Abstract
Next-generation sequencing has enabled patient selection for targeted drugs, some of which have shown remarkable efficacy in cancers that have the cognate molecular signatures. Intriguingly, rapidly emerging data indicate that altered genes representing oncogenic drivers can also be found in sporadic non-malignant conditions, some of which have negligible and/or low potential for transformation to cancer. For instance, activating KRAS mutations are discerned in endometriosis and in brain arteriovenous malformations, inactivating TP53 tumor suppressor mutations in rheumatoid arthritis synovium, and AKT, MAPK, and AMPK pathway gene alterations in the brains of Alzheimer’s disease patients. Furthermore, these types of alterations may also characterize hereditary conditions that result in diverse disabilities and that are associated with a range of lifetime susceptibility to the development of cancer, varying from near universal to no elevated risk. Very recently, the repurposing of targeted cancer drugs for non-malignant conditions that are associated with these genomic alterations has yielded therapeutic successes. For instance, the phenotypic manifestations of CLOVES syndrome, which is characterized by tissue overgrowth and complex vascular anomalies that result from the activation of PIK3CA mutations, can be ameliorated by the PIK3CA inhibitor alpelisib, which was developed and approved for breast cancer. In this review, we discuss the profound implications of finding molecular alterations in non-malignant conditions that are indistinguishable from those driving cancers, with respect to our understanding of the genomic basis of medicine, the potential confounding effects in early cancer detection that relies on sensitive blood tests for oncogenic mutations, and the possibility of reverse repurposing drugs that are used in oncology in order to ameliorate non-malignant illnesses and/or to prevent the emergence of cancer.
Background
In recent years, the rate of development of small molecule and antibody drugs that effectively target oncogenic drivers has increased rapidly [1, 2]. The natural question that emerges is whether or not targeting these genomic alterations in non-malignant illness could also have salutary effects, as there are (i) benign conditions (including but not limited to seborrheic keratosis, endometriosis, arteriovenous malformations in the brain, and Alzheimer’s disease) that arise sporadically and that harbor somatic mutations that are believed to be drivers in cancer (Table 1), and (ii) germline and hereditary phenotypes and somatic mosaic phenotypes that are associated with such mutations (e.g., achondroplasia, neurofibromatosis, CLOVES syndrome, and Proteus syndrome) (Table 2). The benign disorders that harbor putative “oncogenic drivers” have a variable propensity for malignant transformation and, in the case of hereditary conditions that are caused by such mutations, patients have differing vulnerabilities for the development of malignancy, ranging from minimal or no increased risk to a very high lifetime susceptibility to cancer.
Table 1.
Examples of sporadic benign conditions, many with negligible potential for malignant transformation, associated with somatic alterations in driver cancer genes
| Gene | Type of alteration | Benign or premalignant condition | Frequency of alteration in benign condition (%) | Examples of drug(s) that can potentially target the alteration | Examples of malignancies associated with this gene alteration | Mechanism |
|---|---|---|---|---|---|---|
| BRAF | V600E, D594V, V599E | Melanocytic nevi | 70–88% [3–12] | BRAF and/or MEK inhibitors such as dabrafenib and trametanib [13, 14] | Melanoma | RAS-RAF-MEK-ERK pathway upregulation [15] |
| NRAS | Q61K | Giant congenital melanocytic nevi | 6–14% [10, 11] | MEK inhibitors [12] such as trametinib [16] | Melanoma | RAS-RAF-MEK-ERK pathway upregulation [15] |
| Q61K and Q61R | Melanocytic nevi | 70–95% [17, 18] | MEK inhibitors such as trametinib [16] | Melanoma | RAS-RAF-MEK-ERK pathway upregulation [15] | |
| FGFR3 | R248C, S249C, G372C, S373C, A393E, K652E, K652M | Seborrheic keratosis | ∼ 18–85% [19–22] | FGFR inhibitors such as erdafitinib [23] | Urothelial carcinoma | Activation of the FGF/FGFR machinery [24] |
| R248C, G372C, G382R | Epidermal nevi | 33% [25] | FGFR inhibitors such as erdafitinib [23] | Urothelial carcinoma | Activation of the FGF/FGFR machinery [24] | |
| PIK3CA | E542K, E545K, H1047R | Seborrheic keratosis | ∼ 16% [20] | PIK3CA inhibitors such as alpelisib [26] | Breast cancer | PI3K-AKT-mTOR pathway activation |
| M1043V | Endometriosis | ~ 4% [27] | PIK3CA inhibitors such as alpelisib [26] | Breast cancer | PI3K-AKT-mTOR pathway activation | |
| H1047L, H1047R | Normal esophagus mucosa | Not listed [28] | PIK3CA inhibitors such as alpelisib [26] | Breast cancer | PI3K-AKT-mTOR pathway activation | |
| ALK | TPM3-ALK, TPM4-ALK | Inflammatory myofibroblastic tumor | ∼ 50% [29] | ALK inhibitors [30] such as alectinib [31] | Non-small cell lung cancer | ALK pathway activation [32] |
| NOTCH1 | Loci not specified | Aging esophagus | 12–80% [33] | No specific inhibitors approved | Colon cancer | Wnt-beta-catenin pathway activation [34] |
| KRAS | G12V or G12D | Arteriovenous malformations in brain | ∼ 63% [35, 36] | MEK inhibitors such as trametinib [16] | Colorectal and pancreatic cancer | RAS-RAF-MEK-ERK pathway upregulation [15] |
| G12C, G12V, G12A, G12D, G12R | Endometriosis | ~ 21% [27] | MEK inhibitors such as trametinib [16] | Colorectal and pancreatic cancer | RAS-RAF-MEK-ERK pathway upregulation [15] | |
| Q61R | Normal testis | Not listed [28] | MEK inhibitors such as trametinib [16] | Colorectal and pancreatic cancer | RAS-RAF-MEK-ERK pathway upregulation [15] | |
| TP53 | R177S, Q192L, R196*, K139R, H193Y, E224fs, N239S | Rheumatoid arthritis synovium | 17–46% [37, 38] | Bevacizumab may target angiogenesis upregulation that results from TP53 mutations [39] | Serous ovarian cancer (TP53 mutations are common across cancers) | TP53 is a tumor suppressor gene [40] |
| Loci not specified | Aging esophagus | 2–37% [33] | Bevacizumab may target angiogenesis upregulation that results from TP53 mutations [39] | Serous ovarian cancer (TP53 mutations are common across cancers) | TP53 is a tumor suppressor gene [40] | |
| CTNNB1 | T41A and S45P | Desmoid tumor | 88% [41] | COX-2 inhibitors [42] such as celecoxib [43], as well as sorafenib (which can suppress CTNNB1-mediated activation of the WNT pathway) [13, 14, 44] | Adrenocortical cancers | Wnt-beta-catenin pathway activation [45] |
| FGFR2 | Y376C, P286S | Keratinocytic epidermal nevus | 5–10% [46] | FGFR inhibitors such as erdafitinib [23] | Urothelial carcinoma | FGF/FGFR machinery [24] |
| AKT, MAPK, and AMPK pathway genes | – | Alzheimer’s disease | ~ 27% [47] | mTOR inhibitors or MEK inhibitors | Multiple tumor types | Increases tau phosphorylation |
Table 2.
Examples of hereditary germline syndromes and of somatic mosaicism associated with examples of alterations in cancer-driver genes, their relationship with cancer in affected patients, and targeted drugs that might be useful
| Gene | Alteration | Syndrome | Descriptions | Increased incidence of cancer (if yes, most common cancers) | Treatment potentially/theoretically targeting the alteration |
|---|---|---|---|---|---|
| APC | Most common nonsense changes are C>T mutations [48] | Familial adenomatous polyposis [49] | Multiple non-cancerous (benign) growths (polyps) in the colon with strong predisposition to cancer | Yes (colorectal [49, 50]) | Sorafenib and WNT inhibitors [13, 44] |
| ARAF | S214P [51] | Central conducting lymphatic anomaly [52] | Not listed | None found | mTOR inhibitors such as sirolimus [53] or MEK inhibitors such as trametinib [51] |
| BRAF | Q257R, S467A, G596V, V600G | Cardiofaciocutaneous syndrome [54] | Cardiac abnormalities, distinctive craniofacial appearance, and cutaneous abnormalities | Yes (juvenile myelomonocytic leukemia, brain tumors, acute lymphoblastic leukemia, rhabdomyosarcoma, and neuroblastoma [55]) | BRAF inhibitors [9] and/or MEK inhibitors such as dabrafenib [5] and cobimetinib [7] |
| G469E, F595L, L597V | Noonan syndrome [56, 57] | Unusual facial features, short stature, heart defects, bleeding problems, and skeletal malformations | Yes (juvenile myelomonocytic leukemia, brain tumor, acute lymphoblastic leukemia, rhabdomyosarcoma, and neuroblastoma [55]) | – | |
| ERBB4 | R927Q, R1275W | Amyotrophic lateral sclerosis subtype 19 [58] | Degeneration of motor neurons and anterior horns of spinal cord | None found | Pan-ERBB inhibitors such as neratinib [59] will not be effective because the mutations have an inactivating effect |
| FGFR1 | L165S, L191S | Hartsfield syndrome [60] | Holoprosencephaly, ectrodactyly, and cleft lip/palate | None found | These FGFR1 mutations may cause loss of function, so FGFR inhibitors such as erdafitinib [23] will not be effective |
| Multiple loss of function mutations | Kallman syndrome [61] | Hypogonadotropic hypogonadism and impaired sense of smell | None found | – | |
| P252R | Pfeiffer syndrome [62] | Premature fusion of certain skull bones | None found | Gain-of-function alterations and hence may be targeted by FGFR inhibitors such as erdafitinib [23] | |
| FGFR2 | S252W or P253R | Apert syndrome [63] | Premature fusion of certain skull bones (craniosynostosis*) and syndactyly | Hepatoblastoma [64]* | Mutations are gain of function and hence may be targeted by FGFR inhibitors such as erdafitinib [23] |
| Y375C or S372C | Beare-Stevenson cutis gyrata syndrome [65] | Premature fusion of certain skull bones (craniosynostosis*) | Hepatoblastoma [64]* | – | |
| S351C | Pfeiffer syndrome [62] | Premature fusion of certain skull bones (craniosynostosis*) | Hepatoblastoma [64]* | – | |
| FGFR3 | G380R; R248C, G372C, G382R | Achondroplasia [66] | Short-limbed dwarfism | None found | Mutations are gain of function and hence may be targeted by FGFR inhibitors such as erdafitinib [23] |
| N540K | Hypochondroplasia [67] | Short-limbed dwarfism that is milder than achondroplasia | None found | – | |
| D513N | Lacrimo-auriculo-dento-digital syndrome [68] | Abnormal tear production, malformed ears with hearing loss, decreased saliva production, small teeth, and hand deformities | None found | – | |
| P250R | Muenke syndrome [69] | Craniosynostosis*, hearing loss, subtle hand and foot abnormalities, and developmental delay | Hepatoblastoma [64]* | – | |
| R248C, K650E, S249C, Y373C | Thanatophoric dysplasia [70] | Extremely short limbs and folds of extra (redundant) skin on the arms and legs | None found | FGFR3 inhibitor in mice [71] | |
| GNAS | R201C, R201H, Q227L | McCune-Albright syndrome [72] | Abnormal scar-like (fibrous) tissue in their bones, a condition called polyostotic fibrous dysplasia | Yes (breast, thyroid, testicular [73]) | MEK inhibitors [74] such as trametinib [75] |
| HRAS | G12S, G12C | Costello syndrome | Delayed development/intellectual disability, loose folds of skin, unusually flexible joints, and distinctive facial features including a large mouth, heart problems | Yes (juvenile myelomonocytic leukemia, brain tumor, acute lymphoblastic leukemia, rhabdomyosarcoma, and neuroblastoma [55]) | MEK inhibitors [76] such as trametinib [75] |
