Abstract
Phagocytosis is one of the key innate defense mechanisms executed by specialized cells in multicellular animals. Recent evidence suggests that a particular phagocytic receptor expressed by human polymorphonuclear granulocytes, the carcinoembryonic antigen-related cell adhesion molecule 3 (CEACAM3), is one of the fastest-evolving human proteins. In this focused review, we will try to resolve the conundrum why a conserved process such as phagocytosis is conducted by a rapidly changing receptor. Therefore, we will first summarize the biochemical and structural details of this immunoglobulin-related glycoprotein in the context of the human CEACAM family. The function of CEACAM3 for the efficient, opsonin-independent detection and phagocytosis of highly specialized, host-restricted bacteria will be further elaborated. Taking into account the decisive role of CEACAM3 in the interaction with pathogenic bacteria, we will discuss the evolutionary trajectory of the CEACAM3 gene within the primate lineage and highlight the consequences of CEACAM3 polymorphisms in human populations. From a synopsis of these studies, CEACAM3 emerges as an important component of human innate immunity and a prominent example of a dedicated receptor for professional phagocytosis.
Keywords: phagocytosis, CEACAM3, pathogenic bacteria, granulocyte, human innate immunity, ITAM, immunoreceptor tyrosine-based activation motif, signal transduction
Introduction
The ability to detect and phagocytose microbes is vital to protect multicellular organisms against dangerous infections. In mammals, this important function is accomplished by dedicated immune cells, the so-called professional phagocytes, encompassing macrophages, dendritic cells, and polymorphonuclear granulocytes (PMNs). They carry out phagocytosis via two distinct mechanisms: On the one hand, they perform opsonin-independent phagocytosis by utilizing receptors such as mannose receptor, scavenger receptor, Siglecs, DC-SIGN, or Dectin-1, which directly recognize and bind microbial surfaces that expose characteristic molecular patterns, such as glycan structures with terminal mannose or sialic acid residues, or fungal β-glucans (1–3). As such types of glycans are found on various microorganisms, including bacteria, fungi, as well as protozoa, and can also occur on endogenous structures, opsonin-independent receptors often detect a broad and diverse range of particles. On the other hand, professional phagocytes are capable of performing opsonin-dependent phagocytosis. Prominent opsonin-dependent receptors are complement or Fc receptors, which require prior coating of particles with host-derived complement components or specific antibodies before they are able to initiate phagocytosis (4–6). Therefore, opsonin-dependent receptors can be targeted toward specific microbes, but they cannot support phagocytosis in situations where opsonins are either not present or where they fail to mark the microbial surface, for example, in the case of antigenically variable or encapsulated microorganisms.
Recent work has indicated that, at least in primates, a third group of specialized phagocytic receptors operates, which combines pathogen-specific detection with the immediate action of opsonin-independent receptors. The paradigm for this type of phagocytic receptors is the carcinoembryonic antigen-related cell adhesion molecule 3 (CEACAM3). CEACAM3 is a receptor of the immunoglobulin (Ig) superfamily and a member of the CEA subfamily of Ig domain containing cell adhesion molecules (IgCAMs) (Figure 1). In humans, CEACAM3 is selectively expressed by PMNs and plays a prominent role in the opsonin-independent detection and elimination of a small set of human-restricted bacteria. In this review, we will place CEACAM3 in the context of a growing list of bacterial pathogens expressing CEACAM-binding adhesins and discuss the biochemical and functional evidence that this receptor is an effective phagocytosis-initiating protein and granulocyte activator. Further, we will elaborate the evolutionary trajectory of the CEACAM3 gene within the primate lineage and discuss the significance of human CEACAM3 polymorphisms, which appear to accommodate the recognition of variable bacterial surface antigens.
Figure 1.
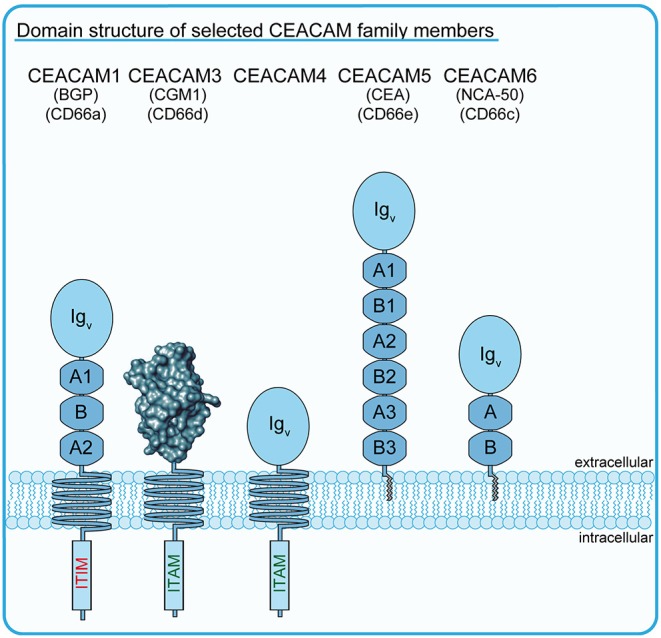
Schematic drawing of selected members of the human CEACAM family. Schematic outline of several members of the human carcinoembryonic antigen (CEA)-related cell adhesion molecule (CEACAM) receptor family. All CEA-related proteins belong to the immunoglobulin (Ig) superfamily and are characterized by the possession of a homologous amino-terminal Ig variable (IgV)-like domain, which is depicted in the case of CEACAM3 as a rendered protein surface according to Bonsor et al. (7). The blue circles indicate the IgV-like domains of CEACAMs other than CEACAM3, while the blue octagons indicate additional Ig constant 2 (IgC2)-like domains occurring in different numbers in particular family members. The transmembrane helices of CEACAM1, CEACAM3, and CEACAM4 connect the extracellular Ig-domains with functional ITIM (CEACAM1), ITAM-like (CEACAM3), or consensus ITAM sequences (CEACAM4). GPI-anchors of CEACAM5/CEA and CEACAM6 are depicted in gray.
CEACAM Family Members and Their Role as Microbial Targets
Upon the identification of carcinoembryonic antigen (CEA) as a prominent surface protein expressed by human colon carcinomas, it was soon realized that antibodies directed against CEA react with numerous other proteins, especially on granulocytes (8, 9). According to their apparent molecular weights, these proteins were initially termed non-specific cross-reacting antigen (NCA) -26, -50, -90, -95, and -160 (10). Screening of a human leukocyte cDNA library with a probe derived from NCA-50 uncovered several transcripts including clone W264 containing a 1259-base pair insert (11). The insert encoded a 252-amino acid protein, which was designated Carcinoembryonic Gene family Member 1a (CGM1a) and later grouped, due to its reactivity with monoclonal antibodies, into the CD66 cluster of differentiation. Besides CGM1a (CD66d), the CD66 antigens comprise biliary glycoprotein (BGP; CD66a), CGM6 (CD66b), NCA-50 (CD66c), and CEA (CD66e), which share 69–92% amino acid sequence identity in their amino-terminal immunoglobulin-variable (IgV)-like domains with CGM1a (11–14). Superposition of known crystal structures of CEA, CD66a, and CD66c reveals that these sequence similarities also translate into high structural conservation between these proteins (7, 15).
The growing awareness of the complexity of the CEA family necessitated a major revision of the nomenclature, which led to CGM1a (CD66d) being renamed CEACAM3 (16) (for current and former nomenclature of CEACAM family members discussed in this review, please consult Figure 1). While CEACAM3 transcripts and protein have only been detected in human granulocytes and myeloid leukemia cells, the other closely related CD66 antigens are either widely expressed on epithelial and hematopoietic cells (CD66a/BGP/CEACAM1 as well as CD66c/NCA-50/CEACAM6) or exclusively expressed by mucosal epithelial cells (CD66e/CEA/CEACAM5) (17–19). Furthermore, CEACAM3 is distinct from other CD66 antigens in that its extracellular part does only comprise a single IgV-like domain and lacks additional Ig constant (IgC)-like domains, which are present in varying numbers (2-6 IgC-like domains) in CEACAM1, CEACAM5, and CEACAM6 (Figure 1) (20). This short stature of CEACAM3 might also be the reason why this receptor does not participate in binding interactions with other CEACAM family members, as the IgC-like domains can stabilize cis- and trans-interactions between CEACAM extracellular domains and thereby contribute to cell–cell adhesion (21, 22). Indeed, CEACAM1, CEA, CEACAM6, and CEACAM8 engage in CEACAM–CEACAM interactions with each other to support cell–cell binding (12, 21–24).
