Abstract
Voltage-gated sodium channels (NaVs) are membrane proteins that are involved in the generation and propagation of action potentials in neurons. Recently, the structure of a complex made of a tetrodotoxin-sensitive (TTX-s) NaV subtype with saxitoxin (STX), a shellfish toxin, was determined. STX potently inhibits TTX-s NaV, and is used as a biological tool to investigate the function of NaVs. More than 50 analogs of STX have been isolated from nature. Among them, zetekitoxin AB (ZTX) has a distinctive chemical structure, and is the most potent inhibitor of NaVs, including tetrodotoxin-resistant (TTX-r) NaV. Despite intensive synthetic studies, total synthesis of ZTX has not yet been achieved. Here, we review recent efforts directed toward the total synthesis of ZTX, including syntheses of 11-saxitoxinethanoic acid (SEA), which is considered a useful synthetic model for ZTX, since it contains a key carbon–carbon bond at the C11 position.
Keywords: saxitoxin, zetekitoxin AB, voltage-gated sodium channel, guanidine alkaloid
1. Introduction
1.1. Voltage-Gated Sodium Channel Isoforms
Voltage-gated sodium channels (NaVs) are membrane proteins involved in neuronal excitation and transmission [1]. Ten subtypes, NaV1.1–1.9 and NaVX, have been identified based on sequence determination (Table 1) [2]. These subtypes can be grouped into two types depending upon their sensitivity to the pufferfish toxin, tetrodotoxin (TTX) [3,4,5,6,7]: tetrodotoxin-sensitive NaVs (TTX-s NaVs 1.1–1.4, 1.6, and 1.7) are significantly inhibited by TTX, while tetrodotoxin-resistant NaVs (TTX-r NaVs 1.5, 1.8, 1.9) are not [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. Subtype-selective modulators of NaVs are required for studies to establish the biological functions of these subtypes. Some of the subtypes are also considered to be potential drug targets; for example, NaV1.7 and 1.8 are potential targets for pain treatment [24,25,26,27,28,29]. Therefore, there is great interest in the development of drugs targeting specific subtypes [30,31].
Table 1.
Isoforms of a voltage-gated sodium channel (NaV) and their classifications.
| NaV Isoform | Primary Locations | Related Diseases | TTX IC50 (nM) |
|---|---|---|---|
| TTX-sensitive | |||
| NaV1.1 | CNS, PNS, heart | Epilepsy | 5.9 |
| NaV1.2 | CNS | Epilepsy | 7.8 |
| NaV1.3 | Embryonic CNS, injured DRG | Nerve injury | 2.0 |
| NaV1.4 | Skeletal muscle | Myotonia | 4.5 |
| NaV1.6 | CNS, PNS, SMCs, DRG | CNS disorders | 3.8 |
| NaV1.7 | PNS, DRG | Pain sensation | 5.5 |
| TTX-resistant | |||
| NaV1.5 | Heart, embryonic CNS | Cardiac arrhythmias | 1970 |
| NaV1.8 | PNS, DRG | Pain sensation | 1330 |
| NaV1.9 | PNS, DRG | Pain sensation | 59,600 |
TTX: tetrodotoxin; CNS: central nervous system; PNS: peripheral nervous system; DRG: dorsal root ganglion; SMCs: smooth muscle cells.
1.2. Saxitoxin As A NaV Modulator
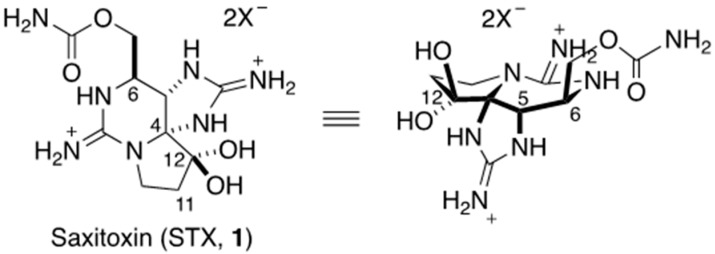
Saxitoxin (STX, 1) is a guanidine alkaloid with potent and specific inhibitory activity towards NaVs (Figure 1) [32,33]. It has long been known as a shellfish toxin. In 1937, Sommer and co-workers found that toxin-free bivalves, including the dinoflagellate Gonyaulax catenella, became poisoned in seawater, and they revealed that the real producer of STX (1) is algae [34,35]. Then, STX (1) was first isolated from Alaska butter clams by Schantz’s group in 1957 [36,37]. Rapport’s group subsequently isolated the same toxin from the same shellfish, and named it saxitoxin [38]. Structural elucidation was troublesome. Initially, tri- or tetracyclic structures were proposed based upon the molecular formula and the presence of two guanidines as functional groups. Finally, the structure of STX (1) was independently determined by the two groups by means of X-ray analysis in 1975 [39,40]. STX (1) consists of ten carbons, seven nitrogens, and four oxygens, and all the carbons except for C11 are connected with heteroatoms. STX (1) contains five- and six-membered cyclic guanidines, which have different pKa values of 8.7 and 12.4, respectively; the five-membered one is less basic, presumably due to its less planar structure [41].
Figure 1.
Structure of saxitoxin (STX, 1).
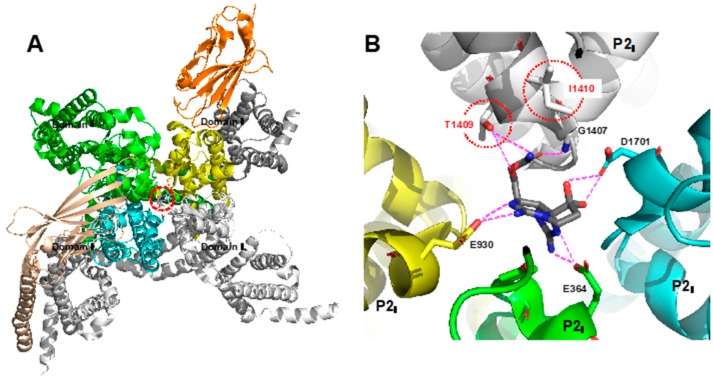
STX (1) binds to the pore-forming region of the alpha-loop of NaV and blocks the influx of sodium cation in a similar manner to tetrodotoxin (TTX) [42,43]. Recently, Yan’s group determined the X-ray structure of the complexes of STX (1) with NaVPas derived from American cockroach and human-derived NaV1.7 by using cryoEM (Figure 2) [44,45]. They found that the carbamoyl group at C13 in STX (1) interacts with Gly1407 and Thr1409 in domain III, the two guanidines interact with Glu364 in domain I and Glu930 in domain II, and the geminal diol interacts with Asp1701 in domain IV. Interestingly, residues 1409 and 1410, located in the P2 loop of domain III in NaV1.7, were mutated to Thr and Ile from Met and Asp, respectively, which may explain why STX (1) has a weaker affinity for NaV1.7 compared with other subtypes (Met1409 and Asp1410 are conserved in the other subtypes of TTX-s) [46,47].
Figure 2.
(A) Top view of the structure of the STX–NaV1.7 complex. (B) Specific interactions in the STX–NaV1.7 complex [45].
1.3. Natural Analogs of Saxitoxin, Including Zetekitoxin AB (ZTX)
To date, more than 50 kinds of natural analogs of saxitoxin have been reported, of which most are modified at N1 (R1), C11 (i.e., R2 and R3), or C13 (R4) in the common structure shown in Figure 3A [48]. For example, neosaxitoxin (neoSTX, 2) is hydroxylated at N1, decarbamoylsaxitoxin (dcSTX, 3) has a hydroxyl group at C13, and gonyautoxins I–III (GTX I–III, 5–7, respectively) have a sulfate ester at C11; all of these analogs show similar NaV-inhibitory activity to STX (1).
Figure 3.
(A) Representative STX derivatives. (B) Structure and NaV-inhibitory activities of zetekitoxin AB (8) [49].
