Abstract
Background: Natural meroterpenes derived from phloroglucinols and β-caryophyllene have shown high inhibitory activity against α-glucosidase or cancer cells, however, the chemical diversity of this type of skeletons in Nature is limited. Methods: To expand the chemical space and explore their inhibitory activities against α-glucosidase (EC 3.2.1.20), we employed β-caryophyllene and some natural moieties (4-hydroxycoumarins, lawsone or syncarpic acid) to synthesize new types of meroterpene-like skeletons. All the products (including side products) were isolated and characterized by NMR, HR-MS, and ECD. Results: In total, 17 products (representing seven scaffolds) were generated through a one-pot procedure. Most products (12 compounds) showed more potential activity (IC50 < 25 μM) than the positive controls (acarbose and genistein, IC50 58.19, and 54.74 μM, respectively). Compound 7 exhibited the most potent inhibition of α-glucosidase (IC50 3.56 μM) in a mixed-type manner. The CD analysis indicated that compound 7 could bind to α-glucosidase and influence the enzyme’s secondary structure. Conclusions: Compound 7 could serve as a new type of template compound to develop α-glucosidase inhibitors. Full investigation of a biomimic reaction can be used as a concise strategy to explore diverse natural-like skeletons and search for novel lead compounds.
Keywords: meroterpene, 4-hydroxycoumarin, β-caryophyllene, lawsone, hetero[4+2] cycloaddition, α-glucosidase, hyperglycemia
1. Introduction
Natural products (NPs) are a diverse family of organic molecules, most of which exhibit significant bioactivities to treat human diseases [1,2,3,4,5,6,7,8]. Thus NPs are an essential source for discovering novel drugs or lead compounds [6]. However, the range of scaffolds readily accessible in Nature is limited [9]. Furthermore, interest in generating novel synthetic scaffolds has recently declined in pharmaceutical research due to the elaborate isolation procedures required or lengthy total synthesis pathways [9]. Recently, there has been increased interest in NP-inspired or NP-like products to improve the chemical diversity of NPs through different routes: (1) diversity-oriented synthesis (DOS) starting from isolated NPs [10,11], (2) expansion of NP space catalysed by uncommon P450 reactions [12], (3) construction of new lead compounds inspired by bioactive NPs [13], (4) recombined NP moieties generated via coupling reactions [14], (5) diversity-enhanced extraction directly from plants [15]. In particular easily accessible NPs can serve as ideal starting scaffolds to efficiently afford diverse NP-like structures [16]. This strategy can avoid lengthy reaction procedures for building complex skeletons.
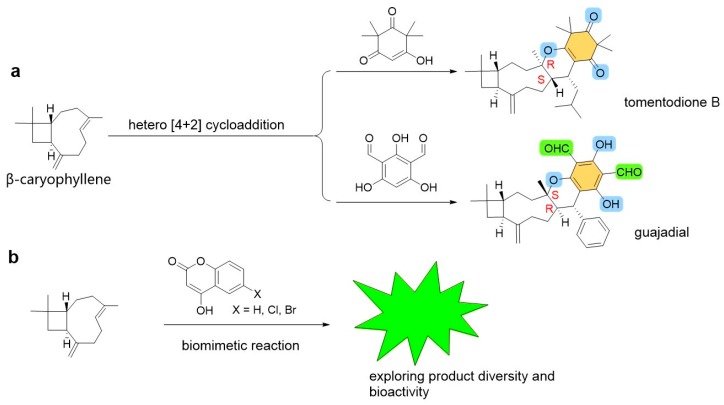
Natural meroterpenes, arising from phloroglucinols, have attracted recent interest in natural products chemistry, total synthesis, and medicinal chemistry due to their special skeletons and bioactivities [17,18,19,20]. Especially the typical skeletons obtained from guava (Psidium guajava) and Rhodomyrtus tomentosa [21,22,23,24,25,26,27,28] contain a phloroglucinol moiety and a sesquiterpene skeleton. They are biogenerated through a hetero [4+2] cycloaddition (Scheme 1a). Some of them possess high activities against cancer cells and α-glucosidase. However, only very limited skeletons were found in Nature so far. In our previous work, some phloroglucinol-like moieties such as lawsone, 2,4,6-trihydroxyisophthalaldehyde [29], and polyphenol moieties [30] could promote α-glucosidase inhibition. The NP 4-hydroxycoumarin is considered as a moiety responsible for α-glucosidase inhibition [31]. Also, the compound can be applied to construct various products due to its reactivity [32]. We thus employed 4-hydroxycoumarin and its halogenated derivatives to explore new meroterpene-like products using the biomimetic reactions (Scheme 1b).
Scheme 1.
(a) Typical meroterpenes and their biogeneration in Nature. (b) A biomimetic strategy to explore NP-like meroterpenes.
2. Results and Discussion
2.1. Synthesis
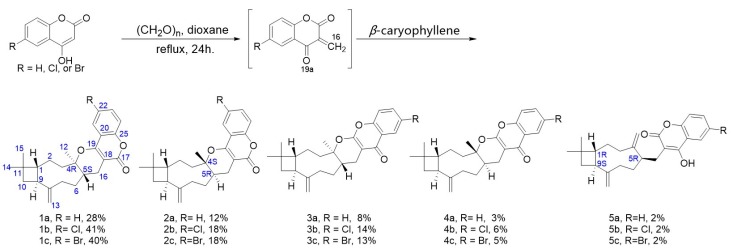
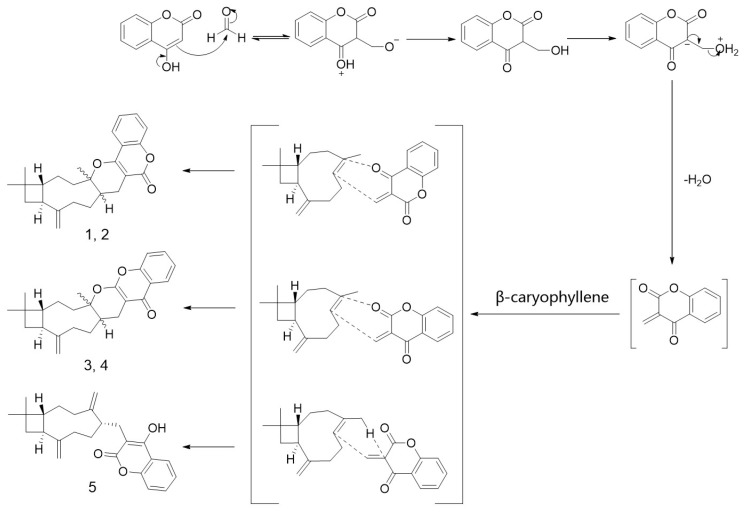
The synthesis was carried out by a one-pot procedure from coumarin, paraformaldehyde, and β-caryophyllene. In total, 15 compounds corresponding to five different skeletons (Scheme 2) were acquired. Due to the electron donation of O-19a in the coumarin building blocks, the major products were obtained from cycloaddition at the 16 and 19a positions (Scheme 2). The product diversity can be rationalized by the reaction mechanism shown in Scheme 3. The reaction starting from the unsubstituted 4-hydroxycoumarin has already been reported [33,34]. However, the reported procedure did not separate the isomer pairs of 1a/2a and 3a/4a since their chromatographic retention times are very close. We optimized the separation to purify these isomers. In the presence of Cl- or Br- substituents on the 4-hydroxycoumarin moiety, the total conversion increased to 81% or 78%, respectively. Generally, the stable conformers of caryophyllene favored generating (4R,5S)-configuration products. However, in the reported total synthesis [35], it was still tricky to obtain enantioselective products. We did not optimize the reaction to promote geneartion of a single target product since the purpose of the reaction in the current work was to acquire diverse skeletons and screen for bioactive products. Thus we explored all the major and side products to get diverse compounds to seek higher bioactivities.
Scheme 2.
One-pot synthesis of meroterpene-like compounds starting from caryophyllene and 4-hydroxycoumarins. Reagents and conditions: (CH2O)n, anhydrous dioxane, reflux, 24 h.
Scheme 3.
A speculated mechanism for the formation of the skeletons 1–5.
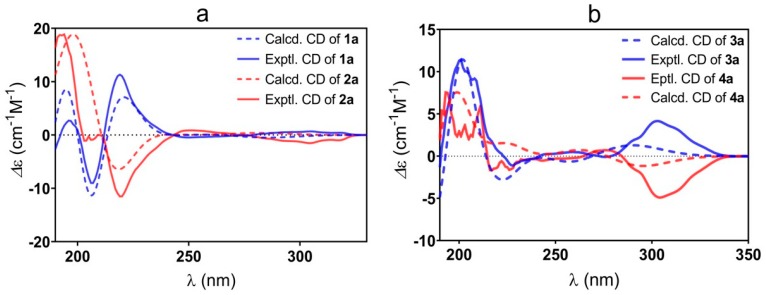
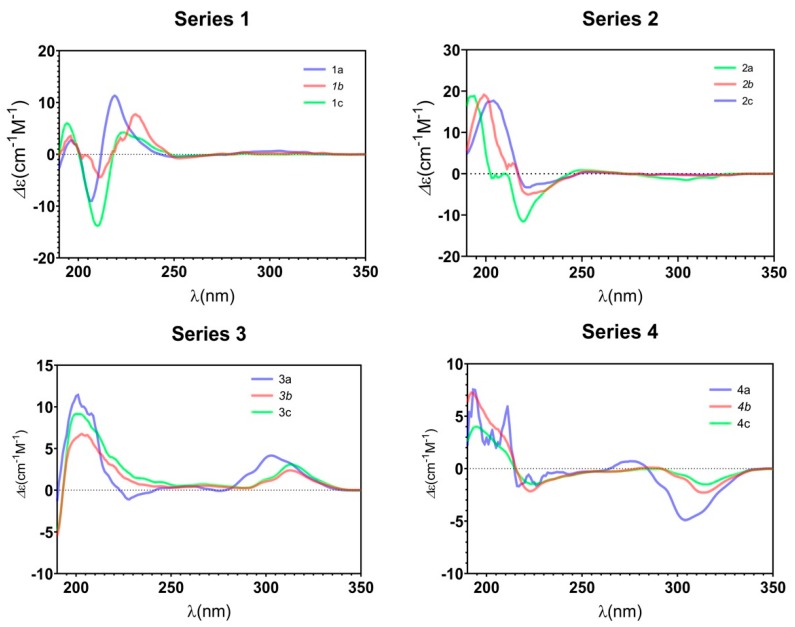
To assign the absolute configurations (ACs) of those newly formed chiral carbons (C-4 and C-5) unambiguously, we carried out ECD calculations [36,37] and compared the calculated curves with the experimental ones. The skeletons 1 and 2 possess the same planar structures but inverse chiralities at C-4 and C-5. Their experimental ECD spectra showed an almost mirror curve from 212 to 320 nm. Comparing their experimental ECD spectra with the calculated curves of the (4S,5R) and (4R,5S)-isomers allowed us to establish the absolute configurations of 1a and 2a (Figure 1a). The ACs of 3a and 4a were also determined by ECD associated with the calculated spectra (Figure 1b). The halogen (Cl or Br)-substituted products b and c in the series 1–4 had the same Cotton effect as the corresponding a products, respectively (Figure 2), which indicated the same configurations of C-4 and C-5 in each set of compounds 1–4.