| IDH2 | R140Q | D-2-hydroxyglutaric aciduria [77] | Delayed development, seizures, weak muscle tone (hypotonia), and abnormalities in the cerebrum | Yes (high-grade glioma [78]) | IDH2 inhibitors such as enasidenib [79] |
| JAK3 | R651W, V599G, W709R | Severe combined immunodeficiency [80] | Lack the necessary immune cells to fight bacteria, viruses, and fungi | None found | Mutations cause loss of function and hence JAK inhibitors such as tofacitinib [81] will not be effective |
| KRAS | P34R | Cardiofaciocutaneous syndrome [54, 82] | Distinctive craniofacial appearance, and cutaneous abnormalities (including but not limited to xerosis, hyperkeratosis, pigmented moles, hemangiomas) | Yes (juvenile myelomonocytic leukemia, brain tumor, acute lymphoblastic leukemia, rhabdomyosarcoma, and neuroblastoma [55]) | MEK inhibitors [83] such as trametinib [75] |
| MET | F841V | DFNB97 hearing loss [84] | Non-syndromic sensorineural hearing loss with prelingual onset | None found | The mutation is damaging, so MET inhibitors such as cabozantinib [85] should not be effective |
| NOTCH1 | C1496Y, D1989N | Adams-Oliver syndrome [86] | Congenital aplasia cutis and malformations of the limbs | None found | Loss-of-function mutations so Notch inhibitors such as LY3039478 [87] will be ineffective |
| NF1 | R304X, Y2264X, R1825W, R1809C, N1229S, D176E | Neurofibromatosis type 1 [88] | Changes in skin coloring (pigmentation) and the growth of benign neoplasms along nerves in the skin, brain, and other parts of the body [89] | Yes (malignant peripheral nerve sheath tumors, optic gliomas, brain tumors, breast cancer [90]) | MEK inhibitors [91] such as trametinib [75] or selumetinib [92] |
| NF2 | L46R, L141P, A211D, K413E, Q324L, and L535P | Neurofibromatosis type 2 [93] | Growth of benign neoplasms in the nervous system; vestibular schwannomas or acoustic neuromas | None found | mTOR inhibitors [94] such as sirolimus [53] |
| RET | P155L, T278A, T278P, D300N, S316I, C620R | Hirschsprung disease [95] | Absence of nerves in distal colon | Yes (medullary thyroid [96, 97]) | Mutations generally cause loss of function, so RET inhibitors such as LOXO-292 [98] or cabozantinib [83] would be ineffective; RET C620R may cause both gain and loss of functions |
| STK11 | 40 different somatic STK11 mutations [99] | Peutz-Jegher syndrome | Gastrointestinal hamartomatous polyps and hyperpigmentation of the lips, buccal mucosa, digits | Yes (gastrointestinal tract, pancreas, cervix, ovary, and breast [100]) | mTOR inhibitors such as everolimus [101] |
| TP53 | Multiple loss of function mutations | Li-Fraumeni [102–105] | Greatly increases the risk of several cancers | Yes (sarcoma, breast, brain, adrenocortical [102]) | Bevacizumab may target angiogenesis associated with TP53 mutations [39] |
| Somatic mosaicism | |||||
| AKT1 | E17K (gain of function) | Proteus syndrome [106] | Overgrowth of the bones, skin, and other tissues | Yes (meningiomas, ovarian cystadenomas, breast cancer, parotid monomorphic adenoma, mesothelioma [107]) | AKT inhibitors such as ipatasertib [108] |
| GNAQ | R183Q | Sturge-Weber syndrome [109] | Port-wine stains affecting the skin, leptomeningeal vascular malformations | None found | Some MEK inhibitors may have activity |
| PIK3CA | E545K | Hemimegalencephaly [110] | Rare neurological condition in which one-half of the brain, or one side of the brain, is abnormally larger than the other | None found | PIK3CA inhibitors such as alpelisib [24] |
| H1047R, C420R, Q542K | CLOVES syndrome [111] | Tissue overgrowth and complex vascular anomalies; CLOVES stands for congenital lipomatous (fatty) overgrowth, vascular malformations, epidermal nevi and scoliosis/skeletal/spinal anomalies | Yes (Wilms tumor [112]) | PIK3CA inhibitors such as alpelisib [26, 113] | |
| H1047R and H1047L | Fibroadipose hyperplasia [114] | Patchy overgrowth of a limb or part/region of the body | None found | PIK3CA or mTOR inhibitors [115] such as alpelisib [26] or everolimus [101] | |
*A recent publication [64] shows that craniosynososis may be associated with increased incidence of hepatoblastoma, although the authors did not define which syndromes were affected
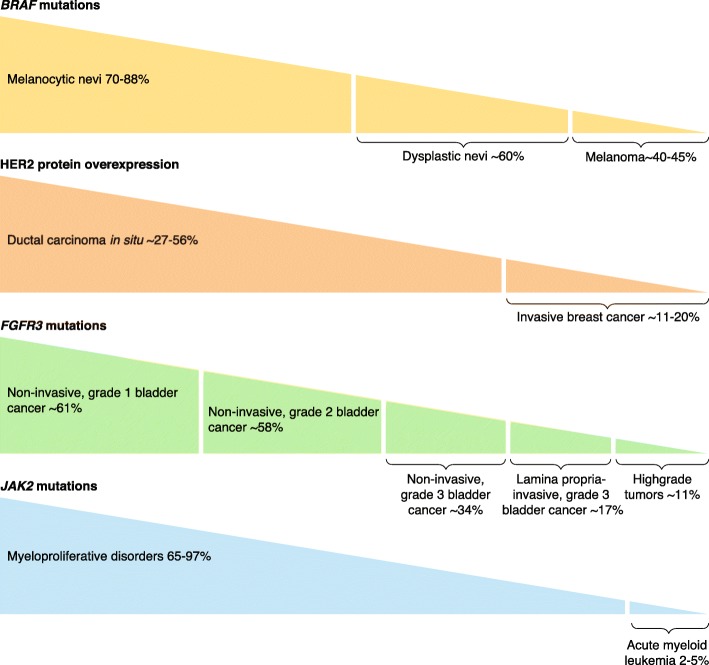
Interestingly, there is also growing evidence that the canonical theory of renegade clonal expansion in carcinogenesis [116] may not be the only manner in which malignant development proceeds. The theory of clonal expansion posits that clones of cells harboring oncogenic drivers will be selected during the development of malignancy because these driver(s) confer a growth advantage. Hence, the percentage of cells with the oncogenic driver(s) will be smaller in premalignant lesions than in lesions that are malignant. However, the opposite is sometimes found (Fig. 1). For instance, BRAF V600E driver mutations are discerned at twice the frequency in benign nevi, which do not transform to melanoma, than in melanoma itself [3, 4, 117]. This paradoxical phenomenon has also been reported in the continuum from benign to malignant in other diseases (Fig. 1).
Fig. 1.
Examples of reverse clonal selection. Aberrant cancer drivers that are paradoxically more frequent in benign or premalignant counterparts than they are in the malignant condition. BRAF mutations included V600E [3, 4, 8, 117–120] and HER2 overexpression [121–123]. FGFR3 mutations included R248C, S249C, and G372C [124–126]. JAK2 mutations included V617F [127–129]. % given is the percentage of cases in which there are alterations (e.g., 70–88% of melanocytic nevi have BRAF mutations)
There are several important consequences of “oncogenic drivers” in benign conditions. First, there are the implications for early detection of cancer based on sensitive blood tests that assess circulating cell-free DNA (cfDNA) [130–132]. If mutations identical to those found in cancer also occur in conditions with no malignant predisposition, their presence may confound the early diagnosis of cancer premise that is the basis of these blood-based screening tests, such as the multi-cancer detection blood test developed by GRAIL that has been granted breakthrough status by the US Food and Drug Administration [133].
Furthermore, as mutations that are indistinguishable from those in cancer exist in benign conditions, and as drugs are available that effectively neutralize the impact of these mutations in cancer, it is plausible that these drugs could be repurposed for illnesses other than cancer. Indeed, several such examples have been established in preclinical models and in patients. For instance, it has recently been demonstrated that increased expression of PARP1, a well-known anti-apoptotic cancer target, plays a role in neuronal cell death in Parkinson’s disease. Consequently, it has been suggested that PARP inhibitors, which have successfully been used to treat BRCA-mutated cancers [134–137], merit examination as candidate drugs in Parkinson’s disease [138]. In BRCA-mutated tumors, repair of double-stranded DNA breaks is deficient. PARP1 is a protein that is important for repairing single-strand breaks; and the suppression of PARP catalytic activity by PARP inhibitors further compromises DNA repair, resulting in tumor cell lethality. In Parkinson’s disease, PARP is elevated and causes alpha-synuclein spread, triggering cell death and Parkinson’s symptoms; theoretically, PARP inhibitors could reverse this process.
Another example in which a drug developed for cancer can be used in a non-cancer condition is provided by CLOVES syndrome, which is caused by mutations in PIK3CA. Patients with CLOVES syndrome, which manifests as congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and scoliosis/skeletal and spinal anomalies, also have a propensity to Wilms tumors [112]. CLOVES syndrome can be treated with the PIK3CA inhibitor alpelisib, which was developed for PIK3CA-mutant breast cancer [113].
In this review, we provide an overview of and update on the rapidly expanding knowledge regarding the conundrum of oncogenic drivers in benign disorders, and we discuss the profound implications of these findings for the treatment of both benign and malignant conditions [139]. First, the ubiquitous finding of oncogenic drivers in non-malignant conditions may prove problematic for the development of sensitive blood tests for early detection of cancer. Second, non-malignant conditions that are caused by actionable oncogenic drivers could potentially be treated with repurposed drugs that have been successfully developed to target and manage cancers harboring those drivers. Examples of such effective repurposing already exist, suggesting that the molecular alterations found in benign disease are indeed drivers of benign disease (as they are in cancer) and not “uninvolved bystanders.” Such strategies are especially important because some of the benign conditions in which these mutations have been found are rare or ultra-rare and present a huge unmet therapeutic need. Importantly, some non-malignant conditions are associated with an increased risk of malignant transformation because of their underlying oncogenic driver. In such conditions, an approach aimed at deploying anti-cancer drugs to target molecular alterations in benign disease might also be exploitable to prevent cancers.
Sporadic benign conditions associated with alterations in “driver” cancer genes
With the advances in next-generation sequencing (NGS) and the resulting identification of driver mutations for various cancers, there has been growing interest in the phenomenon in which well-known cancer-causing genes are altered in benign conditions, some of which have either no (or very limited) potential for malignant transformation (Table 1): (i) FGFR3 activating mutations are well documented to play a major role in the pathogenesis of bladder cancer [124–126], yet they are also found in unrelated conditions such as seborrheic keratosis and epidermal nevi [19–22]; (ii) mutations in the TP53 tumor suppressor gene, which are perhaps the most common alterations in cancer [140], also characterize the synovium of rheumatoid arthritis [37, 38]; (iii) KRAS mutations are found in arteriovenous malformations [35, 36, 141] and in endometriosis [27] (though their functional role is still unclear in these conditions); and (iv) brain somatic mutations in Alzheimer’s disease, in which about 27% of patients (14 of 52) have alterations in genes of the PI3K-AKT, MAPK, and AMPK pathways, are known to contribute to hyper-phosphorylation of tau [47]. Importantly, some of the loci that are mutated in each of these cases do not differ from the loci that are mutated and implicated in cancer. The mechanism by which such mutations cause these benign conditions but fail to cause cancer is unclear, but we hypothesize that aberrant tissue growth that is associated with FGFR3 mutations is dependent on the tissue- or cell-type context of these mutations; when they are found in the epidermis, benign seborrheic keratosis develops [19–22], whereas when they appear in the bladder, cancer develops [124–126]. A similar mechanism could be posited for KRAS mutations and arteriovenous malformations. In the case of TP53 mutations, which are clearly related to the formation of multiple cancers [140], perhaps they induce the inflammatory condition rheumatoid arthritis, rather than cancer, in the synovium [37, 38]. Arthritis might ensue because TP53 mutations upregulate levels of the inflammatory cytokine interleukin-6 (IL-6), a known pathogenic factor in rheumatoid arthritis [142, 143].