As CEACAM3 does not participate in these binding interactions, what could then be the function of this particular CEA-related protein on professional phagocytes? Clearly, the physiological role of CEACAM3 can only be reconciled in light of the fact that several pathogenic bacteria and fungi take advantage of epithelial CEACAMs as preferred docking sites on the mucosa [for review, see (25)]. Indeed, a growing list of pathogens has been found to express dedicated adhesins to specifically connect to human CEACAM family members, such as CEACAM1, CEA, and CEACAM6, which are exposed on the apical surface of human epithelial cells. CEACAM-binding microorganisms comprise Neisseria gonorrhoeae (causative agent of the venereal disease gonorrhea), Neisseria meningitidis (bacterial meningitis), Haemophilus influenzae (pneumonia, bacterial meningitis), Haemophilus aegyptius (purulent conjunctivitis), Helicobacter pylori (chronic gastritis, stomach cancer), Moraxella catarrhalis (otitis media, sinusitis), Fusobacterium nucleatum (periodontal disease), pathogenic Escherichia coli strains (Adherent-invasive E. coli, Diffusely adherent E. coli; involved in Crohn's disease), and the yeast Candida albicans (candidiasis, systemic infections) (26–35). It is important to mention that almost each of these pathogens employs a structurally distinct adhesive protein to bind human CEACAMs, implying that these adhesins have evolved independently multiple times in a striking form of convergent evolution (7, 15, 32, 36–39). Evidently, there must be strong, but not necessarily a uniform selection pressure on these microorganisms to develop CEACAM-binding adhesins. Several non-mutually exclusive explanations have been put forward to explain this exceptional preference of distinct pathogenic microbes to engage human CEACAMs. One finding relates to the fact that CEACAM1, the target of a large fraction of these adhesins, is also expressed by T cells and that major CEACAM1 isoforms have a negative regulatory role in T cell stimulation and proliferation [reviewed in (40)]. A second hypothesis is based on the fact that a unifying theme for all CEACAM-binding microbes is their outstanding ability to colonize, often throughout the lifespan of an individual, the mucosal surface of either the naso-pharynx, the gastrointestinal, or the urogenital tract. The role of CEACAM engagement in mucosal colonization has been best worked out in the case of N. gonorrhoeae and N. meningitidis and demonstrated that both microbes greatly profit from tight association with CEACAMs, which facilitates successful host colonization (41–43). Aside from their role as a handle by which to anchor to the mucosal epithelia, CEACAM engagement allows bacteria to suppress the exfoliation and delamination of superficial epithelial cells, thereby creating a stable foothold on the mucosa (41, 44, 45). It becomes obvious that pathogens can immensely profit, potentially in multiple ways, from engaging CEACAMs on epithelial cells and this nicely explains the prevalence and independent evolution of CEACAM-binding adhesins among human pathogens. However, why is it then that humans rarely succumb to gonococcal infection or develop severe forms of disease after being colonized by N. meningitidis or H. pylori, which are present in a large fraction of the healthy population? Indeed, CEACAM-binding pathogens such as F. nucleatum, M. catarrhalis, N. gonorrhoeae, or H. pylori, despite being able to efficiently colonize the human mucosa, rarely or only in a minority of the cases lead to a fatal outcome. It is exactly in the context of this scenario that we can now appreciate the role of CEACAM3, a CEACAM family member that does not participate in cell–cell interactions, but is present on the surface of professional phagocytes. In particular, the capacity of CEACAM3 to trigger rapid phagocytosis of attached particles and to activate bactericidal mechanisms of granulocytes will be discussed in the next sections, as these features provide major clues to understand the specialized function of this protein.
CEACAM3-Initiated Signal Transduction Leading to Phagocytosis
CEACAM3's notable status within the CEACAM family is not only due to its small extracellular domain and its cell-type-specific expression pattern, but is also based on a particular sequence motif within its cytoplasmic domain. Similar to the prototypic opsonin-dependent phagocytic receptors of the Fc receptor family, the carboxy-terminus of CEACAM3 encompasses an immunoreceptor tyrosine-based activation motif (ITAM) [for review, see (46)]. To be more precise, the motif found in CEACAM3 does not conform perfectly to the consensus ITAM (D/Ex(7)D/ExxYxxI/Lx(6−8)YxxI/L), but resembles an ITAM-like motif, where the carboxy-terminal leucine/isoleucine residue is substituted by methionine (47–49). The presence of this motif and the expression in professional phagocytes already indicate that CEACAM3 might be involved in phagocytosis of CEACAM-binding bacteria. For several CEACAM-binding pathogens, granulocytes play a major role during symptomatic disease. For example, the purulent exudate containing numerous granulocytes with intracellular, gram-negative diplococci is a diagnostic hallmark of gonorrhea (Figures 2A,B). It has long been known that gonococci, which express a CEACAM3-binding adhesin, are recognized and phagocytosed by human granulocytes in an opsonin-independent manner (Figure 2C), while isogenic strains lacking a CEACAM-binding adhesin are hardly recognized under these conditions (26, 27, 50–52). Despite the presence of other CEACAM family members such as CEACAM1 and CEACAM6 on the granulocyte surface and despite the fact that CEACAM3 is expressed at lower levels compared to CEACAM1 and CEACAM6, CEACAM3 is the main driving force behind this rapid and efficient opsonin-independent phagocytosis (53). Evidence for the prominent role of CEACAM3 comes from pharmacological, biochemical, genetic, and microbiological approaches: inhibitors that affect CEACAM1 and CEACAM6-mediated uptake in transfected cell lines (such as cholesterol-depleting agents) do not interfere with the opsonin-independent phagocytosis of CEACAM-binding bacteria by granulocytes (54), while inhibitors or blocking antibodies selectively affecting CEACAM3-mediated uptake in transfected cell lines also disrupt this process in granulocytes (50). Interference with CEACAM3-specific binding partners or signaling processes by transduction of primary human granulocytes with dominant-negative variants also severely compromises the opsonin-independent uptake of CEACAM-binding bacteria. Selective expression of CEACAM3, but not CEACAM1 or CEACAM6, in murine promyelocytic cells can recapitulate major features of neutrophil activation in response to CEACAM-binding bacteria such as oxidative burst and degranulation (55). Furthermore, microbes expressing particular adhesins, which bind CEACAM1, but not CEACAM3, are hardly phagocytosed by primary human granulocytes in the absence of opsonins (56). Therefore, the immediate and dramatic phagocytic response of human granulocytes exposed to CEACAM-binding bacteria can be mainly attributed to CEACAM3.
Figure 2.
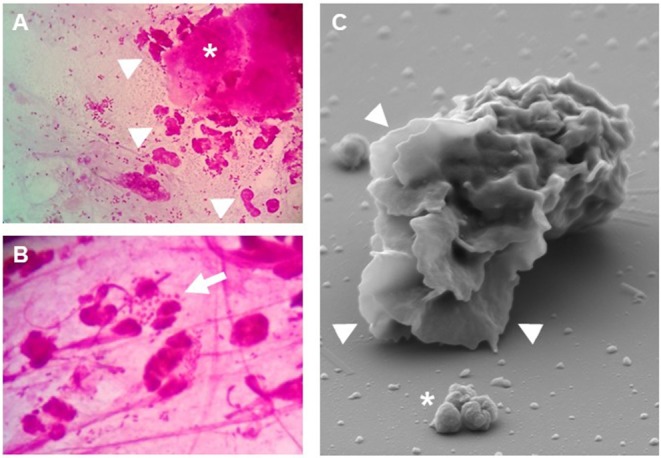
Granulocytes respond to Neisseria gonorrhoeae. (A,B) Gram stain of cervical smears from N. gonorrhoeae-infected women. Even at low magnification (A) the presence of granulocytes (arrowheads) and detached epithelial cells (asterisk) can be readily detected. (B) At higher magnification, granulocytes with phagocytosed diplococci (arrow) as well as extracellular bacteria are visible. (C) Scanning electron microscopy highlights the massive lamellipodia (arrowheads) induced on isolated primary human granulocytes upon exposure to CEACAM3-binding N. gonorrhoeae (asterisk).
A number of studies have addressed the molecular basis of CEACAM3's capability to vigorously trigger opsonin-independent phagocytosis. Most of these investigations, conducted with either transfected human cell lines or primary human granulocytes, have pointed toward a major role of the ITAM-like motif for CEACAM3 functionality in phagocytosis. For example, phosphorylation of the tyrosine residues within this motif (Y230/Y241) is critical for CEACAM3-initiated phagocytosis, as mutation of either tyrosine to a phenylalanine significantly decreases internalization and mutation of both residues results in an additive effect (47, 48, 53, 57). Interestingly, a single tyrosine-to-phenylalanine mutation completely blocked phosphorylation of CEACAM3 (48). Whether this points to a cooperative phosphorylation mechanism requiring both tyrosine residues or is due to inadequate sensitivity of the assay is unclear. Besides the ITAM-like motif, additional structural elements within the cytoplasmic domain possibly contribute to phagocytic signaling as the CEACAM3 Y230F/Y241F double mutant exhibits residual phagocytic activity compared to variants, which lack the complete cytosolic domain (48, 57). In contrast to CEACAM1 and CEACAM6, cholesterol-rich membrane domains (lipid rafts) do not seem to contribute to CEACAM3-mediated phagocytosis, as the CEACAM3-dependent internalization of bacteria is insensitive to severe cholesterol depletion, e.g., by methyl-β-cyclodextrin (54, 58, 59). It has been proposed that a Y-to-F mutation in the ITAM motif generates a binding site for AP-2, which could support an endocytic mode of internalization (48). However, regular endocytosis via AP-2 initiated, clathrin-coated vesicles has an upper size limit of 200 nm (60), implying alternative endocytic processes upon deletion or mutation of the CEACAM3 ITAM-like sequence. Though it is currently unknown which specific cellular processes guide the residual, ITAM-independent internalization of bacteria, the ITAM-dependent events upon CEACAM3 stimulation have been extensively analyzed.