Figure 1.
Experimental and calculated ECD spectra of 1a and 2a (a), 3a, and 4a (b).
Figure 2.
Experimental ECD spectra of compound series 1–4.
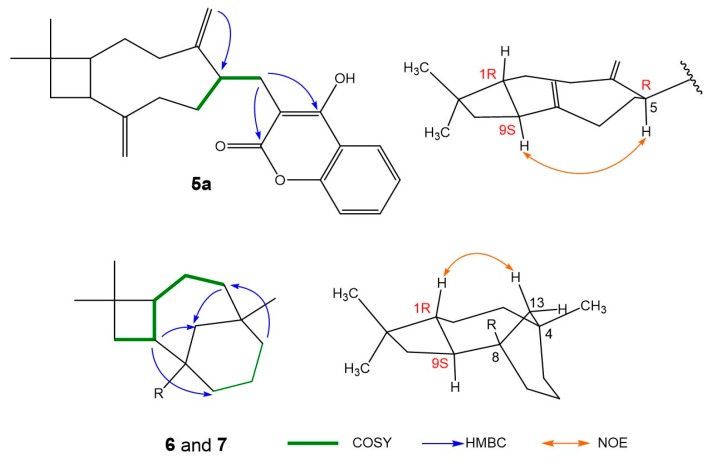
The planar structure of 5a was confirmed by the HMBC correlations, as shown in Figure 3. The chiral C-5 is connected to the chromophore via a flexible CH2 group, thus its configuration cannot be determined from the ECD spectra. However, the chirality of C-1 and C-9 is inherited from β-caryophyllene and remains unchanged during the synthesis procedure. Thus, the NOESY correlation from H-9S to H-5 (as shown in Figure 2) indicated the chiral C-5 was in an (R) configuration. The corresponding 1H- and 13C-NMR data of 5b and 5c when compared with those of 5a indicated that the compounds 5b and 5c possess the same scaffolds and configuration as 5a.
Figure 3.
Selected 2D NMR correlations of compounds 5a, 6, and 7.
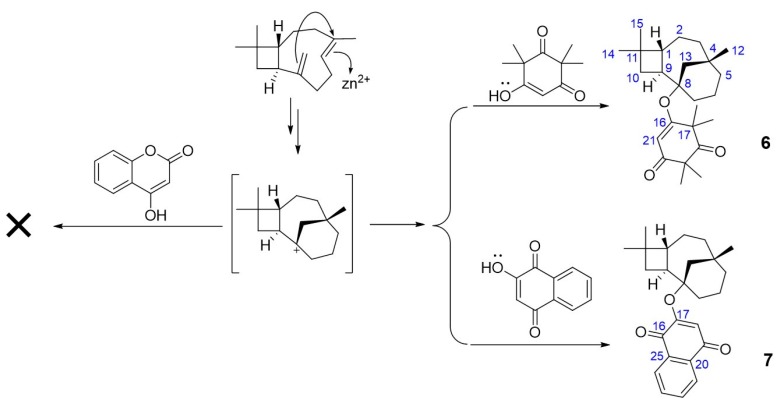
Inspired by the high inhibition against α-glucosidase exhibited by compound 5a (Table 1), we considered that a flexible connection between coumarins and caryophyllene would be beneficial for the bioactivities. To verify our speculation, we attempted to connect 4-hydroxycoumarin and the caryophyllene skeleton via an ether bond. However, according to the GC-EIMS and LC-QTOF-MS/MS analysis of the crude product, none of the expected MS signals were detected (see Figure S1 in Supplementary Materials). Then we used another two similar building blocks (syncarpic acid and lawsone) to replace the 4-hydroxycoumarin to acquire products 6 and 7 (Scheme 4). These two building blocks can also provide hydrogen binding sites. In the presence of ZnI2, the skeleton of caryophyllene rearranged via a transannular reaction to yield a relatively stable carbocation, which was then attached to the hydroxyl to afford compounds 6 and 7.
Table 1.
α-Glucosidase (EC 3.2.1.20) inhibition of highly active compounds in vitro.
| Compd. | IC50 (μM) | Compd. | IC50 (μM) |
|---|---|---|---|
| 2b | 15.56 ± 0.31 | 5a | 9.49 ± 0.03 |
| 2c | 23.62 ± 1.69 | 5b | 11.81 ± 0.03 |
| 3b | 12.04 ± 0.06 | 5c | 12.04 ± 0.10 |
| 3c | 18.09 ± 0.05 | 6 | 8.50 ± 0.24 |
| 4b | 15.35 ± 0.33 | 7 | 3.56 ± 0.24 |
| 4c | 19.99 ± 0.47 | G | 58.19 ± 1.38 |
| A | 54.74 ± 0.16 |
All the other synthesized compounds and starting materials showed weak inhibition (IC50 > 25 μM). G, genistein. A, acarbose.
Scheme 4.
Meroterpene-like compounds coupling caryophyllene with lawsone or syncarpic acid. Reagents and conditions: ZnI2, toluene, 110 °C, 18 h.
The planar structures of 6 and 7 were elucidated according to the 2D NMR correlations, as shown in Figure 3. Since the chirality of C-1(R) and C-9(S) is already known, the newly formed chiral centers (C-4 and C-8) in compounds 6 and 7 were deduced according to the NOESY correlation H-1/H-13 as shown in Figure 3.
2.2. α-Glucosidase Inhibition
α-Glucosidase is a key enzyme for hydrolyzing amylum into glucose, which will raise the glucose levels in the blood [38]. Thus inhibiting this enzyme will postpone glucose absorption, thus lowering postprandial blood glucose levels [39]. To screen for lead compounds, we primarily bioassayed all the compounds and building blocks on α-glucosidase at the 25 and 50 μM level. Those compounds (as listed in Table 1) that showed high inhibition (>50% at 25 μM level) were then subjected to further IC50 evaluation. Compounds 5a, 6, and 7 showed promising inhibition (IC50 < 10 μM).
Interestingly, none of the building blocks, including the coumarins and caryophyllene, showed any inhibitory effects against α-glucosidase. Only when the coumarins were combined with caryophyllene to form meroterpene-like compounds, inhibition was observed. The compound set 1 (1a, 1b, and 1c) all showed weak activities against the bio-target. The compound sets 2–4 showed moderate inhibition against the target protein. The halogen atoms might provide an additional site for hydrogen bonding since compounds 2a, 3a and 4a showed limited inhibition at 25 μM while the compounds 2b and 3b (Cl substituted) and 2c and 3c (Br substituted) all showed moderate activities (IC50 12.0–23.6 μM).
The compound set 5 showed better inhibition than compounds 2–4. These results indicated that a flexible connection between β-caryophyllene and 4-hydroxycoumarin is beneficial to improve the inhibition. Furthermore, the hydroxyl group could be necessary for binding to the target protein. When the rearranged caryophyllene was linked with aromatic ketones through an oxygen bridge (compounds 6 and 7), the product showed better inhibition than the positive control. Among our synthetic products, compound 7 showed the highest inhibition, probably due to the presence of the para-substituted carbonyls and the hydrophobic caryophyllene moiety.
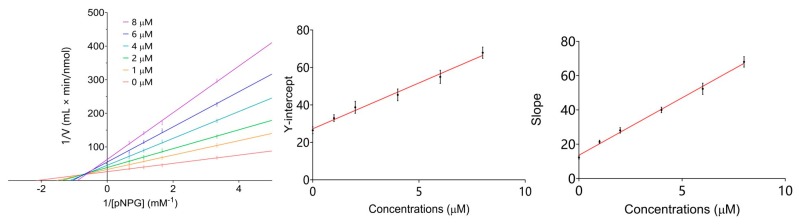
To explore the interaction mechanism of the best inhibitor 7, we carried out the enzyme kinetic study using Lineweaver-Burk plots analysis [40,41]. α-Glucosidase was treated with pNPG and compound 7 at various concentrations. As shown in Figure 4, compound 7 showed a non-competitive type of inhibition against α-glucosidase. Replotting the slope and Y-intercept values taken from each line in the primary Lineweaver–Burk plot allowed extrapolation of the inhibition constants Ki,free (the affinity to the free enzyme) and Ki,bound (the affinity to the complex enzyme-substrate). The Ki,free and Ki,bound values of 7 were 2.03 and 5.57 μM, respectively.
Figure 4.
Lineweaver-Burk plots of compound 7.
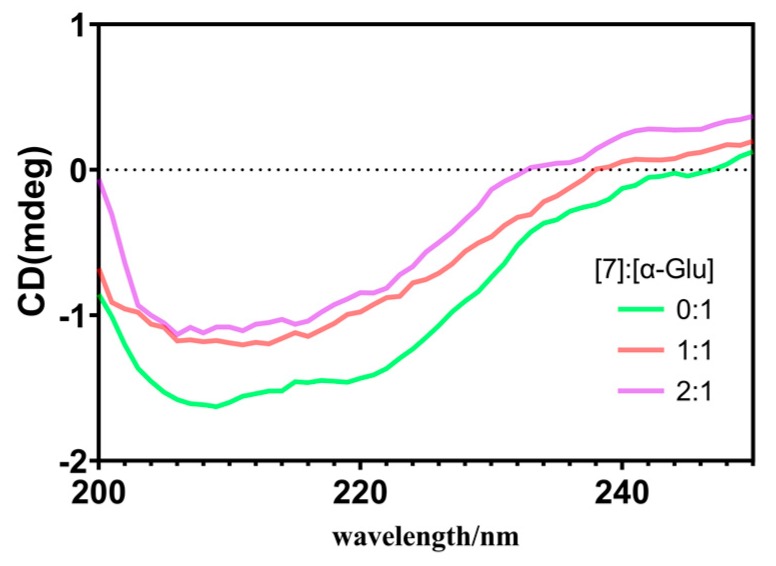
CD spectroscopy is considered as a reliable and sensitive method for monitoring the secondary structural changes of macromolecules interacting with small molecules [42]. The α-glucosidase yield two negative bands at 209 and 219 nm on CD the spectrum which originated from the n π* and π ⟶ π* electron transfer of the α-helix motifs [43,44]. With increasing levels of compound 7 to the enzyme, a regular increasing intensity of both negative bands occurred (as shown in Figure 5). These CD changes indicated that the secondary structure of the target protein was influenced by the small molecule 7.