An example that defies the tissue- or histology-context hypothesis is BRAF V600E, a known oncogenic driver that occurs in around 80% of benign nevi [3, 4]. These nevi are extremely common and are estimated to have a transformation-to-melanoma rate of less than 0.03% for melanocytic nevi [144] and only about 4.8% for dysplastic nevi [145]. Yet, in the setting of melanoma or other tumors, there can be no doubt regarding the oncogenic role of BRAF V600E mutations, based on preclinical modeling [146] and also on the tumor regression that results from the use of antagonists such as BRAF and MEK inhibitors [5, 75]. Explanations for the lack of pathogenicity of BRAF V600E in benign nevi include, but are not limited to the following: (i) RNA silencing, a mechanism whereby deleterious DNA alterations are not expressed at the RNA level [147]; or (ii) the possibility that a “double hit” [148], a concomitant loss of an inhibitor or the activity of a genomic co-factor [149], is necessary in order to initiate carcinogenesis. Another possible mechanism by which oncogenic mutants can exist in normal tissue but not cause cancer is illustrated by a study that showed that normal human esophagus contains TP53-mutant progenitors. Yet, TP53-mutant cells can be displaced from normal tissues through the improvement of the competitive fitness of wild-type progenitors by antioxidants [150].
Both normal aging and specific environmental exposures can also be associated with somatic oncogenic mutations. For instance, in natural aging of the esophagus and in rapidly proliferating tissues such as those in the testes, mutations in known oncogenes such as NOTCH1 [33], PIK3CA [28], TP53 [33], and KRAS [28] may appear. Indeed, in middle-aged and elderly persons, it was found that cell clones containing cancer-associated mutations covered much of the esophageal epithelium, with NOTCH1 and TP53 mutations affecting 12–80% and 2–37% of cells, respectively [33]. The progressive age-related expansion of clones that carry mutations in driver genes in the esophagus can be accelerated substantially by smoking and by alcohol consumption. Although the remodeling of the esophageal epithelium by driver-mutated clones is an inevitable part of normal aging, lifestyle risks may also affect cancer development [151]. Somatic mutations also emerge in skin that is exposed to ultraviolet light [152]. Indeed, aged, sun-exposed skin is a patchwork of thousands of evolving clones with over 25% of cells harboring cancer-causing mutations while preserving epidermal functions. Similarly, age-associated clonal hematopoiesis, which is caused by acquired mutations in myeloid cancer-associated genes such as DNMT3A or TET2, is highly prevalent in the normal population. Its biological impact on hematopoiesis, etiology, and oncogenic risk is poorly delineated at this time [153–156]. Finally, probable driver mutations have been reported in around 1% of normal colorectal crypts in middle-aged adults, indicating that carcinomas are rare outcomes despite a pervasive process of neoplastic change in morphologically normal colorectal tissue [157]. The degree to which the size of the mutant clones influences risk of malignant progression warrants further exploration [158].
A critical question as regards mutations that arise with aging, or as a result of exposure to smoking or other noxious environmental factors, relates to the mechanisms that promote or prevent cancer development. Immune surveillance may play an important role in explaining the presence of oncogenic drivers in benign conditions without progression to malignancy. It could be postulated that natural immune mechanisms may eradicate cells that present neo-antigens derived from these mutations. Failure of this immune surveillance might result in cancer. Indeed, findings in both mouse models of cancer and humans with cancer offer compelling evidence that immune cell types and effector pathways collectively function as potent tumor suppressor mechanisms [159, 160]. Furthermore, it has been shown that the ability of various individuals’ major histocompatibility complexes to present neo-antigens that are produced by the mutanome shapes the mutational landscape in cancers and may predict each patient’s susceptibility to specific tumors [161].
In summary, oncogenic drivers are found in a range of benign conditions as well as in normal tissues, especially with aging. Their limited transformation potential or failure to induce cancers consistently [157] can be hypothesized to be due to several reasons including, but not limited to, tissue and cellular context, a need for genomic driver co-factors or for co-loss of genomic suppressors, the suppressive or competitive growth of progenitors with normal molecular landscapes, the size of the mutant clones, and immune surveillance.
Hereditary conditions that result from germline cancer-related genes have a range of malignant potential
Cancer-associated genes can be altered at the germline level, and yet individuals with these genes may have a wide spectrum of cancer risk, from no increased risk to very high risk (Table 2). It is unclear as to why there is a range of cancer susceptibility, but this range could be related to immune surveillance mechanisms [161]. As an example, patients with “RASopathies” (a group of rare genetic conditions such as cardiofaciocutaneous syndrome and Costello syndrome caused by mutations in genes of the RAS-RAF-MAPK pathway) have an increased risk of juvenile myelomonocytic leukemia, brain tumors, acute lymphoblastic leukemia, rhabdomyosarcoma, and neuroblastoma [55]. These patients do not, however, have increased risk of classic BRAF-mutated melanoma, although ~ 75% of the cardiofaciocutaneous syndromes result from germline BRAF mutations [162], and pigmented nevi are very distinct in this syndrome and help to define it [163].
In other familial syndromes, such as Von Hippel-Lindau, patients harbor a VHL mutation, which has been best defined in clear cell renal cell carcinoma, and subsequently are at significant risk of developing renal cancers [164]. Li-Fraumeni syndrome is another example of a hereditary cancer syndrome in which TP53 mutations predispose patients to cancers of the breast, brain, or adrenocortical organ, or to sarcomas [102]. Further, the APC gene mutation is a well-defined and known cause of familial adenomatous polyposis, and afflicted individuals are at significant risk of developing colorectal carcinoma [49, 50].
On the other hand, there are hereditary conditions caused by “oncogenic driver mutations” that have no clear association with increased cancer risk (although large-scale studies of these diseases are not fully developed and it is conceivable that, with time, some increased cancer risk might be identified). Examples include achondroplasia, hypochondroplasia, lacrimo-auriculo-dento-digital syndrome, and thanatophoric dysplasiam, each of which is attributed to germline FGFR3 mutations that result in their varied phenotypes (Table 2). Patients with neurofibromatosis type 2 also seem to have no clear association with an increased cancer risk [93].
In summary, germline oncogenic mutations are associated with a variety of aberrant phenotypes and a wide spectrum of increased cancer risk (ranging from negligible to very high). The reasons for the variance in vulnerability to malignancies are unclear but could involve the immune machinery [159–161, 165]. It is also possible that heterozygosity may, in some cases, play an antagonistic role in tumor initiation and malignant transformation (even while accelerating the formation of benign neoplasms), as shown for NF1 [166]. Patients who carry some of these germline oncogenic alterations need to be monitored, often throughout their lifespan, for specific cancers on the basis of their diagnosis and the known propensity to malignancy, with cancer risk being determined by epidemiologic studies.
Somatic mosaic conditions that are associated with oncogenic drivers but without clear increased cancer risk
Somatic mosaicism is defined by the occurrence of two genetically distinct populations of cells within an individual, derived from a postzygotic mutation [167]. Unlike inherited mutations, somatic mosaic mutations may affect only a portion or a tissue of the body and are not transmitted to offspring. The phenotypic consequences of somatic mosaicism are dependent upon the biologic impact of the mutation, as well as on the developmental time at which the mutation occurs and the areas of the body that are affected [168].
Several somatic mosaic conditions are associated with gene abnormalities identical to those in cancer but result in a phenotypic presentation other than cancer (Tables 2 and 3). Sturge-Weber syndrome is a neurocutaneous vascular malformation syndrome, characterized by a facial port-wine birthmark, which is associated with choroid “angioma” of the eye and malformed leptomeningeal blood vessels, as well as with seizures, strokes, stroke-like episodes, and neurologic deficits, beginning in infancy [109]. It is caused by a somatic (not heritable) mosaic mutation in GNAQ. This activating mutation in GNAQ (R183Q) results in constitutive overactivation of the Ras-Raf-MEK-ERK pathway and is identical to the GNAQ alteration implicated in uveal melanoma [173, 174]. It has been hypothesized that the occurrence of the GNAQ mutation at a different time in development (in the fetal period or in infancy rather than in adulthood) accounts for its resulting in a vascular malformation rather than a cancer [175].
Table 3.
Examples of sporadic and hereditary conditions and of somatic mosaic non-malignant conditions that have been treated successfully in animal models or in patients by targeting underlying “oncogenic” drivers using drugs, some of which were developed for cancer
| Condition | Underlying molecular defect | Therapy | Result of therapy | Comments | FDA-approved drug: cancers treated |
|---|---|---|---|---|---|
| Sporadic conditions | |||||
| Rheumatoid arthritis | TP53 mutations | Tocilizumab, which is an anti-IL-6 receptor antibody | Decreased incidence of flares, better disease control [169] | Efficacy in humans; TP53 mutations are known to increase IL-6, which mediates inflammation [142] | None |
| Desmoid tumors | CTNNB1 mutations | COX-2 inhibitors and sorafenib | Tumor regression [5, 145, 146] | Efficacy in humans; COX-2 inhibitors and sorafenib can abrogate the activation of the WNT pathway by CTNNB1 alterations [13, 41, 42] |
COX-2 inhibitors: none Sorafenib: renal cell carcinoma, hepatocellular carcinoma |
| Inflammatory myofibroblastic tumors | ALK rearrangements | Crizotinib | Sustained objective responses [30] | Efficacy in humans; crizotinib is a potent ALK inhibitor | Non-small cell lung cancer |
| Schnitzler syndrome | MYD88 L265P mutation | Anakinra, which is an IL-1 antagonist | Complete remission of disease [170] | Efficacy of anankinra in humans | None |
| Neurofibromatosis 1 | NF1 mutations | MEK inhibitor selumetinib | 71% partial response rate for inoperable plexiform neurofibromas [92] | FDA granted breakthrough status for selumetinib for NF1 in 2019 | None |
| Hereditary and somatic mosaic conditions | |||||
| CLOVES syndrome | Mosaic gain-of-function PIK3CA alterations | Alpelisib, which is PIK3CA inhibitor | Improved disease-related symptoms [113] | Efficacy in humans | Hormone-positive, HER2-negative breast cancer |
| Central conducting lymphatic anomaly | Gain-of-function ARAF mutations (MEK or mTOR pathway) | Sirolimus (mTOR inhibitor) or trametinib (MEK inhibitor) |
Resolution of chylous output over the course of a week with removal of chest tube with sirolimus (n = 1) [53] Dramatic clinical improvement, with remodeling of the patient’s lymphatic system and resolution of the lymphatic edema, marked improvement in pulmonary function tests, cessation of supplemental oxygen requirements and near normalization of daily activities with trametinib (n = 1) [51] |
Efficacy in humans |
Sirolimus: none Trametinib: melanoma |
| Fibroadipose hyperplasia | PIK3CA mutations | Sirolimus (mTOR inhibitor) | Stabilization or improvement in disease in patients [115, 171] | Efficacy in humans | None |
| Achondroplasia | FGFR3 mutations | FGFR3 inhibitor in mouse models | Restored size of embryonic achrondroplastic femurs in animals [172] | Animal model efficacy | None |
Fibroadipose hyperplasia is characterized by patchy overgrowth of a limb or of a part or region of the body. It is associated with PIK3CA H1047R mutations, which are implicated in multiple cancers [114, 115, 171]; yet, this condition is not known to associate with cancer, although further longitudinal studies are necessary. Hemimegalencephaly, a condition in which one side of the brain is larger than the other, is also attributed to an activating PIK3CA E545K that is indistinguishable from the alteration observed in several types of malignant neoplasms, but there is no clear cancer risk in hemimegalencephaly [176, 177].
