Abstract
Background
UNAIDS estimates that 34 million people are currently living with the human immunodeficiency virus (HIV) worldwide. Currently recommended regimens for initiating HIV treatment consist of either a non‐nucleoside reverse transcriptase inhibitor (NNRTI) or ritonavir‐boosted protease inhibitor (PI) combined with two nucleoside reverse transcriptase inhibitors (NRTIs). However, there may be some patients for whom NNRTIs and PIs may not be appropriate. This is an update of the review published in the Cochrane Library Issue 3, 2009.
Objectives
To evaluate the effects of any fixed‐dose combination of three NRTIs (co‐formulated abacavir‐lamivudine‐zidovudine) for initial treatment of HIV infection.
Search methods
Between December 2010 and July 2011, we used standard Cochrane methods to search electronic databases and conference proceedings with relevant search terms without limits to language or publication status.
Selection criteria
We selected randomised controlled trials (RCTs) with a minimum follow‐up time of six months which compared co‐formulated abacavir‐lamivudine‐zidovudine with either PI‐based or NNRTI‐based therapy among antiretroviral‐naive HIV‐infected patients aged at least 13 years.
Data collection and analysis
Three authors independently selected eligible studies, assessed risk of bias, and extracted data; resolving discrepancies by consensus. We calculated the risk ratio (RR) or mean difference (MD), as appropriate, with its 95% confidence interval (CI) and conducted meta‐analysis using the random‐effects method because of significant statistical heterogeneity (P<0.1).
Main results
We identified 15 potentially eligible RCTs, four of which met our inclusion criteria. The four included RCTs were conducted in the United States of America (USA); USA, Puerto Rico, Guatemala, Dominican Republic, and Panama; USA and Mexico; and Botswana, respectively. The RCTs compared co‐formulated abacavir‐lamivudine‐zidovudine to treatment based on efavirenz (NNRTI), nelfinavir (PI), atazanavir (PI), and co‐formulated lopinavir‐ritonavir (PI), respectively. Overall, there was no significant difference in virological suppression between co‐formulated abacavir‐lamivudine‐zidovudine and NNRTI‐ or PI‐based therapy (4 trials; 2247 participants: RR 0.73, 95% CI 0.39 to 1.36). However, the results showed significant heterogeneity (I2=79%); with co‐formulated abacavir‐lamivudine‐zidovudine inferior to NNRTI (1 trial, 1147 participants: RR 0.35, 95%CI 0.26 to 0.49) but with a trend towards co‐formulated abacavir‐lamivudine‐zidovudine being superior to PI (3 trials, 1110 participants: RR 1.07, 95%CI 1.00 to 1.16; I2=0%). We found no significant differences between co‐formulated abacavir‐lamivudine‐zidovudine and either PI or NNRTI on CD4+ cell counts (3 trials, 1687 participants: MD ‐0.01, 95%CI ‐0.11 to 0.09; I2=0%), severe adverse events (4 trials: RR 1.22, 95%CI 0.78 to 1.92; I2=62%) and hypersensitivity reactions (4 trials: RR 4.04, 95% CI 0.41 to 40.02; I2=72%). Only two studies involving PIs reported data on the lipid profile. One study found that the mean increase in total cholesterol from baseline to 96 weeks was significantly lower with co‐formulated abacavir‐lamivudine‐zidovudine than with nelfinavir, but there were no differences with triglyceride levels. The second study found the fasting lipid profile to be comparable in both co‐formulated abacavir‐lamivudine‐zidovudine and atazanavir arms at 48 weeks.
The significant heterogeneity of effects for most outcomes evaluated was largely due to differences in the control therapy used in the included trials (i.e. NNRTIs or PIs). Using the GRADE approach, we rated the overall quality of the evidence on the relative effects of co‐formulated abacavir‐lamivudine‐zidovudine for initial treatment of HIV infection as moderate. The main reason for downgrading the quality of the evidence was imprecision of the findings. The estimate of the treatment effect for each outcome has wide confidence intervals, which extend from the fixed‐dose NRTI combination regimen being appreciably better to the regimen being appreciably worse than PI‐ or NNRTI‐based regimens.
Authors' conclusions
This review provides evidence that co‐formulated abacavir‐lamivudine‐zidovudine remains a viable option for initiating antiretroviral therapy, especially in HIV‐infected patients with pre‐existing hyperlipidaemia. The varied geographical locations of the included trials augment the external validity of these findings. We are moderately confident in our estimate of the treatment effects of the triple NRTI regimen as initial therapy for HIV infection. In the context of the GRADE approach, such moderate quality of evidence implies that the true effects of the regimen are likely to be close to the estimate of effects found in this review; but there is a possibility that they could be substantially different. Further research should be geared towards defining the subgroup of HIV patients for whom this regimen will be most beneficial.
Plain language summary
Co‐formulated abacavir‐lamivudine‐zidovudine for treating HIV infection and AIDS
The primary objective of this review was to evaluate the antiviral efficacy of co‐formulated abacavir‐lamivudine‐zidovudine for initial treatment of HIV infection. The secondary objectives were to evaluate the safety and tolerability of the triple drug combination. We identified 15 potentially eligible studies, four of which met our inclusion criteria. Our findings indicate that co‐formulated abacavir‐lamivudine‐zidovudine remains a viable option for initiating antiretroviral therapy, especially in HIV‐infected patients with pre‐existing hyperlipidaemia and those who do not tolerate ritonavir.
Summary of findings
Summary of findings for the main comparison. Co‐formulated abacavir‐lamivudine‐zidovudine compared to NNRTIs or PIs for initial treatment of HIV infection.
| Co‐formulated abacavir‐lamivudine‐zidovudine compared to NNRTIs or PIs for initial treatment of HIV infection and AIDS | |||||
| Patient or population: Antiretroviral‐naive HIV infected patients Settings: Any country setting (i.e. low‐, middle‐, or high‐income) Intervention: Co‐formulated abacavir‐lamivudine‐zidovudine Comparison: Non‐nucleoside reverse transcriptase inhibitor (NNRTI)‐ or protease inhibitor (PI)‐based therapy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| NNRTIs or PIs | Co‐formulated abacavir‐lamivudine‐zidovudine | ||||
| Virologic failure 2 successive HIV‐1 RNA >= 200copies/ml at 16+ weeks after randomisation Follow‐up: mean 48 weeks | 115 per 1000 | 131 per 1000 (64 to 266) | RR 1.14 (0.56 to 2.31) | 1687 (3 studies) | ⊕⊕⊕⊝ moderate1 |
| Virologic suppression Viral load < 50 copies/ml Follow‐up: mean 48 weeks | 732 per 1000 | 710 per 1000 (549 to 915) | RR 0.97 (0.75 to 1.25) | 2247 (4 studies) | ⊕⊕⊕⊝ moderate1 |
| CD4 cell count Follow‐up: mean 48 weeks | The mean CD4 cell count ranged across control groups from 415‐634 cells per cubic millimetres | The mean CD4 cell count in the intervention groups was 0.01 lower (0.11 lower to 0.09 higher) | 1687 (3 studies) | ⊕⊕⊕⊝ moderate1 | |
| Severe adverse events Follow‐up: mean 48 weeks | 116 per 1000 | 142 per 1000 (90 to 223) | RR 1.22 (0.78 to 1.92) | 2247 (4 studies) | ⊕⊕⊕⊝ moderate1 |
| Hypersensitivity reactions Follow‐up: mean 48 weeks | 44 per 1000 | 178 per 1000 (18 to 1000) | RR 4.04 (0.41 to 40.02) | 2247 (4 studies) | ⊕⊕⊕⊝ moderate1 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 The estimate of effect has wide confidence intervals, which extend from appreciable benefit to appreciable harm
Background
The human immunodeficiency virus (HIV) pandemic poses one of the greatest challenges to global public health. In 2011, an estimated 34 million people were living with HIV and 1.7 million died of the acquired immunodeficiency syndrome (AIDS) (UNAIDS 2012). Prevention is commonly advocated to curb the spread of HIV infection, and although preventive methods have considerably slowed the spread of HIV in most parts of the world, people who are already infected need care and treatment.
The goal of antiretroviral therapy is to achieve prolonged suppression of HIV replication. The ideal antiretroviral drugs should be effective in suppressing viral replication, affordable, available in simplified regimens, well tolerated, and have no dietary interactions. The use of monotherapy and dual therapy has often led to mutations and long‐term resistance (Eron 1995; Pialoux 1998; Rutherford 2003), necessitating the development of combination therapy with three drugs taken separately (Carpenter 2000; Hammer 2008). In well‐resourced countries (Ledergerber 1999) and, recently, Brazil (Hacker 2004; Teixeira 2004), antiretroviral therapy has contributed substantially towards delaying HIV progression to AIDS and death. However, these combinations are complex and difficult to take due to high pill burden, stringent intake schedules, and food and fluid restrictions They may also be associated with drug‐drug interactions and numerous side effects, including various lipid abnormalities (Mehta 1997; Gifford 2000). This complexity also makes antiretroviral therapy less accessible to patients in most resource‐constrained regions of the world, which currently are hardest hit by the pandemic, such as sub‐Saharan Africa. This area is inhabited by approximately 10% of the world's population but is home to 60% of all people currently living with HIV (UNAIDS 2012).
Concern over toxicity, adherence, and drug‐drug interactions has led to the development of simpler antiretroviral regimens, including co‐formulated abacavir‐lamivudine‐zidovudine (Anon 2000; Saez‐Llorens 2001). Three NRTIs simplify PI‐based therapy by easing dosing regimens (only one tablet twice daily) and avoiding lipid abnormalities (Seaton 2003). Although treatment simplification could help patients maintain adherence, continued virologic suppression must be ensured. Therefore, clarification of the role of this simplified antiretroviral therapy on prolonged suppression of HIV replication is of considerable importance. Because all three antiretroviral drugs are of the same class, the use of co‐formulated abacavir‐lamivudine‐zidovudine (if proven to be effective) potentially preserves NNRTIs and PIs for later use, thereby avoiding resistance to all classes of antiretroviral agents at the same time, and allows for effective second‐line treatment regimens (Staszewski 2001). There are concerns, however, about hypersensitivity reactions to abacavir (Staszewski 1998). Cross resistance between drugs of the same class should also be considered. Also, entecavir used for hepatitis B virus (HBV) treatment, may select for M184V mutation which confers resistance to lamivudine in individuals co‐infected with HIV and HBV (McMahon 2007).
The aim of this review was to combine all high‐quality RCTs comparing co‐formulated abacavir‐lamivudine‐zidovudine with PI‐ or NNRTI‐based therapy to assess the antiviral potency and tolerability of the simplified triple nucleoside combination in initial therapy for HIV.
Objectives
The primary objective of this review was to evaluate the antiviral efficacy of co‐formulated zidovudine‐lamivudine‐abacavir for initial treatment of HIV infection. The secondary objectives were to evaluate the safety and tolerability of the triple nucleoside combination.
Methods
Criteria for considering studies for this review
Types of studies
Only RCTs with a minimum follow‐up time of six months were included. Six months of treatment was considered enough time to detect significant differences in the suppression of viral activity after initiation of therapy.
Types of participants
HIV‐infected, antiretroviral‐naive patients aged at least 13 years. We chose only studies that focused on adolescents and adults.