Among the STX (1) derivatives, zetekitoxin AB (ZTX, 8) has an unusual structure [49]. ZTX (8) was isolated from skin of the Panamanian dart-poison frog Atelopus zeteki in 1969 by Mosher and co-workers [50,51]. It has extremely potent NaV-inhibitory activity (more than 600-fold greater than that of STX (1)), with IC50 values of 6.1 pM, 65 pM, and 280 pM for NaV1.2, NaV1.4, and TTX-r subtype NaV1.5, respectively [49]. Thus, there is great interest in the mode of action of ZTX (8), but studies are hampered by the fact that Atelopus zeteki is designated as an endangered species. Therefore, a chemical synthesis of ZTX (8) is needed. However, ZTX (8) contains a macrocyclic lactam structure in which isoxazolidine is bridged from C6 to C11, and an N-hydroxycarbamate is linked via a methylene group at N7 [49]. These structural features make ZTX (8) synthetically challenging. So far, several synthetic approaches have been reported, but a total synthesis of 8 has not yet been achieved.
1.4. Scope of This Review
Synthetic studies of STX (1) and its analogs have been extensive, and several total syntheses have been achieved [52,53,54,55,56,57,58,59,60,61], as recently reviewed by Du Bois [62]. Approaches for developing of subtype-selective modulators based on the STX structure have also been explored [25,47,63,64,65]. However, in this review, we focus on recent synthetic work related to ZTX (8). As described above, ZTX (8) has a characteristic macrolactam structure though C6 to C11 with an isoxazolidine ring system, and is structurally quite distinct from other STX analogs. To achieve total synthesis of ZTX (8), two key issues must be addressed: (i) carbon–carbon bond formation at the C11 position in the STX skeleton, and (ii) macrolactam formation of the carboxylic acid at C6 with isoxazolidine nitrogen (Figure 4). Regarding the first issue, the STX derivative 11-saxitoxinethanoic acid (SEA, 9) has been used as a synthetic model for 8, since it also has a carbon–carbon bond at the C11 position. As for the second issue, stereoselective synthesis of disubstituted isoxazolidine and oxidation to carboxylic acid at C13, followed by amide formation with the isoxazolidine, have been examined. First, we will consider recent progress in the total synthesis of SEA (9).
Figure 4.
Key issues in the synthesis of ZTX (8) and 11-saxitoxinethanoic acid (SEA, 9).
2. Development of Carbon–Carbon Linkage at C11 of STX, And Application to The Synthesis of 11-Saxitoxinethanoic Acid (SEA, 9)
The STX analog 11-saxitoxinethanoic acid (SEA, 9) was isolated from Atergatis floridus, an Indo-Pacific crab from the family Xanthidae, by Onoue and co-workers (Figure 5) [66]. SEA (9) has an acetic acid moiety linked to C11 through a carbon–carbon bond, as seen in ZTX (8), and is regarded as a promising synthetic model compound for 8 in terms of construction of the carbon–carbon connection at C11.
Figure 5.
Structure of 11-saxitoxinethanoic acid (SEA, 9) and illustration of the source crab species.
Recently, three total synthesis of SEA (9) were independently reported, including one by our group [67,68,69]. When 9 was first isolated, its toxicity to mice was reported to be 830 μmol/MU, which is similar to that of gonyautoxin II (GTX II, 6) and one-third of that of STX (1), but no information about the NaV-inhibitory activity was provided. After the synthesis of 9, Du Bois and our group independently evaluated the NaV-inhibitory activity of 9. Nagasawa, Yotsu-Yamashita, and co-workers evaluated the NaV-inhibitory activity of SEA (9) by utilizing neuroblastoma Neuro 2A cells, which is known to express NaV1.2, 1.3, 1.4, and 1.7 [70], and found moderate inhibitory activity with an IC50 value of 47.0 ± 1.2 nM (Figure 6B) [67]. Du Bois and co-workers evaluated the inhibitory activity of 9 against NaV1.4, and found that SEA (9) showed similar inhibitory activity to gonyautoxin III (GTX III, 7) (9: IC50 = 17 ± 1.9 nM; GTX III (7): IC50 = 14.9 ± 2.1 nM), even though it was a diastereomeric mixture of α:β = 3:1 at C11 (Figure 6A) [68]. They suggested that the β-form of 9 binds to NaV preferentially, and then the α-form of 9 isomerizes to the β-form, which shows a similar level of inhibitory activity to GTX III (7) (Figure 6C).
Figure 6.
(A) Isomerization of α-SEA to β-SEA. (B) NaV-inhibitory activity of SEA (9) [69,70]. (C) Structure of GTX III (7) and IC50 (NaV1.4).
2.1. Carbon–Carbon Bond Formation at C11 by Mukaiyama Aldol Condensation Reaction, as Applied for The Synthesis of (+)-SEA by Nagasawa’s Group
For the construction of a carbon–carbon bond at C11, Nagasawa and co-workers utilized ketone 10, which was previously developed by their group [67], to install an acetic acid equivalent at C11. They firstly investigated the alkylation reaction of the enolate of ketone 10a with alpha-halo-ethyl acetate. With various bases and halogens, the alkylation did not take place at all, and the starting ketone 10a was recovered. Next, they investigated the Mukaiyama aldol reaction [71,72,73]. Thus, silyl enol ethers 11a and 11b were synthesized from the ketone by reaction with tert-butyldimethylsilyl chloride in the presence of NaHMDS as a base. Then, the Mukaiyama aldol reaction was examined with ethyl glyoxylate under various conditions. Lewis acids, such as TiCl4 or BF3 Et2O [74,75], removed the tert-butoxycarbonyl (Boc) protecting group of guanidine, and no coupling products with ethyl glyoxylate were obtained. In the case of the fluoride anion agent Bu4NF [76], the reaction did not proceed at all. On the other hand, with anhydrous tetrabutyl bisfluorotriphenylphosphine stannate, developed by Raimundo and co-workers [77], the coupling reaction with ethyl glyoxylate proceeded very well to afford the aldol-condensation product 12a a 96% yield (Table 2). Aromatic aldehydes were tolerated, as well as aliphatic aldehydes, and the corresponding aldol condensation products 12a–i were obtained with 42%–80% yield. This reaction afforded mixtures of regioisomers in ratios of 5:1 to >10:1.
Table 2.
Substrate scope of the Mukaiyama aldol condensation reaction of 11a and 11b with aldehydes.

| Entry. | SM | R2 | 12 (E:Z) a | Yield (%) |
|---|---|---|---|---|
| 1 | 11a | CO2Et | 12a (5:1) | 96 |
| 2 | 11b | CO2Et | 12b (5:1) | 85 |
| 3 | 11a | 4-MeC6H4 | 12c (> 1:1) | 45 |
| 4 | 11a | 3-FC6H4 | 12d (> 10:1) | 63 |
| 5 | 11a | 4-ClC6H4 | 12e (> 10:1) | 65 |
| 6 | 11a | 4-NO2C6H4 | 12f (6:1) | 80 |
| 7 | 11a | 2-Furyl | 12g (> 10:1) | 80 |
| 8 | 11a | C6H5 | 12h (> 10:1) | 60 |
| 9 | 11b | C6H5 | 12i (E:Z) a | 42 |
a Ration at C11 were determined by 1H NMR spectroscopy.
With the aldol condensation product 12b in hand, Nagasawa and co-workers went on to achieve a total synthesis of (+)-SEA (9) for the first time (Scheme 1). Thus, selective reduction of the enone moiety in 12b was carried out with L-selectride, and the protecting group of tert-butyldimethylsilyl (TBS) ether was removed with triethylamine trihydrofluoride (3HF-TEA). The resulting alcohol was reacted with trichloroisocyanate, followed by hydrolysis of the trichloroacetyl group with triethylamine in methanol to give carbamoyl 15. After hydrolysis of ethyl ester in 15 with lithium hydroxide, the Boc group was removed with TFA to give (+)-SEA (9).
Scheme 1.
Total synthesis of SEA (9) by Nagasawa’s group.
