Abstract
One of the main characteristics of probiotics is their ability to stimulate and modulate the immune response regardless of their viability. Lactobacillus casei (Lc) can stimulate local and systemic immunity, in addition to the activation of macrophages at sites distant from the intestine. Activated macrophages limit the replication of intracellular protozoa, such as Toxoplasma gondii, through the production of nitric oxide. The present study aimed to evaluate the protection generated by treatment with viable and non-viable Lc in the murine systemic toxoplasmosis model. CD1 male mice were treated with viable Lc (immunobiotic) and non-viable Lc (paraprobiotic), infected with tachyzoites of Toxoplasma gondii RH strain. The reduction of the parasitic load, activation of peritoneal macrophages, inflammatory cytokines, and cell populations was evaluated at 7 days post-infection, in addition to the survival. The immunobiotic and paraprobiotic reduced the parasitic load, but only the immunobiotic increased the activation of peritoneal macrophages, and the production of interferon-gamma (IFN-γ), tumor necrosis factor (TNF), and interleukin-6 (IL-6) while the paraprobiotic increased the production of monocyte chemoattractant protein-1 (MCP-1) and T CD4+CD44+ lymphocytes. Viable and non-viable Lc increases survival but does not prevent the death of animals. The results provide evidence about the remote immunological stimulation of viable and non-viable Lc in an in vivo parasitic model.
Keywords: Lactobacillus casei, immunobiotic, paraprobiotic, activated macrophages, systemic toxoplasmosis, tachyzoites
1. Introduction
Probiotics are defined by Food and Agriculture Organization and World Health Organization as ‘live microorganisms that when administered in adequate amounts confer benefits to the health of the host’ [1]. An important characteristic of probiotics is the ability to stimulate the local (intestinal mucosa) and distant (systemic) immune response; owing to this, they have been attributed with the ‘immunobiotic term’ [2]. However, there is evidence that the immune stimulation of a probiotic microorganism is independent of the cell viability. In this sense, the term paraprobiotic (ghost probiotics) was proposed to all non-viable microbial cells that, when administered in adequate amounts, confer benefits to the host [3].
Multiple species of the genus Lactobacillus have been shown to have the potential to stimulate the innate and adaptive immune response in the host via binding to pattern recognition receptors (PRRs) expressed on immune cells that recognize molecular patterns associated with microorganisms (MAMPs) that triggers the production of cytokines and chemokines [4].
The beneficial effects of Lactobacillus casei Shirota (LcS) have been extensively evaluated in clinical trials, demonstrating protection in infectious (diarrhea) and non-infectious (tumor) pathologies. In addition, the beneficial effects of LcS on allergies, irritable bowel disease (IBD), and autoimmune diseases have been confirmed through experimental models. It suggests that LcS not only increases the host’s immune response against cancer and infections but also controls the excessive immune response to inhibit inflammatory processes [5].
Most of the immunostimulation/immunoregulation functions of LcS are involved in immune response cells, such as macrophages and dendritic cells, that carry out antigen recognition and presentation [6]. In addition, macrophages play an important role in the elimination of intracellular pathogenic microorganisms by activating lithic oxygen and nitrogen mechanisms [7]. It has been shown in the murine model that Lactobacillus casei ATCC 7469 stimulates the production of nitric oxide (NO), and is capable of eliminating intracellular protozoan parasites, such as Trypanosoma cruzi [8], Plasmodium chabaudi [9], and Babesia microti [10].
One of the most complex intracellular protozoa from an immunological point of view is Toxoplasma gondii (T. gondii). This protozoan is the causative agent of toxoplasmosis and it is estimated that around one third of the world’s population are infected, whereas pregnant women and immunosuppressed people are more vulnerable to infection [11]. The spread of the parasite through the use of macrophages and dendritic cells known as a ‘Trojan horse’ and the ability to cross the blood–brain and blood–retinal barrier can cause pathologies known as brain toxoplasmosis and chorioretinitis [12].
The pharmacological treatments used in toxoplasmosis, such as clindamycin, pyrimethamine, sulfadiazine, trimetroprim, and sulfamethoxazole, are specific for the control of the tachyzoite stage. However, these drugs can cause side effects and can deteriorate the health of the host [13]. From this perspective, it is necessary to implement new treatments that offer the patient an ability to control the replication of the parasite, increase the immune response, and ensure the integrity of health, such as biotherapies with probiotics.
The work on the murine model carried out by Martínez-Gómez [14] and Ribeiro [15] showed protection in the chronic phase of infection with the low virulence strain ME49 of T. gondii when administered with L. casei ATCC 7469 and Bifidobacterium animalis subspp. lactis, respectively, reducing the parasitic load of tissue cysts in the brain. However, there is no evidence that assesses the ability to generate protection by probiotics in high-virulence strains of T. gondii infection. Also, in situ stimulation of nitric oxide production in macrophages by immunobiotics/paraprobiotics has not been demonstrated.
The objective of the current work was to evaluate the possible immunological protection mechanism of Lactobacillus casei as an immunobiotic and paraprobiotic against systemic infection caused by T. gondii RH strain in a murine model.
