Abstract
Background
Skeletal muscle cramps are common and often presented to physicians in association with pregnancy, advanced age, exercise or disorders of the motor neuron (such as amyotrophic lateral sclerosis). Magnesium supplements are marketed for the prophylaxis of cramps but the efficacy of magnesium for this indication has never been evaluated by systematic review.
Objectives
To assess the effects of magnesium supplementation compared to no treatment, placebo control or other cramp therapies in people with skeletal muscle cramps.
Search methods
We searched the Cochrane Neuromuscular Disease Group Specialized Register (11 October 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) (2011, Issue 3), MEDLINE (January 1966 to September 2011), EMBASE (January 1980 to September 2011), LILACS (January 1982 to September 2011), CINAHL Plus (January 1937 to September 2011), AMED (January 1985 to October 2011) and SPORTDiscus (January 1975 to September 2011).
Selection criteria
Randomized controlled trials (RCTs) of magnesium supplementation (in any form) to prevent skeletal muscle cramps in any patient group (i.e. all clinical presentations of cramp). We considered comparisons of magnesium with no treatment, placebo control, or other therapy.
Data collection and analysis
Two authors independently selected trials for inclusion and extracted data. Two authors assessed risk of bias. We attempted to contact all study authors and obtained patient level data for three of the included trials, one of which was unpublished. All data on adverse effects were collected from the included RCTs.
Main results
We identified seven trials (five parallel, two cross‐over) enrolling a total of 406 individuals amongst whom 118 cross‐over participants additionally served as their own controls. Three trials enrolled women with pregnancy‐associated leg cramps (N = 202) and four trials enrolled idiopathic cramp sufferers (N = 322 including cross‐over controls). Magnesium was compared to placebo in six trials and to no treatment in one trial.
For idiopathic cramps (largely older adults presumed to have nocturnal leg cramps), differences in measures of cramp frequency, magnesium versus placebo, were small, not statistically significant, and without heterogeneity (I2 = 0%). This includes the primary endpoint, percentage change from baseline in the number of cramps per week at four weeks (‐3.93%, 95% confidence interval (CI) ‐21.12% to 13.26%, moderate quality evidence) and the difference in the number of cramps per week at four weeks (0.01 cramps/week, 95% CI ‐0.52 to 0.55, moderate quality evidence). The percentage of individuals experiencing a 25% or better reduction in cramp rate from baseline was also no different, being 8% lower in the magnesium group (95% CI ‐28% to 12%, moderate quality evidence). Similarly, no statistically significant difference was found at four weeks in measures of cramp intensity (moderate quality evidence) or cramp duration (low quality evidence).
Meta‐analysis was not possible for trials of pregnancy‐associated leg cramps. The single study comparing magnesium to no treatment failed to find statistically significant benefit on a three‐point ordinal scale of overall treatment efficacy. The two trials comparing magnesium to placebo differed in that one trial found no benefit on frequency or intensity measures while the other found benefit for both.
Withdrawals due to adverse events were not significantly different than placebo. While we could not determine the number of subjects with minor adverse events, studies of oral magnesium generally described potential side effects as similar in frequency to placebo.
Authors' conclusions
It is unlikely that magnesium supplementation provides clinically meaningful cramp prophylaxis to older adults experiencing skeletal muscle cramps. In contrast, for those experiencing pregnancy‐associated rest cramps the literature is conflicting and further research in this patient population is needed. We found no randomized controlled trials evaluating magnesium for exercise‐associated muscle cramps or disease state‐associated muscle cramps (for example amyotrophic lateral sclerosis/motor neuron disease).
Plain language summary
Magnesium for muscle cramps
Muscle cramps are common and can occur in a wide range of settings. Older adults and pregnant women commonly complain of leg cramps while they are resting, athletes can cramp when they are pushing the limits of their endurance, and some people develop muscle cramps as a symptom of other medical conditions. One potential treatment that is already being marketed to prevent muscle cramps is magnesium supplementation. Magnesium is a common mineral in our diets and extra oral supplements of this mineral are available either over the Internet or in health food stores and pharmacies (usually in the form of tablets or powders to be dissolved in water). We searched for all high quality published studies evaluating the effectiveness of magnesium to prevent muscle cramps and found four studies in older adults and three studies in pregnant women. There were no studies of people who cramp while exercising and no studies on people who cramp because of underlying medical problems. The four studies in older adults (a total of 322 participants including controls in cross‐over studies) collectively suggest that magnesium is unlikely to provide a meaningful benefit in reducing the frequency or severity of cramps in that population. We consider this evidence to be of moderate quality. In contrast, the three studies in pregnant women (202 participants) are collectively inconclusive since one study found benefit in reducing both cramp frequency and cramp pain while the other two found no benefit. More research on magnesium in pregnant women is needed; however, older adult cramp sufferers appear unlikely to benefit from this therapy. While we could not determine the rate of unwanted side effects, the study withdrawal rates and adverse event discussions suggest the treatment is well tolerated.
Summary of findings
for the main comparison.
| Magnesium for skeletal muscle cramps | ||||||
|
Patient or population: Nonpregnant patients with muscle cramps (largely older adults) Settings: Outpatients recruited through primary care clinics or community advertising Intervention: Magnesium supplements (oral or intravenous) Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Magnesium | |||||
| Percentage change in cramp frequency from baseline at 4 weeks | The mean percentage change in cramp frequency in the control groups was ‐27.8% (i.e. a 27.8% reduction) | The mean percentage change in cramp frequency in the magnesium groups was 3.9% lower | ‐3.9% (‐21.1 to 13.3) |
83 (2 studies) | ⊕⊕⊕⊝ moderate | This difference was neither clinically nor statistically significant. The 95% confidence interval excludes a 25% reduction beyond placebo |
| Percentage of participants with a ≥ 25% reduction in their cramp frequency at 4 weeks | The mean percentage of placebo recipients achieving a 25% or better reduction in the frequency of their cramps was 65.9% | The mean percentage of magnesium recipients achieving a 25% or better reduction in the frequency of their cramps was 8% lower | ‐8% (‐28% to 12%) |
83 (2 studies) | ⊕⊕⊕⊝ moderate | This difference was neither clinically nor statistically significant |
| Number of cramps per week at 4 weeks | The mean number of cramps per week in the placebo groups while on treatment was 4.35 | The mean number of cramps per week in the magnesium groups was 0.01 cramps per week higher | 0.01 cramps per week (‐0.52 to 0.55) |
213 (4 studies) | ⊕⊕⊕⊝ moderate | This difference was neither clinically nor statistically significant. The 95% confidence interval excludes a 1 cramp per week reduction |
| Percentage of participants rating their cramps as moderate or severe (i.e. mean cramp intensity ≥ 2 on the 3 point intensity scale) at 4 weeks | The mean percentage of placebo recipients rating their cramps as moderate or severe was 30% | The mean percentage of magnesium recipients rating their cramps as moderate or severe was 9% greater | 9% (‐7% to 25%) |
91 (2 studies) |
⊕⊕⊕⊝ moderate | This difference was neither clinically nor statistically significant |
| Percentage of participants with the majority of cramp durations ≥ 1 minute at 4 weeks | The mean percentage of placebo recipients with the majority of cramp durations ≥ 1 minute was 22.7% | The mean percentage of magnesium recipients with the majority of cramp durations ≥ 1 minute was 19% greater | 19% (‐7% to 45%) |
46 (1 study) | ⊕⊕⊝⊝ low | This difference was neither clinically nor statistically significant |
| Number of participants with major adverse events | 1 out of 22 | 0 out of 24 | ‐50 per 1000 (‐160 to 70) | 46 (1 study) |
⊕⊝⊝⊝ very low | This difference was neither clinically nor statistically significant |
| Number of participants with minor adverse events | Adverse events were not reported in a way that permitted the number of participants with minor adverse events to be determined. Each study of oral magnesium inferred that side effects were similar in frequency to placebo. Intravenous magnesium was associated with asymptomatic hypotension (3/24 magnesium versus 0/22 placebo recipients), transient light‐headedness (2/24 magnesium versus 0/22 placebo) and burning of the IV site (12/24 magnesium versus 0/22 placebo). | |||||
| CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Downgrading of the quality of evidence is based largely on the number of studies and participants contributing to each estimate. The quality of evidence for the number of participants with major adverse events is considered very low because such events are rare.
Background
Description of the condition
Skeletal muscle cramps are sudden, involuntary, painful and palpable muscle contractions lasting seconds to minutes. Skeltal muscle cramps can be disease‐associated but more commonly occur in the absence of obvious pathology. An understanding of the mechanism behind muscle cramps is lacking but a variety of evidence points either to the lower motor neuron or its distal axonal extensions as the site of origin (Miller 2005). In support of this, neuromuscular diseases associated with cramp are those that involve the lower motor neuron (e.g. amyotrophic lateral sclerosis/motor neuron disease (ALS/MND), recovered poliomyelitis, nerve root compression, polyneuropathies) while diseases of the muscle or central nervous system are not usually associated with cramps. Other conditions associated with cramps include some metabolic diseases (for example liver failure), medications (for example diuretics and inhaled beta‐agonists) and haemodialysis (especially if large volumes of fluid are being removed) (Garrison 2012; McGee 1990). However, cramping is commonly seen in the absence of serious disease and is more frequent in individuals who are either elderly, pregnant, continuing to contract a muscle already at its shortest length, or exercising vigorously.
Exercise‐associated muscle cramps occur either during or immediately following intense exercise, usually in the exercising muscle groups (Schwellnus 2008). In contrast, cramps associated with pregnancy or advanced age occur in the legs or feet during periods of prolonged inactivity, such as while lying in bed at night when they are termed rest cramps, or nocturnal leg cramps. Rest cramps associated with aging are very common in general practice. Within a UK general practice population, roughly one‐third of men and women over the age of 50 reported experiencing a rest cramp during the preceding two months and of those who experienced such cramps, 40% cramped three or more times per week and six per cent cramped nightly (Naylor 1994). Although they can occasionally occur in the same patients and share a similar name, rest cramps and restless leg syndrome should not be confused. Restless leg syndrome is not painful and has no palpable muscle tightening, rather it is an unpleasant sensation of 'needing to move' one's legs that prevents relaxation.
Description of the intervention
Magnesium (Mg) is the fourth most abundant mineral in the human body and a normal component of a typical diet. Foods which are generally high in magnesium include dark green leafy vegetables, legumes, nuts, seeds and unrefined grains. Oral magnesium supplements are also widely available without prescription and many of these are actively marketed for cramp prophylaxis. Such supplements are salts of magnesium and typically combine magnesium with citrate, lactate, gluconate, malate, orotate, chloride, oxide, carbonate, hydroxide, sulphate or combinations of these anions. Most are in tablet form but some are available as liquid suspensions or as powders or crystals to be dissolved in water. Magnesium is absorbed largely in the small bowel by both passive diffusion and by a saturable active transport mechanism that results in a smaller percentage of the ingested oral magnesium being absorbed as dosage increases (Graham 1960; Quamme 2008). As a result, higher doses of oral magnesium salts can potentially lead to diarrhoea because of osmotic retention of fluid within the colon. Some magnesium salts, such as magnesium sulphate and magnesium hydroxide, are commonly employed as laxatives for that reason. Other than the diarrhoea occurring with high doses, oral magnesium supplements are generally considered to be safe and relatively free of adverse effects. Injectable magnesium salts (e.g. magnesium sulphate) are also available and are indicated intravenously (IV) in some countries for the acute management of seizures, especially in pregnancy (eclampsia). Excessively rapid IV magnesium infusion can lead to cardiorespiratory suppression and flaccid paralysis of skeletal muscle (AHFS 2010). Although the IV preparation can be given intramuscularly (IM), it is associated with discomfort at the injection site.
How the intervention might work
The demonstrated efficacy of IV magnesium to prevent eclamptic seizures (Eclampsia Trialists 1995), and the neuromuscular suppression (loss of strength, diminished reflexes) which can manifest when high parenteral doses of magnesium are used (Somjen 1966), both suggest that magnesium could potentially play a role in reducing neuromuscular excitability. Although the mechanism behind skeletal muscle cramps is unclear, if a threshold for depolarization needs to be reached within motor neurons to initiate cramping, anything which conceivably reduces excitability might provide cramp prophylaxis. Hence, if magnesium supplementation were to truly suppress excitable tissue, it might also suppress muscle cramps. This would be consistent with the description of symptoms said to arise from severe magnesium deficiency, which include muscle cramping, though probably as a manifestation of tetany (Bilbey 1996; Hall 1973; Shils 1969).