Figure 5.
CD spectra of α-glucosidase in the presence of compound 7. The concentration of α-glucosidase is 2 μM. The molar ratios of compound 7 to the enzyme were 0:1, 1:1, and 2:1.
3. Materials and Methods
3.1. General Information
Optical rotations were measured on an MCP300 polarimeter (Anton Paar, Graz, Austria) equipped with a thermally jacketed 10 cm cell at 20 °C. Sample concentrations (c) are given in g/100 mL. Melting points were obtained on a MP420 auto melting point system (Hanon, Jinan, China) and are uncorrected. IR spectra were obtained on a Tensor 27 spectrometer (Bruker, Ettlingen, Germany). NMR spectra were recorded in CDCl3 using an AVANCE III 400 MHz spectrometer (Bruker, Ettlingen, Germany). The solvent residual peak was used as an internal reference for chemical shifts (in ppm). Coupling constants, J, are reported in Hertz (Hz). ESI-MS spectra were measured on a LTQ Fleet instrument (Thermo Fisher Scientific, Waltham, MA, USA). HR ESI-MS data were obtained by an Triple TOF 4600 system (AB Sciex, Redwood City, CA, USA). ECD spectra were measured on a Chirascan CD Spectrometer (Applied Photophysics, London, Britain). Column chromatography was performed on silica gel (90–150 μm, Dingkang, Qingdao, China) and Chromatorex C18 gel (40−75 μm, Fuji Silysia Chemical LTD, Aichi-ken, Japan). GF254 plates were used for thin-layer chromatography (TLC). HPLC analysis and preparations were performed on a 1525 instrument (Waters, Milford, MA, USA) equipped with a 250 × 10 mm, 5 μm semi-preparative column (Dr. Maisch GmbH, Ammerbuch, Germany). The reagents 4-hydroxycoumarin (98%), paraformaldehyde (96%), caryophyllene (90% GC) 6-chloro-4-hydroxycoumarin (97%, TCI) and 6-chloro-4-hydroxycoumarin (98%, TCI) were purchased from Energy Chemical Company (Shanghai, China).
3.2. Synthetic Procedures and Product Characterization
3.2.1. Products Starting from 4-Hydroxycoumarin
To a 50 mL reaction tube was added 4-hydroxycoumarin (170 mg, 1.049 mmol) and paraformaldehyde (252 mg). The reaction tube was then flushed with argon and evacuated three times. Anhydrous 1,4-dioxane (5 mL) was added by syringe, and the mixture was stirred under reflux for 1 h. Then anhydrous 1,4-dioxane (2 mL) and β-caryophyllene (630 μL, 3.150 mmol) were injected into the reaction tube. The resulting solution was stirred for 24 h at reflux under an argon atmosphere, after which complete conversion of the starting material was confirmed by TLC inspection. After cooling to rt, the reaction mixture was washed with 10 mL of saturated Na2CO3. The organic layer was collected and removed the solvent under vacuum to yield crude product (869 mg). The crude products were separated on silica gel column (EtOAc in petroleum ether gradient, from 25:1 to 5:1, v/v) to 5 fractions (Fr. A~E). These fractions were further purified by HPLC using the Dr. Maisch GmbH semi-preparative column, eluting with 100% MeCN, to yield compounds 1a (110 mg, 28%), 2a (48 mg, 12%), 3a (31 mg, 8%), 4a (10 mg, 3%) and 5a (7 mg, 2%).
Compound 1a: oil; = −68.00 (c 0.05 in MeCN); UV (MeCN) λmax nm (log ε) 205(4.57), 269 (3.84), 280 (3.91), 304 (3.88), 316 (3.70); IR (KBr, cm−1): 2932, 1712, 1636, 1396, 1219, 1042, 772; 1H-NMR (CDCl3) δ 0.97 (s, 3 H, H3-14), 1.01 (s, 3 H, H3-15), 1.24 (s, 3 H, H3-12), 1.42–1.56 (m, 1 H, H-6a), 1.60–1.64 (m, 1 H, H-2b), 1.67 (d, J = 9.4 Hz, 1 H, H-2a), 1.70–1.74 (m, 1 H, H-1), 1.75–1.82 (m, 1 H, H-6b), 1.83–1.88 (m, 1 H, H-10b), 1.88–1.95 (m, 1 H, 10a), 2.03–2.09 (m, 1 H, H-5), 2.12–2.14 (m, 1 H, H-3a), 2.13–2.17 (m, 1 H, H-16a), 2.17–2.22 (m, 1 H, H-7a), 2.22–2.29 (m, 1 H, H-3b), 2.42–2.46 (m, 1 H, H-7b), 2.47–2.52 (m, 1 H, H-9), 2.59–2.71 (m, 1 H, H-16b), 4.92 (s, 1 H, H-13b), 4.88 (s, 1 H, H-13a), 7.27 (t, J = 7.4 Hz, 1 H, H-22), 7.31 (d, J = 8.2 Hz, 1 H, H-24), 7.49 (td, J = 8.2, 1.2 Hz, 1 H, H-23), 7.76 (dd, J = 7.8, 1.2 Hz, 1 H, H-21); 13C-NMR (CDCl3) δ 21.1(C-14), 22.2(C-12), 22.3(C-2), 26.1(C-16), 30.2(C-15), 33.4(C-6), 33.8(C-11), 34.0(C-5), 35.2 (C-7), 36.5(C-10), 37.8(C-3), 41.5(C-9), 53.5(C-1), 84.4(C-4), 99.8(C-18), 110.6(C-13), 116.1(C-23), 116.5(C-20), 122.3(C-21), 123.6(C-22), 131.1(C-24), 151.8(C-25), 152.5(C-8), 158.8(C-19), 162.9(C-17); HRESIMS m/z [M + H]+ calcd for C25H31O3 379.2273, found 379.2245.
Compound 2a: oil; = +24.80 (c 0.05 in MeCN); UV (MeCN) λmax nm (log ε) 206(4.60), 269 (3.98), 280 (4.05), 304 (4.02), 316 (3.84); IR (KBr, cm−1): 2939, 2864, 1711, 1634, 1395, 1042, 754; 1H-NMR (CDCl3) δ, 0.82–0.91 (m, 1 H, H-6a), 0.95 (s, 3 H, H3-14), 1.00 (s, 3 H, H3-15), 1.16 (s, 3 H, H3-12), 1.40–1.49 (m, 1 H, H-6b), 1.54–1.60 (m, 2 H, H-2a + H-2b), 1.63 (dd, J = 9.2, 2.2 Hz, 1 H, H-1), 1.70 (d, J = 9.8 Hz, 1 H, H-10b), 1.72–1.80 (m, 1 H, H-10a), 1.88 (dd, J = 15.5, 8.0 Hz, 1 H, H-7a), 2.03–2.09 (m, 1 H, H-5), 2.09–2.12 (m, 1 H, H-3a), 2.13–2.18 (m, 1 H, H-16a), 2.30 (dd, J = 15.7, 10.2 Hz, 1 H, H-3b), 2.43–2.52 (m, 1 H, H-7b), 2.62 (q, J = 9.1 Hz, 1 H, H-9), 2.85 (dd, J = 14.9, 2.7 Hz, 1 H, H-16b), 4.75 (s, 1 H, H-13b), 4.84 (s, 1 H, H-13a), 7.26 (t, J = 7.4 Hz, 1 H, H-22), 7.31 (d, J = 8.2 Hz, 1 H, H-24), 7.50 (td, J = 7.8, 1.0 Hz, 1 H, H-23), 7.77 (dd, J = 8.0, 1.4 Hz, 1 H, H-21); 13C-NMR (CDCl3) δ 20.0 (C-14), 22.5 (C-12), 22.8 (C-2), 25.3 (C-16), 29.7 (C-15), 33.4 (C-6), 33.7 (C-11), 34.8 (C-5), 36.5 (C-7), 38.5 (C-10), 38.9 (C-3), 42.5 (C-9), 56.3 (C-1), 84.4 (C-4), 99.8 (C-18), 110.1 (C-13), 116.1 (C-23), 116.5 (C-20), 122.4 (C-21), 123.6 (C-22), 131.2 (C-23), 152.5 (C-25), 154.5 (C-8), 159.1 (C-19), 162.9 (C-17); HRESIMS m/z [M + Na]+ calcd for C25H30O3Na 401.2093, found 401.2068.
Compound 3a: oil; = +15.60 (c 0.05 in MeCN); UV (MeCN) λmax nm (log ε) 204(4.58), 223 (4.43), 274 (4.16), 286 (4.09); IR (KBr, cm−1): 2934, 2864, 1568, 1420, 760; 1H-NMR (CDCl3) δ 1.00 (s, 6 H, H3-14, 15), 1.29 (s, 3 H, H3-12), 1.41–1.52 (m, 1 H, H-6a), 1.59–1.67 (m, 2 H, H-2a + H-2b), 1.68–1.76 (m, 2 H, H-1 + H-6b), 1.81–1.88 (m, 1 H, H-10b), 1.88–1.94 (m, 1 H, H-10a), 1.99 (ddd, J = 15.5, 6.3, 2.5 Hz, 1 H, H-5), 2.11–2.13 (m, 1 H, H-3a), 2.14–2.15 (m, 1 H, H-16a), 2.15–2.24 (m, 2 H, H-3b + H-7a), 2.40–2.50 (m, 2 H, H-7b + H-9), 2.74–2.84 (m, 1 H, H-16b), 4.87 (s, 1 H, H-13a), 4.91 (s, 1 H, H-13b), 7.35 (br. s, 1 H, H-24), 7.36 (br. s, 1 H, H-22), 7.57 (td, J = 7.7, 1.8 Hz, 1 H, H-23), 8.15–8.21 (m, 1 H, H-21); 13C-NMR (CDCl3) δ 21.0 (C-14), 22.1 (C-12), 22.3 (C-2), 24.8 (C-16), 30.2 (C-15), 32.9 (C-6), 33.9 (C-5), 34.3 (C-11), 35.1 (C-7), 36.5 (C-10), 37.8 (C-3), 41.4 (C-9), 53.5 (C-1), 88.5 (C-4), 95.5 (C-18), 109.7 (C-13), 117.0 (C-24), 122.7 (C-22), 124.7 (C-20), 125.7 (C-21), 132.5 (C-23), 151.7 (C-25), 153.2 (C-8), 162.8 (C-17), 177.3 (C-19); HRESIMS m/z [M + H]+ calcd for C25H31O3 379.2273, found 379.2265.