Types of interventions
Treatment of HIV infection with co‐formulated abacavir‐lamivudine‐zidovudine as initial therapy compared with treatment based on PIs or NNRTIs
Types of outcome measures
The primary outcome measure was suppression of viral activity, as defined by the authors.
The secondary outcome measures included: 1‐ CD4 cell count 2‐ Severe adverse events 3‐ Clinical lipodystrophy manifestations 4‐ Total cholesterol 5‐ Triglyceride level 6‐ Treatment adherence
Search methods for identification of studies
See: HIV/AIDS Collaborative Review Group search strategy.
Between February 2008 and May 2009, we searched PubMed, EMBASE, Cochrane Database of Systematic Reviews, and the York Database of Abstracts of Reviews of Effectiveness (DARE) for previous reviews and meta‐analyses of antiretroviral therapy for treatment of HIV that included co‐formulated abacavir‐lamivudine‐zidovudine; and searched the references of these reviews for reports of eligible trials. We then carried out an exhaustive search of the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, EMBASE, NLM GATEWAY, and AIDSearch, for randomised controlled trials of co‐formulated abacavir‐lamivudine‐zidovudine for initial treatment of HIV, using standardised methodological filters (Higgins 2011) where appropriate. We also searched reference lists of identified articles.There were no time or language restrictions to our search.
We updated the search in December 2010 by searching EMBASE, ISI Web of Science, PsycINFO, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform (http://www.who.int/ictrp/search/en/). In addition, in July 2011, we finalised the update by searching CENTRAL, and PubMed.
See Appendix 1 for all search strategies.
Data collection and analysis
See: Cochrane HIV/AIDS Group methods used in reviews.
Selection of studies
The Trials Search Coordinator of the Cochrane HIV/AIDS Group (http://www.igh.org/Cochrane/) conducted the electronic database searches. For the original version of the review and this update, three authors (MS, EJK, and CSW) independently conducted the selection of potentially relevant studies by scanning the titles and abstracts of all material downloaded from the electronic searches. Irrelevant reports were discarded and the full articles were obtained for all potentially relevant or uncertain reports. From this pool of potentially eligible studies, we selected studies for inclusion in the review if they were RCTs (study design) comparing any fixed‐dose combination of abacavir, lamivudine and zidovudine (NRTI) with PI‐ or NNRTI‐based antiretroviral therapy (intervention) in antiretroviral‐naive, HIV‐infected adults (participants). Disagreements between the review authors were resolved by discussion and consensus. When no consensus could be reached, SMA and JS arbitrated.
Data extraction and management
The three authors (MS, EJK, and CSW) extracted data independently using pre‐established data collection forms. We extracted information from included studies on study details (i.e. how the allocation sequence was generated, method used to conceal treatment allocation, blinding of those receiving and providing care and those assessing outcomes, losses to follow‐up and how they were handled), participant characteristics (i.e. setting, number of patients randomised, baseline HIV‐1 RNA and CD4 cell levels), interventions (i.e. treatment and control, length of treatment), and outcomes (virological failure/suppression, CD4+ cell count, cholesterol level, clinical lipodystrophy manifestations, other side effects). Disagreements between the review authors were resolved by discussion and consensus. When no consensus could be reached, SMA and JS arbitrated.
Assessment of risk of bias in included studies
We assessed risk of bias as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Three review authors (MS, EJK, and CSW) independently assessed the risk of bias in each included study by addressing seven specific domains, namely, random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and ‘other issues'. For each included trial, the authors independently described what the study authors reported that they did for each domain and then made a decision relating to the risk of bias for that domain by assigning a judgement of 'Low risk' of bias, 'High risk' of bias, or 'Unclear risk' of bias. The review authors compared the results of their independent assessments of risk of bias and resolved any discrepancies by discussion and consensus. When no consensus could be reached, SMA and JS arbitrated.
For study selection, data extraction, and risk of bias assessment we were not blinded to the names of the trial authors, their institutions, nor the journals of publication.
Data synthesis
We undertook meta‐analysis using RevMan 5. We analysed all participants in the groups to which they were randomised, irrespective of whether they received the allocated intervention, and assessed heterogeneity between study results using the chi‐square test of homogeneity, with significance defined at the 10% level (www.cochrane‐handbook.org). We expressed each trial result as a risk ratio (RR) for dichotomous data and mean difference (MD) for continuous data, with 95% confidence intervals (CIs), and combined the results using the random‐effects method because of significant heterogeneity. We also used the I2 statistic to describe the percentage of between‐study variability in effect estimates, which is attributable to true heterogeneity rather than chance (Higgins 2011).
We used the GRADE method to rate the quality of evidence on the effectiveness of the triple NRTI regimen (Guyatt 2008; Balshem 2011), and have presented these ratings in Table 1. The GRADE approach results in an assessment of the quality of a body of evidence as high, moderate, low, or very low. High quality evidence implies that “further research is very unlikely to change our confidence in the estimate of effect”. Moderate quality evidence means “further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate”. Evidence is considered of low quality if “further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate”, and very low quality if “we have very little confidence in the effect estimate”. In this review we considered five factors when grading the quality of evidence on the relative effects of fixed‐dose NRTI regimen for initiating HIV treatment; namely, risk of bias in included RCTs, unexplained heterogeneity of effects, indirectness of the evidence, imprecision of the findings, and possibility of publication bias. Regarding risk of bias, we were most concerned with lack of allocation concealment, lack of blinding of outcome assessment, and a large loss to follow‐up. Heterogeneity of effects across studies for which there were no compelling explanations would also have reduced our confidence in the evidence. Indirectness refers to differences between the population, intervention, comparison group and outcome of interest to us, and those reported by the included RCTs. For imprecision, if we found that studies included relatively few participants and few events and thus had estimates of effects with wide confidence intervals, we rated down the quality of the evidence. Finally, we would also have rated down the quality of evidence if there was a high likelihood of publication bias (Balshem 2011).
Results
Description of studies
After scanning the titles and abstracts of all material obtained from the searches conducted from February 2008 to July 2011 (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11), and discarding clearly irrelevant reports, we obtained 15 potentially eligible studies. We reviewed the full‐text articles of the 15 randomised controlled trials (Gulick 2004; Kumar 2006; Kumar 2009; Shapiro 2010; Ait‐Khaled 2002; Cahn 2004; d'Ettorre 2009; Feinberg 2003; Matheron 2003; Munderi 2010; Ndembi 2010; Shao 2009; Sprenger 2010; Staszewski 2001; Vibhagool 2004) and four met our inclusion criteria (Gulick 2004; Kumar 2006; Kumar 2009; Shapiro 2010).
The Gulick 2004 trial recruited 1147 participants from 33 units of The AIDs Clinical Trials Group (ACTG) in the United States of America (USA); the Kumar 2006 trial recruited 261 participants from 34 sites in the USA, Puerto Rico, Guatemala, Dominican Republic, and Panama; the Kumar 2009 study recruited 279 participants from 46 sites in the USA and Mexico; and the Shapiro 2010 trial recruited 560 women in both urban and rural areas in Botswana. The four trials only included participants who were antiretroviral‐naive. The Gulick 2004 and Kumar 2009 trials recruited predominantly male participants (81% and 79%, respectively), while only 50% of participants in the Kumar 2006 trial were men. Finally, all participants in the Shapiro 2010 trial were women.
Participants in the Gulick 2004 trial had mean age 38 years (SD 9 years), were 40% white, 36% African American, and 21% Hispanic and had mean HIV‐1 RNA level of 4.85 log10 copies/mL (SD 0.70) and mean CD4+ cell count of 234 cells/mm3 (SD 187). Participants in the Kumar 2006 trial had median age 34 years (range 18‐60 years), were 21% white, 40% African American, and 37% Hispanic, and had median HIV‐1 RNA level of 4.44 log10 copies/mL (range 2.23 to 5.77) and median CD4 cell count of 339 cells/mm3 (range 19 to 1269). Participants in the Kumar 2009 study had median age 37 years (range 18 to 68 years), HIV‐1 RNA levels between 2.3 and 5.6 log10 copies/mL, and CD4+ cell counts from 103 to 889 cells/mm3. Participants in the Shapiro 2010 trial were pregnant women, aged 18 years or older, were presumably all black Africans, and had median HIV‐1 RNA levels of 13,300 copies/mL in the NRTI arm and 9,100 in the PI arm, and a CD4+ cell count of at least 200 cells/mm3 (median 393 cells/mm3 in the NRTI arm and 403 cells/mm3 in the PI arm).
The participants in the Gulick 2004 trial were randomised to either zidovudine (ZDV)‐lamivudine(3TC)‐abacavir (ABC) [Trizivir®], or ZDV‐3TC [Combivir®] + efavirenz [a NNRTI] or Trizivir® + efavirenz. Participants took a total of seven pills per day, including placebo tablets, divided into two doses. In the Kumar 2006 trial, participants were assigned to either Trizivir® twice daily, or Combivir® + nelfinavir [a PI] 1250 mg twice daily, or stavudine [d4T] 40 mg + 3TC 150 mg + nelfinavir 1250 mg twice daily. In Kumar 2009, participants were randomised to receive either Trizivir® or atazanavir plus lamivudine and zidovudine. In Shapiro 2010, participants were randomised to receive Trizivir® twice daily in the NRTI arm, or 400 mg of lopinavir and 100 mg of ritonavir [co‐formulated as Kaletra®] twice daily in the PI arm. The Shaipro 2010 trial also had a third group of participants (observational arm) who received Combivir® twice daily. This observational arm was not included in our analysis.
Risk of bias in included studies
Our judgements about the risk of bias in each included study are summarised in Figure 1 and Figure 2.
1.
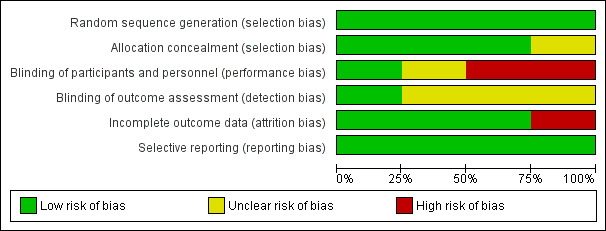
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
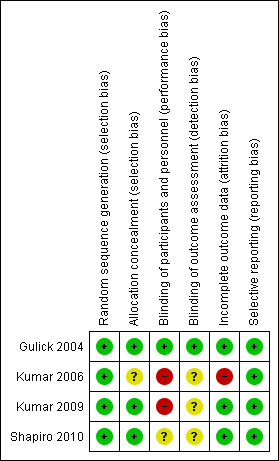
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Generation of allocation sequence
Three trials did not provide an adequate description of the methods used for generating the allocation sequence, but all were described as randomised [Gulick 2004; Kumar 2006; Kumar 2009]. However, the equal allocation of participants suggests that a computer‐generated block randomisation process was used. In the Shapiro 2010 trial, participants were randomly assigned to treatment groups based upon computer‐generated lists (Shapiro 2010).
Allo cation concealment
The Gulick 2004, Kumar 2009, and Shapiro 2010 trials used central randomisation, suggesting that allocation concealment in all three trials was adequate. The Kumar 2006 trial did not provide sufficient detail to describe the allocation concealment process.