Why it is important to do this review
The only intervention for cramp prophylaxis whose (modest) efficacy is supported by systematic review is the antimalarial drug quinine. Compared to placebo, over a two‐week interval quinine significantly reduced cramp number by 28%, cramp intensity by 10% and number of cramp days by 20% (El‐Tawil 2011). Cramp duration remained unchanged. Unfortunately, quinine has also been associated with significant hematologic and cardiac toxicity and its use as an off‐label cramp prophylactic has been actively discouraged by multiple drug regulatory agencies such as the US Food and Drug Administration (FDA) and its counterparts in Australia and New Zealand (ADRAC 2002; FDA 2006; Medsafe 2007). The commonest non‐prescription alternative to quinine for the prophylaxis of cramps, widely marketed over the Internet and readily available in both pharmacies and health food stores, is oral magnesium supplementation. We are aware of several studies employing oral magnesium for rest cramp prophylaxis in both pregnant and elderly populations. We are also aware that these data conflict to some degree. Given how common skeletal muscle cramps are, especially in older adults, an effective and safe alternative to quinine is needed. With this review we hope to determine, in any setting in which skeletal muscle cramps occur, whether magnesium supplementation provides effective cramp prophylaxis. This question has not yet been addressed by systematic review and our review will supplement more general pre‐existing systematic reviews of cramp therapeutics ((Young 2002 exploring the prophylaxis of pregnancy‐associated muscle cramps, and Katzberg 2010 exploring the prophylaxis of idiopathic cramps).
Objectives
To assess the effects of magnesium supplementation compared to no treatment, placebo control or other cramp therapies in people with symptomatic skeletal muscle cramps.
Methods
Criteria for considering studies for this review
Types of studies
Open label, single blind or double blind randomized controlled trials (RCTs) (including parallel group or cross‐over trials).
Types of participants
People in any age group with all forms of skeletal muscle cramp, whether idiopathic or disease‐associated, and in any body part.
Participants potentially included (but were not limited to) those with nocturnal leg cramps, pregnancy‐associated leg cramps, exercise‐associated cramps and disease state‐associated cramps such as those associated with ALS/MND, haemodialysis or liver failure.
Types of interventions
Magnesium salts and combinations of salts (e.g. magnesium citrate, lactate, gluconate, malate, orotate, chloride, oxide, carbonate, hydroxide or sulphate) administered orally or parenterally (IM or IV) at any dose. We excluded trials if the intervention combined magnesium salts with other active ingredients unless the same intervention was given to both groups.
Valid comparators included placebo, no treatment or other cramp therapies (e.g. prophylactic stretching, quinine, calcium channel blockers, sodium channel blockers, electrolyte supplements or supplemental hydration).
Types of outcome measures
Primary outcomes
The primary outcome measure was the percentage reduction from baseline in the number of muscle cramps per week at four weeks. We also reported the same measure at 12 weeks as a secondary outcome.
We chose per cent reduction from baseline as the primary outcome because we believe it to be the most clinically relevant outcome measure and because the effect of cramp treatments in general is more likely to be proportional to baseline cramp rate than to be additive (i.e. a patient with 20 cramps per week and a patient with two cramps per week who receive benefit from a therapy are more likely to see a similar percentage reduction in cramps than to share a similar absolute reduction in the number of cramps per week).
Secondary outcomes
1) Percentage of subjects with at least a 25% reduction from baseline in the number of muscle cramps per week at four weeks and 12 weeks.
This was chosen as a secondary outcome since most therapies only work in a subset of individuals. Hence, it is useful to know how many people receive what we believe to be the minimally important clinical difference (a 25% reduction in cramp rate).
2) Number of cramps per week at four weeks and 12 weeks.
We chose this as a secondary outcome to improve the ability to pool results if studies did not report baseline cramp rates. We also chose this measure because percentage change in cramp rate (the primary outcome) can have low power when the correlation between baseline and post‐treatment measures is low (i.e. Pearson correlation coefficient less than 0.5) and in this low range of correlation the difference between treatment groups at the end of treatment offers better power than either percentage change or absolute change from baseline (Vickers 2001). In a recent RCT that we conducted (five consecutive days of IV magnesium versus IV placebo infusions in nocturnal leg cramp sufferers with comparison of change in cramp rate from baseline at days 30 and 90), we found the correlation coefficient between baseline and post‐treatment cramps to be approximately 0.5 (Garrison 2011).
3) Cramp intensity (pain) on a three‐point scale at four weeks and 12 weeks.
The mean of all cramp intensities (if cramps were individually rated) or the global assessment of cramp pain while on treatment were translated into a three‐point scale representing cramp intensity, where 1 = mild, 2 = moderate, 3 = severe. We analysed cramp intensity by looking at mean values and also by looking at the number of individuals rating their cramps as moderate or severe (i.e. a score of at least two on the three‐point intensity scale).
4) Cramp duration on a three‐point scale at four weeks and 12 weeks.
We translated the mean of all cramp durations (if cramps were individually rated) or the global assessment of cramp duration while on treatment into a three‐point scale representing cramp duration where one is equal to less than one minute; two is equal to one to five minutes; and three is equal to more than five minutes. We analysed cramp duration by looking at the number of individuals rating their cramps as lasting more than one minute (i.e. a score of at least two on the three‐point duration scale).
5) Treatment withdrawals due to adverse events.
6) Number of subjects reporting minor adverse events (minor adverse events being symptoms not requiring medical treatment, e.g. diarrhoea).
7) Number of subjects reporting major adverse events (major adverse events being death, hospitalization and or symptoms requiring medical treatment).
Main outcomes for 'Summary of findings' table
We included a 'Summary of findings' table that presented our assessments of the quality of the evidence for the following outcomes.
Percentage change in cramp frequency from baseline at 4 weeks.
Percentage of participants with a ≥ 25% reduction in their cramp frequency at 4 weeks.
Number of cramps per week at 4 weeks.
Percentage of participants rating their cramps as moderate or severe (i.e. mean cramp intensity ≥ 2 on the 3 point intensity scale) at 4 weeks.
Percentage of participants with the majority of cramp durations ≥ 1 minute at 4 weeks.
Number of participants with major adverse events.
Number of participants with minor adverse events.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Neuromuscular Disease Group Specialized Register (11 October 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 3), MEDLINE (January 1966 to September 2011), EMBASE (January 1980 to September 2011), LILACS (January 1982 to September 2011), CINAHL Plus (January 1937 to September 2011), AMED (January 1985 to October 2011) and SPORTDiscus (January 1975 to September 2011).
We used the following terms: muscle cramp(s); muscle spasm(s); muscle contraction(s); charlie horse(s); charley horse(s); EAMC(s); Magnesium; Mg2. EAMC is a commonly used sports medicine acronym for "exercise associated muscle cramp" and charlie (or charley) horse is a lay term for muscle cramps.
The detailed search strategies are in the appendices: CENTRAL Appendix 1, MEDLINE Appendix 2, EMBASE Appendix 3, LILACS Appendix 4, CINAHL Plus Appendix 5, AMED Appendix 6 and SPORTDiscus Appendix 7.
Searching other resources
We checked all references in the identified trials and contacted the authors to identify any additional published or unpublished data. We contacted relevant pharmaceutical manufacturers to request any unpublished trials that might be in their possession. We also searched the International Clinical Trials Registry Platform (WHO‐ICTRP) in an attempt to uncover unpublished trials, searched ISI Web of Science for papers citing the studies included in this review, and contacted the FDA to ask if they had any related clinical trial information in their possession.
Data collection and analysis
Selection of studies
Two review authors independently examined the titles and abstracts of all articles identified by the search algorithm, obtained the full text of all potentially relevant studies and determined which studies met the inclusion criteria. A third author was available to adjudicate any disagreements regarding study inclusion.
Data extraction and management
Two authors independently rated the risk of bias of each study and extracted data onto specially designed forms. S Garrison entered the data into RevMan and a second author independently checked data entry.
Assessment of risk of bias in included studies
Two authors assessed the risk of bias in the included studies using The Cochrane Collaboration's recommended tool (Higgins 2008). We documented the risk of bias within each study according to seven domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, use of a cramp diary and 'other sources of bias'. We rated each domain as at high, low or unclear risk of bias. Use of a cramp diary was included as a dimension for bias in this review since patient recollection at the time of an exit interview is open to more bias, especially if blinding is poor. Two authors of this review (Garrison and Khan) were also co‐authors of one of the included trials. Neither of these authors participated in the bias rating of their own trial.
Measures of treatment effect
Diary recording of individual cramps, along with their intensity and duration, was the preferred measure of cramps, but we accepted any means of recording cramp data (e.g. subject recollection at the time of reassessment) from which the number of cramps per week could be calculated. In order to combine results, the number of cramps per week needed to be reported and calculable on a continuous scale. We did not use cramp frequency data for meta‐analysis if it was reported in category ranges such as 'less than two cramps per week' or 'more than seven cramps per week'.
For duration and intensity of cramps, either an average of the duration and intensity of each cramp over the treatment period, or a global assessment by each subject regarding duration and intensity was acceptable so long as it could be meaningfully translated into the three‐point scales for intensity and duration outlined above.
Unit of analysis issues
For each included study the unit of analysis and the unit of randomization (expected to be the patient) needed to match to prevent the introduction of bias.
Dealing with missing data
We requested any missing data from the study authors. Where studies measured cramp rate, intensity or duration in categories that did not allow us to meaningfully convert cramp rate into a continuous number of cramps per week (or intensity and duration into our three‐point scales), we described and discussed these studies but did not include them in the meta‐analysis.
Assessment of heterogeneity
We assessed heterogeneity by using a Chi2 test on n‐1 degrees of freedom and by calculating the I2 statistic.
Assessment of reporting biases
We planned to use a funnel plot to assess publication bias but there were too few studies for this to be meaningful.
Data synthesis
Our inclusion criteria encompassed a wide range of diagnoses (e.g. cramps associated with exercise, pregnancy, aging or disease states such as ALS/MND) for which a variation in treatment effect was certainly possible. However, combining such disparate patient populations using a random‐effects model and producing an overall treatment effect for magnesium across all populations could have been misleading and not properly address the clinical question, which should clearly account for the patients' clinical setting. Accordingly, we undertook a fixed‐effect analysis within each of the clearly defined clinical settings for which data were available (these being pregnancy and age‐associated leg cramps) and did not provide meta‐analysis across all patient groups. Any across group comparison was descriptive (qualitative) in nature. For meta‐analysis within each clinical setting we calculated the fixed‐effect statistic I2 and, if it exceeded 25%, a sensitivity analysis was conducted. We combined trial data identified for inclusion in this review using the Cochrane statistical package, Review Manager 5.1 (RevMan). We combined continuous outcomes using the generic inverse variance method (GIV) which allowed paired data from cross‐over trials (in which subjects serve as their own controls) to be combined with two‐group parallel studies. We obtained standard error estimates for included studies from intervention and control group means and standard deviations when unpaired t‐tests were applied (parallel group trials), and from the mean difference between groups and P value for the difference when paired t‐tests were used (cross‐over trials).
Subgroup analysis and investigation of heterogeneity
An insufficient number of studies was available to permit meaningful subgroup analysis at this time.
We planned, if a sufficient number of studies had been found, to use subgroup analysis to search for variation in treatment effect and to explore possible sources of heterogeneity. Subgroups related to clinical presentation were to include gender and cramp frequency with a "high frequency" versus "low frequency" subgroup defined as averaging one or more cramps per day at baseline. Subgroups related to method of treatment were to include route of administration (oral versus parenteral), formulation (grouped by the specific magnesium salt used) and duration of therapy (four weeks or less, four to eight weeks, more than eight weeks). We intended to view results from these subgroup analyses merely as hypothesis generating.
Sensitivity analysis
Heterogeneity existed only in the trials of magnesium versus placebo for the prophylaxis of pregnancy‐associated leg cramps (two studies). Neither of these trials had reported outcomes in a way that permitted data pooling. We discussed differences in study design that could have led to their discrepant results qualitatively.
Results
Description of studies
Results of the search
Search results from MEDLINE, EMBASE, CENTRAL, CINAHL Plus, SPORTDiscus, Cochrane Neuromuscular Disease Group Specialized Register, AMED and LILACS revealed 82, 57, 16, 14, 14, 4, 1 and 1 papers, respectively. Thirty‐three titles were relevant to the topic and we analyzed the abstracts of these papers. The authors reviewed full texts of 11 studies. We excluded five (see Characteristics of excluded studies) leaving six studies that met our inclusion criteria. Additionally, we uncovered one unpublished eligible RCT by a search of the International Clinical Trials Registry Platform (WHO‐ICTRP), bringing the total of included studies to seven. Contacting the FDA, Health Canada and relevant manufacturers revealed no further studies, nor did searching ISI Web of Science for papers citing the studies included in this review. Examining reference lists of all included papers and relevant reviews revealed two studies whose full papers were obtained but which we excluded. See Table 4 for a tabulated summary of the included trials and Figure 1 for the study flow diagram. All included trials are in English but no exclusions were made based on language.
