Abstract
Empathy is a multidimensional paradigm, and there currently is a lack of scientific consensus in its definition. In this paper, we review the possibility of compromising data during behavioral neuroscience experiments, including but not limited to those who study empathy. The experimental protocols can affect, and be affected by, empathy and related processes at multiple levels. We discuss several points to help researchers develop a successful translational pathway for behavioral research on empathy. Despite varying in their focus with no widely accepted model, current rodent models on empathy have provided sound translational explanations for many neuropsychiatric proof-of-concepts to date. Research has shown that empathy can be influenced by many parameters, some of which are to be reviewed in this paper. We emphasize the future importance of consistency in modeling proof of concept; efforts to create a multidisciplinary group which would include both bench scientists and clinicians with expertise in neuropsychiatry, and the consideration of empathy as an independent variable in animal behavioral experimental designs which is not the mainstream practice at present.
Keywords: Empathy, oxytocin, stress, sex difference, microbiota
INTRODUCTION
Empathy is a multidimensional concept with no mutually agreed upon scientific definition; different researchers may use the term differently. It may refer both to sharing/adopting, and understanding the emotional state of others (1, 2). Nevertheless, mainly accepted practical classification is: cognitive (top-down), and emotional/affective-used interchangeably in this paper-(bottom-up) empathy. Behavioral patterns appear to change from simple observation without a need for explicit cognitive processing (bottom-up; affective) such as emotional contagion, motor mimicry; to cognitive skill-requiring behaviors (top-down; cognitive empathy) such as perspective acquisition and targeted assistance, as the individual becomes evolutionally more complex. First one is mainly an unconscious emergence triggered by mimics and the act of mirroring; while the latter is a conscious process of recognizing the physical and emotional state of the others, and interpreting this knowledge (3, 4). It is important to note that shared processing is required for both forms of empathy (5, 6).
Positive social behavior and helping behaviors have been observed in rodents (7, 8). With empathy-like behaviors a seen in a wide range of populations from mice to elephants, investigations focus more on by which mechanisms empathy occurs, rather than whether or not empathy exists (9). There are various working hypotheses such as perception-action model (PAM), and the self to other model of empathy (SOME), which are important to mention, yet are out of the scope of this paper (10).
Successful relationships are necessary for survival, reproduction, reaching resources, and achieving social status in the group. In this context, empathy and empathy-like behaviors appear evolutionarily protected (1). In mammals, the empathy response is thought to phylogenetically originate from caring for the offspring, a behavior characteristic for mammals, yet observed in other species; such as birds, reptiles, and fish (11–13). From an experimental point of view, empathy has so far been modeled in rodents and primates. Current rodent models on empathy vary in their focus, as will be detailed below, and no widely accepted model exists (14, 15).
THE NEUROBIOLOGY OF EMPATHY
Functional neuroimaging studies have revealed a loop that responds to others’ perception of distressing situations in humans: anterior insula, dorsal anterior cingulate cortex, anterior midcingulate cortex, supplementary motor area, amygdala, brainstem and periaquaductal gray (16). Animal studies have shown that the ability to share and be influenced by others’ emotions are organized by regions including brain stem, the preoptic area of the thalamus, and paralimbic regions; which also play a role in attachment (17, 18). Cognitive and affective empathy are shown to follow mainly different neurocircuitry. Cognitive empathy engages the ventromedial prefrontal cortex, temporoparietal junction and hippocampal regions of the brain called the “memory network” (19, 20) while affective empathy engages inferior parietal lobule and amygdala which is called “the mirror neuron system” (21, 22). Despite this practical classification of empathy; it has been shown that shared processing is required for both forms of empathy (5, 6). The limbic system is particularly important for emotional, while frontoparietal networks, in connection with superior temporal cortex activation, are necessary for motor display. These neurons discharge during both observation and execution of motor action (23). Any experimental plan involving empathy-associated brain regions is likely to be influenced by the empathy behavior that may occur between animals during the experiment.
Despite relative limitation of data in neurochemical underpinnings of empathy, when comparing to that of neuroimaging, studies have been promising. Oxytocin and arginine vasopressin have been shown to play an important role in social signaling of vast evolutionary process from fish to primates (24). Animal studies have demonstrated the role of these two social neuropeptides in emotional behaviors such as parenting and aggression (25). Both of these peptides, which are administered intranasally in humans, have shown to promote attachment, trust, empathy, generosity and positive social engagement (26). The epigenetic effects, such as early parenting experiences, permanently alter oxytocin and vasopressin expression and the neural architecture of empathy (27, 28). Although both peptides have similar neuroendocrine effects; oxytocin has been more studied. Oxytocin receptor gene polymorphism has been found to have a significant relation with emotion-recognition scores (29), the interaction of high plasma oxytocin and low-risk alleles of CD38, an ectoenzyme that mediates the release of oxytocin, predicted longer parental touch, and increased duration of parent-infant gaze synchrony (30). Positive correlation between plasma oxytocin levels, both during and after pregnancy, and mother-infant bonding has been shown (31). Nasal oxytocin administration is found to increase emotion recognition accuracy (32, 33), although controversy exists (34, 35). In a recent study, intranasal oxytocin and vasopressin on parental caregiving were investigated; while no significant caregiving change was observed in correlation with oxytocin, vasopressin caused increased caregiving behavior in men (36). Intranasal vasopressin is shown to increase empathic concern and experience of increased paternal warmth during childhood (28). Paternal behavior related hormone Arginine vasopressin is found to be related with aggression (37) while relation between vasopressin 1B receptor polymorphism and affective empathy is shown (38). Maternal deprivation increases vasopressin receptors, while decreases oxytocin receptors in the brain (27). Serotonergic system’s involvement in affective regulation is well known. Stimulation of 5-HT2A/1A serotonergic receptor reduced social pain processing in association with changes in self-experience, decreased cognitive empathy, but increased emotional empathy (39). These findings suggest that serotonergic system is involved in emotional sharing (40). Evidence suggests that dopaminergic system is also involved in empathic development; D4 receptors have been linked with cognitive empathy and longitudinal development of infant temperament (41, 42). It has also has been shown that oxytocin facilitates mating-induced pair bonds in adults through interaction with the mesolimbic dopaminergic system (43). Furthermore, early caregiving experiences can have an impact on hypothalamic-pituitary-adrenal (HPA) system (44), and it was shown that early maternal deprivation negatively affects HPA system and causes increased anxiety in offsprings later in life (45). Early caregiving reduces corticosterone responses to stress in rats; increasing hippocampal corticosterone sensitivity while decreasing it at the hypothalamic level. These findings are particularly important in view of other study results indicating a correlation between increased cortisol levels being associated with decreased empathy (46). Testosterone has been found to reduce empathy and compassionate behavior both in men (47) and women (48).