Blinding
In the Gulick 2004 trial, participants, providers, and outcome assessors were all blinded. In the Kumar 2006 and Kumar 2009 trials, the participants and providers were not blinded, but it was not clear if the outcome assessors were blinded. In the Shapiro 2010 trial, no details were given about blinding of participants, providers, or outcome assessors.
Loss to follow‐up
When the triple‐nucleoside arm was stopped in the Gulick 2004 trial after a median of 32 weeks, 83 participants (7%) had discontinued the study for various reasons, including withdrawal of consent (2%) and loss to follow‐up (2%). In the Kumar 2006 trial, loss to follow‐up at 96 weeks was 26.4% for Trizivir®, 24.2% for COM/NFV, and 14.5% for d4T/3TC/NFV groups. In the Kumar 2009 trial, 9% and 10% of the participants were lost to follow‐up in the Trizivir® and ATV + 3TC/ZDV arms, respectively. In the Shapiro 2010 trial 15(5.2%) women in the Trizivir® and 13(5.1%) in Kaletra® arms left the study for reasons that are not stated.
Effects of interventions
See: Table 1
There was significant heterogeneity between the included trials in the incidence of virological failure (3 trials, 1687 participants, heterogeneity P=0.009, I2=79%; Analysis 1.1). The Kumar studies (Kumar 2006; Kumar 2009) did not find a significant difference in the incidence of virological failure between participants on NRTIs and those on a PI (i.e. nelfinavir or atazanavir) (two trials, 540 participants: RR 0.82, 95% CI 0.50 to 1.36; heterogeneity P=0.21, I2=35%). Gulick and colleagues found that participants on NRTIs had a significantly higher incidence of virological failure than did those on the NNRTI efavirenz (1 trial, 1147 participants: RR 1.93, 95% CI 1.46 to 2.55). Overall, there was no significant difference between the participants on NRTIs and those on either PI‐based or NNRTI‐based therapy (RR 1.14, 95% CI 0.56 to 2.32).
1.1. Analysis.
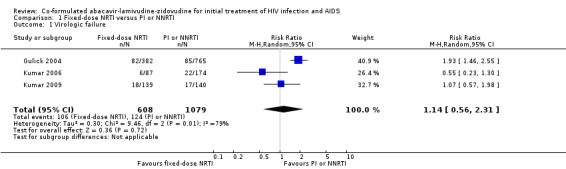
Comparison 1 Fixed‐dose NRTI versus PI or NNRTI, Outcome 1 Virologic failure.
Whatever the definition of virological suppression considered, there was significant heterogeneity between the four studies (heterogeneity P<0.00001, I2=93% for viral load<50copies/ml; heterogeneity P=0.0002, I2=85% for viral load <400 copies/mL) with Kumar 2006, Kumar 2009 and Shapiro 2010 finding no significant differences between comparison groups and Gulick 2004 finding NRTIs to be inferior to efavirenz. For viral load of <50 copies/mL, the risk ratios were 1.15 (0.83 to 1.59) in the Kumar 2006 trial, 1.03 (0.85 to 1.25) in the Kumar 2009 trial, 0.73 (0.67 to 0.80) in the Gulick 2004 trial, 1.08 (0.99 to 1.17) in the Shapiro 2010 trial, and 0.97 (0.75 to 1.25) overall (Analysis 1.3). For viral load of <400 copies/mL, the risk ratios were 1.10 (0.65 to 1.84) in the Kumar 2006 trial, 0.96 (0.58 to 1.58) in the Kumar 2009 trial, 0.35 (0..26 to 0.49) in the Gulick 2004 trial, and 0.73 (0.39 to 1.36) overall (Analysis 1.2).
1.3. Analysis.
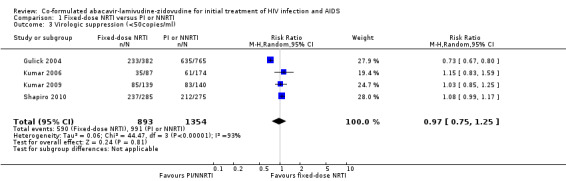
Comparison 1 Fixed‐dose NRTI versus PI or NNRTI, Outcome 3 Virologic suppression (<50copies/ml).
1.2. Analysis.
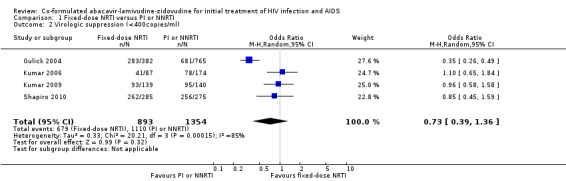
Comparison 1 Fixed‐dose NRTI versus PI or NNRTI, Outcome 2 Virologic suppression (<400copies/ml).
We found no significant differences between NRTIs and either PIs or NNRTIs on CD4+ cell counts (3 trials, 1687 participants: mean difference ‐0.01, 95% CI ‐0.11 to 0.09, I2=0%: Analysis 1.4), the incidence of severe adverse events (4 trials; 2247 participants: RR 1.22, 95% CI 0.78 to 1.92, I2=62%; Analysis 1.5) and hypersensitivity reactions (RR 4.04, 95% CI 0.41 to 40.02, I2=72%; Analysis 1.6). The Shapiro trial did not encounter a hypersensitivity reaction in any treatment group.
1.4. Analysis.
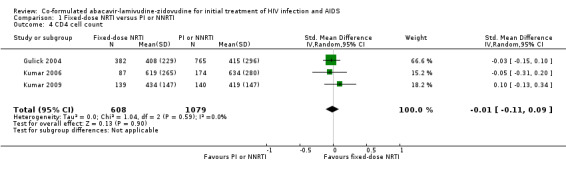
Comparison 1 Fixed‐dose NRTI versus PI or NNRTI, Outcome 4 CD4 cell count.
1.5. Analysis.
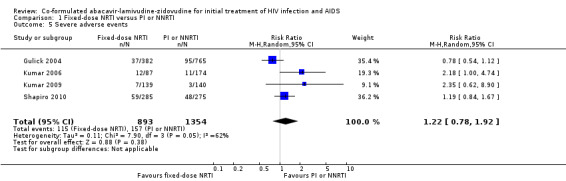
Comparison 1 Fixed‐dose NRTI versus PI or NNRTI, Outcome 5 Severe adverse events.
1.6. Analysis.
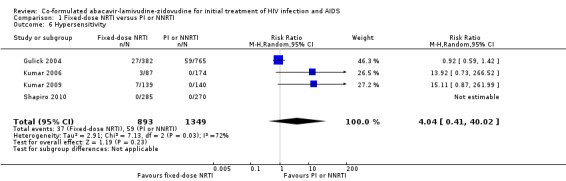
Comparison 1 Fixed‐dose NRTI versus PI or NNRTI, Outcome 6 Hypersensitivity.
Gulick 2004 and Shapiro 2010 did not report on lipid levels. At week 96, Kumar 2006 found the least squares means increase in total cholesterol from baseline was significantly lower with NRTIs than with nelfinavir. Kumar 2006 also found that mean total cholesterol remained below 200mg/dL only in the NRTI group, and the proportion of patients with total cholesterol levels more than 200mg/dL after 96 weeks of treatment was significantly lower in the NRTI group. At 96 weeks, Kumar 2006 found no significant differences between the comparison groups in least squares means triglyceride levels and least squares means change from baseline in triglyceride levels. At 48 weeks, Kumar 2009 found the fasting lipids to be comparable in both the NRTI and atazanavir arms.
Using the GRADE approach (Balshem 2011), we rated the quality of the evidence on the relative effects of co‐formulated abacavir‐lamivudine‐zidovudine for initial treatment of HIV infection as moderate outcome evaluated (Table 1).
Discussion
The large Gulick 2004 trial found the co‐formulated abacavir‐lamivudine‐zidovudine regimen to be virologically inferior to a regimen containing efavirenz and two or three nucleoside analogues after 32 weeks; Kumar 2006 and Kumar 2009 found the triple nucleoside fixed‐dose combination to be equivalent to nelfinavir‐ and atazanavir‐based regimens in maintaining virological suppression over 96 weeks and 48 weeks, respectively; but Shapiro 2010 found viral suppression to be relatively superior in the co‐formulated abacavir‐lamivudine‐zidovudine arm compared to the co‐formulated lopinavir‐ritonavir arm after six months of therapy. The significant heterogeneity of effects was largely due to differences in the control therapy used in the included trials (i.e. NNRTIs or PIs). Pooling the four trials, we did not find significant differences in virological suppression between initiating treatment with the triple nucleoside fixed‐dose combination (NRTI) and therapy based on efavirenz (NNRTI), lopinavir‐ritonavir (PI), nelfinavir (PI), or atazanavir (PI). In addition, the triple nucleoside fixed‐dose combination regimen was well tolerated and had no deleterious effects on the lipid profile. Using the GRADE approach (Balshem 2011), we rated the overall quality of the evidence on the relative effects of the fixed‐dose NRTI regimen for initiating HIV treatment as moderate. The main reason for downgrading the quality of the evidence was imprecision of the findings. The estimate of the treatment effect for each outcome has wide confidence intervals, which extend from the fixed‐dose NRTI combination regimen being appreciably better to the regimen being appreciably worse than PI‐ or NNRTI‐based regimens (Table 1).
The Shapiro 2010 trial examined the use of Trizivir® (NRTI) or Kaletra® (PI) as first‐line therapy in HIV‐infected pregnant women. Eventhough the rate of viral suppression after six months of follow‐up (up till postpartum period) did not show any difference between the interventions, there was a significant increase in viral suppression to below 50 copies/ml with Trizivir® compared to Keletra® at delivery (81% and 69%, respectively). This difference was not observed when the viral set point was raised to 400 copies/ml (Shapiro 2010).
The Kumar 2006 study compared NRTI with a PI (nelfinavir) which is no longer a component of initial recommended regimen. The comparator nelfinavir has been shown to be inferior to current PI regimens both in tolerability and virological suppression and is no longer a preferred treatment option (Moore 2006). There is considerable heterogeneity amongst PIs as far as tolerability is concerned, with newer members of the class, such as atazanavir, very suitable for individuals with hyperlipidaemia (Kumar 2009). Ritonavir‐boosted PIs are now routinely used to initiate therapy (Ananworanich 2008; Hammer 2008; Potard 2007). Ritonavir was not included in any of the comparator arms of either the Kumar or Gulick studies but was included in the Shapiro trial. However, ritonavir may not be appropriate for some HIV‐infected patients, such as those with pre‐existing hyperlipidaemia, metabolic syndrome, underlying severe depression, and intolerance of ritonavir. For the latter, it is important to have a treatment regimen that is both efficacious and safe (Kumar 2009).
