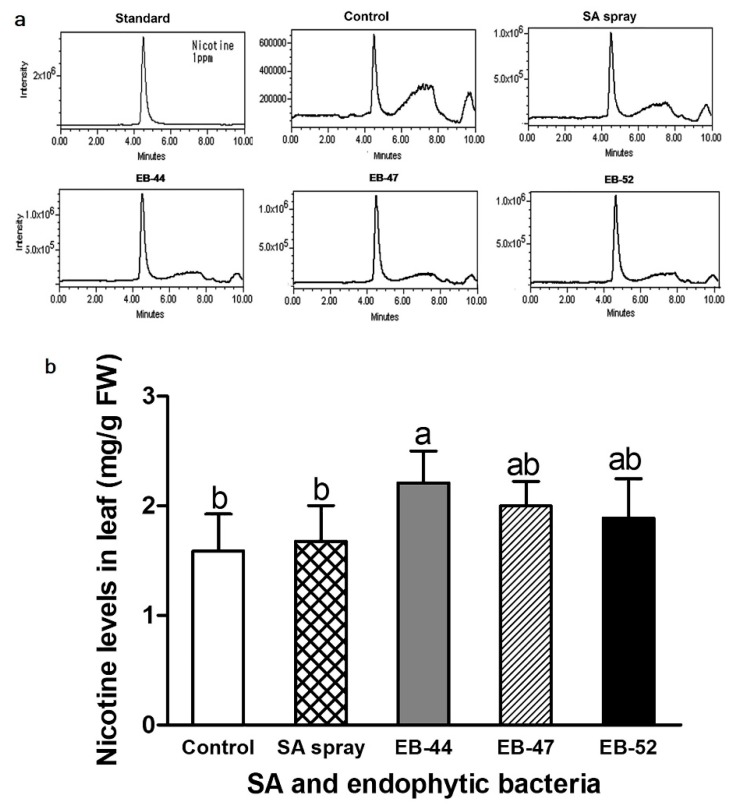
All leaf extracts from N. tabacum grown from seeds treated with SA-producing EB, non-SA-producing EB, or 10 mM MgCl2 exhibited LC peaks at 4.49 min (Figure 6a), exactly matching the retention time of the nicotine standard. Under glasshouse growth conditions, leaf nicotine concentrations increased 1.4, 1.3, 1.2, and 1.1-fold in EB-44, EB-47, EB-52, and SA-treated plants, respectively (Figure 6b).
Figure 6.
Liquid chromatography–mass spectrometry (LC–MS) analysis for the identification and quantification of nicotine levels in the leaves of Nicotiana tabacum treated with salicylic acid (SA)-producing endophytic bacteria (EB) or SA sprayed on plant leaves. (a) Completely matched LC–MS chromatograms of the standard sample with the nicotine peak at 4.49 min and the EB-44 sample with the peak at 4.49. (b) Nicotine content in the leaves of N. tabacum. Data represent means ± standard deviations of four independent replications for each treatment. Means with different letters are significantly different at p < 0.05 by Duncan’s multiple range test. FW = fresh weight.
4. Discussion
We aimed to determine the effects of SA-producing EB on the resistance of two host tobacco species against tobacco wildfire disease, one of the most devastating diseases affecting tobacco and for which no effective control measures currently exist. Endophytic bacteria are known as bioprospecting microorganisms owing to their ability to synthesize novel bioactive compounds that can be used by plants for defence against pathogens [40]. In total, 134 EB isolates were obtained from the leaves, fruits, seeds, and cones of six plant species; namely: M. glyptostroboides, G. biloba, T. brevifolia, P. densiflora, S. babylonica, and S. chaenomeloides. The bacterial population densities, shown as CFUs per gram of fresh weight, varied depending on the plant species and tissue type (Table 1). Variations in bacterial population densities have been reported to depend on plant species, tissue type, location, and environmental conditions [41,42,43]. In our studied plant species, the highest EB density was found in leaves, except for G. biloba, where the EB density in the fruits was 33-fold higher than that in the leaves (Table 1).