1. Table 1. Study design of the seven included trials.
| Study | Number/design/clinical Setting | Mean age (years) | % Female | Magnesium dose and route of administration | Frequency of administration | Treatment and assessment periods (days) | Washout period (days) | Comparator |
| Dahle 1995 | N = 73 Parallel Pregnancy |
Not given (child‐ bearing years) |
100% | 5 mmol combination Mg lactate + Mg citrate (122 mg elemental Mg) taken orally | Once each morning and twice each evening | Treatment 21 Assessment 21 |
Not applicable | Matched placebo tablet |
| Frusso 1999 | N = 45 Cross‐over Idiopathic |
61.6 | 73.3% | Mg citrate 900 mg tablet (100 mg elemental Mg) taken orally | Twice daily | Treatment 28 Assessment 28 |
28 | Matched placebo tablet |
| Garrison 2011 | N = 46 Parallel Idiopathic |
69.3 | 69.6% | 20 mmol Mg sulfate (486 mg elemental Mg) given intravenously | Once daily over 4 hrs on 5 consecutive days | Treatment 5 Assessment 90 |
Not applicable | Matched placebo solution |
| Nygaard 2008 | N = 45 Parallel Pregnancy |
30.9 | 100% | Mg lactate and Mg citrate chewable tablets containing 122 mg elemental Mg taken orally | Once each morning and twice each evening | Treatment 14 Assessment 14 |
Not applicable | Matched placebo tablet |
| Roffe 2002 | N = 73 Cross‐over Idiopathic |
62.9 | 54.3% | 1830 mg of tri‐magnesium dicitrate powder (300 mg elemental Mg) poured from a sachet into a glass of water taken orally | Once each evening | Treatment 42 Assessment during last 28 days of treatment |
First 14 days of second treatment period considered as washout |
Matched placebo powder |
| Rosenbaum 2011 | N = 40 Parallel Idiopathic |
66.6 | 57.5% | Slow release tablet of Mg lactate containing 84 mg of elemental Mg taken orally |
Two tablets twice daily | Treatment 30 Assessment 30 |
Not applicable | Matched placebo tablet |
| Sohrabvand 2006 | N = 84 Parallel Pregnancy |
Not given (child‐ bearing years) |
100% | 7.5 mmol magnesium aspartate (182 mg elemental Mg) taken orally. Unclear if tablet or powder / solution |
Twice daily | Treatment 14 Assessment 28 |
Not applicable | 3 different comparators 1) No treatment 2) 500 mg calcium carbonate tablet once daily 3)100 mg of thiamine (vit B1) plus 40 mg of pyridoxine (vit B6) once daily |
vit B1: vitamin B1
1.
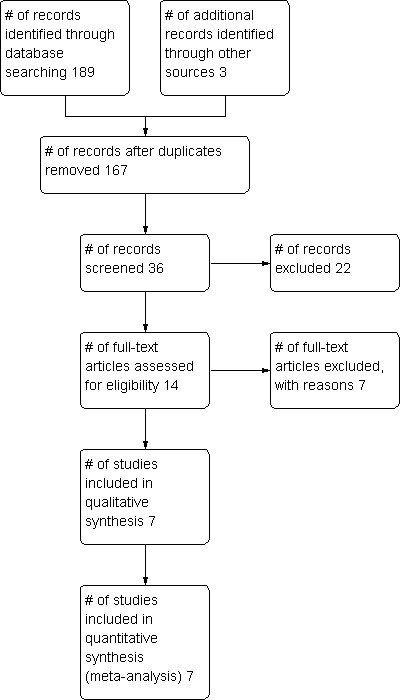
Study flow diagram.
Included studies
Magnesium was generally compared to placebo (six trials) although one trial (Sohrabvand 2006) with four parallel treatment arms compared no treatment to magnesium, calcium carbonate and a combined supplement of vitamins B1 and B6. Magnesium was given orally in all but one trial, where it was administered as a series of intravenous infusions (Garrison 2011). Oral magnesium was given either once at night (Roffe 2002) or twice daily (Dahle 1995; Frusso 1999; ; Nygaard 2008; Rosenbaum 2011; Sohrabvand 2006), with larger doses at night in two studies (Dahle 1995; Nygaard 2008). The amount of elemental magnesium administered daily through the various oral protocols included 366 mg from "primarily magnesium lactate and magnesium citrate" tablets (Dahle 1995; Nygaard 2008), 200 mg from magnesium citrate tablets (Frusso 1999), 300 mg from tri‐magnesium dicitrate powder dissolved in water (Roffe 2002), 336 mg from magnesium lactate as slow release tablets (Rosenbaum 2011), and 364 mg from magnesium aspartate (Sohrabvand 2006) (unclear if tablet or powder). The durations of treatment for oral magnesium ranged from 14 to 42 days, with total oral doses of elemental magnesium over the entire treatment period ranging from 5096 mg to 12,600 mg. The study (Garrison 2011) providing magnesium intravenously gave 20 mmol of magnesium sulfate (486 mg of elemental magnesium) as an infusion over four hours on five consecutive days. This provided a total treatment dose of 2430 mg of elemental magnesium, although with presumably different (higher) bioavailability. This trial recorded cramps for 90 days post infusions but all other trials recorded cramps over two to four weeks. Five of the studies were parallel in design and two were cross‐over. Three studies involved treatment of pregnancy‐associated leg cramps (Dahle 1995; Nygaard 2008; Sohrabvand 2006) and the remaining four involved the treatment of idiopathic cramps in older adults (most of whom are presumed to have been suffering nocturnal leg cramps). One of the trials was unpublished (Rosenbaum 2011) but some patient level data were made available to us. Two other studies (Garrison 2011; Roffe 2002) also made patient level data available, although the data from Roffe did not include noncompleters. No studies investigating exercise or disease state associated cramps were found.
A total of 406 participants were enrolled in these trials. Of these, 118 were cross‐over subjects and additionally formed their own controls. All trials were small, varying from 40 to 84 participants. All subjects were community dwelling. Most were outpatients recruited from primary care or maternity clinics, although some were recruited by newspaper or radio advertizement. Subjects in the idiopathic cramp trials were 64.8 years of age on average and 62.8% female. Only one of the three pregnancy trials provided data on mean age (30.9 years).
Cross‐over trials can present difficulties in meta‐analysis. One of the cross‐over trials (Frusso 1999) included a 28‐day washout period. The other (Roffe 2002) did not have a formal washout period but evaluated outcomes only in the last four weeks of each of two sequential six‐week treatment periods. This effectively gave a two‐week washout to those who started on magnesium and an extra two weeks of magnesium therapy (while on magnesium) prior to each evaluation period. The author of this trial kindly provided us with patient level data to permit data pooling. It is our opinion that this cross‐over trial demonstrated a large difference in treatment effect depending on the sequence of treatments. Of 17 subjects receiving the sequence magnesium followed by placebo, eight favored magnesium, seven favored placebo and two were unchanged. In contrast, of 29 subjects receiving the sequence placebo followed by magnesium, 21 favored magnesium, five favored placebo and three were unchanged. It is unclear how much of this difference was due to the period effect and how much was due either to the high rate of noncompleters (27 of 73 subjects did not complete the trial), the potential for carry‐over or the potential for unblinding. As a result of this sequence order effect we chose to minimize these potential sources of bias by using only data from the first period of this study.
Excluded studies
Excluded trials were either uncontrolled, did not have a magnesium treatment arm, or did not measure outcomes relevant to cramping.
Risk of bias in included studies
We carried out the 'Risk of bias' assessment as outlined in the methods and summarized our assessments in Figure 2. There was considerable variability in the quality of included trials.
2.
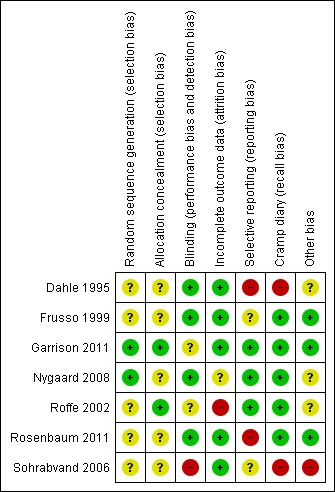
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The risk of selection bias (randomization or allocation) was unclear (though likely adequate) in six of seven trials, largely because of inadequate description of methods in the manuscripts and our inability to obtain responses from some authors. In one cross‐over trial (Roffe 2002) the manufacturer provided randomization in large blocks which were either unbalanced initially or became unbalanced because of noncompleters (17 subjects were randomized to placebo followed by magnesium and 29 to magnesium followed by placebo). Since the treatment effect in this trial varied depending on the sequence order of treatment, this imbalance in sequence allocation was an important potential source of bias and we pooled data only for the first treatment period.
Blinding
Blinding was low risk in four of seven trials. In one trial (Garrison 2011) it was unclear because of a greater likelihood of magnesium recipients experiencing a burning sensation at the IV site during intravenous infusion. In one cross‐over trial (Roffe 2002) it was unclear because no description was given as to whether magnesium and placebo solutions could be distinguished by taste. The risk of bias was high in one trial (Sohrabvand 2006) because blinding was not possible (open label).
Incomplete outcome data
Attrition bias was low risk in five of the seven studies. It was unclear in one study (Nygaard 2008) with a 15.6% dropout rate and high risk in one study (Roffe 2002) with a 37% dropout rate.
Selective reporting
In three of the seven studies reporting bias was considered to be low. This included two studies (Garrison 2011; Roffe 2002) whose manuscripts did report selectively (that is, both reported that some secondary outcomes were not statistically significant without providing actual numbers) but whose authors made the patient level data available to the authors of this review to allow for the calculation of these outcomes. Studies at greater than low risk included two studies (Frusso 1999; Sohrabvand 2006) whose risk was unclear because of inconsistencies in reporting, one unpublished study (Rosenbaum 2011) whose risk was high because only a subset of outcomes was available and one study (Dahle 1995) whose risk was high because it was unclear how well the outcomes were predefined (there was no description of outcomes by primary and secondary and the outcomes were incompletely described in the methods).
Other potential sources of bias
Two of the three trials in pregnant women (Dahle 1995; Sohrabvand 2006) were viewed as having a high risk of bias for not using cramp diaries (instead recalling cramp frequency at the time of exit interviews) and one trial was judged to be at high risk of bias for being extremely under reported (Sohrabvand 2006). Most studies also had one or more additional sources of bias but nothing in common with other trials or viewed as a fatal flaw.
Effects of interventions
See: Table 1
Measures of cramp frequency
While all studies attempted to measure the cramp frequency, or change in cramp frequency, the three trials in pregnant women all used frequency measures which prevented data pooling; only one (Nygaard 2008) used a cramp diary. Cramp frequency measures that could not be pooled included cramp frequency on a five point ordinal scale (Dahle 1995), change in cramp frequency on a three point ordinal scale (Sohrabvand 2006), and number of days and nights in which cramps occurred (Nygaard 2008). In contrast, all four idiopathic rest cramp trials used cramp diaries and recorded the occurrence of each cramp, permitting analysis of cramp frequency as a continuous variable. The resulting pooled estimates of cramp frequency measures include data from two trials rated as having a high risk of bias, either due to a high dropout rate (Roffe 2002) or selective reporting (Rosenbaum 2011).
Percentage reduction from baseline in the number of muscle cramps per week at four weeks (the primary outcome) and at 12 weeks (a secondary outcome)
Magnesium versus placebo
Pregnancy‐associated cramps
None of the pregnancy trials determined the baseline cramp rate needed to calculate a percentage change.
Idiopathic rest cramps
At four weeks
Two of the four idiopathic rest cramp trials measured the baseline cramp rate, either over 30 days pre‐treatment (Garrison 2011) or seven days pre‐treatment (Rosenbaum 2011). Pooling these two studies provided a statistically nonsignificant difference (‐3.93% (95% CI ‐21.12 to 13.26) in the percentage change in cramp rate, magnesium versus placebo (Figure 3, Analysis 1.1). There was no evidence of heterogeneity (I2 = 0%) and the resulting 95% CI excludes a 25% reduction in cramp rate over placebo.
3.