Treatment of antiretroviral‐naive HIV‐infected patients requires regimens that have the potential to be used for a long period without the fear of mutations often associated with failing regimens. Treatment with co‐formulated abacavir‐lamivudine‐zidovudine offers the opportunity for patients to switch over to other antiretroviral classes in case of treatment failure. Patients failing on co‐formulated abacavir‐lamivudine‐zidovudine are unlikely to be associated with emergence of multi‐NRTI resistance (Shaefer 2004). However, components of the fixed‐dose combination regimen have been associated with certain side effects (Shaefer 2004). Zidovudine may cause anaemia in some patients, lamivudine is associated with gastrointestinal adverse events, while abacavir is commonly associated with hypersensitivity reactions. Recent studies have shown that abacavir is associated with fatal hypersensitivity reactions in patients with a rare human leukocyte antigen (HLA) allele, HLA‐B*5701 (Mallal 2008; Hughes 2008; Saag 2008). This suggests the need for genetic screening in individuals receiving abacavir‐based therapy to reduce the risk of hypersensitivity reactions associated with the drug, which are often characterized by two or more clinical signs or symptoms that can include fever, rash, gastrointestinal symptoms, respiratory symptoms, and constitutional symptoms. Shapiro and colleagues did not observe any abacavir‐related hypersensitivity reactions in their trial conducted in Botswana as none of their participants tested positive for HLA‐B*5701 (Shapiro 2010).
Our findings indicate that the triple nucleoside fixed‐dose combination remains a viable option for initiating anti‐retroviral therapy, especially in HIV‐infected patients with pre‐existing hyperlipidaemia; possibly preventing exacerbation of the condition and obviating the need for antihyperlipidaemic agents and their incumbent drug interactions. Like any other antiretroviral therapy, constant monitoring of patients receiving this combination drug is advised to detect any resistance or side effects that may be attributed to abacavir, zidovudine, or lamivudine.
Publication and language biases are potential threats to all systematic reviews. We did not restrict our search to any language or publication status (published or unpublished). We are therefore confident that we have identified all existing randomised controlled trials relevant to our question but cannot rule out the possibility that there are additional trials that are unpublished or published in sources not accessible to our search.
Authors' conclusions
Implications for practice.
We found that co‐formulated abacavir‐lamivudine‐zidovudine remains a viable option for initiating anti‐retroviral therapy, especially in HIV‐infected patients with pre‐existing hyperlipidaemia and those who do not tolerate ritonavir. The varied geographical locations of the included trials augment the external validity of our findings. We are moderately confident in our estimate of the treatment effects of the triple NRTI regimen as initial therapy for HIV infection. In the context of the GRADE approach, such moderate quality of evidence implies that the true effects of the regimen are likely to be close to the estimate of effects found in this review.
Implications for research.
There is a need for antiretroviral treatment programmes to have robust monitoring systems capable of identifying patients most likely to develop severe adverse events, viral resistance, and mutations. Further research on co‐formulated abacavir‐lamivudine‐zidovudine should be geared towards defining the subgroup of HIV patients for whom this regimen will be most beneficial.
Feedback
Feedback from Erin Ready, BSc(Pharm), ACPR; Julian Lee, BSc(Pharm), ACPR; Dr. Aaron Tejani, Lower Mainland Pharmacy Services, Medication Use Evaluation Coordinator, 14 December 2014
Summary
We question the ability to make a decisive conclusion with regards to the efficacy of this regimen, given the degree of heterogeneity present in the review (Analysis 1.1, I2 = 79%; Analysis 1.2, I2 = 85%; Analysis 1.3, I2 = 93%). It is stated that the heterogeneity observed in the four studies is primarily a result of differences in the comparator regimens of the included trials (i.e. NNRTI‐ vs. PI‐based regimens). However, other possible explanations for the heterogeneity observed are not explored. Of note, there is great diversity in the study populations, which could be a significant source of heterogeneity. Shapiro 2010 studied a population made up exclusively of pregnant and breastfeeding women in Botswana, whereas the studied populations in Gulick 2004 and Kumar 2009 were predominantly made up of males in North America. A minority of patients in Kumar 2006, Kumar 2009, & Shapiro 2010 had baseline viral loads of > 100 000 copies/mL, whereas Gulick 2004 included a greater proportion of patients with levels above 100 000 copies/mL. Given this diversity in study populations, along with the overall substantial heterogeneity measured, it is perplexing that the review authors were able to draw any decisive conclusion, let alone one that is seemingly to be applied to the general population of HIV patients. In the context of these shortcomings, we feel it is unsubstantiated to broadly conclude that co‐formulated abacavir‐lamivudine‐zidovudine remains a ‘viable’ option. Additionally, we found the call for further research ‘geared towards defining the subgroup of HIV patients for whom this regimen will be most beneficial’ a concerning statement, as it implies that this co‐formulated triple nucleoside regimen is beneficial for all subgroups of patients. This cannot and should not be inferred from this review, especially given the wide diversity in findings from the pooled results. These range from suggesting that this triple nucleoside regimen is significantly inferior in some populations (Gulick 2004), to suggesting it is no different than the PI‐ or NNRTI‐based comparator regimens. A more objective conclusion would be that, based on the results of this review, one is unable to conclude that co‐formulated abacavir‐lamivudine‐zidovudine is a viable option in all patients.
In addition, the title of this review implies an inherent difference between co‐formulated abacavir‐lamivudine‐zidovudine and the same drugs at the same doses formulated separately. Eight of eleven excluded trials were excluded because these three medications were not co‐formulated, yet a difference between the two formulations was not assessed for or even justified in this review. Rather than omitting such a substantial amount of efficacy and safety data, a subgroup analysis could easily be performed to explore whether or not there is a true difference in the behaviour of the fixed‐dose and separate‐dose formulations. The absence of this data, unjustified, impacts confidence in the conclusions reached, as these conclusions may have differed had these additional eight trials been included. We suggest the review be amended to include an analysis of the eight trials excluded on the basis of not being fixed‐dose formulations and, if pertinent, the title of the review changed to reflect its broadened scope.
In addition to concerns about the conclusions made with regards to this regimen’s efficacy, we wish to address those made regarding its safety. These conclusions were made based on the finding of no significant difference in the incidence of serious adverse events and hypersensitivity reactions between the abacavir‐lamivudine‐zidovudine regimen and the comparator regimens. We acknowledge that the review authors go on to note the known risks of harm secondary to these agents, such as zidovudine‐induced anemia and abacavir‐induced hypersensitivity reactions, and that the authors also advocate for ‘constant monitoring’ of patients on co‐formulated abacavir‐lamivudine‐zidovudine in order to detect any side effects associated with this regimen. However, their overall conclusion that co‐formulated abacavir‐lamivudine‐zidovudine ‘remains a viable option for initiating anti‐retroviral therapy’ is misleading. In the RCTs included in the review, co‐formulated abacavir‐lamivudine‐zidovudine is compared to regimens that all include at least one agent (i.e. AZT, d4T, NFV, and/or LPV/r) that is no longer recommended by North American guidelines as initial therapy due to toxicity concerns(1,2). Although the World Health Organization’s guidelines continue to list AZT as an alternative agent if first‐line TDF + 3TC (or FTC) is contraindicated or unavailable, it is noted that AZT is not a preferred first‐line agent(3). To prevent the false impression that co‐formulated abacavir‐lamivudine‐zidovudine is ‘safe’ simply because it was not found to cause more harm than its AZT‐ or d4T‐based comparators, we suggest that a qualifying statement describing the relative safety of zidovudine compared to first‐line NRTIs be included with the discussion of this result.
We additionally feel the risk of emergent NRTI resistance following failure of the abacavir‐lamivudine‐zidovudine regimen was not explored in an unbiased manner. Resistance is an important outcome at time of virological failure, as resistance has important implications on future available regimens. This outcome seems particularly important for patients initiating on triple NRTI regimens for the purpose of avoiding adverse effects, as the true benefits this regimen offers may be overstated if a consequence of virological failure will be the need to switch to a more complex regimen made up of agents initially avoided. The lone mention of resistance in this review is limited to the discussion section, when it is noted that the emergence of multi‐NRTI resistance is ‘unlikely’ in the event of patients failing co‐formulated abacavir‐lamivudine‐zidovudine. This statement was made not based on evaluations by the review authors themselves, but instead appears to be referenced from an opinion article authored by an employee of the industry that patented the co‐formulation(4). Additionally, no context is provided as to how multi‐NRTI resistance is being defined or measured, leaving it unclear as to whether this statement is referring to the likelihood of developing multiple mutations associated with resistance to more than one NRTI, mutations associated with multi‐NRTI resistance, or phenotypic evidence of multi‐NRTI resistance. Furthermore, the author of the opinion article in question evaluated resistance in a clinical trial setting, which underestimates the risk of resistance relative to the real‐world setting(5,6). Thus, we suggest revisiting the topic of NRTI resistance to incorporate the objective evidence available and address the possibility of the emergence of multi‐NRTI resistance following triple nucleoside regimen failure in a more in‐depth and impartial manner. We recommend including the development of resistance as a secondary outcome in this review so as to encourage a more rigorous investigation of the risk of resistance as documented in the available literature. At the very least, we recommend an explicit explanation be made with regards to how multi‐NRTI resistance is defined and measured.
Our final comment pertains to the analysis of the risk of bias in this review. The review restricted its analysis of bias to allocation concealment, blinding, and loss to follow‐up. By restricting the analysis to these elements, several other highly relevant potential sources of bias were neglected. Specifically, the role of industry funding is an element of bias that is relevant to this review and has the potential to have important implications on the interpretation of its results. In a review of four RCTs with inconsistent direction of results, it seems particularly prudent to note that two of the three trials that concluded no difference between co‐formulated abacavir‐lamivudine‐zidovudine and NNRTI‐ or PI‐based regimens appear to contain significant potential for industry bias. Kumar 2006 was funded by the company that patented co‐formulated abacavir‐lamivudine‐zidovudine, and Kumar 2009 was conceived and designed by two employees and one former employee of this company, all of whom went on to analyze and interpret the data, and eventually develop the manuscript. Gulick 2004 and Shapiro 2010 each have at least one author who declares industry conflicts; however, the trials appear to be independently funded. It has been reported that industry‐sponsored drug trials are more likely to produce statistically significant results in favour of the industry’s product(7). In fact, a Cochrane review on industry sponsorship and research outcome concluded that the factors included in Cochrane’s risk of bias assessment tool are likely insufficient to capture the full effect of bias that industry sponsorship may impose on trials, suggesting that industry sponsorship should be discussed as a distinct factor when analyzing trials for risk of bias(8). A sensitivity analysis of industry‐funded vs. independently funded studies whose authors declare conflicts of interest may provide some meaningful insight into the influence of industry sponsorship bias in this review.
Erin Ready, BSc(Pharm), ACPR
Julian Lee, BSc(Pharm), ACPR
Dr. Aaron Tejani, Lower Mainland Pharmacy Services, Medication Use Evaluation Coordinator
References:
1. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV‐1‐infected adults and adolescents. Department of Health and Human Services. [Internet]. 2014. [cited November 19, 2014]. Available from:http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf
2. Montaner J, Guillemi S, Harris M, editors on behalf of the Therapeutics Guidelines Committee, British Columbia Centre for Excellence in HIV/AIDS. Therapeutic Guidelines: Antiretroviral (ARV) Treatment of Adult HIV Infection. [Internet]. February 2013. [cited November 19, 2014]. Available from:
http://www.cfenet.ubc.ca/sites/default/files/uploads/Therapeutic%20Guidelines%202013‐Feb‐final.pdf
3. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. [Internet]. June 2013. [cited November 20, 2014]. Available from:
http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1
4. Shaefer MS Abacavir/lamivudine/zidovudine continues to be a valid and useful antiretroviral regimen. Annals of Pharmacotherapy. 2004. 38(7‐8), 1314–1316.
