Abstract
Background
Shigella continues to be important causes of acute pediatric diarrhea worldwide. Shigella produces numerous virulence factors involved in colonization and invasion into epithelial cells which eventually result in the disease. The present study was conducted to evaluate the prevalence of virulence genes and to investigate antibiotic resistance profiles among Shigella isolates obtained from pediatric patients in Iran.
Methods
A total of 141 Shigella isolates were collected between March 2017 and September 2018 from stool of children under 14 who were suspected to have shigellosis. Shigella isolates were identified using standard microbiological and serological tests and antimicrobial susceptibility testing was carried out via Kirby–Bauer disk diffusion method. In addition, the presence of seven virulence determinants including ipaH, ipaB, ipaC, ipaD, ipgD, sen, and virA were evaluated using PCR.
Results
S. sonnei (78.7%) was the most prevalent shigella spp. among children with shigellosis followed by S. flexneri (19.9%) and S. boydii (1.4%). Antimicrobial susceptibility testing revealed that most of the isolates were considered as multidrug-resistant (MDR) strains. Our findings also showed a high resistance rate against trimethoprim-sulfamethoxazole in Shigella isolates. The prevalence of ipaH, ipaC, sen, ipaD, virA, ipaB, and ipgD were 100%, 95.7%, 95.7%, 94.3%, 93.6%, 92.9%, and 80.8%, respectively.
Conclusion
The current study revealed that S. sonnei was the predominant species isolated from children with shigellosis in Iran. Our results also indicated a high distribution of type III secretion system effector protein-encoding genes and high multidrug-resistance among shigella spp. in Iran. Therefore, it is suggested that antimicrobial susceptibility testing be performed prior to antibiotic prescription.
Keywords: Shigella, virulence factors, type III secretion system, ipaH, ipaB, virA
Introduction
Shigellosis is an infectious disease ranging from mild diarrhea to severe dysentery. This infection has long been considered as a public health issue predominantly in developing countries due to overcrowding and poor sanitation.1 According to the World Health Organization (WHO), approximately 191 million shigellosis cases were reported in 2010 worldwide.2 As a matter of fact, infants and young children suffering from shigellosis have a more severe illness and a greater risk of death. Accordingly, it is estimated that approximately 28,000 deaths occur annually among children under five due to this acute bacterial infection.3 Moreover, Rogawski et al recently reported that subclinical and non-diarrhoeal infection with Shigella has a substantial negative association with linear growth, which was sustained during the first two years of life in two-year-old children.4 The major environmental factors contributing to shigellosis outbreaks are low hygienic and poor conditions, deficiency of drinking water, inadequate medical care, and malnutrition in the undeveloped regions.5
Shigellosis is caused by the members of shigella species including Shigella dysenteriae, Shigella flexneri, Shigella sonnei, and Shigella boydii.6 S. dysenteriae and S. flexneri are principally responsible for epidemic and endemic shigellosis, respectively, with high transmission rates and significant mortality rates in low-income countries whereas S. sonnei and S. boydii generally cause a relatively mild self-limiting watery diarrhea.7 The ability of shigella spp. to resist against high-level stomach acid and to produce several key virulence traits help the bacteria to survive in gastrointestinal tract, which makes it highly infectious with only 10–100 viable bacterial cells necessary to cause disease.8 The predominant shigella strains producing shigellosis vary in different geographical locations. Accordingly, S. flexneri is isolated most frequently in resource-poor countries, while S. dysenteriae was traditionally responsible for large epidemics, and yet is now rarely identified. S. boydii frequently reported from India, and S. sonnei, which has historically been more frequently isolated in developed countries, are undergoing a significant and rapid expansion across developing regions in Latin America, Asia, and the Middle East.7
Species of the genus Shigella could express numerous virulence factors that contribute to the pathobiology of shigellosis. The ipa and mxi-spa loci code various mediators and virulence factors that play significant roles to facilitate cell invasion, intracellular diffusion, and cell death in the bowel epithelium.7,9 The Ipa proteins have been demonstrated in the development of shigellosis and are acknowledged with respect to their associations with each other, their extracellular secretion, and target eukaryotic cells and their components.3 The components of Ipa complex, e.g. IpaA, IpaB, IpaC, and IpaD, are required in the invasion process, which are injected upon contact with host cells using a type III secretion apparatus.7
Oral rehydration and antimicrobial therapy are recommended treatments for this illness; however, recent reports have determined that the rate of antimicrobial resistance for Shigella spp. is increasing.10,11 The growing emergence of resistant clones of Shigella against antimicrobial agents used in clinical practice, including ciprofloxacin, azithromycin, and ceftriaxone, raises serious concerns for antimicrobial therapy of shigellosis.12
To the best of our knowledge, limited numbers of studies have been conducted to investigate antimicrobial resistance and virulence profiles among Shigella isolates from infected children in Iran. Therefore, the aim of the current study was to evaluate antibiotic resistance profiles and virulence-associated genes of shigella spp. isolated from children with shigellosis referred to Children’s Medical Center in Tehran, Iran.