Compound 4a: oil; = −79.39 (c 0.05 in MeCN); UV (MeCN) λmax nm (log ε) 206(4.61), 223 (4.53), 274 (4.29), 286 (4.21); IR (KBr, cm−1): 2936, 2860, 1624, 1568, 1420, 1261, 760; 1H-NMR (CDCl3) δ 0.99 (s, 3 H, H3-14), 1.01 (s, 3 H, H3-15), 1.23 (s, 3 H, H3-12), 1.53–1.57 (m, 2 H, H-6a + H-6b), 1.59–1.65 (m, 2 H, H-2a + H-2b), 1.71 (m, 1 H, H-1), 1.77 (m, 1 H, H-10a), 1.78–1.82 (m, 1 H, H-10b), 1.86 (td, J = 7.8, 1.2 Hz, 1 H, H-5), 2.04–2.10 (m, 1 H, H-3a), 2.11–2.18 (m, 2 H, H-7a + H-16a), 2.21–2.30 (m, 1 H, H-3b), 2.43–2.52 (m, 1 H, H-7b), 2.59–2.70 (q, J = 7.8 Hz, 1 H, H-9), 3.02 (dd, J = 15.1, 4.1 Hz, 1 H, 16b), 4.76 (br. t, J = 1.6 Hz, 1 H, H-13a), 4.85 (br. d, J = 1.6 Hz, 1 H, H-13b), 7.35–7.41 (m, 2 H, H-24 + H-22), 7.60 (ddd, J = 8.4, 7.0, 1.8 Hz, 1 H, H-23), 8.21 (dt, J = 8.2, 0.8 Hz, 1 H, H-21); 13C-NMR (CDCl3) δ 20.0 (C-14), 22.6 (C-12), 22.9 (C-2), 24.0 (C-16), 29.7 (C-15), 33.2 (C-6), 33.4 (C-11), 35.2 (C-5), 36.6 (C-7), 38.5 (C-10), 38.7 (C-3), 42.5 (C-9), 56.4 (C-1), 88.6 (C-4), 95.5 (C-18), 110.2 (C-13), 117.1 (C-24), 122.7 (C-22), 124.8 (C-20), 125.7 (C-21), 132.6 (C-23), 153.3 (C-25), 154.5 (C-8), 163.0 (C-17), 177.4 (C-19); HRESIMS m/z [M + H]+ calcd. for C25H31O3 379.2273, found 379.2248.
Compound 5a: oil; = −10.20 (c 0.05 in MeCN); UV (MeCN) λmax nm (log ε) 202(4.43), 254 (3.89), 311 (3.38); IR (KBr, cm−1):3470, 2934, 1630, 1524, 776; 1H-NMR (CDCl3) δ 0.92 (s, 3 H, H3-14), 0.93 (s, 3 H, H3-15), 1.24–1.29 (m, 2 H, H-6a + H-6b), 1.46–1.55 (m, 2 H, H-2a + H-2b), 1.57–1.60 (m, 1 H, H-1), 1.70–1.74 (m, 1 H, H-10b), 1.78–1.85 (m, 3 H, h-3a, 5, 10a), 2.25 (m, 1 H, H-16a), 2.27–2.38 (m, 2 H, H-3b + H-7a), 2.41–2.46 (m, 2 H, H-7b + H-9), 2.90 (d, J = 9.8 Hz, 1 H, H-16b), 4.91 (s, 1 H, H-12a), 4.93 (s, 1 H, H-12b), 5.15 (s, 1 H, H-13a), 5.19 (s, 1 H, H-13b), 7.01 (s, 1 H, HO-19), 7.25–7.28 (overlapped, 1 H, H-21), 7.29–7.34 (overlapped, 1 H, H-24), 7.52 (ddd, J = 8.4, 7.2, 1.6 Hz, 1 H, H-22), 7.80 (dd, J = 7.8, 1.6 Hz, 1 H, H-23); 13C-NMR (CDCl3) δ ppm 21.7(C-14), 29.7(C-15), 32.7(C-2), 33.0(C-16), 33.2(C-11), 35.9(C-6), 36.0(C-7), 36.5(C-10), 40.5(C-3), 41.9(C-9), 47.6(C-5), 54.5(C-1), 104.1(C-18), 109.6(C-12), 110.2(C-13), 115.4(C-20), 116.4(C-24), 123.1(C-21), 123.8(C-22), 131.7(C-23), 152.0(C-25), 152.4(C-8), 160.0(C-4), 161.0(C-19), 163.4(C-17); HRESIMS m/z [M + H]+ calcd. for C25H31O3 379.2273, found 379.2256.
3.2.2. Products Starting from 6-Chloro-4-Hydroxycoumarin
The procedure was the same as the above reaction. Paraformaldehyde (248 mg), 6-chloro-4-hydroxycoumarin (193 mg, 0.982 mmol) and β-caryophyllene (690 μL, 3.450 mmol) were used as starting materials and 919 mg of crude product were obtained from the reaction. After the same separation and purification procedure as above, five products were isolated: 1b (41%, 168 mg), 2b (18%, 71 mg), 3b (14%, 59 mg), 4b (6%, 25 mg), 5b (2%, 7 mg).
Compound 1b: oil; = −176.19 (c 0.05 in MeCN); UV (MeCN) λmax nm (log ε) 204(4.62), 273 (4.12), 285 (4.15), 311 (4.12), 325 (4.00); IR (KBr, cm−1): 2936, 1717, 1636, 1385, 758; 1H-NMR (CDCl3) δ 0.99 (s, 3 H, H3-14), 1.02 (s, 3 H, H3-15), 1.24 (s, 3 H, H3-12), 1.46–1.56 (m, 1 H, H-6a), 1.59–1.65 (m, 2 H, H-2a + H-2b), 1.70 (d, J = 10.2 Hz, 1 H, H-1), 1.76–1.87 (m, 2 H, H-6b + H-10b), 1.88–1.94 (m, 1 H, H-10a), 2.02–2.08 (m, 1 H, H-5), 2.09–2.14 (m, 2 H, H-3a + H-16a), 2.15–2.20 (m, 1 H, H-7a), 2.22–2.29 (m, 1 H, H-3b), 2.42–2.51 (m, 2 H, H-7b + H-9), 2.58–2.70 (m, 1 H, H-16b), 4.89 (s, 1 H, H-13a), 4.92 (s, 1 H, H-13b), 7.25 (d, J = 8.6 Hz, 1 H, H-24), 7.44 (dd, J = 8.8, 2.5 Hz, 1 H, H-23), 7.71 (d, J = 2.3 Hz, 1 H, H-21); 13C-NMR (CDCl3) δ ppm 21.1(C-14), 22.2(C-12), 22.3(C-2), 26.1(C-16), 30.2(C-15), 33.4(C-6), 33.9(C-11), 33.9(C-5) 35.2(C-7), 36.5(C-10), 37.8(C-3), 41.4(C-9), 53.6(C-1), 84.9(C-4), 100.7(C-18), 110.7(C-13), 117.2(C-20), 118.0(C-24), 121.9(C-21), 129.1(C-22), 131.1(C-23), 150.8(C-25), 151.8(C-8), 157.7(C-19), 162.4(C-17); HRESIMS m/z [M + H]+ calcd for C25H30ClO3 413.1883, found 413.1864.
Compound 2b: white solid, mp 161.4–163.2 °C (CHCl3); = +72.39 (c 0.05 in MeCN); UV (MeCN) λmax nm (log ε) 209(4.61), 273 (3.93), 285 (3.95), 311 (3.93), 325 (3.81); IR (KBr, cm−1): 2936, 1715, 1634, 1385, 1042, 814; 1H-NMR (CDCl3) δ 0.97 (s, 3 H, H3-14), 1.02 (s, 3 H, H3-15), 1.17 (s, 3 H, H3-12), 1.50–1.58 (m, 2 H, H-6a + H-6b), 1.61 (d, J = 14.9 Hz, 2 H, H-2a + H-2b), 1.63–1.68 (m, 1 H, H-1), 1.69–1.73 (m, 1 H, H-10a), 1.75–1.79 (m, 1 H, H-10b), 1.89 (dd, J = 15.7, 8.6 Hz, 1 H, H-5), 2.08–2.18 (m, 3 H, H-3a + H-7a + H-16a), 2.31 (dd, J = 15.5, 10.4 Hz, 1 H, H-3b), 2.43–2.53 (m, 1 H, H-7b), 2.63 (q, J = 9.1 Hz, 1 H, H-9), 2.85 (dd, J = 15.5, 3.3 Hz, 1 H, H-16b), 4.77 (s, 1 H, H-13a), 4.85 (s, 1 H, H-13b), 7.26 (d, J = 9.0 Hz, 1 H, H-24), 7.45 (dd, J = 8.8, 2.5 Hz, 1 H, H-23), 7.72 (d, J = 2.7 Hz, 1 H, H-21); 13C-NMR (CDCl3) δ 20.0 (C-14), 22.6 (C-12), 22.8 (C-2), 25.3 (C-16), 29.7 (C-15), 33.4 (C-11), 33.7 (C-6), 34.8 (C-5), 36.5 (C-7), 38.5 (C-10), 38.8 (C-3), 42.5 (C-9), 56.3 (C-1), 85.0 (C-4), 100.7 (C-18), 110.2 (C-13), 117.2 (C-20), 118.0 (C-24), 122.0 (C-21), 129.1 (C-22), 131.2 (C-23), 150.8 (C-25), 154.4 (C-8), 158.1 (C-19), 162.3 (C-17); HRESIMS m/z [M + H]+ calcd for C25H30ClO3 413.1883, found 413.1881.