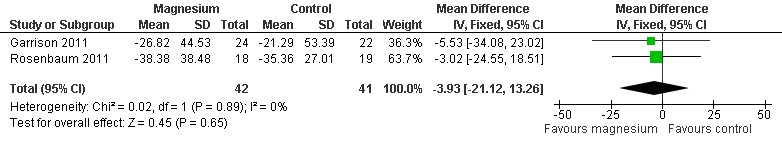
Forest plot of comparison: 1 Idiopathic rest cramps, magnesium versus placebo, outcome: 1.1 % Change in cramp frequency from baseline at 4 weeks.
1.1. Analysis.
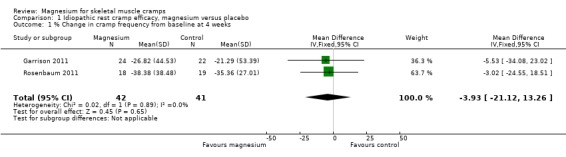
Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 1 % Change in cramp frequency from baseline at 4 weeks.
At 12 weeks
Only one study (Garrison 2011) had data to 12 weeks and found a nonsignificant mean difference, magnesium versus placebo of ‐12.09% (95% CI ‐40.22 to 16.04) (Analysis 1.2).
1.2. Analysis.
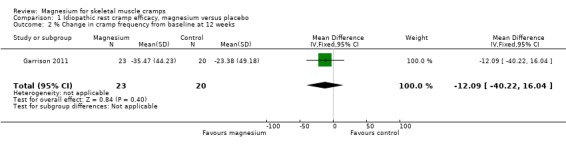
Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 2 % Change in cramp frequency from baseline at 12 weeks.
Percentage of participants with ≥ 25% reduction in cramp frequency at four weeks and at 12 weeks
Magnesium versus placebo
Pregnancy‐associated cramps
None of the pregnancy trials determined the baseline cramp rate needed to calculate a percentage change.
Idiopathic rest cramps
At four weeks
Pooling results from the only two trials with baseline measures (Garrison 2011; Rosenbaum 2011) showed 65.9% of participants in the placebo group to have achieved a 25% or better reduction in cramp frequency, with the magnesium group being a nonsignificant 8% lower (95% CI ‐28% to 12%) (Figure 4). There was no evidence of heterogeneity (I2 = 0%) (Analysis 1.3).
4.
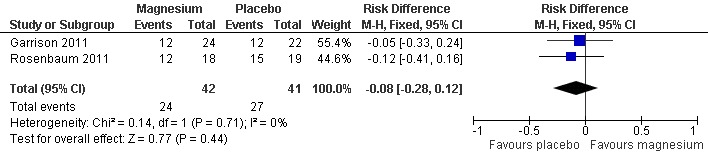
Forest plot of comparison: 1 Idiopathic rest cramp efficacy, magnesium versus placebo, outcome: 1.3 Proportion of subjects with a ≥ 25% reduction in cramp frequency at 4 weeks.
1.3. Analysis.
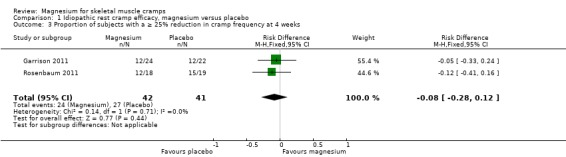
Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 3 Proportion of subjects with a ≥ 25% reduction in cramp frequency at 4 weeks.
At 12 weeks
The single study to 12 weeks (Garrison 2011) found the percentage of subjects achieving a 25% or better reduction in the frequency of cramps to be a nonsignificant 11% higher (95% CI ‐19% to 41%) in the magnesium group (Analysis 1.4).
1.4. Analysis.
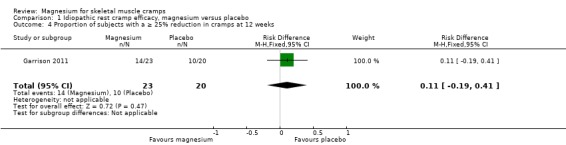
Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 4 Proportion of subjects with a ≥ 25% reduction in cramps at 12 weeks.
Number of cramps per week at four weeks and at 12 weeks
Magnesium versus placebo
Pregnancy‐associated cramps
At four weeks
There were two trials evaluating the frequency of leg cramps, magnesium versus placebo, in pregnant women. The earliest (Dahle 1995) was the first published RCT of magnesium in cramping (in any setting) and the only trial to show statistically significant benefit for reducing the frequency of cramps. It measured cramp frequency on a five‐point ordinal scale (daily, every other day, twice a week, once a week, never) and also measured patient evaluation of treatment effect on a five‐point ordinal scale (entirely free of symptoms, considerably improved, unchanged, worsened, considerably worsened). This study reported a reduction in the frequency of symptoms "from the initial average of every other day, to every 3 days in the placebo group and one to two times a week in the magnesium group (P < 0.05)". The way in which this result is reported is problematic in that "every 3 days" and "one to two times a week" do not belong to the 5 point ordinal scale used to measure this outcome. Dahle 1995 also reported benefit in patient evaluation of treatment effect in that "the magnesium group indicated that they had to a significantly greater extent "improved considerably" or "become asymptomatic" compared with the placebo group (P = 0.0002)" (17/34 magnesium and 11/35 placebo recipients improving considerably, and 10/34 magnesium and 3/35 placebo recipients becoming asymptomatic). In contrast, the subsequent trial (Nygaard 2008) measured the mean number of days and nights with leg cramps present and found no significant benefit with 7.7 ± 4.7 (SD) days and nights of cramping over two weeks in the placebo group and 9.5 ± 5.1 in the magnesium group (P = 0.27).
At 12 weeks
There were no trials in pregnant women longer than three weeks' duration.
Idiopathic rest cramps
At four weeks
Cramps per week on treatment was available as an outcome for all four of the idiopathic rest cramp trials and provided a nonsignificant pooled estimate (N = 216) for the MD in the number of cramps per week of 0.01 cramps per week (95% CI ‐0.52 to 0.55) (Figure 5, Analysis 1.5). There was no evidence of heterogeneity (I2 = 0) and the CI excluded a one cramp per week reduction. As mentioned under Included studies, only the first period of Roffe 2002 was used because of an unbalanced randomization and a difference in benefit depending on treatment order.
5.
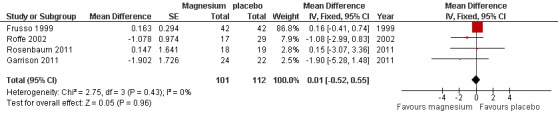
Forest plot of comparison: 1 Idiopathic rest cramps, magnesium versus placebo, outcome: 1.6 Number of cramps per week at 4 weeks.
1.5. Analysis.
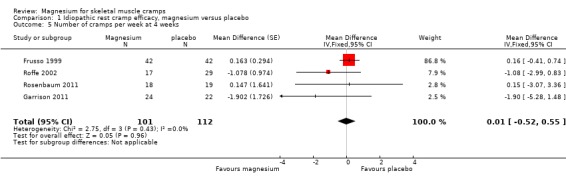
Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 5 Number of cramps per week at 4 weeks.
At 12 weeks
The single study (Garrison 2011) to 12 weeks found a nonsignificant MD in the number of cramps per week of ‐0.84 (95% CI ‐3.23 to 1.55) (Analysis 1.6).
1.6. Analysis.
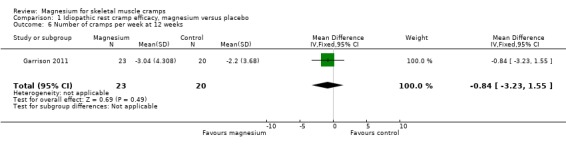
Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 6 Number of cramps per week at 12 weeks.
No treatment versus magnesium, calcium carbonate and a combination vitamin B1 / B6 supplement
Pregnancy‐associated cramps
At four weeks
The single study in any clinical setting (Sohrabvand 2006) to use a comparator other than placebo used four parallel treatment arms to compare no treatment with either 182 mg elemental magnesium twice daily (from magnesium aspartate), 500 mg of calcium carbonate once daily or 100 mg of thiamine (vitamin B1) combined with 40 mg of pyridoxine (vitamin B6) once daily in a group of pregnant Iranian women. This trial had an unusual design with the intervention being given over two weeks but outcomes assessed over four weeks. The outcome for this study was "change in muscle spasms" on a three‐point ordinal scale with "no change", "relative improvement", and "absolute improvement" clarified with the authors to mean no improvement, partial resolution and complete resolution of cramping. A multinomial regression test was applied to each of the three possible response categories to see if any of the active treatment arms differed from no treatment. Significantly more women reported absolute improvement in both the B vitamin and calcium arms, but not in the magnesium arm. Neither was there a difference in relative improvement between no treatment and the magnesium arm. Comparisons between the active intervention arms were not made.
Measures of cramp intensity (pain)
Cramp intensity (pain) on a three‐point scale (1 = mild, 2 = moderate, 3 = severe) at four weeks and at 12 weeks
Magnesium versus placebo
Pregnancy‐associated cramps
Although two of the three trials in pregnant women recorded cramp severity on scales which could potentially have been transformed into our three‐point scale, neither reported results in a manner that allowed us to do so. In Dahle 1995 cramp intensity was recorded on a 0 to 100 mm visual analog scale (VAS) but mean scores were reported without SD along with P value thresholds for the difference in change from baseline within and between groups. These results were reported as follows: "Subjectively (sic) experienced distress according to the VAS was reduced from 68.2 mm before to 47.8 mm after treatment (P < 0.05) in the placebo group and from 70.4 mm to 30.3 mm (P < 0.001) in the magnesium group. The reduction of distress in the magnesium group was significantly greater (P < 0.05) than in the placebo group." In Nygaard 2008 the intensity of cramping during each nighttime and each daytime were recorded on a zero to four intensity scale (zero = no pain, one = light pain, two = medium pain, three = strong pain, four = severe pain) and added together over the two‐week assessment period. The mean of each patients summed intensity scores was 11.4 ± 8.5 (SD) for placebo and 13.2 ± 6.5 for magnesium with a nonsignificant P value for the difference (P = 0.46).
Idiopathic rest cramps
At four weeks
Pooling results from the three trials with available data (Frusso 1999; Garrison 2011; first period of Roffe 2002) shows a statistically nonsignificant MD of ‐0.04 (95% CI ‐0.18 to 0.11) on a three‐point intensity scale (I2 = 5%) (Figure 6, Analysis 1.7).
6.
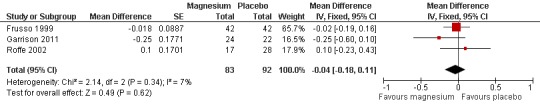
Forest plot of comparison: 1 Idiopathic rest cramp efficacy, magnesium versus placebo, outcome: 1.7 Cramp intensity (pain) on a 3 point scale (1 = mild, 2 = moderate, 3 = severe) at 4 weeks.
1.7. Analysis.
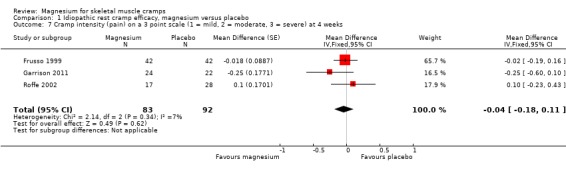
Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 7 Cramp intensity (pain) on a 3 point scale (1 = mild, 2 = moderate, 3 = severe) at 4 weeks.
At 12 weeks
The single study with 12‐week data (Garrison 2011) showed a statistically nonsignificant MD of ‐0.18 (95% CI ‐0.55 to 0.19) on a three‐point intensity scale (Analysis 1.8).
1.8. Analysis.
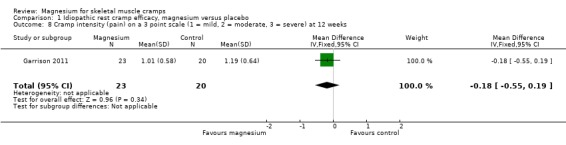
Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 8 Cramp intensity (pain) on a 3 point scale (1 = mild, 2 = moderate, 3 = severe) at 12 weeks.
Percentage of subjects rating their cramps as moderate to severe (i.e. with mean cramp intensity of two or more on the three‐point intensity scale) at four weeks and at 12 weeks
Magnesium versus placebo
Pregnancy‐associated cramps
None of the studies in pregnant women reported results in this way and patient level data were unavailable.