Empathy behavior among subjects may affect, and be affected by, empathy-related neurotransmitters.
EXAMPLES OF RODENT MODELS OF EMPATHY
Measuring response to pain or stress as an emotional contagion could be considered as the main theme for first experimental models of empathy in laboratory animals. (49) Initial rodent empathy models focused on ultrasonic vocalizations of rats that are under stress. Ultrasonic vocalizations were considered as a potential method of assessing emotional contagion (50). Audible and ultrasonic vocalizations (USV) of rodents are produced to reflect a variety of emotional states. In 1991, Blanchard et al demonstrated that ultrasonic vocalizations (USV) in 22 kHz were emitted by rats to communicate a presentation of a predator (51) and several other following studies later investigated the relationship between social rat behavior and USV (52–54). In 2011 Atsak et al. proposed a rat empathy model based on USV and freezing response to foot shock (55). One study used consolation test as a rodent empathy model (56). Many other rodent models so far have persistently attempted to measure emotional contagion elicited by painful or fearful stimuli (14). The first studies on this subject, as in humans, are based on the measurement of pain or stress response as an emotional transmission of laboratory animals (57).
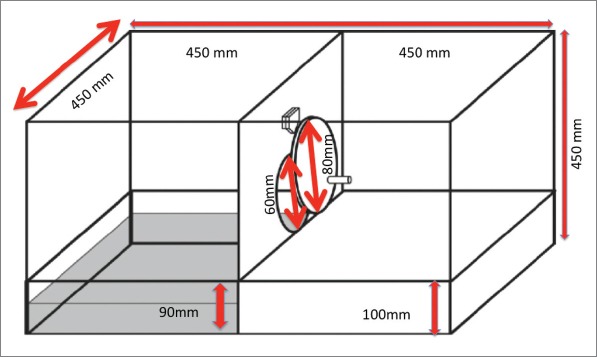
The last step was to measure empathy in rodents through equipment. More recently Sato et al developed the “Helping Behavior Test Equipment” to measure empathy in rodents which is based on the “rescue” of the endangered partner (15). This test protocol consists of two phases; door-opening session and control test. Helper rat learns to open the door between pool area and ground area during door-opening session. This training session continues for 12 days. This follows a three-day period for the control test session. Each experimental session continues for 300 seconds. Before all test period, the rats participating in the experiment should be separated as two rats per cage for 14 days to acclimate cage mate. If the cage has more than two rats, they learn to open the door faster than one. We slightly modified “Helping Behavior Test Equipment” in our experiments by doubling the depth of the rescue (45 mm vs 90 mm) (Fig. 1).
Figure 1.
Lu et al. used an experimental model where rats were assigned to different groups in pairs; pain was induced in one of each while the cage-mate was allowed to witness and interact whose behavior was analyzed (59).
VARIOUS AREAS FOR TRANSLATIONAL CONSIDERATION
Empathy and Stress
Stress is known to be associated with homeostatic imbalance and activation of HPA in both humans and rodents. As a response to stress, hypothalamus secretes corticotropin releasing factor (CRF/CRH), which triggers a release of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland; as a result, glucocorticoids are released by the adrenal cortex into the blood stream. A negative feedback system terminates this cascade by inhibiting CRF production from the hypothalamus. This is an acute response to stress and is considered crucial for homeostatic balance (60). It has been suggested that stress may cause retraction from social interactions, irritability and hostility which also could increase predisposition to antisocial behaviors (61). In a recent study involving male rats, three hours of immobilization has been found to reduce aggression among cage-mates, increase huddling and resource sharing, conversely as a more threatening stressor. Interestingly in the same experiment predator odor stress has disrupted this social bonding (62). Steinbeis et al. found stress-reactive cortisol levels had no effect on trust behavior, higher baseline cortisol was correlated with greater trust (63). In our recent study, low intensity stress improved empathic behavior whereas high intensity stress did not. Our group demonstrated that low intensity stress caused a surge in vasopressin levels in both prefrontal cortex and amygdalae; oxytocin was only found to be increased in the prefrontal cortex (58).
Another study suggested that stress caused false interpretation of faces with averted gaze direction as making eye contact more often than did controls, independent of the expressed emotion. These results suggest that a stress-induced raise in cortisol level increases the sense of being watched (64). Vinkers et al. found that stress was associated with reduced generosity (65). It was suggested that serotonin transporter polymorphism is associated with biological stress reactivity and with lower rates of helping others in humans (66, 67). Several human studies suggested a positive correlation between acute psychological stress and prosocial behavior (68–70).
In an interesting experiment involving both humans and mice demonstrated that blockade of glucocorticoid synthesis or receptors for adrenal stress hormones elicits the expression of emotional contagion in strangers of both species. Authors brought up an evolutionary perspective and speculated that emotional contagion is prevented by the stress of a social interaction with an unfamiliar member of the species, and which can be stimulated by blocking the endocrine stress response (71). Consistent with this results, another study demonstrated that familiarity reduced the stress response in mice and enabled the emotional contagion (57). However, results of one human study found that social exclusion was associated with a reduction in cortisol, and social inclusion with an increase in cortisol (72).
It appears that in a stressful situation, automatic response is enhanced and control mechanisms are suppressed (73). In a recent study, stress was found to effect bottom-up and top-down components of empathy in opposing ways. Whether stress is beneficial or harmful for social interaction and helping behavior, depends on the complexity of the social situation. When only automatic response is necessary, stress may enhance this automatic helping response, however when the social situation is more complex, empathy may be affected negatively (74). It has also been suggested that psychosocial stress has a conflicting impact on two separate subconcepts of empathy. As a response to stress, emotional empathy was enhanced while cognitive empathy did not differ in young healthy men (75). Patients with post-traumatic stress disorder are shown to have decreased emotional and cognitive empathic abilities (76).
It has been reported that stress may have a gender dependent impact on empathy. Tomova et al. (77) investigated the effect of stress on self-other distinction which is thought to be important for perspective taking (higher form of empathy) in humans, and found opposing effects in the two genders; women showed increase in self-other distinction, while men showed decrease. Gonzalez et al. (78) found after psychosocial stress, empathy for pain was higher in both sexes but late event-related potentials in electroencephalography showed sex dependent changes.