Compound 3b: oil; = +22.60 (c 0.05 in MeCN); UV (MeCN) λmax nm (log ε) 211(4.45), 282 (3.88); IR (KBr, cm−1): 2928, 1624, 1564, 1447, 772; 1H-NMR (CDCl3) δ 1.00 (s, 6 H, H3-14, 15), 1.29 (s, 3 H, H3-12), 1.43–1.52 (m, 1 H, H-6a), 1.61–1.73 (m, 4 H, H-1 + H-2a + H-2b + H-6b), 1.78–1.87 (m, 1 H, H-10a), 1.91 (td, J = 10.2, 5.5 Hz, 1 H, H-10b), 2.00 (ddd, J = 15.6, 6.4, 2.3 Hz, 1 H, H-5), 2.10–2.21 (m, 4 H, H-3a + H-3b + H-7a + H-16a), 2.40–2.51 (m, 2 H, H-7b + H-9), 2.73–2.84 (m, 1 H, H-16b), 4.88 (s, 1 H, H-13a), 4.92 (s, 1 H, H-13b), 7.25–7.34 (m, 1 H, H-24), 7.52 (dd, J = 8.8, 2.5 Hz, 1 H, H-23), 8.14 (d, J = 2.3 Hz, 1 H, H-21); 13C-NMR (CDCl3) δ 21.0 (C-14), 22.1 (C-12), 22.3 (C-2), 24.7 (C-16), 30.2 (C-15), 32.9 (C-6), 33.9 (C-11), 34.2 (C-5), 35.1 (C-7), 36.4 (C-10), 37.8 (C-3), 41.4 (C-9), 53.6 (C-1), 89.0 (C-4), 95.7 (C-18), 110.8 (C-13), 118.7 (C-24), 123.7 (C-20), 125.2 (C-21), 130.5 (C-22), 132.6 (C-23), 151.5 (C-25), 151.6 (C-8), 162.9 (C-17), 176.1(C-19); HRESIMS m/z [M + H]+ calcd for C25H30ClO3 413.1883, found 413.1862.
Compound 4b: white solid, mp 137.0–139.4 °C (CHCl3); = −44.40 (c 0.05 in MeCN); UV (MeCN) λmax nm (log ε) 211(4.47), 282 (3.90); IR (KBr, cm−1): 2957, 1624, 1562, 1447, 1259, 808; 1H-NMR (CDCl3) δ 0.99 (s, 3 H, H3-14), 1.01 (s, 3 H, H3-15), 1.23 (s, 3 H, H3-12), 1.53–1.57 (m, 2 H, H-6a + H-6b), 1.58–1.68 (m, 3 H, H-1 + H-2a + H-2b), 1.71 (d, J = 10.2 Hz, 1 H, H-10a), 1.75–1.79 (m, 1 H, H-10b), 1.82–1.89 (m, 1 H, H-5), 2.09–2.19 (m, 3 H, H-3a + H-7a + H-16a), 2.25 (dd, J = 15.8, 10.0 Hz, 1 H, H-3b), 2.42–2.51 (m, 1 H, H-7b), 2.64 (q, J = 9.0 Hz, 1 H, H-9), 2.99 (dd, J = 15.1, 4.1 Hz, 1 H, H-16b), 4.76 (br. s, 1 H, H-13a), 4.85 (br. s, 1 H, H-13b), 7.33 (d, J = 9.0 Hz, 1 H, H-24), 7.53 (dd, J = 8.8, 2.5 Hz, 1 H, H-23), 8.16 (d, J = 2.7 Hz, 1 H, H-21); 13C-NMR (CDCl3) δ 20.0 (C-14), 22.6 (C-12), 22.9 (C-2), 23.9 (C-16), 29.7 (C-15), 33.2 (C-6), 33.4 (C-11), 35.1 (C-5), 36.5 (C-7), 38.5 (C-10), 38.7 (C-3), 42.5 (C-9), 56.2 (C-1), 89.0 (C-4), 95.7 (C-18), 110.3 (C-13), 118.7 (C-24), 123.7 (C-20), 125.2 (C-21), 130.6 (C-22), 132.6 (C-23), 151.5 (C-25), 154.4 (C-8), 163.1 (C-17), 176.1 (C-19); HRESIMS m/z [M + H]+ calcd for C25H30ClO3 413.1883, found 413.1874.
Compound 5b: oil; = −6.80 (c 0.05 in MeCN); UV (MeCN) λmax nm (log ε) 201(4.05), 219 (4.02), 319 (2.97); IR (KBr, cm−1): 3285, 2934, 2864, 1711, 1638, 1466, 1277, 758; 1H-NMR (CDCl3) δ 0.93 (s, 6 H, H3-14, 15), 1.25–1.33 (m, 2 H, H-6a + H-6b), 1.41–1.50 (m, 2 H, H-2a + H-2b), 1.56–1.59 (m, 1 H, H-1), 1.71–1.73 (m, 1 H, H-10a), 1.81 (m, 3 H, H-3a + H-5 + H-10b), 2.22–2.28 (m, 1 H, H-16a), 2.31–2.40 (m, 2 H, H-3b, 7a), 2.41–2.45 (m, 2 H, H-7b + H-9), 2.89 (m, J = 10.2 Hz, 1 H, H-16b), 4.90 (s, 1 H, H-13a), 4.93 (s, 1 H, H-13b), 5.16 (s, 1 H, H-12a), 5.19 (s, 1 H, H-12b), 7.10 (s, 1 H, HO-10), 7.25 (d, J = 9.0 Hz, 1 H, H-24), 7.46 (dd, J = 8.6, 2.3 Hz, 1 H, H-23), 7.77 (d, J = 2.3 Hz, 1 H, H-21); 3C-NMR (CDCl3) δ 21.6 (C-14), 29.7 (C-15), 32.7 (C-2), 32.9 (C-16), 33.2 (C-11), 35.9 (C-6), 36.0 (C-7), 36.4 (C-10), 40.6 (C-3), 41.9 (C-9), 47.6 (C-5), 54.5 (C-1), 105.0 (C-18), 109.6 (C-12), 110.3 (C-13), 116.6 (C-20), 117.8 (C-24), 122.8 (C-21), 130.9 (C-22), 131.7 (C-23), 150.7 (C-25), 152.0 (C-8), 159.0 (C-4), 161.0 (C-19), 162.9 (C-17); HRESIMS m/z [M + H]+ calcd for C25H30ClO3 413.1883, found 413.1868.
3.2.3. Products Starting from 6-Bromo-4-Hydroxycoumarin
The procedure was the same as the above reaction. Paraformaldehyde (203 mg), 6-bromo-4-hydroxycoumarin (188 mg, 0.780 mmol) and β-caryophyllene (565 μL, 2.825 mmol) were used as starting materials and 772 mg of crude product was obtained from the reaction. After the same separation and purification procedure as above, five products were separated: 1c (41%, 142 mg), 2c (18%, 63 mg), 3c (14%, 48 mg), 4c (5%, 18 mg), 5c (2%, 8 mg).
Compound 1c: oil; = −90.99 (c 0.05 in MeCN); UV (MeCN) λmax nm (log ε) 210(4.55), 274 (3.82), 286 (3.84), 312 (3.81), 325 (3.71); IR (KBr, cm−1): 2936, 1715, 1634, 1385, 764; 1H-NMR (CDCl3) δ 0.99 (s, 3 H, H3-14), 1.02 (s, 3 H, H3-15), 1.23 (s, 3 H, H3-12), 1.51 (ddd, J = 10.2, 7.8, 3.1 Hz, 1 H, H-6a), 1.62–1.69 (m, 2 H, H-2a + H-2b), 1.70–1.74 (m, 1 H, H-1), 1.75–1.80 (m, 1 H, H-6b), 1.81–1.86 (m, 1 H, H-10a), 1.87–1.93 (m, 1 H, H-10b), 2.02–2.08 (m, 1 H, H-5), 2019–2.12 (m, 1 H, H-3a), 2.12–2.15 (m, 1 H, H-16a), 2.18–2.21 (m, 1 H, H-7a), 2.21–2.29 (m, 1 H, H-3b), 2.42–2.51 (m, 2 H, H-7b + H-9), 2.59–2.70 (m, 1 H, H-16b), 4.88 (s, 1 H, H-13a), 4.92 (s, 1 H, H-13b), 7.18 (d, J = 9.0 Hz, 1 H, H-24), 7.57 (dd, J = 8.6, 2.3 Hz, 1 H, H-23), 7.85 (d, J = 2.3 Hz, 1 H, H-21); 13C-NMR (CDCl3) δ 21.1 (C-14), 22.2 (C-12), 22.3 (C-2), 26.1 (C-16), 30.2 (C-15), 33.4 (C-6), 33.8 (C-5), 33.9 (C-11), 35.2 (C-7), 36.5 (C-10), 37.8 (C-3), 41.4 (C-9), 53.6 (C-1), 85.0 (C-4), 100.7 (C-18), 110.7(C-13), 116.5(C-22), 117.7(C-24), 118.3(C-20), 124.9(C-21), 133.9(C-23), 151.3(C-25), 151.8(C-8), 157.6(C-19), 162.3(C-17); HRESIMS m/z [M + Na]+ calcd for C25H29BrO3Na 479.1198, found 479.1184.
Compound 2c: white solid, mp 149.0–151.4 °C (CHCl3); = +67.40 (c 0.05 in MeCN); UV (MeCN) λmax nm (log ε) 210(4.58), 274 (3.85), 285 (3.87), 312 (3.84), 325 (3.74); IR (KBr, cm−1): 2934, 1715, 1634, 1363, 812; 1H-NMR (CDCl3) δ 0.97 (s, 3 H, H3-14), 1.02 (s, 3 H, H3-15), 1.16 (s, 3 H, H3-12), 1.40–1.47 (m, 1 H, H-6a), 1.55–1.60 (m, 2 H, H-2a + H-2b), 1.61–1.63 (m, 1 H, H-1), 1.64–1.68 (m, 1 H, H-6b), 1.68–1.73 (m, 1 H, H-10a), 1.74–1.81 (m, 1 H, H-10b), 1.89 (dd, J = 15.7, 8.2 Hz, 1 H, H-5), 2.03–2.09 (m, 1 H, H-3a), 2.11–2.17 (m, 2 H, H-7a + H-16a), 2.31 (dd, J = 15.7, 10.2 Hz, 1 H, H-3b), 2.43–2.53 (m, 1 H, H-7b), 2.62 (q, J = 9.0 Hz, 1 H, H-9), 2.85 (dd, J = 15.7, 3.1 Hz, 1 H, H-16b), 4.76 (s, 1 H, H-13a), 4.85 (s, 1 H, H-13b), 7.20 (d, J = 8.6 Hz, 1 H, H-24), 7.59 (dd, J = 9.0, 2.3 Hz, 1 H, H-23), 7.86 (d, J = 2.3 Hz, 1 H, H-21); 13C-NMR (CDCl3) δ 20.0 (C-14), 22.6 (C-12), 22.9 (C-2), 25.3 (C-16), 29.7 (C-15), 33.4 (C-11), 33.7 (C-6), 34.8 (C-5), 36.5 (C-7), 38.5 (C-10), 38.8 (C-3), 42.5 (C-9), 56.3 (C-1), 85.0 (C-4), 100.7 (C-18), 110.2 (C-13), 116.5 (C-22), 117.6 (C-24), 118.3 (C-20), 125.1 (C-21), 134.0 (C-23), 151.3 (C-25), 154.4 (C-8), 158.0 (C-19), 162.3 (C-17); HRESIMS m/z [M + H]+ calcd for C25H30BrO3 457.1378, found 457.1360.