Analysis by HPLC confirmed that 27 EB isolates could produce SA, with EB-44 producing the highest quantity (Figure 1 and Supplementary Table S1). In bacteria, SA is biosynthesized from chorismate via two reactions catalysed by isochorismate synthase and isochorismate pyruvate lyase [44]. We also quantified SA levels in the EB host plants to determine whether they accumulated SA. Among the host plants, S. babylonica produced the most SA (966.50 µg/g FW). The number of SA-producing EB isolates was strongly correlated with SA contents in the leaves of the host plants.
Nicotiana benthamiana plants grown from seeds soaked in SA-producing EB-44 and EB-47 accumulated higher levels of SA than those treated with non-SA producing EB-52 or control plants (Figure 2). Natural synthesis of SA in N. tabacum and Solanum tuberosum has been reported to be less than 100 ng and 10 µg/g fresh weight, respectively [45,46,47,48]. Nicotiana tabacum plants treated with SA-producing S. marcescens 90-166 accumulated markedly higher levels of SA [10], which may be related to increased resistance against pathogen-induced diseases [49].
Endophytic bacteria are known for their advantageous relationships with host plants and antagonistic activities against plant pathogens [50]. In our plant disease assays, inoculation of N. benthamiana and N. tabacum with EB-44 elicited a greater reduction in Pst-induced disease symptoms than the control treatment with 10 mM MgCl2 (Figure 2 and Figure 3). Reduced Botrytis cinerea-induced necrosis has been observed in beans in the presence of SA-producing P. aeruginosa 7NSK2 [9,51], while N. tabacum plants show significant resistance against tobacco necrosis virus when inoculated with the SA-producing P. fluorescens CHA0 strain [9,51]. Endophytic bacteria mediate increased host resistance to pathogen-induced disease in association with pathogen-induced SAR [52]. SA is converted to methyl salicylate by the action of SA carboxyl methyltransferase, which acts as an important long-distance signal mediating SAR [53,54].
In addition to the effect on SA accumulation, EB-44 and EB-47 treatment increased height, weight, and leaf number in the N. benthamiana and N. tabacum hosts (Figure 4). These effects could be mediated either by SA produced by the EB or by a growth-promoting effect of the EB themselves. In our study, exogenous SA application also exerted a positive effect on the growth of N. tabacum plants (Figure 4), indicating that growth promotion may be mediated by the higher levels of SA generated by SA-producing EB. Several studies have reported that exogenous SA application increases the growth of soybeans, Arabidopsis, and chamomile [55,56,57]. However, the effect of SA on plant growth is concentration-dependent, and at high concentrations, SA can also inhibit plant growth [57].
Nicotine is a naturally occurring pyridine alkaloid in Nicotiana species [24,58,59]. In addition to increasing the disease resistance in N. benthamiana and N. tabacum, EB-44 also induced the highest nicotine concentrations of all the treatments (Figure 5 and Figure 6). To the best of our knowledge, this is the first report on enhanced nicotine accumulation induced by SA-producing EB. Tobacco plants inoculated with Pst and P. syringae pv. tomato and infected with tobacco mosaic virus displayed increased nicotine accumulation [27,28,29]. Infection with Pst leads to the production of pipecolic acid in tobacco, triggering SA biosynthesis and increased nicotine accumulation [29].
Salicylic acid is widely used as an elicitor to stimulate the synthesis of secondary metabolites [60]. The accumulation of nicotine and other related alkaloids in tobacco can also be affected by environmental factors and levels of plant hormones, including that of JA [61,62,63]. Nicotine accumulation was shown to be inhibited by SA application, whereas it was enhanced by wounding in tobacco plants [64]. In contrast, in our study, we found that SA application induced nicotine accumulation, possibly because nicotine synthesis in tobacco plants is regulated by multiple signalling molecules, including JA and/or auxin [30], and a high SA concentration has been reported to inhibit JA signal transduction [65]. Alternatively, SA-producing EB might induce the production of some amino acids in tobacco plants that lead to increased nicotine and SA synthesis.