Materials and Methods
Bacterial Isolation and Serotyping
Diarrheal stool or rectal samples were obtained from children under 14 suspected to have shigellosis referred to Children’s Medical Center in Tehran during 2018 and 2019. The samples were inoculated into MacConkey (MAC) (Mast, Bootle, UK) and xylose lysine deoxycholate media (XLD) (Mast, Bootle, UK) and then the media was incubated for 18 to 24 h at 37°C. Conventional biochemical tests, including triple sugar iron agar (TSI), ornithine decarboxylase (ODC), and O-Nitrophenyl-β-D-galactopyranoside (ONPG) were run for suspected colonies to identify Shigella isolates. In order to determine the Shigella serogroups, latex agglutination serotyping was carried out using the Shigella antisera purchased from Statens Serum Institut (Statens Serum Institut, Denmark) according to the manufacturer’s protocol.
The Shigella isolates were kept in Trypticase Soy Broth (TSB) (Fluka, USA) supplemented with 15% glycerol and stored at −70°C for further experiments.
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility pattern of all Shigella isolates was determined using Kirby-Bauer disk diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) 2018 recommendations.13 We selected the antimicrobial agents based on CLSI 2018 and prescription of physicians in Iran. The antimicrobial agents were as follows: ampicillin (AMP), azithromycin (AZT), ciprofloxacin (CIP), nalidixic acid (NA), Trimethoprim-sulfamethoxazole (SXT), cefixime (CFM), cefotaxime (CTX), minocycline (MN), and levofloxacin (LEV) (Mast, Bootle, UK). In addition, the minimum inhibitory concentration (MIC) for the two most important antibiotics, including CIP and AZT was determined using microdilution broth method. S. flexneri ATCC 12022 was used as a quality control in the antimicrobial susceptibility testing. In the present study, the multidrug-resistant (MDR) isolates were considered as those resistant to at least one member of three different classes of antibiotics.
Molecular Assay for Virulence Genes
Total genomic DNA was extracted using High Pure Isolation Kit (Roche, Mannheim, Germany) according to the manufacturer’s instruction. The quality and quantity of extracted DNA was assessed via NanoDrup (Denovix, Wilmington, USA)
The presence of the virulence genes encoding T3SS effector proteins, including ipaH, ipaB, ipaC, ipaD, ipgD, sen, and virA, was investigated using polymerase chain reaction (PCR) using specific primers (Table S1 and Figures S1–S7). Each PCR reaction was performed in a total volume of 25 μL containing 12.5 μL of 2X master mix (Genet bio, Korea), 1μL (10 pM/µL) of each primer, 8.5 μL of distilled water, and 2 μL of DNA (10 ng) template. The cycling programs were preceded by 5 min at 95° C and consisted of 35 cycles of 94° C for 1 min, 1 min annealing at specific temperature for each primer set (Table S1), and 72° C for 1 min, followed by a final extension step at 72° C for 5 min. PCR amplicons were separated using 1.2% agarose gel and visualized by staining with safe stain (CinnaGen, Iran). S. flexneri ATCC 12022 was used as positive control.
Statistical Analysis
For data analysis, SPSS (version 20.0) was used (SPSS Inc., Chicago, IL, USA). The correlation between virulence-associated genes and antibiotic-resistant profiles was assessed running Chi-square exact test. P-values<0.05 were considered as statistically significant.
Results
Shigella Isolation and Speciation
In the present study, 59.6% (n=84) of the subjects were male and 40.4% (n=57) were female, ranging from 2 months to 14 years of age with the majority of the pediatric patients (56%, n=79) being under 5 years old. During an 18-month period, 141 isolates were identified as Shigella spp., revealing S. sonnei as the most prevalent species (78.7%, n=111), followed by S. flexneri (19.9%, n=28) and S. boydii (1.4%, n= 2). In the current study, no isolate was identified as S. dysenteriae.