Genetic, biochemical, and pharmacological evidence supports a major role for kinases of the Src family in CEACAM3 phosphorylation (Figure 3A). Indeed, the local clustering of CEACAM3 by the multivalent bacteria triggers recruitment and activation of several members of the Src family tyrosine kinases, including Hck and Fgr in granulocytes, while in transfected cell lines, Src, Yes, and Fyn might take over the respective role (48, 52, 61, 62). Due to acyl modification, Src family kinases are constitutively associated with the cytoplasmic leaflet of membranes and are therefore in a prime position to initially phosphorylate the ITAM tyrosine residues. Although the tyrosine kinase Syk is also recruited to nascent gonococci-containing phagosomes in an ITAM-dependent fashion, pharmacological inhibition of Syk did not reduce bacterial internalization (63). Only when polystyrol beads larger than 5 μm (a typical gonococcal diplococcus is about 1–2 μm in size) were coated with anti-CEACAM IgG and used as bacterial surrogate did Syk augment internalization. The fact that Syk facilitates phagocytosis depending on particle size is not unique to CEACAM3 as the same phenomenon has been observed for FcγR-mediated phagocytosis (64). Therefore, Syk is dispensable for internalization of gonococci, but it clearly does promote downstream bactericidal activity by enhancing the oxidative burst, degranulation, and the NF-κB-mediated inflammatory response (63).
Figure 3.
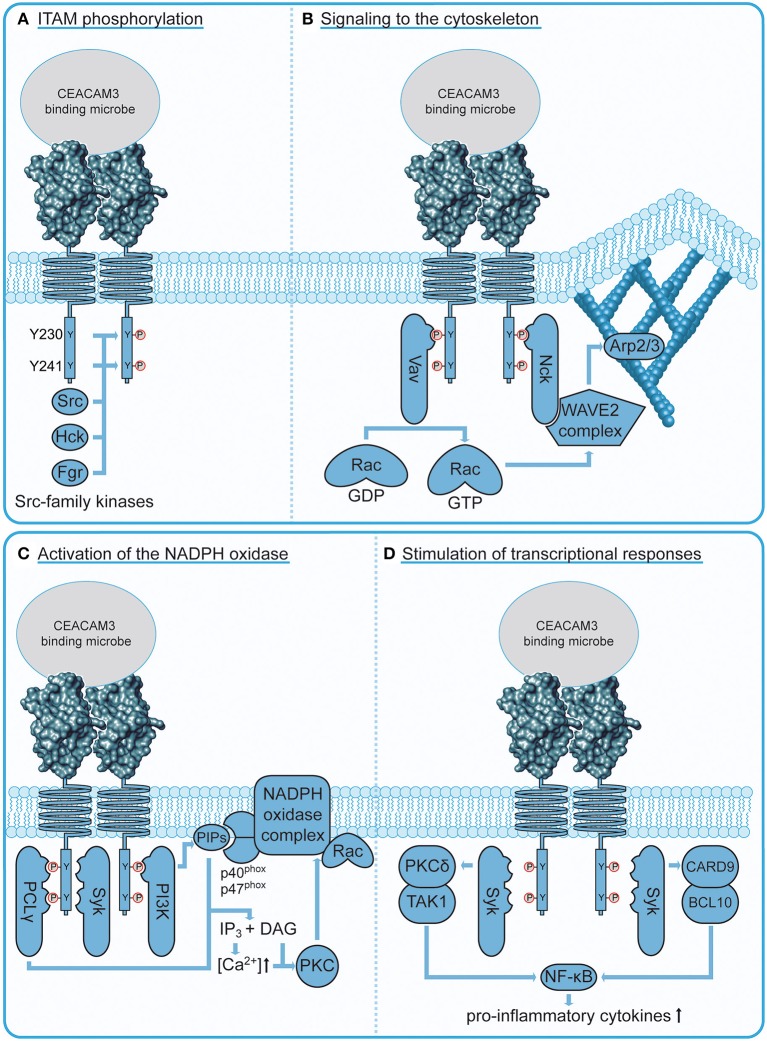
CEACAM3 signaling connections. (A) Phosphorylation of CEACAM3 upon bacterial engagement. Localized clustering of CEACAM3 receptors by multivalent, CEACAM-binding bacteria trigger recruitment and activation of several Src family tyrosine kinases, which in turn phosphorylate the ITAM-like sequence in the cytoplasmic domain of the receptor at two tyrosine residues (Y230 and Y241). This phosphorylation event is critical for downstream signaling processes. (B) CEACAM3 signaling to the cytoskeleton. The adaptor protein Nck binds via its SH2 domain to the membrane proximal CEACAM3 phosphotyrosine residue pY230 and recruits the WAVE2 complex to sites of bacterial attachment. Activation of this complex requires a localized increase in GTP-loaded Rac, which is orchestrated by recruitment of the Rac guanine nucleotide exchange factor Vav. Similar to Nck, Vav binds via its SH2 domain to CEACAM3 pY230. Activation of the WAVE2 complex leads to localized activation of Arp2/3 at the site of infection resulting in particle engulfment by actin cytoskeleton-based lamellipodial protrusions. (C) CEACAM3 signaling to the NADPH oxidase. Residue pY230 serves as an interaction platform for the regulatory subunit of phosphatidylinositol 3′ kinase (PI3K). PI3Ks are responsible for the generation of phosphoinositides (PIPs), which regulate the oxidative burst by recruitment of NADPH oxidase subunits p40phox and p47phox. In addition, PIP hydrolysis by CEACAM3-recruited phospholipase C γ (PLCγ) releases the second messengers inositol-(1,4,5)-trisphosphate (IP3) and diacyl glycerol (DAG). Together, they activate the protein kinase C (PKC), which further stimulates the NADPH oxidase complex. (D) CEACAM3 stimulation of neutrophil transcriptional response. CEACAM3 can trigger the NF-κB-mediated transcriptional regulation of IL-8 and other neutrophil chemotactic factors. CEACAM3-initiated NF-κB activation occurs via two distinct routes: the PKCδ/TAK1 pathway and the CARD9/BCL10 pathway depend on Syk localization to phosphorylated CEACAM3.
Upon phosphorylation, the ITAM-like motif in CEACAM3 creates a platform for effectors that drive cytoskeletal remodeling required for phagocytic cup formation (Figure 3B). The adaptor molecule Nck binds CEACAM3 in a phosphorylation-dependent manner via its SH2 domain and recruits the WAVE2 complex to sites of bacterial attachment (65). WAVE2 is part of a multiprotein complex that activates Arp2/3-mediated actin nucleation, which drives lamellipodia extension [reviewed in (66)]. Indeed, ablation of Nck or inhibition of WAVE2 impedes lamellipodia formation and bacterial uptake (65). The WAVE2 complex is a coincidence detector, which is activated by the local and temporal co-occurrence of protein tyrosine phosphorylation, acidic phospholipids, and activation of the small GTPase Rac (67). Importantly, all of these events are concomitantly initiated by CEACAM3 engagement. Acidic phospholipids such as phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) and phosphatidylinositol-(3)-phosphate (PI3P) sequentially accumulate at the phagocytic cup during engulfment (59, 68, 69). WAVE2 activation strictly depends on the recruitment of the small Rho GTPase, Rac, which is crucial for remodeling of the cytoskeleton (70). Rac1 is also essential for CEACAM3-mediated phagocytosis and is locally and transiently activated at the phagocytic cup (52, 53, 57). Activation of Rac is catalyzed by specific guanine nucleotide exchange factors (GEFs) that exchange Rac1-bound GDP for GTP and CEACAM3 aggregation triggers Rac GTP loading by the GEF Vav (61). Interestingly, Vav itself is activated by Src family kinases (71, 72) and the Vav SH2 domain can interact directly with the phosphorylated Y230 within the ITAM-like motif of CEACAM3 (61). As most other GEFs bind via their pleckstrin homology domain (PH domain) to phosphoinositides, their recruitment is intrinsically dependent on PI3K activity. In contrast, Vav's direct interaction with phosphorylated CEACAM3 could render this process PI3K-independent. Indeed, phosphoinositide 3′ kinase (PI3K) activity is dispensable for CEACAM3-mediated internalization of gonococci (68, 73). The direct interaction between phosphorylated CEACAM3 and a Rac GEF as well as the direct linkage to the WAVE complex via Nck could explain why CEACAM3-mediated phagocytosis is particularly efficient and rapid (69). In vitro, ~90% of primary human granulocytes have internalized multiple CEACAM-binding gonococci within 20 min of infection, while CEACAM-non-binding gonococci remain almost untouched (48, 69). These studies of CEACAM3-initiated signal transduction have also indicated that the ability of this receptor to trigger phagocytosis relies on cellular constituents, such as Src family kinases, Vav, Rac, and Nck-WAVE, which can be found in almost any mammalian cell type. Maybe this universality of CEACAM3 downstream connections explains why transfection of CEACAM3 cDNA in diverse cell types, from cervical epithelial cells to mouse fibroblasts, is sufficient to convert such non-professional phagocytes into cells avidly and efficiently internalizing CEACAM-binding bacteria. The generic nature of these processes also indicates that studies of CEACAM3-initiated phagocytosis are helpful to delineate the core elements necessary for productive internalization of particles.