Empathy and Pain
Pain has long been considered a common and fundamental concept in assessment of empathy in both mice and rats (14). In humans, bilateral anterior insula (AI), rostral anterior cingulate cortex (ACC), brainstem, and cerebellum were found to be commonly activated both subjects received pain themselves and also when their loved ones experienced pain. Wang et al. conducted a study in humans suggested that more cognitive attentional efforts are required to judge a stranger’s happiness than a friend’s happiness, yet the opposite was found to be true for judging pain of others (16, 79, 80). Mischkowski et al. conducted an experiment, which demonstrated that, a pain reliever; acetaminophen reduced empathic response in undergraduate students (81).
Gender Impact on Empathy
Evidence suggests that there are differences in empathic abilities between men and women and these differences have been found to be consistent across lifespan. Literature has consistently suggested that women have better empathic abilities than men (82). Women are faster in recognizing facial expression, emotional body language, more sensitive to baby voice, more experientially reactive to negative, but not positive, emotional pictures compared to men. Men, on the other hand, seem to show better skills in cognitive empathy while women performed better in emotional empathy (23, 83, 84). While women are more successful in recognizing angry and neutral body language, men have been found to better recognize the happy body language (85). In mice, females were shown to have greater sensitivity to other’s pain compared to males (86). In another mice study, female mice were found to be more likely than male mice to approach cage-mates who were restrained and in pain, compared to an unaffected cage-mate and females did not respond to unfamiliar mice in pain (87). In rats, increased level of activation in the lateral and central amygdala, prelimbic (PL) and infralimbic (IL) parts of the prefrontal cortex in the males who observed emotionally aroused others and such activation in these regions could not be shown in female observers (88). In a recent mice study, empathic fear response was found to be significantly different among different mice strains (89).
Empathy and the Intestinal Microbiota
The intestinal microbiota have been a focus of interest in recent years as it has potentially significant interactions with many hormones and peptides of great importance in several neuropsychiatric disorders. These include, but are not limited to, serotonin, dopamine, norepinephrine, gamma-amino butyric acid (GABA) and oxytocin. Interestingly, Lactobacillus reuteri, a member of microbiota, is known to induce oxytocin production (90, 91). Lactabacillus and Bifidobacterium are known to modulate stress response, whereas presence of Campylobacter jejuni and E. C. oli is linked with anxiety states/disorders (92–94). It has also been shown that gut permeability and microbiota composition are altered; Bacteriodetes and Firmicutes were decreased in patients with major depressive disorder (95, 96).
Recently Desbonnet et al. showed that antibiotic usage caused to significant decrease in oxytocin and vasopressin levels in mice hypothalamus (97). It was suggested that gut microbes interact to regulate these neuropeptides. In addition, the relation between gut microbiota and empathy has been shown in germ free mice, it is found that social interaction and social memory are impaired in germ-free mice (98). Feeding of Lactobacillus reuteri, a member of gut microbiota is cause to increase hypothalamic oxytocin levels (99). Another study has shown stress altered gut microbiota composition correlates positively with oxytocin levels (100). It is suggested that vagal signaling mediate all of these alterations, interrupting the positive effects of Lactobacillus on host plasma and hypothalamic oxytocin (99).
Empathy and Animal Models of Psychiatric Disorders
Oxytocin is considered to be the most important hormone modulator in empathy and has been studied in several psychiatric disorders, including anxiety, depression, post-traumatic stress disorder (PTSD), autism, psychotic disorders, anti-social, narcissistic and borderline personality disorders.
In human studies, anxiety has been shown to enhance helping behavior and empathy (101). Gottschalk and Domschke suggested oxytocin as a biomarker in anxiety spectrum disorders (102). People with PSTD was consistently found to have decreased empathy (103, 104) and regions associated with empathy (amygdala, prefrontal cortex, nucleus accumbens and hippocampus) have been found to have altered activity in PTSD patients (105). Intranasal oxytocin was shown to improve empathy in PTSD patients (106). Ben-Ami Bartal et al. showed that administration of a benzodiazepine (midazolam) impaired helping behavior in rats (107). Benzodiazepines are known to downregulate oxytocin transmission, which is linked to empathy, and reducing anxiety is also linked to deactivating HPA axis and sympathetic system. (108). In another study, a chemosensory stimulus was found to initiate empathy related behavior by activating insula region of the brain (109).
Clinical depression is associated with decreased social awareness and reduced empathic abilities (110). One proposed mechanism is the impaired of HPA axis function and elevated blood cortisol which is associated with depression (111). It is also known that stress induced cortisol surge suppresses oxytocin secretion. Administration of oxytocin was also shown to decreased activity of HPA (112). Oxytocin levels were found to be decreased in depressed women, yet in men it was unchanged (113). Vasopressin, another peptide, has been associated with empathy and lack of vasopressin is also interestingly linked to depression in rodent studies (114, 115).
The plasma oxytocin hormone levels were found to be very low in autism (116). Oxytocin receptor gene polymorphism was found to be associated with autism spectrum disorders (117) and furthermore oxytocin was considered as a potential treatment avenue in autism. Interestingly, oxytocin improved repetitive behaviors (118) and social cognition in autism (119–121).
Schizophrenic patients have been found to have significant deficits in affective empathy (122). It has also been demonstrated that plasma oxytocin levels were found to be decreased in schizophrenic patients (123, 124). Dopamine hypothesis of psychosis is known and dopaminergic receptors exist on oxytocinergic neurons (125). Additionally, oxytocinergic receptors are found in mesocorticodopaminergic area (126, 127).
Anti-social personality disorder (sociopathy) has been most commonly associated with decreased empathic ability (128). This was partially explained by variability in brain morphometry as a factor for psychopaths’ impaired ability to recognize emotional face expressions (129). There has been some evidence linking down-regulated or impaired oxytocin system activity with increased aggression and chronic enhancement of brain oxytocin has been associated with anti-aggressive and pro-social exploratory behavior (130–132). In borderline personality disorder both emotional and cognitive empathy were shown to be reduced (133). It is shown that plasma oxytocin levels reduced in female borderline personality disorder patients (134). In another study, single dose intranasal oxytocin caused to normalize social behaviors via decreased amygdala hypersensitivity (135). Narcissistic personality disorder has also been strongly associated with lack of empathy, which is indeed one of its diagnostic criteria (136, 137).