5. Sabin, C. Antiretroviral resistance in clinical practice. Geretti, AM, editor. [Internet]. London: Mediscript; 2006. Chapter 14: Database analyses of predictors of resistance. [cited November 18, 2014]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK2246/
6. Clotet, B., Hill, A., van Delft, Y., Gupta, R. K., & Moecklinghoff, C. Interpretation of Resistance Data from Randomized Trials of First‐Line Antiretroviral Treatment. AIDS Reviews. 2012. 14: 247‐255.
7. Bhandari, M., Busse, J. W., Jackowski, D., Montori, V. M., Schunemann, H., Sprague, S., et al. (2004). Association between industry funding and statistically significant pro‐industry findings in medical and surgical randomized trials. CMAJ. 17 February 2004.170(4): 477‐80.
8. Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews [Internet] 2012, Issue 12. Art. No.: MR000033.DOI: 10.1002/14651858.MR000033.pub2.
We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Reply
The editors are grateful to the contributors for their correspondence. We acknowledge the issues they raise and believe that a full update of this review is required to address them. Readers should take into account these comments in assessing the limitations of the current version of the review and in interpreting the findings they present.
Contributors
Erin Ready provided this feedback. CIDG editors (Paul Garner, David Sinclair, Tamara Kredo and Olalekan Uthman) examined the feedback and formulated the response.
What's new
| Date | Event | Description |
|---|---|---|
| 18 March 2016 | Amended | Corrected names and affiliations of feedback contributors. |
History
Protocol first published: Issue 4, 2005 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 3 March 2016 | Amended | feedback incorporated |
| 3 March 2016 | Feedback has been incorporated | The editors are grateful to the contributors for their correspondence. We acknowledge the issues they raise and believe that a full update of this review is required to address them. Readers should take into account these comments in assessing the limitations of the current version of the review and in interpreting the findings they present. |
| 29 January 2013 | New citation required and conclusions have changed | One new trial found (Shapiro 2010) and included. Title and conclusions changed. |
| 29 January 2013 | New search has been performed | Update of review. |
| 24 June 2008 | New citation required and minor changes | Substantive amendment |
Acknowledgements
We thank Dr. Judith Shang for her work on the original version of this review.
Appendices
Appendix 1. Search strategies
1 Search strategy for CENTRAL: May 2009
| Query | |
| #1 | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (VIRAL SEXUALLY TRANSMITTED DISEASES) |
| #2 | (HIGHLY ACTIVE ANTIRETROVIRAL THERAPY) OR (ANTI‐RETROVIRAL AGENTS) OR (ANTIVIRAL AGENTS) OR ((ANTI) AND (HIV)) OR ANTIRETROVIRAL* OR ((ANTI) AND (RETROVIRAL*)) OR HAART OR ((ANTI) AND (ACQUIRED IMMUNODEFICIENCY)) OR ((ANTI) AND (ACQUIRED IMMUNEDEFICIENCY)) OR ((ANTI) AND (ACQUIRED IMMUNO‐DEFICIENCY)) OR ((ANTI) AND (ACQUIRED IMMUNE‐DEFICIENCY)) OR ((ANTI) AND (ACQUIRED IMMUN*) AND (DEFICIENCY)) |
| #3 | (ZIDOVUDINE AND LAMIVUDINE AND ABACAVIR) OR TRIZIVIR |
| #4 | (#1 AND #2 AND #3) |
Footnotes
2 Search strategy for PubMed: May 2009
| Query | |
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MESH:NoExp] |
| #2 | Search "Antiretroviral Therapy, Highly Active"[MeSH] OR "Anti‐Retroviral Agents"[MeSH] OR "Antiviral Agents"[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])) OR ((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])) |
| #3 | Search TRIZIVIR |
| #4 | Search ZIDOVUDINE AND LAMIVUDINE AND ABACAVIR |
| #5 | Search #3 OR #4 |
| #6 | Search randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) |
| #7 | Search #1 AND #2 AND #5 AND #6 |
Footnotes
3 Search string for EMBASE: February 2008
| Query | |
| #1 | ('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection') OR (('human immunodeficiency virus'/exp OR 'human immunodeficiency virus')) OR (('b cell lymphoma'/de OR 'b cell lymphoma')) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab) OR ('human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab) OR ('acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab) OR ('acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab) OR ('acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab) OR ('acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab) |
| #2 | ('human immunodeficiency virus vaccine'/de OR 'human immunodeficiency virus vaccine') OR ('anti human immunedeficiency':ti OR 'anti human immunedeficiency':ab) OR ('anti human immunodeficiency':ti OR 'anti human immunodeficiency':ab) OR ('anti human immuno‐deficiency':ti OR 'anti human immuno‐deficiency':ab) OR ('anti human immune‐deficiency':ti OR 'anti human immune‐deficiency':ab) OR ('anti acquired immune‐deficiency':ti OR 'anti acquired immune‐deficiency':ab) OR ('anti acquired immunedeficiency':ti OR 'anti acquired immunedeficiency':ab) OR ('anti acquired immunodeficiency':ti OR 'anti acquired immunodeficiency':ab) OR ('anti acquired immuno‐deficiency':ti OR 'anti acquired immuno‐deficiency':ab) OR ('anti hiv':ti OR 'anti hiv':ab) OR (antiretrovir*:ti OR antiretrovir*:ab) OR ('anti retroviral':ti OR 'anti retroviral':ab OR 'anti retrovirals':ti OR 'anti retrovirals':ab OR 'anti retrovirus':ti OR 'anti retrovirus':ab) OR (haart:ti OR haart:ab) OR ('aids vaccine':ti OR 'aids vaccine':ab OR 'aids vaccines':ti OR 'aids vaccines':ab) OR (('anti human immunodeficiency virus agent'/de OR 'anti human immunodeficiency virus agent')) OR (('antiretrovirus agent'/de OR 'antiretrovirus agent')) OR (('antivirus agent'/de OR 'antivirus agent')) OR (('highly active antiretroviral therapy'/de OR 'highly active antiretroviral therapy')) |
| #3 | ((random*:ti OR random*:ab) OR (factorial*:ti OR factorial*:ab) OR (cross?over*:ti OR cross?over*:ab OR crossover*:ti OR crossover*:ab) OR (placebo*:ti OR placebo*:ab) OR ((doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab)) OR ((singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab)) OR (assign*:ti OR assign*:ab) OR (allocat*:ti OR allocat*:ab) OR (volunteer*:ti OR volunteer*:ab) OR (('crossover procedure'/de OR 'crossover procedure')) OR (('double‐blind procedure'/de OR 'double‐blind procedure')) OR (('single‐blind procedure'/de OR 'single‐blind procedure')) OR (('randomized controlled trial'/de OR 'randomized controlled trial'))) |
| #4 | 'trizivir'/de OR 'trizivir' |
| #5 | ('zidovudine'/de OR 'zidovudine') AND ('lamivudine'/de OR 'lamivudine') AND ('abacavir'/de OR 'abacavir') |
| #6 | #4 OR #5 |
| #7 | #1 AND #2 AND #3 AND #6 |
Footnotes
4 Search strategy for NLM Gateway: February 2008
| Query | |
| #1 | Search: ((HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw]))) OR (acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MESH:NoExp])) AND ((Antiretroviral Therapy, Highly Active[MeSH] OR Anti‐Retroviral Agents[MeSH] OR Antiviral Agents[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw]) OR (((anti) AND (acquired immunodeficiency[tw])) OR ((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])))) AND ((TRIZIVIR) OR (ZIDOVUDINE AND LAMIVUDINE AND ABACAVIR)) AND ((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw]))) OR (( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]))) Limit: 1980:2008, AIDS |
Footnotes
5 Search strategy for AIDSearch: February 2008
| Query | |
| #1 | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL) |
| #2 | ((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR ("CLINICAL TRIAL") OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL* AND (MASK* OR BLIND* )) OR PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) NOT (ANIMALS NOT HUMAN ) |
| #3 | #1 AND #2 |
| #4 | (HIGHLY ACTIVE ANTIRETROVIRAL THERAPY) OR (ANTI‐RETROVIRAL AGENTS) OR (ANTIVIRAL AGENTS) OR ((ANTI) AND (HIV)) OR ANTIRETROVIRAL* OR ((ANTI) AND (RETROVIRAL*)) OR HAART OR ((ANTI) AND (ACQUIRED IMMUNODEFICIENCY)) OR ((ANTI) AND (ACQUIRED IMMUNEDEFICIENCY)) OR ((ANTI) AND (ACQUIRED IMMUNO‐DEFICIENCY)) OR ((ANTI) AND (ACQUIRED IMMUNE‐DEFICIENCY)) OR ((ANTI) AND (ACQUIRED IMMUN*) AND (DEFICIENCY)) |
| #5 | (ZIDOVUDINE AND LAMIVUDINE AND ABACAVIR) OR TRIZIVIR |
| #6 | #3 AND #4 AND #5 |
| #7 | #6 NOT (ANIMAL NOT HUMAN) |
Footnotes
6 Search strategy for NLM Gateway: February 2008
| Query | |
| #1 | Search: ((HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw]))) OR (acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MESH:NoExp])) AND ((Antiretroviral Therapy, Highly Active[MeSH] OR Anti‐Retroviral Agents[MeSH] OR Antiviral Agents[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw]) OR (((anti) AND (acquired immunodeficiency[tw])) OR ((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])))) AND ((TRIZIVIR) OR (ZIDOVUDINE AND LAMIVUDINE AND ABACAVIR)) AND ((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw]))) OR (( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]))) Limit: 1980:2008, AIDS |
Footnotes
7 Search strategy for CENTRAL: July 2011
| Query | |
| #1 | MeSH descriptor HIV Infections explode all trees |
| #2 | MeSH descriptor HIV explode all trees |
| #3 | hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR HIV INFECT* OR HUMAN IMMUNODEFICIENCY VIRUS OR HUMAN IMMUNEDEFICIENCY VIRUS OR HUMAN IMMUNE‐DEFICIENCY VIRUS OR HUMAN IMMUNO‐DEFICIENCY VIRUS OR HUMAN IMMUN* DEFICIENCY VIRUS OR ACQUIRED IMMUNODEFICIENCY SYNDROME OR ACQUIRED IMMUNEDEFICIENCY SYNDROME OR ACQUIRED IMMUNO‐DEFICIENCY SYNDROME OR ACQUIRED IMMUNE‐DEFICIENCY SYNDROME OR ACQUIRED IMMUN* DEFICIENCY SYNDROME |
| #4 | MeSH descriptor Lymphoma, AIDS‐Related, this term only |
| #5 | MeSH descriptor Sexually Transmitted Diseases, Viral, this term only |
| #6 | (#1 OR #2 OR #3 OR #4 OR #5) |
| #7 | MeSH descriptor Antiretroviral Therapy, Highly Active, this term only |
