Key Teaching Points.
-
•
The TightRail rotating mechanical dilator sheath (Spectranetics Corp, Colorado Springs, CO) has emerged as a novel tool for extraction of transvenous implantable cardioverter-defibrillator (ICD) leads.
-
•
Its use for extraction of subcutaneous ICD (S-ICD) systems has not been reported before.
-
•
To the best of our knowledge, this is the first case reporting use of a mechanical dilator sheath for successful extraction of a subcutaneous coil of an S-ICD.
Introduction
Over the last decade, subcutaneous implantable cardioverter-defibrillator (S-ICD) implantation has gained significant momentum for the prevention of sudden cardiac death. This device has been implanted in more than 19,000 patients worldwide.1 Given their ability to deliver defibrillation while leaving the heart and vasculature untouched, S-ICDs have emerged as an appealing alternative to conventional transvenous ICDs. Supporting the notion that transvenous leads are the “Achilles heel” of traditional ICDs, a recent meta-analysis2 demonstrated a significantly lower rate of lead-related complications in patients implanted with S-ICDs compared to transvenous ICDs. However, acute and chronic complications still do occur with S-ICDs, sometimes requiring device extraction. There is an increasing necessity to establish safe and effective methods for device extraction in the event of untoward complications.
Currently, data describing the challenges encountered during S-ICD extraction are scarce. Of the various components, extraction of the subcutaneous coil can be particularly difficult. The TightRail (Spectranetics Corp, Colorado Springs, CO) rotating mechanical dilator has emerged as a novel tool for the extraction of transvenous ICD and pacemaker leads in the past 5 years, with early reports revealing encouraging results.3, 4 The purpose of this case report is to describe the use of a mechanical dilator sheath for extraction of an S-ICD coil. To our knowledge, this is the first case in the literature reporting the safety and efficacy of TightRail for successful extraction of a chronically implanted S-ICD coil.
Case report
A 37-year-old man was referred to the electrophysiology service at our institution for implantation of an ICD for primary prevention. He had a medical history of morbid obesity, TTN gene mutation, and dilated cardiomyopathy with ejection fraction of 15%. His family history was significant for sudden cardiac death (father) and myocarditis (brother). Heart failure was deemed to be nonischemic in etiology as his Agatston coronary artery calcium score was noted to be zero on coronary computed tomography angiography. His electrocardiogram revealed QRS duration of 76 milliseconds. Given his young age and no indication for cardiac resynchronization therapy, the patient was implanted with a Boston Scientific EMBLEM S-ICD (Boston Scientific, Marlborough, MA).
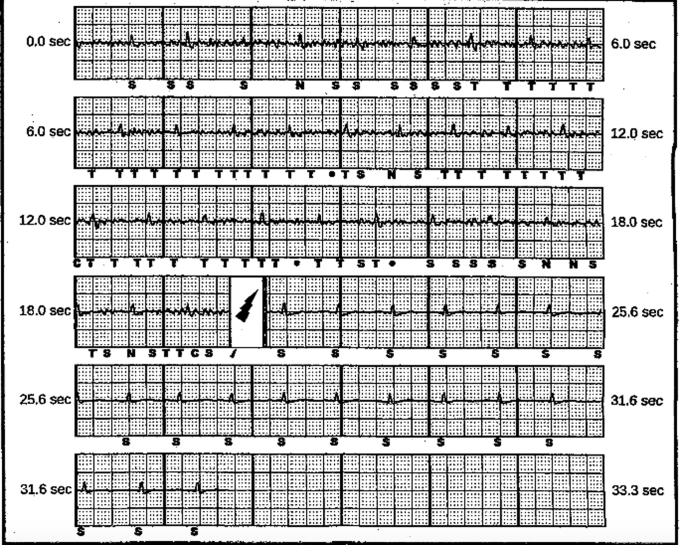
Approximately 17 months after initial implantation, the patient sustained 2 shocks from his device without any preceding palpitations, chest pain, lightheadedness, or shortness of breath. The first shock was delivered when the patient lifted his arms over his head after watching his favorite wrestler win a tournament. On his way to the hospital, the device fired again. Device interrogation revealed 2 inappropriate shocks, both of which were triggered by noise (Figure 1). Impedance was also noted to be elevated (150 ohms). The sensing configuration was programmed to the primary vector at the time of shock delivery. Oversensing was reproducible with movements such as elevating the left arm above his head and with isometric maneuvers. Alternate and secondary vectors were tested and maneuvers in these sensing configurations reproduced noise. Neither chest radiograph nor computed tomography scan revealed any signs of S-ICD lead fracture. The high impedance was deemed to be secondary to poor contact with the fascial plane, but the etiology of the noise was unclear. At this point, therapies were turned off, yet the continued need for ICD therapy was deemed necessary owing to the patient’s medical history. Given the high risk of recurrent oversensing and inappropriate shocks, extraction of his S-ICD and consequent insertion of a transvenous ICD was recommended.