2.2. Carbon–Carbon Bond Formation At C11 by Stille Coupling Reaction, As Applied for The Synthesis of (+)-SEA by Du Bois’ Group
Another approach for the construction of the carbon–carbon bond at C11 in STX was explored by Du Bois and co-workers, who employed Stille coupling reaction conditions [68]. They firstly examined the coupling reaction of zinc enolate of ethyl acetate or the stannane enolate of ethyl acetate-type agents with vinyl halide 17, which was prepared from 20, developed by their group (Scheme 2), in the presence of palladium catalyst (Table 3, entries 1 and 2) [78,79,80,81,82,83]. Under the conditions examined, decomposition of the starting substrate was observed in the case of zinc agent, and no reaction occurred with the stannane agent. Then they examined the Stille coupling reaction, using vinyl stannane for the construction of the carbon–carbon bond at C11 [84]. A Stille coupling reaction of vinyl iodide 17 with tributyl(vinyl)tin was examined in the presence of a catalytic amount of Pd(PPh3)4, with CuI as an additive (a standard condition). Unfortunately, only a trace amount of the corresponding coupling product of 18c was obtained (entry 3). Then, they changed vinyl stannane to cis-tributyl (2-ethoxyvinyl) tin, and included LiCl as an additional additive. Under these conditions, the corresponding coupling product 18d was obtained with 67% yield (entry 4) [85,86]. Interestingly, poor reproducibility or low yield of the coupling reaction was observed when they used a highly oxidized vinyl stannane agent, tributyl(2,2-diethoxyvinyl)stannane (entry 5). This issue was successfully overcome by switching from CuI to copper(I) thiophene-2-carboxylate (CuTC), and 19 was obtained with 60% yield and with good reproducibility (entry 6) [87].
Scheme 2.
Introduction of substituent at C11 by Stille coupling, leading to total synthesis of (+)-SEA (9) by Du Bois’ group.
Table 3.
Stille-based cross-coupling conditions with iodoenaminone 17.

| Entry | Conditions | R | Result |
|---|---|---|---|
| 1 | CH2C(OZnBr)OtBu, Pd2(dba)3/dppf, THF | CH2CO2tBu | 18a (decomp.) |
| 2 | CH2C(OSnnBu3)OEt, PdCl2(P(o-tol)3)2, CuF2 | CH2CO2Et | 18b (N.R.) |
| 3 | nBu3SnCH=CH2, Pd(PPh3)4, CuI | CH=CH2 | 18c (< 5%) |
| 4 | nBu3SnCH=CH(OEt), Pd(PPh3)4, CuCl, LiCl, THF | CH=CH(OEt) | 18d (67%) |
| 5 | nBu3SnCH=C(OEt)2, Pd(PPh3)4, CuCl, LiCl, THF | CH=C(OEt)2 | 19 (0–40%) |
| 6 | nBu3SnCH=C(OEt)2, Pd(PPh3)4, CuTC, THF | CH=C(OEt)2 | 19 (60%) |
CuTC = Copper (I) thiophene-2-carboxylate.
Based upon the Stille coupling strategy, Du Bois and co-workers achieved a total synthesis of SEA (9), as shown in Scheme 2, including the synthesis of vinyl halide 17 as a substrate for the Stille coupling reaction. Firstly, vinyl halide 17 was synthesized from 20 via Mislow–Evans [2,3] rearrangement: bisguanidine 20 was converted to N,S-acetal 21 by reaction with benzenethiol in the presence of BF3·Et2O with 84% yield. Upon treatment of 21 with urea–hydrogen peroxide (UHP), the Mislow–Evans [2,3] rearrangement reaction [88,89] took place under heating in the presence of sodium benzenthiolate, and allylic alcohol 23 was obtained with 81% yield in two steps. After oxidation of the alcohol with Dess–Martin periodinate, the resulting enone 24 was reacted with iodine in the presence of pyridine to give vinyl iodide 17 [90,91], which was further elaborated to 19 by Stille coupling reaction with 25 with 60% yield. Then, the double bond in enone 19 was hydrogenated under high pressure in the presence of Crabtree catalyst 26. Deprotection of the tert-butyldiphenylchlorosilane (TBDPS) ether in 27 with tetrabutylammonium (TBAF) was followed by installation of a carbamoyl group on the resulting hydroxyl group. Finally, deprotection of Tces and Troc and hydrolysis of the ester group were carried out to give (+)-SEA (9).
2.3. Carbon–Carbon Bond Formation at C11 by C-Alkylation, As Applied for The Synthesis of (+)-SEA by Looper’s Group
In 2019, Looper and co-workers successfully constructed a carbon–carbon bond at C11 in STX, and reported a total synthesis of SEA (9) [69]. They initially examined C-alkylation with ketone 28 and electrophiles in the presence of variety of bases, such as lithium bis(trimethylsilyi)amide (LHMDS), lithium diisopropyl amide (LDA), potassium bis(trimethylsilyi)amide (KHMDS) and sodium bis(trimethylsilyi)amide (NaHMDS). In addition, they examined various electrophiles (haloacetates, allylic halides, and propargylic halides), but no reaction took place, as Nagasawa and co-workers had found (Scheme 3) [67].
Scheme 3.
Examination of carbon–carbon bond construction at C11 by alkylation with ketone 28 in the presence of bases.
On the other hand, they found that C-alkylation took place upon reaction of ketone 28 and tert-butyl bromoacetate via the generation of zinc enolate by reaction with LiHMDS in the presence of Et2Zn, affording a mixture of 29a and its Boc-deprotected derivative 29b with 60% yield (based on the starting material) (Scheme 4). By means of this alkylation strategy, they succeeded in synthesizing ZTX (9) as follows. Deprotection of TBDPS ether in 29 with TBAF followed by carbamoylation of the resulting alcohol resulted in 30. Finally, total synthesis of SEA (9) was achieved by reaction with TFA to hydrolyze the ester and deprotect the Boc and DPM groups.
Scheme 4.
Carbon–carbon bond formation at C11 by C-alkylation, and total synthesis of (+)-SEA (9) by Looper’s group.
2.4. NaV-Inhibitory Activity of Synthesized, C11-Substituted Saxitoxin Analogs
Based on the method described above for constructing a carbon–carbon bond at C11 in STX, Nagasawa and co-workers synthesized a series of STX analogs bearing substituents at C11, and evaluated the NaV-inhibitory activity of these analogs at the cellular level [67].
Beside SEA (9), they synthesized dicarbamoyl SEA (dcSEA, 31), 11-saxitoxin ethyl ethanoate (SEE, 32), and 11-benzylidene STX (33a), and evaluated their NaV-inhibitory activity in mouse neuroblastoma Neuro 2A cells, which is known to express NaV1.2, 1.3, 1.4, and 1.7 [70]. SEA (9) showed potent inhibitory activity with an IC50 value of 47 ± 12 nM, which is twice as potent as decarbamoyl saxitoxin (dcSTX (3), IC50 = 89 ± 36 M) (Figure 7, Table 4). The dcSEA (31) and SEE (32) showed IC50 values of 5700 ± 3.1 and 185 ± 74 nM, respectively. Interestingly, 11-benzylidene STX (33a) was a potent inhibitor, with an IC50 value of 16.0 ± 6.9 nM. Although the inhibition mode of 33a has not been clarified yet, the non-hydrated keto group at C12 in 33 might bind efficiently with NaV, resulting in potent inhibitory activity.
Figure 7.
Structures of 9, 31, 32, and 33a.
Table 4.
NaV-inhibitory activity of 9, 31, 32, and 33a in a cell-based assay with Neuro 2A cells.
| Compound | IC50 (mean ± SD) (nM) | n |
|---|---|---|
| dcSTX (3) | 89 ± 36 | 3 |
| SEA (9) | 47 ± 12 | 3 |
| dcSEA (31) | 5700 ± 3.1 | 3 |
| SEE (32) | 185 ± 74 | 4 |
| 11-benzylidene STX (33a) | 16 ± 6.9 | 5 |
Next, they further synthesized 11-substituted STX analogs 33b–f, and elucidated their subtype selectivity towards NaV1.2, 1.5, and 1.7, using the whole-cell patch-clamp recording method (Figure 8, Table 5) [92]. They found that 11-fluorobenzylidene STX (33c) showed selective and potent inhibitory activity against NaV1.2 (IC50 = 7.7 ± 1.6 nM), compared to the other subtypes tested. 11-Benzylidene STX (33a) and 11- nitrobenzylidene STX (33d) showed potent inhibitory activity against NaV1.5, with IC50 values of 94.1 ± 12.0 nM and 50.9 ± 7.8 nM, respectively. These compounds are the most potent TTX-r modulators among STX derivatives so far reported, except for ZTX (8) [49].
Figure 8.
Structures of 33a–f.
Table 5.