DISCUSSION AND FUTURE CHALLENGES
There are many animal models for many psychiatric disorders which have significantly helped clinical researchers to understand the disease perspective of these problems. Empathy, in a broader sense, is a construct with promising translational utilization, which may have diagnostic and therapeutic implications for many psychiatric disorders. So a convincing laboratory proof of concept model is needed. It is our belief that concept of empathy is a particularly challenging area to define and research in the laboratory, and we would like to focus on several points to be discussed to help both bench and clinical researchers develop a successful translational pathway.
Empathy refers to an abstract ability has been defined in many different layers, among the most widely accepted is de Waal’s multi-level conceptualization (1, 2), which considers emotional contagion as the central concept that is observed in all non-human animals and considered to have evolutionary continuity. No matter how we attempt to measure empathy, we will be limited by our definitions so it is of crucial importance to define a bench concept. Current rodent literature includes several different models and there are differences in methods and definitions. Consistency will be the key for future success of the translational animal models.
From a psychiatric point of view, it can be argued that most apparent societal burden of empathic impairment is antisocial behavior, which manifests in different forms in psychiatric practice. Having strong biological roots, antisocial personality disorder, also known as sociopathy, may also have the potential to serve as a prototype disease model for a primary empathic deficiency disorder, which can open up new avenues of intervention to build up prosocial helping behavior while decreasing criminal involvement and aggression. Oxytocin has been in the center of focus in pharmacological attempts to improve empathic abilities with conflicting results. Transcranial magnetic stimulation has been also used as a non-pharmacological modality with limited success (138). Oxytocin is affected many different conditions such as stress, drugs, metabolic changes, microbiota changes, psychiatric disorders. Also oxytocin is modulating different physiological processes, including immune-related processes (99).
In this review, we believe we have reported enough evidence for the consideration of empathy as an independent variable in experimental designs, which is obviously disregarded in the mainstream bench models. One would wonder whether this may compromise the experimental processes by mechanisms as simple as harboring of rodents in conditions permissive of social contagion. Not to mention that gender differences in empathy may be further complicating the matter by which gender of the experimental animal to be used in the behavioral test. Particularly vulnerable to empathic component are the experimental stress models.
To conclude, we will emphasize the future importance of consistency in modeling proof of concept; efforts to create a multidisciplinary group which would include both bench scientists and clinicians with expertise in neuropsychiatry; and the consideration of empathy as an individual element in animal experimental designs.
Footnotes
Peer-review: Externally peer-reviewed.
Conflict of Interest: The authors declare no conflict of interest.
Financial Disclosure: No.
REFERENCES
- 1.Preston SD, de Waal FB. Empathy:Its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. discussion –71. [DOI] [PubMed] [Google Scholar]
- 2.de Waal FBM, Preston SD. Mammalian empathy:behavioural manifestations and neural basis. Nat Rev Neurosci. 2017;18:498–509. doi: 10.1038/nrn.2017.72. [DOI] [PubMed] [Google Scholar]
- 3.Keysers C, Fadiga L. The mirror neuron system:new frontiers. Soc Neurosci. 2008;3:193–8. doi: 10.1080/17470910802408513. [DOI] [PubMed] [Google Scholar]
- 4.Prguda E, Neumann DL. Inter-human and animal-directed empathy:a test for evolutionary biases in empathetic responding. Behav Processes. 2014;108:80–86. doi: 10.1016/j.beproc.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Lamm C, Nusbaum HC, Meltzoff AN, Decety J. What are you feeling?Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS One. 2007;2:e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaki J, Ochsner KN. The neuroscience of empathy:progress, pitfalls and promise. Nature Neurosci. 2012;15:675–680. doi: 10.1038/nn.3085. [DOI] [PubMed] [Google Scholar]
- 7.Jeon D, Shin HS. A mouse model for observational fear learning and the empathetic response. Curr Prot Neurosci. 2011;57:8.27.1–8.27.9. doi: 10.1002/0471142301.ns0827s57. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PloS One. 2009;4:e4387. doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sivaselvachandran S, Acland EL, Abdallah S, Martin LJ. Behavioral and mechanistic insight into rodent empathy. Neurosci Biobehav Rev. 2018;91:130–137. doi: 10.1016/j.neubiorev.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Bird G, Viding E. The self to other model of empathy:providing a new framework for understanding empathy impairments in psychopathy, autism, and alexithymia. Neurosci Biobehav Rev. 2014;47:520–532. doi: 10.1016/j.neubiorev.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Clutton-Brock TH. The Evolution of Parental Care. USA: Princeton University Press; 1991. [Google Scholar]
- 12.Cockburn A. Prevalence of different modes of parental care in birds. Proc Biol Sci. 2006;273:1375–1383. doi: 10.1098/rspb.2005.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin NB, Balshine-Earn S, Reynolds JD. Evolutionary transitions in parental care in cichlid fish. Proc Biol Sci. 1998;265:2265–2272. [Google Scholar]
- 14.Meyza KZ, Bartal IB, Monfils MH, Panksepp JB, Knapska E. The roots of empathy:Through the lens of rodent models. Neurosci Biobehav Rev. 2017;76:216–234. doi: 10.1016/j.neubiorev.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato N, Tan L, Tate K, Okada M. Rats demonstrate helping behavior toward a soaked conspecific. Anim Cogn. 2015;18:1039–1047. doi: 10.1007/s10071-015-0872-2. [DOI] [PubMed] [Google Scholar]
- 16.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Panksepp J. Affective Neuroscience:The Foundations of Human and Animal Emotions. New York: Oxford University Press; 1998. [Google Scholar]