Antimicrobial Resistance Profiles of Shigella spp
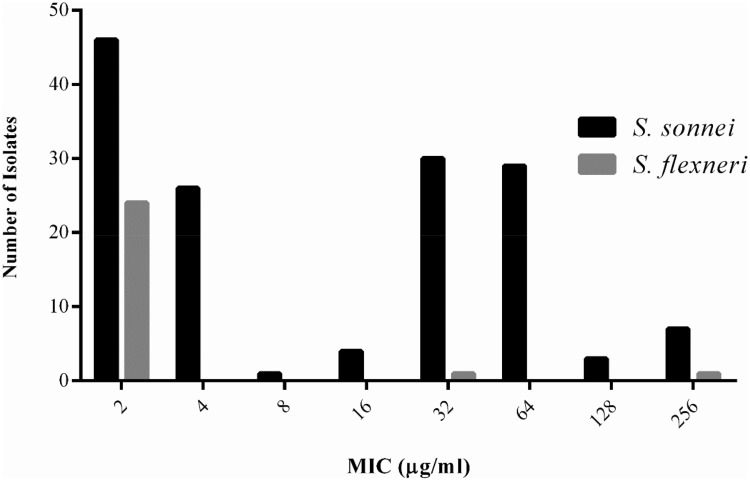
The majority (99.29%) of isolates exhibited the phenotypic resistance to at least one tested drug. A total of 101 (71.6%) shigella isolates were considered as MDR strains, including 86 (77.5%) out of 111 S. sonnei, 13 (46.4%) out of 28 S. flexneri, and 2 out of 2 S. boydii (100%). S. sonnei strains indicated a significantly higher prevalence of MDR compared with S. flexneri species (χ2, P < 0.05). The highest rate of resistance was against SXT (98.5%, 139/141) followed by NA (82.3%, 116/141), AMP (73.0%, 103/141), MN (65.9%, 93/141), CTX (59.6%, 84/141), CFM (57.4%, 81/141), and AZT (47.5%, 67/141) (Table 1). The lowest rate of resistance was observed for LEV (11.3%, 16/141) and CIP (5.7%, 8/141). The antibiotic-resistant profiles are shown in Table 2. The MIC50 of AZT and CIP for S. sonnei were 32 µg/mL and 2 µg/mL, and MIC90 of AZT and CIP for S. sonnei were 64 µg/mL and 2 µg/mL, respectively. In addition, MIC50 and MIC90 of AZT and CIP for S. flexneri were 2 µg/mL. Figure 1 displays the MIC distribution of AZT against clinical isolates of Shigella recovered from pediatric patients. Moreover, S. sonnei isolates showed a significantly higher resistance to various antibiotics, including CTX, NA, CFM, MN, CIP and AZT, compared with that observed for the S. flexneri isolates (χ2 analysis, P < 0.05).
Table 1.
Antimicrobial Resistance of Shigella Isolates from Pediatric Patients in Iran
| Antibiotics | S. sonnei No. (%), n = 111 | S. flexneri No. (%), n = 28 | p-value | S. boydii No. (%), n = 2 | Total of Shigella Strains No. (%), n =141 |
|---|---|---|---|---|---|
| CTX | 70 (63.1) | 12 (42.8) | 0.004 | 2 (100.0) | 84 (59.6) |
| NA | 104 (93.7) | 10 (35.7) | 0.00001 | 2 (100.0) | 116 (82.3) |
| AMP | 74 (66.7) | 27 (96.4) | 0.00001 | 2 (100.0) | 103 (73.0) |
| SXT | 111 (100.0) | 26 (92.8) | 0.03 | 2 (100.0) | 139 (98.5) |
| LEV | 12 (10.8) | 4 (14.3) | 0.521 | 0 (00.0) | 16 (11.3) |
| CFM | 69 (62.1) | 13 (46.4) | 0.023 | 2 (100.0) | 84 (59.6) |
| MN | 86 (77.5) | 7 (25) | 0.00001 | 0 (00.0) | 93 (65.9) |
| CIP | 3 (2.7) | 5 (17.8) | 0.0005 | 0 (00.0) | 8 (5.7) |
| AZT | 65 (58.6) | 2 (7.1) | 0.00001 | 0 (00.0) | 67 (47.5) |
| MDR | 86 (77.5) | 13 (46.4) | 0.00001 | 2 (100.0) | 101 (71.6) |
Note: Statistically significant values (P<0.05) are presented in bold.
Abbreviations: AMP, ampicillin; AZT, azithromycin; CFM, cefixime; CIP, ciprofloxacin; CTX, cefotaxime; LEV, levofloxacin; NA, nalidixic acid; MDR, multidrug resistance; MN, minocycline; SXT, trimethoprim-sulfamethoxazole.