CEACAM3-Initiated Signal Transduction Beyond Phagocytosis
In professional phagocytes, phagocytosis is tightly coupled to downstream bactericidal processes. One central regulatory switch in this regard is the GTPase Rac, which not only regulates cytoskeletal remodeling, but is also an essential subunit of the NADPH oxidase, the enzyme complex generating the microbiocidal oxidative burst (Figure 3C). In contrast to the PI3K-independent Rac activation during engulfment described above, the CEACAM3-induced oxidative burst strictly depends on PI3K activity (73). Since there is only a partial temporal overlap between particle engulfment and the oxidative burst, it is possible that Vav-mediated GTP-loading of Rac during engulfment is disconnected from a putative phosphoinositide-dependent GEF, which might activate Rac later during the induction of an oxidative burst. However, PI3K-generated phosphoinositides are required at additional regulatory steps during NADPH oxidase assembly, such as the recruitment of NADPH oxidase subunits p40phox and p47phox through their PI3P-binding PX domains (74). Phosphoinositides also serve as substrate for various lipid phosphatases and phospholipases. Interestingly, the SH2 domain of phospholipase C γ (PLCγ) can bind CEACAM3 in vitro and the isolated SH2 domain is enriched around gonococci-containing phagosomes (48, 75). PLCγ-mediated hydrolysis of phosphatidylinositol-(4,5)-bisphosphate (PIP2) produces the second messengers diacyl glycerol (DAG) and inositol-(1,4,5)-trisphosphate (IP3), which in turn trigger increases in cytosolic Ca2+ and PKC activation. Indeed, intracellular Ca2+ levels in PMNs rapidly rise upon CEACAM3 engagement and this process does not occur in cells lacking PLCγ (47). Accordingly, upstream PLCγ activity might be required to allow PKC-mediated phosphorylation of multiple NADPH oxidase subunits, which represents an important regulatory step in activation of the oxidative burst [reviewed in (76)].
Though both tyrosine residues within the ITAM-like sequence of CEACAM3 seem to be functionally relevant, the biochemical assays conducted so far have pointed toward the Y230 residue as the central hub for interactions with SH2 domain-containing proteins. This conclusion is based on binding studies with synthetic phospho-peptides and pull-down experiments with recombinant proteins, which demonstrate that the SH2 domains of Src family kinases, PI3K, Nck, or Vav selectively bind to phospho-Y230. Therefore, the CEACAM3 ITAM-like sequence has been likened to a so-called HemITAM sequence found, for example, in the macrophage receptor Dectin-1, where also a single tyrosine residue conveys the phagocytic function (77, 78). Interestingly, the only known negative regulator of CEACAM3-mediated signaling, the adaptor protein Grb14, also targets phospho-Y230 (79). One can easily envision that Grb14 restricts access for other SH2 domain-containing effector proteins and thereby interferes with CEACAM3-mediated phagocytosis. However, the idea that multiple proteins compete for phospho-Y230 of CEACAM3 immediately begs the question how these binding events can be coordinated to allow a productive and orchestrated cellular response. In the future, time-resolved analysis of the various SH2 domain-mediated binding events upon bacterial CEACAM3 engagement might help to answer this question. Nevertheless, the emerging overall picture of CEACAM3 phosphorylation-initiated events depicts Y230 within the ITAM-like motif as the minimal structural feature, which directly links CEACAM3 engagement with cytoskeletal remodeling as well as with the initiation of bactericidal responses.
It is interesting to mention that there is a further member of the CEACAM family, CEACAM4, which has a domain architecture similar to CEACAM3, which is selectively expressed in granulocytes, and which harbors a consensus ITAM (D/ExxYxxLx(6−8)YxxI) (Figure 1). CEACAM4 is an orphan receptor, as neither an endogenous nor a microbial ligand for this membrane protein has been detected. However, the cytoplasmic domain of CEACAM4 can trigger particle uptake, with both ITAM-embedded tyrosine residues engaging in SH2 domain interactions (80). Therefore, the human CEACAM family appears to harbor additional phagocytic receptors that might function to eliminate microorganisms in an opsonin-independent manner.
Although the main task of PMNs is to clear pathogens through their bactericidal capabilities, there is mounting evidence that they can also shape the inflammatory response [reviewed in (81, 82)]. Indeed, CEACAM3 activation can trigger an inflammatory program in PMNs (Figure 3D). CEACAM3 binding by Moxarella catarrhalis activates the CARD9/BCL10 pathway resulting in NF-κB-mediated expression of the potent neutrophil chemotactic factor, interleukin-8 (IL-8) (83). As CEACAM3 expression is restricted to human neutrophils, its contribution to inflammatory responses in an organismal context are difficult to study. Sintsova et al. made use of a transgenic mouse model that harbors a 187-kb human bacterial artificial chromosome encoding CEACAM3, CEACAM5, CEACAM6, and CEACAM7 (CEABAC mice) (84) to study the CEACAM-mediated response to infection with N. gonorrhoeae (85). Global expression profiles were generated from both WT and transgenic neutrophils infected with CEACAM-binding gonococci to discern CEACAM-dependent signatures. A pronounced upregulation of pro-inflammatory cytokines was observed in neutrophils from CEABAC mice, which depends on p38 MAPK activity and the PKCδ/TAK1/NF-κB axis (85). Though the used transgenic neutrophils and primary human granulocytes express other CEACAMs, which could be engaged by CEACAM-binding bacteria (such as CEACAM6 in the case of transgenic murine neutrophils or CEACAM1 and CEACAM6 in the case of primary human neutrophils), inhibitor studies point to CEACAM3-ITAM signaling as the main contributor to neutrophil-initiated inflammatory responses. In vivo, infection of CEACAM-transgenic, but not wild-type mice with N. gonorrhoeae led to increased neutrophil infiltration and increased levels of neutrophil-derived IL-1β and MIP-1α. Accordingly, CEACAM3 signaling on the one hand helps to limit gonococcal survival, but also initiates a vicious cycle, where the bacteria-triggered release of chemotactic cytokines leads to increased neutrophil influx and potentiates the risk of severe damage to the infected tissue (86). In this light, it will be important to understand how CEACAM3 signaling is kept in check to prevent unrestrained inflammatory signaling. In contrast to the initiation of CEACAM3 signaling, surprisingly little is known about its termination. The negative regulatory role of the adaptor protein Grb14 (79) has already been discussed above. Furthermore, phosphorylation of CEACAM3 appears to be counteracted by the cytoplasmic protein tyrosine phosphatase SHP-1, which most likely constrains CEACAM3 effector functions by compromising ITAM functionality (87). Interestingly, neutrophils also express CEACAM1, which contains an immunoreceptor tyrosine-based inhibitory motif (ITIM). However, co-recruitment of CEACAM1 does not seem to have an inhibitory effect on CEACAM3 phagocytic activity (55). Further research is required to address mechanisms that could regulate CEACAM3 activity, including intracellular trafficking of CEACAM3 and cooperation with other phagocytic receptors (e.g., Fc-γ and complement receptors). Considering the potential detrimental effects of excessive CEACAM3 activation, it is highly likely that in human PMNs, additional negative modulators of CEACAM3 signaling operate.