2. Materials and Methods
2.1. Bacterial Culture and Preparation of Heat Killed Bacteria
The probiotic bacterium Lactobacillus casei (Lc) was obtained from the commercial drink Yakult (Yakult®, Mexico). L. casei was identified by 16S rRNA sequencing. The bacterium was grown in MRS broth (de Man-Rogosa-Sharpe broth, BDL, Franklin Lakes, NJ, USA) at 37 °C at 5% CO2 for 24 h. Briefly, the bacteria were harvested and washed twice with sterile phosphate buffered saline (PBS) at 2700× g for 15 min and the viable count was made on MRS agar plates at 37 °C with 5% CO2 for 24 to 48 h. Colony forming units (CFUs) were quantified and adjusted to 1 × 109 CFU/mL in PBS at pH 7.2; these bacteria were called immunobiotics (IBs). From the same vials adjusted to 1 × 109 CFU/mL, they were subjected to heat treatment at 100 °C for 30 min, immediately after they were placed in an ice bath (thermal shock) [16]. The inactivation was confirmed by the absence of colonies in MRS agar plates; the heat-inactivated bacteria were called paraprobiotics (PPs).
2.2. T. gondii RH
The RH strain of T. gondii was maintained in male CD1 mice (12 weeks old) inoculated intraperitoneally with tachyzoites [17]. At 3 days post-infection, a peritoneal wash with sterile PBS was performed and the suspension obtained was centrifuged at 1000× g for 10 min. The cell package was resuspended in sterile PBS and the tachyzoites were counted in a Neubauer chamber.
2.3. Animal Model
Ethical Committee of Instituto de Oftalmología “Fundación de Asistencia Privada Conde de Valenciana” (CONBIOETICA-09-CEI-023-20160830), approved the use of animals in this study. All animals were treated according to the ARVO statement for the Use of Animals in Ophthalmology and Vision Research. Male CD1 mice aged 8 to 10 weeks and weighing between 28 and 31 g were used in this study and were obtained from the vivarium of the National School of Biological Sciences, National Polytechnic Institute (Mexico City, Mexico). All animals were free of specific parasites and were kept in normal conditions of room temperature, 12/12 h light cycle, and subjected to ad libitum fed.
2.4. Experimental Design
A batch of 68 male CD1 mice were randomly divided into 4 groups. Three groups were infected with T. gondii RH and received a treatment with PBS (n = 22), immunobiotics (n = 20), or paraprobiotics (n = 20). Additionally, a group of uninfected and untreated animals were used as a control group (n = 4). The treatment of the animals began on day 0 and concluded on day 14 of the experiment by administering daily intragastrically 100 μL of PBS, 1 × 109 CFU/100 μL of alive Lc (IBs), or 1 × 109 UFC/100 μL of dead Lc (PPs). On day 7, the mice were infected intraperitoneally with 1000 tachyzoites of the T. gondii RH strain. Seven to 9 mice per group were sacrificed in a lethal chamber on day 14 of starting treatment, that is, on the 7th day post-infection. The rest of the animals (12 to 13 mice per group) were monitored for survival once the treatment was complete.
2.5. Evaluation of Load Tachyzoites T. gondii RH
A first peritoneal wash was performed to obtain ascites fluid with 2 mL of cold sterile PBS, and 1 mL was extracted. It was deposited in a 1.5-mL Eppendorf tube and placed in refrigeration. Then, a second peritoneal wash was performed with 3 mL of cold sterile PBS, and approximately 3 mL of ascites fluid was removed and placed in a Vacutainer tube with heparin (Franklin Lake, NJ, USA). Eppendorf tubes were centrifuged at 3000× g for 10 min, and then the cell-free supernatant was removed and stored at −70 °C for the evaluation of inflammatory cytokines. The cell packet was collected and transferred to the tube containing 3 mL of ascites fluid, centrifuged at 3000× g for 10 min and the supernatant discarded, and the cell packet was resuspended in 1 mL of sterile PBS. A 1:10 dilution was performed in PBS and the peritoneal exudate cells (PECs) and tachyzoites were quantified in a Neubauer chamber.
2.6. In Vivo Cytokine Production
Cytokine concentrations were determined in ascites fluid from CD1 mice. Briefly, the mice were euthanized in a CO2 chamber, 2 mL of sterile PBS was inoculated, and a peritoneal wash was performed. The fluid was centrifuged at 10,000× g for 10 min at 4 °C. The supernatant was frozen at −70 °C until use. Liquid concentrations of interferon-gamma (IFN-γ), tumor necrosis factor (TNF), monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6), interleukin-10 (IL-10), and interleukin-12 (IL-12) were quantified with the BD Cytometric Bead Array (CBA) commercial Mouse Inflammation Kit as described in the manufacturer’s protocol. The analysis was performed on a flow cytometer FACS Canto II (BD).
2.7. Determination of Intracellular Nitric Oxide in Peritoneal Macrophages
Peritoneal exudate cells were obtained from the ascites liquid. A 50-μL aliquot was taken from the sample and centrifuged at 3000× g for 5 min, then the supernatant was removed and 10 µL of the working solution of the 4-amino-5-methylamino-2’, 7’-difluorofluorescein diacetate (DAF-FM DA) (Thermo Scientific, Eugene, OR, USA) fluorescent probe was deposited at a concentration of 5 µM, and incubated at 37 °C for 30 min in the dark. Then, 500 μL of sterile PBS were added and centrifuged at 3000× g for 5 min, the supernatant was discarded, 500 µL of PBS were added, and it was incubated at 37 °C for 15 min in the dark. It was then centrifuged at 3000× g for 5 min, and the supernatant was discarded [18]. The cell packet labeled with the DAF-FM DA probe was prepared for peritoneal macrophage labeling. In total, 4 µL of the anti-F4/80 monoclonal antibody labeled with APC (Biolegend, San Diego, CA, USA) was deposited and incubated for 30 min at ambient temperature in darkness. Subsequently, 500 μL of FACSFlow was added, centrifuged at 3000× g for 5 min, and the supernatant was removed. The cell packet was resuspended in 200 μL of FACSFlow to be evaluated in the FACS Canto II (BD) flow cytometer.