Idiopathic rest cramps
At four weeks
Results were not reported in this manner but patient level data were available from two studies (Garrison 2011; first period of Roffe 2002) to allow this statistic to be calculated. Pooled results showed the mean percentage of placebo recipients rating their cramps as moderate or severe (i.e. two or three on the three‐point scale) to be 30%, with the mean percentage of magnesium recipients rating their cramps as moderate or severe being a nonsignificant 9% greater (95% CI ‐7% to 25%) (I2 = 79%) (Analysis 1.9). Heterogeneity could have been high in this analysis because of what appear to be very different patient populations. Only 3 of 46 patients (6.5%) in Garrison 2011 rated their cramps as moderate to severe compared to 25 of 45 (56%) in Roffe 2002. This difference in patient population might be accounted for by differences in recruitment methods since Roffe 2002 recruited 100% of patients from community advertising while Garrison 2011 recruited half from advertising and half from GP referral. The higher dropout rate in Roffe 2002 (37% versus 0%) might also have contributed if participants who would have rated their cramps as less severe were more likely to drop out of the trial.
1.9. Analysis.
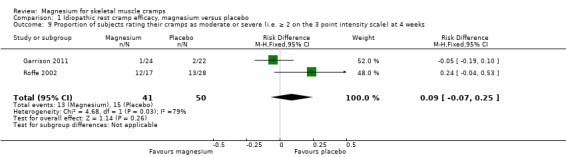
Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 9 Proportion of subjects rating their cramps as moderate or severe (i.e. ≥ 2 on the 3 point intensity scale) at 4 weeks.
At 12 weeks
The single study with 12‐week data (Garrison 2011) found that 10% of placebo recipients rated their cramps as moderate or severe, with the magnesium group being a nonsignificant 6% lower (95% CI ‐21% to 10%) (Analysis 1.10).
1.10. Analysis.
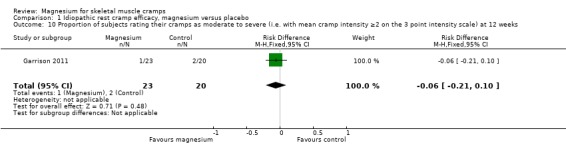
Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 10 Proportion of subjects rating their cramps as moderate to severe (i.e. with mean cramp intensity ≥2 on the 3 point intensity scale) at 12 weeks.
Measures of cramp duration
Number of subjects with majority of cramp durations of 1 min or more at four weeks and at 12 weeks
Magnesium versus placebo
Pregnancy‐associated cramps
None of the studies in pregnant women recorded the duration of cramps.
Idiopathic rest cramps
At four weeks
Three idiopathic cramp trials measured cramp duration. Frusso 1999 used a four‐point ordinal scale divided into less than five min, 5 to 10 min, 10 to 30 min and over 30 min. Garrison 2011 used a three‐point ordinal scale divided into less than 1 min, one to five min and over five min. Roffe 2002 used a three‐point ordinal scale divided into short, medium and long. All three studies reported no statistically significant difference in duration. The single study (Garrison 2011) that recorded duration using a one min cut‐off found the mean percentage of placebo recipients with a majority of cramp durations of one min or more to be 22.7%. The mean percentage of magnesium recipients with a majority of cramp durations of one min or more was a nonsignificant 19% greater (95% CI ‐7% to 45%) (Analysis 1.11).
1.11. Analysis.
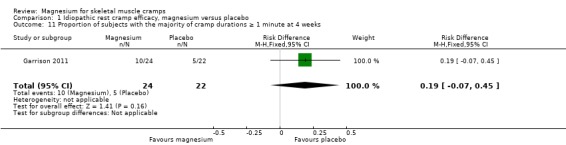
Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 11 Proportion of subjects with the majority of cramp durations ≥ 1 minute at 4 weeks.
At 12 weeks
The single study with 12‐week data (Garrison 2011) found the placebo group to have 25% of participants reporting the majority of their cramps as lasting one minute or more, with the magnesium group being a nonsignificant 14% greater (95% CI ‐13% to 42%) (Analysis 1.12).
1.12. Analysis.
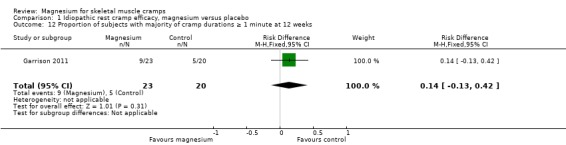
Comparison 1 Idiopathic rest cramp efficacy, magnesium versus placebo, Outcome 12 Proportion of subjects with majority of cramp durations ≥ 1 minute at 12 weeks.
Measures of safety and tolerability
Treatment withdrawals due to adverse events
Magnesium versus placebo
Most studies had omissions or inconsistencies which made the determination of the number of withdrawals due to adverse effects difficult. Because we believed that the difference in withdrawals due to adverse events and the difference in total withdrawals between groups would be estimating the same parameter, we used total withdrawals for each group whenever it was unclear how many patients withdrew due to adverse effects. Neither of the cross‐over studies (Frusso 1999; Roffe 2002) could be used to estimate withdrawals due to adverse effects since information on patient experience in each period was not available (i.e. would patients withdrawing in one period also have withdrawn in the other?). Although we had patient level data for Roffe 2002, we could not use data from the first period to estimate withdrawals since the data provided to us only included patients completing both study periods. Determined in this way, and using only parallel studies, the percentage difference in withdrawals across all studies (which we presume to be due to adverse events) is, magnesium versus placebo, ‐3% (95% CI ‐10% to 3%) (Analysis 2.1).
2.1. Analysis.
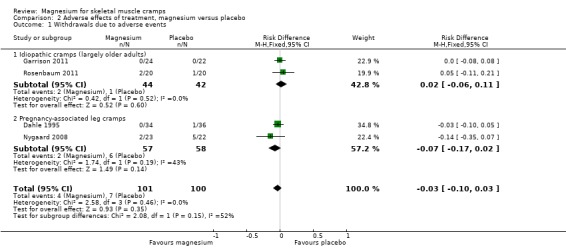
Comparison 2 Adverse effects of treatment, magnesium versus placebo, Outcome 1 Withdrawals due to adverse events.
Number of subjects reporting minor adverse events
Magnesium versus placebo
Adverse events were reported in most studies but usually not in a way which allowed us to determine the number of subjects reporting minor adverse events.
Pregnancy‐associated cramps
In Nygaard 2008, 6/23 patients (26.1%) in the magnesium arm and 6/22 patients (27.3%) in the placebo arm reported adverse events which were lumped together as gastrointestinal in nature (nausea, flatulence, diarrhoea, intestinal air). Dahle 1995 only noted adverse events as being infrequent (without specifying placebo versus magnesium) and consisting of "primarily slight or initial nausea".
Idiopathic rest cramps
Frusso 1999 reported diarrhoea, nausea or vomiting as occurring in 10.7% of magnesium recipients and 10.1% of placebo recipients. Roffe 2002 and Garrison 2011 reported the number of patients with different specific side effects but not the number of subjects with any side effect (i.e. the same subjects may be counted more than once). In Roffe 2002 diarrhoea occurred in 30% on magnesium and 17% on placebo. Constipation occurred in 13% on magnesium and 24% on placebo. Nausea, indigestion or flatulence occurred in two magnesium and four placebo subjects. Other side effects included skin peeling (one on magnesium), bruising (one on placebo) and headaches (one on placebo). Garrison 2011 was different, in that it was a study of magnesium given intravenously. Side effects included asymptomatic hypotension (reported by the study nurse) in 3/24 magnesium versus 0/22 placebo participants and facial flushing being noted in 9/24 magnesium and 7/22 placebo recipients but not generally complained of by participants. Two magnesium recipients noted transient light‐headedness several hours after the infusions on day three and day four and more magnesium recipients noted burning of the IV site (12/24 versus 0/22) with 5/24 magnesium participants receiving some piggybacked extra dilution of the IV solution to improve tolerability.
Number of subjects reporting major adverse effects
Magnesium versus placebo
Pregnancy‐associated cramps
Nygaard 2008 reported no major adverse events during the study. The other studies do not state this outcome explicitly.
Idiopathic rest cramps
Garrison 2011 and Roffe 2002 both reported one stroke that occurred in the placebo arms but reported no other major adverse events. These data were not available from Rosenbaum 2011, and Frusso 1999 did not explicitly state whether major adverse events occurred. Being a cross‐over trial without data on all patients for both periods, Roffe 2002 could not be combined with Garrison 2011 to improve the estimate of major adverse event rates.
Discussion
Magnesium versus placebo
Idiopathic rest cramps
There is moderate quality evidence that magnesium supplementation does not offer a clinically important benefit over placebo in the prophylaxis of idiopathic cramps in older adults (most of whom are presumed to have been experiencing nocturnal leg cramps). The difference in percentage change in cramps per week from baseline at four weeks was small and nonsignificant (‐3.93%, 95% CI ‐21.12 to 13.26) and the CI excludes a 25% reduction (the difference we predefined as being clinically important). In addition, while medications are often only effective in a subset of patients, the percentage of patients obtaining a 25% or greater reduction from baseline in the number of cramps per week at four weeks did not even trend in favor of magnesium (‐8%, 95% CI ‐28% to 12%). This makes it less likely that a meaningful subset of patients receiving a greater than 25% benefit is being missed by averaging their results in with nonresponders. Cramp frequency was also measured looking at the number of cramps per week on treatment at four weeks. This measure allowed pooling of the largest number of studies (four studies, N = 216) and similarly found no significant reduction in the change in cramps per week on treatment (0.01 cramps per week, 95% CI ‐0.52 to 0.55) with a CI that excludes a one cramp per week reduction.
Similarly, the MD in cramp intensity on a three‐point scale at four weeks was no different (‐0.04, 95% CI ‐0.21 to 0.13), nor was the mean percentage of patients rating their cramps as moderate or severe, which was a nonsignificant nine per cent greater (95% CI ‐7% to 25%) in the magnesium group. Only one study reported cramp duration in a format that allowed us to determine the number of subjects with the majority of cramp durations longer than one minute. In this study, the mean percentage of participants having the majority of their cramp durations lasting one minute or longer was a nonsignificant 19% greater (95% CI ‐7% to 45%) in the magnesium group. Two other studies reported cramp duration in different formats and in neither was there a significant difference. All the above outcomes were also sought at 12 weeks but only one study had 12‐week data. The results of this single study at 12 weeks were not materially different than the four‐week results above.
Adverse events and withdrawals due to adverse events were poorly reported in most trials. Supplementing the number of withdrawals due to adverse events with total withdrawals, we found a nonsignificant difference for the number of withdrawals due to adverse events of ‐3% (95% CI ‐10% to 3%). Two major adverse events (strokes) occurred in the placebo groups and none in the magnesium group. The number of patients with minor adverse events could not be meaningfully estimated. Qualitatively it appears that the frequency of side effects from oral magnesium are low and minimally different from placebo (with the possible exception of diarrhoea). Intravenous magnesium appeared to cause a burning sensation at the IV site in half of patients (compared to none in the placebo group) and a small subset of patients had either asymptomatic hypotension or light‐headedness.
Pregnancy‐associated cramps
It is unclear whether magnesium supplementation can provide an advantage over placebo in the prophylaxis of pregnancy‐associated cramps since the only two relevant studies were discordant and did not report results in a way that enabled us to pool their data. Dahle 1995 reported a reduction in cramp frequency on a 5‐point ordinal scale, a reduction in cramp intensity on a VAS, and a more favorable global assessment of treatment effect also on a 5‐point ordinal scale. In contrast, the subsequent trial (Nygaard 2008) measured the mean number of days and nights with leg cramps present and found no significant benefit (7.7 ± 4.7 (SD) days and nights of cramping over two weeks in the placebo group and 9.5 ± 5.1 in the magnesium group, P = 0.27). Cramp intensity (5‐point ordinal scale) was also not significantly different. Neither of these studies reported the duration of cramps. Nygaard 2008 reported the number of subjects with minor adverse events to be the same (six subjects) in placebo and magnesium arms and noted no major adverse events. Dahle 1995 reported only that side effects were infrequent and that one patient withdrew from the placebo group because of nausea.
Both of these trials were parallel double blind RCTs in Scandinavian maternity clinic patients and both used the same intervention, a chewable tablet containing 122 mg elemental magnesium "primarily as Mg lactate or Mg citrate" taken once each morning and twice each evening (366 mg daily). Despite these similarities they were discrepant. Factors which might have prevented Nygaard 2008 from showing benefit include less power (although there was not even a trend to benefit) due to fewer subjects (45 versus 73), a shorter period of therapy (two weeks versus three) and the lack of baseline measures of cramp frequency. The lack of baseline measures is particularly important since the outcome was the number of cramps during the treatment period and it is not known if the mean baseline cramp rates were equal. Conversely, the trial design of Dahle 1995 might have biased towards benefit in that outcomes were not well defined (in particular no primary outcome was identified), randomization and allocation concealment were not described and no cramp diary was used.