Table 2.
Antibiotic Resistance Profiles of Shigella spp. from Pediatric Diarrhetic Patients
| Antibiotics Profiles | Age | Total No. | |||||
|---|---|---|---|---|---|---|---|
| 2 Months to 5 Years | 6 to14 Years | ||||||
| S. sonnei No. (%) | S. flexneri No. (%) | S. boydii No. (%) | S. sonnei No. (%) | S. flexneri No. (%) | S. boydii No. (%) | ||
| CIP/AZT/CFM/LEV/MN/CTX/NA/AM/SXT | 4 | _ | _ | 5 | _ | _ | 9 |
| AZT/CFM/CTX/MN/NA/AM/SXT/LEV | 20 | _ | _ | 21 | _ | _ | 41 |
| AZT/CFM/MN/CTX/NA/AM/SXT | _ | _ | _ | 3 | _ | _ | 3 |
| SXT/CTX/NA/MN/AM/ | 2 | 5 | 1 | _ | 1 | _ | 9 |
| SXT/MN/NA | 17 | _ | _ | 7 | _ | _ | 24 |
Abbreviations: AMP, ampicillin; AZT, azithromycin; CFM, cefixime; CIP, ciprofloxacin; CTX, cefotaxime; LEV, levofloxacin; NA, nalidixic acid; MDR, multidrug resistance; MN, minocycline; SXT, trimethoprim-sulfamethoxazole.
Figure 1.
The minimum inhibitory concentration (MIC) of azithromycin for clinical isolates of Shigella recovered from pediatric patients in Tehran, Iran.
Virulence Genes Profiles
The ipaH was detected as the most frequent virulence factor, which was found in all Shigella strains, while the ipgD was considered as the less common virulence determinant detected in 114 (80.8%) strains (Table 3). Overall, 13 virulence profiles were detected in the present study. A total of 113 isolates harbored all seven virulence genes whereas 13 isolates were shown to possess all virulence genes except for ipgD. Moreover, seven unique profiles were discovered in seven samples. Additionally, 130 strains were identified to harbor at least 5 virulence genes. The virulence profiles of the isolated Shigella are shown in Table 4.
Table 3.
Distribution of Virulence Factor Genes in 141 Shigella spp. Isolated from Pediatric Patients
| Virulence Genes | S. sonnei No. (%), n = 111 | S. flexneri No. (%), n = 28 | S. boydii No. (%), n = 2 | Total of Shigella Strains No. (%), n = 141 |
|---|---|---|---|---|
| ipaH | 111 (100.0) | 28 (100.0) | 2 (100.0) | 141 (100.0) |
| ipaB | 101 (90.1) | 28 (100.0) | 2 (100.0) | 131 (92.9) |
| ipaC | 105 (94.6) | 28 (100.0) | 2 (100.0) | 135 (95.7) |
| ipaD | 103 (92.8) | 28 (100.0) | 2 (100.0) | 133 (94.3) |
| ipgD | 88 (79.3) | 24 (85.7) | 2 (100.0) | 114 (80.8) |
| Sen | 107 (96.4) | 26 (92.8) | 2 (100.0) | 135 (95.7) |
| virA | 103 (92.8) | 27 (96.4) | 2 (100.0) | 132 (93.6) |
Table 4.
Virulence Determinant Profiles of the Shigella spp. Isolated from Pediatric Patients
| Shigella spp. | ipaH | ipaB | ipaC | ipaD | ipgD | Sen | virA | No. (%) |
|---|---|---|---|---|---|---|---|---|
| S. sonnei, n = 111 | + | + | + | + | + | + | + | 86 (77.5) |
| + | + | + | + | – | + | + | 12 (10.8) | |
| + | + | – | + | – | + | + | 2 (1.8) | |
| + | – | + | – | – | + | – | 3 (2.7) | |
| + | – | – | – | – | + | + | 2 (1.8) | |
| + | – | – | + | – | – | – | 2 (1.8) | |
| + | + | + | – | + | – | – | 1 (0.9) | |
| + | – | + | + | + | + | + | 1 (0.9) | |
| + | – | + | – | – | + | + | 1 (0.9) | |
| + | – | + | – | – | – | – | 1 (0.9) | |
| S. flexneri, n = 28 | + | + | + | + | + | + | + | 24 (85.7) |
| + | – | + | + | – | + | + | 1 (86.0) | |
| + | + | + | + | – | – | + | 1 (3.5) | |
| + | + | + | + | – | + | + | 1 (3.5) | |
| + | + | + | – | – | – | + | 1 (3.5) | |
| S. boydii, n = 2 | + | + | + | + | + | + | + | 2 (100.0) |
Our experiments showed that five different virulence profiles were present among 28 S. flexneri (Table 4). In addition, two S. boydii isolates possessed all seven virulence genes.