2.8. Determination of Lymphocytes Populations in Mice Spleen
The spleen was divided between 2 slides with a frosted edge, with the homogenate being resuspended in 10 mL of PBS, and centrifuged at 500× g for 10 min at 4 °C. The supernatant was removed and 5 mL of lysis buffer for red blood cells (NH4Cl 0.155 M, NaHCO3 0.01 M, EDTA 0.1 mM) were added, homogenized, and incubated at room temperature for 5 min. Two washes were made with 5 mL of PBS and centrifuged at 500× g for 10 min at 4 °C. The supernatant was then discarded and 1 mL of sterile PBS was added. Splenocytes were counted in a Neubauer chamber and adjusted to 1 × 106 cells/mL. Subsequently, the cells were labeled with anti-CD22 monoclonal antibodies conjugated with FITC, anti-CD335 conjugated with PE/Cy7, anti-CD3 conjugated with PE, anti-CD4 conjugated with FITC, anti-CD8 conjugated with APC, anti-CD25 conjugated with PE, and anti-CD44 conjugated with PE/Cy7 (all from BioLegend, San Diego, CA, USA) at room temperature for 30 min in darkness [19]. A FACSFlow wash was performed, and finally, 200 µL of FACSFlow were added. The analysis was carried out on a FACS Canto II flow cytometer (Becton Dickinson). A total of 30,000 events were captured using the BD FACSDIVA software and analyzed with the Flowjo X software [20].
2.9. Statistical Analysis
GraphPad Prism® version 6.0 software for Mac was used and the one-way Tukey’s multiple comparisons test was applied. Survival data were presented in a Kaplan–Meier survival curve. A 95% confidence interval was considered significant (p < 0.05).
3. Results
3.1. Clinical Evaluation of Organs
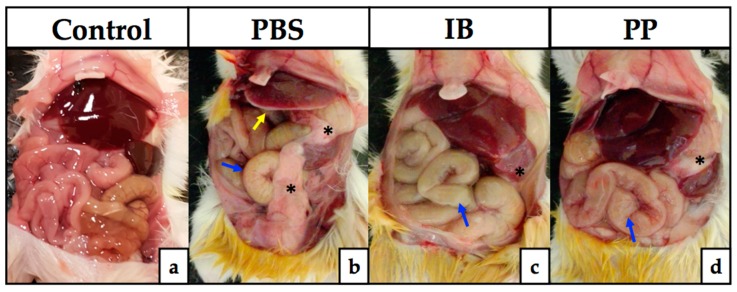
To demonstrate the degree of damage generated by intraperitoneal inoculation of tachyzoites of T. gondii RH, the peritoneum organs were exposed. The morphology and typical appearance of the peritoneal organs of healthy CD1 mice showed a total absence of a pathological process (Figure 1a). All groups that were infected with T. gondii showed different degrees of organ lesions, with abnormalities in the PBS-treated group being more evident (Figure 1b), with the appearance of necrotic foci in the liver (yellow arrow), bowel inflammation (blue arrow), and fibrosis around the spleen that extended to partially cover the intestines (black asterisks). The groups treated with immunobiotics (Figure 1c) and paraprobiotics (Figure 1d) showed inflammation of the intestines (red arrows) and poor fibrosis, which covered the spleen only (blue asterisks), with a loss of normal liver coloration.
Figure 1.
Clinical evaluation of the pathology in peritoneum organs. The development of inflammation of the intestine (blue arrow), appearance of necrotic foci in the liver (yellow arrow), and fibrosis formation around the spleen (black asterisks) are signs of parasite infection on the seventh day post-infection, being more evident in the group treated with PBS (b) with respect to those treated with viable Lactobacillus casei (c) and non-viable Lc (d), when compared to healthy mice (a). PBS) phosphate buffered saline; IB) immunobiotic; PP) paraprobiotic.
3.2. Evaluation of the Parasitic Load in Peritoneal Exudate
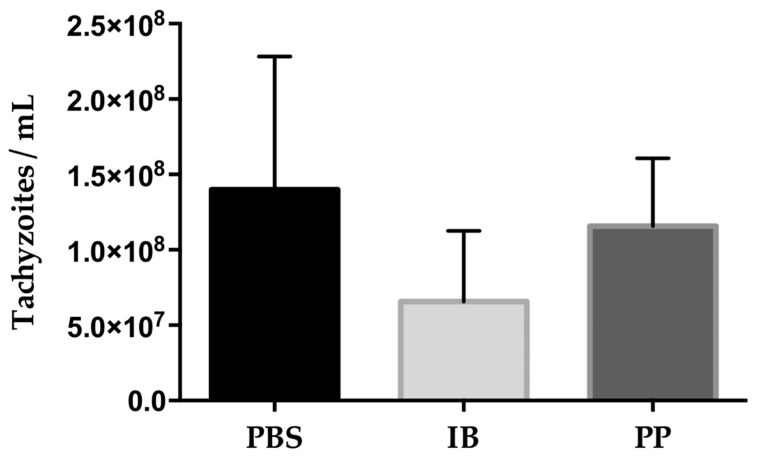
The quantification of tachyzoites in peritoneal exudate was evaluated at 7 days post-infection in infected mice. The control group that received the PBS treatment showed the highest amount of tachyzoites (1.39816667 × 108) while the groups that received the Lactobacillus treatment reduced the parasitic load of tachyzoites, 6.5725 × 107 with immunobiotic and 1.15628571 × 108 with paraprobiotic. The reduction of the parasitic face in the immunobiotic group was 53% while the group treated with paraprobiotic was 17.3% with respect to the control group (Figure 2). The quantification of peritoneal exudate cells (PECs) was performed in the peritoneal exudate of infected mice and the relationship with respect to the amount of tachyzoites present was investigated, showing a greater amount of tachyzoites per PEC in the control group (1:47.47). In the groups treated with immunobiotic, the ratio was 1:9.12 and those treated with paraprobiotic was 1:17.5.
