Abstract
The vasculature not only transports oxygenated blood, metabolites, and waste products but also serves as a conduit for hormonal communication between distant tissues. Therefore, it is important to maintain homeostasis within the vasculature. Recent studies have greatly expanded our understanding of the regulation of vasculature development and vascular-related diseases at the epigenetic level, including by protein posttranslational modifications, DNA methylation, and noncoding RNAs. Integrating epigenetic mechanisms into the pathophysiologic conceptualization of complex and multifactorial vascular-related diseases may provide promising therapeutic approaches. Several reviews have presented detailed discussions of epigenetic mechanisms not including histone methylation in vascular biology. In this review, we primarily discuss histone methylation in vascular development and maturity, and in vascular diseases.
Keywords: Histone methylation, Histone methyltransferase, Demethylase, Atherosclerosis, Intimal hyperplasia, Aortic dissection/aneurysm, Pulmonary arterial hypertension, Diabetic angiopathy, Cancer angiogenesis
The vasculature, which consists of arterial, venous, and interconnecting capillary beds, is formed through vasculogenesis or angiogenesis during embryogenesis. The walls of the vessels are composed of endothelial cells, mural cells, and the extracellular matrix (ECM). The origin, number, type, and organization of mural cells depend on the location of the vessel and its function. For example, the smooth muscle cells (SMCs) of the ascending and arch portions of the aorta originate from the neural crest, while the SMCs of the descending thoracic aorta are contributed by somite-derived cells [1]. The vasculature, a highly branched, tree-like, tubular network, not only transports oxygenated blood, metabolites, and waste products but also serves as a conduit for hormonal communication between distant tissues. Furthermore, the vasculature facilitates rapid deployment of immune responses to distal sites within the body [2]. Maintaining vascular biologic homeostasis is essential for the body; once this balance is disrupted, the vasculature will suffer from dysplasia or diseases, such as angiodysplasia [3], aortic aneurysm/dissection [4], atherosclerosis [5, 6], pulmonary arterial hypertension [7], diabetic angiopathy [8], or arteritis [9]. Multiple mechanisms are involved in the shift from the physiological status to the pathological state of the vasculature. Among them, epigenetic mechanisms (e.g., posttranslational modification, RNA methylation, DNA methylation, and miRNA) play an indispensable role during these processes [10, 11]. Several published reviews have summarized epigenetic regulation in vascular biology; in particular, noncoding RNAs, DNA methylation, and protein acetylation and phosphorylation have been widelydiscussed [12–14]. In recent years, m6A RNA methylation has emerged as anew research field, but the functions of m6A RNA methylation in vascular development and vascular diseases remain to be revealed. Incontrast, histone methylation has been investigated extensively in vascular biology after the discoveries of the first histone methyltransferase (HMT) in 2000 and the first histone demethylase in 2004 [15, 16]. Therefore, in the present review, we focus only on histone methylation and systematically summarize the research on the roles of histone methylation and mechanisms by which it is involved in vascular development and diseases.
Histone methylation
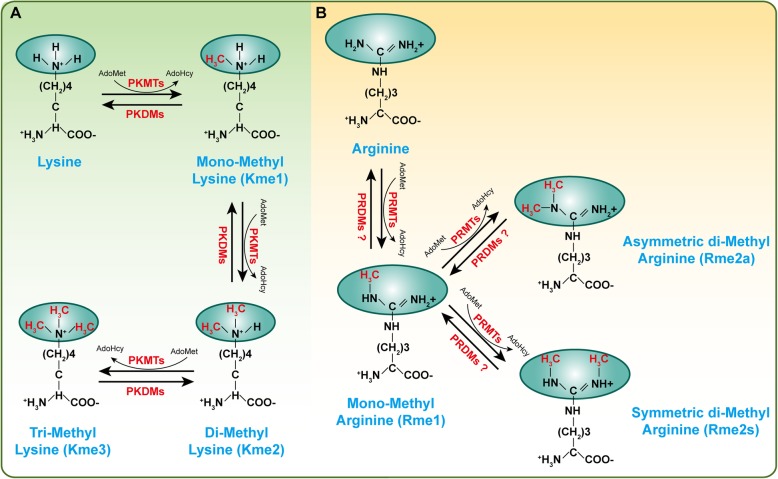
Histone methylation, a reversible posttranslational modification, is written by HMTs and erased by histone demethylases (HDMTs) [17]. To date, two main types of histone methylation have been identified: methylation on lysine and arginine residues. Correspondingly, HMTs have been divided into two categories: protein lysine methyltransferases (PKMTs) and protein arginine methyltransferases (PRMTs) [18, 19]. The ε-amine group of lysine can be marked with monomethylation (me1), dimethylation (me2), and trimethylation (me3) by suppressor of variegation, enhancer of Zeste, Trithorax (SET) domain-containing PKMTs or non-SET-domain-containing PKMTs [18, 20, 21] (Fig. 1a). In contrast, arginine is methylated by PRMTs at ω-amino groups, which appeared as monomethylation (MMA, Rme1), symmetric dimethylarginine (SDMA, Rme2s), and asymmetric dimethylarginine (ADMA, Rme2a) (Fig. 1b) [22]. S-Adenosyl-l-methionine (AdoMet), the primary methyl group donor, interacts with PKMTs or PRMTs to transfer methyl groups to the lysine or arginine residues (Fig. 1) [23]. A variety of substrates can be methylated by HMTs, with canonical substrates being histones, such as H3K27, H3K4, H3K9, H4K20, and H3R17 [24–27]. However, with further research, an increasing number of nonhistone proteins (e.g., p53, Rb, and Hsp90) have been found to be methylated by HMTs [28, 29]. Methylation on nonhistone proteins is associated with other post-translational modifications (PTMs), such as phosphorylation and acetylation, which affects the activity or stability of proteins [30–32]. In recent years, many studies have revealed that histone methylation is involved in and indispensable for the development of a variety of vascular diseases. In this review, we discuss the role of histone methylation on vascular development and maturity, atherosclerosis and vascular intimal hyperplasia, acute thoracic aortic syndromes and aortic aneurysms, pulmonary arterial hypertension, diabetic angiopathy, endothelial dysfunction, and other forms of vasculopathy.
Fig. 1.
A schematic diagram of histone methylation on lysine or arginine residues. Protein can be methylated by methyltransferases and S-adenosyl-l-methionine (AdoMet) is used as the primary methylgroup donor, while these modifications are reversible and can be erased by demethylases. a Protein lysine methyltransferases (PKMTs) catalyze monomethylation (Kme1), dimethylation (Kme2) and trimethylation (Kme3) of proteins on the ε-amine group of lysine. b Protein arginine methyltransferases (PRMTs) methylate the ω-amino group of arginine residues, resulting in either monomethylated (Rme1) or symmetric (Rme2s) or asymmetric (Rme2a) dimethylation. PKDMs protein lysine demethylases, PRDMs protein arginine demethylases
Histone methylation in vascular development and maturity
Defects in placental vascular development cause embryonic death and abnormal organogenesis, negatively affect fetal growth, or confer a higher risk of disease during postnatal life [33]. Vascular remodeling is an important pregnancy-associated adaptation in hemochorial placentation, and the most common cause of placental dysfunction is the failure of vascular remodeling by extravillous trophoblast [34]. As reported by Rodesch et al. in 1992, they found that relatively hypoxic environment within the intervillous space of placenta (varies between 2 and 8%) than endometrial oxygen tension during early implantation [35, 36]. This environment is thought to facilitate the villous capillary network continued sprouting and remodeling throughout gestation [37]. The HIF signaling is a classic oxygen-sensitive pathway to regulate angiogenesis under hypoxic environments. Hypoxia activates Hif-dependent expression of lysine demethylase 3A (Kdm3a) which demethylates H3K9 to accelerate Mmp12 expression to facilitate trophoblast invasion and uterine vascular remodeling [38].
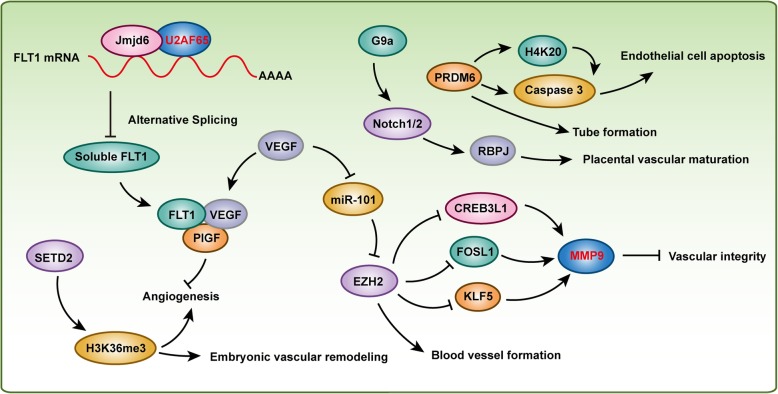
In mice in which the Flk1 (also known as Vegfr2) gene was targeted for disruption, the absence of both endothelial and hematopoietic development was detected, and the mice died in utero on E8.0-E9.0, indicating that Flk1was required in the earliest stages of hematovascular development [39]. Histone-lysine N-methyltransferase Prdm6 is enriched in Flk1(+) hematovascular precursor cells [40]. In mouse embryonic endothelial cells, overexpression of Prdm6 induced apoptosis by activating caspase-3 and inducing G1 arrest and resulted in inhibited tube formation, which indicated that Prdm6 may play a role in vascular cell precursor differentiation and survival [40]. Flt1 (also known as Vegfr1), an important paralog of Flk1, was reported to be regulated by histone arginine demethylase Jmjd6 which controlled angiogenic sprouting [41]. Jmjd6 interacted with splicing factor U2af65 to alter the splicing of Flt1, affecting the levels of the soluble form of Flt1, which was subsequently bound to Vegf and placental growth factor (Plgf) to regulate angiogenesis [41]. VEGF treatment inhibited miR-101 expression in endothelial cells, and miR-101 targeted Ezh2, which methylated histone H3 lysine 27 (H3K27), suppressing gene expression. Furthermore, systemic administration of DZNep to inhibit Ezh2 reduced the number of blood vessels in a subcutaneous glioblastoma mouse model [42]. In addition, Ezh2 inhibited Creb3l1, Fosl1, Klf5, and Mmp9 in endothelial cells to maintain the integrity of the developing vasculature [43]. MMP9 was also elevated significantly in blood samples from acute aortic dissection (AAD) patients, and the incidence of AAD was reduced significantly, by 40%, following the administration of an MMP inhibitor and was almost completely blocked in Mmp9−/− mice [44]. More importantly, recent results from our studies demonstrated that Ezh2 was involved in AAD by inhibiting the autophagic cell death that was regulated by the Atg5, Atg7, and Mek1/2-Erk1/2 signaling pathway [24]. Histone methyltransferase G9a was reported to activate Notch pathway effectors (e.g., Rbpj) to control placental vascular maturation, and G9a and RBPJ were downregulated in human placentae from intrauterine growth restriction-affected pregnancies [33]. Given that the expression of Jagged1, a ligand involved in Notch signaling, was linked to increased circulating plasma VEGF in giant cell arteritis patient blood vessels, VEGF enhanced Jagged1 expression and vessel wall inflammation in mice which were implanted with patient peripheral blood mononuclear cells and human arteries [45]. Furthermore, Spuul et al. demonstrated that VEGF/Notch signaling regulates the formation of functional podosomes in endothelial cells to promote retinal neovascularization [46]. However, how histone methylation and its corresponding HMTs or HDMTs cooperates with VEGF/Notch signaling to regulate vascular development and maturity need further research. In addition, HYPB (also known as SETD2 and KMT3A) is a histone H3 lysine 36 (H3K36)-specific methyltransferase [27]. Homozygous disruption of Hypb resulted in embryonic lethality at E10.5-E11.5 due to severe vascular defects in the embryo, yolk sac, and placenta that was mediated by impaired H3K36 trimethylation but not monomethylation or dimethylation [3]. In early mammalian erythropoiesis, histone methyltransferase Dot1l plays a critical role in controlling the number of circulating erythroid and myeloid cells, as indicated by Dot1l-mutant mice that developed more slowly and died between E10.5 and E13.5, displaying profound anemia, which was especially apparent in the small vessels of the yolk sac. These effects were induced by inhibiting Gata2 expression while enhancing PU.1 levels [47]. The findings from these aforementioned studies indicate that histone methylation plays an essential role in vascular development and maturity (Fig. 2). However, more investigation is needed to uncover whether other HMTs or HDMTs regulate angiogenesis, and more importantly, additional vascular system-specific HMT- and HDMT-knockout animal models should be used to interpret HMT and HDMT function in vascular development. In addition, ascertaining whether nonhistone proteins take part in these biological processes would be a valuable undertaking.