Discussion
Due to the limited information available about antibiotic-resistant and virulence factor profiles of Shigella in Iran, the present epidemiological study was conducted to characterize antimicrobial susceptibility and virulence-associated genes of shigella isolates from pediatric patients in Tehran, Iran. In the current study, the serotyping findings indicated that S. sonnei was the most predominant species, which is consistent with the findings reported by Yaghoubi et al14 in Tehran, Iran. However, Moosavian et al in Ahvaz15 and Hosseini et al in Kerman16 previously reported that S. flexneri was the most common species. These variations could be due to the differences between these studies in terms of geographical and socio-economic conditions as well as the groups of individuals studied.
Our study revealed that 15% of Shigella isolates were non-susceptible to all of the tested antibiotics. A majority of S. flexneri (92.8%) and all of S. sonnei were resistant to SXT, a finding which was similar to those reported by other groups in Iran15,17,18 and in other countries19,20 and opposite to that reported by Moosavian et al in Iran.15 Moreover, in the current study S. flexneri showed high resistance to AMP that is consistent with those previously reported by Iranian authors.16,21,22 Moreover, a high-level resistance was observed against AMP, NA, MN, CTX, CFM, and AZT among Shigella isolates. Until a few years ago, AMP and SXT were the first choice for treatment of shigellosis and were used to improve the dysenteric syndrome; however, the prescription of these drugs is currently not suggested anymore due to development of resistant strains.23
Furthermore, in the current study, medium-level resistance was observed against AZT, that is consistent with that found by Barak et al in Ardebil, Iran18 but different from some reports in other studies.20,24 In our study, the high level of antibiotic susceptibility of shigella spp. to CIP is in agreement with those reported in some studies in Iran and other countries.22,25,26 In shigellosis, depending on the severity of symptoms, the medical specialist may prescribe antibiotics to deal with it. CIP is a preferred treatment for adults, but because of potential toxicity for children below 18 years of age, it is relatively unsafe to use for this age group. Accordingly, AZT is currently the mainstay antibiotic for the treatment of shigellosis in children in Iran;24 however, in the present study, approximately half of shigella spp., especially S. sonnei, were found to be resistant to AZT. According to the WHO, AZT is recommended as a second-line treatment in patients who have severe cases of shigellosis.27
Unfortunately, high rates of MDR profiles were observed in the current study with 88 (62.4%) isolates resistant to at least four antibiotics. The misuse and unreasonable administration of antibiotics could induce the development of antibiotic resistance.28 To reduce the emergence of resistance, antibiotics should be avoided for treating Shigella infections, except in patients with severe shigellosis or cases at risk for systemic infection.29 In our study, nine (6.3%) S. sonnei were non-susceptible to all of the tested antibiotics. Our analysis also showed that the multidrug resistance was more frequent among S. sonnei as compared to S. flexneri. Several studies have described that the antimicrobial resistance levels are different between Shigella spp.19,30 Thus, healthcare providers are suggested to order susceptibility testing so as to determine which antibiotics are preferred rather than pursuing the empirical treatment.31
Each Shigella species possess a large virulence plasmid and single circular chromosome. There are several lines of evidence indicating that type III secretion system (T3SS) as well as ipa and ipg genes are necessary for invasion to epithelial cells and development of shigellosis. These factors are encoded in the ipa-mxi-spa region by the virulence plasmid.7 The IpaH, which encodes invasion plasmid antigen H (IpaH), is commonly used for molecular identification of Shigella spp. using PCR assays. There are 4 to 10 copies of ipaH in the chromosome of the bacterium as well as in invasion plasmids. In our study, all isolates harbored ipaH, which was described previously in other studies.6,32,33 ipaB, ipaC, and ipaD are essential virulence factors to develop shigellosis, because they are necessary for invasion and intracellular survival. Furthermore, they regulate secretion and translocation of some other effector proteins and play a principle role in the intracellular actin polymerization and depolymerization.32 Most of Shigella isolates in the current study possessed ipaB, ipaC, and ipaD and these genes were positive in all S. flexneri and S. boydii isolates. Another T3SS effector is invasion plasmid gene D (ipgD) that stops T cell migration during infection and then blocks the release of ATP to reduce inflammation. It seems that ipgD plays an important role in evading the immune system.34 Our study also showed that ipgD was detected at the lowest rate among clinical isolates of Shigella spp., which was more frequent in S. flexneri than in S. sonnei isolates. This finding is in accordance with the results reported by Lluque et al.32 The sen gene encodes enterotoxin 2 (ShET2) and is located in a 140 MDa plasmid in Shigella strains. It has long been described that ShET2 is responsible for water and electrolyte fluxes in early watery phase of shigellosis in intestine. Therefore, the symptoms of dehydration are closely associated with ShET2 activity.35 In the study conducted by Yaghoubi et al, it was reported that only a few clinical isolates of S. sonnei possess ShET2, which is in contrast to our finding.14 The virA in shigella spp. is located in the virulence plasmid and is involved in intracellular spreading and invasion of bacteria, and is responsible for the formation of entry region.36 In many previous studies, it was reported that all Shigella isolates harbored virA14–37 and, correspondingly, a high rate of virA was detected in our samples.