NaV-inhibitory activities of 33a–f, using whole-cell, patch-clamp recording.
| Compound | hNaV1.2 | hNaV1.5 | hNaV1.7 |
|---|---|---|---|
| 11-benzylidene STX (33a) | 5.2 ± 6.0 | 94.1 ± 12.0 | 124.1 ± 20.6 |
| 11-methylbenzylidene STX (33b) | 22.9 ± 8.6 | >300 | >300 |
| 11-fluorobenzylidene STX (33c) | 7.7 ± 1.6 | >300 | >300 |
| 11-nitrobenzylidene STX (33d) | 8.79 ± 0.96 | 50.9 ± 7.8 | >300 |
| 11-furfuryl STX (33e) | 542.7 ± 65.7 | >300 | >300 |
| 11-metoxybenzylidene STX (33f) | 45.0 ±2.72 | >300 | >300 |
IC50 (mean ± SD) (nM).
3. Stereoselective Synthesis of The Isoxazolidine Moiety of ZTX (8), And Its Introduction at C13 in A Model Compound
As described in the introduction, ZTX (8) has a characteristic macrolactam structure from C6 to C11, involving an isoxazolidine ring system. Thus, stereoselective synthesis of the di-substituted isoxazolidine unit in ZTX (8) has been examined. In the paper reporting the isolation of 8 in 2004, the amide carbonyl group in ZTX (8) at C13 appeared at 156.5 ppm in the 13C nuclear magnetic resonance (NMR) spectrum, which is a higher chemical shift compared to other amide carbonyls [49]. This interesting observation might be attributed to the unusual macrolactam structure in ZTX (8), and synthetic studies of model compounds have been carried out to understand the origin of this unusual chemical shift. In the following section, we discuss the stereoselective isoxazolidine syntheses reported by Nishikawa’s [93] and Lopper’s groups [94].
3.1. Synthesis of The Isoxazolidine Part of Zetekitoxin (8) from D-ribose by Nishikawa And Co-workers
In 2009, Nishikawa and co-workers reported the stereoselective synthesis of isoxazolidine 42 from D-ribose (34) (Scheme 5) [93]. They firstly synthesized nitroolefin 36 from aldehyde 35, which was derived from D-ribose (34) by means of a Henry reaction followed by dehydration with mesylation. After reduction of the double bond in 36 with NaBH4, the resulting nitroalkane 37 was treated with Boc2O to produce dihydrooxazole 39a and 39b with 86% yield, as a diastereomeric mixture at C16 in a ratio of 3:1. In this reaction, nitrile oxide 38 was generated first, and a 1,3-dipolar cyclization reaction occurred simultaneously. The major transition state model is shown in Scheme 5. The major diastereomer 39a was reduced stereoselectively with NaBH3CN to isoxazolidine 40. After acetylation of the amine in 40, isoxazolidine 42, which has the same stereochemistry as ZTX at C15 and C16, was obtained in three steps: (1) deprotection of acetonide with TFA, (2) oxidative cleavage of diol with NaIO4, and (3) reduction of the resulting aldehyde with NaBH4.
Scheme 5.
Synthesis of the isoxazolidine 42 by Nishikawa’s group.
3.2. Stereoselective Synthesis of The Isoxazolidine Part from Methyl α-d-glucopyranoside by Lopper and Co-Workers
In 2015, Lopper and co-workers reported a synthesis of isoxazolidine 59 (Scheme 6) [94]. Aldehyde 45 was synthesized from commercially available methyl α-d-glucopyranoside (43) by the iodination of 44 with iodine and PPh3, acetylation of the hydroxyl group, and reductive cleavage of the pyran ring with zinc in acetic acid [95]. Then, intramolecular 1,3-dipolar reaction of the terminal olefin with nitrone, which was generated from aldehyde 45 by reaction with hydroxylamine 46, took place stereoselectively to afford 48 via 47 with 52% yield. After deprotection of acetate in 48 with sodium methoxide [96,97], the resulting triol 49 was treated with NaOI4 followed by LiAlH4 to give diol 51 with 70% yield in two steps [98,99]. The isoxazolidine synthon 59 in ZTX (8) was synthesized from diol 51 in seven steps by selective functionalization of the two hydroxyl groups, followed by N-acylation.
Scheme 6.
Synthesis of N-acyl isoxazolidine 59 by Looper’s group.
3.3. Comparison of the Chemical Shift at C13 in Zetekitoxin (8) with Those in Some Synthetic Models
As discussed above, the 13C NMR chemical shift of the carbonyl group at C13 in ZTX (8) has been observed at 156.5 ppm [49], which is a higher value compared with usual amide carbonyl groups (170–175 ppm). To address the issue, Nishikawa’s and Looper’s groups independently examined the 13C chemical shifts of the carbonyl group at C13 in some model compounds (Figure 9) [93,94]. Simple N-acyl isoxazolidine models 60, 42, and 59 showed chemical shifts of 171.0, 172.7, 171.0 ppm, respectively, which are quite similar to those of regular cyclic N-acyl amides. However, model compounds 61–63 bearing alpha-guanidinoacetyl amide groups showed chemical shifts of 166.0, 168.3, and 167.0 ppm, respectively, being shifted ca. 5 ppm upfield compared to the other simple models.
Figure 9.
13C-NMR chemical shifts of ZTX (8) and model compounds.
Nagasawa and co-workers examined the chemical shift at C13 of 70, which has an STX skeleton; its synthesis is depicted in Scheme 7 [100]. They firstly aimed to obtain carboxylic acid 66 from alcohol 64 by oxidation. They examined various oxidants and conditions, but it appeared that the hydroxyl group in 64 was unreactive due to its axial orientation, and no reaction occurred, or unexpected side reactions proceeded. Finally, they found that 2-azaadamantane N-oxyl (AZADO)–NaClO and NaClO2 [101,102] were effective, resulting in carboxylic acid 66, which was obtained with 79% yield after TMSCHN2 treatment of the crude carboxylic acid 66 to hydrolyze the methyl ester 67. Condensation of carboxylic acid 66 with isoxazolidine 40 [93] in the presence of 4-(4, 6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM,68) [103], followed by deprotection of the Boc group and acetal with TFA, gave amide 70 in 98% yield. Unfortunately, the chemical shift of the carbonyl group in 70 was observed at 166.1 ppm, slightly higher than that of 62 or 63, but still lower than that of ZTX (8). The chemical shift in ZTX (8) may reflect the characteristic spatial structure associated with the presence of the macrolactam moiety.
Scheme 7.
Oxidation at C13 and introduction of the isoxazolidine motif.
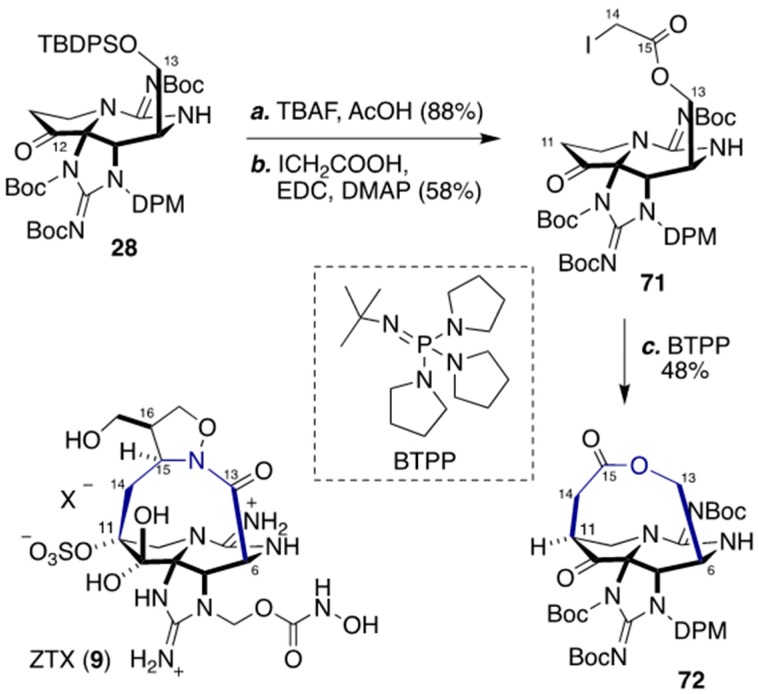
4. Synthesis of The Characteristic Macrocyclic Structure of ZTX (8) by Looper’s Group
Looper and co-workers have reported macrocyclic compound 72 as a model for ZTX (8) (Scheme 8) [71]. After deprotection of the TBDPS ether group at C13 with TBAF, the resulting alcohol was reacted with iodoacetic acid in the presence of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) and N,N-dimethyl-4-aminopyridine (DMAP) to give iodoester 71 with 58% yield. When iodoester 71 was treated with a strong base, tert-butylimino-tri(pyrrolidino)phosphorane (BTPP), intramolecular alkylation proceeded at C11, and the corresponding macrolactone 72 was obtained in 48% yield. It should be possible to construct the macrolactam structure of 8 via a similar strategy, and this should also resolve the chemical shift issue in ZTX (8).