Fig. 2.
Histone methylation regulates vascular development and maturity. Histone arginine demethylase Jmjd6 and histone methyltransferases SETD2, EZH2, G9a, and PRDM6 are involved in vascular development and maturity
Histone methylation in atherosclerosis and vascular intimal hyperplasia
Atherosclerosis, one of the primary causes of cardiovascular death worldwide, is initiated by endothelial dysfunction and lipid accumulation [5, 48], and it is characterized by fibrotic cell proliferation, chronic inflammation, lipid accumulation, and immune disorder in the vessel wall [49]. Vascular SMCs have been found to contribute to atherosclerotic plaque formation through proliferation, migration, and apoptosis, and they are involved in inflammation, extracellular matrix synthesis, and foam cell formation through cholesterol uptake [50]. Vulnerable plaques are prone to rupture after the atheromatous plaques develop into an advanced stage, which leads to acute cardiovascular events, including ischemic stroke and myocardial infarction [49]. Although the research is still in its infancy, emerging evidence is elucidating the role of epigenetic mechanisms in atherosclerosis. In this review, we focus on discussing histone methylation in atherosclerosis (Fig. 3). For reviews on other epigenetic mechanisms, the reader is referred to a review by Xu et al. [49].
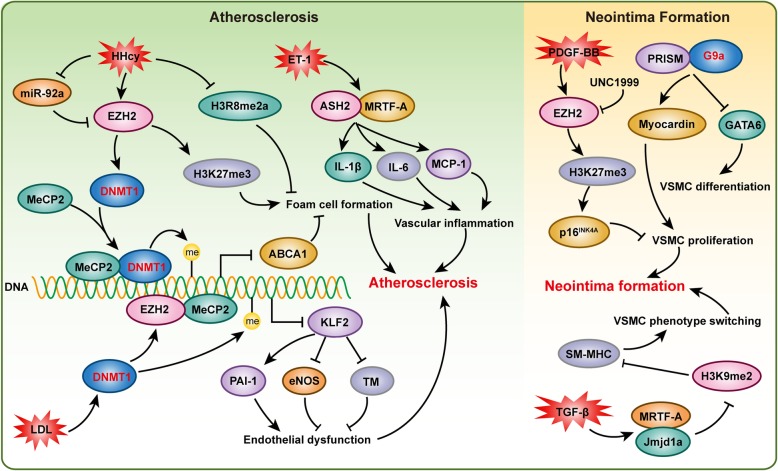
Fig. 3.
Histone methylation is critical for atherosclerosis and neointima formation. Histone methyltransferases EZH2 and ASH2 participate in atherosclerosis via regulating endothelial dysfunction, foam cell formation, and vascular inflammation, respectively. Histone methyltransferases EZH2 and G9a and demethylase Jmjd1A are involved in neointima formation by affecting vascular smooth muscle cell (VSMC) proliferation, differentiation and phenotype switching
Several studies have investigated global histone methylation in human atherosclerotic plaques [6, 51, 52]. Greißel et al. demonstrated that global H3K9me2 and H3K27me2 were significantly decreased in atherosclerotic lesions, while comparable H3K4me2 levels were identified in atherosclerotic and healthy carotid arteries [6]. Interestingly, the immunohistochemistry results revealed increased H3K4me2 levels but decreased H3K9me2 levels in VSMCs, as well as reduced H3K9me2 and H3K27me2 levels in inflammatory cells. Paradoxically, the expression of the corresponding histone methyltransferases MLL2 and G9a was increased in advanced atherosclerosis compared to early atherosclerosis [6]. In addition, this research group also demonstrated that H3K4 methylation and H3K9 acetylation were significantly associated with the severity of atherosclerosis [52]. Similarly, Wierda et al. also demonstrated that the global level of H3K27me3 was reduced in vessels with advanced atherosclerotic plaques, but this reduction in H3K27me3 level was not accompanied by alterations in the corresponding histone methyltransferase EZH2 or demethylase JMJD3 [51]. These results indicated that H3K9 and H3K27 demethylation was critical for atherosclerotic plaque formation. Ezh2 the methyltransferase corresponding to H3K27 promoted foam cell formation and the development of atherosclerosis in ApoE−/− mice. Mechanistically, Ezh2 induced DNA methyltransferase 1 (Dnmt1) expression, methyl CpG-binding protein-2 (MeCP2) recruitment, and the binding of Dnmt1 and MeCP2 to the ATP-binding cassette transporter A1 (Abca1) promoter, thereby promoting Abca1 gene DNA methylation, which inhibited Abca1 expression and accelerated atherosclerosis [53]. Elevated low-density lipoprotein (LDL) levels are a major risk factor for atherosclerosis development. Increased LDL induces endothelial Dnmt1 expression and DNA methyltransferase activity and stimulated binding of MeCP2 and EZH2, which resulted in myocyte enhancing factor-2 (MEF2) dissociation from the KLF2 promoter to suppress KLF2 expression in endothelial cells. Decreased KLF2 led to thrombomodulin and endothelial nitric oxide synthase (eNOS) expression suppression and to PAI-1 activation, which impaired endothelial function [54]. Hyperhomocysteinemia (HHcy) is another independent risk factor for atherosclerosis. After ApoE−/− mice were challenged with a high-methionine diet for 16 weeks, the levels of Ezh2 and H3K27me3 were increased in their aortas, which promoted the accumulation of total cholesterol and triglycerides in foam cells, and miR-92a inhibited this HHcy-mediated lipid metabolism disorders by targeting Ezh2 [55]. These studies indicated that Ezh2 and Dnmt1 could form a positive feedback regulation fashion. On the one hand, they regulate the formation of foam cells by inhibiting ATP-binding cassette transporter A1 (ABCA1); on the other hand, they affect endothelial dysfunction by suppressing KLF2, and jointly promote the formation of atherosclerosis. It is also a model of the interconnection between histone methylation and DNA methylation. In an animal model of diet-induced HHcy, Esse et al. showed that severe HHcy disrupted global protein arginine methylation in a tissue-specific manner, especially the H3R8me2a mark, the level of which was profoundly decreased [56]. In addition, histone-arginine methyltransferase Prmt4 and demethylase Jmjd6 participated with low-density lipoprotein receptor-related protein 6 (Lrp6) to promote arteriosclerotic calcification in diabetic Ldlr−/− mice [57]. ASH2, a histone methyltransferase complex subunit, interacted with MRTF-A to transactivate pro-inflammatory genes in VSMCs in response to endothelin (ET-1) treatment [58].
Angioplasty and coronary artery bypass grafting are highly effective treatment for narrowed coronary arteries due to atherosclerosis. However, restenosis resulting from neointima hyperplasia after angioplasty greatly dampens the satisfactory prognosis of the atherosclerosis for patients [59]. Recent research advances have indicated that histone methylation is critical for regulating neointima hyperplasia (Fig. 3). For example, Liang et al. showed that PDGF-BB markedly increased H3K27me3 and Ezh2 levels. Inhibition of Ezh2/1 activity by UNC1999 significantly suppressed PDGF-BB-induced VSMC proliferation and neointima formation following wire-guided common carotid injury, which was mediated by increasing the transcription of the cyclin-dependent kinase inhibitor p16INK4A [59]. Knockdown of Jmjd1a in primary rat aortic SMCs attenuated TGF-β-induced upregulation of endogenous SM myosin heavy chain expression by interacting with MRTF-A and regulating H3K9me2 levels to affect VSMC phenotype switching [60]. PRISM interacted with G9a histone methyltransferase and class I histone deacetylases to induce genes associated with the proliferative smooth muscle phenotype while repressing regulators of differentiation, including myocardin and GATA-6 in primary VSMCs [61]. H3K27me3 and H3K4me2 were reportedly involved in neointima formation by regulating Myh11, Acta2, Cnn1, and Sm22 or Vcam-1 expression [62, 63].
Although several kinds of HMTs and HDMTs were found to have changed expression levels during atherosclerosis or neointima formation, thereby affecting histone methylation levels, the potential roles of HMTs and HDMTs in atherosclerosis and neointima formation require further investigation. As many inhibitors targeting HMTs or HDMTs have been developed, with some in ongoing clinical trials for treating cancer, it is urgent to verify whether these inhibitors have the potential to reverse atherosclerosis or neointima formation in the near future.
Histone methylation in acute thoracic aortic syndromes and aortic aneurysm
According to 2014 ESC guidelines on the diagnosis and treatment of aortic diseases, acute thoracic aortic syndromes (AASs) which include intramural hematoma (IMH), penetrating aortic ulcer (PAU), aortic dissection (AD), and thoracic aortic rupture are defined as emergency conditions with similar clinical characteristics involving the aorta [64]. Among them, AD is the disease that has been extensively investigated. AD is a life-threatening disease with an incidence of six per hundred thousand persons per year [65]. Furthermore, 50% of patients with acute type A AD who do not receive surgery die within the first 48 h of the event [64]. The pathological features of AD are characterized by an enlarged and degenerative medial layer, vascular smooth muscle cell (VSMC) loss or dysfunction, proteoglycan accumulation, and collagen and elastic fiber cross-linked disorder and fragmentation [66]. Our recent results demonstrated that EZH2, a methyltransferase for H3K27 dimethylation and trimethylation, was downregulated in the aortic wall of patients with AD compared with the levels in the normal controls [24]. Most importantly, EZH2 negatively regulated autophagosome formation by inhibiting ATG5 and ATG7 expression and the MEK1/2-ERK1/2 signaling pathway to prevent autophagic death of VSMCs. In addition, we also found that the protein levels of H3K9me2 and H3K23me1 were upregulated, while H4K20me2 was downregulated in the aorta samples of AD patients [67]. For abdominal aortic aneurysm (AAA), Jones et al. identified four new AAA-specific risk loci, including 1q32.3 (SMYD2), 13q12.11 (LINC00540), 20q13.12 (near PCIF1/MMP9/ZNF335), and 21q22.2 (ERG), via a meta-analysis of 6 genome-wide-associated study data sets and a validation study with a total of 10,204 cases and 107,766 controls [68]. Furthermore, Toghill et al. revealed that, in aortic tissues of AAA patients, the SMYD2 promoter was hypo-methylated and SMYD2 was downregulated compared to the methylation and expression levels of the respective controls [69]. These two related studies highlight the role of SMYD2 in AAA, but further investigation is needed to uncover its exact role and mechanisms. In addition, in human thoracic aortic aneurysms (TAAs), SMAD2 was upregulated, compared the level in normal aortas, and H3K9/14 acetylation and H3K4 methylation were involved in SMAD2 overexpression in TAAs [70].