CEACAM3 Evolution—a Red Queen Scenario at Work
Based on the functional studies summarized in the previous sections, it is safe to conclude that CEACAM3 represents an effective detector and eliminator of CEACAM-binding bacteria. In its capacity as a phagocytosis-promoting receptor, CEACAM3 can take care of pathogens expressing CEACAM-binding adhesins designed to exploit the human receptors for mucosal colonization. Thereby, CEACAM3 might help to establish a truce between host and pathogen, which could be one of the reasons why CEACAM-binding pathogens are contained in most instances. Looking at this reality from the viewpoint of a microbe, wouldn't it then be smart to avoid expressing a CEACAM3-binding adhesin in the first place? Obviously, bacteria unanimously answered this question with “Yes,” as they appear to optimize their adhesins to allow binding to epithelial CEACAMs such as CEACAM1 and CEA, while evading CEACAM3 recognition. Indeed, most CEACAM-binding bacterial adhesins analyzed in this regard show selective binding to either CEACAM1 or CEA. This is true for the CEACAM-binding Opa protein adhesins of N. gonorrhoeae and N. meningitidis as well as for their functional homolog, the OMP P1 adhesin of H. influenzae, which have been studied most thoroughly in this context. For example, a single gonococcal strain encodes 11 distinct Opa adhesins and the complete compendium of Opa proteins has been functionally tested for binding to CEACAM family members in the case of N. gonorrhoeae strains MS11 and VP1. In MS11, 10 out of 11 Opa adhesins bind CEA, while only three of these are also able to be recognized by CEACAM3 (50, 51, 88). For strain VP1, 8 out of 10 tested Opa adhesins bound to CEA or CEACAM1, while only a single Opa protein associated with CEACAM3 (56). In a complementary approach, Sintsova et al. screened a large collection of primary isolates from gonorrhea patients for their CEACAM-binding capacity. Instead of trying to analyze the complete Opa repertoire of each of these strains, the authors used low passage isolates, which are thought to continue to express in vitro the Opa variant previously selected for and expressed in vivo (89). Also in this study, the overwhelming majority with 74% or 80% of the strains bound CEA or CEACAM1, respectively, and only 27% were found to also recognize CEACAM3 (89). While most strains bound CEA and/or CEACAM1, but not CEACAM3, not a single strain could be observed, which showed a reverse binding pattern: recognizing CEACAM3, but not recognizing an epithelial CEA family member (89). An even more skewed situation is found in N. meningitidis, where 11 of 13 Opa proteins of serogroup A, B, and C strains, which have been tested in binding assays with different CEACAMs, associated with either CEA or CEACAM1, but none of those Opa proteins associated with CEACAM3 (90, 91). Similarly, all of the OMP P1 adhesins derived from 13 different strains of H. influenzae bound to CEACAM1, but none of those adhesins was recognized by CEACAM3 (32). Accordingly, bacteria seem to optimize their adhesins to discriminate between these closely related CEACAMs and to avoid recognition by CEACAM3.
If there is such a directed evolution on the side of bacterial CEACAM-binding adhesins, is there a discernable adaptation on the host side? The increasing availability of human and other primate genomic information has now allowed an unraveling of the evolutionary context of CEACAM3. It has been recognized before that homologs of several human CEACAMs are absent from rodents. More specifically, a CEACAM3 ortholog could not be identified even in Old World primates, such as baboon or African green monkey, by homology searches based on the extracellular IgV-like domain (92). Large-scale genome comparisons between closely related primate species have revealed a high degree of non-synonymous vs. synonymous nucleotide changes in ortholog genes of CEACAM family members (93, 94). Interestingly, the bacterial receptors within the CEACAM family (CEACAM1, CEACAM3, CEA, and CEACAM6) show an exceptionally strong signature of positive selection, suggesting that they belong to the fastest-evolving human genes. In particular, the CEACAM3 gene appears to be a recent evolutionary invention. Though ITAM-sequence containing CEACAM-related genes have been described for various mammals (95–97), a CEACAM3 gene with its specific exon/intron structure and the characteristic ITAM-like sequence seems to occur only after the emergence of Old World monkeys (around 35 million years ago) (98). Indeed, a CEACAM3 ortholog has only been detected at the syntenic locus in the genomes of baboon, macaque, orangutan, gorilla, chimpanzee, and humans (94). Due to uncertainties in the assembly of some genomes such as tarsier, lemurs, and lories, gene synteny is not a valid criterion to rule out the existence of CEACAM3 orthologs in lower primates. However, CEACAM3 orthologs share several discriminative features such as protein length or the presence of the characteristic HemITAM, which does not seem to occur in any lower primate genome supporting the idea that CEACAM3 emerged late in primate evolution. Despite the absence of a CEACAM3 gene, ITAM-containing CEACAM transcripts are present in lower primates, such as transcript XR_001153184.2 in the mouse lemur. The ITAM encoded by XR_001153184.2 perfectly matches the ITAM consensus (YxxL−7AS–YxxI) as well as the sequence found in human CEACAM4 (80). Therefore, this transcript could originate from an ancestral primate CEACAM4 ortholog. It is plausible that CEACAM3 is the result of gene duplication and recombination between an ancestral CEACAM1 gene (providing the IgV domain encoding exon) and an ancestral lower primate CEACAM4 gene (providing the transmembrane and the intracellular domain encoding exons) (99). Indeed, the 3′ end of the large intron 2 of the CEACAM3 gene bears similarities to intron 2 of the CEACAM4 gene, supporting the idea that such a recombination event occurred at the advent of higher primates (99). Together, the genomic evidence suggests that CEACAM3, the specialized phagocytic receptor for CEACAM-binding bacteria, emerged relatively late during primate evolution, expanding the human innate immunity arsenal.
An interesting corollary of this continuing bacteria–host co-evolution can be seen by the occurrence of CEACAM3 polymorphisms in the human population. In fact, while people outside of Africa mainly express the common CEACAM3 allele, particular ethnic groups harbor CEACAM3 variants. For example, in several groups with African ancestry, a third of the population expresses a distinct CEACAM3 allele (minor CEACAM3 allele) (94). This minor allele carries four non-synonymous single-nucleotide changes, which convert the CEACAM3-IgV domain amino acid sequence into a near replica of the CEACAM1 IgV domain. Modeling of the polymorphic CEACAM3 IgV domain according to the known CEACAM3 crystal structure (7) reveals that these residues are surface exposed and three of the modified amino acids contribute to the CEACAM3 binding interface for various bacterial adhesins (94). It comes as no surprise that this minor CEACAM3 allele, which now mimics CEACAM1, shows a striking gain of function in that it recognizes additional bacterial adhesins, such as the OMP P1 protein of H. influenzae, which escape detection by the common CEACAM3 allele (94).
These recent studies have shed light on an ongoing arms race between several host-restricted CEACAM-binding pathogens and the human immune system. In a type of Red-Queen scenario, the diverse CEACAM-binding pathogens need to optimize their adhesins to escape CEACAM3 detection, while still retaining their ability to bind mucosal CEACAMs. As a consequence, human CEACAM3 rapidly evolves to keep up its function. Viewed from the angle of bacterial adhesin binding to distinct CEACAMs as well as from the perspective of human genomic variation, the phagocytic receptor CEACAM3 appears as a central element in the innate defense against this group of extremely specialized bacteria. However, one has to acknowledge that CEACAM binding preferences of bacterial adhesins as well as analyses of CEACAM3 evolution only provide circumstantial evidence for the importance of this innate immune receptor. Accordingly, a more direct test of CEACAM3 function in the context of innate immune responses within the intact organism would be desirable. Unfortunately, the standard genetic approach of employing knock-out animals, such as a “CEACAM3-knock-out” mouse, is not an option in this case. Moreover, CEACAM3-transgenic mice with granulocyte-selective expression of CEACAM3, in the absence of other CEACAM family members, do not exist currently.
In view of the increasing availability of human genomic information and corresponding medical records, it does not appear unrealistic to foresee a future, where novel insight into CEACAM3 function might come directly from combined genomic–phenotypic studies in humans (100). Indeed, the occurrence of an unexpected high proportion of loss-of-function alleles in particular human populations (101) could help to reveal plausible connections between CEACAM3 deficiency and susceptibility for particular infectious diseases. Despite the inherent limitations of research on a primate-only protein, the study of the phagocytic receptor CEACAM3 will surely continue to unearth fascinating aspects of human biology and of our constant and challenging interplay with the microbial world.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are indebted to Michael Laumann (Electron Microscopy Service; University of Konstanz, Germany) for scanning electron microscopy and Nathlee Abbai (University KwaZulu-Natal, Durban, South Africa) for providing histological samples.
Glossary
Abbreviations
- CEA
carcinoembryonic antigen
- CEACAM
CEA-related cell adhesion molecule
- Ig
immunoglobulin
- ITAM
immunoreceptor tyrosine-based activation motif.
Footnotes
Funding. Work by the authors has been supported by the DFG (Ha 2856/10-1 and Ha 2856/11-1) and Baden-Württemberg Stiftung (BWST_WSF-020) to CH.