Scheme 8.
Synthesis of macrolactone 72 via intramolecular alkylation by Looper’s group.
5. Conclusions
Here, we have reviewed recent progress towards the total synthesis of zetekitoxin AB (8, ZTX). Although this goal still remains elusive, there have been some significant synthetic advances in the construction of characteristic structures of ZTX, such as (i) the carbon–carbon bond at C11 in the STX structure, (ii) stereoselective construction of the substituted isoxazolidine moiety at C15 and C16, and (iii) the macrocyclic structure from C6 to C11. Since ZTX has potent inhibitory activity, even towards tetrodotoxin-resistant (TTX-r) NaVs, a total synthesis of ZTX and its analogs is expected to provide useful tools for chemical biological studies of NaVs, overcoming the severely restricted availability of natural ZTX.
Acknowledgments
K.A. thanks SUNBOR for providing a scholarship.
Author Contributions
Conceived and designed the story of manuscript, K.N.; writing–original draft preparation, K.N. and M.O.; writing—review and editing, K.A., H.I., M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Grants-in-Aid for Scientific Research on Innovative Areas “Middle Molecular Strategy” (18H04387 to K.N.), Grants-in-Aid for Scientific Research (B) (JP26282214 to K.N.), and the A3-foresight program. M.O. thanks the Japan Society for the Promotion of Science (JSPS), KAKENHI Grant Number 18K14210. This work was inspired by the international and interdisciplinary environment of the JSPS Asian CORE Program of ACBI (Asian Chemical Biology Initiative).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hodgkin A.L., Huxley A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldin A.L., Barchi R.L., Caldwell J.H., Hofmann F., Howe J.R., Hunter J.C., Kallen R.G., Mandel G., Meisler M.H., Netter Y.B., et al. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28:365–368. doi: 10.1016/S0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 3.Narahashi T. Tetrodotoxin. Proc. Jpn. Acad. Ser. B. 2008;84:147–154. doi: 10.2183/pjab.84.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishikawa T., Isobe M. Synthesis of Tetrodotoxin, a Classic but Still Fascinating Natural Product. Chem. Rec. 2013;13:286–302. doi: 10.1002/tcr.201200025. [DOI] [PubMed] [Google Scholar]
- 5.Moczydlowski E.G. The molecular mystique of tetrodotoxin. Toxicon. 2013;63:165–183. doi: 10.1016/j.toxicon.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Fozzard H.A., Lipkind G.M. The Tetrodotoxin Binding Site Is within the Outer Vestibule of the Sodium Channel. Mar. Drugs. 2010;8:219–234. doi: 10.3390/md8020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee C.H., Ruben P.C. Interaction between voltage-gated sodium channels and the neurotoxin, tetrodotoxin. Channels. 2008;2:407–412. doi: 10.4161/chan.2.6.7429. [DOI] [PubMed] [Google Scholar]
- 8.Clare J.J., Tate S.N., Nobbs M., Romanos M.A. Voltage-gated sodium channels as therapeutic targets. Drug Discov. Today. 2000;5:506–520. doi: 10.1016/S1359-6446(00)01570-1. [DOI] [PubMed] [Google Scholar]
- 9.Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986;320:188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- 10.Meadows L.S., Chen Y.H., Powell A.J., Clare J.J., Ragsdale D.S. Functional modulation of human brain NaV1.3 sodium channels, expressed in mammalian cells, by auxiliary beta 1, beta 2 and beta 3 subunits. Neuroscience. 2002;114:745–753. doi: 10.1016/S0306-4522(02)00242-7. [DOI] [PubMed] [Google Scholar]
- 11.Trimmer J.S., Cooperman S.S., Tomiko S.A., Zhou J., Crean S.M., Boyle M.B., Kallen R.G., Sheng Z., Barchi R.L., Sigworth F.J. Primary structure and functional expression of a mammalian skeletal muscle sodium channel. Neuron. 1989;3:33–49. doi: 10.1016/0896-6273(89)90113-X. [DOI] [PubMed] [Google Scholar]
- 12.Chahine M., Bennett P.B., George A.L., Jr., Horn R. Functional expression and properties of the human skeletal muscle sodium channel. Pflug. Arch. Eur. J. Physiol. 1994;427:136–142. doi: 10.1007/BF00585952. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich P.S., McGivern J.G., Delgado S.G., Koch B.D., Eglen R.M., Hunter J.C., Sangameswaran L. Functional analysis of a voltage-gated sodium channel and its splice variant from rat dorsal root ganglia. J. Neurochem. 1998;70:2262–2272. doi: 10.1046/j.1471-4159.1998.70062262.x. [DOI] [PubMed] [Google Scholar]
- 14.Smith M.R., Smith R.D., Plummer N.W., Meisler M.H., Goldin A.L. Functional Analysis of the Mouse Scn8a Sodium Channel. J. Neurosci. 1998;18:6093–6102. doi: 10.1523/JNEUROSCI.18-16-06093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klugbauer N., Lacinova L., Flockerzi V., Hofmann F. Structure and functional expression of a new member of the tetrodotoxin-sensitive voltage-activated sodium channel family from human neuroendocrine cells. EMBO J. 1995;14:1084–1090. doi: 10.1002/j.1460-2075.1995.tb07091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummins T.R., Howe J.R., Waxman S.G. Slow Closed-State Inactivation: A Novel Mechanism Underlying Ramp Currents in Cells Expressing the hNE/PN1 Sodium Channel. J. Neurosci. 1998;18:9607–9619. doi: 10.1523/JNEUROSCI.18-23-09607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sangameswaran L., Fish L.M., Koch B.D., Rabert D.K., Delgado S.G., Ilnicka M., Jakeman L.B., Novakovic S., Wong K., Sze P., et al. A Novel Tetrodotoxin-sensitive, Voltage-gated Sodium Channel Expressed in Rat and Human Dorsal Root Ganglia. J. Biol. Chem. 1997;272:14805–14809. doi: 10.1074/jbc.272.23.14805. [DOI] [PubMed] [Google Scholar]
- 18.Santarelli V.P., Eastwood A.L., Dougherty D.A., Horn R., Ahern C.A. A cation-pi interaction discriminates among sodium channels that are either sensitive or resistant to tetrodotoxin block. J. Biol. Chem. 2007;282:8044–8051. doi: 10.1074/jbc.M611334200. [DOI] [PubMed] [Google Scholar]
- 19.Satin J., Kyle J.W., Chen M., Bell P., Cribbs L.L., Fozzard H.A., Rogart R.B. A Mutant of TTX-Resistant Cardiac Sodium Channels with TTX-Sensitive Properties. Science. 1992;256:1202–1205. doi: 10.1126/science.256.5060.1202. [DOI] [PubMed] [Google Scholar]
- 20.Sangameswaran L., Delgado S.G., Fish L.M., Koch B.D., Jakeman L.B., Stewart G.R., Sze P., Hunter J.C., Eglen R.M., Herman R.C. Structure and Function of a Novel Voltage-gated, Tetrodotoxin-resistant Sodium Channel Specific to Sensory Neurons. J. Biol. Chem. 1996;271:5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- 21.Akopian A.N., Sivilotti L., Wood J.N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- 22.Dib-Hajj S.D., Tyrrell L., Black J.A., Waxman S.G. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc. Natl. Acad. Sci. USA. 1998;95:8963–8968. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dib-Hajj S., Black J.A., Cummins T.R., Waxman S.G. NaN/NaV1.9: A sodium channel with unique properties. Trends Neurosci. 2002;25:253–259. doi: 10.1016/S0166-2236(02)02150-1. [DOI] [PubMed] [Google Scholar]