Hypertension is identified as the most common risk factor associated with AD, as it was observed in 65–75% of the individuals with AD [64, 71]. Thus, the prevention and control of hypertension are critical ways to prevent and treat AD. It is well known that renin-angiotensin-aldosterone system (RAAS) dysregulation plays a crucial role in the development of hypertension; thus, the epigenetic regulation of RAAS-regulated genes has been extensively studied in hypertensive models [72, 73]. For example, in the aortas of spontaneously hypertensive rats (SHRs), enrichment of H3K4me3 but a decrease in H3K9me2 level was found at the angiotensin-converting enzyme 1 (Ace1) promoter, which is associated with Ace1 upregulation [74]. Downregulation of the hydroxysteroid dehydrogenase-11β2 enzyme (Hsd11b2), a gene related to renal sodium balance, was associated with a decrease in H3K36me3 in SHRs [75]. Furthermore, higher levels of H4ac and H3K4me3, but lower levels of H3K27me3 and H3K9me3 at atrial natriuretic peptide (Anp) and brain natriuretic peptide (Bnp) gene promoters accelerated Anp and Bnp expression to regulate heart damage in the SHRs [75, 76].
The eNOS (also known as NOS3), constitutively expressed in vascular endothelial cells, plays a key role in vascular wall homeostasis and the regulation of vasomotor tone [77]. eNOS is critical for most vasoprotective molecule nitric oxide production, and vascular nitric oxide dilates all types of blood vessels by stimulating soluble guanylyl cyclase and increasing cyclic guanosine monophosphate (cGMP) levels in VSMCs [78]. In endothelial cells, H3K9ac, H4K12ac, H3K4me2, and H3K4me3 are enriched at the eNOS proximal promoter to regulate the basal expression of eNOS [77]. Lysine-specific demethylase-1 (LSD1) demethylates H3K4 and H3K9 to alter gene transcription. Heterozygous Lsd1-knockout mice (Lsd1+/−) had higher blood pressure than wild-type (WT) mice on a liberal salt diet but not on a salt-restricted diet [79]. In Lsd1+/− mice, RAAS was suppressed, as shown by plasma renin activity and plasma levels and urinary excretion of aldosterone wad lower in Lsd1+/− mice than in WT mice. Furthermore, decreased eNOS and guanylate cyclase expression indicated enhanced vascular contraction and reduced relaxation via the NO-cGMP pathway in the Lsd1+/− mice on a liberal salt diet [79]. Endothelin-1, a potent vasoconstrictor derived from vascular endothelium, was induced by angiotensin II, which was accompanied by the accumulation of H3K4me3 on its promoter [80]. Under angiotensin II treatment, Suv, Ez, and Trithorax domain 1 (Set1), a histone H3K4 tri-methyltransferase, was recruited to the promoter of endothelin-1 by activating protein 1 (Ap1) to methylate H3K4, and in synergy with Ap1, to activate endothelin-1 transcription. Increased endothelin-1 expression resulted in vasoconstriction and elevated blood pressure, thereby contributing to angiotensin II-induced cardiac hypertrophy [80].
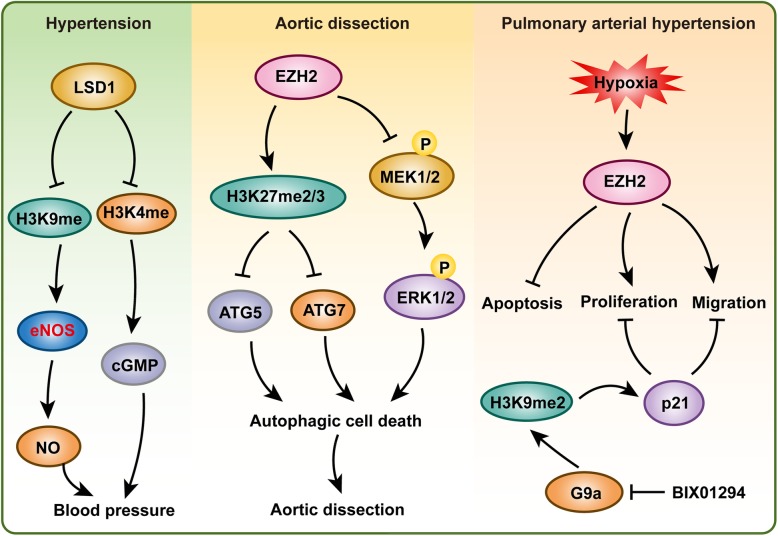
These results indicate that histone methylation is critical for AD, AAA, and TAA formation and VSMC survival, as well as being a risk factor hypertension (Fig. 4). However, the importance of histone methylation in aortic dissection has obviously been underrated, and more attention should be paid to this research field.
Fig. 4.
Histone methylation plays a role in hypertension, aortic dissection, and pulmonary arterial hypertension. Histone demethylase LSD1 was reported to regulate blood pressure. EZH2 inhibits autophagic death of VSMC to suppress aortic dissection by regulating ATG5 and ATG7 expression and MEK-ERK1/2 signaling pathway. In addition, EZH2 and G9a play a critical role in pulmonary arterial smooth muscle cells to affect pulmonary arterial hypertension
Histone methylation in pulmonary arterial hypertension
Pulmonary hypertension (PH) is defined as a resting mean pulmonary artery pressure (mPAP) greater than or equal to 25 mmHg [81]. Pulmonary arterial hypertension (PAH) should meet the following criteria: pulmonary capillary wedge pressure (PCWP) that is below 15 mmHg, PVR ≥ 3 Wood units, and mPAP ≥ 25 mmHg, in the absence of more prevalent causes of pulmonary hypertension, such as chronic lung disease, left heart disease, or venous thromboembolism [81, 82]. The incidence of PAH ranges from 2 to 7.6 cases per million adults per year and is four-fold higher in women than in men [81, 83]. The median survival is now 6 years, and 1-year survival rates are up to 90%, but survival is paradoxically worse in men with PAH [84, 85]. Fourteen PAH-specific therapies that target four relevant molecular pathways (voltage gated, L-type calcium channels, nitric oxide/cGMP, endothelin, and prostacyclin) are available for PAH [81, 86]. However, current therapies for PAH improve the quality of life but do not decrease the mortality of the patients [81, 87]. Thus, a better understanding of PAH pathogenesis contributes to the identification of new targets for therapy. The pathological features of PAH include augmented vasoconstriction, vascular obstruction, vascular stiffening, endothelial dysfunction, inflammation, fibrosis, and right ventricular failure [88, 89]. Mechanisms that drive pathological vascular remodeling in the lungs of patients with PAH include cellular, genetic, and epigenetic changes. Published studies have largely focused on the role of the genetic component in the development of PAH, and the most common genetic mechanism is mutation in bone morphogenetic protein receptor 2 (BMPR2) [7], while the means of epigenetic alterations such as DNA methylation, noncoding RNAs, and histone methylation and acetylation in PAH are currently receiving increasing attention [89].
Excessive proliferation and resistance to apoptosis of the pulmonary artery smooth muscle cells (PASMCs) contribute to the reduction in arterial compliance and increased vascular resistance and blood pressure in PAH patients [89]. Therefore, maintaining homeostasis of PASMCs is critical for the prevention and treatment of PAH. Several studies have demonstrated that histone methylation plays a vital role in PASMCs and PAH [90–92]. Histone lysine methyltransferase G9a is a key enzyme for generating H3K9me2, which is an epigenetic mark of gene suppression [93]. BIX-01294, a specific inhibitor of G9a, inhibited the proliferation of fetal PASMCs and led to cell cycle arrest in the G1 phase by inducing p21 expression. In addition, the migration and contractility of fetal PASMCs were also suppressed by BIX-01294 [90]. In a hypoxia-induced PAH mouse model, Ezh2 protein expression was positively correlated with an increase in right ventricular systolic pressure and right ventricular hypertrophy. More importantly, overexpression of Ezh2 enhanced the proliferation and migration, but reduced the apoptosis, of human PASMCs to a greater extent than GFP transfection [91]. Using a transverse aortic constriction (TAC)-induced PAH mouse model, Shi et al. also demonstrated that Ezh2 expression levels increased in PAH mice compared with the levels in the sham control mice, and this increase was accompanied by ROS deposition [92]. Furthermore, EPZ005687, a selective inhibitor of Ezh2, significantly inhibited the development of TAC-induced PAH by suppressing oxidative stress in the lung [92].
Tremendous advances have been made in elucidating the epigenetic mechanisms of PAH, but the importance of histone methylation on PAH has only recently been appreciated by researchers (Fig. 4). Studies of G9a and Ezh2 on PAH indicated that histone methylation plays an essential role in PASMC proliferation and PAH. More importantly, many inhibitors targeting protein methyltransferases or demethylases have been developed, and some of them have been used in clinical trials for treating cancer or other diseases, for example, a phase II multicenter clinical trial of tazemetostat (inhibitor of EZH2) for adult subjects with INI1-negative tumors or relapsed/refractory synovial sarcoma is in the recruiting phase (ClinicalTrials.gov Identifier: NCT02601950). Therefore, further clarifying the role and molecular mechanisms of histone methylation on PAH will likely accelerate the application of inhibitors of protein methyltransferases or demethylases in the treatment of PAH. Unfortunately, despite recent advances in epigenetics, the identification of clinical epigenetic-based therapies, especially those targeting histone methylation with effective reversibility, or a cure for PAH remains a challenge for future research.
Histone methylation in diabetic angiopathy
Vascular disorders, one of the main complications of diabetes mellitus, constitute the leading cause of morbidity and mortality in patients with diabetes mellitus [94]. Interestingly, the vascular complications often persist and may progress despite improved glucose control, possibly as a result of prior episodes of hyperglycemia, in a process typically referred to as either “hyperglycemic memory” or the legacy effect [95–97]. This poorly understood “hyperglycemic memory” phenomenon poses major challenges in treating diabetes. Recent studies have demonstrated a link between epigenetic changes such as chromatin histone lysine methylation and vascular complications of diabetes (Fig. 5).
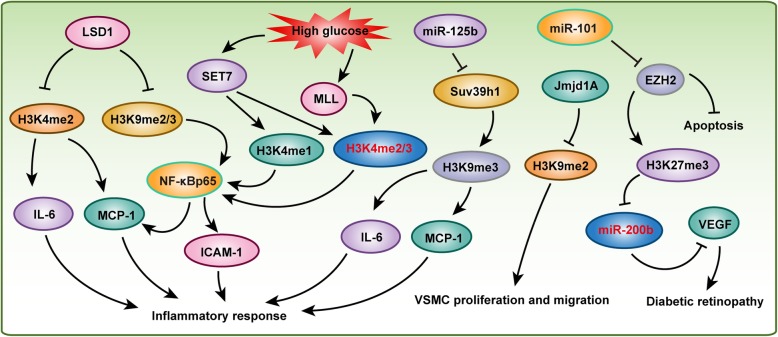
Fig. 5.