Figure 1.
Stored electrogram of primary vector recording from subcutaneous implantable cardioverter-defibrillator. The patient received a shock from oversensing of non-QRS when he lifted his arms over his head. The 2 shock delivery tracings appeared similar.
The patient underwent S-ICD system extraction 506 days after initial implantation. The procedure was carried out under general anesthesia. Sharp and blunt dissection techniques were used to remove the pulse generator from the pocket and detach it from the lead. The suture sleeve was identified and freed from surrounding tissues. Following this, the lead was transected at the subxiphoid site. There was no difficulty in removing the segment of the lead between the pulse generator and the subxiphoid site. Removal of the subcutaneous coil proved to be challenging. The lead had been previously placed via the 3-incision technique; the upper sternal incision was opened and the suture was cut. Sharp and blunt dissection was performed along the coil at both the subxiphoid and upper sternal sites. Despite this, the subcutaneous coil could not be freed from the surrounding tissue. Consequently, a Bulldog lead extender (Cook Medical LLC, Bloomington, IN) and an Amplatz Goose Neck snare (Medtronic, Minneapolis, MN) were utilized to support the use of a TightRail rotating mechanical dilator sheath (Figure 2). With the appropriate support, the TightRail mechanical sheath was then advanced serially over the coil (Figure 3). The subcutaneous coil was then pulled back into the sheath and successfully extracted. No apparent complications were noted. The wounds were irrigated and closed in the routine manner. Subsequently, a single-chamber ICD was successfully implanted through the cephalic vein.
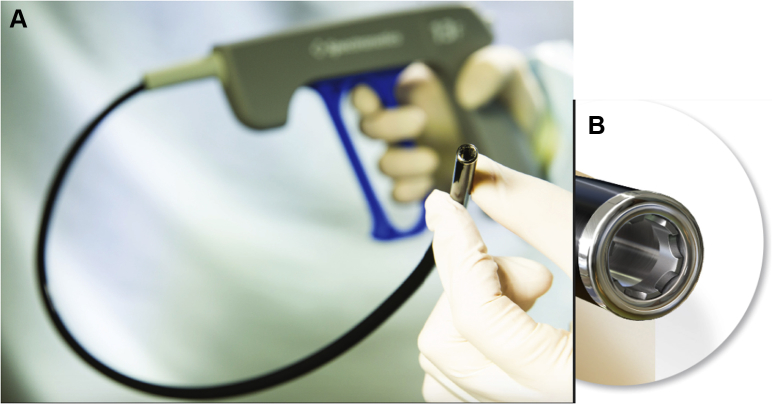
Figure 2.
TightRail mechanical dilator sheath (Spectranetics Corp, Colorado Springs, CO) components illustrated. A: Flexible inner sheath, proximal handheld drive mechanism. B: Dilating metal blade at distal tip.
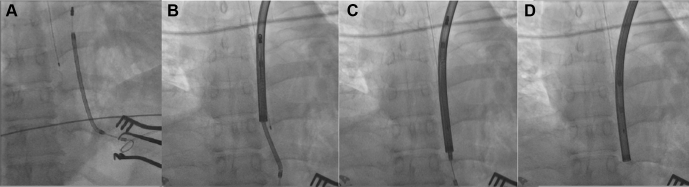
Figure 3.