- 18.Watt DF. The centrecephalon and thalamocortical integration:neglected contributions of periaqueductal gray. Conscious Emot. 2000;1:91–114. [Google Scholar]
- 19.Schnell K, Bluschke S, Konradt B, Walter H. Functional relations of empathy and mentalizing:an fMRI study on the neural basis of cognitive empathy. Neuroimage. 2011;54:1743–1754. doi: 10.1016/j.neuroimage.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Shamay-Tsoory SG. The neural bases for empathy. The Neuroscientist. 2011;17:18–24. doi: 10.1177/1073858410379268. [DOI] [PubMed] [Google Scholar]
- 21.Cox CL, Uddin LQ, Di Martino A, Castellanos FX, Milham MP, Kelly C. The balance between feeling and knowing:affective and cognitive empathy are reflected in the brain's intrinsic functional dynamics. Soc Cogn Affect Neurosci. 2012;7:727–737. doi: 10.1093/scan/nsr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shamay-Tsoory S, Tomer R, Goldsher D, Berger B, Aharon-Peretz J. Impairment in cognitive and affective empathy in patients with brain lesions:anatomical and cognitive correlates. J Clin Exp Neuropsychol. 2004;26:1113–1127. doi: 10.1080/13803390490515531. [DOI] [PubMed] [Google Scholar]
- 23.Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- 24.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 25.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 26.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain:social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 27.Lukas M, Bredewold R, Neumann ID, Veenema AH. Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats. Neuropharmacology. 2010;58:78–87. doi: 10.1016/j.neuropharm.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Tabak BA, Meyer ML, Castle E, Dutcher JM, Irwin MR, Han JH, Lieberman MD, Eisenberger NI. Vasopressin, but not oxytocin, increases empathic concern among individuals who received higher levels of paternal warmth:A randomized controlled trial. Psychoneuroendocrinology. 2015;51:253–261. doi: 10.1016/j.psyneuen.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melchers M, Montag C, Markett S, Reuter M. Relationship between oxytocin receptor genotype and recognition of facial emotion. Behav Neurosci. 2013;127:780–787. doi: 10.1037/a0033748. [DOI] [PubMed] [Google Scholar]
- 30.Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, Shalev I, Ebstein RP. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol Psychiatry. 2012;72:175–181. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 31.Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation:plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 32.Gamer M, Zurowski B, Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci U S A. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahrestani S, Kemp AH, Guastella AJ. The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans:a meta-analysis. Neuropsychopharmacology. 2013;38:1929–1936. doi: 10.1038/npp.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guastella AJ, Carson DS, Dadds MR, Mitchell PB, Cox RE. Does oxytocin influence the early detection of angry and happy faces?Psychoneuroendocrinology. 2009;34:220–225. doi: 10.1016/j.psyneuen.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Lischke A, Berger C, Prehn K, Heinrichs M, Herpertz SC, Domes G. Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology. 2012;37:475–481. doi: 10.1016/j.psyneuen.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Cohen-Bendahan CC, Beijers R, van Doornen LJ, de Weerth C. Explicit and implicit caregiving interests in expectant fathers:Do endogenous and exogenous oxytocin and vasopressin matter?Infant Behav Dev. 2015;41:26–37. doi: 10.1016/j.infbeh.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Bos PA, Panksepp J, Bluthe RM, van Honk J. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior:a review of single administration studies. Front Neuroendocrinol. 2012;33:17–35. doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Wu N, Shang S, Su Y. The arginine vasopressin V1b receptor gene and prosociality:Mediation role of emotional empathy. Psych J. 2015;4:160–165. doi: 10.1002/pchj.102. [DOI] [PubMed] [Google Scholar]
- 39.Preller KH, Pokorny T, Hock A, Kraehenmann R, Stampfli P, Seifritz E, Scheidegger M, Vollenweider FX. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci U S A. 2016;113:5119–5124. doi: 10.1073/pnas.1524187113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsunaga M, Ishii K, Ohtsubo Y, Noguchi Y, Ochi M, Yamasue H. Association between salivary serotonin and the social sharing of happiness. PLoS One. 2017;12:e0180391. doi: 10.1371/journal.pone.0180391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmboe K, Nemoda Z, Fearon RM, Sasvari-Szekely M, Johnson MH. Dopamine D4 receptor and serotonin transporter gene effects on the longitudinal development of infant temperament. Genes Brain Behav. 2011;10:513–522. doi: 10.1111/j.1601-183X.2010.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uzefovsky F, Shalev I, Israel S, Edelman S, Raz Y, Perach-Barzilay N, Mankuta D, SG Tsoory S, Knafo A, Ebstein RP. The dopamine D4 receptor gene shows a gender-sensitive association with cognitive empathy:evidence from two independent samples. Emotion. 2014;14:712–721. doi: 10.1037/a0036555. [DOI] [PubMed] [Google Scholar]
- 43.Bosch OJ, Young LJ. Oxytocin and Social Relationships:From Attachment to Bond Disruption. Curr Top Behav Neurosci. 2018;35:97–117. doi: 10.1007/7854_2017_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 45.Uysal N, Sisman AR, Dayi A, Aksu I, Cetin F, Gencoglu C, Tas A, Buyuk E. Maternal exercise decreases maternal deprivation induced anxiety of pups and correlates to increased prefrontal cortex BDNF and VEGF. Neurosci Lett. 2011;505:273–278. doi: 10.1016/j.neulet.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 46.Johnson MM, Caron KM, Mikolajewski AJ, Shirtcliff EA, Eckel LA, Taylor J. Psychopathic traits, empathy, and aggression are differentially related to cortisol awakening response. J Psychopathol Behav. 2014;36:380–388. [Google Scholar]
- 47.Durdiaková J, Celec P, Laznibatová J, Minárik G, Lakatošová S, Kubranská A, Ostatníková D. Differences in salivary testosterone, digit ratio and empathy between intellectually gifted and control boys. Intelligence. 2015;48:76–84. [Google Scholar]