Histone methylation participates in diabetic angiopathy. Histone demethylase LSD1 and histone methyltransferases SET7, MLL, and Suv39h1 play critical roles in regulating vascular inflammatory response associated to diabetes mellitus. Jmjd1A and EZH2 are involved in VSMC proliferation, migration, or apoptosis respectively
Compelling data have shown that a high glucose-induced inflammatory process plays an important role in diabetes and cardiovascular diseases [98–100]. NF-κB signaling is one of the most important pathways regulating inflammation via initiating inflammatory factors and cytokine expression. Transient hyperglycemia stimulation induced sustained upregulation of the NF-κBp65 gene, which is associated with increased H3K4me1 and decreased H3K9me2 and H3K9me3 on the NF-κBp65 promoter in aortic endothelial cells [97]. Histone methyltransferases SET7 and LSD1 mediated H3K4 mono-methylation and H3K9me2/3 demethylation, respectively [97]. Moreover, increased NF-κBp65 significantly promoted inflammatory factor monocyte chemoattractant protein-1 (MCP-1) expression [97]. Han et al. also demonstrated that, in EA.hy926 (a human umbilical vein cell line) cells treated with high glucose, H3K4me2 and H3K4me3 marks were enriched on the promotor of the MCP-1 gene [101]. Furthermore, they found that the histone methyltransferases MLL and SET7, which catalyze H3K4 methylation, were increased on the MCP-1 promotor, while the demethylase LSD1 was decreased in endothelial cells challenged with high glucose [101]. In peripheral blood monocytes (PBMs) isolated from 44 T2DM patients and 24 age-matched controls, the T2DM patients showed higher SET7 expression levels than were shown by the controls, and SET7 methylated H3K4me1 on the promoter of NF-κBp65 to accelerate its expression, resulting in ICAM-1 and MCP-1 secretion into plasma to induce oxidative stress and the inflammatory response [102]. Similarly, in human aortic endothelial cells (HAECs), knockdown of SET7 reduced H3K4me1 mark and abolished NF-kB-dependent oxidant and inflammatory signaling [102]. These studies indicated that SET7 plays a pivotal role in glucose-mediated inflammatory response and is therefore a candidate gene for the induction of diabetic vascular complications. In addition, Lsd1, which demethylates H3K4, was significantly decreased in db/db mice compared with the level in their counterparts, while H3K4me2 was elevated at the promoters of the inflammatory genes Mcp-1 and Il-6 in db/db VSMCs. Silencing of Lsd1 facilitated inflammatory gene expression and enhanced VSMC-monocyte binding in nondiabetic VSMCs. In contrast, overexpression of Lsd1 inhibited these effects [103]. NADPH oxidase 4 (Nox4) and eNOS, which are important enzymatic sources of reactive oxygen species (ROS) in diabetic vasculature, were regulated by H3K4me1, H3K9me2, and H3K9me3 resulting in endothelial dysfunction [104].
As H3K9 methylation levels are elevated upon high glucose stimulation, its methyltransferases Suv39h1/2 were also reported to be involved in vascular complications of diabetes [105, 106]. For example, in vascular smooth muscle cells (MVSMCs) from type 2 diabetic db/db mice, miR-125b, which targets Suv39h1, was upregulated, while the Suv39h1 protein level was lower than that in the db/+ controls [105]. Knocking down Suv39h1 in normal human VSMCs increased inflammatory gene expression by decreasing H3K9me3 occupancy at its promoter. In contrast, overexpression of Suv39h1 in db/db VSMCs reversed this diabetic phenotype [106]. Furthermore, miR-125b mimics increased the expression of the inflammatory genes Mcp-1 and Il-6 by targeting Suv39h1 to reduce H3K9me3 mark at their promoters in nondiabetic cells [105]. In addition, the minor T allele of the exonic SNP rs17353856 in Suv39h2 (a member of the Suv39h1 family) was associated with diabetic retinopathy and cardiovascular disease in the FinnDiane cohort [107]. JMJD1A is the demethylase of H3K9me2, and H3K9me2 decreases when JMJD1A is elevated in diabetic vessels [108]. Jmjd1a promoted high glucose and Ang II-induced proliferation and migration of VSMCs. Moreover, Jmjd1a overexpression accelerated balloon injury-induced neointima formation in diabetic rats in which glucose was not controlled, and this effect was mediated by the Rho/ROCK and Ang II/AGTR1 pathways [108]. Interestingly, in brown adipocytes, Jmjd1a was phosphorylated at S265 by protein kinase A (PKA) to increase its interaction with the SWI/SNF nucleosome remodeling complex and DNA-bound Pparγ, thereby activating the β1-adrenergic receptor gene (Adrb1) and its downstream targets, including Ucp1. Unexpectedly, this rapid gene induction was found to be dependent on S265 phosphorylation of Jmjd1a but not on its demethylation activity [109].
H3K27me3 methylated by Polycomb repressive complex 2 (PRC2) is one of the most widely studied histone marks. In human retinal microvascular endothelial cells, PRC2 methylated H3K27me3 to inhibit miR-200b which targeted to vascular endothelial growth factor (VEGF) under high glucose conditions. Increased VEGF increased ocular permeability and neovascularization and accelerated the development of diabetic retinopathy [110]. EZH2 is the main active subunit of PRC2 that initiates and maintains H3K27me3. In human fetal endothelial cells (ECs) of the umbilical cord vein (HUVECs) in gestational diabetes mellitus patients, miR-101 was upregulated, leading to H3K27me3 downregulation by targeting EZH2 [111]. Interestingly, both gestational diabetes mellitus and high glucose could reduce EZH2 binding to the miR-101 locus in HUVECs, and EZH2 overexpression decreased the relative apoptotic activity and increased the migratory capacity of the HUVECs exposed to gestational diabetes mellitus [111]. These results indicate that EZH2-miR-101 creates a positive feedback loop that regulates endothelial cell dysfunction in gestational diabetes mellitus.
Histone methylation in endothelial cell dysfunction
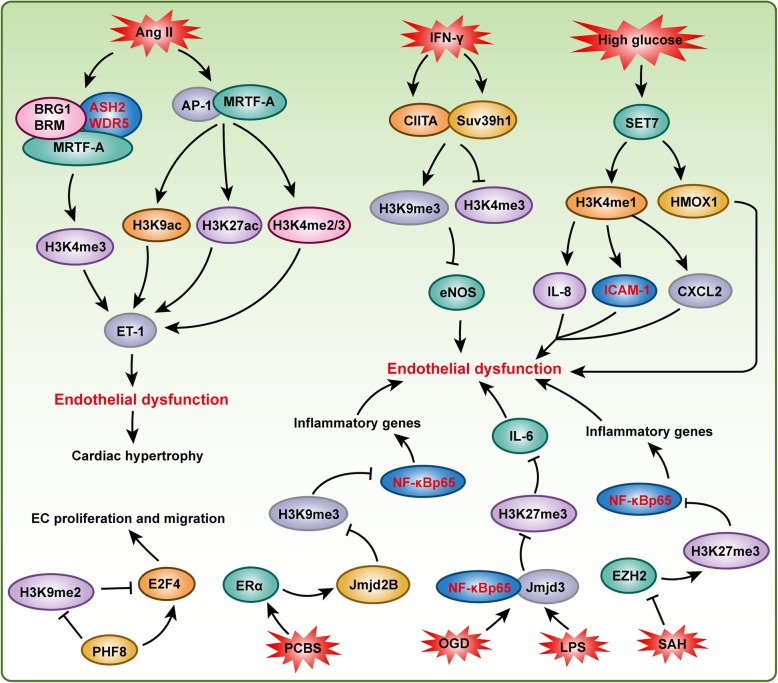
Vascular EC dysfunction is one of the major causes of cardiovascular disease, such as hypertension, cardiac remodeling, and diabetic cardiomyopathy. Epigenetic mechanisms, especially histone methylation, play essential roles in regulating the function of ECs and their homeostasis (Fig. 6). eNOS is constitutively expressed in ECs, and it plays a critical role in vascular wall homeostasis and the regulation of vasomotor tone. Thus, clarifying the mechanisms regulating eNOS expression in ECs is essential to understand the way these mechanisms may be perturbed in vascular biology. The expression level of eNOS is reduced when ECs are treated with IFN-γ, and the complex formed by class II trans-activator (CIITA) and Suv39h1 directly binds to the proximal eNOS promoter to repress transcription, and H3K9me3, which is induced by Suv39h1, mediates IFN-γ-induced eNOS repression [112]. In addition to methylated H3K9, H3K9ac, H4K12ac, H3K4me2, and H3K4me3 also participate in the regulation of eNOS expression in ECs [77]. In contrast to eNOS, endothelin (ET-1) is clearly the most potent vasoconstrictor. In response to Ang II stimulation, myocardin-related transcription factor A (MRTF-A) is recruited to the ET-1 promoter by c-Jun/c-Fos (AP-1), which alters the chromatin structure by modulating H3K9ac, H3K27ac, and H3K4me2/3 on the ET-1 promoter [113]. Further investigation indicated that the Brg1/Brm and Ash2/Wdr5 complexes are recruited by MRTF-A to catalyze H3K4 methylation on the ET-1 promoter, which induces ET-1 transactivation in ECs to accelerate Ang II-induced cardiac hypertrophy and fibrosis [114].
Fig. 6.
Histone methylation is important for maintaining endothelial cell homeostasis. Under stimulation of many stresses, such as IFN-γ, LPS, and high glucose, the function of endothelial cells were regulated by many histone methyltransferases (e.g., Suv39h1, SET7, and EZH2) and demethylases (e.g., Jmjd2B, Jmjd3, and PHF8)
High levels of glucose have been found to result in pathophysiological changes of vascular cells, contributing to accelerated atherosclerosis and other vascular complications associated with diabetes, and epigenetic changes have been implicated in the persisting vascular effects of hyperglycemia [115]. For example, in response to hyperglycemia, histone methyltransferase Setd7 protein accumulates in the nucleus of ECs, which promotes Il-8, Icam1, and Cxcl2 expression in an H3K4me1-dependent manner, and inhibits Hmox1 expression in an H3K4me1-independent fashion to regulate “hyperglycemic memory” [115]. In ECs with oxygen-glucose deprivation/reperfusion injury, histone H3K27me3 demethylase Jmjd3 expression is upregulated, and the increase in Jmjd3 leads to greater Jmjd3 interactions with Nf-κb (p65/p50) and CCAAT-enhancer-binding protein β at the Il-6 gene promoter, which decreases H3K27me3 levels to promote Il-6 expression to regulate the inflammatory response [116]. Similarly, LPS treatment promotes Jmjd3 expression in ECs to activate the expression of target genes by synergizing with Nf-κb and demethylation of H3K27me3 [117]. Ezh2, the methyltransferase that targets H3K27, was suppressed by excess S-adenosylhomocysteine (SAH) in the ECs, and decreased Ezh2 contributes to Nf-κb activation and the consequent vascular inflammatory response [118]. Environmental pollutants were reported to increase the incidence rates of cardiovascular diseases, while the underlying epigenetic mechanisms were largely unknown. Liu et al. treated ECs with polychlorinated biphenyls (PCBs), which are common environmental pollutants, and the coplanar PCBs induced not only Nf-κb signaling and Nf-κb target inflammatory gene activation but also histone H3K9me3 demethylase jumonji domain-containing protein 2B (Jmjd2b) expression. The increased accumulation of Jmjd2b on the p65 promoter led to the demethylation of the H3K9me3 repression mark and to the observed upregulation of p65 and associated inflammatory genes [119]. Another demethylase, histone plant homeodomain finger protein 8 (PHF8), catalyzed the removal of methyl groups from H3K9 and H4K20. In ECs, PHF8 maintained E2F4 expression by demethylating H3K9me2 at the E2F4 transcriptional start site to facilitate endothelial cell proliferation, survival, and the capacity for migration and development of capillary-like structures [120]. G9a is the methyltransferase that targets H3K9, and inhibition of G9a activity by BIX-01294 or knockdown by shRNA attenuates the proliferation of human microvascular ECs, and arresting them in the G1 phase of the cell cycle by regulating the phosphorylation of CHK1 [121]. In addition, histone methyltransferase MLL contributes to endothelial-cell sprout formation by regulating HoxA9 and EphB4 expression [122].
Histone methylation in tumor angiogenesis
It is well known that angiogenesis is a main contributor to tumor growth and the metastatic process. Therefore, approximately half a century ago, some scholars proposed the concept of inhibiting tumor angiogenesis for treating solid tumors. The anti-angiogenic drugs were expected to decrease or even block the oxygen and nutritional supply of tumor and then to arrest tumor growth, and displayed minimal toxic side effects to healthy tissues at the same time. Given that VEGFA is the most important regulator of tumor angiogenesis, Bevacizumab (Avastin), a humanized monoclonal anti-VEGFA antibody, is a typical example of anti-tumor angiogenesis and it is now used as anti-angiogenic drug in several forms of cancers, including breast, colorectal, and lung cancers [123]. Thus, the mechanisms that regulates the expression or activating of VEGFA are critical for regulating tumor angiogenesis. Importantly, histone methylation and its responsible methyltransferases or demethylases are indispensable for VEGFA and its receptors regulation and tumor angiogenesis.