In summary, as for conditions that are associated with germline mutations, conditions caused by somatic mosaic mutations may be associated with aberrant tissue growth and with a range of cancer risks (Table 2). Cancer risk may relate to the actual mutation involved, tissues affected and developmental period, and to other poorly studied factors such as immune function. Because these conditions are very rare, it is conceivable that more in-depth investigations of them will reveal some increased cancer risks, even in those conditions that are currently not believed to carry such a risk. Epidemiological surveys are needed in order to define cancer risk in these disorders fully. However, such studies may be challenging because of the rarity of the disorders. Finally, for patients who have elevated cancer risk, lifetime monitoring for the specific cancers that are most likely to occur is needed.
The paradox of reverse clonal evolution and selection
The classic theory of clonal evolution and selection posits that driver alterations cause cancer progression from benign to premalignant lesions and then to invasive malignancy (Fig. 1). Indeed, cancers are believed to evolve by a reiterative process of clonal expansion, genetic diversification, and clonal selection within the adaptive backgrounds of tissue bionetworks [178]. Clonal evolution involves the interplay of advantageous or “driver” alterations that give a cancer cell a fundamental growth advantage, genomic alterations that enhance the rate of other DNA changes by creating genomic instability (“mutator” genes), neutral or “passenger” (hitchhiker) gene alterations that do not directly determine cancer development, and modifications to the tumor habitat that refashion the fitness effects of each of these abnormalities [179–181]. The dynamics are complex, with highly variable configurations of genetic diversity and ensuing clonal architecture. Further, evolutionary selection pressures that operate at a multicellular level—and therefore can be distinct from the clonal events that drive initiation and the benign-to-malignant transition—govern late-stage tumor progression and metastases [116, 182]. These issues are important because therapeutic interventions are aimed at driver alterations, which must be distinguished from passenger mutations. It has been previously assumed that hotspots, meaning sites in the genome that are prone to mutations across multiple tumors, are drivers of tumorigenesis; however, it has been demonstrated more recently that many hotspot mutations represent passenger events, recurring at sites that are simply more predisposed to mutation [183]. Impacting driver mutations may decimate cancer clones and their ecosystems, but may also provide potent selective pressure for the emergence and/or expansion of resistant molecular alterations [116].
A canonical understanding of clonal evolution and selection suggests that driver alterations should appear more frequently as the continuum progresses from benign to premalignant to malignant neoplasm. Traditionally, it would be assumed that, for example, a BRAF V600E mutation—identified as a known driver of melanoma on the basis that mutated BRAF proteins have elevated kinase activity and are transforming in NIH3T3 cells [117]—would be found most abundantly in melanomas rather than in dysplastic or benign nevi. On the contrary, however, the incidence of the BRAF V600E mutation in benign nevi and premalignant conditions or dysplastic nevi is more frequent (~ 70–88% and ~ 60%, respectively) than in melanoma (~ 40–45%) (Fig. 1), despite the fact that the conversion rate of benign nevi to melanoma is negligible [144]. Another example that contradicts the classic theory of clonal expansion is HER2 overexpression, a clearly druggable driver of breast malignancies, which is nonetheless identified more commonly in ductal carcinoma in situ (~ 27–56%) than in invasive mammary cancers (~ 11–20%) [121–123]. Similarly, grade of bladder cancer is inversely related to the frequency of driver FGFR3 mutations. As successive grades are diagnosed, the incidence of FGFR3 mutations decreases: non-invasive, grade 1 bladder cancer has the most frequent occurrence of FGFR3 mutations (~ 61%), then non-invasive, grade 2 bladder cancer (~ 58%), followed by non-invasive, grade 3 bladder cancer (~ 34%), lamina propria-invasive grade 3 (~ 17%), and, last, high-grade tumors, which demonstrate FGFR3 mutations in only about 11% of cases [124–126]. This paradoxical phenomenon is also seen in hematologic malignancies. JAK2 mutations are found in the majority of myeloproliferative disorders (65–97%), but rarely in acute myeloid leukemias (2–5%) [127–129, 184, 185]. In each of the examples mentioned above, there can be little question regarding the driver role of these alterations because of the efficacy of drugs developed against them in achieving tumor regression.
The mechanism that underlies the paradoxical decrease in the frequency of driver alterations with malignant progression is unknown. However, the phenomenon is especially pertinent to therapeutic drug development because it is critical that one does not assume that a mutation or other alteration is a passenger just because it is more frequently found in the benign counterpart of an invasive cancer. Had such an assumption been made, BRAF inhibitors would not have been developed for melanoma. Another question is how oncogenic drivers that are less frequent in malignant disease than in benign disease act to impart the oncogenic phenotype in the malignancy, but not in the benign lesions. Perhaps the driver alteration acts in an oncogenic capacity only when a required co-factor or co-alteration is in place, or perhaps the suppression of an endogenous inhibitor is required in order for the malignancy to emerge [186]. Preclinical and ex vivo studies examining the functional effects of mutations in various tissue contexts and with different co-alterations can be performed with a variety of techniques, including patient-derived cell cultures that serve as avatars [187]. These studies may provide a biologic understanding of the role of these mutations in determining the aggressiveness of a tumor, and whether or not malignant transformation takes place.
Therapeutic implications of oncogenic drivers in non-malignant conditions
In many instances, there are approved drugs that specifically target a gene mutation product and are readily available for use in the setting of a malignancy. Using the same gene-targeting paradigm and shifting it towards sporadic benign diseases, hereditary conditions or somatic mosaic syndromes that carry the cognate driver genomic aberration (regardless of their malignant potential) could offer innovative treatments for these conditions, perhaps reversing their phenotype. Factors that would need to be considered would be the potency of the agent against the genomic target and its potential toxicity. For disorders that have potential for malignant transformation, it is conceivable that the use of such targeted agents might also attenuate the risk of developing cancer.
Repurposing cancer drugs for sporadic conditions
Several examples now exist to demonstrate how the targeting of genomic drivers in benign illnesses can alleviate disease, and to show that drugs that were developed for illnesses on the neoplastic spectrum can be used (Table 3). For instance, tocilizumab is an anti-IL-6-receptor monoclonal antibody approved for use in rheumatoid arthritis and also developed for the treatment of Castleman disease, a lymphoma-like condition [169]. TP53 mutations, which are known to occur in the synovium in rheumatoid arthritis [37, 38], upregulate IL-6 levels [142, 143], perhaps mediating the inflammation of arthritis and explaining the efficacy of tocilizumab in this condition. Desmoid tumors provide another example; these neoplasms are an aggressive fibromatosis that have similarities to fibrosarcoma but are considered benign because they do not metastasize. They are characterized by CTNNB1 mutations [41], which are known to activate the WNT pathway [13]. They can be treated with COX-2 inhibitors such as celecoxib (approved for familial adenomatosis polyposis, which predisposes carriers to colorectal cancer) and/or with sorafenib (approved for several types of cancer), both of which suppress the WNT pathway [14, 42, 43].
Another example is inflammatory myofibroblastic tumor, which is an uncommon, usually benign neoplasm composed of myofibroblastic spindle cells with an inflammatory infiltrate. Approximately half of inflammatory myofibroblastic tumors carry rearrangements of the anaplastic lymphoma kinase gene locus (ALK) on chromosome 2p23, causing aberrant ALK expression. After the initial report of a striking response to treatment with the ALK inhibitor crizotinib (approved for lung cancers with ALK rearrangements) in a patient suffering from an ALK-rearranged inflammatory myofibroblastic tumor [30], a larger study showed that six of 12 ALK-positive patients (50%) achieved an objective response with crizotinib [188].
Finally, in Schnitzler syndrome, a rare auto-inflammatory disease that often presents with urticarial rash, fever, lymphadenopathy, musculoskeletal pain, and thrombosis and that is attributed to cytokine dysregulation involving IL-1β and the inflammasome pathway, there is evidence that blocking IL-1 can lead to significant disease control [170]. We previously described a patient with Schnitzler syndrome and a MYD88 mutation; the latter is classically discerned in Waldenström macroglobulinemia. Treatment with anakinra, an IL-1 receptor antagonist (IL-1RA), resulted in a durable response [170]. This beneficial effect may be due to the fact that MYD88 plays an important role in IL-1 signaling, mediating the association between IL-1R- and the IL-1R-associated kinase (IRAK) [189].
Theoretical examples also exist. For instance, drugs that target PIK3CA or MEK signals, such as alpelisib or trametinib, respectively, may theoretically offer new options for women suffering with endometriosis, which harbors mutations in PIK3CA or KRAS [27]. In sporadic brain arteriovenous malformations (AVMs) that are caused by KRAS mutations, using agents that inhibit the MAP-ERK pathway could also offer potential therapy for patients, at least in theory [35]. These AVMs have potential to rupture and cause significant morbidity in these patients.
Taken together, these observations suggest that drugs that impact driver molecular alterations or their downstream effectors can be repurposed to treat a variety of benign, sporadic illnesses, and that such new uses merit investigation in clinical trials that select drugs for non-malignant conditions on the basis of their somatic alterations. Nevertheless, several caveats would need to be considered. These include the possibility that the drug action might depend on tissue context and that potential side effects might attenuate the ability to administer the drug to patients who are afflicted with non-malignant conditions.
Repurposing cancer drugs for somatic mosaic and germline conditions
Gene-product targeted drugs may also be beneficial in hereditary or somatic mosaic conditions (Table 3). A dramatic example is provided by CLOVES syndrome (congenital lipomatous overgrowth, vascular malformations, epidermal nevi, scoliosis/skeletal, and spinal syndrome), which is a disorder that results from somatic, mosaic gain-of-function mutations of the PIK3CA gene and that belongs to the spectrum of PIK3CA-related overgrowth syndromes. Previously, this ultra-rare condition had no specific treatment and a poor survival rate. Use of the PIK3CA inhibitor alpelisib improved disease-related symptoms in all of the 19 patients that received the drug [113]. Intractable vascular tumors became smaller, congestive heart failure was improved, hemihypertrophy was reduced, and scoliosis was attenuated. The treatment was not associated with significant toxicity at doses of alpelisib of 250 mg by mouth per day in adults taken for a period of up to 18 months (the approved dose for breast cancer starts at 300 mg per day); children received 50 mg per day with excellent tolerance.
A second illustration of the repurposing of medications has been described in patients with central conducting lymphatic anomaly, in which aberrations can occur along the MAPK or mTOR pathways [52]. The use of sirolimus (a mTOR inhibitor) [53] or trametinib (a MEK inhibitor) [51] provided significant benefit and attenuation of disease in treated patients. For example, a patient given sirolimus, who required a chest tube for the abundant output of chylous effusion, attained a complete resolution of chylous output and no longer required the chest tube [53]. In the patient treated with trametinib, there was resolution of the lymphatic edema, improvement on pulmonary function tests so that the patient no longer required supplemental oxygen, and significant improvement in functional status [51]. In other words, the phenotype of these genetic disorders was reversed by precise targeting of the molecular abnormality using a drug developed for cancer.