In conclusion, our study reports novel roles for EB, including in SA production; promotion of SA biosynthesis and disease resistance in inoculated plants; and production and increased accumulation of nicotine in tobacco. The SA-producing isolate EB-44 (P. tremae) was the most effective at suppressing tobacco wildfire disease and has potential for use as an alternative, eco-friendly control measure for this disease. Our results indicated that P. tremae showed the most promise in this role, possibly because it contains antibacterial compounds. To the best of our knowledge, the increased accumulation of nicotine in tobacco plants observed in this study is a new EB-associated phenomenon. Further studies are needed to reveal the mechanism underlying EB-mediated enhancement of nicotine accumulation in tobacco; in addition, the antibacterial compounds produced by this EB should also be identified for further formulation and future applications in the field.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/2076-2607/8/1/31/s1. Table S1: Quantification of salicylic acid production by endophytic bacteria isolated from various plant species based on HPLC method.
Author Contributions
M.N.I., M.S.A., and S.-J.C. performed the experiments. M.N.I., Y.P., and K.-H.B. designed the experiments and interpreted the data. M.N.I., M.S.A., and K.-H.B. prepared the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET) through the Agri-Bio Industry Technology Development Program funded by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (117044-3).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kloepper J.W., Beauchamp C.J. A review of issues related to measuring colonization of plant roots by bacteria. Can. J. Microbiol. 1992;38:1219–1232. doi: 10.1139/m92-202. [DOI] [Google Scholar]
- 2.Meguro A., Ohmura Y., Hasegawa S., Shimizu M., Nishimura T., Kunoh H. An endophytic actinomycete, Streptomyces sp. MBR-52, that accelerates emergence and elongation of plant adventitious roots. Actinomycetologica. 2006;20:1–9. doi: 10.3209/saj.20.1. [DOI] [Google Scholar]
- 3.Gibert A., Volaire F., Barre P., Hazard L. A fungal endophyte reinforces population adaptive differentiation in its host grass species. New Phytol. 2012;194:561–571. doi: 10.1111/j.1469-8137.2012.04073.x. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu M., Nakagawa Y., Sato Y., Furumai T., Igarashi Y., Onaka H., Yoshida R., Kunoh H. Studies on Endophytic Actinomycetes (I) Streptomyces sp. isolated from Rhododendron and Its antifungal activity. J. Gen. Plant Pathol. 2000;66:360–366. doi: 10.1007/PL00012978. [DOI] [Google Scholar]
- 5.Rodriguez R., Redman R. More than 400 million years of evolution and some plants still can’t make it on their own: Plant stress tolerance via fungal symbiosis. J. Exp. Bot. 2008;59:1109–1114. doi: 10.1093/jxb/erm342. [DOI] [PubMed] [Google Scholar]
- 6.Conn V.M., Walker A.R., Franco C.M.M. Endophytic actinobacteria induce defense pathway in Arab. thaliana. Mol. Plant Microbe Interact. 2008;21:208–218. doi: 10.1094/MPMI-21-2-0208. [DOI] [PubMed] [Google Scholar]
- 7.Khalaf E.M., Raizada M.N. Bacterial seed endophytes of domesticated cucurbits antagonize fungal and oomycete pathogens including powdery mildew. Front. Microbiol. 2018;9:1–18. doi: 10.3389/fmicb.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terhonen E., Blumenstein K., Kovalchuk A., Asiegbu F.O. Forest tree microbiomes and associated fungal endophytes: Functional roles and impact on forest health. Forests. 2019;10:1–33. doi: 10.3390/f10010042. [DOI] [Google Scholar]