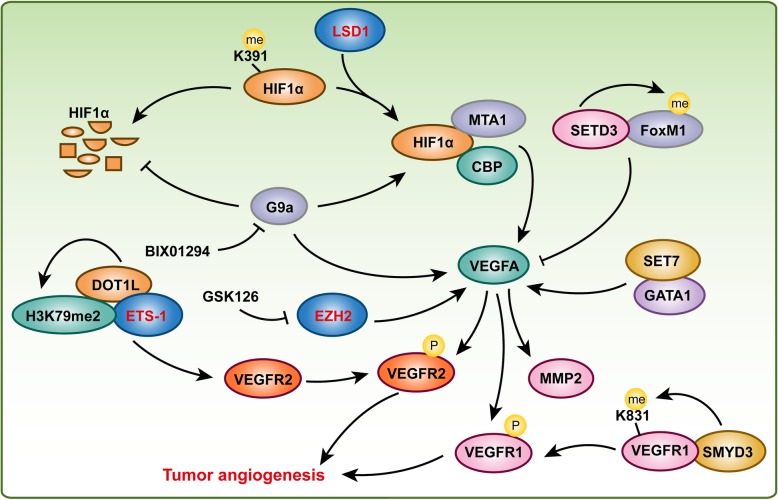
It is reported that histone methyltransferase Dot1l deletion results in embryonic lethality and cardiovascular defects including decreased vasculature [47]. In HUVECs, knockdown of DOT1L results in decreased cell viability, migration, tube formation, and capillary sprout formation, as well as reduced formation of functional vascular networks in vivo, which was mediated by H3K79me2 and cooperating with transcription factor ETS-1 to regulate VEGFR2 expression [124]. In breast cancer patients, histone methyltransferase SET7 and transcription factor GATA1 expression levels were upregulated and positively correlated with VEGFA expression and microvessel number. Furthermore, SET7 associates with GATA1 to promote VEGFA transcription and breast tumor angiogenesis [125]. However, by using ProtoArray system, Cohn et al. identified 172 new SETD3 interacting proteins, and further investigation found that SETD3 binds and methylates the transcription factor FoxM1 to inhibit VEGFA expression under hypoxia [126]. In addition, GSK126, an EZH2 inhibitor, inhibits gastric cancer and lung adenocarcinoma cell migration and angiogenesis in solid tumor cell lines through downregulation of VEGFA expression [127]. In addition to the regulation of VEGFA or its receptor expression, HMTs also regulate PTMs of VEGFR1 or alternative splicing of VEGFA to affect tumor angiogenesis. For example, histone methyltransferase SMYD3 expression level was elevated in colorectal, hepatocellular, and breast carcinomas, and elevated SMYD3 interacts with VEGFR1 to methylate VEGFR1 at its lysine 831. Furthermore, methylation of VEGFR1 enhanced its kinase activity in cells [128]. The H3K9 methyltransferase G9a was reported to regulate the alternative splicing of VEGFA (exclusion of VEGFA exon 6a) via interacting with chromatin modulator HP1γ and methylated H3K9 to recruit splicing regulator SRSF1, but this kind of alternative splicing did not alter total VEGFA mRNA levels [129].
HIF1α is another key regulator of tumor growth and angiogenesis as a transcriptional regulator of VEGFA [130]. The stability and function of the HIF1α protein are also affected by methylation. BIX01294, a G9a-specific inhibitor, decreased expression levels of HIF1α, VEGFA, proline hydroxylase 2 (PHD2), hydroxylated HIF1α and von Hippel-Lindau protein (pVHL), as well as shortened the half-life of HIF1α in HepG2 human hepatocellular carcinoma cells under hypoxic conditions. Furthermore, BIX01294 suppressed VEGFA-induced MMP2 activity and phosphorylation of VEGFR2, focal adhesion kinase (FAK), and paxillin in HUVECs [131]. These results indicated that histone methyltransferase G9a could facilitate HIF1α stability and VEGFA-induced angiogenesis. In prostate cancer, elevated expression of LSD1 correlates with prostate cancer recurrence and with increased VEGFA expression, and knockdown of LSD1 in prostate cancer cells decreases VEGFA expression [132]. Importantly, LSD1 demethylates HIF1α at lysine 391 to protect HIF1α against ubiquitin-mediated protein degradation. HIF1α stabilized by LSD1 cooperates with CBP and MTA1 to enhance VEGFA-induced tumor angiogenesis [130].
These studies indicated that HMTs and HDMTs not only regulate VEGFA and HIF1α expression but also involve in their PTMs, activity, and stability to affect tumor angiogenesis (Fig. 7).
Fig. 7.
Histone methyltransferases and demethylases are involved in tumor angiogenesis. HIF signaling pathway and VEGFA signaling pathway play a central role in tumor angiogenesis. LSD1 and G9a could promote HIF1α expression and increase its stability, which subsequently accelerates VEGFA and its downstream genes expression, and activates VEGFA signaling pathway to regulate tumor angiogenesis. In addition, EZH2, DOT1L, SMYD3, SETD3 and SET7 are also involved in regulating VEGFA expression or VEGFA signaling pathway during tumor angiogenesis
Histone methylation in other forms of vasculopathy
In addition to the aforementioned vascular diseases, histone methylation is also involved in other forms of vasculopathy. For example, Chen et al. reported that, in indoxyl sulfate-induced VSMCs, the characteristics of osteoblastic differentiation and calcification are manifested with the downregulation of the expression of histone methyltransferase Set7/9 and with autophagy activation, which indicates that Set7/9 downregulation and autophagy activation may be the key mechanisms of indoxyl sulfate-induced vascular calcification in chronic kidney disease [133]. Intercellular adhesion molecule 1 (Icam1) mediates the adhesion and transmigration of leukocytes across the endothelium to promote inflammation in the vasculature. In human brain microvascular endothelial cells and mouse brain microvessels, the pro-inflammatory cytokine Tnf-α dramatically increases Icam1 mRNA and protein levels by regulating H3K9me2, which is achieved by treatments with histone methyltransferase G9a and demethylase Kdm4b. Moreover, G9a overexpression or Icam1 or Kdm4b depletion reduces inflammation-induced leukocyte extravasation, which indicates that blocking Icam1 or Kdm4b may offer a novel therapeutic approach for treating brain diseases [134]. Anti-neutrophil cytoplasmic autoantibody-associated vasculitis (AAV) is a systemic autoimmune disease characterized by destructive vascular inflammation, which is associated with autoantibodies directed against the neutrophil granule proteins myeloperoxidase (MPO) or proteinase 3 (PR3). H3K9 methylation and its corresponding methyltransferases EHMT1 and EHMT2 were depleted most extensively at the MPO and PR3 genes, while H3K4 methylation and H4K16 acetylation were enriched at the MPO and PR3 genes in patients with active disease [135]. In addition, Karnewar et al. demonstrated that H3K79me was involved in metformin-regulated mitochondrial biogenesis and senescence in age-associated vascular dysfunction [136].
Conclusion and perspective
In this review, we highlight the role of histone methylation in the vascular development and vascular-related diseases, such as aortic dissection and pulmonary arterial hypertension. Currently, our understanding of histone methylation in vascular biology is rudimentary, but the observations presented in this review offer a broad base for further discovery. Although great progress has been made in the field of histone methylation in vascular biology, it is important to raise a few points. First, the published studies primarily focused on a few molecules related to histone methylation, such as EZH2, G9a, and LSD1, but did not clarify the roles of other HMTs and HDMTs. Second, few nonhistone targets that mediate the function of HMTs and HDMTs in vascular biology have been identified; however, nonhistone proteins are commonly methylated by HMTs in other biological processes (e.g., cancer). Methylation of the nonhistone protein not only affects protein activity and stability but also interacts with other posttranslational modifications to regulate its function; therefore, the discovery of more methylation signaling pathways in vascular biology is important. Third, do HMTs or HDMTs function in vascular biology independent of their methyltransferase or demethylase activity? Fourth, more conditional knockout animal models rather than global knockout models should be used to investigate the roles and mechanisms of HMTs and HDMTs in vascular biology in the future. Fifth, S-adenosylmethionine (SAM), the methyl-donating substrate of histone methyltransferases, and S-adenosylhomocysteine (SAH) link one-carbon metabolism to methylation status. Extensive research demonstrated that one carbon metabolism is closely related to histone methylation, and they play critical roles in embryonic development, cancer, and neurodegenerative diseases. However, there is almost no study published that tried to investigate how one carbon metabolism works together with histone methylation to affect vascular biology or diseases. Thus, more efforts should be pained to delve into this new field, which may open new pathways for pharmacological intervention in vascular diseases. Sixth, some inhibitors of HMTs or HDMTs may have the potential to reverse pathological vascular changes, and more attention should be paid to the clinical application of these inhibitors. We suspect that inhibitors of HMTs and HDMTs have great potential to remedy vascular-related diseases. Nevertheless, although more of these inhibitors are likely to be developed, the issue of specificity may be a limiting factor for their safe and efficacious widespread use.
Acknowledgments
We apologize to authors whose papers could not be cited due to space restrictions.
Availability of data and material
Not applicable.
Abbreviations
- AAA
Abdominal aortic aneurysm
- AAD
Acute aortic dissection
- AAS
Acute thoracic aortic syndromes
- AAV
Anti-neutrophil cytoplasmic autoantibody-associated vasculitis
- ABCA1
ATP-binding cassette transporter A1
- ACE1
Angiotensin-converting enzyme 1
- AD
Aortic dissection
- ADMA
Asymmetric dimethylarginine
- AdoMet
S-Adenosyl-l-methionine
- Adrb1
β1-adrenergic receptor gene
- ANP
Atrial natriuretic peptide
- AP1
Activating protein 1
- BMPR2
Bone morphogenetic protein receptor 2
- BNP
Brain natriuretic peptide
- cGMP
Cyclic guanosine monophosphate
- DNMT1
DNA methyltransferase 1
- ECM
Extracellular matrix
- ECs
Endothelial cells
- eNOS
Endothelial NO synthase
- ET-1
Endothelin
- HAECs
Human aortic endothelial cells
- HDMTs
Histone demethylases
- HHcy
Hyperhomocysteinemia
- HMT
Histone methyltransferase
- HSD11B2
Hydroxysteroid dehydrogenase-11β2 enzyme
- ICAM1
Intercellular adhesion molecule 1
- IMH
Intramural hematoma
- JMJD2B
Jumonji domain-containing protein 2B
- LDL
Low-density lipoprotein
- LRP6
Low-density lipoprotein receptor-related protein 6
- LSD1
Lysine-specific demethylase-1
- MCP-1
Monocyte chemoattractant protein-1
- MeCP2
Methyl CpG-binding protein-2
- MEF2
Myocyte enhancing factor-2
- MMA
Monomethylation
- mPAP
Mean pulmonary artery pressure
- MPO
Myeloperoxidase
- MRTF-A
Myocardin-related transcription factor A
- Nox4
NADPH oxidase 4
- PAH
Pulmonary arterial hypertension
- PASMCs
Pulmonary artery smooth muscle cells
- PAU
Penetrating aortic ulcer
- PBM
Peripheral blood monocytes
- PCBs
Polychlorinated biphenyls
- PCWP
Pulmonary capillary wedge pressure
- PH
Pulmonary hypertension
- PHF8
Plant homeodomain finger protein 8
- PKA
Protein kinase A
- PKMTs
Protein lysine methyltransferases
- PlGF
Placental growth factor
- PR3
Proteinase 3
- PRC2
Polycomb repressive complex 2
- PRMTs
Protein arginine methyltransferases
- RAAS
Renin-angiotensin-aldosterone system
- ROS
Reactive oxygen species
- SAH
S-adenosylhomocysteine
- SDMA
Symmetric dimethylarginine
- SET
Suppressor of variegation, enhancer of Zeste, Trithorax
- SET1
Suv, Ez, and Trithorax domain 1
- SHR
Spontaneously hypertensive rat
- SMCs
Smooth muscle cells
- TAAs
Thoracic aortic aneurysms
- TAC
Transverse aortic constriction
- VEGF
Vascular endothelial growth factor
Authors’ contributions
All authors searched for literature and wrote and edited the manuscript. XW, XHZ, and DSJ provided funding support. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (NO. 81600188, NO. 81670050, NO. 81873456).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiang Wei and Xin Yi contributed equally to this work.