Figure 2.
Quantification of T. gondii RH tachyzoites in peritoneal fluid. The parasitic load was reduced by 53% in the group treated with immunobiotic and 17.3% in those treated with paraprobiotic compared to the control group treated with PBS. Data were combined from two individual experiments with three to five mice per group. Values represent the means ± SD. No significant difference between treatments. PBS) phosphate buffered saline; IB) immunobiotic; PP) paraprobiotic.
3.3. Percentage of Activated Macrophages and Production of Intracellular Nitric Oxide
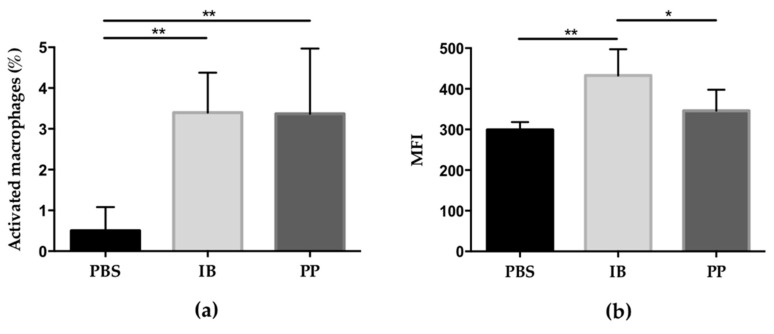
The percentage of activated macrophages in the peritoneal cavity of mice infected with T. gondii was determined. Those cells that expressed double labeling with the anti-F4/80 monoclonal antibody and the DAF-FM DA probe were selected. It was observed that the number of activated macrophages of the control group was less than 1% while in the groups treated with lactobacilli, the percentage was greater than 3%, with there being a significant difference with respect to the control group (Figure 3a). The amount of nitric oxide produced intracellularly was evaluated by measuring the average fluorescence intensity (MFI) generated by the DAF-FM DA probe, which is directly proportional. The macrophages that produced the greatest amount of intracellular nitric oxide were from the mice treated with the immunobiotic with respect to the mice treated with paraprobiotic and PBS (significant difference) while the group treated with paraprobiotic generated higher NO production compared to the control group, although this was not significant (Figure 3b).
Figure 3.
Evaluation of the activation of peritoneal macrophages and production of intracellular nitric oxide in CD1 mice infected with T. gondii RH: (a) the percentage of activated macrophages in the peritoneal cavity is increased in treatments with lactobacilli, regardless of whether they are alive or dead, compared to the control group; (b) there is an increase in the production of nitric oxide in the group treated with immunobiotic compared to the other two groups. Data were combined from two individual experiments with three to five mice per group. Values represent the means ± SD. * p < 0.05; ** p < 0.01. PBS) phosphate buffered saline; IB) immunobiotic; PP) paraprobiotic; MFI) mean fluorescence intensity.
3.4. Quantification of Cytokines in Ascites Fluid
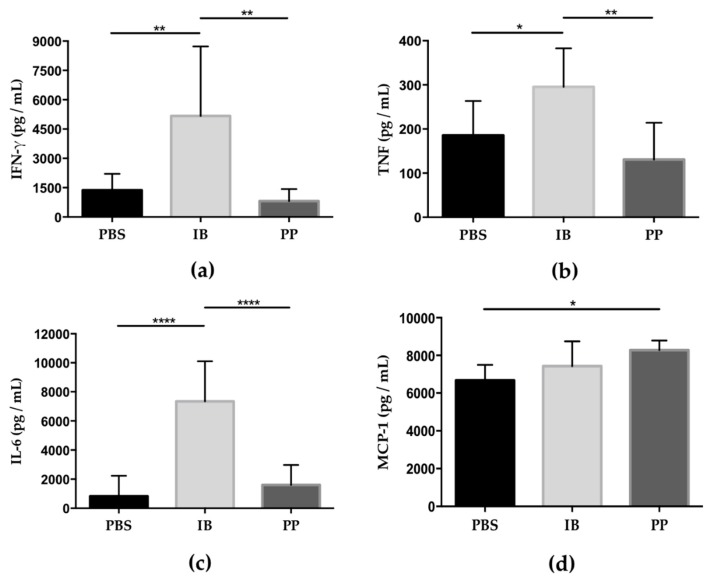
Proinflammatory cytokine levels in the peritoneal fluid were determined at 7 days post-infection. The levels of IFN-γ, TNF, and IL-6 were significantly increased in mice treated with immunobiotics compared to the groups treated with paraprobiotic and PBS (Figure 4a–c). However, chemokine MCP-1 was observed as significantly increased only in the group treated with paraprobiotic compared to the control group (Figure 4d). The cytokines IL-10 and IL-12 showed no significant differences between the groups.