- 9.De Meyer G., Höfte M. Salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 induces resistance to leaf infection by Botrytis cinerea on bean. Phytopathology. 1997;87:588–593. doi: 10.1094/PHYTO.1997.87.6.588. [DOI] [PubMed] [Google Scholar]
- 10.Press C.M., Wilson M., Tuzun S., Kloepper J.W. Salicylic acid produced by Serratia marcescens 90-166 is not the primary determinant of induced systemic resistance in Cucumber or Tobacco. Mol. Plant Microbe Interact. 1997;10:761–768. doi: 10.1094/MPMI.1997.10.6.761. [DOI] [Google Scholar]
- 11.An C., Mou Z. Salicylic acid and its function in plant immunity. J. Integr. Plant Biol. 2011;53:412–428. doi: 10.1111/j.1744-7909.2011.01043.x. [DOI] [PubMed] [Google Scholar]
- 12.Anand A., Uppalapati S.R., Ryu C.M., Allen S.N., Kang L., Tang Y., Mysore K.S. Salicylic acid and systemic acquired resistance play a role in attenuating crown gall disease caused by Agrobacterium tumefaciens. Plant Physiol. 2008;146:703–715. doi: 10.1104/pp.107.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denancé N., Sánchez-Vallet A., Goffner D., Molina A. Disease resistance or growth: The role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 2013;4:1–12. doi: 10.3389/fpls.2013.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang D.L., Yang Y., He Z. Roles of plant hormones and their interplay in rice immunity. Mol. Plant. 2013;6:675–685. doi: 10.1093/mp/sst056. [DOI] [PubMed] [Google Scholar]
- 15.Turner J.G., Debbage J.M. Tabtoxin-induced symptoms are associated with the accumulation of ammonia formed during photorespiration. Physiol. Plant Pathol. 1982;20:223–233. doi: 10.1016/0048-4059(82)90087-X. [DOI] [Google Scholar]
- 16.Studholme D.J., Ibanez S.G., MacLean D., Dangl J.L., Chang J.H., Rathjen J.P. A draft genome sequence and functional screen reveals the repertoire of type III secreted proteins of Pseudomonas syringae pathovar tabaci 11528. BMC Genom. 2009;10:1–19. doi: 10.1186/1471-2164-10-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng D.D., Zhang Z.S., Sun X.B., Zhao M., Sun G.Y., Chow W.S. Photoinhibition and photoinhibition-like damage to the photosynthetic apparatus in tobacco leaves induced by pseudomonas syringae pv. tabaci under light and dark conditions. BMC Plant Biol. 2016;16:1–11. doi: 10.1186/s12870-016-0723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoemaker P.B. Wildfire and Angular Leaf Spot. In: Shew H.D., Lucas G.B., editors. Compendium of Tobacco Diseases. APS Press; Saint Paul, MN, USA: 1991. pp. 30–32. [Google Scholar]
- 19.Valleau W.D. Breeding tobacco for disease resistance. Econ. Bot. 1952;6:69–102. doi: 10.1007/BF02859199. [DOI] [Google Scholar]
- 20.McManus P.S., Stockwell V.O., Sundin G.W., Jones A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002;40:443–465. doi: 10.1146/annurev.phyto.40.120301.093927. [DOI] [PubMed] [Google Scholar]
- 21.Carter A.P., Clemons W.M., Brodersen D.E., Morgan-Warren R.J., Wimberly B.T., Ramakrishnan V. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature. 2000;407:340–348. doi: 10.1038/35030019. [DOI] [PubMed] [Google Scholar]
- 22.Hyun J.W., Kim H.J., Yi P.H., Hwang R.Y., Park E.W. Mode of action of streptomycin resistance in the citrus canker pathogen (Xanthomonas smithii subsp. citri) in Jeju island. Plant Pathol. J. 2012;28:207–211. doi: 10.5423/PPJ.2012.28.2.207. [DOI] [Google Scholar]