Another example pertinent to the repurposing of drugs for benign illness pertains to NF1, a gene whose aberration activates the MEK pathway. Neurofibromatosis-1 is a hereditary condition caused by germline NF1 mutations; it manifests mainly with non-malignant neurofibromas, which nonetheless cause functional disabilities. Recently, the MEK inhibitor selumetinib was given Breakthrough Status by the FDA for this condition because of a ~ 70% response rate in children with neurofibromatosis-1 and inoperable plexiform neurofibromas [92]. Of interest, NF1 mutations may also be found in melanoma, but some studies suggest that targeting them with MEK inhibitors would be ineffective (though there may be exceptions) [190]. Melanomas with NF1 mutations may not respond to MEK inhibitors (although neurofibromatosis is responsive) because melanomas tend to have important co-alterations, whereas neurofibromatosis is driven only by NF1 alterations [191, 192].
Finally, targeting activating FGFR3 mutations in achondroplasia with FGFR inhibitors is another example worth noting, although the data here are from animal models only [23]. In a mouse model with FGFR3-mutated skeletal cells, use of an FGFR3 inhibitor led to restoration in the size of achrondroplastic femurs [172]. FGFR mutations cause multiple skeletal disorders and also play a role in certain cancers. Targeting these mutations could potentially abrogate the skeletal anomalies seen in these hereditary conditions. However, if the lack of increased cancer risk in these patients is due to a compensatory factor that develops in the presence of germline activated FGFR3, and if this compensatory factor is attenuated in the presence of FGFR inhibitors given during early life stages, it would be important to take into consideration the theoretical possibility of a later cancer risk if these FGFR3 inhibitors were discontinued [193].
Confounding the holy grail—early detection of cancer with blood tests
In recent years, liquid biopsy to detect cfDNA or circulating tumor DNA (ctDNA) has emerged as an attractive non-invasive methodology to discern cancer-specific genomic aberrations in plasma. Numerous studies have reported the utility of ctDNA in advanced cancer [194–197]. In particular, ctDNA assays can capture a more global portrait of tumor heterogeneity than that provided by tissue DNA (which reflects the small piece of tissue that is biopsied rather than DNA shed from both primary and multiple metastatic sites [198]); therefore, ctDNA can be exploited to monitor tumor response and resistance.
Recently, ctDNA analysis has also been proposed as a promising future tool for the identification of early neoplasms as part of cancer screening. As the average amount of mutated DNA in plasma is very low (about 0.4% even in metastatic malignancies), exceedingly sensitive technologies must be developed; further, in cancer patients with low tumor burden, ctDNA is difficult to detect [130, 199]. Hence, in patients without known tumors who are being screened, the levels of ctDNA may be very, very low. Yet, increased sensitivity of ctDNA tests is a two-edged sword. It is plausible that with overly sensitive tests, molecular alterations from benign lesions would be picked up in cfDNA. Being able to differentiate between these sources of ctDNA and to determine thresholds that correspond to levels of concern for screening tools are areas of continuing development [200]. It is also possible that serial tests may need to be conducted and that increasing ctDNA levels with time might be the trigger for further work up for cancer. In addition, as cancers are heterogeneous at the molecular level, any screening blood test would need to assay multiple gene targets in order to increase the chances of finding a cancer.
Of significant interest, non-invasive prenatal testing, which uses cfDNA as an analyte to detect copy-number alterations in the fetal genome (by testing maternal blood), can detect early cancers in pregnant women. In one study, an abnormal genomic profile not consistent with fetal abnormalities was identified in about 10 out of 100,000 cases; a significant subset of these observations (18 of 43; 41.9%) was attributed to mostly unsuspected maternal malignant neoplasms [201]. These findings substantiate the claim that sensitive cfDNA screening may be exploitable as a cancer biomarker for the early detection of malignant disease.
In addition to cfDNA or ctDNA, other components of tumors that are shed into the circulation may be important for early detection: circulating tumor cells or extracellular vesicles. Indeed, these tumor components have been informative for early recognition of relapse, albeit of advanced tumors [202].
For the identification of early cancer, strategies for analysis are in principle relatively similar to those for advanced disease. However, beyond the sensitivity issues discussed above (i.e., very early-stage (asymptomatic) tumors may not release enough ctDNA to be detectable in a typical blood draw), the challenges with these techniques are considerable. For instance, white blood cells are a major source of cfDNA in blood, and it is crucial to distinguish acquired mutations in leukocytes (benign clonal hematopoiesis that increases with age [203]) from incipient invasive cancer. Further, “oncogenic” mutations can be found in healthy individuals, including in their cfDNA, and can be indistinguishable from those associated with cancer [130]. Therefore, caution needs to be applied when interpreting results from mutation-based early detection tools, as both false negatives (resulting from lack of sensitivity) and false positives (resulting from the detection of shed DNA from benign lesions that harbor oncogenic mutations) could confound the interpretation of these tests. Other methods being explored to screen for cancers using blood-based methods include the use of autoantibodies [204–208] and tumor-associated antigens [209]. As regards technologies that use circulating tumor cells or extracellular vesicles, in addition to the low volume of the aberrations in the blood, theoretically confounding phenomena must be addressed. These might include the rate of clearance in patients with renal or hepatic impairments, stability in the bloodstream, diurnal or other biologic influences on time of collection, the effects of smoking, pregnancy, and other inflammatory conditions, and clonal expansions of non-tumors.
Other technologies, including gene and protein expression signatures [210–214], have also been developed to help to decipher the code that differentiates benign and cancerous molecular anomalies. Intriguingly, there are models that predict (with up to 90% accuracy) the pattern of epigenetic changes found on circulating DNA in the bloodstream that imply malignancy versus those that do not [215]. Indeed, there is evidence that the methyl clusters that occur on the cancer DNA not only help to identify cancer DNA, but are major contributors to carcinogenesis [215].
In summary, myriad blood-based assays are being developed for early detection of cancer. They include tests of ctDNA mutations or methylation patterns as well as interrogation of exosomes or circulating tumor cells. Validating these biomarkers will probably require serial follow-up to discern an increasing level of abnormality and will also need threshold trigger values for imaging patients in order to confirm the presence of cancer.
Perspective and future directions
The rapid expansion of the use of NGS in cancer clinical care and research has resulted in significant improvement in outlook for a subset of malignancies [216–218]. Indeed, genomic markers can drive new clinical trials of both gene- and immune-targeted agents [219–225]. Relatively new, however, is the emergence of data showing that non-cancerous illnesses also have genomic markers, and intriguingly, that some of these molecular alterations are indistinguishable from those considered oncogenic drivers for certain malignancies. Further large-scale studies across benign conditions may provide insight into crucial, subtle differences in the molecular landscape that enable the same “driver” to navigate towards two different “destinations”—that is, benign versus malignant disease. Identifying potential co-alterations may be key; alternatively, it may be that tissue of origin or histologic context is critical or that immune function shapes the outcome.
A wide variety of sporadic, mosaic, and hereditary conditions can be characterized by “oncogenic” aberrations, including conditions that have negligible malignant potential (Tables 1, 2, and 3). Furthermore, there are now several examples of the paradox of decreasing frequency of the “oncogenic driver” as the condition progresses from benign to premalignant to malignant (Fig. 1). Importantly, recent RNA sequence analysis also identified the somatic clonal expansion of mutations associated with cancer across normal tissues, most commonly in the lung, skin, and esophagus; the number of mutations correlated with age and with tissue proliferation rate [28]. The presence of these molecular abnormalities in benign conditions may confound efforts to detect cancer event cascades early through the use of blood tests. Serial blood tests may need to be done, with increasing levels of the biomarker being indicative of a cancer concern.
Of significant future interest is the potential to repurpose drugs used in cancer for non-malignant illnesses that harbor actionable genomic alterations and/or to prevent the development of cancer in conditions and syndromes where there is a predisposition to malignancy. The use of open-label basket clinical trials, in which patients are matched with drugs on the basis of a genomic aberration (regardless of histology), has been effective in a variety of cancer settings [16, 226–229]; similar approaches could conceivably be taken in benign conditions, for which trials that are disease agnostic could be developed and drug choice would be dictated by the genomic aberration. Alternatively, individual sequencing studies of somatic or germline tissue may define the treatment prosecution strategy on an N-of-one basis in selected non-malignant diseases, as it is beginning to do in malignancy [223]. Regardless, patients would require close follow-up to determine whether their cancer risk was modified by the use of matched targeted agents, and functional studies on tissues might help to identify those conditions that are most likely to respond to cognate compounds. Finally, moving forward in this field will require multidisciplinary collaborative teams with expertise in the benign conditions, their malignant counterparts, and targeted drugs and genomics, as well as translational scientists to bridge the emerging preclinical and clinical data.
Acknowledgements
Funded in part by the Joan and Irwin Jacobs Fund, and by National Cancer Institute grants P30 CA023100 (RK).
Abbreviations
- ALK
Anaplastic lymphoma kinase gene locus
- AVM
Arteriovenous malformation
- cfDNA
Circulating cell-free DNA
- ctDNA
Circulating tumor DNA
- FDA
US Food and Drug Administration
- IL
Interleukin
- NGS
Next-generation sequencing
Authors’ contributions
JJA, SK, and RK conducted the literature review. JJA and RK drafted the manuscript. All authors read and approved the final manuscript.
Competing interests
JJA and SML declare that they have no competing interests. SK serves as a consultant for Foundation Medicine. RK has received research funding from Incyte, Genentech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, Guardant Health, Grifols, and Konica Minolta, as well as consultant fees from LOXO, X-Biotech, Actuate Therapeutics, Genentech, and NeoMed. She receives speaker fees from Roche and has an equity interest in IDbyDNA and Curematch, Inc.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jacob Adashek and Shumei Kato contributed equally to this work and should be considered co-first authors.
Scott Lippman and Razelle Kurzrock contributed equally to this work and should be considered senior/last authors.