Figure 4.
Cytokine levels in the ascites fluid of mice infected with T. gondii. Concentrations levels of interferon-gamma (FN-γ), tumor necrosis factor (TNF), and interleukin-6 (IL-6) were significantly elevated in mice treated with immunobiotic (IB) compared to mice in the control group (PBS) and paraprobiotic (PP) but without significant differences between the PBS and PP groups (a–c). The administration of the paraprobiotic treatment significantly increased the concentration of monocyte chemoattractant protein-1 (MCP-1) compared to the group treated with PBS, however, no significant difference between the PP and IB groups or between the IB and PBS groups (d). Data were combined from two individual experiments with three to five mice per group. Values represent the means ± SD. * p < 0.05; ** p < 0.01; **** p < 0.0001. PBS) phosphate buffered saline; IB) immunobiotic; PP) paraprobiotic.
3.5. Determination of Spleen Cell Populations
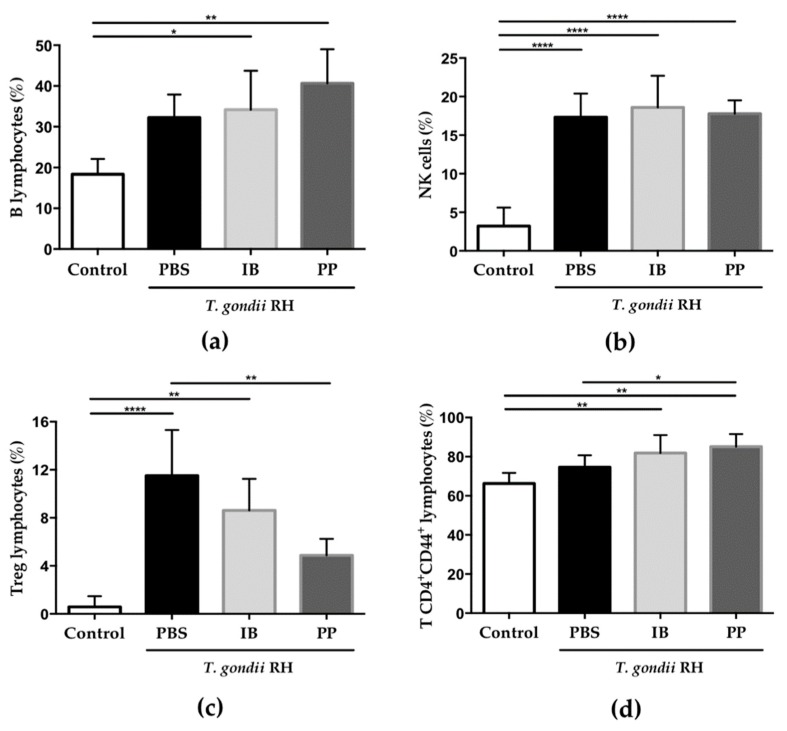
Since the immune response during infection with T. gondii involves cell populations that must be mobilized from secondary lymphoid organs, some of the most important lymphocyte populations in the spleen were evaluated. The presence of the protozoan increased the percentage of B lymphocytes (Figure 5a) and Natural Killer cells (Figure 5b) while regulatory T lymphocytes (Figure 5c) and the subpopulation of T CD4+CD44+ lymphocytes (Figure 5d) also showed a significant difference between the treated groups.
Figure 5.
Determination of the percentage of the spleen cell populations on the seventh day post-infection with T. gondii RH. Splenocytes were labeled with different monoclonal antibodies for B lymphocytes (a), Natural Killer cells (b), regulatory T lymphocytes (c), and T CD4+CD44+ lymphocytes (d) Data were combined from two individual experiments with three to five mice per group. Values represent the means ± SD. * p < 0.05; ** p < 0.01; **** p < 0.0001. PBS) phosphate buffered saline; IB) immunobiotic; PP) paraprobiotic. PBS) phosphate buffered saline; IB) immunobiotic; PP) paraprobiotic.
3.6. Survival of CD1 Mice to Infection with T. gondii
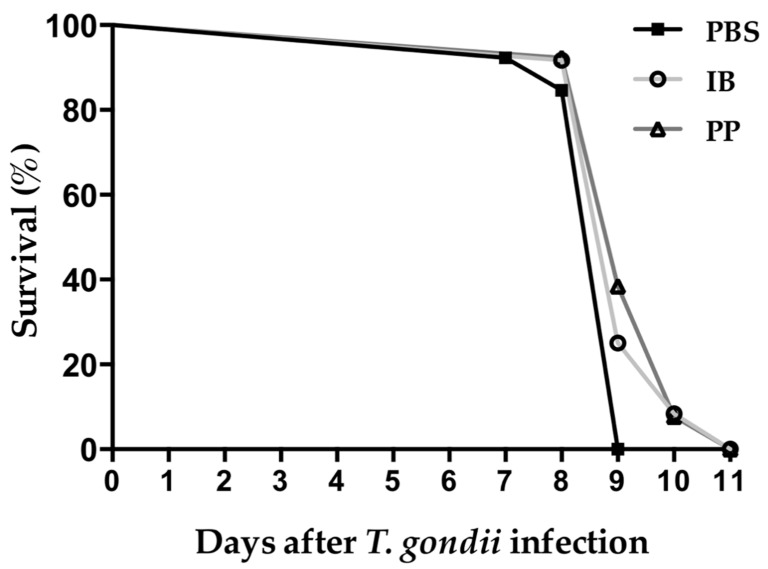
We evaluated the protective capacity of viable and non-viable Lc during a complete course of infection with tachyzoites of T. gondii RH. The mice that received the vehicle (PBS) showed signs of piloerection, overcrowding, and hyperventilation from 4 days post-infection, with all animals treated with PBS succumbing at 9 days post-infection. The mice that were treated with lactobacilli had the same signs until day 6 post-infection. At 9 days, there was 25% and 38.5% survival in mice treated with viable Lc and non-viable Lc, respectively. In the end, mice treated with lactobacilli succumbed to infection at day 11 post-infection (Figure 6).