References
- 1.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. (2002) 20:825–52. 10.1146/annurev.immunol.20.103001.114744 [DOI] [PubMed] [Google Scholar]
- 2.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. (2007) 7:255–66. 10.1038/nri2056 [DOI] [PubMed] [Google Scholar]
- 3.Goyal S, Castrillon-Betancur JC, Klaile E, Slevogt H. The interaction of human pathogenic fungi with C-type lectin receptors. Front Immunol. (2018) 9:1261. 10.3389/fimmu.2018.01261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joshi T, Butchar JP, Tridandapani S. Fcgamma receptor signaling in phagocytes. Int J Hematol. (2006) 84:210–6. 10.1532/IJH97.06140 [DOI] [PubMed] [Google Scholar]
- 5.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. (2008) 8:34–47. 10.1038/nri2206 [DOI] [PubMed] [Google Scholar]
- 6.Degn SE, Thiel S. Humoral pattern recognition and the complement system. Scand J Immunol. (2013) 78:181–93. 10.1111/sji.12070 [DOI] [PubMed] [Google Scholar]
- 7.Bonsor DA, Zhao Q, Schmidinger B, Weiss E, Wang J, Deredge D, et al. The Helicobacter pylori adhesin protein HopQ exploits the dimer interface of human CEACAMs to facilitate translocation of the oncoprotein CagA. EMBO J. (2018) 37:e98664. 10.15252/embj.201798664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. (1965) 122:467–81. 10.1084/jem.122.3.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goleski CA, Rule AH, Janowitz HD. Cea-like antigens - presence in tumor, normal colon and meconium extracts. Federat Proc. (1972) 31:A639 [Google Scholar]
- 10.Kuroki M, Matsuo Y, Matsuoka Y. Nonspecific cross-reacting antigen (NCA) expressed by human granulocytes: six species with different peptide sizes and membrane anchoring forms. Biochem Biophys Res Commun. (1990) 166:701–8. 10.1016/0006-291X(90)90866-L [DOI] [PubMed] [Google Scholar]
- 11.Kuroki M, Arakawa F, Matsuo Y, Oikawa S, Misumi Y, Nakazato H, et al. Molecular cloning of nonspecific cross-reacting antigens in human granulocytes. J Biol Chem. (1991) 266:11810–7. [PubMed] [Google Scholar]
- 12.Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. (1989) 57:327–34. 10.1016/0092-8674(89)90970-7 [DOI] [PubMed] [Google Scholar]
- 13.Oikawa S, Inuzuka C, Kuroki M, Matsuoka Y, Kosaki G, Nakazato H. Cell adhesion activity of non-specific cross-reacting antigen (NCA) and carcinoembryonic antigen (CEA) expressed on CHO cell surface: homophilic and heterophilic adhesion. Biochem Biophys Res Commun. (1989) 164:39–45. 10.1016/0006-291X(89)91679-3 [DOI] [PubMed] [Google Scholar]
- 14.Oikawa S, Inuzuka C, Kuroki M, Arakawa F, Matsuoka Y, Kosaki G, et al. A specific heterotypic cell adhesion activity between members of carcinoembryonic antigen family, W272 and NCA, is mediated by N-domains. J Biol Chem. (1991) 266:7995–8001. [PubMed] [Google Scholar]
- 15.Moonens K, Hamway Y, Neddermann M, Reschke M, Tegtmeyer N, Kruse T, et al. (2018). Helicobacter pylori adhesin HopQ disrupts trans dimerization in human CEACAMs. EMBO J. 37:e98665. 10.15252/embj.201798665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, et al. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. (1999) 252:243–9. 10.1006/excr.1999.4610 [DOI] [PubMed] [Google Scholar]
- 17.Thompson J, Zimmermann W. The carcinoembryonic antigen gene family: structure, expression and evolution. Tumour Biol. (1988) 9:63–83. 10.1159/000217547 [DOI] [PubMed] [Google Scholar]
- 18.Nagel G, Grunert F, Kuijpers TW, Watt SM, Thompson J, Zimmermann W. Genomic organization, splice variants and expression of CGM1, a CD66-related member of the carcinoembryonic antigen gene family. Eur. J Biochem. (1993) 214:27–35. 10.1111/j.1432-1033.1993.tb17892.x [DOI] [PubMed] [Google Scholar]
- 19.Kuroki M, Yamanaka T, Matsuo Y, Oikawa S, Nakazato H, Matsuoka Y. Immunochemical analysis of carcinoembryonic antigen (CEA)- related antigens differentially localized in intracellular granules of human neutrophils. Immunol Invest. (1995) 24:829–43. 10.3109/08820139509060710 [DOI] [PubMed] [Google Scholar]
- 20.Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. (1991) 5:344–66. 10.1002/jcla.1860050510 [DOI] [PubMed] [Google Scholar]
- 21.Zhou H, Fuks A, Alcaraz G, Bolling TJ, Stanners CP. Homophilic adhesion between Ig superfamily carcinoembryonic antigen molecules involves double reciprocal bonds. J Cell Biol. (1993) 122:951–60. 10.1083/jcb.122.4.951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watt SM, Teixeira AM, Zhou GQ, Doyonnas R, Zhang Y, Grunert F, et al. Homophilic adhesion of human CEACAM1 involves N-terminal domain interactions: structural analysis of the binding site. Blood. (2001) 98:1469–79. 10.1182/blood.V98.5.1469 [DOI] [PubMed] [Google Scholar]
- 23.Teixeira AM, Fawcett J, Simmons DL, Watt SM. The N-domain of the biliary glycoprotein (BGP) adhesion molecule mediates homotypic binding: domain interactions and epitope analysis of BGPc. Blood. (1994) 84:211–9. 10.1182/blood.V84.1.211.bloodjournal841211 [DOI] [PubMed] [Google Scholar]
- 24.Wikstrom K, Kjellstrom G, Obrink B. Homophilic intercellular adhesion mediated by C-CAM is due to a domain 1-domain 1 reciprocal binding. Exp Cell Res. (1996) 227:360–6. 10.1006/excr.1996.0285 [DOI] [PubMed] [Google Scholar]
- 25.Tchoupa AK, Schuhmacher T, Hauck CR. Signaling by epithelial members of the CEACAM family - mucosal docking sites for pathogenic bacteria. Cell Commun Signal. (2014) 12:27. 10.1186/1478-811X-12-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen T, Gotschlich EC. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc Natl Acad Sci USA. (1996) 93:14851–6. 10.1073/pnas.93.25.14851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virji M, Makepeace K, Ferguson DJP, Watt SM. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol Microbiol. (1996) 22:941–50. 10.1046/j.1365-2958.1996.01551.x [DOI] [PubMed] [Google Scholar]
- 28.Virji M, Watt SM, Barker S, Makepeace K, Doyonnas R. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol Microbiol. (1996) 22:929–39. 10.1046/j.1365-2958.1996.01548.x [DOI] [PubMed] [Google Scholar]
- 29.Hill DJ, Virji M. A novel cell-binding mechanism of Moraxella catarrhalis ubiquitous surface protein UspA: specific targeting of the N-domain of carcinoembryonic antigen-related cell adhesion molecules by UspA1. Mol Microbiol. (2003) 48:117–29. 10.1046/j.1365-2958.2003.03433.x [DOI] [PubMed] [Google Scholar]
- 30.Berger CN, Billker O, Meyer TF, Servin AL, Kansau I. Differential recognition of members of the carcinoembryonic antigen family by Afa/Dr adhesins of diffusely adhering Escherichia coli (Afa/Dr DAEC). Mol Microbiol. (2004) 52:963–83. 10.1111/j.1365-2958.2004.04033.x [DOI] [PubMed] [Google Scholar]
- 31.Barnich N, Carvalho FA, Glasser AL, Darcha C, Jantscheff P, Allez M, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. (2007) 117:1566–74. 10.1172/JCI30504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tchoupa AK, Lichtenegger S, Reidl J, Hauck CR. Outer membrane protein P1 is the CEACAM-binding adhesin of Haemophilus influenzae. Mol Microbiol. (2015) 98:440–55. 10.1111/mmi.13134 [DOI] [PubMed] [Google Scholar]
- 33.Koniger V, Holsten L, Harrison U, Busch B, Loell E, Zhao Q, et al. Helicobacter pylori exploits human CEACAMs via HopQ for adherence and translocation of CagA. Nat Microbiol. (2016) 2:16188 10.1038/nmicrobiol.2016.188 [DOI] [PubMed] [Google Scholar]
- 34.Klaile E, Muller MM, Schafer MR, Clauder AK, Feer S, Heyl KA, et al. Binding of Candida albicans to human CEACAM1 and CEACAM6 modulates the inflammatory response of intestinal epithelial cells. MBio. (2017) 8:e02142–e02116. 10.1128/mBio.02142-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brewer ML, Dymock D, Brady RL, Singer BB, Virji M, Hill DJ. Fusobacterium spp. target human CEACAM1 via the trimeric autotransporter adhesin CbpF. J Oral Microbiol. (2019) 11:1565043. 10.1080/20002297.2018.1565043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malorny B, Morelli G, Kusecek B, Kolberg J, Achtman M. Sequence diversity, predicted two-dimensional protein structure, and epitope mapping of neisserial Opa proteins. J Bacteriol. (1998) 180:1323–30. 10.1128/JB.180.5.1323-1330.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Jonge MI, Bos MP, Hamstra HJ, Jiskoot W, Van Ulsen P, Tommassen J, et al. Conformational analysis of opacity proteins from Neisseria meningitidis. Eur J Biochem. (2002) 269:5215–23. 10.1046/j.1432-1033.2002.03228.x [DOI] [PubMed] [Google Scholar]