- 48.Van Honk J, Schutter DJ, Bos PA, Kruijt A-W, Lentjes EG, Baron-Cohen S. Testosterone administration impairs cognitive empathy in women depending on second-to-fourth digit ratio. Proc Nat Acad Sci. 2011;108:3448–3452. doi: 10.1073/pnas.1011891108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Batson CD. These things called empathy:eight related but distinct phenomena. In: Decety J, Ickes W, editors. The Social Neuroscience of Empathy. Published to MIT Press Scholarship Online; 2013. [Google Scholar]
- 50.Saito Y, Yuki S, Seki Y, Kagawa H, Okanoya K. Cognitive bias in rats evoked by ultrasonic vocalizations suggests emotional contagion. Behav Processes. 2016;132:5–11. doi: 10.1016/j.beproc.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol Behav. 1991;50:967–972. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- 52.Burgdorf J, Panksepp J, Brudzynski SM, Kroes R, Moskal JR. Breeding for 50-kHz positive affective vocalization in rats. Behav Genet. 2005;35:67–72. doi: 10.1007/s10519-004-0856-5. [DOI] [PubMed] [Google Scholar]
- 53.Brudzynski SM, Chiu EM. Behavioural responses of laboratory rats to playback of 22 kHz ultrasonic calls. Physiol Behav. 1995;57:1039–1044. doi: 10.1016/0031-9384(95)00003-2. [DOI] [PubMed] [Google Scholar]
- 54.Panksepp J, Burgdorf J. 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats:effects of social housing and genetic variables. Behav Brain Res. 2000;115:25–38. doi: 10.1016/s0166-4328(00)00238-2. [DOI] [PubMed] [Google Scholar]
- 55.Atsak P, Orre M, Bakker P, Cerliani L, Roozendaal B, Gazzola V, Moita M, Keysers C. Experience modulates vicarious freezing in rats:a model for empathy. PLoS One. 2011;6:e21855. doi: 10.1371/journal.pone.0021855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351:375–378. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez-Liencres C, Juckel G, Tas C, Friebe A, Brune M. Emotional contagion in mice:the role of familiarity. Behav Brain Res. 2014;263:16–21. doi: 10.1016/j.bbr.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 58.Karakilic A, Kizildag S, Kandis S, Guvendi G, Koc B, Camsari GB, Camsari UM, Ates M, Arda SG, Uysal N. The effects of acute foot shock stress on empathy levels in rats. Behav Brain Res. 2018;349:31–36. doi: 10.1016/j.bbr.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 59.Lu YF, Yang Y, Li CL, Wang Y, Li Z, Chen J. The Locus Coeruleus-Norepinephrine System Mediates Empathy for Pain through Selective Up-Regulation of P2X3 Receptor in Dorsal Root Ganglia in Rats. Front Neural Circuits. 2017;11:66. doi: 10.3389/fncir.2017.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bale TL, Vale WW. CRF and CRF receptors:role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 61.Sandi C, Haller J. Stress and the social brain:behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015;16:290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- 62.Muroy SE, Long KL, Kaufer D, Kirby ED. Moderate Stress-Induced Social Bonding and Oxytocin Signaling are Disrupted by Predator Odor in Male Rats. Neuropsychopharmacology. 2016;41:2160–2170. doi: 10.1038/npp.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steinbeis N, Engert V, Linz R, Singer T. The effects of stress and affiliation on social decision-making:Investigating the tend-and-befriend pattern. Psychoneuroendocrinology. 2015;62:138–148. doi: 10.1016/j.psyneuen.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 64.Rimmele U, Lobmaier JS. Stress increases the feeling of being looked at. Psychoneuroendocrinology. 2012;37:292–298. doi: 10.1016/j.psyneuen.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 65.Vinkers CH, Zorn JV, Cornelisse S, Koot S, Houtepen LC, Olivier B, Verster JC, Kahn RS, Boks MPM, Kalenscher T, Joëls M. Time-dependent changes in altruistic punishment following stress. Psychoneuroendocrinology. 2013;38:1467–1475. doi: 10.1016/j.psyneuen.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 66.Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity:a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stoltenberg SF, Christ CC, Carlo G. Afraid to help:Social anxiety partially mediates the association between 5-HTTLPR triallelic genotype and prosocial behavior. Social Neuroscience. 2013;8:400–406. doi: 10.1080/17470919.2013.807874. [DOI] [PubMed] [Google Scholar]
- 68.von Dawans B, Fischbacher U, Kirschbaum C, Fehr E, Heinrichs M. The social dimension of stress reactivity:acute stress increases prosocial behavior in humans. Psychol Sci. 2012;23:651–660. doi: 10.1177/0956797611431576. [DOI] [PubMed] [Google Scholar]
- 69.Buchanan TW, Preston SD. Stress leads to prosocial action in immediate need situations. Front Behav Neurosci. 2014;8:5. doi: 10.3389/fnbeh.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Margittai Z, Strombach T, van Wingerden M, Joels M, Schwabe L, Kalenscher T. A friend in need:Time-dependent effects of stress on social discounting in men. Horm Behav. 2015;73:75–82. doi: 10.1016/j.yhbeh.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 71.Martin LJ, Hathaway G, Isbester K, Mirali S, Acland EL, Niederstrasser N, Slepian PM, Trost Z, Bartz JA, Sapolsky RM, Sternberg WF, Levitin DJ, Mogil JS. Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Curr Biol. 2015;25:326–332. doi: 10.1016/j.cub.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 72.Bass EC, Stednitz SJ, Simonson K, Shen T, Gahtan E. Physiological stress reactivity and empathy following social exclusion:A test of the defensive emotional analgesia hypothesis. Soc Neurosci. 2014;9:504–513. doi: 10.1080/17470919.2014.929533. [DOI] [PubMed] [Google Scholar]
- 73.Phelps EA, Lempert KM, Sokol-Hessner P. Emotion and decision making:multiple modulatory neural circuits. Annu Rev Neurosci. 2014;37:263–287. doi: 10.1146/annurev-neuro-071013-014119. [DOI] [PubMed] [Google Scholar]
- 74.Tomova L, Majdandzic J, Hummer A, Windischberger C, Heinrichs M, Lamm C. Increased neural responses to empathy for pain might explain how acute stress increases prosociality. Soc Cogn Affect Neurosci. 2017;12:401–408. doi: 10.1093/scan/nsw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolf OT, Schulte JM, Drimalla H, Hamacher-Dang TC, Knoch D, Dziobek I. Enhanced emotional empathy after psychosocial stress in young healthy men. Stress. 2015;18:631–637. doi: 10.3109/10253890.2015.1078787. [DOI] [PubMed] [Google Scholar]
- 76.Palgi S, Klein E, Shamay-Tsoory S. The role of oxytocin in empathy in PTSD. Psychol Trauma. 2017;9:70–75. doi: 10.1037/tra0000142. [DOI] [PubMed] [Google Scholar]