| #8 | MeSH descriptor Anti‐HIV Agents explode all trees |
| #9 | MeSH descriptor Antiviral Agents, this term only |
| #10 | MeSH descriptor AIDS Vaccines, this term only |
| #11 | ANTI HIV OR ANTIRETROVIRAL* OR ANTI RETROVIRAL* OR AIDS VACCIN* |
| #12 | (#7 OR #8 OR #9 OR #10 OR #11) |
| #13 | abacavir OR ziagen |
| #14 | lamivudine OR epivir OR 3TC |
| #15 | zidovudine OR retrovir OR AZT |
| #16 | (#13 AND #14 AND #15) |
| #17 | trizivir OR TZV |
| #18 | (#16 OR #17) |
| #19 | (#6 AND #12 AND #18) |
| #20 | (#6 AND #12 AND #18), from 2008 to 2011 |
Footnotes
8 Search strategy for PubMed: July 2011
| Query | |
| #11 | Search #1 AND #2 AND #3 AND #9 Limits: Publication Date from 2008/02/29 to 2011/07/15 |
| #10 | Search #1 AND #2 AND #3 AND #9 |
| #9 | Search #7 OR #8 |
| #8 | Search trizivir OR TZV |
| #7 | Search #4 AND #5 AND #6 |
| #6 | Search zidovudine OR retrovir OR AZT |
| #5 | Search lamivudine OR epivir OR 3TC |
| #4 | Search abacavir OR ziagen |
| #3 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) |
| #2 | Search “Antiretroviral Therapy, Highly Active”[MeSH] OR “Anti‐Retroviral Agents”[MeSH] OR “Antiviral Agents”[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])) OR ((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])) |
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MESH:NoExp] |
Footnotes
9 Search string for EMBASE: December 2010
| Query | |
| #13 | #2 AND #3 AND #4 AND #11 AND [embase]/lim AND [29‐2‐2008]/sd NOT [13‐12‐2010]/sd |
| #12 | #2 AND #3 AND #4 AND #11 |
| #11 | #9 OR #10 |
| #10 | 'trizivir'/de OR trizivir OR tzv |
| #9 | #6 AND #7 AND #8 |
| #8 | 'zidovudine'/de OR zidovudine OR 'retrovir'/de OR retrovir OR 'azt'/de OR azt |
| #7 | 'lamivudine'/de OR lamivudine OR 'epivir'/de OR epivir OR '3tc'/de OR 3tc |
| #6 | 'abacavir'/de OR abacavir OR 'ziagen'/de OR ziagen |
| #4 | random*:ti OR random*:ab OR factorial*:ti OR factorial*:ab OR cross?over*:ti OR cross?over*:ab OR crossover*:ti OR crossover*:ab OR placebo*:ti OR placebo*:ab OR (doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab) OR (singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab) OR assign*:ti OR assign*:ab OR allocat*:ti OR allocat*:ab OR volunteer*:ti OR volunteer*:ab OR 'crossover procedure'/exp OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/exp OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/exp OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR 'randomized controlled trial'/exp OR 'randomized controlled trial'/de OR 'randomized controlled trial' |
| #3 | 'human immunodeficiency virus vaccine'/de OR 'human immunodeficiency virus vaccine' OR 'anti human immunedeficiency':ti OR 'anti human immunedeficiency':ab OR 'anti human immunodeficiency':ti OR 'anti human immunodeficiency':ab OR 'anti human immuno‐deficiency':ti OR 'anti human immuno‐deficiency':ab OR 'anti human immune‐deficiency':ti OR 'anti human immune‐deficiency':ab OR 'anti acquired immune‐deficiency':ti OR 'anti acquired immune‐deficiency':ab OR 'anti acquired immunedeficiency':ti OR 'anti acquired immunedeficiency':ab OR 'anti acquired immunodeficiency':ti OR 'anti acquired immunodeficiency':ab OR 'anti acquired immuno‐deficiency':ti OR 'anti acquired immuno‐deficiency':ab OR 'anti hiv':ti OR 'anti hiv':ab OR antiretrovir*:ti OR antiretrovir*:ab OR 'anti retroviral':ti OR 'anti retroviral':ab OR 'anti retrovirals':ti OR 'anti retrovirals':ab OR 'anti retrovirus':ti OR 'anti retrovirus':ab OR haart:ti OR haart:ab OR 'aids vaccine':ti OR 'aids vaccine':ab OR 'aids vaccines':ti OR 'aids vaccines':ab OR 'anti human immunodeficiency virus agent'/de OR 'anti human immunodeficiency virus agent' OR 'antiretrovirus agent'/de OR 'antiretrovirus agent' OR 'antivirus agent'/de OR 'antivirus agent' OR 'highly active antiretroviral therapy'/de OR 'highly active antiretroviral therapy' |
| #2 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus' OR hiv:ti OR hiv:ab OR 'hiv‐1':ti OR 'hiv‐1':ab OR 'hiv‐2':ti OR 'hiv‐2':ab OR 'human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab OR 'human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab OR 'human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab OR 'human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab OR 'acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab OR 'acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab OR 'acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab OR 'acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab |
Footnotes
10 Search string for ISI Web of Science: December 2010
| Query | |
| # 10 | #9 AND #3 AND #2 AND #1 |
| # 9 | #8 OR #7 |
| # 8 | TS=(trizivir OR TZV) |
| # 7 | #6 AND #5 AND #4 |
| # 6 | TS=(zidovudine OR retrovir OR AZT) |
| # 5 | TS=(lamivudine OR epivir OR 3TC) |
| # 4 | TS=(abacavir OR ziagen) |
| # 3 | TS=(random* OR "clinical trial") |
| # 2 | TS=("antiretroviral therapy" OR "anti‐retroviral therapy" OR HAART) |
| # 1 | TS=(HIV OR HIV/AIDS OR "human immun*" OR "acquired immun*") |
Footnotes
11 Search string for PsycINFO: December 2010
| Query | |
| # 10 | #9 AND #3 AND #2 AND #1 |
| # 9 | #8 OR #7 |
| # 8 | KW=(trizivir OR TZV) |
| # 7 | #6 AND #5 AND #4 |
| # 6 | KW=(zidovudine OR retrovir OR AZT) |
| # 5 | KW=(lamivudine OR epivir OR 3TC) |
| # 4 | KW=(abacavir OR ziagen) |
| # 3 | KW=(random* OR "clinical trial") |
| # 2 | KW=("antiretroviral therapy" OR "anti‐retroviral therapy" OR HAART) |
| # 1 | KW=(HIV OR HIV/AIDS OR "human immun*" OR "acquired immun*") |
Footnotes
Data and analyses
Comparison 1. Fixed‐dose NRTI versus PI or NNRTI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Virologic failure | 3 | 1687 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.56, 2.31] |
| 2 Virologic suppression (<400copies/ml) | 4 | 2247 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.39, 1.36] |
| 3 Virologic suppression (<50copies/ml) | 4 | 2247 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.75, 1.25] |
| 4 CD4 cell count | 3 | 1687 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 5 Severe adverse events | 4 | 2247 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.78, 1.92] |
| 6 Hypersensitivity | 4 | 2242 | Risk Ratio (M‐H, Random, 95% CI) | 4.04 [0.41, 40.02] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gulick 2004.
| Methods | Sequence generation: Patients were randomly assigned with equal opportunity to the treatment arms. Treatment allocation was stratified by screening HIV‐1 RNA levels (<100,000 copies/ml or >=100,000 copies/mL) Allocation concealment: Adequate (central remote randomisation) Blinding: Participants, providers, and assessors all blinded. Loss‐to‐follow‐up: When the triple nucleoside arm was stopped, after a median of 32 weeks, 83 participants (7%) had discontinued the study for various reasons including withdrawal of consent (n=21) and loss to follow‐up (n=21). Analysis: performed on an intention‐to‐treat basis and included all follow‐up data. | |
| Participants | Antiretroviral‐naive HIV‐1‐infected adults recruited from 33 units of The AIDs Clinical Trials Group (ACTG) in the US. Exclusion criteria: Immunomodulator investigational therapy or vaccines within previous 30 days, weight less than 40kg, pregnancy, or lactation. N=1147 Male 81%, mean age 38 (SD 9) years, whites 40%, blacks 36%, Hispanics 21%, mean HIV‐1 RNA level 4.85 log(10) copies/mL [SD 0.70), mean CD4 cell count = 234 cells/mm3 (SD187). No significant levels between treatment arms. | |
| Interventions | Eligible subjects were randomly allocated to one of three regimens given orally at standard doses and intervals: Regimen A: zidovudine (ZDV)–lamivudine(3TC)– abacavir (ABC) [Trizivir]. Regimen B: ZDV–3TC [Combivir] + efavirenz Regimen C: ZDV–3TC–ABC + efavirenz. Participants took a total of seven pills per day (including placebos), divided into two doses. In the event of treatment‐limiting toxic effects of study drugs, the identity of the implicated drug was allowed to be revealed and substitution of another drug in the same class was permitted. Stavudine could be substituted for ZDV, didanosine could be substituted for ABC, and nevirapine could be substituted for efavirenz.. | |
| Outcomes | 1. Virologic failure i.e. two successive HIV‐1 RNA values of 200 or more copies/ml at least 16 weeks after randomisation. 2. HIV‐1 RNA level of less than 200 copies/ml and with a level below 50 copies/ml. 3. Change in CD4 cell count from base line 4. Adverse events | |
| Notes | The study was reviewed annually for safety and efficacy by the data and safety monitoring board. The second annual review showed differences between the triple‐nucleoside regimen and each of the efavirenz‐containing regimens that met prespecified stopping guidelines, and the DSMB recommended stopping the triple‐nucleoside portion of the study, continuing double‐blind follow‐up of the other two groups, and analysing and presenting the results with the data for the triple‐nucleoside group compared with the pooled data from the efavirenz groups. At the time of stopping the triple‐nucleoside arm, the median duration of follow‐up was 32 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned with equal opportunity to the treatment arms. Treatment allocation was stratified by screening HIV‐1 RNA levels. Such an elaborate randomisation sequence is likely to have been computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Central remote randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and providers blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors all blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | When the triple‐nucleoside arm was stopped in the Gulick 2004 trial after a median of 32 weeks, 83 participants (7%) had discontinued the study for various reasons, including withdrawal of consent (2%) and loss to follow‐up (2%). |
| Selective reporting (reporting bias) | Low risk | No |
Kumar 2006.