- 24.McKerrall S.J., Sutherlin D.P. NaV1.7 inhibitors for the treatment of chronic pain. Bioorg. Med. Chem. Lett. 2018;28:3141–3149. doi: 10.1016/j.bmcl.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Mulcahy J.V., Pajouhesh H., Beckley J.T., Delwig A., Du Bois J., Hunter J.C. Challenges and Opportunities for Therapeutics Targeting the Voltage-Gated Sodium Channel Isoform NaV1.7. J. Med. Chem. 2019;62:8695–8710. doi: 10.1021/acs.jmedchem.8b01906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagal S.K., Kemp M.L., Bungay P.J., Hay T.L., Murata Y., Payne C.E., Stevens E.B., Brown A., Blakemore D.C., Corbett M.S., et al. Discovery and optimisation of potent and highly subtype selective NaV1.8 inhibitors with reduced cardiovascular liabilities. Med. Chem. Comm. 2016;7:1925–1931. doi: 10.1039/C6MD00281A. [DOI] [Google Scholar]
- 27.Kort M.E., Atkinson R.N., Thomas J.B., Drizin I., Johnson M.S., Secrest M.A., Gregg R.J., Scanio M.J., Shi L., Hakeem A.H., et al. Subtype-selective NaV1.8 sodium channel blockers: Identification of potent, orally active nicotinamide derivatives. Bioorg. Med. Chem. Let. 2010;20:6812–6815. doi: 10.1016/j.bmcl.2010.08.121. [DOI] [PubMed] [Google Scholar]
- 28.Jarvis M.F., Honore P., Shieh C.C., Chapman M., Joshi S., Zhang X.F., Kort M., Carroll W., Marron B., Atkinson R., et al. A-803467, a potent and selective NaV1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc. Natl. Acad. Sci. USA. 2007;104:8520–8525. doi: 10.1073/pnas.0611364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kort M.E., Drizin I., Gregg R.J., Scanio M.J., Shi L., Gross M.F., Atkinson R.N., Johnson M.S., Pacofsky G.J., Thomas J.B., et al. Discovery and biological evaluation of 5-aryl-2-furfuramides, potent and selective blockers of the NaV1.8 sodium channel with efficacy in models of neuropathic and inflammatory pain. J. Med. Chem. 2008;51:407–416. doi: 10.1021/jm070637u. [DOI] [PubMed] [Google Scholar]
- 30.Priest B.T., Kaczorowski G.J. Subtype-selective sodium channel blockers promise a new era of pain research. Proc. Natl. Acad. Sci. USA. 2007;104:8205–8206. doi: 10.1073/pnas.0703091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.England S., de Groot M.J. Subtype-selective targeting of voltage-gated sodium channels. Br. J. Pharm. 2009;158:1413–1425. doi: 10.1111/j.1476-5381.2009.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llewellyn L.E. Saxitoxin, a toxic marine natural product that targets a multitude of receptors. Nat. Prod. Rep. 2006;23:200–222. doi: 10.1039/b501296c. [DOI] [PubMed] [Google Scholar]
- 33.Cusick K.D., Sayler G.S. An Overview on the Marine Neurotoxin, Saxitoxin: Genetics, Molecular Targets, Methods of Detection and Ecological Functions. Mar. Drugs. 2013;11:991–1018. doi: 10.3390/md11040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommer H., Meyer G.F. Paralytic shellfish poisoning. Arch. Pathol. 1937;24:560–598. [Google Scholar]
- 35.Sommer H., Whedon W.F., Kofoid C.A., Strohler R. Relation of paralytic shellfish poison to certain plankton organisms of the genus Gonyaulax. Arch. Pathol. 1937;24:537–559. [Google Scholar]
- 36.Schantz E.J., Mold J.D., Stanger D.W., Shavel J., Riel F.J., Bowden J.P., Lynch J.M., Wyler R.S., Riegel B., Sommer H. Paralytic Shellfish Poison. VI. A Procedure for the Isolation and Purification of the Poison from Toxic Clam and Mussel Tissues. J. Am. Chem. Soc. 1957;79:5230–5235. doi: 10.1021/ja01576a044. [DOI] [Google Scholar]
- 37.Mold J.D., Bowden J.P., Stanger D.W., Maurer J.E., Lynch J.M., Wyler R.S., Schantz E.J., Riegel B. Paralytic Shellfish Poison. VII. Evidence for the Purity of the Poison Isolated from Toxic Clams and Mussels. J. Am. Chem. Soc. 1957;79:5235–5238. doi: 10.1021/ja01576a045. [DOI] [Google Scholar]
- 38.Schuett W., Rapoport H. Saxitoxin, the Paralytic Shellfish Poison. Degradation to a Pyrrolopyrimidine. J. Am. Chem. Soc. 1962;84:2266–2267. doi: 10.1021/ja00870a056. [DOI] [Google Scholar]
- 39.Russell F.E. Comparative pharmacology of some animal toxins. Fed. Proc. 1967;26:1206–1224. [PubMed] [Google Scholar]
- 40.Bordner J., Thiessen W.E., Bates H.A., Rapoport H. Structure of a crystalline derivative of saxitoxin. Structure of saxitoxin. J. Am. Chem. Soc. 1975;97:6008–6012. doi: 10.1021/ja00854a009. [DOI] [PubMed] [Google Scholar]
- 41.Rogers P.S., Rapoport H. The pKa’s of saxitoxin. J. Am. Chem. Soc. 1980;102:7335–7339. doi: 10.1021/ja00544a030. [DOI] [Google Scholar]
- 42.Henderson R., Ritchie J.M., Strichartz G.R. Evidence that tetrodotoxin and saxitoxin act at a metal cation binding site in the sodium channels of nerve membrane. Proc. Natl. Acad. Sci. USA. 1974;71:3936–3940. doi: 10.1073/pnas.71.10.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hille B. The receptor for tetrodotoxin and saxitoxin. A structural hypothesis. Biophys. J. 1975;15:615–619. doi: 10.1016/S0006-3495(75)85842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan X., Li Z., Zhou Q., Shen H., Wu K., Huang X., Chen J., Zhang J., Zhu X., Lei J., et al. Structural basis for the modulation of voltage-gated sodium channels by animal toxins. Science. 2018;362:eaau2596. doi: 10.1126/science.aau2596. [DOI] [PubMed] [Google Scholar]
- 45.Shen H., Liu D., Wu K., Lei J., Yan N. Structures of human NaV1.7 channel in complex with auxiliary subunits and animal toxins. Science. 2019;363:1303–1308. doi: 10.1126/science.aaw2493. [DOI] [PubMed] [Google Scholar]
- 46.Walker J.R., Novick P.A., Parsons W.H., McGregor M., Zablocki J., Pande V.S., Du Bois J. Marked difference in saxitoxin and tetrodotoxin affinity for the human nociceptive voltage-gated sodium channel (NaV1.7) Proc. Natl. Acad. Sci. USA. 2012;109:18102–18107. doi: 10.1073/pnas.1206952109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas-Tran R., Du Bois J. Mutant cycle analysis with modified saxitoxins reveals specific interactions critical to attaining high-affinity inhibition of hNaV1.7. Proc. Natl. Acad. Sci. USA. 2016;113:5856–5861. doi: 10.1073/pnas.1603486113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiese M., D’Agostino P.M., Mihali T.K., Moffitt M.C., Neilan B.A. Neurotoxic Alkaloids: Saxitoxin and Its Analogs. Mar. Drugs. 2010;8:2185–2211. doi: 10.3390/md8072185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yotsu-Yamashita M., Kim Y.H., Dudley S.C., Jr., Choudhary G., Pfahnl A., Oshima Y., Daly J.W. The structure of zetekitoxin AB, a saxitoxin analog from the Panamanian golden frog Atelopus zeteki: A potent sodium-channel blocker. Proc. Natl. Acad. Sci. USA. 2004;101:4346–4351. doi: 10.1073/pnas.0400368101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuhrman F.A., Fuhrman G.J., Mosher H.S. Toxin from Skin of Frogs of the Genus Atelopus: Differentiation from Dendrobatid Toxins. Science. 1969;165:1376–1377. doi: 10.1126/science.165.3900.1376. [DOI] [PubMed] [Google Scholar]