References
- 1.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27(6):1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 2.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104(5):576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 3.Hu M, Sun XJ, Zhang YL, Kuang Y, Hu CQ, Wu WL, et al. Histone h3 lysine 36 methyltransferase hypb/setd2 is required for embryonic vascular remodeling. Proc Natl Acad Sci U S A. 2010;107(7):2956–2961. doi: 10.1073/pnas.0915033107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang H, Xia Q, Xin S, Lun Y, Song J, Tang D, et al. Abnormal epigenetic modifications in peripheral t cells from patients with abdominal aortic aneurysm are correlated with disease development. J Vasc Res. 2015;52(6):404–413. doi: 10.1159/000445771. [DOI] [PubMed] [Google Scholar]
- 5.Findeisen HM, Kahles FK, Bruemmer D. Epigenetic regulation of vascular smooth muscle cell function in atherosclerosis. Current Atherosclerosis Reports. 2013;15(4):319. doi: 10.1007/s11883-013-0319-7. [DOI] [PubMed] [Google Scholar]
- 6.Greissel A, Culmes M, Napieralski R, Wagner E, Gebhard H, Schmitt M, et al. Alternation of histone and DNA methylation in human atherosclerotic carotid plaques. Thromb Haemost. 2015;114(2):390–402. doi: 10.1160/TH14-10-0852. [DOI] [PubMed] [Google Scholar]
- 7.Pullamsetti SS, Perros F, Chelladurai P, Yuan J, Stenmark K. Transcription factors, transcriptional coregulators, and epigenetic modulation in the control of pulmonary vascular cell phenotype: Therapeutic implications for pulmonary hypertension (2015 grover conference series) Pulm Circ. 2016;6(4):448–464. doi: 10.1086/688908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costantino S, Ambrosini S, Paneni F. The epigenetic landscape in the cardiovascular complications of diabetes. J Endocrinol Invest. 2019;42(5):505–511. doi: 10.1007/s40618-018-0956-3. [DOI] [PubMed] [Google Scholar]
- 9.Lazarewicz K, Watson P. Giant cell arteritis. BMJ. 2019;365:l1964. doi: 10.1136/bmj.l1964. [DOI] [PubMed] [Google Scholar]
- 10.Zarzour A, Kim HW, Weintraub NL. Epigenetic regulation of vascular diseases. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39(6):984–990. doi: 10.1161/ATVBAHA.119.312193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan MS, Marsden PA. Epigenetics in the vascular endothelium. Arteriosclerosis, Thrombosis, and Vascular Biology. 2015;35(11):2297–2306. doi: 10.1161/ATVBAHA.115.305043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khyzha N, Alizada A, Wilson MD, Fish JE. Epigenetics of atherosclerosis: emerging mechanisms and methods. Trends Mol Med. 2017;23(4):332–347. doi: 10.1016/j.molmed.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- 14.Liang M. Epigenetic mechanisms and hypertension. Hypertension. 2018;72(6):1244–1254. doi: 10.1161/HYPERTENSIONAHA.118.11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rea S, Eisenhaber F, O'Carroll D, Strahl BD, Sun ZW, Schmid M, et al. Regulation of chromatin structure by site-specific histone h3 methyltransferases. Nature. 2000;406(6796):593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, et al. Histone demethylation mediated by the nuclear amine oxidase homolog lsd1. Cell. 2004;119(7):941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Kaniskan HU, Martini ML, Jin J. Inhibitors of protein methyltransferases and demethylases. Chem Rev. 2018;118(3):989–1068. doi: 10.1021/acs.chemrev.6b00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greer EL, Shi Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13(5):343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi X, Jiang XJ, Fang ZM. Histone methyltransferase smyd2: Ubiquitous regulator of disease. Clin Epigenetics. 2019;11(1):112. doi: 10.1186/s13148-019-0711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamamoto R, Saloura V, Nakamura Y. Critical roles of non-histone protein lysine methylation in human tumorigenesis. Nat Rev Cancer. 2015;15(2):110–124. doi: 10.1038/nrc3884. [DOI] [PubMed] [Google Scholar]
- 21.Spellmon N, Holcomb J, Trescott L, Sirinupong N, Yang Z. Structure and function of set and mynd domain-containing proteins. Int J Mol Sci. 2015;16(1):1406–1428. doi: 10.3390/ijms16011406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wesche J, Kuhn S, Kessler BM, Salton M, Wolf A. Protein arginine methylation: a PROMINENT modification and its demethylation. Cell Mol Life Sci. 2017;74(18):3305–3315. doi: 10.1007/s00018-017-2515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dillon SC, Zhang X, Trievel RC, Cheng X. The set-domain protein superfamily: protein lysine methyltransferases. Genome Biol. 2005;6(8):227. doi: 10.1186/gb-2005-6-8-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li R, Yi X, Wei X, Huo B, Guo X, Cheng C, et al. Ezh2 inhibits autophagic cell death of aortic vascular smooth muscle cells to affect aortic dissection. Cell Death Dis. 2018;9(2):180. doi: 10.1038/s41419-017-0213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi X, Jiang X, Li X, Jiang DS. Histone lysine methylation and congenital heart disease: from bench to bedside (review) Int J Mol Med. 2017;40(4):953–964. doi: 10.3892/ijmm.2017.3115. [DOI] [PubMed] [Google Scholar]
- 26.Jiang DS, Yi X, Li R, Su YS, Wang J, Chen ML, et al. The histone methyltransferase mixed lineage leukemia (mll) 3 may play a potential role on clinical dilated cardiomyopathy. Mol Med. 2017;23:196–203. doi: 10.2119/molmed.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi X, Tao Y, Lin X, Dai Y, Yang T, Yue X, et al. Histone methyltransferase setd2 is critical for the proliferation and differentiation of myoblasts. Biochim Biophys Acta Mol Cell Res. 2017;1864(4):697–707. doi: 10.1016/j.bbamcr.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yi X, Jiang XJ, Li XY, Jiang DS. Histone methyltransferases: novel targets for tumor and developmental defects. Am J Transl Res. 2015;7(11):2159–2175. [PMC free article] [PubMed] [Google Scholar]
- 29.Li R, Wei X, Jiang DS. Protein methylation functions as the posttranslational modification switch to regulate autophagy. Cell Mol Life Sci. 2019;76(19):3711–3722. doi: 10.1007/s00018-019-03161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao S, Wang Z, Wang W, Hu X, Chen P, Li J, et al. The lysine methyltransferase smyd2 methylates the kinase domain of type ii receptor bmpr2 and stimulates bone morphogenetic protein signaling. J Biol Chem. 2017;292(30):12702–12712. doi: 10.1074/jbc.M117.776278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia M, Liu J, Wu X, Liu S, Li G, Han C, et al. Histone methyltransferase ash1l suppresses interleukin-6 production and inflammatory autoimmune diseases by inducing the ubiquitin-editing enzyme a20. Immunity. 2013;39(3):470–481. doi: 10.1016/j.immuni.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Hirata Y, Katagiri K, Nagaoka K, Morishita T, Kudoh Y, Hatta T, et al. Trim48 promotes ask1 activation and cell death through ubiquitination-dependent degradation of the ask1-negative regulator prmt1. Cell Rep. 2017;21(9):2447–2457. doi: 10.1016/j.celrep.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Chi L, Ahmed A, Roy AR, Vuong S, Cahill LS, Caporiccio L, et al. G9a controls placental vascular maturation by activating the notch pathway. Development. 2017;144(11):1976–1987. doi: 10.1242/dev.148916. [DOI] [PubMed] [Google Scholar]
- 34.Chang CW, Wakeland AK, Parast MM. Trophoblast lineage specification, differentiation and their regulation by oxygen tension. J Endocrinol. 2018;236(1):R43–R56. doi: 10.1530/JOE-17-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80(2):283–285. [PubMed] [Google Scholar]
- 36.Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157(6):2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burton GJ, Fowden AL. The placenta: a multifaceted, transient organ. Philos Trans R Soc Lond B Biol Sci. 2015;370(1663):20140066. doi: 10.1098/rstb.2014.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakraborty D, Cui W, Rosario GX, Scott RL, Dhakal P, Renaud SJ, et al. Hif-kdm3a-mmp12 regulatory circuit ensures trophoblast plasticity and placental adaptations to hypoxia. Proc Natl Acad Sci U S A. 2016;113(46):E7212–E7E21. doi: 10.1073/pnas.1612626113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, et al. Failure of blood-island formation and vasculogenesis in flk-1-deficient mice. Nature. 1995;376(6535):62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Ferguson JE, 3rd, Wang H, Kelley R, Ren R, McDonough H, et al. Prdm6 is enriched in vascular precursors during development and inhibits endothelial cell proliferation, survival, and differentiation. J Mol Cell Cardiol. 2008;44(1):47–58. doi: 10.1016/j.yjmcc.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boeckel JN, Guarani V, Koyanagi M, Roexe T, Lengeling A, Schermuly RT, et al. Jumonji domain-containing protein 6 (jmjd6) is required for angiogenic sprouting and regulates splicing of vegf-receptor 1. Proc Natl Acad Sci U S A. 2011;108(8):3276–3281. doi: 10.1073/pnas.1008098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smits M, Mir SE, Nilsson RJ, van der Stoop PM, Niers JM, Marquez VE, et al. Down-regulation of mir-101 in endothelial cells promotes blood vessel formation through reduced repression of ezh2. PLoS One. 2011;6(1):e16282. doi: 10.1371/journal.pone.0016282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delgado-Olguin P, Dang LT, He D, Thomas S, Chi L, Sukonnik T, et al. Ezh2-mediated repression of a transcriptional pathway upstream of mmp9 maintains integrity of the developing vasculature. Development. 2014;141(23):4610–4617. doi: 10.1242/dev.112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurihara T, Shimizu-Hirota R, Shimoda M, Adachi T, Shimizu H, Weiss SJ, et al. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation. 2012;126(25):3070–3080. doi: 10.1161/CIRCULATIONAHA.112.097097. [DOI] [PubMed] [Google Scholar]
- 45.Wen Z, Shen Y, Berry G, Shahram F, Li Y, Watanabe R, et al. The microvascular niche instructs t cells in large vessel vasculitis via the vegf-jagged1-notch pathway. Sci Transl Med. 2017;9(399):eaal3322. doi: 10.1126/scitranslmed.aal3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spuul P, Daubon T, Pitter B, Alonso F, Fremaux I, Kramer I, et al. Vegf-a/notch-induced podosomes proteolyse basement membrane collagen-iv during retinal sprouting angiogenesis. Cell Rep. 2016;17(2):484–500. doi: 10.1016/j.celrep.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Feng Y, Yang Y, Ortega MM, Copeland JN, Zhang M, Jacob JB, et al. Early mammalian erythropoiesis requires the dot1l methyltransferase. Blood. 2010;116(22):4483–4491. doi: 10.1182/blood-2010-03-276501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 49.Xu S, Kamato D, Little PJ, Nakagawa S, Pelisek J, Jin ZG. Targeting epigenetics and non-coding rnas in atherosclerosis: From mechanisms to therapeutics. Pharmacol Ther. 2019;196:15–43. doi: 10.1016/j.pharmthera.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28(5):812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wierda RJ, Rietveld IM, van Eggermond MC, Belien JA, van Zwet EW, Lindeman JH, et al. Global histone h3 lysine 27 triple methylation levels are reduced in vessels with advanced atherosclerotic plaques. Life Sci. 2015;129:3–9. doi: 10.1016/j.lfs.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 52.Greissel A, Culmes M, Burgkart R, Zimmermann A, Eckstein HH, Zernecke A, et al. Histone acetylation and methylation significantly change with severity of atherosclerosis in human carotid plaques. Cardiovasc Pathol. 2016;25(2):79–86. doi: 10.1016/j.carpath.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Lv YC, Tang YY, Zhang P, Wan W, Yao F, He PP, et al. Histone methyltransferase enhancer of zeste homolog 2-mediated abca1 promoter DNA methylation contributes to the progression of atherosclerosis. PLoS One. 2016;11(6):e0157265. doi: 10.1371/journal.pone.0157265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar A, Kumar S, Vikram A, Hoffman TA, Naqvi A, Lewarchik CM, et al. Histone and DNA methylation-mediated epigenetic downregulation of endothelial kruppel-like factor 2 by low-density lipoprotein cholesterol. Arterioscler Thromb Vasc Biol. 2013;33(8):1936–1942. doi: 10.1161/ATVBAHA.113.301765. [DOI] [PubMed] [Google Scholar]