In addition, our results revealed that 130 (92.2%) cases had at least 5 T3SS effector protein-encoding genes, which suggests the ability of these Shigella isolates to induce inflammatory statement and diarrhea. However, it should be pointed out that the pathogenicity of Shigella spp. is also associated with the immunity of patients and the number of infected bacteria.38
Our study has also some limitations that should be taken into consideration prior to any generalization, including requirement of validation by appropriate techniques addressing virulence factor expression in clinical isolates of Shigella, which can provide a deeper insight into the actual function of the genes.
In the current study, we provided some information about the prevalence of Shigella spp. and also the distribution of some relevant virulence genes among pediatric patients in Tehran, Iran. Our results showed that S. sonnei was the predominant species among children. Most of the Shigella isolates have all of the seven T3SS effector genes. Among the tested antibiotics, CIP and LEV were found to be the most effective for both S. sonnei and S. flexneri; however, CIP is contraindicated in young children due to its adverse effects. Consequently, AZT is still recommended as a good choice for the treatment of S. flexneri severe pediatric shigellosis in Iran. Accordingly, accurate identification of Shigella species and antimicrobial susceptibility testing are recommended to avoid empirical treatment.
Acknowledgments
We express special thanks to members of the Department of Microbiology and Department of Immunology of Shahid Beheshti University of Medical Sciences, Tehran, Iran. This manuscript is based on the PhD dissertation of Mohammadmahdi Karimi-Yazdi submitted at Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Funding Statement
This scientific research was granted by Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant number: 14809).
Abbreviations
ipa, invasion plasmid antigen; ShET-2, Shigella enterotoxin-2; ipgD, invasion plasmid gene D; MDR, multidrug-resistant; MIC, minimum inhibitory concentration; PCR, polymerase chain reaction; T3SS, Type 3 secretion system.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee at the School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Informed consent was obtained from all enrolled patients from parents and/or legal guardians.
Data Sharing Statement
The datasets analyzed during this study are available from the corresponding author on reasonable request.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Navaneethan U, Giannella RA. Mechanisms of infectious diarrhea. Nat Clin Pract Gastroenterol Hepatol. 2008;5:637–647. doi: 10.1038/ncpgasthep1264 [DOI] [PubMed] [Google Scholar]
- 2.Organization WH. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. World Health Organization; 2015. [Google Scholar]
- 3.Mani S, Wierzba T, Walker RI. Status of vaccine research and development for Shigella. Vaccine. 2016;34:2887–2894. doi: 10.1016/j.vaccine.2016.02.075 [DOI] [PubMed] [Google Scholar]
- 4.Rogawski ET, Liu J, Platts-Mills JA, et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health. 2018;6:e1319–e1328. doi: 10.1016/S2214-109X(18)30351-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nygren BL, Schilling KA, Blanton EM, Silk BJ, Cole DJ, Mintz ED. Foodborne outbreaks of shigellosis in the USA, 1998–2008. Epidemiol Infect. 2013;141:233–241. doi: 10.1017/S0950268812000222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan W, Qian H, Shang W, et al. Low distribution of genes encoding virulence factors in Shigella flexneri serotypes 1b clinical isolates from eastern Chinese populations. Gut Pathog. 2017;9:76. doi: 10.1186/s13099-017-0222-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattock E, Blocker AJ. How do the virulence factors of Shigella work together to cause disease? Front Cell Infect Microbiol. 2017;7:64. doi: 10.3389/fcimb.2017.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lima IF, Havt A, Lima AA. Update on molecular epidemiology of Shigella infection. Curr Opin Gastroenterol. 2015;31:30–37. doi: 10.1097/MOG.0000000000000136 [DOI] [PubMed] [Google Scholar]