Magnesium versus other therapies (no treatment)
Pregnancy‐associated cramps
The single study (Sohrabvand 2006) to use a comparator other than placebo used four parallel treatment arms to compare no treatment with either oral magnesium, calcium carbonate or a combined vitamin B1 and B6 supplement. The outcome for this study was the patients' reporting of either no improvement, partial resolution or complete resolution of cramping. A multinomial regression test was applied to each of the three possible response categories to see if any of the active treatment arms differed from no treatment. Significantly more women reported absolute improvement in the B vitamin and calcium arms, but not in the magnesium arm. Neither was there a significant difference in relative improvement between no treatment and the magnesium arm. Comparisons between the active intervention arms were not made. It would not be unusual for placebo to show benefit from baseline in cramp trials. One might speculate that the same "placebo effect" benefit might be seen for any active intervention over no treatment, which makes the benefit seen for B vitamins and calcium over no treatment in this trial unreliable. However, magnesium was certainly no better than calcium or vitamin B supplements and was alone in not reaching significance for benefit against no treatment. Collectively, these results do not support a clinically important benefit for magnesium over no treatment.
Effect of dose, duration and route of administration
Serum magnesium levels are known to correlate poorly with tissue magnesium, making it difficult to detect patients with magnesium deficiency in the clinic. In theory, if magnesium deficiency played a role in skeletal muscle cramping, either the duration of therapy or the total dose provided over the course of the trial might be an important variable (since a deficit might be better replaced in longer duration, higher total dose trials). However, the differences in dosing and duration for the oral magnesium trials is not that great, with the idiopathic rest cramp trials using 200 mg to 336 mg of elemental magnesium daily over four to six weeks and the pregnancy‐associated cramp trials using either 364 or 366 mg daily over two to three weeks. No obvious pattern is present, but the oral trials are too few and too close in dose and duration to be able to detect any meaningful pattern. However, one trial (Garrison 2011) used a series of slow IV infusions of magnesium to improve delivery and simultaneously measured 24‐hour urinary magnesium excretion to determine the extent to which patients were retaining magnesium. Measuring the percentage retention of the infused magnesium was felt to be important because it had been used as a tool to predict total body magnesium deficiency (retention of magnesium suggesting the presence of deficiency) (al‐Ghamdi 1994; Cohen 1990; Lim 1972; Quamme 1993; Ryzen 1985), and because there is a strong positive correlation between the total amount of IV magnesium retained during replacement therapy and the rise in intracellular magnesium on skeletal muscle biopsy (Lim 1972). In this trial of IV magnesium, no correlation was found between percent retention of magnesium on day one of infusions and the reduction in the number of cramps per week from baseline. On its own, this finding argues against a therapeutic benefit from magnesium in providing cramp prophylaxis.
Potential biases in the review process
Data were available from all relevant RCTs identified for inclusion in this trial. This includes patient level data for three of the four idiopathic (older adult) cramp trials, including one cross‐over trial (Roffe 2002) where patient level data were used (by looking at the first period only) to reduce potential bias from an unbalanced randomization and a strong treatment order effect. Although we were able to identify one relatively recent (2011) unpublished trial using a clinical trial registry, we could be missing other unpublished RCTs that predate trial registration. However, we would expect such missing trials to be less likely to have demonstrated a significant benefit because of publication bias. All of the included trials were fairly small, ranging from 40 to 73 subjects, and all had some degree of methodological limitations (Figure 2). However, heterogeneity was low for all but one analysis (percentage of subjects rating their cramps as moderate or severe) where a difference in pain scores may have resulted from differences in the method of recruitment.
Authors' conclusions
Implications for practice.
It is unlikely that magnesium supplementation provides clinically meaningful cramp prophylaxis to older adults experiencing skeletal muscle cramps. This is supported by evidence of moderate quality. In contrast, for women suffering pregnancy‐associated rest cramps the literature is conflicting and unclear. No RCTs evaluating magnesium for exercise‐associated muscle cramps or disease state‐associated muscle cramps have been conducted.
Implications for research.
Given the low probability of benefit in older adult cramp sufferers, investigators may be less inclined to pursue the evaluation of magnesium for other cramp indications. However, there is conflicting evidence surrounding the benefit of magnesium for pregnancy‐associated leg cramps and it is conceivable that magnesium could have differing efficacies in metabolically distinct populations. To resolve the uncertainty surrounding the role of magnesium in pregnant women, parallel‐group blinded placebo‐controlled RCTs of magnesium in that population are needed. Trialists should measure cramp rates as a continuous variable (for example number of cramps on treatment or change from baseline) to permit pooling of data. If not using change from baseline, they should also consider stratifying the study randomization by baseline cramp rate to help prevent an unequal distribution of cramp frequencies invalidating their findings.
Acknowledgements
The Cochrane Neuromuscular Disease Group editorial base is supported by the MRC Centre for Neuromuscular Disease, the Motor Neurone Disease Association and the Muscular Dystrophy Campaign. Penelope M. A. Brasher, PhD, provided statistical support for this review and Shannon Long, MLIS, assisted with literature retrieval.
Appendices
Appendix 1. CENTRAL search strategy
#1"muscle cramp*" or "muscle spasm*" or "muscle contraction*" #2"charley horse*" or "charlie horse*" #3eamc #4"exercise associated muscle cramp*" #5(#1 OR #2 OR #3 OR #4) #6magnesium or mg2 #7(#5 AND #6)
Appendix 2. MEDLINE (OvidSP) search strategy
1 randomized controlled trial.pt. (319110) 2 controlled clinical trial.pt. (83678) 3 randomized.ab. (224381) 4 placebo.ab. (129163) 5 drug therapy.fs. (1505532) 6 randomly.ab. (161540) 7 trial.ab. (232182) 8 groups.ab. (1070965) 9 or/1‐8 (2788051) 10 exp animals/ not humans.sh. (3698786) 11 9 not 10 (2365479) 12 Muscle Cramp$.mp. (2287) 13 muscle spasm$.mp. (1208) 14 Muscle Contraction$.mp. (90622) 15 (charley horse$ or charlie horse$).mp. (3) 16 eamc.mp. (13) 17 exercise associated muscle cramp$.mp. (14) 18 or/12‐17 (93884) 19 (magnesium or mg2).mp. (96848) 20 11 and 18 and 19 (84) 21 remove duplicates from 20 (82)
Appendix 3. EMBASE (OvidSP) search strategy
1 crossover‐procedure/ (30907) 2 double‐blind procedure/ (100996) 3 randomized controlled trial/ (290224) 4 single‐blind procedure/ (14260) 5 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. (1024327) 6 or/1‐5 (1094729) 7 exp animals/ (1655604) 8 exp humans/ (12628304) 9 7 not (7 and 8) (1260032) 10 6 not 9 (1058983) 11 limit 10 to embase (858133) 12 (muscle cramp$ or muscle spasm$ or muscle contraction$).mp. (84647) 13 (charley horse$ or charlie horse$).mp. (3) 14 eamc.mp. (17) 15 exercise associated muscle cramp$.mp. (20) 16 or/12‐15 (84654) 17 (magnesium or mg2).mp. (124341) 18 11 and 16 and 17 (57) 19 remove duplicates from 18 (57)
Appendix 4. LILACS search strategy
muscle cramp$ or muscle spasm$ or muscle contraction$ or charley horse$ or charlie horse$ or eamc or exercise associated muscle cramp$ [Words] and magnesium or mg2 [Words] and ((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal))) [Words]
Appendix 5. CINAHL Plus (EBSCOhost) search strategy
S24 S18 and S22 and S23 S23 magnesium or mg2 S22 s19 or s20 or s21 S21 eamc or exercise associated muscle cramp* S20 charley horse* or charlie horse* S19 muscle cramp* or muscle spasm* or muscle contraction* S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 S17 ABAB design* S16 TI random* or AB random* S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) S13 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) S11 PT ("clinical trial" or "systematic review") S10 (MH "Factorial Design") S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") S8 (MH "Meta Analysis") S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") S6 (MH "Quasi‐Experimental Studies") S5 (MH "Placebos") S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") S3 (MH "Clinical Trials+") S2 (MH "Crossover Design") S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample")
Appendix 6. AMED (OvidSP) search strategy
1 Randomized controlled trials/ (1462) 2 Random allocation/ (299) 3 Double blind method/ (424) 4 Single‐Blind Method/ (21) 5 exp Clinical Trials/ (3109) 6 (clin$ adj25 trial$).tw. (5273) 7 ((singl$ or doubl$ or treb$ or trip$) adj25 (blind$ or mask$ or dummy)).tw. (2166) 8 placebos/ (514) 9 placebo$.tw. (2448) 10 random$.tw. (12220) 11 research design/ (1663) 12 Prospective Studies/ (396) 13 meta analysis/ (106) 14 (meta?analys$ or systematic review$).tw. (1668) 15 control$.tw. (26563) 16 (multicenter or multicentre).tw. (701) 17 ((study or studies or design$) adj25 (factorial or prospective or intervention or crossover or cross‐over or quasi‐experiment$)).tw. (9356) 18 or/1‐17 (40966) 19 (cramp$ or spasm$ or contraction$).mp. (5288) 20 (charley horse$ or charlie horse$).mp. (0) 21 eamc.mp. (1) 22 exercise associated muscle cramp$.mp. (3) 23 or/19‐22 (5288) 24 (magnesium or mg2).mp. (229) 25 18 and 23 and 24 (1)
Appendix 7. SPORTDiscus (EBSCOHost) search strategy
S11 S5 and S9 and S10 S10 magnesium or mg2 S9 S6 or S7 or S8 S8 eamc S7 charley horse* or charlie horse* S6 cramp* or spasm* or contraction* S5 S1 or S2 or S3 or S4 S4 cross?over or placebo* or control* or factorial or sham? or dummy S3 (single* or doubl* or tripl* or trebl*) and (blind* or mask*) S2 clinical trial* S1 randomi*
Data and analyses
Comparison 1. Idiopathic rest cramp efficacy, magnesium versus placebo.
Comparison 2. Adverse effects of treatment, magnesium versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawals due to adverse events | 4 | 201 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.10, 0.03] |
| 1.1 Idiopathic cramps (largely older adults) | 2 | 86 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.06, 0.11] |
| 1.2 Pregnancy‐associated leg cramps | 2 | 115 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.17, 0.02] |
| 2 Number of subjects with major adverse events | 2 | 91 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.05] |
| 2.1 Idiopathic cramps (largely older adults) | 1 | 46 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.05 [‐0.16, 0.07] |
| 2.2 Pregnancy‐associated leg cramps | 1 | 45 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.08, 0.08] |
| 3 Number of subjects with minor adverse events | 1 | 45 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.27, 0.25] |
| 3.1 Idiopathic cramps (largely older adults) | 0 | 0 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Pregnancy‐associated leg cramps | 1 | 45 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.27, 0.25] |
2.2. Analysis.
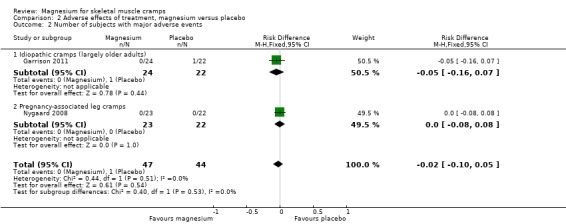
Comparison 2 Adverse effects of treatment, magnesium versus placebo, Outcome 2 Number of subjects with major adverse events.