| Methods | Sequence generation: Patients were "randomized 1:1:1" suggesting block randomisation, but no detail of method of generating the randomisation sequence was given. Allocation concealment: Not described. Blinding: Participants ‐ No. Providers ‐ No. Assessors ‐ Unclear. Loss to follow‐up: 26.4%(23/87) for Trizivir, 24.2% (22/91) in COM/NFV, and 14.5%(12/83) in d4T/3TC/NFV groups. Analysis: performed on an intention‐to‐treat basis. | |
| Participants | Partcipants recruited from 34 outpatient sites in USA, Puerto Rico, Guatemala, Dominican Republic & Panama. Inclusion criteria: Documented HIV infection; naive or limited experience with antiretroviral therapy; age >= 18 years; CD4+ count > 50 cells/microL; 1000 copies/ml < HIV‐1 RNA < 200,000 copies/ml. Exclusion criteria: pregnancy, lactation, , no antihyperlipidaemic or antidiabetic medications. N=261 Male 50%, median age 34 (range 18‐60) years , Whites 20.9%, Blacks 39.8%, Hispanics 37.0%, median HIV‐1 RNA level 4.44 log(10) copies/ml [range2.23‐5.77), median CD4 cell count = 339 cells/mm3 (range19‐1269), median total cholesterol 163mg/dl (92‐267), median triglycerides 107 mg/dl (range38‐597) No significant levels between treatment arms. | |
| Interventions | Patients meeting entry criteria were randomised 1:1:1 to: Regimen A: Trizivir twice daily. Regimen B: Combivir + nelfinavir 1250 twice daily. Regimen C: Stavudine 40 mg + 3TC 150 mg + nelfinavir 1250 mg twice daily. At enrolment participants were stratified into two groups based on their screening plasma HIV‐1 RNA level: <1000–100,000 copies/mL or >100,000–200,000 copies/mL. | |
| Outcomes | 1. Change from baseline in LDL cholesterol. 2. Virologic failure i.e. two successive HIV‐1 RNA values of 200 or more copies/ml at least 16 weeks after randomisation. 2. HIV‐1 RNA level of less than 200 copies/ml and with a level below 50 copies/ml. 3. Change in CD4 cell count from base line 4. Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were "randomized 1:1:1" suggesting block randomisation, and presumable computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and providers not blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 26.4% for Trizivir, 24.2% in COM/NFV, and 14.5% in d4T/3TC/NFV groups. |
| Selective reporting (reporting bias) | Low risk | No |
Kumar 2009.
| Methods | Sequence generation: Patients were "randomized 1:1:1" suggesting block randomisation, but no detail of method of generating the randomisation sequence was given. Allocation concealment: Adequate (central randomisation). Blinding: No blinding Loss to follow‐up: 9% (12/138) in the ABC/3TC/ZDV and 10% (14/140) in the ATV + 3TC/ZDV groups Analysis: Performed on an intent‐to‐treat exposed basis |
|
| Participants | 279 subjects recruited between May 2004 and March 2005 from 46 sites in USA and Mexico. Inclusion Criteria: HIV‐1 infection, 18 years or older, ART‐naive, and plasma HIV‐1 RNA >=5000 but <200,000c/ML and CD4+ cell count >= 100 cells/mm3. Exclusion criteria: Patients were excluded if they had medical conditions or required medications that could compromise their safety or interfere with drug absorption, if they had protocol‐specific abnormal laboratory values. N=279 79% male and racially diverse (>50% non‐white race or ethnicity), 82% had HIV‐1 RNA <100,000c/mL at baseline. |
|
| Interventions | Patients meeting inclusion criteria were randomized 1:1 to receive ABC/3TC/ZDV (Trizivir®) twice daily or ATV (once daily) + 3TC/ZDV (twice daily). | |
| Outcomes | 1. HIV‐1 viral load 2. CD4+/CD8+ lymphocyte subsets 3. Clinical chemistry 4. Hematology 5. Serum lipid panels 6. Insulin 7. Hemoglobin |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were "randomized 1:1:1" suggesting block randomisation, and presumable computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding: No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 9% in the ABC/3TC/ZDV and 10% in the ATV + 3TC/ZDV groups |
| Selective reporting (reporting bias) | Low risk | No |
Shapiro 2010.
| Methods | Sequence generation: Computer‐generated randomisation sequence Allocation concealment: Patients were randomised 1:1:1 to Trizivir, Kaletra or Combivir by block permutation according to clinical site. Randomisation was assigned by calling the Data Management Centre in Gaborone (Central randomisation). Blinding: Not described Loss to follow‐up: 15/285 in Trizivir group, 13/275 in kaletra group, and 5/170 in observational group left the study but reasons not given. Analysis: Not mentioned |
|
| Participants | 560 pregnant women with HIV‐1 infection were recruited between 2006 and 2008 in Botswana. Inclusion criteria included confirmed HIV‐1 infection, age at least 18yrs, 26 to 34 weeks of gestation, haemoglobin level of at least 8.0g/deciliter, absolute neutrophil count of at least 1000 cells per cubic millimeter, alanine aminotransferase and aspartate aminotransferase levels at most 2 times the upper limit of normal and women who preferred to exclusively feed their babies by formula were excluded. |
|
| Interventions | Patients meeting inclusion criteria were randomised 1:1 to receive ABC/3TC/ZDV (Trizivir®) twice daily or co‐formulated lopinavir and ritonavir (Kaletra) twice daily + 3TC/ZDV (Combivir) twice daily. | |
| Outcomes | HIV viral load(viral suppression to <400 and <50 copies/ml) Mother‐to‐child transmission intrapartum and postpartum Adverse events |
|
| Notes | Study protocol available at: http://www.nejm.org/doi/suppl/10.1056/NEJMoa0907736/suppl_file/nejmoa0907736_protocol.pdf | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All randomisation assignments were made based upon computer‐generated lists |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 5.2% in the Trizivir® and 5.1% in Keletra® arms left the study for reasons that are not stated. |
| Selective reporting (reporting bias) | Low risk | No |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ait‐Khaled 2002 | Abacavir, lamivudine, zidovudine not used as a fixed‐dose combination. |
| Cahn 2004 | Abacavir, lamivudine, zidovudine not used as a fixed‐dose combination. |
| d'Ettorre 2009 | Abstract and article not available and authors did not respond to article request. |
| Feinberg 2003 | Abacavir, lamivudine, zidovudine not used as a fixed‐dose combination. |
| Matheron 2003 | Abacavir, lamivudine, zidovudine not used as a fixed‐dose combination. |
| Munderi 2010 | Abacavir, lamivudine, zidovudine not used as a fixed‐dose combination. |
| Ndembi 2010 | Abacavir, lamivudine, zidovudine not used as a fixed‐dose combination. |
| Shao 2009 | Co‐formulated abacavir‐lamivudine‐zidovudine not compared to PI or NNRTI regimens |
| Sprenger 2010 | Co‐formulated abacavir‐lamivudine‐zidovudine used as maintenance therapy |
| Staszewski 2001 | Abacavir, lamivudine, zidovudine not used as a fixed‐dose combination. |
| Vibhagool 2004 | Abacavir, lamivudine, zidovudine not used as a fixed‐dose combination. |
Contributions of authors
MS led the preparation of the original review and the current update. MS, EJK, and CSW assessed the eligibility of identified studies and extracted data from included studies. SMA and JS arbitrated (in the original review) when MS, EJK and CSW could not reach a consensus. MS and CSW wrote the first draft of the review, and all authors commented on the review and approved the final version.
Sources of support
Internal sources
University of Cape Town (MSS, CSW), South Africa.
University of Yaounde I (JDS), Cameroon.
South African Medical Research Council (CSW), South Africa.
Liverpool School of Tropical Medicine (EJK), UK.
External sources
No sources of support supplied
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Gulick 2004 {published and unpublished data}
- Gulick RM, Ribaudo HJ, Shikuma CM, Lustgarten S, Squires KE, Meyer WA 3rd, Acosta EP, Schackman BR, Pilcher CD, Murphy RL, Maher WE, Witt MD, Reichman RC, Snyder S, Klingman KL, Kuritzkes DR, AIDS Clinical Trials Group Study A5095 Team. Triple‐nucleoside regimens versus efavirenz‐containing regimens for the initial treatment of HIV‐1 infection. N Engl J Med 2004;350:1850‐61. [DOI] [PubMed] [Google Scholar]
Kumar 2006 {published data only}
- Kumar PN, Rodriguez‐French A, Thompson MA, Tashima KT, Averitt D, Wannamaker PG, Williams VC, Shaefer MS, Pakes GE, Pappa KA, ESS40002 Study Team. A prospective, 96‐week study of the impact of Trizivir, Combivir/nelfinavir, and lamivudine/stavudine/nelfinavir on lipids, metabolic parameters and efficacy in antiretroviral‐naive patients: effect of sex and ethnicity. HIV Med 2006;7:85‐98. [DOI] [PubMed] [Google Scholar]
Kumar 2009 {published data only}
- Kumar PN, Salvado P, LaMarca A, DeJesus E, Patel P, McClernon D, Florance A, Shaefer MS. A randomized, controlled trial of initial anti‐retroviral therapy with abacavir/Lamivudine/zidovudine twice‐daily compared to atazanavir once‐daily with lamivudine/zidovudine twice‐daily in HIV‐infected patients over 48 weeks (ESSI00327, the ACTION Study). AIDS Research and Therapy 2009;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
Shapiro 2010 {published data only}
- Lopinavir/Ritonavir/Combivir vs. Abacavir/Zidovudine/Lamivudine for Virologic Efficacy and the Prevention of Mother‐to‐Child HIV Transmission Among Breastfeeding Women With CD4 Counts Greater Than or Equal to 200 Cells/mm3 in Botswana. http://clinicaltrials.gov/show/NCT00270296.