- 77.Tomova L, von Dawans B, Heinrichs M, Silani G, Lamm C. Is stress affecting our ability to tune into others?Evidence for gender differences in the effects of stress on self-other distinction. Psychoneuroendocrinology. 2014;43:95–104. doi: 10.1016/j.psyneuen.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 78.Gonzalez-Liencres C, Breidenstein A, Wolf OT, Brüne M. Sex-dependent effects of stress on brain correlates to empathy for pain. Int J Psychophysiol. 2016;105:47–56. doi: 10.1016/j.ijpsycho.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Song J, Guo F, Zhang Z, Yuan S, Cacioppo S. Spatiotemporal Brain Dynamics of Empathy for Pain and Happiness in Friendship. Front Behav Neurosci. 2016;10:45. doi: 10.3389/fnbeh.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 81.Mischkowski D, Crocker J, Way BM. From painkiller to empathy killer:acetaminophen (paracetamol) reduces empathy for pain. Soc Cogn Affect Neurosci. 2016;11:1345–1353. doi: 10.1093/scan/nsw057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baron-Cohen S, Wheelwright S. The empathy quotient:an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. 2004;34:163–175. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- 83.Gard MG, Kring AM. Sex differences in the time course of emotion. Emotion. 2007;7:429–437. doi: 10.1037/1528-3542.7.2.429. [DOI] [PubMed] [Google Scholar]
- 84.Christov-Moore L, Simpson EA, Coude G, Grigaityte K, Iacoboni M, Ferrari PF. Empathy:gender effects in brain and behavior. Neurosci Biobehav Rev. 2014;46:604–627. doi: 10.1016/j.neubiorev.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sokolov AA, Kruger S, Enck P, Krageloh-Mann I, Pavlova MA. Gender affects body language reading. Front Psychol. 2011;2:16. doi: 10.3389/fpsyg.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, Chanda ML, Levitin DJ, Mogil JS. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- 87.Langford DJ, Tuttle AH, Brown K, Deschenes S, Fischer DB, Mutso A, Root KC, Sotocinal SG, Stern MA, Mogil JS, Sternberg WF. Social approach to pain in laboratory mice. Soc Neurosci. 2010;5:163–170. doi: 10.1080/17470910903216609. [DOI] [PubMed] [Google Scholar]
- 88.Mikosz M, Nowak A, Werka T, Knapska E. Sex differences in social modulation of learning in rats. Sci Rep. 2015;5:18114. doi: 10.1038/srep18114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keum S, Park J, Kim A, Park J, Kim KK, Jeong J, Shin HS. Variability in empathic fear response among 11 inbred strains of mice. Genes Brain Behav. 2016;15:231–242. doi: 10.1111/gbb.12278. [DOI] [PubMed] [Google Scholar]
- 90.Ibrahim YM, Kearney SM, Levkovich T, Springer A, Mirabal S, Poutahidis T, Varian BJ, Lakritz JR, Alm EJ, Erdman SE. Maternal gut microbes control offspring sex and survival. J Probiotics Health. 2015;2:120. [Google Scholar]
- 91.Erdman SE, Poutahidis T. Microbes and Oxytocin:Benefits for Host Physiology and Behavior. Int Rev Neurobiol. 2016;131:91–126. doi: 10.1016/bs.irn.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 92.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, Cazaubiel JM. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 93.Goehler LE, Park SM, Opitz N, Lyte M, Gaykema RP. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard:possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav Immun. 2008;22:354–366. doi: 10.1016/j.bbi.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Macedo D, Filho A, Soares de Sousa CN, Quevedo J, Barichello T, Junior HVN, de Lucena DF. Antidepressants, antimicrobials or both?Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J Affect Disord. 2017;208:22–32. doi: 10.1016/j.jad.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 96.Evrensel A, Ceylan ME. The Gut-Brain Axis:The Missing Link in Depression. Clin Psychopharmacol Neurosci. 2015;13:239–244. doi: 10.9758/cpn.2015.13.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Desbonnet L, Clarke G, Traplin A, O'Sullivan O, Crispie F, Moloney RD, Cotter PD, Dinan TG, Cryan JF. Gut microbiota depletion from early adolescence in mice:Implications for brain and behaviour. Brain Behav Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 98.Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19:146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Varian BJ, Poutahidis T, DiBenedictis BT, Levkovich T, Ibrahim Y, Didyk E, Shikhman L, Cheung HK, Hardas A, Ricciardi CE, Kolandaivelu K, Veenema AH, Alm EJ, Erdman SE. Microbial lysate upregulates host oxytocin. Brain Behav Immun. 2017;61:36–49. doi: 10.1016/j.bbi.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Farshim P, Walton G, Chakrabarti B, Givens I, Saddy D, Kitchen I, Swann JR, Bailey A. Maternal Weaning Modulates Emotional Behavior and Regulates the Gut-Brain Axis. Sci Rep. 2016;6:21958. doi: 10.1038/srep21958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shu J, Hassell S, Weber J, Ochsner KN, Mobbs D. The role of empathy in experiencing vicarious anxiety. J Exp Psychol Gen. 2017;146:1164–1188. doi: 10.1037/xge0000335. [DOI] [PubMed] [Google Scholar]
- 102.Gottschalk MG, Domschke K. Oxytocin and Anxiety Disorders. Curr Top Behav Neurosci. 2018;35:467–498. doi: 10.1007/7854_2017_25. [DOI] [PubMed] [Google Scholar]
- 103.Mazza M, Pino MC, Tempesta D, Catalucci A, Masciocchi C, Ferrara M. Neural activity related to emotional and empathic deficits in subjects with post-traumatic stress disorder who survived the L'Aquila (Central Italy) 2009 earthquake. Epidemiol Prev. 2016;40:42–44. doi: 10.19191/EP16.2S1.P042.046. [DOI] [PubMed] [Google Scholar]
- 104.Charuvastra A, Cloitre M. Social bonds and posttraumatic stress disorder. Annu Rev Psychol. 2008;59:301–328. doi: 10.1146/annurev.psych.58.110405.085650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Palgi S, Klein E, Shamay-Tsoory SG. Oxytocin improves compassion toward women among patients with PTSD. Psychoneuroendocrinology. 2016;64:143–149. doi: 10.1016/j.psyneuen.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 107.Ben-Ami Bartal I, Shan H, Molasky NM, Murray TM, Williams JZ, Decety J, Mason P. Anxiolytic Treatment Impairs Helping Behavior in Rats. Front Psychol. 2016;7:850. doi: 10.3389/fpsyg.2016.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yagi K, Onaka T. A benzodiazepine, chlordiazepoxide, blocks vasopressin and oxytocin release after footshocks but not osmotic stimulus in the rat. Neurosci Lett. 1996;203:49–52. doi: 10.1016/0304-3940(95)12262-1. [DOI] [PubMed] [Google Scholar]