- 51.Shindelman J., Mosher H.S., Fuhrman F.A. Atelopidtoxin from the Panamanian frog, Atelopus Zeteki. Toxicon. 1969;7:315–319. doi: 10.1016/0041-0101(69)90031-2. [DOI] [PubMed] [Google Scholar]
- 52.Tanino H., Nakada T., Kaneko Y., Kishi Y. A stereospecific total synthesis of dl-saxitoxin. J. Am. Chem. Soc. 1977;99:2818–2819. doi: 10.1021/ja00450a079. [DOI] [PubMed] [Google Scholar]
- 53.Jacobi P.A., Martineli M.J., Polanc S. Total synthesis of (±)-saxitoxin. J. Am. Chem. Soc. 1984;106:5594–5598. doi: 10.1021/ja00331a032. [DOI] [Google Scholar]
- 54.Fleming J.J., Du Bois J. A Synthesis of (+)-Saxitoxin. J. Am. Chem. Soc. 2006;128:3926–3927. doi: 10.1021/ja0608545. [DOI] [PubMed] [Google Scholar]
- 55.Iwamoto O., Koshino H., Hashizume D., Nagasawa K. Total synthesis of (−)-decarbamoyloxysaxitoxin. Angew. Chem. Int. Ed. 2007;46:8625–8628. doi: 10.1002/anie.200703326. [DOI] [PubMed] [Google Scholar]
- 56.Mulcahy J.V., Du Bois J. A Stereoselective Synthesis of (+)-Gonyautoxin 3. J. Am. Chem. Soc. 2008;130:12630–12631. doi: 10.1021/ja805651g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iwamoto O., Shinohara R., Nagasawa K. Total Synthesis of (−)- and (+)-Decarbamoyloxysaxitoxin and (+)-Saxitoxin. Chem. Asian J. 2009;4:277–285. doi: 10.1002/asia.200800382. [DOI] [PubMed] [Google Scholar]
- 58.Iwamoto O., Nagasawa K. Total Synthesis of (+)-Decarbamoylsaxitoxin and (+)-Gonyautoxin 3. Org. Lett. 2010;12:2150–2153. doi: 10.1021/ol1006696. [DOI] [PubMed] [Google Scholar]
- 59.Sawayama Y., Nishikawa T. A Synthetic Route to the Saxitoxin Skeleton: Synthesis of Decarbamoyl α-Saxitoxinol, an Analogue of Saxitoxin Produc ed by the Cyanobacterium Lyngbya wollei. Angew. Chem. Int. Ed. 2011;50:7176–7178. doi: 10.1002/anie.201102494. [DOI] [PubMed] [Google Scholar]
- 60.Bhonde V.R., Looper R.E. A Stereocontrolled Synthesis of (+)-Saxitoxin. J. Am. Chem. Soc. 2011;133:20172–20174. doi: 10.1021/ja2098063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mulcahy J.V., Walker J.R., Merit J.E., Whitehead A., Du Bois J. Synthesis of the Paralytic Shellfish Poisons (+)-Gonyautoxin 2, (+)-Gonyautoxin 3, and (+)-11,11-Dihydroxysaxitoxin. J. Am. Chem. Soc. 2016;138:5994–6001. doi: 10.1021/jacs.6b02343. [DOI] [PubMed] [Google Scholar]
- 62.Thottumkara A.P., Parsons W.H., Du Bois J. Saxitoxin. Angew. Chem. Int. Ed. 2014;53:5760–5784. doi: 10.1002/anie.201308235. [DOI] [PubMed] [Google Scholar]
- 63.Andresen B.M., Du Bois J. De Novo Synthesis of Modified Saxitoxins for Sodium Ion Channel Study. J. Am. Chem. Soc. 2009;131:12524–12525. doi: 10.1021/ja904179f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parsons W.H., Du Bois J. Maleimide Conjugates of Saxitoxin as Covalent Inhibitors of Voltage-Gated Sodium Channels. J. Am. Chem. Soc. 2013;135:10582–10585. doi: 10.1021/ja4019644. [DOI] [PubMed] [Google Scholar]
- 65.Akimoto T., Masuda A., Yotsu-Mari M., Hirokawa T., Nagasawa K. Synthesis of saxitoxin derivatives bearing guanidine and urea groups at C13 and evaluation of their inhibitory activity on voltage-gated sodium channels. Org. Biomol. Chem. 2013;11:6642–6649. doi: 10.1039/c3ob41398e. [DOI] [PubMed] [Google Scholar]
- 66.Arakawa O., Nishio S., Noguchi T., Shida Y., Onoue Y. A new saxitoxin analogue from a xanthid crab Atergatis Floridus. Toxicon. 1995;12:1577–1584. doi: 10.1016/0041-0101(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 67.Wang C., Oki M., Nishikawa T., Harada D., Yotsu-Yamashita M., Nagasawa K. Total Synthesis of 11-Saxitoxinethanoic Acid and Evaluation of its Inhibitory Activity on Voltage-gated Sodium Channels. Angew. Chem. Int. Ed. 2016;55:11600–11603. doi: 10.1002/anie.201604155. [DOI] [PubMed] [Google Scholar]
- 68.Walker R.J., Merit E.J., Thomas-Tran R., Tang D.T.Y., Du Bois J. Divergent Synthesis of Natural Derivatives of (+)-Saxitoxin Including 11-Saxitoxinethanoic Acid. Angew. Chem. Int. Ed. 2018;58:1689–1693. doi: 10.1002/anie.201811717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paladugu S.R., James C.K., Looper R.E. A Direct C11 Alkylation Strategy on the Saxitoxin Core: A Synthesis of (+)-11-Saxitoxinethanoic Acid. Org. Lett. 2019;21:7999–8002. doi: 10.1021/acs.orglett.9b02986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lou J.-Y., Laezza F., Gerber B.R., Xiao M.L., Yamada K.A., Hartmann H., Craig A.M., Nerbonne J.M., Ornitz D.M. Fibroblast growth factor 14 is an intracellular modulator of voltage-gated sodium channels. J. Physiol. 2005;569:179–193. doi: 10.1113/jphysiol.2005.097220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsuo J., Murakami M. The Mukaiyama Aldol Reaction: 40 Years of Continuous Development. Angew. Chem. Int. Ed. 2013;52:9109–9118. doi: 10.1002/anie.201303192. [DOI] [PubMed] [Google Scholar]
- 72.Mase N., Hayashi Y. The Aldol Reaction: Organocatalysis Approach. In: Knochel P., Molander G.A., editors. Comprehensive Organic Synthesis. 2nd ed. Volume 2. Elsevier; Amsterdam, The Netherlands: 2014. pp. 273–339. [Google Scholar]
- 73.Hosokawa S. Recent development of vinylogous Mukaiyama aldol reactions. Tetrahedron Lett. 2018;59:77–88. doi: 10.1016/j.tetlet.2017.11.056. [DOI] [Google Scholar]
- 74.Mukaiyama T., Narasaka K., Banno K. New aldol type reaction. Chem. Lett. 1973:1011–1014. [Google Scholar]
- 75.Mukaiyama T., Banno K., Narasaka K. New cross-aldol reactions. Reactions of silyl enol ethers with carbonyl compounds activated by titanium tetrachloride. J. Am. Chem. Soc. 1974;96:7503–7509. doi: 10.1021/ja00831a019. [DOI] [Google Scholar]
- 76.Noyori R., Yokoyama K., Sakata J., Kuwajima I., Nakamura E., Shimizu M. Fluoride ion catalyzed aldol reaction between enol silyl ethers and carbonyl compounds. J. Am. Chem. Soc. 1977;99:1265–1267. doi: 10.1021/ja00446a049. [DOI] [Google Scholar]