- 23.Qin C., Tao J., Liu T., Liu Y., Xiao N., Li T., Gu Y., Yin H., Meng D. Responses of phyllosphere microbiota and plant health to application of two different biocontrol agents. AMB Express. 2019;9 doi: 10.1186/s13568-019-0765-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doolittle D.J., Winegar R., Lee C.K., Caldwell W.S., Hayes A.W., De Bethizy J.D. The genotoxic potential of nicotine and its major metabolites. Mutat. Res. 1995;344:95–102. doi: 10.1016/0165-1218(95)00037-2. [DOI] [PubMed] [Google Scholar]
- 25.Bush L.P., Fannin R.L., Chelvarajan H.R.B. Biosynthesis and metabolism of nicotine and related alkaloids. In: Garrod J.W., Wahren J., editors. Nicotine and Related Alkaloids: Absorbtion, Distribution, Metabolism and Excretion. Springer; Dordrecht, The Netherlands: 1993. pp. 1–30. [Google Scholar]
- 26.Wink M.W. Modes of action of alkaloids. In: Robers M.F., Wink M., editors. Alkaloids: Biochemistry, Ecology and Medicinal Applications. Plenum Press; New York, NY, USA: 1998. pp. 301–3325. [Google Scholar]
- 27.Ziebell H., Murphy A.M., Groen S.C., Tungadi T., Westwood J.H., Lewsey M.G., Moulin M., Leczkowski A., Smith A.G., Stevens M., et al. Cucumber mosaic virus and its 2b RNA silencing suppressor modify plant-aphid interactions in tobacco. Sci. Rep. 2011;1:1–7. doi: 10.1038/srep00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L., Oh Y., Li H., Baldwin I.T., Galis I. Alternative oxidase in resistance to biotic stresses: Nicotiana attenuata AOX contributes to resistance to a pathogen and a piercing-sucking insect but not manduca sexta larvae. Plant Physiol. 2012;160:1453–1467. doi: 10.1104/pp.112.200865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel-Adghough D., Stahl E., Návarová H., Zeier J. Pipecolic acid enhances resistance to bacterial infection and primes salicylic acid and nicotine accumulation in tobacco. Plant Signal. Behav. 2013;8:e26366. doi: 10.4161/psb.26366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C., Teng W., Shi Q., Zhang F. Multiple signals regulate nicotine synthesis in tobacco plant. Plant Signal. Behav. 2007;2:280–281. doi: 10.4161/psb.2.4.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruan A., Min H., Peng X., Huang Z. Isolation and characterization of Pseudomonas sp. strain HF-1, capable of degrading nicotine. Res. Microbiol. 2005;156:700–706. doi: 10.1016/j.resmic.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Chen C., Li X., Yang J., Gong X., Li B., Zhang K.Q. Isolation of nicotine-degrading bacterium Pseudomonas sp. Nic22, and its potential application in tobacco processing. Int. Biodeterior. Biodegrad. 2008;62:226–231. doi: 10.1016/j.ibiod.2008.01.012. [DOI] [Google Scholar]
- 33.Yuan Y.J., Lu Z.X., Wu N., Huang L.J., Lü F.X., Bie X.M. Isolation and preliminary characterization of a novel nicotine-degrading bacterium, Ochrobactrum intermedium DN2. Int. Biodeterior. Biodegrad. 2005;56:45–50. doi: 10.1016/j.ibiod.2005.04.002. [DOI] [Google Scholar]
- 34.Gong X.-W., Yang J.-K., Duan Y.-Q., Dong J.-Y., Zhe W., Wang L., Li Q.-H., Zhang K.-Q. Isolation and characterization of Rhodococcus sp. Y22 and its potential application to tobacco processing. Res. Microbiol. 2009;160:200–204. doi: 10.1016/j.resmic.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Wang S.N., Liu Z., Xu P. Biodegradation of nicotine by a newly isolated Agrobacterium sp. strain S33. J. Appl. Microbiol. 2009;107:838–847. doi: 10.1111/j.1365-2672.2009.04259.x. [DOI] [PubMed] [Google Scholar]