- 109.Prehn-Kristensen A, Wiesner C, Bergmann TO, Wolff S, Jansen O, Mehdorn HM, Ferstl R, Pause BM. Induction of empathy by the smell of anxiety. PLoS One. 2009;4:e5987. doi: 10.1371/journal.pone.0005987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kupferberg A, Bicks L, Hasler G. Social functioning in major depressive disorder. Neurosci Biobehav Rev. 2016;69:313–332. doi: 10.1016/j.neubiorev.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 111.Doolin K, Farrell C, Tozzi L, Harkin A, Frodl T, O'Keane V. Diurnal Hypothalamic-Pituitary-Adrenal Axis Measures and Inflammatory Marker Correlates in Major Depressive Disorder. Int J Mol Sci. 2017;18:2226. doi: 10.3390/ijms18102226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cardoso C, Ellenbogen MA, Orlando MA, Bacon SL, Joober R. Intranasal oxytocin attenuates the cortisol response to physical stress:a dose-response study. Psychoneuroendocrinology. 2013;38:399–407. doi: 10.1016/j.psyneuen.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 113.Yuen KW, Garner JP, Carson DS, Keller J, Lembke A, Hyde SA, Kenna HA, Tennakoon L, Schatzberg AF, Parker KJ. Plasma oxytocin concentrations are lower in depressed vs. healthy control women and are independent of cortisol. J Psychiatr Res. 2014;51:30–36. doi: 10.1016/j.jpsychires.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roper J, O'Carroll AM, Young W, 3rd, Lolait S. The vasopressin Avpr1b receptor:molecular and pharmacological studies. Stress. 2011;14:98–115. doi: 10.3109/10253890.2010.512376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ebstein RP, Knafo A, Mankuta D, Chew SH, Lai PS. The contributions of oxytocin and vasopressin pathway genes to human behavior. Horm Behav. 2012;61:359–379. doi: 10.1016/j.yhbeh.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 116.Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Biol Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- 117.LoParo D, Waldman ID. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder:a meta-analysis. Mol Psychiatry. 2015;20:640–646. doi: 10.1038/mp.2014.77. [DOI] [PubMed] [Google Scholar]
- 118.Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- 119.Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 120.Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 121.Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci U S A. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bonfils KA, Lysaker PH, Minor KS, Salyers MP. Empathy in schizophrenia:A meta-analysis of the Interpersonal Reactivity Index. Psychiatry Res. 2017;249:293–303. doi: 10.1016/j.psychres.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 123.Keech B, Crowe S, Hocking DR. Intranasal oxytocin, social cognition and neurodevelopmental disorders:A meta-analysis. Psychoneuroendocrinology. 2017;87:9–19. doi: 10.1016/j.psyneuen.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 124.Keri S, Kiss I, Kelemen O. Sharing secrets:oxytocin and trust in schizophrenia. Soc Neurosci. 2009;4:287–293. doi: 10.1080/17470910802319710. [DOI] [PubMed] [Google Scholar]
- 125.Baskerville TA, Allard J, Wayman C, Douglas AJ. Dopamine-oxytocin interactions in penile erection. Eur J Neurosci. 2009;30:2151–2164. doi: 10.1111/j.1460-9568.2009.06999.x. [DOI] [PubMed] [Google Scholar]
- 126.Feifel D, Reza T. Oxytocin modulates psychotomimetic-induced deficits in sensorimotor gating. Psychopharmacology (Berl) 1999;141:93–98. doi: 10.1007/s002130050811. [DOI] [PubMed] [Google Scholar]
- 127.Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68:678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 128.Warrier V, Grasby KL, zefovsky F, Toro R, Smith P, Chakrabarti B, Khadake J, Mawbey-Adamson E, Litterman N9, Hottenga JJ1, Lubke G, Boomsma DI, Martin NG, Hatemi PK, Medland SE, Hinds DA, Bourgeron T, Baron-Cohen S. Genome-wide meta-analysis of cognitive empathy:heritability, and correlates with sex, neuropsychiatric conditions and cognition. Mol Psychiatry. 2018;23:1402–1409. doi: 10.1038/mp.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pera-Guardiola V, Contreras-Rodriguez O, Batalla I, Kosson D, Menchon JM, Pifarre J, Bosque J, Cardoner N, Mas CS. Brain Structural Correlates of Emotion Recognition in Psychopaths. PLoS One. 2016;11:e0149807. doi: 10.1371/journal.pone.0149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.de Jong TR, Neumann ID. Oxytocin and Aggression. Curr Top Behav Neurosci. 2018;35:175–192. doi: 10.1007/7854_2017_13. [DOI] [PubMed] [Google Scholar]
- 131.Alcorn JL, 3rd, Rathnayaka N, Swann AC, Moeller FG, Lane SD. Effects of Intranasal Oxytocin on Aggressive Responding in Antisocial Personality Disorder. Psychol Rec. 2015;65:691–703. doi: 10.1007/s40732-015-0139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Calcagnoli F, Meyer N, de Boer SF, Althaus M, Koolhaas JM. Chronic enhancement of brain oxytocin levels causes enduring anti-aggressive and pro-social explorative behavioral effects in male rats. Horm Behav. 2014;65:427–433. doi: 10.1016/j.yhbeh.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 133.Roepke S, Vater A, Preissler S, Heekeren HR, Dziobek I. Social cognition in borderline personality disorder. Front Neurosci. 2012;6:195. doi: 10.3389/fnins.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bertsch K, Schmidinger I, Neumann ID, Herpertz SC. Reduced plasma oxytocin levels in female patients with borderline personality disorder. Horm Behav. 2013;63:424–429. doi: 10.1016/j.yhbeh.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 135.Bertsch K, Gamer M, Schmidt B, Schmidinger I, Walther S, Kastel T, Schnell K, Buchel C, Domes G, Herpertz SC. Oxytocin and reduction of social threat hypersensitivity in women with borderline personality disorder. Am J Psychiatry. 2013;170:1169–1177. doi: 10.1176/appi.ajp.2013.13020263. [DOI] [PubMed] [Google Scholar]
- 136.Baskin-Sommers A, Krusemark E, Ronningstam E. Empathy in narcissistic personality disorder:from clinical and empirical perspectives. Personal Disord. 2014;5:323–333. doi: 10.1037/per0000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lehmann V, Huis in't Veld EM, Vingerhoets AJ. The human and animal baby schema effect:correlates of individual differences. Behav Processes. 2013;94:99–108. doi: 10.1016/j.beproc.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 138.Yang CC, Khalifa N, Vollm B. The effects of repetitive transcranial magnetic stimulation on empathy:a systematic review and meta-analysis. Psychol Med. 2017;48:737–750. doi: 10.1017/S003329171700232X. [DOI] [PubMed] [Google Scholar]