Figure 6.
Survival of CD1 mice after infection with T. gondii. The mice were infected with 1000 tachyzoites of the RH strain of T. gondii intraperitoneally. All of the mice that were treated with PBS died on day 9 post-infection while all mice treated with immunobiotic and paraprobiotic died on day 11 post-infection. Data were combined from two individual experiments with six to seven animals per group. PBS) phosphate buffered saline; IB) immunobiotic; PP) paraprobiotic.
4. Discussion
The work shows anatomical, parasitological, and immunological evidence of the ability of live (immunobiotic) and dead (paraprobiotic) lactobacilli to stimulate the systemic immune system to control replication and pathology caused by infection with the protozoan T. gondii. The complexity and speed of replication of intracellular protozoa have made it difficult for patients to have effective and safe treatments to date. However, probiotics arise as a possibility that should be evaluated in in vivo models and demonstrate evidence of their benefit to health.
The systemic toxoplasmosis model caused by T. gondii RH allowed us to have a possible overview of the immunological mechanisms that a commercial probiotic, such as Lactobacillus casei, can carry out regardless of its viability.
The effect of probiotic lactobacilli goes beyond the gastrointestinal tract. It has been shown that the induced immune response in the small intestine can be extended to the systemic immune system and reach distant sites through lymphatic and blood circulation, which connects the intestinal immune system with the systemic immune system [21,22].
The administration of viable and non-viable Lc increased the microbicidal activity of peritoneal macrophages on the tachyzoites of T. gondii RH, as well as the cytokine production capacity.
This effect is closely related to the parasitic load, where the mice treated with immunobiotics presented the least amount of free tachyzoites in relation to the control group. High concentrations of IFN-γ and TNF stimulate the activation of peritoneal macrophages as well as the production of intracellular nitric oxide in mice treated with immunobiotics. There are in vitro and in vivo reports that some species of lactobacilli, including Lactobacillus casei, stimulate the production of proinflammatory cytokines IFN-γ and TNF [23]. IFN-γ, like TNF, play an important role in the resistance to infection of T. gondii due to the ability of macrophage activation [24]. IFN-γ is produced primarily by NK cells, T CD4+ cells, and T CD8+ cells in response to the antigenic recognition of glycosylphosphatidylinositol by Toll-like receptor 2 and 4 (TLR2 and TLR4), as well as to the profilin-like protein that is recognized by TLR11 [25]. IFN-γ has a pleiotropic effect on infected cells, mainly macrophages that result in the reduction of protozoan replication by inducing the expression of 2,3 dioxygenase indolamine. It depletes tryptophan, which is an essential amino acid for growth of T. gondii, increases the expression of inducible nitric oxide synthase (iNOS) through the production of NO, and depletes arginine, which is another essential amino acid for the parasite, and finally, the expression of immunity-related GTPases (IRGs) and guanylate-binding proteins (GBPs) that are responsible for the destruction of the parasitophorous vacuole [26].
Macrophages play a key role in controlling the replication of tachyzoites of T. gondii. One of the main microbicidal mechanisms of activated macrophages is the production of nitric oxide. However, it is known that T. gondii has the ability to evade the cytotoxic effect of nitric oxide by inhibiting the production of NO in mouse activated macrophages [27,28]. Cabral [29] suggest that inhibition of NO production in activated macrophages infected with T. gondii is a general phenomenon, but the suppression of iNOS varies depending on the macrophage cell line. The increase in the percentage of activated macrophages, as well as the production of intracellular nitric oxide in mice treated with immunobiotics, show that inhibition of NO production by T. gondii can be reversed after administration of viable Lc, thereby increasing the microbicidal capacity of activated macrophages.
Interestingly, spleen cell populations increased when mice were infected with T. gondii, however, regulatory T lymphocytes were found to be decreased and T CD4+CD44+ cells increased in the groups treated with Lc relative to the control group. IL-10 is the main cytokine that regulates the inflammatory process generated by T. gondii, so increasing the population of this cell is indicative of the severe immunopathology carried out in the peritoneal organs of the control group [30]. For T CD4+CD44+ lymphocytes, it has been shown that they have the ability to mobilize towards the flash point [31,32].
The prolongation of the survival of the mice treated with Lc showed that there is a greater control and elimination of tachyzoites of T. gondii, but the inflammatory process is so severe that the immunopathology caused led to the death of the mice treated with Lc, and this is due to the high replication of the parasite, where the T. gondii strain RH had a doubling time of approximately 5 h [33].