2.3. Analysis.
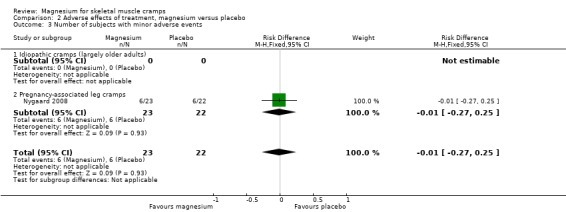
Comparison 2 Adverse effects of treatment, magnesium versus placebo, Outcome 3 Number of subjects with minor adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dahle 1995.
| Methods | Double‐blind, parallel group RCT | |
| Participants | 73 pregnant women (mean 29 wk gestation) with rest cramps and no previous cramp treatment. Recruitment from Swedish prenatal care clinics. | |
| Interventions | Either a chewable tablet containing 122 mg elemental magnesium ("primarily as Mg lactate or Mg citrate"), or matched placebo tablet, taken once each morning and twice each evening for 3 weeks | |
| Outcomes | Primary outcome unclear. Change in cramp frequency on a 5‐point ordinal scale. Time of day cramps occurred on a 4‐point nominal scale. Presence of symptoms the day after a night of cramping on a 3‐point ordinal scale. Global patient assessment of treatment effect on a 5‐point ordinal scale. Cramp intensity on a visual analog scale (VAS). Serum magnesium and calcium and 24‐h urinary magnesium and calcium excretion | |
| Notes | Published. Manufacturer sponsored. Did laboratory tests at only one of the two centres | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described ("The patients were then randomly allocated to either magnesium or placebo") |
| Allocation concealment (selection bias) | Unclear risk | Not described. See above for only quote |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “A magnesium‐placebo tablet batch of 90 numbered bottles was prepared by ACO Lakemedel...” Comment: Probably satisfactory, although pills were not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/73 subjects dropped out of the study and were excluded from the analysis. Reasons for dropout were well described but treatment group was not identified. One placebo patient withdrew from treatment but appears (unclear) to have been included in the analysis. Comment: Probably adequate as total number of dropouts was small. |
| Selective reporting (reporting bias) | High risk | No description of outcomes by primary and secondary, and outcomes were incompletely described in methods, i.e. only in the results is it evident that before and after comparisons, mean differences and numbers attaining specific cut‐offs are used. Unclear how well outcomes were predefined. Inadequate reporting: no actual numbers for many P values. This study also reported a reduction in cramp frequency "from the initial average of every other day, to every 3 days in the placebo group and one to two times a week in the magnesium group (P < 0.05)". However, "every 3 days" and "one to two times a week" do not belong to the 5‐point ordinal scale used to measure this outcome (daily, every other day, twice a week, once a week, never). |
| Cramp diary (recall bias) | High risk | No diary used |
| Other bias | Unclear risk | Subjects treated differently at each site (one used laboratory testing, the other did not). |
Frusso 1999.
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 45 non‐pregnant rest cramp sufferers > 18 years (mean age 61.6 years) having a normal neurologic exam and at least 6 leg cramps in a 4‐week placebo run‐in. Recruitment from a single large university‐based Argentinean family practice clinic. | |
| Interventions | Magnesium citrate 900 mg pill (approx. 100 mg elemental magnesium) twice daily or similar tasting and appearing placebo, each for 4 weeks. Four‐week placebo run‐in and 4‐week washout between treatments. | |
| Outcomes | Primary: Number of cramps in treatment period. Secondary: Cramp duration by 4 ordinal categories (< 5 minutes, 5 to 10 minutes, 10 to 30 minutes, > 30 minutes). Cramp intensity by “analog scale”. Sleep disturbance on a 0 to 10 scale with 0 = “no sleep disturbance” and 10 = “could not sleep because of the cramps”. Adverse events. | |
| Notes | Published. Independent funding. The 4‐week placebo run‐in was pre‐randomization. Unclear what the range for the analog scale of intensity is (assumed 0 to 10). Cramp duration was recorded by ordinal category but reported with a mean and standard deviation in minutes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients randomly received magnesium or placebo...” Comment: unclear how randomization was performed |
| Allocation concealment (selection bias) | Unclear risk | Quote: “The codes were inside a sealed envelope opened at the end of the analysis.” Comment: Unclear who allocated subjects and maintained the blinding |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “Each pill contained 900 mg of magnesium citrate or matched placebo (same appearance and taste).” Comment: Satisfactory blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/45 subjects withdrew with reasons given. It is not stated which intervention they were receiving at the time or how their data were dealt with. Comment: Probably satisfactory as the number of dropouts was small. |
| Selective reporting (reporting bias) | Unclear risk | No indication of selective reporting for clinical endpoints (although urine for magnesium was collected and not reported). Duration of cramps was measured on a 4‐point ordinal scale but results were reported with the mean duration and standard deviation measured in minutes as though they were a continuous variable |
| Cramp diary (recall bias) | Low risk | Diary used |
| Other bias | Low risk | No obvious other bias |
Garrison 2011.
| Methods | Double‐blind, parallel group RCT | |
| Participants | 46 non‐pregnant rest cramp sufferers (mean age 69.3 yrs) with at least 8 cramps in a 30 day baseline diary. Recruitment from posters and pamphlets in 21 Canadian (Richmond BC) family practitioner offices and also by newspaper advertizement | |
| Interventions | 5 days consecutive 4 hour intravenous infusions of 250 ml D5W (5% dextrose in water) either with (treatment group) or without (control group) 20 mmol of magnesium sulfate added (20 mmol = 486 mg elemental magnesium). Indistinguishable. | |
| Outcomes | Primary: Change in the number of cramps per week from baseline at 30 days. Secondary: Change in the number of cramps per week from baseline at 90 days. Percentage change in cramps / wk. Cramp pain (1 to 10 interval scale). Cramp duration on a 3‐point ordinal scale (1 = < 1 minute, 2 = 1‐5 minutes, 3 = > 5 minutes). 24‐h urinary magnesium on days 1 and 5 to determine % retention of infused magnesium. | |
| Notes | Published. Independent funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization, using a computer generated random allocation sequence without any blocking or stratification was carried out by the hospital pharmacist dispensing the study drugs according to a series of opaque allocation envelopes kept in the pharmacy." Comment: Satisfactory randomization |
| Allocation concealment (selection bias) | Low risk | Quote: "All investigators, study nurses and subjects were blinded as to treatment allocation." Comment: Satisfactory allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: 1) "Active and Placebo solutions were indistinguishably clear and colorless." 2) "Subjects had been told that IV site discomfort was possible with both placebo and Mg infusions. While generally it was considered that blinding was reasonable, the sensation of burning at the IV site, coupled with the additional saline dilution in some Mg subjects, could have compromised the blind to some extent (presumably favouring the intervention)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs or losses to follow‐up. Analysis was intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | Did not process urine samples for magnesium on those getting placebo (although did a reasonable job collecting urine samples from all patients to make sure the blinding was not broken). Severity and duration of cramps were described only as not being different (i.e. no numbers given), however, these data were made available by the authors. |
| Cramp diary (recall bias) | Low risk | Diary used |
| Other bias | Low risk | No obvious other bias |
Nygaard 2008.
| Methods | Double‐blind, parallel group RCT | |
| Participants | 45 pregnant women with rest cramps and no previous cramp treatment. Recruitment by pamphlets provided to pregnant Norwegian women undergoing 18 week ultrasound | |
| Interventions | Either a chewable tablet containing 122 mg elemental magnesium ("primarily as Mg lactate and Mg citrate"), or a matched placebo tablet, taken once each morning and twice each evening for 2 weeks | |
| Outcomes | Number of days or nights in which cramps occurred over 2 weeks. Degree of cramp pain on a 5‐point ordinal scale. Side effects. Serum magnesium and calcium and 24‐h urinary magnesium on days 1 and 15 | |
| Notes | Published. Source of funding not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomization program was provided by Medstat Research AS.” Comment: Probably adequate |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation method given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: “Both groups received a plastic container with the trial medication, 42 chewable tablets...”, containing either magnesium or placebo, both provided by the manufacturer Comment: Probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7/45 women (15.6%) dropped out (2 from the treatment arm and 5 from control). Reasons were given and most were unrelated to potential drug effects. None of the 7 were included in the analysis because of a lack of data. |
| Selective reporting (reporting bias) | Low risk | Primary outcome assumed to be the number of days and nights with cramping but not explicitly stated. All outcomes reported. |
| Cramp diary (recall bias) | Low risk | Diary used |
| Other bias | Unclear risk | Frequency of cramping at baseline was not assessed, making it impossible to tell if the group was imbalanced in this important baseline characteristic |
Roffe 2002.
| Methods | Double blind RCT of cross‐over design | |
| Participants | 73 non‐pregnant rest cramp sufferers (mean age 63 yrs), having at least 2 cramps per week. Recruitment by community advertizement in a UK population | |
| Interventions | Either 1830 mg of tri‐magnesium dicitrate powder (300 mg elemental magnesium) poured from a sachet into a glass of water, or matched placebo powder, taken orally each night for 6 weeks before switching to the alternate therapy. 2 week magnesium free run‐in and effectively a 2 week washout between treatments since only the last 4 weeks of each 6 weeks on treatment was used for outcome assessment | |
| Outcomes | Number of cramps during the last 4 weeks of each treatment period. Severity of cramps (mild, moderate, severe). Duration of cramps (short, medium, long). Self reported assessment of treatment effectiveness (yes, no) | |
| Notes | Published. Manufacturer sponsored. Only data from the first period have been used in this review because large differences in treatment effect are seen depending on the sequence in which treatment is given. Patient level data provided by the principle investigator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The manufacturer provided centralized randomization for the trial in large blocks of 10. Specifics regarding the sequence generation were not given. The resulting allocation was unequal with more subjects included in the analysis receiving magnesium second (29 vs 17). |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation code was not known to the investigators who gave out the sachets. The code remained concealed from everyone except the pharmacist who prepared the sachets..." Comment: Satisfactory concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No description of whether the magnesium and placebo suspensions tasted different |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for dropout documented, but 27 of 73 subjects (37%) did not complete the study |
| Selective reporting (reporting bias) | Low risk | Severity and duration of cramps were described only as not being different (i.e. no numbers given), however, these data were provided to us by the authors. |
| Cramp diary (recall bias) | Low risk | Diary used |
| Other bias | Unclear risk | Manufacturer played an active role in the trial. There was a large difference in treatment effect depending on the sequence of treatments (much greater benefit if treatment was received in the order placebo→magnesium). Unclear if this difference was due entirely to period effect or if noncompleters, the potential for carry‐over or unblinding contributed. This difference in benefit resulting from treatment order was important since the randomization was unbalanced (many more subjects receiving the placebo→magnesium sequence). |
Rosenbaum 2011.
| Methods | Double blind, parallel group RCT | |
| Participants | 40 non‐pregnant rest cramp sufferers (45 to 80 years of age) with normal renal function having at least 2 cramps per week that were rated 5 or more on a 0‐10 pain scale. Recruitment by radio advertisement in an American (State of Michigan) population. | |
| Interventions | Either 168 mg elemental magnesium from slow release magnesium lactate tablets (MagTabSR) or matching placebo tablets taken orally twice daily for 30 days. | |
| Outcomes | Frequency, duration and severity of leg cramps captured daily x 1 wk pre‐intervention and daily during the 30 days of intervention (via diary recording of cramps and sleep disturbance). Pittsburgh Sleep Quality questionnaire also administered pre‐ and post‐intervention. | |
| Notes | Unpublished. Sponsorship not provided. Incomplete results reporting. Patient level data for cramp frequency were kindly provided by study statistician. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomized but details not provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Masking: Double Blind (Subject, Investigator, Outcomes Assessor)". No details provided. Probably adequate. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small number of dropouts, two from magnesium and one from placebo. Reasons not provided. |
| Selective reporting (reporting bias) | High risk | Patient level data were provided to us but were only available for a subset of the outcomes. |
| Cramp diary (recall bias) | Low risk | Diary used |
| Other bias | Low risk | No obvious other bias |
Sohrabvand 2006.
| Methods | Open label RCT with 4 parallel treatment groups | |
| Participants | 84 pregnant women. Recruitment method (Iranian women) not provided. | |
| Interventions | Group 1: 500 mg calcium carbonate tablet once daily Group 2: 7.5 mmol magnesium aspartate (182 mg elemental magnesium) twice daily Group 3: 100 mg of thiamine (vitamin B1) plus 40 mg of pyridoxine (vitamin B6) once daily Group 4: No treatment |
|
| Outcomes | "Change in muscle spasms" on a 3‐point ordinal scale (no change, "relative improvement", or "absolute improvement") | |
| Notes | Unusual design. Each treatment was given over two weeks but efficacy was assessed at 4 weeks. Published as a "brief communication" (letter) only. Funding source not provided. No definition of relative and absolute improvement was given in the manuscript but this was confirmed with the author to mean partial and complete resolution of the overall cramp burden (which presumably takes into account both intensity and frequency). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No details regarding flow of patients in the manuscript but author communication suggests no dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome not identified (though only one outcome reported). Table 2 showed statistical significance in total improvement for groups 2 and 3 compared to group 4 but in the text it stated groups 1 and 3 (which is supported by the CI results). |
| Cramp diary (recall bias) | High risk | Specifics were not given but there appeared to have only been a qualitative assessment of the change in cramps upon study completion |
| Other bias | High risk | Baseline characteristics were said to be not significantly different but they were not provided. Unclear who rated the degree of improvement (patient or physician).Trial was very under reported. Outcomes were grouped in an impractical way |
CI: confidence interval IV: intravenous RCT: randomized controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aagaard 2005 | This RCT looked at muscle strength, muscle mass and muscle magnesium content. It did not look at measures of muscle cramping |
| Bachem 1986 | No control group. Article in German with English abstract. Methods translated |
| Bartl 1982 | Did not appear to be randomized. Evaluated serum magnesium levels in pregnant cramp sufferers before and after magnesium supplementation. Did not evaluate changes in muscle cramping. Article in German with English abstract. Methods translated |
| Hammar 1987 | No magnesium treatment arm |
| Häringer 1981 | No control group. Article in German. Abstract and Methods translated |
| Riss 1983 | Uncontrolled. Article in German with English abstract |
| Weller 1998 | This RCT looked at exercise performance and magnesium concentration in various tissues. It did not look at measures of muscle cramping |
RCT: randomized controlled trial
Differences between protocol and review
Searching other resources
We ultimately carried out three additional approaches to obtaining studies for our review that we had not pre‐identified in our study protocol. As a result we added a sentence to the end of the "Searching other resources" section of the protocol which states: "We also searched the International Clinical Trials Registry Platform (WHO‐ICTRP) in an attempt to uncover unpublished trials, searched ISI Web of Science for papers citing the studies included in this review, and contacted the FDA to ask if they had any related clinical trial information in their possession."