- 38.Conners R, Hill DJ, Borodina E, Agnew C, Daniell SJ, Burton NM, et al. The Moraxella adhesin UspA1 binds to its human CEACAM1 receptor by a deformable trimeric coiled-coil. EMBO J. (2008) 27:1779–89. 10.1038/emboj.2008.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korotkova N, Yang Y, Le Trong I, Cota E, Demeler B, Marchant J, et al. Binding of Dr adhesins of Escherichia coli to carcinoembryonic antigen triggers receptor dissociation. Mol Microbiol. (2008) 67:420–34. 10.1111/j.1365-2958.2007.06054.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. (2006) 6:433–46. 10.1038/nri1864 [DOI] [PubMed] [Google Scholar]
- 41.Muenzner P, Bachmann V, Hentschel J, Zimmermann W, Hauck CR. Human-restricted bacterial pathogens block shedding of epithelial cells by stimulating integrin activation. Science. (2010) 329:1197–201. 10.1126/science.1190892 [DOI] [PubMed] [Google Scholar]
- 42.Johswich KO, McCaw SE, Islam E, Sintsova A, Gu A, Shively JE, et al. In vivo adaptation and persistence of Neisseria meningitidis within the nasopharyngeal mucosa. PLoS Pathog. (2013) 9:e1003509. 10.1371/journal.ppat.1003509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Islam EA, Anipindi VC, Francis I, Shaik-Dasthagirisaheb Y, Xu S, Leung N, et al. Specific binding to differentially expressed human Carcinoembryonic Antigen-related cell adhesion molecules determines the outcome of Neisseria gonorrhoeae infections along the female reproductive tract. Infect Immun. (2018) 86:e00092–e00018. 10.1128/IAI.00092-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muenzner P, Rohde M, Kneitz S, Hauck CR. CEACAM engagement by human pathogens enhances cell adhesion and counteracts bacteria-induced detachment of epithelial cells. J Cell Biol. (2005) 170:825–36. 10.1083/jcb.200412151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muenzner P, Kengmo Tchoupa A, Klauser B, Brunner T, Putze J, Dobrindt U, et al. Uropathogenic E. coli exploit CEA to promote colonization of the urogenital tract mucosa. PLoS Pathog. (2016) 12:e1005608. 10.1371/journal.ppat.1005608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. (1994) 76:263–74. 10.1016/0092-8674(94)90334-4 [DOI] [PubMed] [Google Scholar]
- 47.Chen T, Bolland S, Chen I, Parker J, Pantelic M, Grunert F, et al. The CGM1a (CEACAM3/CD66d)-mediated phagocytic pathway of Neisseria gonorrhoeae expressing opacity proteins is also the pathway to cell death. J Biol Chem. (2001) 276:17413–9. 10.1074/jbc.M010609200 [DOI] [PubMed] [Google Scholar]
- 48.McCaw SE, Schneider J, Liao EH, Zimmermann W, Gray-Owen SD. Immunoreceptor tyrosine-based activation motif phosphorylation during engulfment of Neisseria gonorrhoeae by the neutrophil-restricted CEACAM3 (CD66d) receptor. Mol Microbiol. (2003) 49:623–37. 10.1046/j.1365-2958.2003.03591.x [DOI] [PubMed] [Google Scholar]
- 49.Buntru A, Roth A, Nyffenegger-Jann NJ, Hauck CR. HemITAM signaling by CEACAM3, a human granulocyte receptor recognizing bacterial pathogens. Arch Biochem Biophys. (2012) 524:77–83. 10.1016/j.abb.2012.03.020 [DOI] [PubMed] [Google Scholar]
- 50.Gray-Owen SD, Dehio C, Haude A, Grunert F, Meyer TF. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. (1997) 16:3435–45. 10.1093/emboj/16.12.3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gray-Owen SD, Lorenzen DR, Haude A, Meyer TF, Dehio C. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol Microbiol. (1997) 26:971–80. 10.1046/j.1365-2958.1997.6342006.x [DOI] [PubMed] [Google Scholar]
- 52.Hauck CR, Meyer TF, Lang F, Gulbins E. CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J. (1998) 17:443–54. 10.1093/emboj/17.2.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmitter T, Agerer F, Peterson L, Muenzner P, Hauck CR. Granulocyte CEACAM3 is a phagocytic receptor of the innate immune system that mediates recognition and elimination of human-specific pathogens. J Exp Med. (2004) 199:35–46. 10.1084/jem.20030204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmitter T, Pils S, Weibel S, Agerer F, Buntru A, Kopp K, et al. Opa proteins of pathogenic Neisseriae initiate Src-kinase-dependent or lipid raft-mediated uptake via distinct human CEACAM isoforms. Infect Immun. (2007) 75:4116–26. 10.1128/IAI.01835-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarantis H, Gray-Owen SD. Defining the roles of human carcinoembryonic antigen-related cellular adhesion molecules during neutrophil responses to Neisseria gonorrhoeae. Infect Immun. (2012) 80:345–58. 10.1128/IAI.05702-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roth A, Mattheis C, Münzner P, Unemo M, Hauck CR. Innate recognition by neutrophil granulocytes differs between Neisseria gonorrhoeae strains causing local or disseminating infections. Infect Immun. (2013) 81:2358–70. 10.1128/IAI.00128-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Billker O, Popp A, Brinkmann V, Wenig G, Schneider J, Caron E, et al. Distinct mechanisms of internalization of Neisseria gonorrhoeae by members of the CEACAM receptor family involving Rac1- and Cdc42- dependent and -independent pathways. EMBO J. (2002) 21:560–71. 10.1093/emboj/21.4.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muenzner P, Bachmann V, Kuespert K, Hauck CR. The CEACAM1 transmembrane domain, but not the cytoplasmic domain, directs internalization of human pathogens via membrane-microdomains. Cell Microbiol. (2008) 10:1074–92. 10.1111/j.1462-5822.2007.01106.x [DOI] [PubMed] [Google Scholar]
- 59.Voges M, Bachmann V, Naujoks J, Kopp K, Hauck CR. Extracellular IgC2-like domains of CEACAMS mediate PI3K sensitivity during uptake of pathogens. PLoS ONE. (2012) 7:e39908. 10.1371/journal.pone.0039908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. (2004) 377:159–69. 10.1042/bj20031253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmitter T, Pils S, Sakk V, Frank R, Fischer KD, Hauck CR. The granulocyte receptor CEACAM3 directly associates with Vav to promote phagocytosis of human pathogens. J Immunol. (2007) 178:3797–805. 10.4049/jimmunol.178.6.3797 [DOI] [PubMed] [Google Scholar]
- 62.Buntru A, Zimmermann T, Hauck CR. FRET-based subcellular visualization of pathogen-induced host receptor signalling. BMC Biol. (2009) 7:81 10.1186/1741-7007-7-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarantis H, Gray-Owen SD. The specific innate immune receptor CEACAM3 triggers neutrophil bactericidal activities via a Syk kinase-dependent pathway. Cell Microbiol. (2007) 9:2167–80. 10.1111/j.1462-5822.2007.00947.x [DOI] [PubMed] [Google Scholar]
- 64.Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, et al. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med. (1997) 186:1027–39. 10.1084/jem.186.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pils S, Kopp K, Peterson L, Delgado-Tascon J, Nyffenegger-Jann NJ, Hauck CR. The adaptor molecule Nck localizes the WAVE complex to promote actin polymerization during CEACAM3-mediated phagocytosis of bacteria. PLoS ONE. (2012) 7:e32808. 10.1371/journal.pone.0032808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rougerie P, Miskolci V, Cox D. Generation of membrane structures during phagocytosis and chemotaxis of macrophages: role and regulation of the actin cytoskeleton. Immunol Rev. (2013) 256:222–39. 10.1111/imr.12118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lebensohn AM, Kirschner MW. Activation of the WAVE complex by coincident signals controls actin assembly. Mol Cell. (2009) 36:512–24. 10.1016/j.molcel.2009.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Booth JW, Telio D, Liao EH, McCaw SE, Matsuo T, Grinstein S, et al. Phosphatidylinositol 3-kinases in carcinoembryonic antigen-related cellular adhesion molecule-mediated internalization of Neisseria gonorrhoeae. J Biol Chem. (2003) 278:14037–45. 10.1074/jbc.M211879200 [DOI] [PubMed] [Google Scholar]