5. Conclusions
This is the first study carried out that demonstrates the remote immunostimulation of a viable and non-viable probiotic on a murine systemic toxoplasmosis model. The results demonstrate that regardless of the viability of Lactobacillus casei, these can activate macrophages and destroy tachyzoites by producing intracellular nitric oxide. This study lays the groundwork to investigate the molecular mechanism of immunostimulation of paraprobiotics on lethal systemic parasitic infections.
Acknowledgments
The authors thanks to María de la Luz Galván Ramírez by donating the T. gondii RH strain. To M.V.Z. Mónica Lascari Vargas by donating CD1 mice strain. To QFBT Pablo Franco Mendoza for his technical support.
Author Contributions
Conceptualization, A.G.S.-L. and V.M.B.-d.L.; Formal analysis, A.G.S.-L. and V.M.B.-d.L.; Funding acquisition, V.M.B.-d.L.; Investigation, A.R.-C. and V.M.B.-d.L.; Methodology, A.G.S.-L. and A.R.-C.; Writing—original draft, A.G.S.-L. and V.M.B.-d.L.; Writing—review and editing, J.A.B.-L. All authors have read and agreed to the published version of the manuscript.
Funding
Angel Gustavo Salas-Lais received academic and research support of the Posgrado en Biomedicina y Biotecnología Molecular, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional (IPN), and received grants from CONACyT 594529.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Reid G., Food and Agricultural Organization of the United Nation. WHO The importance of guidelines in the development and application of probiotics. Curr. Pharm. Des. 2005;11:11–16. doi: 10.2174/1381612053382395. [DOI] [PubMed] [Google Scholar]
- 2.Clancy R. Immunobiotic and the probiotic evolution. FEMS Immunol. Med. Microbiol. 2003;38:9–12. doi: 10.1016/S0928-8244(03)00147-0. [DOI] [PubMed] [Google Scholar]
- 3.Taverniti V., Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept) Genes Nutr. 2011;6:261–274. doi: 10.1007/s12263-011-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wells J.M. Immunomodulatory mechanism of lactobacilli. Microb. Cell Fact. 2011;10:1–15. doi: 10.1186/1475-2859-10-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shida K., Nanno M., Nagata S. Flexible cytokine production by macrophages and T cells in response to probiotic bacteria. Gut Microb. 2011;2:109–114. doi: 10.4161/gmic.2.2.15661. [DOI] [PubMed] [Google Scholar]
- 6.Gaudino S.J., Kumar P. Cross-talk between antigen presenting cells and T cells impacts intestinal homeostasis, bacterial infections, and tumorigenesis. Front. Immunol. 2019;10:360. doi: 10.3389/fimmu.2019.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habil N., Beal J., Foey A.D. Lactobacillus casei strain Shirota selectively modulates macrophage subset cytokine production. Int. J. Probiotics Prebiotics. 2012;7:1–12. [Google Scholar]
- 8.Garfias C.R.B., Álvarez M.C.T., Gómez F.M. The inoculation of Lactobacillus casei in NIH mice induces a protective response against Trypanosoma cruzi (Ninoa Strain) infection. Vet. Méx. 2008;39:139–144. [Google Scholar]
- 9.Martínez-Gómez F., Ixta-Rodríguez O., Aguilar-Figueroa B., Hernández-Cruz R., Monroy-Ostria A. Lactobacillus casei ssp. rhamnosus enhances nonspecific protection against Plasmodium chabaudi AS in mice. Salud Publica Mex. 2006;48:498–503. doi: 10.1590/s0036-36342006000600008. [DOI] [PubMed] [Google Scholar]
- 10.Bautista-Garfias C.R., Gómez M.B., Aguilar B.R., Ixta O., Martínez F., Mosqueda J. The treatment of mice with Lactobacillus casei induces protection against Babesia microti infection. Parasitol. Res. 2005;97:472–477. doi: 10.1007/s00436-005-1475-7. [DOI] [PubMed] [Google Scholar]
- 11.Munoz M., Liesenfeld O., Heimesaat M.M. Immunology of Toxoplasma gondii. Immunol. Rev. 2011;240:269–285. doi: 10.1111/j.1600-065X.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 12.Lambert H., Barragan A. Modelling parasite dissemination: host cell subversion and immune evasion by Toxoplasma gondii. Cell. Microbiol. 2010;12:292–300. doi: 10.1111/j.1462-5822.2009.01417.x. [DOI] [PubMed] [Google Scholar]
- 13.Galván-Ramírez M., Mondragón-Flores R. Toxoplasmosis Humana. 1st ed. Ecorfan; Mexico City, Mexico: 2017. [Google Scholar]
- 14.Martínez-Gómez F., García-González L.F., Mondragón-Flores R., Bautista-Garfias C.R. Protection against Toxoplasma gondii brain cyst formation in mice immunized with Toxoplasma gondii cytoskeleton proteins and Lactobacillus casei as adjuvant. Vet. Parasitol. 2009;160:311–315. doi: 10.1016/j.vetpar.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro C.M., Zorgi N.E., Meireles L.R., Garcia J.L., Andrade-Junior H.F. CD19 lymphocyte proliferation induced by Bifidobacterium animalis subsp. lactis in C57BL/6 mice experimentally infected with Toxoplasma gondii. Rev. Inst. Med. Trop. Sao Paulo. 2016;58:26. doi: 10.1590/S1678-9946201658026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C.Y., Tsen H.H., Lin C.L., Lin C.K., Chuang L.T., Chen C.S., Chiang Y.C. Enhancement of the immune response against Salmonella infection of mice by heat-killed multispecies combinations of lactic acid bacteria. J. Med. Microbiol. 2013;62:1657–1664. doi: 10.1099/jmm.0.061010-0. [DOI] [PubMed] [Google Scholar]