- 55.Xiaoling Y, Li Z, ShuQiang L, Shengchao M, Anning Y, Ning D, et al. Hyperhomocysteinemia in apoe-/- mice leads to overexpression of enhancer of zeste homolog 2 via mir-92a regulation. PLoS One. 2016;11(12):e0167744. doi: 10.1371/journal.pone.0167744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Esse R, Florindo C, Imbard A, Rocha MS, de Vriese AS, Smulders YM, et al. Global protein and histone arginine methylation are affected in a tissue-specific manner in a rat model of diet-induced hyperhomocysteinemia. Biochim Biophys Acta. 2013;1832(10):1708–1714. doi: 10.1016/j.bbadis.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 57.Cheng SL, Ramachandran B, Behrmann A, Shao JS, Mead M, Smith C, et al. Vascular smooth muscle lrp6 limits arteriosclerotic calcification in diabetic ldlr-/- mice by restraining noncanonical wnt signals. Circ Res. 2015;117(2):142–156. doi: 10.1161/CIRCRESAHA.117.306712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y, Cheng X, Tian W, Zhou B, Wu X, Xu H, et al. Mrtf-a steers an epigenetic complex to activate endothelin-induced pro-inflammatory transcription in vascular smooth muscle cells. Nucleic Acids Res. 2014;42(16):10460–10472. doi: 10.1093/nar/gku776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang J, Li Q, Cai W, Zhang X, Yang B, Li X, et al. Inhibition of polycomb repressor complex 2 ameliorates neointimal hyperplasia by suppressing trimethylation of h3k27 in vascular smooth muscle cells. Br J Pharmacol. 2019;176(17):3206–3219. doi: 10.1111/bph.14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lockman K, Taylor JM, Mack CP. The histone demethylase, jmjd1a, interacts with the myocardin factors to regulate smc differentiation marker gene expression. Circ Res. 2007;101(12):e115–e123. doi: 10.1161/CIRCRESAHA.107.164178. [DOI] [PubMed] [Google Scholar]
- 61.Davis CA, Haberland M, Arnold MA, Sutherland LB, McDonald OG, Richardson JA, et al. Prism/prdm6, a transcriptional repressor that promotes the proliferative gene program in smooth muscle cells. Mol Cell Biol. 2006;26(7):2626–2636. doi: 10.1128/MCB.26.7.2626-2636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elia L, Kunderfranco P, Carullo P, Vacchiano M, Farina FM, Hall IF, et al. Uhrf1 epigenetically orchestrates smooth muscle cell plasticity in arterial disease. J Clin Invest. 2018;128(6):2473–2486. doi: 10.1172/JCI96121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lehrke M, Kahles F, Makowska A, Tilstam PV, Diebold S, Marx J, et al. Pde4 inhibition reduces neointima formation and inhibits vcam-1 expression and histone methylation in an epac-dependent manner. J Mol Cell Cardiol. 2015;81:23–33. doi: 10.1016/j.yjmcc.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 64.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 esc guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the european society of cardiology (esc) Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 65.Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM, et al. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the oxford vascular study. Circulation. 2013;127(20):2031–2037. doi: 10.1161/CIRCULATIONAHA.112.000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oller J, Mendez-Barbero N, Ruiz EJ, Villahoz S, Renard M, Canelas LI, et al. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease adamts1 and in a mouse model of marfan syndrome. Nat Med. 2017;23(2):200–212. doi: 10.1038/nm.4266. [DOI] [PubMed] [Google Scholar]
- 67.Guo X, Fang ZM, Wei X, Huo B, Yi X, Cheng C, et al. Hdac6 is associated with the formation of aortic dissection in human. Mol Med. 2019;25(1):10. doi: 10.1186/s10020-019-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones GT, Tromp G, Kuivaniemi H, Gretarsdottir S, Baas AF, Giusti B, et al. Meta-analysis of genome-wide association studies for abdominal aortic aneurysm identifies four new disease-specific risk loci. Circ Res. 2017;120(2):341–353. doi: 10.1161/CIRCRESAHA.116.308765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Toghill BJ, Saratzis A, Freeman PJ, Sylvius N, collaborators U, Bown MJ. Smyd2 promoter DNA methylation is associated with abdominal aortic aneurysm (aaa) and smyd2 expression in vascular smooth muscle cells. Clin Epigenetics 2018;10:29. [DOI] [PMC free article] [PubMed]
- 70.Gomez D, Coyet A, Ollivier V, Jeunemaitre X, Jondeau G, Michel JB, et al. Epigenetic control of vascular smooth muscle cells in marfan and non-marfan thoracic aortic aneurysms. Cardiovasc Res. 2011;89(2):446–456. doi: 10.1093/cvr/cvq291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Januzzi JL, Eagle KA, Cooper JV, Fang J, Sechtem U, Myrmel T, et al. Acute aortic dissection presenting with congestive heart failure: results from the international registry of acute aortic dissection. J Am Coll Cardiol. 2005;46(4):733–735. doi: 10.1016/j.jacc.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 72.Stoll S, Wang C, Qiu H. DNA methylation and histone modification in hypertension. Int J Mol Sci. 2018;19(4):1174. doi: 10.3390/ijms19041174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wise IA, Charchar FJ. Epigenetic modifications in essential hypertension. Int J Mol Sci. 2016;17(4):451. doi: 10.3390/ijms17040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee HA, Cho HM, Lee DY, Kim KC, Han HS, Kim IK. Tissue-specific upregulation of angiotensin-converting enzyme 1 in spontaneously hypertensive rats through histone code modifications. Hypertension. 2012;59(3):621–626. doi: 10.1161/HYPERTENSIONAHA.111.182428. [DOI] [PubMed] [Google Scholar]
- 75.Arif M, Sadayappan S, Becker RC, Martin LJ, Urbina EM. Epigenetic modification: a regulatory mechanism in essential hypertension. Hypertens Res. 2019;42(8):1099–1113. doi: 10.1038/s41440-019-0248-0. [DOI] [PubMed] [Google Scholar]
- 76.Mehrotra A, Joe B, de la Serna IL. Swi/snf chromatin remodeling enzymes are associated with cardiac hypertrophy in a genetic rat model of hypertension. J Cell Physiol. 2013;228(12):2337–2342. doi: 10.1002/jcp.24404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fish JE, Matouk CC, Rachlis A, Lin S, Tai SC, D'Abreo C, et al. The expression of endothelial nitric-oxide synthase is controlled by a cell-specific histone code. J Biol Chem. 2005;280(26):24824–24838. doi: 10.1074/jbc.M502115200. [DOI] [PubMed] [Google Scholar]
- 78.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation. 2006;113(13):1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 79.Pojoga LH, Williams JS, Yao TM, Kumar A, Raffetto JD. do Nascimento GR et al. Histone demethylase lsd1 deficiency during high-salt diet is associated with enhanced vascular contraction, altered no-cgmp relaxation pathway, and hypertension. Am J Physiol Heart Circ Physiol. 2011;301(5):H1862–H1871. doi: 10.1152/ajpheart.00513.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu L, Yang G, Weng X, Liang P, Li L, Li J, et al. Histone methyltransferase set1 mediates angiotensin ii-induced endothelin-1 transcription and cardiac hypertrophy in mice. Arterioscler Thromb Vasc Biol. 2015;35(5):1207–1217. doi: 10.1161/ATVBAHA.115.305230. [DOI] [PubMed] [Google Scholar]
- 81.Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ. 2018;360:j5492. doi: 10.1136/bmj.j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 83.Jacobs W, van de Veerdonk MC, Trip P, de Man F, Heymans MW, Marcus JT, et al. The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest. 2014;145(6):1230–1236. doi: 10.1378/chest.13-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122(2):156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 85.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management (reveal) Circulation. 2010;122(2):164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 86.Tuder RM, Archer SL, Dorfmuller P, Erzurum SC, Guignabert C, Michelakis E, et al. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D4–12. doi: 10.1016/j.jacc.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334(5):296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 88.Ataya A, Patel S, Cope J, Alnuaimat H. Pulmonary arterial hypertension and associated conditions. Dis Mon. 2016;62(11):379–402. doi: 10.1016/j.disamonth.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 89.Luna RCP, de Oliveira Y, Lisboa JVC, Chaves TR, de Araujo TAM, de Sousa EE, et al. Insights on the epigenetic mechanisms underlying pulmonary arterial hypertension. Braz J Med Biol Res. 2018;51(12):e7437. doi: 10.1590/1414-431x20187437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang Q, Lu Z, Singh D, Raj JU. Bix-01294 treatment blocks cell proliferation, migration and contractility in ovine foetal pulmonary arterial smooth muscle cells. Cell Proliferation. 2012;45(4):335–344. doi: 10.1111/j.1365-2184.2012.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aljubran SA, Cox R, Jr, Tamarapu Parthasarathy P, Kollongod Ramanathan G, Rajanbabu V, Bao H, et al. Enhancer of zeste homolog 2 induces pulmonary artery smooth muscle cell proliferation. PLoS One. 2012;7(5):e37712. doi: 10.1371/journal.pone.0037712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi ZL, Fang K, Li ZH, Ren DH, Zhang JY, Sun J. Ezh2 inhibition ameliorates transverse aortic constriction-induced pulmonary arterial hypertension in mice. Can Respir J. 2018;2018:9174926. doi: 10.1155/2018/9174926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Segovia C, San Jose-Eneriz E, Munera-Maravilla E, Martinez-Fernandez M, Garate L, Miranda E, et al. Inhibition of a g9a/dnmt network triggers immune-mediated bladder cancer regression. Nat Med. 2019;25(7):1073–1081. doi: 10.1038/s41591-019-0499-y. [DOI] [PubMed] [Google Scholar]
- 94.Cooper ME, Johnston CI. Optimizing treatment of hypertension in patients with diabetes. JAMA. 2000;283(24):3177–3179. doi: 10.1001/jama.283.24.3177. [DOI] [PubMed] [Google Scholar]
- 95.El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205(10):2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chalmers J, Cooper ME. Ukpds and the legacy effect. N Engl J Med. 2008;359(15):1618–1620. doi: 10.1056/NEJMe0807625. [DOI] [PubMed] [Google Scholar]