A: Fluoroscopic view of the subcutaneous implantable cardioverter-defibrillator lead before extraction. B–D: Lead covered by TightRail sheath (Spectranetics Corp, Colorado Springs, CO) followed by serial advancement and successful extraction, without any damage to the lead.
Discussion
To the best of our knowledge, this is the first report of the successful use of a mechanical dilator sheath for the extraction of a subcutaneous coil of the S-ICD system.
The Boston Scientific S-ICD system received the CE mark in 2008, followed by U.S. Food and Drug Administration approval in 2012. Initial prospective studies were promising,5, 6, 7 and PRAETORIAN,8 a randomized, multicenter trial, is currently underway. Subsequently, the use of S-ICDs in clinical practice has increased significantly since their inception 10 years ago. However, a certain proportion of these devices will inevitably need to be explanted owing to acute and chronic complications. A recently published systematic review of 16 studies involving 5380 implants reported an explant rate of 3.8%.9 The most common cause of S-ICD extraction is device infection; more rarely, the cause of extraction is need for antitachycardia pacing, antibradycardia pacing, and cardiac resynchronization therapy.5, 6, 7 Inappropriate shocks are an uncommon indication for device extraction, but this issue remains an important cause of morbidity related to S-ICDs.
Manual traction is an effective method for ICD lead extraction for leads that have been implanted recently; however, it might not be sufficient for chronically implanted leads. The body demonstrates a foreign body response to ICD leads, manifested as formation of adhesions. These adhesions tend to be dense in chronically implanted leads, and they are sometimes present not just at the tip, but along the entire length of the lead. This response appears more pronounced in younger individuals, who form a significant portion of patients receiving S-ICDs. To remove chronically implanted S-ICD leads in a safe and efficacious manner, it becomes important to broaden and refine the array of tools available for extraction.
The TightRail system is a recently developed hand-powered device. It was primarily designed to facilitate percutaneous extraction of indwelling ICD and pacemaker leads from the vasculature. Its use has been reported even as a first-line extraction tool, with a success rate of >97%.2 TightRail is typically utilized in conjunction with conventional tools used to provide traction, such as locking stylets. However, since the S-ICD coil has no lumen, a Bulldog lead extender and Amplatz snare were used to provide support to the mechanical dilator sheath in our case. The components of this system include a rotating inner sheath, a static outer sheath, a dilating metal blade at the distal tip, and a proximal handheld drive mechanism (Figure 3). The inner sheath moves over the lead body when the operator pulls the handle. The dilating metal blade remains shielded until activated. It has a bidirectional rotating mechanism, rotating 574° with each full trigger activation, 287° clockwise and 287° counterclockwise. This occurs while extending the blade just 0.5 mm, cutting fibrous attachments surrounding the lead. Once the surrounding attachments are cut, the outer sheath is advanced until a different area of attachment is encountered. Once the lead is free, it can be extracted through the inner sheath.
A report on 21 patients with subcutaneous shocking coils (6996SQ Medtronic) undergoing extraction was published recently.10 In the absence of other major studies, this report could be used as a surrogate for S-ICD lead extraction. The majority of included patients underwent successful extraction by accessing the device pocket followed by tie-down and manual traction. However, 3 patients had dense adhesions and required further incisions beyond device pocket and suture sleeve sites to complete extraction of the lead. One procedure required the use of a laser sheath to break adhesions and facilitate removal of a 63-day-old S-ICD lead. A direct correlation was also noted between the dwell-in time of the S-ICD and procedural time (<1 year, mean: 149 ± 63 minutes vs >1 year, mean: 201 ± 112 minutes). With the use of the TightRail in our case, the device removal procedure time of 103 minutes was comparable to the time reported in the prior study. Furthermore, a retrospective analysis comparing laser and mechanical approaches for chronically implanted transvenous lead removal favored the use of a mechanical approach in terms of clinical success and cost-effectiveness.11 However, there are no data comparing these 2 approaches for removal of S-ICD leads. Overall, the success rate of the TightRail system has been comparable to prior established methods of transvenous lead extraction.12, 13, 14 We believe this tool can also be considered as an option for challenging cases of S-ICD coil extraction.