- 9.Menard R, Prevost MC, Gounon P, Sansonetti P, Dehio C. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc Natl Acad Sci U S A. 1996;93:1254–1258. doi: 10.1073/pnas.93.3.1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotloff KL, Riddle MS, Platts-Mills JA, Pavlinac P, Zaidi AK. Shigellosis. Lancet. 2018;391:801–812. doi: 10.1016/S0140-6736(17)33296-8 [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Ma Q, Hao R, et al. Antimicrobial resistance and genetic characterization of Shigella spp. in Shanxi Province, China, during 2006–2016. BMC Microbiol. 2019;19:116. doi: 10.1186/s12866-019-1495-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klontz KC, Singh N. Treatment of drug-resistant Shigella infections. Expert Rev Anti Infect Ther. 2015;13:69–80. doi: 10.1586/14787210.2015.983902 [DOI] [PubMed] [Google Scholar]
- 13.CaLSI C. Performance Standards for Antimicrobial Susceptibility Testing: Approved Twenty-: Document M100-S28. Wayne, PA, USA: CLSI; 2018:2018. [Google Scholar]
- 14.Yaghoubi S, Ranjbar R, Dallal MMS, Fard SY, Shirazi MH, Mahmoudi M. Profiling of virulence-associated factors in Shigella species isolated from acute pediatric diarrheal samples in Tehran, Iran. Osong Public Health Res Perspect. 2017;8:220–226. doi: 10.24171/j.phrp.2017.8.3.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moosavian M, Ghaderiyan GH, Shahin M, Navidifar T. First investigation of the presence of SPATE genes in Shigella species isolated from children with diarrhea infection in Ahvaz, southwest Iran. Infect Drug Resist. 2019;12:795–804. doi: 10.2147/IDR.S194740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosseini Nave H, Mansouri S, Sadeghi A, Moradi M. Molecular diagnosis and anti-microbial resistance patterns among Shigella spp. isolated from patients with diarrhea. Gastroenterol Hepatol Bed Bench. 2016;9:205–210. [PMC free article] [PubMed] [Google Scholar]
- 17.Mardaneh J, Abbas Poor S, Afrugh P. Prevalence of Shigella species and antimicrobial resistance patterns of isolated strains from infected pediatrics in Tehran. Int J Enteric Pathog. 2013;1:28–31. doi: 10.17795/ijep [DOI] [Google Scholar]
- 18.Barak M, Arzanlou M, Babapour B, Ghorbani L. Antibiotic resistance pattern of bacterial enteritis among hospitalized children in ardabil: a single center experience. Int J Adv Med. 2016;3:989–993. doi: 10.18203/2349-3933. [DOI] [Google Scholar]
- 19.Brown JD, Willcox SJ, Franklin N, et al. Shigella species epidemiology and antimicrobial susceptibility: the implications of emerging azithromycin resistance for guiding treatment, guidelines and breakpoints. J Antimicrob Chemother. 2017;72:3181–3186. doi: 10.1093/jac/dkx268 [DOI] [PubMed] [Google Scholar]
- 20.Heffernan H, Woodhouse R, Hewison C, Sherwood J. Antimicrobial resistance among Shigella in New Zealand. N Z Med J. 2018;131:56–62. [PubMed] [Google Scholar]
- 21.Pourakbari B, Mamishi S, Mashoori N, et al. Frequency and antimicrobial susceptibility of Shigella species isolated in Children Medical Center Hospital, Tehran, Iran, 2001–2006. Braz J Infect Dis. 2010;14:153–157. doi: 10.1016/S1413-8670(10)70029-5 [DOI] [PubMed] [Google Scholar]
- 22.Jomezadeh N, Babamoradi S, Kalantar E, Javaherizadeh H. Isolation and antibiotic susceptibility of Shigella species from stool samples among hospitalized children in Abadan, Iran. Gastroenterol Hepatol Bed Bench. 2014;7:218–223. [PMC free article] [PubMed] [Google Scholar]
- 23.Boumghar-Bourtchai L, Mariani-Kurkdjian P, Bingen E, et al. Macrolide-resistant Shigella sonnei. Emerg Infect Dis. 2008;14:1297–1299. doi: 10.3201/eid1408.080147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikfar R, Shamsizadeh A, Darbor M, Khaghani S, Moghaddam M. A study of prevalence of Shigella species and antimicrobial resistance patterns in paediatric medical center, Ahvaz, Iran. Iran J Microbiol. 2017;9:277. [PMC free article] [PubMed] [Google Scholar]