- 97.Brasacchio D, Okabe J, Tikellis C, Balcerczyk A, George P, Baker EK, et al. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes. 2009;58(5):1229–1236. doi: 10.2337/db08-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taube A, Schlich R, Sell H, Eckardt K, Eckel J. Inflammation and metabolic dysfunction: Links to cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2012;302(11):H2148–H2165. doi: 10.1152/ajpheart.00907.2011. [DOI] [PubMed] [Google Scholar]
- 99.Goldfine AB, Shoelson SE. Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk. J Clin Invest. 2017;127(1):83–93. doi: 10.1172/JCI88884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pollack RM, Donath MY, LeRoith D, Leibowitz G. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care. 2016;39(Suppl 2):S244–S252. doi: 10.2337/dcS15-3015. [DOI] [PubMed] [Google Scholar]
- 101.Han P, Gao D, Zhang W, Liu S, Yang S, Li X. Puerarin suppresses high glucose-induced mcp-1 expression via modulating histone methylation in cultured endothelial cells. Life Sciences. 2015;130:103–107. doi: 10.1016/j.lfs.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 102.Paneni F, Costantino S, Battista R, Castello L, Capretti G, Chiandotto S, et al. Adverse epigenetic signatures by histone methyltransferase set7 contribute to vascular dysfunction in patients with type 2 diabetes mellitus. Circ Cardiovasc Genet. 2015;8(1):150–158. doi: 10.1161/CIRCGENETICS.114.000671. [DOI] [PubMed] [Google Scholar]
- 103.Reddy MA, Villeneuve LM, Wang M, Lanting L, Natarajan R. Role of the lysine-specific demethylase 1 in the proinflammatory phenotype of vascular smooth muscle cells of diabetic mice. Circ Res. 2008;103(6):615–623. doi: 10.1161/CIRCRESAHA.108.175190. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Liao Y, Gou L, Chen L, Zhong X, Zhang D, Zhu H, et al. Nadph oxidase 4 and endothelial nitric oxide synthase contribute to endothelial dysfunction mediated by histone methylations in metabolic memory. Free Radical Biology and Medicine. 2018;115:383–394. doi: 10.1016/j.freeradbiomed.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 105.Villeneuve LM, Kato M, Reddy MA, Wang M, Lanting L, Natarajan R. Enhanced levels of microrna-125b in vascular smooth muscle cells of diabetic db/db mice lead to increased inflammatory gene expression by targeting the histone methyltransferase suv39h1. Diabetes. 2010;59(11):2904–2915. doi: 10.2337/db10-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R. Epigenetic histone h3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci U S A. 2008;105(26):9047–9052. doi: 10.1073/pnas.0803623105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Syreeni A, El-Osta A, Forsblom C, Sandholm N, Parkkonen M, Tarnow L, et al. Genetic examination of setd7 and suv39h1/h2 methyltransferases and the risk of diabetes complications in patients with type 1 diabetes. Diabetes. 2011;60(11):3073–3080. doi: 10.2337/db11-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen J, Zhang J, Yang J, Xu L, Hu Q, Xu C, et al. Histone demethylase kdm3a, a novel regulator of vascular smooth muscle cells, controls vascular neointimal hyperplasia in diabetic rats. Atherosclerosis. 2017;257:152–163. doi: 10.1016/j.atherosclerosis.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 109.Abe Y, Rozqie R, Matsumura Y, Kawamura T, Nakaki R, Tsurutani Y, et al. Jmjd1a is a signal-sensing scaffold that regulates acute chromatin dynamics via swi/snf association for thermogenesis. Nat Commun. 2015;6:7052. doi: 10.1038/ncomms8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wen R, Ruiz MA, Feng B, Chakrabarti S. Polycomb repressive complex 2 regulates mir-200b in retinal endothelial cells: Potential relevance in diabetic retinopathy. Plos One. 2015;10(4):e0123987. doi: 10.1371/journal.pone.0123987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Floris I, Descamps B, Vardeu A, Mitic T, Posadino AM, Shantikumar S, et al. Gestational diabetes mellitus impairs fetal endothelial cell functions through a mechanism involving microrna-101 and histone methyltransferase enhancer of zester homolog-2. Arterioscler Thromb Vasc Biol. 2015;35(3):664–674. doi: 10.1161/ATVBAHA.114.304730. [DOI] [PubMed] [Google Scholar]
- 112.Weng X, Zhang Y, Li Z, Yu L, Xu F, Fang M, et al. Class ii transactivator (ciita) mediates ifn-gamma induced enos repression by enlisting suv39h1. Biochim Biophys Acta Gene Regul Mech. 2019;1862(2):163–172. doi: 10.1016/j.bbagrm.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 113.Weng X, Yu L, Liang P, Chen D, Cheng X, Yang Y, et al. Endothelial mrtf-a mediates angiotensin ii induced cardiac hypertrophy. J Mol Cell Cardiol. 2015;80:23–33. doi: 10.1016/j.yjmcc.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 114.Weng X, Yu L, Liang P, Li L, Dai X, Zhou B, et al. A crosstalk between chromatin remodeling and histone h3k4 methyltransferase complexes in endothelial cells regulates angiotensin ii-induced cardiac hypertrophy. J Mol Cell Cardiol. 2015;82:48–58. doi: 10.1016/j.yjmcc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 115.Okabe J, Orlowski C, Balcerczyk A, Tikellis C, Thomas MC, Cooper ME, et al. Distinguishing hyperglycemic changes by set7 in vascular endothelial cells. Circ Res. 2012;110(8):1067–1076. doi: 10.1161/CIRCRESAHA.112.266171. [DOI] [PubMed] [Google Scholar]
- 116.Lee K, Na W, Lee JY, Na J, Cho H, Wu H, et al. Molecular mechanism of jmjd3-mediated interleukin-6 gene regulation in endothelial cells underlying spinal cord injury. J Neurochem. 2012;122(2):272–282. doi: 10.1111/j.1471-4159.2012.07786.x. [DOI] [PubMed] [Google Scholar]
- 117.Yu S, Chen X, Xiu M, He F, Xing J, Min D, et al. The regulation of jmjd3 upon the expression of nf-kappab downstream inflammatory genes in lps activated vascular endothelial cells. Biochem Biophys Res Commun. 2017;485(1):62–68. doi: 10.1016/j.bbrc.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 118.Barroso M, Kao D, Blom HJ. Tavares de Almeida I, Castro R, Loscalzo J et al. S-adenosylhomocysteine induces inflammation through nfkb: A possible role for ezh2 in endothelial cell activation. Biochim Biophys Acta. 2016;1862(1):82–92. doi: 10.1016/j.bbadis.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu D, Perkins JT, Petriello MC, Hennig B. Exposure to coplanar pcbs induces endothelial cell inflammation through epigenetic regulation of nf-kappab subunit p65. Toxicol Appl Pharmacol. 2015;289(3):457–465. doi: 10.1016/j.taap.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gu L, Hitzel J, Moll F, Kruse C, Malik RA, Preussner J, et al. The histone demethylase phf8 is essential for endothelial cell migration. PLoS One. 2016;11(1):e0146645. doi: 10.1371/journal.pone.0146645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wojtala M, Macierzynska-Piotrowska E, Rybaczek D, Pirola L, Balcerczyk A. Pharmacological and transcriptional inhibition of the g9a histone methyltransferase suppresses proliferation and modulates redox homeostasis in human microvascular endothelial cells. Pharmacol Res. 2018;128:252–263. doi: 10.1016/j.phrs.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 122.Diehl F, Rossig L, Zeiher AM, Dimmeler S, Urbich C. The histone methyltransferase mll is an upstream regulator of endothelial-cell sprout formation. Blood. 2007;109(4):1472–1478. doi: 10.1182/blood-2006-08-039651. [DOI] [PubMed] [Google Scholar]
- 123.Pirola Luciano, Ciesielski Oskar, Balcerczyk Aneta. The Methylation Status of the Epigenome: Its Emerging Role in the Regulation of Tumor Angiogenesis and Tumor Growth, and Potential for Drug Targeting. Cancers. 2018;10(8):268. doi: 10.3390/cancers10080268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Duan Y, Wu X, Zhao Q, Gao J, Huo D, Liu X, et al. Dot1l promotes angiogenesis through cooperative regulation of vegfr2 with ets-1. Oncotarget. 2016;7(43):69674–69687. doi: 10.18632/oncotarget.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Y, Liu J, Lin J, Zhou L, Song Y, Wei B, et al. The transcription factor gata1 and the histone methyltransferase set7 interact to promote vegf-mediated angiogenesis and tumor growth and predict clinical outcome of breast cancer. Oncotarget. 2016;7(9):9859–9875. doi: 10.18632/oncotarget.7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cohn O, Feldman M, Weil L, Kublanovsky M, Levy D. Chromatin associated setd3 negatively regulates vegf expression. Sci Rep. 2016;6:37115. doi: 10.1038/srep37115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen YT, Zhu F, Lin WR, Ying RB, Yang YP, Zeng LH. The novel ezh2 inhibitor, gsk126, suppresses cell migration and angiogenesis via down-regulating vegf-a. Cancer Chemother Pharmacol. 2016;77(4):757–765. doi: 10.1007/s00280-016-2990-1. [DOI] [PubMed] [Google Scholar]
- 128.Kunizaki M, Hamamoto R, Silva FP, Yamaguchi K, Nagayasu T, Shibuya M, et al. The lysine 831 of vascular endothelial growth factor receptor 1 is a novel target of methylation by smyd3. Cancer Res. 2007;67(22):10759–10765. doi: 10.1158/0008-5472.CAN-07-1132. [DOI] [PubMed] [Google Scholar]
- 129.Salton M, Voss TC, Misteli T. Identification by high-throughput imaging of the histone methyltransferase ehmt2 as an epigenetic regulator of vegfa alternative splicing. Nucleic Acids Res. 2014;42(22):13662–13673. doi: 10.1093/nar/gku1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee JY, Park JH, Choi HJ, Won HY, Joo HS, Shin DH, et al. Lsd1 demethylates hif1alpha to inhibit hydroxylation and ubiquitin-mediated degradation in tumor angiogenesis. Oncogene. 2017;36(39):5512–5521. doi: 10.1038/onc.2017.158. [DOI] [PubMed] [Google Scholar]
- 131.Oh SY, Seok JY, Choi YS, Lee SH, Bae JS, Lee YM. The histone methyltransferase inhibitor bix01294 inhibits hif-1alpha stability and angiogenesis. Mol Cells. 2015;38(6):528–534. doi: 10.14348/molcells.2015.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kashyap V, Ahmad S, Nilsson EM, Helczynski L, Kenna S, Persson JL, et al. The lysine specific demethylase-1 (lsd1/kdm1a) regulates vegf-a expression in prostate cancer. Mol Oncol. 2013;7(3):555–566. doi: 10.1016/j.molonc.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen J, Gu Y, Zhang H, Ning Y, Song N, Hu J, et al. Amelioration of uremic toxin indoxyl sulfate-induced osteoblastic calcification by set domain containing lysine methyltransferase 7/9 protein. Nephron. 2019;141(4):287–294. doi: 10.1159/000495885. [DOI] [PubMed] [Google Scholar]
- 134.Choi JY, Yoon SS, Kim SE, Ahn JS. Kdm4b histone demethylase and g9a regulate expression of vascular adhesion proteins in cerebral microvessels. Sci Rep. 2017;7:45005. doi: 10.1038/srep45005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang J, Ge H, Poulton CJ, Hogan SL, Hu Y, Jones BE, et al. Histone modification signature at myeloperoxidase and proteinase 3 in patients with anti-neutrophil cytoplasmic autoantibody-associated vasculitis. Clin Epigenetics. 2016;8:85. doi: 10.1186/s13148-016-0251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Karnewar S, Neeli PK, Panuganti D, Kotagiri S, Mallappa S, Jain N, et al. Metformin regulates mitochondrial biogenesis and senescence through ampk mediated h3k79 methylation: relevance in age-associated vascular dysfunction. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 Pt A):1115–1128. doi: 10.1016/j.bbadis.2018.01.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.