References
- 1.Dang CV, Reddy EP, Shokat KM, Soucek L. Drugging the 'undruggable' cancer targets. Nat Rev Cancer. 2017;17:502–508. doi: 10.1038/nrc.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seebacher NA, Stacy AE, Porter GM, Merlot AM. Clinical development of targeted and immune based anti-cancer therapies. J Exp Clin Cancer Res. 2019;38:156. doi: 10.1186/s13046-019-1094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar R, Angelini S, Snellman E, Hemminki K. BRAF mutations are common somatic events in melanocytic nevi. J Invest Dermatol. 2004;122:342–348. doi: 10.1046/j.0022-202X.2004.22225.x. [DOI] [PubMed] [Google Scholar]
- 4.Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 5.Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893–1901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen FW, Tseng D, Reddy S, Daud AI, Swetter SM. Involution of eruptive melanocytic nevi on combination BRAF and MEK inhibitor therapy. JAMA Dermatol. 2014;150:1209–1212. doi: 10.1001/jamadermatol.2014.838. [DOI] [PubMed] [Google Scholar]
- 7.Wong H, Vernillet L, Peterson A, Ware JA, Lee L, Martini JF, et al. Bridging the gap between preclinical and clinical studies using pharmacokinetic-pharmacodynamic modeling: an analysis of GDC-0973, a MEK inhibitor. Clin Cancer Res. 2012;18:3090–3099. doi: 10.1158/1078-0432.CCR-12-0445. [DOI] [PubMed] [Google Scholar]
- 8.Saroufim M, Novy M, Taraif S, Habib RH, Loya A, Rauscher B, et al. BRAF mutational epidemiology in dysplastic nevi: does different solar UV radiation exposure matter? J Eur Acad Dermatol Venereol. 2014;28:615–625. doi: 10.1111/jdv.12148. [DOI] [PubMed] [Google Scholar]
- 9.McClenahan P, Lin LL, Tan JM, Flewell-Smith R, Schaider H, Jagirdar K, et al. BRAFV600E mutation status of involuting and stable nevi in dabrafenib therapy with or without trametinib. JAMA Dermatol. 2014;150:1079–1082. doi: 10.1001/jamadermatol.2014.436. [DOI] [PubMed] [Google Scholar]
- 10.Poynter JN, Elder JT, Fullen DR, Nair RP, Soengas MS, Johnson TM, et al. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma Res. 2006;16:267–273. doi: 10.1097/01.cmr.0000222600.73179.f3. [DOI] [PubMed] [Google Scholar]
- 11.Tschandl P, Berghoff AS, Preusser M, Burgstaller-Muehlbacher S, Pehamberger H, Okamoto I, Kittler H. NRAS and BRAF mutations in melanoma-associated nevi and uninvolved nevi. PLoS One. 2013;8:e69639. doi: 10.1371/journal.pone.0069639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Recio A, Sánchez-Moya AI, Félix V, Campos Y. Congenital melanocytic nevus syndrome: a case series. Actas Dermosifiliogr. 2017;108:e57–e62. doi: 10.1016/j.ad.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gounder MM, Mahoney MR, Van Tine BA, Ravi V, Attia S, Deshpande HA, et al. Sorafenib for advanced and refractory desmoid tumors. N Engl J Med. 2018;379:2417–2428. doi: 10.1056/NEJMoa1805052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, et al. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- 16.Drilon Alexander E., Subbiah Vivek, Oxnard Geoffrey R., Bauer Todd Michael, Velcheti Vamsidhar, Lakhani Nehal J., Besse Benjamin, Park Keunchil, Patel Jyoti D., Cabanillas Maria E., Johnson Melissa Lynne, Reckamp Karen L., Boni Valentina, Loong Herbert H. F., Schlumberger Martin, Solomon Ben, Cruickshank Scott, Rothenberg Stephen M., Shah Manisha H., Wirth Lori J. A phase 1 study of LOXO-292, a potent and highly selective RET inhibitor, in patients with RET-altered cancers. Journal of Clinical Oncology. 2018;36(15_suppl):102–102. doi: 10.1200/JCO.2018.36.15_suppl.102. [DOI] [Google Scholar]
- 17.Bauer J, Curtin JA, Pinkel D, Bastian BC. Congenital melanocytic nevi frequently harbor NRAS mutations but no BRAF mutations. J Invest Dermatol. 2007;127:179–182. doi: 10.1038/sj.jid.5700490. [DOI] [PubMed] [Google Scholar]
- 18.Charbel C, Fontaine RH, Malouf GG, Picard A, Kadlub N, How-Kit A, et al. NRAS mutation is the sole recurrent somatic mutation in large congenital melanocytic nevi. J Invest Dermatol. 2014;134:1067–1074. doi: 10.1038/jid.2013.429. [DOI] [PubMed] [Google Scholar]
- 19.Hafner C, Hartmann A, van Oers JM, Stoehr R, Zwarthoff EC, Hofstaedter F, et al. FGFR3 mutations in seborrheic keratoses are already present in flat lesions and associated with age and localization. Mod Pathol. 2007;20:895–903. doi: 10.1038/modpathol.3800837. [DOI] [PubMed] [Google Scholar]
- 20.Hafner C, Lopez-Knowles E, Luis NM, Toll A, Baselga E, Fernández-Casado A, et al. Oncogenic PIK3CA mutations occur in epidermal nevi and seborrheic keratoses with a characteristic mutation pattern. Proc Natl Acad Sci U S A. 2007;104:13450–13454. doi: 10.1073/pnas.0705218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hafner C, van Oers JM, Hartmann A, Landthaler M, Stoehr R, Blaszyk H, et al. High frequency of FGFR3 mutations in adenoid seborrheic keratoses. J Invest Dermatol. 2006;126:2404–2407. doi: 10.1038/sj.jid.5700422. [DOI] [PubMed] [Google Scholar]
- 22.Hida Y, Kubo Y, Arase S. Activation of fibroblast growth factor receptor 3 and oncogene-induced senescence in skin tumours. Br J Dermatol. 2009;160:1258–1263. doi: 10.1111/j.1365-2133.2009.09068.x. [DOI] [PubMed] [Google Scholar]
- 23.Tabernero J, Bahleda R, Dienstmann R, Infante JR, Mita A, Italiano A, et al. Phase I dose-escalation study of JNJ-42756493, an oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2015;33:3401–3408. doi: 10.1200/JCO.2014.60.7341. [DOI] [PubMed] [Google Scholar]
- 24.Maehara O, Suda G, Natsuizaka M, Ohnishi S, Komatsu Y, Sato F, et al. Fibroblast growth factor-2-mediated FGFR/Erk signaling supports maintenance of cancer stem-like cells in esophageal squamous cell carcinoma. Carcinogenesis. 2017;38:1073–1083. doi: 10.1093/carcin/bgx095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hafner C, van Oers JM, Vogt T, Landthaler M, Stoehr R, Blaszyk H, et al. Mosaicism of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest. 2006;116:2201–2207. doi: 10.1172/JCI28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furet P, Guagnano V, Fairhurst RA, Imbach-Weese P, Bruce I, Knapp M, et al. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg Med Chem Lett. 2013;23:3741–3748. doi: 10.1016/j.bmcl.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noë M, Horlings HM, et al. Cancer-associated mutations in endometriosis without cancer. N Engl J Med. 2017;376:1835–1848. doi: 10.1056/NEJMoa1614814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yizhak Keren, Aguet François, Kim Jaegil, Hess Julian M., Kübler Kirsten, Grimsby Jonna, Frazer Ruslana, Zhang Hailei, Haradhvala Nicholas J., Rosebrock Daniel, Livitz Dimitri, Li Xiao, Arich-Landkof Eila, Shoresh Noam, Stewart Chip, Segrè Ayellet V., Branton Philip A., Polak Paz, Ardlie Kristin G., Getz Gad. RNA sequence analysis reveals macroscopic somatic clonal expansion across normal tissues. Science. 2019;364(6444):eaaw0726. doi: 10.1126/science.aaw0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrence B, Perez-Atayde A, Hibbard MK, Rubin BP, Dal Cin P, Pinkus JL, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol. 2000;157:377–384. doi: 10.1016/S0002-9440(10)64550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butrynski JE, D'Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, Fukami TA, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19:679–690. doi: 10.1016/j.ccr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Delisle L, Pierre-Eugene C, Louis-Brennetot C, Surdez D, Raynal V, Baulande S, et al. Activated ALK signals through the ERK-ETV5-RET pathway to drive neuroblastoma oncogenesis. Oncogene. 2018;37:1417–1429. doi: 10.1038/s41388-017-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martincorena I, Fowler JC, Wabik A, Lawson ARJ, Abascal F, Hall MWJ, et al. Somatic mutant clones colonize the human esophagus with age. Science. 2018;362:911–917. doi: 10.1126/science.aau3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishiguro H, Okubo T, Kuwabara Y, Kimura M, Mitsui A, Sugito N, et al. NOTCH1 activates the Wnt/beta-catenin signaling pathway in colon cancer. Oncotarget. 2017;8:60378–60389. doi: 10.18632/oncotarget.19534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikolaev SI, Vetiska S, Bonilla X, Boudreau E, Jauhiainen S, Rezai Jahromi B, et al. Somatic activating KRAS mutations in arteriovenous malformations of the brain. N Engl J Med. 2018;378:250–261. doi: 10.1056/NEJMoa1709449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oka M, Kushamae M, Aoki T, Yamaguchi T, Kitazato K, Abekura Y, et al. KRAS G12D or G12V mutation in human brain arteriovenous malformations. World Neurosurg. 2019;126:e1365–e1373. doi: 10.1016/j.wneu.2019.03.105. [DOI] [PubMed] [Google Scholar]
- 37.Firestein GS, Echeverri F, Yeo M, Zvaifler NJ, Green DR. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc Natl Acad Sci U S A. 1997;94:10895–10900. doi: 10.1073/pnas.94.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, Olivier M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 39.Schwaederle M, Lazar V, Validire P, Hansson J, Lacroix L, Soria JC, et al. VEGF-A expression correlates with TP53 mutations in non-small cell lung cancer: implications for antiangiogenesis therapy. Cancer Res. 2015;75:1187–1190. doi: 10.1158/0008-5472.CAN-14-2305. [DOI] [PubMed] [Google Scholar]
- 40.Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 41.Le Guellec S, Soubeyran I, Rochaix P, Filleron T, Neuville A, Hostein I, Coindre JM. CTNNB1 mutation analysis is a useful tool for the diagnosis of desmoid tumors: a study of 260 desmoid tumors and 191 potential morphologic mimics. Mod Pathol. 2012;25:1551–1558. doi: 10.1038/modpathol.2012.115. [DOI] [PubMed] [Google Scholar]
- 42.Yang S, Wang X, Jiang H, Wang Y, Li Z, Lu H. Effective treatment of aggressive fibromatosis with celecoxib guided by genetic testing. Cancer Biol Ther. 2017;18:757–760. doi: 10.1080/15384047.2017.1373215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl] benze nesulfonamide (SC-58635, celecoxib) J Med Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 44.Lachenmayer A, Alsinet C, Savic R, Cabellos L, Toffanin S, Hoshida Y, et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res. 2012;18:4997–5007. doi: 10.1158/1078-0432.CCR-11-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanabe S, Kawabata T, Aoyagi K, Yokozaki H, Sasaki H. Gene expression and pathway analysis of CTNNB1 in cancer and stem cells. World J Stem Cells. 2016;8:384–395. doi: 10.4252/wjsc.v8.i11.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toll A, Fernandez LC, Pons T, Groesser L, Sagrera A, Carrillo-de Santa Pau E, et al. Somatic embryonic FGFR2 mutations in keratinocytic epidermal nevi. J Invest Dermatol. 2016;136:1718–1721. doi: 10.1016/j.jid.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 47.Park JS, Lee J, Jung ES, Kim MH, Kim IB, Son H, et al. Brain somatic mutations observed in Alzheimer's disease associated with aging and dysregulation of tau phosphorylation. Nat Commun. 2019;10:3090. doi: 10.1038/s41467-019-11000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leoz ML, Carballal S, Moreira L, Ocaña T, Balaguer F. The genetic basis of familial adenomatous polyposis and its implications for clinical practice and risk management. Appl Clin Genet. 2015;8:95–107. doi: 10.2147/TACG.S51484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jasperson KW, Patel SG, Ahnen DJ. APC-associated polyposis conditions. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al., editors. GeneReviews. Seattle: University of Washington; 1998.