- 25.Esmaeili Dooki MR, Rajabnia R, Barari Sawadkohi R, Mosaiebnia Gatabi Z, Poornasrollah M, Mirzapour M. Bacterial entropathogens and antimicrobial susceptibility in children with acute diarrhea in Babol, Iran. Caspian J Intern Med. 2014;5(1):30–34. [PMC free article] [PubMed] [Google Scholar]
- 26.Terfassa A, Jida M. Prevalence and antibiotics susceptibility pattern of Salmonella and Shigella species among diarrheal patients attending Nekemte Referral Hospital, Oromia, Ethiopia. Int J Microbiol. 2018;2018:9214689. doi: 10.1155/2018/9214689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC).Outbreaks of multidrug-resistant Shigella sonnei gastroenteritis associated with day care centers–Kansas, Kentucky, and Missouri, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:1068–1071. [PubMed] [Google Scholar]
- 28.Chang Q, Wang W, Regev-Yochay G, Lipsitch M, Hanage WP. Antibiotics in agriculture and the risk to human health: how worried should we be? Evol Appl. 2015;8:240–247. doi: 10.1111/eva.2015.8.issue-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray K, Reddy V, Kornblum JS, et al. Increasing antibiotic resistance in Shigella spp. from infected New York city residents, New York, USA. Emerg Infect Dis. 2017;23:332. doi: 10.3201/eid2302.161203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lane CR, Sutton B, Valcanis M, et al. Travel destinations and sexual behavior as indicators of antibiotic resistant Shigella strains–Victoria, Australia. Clin Infect Dis. 2016;62:722–729. doi: 10.1093/cid/civ1018 [DOI] [PubMed] [Google Scholar]
- 31.Liu G, Qian H, Tang B, et al. Prevalence and characterisation of third-generation cephalosporin-resistant Shigella flexneri isolates from Jiangsu Province, China, 2013–2015. J Glob Antimicrob Resist. 2018;15:283–287. doi: 10.1016/j.jgar.2018.08.012 [DOI] [PubMed] [Google Scholar]
- 32.Lluque A, Mosquito S, Gomes C, et al. Virulence factors and mechanisms of antimicrobial resistance in Shigella strains from periurban areas of Lima (Peru). Int J Med Microbiol. 2015;305:480–490. doi: 10.1016/j.ijmm.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farshad S, Ranjbar R, Hosseini M. Molecular genotyping of Shigella sonnei strains isolated from children with bloody diarrhea using pulsed field gel electrophoresis on the total genome and PCR-RFLP of IpaH and IpaBCD genes. Jundishapur J Microbiol. 2015;8:e14004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellouk N, Weiner A, Aulner N, et al. Shigella subverts the host recycling compartment to rupture its vacuole. Cell Host Microbe. 2014;16:517–530. doi: 10.1016/j.chom.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 35.da Cruz CB, de Souza MC, Serra PT, et al. Virulence factors associated with pediatric shigellosis in Brazilian Amazon. Biomed Res Int. 2014;2014:539697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casabonne C, Gonzalez A, Aquili V, Balague C. Prevalence and virulence genes of Shigella spp. Isolated from patients with Diarrhea in Rosario, Argentina. Jpn J Infect Dis. 2016;69:477–481. doi: 10.7883/yoken.JJID.2015.459 [DOI] [PubMed] [Google Scholar]
- 37.Zhang CL, Liu QZ, Wang J, Chu X, Shen LM, Guo YY. Epidemic and virulence characteristic of Shigella spp. with extended-spectrum cephalosporin resistance in Xiaoshan District, Hangzhou, China. BMC Infect Dis. 2014;14:260. doi: 10.1186/1471-2334-14-260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phalipon A, Sansonetti PJ. Shigella’s ways of manipulating the host intestinal innate and adaptive immune system: a tool box for survival? Immunol Cell Biol. 2007;85:119–129. doi: 10.1038/sj.icb7100025 [DOI] [PubMed] [Google Scholar]
- 39.Sangeetha A, Parija SC, Mandal J, Krishnamurthy S. Clinical and microbiological profiles of shigellosis in children. J Health Popul Nutr. 2014;32:580. [PMC free article] [PubMed] [Google Scholar]