- Powis KM, Smeaton L, Ogwu A, Lockman S, Dryden‐Peterson S, Widenfelt E, Leidner J, Makhema J, Essex M, Shapiro RL. Effects of in utero antiretroviral exposure on longitudinal growth of HIV‐exposed uninfected infants in Botswana. J Acquir Immune Defic Syndr 2011;56:131‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, . Moffat C, Makhema J, Moyo S, McIntosh K, Widenfelt E, Leidner J, Powis K, Asmelash A, Tumbare E, Awerki S, Sharma U, Hendelsman E, Mburu K, Jayeoba O, Moko E, Souda S, Lubega E, Akhtar M, Wester C, Tuomola R, Snowden W, Martinez‐Tristani M, Mazhani L, Essex M. Antiretroviral regimens in pregnancy and breast‐feeding in Botswana. N Engl J Med 2010;362:2282‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ait‐Khaled 2002 {published data only}
- Ait‐Khaled M, Rakik A, Griffin P, Cutrell A, Fischl MA, Clumeck N, Greenberg SB, Rubio R, Peters BS, Pulido F, Gould J, Pearce G, Spreen W, Tisdale M, Lafon S, CNA3003 International Study Team. Mutations in HIV‐1 reverse transcriptase during therapy with abacavir, lamivudine and zidovudine in HIV‐1‐infected adults with no prior antiretroviral therapy. Antivir Ther 2002;7:43‐51. [PubMed] [Google Scholar]
Cahn 2004 {published data only}
- Cahn P, Vibhagool A, Schechter M, Soto‐Ramirez L, Carosi G, Smaill F, Jordan JC, Pharo CE, Thomas NE, Steel HM. Predictors of adherence and virologic outcome in HIV‐infected patients treated with abacavir‐ or indinavir‐based triple combination HAART also containing lamivudine/zidovudine. Curr Med Res Opin 2004;20:1115‐23. [DOI] [PubMed] [Google Scholar]
d'Ettorre 2009 {published data only}
- d'Ettorre G, Ceccarelli G, Turriziani O, Andreotti M, Massetti P, Rizza C, Vella S, Antonelli G, Mastroianni CM, Vullo V. The absence of HIV mutations in cerebrospinal fluid and in peripheral blood mononuclear cells during a regimen containing Trizivir plus tenofovir.. Journal of Chemotherapy 2009;21:455‐457. [DOI] [PubMed] [Google Scholar]
Feinberg 2003 {published data only}
- Feinberg J. Meeting notes from the 2nd International AIDS Society Conference on HIV Pathogenesis and Treatment. Trizivir vs. efavirenz: results from ACTG 5095. AIDS Clin Care 2003;15:78‐79. [PubMed] [Google Scholar]
Matheron 2003 {published data only}
- Matheron S, Descamps D, Boué F, Livrozet JM, Lafeuillade A, Aquilina C, Troisvallets D, Goetschel A, Brun‐Vezinet F, Mamet JP, Thiaux C, CNA3007 Study Group. Triple nucleoside combination zidovudine/lamivudine/abacavir versus zidovudine/lamivudine/nelfinavir as first‐line therapy in HIV‐1‐infected adults: a randomized trial. Antivir Ther 2003;8:163‐71. [PubMed] [Google Scholar]
Munderi 2010 {published data only}
- Munderi P, Walker AS, Kityo C, Babiker AG, Ssali F, Reid A, Darbyshire JH, Grosskurth H, Mugyenyi P, Gibb DM, Gilks CF. Nevirapine/zidovudine/lamivudine has superior immunological and virological responses not reflected in clinical outcomes in a 48‐week randomized comparison with abacavir/zidovudine/lamivudine in HIV‐infected Ugandan adults with low CD4 cell counts. HIV Medicine 2010;11:334‐44. [DOI] [PubMed] [Google Scholar]
Ndembi 2010 {published data only}
- Ndembi N, Goodall RL, Dunn DT, McCormick A, Burke A, Lyagoba F, Munderi P, Katundu P, Kityo C, Robertson V, Yirrell DL, Walker AS, Gibb DM, Gilks CF, Kaleebu P, Pillay D. Viral rebound and emergence of drug resistance in the absence of viral load testing: a randomized comparison between zidovudine‐lamivudine plus Nevirapine and zidovudine‐lamivudine plus Abacavir. Journal of Infectious Disease 2010;201:106‐13. [DOI] [PubMed] [Google Scholar]
Shao 2009 {published data only}
- Shao HJ, Crump JA, Ramadhani HO, Uiso LO, Ole‐Nguyaine S, Moon AM, Kiwera RA, Woods CW, Shao JF, Bartlett JA, Thielman NM. Early versus delayed fixed dose combination abacavir/lamivudine/zidovudine in patients with HIV and tuberculosis in Tanzania. AIDS Research and Human Retroviruses 2009;25:1277‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sprenger 2010 {published data only}
- Sprenger HG, Langebeek N, Mulder PG, Napel CH, Vriesendorp R, Hoepelman AI, Legrand JC, Koopmans PP, Kasteren ME, Bravenboer B, Kate RW, Groeneveld PH, Werf TS, Gisolf EH, Richter C. Abacavir/lamivudine/zidovudine maintenance after standard induction in antiretroviral therapy‐naïve patients: FREE randomized trial interim results. AIDS Patient Care and STDS 2010;24:361‐66. [DOI] [PubMed] [Google Scholar]
Staszewski 2001 {published data only}
- Staszewski S, Keiser P, Montaner J, Raffi F, Gathe J, Brotas V, Hicks C, Hammer SM, Cooper D, Johnson M, Tortell S, Cutrell A, Thorborn D, Isaacs R, Hetherington S, Steel H, Spreen W, CNAAB3005 International Study Team. Abacavir‐lamivudine‐zidovudine vs indinavir‐lamivudine‐zidovudine in antiretroviral‐naive HIV‐infected adults: A randomized equivalence trial. JAMA 2001;285:1155‐63. [DOI] [PubMed] [Google Scholar]
Vibhagool 2004 {published data only}
- Vibhagool A, Cahn P, Schechter M, Smaill F, Soto‐Ramirez L, Carosi G, Montroni M, Pharo CE, Jordan JC, Thomas NE, Pearce G. Triple nucleoside treatment with abacavir plus the lamivudine/zidovudine combination tablet (COM) compared to indinavir/COM in antiretroviral therapy‐naïve adults: results of a 48‐week open‐label, equivalence trial (CNA3014). Curr Med Res Opin 2004;20:1103‐14. [DOI] [PubMed] [Google Scholar]
Additional references
Ananworanich 2008
- Ananworanich J, Gayet‐Ageron A, Ruxrungtham K, Chetchotisakd P, Prasithsirikul W, Kiertiburanakul S, Munsakul W, Raksakulkarn P, Tansuphasawadikul S, LeBraz M, Jupimai T, Ubolyam S, Schutz M, Hirschel B. Long‐term efficacy and safety of first‐line therapy with once‐daily saquinavir/ritonavir. Antivir Ther 2008;13:375‐80. [PubMed] [Google Scholar]
Anon 2000
- Anon. Anti‐HIV agents. Trizivir‐‐three drugs in one pill. Treatmentupdate 2000;12:1‐2. [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines 3: rating the quality of evidence ‐ introduction. Journal of Clinical Epidemiology 2011;64:401‐6. [DOI] [PubMed] [Google Scholar]
Carpenter 2000
- Carpenter CC, Cooper DA, Fischl MA, et al. Anti‐retroviral therapy in adults: updated recommendations of the International AIDS Society‐USA Panel. JAMA 2000;283:381‐90. [DOI] [PubMed] [Google Scholar]
Eron 1995
- Eron JJ, Benoit SL, Jemsek J, et al. Treatment with lamivudine, zidovudine, or both in HIV‐positive patients with 200 to 500 CD4+ cells per cubic millimeter. North American HIV Working Party. N Eng J Med 1995;333:1662‐69. [DOI] [PubMed] [Google Scholar]
Gifford 2000
- Gifford AL, Bormann JE, Shivley MJ, Wright BC, Richman DD, Bozette SA. Predictors of self‐reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. J Acquir Immune Defic Syndr 2000;23:386‐95. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hacker 2004
- Hacker MA, Petersen ML, Enriquez M, Bastos FI. Highly active antiretroviral therapy in Brazil: the challenge of universal access in a context of social inequality. Rev Panam Salud Publica 2004;16:78‐83. [DOI] [PubMed] [Google Scholar]
Hammer 2008
- Hammer SM, Eron JJ Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JS, Richman DD, Yeni PG, Volberding PA, International AIDS Society‐USA. Antiretroviral treatment for adult HIV infection in 2008: updated recommendations of the International AIDS Society‐USA Panel. JAMA 2008;300:555‐70. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Hughes 2008
- Hughes CA, Foisy MM, Dewhurst N, Higgins N, Robinson L, Kelly DV, Lechelt KE. Abacavir hypersensitivity reaction: an update. Ann Pharmacother 2008;42:387‐96. [DOI] [PubMed] [Google Scholar]
Ledergerber 1999
- Ledergerber B, Egger M, Opravil M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV‐1 patients: a prospective cohort study. Lancet 1999;353:863‐68. [DOI] [PubMed] [Google Scholar]
Mallal 2008
- Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, Jagel‐Guedes E, Rugina S, Kozyrev O, Cid JF, Hay P, Nolan D, Hughes S, Hughes A, Ryan S, Fitch N, Thorborn D, Benbow A. HLA‐B*5701 screening for hypersensitivity to abacavir. N Engl J Med 2008;358:568‐79. [DOI] [PubMed] [Google Scholar]
McMahon 2007
- McMahon MA, Jilek BL, Brennan TP, Shen L, Zhou Y, Wind‐Rotolo M, Xing S, Bhat S, Hale B, Hegarty R, Chong CR, Liu JO, Siliciano RF, Thio CL. The HBV drug entecavir ‐ effects on HIV‐1 replication and resistance. New England journal of Medicine 2007;356(25):2614‐2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehta 1997
- Mehta S, Moore RD, Graham NMH. Potential factors affecting adherence with HIV therapy. AIDS 1997;11:1665‐70. [DOI] [PubMed] [Google Scholar]
Moore 2006
- Moore DM, Hogg RS, Yip B, Wood E, Harris M, Montaner JS. Regimen‐dependent variations in adherence to therapy and virological suppression in patients initiating protease inhibitor‐based highly active antiretroviral therapy. HIV Med 2006;7:311‐16. [DOI] [PubMed] [Google Scholar]
Pialoux 1998
- Pialoux G, Raffi F, Brun VF, et al. A randomized trial of three maintenance regimens given after three months of induction therapy with zidovudine, lamivudine, and indinavir in previously untreated HIV‐1‐infected patients. N Engl J Med 1998;339:1269‐76. [DOI] [PubMed] [Google Scholar]
Potard 2007
- Potard V, Rey D, Mokhtari S, Frixon‐Marin V, Pradier C, Rozenbaum W, Brun‐Vezinet F, Costagliola D. First‐line highly active antiretroviral regimens in 2001‐2002 in the French Hospital Database on HIV: combination prescribed and biological outcomes. Antivir Ther 2007;12:317‐24. [PubMed] [Google Scholar]
Rutherford 2003
- Rutherford GW, Sangani PR, Kennedy GE. Three‐ or four‐ versus two‐drug antiretroviral maintenance regimens for HIV infection. Cochrane Database of Systematic Reviews 2003, Issue 4. [CD002037] [DOI] [PubMed] [Google Scholar]
Saag 2008
- Saag M, Balu R, Phillips E, Brachman P, Martorell C, Burman W, Stancil B, Mosteller M, Brothers C, Wannamaker P, Hughes A, Sutherland‐Phillips D, Mallal S, Shaefer M. High sensitivity of human leukocyte antigen‐b*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis 2008;46:1111‐18. [DOI] [PubMed] [Google Scholar]
Saez‐Llorens 2001
- Sáez‐Llorens X, Nelson RP Jr, Emmanuel P, Wiznia A, Mitchell C, Church JA, Sleasman J, Dyke R, Richardson CG, Cutrell A, Spreen W, Hetherington S. A randomised, double‐blind study of triple nucleoside therapy of abacavir, lamivudine, and zidovudine versus lamivudine and zidovudine in previously treated human immunodeficiency virus type 1‐infected children. Pediatrics 2001;107:1‐11. [DOI] [PubMed] [Google Scholar]
Seaton 2003
- Seaton ORA, Fox R, Bodasing N, Peters SE, Gourlay Y. Effect of co‐formulated zidovudine, lamivudine and abacavir (Trizivir) on antiretroviral‐naive patients presenting with advanced HIV‐1 infection. AIDS 2003;17:445‐47. [DOI] [PubMed] [Google Scholar]
Shaefer 2004
- Shaefer MS. Abacavir/Lamivudine/Zidovudine continues to be a valid and useful antiretroviral regimen. Ann Pharmacother 2004;38:1314‐16. [DOI] [PubMed] [Google Scholar]
Staszewski 1998
- Staszewski S, Katlama C, Harrer T, Massip P, Yeni P, Cutrell A, Tortell SM, Harrigan RP, Steel H, Lanier RE, Pearce G. A dose‐ranging study to evaluate the safety and efficacy of abacavir alone or in combination with zidovudine and lamivudine in antiretroviral treatment‐naive subjects. AIDS 1998;12:F197‐202. [DOI] [PubMed] [Google Scholar]
Teixeira 2004
- Teixeira PR, Vitoria MA, Barcarolo J. Antiretroviral treatment in resource‐poor settings: the Brazilian experience. AIDS 2004;18(Supplement 3):S5‐7. [DOI] [PubMed] [Google Scholar]
UNAIDS 2012
- UNAIDS. Report on the global AIDS epidemic: Together we will end AIDS. Geneva: Joint United Nations Programme on HIV/AIDS 2012.