- 36.Costa L.E.D.O., de Queiroz M.V., Borges A.C., de Moraes C.A., de Araújo E.F. Isolation and characterization of endophytic bacteria isolated from the leaves of the common bean (Phaseolus vulgaris) Braz. J. Microbiol. 2012;43:1562–1575. doi: 10.1590/S1517-83822012000400041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Susilowati R., Sabdono A., Widowati I. Isolation and characterization of bacteria associated with brown algae Sargassum spp. from Panjang island and their antibacterial activities. Procedia Environ. Sci. 2015;23:240–246. doi: 10.1016/j.proenv.2015.01.036. [DOI] [Google Scholar]
- 39.Dhakal R., Park E., Lee S.W., Baek K.H. Soybean (Glycine max L. Merr.) sprouts germinated under red light irradiation induce disease resistance against bacterial rotting disease. PLoS ONE. 2015;10:1–14. doi: 10.1371/journal.pone.0117712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strobel G., Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003;67:491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quadt-Hallmann A., Kloepper J.W., Benhamou N. Bacterial endophytes in cotton: Mechanisms of entering the plant. Can. J. Microbiol. 1997;43:577–582. doi: 10.1139/m97-081. [DOI] [Google Scholar]
- 42.Hallmann J., Quadt-Hallmann A., Miller W.G., Sikora R.A., Lindow S.E. Endophytic colonization of plants by the biocontrol agent Rhizobium etli G12 in relation to Meloidogyne incognita infection. Phytopathology. 2002;91:415–422. doi: 10.1094/PHYTO.2001.91.4.415. [DOI] [PubMed] [Google Scholar]
- 43.Sturz A.V., Nowak J. Endophytic communities of rhizobacteria and the strategies required to create yield enhancing associations with crops. Appl. Soil Ecol. 2000;15:183–190. doi: 10.1016/S0929-1393(00)00094-9. [DOI] [Google Scholar]
- 44.Serino L., Reimmann C., Baur H., Beyeler M., Visca P., Haas D. Structural genes for salicylate biosynthesis from chorismate in Pseudomonas aeruginosa. Mol. Gen. Genet. 1995;249:217–228. doi: 10.1007/BF00290369. [DOI] [PubMed] [Google Scholar]
- 45.Yalpani N., Silverman P., Wilson T.M.A., Kleier D.A., Raskin L. Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. Plant Cell. 1991;3:809–818. doi: 10.1105/tpc.3.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yalpani N., Shulaev V., Raskin I. Endogenous salicylic acid levels correlate with accumulation of pathogenesis-related proteins and virus resistance in tobacco. Phytopathology. 1993;83:702–708. doi: 10.1094/Phyto-83-702. [DOI] [Google Scholar]
- 47.Coquoz J.-L., Buchala A., Métraux J.-P. The biosynthesis of salicylic acid in potato plants. Plant Physiol. 1998;117:1095–1101. doi: 10.1104/pp.117.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Navarre D.A., Mayo D. Differential characteristics of salicylic acid-mediated signaling in potato. Physiol. Mol. Plant Pathol. 2004;64:179–188. doi: 10.1016/j.pmpp.2004.09.001. [DOI] [Google Scholar]
- 49.Delaney T.P., Uknes S., Vernooij B., Friedrich L., Weymann K., Negrotto D., Gaffney T., Gut-Rella M., Kessmann H., Ward E., et al. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- 50.Rosenblueth M., Martínez-Romero E. Bacterial endophytes and their interactions with hosts. Mol. Plant Microbe Interact. 2006;19:827–837. doi: 10.1094/MPMI-19-0827. [DOI] [PubMed] [Google Scholar]