Compound 3c: oil; = +34.20 (c 0.05 in MeCN); UV (MeCN) λmax nm (log ε) 212(4.56), 282 (4.00); IR (KBr, cm−1): 2936, 1624, 1562, 1445, 1396, 756; 1H-NMR (CDCl3) δ 1.01 (s, 6 H, H3-14 + H3-15), 1.29 (s, 3 H, H3-12), 1.42–1.53 (m, 1 H, H-6a), 1.62–1.72 (m, 4 H, H-1 + H-2a + H-2b + H-6b), 1.77–1.87 (m, 1 H, H-10a), 1.92 (td, J = 10.2, 5.5 Hz, 1 H, H-10b), 2.00 (ddd, J = 15.3, 6.3, 2.3 Hz, 1 H, H-5), 2.11–2.20 (m, 4 H, H-3a + H-3b + H-7a + H-16a), 2.41–2.50 (m, 2 H, H-7b + H-9), 2.73–2.84 (m, 1 H, H-16b), 4.88 (s, 1 H, H-13a), 4.92 (s, 1 H, H-13b), 7.25 (d, J = 8.6 Hz, 1 H, H-24), 7.66 (dd, J = 9.0, 2.3 Hz, 1 H, H-23), 8.30 (d, J = 2.3 Hz, 1 H, H-21); 13C-NMR (CDCl3) δ 21.0 (C-14), 22.1 (C-12), 22.3 (C-2), 24.7 (C-16), 30.2 (C-15), 32.9 (C-6), 33.9 (C-11), 34.2 (C-5), 35.1 (C-7), 36.4 (C-10), 37.8 (C-3), 41.4 (C-9), 53.6 (C-1), 89.1 (C-4), 95.7 (C-18), 110.8 (C-13), 118.0 (C-24), 119.0 (C-22), 124.1 (C-20), 128.3 (C-21), 135.4 (C-23), 151.6 (C-25), 151.9 (C-8), 162.9 (C-17), 175.9 (C-19); HRESIMS m/z [M + H]+ calcd for C25H30BrO3 457.1378, found 457.1357.
Compound 4c: oil; = −29.00 (c 0.05 in MeCN); UV (MeCN) λmax nm (log ε) 212(4.26), 282 (3.70); IR (KBr, cm−1): 2941, 1622, 1562, 1447, 768; 1H-NMR (CDCl3) δ 0.98 (s, 3 H, H3-14), 0.99 (s, 3 H, H3-15), 1.22 (s, 3 H, H3-12), 1.50–1.57 (m, 3 H, H-2a + H-2b + H-6a), 1.58–1.60 (m, 1 H, H-6b), 1.60–1.63 (m, 1 H, H-1), 1.67–1.78 (m, 2 H, H-10a, 10b), 1.82–1.88 (m, 1 H, H-5), 2.09–2.18 (m, 3 H, H-3a + H-7a + H-16a), 2.24 (dd, J = 15.7, 9.4 Hz, 1 H, H-3b), 2.41–2.50 (m, 1 H, H-7b), 2.63 (q, J = 9.4 Hz, 1 H, H-9), 2.98 (dd, J = 15.3, 4.3 Hz, 1 H, H-16b), 4.75 (br. t, J = 1.6, 1.6 Hz, 1 H, H-13a), 4.84 (br. d, J = 0.8 Hz, 1 H, H-13b), 7.25 (d, J = 5.1 Hz, 1 H, H-24), 7.66 (dd, J = 8.6, 2.3 Hz, 1 H, H-23), 8.31 (d, J = 2.3 Hz, 1 H, h-21); 13C-NMR (CDCl3) δ 19.7 (C-14), 22.3 (C-12), 22.6 (C-2), 23.6 (C-16), 29.4 (C-15), 32.9 (C-6), 33.1 (C-11), 34.8 (C-5), 36.2 (C-7), 38.2 (C-10), 38.4 (C-3), 42.2 (C-9), 55.9 (C-1), 88.8 (C-4), 95.5 (C-18), 110.0 (C-13), 117.7 (C-24), 118.7 (C-22), 123.8 (C-20), 128.1 (C-21), 135.1 (C-23), 151.7 (C-25), 154.0 (C-8), 162.7 (C-17), 175.7 (C-19); HRESIMS m/z [M + H]+ calcd for C25H30BrO3 457.1378, found 457.1367.
Compound 5c: oil; = −5.40 (c 0.05 in MeCN); UV (MeCN) λmax nm (log ε) 203(4.14), 222 (4.12), 318 (3.07); IR (KBr, cm−1): 3659, 2934, 1713, 1464, 1275, 770; 1H-NMR (CDCl3) δ 0.93 (s, 6 H, H3-14, 15), 1.25–1.35 (m, 2 H, H-6a, 6b), 1.49–1.60 (m, 3 H, H-1 + H-2a + H-2b), 1.70–1.73 (m, 1 H, H-10a), 1.49–1.84 (m, 3 H, H-3a + H-5 + H-10b), 2.21–2.29 (m, 1 H, H-16a), 2.36 (d, J = 3.5 Hz, 1 H, H-7a), 2.41–2.48 (m, 3 H, H-3b + H-7b + H-9), 2.88 (d, J = 10.2 Hz, 1 H, H-16b), 4.90 (s, 1 H, H-13a), 4.92 (s, 1 H, H-13b), 5.16 (s, 1 H, H-12a), 5.19 (s, 1 H, H-12b), 7.12 (br. s., 1 H, HO-19), 7.20 (d, J = 9.0 Hz, 1 H, H-24), 7.60 (dd, J = 8.8, 2.2 Hz, 1 H, H-23), 7.93 (d, J = 2.3 Hz, 1 H, H-21); 13C-NMR (CDCl3) δ 21.6 (C-14), 29.7 (C-15), 32.7 (C-2), 32.9 (C-16), 33.1 (C-11), 35.8 (C-6), 36.0 (C-7), 36.4 (C-10), 40.5 (C-3), 41.8 (C-9), 47.5 (C-5), 54.5 (C-1), 105.0 (C-18), 109.6 (C-12), 110.3 (C-13), 116.6 (C-20), 117.0 (C-22), 118.1 (C-24), 125.8 (C-21), 134.5 (C-23), 151.2 (C-25), 152.0 (C-8), 158.9 (C-4), 160.9 (C-19), 162.8 (C-17); HRESIMS m/z [M + H]+ calcd for C25H30BrO3 457.1378, found 457.1356.
3.2.4. Product 6 Starting from Syncarpic Acid
Syncarpic acid was synthesized from 2′,4′,6′-trihydroxyacetophenone according to our previously reported procedure [29]. Then, to a dry reaction tube, was added syncarpic acid (50 mg, 0.275 mmol), ZnI2 (53 mg, 0.166 mmol), anhydrous toluene (3 mL) and β-caryophyllene (168 mg, 0.824 mmol). After stirring for 18 h at 110 °C, the mixture was concentrated. To the residue was added 5 mL water and the mixture was then extracted with EtOAc (10 mL) three times. The organic layers were combined, dried over Na2SO4 and the solvent removed under reduced pressure to yield the crude product, which was then separated on a silica gel column (petroleum ether-EtOAc 50:1) to afford oily compound 6 (38 mg, 36%).
Compound 6: oil; = +135.6 (c 0.05 in MeCN); IR (KBr, cm−1): 3096, 3058, 1704, 1648, 1510, 1202; 1H- NMR (CDCl3) δ 0.95 (s, 6 H, H3-12, 15), 0.97 (s, 3 H, H3-14), 1.13(m, 1 H, H-10a), 1.19–1.28 (m, 1 H, H-5a), 1.30 (s, 6 H, Me-17), 1.34 (s, 3 H, H3-19a), 1.35–1.40 (m, 1 H, H-13a), 1.41 (s, 3 H, Me-19b), 1.43–1.57 (m, 4 H, H-3a + H-5b + H-7a), 1.66–1.76 (m, 2 H, H-6a + H-6b), 1.79–1.88 (m, 1 H, H-7b), 2.05 (dd, 13.3, 1.6, 1 H, H-13b), 2.27–2.43 (m, 2 H, H-9 + H-10b), 5.37(s, 1 H, H-21); 13C-NMR (CDCl3) δ 20.6 (C-14), 20.8 (C-6), 24.3 (Me-17), 24.4 (C-2), 24.8 (Me-19), 25.6 (Me-17), 25.7 (Me-19), 30.6 (C-15), 34.1 (C-12), 34.7 (C-4), 34.9 (C-11), 35.6 (C-10), 36.1 (C-3), 39.9 (C-5), 40.5 (C-7), 44.4 (C-9), 47.5 (C-13), 47.7 (C-1), 48.8 (C-17), 55.2 (C-19), 85.3 (C-8), 104.7 (C-21), 174.6 (C-16), 199.1 (C-20), 214.1 (C-18); HR ESIMS m/z [M + Na]+ calcd for C25H38O3Na 409.2718, found 409.2705.
3.2.5. Product 7 Starting from Lawsone
To a dry reaction tube, was added lawsone (50 mg, 0.287 mmol), ZnI2 (55 mg, 0.172 mmol), anhydrous toluene (3 mL) and β-caryophyllene (176 mg, 0.863 mmol). After stirring for 18 h at 110 °C, the mixture was concentrated. Using the same procedure as above, the residue afforded compound 7 (35 mg, 32%) as an oil.