- 17.Sudan V., Tewari A.K., Singh H., Singh R. Pathobiology of human RH strain induced experimental toxoplasmosis in murine model. J. Parasit. Dis. 2016;40:840–844. doi: 10.1007/s12639-014-0589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López-Figueroa M.O., Caamaño C., Marin R., Guerra B., Alonso R., Morano M.I., Akil H., Watson S.J. Characterization of basal nitric oxide production in living cells. Biochim. Biophys. Acta. 2001;1540:253–264. doi: 10.1016/S0167-4889(01)00138-0. [DOI] [PubMed] [Google Scholar]
- 19.Terrazas C.A., Juarez I., Terrazas L.I., Saavedra R., Calleja E.A., Rodriguez-Sosa M. Toxoplasma gondii: Impaired maturation and pro-inflammatory response of dendritic cells in MIF-deficient mice favors susceptibility to infection. Exp. Parasitol. 2010;126:348–358. doi: 10.1016/j.exppara.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Lee H., Sun Y., Patti-Diaz L., Hendrick M., Ehrhardt A.G. High-throughput analysis of clinical flow cytometry data by automated gating. Bioinform. Biol. Insights. 2019;13:1–9. doi: 10.1177/1177932219838851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemgang T.S., Kapila S., Shanmugam V.P., Kapila R. Cross-talk between probiotic lactobacilli and host immune system. J. Appl. Microbiol. 2014;117:303–319. doi: 10.1111/jam.12521. [DOI] [PubMed] [Google Scholar]
- 22.Czerkinsky C., Holmgren J. Mucosal delivery routes for optimal immunization: targeting immunity to the right tissues. Curr. Top. Microbiol. Immunol. 2012;354:1–18. doi: 10.1007/82_2010_112. [DOI] [PubMed] [Google Scholar]
- 23.Marranzino G., Villena J., Salva S., Alvarez S. Stimulation of macrophages by immunobiotic Lactobacillus strains: influence beyond the intestinal tract. Microbiol. Immunol. 2012;56:771–781. doi: 10.1111/j.1348-0421.2012.00495.x. [DOI] [PubMed] [Google Scholar]
- 24.Miller C.M., Bolter N.R., Ikin R.J., Smith N.C. The immunobiology of the innate response to Toxoplasma gondii. Int. J. Parasitol. 2009;39:23–39. doi: 10.1016/j.ijpara.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Debierre-Grockiego F., Campos M.A., Azzouz N., Schmidt J., Bieker U., Garcia-Resende M., Santos-Mansur D., Weingart R., Schmindt R.R., Golenbock D.T., et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J. Immunol. 2007;179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- 26.Yarovinsky F. Innate immunity of Toxoplasma gondii infection. Nat. Rev. Immunol. 2014;14:109–121. doi: 10.1038/nri3598. [DOI] [PubMed] [Google Scholar]
- 27.Seabra S.H., de Souza W., DaMatta R.A. Toxoplasma gondii partially inhibits nitric oxide production of activated murine macrophages. Exp. Parasitol. 2002;100:62–70. doi: 10.1006/expr.2001.4675. [DOI] [PubMed] [Google Scholar]
- 28.Lüder C.G., Algner M., Lang C., Bleicher N., Gross U. Reduced expression of the inducible nitric oxide synthase after infection with Toxoplasma gondii facilitates parasite replication in activated murine macrophages. Int. J. Parasitol. 2003;33:833–844. doi: 10.1016/S0020-7519(03)00092-4. [DOI] [PubMed] [Google Scholar]
- 29.Cabral G.R.A., Wang Z.T., Sibley L.D., DaMatta R.A. Inhibition of nitric oxide production in activated macrophages caused by Toxoplasma gondii infection occurs by distinct mechanisms in different mouse macrophage cell lines. Front. Microbiol. 2018;9:1936. doi: 10.3389/fmicb.2018.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morampudi V., De Craeye S., Le Moine A., Detienne S., Braun M.Y., D’Souza S. Partial depletion of CD4+CD25+Foxp3+ T regulatory cells significantly increases morbidity during acute phase Toxoplasma gondii infection in resistant BALB/c mice. Microbes Infect. 2011;13:394–404. doi: 10.1016/j.micinf.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Baaten B.J.G., Li C.R., Bradley L.M. Multifaceted regulation of T cells by CD44. Commun. Integr. Biol. 2010;3:508–512. doi: 10.4161/cib.3.6.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu T., Soong L., Liu G., König R., Chopra A.K. CD44 expression positively correlates with Foxp3 expression and suppressive function of CD4+Treg cells. Biol. Dir. 2009;4:40. doi: 10.1186/1745-6150-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lüder C.G.K., Rahman T. Impact of the host on Toxoplasma stage differentiation. Microb. Cell. 2017;4:203–211. doi: 10.15698/mic2017.07.579. [DOI] [PMC free article] [PubMed] [Google Scholar]