'Summary of findings' table addition
Under the heading 'Secondary outcomes' in the study protocol we had pre‐identified our plans for a 'Summary of findings table' with several elements. Since considerably more poolable data were available for the outcome "Number of cramps per week at four weeks" (i.e. there were four poolable studies, compared to just two for the other frequency estimates) we chose to add this outcome to the 'Summary of findings' table. We did this because we believed number of cramps per week at four weeks to be the most reliable measure of cramp frequency given the greater amount of available data.
Number of subjects reporting minor adverse events
Although we wished to determine the number of subjects reporting any minor adverse events this was not possible from the trial descriptions, which typically described the occurrence of adverse events but not the number of subjects with one or more adverse events (i.e. the same patient could contribute to the reporting of multiple adverse events). As a result, our description of minor adverse events was qualitative.
Contributions of authors
Protocol Stage: Dr Garrison drafted the protocol and the other authors commented and approved the final text.
Review Stage: Dr Garrison and Dr Sekhon selected studies for inclusion. Dr Garrison and Dr Allan extracted data. Drs Garrison, Allan and Musini rated studies for bias (Dr Garrison did not participate in the rating of his own trial). Dr Garrison performed all data analysis and wrote the draft manuscript. All authors commented on and approved the final text.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Canadian Institutes of Health Research ‐ Doctoral Research Award in the Area of Research on Aging, Canada.
Trainee Stipend (Dr. Garrison)
Declarations of interest
Drs Garrison and Khan are co‐authors of a RCT which was included in this review. None of the co‐authors of this review have links, financial or otherwise, to any company or special interest group that is involved in the marketing of magnesium or other cramp related therapies.
New
References
References to studies included in this review
Dahle 1995 {published data only (unpublished sought but not used)}
- Dahle LO, Berg G, Hammar M, Hurtig M, Larsson L. The effect of oral magnesium substitution on pregnancy‐induced leg cramps. American Journal of Obstetrics and Gynecology 1995;173(1):175‐80. [PUBMED: 7631676] [DOI] [PubMed] [Google Scholar]
Frusso 1999 {published data only (unpublished sought but not used)}
- Frusso R, Zárate M, Augustovski F, Rubinstein A. Magnesium for the treatment of nocturnal leg cramps: a crossover randomized trial. Journal of Family Practice 1999;48(11):868‐71. [PUBMED: 10907623] [PubMed] [Google Scholar]
Garrison 2011 {published and unpublished data}
- Garrison SR, Birmingham CL, Koehler BE, McCollom RA, Khan KM. The effect of magnesium infusion on rest cramps: randomized controlled trial. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2011;66(6):661‐6. [PUBMED: 21289017] [DOI] [PubMed] [Google Scholar]
Nygaard 2008 {published data only (unpublished sought but not used)}
- Nygaard IH, Valbø A, Pethick SV, Bøhmer T. Does oral magnesium substitution relieve pregnancy‐induced leg cramps?. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2008;141(1):23‐6. [PUBMED: 18768245] [DOI] [PubMed] [Google Scholar]
Roffe 2002 {published and unpublished data}
- Roffe C, Sills S, Crome P, Jones P. Randomised, cross‐over, placebo controlled trial of magnesium citrate in the treatment of chronic persistent leg cramps. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 2002;8(5):CR326‐30. [PUBMED: 12011773] [PubMed] [Google Scholar]
Rosenbaum 2011 {unpublished data only}
- Rosenbaum L. Beneficial effects of magnesium supplementation on idiopathic muscle cramps. ClinicalTrials.gov Identifier: NCT00963638.
Sohrabvand 2006 {published data only (unpublished sought but not used)}
- Sohrabvand F, Shariat M, Haghollahi F. Vitamin B supplementation for leg cramps during pregnancy. International Journal of Gynaecology and Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics 2006;95(1):48‐9. [PUBMED: 16919630] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aagaard 2005 {published data only}
- Aagaard NK, Andersen H, Vilstrup H, Clausen T, Jakobsen J, Dorup I. Magnesium supplementation and muscle function in patients with alcoholic liver disease: a randomized, placebo‐controlled trial. Scandinavian Journal of Gastroenterology 2005;40(8):972‐9. [PUBMED: 16173138] [DOI] [PubMed] [Google Scholar]
Bachem 1986 {published data only}
- Bachem MG, Scheffler K, Jastram U, Pfeiffer EF. Effectiveness of oral magnesium substitution in type I diabetic patients with nocturnal leg cramps [Effektivität einer peroralen Magnesiumsubstitution bei Typ‐I‐Diabetikern mit nächtlichen Wadenkrämpfen]. Magnesium‐Bulletin 1986;8(3):280‐3. [Google Scholar]
Bartl 1982 {published data only}
- Bartl W, Riss P. Pathophysiology and therapy of magnesium deficiency in pregnancy [Zur Pathophysiologie und Therapie des Magnesiummangels in der Schwangerschaft]. Zeitschrift für Geburtshilfe und Perinatologie 1982;186(6):335‐7. [PubMed] [Google Scholar]
Hammar 1987 {published data only}
- Hammar M, Berg G, Solheim F, Larsson L. Calcium and magnesium status in pregnant women. A comparison between treatment with calcium and vitamin C in pregnant women with leg cramps. International Journal for Vitamin and Nutritional Research 1987;57(2):179‐83. [PUBMED: 3308737] [PubMed] [Google Scholar]
Häringer 1981 {published data only}
- Häringer E. Calf cramps at night: A deficiency of magnesium? [Wadenkrämpfe in der Nacht: Fehlt Magnesium?]. Ärtzliche Praxis 1981;33:2653‐4. [Google Scholar]
Riss 1983 {published data only}
- Riss P, Bartl W, Jelincic D. Clinical aspects and treatment of calf muscle cramps during pregnancy [Zur Klinik und Therapie von Wadenkrämpfen in der Schwangerschaft]. Geburtshilfe und Frauenheilkunde 1983;43(5):329‐31. [PUBMED: 6553557] [DOI] [PubMed] [Google Scholar]
Weller 1998 {published data only}
Additional references
ADRAC 2002
- Adverse Drug Reactions Advisory Committee. Quinine and profound thrombocytopenia. Australian Adverse Drug Reactions Bulletin 2002;21(3):10. [Google Scholar]
AHFS 2010
- Magnesium sulfate. In: McEvoy GK editor(s). American Hospital Formulary Service (AHFS) Drug Information. 2010. Bethesda, MD: American Society of Health‐System Pharmacists, 2010:2282‐5. [Google Scholar]
al‐Ghamdi 1994
- al‐Ghamdi SM, Cameron EC, Sutton RA. Magnesium deficiency: pathophysiologic and clinical overview. American Journal of Kidney Diseases 1994;24(5):737‐52. [DOI] [PubMed] [Google Scholar]
Bilbey 1996
- Bilbey DL, Prabhakaran VM. Muscle cramps and magnesium deficiency: case reports. Canadian Family Physician 1996;42:1348‐51. [PMC free article] [PubMed] [Google Scholar]
Cohen 1990
- Cohen L, Laor A. Correlation between bone magnesium concentration and magnesium retention in the intravenous magnesium load test. Magnesium Research 1990;3(4):271‐4. [PubMed] [Google Scholar]
Eclampsia Trialists 1995
- The Eclampsia Trial Collaborative Group. Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet 1995;345(8963):1455‐63. [PubMed] [Google Scholar]
El‐Tawil 2011
- El‐Tawil S, Al Musa T, Valli H, Lunn MPT, El‐Tawil T, Weber M. Quinine for muscle cramps. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD005044.pub2] [DOI] [PubMed] [Google Scholar]
FDA 2006
- Drug products containing quinine; enforcement action dates. Federal Register 2006; Vol. 71, issue 241:75557–60.
Garrison 2012
- Garrison SR, Dormuth CR, Morrow RL, Carney GA, Khan KM. Nocturnal leg cramps and prescription use that precedes them: a sequence symmetry analysis. Archives of Internal Medicine 2012;172(2):120‐6. [DOI] [PubMed] [Google Scholar]
Graham 1960
- Graham LA, Caesar JJ, Burgen AS. Gastrointestinal absorption and excretion of Mg 28 in man. Metabolism 1960;9:646‐59. [PubMed] [Google Scholar]
Hall 1973
- Hall RC, Joffe JR. Hypomagnesemia. Physical and psychiatric symptoms. JAMA 1973;224(13):1749‐51. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Katzberg 2010
- Katzberg HD, Khan AH, So YT. Assessment: symptomatic treatment for muscle cramps (an evidence‐based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2010;74(8):691‐6. [DOI] [PubMed] [Google Scholar]
Lim 1972
- Lim P, Jacob E. Magnesium status of alcoholic patients. Metabolism 1972;21(11):1045‐51. [DOI] [PubMed] [Google Scholar]
McGee 1990
- McGee SR. Muscle cramps. Archives of Internal Medicine 1990;150(3):511‐8. [PubMed] [Google Scholar]
Medsafe 2007
- Medsafe Pharmacovigilance Team, New Zealand Medicines and Medical Devices Safety Authority. Quinine ‐ not for leg cramps anymore. Prescriber Update 2007;28(1):2‐6. [Google Scholar]
Miller 2005
- Miller TM, Layzer RB. Muscle cramps. Muscle and Nerve 2005;32(4):431‐42. [DOI] [PubMed] [Google Scholar]
Naylor 1994
- Naylor JR, Young JB. A general population survey of rest cramps. Age and Ageing 1994;23(5):418‐20. [DOI] [PubMed] [Google Scholar]
Quamme 1993
- Quamme GA. Laboratory evaluation of magnesium status. Renal function and free intracellular magnesium concentration. Clinics in Laboratory Medicine 1993;13(1):209‐23. [PubMed] [Google Scholar]
Quamme 2008
- Quamme GA. Recent developments in intestinal magnesium absorption. Current Opinion in Gastroenterology 2008;24(2):230‐5. [DOI] [PubMed] [Google Scholar]
Ryzen 1985
- Ryzen E, Elbaum N, Singer FR, Rude RK. Parenteral magnesium tolerance testing in the evaluation of magnesium deficiency. Magnesium 1985;4(2‐3):137‐47. [PubMed] [Google Scholar]
Schwellnus 2008
- Schwellnus MP, Drew N, Collins M. Muscle cramping in athletes‐‐risk factors, clinical assessment, and management. Clinics in Sports Medicine 2008;27(1):183‐94, ix‐x. [DOI] [PubMed] [Google Scholar]
Shils 1969
- Shils ME. Experimental human magnesium depletion. Medicine (Baltimore) 1969;48(1):61‐85. [DOI] [PubMed] [Google Scholar]
Somjen 1966
- Somjen G, Hilmy M, Stephen CR. Failure to anesthetize human subjects by intravenous administration of magnesium sulfate. Journal of Pharmacology and Experimental Therapeutics 1966;154(3):652‐9. [PubMed] [Google Scholar]
Vickers 2001
- Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Medical Research Methodology 2001;1:6. [DOI: 10.1186/1471-2288-1-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2002
- Young G, Jewell D. Interventions for leg cramps in pregnancy. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD000121] [DOI] [PubMed] [Google Scholar]