Compound 7: oil; = +92.4 (c 0.05 in MeCN); IR (KBr, cm−1): 3092, 3036, 1726, 1652, 1326, 1216, 996; 1H-NMR (CDCl3) δ 0.95 (s, 3 H, H3-14), 0.98 (s, 3 H, H3-12), 1.00 (s, 3 H, H3-15), 1.16 (dd, J = 7.8, 6.3 Hz, 1 H, H-10a), 1.30–1.34 (m, 1 H, H-5a), 1.35–1.41 (m, 1 H, H-2a), 1.45–1.49 (m, 1 H, H-10b), 1.52–1.60 (m, 4 H, H-2b + H-3a + H-5b + H-7a), 1.63 (s, 1 H, H-13a), 1.71–1.79 (m, 2 H, H-6a + H-6b), 1.85 (dd, J = 9.8, 7.4 Hz, 1 H, H-7b), 1.97 (ddd, J = 11.5, 9.6, 3.9 Hz, 1 H, H-1), 2.23–2.29 (m, 1 H, H-13b), 2.36–2.45 (m, 2 H, H-3b + H-9), 6.11 (s, 1 H, H-18), 7.65–7.74 (m, 2 H, H-22 + H-23), 8.03–8.06 (m, 1 H, H-24), 8.08–8.12 (m, 1 H, H-21); 13C-NMR (CDCl3) δ 20.5 (C-14), 20.5 (C-6), 24.1 (C-2), 30.0 (C-15), 33.6 (C-12), 34.6 (C-4), 34.6 (C-11), 35.1 (C-10), 35.6 (C-3), 39.5 (C-5), 40.3 (C-7), 44.3 (C-9), 47.0 (C-13), 47.2 (C-1), 86.1 (C-8), 114.7 (C-18), 125.9 (C-24), 126.6 (C-21), 131.4 (C-25), 131.9 (C-20), 133.0 (C-23), 133.9 (C-22), 156.8 (C-17), 181.0 (C-16), 185.0 (C-19); HR ESIMS m/z [M + Na]+ calcd for C25H30O3Na 401.2092, found 401.2091.
3.3. ECD Calculation Method
The calculation was performed as our previously reported procedure [45,46]. All the conformers of every calculated compound were searched by Conflex using the MMFF94s force field [47,48]. Further optimization was performed at B3LYP/6-31 + G(d,p) level in Gaussian 09 package [49]. The theoretical CD spectra were calculated by cam-B3LYP/TZVP and added in SpecDis [50] according to their Boltzmann-calculated distributions.
3.4. α-Glucosidase Inhibitory Assay
The α-glucosidase inhibitory assay of the synthesized compounds was performed as previously reported procedure [45]. α-Glucosidase (EC 3.2.1.20) isolated from Saccharomyces cerevisiae was purchased from Sigma (St. Louis, MO, USA). The enzyme was dissolved in 200 μL of 10 mM phosphate buffer (pH 6.80) and incubated with 12 μL of the test compound in DMSO at 37 °C for 5 min. Then the enzymatic reaction was started by the addition of 36 μL of 4-nitrophenyl α-D-glucopyranoside (pNPG) and kept under 37 °C for 40 min. The amount of released 4-nitrophenol was determined according to the absorbance at 400 nm. The primary screening was carried out at two concentrations (25 and 50 μM). While the IC50 assay was performed with five different concentrations around the IC50 values. In each set of experiments, the assay was performed in triplicate. The percentage inhibition of α-glucosidase activity was calculated via the following formula: Inhibition ratio (%) = 100 × (Acontrol − Asample)/Acontrol. The IC50 values were calculated in Prism 7 using a nonlinear regression method with the normalized response and variable slope.
3.5. Kinetic Analysis of α-Glucosidase Inhibition
The kinetic parameters of α-glucosidase inhibition by compound were evaluated by the Lineweaver-Burk plots and its secondary plots. The double-reciprocal plots were constructed with enzyme reaction initial velocity (V) versus substrate (S) concentration (1/v vs. 1/[S]) in the absence (control) or presence of compound 9 at different levels (0–8 μM). The initial rate was measured by stopping the reaction after 2 min. The type of inhibition, Km, and Vmax values were determined from the plots. Slopes and Y-intercepts of these reciprocal plots were also replotted against the inhibitor concentration, respectively. Data analysis was performed by the Prism software.
3.6. Circular Dichroism Measurement of Inhibitor-Enzyme Complex
CD spectra (190–250 nm) of α-glucosidase with and without compound 7 were recorded on a Chirascan CD spectrometer at room temperature. All the CD spectra were corrected with the buffer signal under constant nitrogen flush. The concentration of α-glucosidase was 2.0 μM, and the molar ratios of compound 7 to α-glucosidase were set to 0:1, 1:1, and 2:1.
4. Conclusions
In summary, we use a biomimetic hetero-Diels-Alder reaction as a tool for generating NP-like skeletons. Although the yields and stereoselectivities of these reactions were very limited, the product diversity provided a chance to explore new NP-like scaffolds. These reactions starting from 4-hydroxycoumarins and β-caryophyllene could provide a facile way to acquire meroterpene-like skeletons. All 17 products, representing seven different scaffolds, provided candidates to identify potentially new chemotypes. Due to the limited stereoselectivity of the construction reaction, all the ACs of products were determined unambiguously by ECD calculations. Inspired by the inhibition of compound 5a, we furtherly linked β-caryophyllene and lawsone (or syncarpic acid) via an ether connection to yield compound 6 and 7. Compounds 5a, 6, and 7 showed interesting potential activities (IC50 < 10 μM). They can be considered novel scaffolds for the design of α-glucosidase inhibitors. Furthermore, side products from biomimetic reactions can provide diverse NP-like scaffolds, and will favor searching for lead candidates for drug discovery. Since a hydrogen bonding site is essential for inhibition, we will further explore the chemical space in the future based on natural polyphenol moieties. Also, the inhibition mechanism should be further investigated based on a more promising molecule.
Supplementary Materials
The following are available online. Figure S1: GC-EI MS and LC-ESI-MS/MS analysis, Figure S2: NMR and HR-MS spectra.
Author Contributions
Formal analysis, S.-J.M. and Q.Z.; Investigation, D.-W.Y., C.-D.H., H.-H.Z., N.Z. and X.-L.F.; Writing—review & editing, A.-L.Z. and Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (30971882).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of all compounds involved in this paper are available from the authors.
References
- 1.Ertl P., Schuhmann T. A systematic cheminformatics analysis of functional groups occurring in natural products. J. Nat. Prod. 2019;82:1258–1263. doi: 10.1021/acs.jnatprod.8b01022. [DOI] [PubMed] [Google Scholar]
- 2.Gao H., Li G., Lou H.-X. Structural diversity and biological activities of novel secondary metabolites from endophytes. Molecules. 2018;23:646. doi: 10.3390/molecules23030646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shan M., Yu S., Yan H., Guo S., Xiao W., Wang Z., Zhang L., Ding A., Wu Q., Li S.F.Y. A review on the phytochemistry, pharmacology, pharmacokinetics and toxicology of geniposide, a natural product. Molecules. 2017;22:1689. doi: 10.3390/molecules22101689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen J., Zhou Q., Li P., Wang Z., Liu S., He C., Zhang C., Xiao P. Update on phytochemistry and pharmacology of naturally occurring resveratrol oligomers. Molecules. 2017;22:2050. doi: 10.3390/molecules22122050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y., Jing S.-X., Luo S.-H., Li S.-H. Non-volatile natural products in plant glandular trichomes: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2019;36:626–665. doi: 10.1039/C8NP00077H. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues T., Reker D., Schneider P., Schneider G. Counting on natural products for drug design. Nat. Chem. 2016;8:531–541. doi: 10.1038/nchem.2479. [DOI] [PubMed] [Google Scholar]
- 7.Stratton C.F., Newman D.J., Tan D.S. Cheminformatic comparison of approved drugs from natural product versus synthetic origins. Bioorg. Med. Chem. Lett. 2015;25:4802–4807. doi: 10.1016/j.bmcl.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao J.-M., Yang S.-X., Qin J.-C. Azaphilones: Chemistry and biology. Chem. Rev. 2013;113:4755–4811. doi: 10.1021/cr300402y. [DOI] [PubMed] [Google Scholar]
- 9.Pye C.R., Bertin M.J., Lokey R.S., Gerwick W.H., Linington R.G. Retrospective analysis of natural products provides insights for future discovery trends. Proc. Natl. Acad. Sci. USA. 2017;114:5601–5606. doi: 10.1073/pnas.1614680114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L., Chen Z., Zhang X., Jia Y. Divergent strategy in natural product total synthesis. Chem. Rev. 2018;118:3752–3832. doi: 10.1021/acs.chemrev.7b00653. [DOI] [PubMed] [Google Scholar]
- 11.Crossley S.W.M., Barabé F., Shenvi R.A. Simple, chemoselective, catalytic olefin isomerization. J. Am. Chem. Soc. 2014;136:16788–16791. doi: 10.1021/ja5105602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X., Li S. Expansion of chemical space for natural products by uncommon P450 reactions. Nat. Prod. Rep. 2017;34:1061–1089. doi: 10.1039/C7NP00028F. [DOI] [PubMed] [Google Scholar]
- 13.Abouelhassan Y., Garrison A.T., Yang H., Chavez-Riveros A., Burch G.M., Huigens R.W. Recent progress in natural-product-inspired programs aimed to address antibiotic resistance and tolerance. J. Med. Chem. 2019;62:7618–7642. doi: 10.1021/acs.jmedchem.9b00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinfe H.H. Versatility of glycals in synthetic organic chemistry: Coupling reactions, diversity oriented synthesis and natural product synthesis. Org. Biomol. Chem. 2019;17:4153–4182. doi: 10.1039/C9OB00343F. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi H., Sakurai K., Oshima Y. Development of diversity-enhanced extracts of curcuma zedoaria and their new sesquiterpene-like compounds. Org. Lett. 2014;16:1916–1919. doi: 10.1021/ol5004324. [DOI] [PubMed] [Google Scholar]