- 50.Nieuwenhuis MH, Lefevre JH, Bulow S, Järvinen H, Bertario L, Kernéis S, et al. Family history, surgery, and APC mutation are risk factors for desmoid tumors in familial adenomatous polyposis: an international cohort study. Dis Colon Rectum. 2011;54:1229–1234. doi: 10.1097/DCR.0b013e318227e4e8. [DOI] [PubMed] [Google Scholar]
- 51.Li D, March ME, Gutierrez-Uzquiza A, Kao C, Seiler C, Pinto E, et al. ARAF recurrent mutation causes central conducting lymphatic anomaly treatable with a MEK inhibitor. Nat Med. 2019;25:1116–1122. doi: 10.1038/s41591-019-0479-2. [DOI] [PubMed] [Google Scholar]
- 52.Li D, Wenger TL, Seiler C, March ME, Gutierrez-Uzquiza A, Kao C, et al. Pathogenic variant in EPHB4 results in central conducting lymphatic anomaly. Hum Mol Genet. 2018;27:3233–3245. doi: 10.1093/hmg/ddy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCormick Andrew, Rosenberg Stacy, Tier Katherine, Balest Arcangela. A Case of a Central Conducting Lymphatic Anomaly Responsive to Sirolimus. Pediatrics. 2015;137(1):e20152694. doi: 10.1542/peds.2015-2694. [DOI] [PubMed] [Google Scholar]
- 54.Rauen KA, et al. Cardiofaciocutaneous syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, LJH B, Stephens K, et al., editors. GeneReviews. Seattle: University of Washington; 2007. [Google Scholar]
- 55.Kratz CP, Franke L, Peters H, Kohlschmidt N, Kazmierczak B, Finckh U, et al. Cancer spectrum and frequency among children with Noonan, Costello, and cardio-facio-cutaneous syndromes. Br J Cancer. 2015;112:1392–1397. doi: 10.1038/bjc.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hussain MR, Baig M, Mohamoud HS, Ulhaq Z, Hoessli DC, Khogeer GS, et al. BRAF gene: from human cancers to developmental syndromes. Saudi J Biol Sci. 2015;22:359–373. doi: 10.1016/j.sjbs.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarkozy A, Carta C, Moretti S, Zampino G, Digilio MC, Pantaleoni F, et al. Germline BRAF mutations in Noonan, LEOPARD, and cardiofaciocutaneous syndromes: molecular diversity and associated phenotypic spectrum. Hum Mutat. 2009;30:695–702. doi: 10.1002/humu.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi Y, Fukuda Y, Yoshimura J, Toyoda A, Kurppa K, Moritoyo H, et al. ERBB4 mutations that disrupt the neuregulin-ErbB4 pathway cause amyotrophic lateral sclerosis type 19. Am J Hum Genet. 2013;93:900–905. doi: 10.1016/j.ajhg.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong KK, Fracasso PM, Bukowski RM, Lynch TJ, Munster PN, Shapiro GI, et al. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res. 2009;15:2552–2558. doi: 10.1158/1078-0432.CCR-08-1978. [DOI] [PubMed] [Google Scholar]
- 60.Takagi M, Miyoshi T, Nagashima Y, Shibata N, Yagi H, Fukuzawa R, Hasegawa T. Novel heterozygous mutation in the extracellular domain of FGFR1 associated with Hartsfield syndrome. Hum Genome Var. 2016;3:16034. doi: 10.1038/hgv.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonçalves C, Bastos M, Pignatelli D, Borges T, Aragüés JM, Fonseca F, et al. Novel FGFR1 mutations in Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism: evidence for the involvement of an alternatively spliced isoform. Fertil Steril. 2015;104:1261–1267. doi: 10.1016/j.fertnstert.2015.07.1142. [DOI] [PubMed] [Google Scholar]
- 62.Chokdeemboon C, Mahatumarat C, Rojvachiranonda N, Tongkobpetch S, Suphapeetiporn K, Shotelersuk V. FGFR1 and FGFR2 mutations in Pfeiffer syndrome. J Craniofac Surg. 2013;24:150–152. doi: 10.1097/SCS.0b013e3182646454. [DOI] [PubMed] [Google Scholar]
- 63.Bochukova EG, Roscioli T, Hedges DJ, Taylor IB, Johnson D, David DJ, et al. Rare mutations of FGFR2 causing apert syndrome: identification of the first partial gene deletion, and an Alu element insertion from a new subfamily. Hum Mutat. 2009;30:204–211. doi: 10.1002/humu.20825. [DOI] [PubMed] [Google Scholar]
- 64.Lupo Philip J., Schraw Jeremy M., Desrosiers Tania A., Nembhard Wendy N., Langlois Peter H., Canfield Mark A., Copeland Glenn, Meyer Robert E., Brown Austin L., Chambers Tiffany M., Sok Pagna, Danysh Heather E., Carozza Susan E., Sisoudiya Saumya D., Hilsenbeck Susan G., Janitz Amanda E., Oster Matthew E., Scheuerle Angela E., Schiffman Joshua D., Luo Chunqiao, Mian Amir, Mueller Beth A., Huff Chad D., Rasmussen Sonja A., Scheurer Michael E., Plon Sharon E. Association Between Birth Defects and Cancer Risk Among Children and Adolescents in a Population-Based Assessment of 10 Million Live Births. JAMA Oncology. 2019;5(8):1150. doi: 10.1001/jamaoncol.2019.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Przylepa KA, Paznekas W, Zhang M, Golabi M, Bias W, Bamshad MJ, et al. Fibroblast growth factor receptor 2 mutations in Beare-Stevenson cutis gyrata syndrome. Nat Genet. 1996;13:492–494. doi: 10.1038/ng0896-492. [DOI] [PubMed] [Google Scholar]
- 66.Bellus GA, Hefferon TW, Ortiz de Luna RI, Hecht JT, Horton WA, Machado M, et al. Achondroplasia is defined by recurrent G380R mutations of FGFR3. Am J Hum Genet. 1995;56:368–373. [PMC free article] [PubMed] [Google Scholar]
- 67.Prinos P, Costa T, Sommer A, Kilpatrick MW, Tsipouras P. A common FGFR3 gene mutation in hypochondroplasia. Hum Mol Genet. 1995;4:2097–2101. doi: 10.1093/hmg/4.11.2097. [DOI] [PubMed] [Google Scholar]
- 68.Shams I, Rohmann E, Eswarakumar VP, Lew ED, Yuzawa S, Wollnik B, et al. Lacrimo-auriculo-dento-digital syndrome is caused by reduced activity of the fibroblast growth factor 10 (FGF10)-FGF receptor 2 signaling pathway. Mol Cell Biol. 2007;27:6903–6912. doi: 10.1128/MCB.00544-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kruszka P, Addissie YA, Agochukwu NB, Doherty ES, Muenke M, et al. Muenke syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, LJH B, Stephens K, et al., editors. GeneReviews. Seattle: University of Washington; 2006. [Google Scholar]
- 70.Pannier S, Martinovic J, Heuertz S, Delezoide AL, Munnich A, Schibler L, et al. Thanatophoric dysplasia caused by double missense FGFR3 mutations. Am J Med Genet A. 2009;149A:1296–1301. doi: 10.1002/ajmg.a.32880. [DOI] [PubMed] [Google Scholar]
- 71.Jin M, Yu Y, Qi H, Xie Y, Su N, Wang X, et al. A novel FGFR3-binding peptide inhibits FGFR3 signaling and reverses the lethal phenotype of mice mimicking human thanatophoric dysplasia. Hum Mol Genet. 2012;21:5443–5455. doi: 10.1093/hmg/dds390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boyce AM, Florenzano P, de Castro LF, Collins MT, et al. Fibrous dysplasia/McCune-Albright Syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, LJH B, Stephens K, et al., editors. GeneReviews. Seattle: University of Washington; 2015. [Google Scholar]
- 73.Dumitrescu CE, Collins MT. McCune-Albright syndrome. Orphanet J Rare Dis. 2008;3:12. doi: 10.1186/1750-1172-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khalili JS, Yu X, Wang J, Hayes BC, Davies MA, Lizee G, et al. Combination small molecule MEK and PI3K inhibition enhances uveal melanoma cell death in a mutant GNAQ- and GNA11-dependent manner. Clin Cancer Res. 2012;18:4345–4355. doi: 10.1158/1078-0432.CCR-11-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Falchook GS, Lewis KD, Infante JR, Gordon MS, Vogelzang NJ, DeMarini DJ, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:782–789. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kiessling MK, Curioni-Fontecedro A, Samaras P, Atrott K, Cosin-Roger J, Lang S, et al. Mutant HRAS as novel target for MEK and mTOR inhibitors. Oncotarget. 2015;6:42183–42196. doi: 10.18632/oncotarget.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kranendijk M, Struys EA, van Schaftingen E, Gibson KM, Kanhai WA, van der Knaap MS, et al. IDH2 mutations in patients with D-2-hydroxyglutaric aciduria. Science. 2010;330:336. doi: 10.1126/science.1192632. [DOI] [PubMed] [Google Scholar]
- 78.Patay Z, Orr BA, Shulkin BL, Hwang SN, Ying Y, Broniscer A, et al. Successive distinct high-grade gliomas in L-2-hydroxyglutaric aciduria. J Inherit Metab Dis. 2015;38:273–277. doi: 10.1007/s10545-014-9782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yen K, Travins J, Wang F, David MD, Artin E, Straley K, et al. AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov. 2017;7:478–493. doi: 10.1158/2159-8290.CD-16-1034. [DOI] [PubMed] [Google Scholar]
- 80.Di Matteo G, Chiriaco M, Scarselli A, Cifaldi C, Livadiotti S, Di Cesare S, et al. JAK3 mutations in Italian patients affected by SCID: new molecular aspects of a long-known gene. Mol Genet Genomic Med. 2018;6:713–721. doi: 10.1002/mgg3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 82.Schubbert S, Zenker M, Rowe SL, Böll S, Klein C, Bollag G, et al. Germline KRAS mutations cause Noonan syndrome. Nat Genet. 2006;38:331–336. doi: 10.1038/ng1748. [DOI] [PubMed] [Google Scholar]
- 83.Sun C, Hobor S, Bertotti A, Zecchin D, Huang S, Galimi F, et al. Intrinsic resistance to MEK inhibition in KRAS mutant lung and colon cancer through transcriptional induction of ERBB3. Cell Rep. 2014;7:86–93. doi: 10.1016/j.celrep.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 84.Mujtaba G, Schultz JM, Imtiaz A, Morell RJ, Friedman TB, Naz S. A mutation of MET, encoding hepatocyte growth factor receptor, is associated with human DFNB97 hearing loss. J Med Genet. 2015;52:548–552. doi: 10.1136/jmedgenet-2015-103023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29:2660–2666. doi: 10.1200/JCO.2010.32.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stittrich AB, Lehman A, Bodian DL, Ashworth J, Zong Z, Li H, et al. Mutations in NOTCH1 cause Adams-Oliver syndrome. Am J Hum Genet. 2014;95:275–284. doi: 10.1016/j.ajhg.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Massard C, Azaro A, Soria JC, Lassen U, Le Tourneau C, Sarker D, et al. First-in-human study of LY3039478, an oral notch signaling inhibitor in advanced or metastatic cancer. Ann Oncol. 2018;29:1911–1917. doi: 10.1093/annonc/mdy244. [DOI] [PubMed] [Google Scholar]
- 88.Ars E, Kruyer H, Morell M, Pros E, Serra E, Ravella A, et al. Recurrent mutations in the NF1 gene are common among neurofibromatosis type 1 patients. J Med Genet. 2003;40:e82. doi: 10.1136/jmg.40.6.e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schroeder RD, Angelo LS, Kurzrock R. NF2/merlin in hereditary neurofibromatosis 2 versus cancer: biologic mechanisms and clinical associations. Oncotarget. 2014;5:67–77. doi: 10.18632/oncotarget.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Friedman JM, et al. Neurofibromatosis 1. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, LJH B, Stephens K, et al., editors. GeneReviews. Seattle: University of Washington; 1998. [Google Scholar]
- 91.Papalia H, Audic F, Riviere GR, Verschuur A, André N. Quick and sustained clinical response to MEK inhibitor I in a NF1 patient with neurofibromas. Ecancermedicalscience. 2018;12:862. doi: 10.3332/ecancer.2018.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gross Andrea M., Wolters Pamela, Baldwin Andrea, Dombi Eva, Fisher Michael J., Weiss Brian D., Kim AeRang, Blakeley Jaishri O'Neill, Whitcomb Patricia, Holmblad Marielle, Martin Staci, Roderick Marie Claire, Paul Scott M., Therrien Janet, Heisey Kara, Doyle Austin, Smith Malcolm A., Glod John, Steinberg Seth M., Widemann Brigitte C. SPRINT: Phase II study of the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886) in children with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas (PN) Journal of Clinical Oncology. 2018;36(15_suppl):10503–10503. doi: 10.1200/JCO.2018.36.15_suppl.10503. [DOI] [Google Scholar]
- 93.Yang C, Asthagiri AR, Iyer RR, Lu J, Xu DS, Ksendzovsky A, et al. Missense mutations in the NF2 gene result in the quantitative loss of merlin protein and minimally affect protein intrinsic function. Proc Natl Acad Sci U S A. 2011;108:4980–4985. doi: 10.1073/pnas.1102198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Subbiah V, Slopis J, Hong DS, Ketonen LM, Hamilton J, McCutcheon IE, Kurzrock R. Treatment of patients with advanced neurofibromatosis type 2 with novel molecularly targeted therapies: from bench to bedside. J Clin Oncol. 2012;30:e64–e68. doi: 10.1200/JCO.2011.38.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.So MT, Leon TY, Cheng G, Tang CS, Miao XP, Cornes BK, et al. RET mutational spectrum in Hirschsprung disease: evaluation of 601 Chinese patients. PLoS One. 2011;6:e28986. doi: 10.1371/journal.pone.0028986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Skába R, Dvoráková S, Václavíková E, Vlcek P, Frantlová M, Bendlová B. The risk of medullary thyroid carcinoma in patients with Hirschsprung's disease. Pediatr Surg Int. 2006;22:991–995. doi: 10.1007/s00383-006-1785-6. [DOI] [PubMed] [Google Scholar]
- 97.Sijmons RH, Hofstra RM, Wijburg FA, Links TP, Zwierstra RP, Vermey A, et al. Oncological implications of RET gene mutations in Hirschsprung's disease. Gut. 1998;43:542–547. doi: 10.1136/gut.43.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Subbiah V, Velcheti V, Tuch BB, Ebata K, Busaidy NL, Cabanillas ME, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol. 2018;29:1869–1876. doi: 10.1093/annonc/mdy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Launonen V. Mutations in the human LKB1/STK11 gene. Hum Mutat. 2005;26:291–297. doi: 10.1002/humu.20222. [DOI] [PubMed] [Google Scholar]
- 100.van Lier MG, Wagner A, Mathus-Vliegen EM, Kuipers EJ, Steyerberg EW, van Leerdam ME. High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. Am J Gastroenterol. 2010;105:1258–1264. doi: 10.1038/ajg.2009.725. [DOI] [PubMed] [Google Scholar]
- 101.Schuler W, Sedrani R, Cottens S, Häberlin B, Schulz M, Schuurman HJ, et al. SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo. Transplantation. 1997;64:36–42. doi: 10.1097/00007890-199707150-00008. [DOI] [PubMed] [Google Scholar]
- 102.Schneider K, Zelley K, Nichols KE, Garber J. Li-Fraumeni syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al., editors. GeneReviews. Seattle: University of Washington; 1999.