- 51.Maurhofer M., Hase C., Meuwly P., Metraux J.P., Defago G. Induction of systemic resistance of tobacco to tobacco necrosis virus by the root-colonizing Pseudomonas fluorescens strain CHA0: Influence of the gacA gene and of pyoverdine production. Phytopathology. 1994;84:139–146. doi: 10.1094/Phyto-84-139. [DOI] [Google Scholar]
- 52.Chasan R. SA: Source or signal for SAR? Plant Cell. 1995;7:1519–1521. doi: 10.1105/tpc.7.10.1519. [DOI] [Google Scholar]
- 53.Shulaev V., Silverman P., Raskin I. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature. 1997;385:718–721. doi: 10.1038/385718a0. [DOI] [Google Scholar]
- 54.Park S.W., Kaimoyo E., Kumar D., Mosher S., Klessig D.F. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science. 2007;318:113–116. doi: 10.1126/science.1147113. [DOI] [PubMed] [Google Scholar]
- 55.Santner A., Calderon-Villalobos L.I.A., Estelle M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009;5:301–307. doi: 10.1038/nchembio.165. [DOI] [PubMed] [Google Scholar]
- 56.Wolters H., Jürgens G. Survival of the flexible: Hormonal growth control and adaptation in plant development. Nat. Rev. Genet. 2009;10:305–317. doi: 10.1038/nrg2558. [DOI] [PubMed] [Google Scholar]
- 57.Kováčik J., Grúz J., Bačkor M., Strnad M., Repčák M. Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep. 2009;28:135–143. doi: 10.1007/s00299-008-0627-5. [DOI] [PubMed] [Google Scholar]
- 58.Siegmund B., Leitner E., Pfannhauser W. Determination of the nicotine content of various edible nightshades (Solanaceae) and their products and estimation of the associated dietary nicotine intake. J. Agric. Food Chem. 1999;47:3113–3120. doi: 10.1021/jf990089w. [DOI] [PubMed] [Google Scholar]
- 59.Cai B., Jack A.M., Lewis R.S., Dewey R.E., Bush L.P. Nicotine biosynthesis, metabolism and translocation in tobacco as determined by nicotine demethylase mutants. Phytochemistry. 2013;95:188–196. doi: 10.1016/j.phytochem.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 60.Li X., Guo H., Qi Y., Liu H., Zhang X., Ma P., Liang Z., Dong J. Salicylic acid-induced cytosolic acidification increases the accumulation of phenolic acids in Salvia miltiorrhiza cells. Plant Cell Tissue Organ Cult. (PCTOC) 2016;126:333–341. doi: 10.1007/s11240-016-1001-x. [DOI] [Google Scholar]
- 61.De Luca V., St Pierre B. The cell and developmental biology of alkaloid biosynthesis. Trends Plant Sci. 2000;5:168–173. doi: 10.1016/S1360-1385(00)01575-2. [DOI] [PubMed] [Google Scholar]
- 62.Shoji T., Yamada Y., Hashimoto T. Jasmonate induction of putrescine N-methyltransferase genes in the root of Nicotiana sylvestris. Plant Cell Physiol. 2000;41:831–839. doi: 10.1093/pcp/pcd001. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H.-B., Bokowiec M.T., Rushton P.J., Han S.-C., Timko M.P. Tobacco transcription factors NtMYC2a and NtMYC2b form nuclear complexes with the NtJAZ1 repressor and regulate multiple jasmonate-inducible steps in nicotine biosynthesis. Mol. Plant. 2012;5:73–84. doi: 10.1093/mp/ssr056. [DOI] [PubMed] [Google Scholar]
- 64.Nugroho L.H., Peltenburg-Looman A.M.G., De Vos H., Verberne M.C., Verpoorte R. Nicotine and related alkaloids accumulation in constitutive salicylic acid producing tobacco plants. Plant Sci. 2002;162:575–581. doi: 10.1016/S0168-9452(01)00596-9. [DOI] [Google Scholar]
- 65.Preston C.A., Lewandowski C., Enyedi A.J., Baldwin I.T. Tobacco mosaic virus inoculation inhibits wound-induced jasmonic acid-mediated responses within but not between plants. Planta. 1999;209:87–95. doi: 10.1007/s004250050609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.