- 16.Barnes E.C., Kumar R., Davis R.A. The use of isolated natural products as scaffolds for the generation of chemically diverse screening libraries for drug discovery. Nat. Prod. Rep. 2016;33:372–381. doi: 10.1039/C5NP00121H. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H., Zhao Z., Chen J., Bai X., Wang H. Tricycloalternarene analogs from a symbiotic Fungus aspergillus sp. d and their antimicrobial and cytotoxic effects. Molecules. 2018;23:855. doi: 10.3390/molecules23040855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu R., Le Z., Wang Z., Tian S., Xue Y., Chen Y., Hu L., Zhang Y. Hyperjaponol h, a new bioactive filicinic acid-based meroterpenoid from Hypericum japonicum thunb. ex murray. Molecules. 2018;23:683. doi: 10.3390/molecules23030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamed A., Abdel-Razek A.S., Frese M., Stammler H.G., El-Haddad A.F., Ibrahim T.M.A., Sewald N., Shaaban M. Terretonin n: A new meroterpenoid from Nocardiopsis sp. Molecules. 2018;23:299. doi: 10.3390/molecules23020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng L.-Z., Qin F.-Y., Ma X.-C., Wang S.-M., Yan Y.-M., Cheng Y.-X. Cytotoxic and n-acetyltransferase inhibitory meroterpenoids from Ganoderma cochlear. Molecules. 2018;23:1797. doi: 10.3390/molecules23071797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y.-B., Li W., Jiang L., Yang L., Chen N.-H., Wu Z.-N., Li Y.-L., Wang G.-C. Cytotoxic and anti-inflammatory active phloroglucinol derivatives from Rhodomyrtus tomentosa. Phytochemistry. 2018;153:111–119. doi: 10.1016/j.phytochem.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y.-L., Zhou X.-W., Wu L., Wang X.-B., Yang M.-H., Luo J., Luo J.-G., Kong L.-Y. Isolation, structure elucidation, and absolute configuration of syncarpic acid-conjugated terpenoids from Rhodomyrtus tomentosa. J. Nat. Prod. 2017;80:989–998. doi: 10.1021/acs.jnatprod.6b01005. [DOI] [PubMed] [Google Scholar]
- 23.Qin X.-J., Yu Q., Yan H., Khan A., Feng M.-Y., Li P.-P., Hao X.-J., An L.-K., Liu H.-Y. Meroterpenoids with antitumor activities from guava (Psidium guajava) J. Agric. Food Chem. 2017;65:4993–4999. doi: 10.1021/acs.jafc.7b01762. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y.-L., Chen C., Wang X.-B., Wu L., Yang M.-H., Luo J., Zhang C., Sun H.-B., Luo J.-G., Kong L.-Y. Rhodomyrtials a and b, two meroterpenoids with a triketone-sesquiterpene-triketone skeleton from Rhodomyrtus tomentosa: Structural elucidation and biomimetic synthesis. Org. Lett. 2016;18:4068–4071. doi: 10.1021/acs.orglett.6b01944. [DOI] [PubMed] [Google Scholar]
- 25.Liu H.-X., Chen K., Tang G.-H., Yuan Y.-F., Tan H.-B., Qiu S.-X. Isolation and biomimetic total synthesis of tomentodiones A-B, terpenoid-conjugated phloroglucinols from the leaves of Rhodomyrtus tomentosa. RSC Adv. 2016;6:48231–48236. doi: 10.1039/C6RA08776K. [DOI] [Google Scholar]
- 26.Li C.-J., Ma J., Sun H., Zhang D., Zhang D.-M. Guajavadimer a, a dimeric caryophyllene-derived meroterpenoid with a new carbon skeleton from the leaves of Psidium guajava. Org. Lett. 2016;18:168–171. doi: 10.1021/acs.orglett.5b03117. [DOI] [PubMed] [Google Scholar]
- 27.Jian Y.-Q., Huang X.-J., Zhang D.-M., Jiang R.-W., Chen M.-F., Zhao B.-X., Wang Y., Ye W.-C. Guapsidial a and guadials b and c: Three new meroterpenoids with unusual skeletons from the leaves of Psidium guajava. Chem. Eur. J. 2015;21:9022–9027. doi: 10.1002/chem.201500533. [DOI] [PubMed] [Google Scholar]
- 28.Yang X.-L., Hsieh K.-L., Liu J.-K. Guajadial: An unusual meroterpenoid from guava leaves Psidium guajava. Org. Lett. 2007;9:5135–5138. doi: 10.1021/ol702537q. [DOI] [PubMed] [Google Scholar]
- 29.Ma S.-J., Yu J., Yan D.-W., Wang D.-C., Gao J.-M., Zhang Q. Meroterpene-like compounds derived from β-caryophyllene as potent α-glucosidase inhibitors. Org. Biomol. Chem. 2018;16:9454–9460. doi: 10.1039/C8OB02687D. [DOI] [PubMed] [Google Scholar]
- 30.Wei J., Zhang X.-Y., Deng S., Cao L., Xue Q.-H., Gao J.-M. α-Glucosidase inhibitors and phytotoxins from Streptomyces xanthophaeus. Nat. Prod. Res. 2017;31:2062–2066. doi: 10.1080/14786419.2016.1269100. [DOI] [PubMed] [Google Scholar]
- 31.Adib M., Peytam F., Rahmanian-Jazi M., Mohammadi-Khanaposhtani M., Mahernia S., Bijanzadeh H.R., Jahani M., Imanparast S., Faramarzi M.A., Mahdavi M., et al. Design, synthesis and in vitro α-glucosidase inhibition of novel coumarin-pyridines as potent antidiabetic agents. New J. Chem. 2018;42:17268–17278. doi: 10.1039/C8NJ02495B. [DOI] [Google Scholar]
- 32.Yin Y., Sha S., Wu X., Wang S.-F., Qiao F., Song Z.-C., Zhu H.-L. Development of novel chromeno[4,3-c]pyrazol-4(2H)-one derivates bearing sulfonylpiperazine as antitumor inhibitors targeting PI3Kα. Eur. J. Med. Chem. 2019;182:111630. doi: 10.1016/j.ejmech.2019.111630. [DOI] [PubMed] [Google Scholar]
- 33.Annunziata R., Raimondi L., Appendino G., Cravotto G., Palmisano G. The chemistry of coumarin derivatives. VI. Structural determination of coumarin derivatives. Diels-Alder adducts using 3-methylene-2,4-chromanedione as trapping agent. Gazz. Chim. Ital. 1995;125:465–477. [Google Scholar]
- 34.Appendino G., Cravotto G., Toma L., Annunziata R., Palmisano G. The chemistry of coumarin derivatives. part vi. diels-alder trapping of 3-methylene-2,4-chromandione. a new entry to substituted pyrano[3,2-c]coumarins. J. Org. Chem. 1994;59:5556–5564. doi: 10.1021/jo00098a013. [DOI] [Google Scholar]
- 35.Lam H.C., Spence J.T.J., George J.H. Biomimetic total synthesis of hyperjapones a–e and hyperjaponols a and c. Angew. Chem. Int. Ed. 2016;55:10368–10371. doi: 10.1002/anie.201606091. [DOI] [PubMed] [Google Scholar]
- 36.Cerra B., Carotti A., Passeri D., Sardella R., Moroni G., Di Michele A., Macchiarulo A., Pellicciari R., Gioiello A. Exploiting chemical toolboxes for the expedited generation of tetracyclic quinolines as a novel class of pxr agonists. ACS Med. Chem. Lett. 2019;10:677–681. doi: 10.1021/acsmedchemlett.8b00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrovic A.G., Navarro-Vazquez A., Alonso-Gomez J.L. From relative to absolute configuration of complex natural products. Interplay between NMR, ECD, VCD, and ORD assisted by ab initio calculations. Curr. Org. Chem. 2010;14:1612–1628. doi: 10.2174/138527210793563215. [DOI] [Google Scholar]
- 38.Oboh G., Isaac A.T., Akinyemi A.J., Ajani R.A. Inhibition of Key Enzymes Linked to Type 2 Diabetes and Sodium Nitroprusside Induced Lipid Peroxidation in Rats’ Pancreas by Phenolic Extracts of Avocado Pear Leaves and Fruit. Int. J. Biomed. Sci. 2014;10:208–216. [PMC free article] [PubMed] [Google Scholar]
- 39.Chiasson J.-L. The efficacy of acarbose in the treatment of patients with non–insulin-dependent diabetes mellitus: A multicenter, controlled clinical trial. Ann. Intern. Med. 1994;121:928–935. doi: 10.7326/0003-4819-121-12-199412150-00004. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X.-Q., Mou X.-F., Mao N., Hao J.-J., Liu M., Zheng J.-Y., Wang C.-Y., Gu Y.-C., Shao C.-L. Design, semisynthesis, α-glucosidase inhibitory, cytotoxic, and antibacterial activities of p-terphenyl derivatives. Eur. J. Med. Chem. 2018;146:232–244. doi: 10.1016/j.ejmech.2018.01.057. [DOI] [PubMed] [Google Scholar]
- 41.Tavani C., Bianchi L., De Palma A., Passeri G.I., Punzi G., Pierri C.L., Lovece A., Cavalluzzi M.M., Franchini C., Lentini G., et al. Nitro-substituted tetrahydroindolizines and homologs: Design, kinetics, and mechanism of α-glucosidase inhibition. Bioorg. Med. Chem. Lett. 2017;27:3980–3986. doi: 10.1016/j.bmcl.2017.07.068. [DOI] [PubMed] [Google Scholar]
- 42.Dan W.-J., Zhang Q., Zhang F., Wang W.-W., Gao J.-M. Benzonate derivatives of acetophenone as potent α-glucosidase inhibitors: Synthesis, structure–activity relationship and mechanism. J. Enzym. Inhib. Med. Chem. 2019;34:937–945. doi: 10.1080/14756366.2019.1604519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding H., Wu X., Pan J., Hu X., Gong D., Zhang G. New Insights into the Inhibition Mechanism of Betulinic Acid on α-Glucosidase. J. Agric. Food Chem. 2018;66:7065–7075. doi: 10.1021/acs.jafc.8b02992. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimizu M., Tajima Y., Matsuzawa F., Aikawa S.-I., Iwamoto K., Kobayashi T., Edmunds T., Fujishima K., Tsuji D., Itoh K., et al. Binding parameters and thermodynamics of the interaction of imino sugars with a recombinant human acid alpha-glucosidase (alglucosidase alfa): Insight into the complex formation mechanism. Clin. Chim. Acta Int. J. Clin. Chem. 2008;391:68–73. doi: 10.1016/j.cca.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q., Tang H.-Y., Chen M., Yu J., Li H., Gao J.-M. Natural product driven diversity via skeletal remodeling of caryophyllene β-lactam. Org. Biomol. Chem. 2017;15:4456–4463. doi: 10.1039/C7OB00741H. [DOI] [PubMed] [Google Scholar]
- 46.Ma S.-J., Yu J., Fan H.-F., Li Z.-H., Zhang A.-L., Zhang Q. Exploring sesquiterpene alkaloid-like scaffolds via Beckmann-transannular remodelling of beta-caryophyllene. RSC Adv. 2017;7:40510–40516. doi: 10.1039/C7RA08196K. [DOI] [Google Scholar]
- 47.CONFLEX. Conflex Corp.; Tokyo-Yokohama, Japan: 2010. [Google Scholar]
- 48.Gotō H., Ōsawa E. An efficient algorithm for searching low-energy conformers of cyclic and acyclic molecules. J. Chem. Soc. Perkin Trans. 1993;2:187–198. doi: 10.1039/P29930000187. [DOI] [Google Scholar]
- 49.Gaussian 09. Gaussian, Inc.; Wallingford, CT, USA: 2013. [Google Scholar]
- 50.Bruhn T., Schaumlöffel A., Hemberger Y., Bringmann G. Specdis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality. 2013;25:243–249. doi: 10.1002/chir.22138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.