- 69.McCaw SE, Liao EH, Gray-Owen SD. Engulfment of Neisseria gonorrhoeae: revealing distinct processes of bacterial entry by individual carcinoembryonic antigen-related cellular adhesion molecule family receptors. Infect Immun. (2004) 72:2742–52. 10.1128/IAI.72.5.2742-2752.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sit ST, Manser E. Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci. (2011) 124:679–83. 10.1242/jcs.064964 [DOI] [PubMed] [Google Scholar]
- 71.English BK, Orlicek SL, Mei Z, Meals EA. Bacterial LPS and IFN-gamma trigger the tyrosine phosphorylation of vav in macrophages: evidence for involvement of the hck tyrosine kinase. J Leukoc Biol. (1997) 62:859–64. 10.1002/jlb.62.6.859 [DOI] [PubMed] [Google Scholar]
- 72.Fumagalli L, Zhang H, Baruzzi A, Lowell CA, Berton G. The Src family kinases Hck and Fgr regulate neutrophil responses to N-formyl-methionyl-leucyl-phenylalanine. J Immunol. (2007) 178:3874–85. 10.4049/jimmunol.178.6.3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buntru A, Kopp K, Voges M, Frank R, Bachmann V, Hauck CR. Phosphatidylinositol-3' kinase activity is critical for initiating the oxidative burst and bacterial destruction during CEACAM3-mediated phagocytosis. J Biol Chem. (2011) 286:9555–66. 10.1074/jbc.M110.216085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhan Y, Virbasius JV, Song X, Pomerleau DP, Zhou GW. The p40phox and p47phox PX domains of NADPH oxidase target cell membranes via direct and indirect recruitment by phosphoinositides. J Biol Chem. (2002) 277:4512–8. 10.1074/jbc.M109520200 [DOI] [PubMed] [Google Scholar]
- 75.Streichert T, Ebrahimnejad A, Ganzer S, Flayeh R, Wagener C, Brummer J. The microbial receptor CEACAM3 is linked to the calprotectin complex in granulocytes. Biochem Biophys Res Commun. (2001) 289:191–7. 10.1006/bbrc.2001.5955 [DOI] [PubMed] [Google Scholar]
- 76.Belambri SA, Rolas L, Raad H, Hurtado-Nedelec M, Dang PM, El-Benna J. NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits. Eur J Clin Invest. (2018) 48(Suppl. 2):e12951. 10.1111/eci.12951 [DOI] [PubMed] [Google Scholar]
- 77.Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. (2005) 22:507–17. 10.1016/j.immuni.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 78.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. (2005) 106:2543–50. 10.1182/blood-2005-03-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kopp K, Buntru A, Pils S, Zimmermann T, Frank R, Zumbusch A, et al. GRB14 is a negative regulator of CEACAM3-mediated phagocytosis of pathogenic bacteria. J Biol Chem. (2012) 287:39158–70. 10.1074/jbc.M112.395228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Delgado Tascon J, Adrian J, Kopp K, Scholz P, Tschan MP, Kuespert K, et al. The granulocyte orphan receptor CEACAM4 is able to trigger phagocytosis of bacteria. J Leukoc Biol. (2015) 97:521–31. 10.1189/jlb.2AB0813-449RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. (2006) 6:173–82. 10.1038/nri1785 [DOI] [PubMed] [Google Scholar]
- 82.Thomas CJ, Schroder K. Pattern recognition receptor function in neutrophils. Trends Immunol. (2013) 34:317–28. 10.1016/j.it.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 83.Heinrich A, Heyl KA, Klaile E, Muller MM, Klassert TE, Wiessner A, et al. Moraxella catarrhalis induces CEACAM3-Syk-CARD9-dependent activation of human granulocytes. Cell Microbiol. (2016) 18:1570–82. 10.1111/cmi.12597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chan CH, Stanners CP. Novel mouse model for carcinoembryonic antigen-based therapy. Mol Ther. (2004) 9:775–85. 10.1016/j.ymthe.2004.03.009 [DOI] [PubMed] [Google Scholar]
- 85.Sintsova A, Sarantis H, Islam EA, Sun CX, Amin M, Chan CH, et al. Global analysis of neutrophil responses to Neisseria gonorrhoeae reveals a self-propagating inflammatory program. PLoS Pathog. (2014) 10:e1004341. 10.1371/journal.ppat.1004341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stevens JS, Criss AK. Pathogenesis of Neisseria gonorrhoeae in the female reproductive tract: neutrophilic host response, sustained infection, and clinical sequelae. Curr Opin Hematol. (2018) 25:13–21. 10.1097/MOH.0000000000000394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hauck CR, Gulbins E, Lang F, Meyer TF. Tyrosine phosphatase SHP-1 is involved in the opsonin-independent phagocytosis of Opa52-expressing Neisseria gonorrhoeae. Infect Immun. (1999) 67:5490–4. 10.1128/IAI.67.10.5490-5494.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bos MP, Grunert F, Belland RJ. Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae. Infect Immun. (1997) 65:2353–61. 10.1128/IAI.65.6.2353-2361.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sintsova A, Wong H, Macdonald KS, Kaul R, Virji M, Gray-Owen SD. Selection for a CEACAM receptor-specific binding phenotype during Neisseria gonorrhoeae infection of the human genital tract. Infect Immun. (2015) 83:1372–83. 10.1128/IAI.03123-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muenzner P, Dehio C, Fujiwara T, Achtman M, Meyer TF, Gray-Owen SD. Carcinoembryonic antigen family receptor specificity of Neisseria meningitidis Opa variants influences adherence to and invasion of proinflammatory cytokine-activated endothelial cells. Infect Immun. (2000) 68:3601–7. 10.1128/IAI.68.6.3601-3607.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Jonge MI, Hamstra HJ, Van Alphen L, Dankert J, Van Der Ley P. Mapping the binding domains on meningococcal Opa proteins for CEACAM1 and CEA receptors. Mol Microbiol. (2003) 50:1005–15. 10.1046/j.1365-2958.2003.03749.x [DOI] [PubMed] [Google Scholar]
- 92.Zhou GQ, Zhang Y, Hammarstrom S. The carcinoembryonic antigen (CEA) gene family in non-human primates. Gene. (2001) 264:105–12. 10.1016/S0378-1119(00)00595-3 [DOI] [PubMed] [Google Scholar]
- 93.Rhesus Macaque Genome Sequencing and Analysis Consortium. Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science. (2007) 316:222–34. 10.1126/science.1139247 [DOI] [PubMed] [Google Scholar]
- 94.Adrian J, Bonsignore P, Hammer S, Frickey T, Hauck CR. Adaptation to host-specific bacterial pathogens drives rapid evolution of a human innate immune receptor. Curr Biol. (2019) 29:616–630 e615. 10.1016/j.cub.2019.01.058 [DOI] [PubMed] [Google Scholar]
- 95.Kammerer R, Zimmermann W. Coevolution of activating and inhibitory receptors within mammalian carcinoembryonic antigen (CEA) families. BMC Biol. (2010) 8:12 10.1186/1741-7007-8-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kammerer R, Mansfeld M, Hanske J, Missbach S, He X, Kollner B, et al. Recent expansion and adaptive evolution of the carcinoembryonic antigen family in bats of the Yangochiroptera subgroup. BMC Genomics. (2017) 18:717. 10.1186/s12864-017-4106-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Missbach S, Aleksic D, Blaschke L, Hassemer T, Lee KJ, Mansfeld M, et al. Alternative splicing after gene duplication drives CEACAM1-paralog diversification in the horse. BMC Evol Biol. (2018) 18:32. 10.1186/s12862-018-1145-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bond M, Tejedor MF, Campbell KE, Jr, Chornogubsky L, Novo N, Goin F. Eocene primates of South America and the African origins of New World monkeys. Nature. (2015) 520:538–41. 10.1038/nature14120 [DOI] [PubMed] [Google Scholar]
- 99.Pils S, Gerrard D, Meyer A, Hauck CR. CEACAM3: an innate immune receptor directed against human-resticted bacterial pathogens. Intl J Med Microbiol. (2008) 298:553–60. 10.1016/j.ijmm.2008.04.005 [DOI] [PubMed] [Google Scholar]
- 100.Fitzgerald G, Botstein D, Califf R, Collins R, Peters K, Van Bruggen N, et al. The future of humans as model organisms. Science. (2018) 361:552–3. 10.1126/science.aau7779 [DOI] [PubMed] [Google Scholar]
- 101.Saleheen D, Natarajan P, Armean IM, Zhao W, Rasheed A, Khetarpal SA, et al. Human knockouts and phenotypic analysis in a cohort with a high rate of consanguinity. Nature. (2017) 544:235–9. 10.1038/nature22034 [DOI] [PMC free article] [PubMed] [Google Scholar]