- 77.Gingras M., Chabre Y.M., Raimundo J.-M. Tetrabutylammonium Difluorotriphenylstannate [Bu4N] [Ph3SnF2]: Delivering Carbon or Fluorine Ligands via Hypercoordination. Synthesis. 2006;1:182–185. doi: 10.1055/s-2005-921747. [DOI] [Google Scholar]
- 78.Hama T., Liu X., Culkin D.A., Hartwig J.F. Palladium-Catalyzed α-Arylation of Esters and Amides under More Neutral Conditions. J. Am. Chem. Soc. 2003;125:11176–11177. doi: 10.1021/ja036792p. [DOI] [PubMed] [Google Scholar]
- 79.Moradi W.A., Buchwald S.L. Palladium-Catalyzed α-Arylation of Esters. J. Am. Chem. Soc. 2001;123:7996–8002. doi: 10.1021/ja010797+. [DOI] [PubMed] [Google Scholar]
- 80.Magauer T., Mulzer J., Tiefenbacher K. Total Syntheses of (+)-Echinopine A and B: Determination of Absolute Stereochemistry. Org. Lett. 2009;11:5306–5309. doi: 10.1021/ol902263k. [DOI] [PubMed] [Google Scholar]
- 81.Xiao Q., Ren W.-W., Chen Z.-X., Sun T.-W., Li Y., Ye Q.-D., Gong J.-X., Meng F.-K., You L., Liu Y.-F., et al. Diastereoselective Total Synthesis of (±)-Schindilactone A. Angew. Chem. Int. Ed. 2011;50:7373–7377. doi: 10.1002/anie.201103088. [DOI] [PubMed] [Google Scholar]
- 82.Negishi E. Novel and selective α-substitution of ketones and other carbonyl compounds based on Pd-catalyzed cross coupling of α-unsaturated carbonyl derivatives containing α-halogen or α-metal groups. J. Organomet. Chem. 1999;576:179–194. doi: 10.1016/S0022-328X(98)01057-2. [DOI] [Google Scholar]
- 83.Johnson C.R., Adams J.P., Braun M.P., Senanayake C.B.W. Modified stille coupling utilizing α-iodoenones. Tetrahedron Lett. 1992;33:919–922. doi: 10.1016/S0040-4039(00)91576-4. [DOI] [Google Scholar]
- 84.Cordvilla C., Bartolome C., Martinez-Ilarduya J.M., Espinet P. The Stille Reaction, 38 Years Later. ACS Catal. 2015;5:3040–3053. doi: 10.1021/acscatal.5b00448. [DOI] [Google Scholar]
- 85.Han X., Stoltz B., Corey E.J. Cuprous Chloride Accelerated Stille Reactions. A General and Effective Coupling System for Sterically Congested Substrates and for Enantioselective Synthesis. J. Am. Chem. Soc. 1999;121:7600–7605. doi: 10.1021/ja991500z. [DOI] [Google Scholar]
- 86.Devlin A.S., Du Bois J. Modular synthesis of the pentacyclic core of batrachotoxin and select batrachotoxin analogue designs. Chem. Sci. 2013;4:1059–1063. doi: 10.1039/C2SC21723F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Allred G.D., Liebeskind L.S. Copper-Mediated Cross-Coupling of Organostannanes with Organic Iodides at or below Room Temperature. J. Am. Chem. Soc. 1996;118:2748–2749. doi: 10.1021/ja9541239. [DOI] [Google Scholar]
- 88.Colomer I., Velado M., Fe rnandez de la Pradilla R., Viso A. From Allylic Sulfoxides to Allylic Sulfenates: Fifty Years of a Never-Ending [2,3]-Sigmatropic Rearrangement. Chem. Rev. 2017;117:14201–14243. doi: 10.1021/acs.chemrev.7b00428. [DOI] [PubMed] [Google Scholar]
- 89.Rojas C.M. The Mislow-Evans rearrangement. In: Rojas C.M., editor. Molecular Rearrangements in Organic Synthesis. John Wiley & Sons; Hoboken, NJ, USA: 2016. [DOI] [Google Scholar]
- 90.Johnson C.R., Adams J.P., Braun M.P., Senanayake C.B.W., Wovkulich P.M., Uskokovic M.R. Direct α-iodination of cycloalkenones. Tetrahedron Lett. 1992;33:917–918. doi: 10.1016/S0040-4039(00)91575-2. [DOI] [Google Scholar]
- 91.Djuardi E., Bovonsombat P., McNelis E. Formations of α-Iodoenones by Iodine and Catalytic Amounts of Amines. Synth. Commun. 1997;27:2497–2503. doi: 10.1080/00397919708004113. [DOI] [Google Scholar]
- 92.Adachi K., Yamada T., Ishizuka H., Oki M., Tsunogae S., Shimada N., Chiba O., Orihara T., Hidaka M., Hirokawa T., et al. Synthesis of C12-keto saxitoxin derivatives with unusual inhibitory activity against voltage-gated sodium channels. Chem. Eur. J. 2019 doi: 10.1002/chem.201904184. [DOI] [PubMed] [Google Scholar]
- 93.Nishikawa T., Urabe D., Isobe M. Syntheses of N-Acylisoxazolidine Derivatives, Related to a Partial Structure Found in Zetekitoxin AB, a Golden Frog Poison. Heterocycles. 2009;79:379–385. doi: 10.3987/COM-08-S(D)44. [DOI] [Google Scholar]
- 94.Paladugu S.R., Looper R.E. Preparation of a 1,2-isoxazolidine synthon for the synthesis of zetekitoxin AB. Tetrahedron Lett. 2015;56:6332–6334. doi: 10.1016/j.tetlet.2015.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bernet B., Vasella A. Carbocyclische Verbindungen aus Monosacchariden. II. Umsetzungen in der Mannosereihe. Helv. Chim. Acta. 1979;62:2400–2410. doi: 10.1002/hlca.19790620736. [DOI] [Google Scholar]
- 96.Ferrier R.J., Furneaux R.H., Prasit P., Tyler P.C. Functionalised carbocycles from carbohydrates. Part 2. The synthesis of 3-oxa-2-azabicyclo[3.3.0]octanes. X-Ray crystal structure of (1R,5S)-6-exo,7-endo,8-exo-triacetoxy-N-methyl-4-endo-phenylthio-3-oxa-2-azabicyclo[3.3.0]octane. J. Chem. Soc. Perkin Trans. 1. 1983;1:1621–1628. doi: 10.1039/p19830001621. [DOI] [Google Scholar]
- 97.Dransfield P.J., Moutel S., Shipman M., Sik V. Stereocontrolled synthesis of polyhydroxylated hexahydro-1H-cyclopent[c]isoxazoles by intramolecular oxime olefin cycloadditions: An approach to aminocyclopentitols. J. Chem. Soc. Perkin Trans. 1. 1999;1:3349–3355. doi: 10.1039/a905706d. [DOI] [Google Scholar]
- 98.Ogawa S., Orihara M. Synthesis of the penta-N,O-acetyl derivatives of some pseudo-3-amino-3-deoxy-dl-hexopyranoses a and -dl-hexopyranosylamine derivative. Carbohydr. Res. 1989;189:323–330. doi: 10.1016/0008-6215(89)84108-4. [DOI] [Google Scholar]
- 99.Baumgartner H., O’Sullivan A.C., Schneider J. The Synthesis of Oxa-Analogs of the Kainoid Family. Heterocycles. 1997;45:1537–1549. [Google Scholar]
- 100.Nishikawa T., Wang C., Akimoto T., Koshino H., Nagasawa K. Synthesis of an advance model of zetekitoxin AB focusing on N-acylisoxazolidine amide structure corresponding to C13–C17. Asian J. Org. Chem. 2014;3:1308–1311. doi: 10.1002/ajoc.201402206. [DOI] [Google Scholar]
- 101.Shibuya M., Tomizawa M., Suzuki I., Iwabuchi Y. 2-Azaadamantane N-Oxyl (AZADO) and 1-Me-AZADO: Highly Efficient Organocatalysts for Oxidation of Alcohols. J. Am. Chem. Soc. 2006;128:8412–8413. doi: 10.1021/ja0620336. [DOI] [PubMed] [Google Scholar]
- 102.Shibuya M., Sato T., Tomizawa M., Iwabuchi Y. Oxoammonium salt/NaClO2: An expedient, catalytic system for one-pot oxidation of primary alcohols to carboxylic acids with broad substrate applicability. Chem. Commun. 2009;13:1739–1741. doi: 10.1039/b822944a. [DOI] [PubMed] [Google Scholar]
- 103.Kunishima M., Kawachi C., Hioki K., Tani S. Formation of carboxamides by direct condensation of carboxylic acids and amines in alcohols using a new alcohol- and water-soluble condensing agent: DMT-MM. Tetrahedron. 2001;57:1551–1558. doi: 10.1016/S0040-4020(00)01137-6. [DOI] [Google Scholar]