Conclusion
This case report highlights the first successful use of a mechanical dilator sheath for the extraction of a chronically implanted S-ICD coil. Further study of this system in a larger patient cohort to assess its success and complication rates in relation to existing extraction tools is warranted.
References
- 1.Boston Scientific CRM Product Performance Report. June 2016. http://www.bostonscientific.com/content/dam/bostonscientific/quality/ppr/2016/Q3/Product%20Performance%20Report%20Q3%202016%20Rev%20B.pdf Available at: Accessed February 10, 2019.
- 2.Basu-Ray I., Liu J., Jia X. Subcutaneous versus transvenous implantable defibrillator therapy: A meta-analysis of case-control studies. JACC Clin Electrophysiol. 2017;3:1475–1483. doi: 10.1016/j.jacep.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Aytemir K., Yorgun H., Canpolat U. Initial experience with the TightRail Rotating Mechanical Dilator Sheath for transvenous lead extraction. Europace. 2016;18:1043–1048. doi: 10.1093/europace/euv245. [DOI] [PubMed] [Google Scholar]
- 4.Aytemir K., Kaya B., Sahiner L. Transvenous lead extraction by using Tight RailTM mechanical dilator sheath: single center experience. Acta Medica. 2017;48:6–11. [Google Scholar]
- 5.Weiss R., Knight B.P., Gold M.R. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation. 2013;128:944–953. doi: 10.1161/CIRCULATIONAHA.113.003042. [DOI] [PubMed] [Google Scholar]
- 6.Gold M.R., Aasbo J.D., El-Chami M.F. Subcutaneous implantable cardioverter-defibrillator Post-Approval Study: clinical characteristics and perioperative results. Heart Rhythm. 2017;14:1456–1463. doi: 10.1016/j.hrthm.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Boersma L., Barr C., Knops R. Implant and midterm outcomes of the subcutaneous implantable cardioverter-defibrillator registry: The EFFORTLESS Study. J Am Coll Cardiol. 2017;70:830–841. doi: 10.1016/j.jacc.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 8.Olde Nordkamp L.R., Knops R.E., Bardy G.H. Rationale and design of the PRAETORIAN trial: a Prospective, RAndomizEd comparison of subcuTaneOus and tRansvenous ImplANtable cardioverter-defibrillator therapy. Am Heart J. 2012;163:753–760 e752. doi: 10.1016/j.ahj.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Chue C.C., Kwok C.S., Wong C.W. Efficacy and safety of the subcutaneous implantable cardioverter debrillator: a systematic review. Heart. 2017;103:1315–1322. doi: 10.1136/heartjnl-2016-310852. [DOI] [PubMed] [Google Scholar]
- 10.Nakhla S., Hussein A.A., Brunner M.P., Wazni O., Wilkoff B.L., Tarakji K.G. Removal of subcutaneous defibrillator shocking coils: lessons to learn for future extraction of subcutaneous defibrillator systems. Pacing Clin Electrophysiol. 2018;41:1341–1344. doi: 10.1111/pace.13481. [DOI] [PubMed] [Google Scholar]
- 11.Starck C.T., Rodriguez H., Hurlimann D. Transvenous lead extractions: comparison of laser vs. mechanical approach. Europace. 2013;15:1636–1641. doi: 10.1093/europace/eut086. [DOI] [PubMed] [Google Scholar]
- 12.Wazni O., Epstein L.M., Carrillo R.G. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol. 2010;55:579–586. doi: 10.1016/j.jacc.2009.08.070. [DOI] [PubMed] [Google Scholar]
- 13.Wilkoff B.L., Byrd C.L., Love C.J. Pacemaker lead extraction with the laser sheath: results of the pacing lead extraction with the excimer sheath (PLEXES) trial. J Am Coll Cardiol. 1999;33:1671–1676. doi: 10.1016/s0735-1097(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 14.Oto A., Aytemir K., Canpolat U. Evolution in transvenous extraction of pacemaker and implantable cardioverter defibrillator leads using a mechanical dilator sheath. Pacing Clin Electrophysiol. 2012;35:834–840. doi: 10.1111/j.1540-8159.2012.03385.x. [DOI] [PubMed] [Google Scholar]