- 103.Li FP, Fraumeni JF., Jr Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med. 1969;71:747–752. doi: 10.7326/0003-4819-71-4-747. [DOI] [PubMed] [Google Scholar]
- 104.Borges LM, Ayres FM. R337H mutation of the TP53 gene as a clinical marker in cancer patients: a systematic review of literature. Genet Mol Res. 2015;14:17034–17043. doi: 10.4238/2015.December.16.4. [DOI] [PubMed] [Google Scholar]
- 105.Malkin D. Li-Fraumeni syndrome. Genes Cancer. 2011;2:475–484. doi: 10.1177/1947601911413466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lindhurst MJ, Sapp JC, Teer JK, Johnston JJ, Finn EM, Peters K, et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N Engl J Med. 2011;365:611–619. doi: 10.1056/NEJMoa1104017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Biesecker LG, Sapp JC, et al. Proteus syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al., editors. GeneReviews. Seattle: University of Washington; 2019. [PubMed] [Google Scholar]
- 108.Saura C, Roda D, Roselló S, Oliveira M, Macarulla T, Pérez-Fidalgo JA, et al. A first-in-human phase I study of the ATP-competitive AKT inhibitor ipatasertib demonstrates robust and safe targeting of AKT in patients with solid tumors. Cancer Discov. 2017;7:102–113. doi: 10.1158/2159-8290.CD-16-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shirley MD, Tang H, Gallione CJ, Baugher JD, Frelin LP, Cohen B, et al. Sturge-weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971–1979. doi: 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.D'Gama AM, Geng Y, Couto JA, Martin B, Boyle EA, LaCoursiere CM, et al. Mammalian target of rapamycin pathway mutations cause hemimegalencephaly and focal cortical dysplasia. Ann Neurol. 2015;77:720–725. doi: 10.1002/ana.24357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martinez-Lopez A, Blasco-Morente G, Perez-Lopez I, Herrera-Garcia JD, Luque-Valenzuela M, Sanchez-Cano D, et al. CLOVES syndrome: review of a PIK3CA-related overgrowth spectrum (PROS) Clin Genet. 2017;91:14–21. doi: 10.1111/cge.12832. [DOI] [PubMed] [Google Scholar]
- 112.Peterman Caitlin M., Fevurly R. Dawn, Alomari Ahmad I., Trenor Cameron C., Adams Denise M., Vadeboncoeur Sophie, Liang Marilyn G., Greene Arin K., Mulliken John B., Fishman Steven J. Sonographic screening for Wilms tumor in children with CLOVES syndrome. Pediatric Blood & Cancer. 2017;64(12):e26684. doi: 10.1002/pbc.26684. [DOI] [PubMed] [Google Scholar]
- 113.Venot Q, Blanc T, Rabia SH, Berteloot L, Ladraa S, Duong JP, et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature. 2018;558:540–546. doi: 10.1038/s41586-018-0217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lindhurst MJ, Parker VE, Payne F, Sapp JC, Rudge S, Harris J, et al. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat Genet. 2012;44:928–933. doi: 10.1038/ng.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parker VER, Keppler-Noreuil KM, Faivre L, Luu M, Oden NL, De Silva L, et al. Safety and efficacy of low-dose sirolimus in the PIK3CA-related overgrowth spectrum. Genet Med. 2019;21:1189–1198. doi: 10.1038/s41436-018-0297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 118.Mar VJ, Liu W, Devitt B, Wong SQ, Dobrovic A, McArthur GA, et al. The role of BRAF mutations in primary melanoma growth rate and survival. Br J Dermatol. 2015;173:76–82. doi: 10.1111/bjd.13756. [DOI] [PubMed] [Google Scholar]
- 119.Turski ML, Vidwans SJ, Janku F, Garrido-Laguna I, Munoz J, Schwab R, et al. Genomically driven tumors and actionability across histologies: BRAF-mutant cancers as a paradigm. Mol Cancer Ther. 2016;15:533–547. doi: 10.1158/1535-7163.MCT-15-0643. [DOI] [PubMed] [Google Scholar]
- 120.Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 121.Allred DC, Clark GM, Molina R, Tandon AK, Schnitt SJ, Gilchrist KW, et al. Overexpression of HER-2/neu and its relationship with other prognostic factors change during the progression of in situ to invasive breast cancer. Hum Pathol. 1992;23:974–979. doi: 10.1016/0046-8177(92)90257-4. [DOI] [PubMed] [Google Scholar]
- 122.Rakovitch E, Nofech-Mozes S, Hanna W, Narod S, Thiruchelvam D, Saskin R, et al. HER2/neu and Ki-67 expression predict non-invasive recurrence following breast-conserving therapy for ductal carcinoma in situ. Br J Cancer. 2012;106:1160–1165. doi: 10.1038/bjc.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Williams KE, Barnes NL, Cramer A, Johnson R, Cheema K, Morris J, et al. Molecular phenotypes of DCIS predict overall and invasive recurrence. Ann Oncol. 2015;26:1019–1025. doi: 10.1093/annonc/mdv062. [DOI] [PubMed] [Google Scholar]
- 124.Cancer Genome Atlas Research Network Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, Sastre-Garau X, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18–20. doi: 10.1038/12615. [DOI] [PubMed] [Google Scholar]
- 126.Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR landscape in cancer: analysis of 4853 tumors by next generation sequencing. Clin Cancer Res. 2016;22:259–267. doi: 10.1158/1078-0432.CCR-14-3212. [DOI] [PubMed] [Google Scholar]
- 127.Tefferi A, Lasho TL, Gilliland G. JAK2 mutations in myeloproliferative disorders. N Engl J Med. 2005;353:1416–1417. doi: 10.1056/NEJMc051878. [DOI] [PubMed] [Google Scholar]
- 128.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 129.Beer PA, Delhommeau F, LeCouedic JP, Dawson MA, Chen E, Bareford D, et al. Two routes to leukemic transformation after a JAK2 mutation-positive myeloproliferative neoplasm. Blood. 2010;115:2891–2900. doi: 10.1182/blood-2009-08-236596. [DOI] [PubMed] [Google Scholar]
- 130.Fiala C, Diamandis EP. Utility of circulating tumor DNA in cancer diagnostics with emphasis on early detection. BMC Med. 2018;16:166. doi: 10.1186/s12916-018-1157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang X, Shi XQ, Zeng PW, Mo FM, Chen ZH. Circulating cell free DNA as the diagnostic marker for colorectal cancer: a systematic review and meta-analysis. Oncotarget. 2018;9:24514–24524. doi: 10.18632/oncotarget.25314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu D, Tong Y, Guo X, Feng L, Jiang Z, Ying S, et al. Diagnostic value of concentration of circulating cell-free DNA in breast cancer: a meta-analysis. Front Oncol. 2019;9:95. doi: 10.3389/fonc.2019.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Filippova Darya, Larson Matthew H., Maher M. Cyrus, Calef Robert, Pimentel Monica, Zhou Yiqi, Newman Joshua, Gross Samuel, Nicula Virgil, Liu Ting-Chun, Yakym Christopher, Berman Jennifer, Aravanis Alex, Jamshidi Arash. The Circulating Cell-free Genome Atlas (CCGA) Study: Size selection of cell-free DNA (cfDNA) fragments. Journal of Clinical Oncology. 2019;37(15_suppl):3103–3103. doi: 10.1200/JCO.2019.37.15_suppl.3103. [DOI] [Google Scholar]
- 134.Kim G, Ison G, McKee AE, Zhang H, Tang S, Gwise T, et al. FDA approval summary: olaparib monotherapy in patients with deleterious germline BRCA-mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clin Cancer Res. 2015;21:4257–4261. doi: 10.1158/1078-0432.CCR-15-0887. [DOI] [PubMed] [Google Scholar]
- 135.Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 136.Tung Nadine M., Im Seock-Ah, Senkus-Konefka Elzbieta, Xu Binghe, Domchek Susan M., Masuda Norikazu, Li Wei, Armstrong Anne Caroline, Conte Pier Franco, Wu Wenting, Goessl Carsten Dietrich, Runswick Sarah, Robson Mark E. Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer (OlympiAD): Efficacy in patients with visceral metastases. Journal of Clinical Oncology. 2018;36(15_suppl):1052–1052. doi: 10.1200/JCO.2018.36.15_suppl.1052. [DOI] [Google Scholar]
- 137.Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Olsen AL, Feany MB. PARP inhibitors and Parkinson's disease. N Engl J Med. 2019;380:492–494. doi: 10.1056/NEJMcibr1814680. [DOI] [PubMed] [Google Scholar]
- 139.Kato Shumei, Lippman Scott M., Flaherty Keith T., Kurzrock Razelle. The Conundrum of Genetic “Drivers” in Benign Conditions. Journal of the National Cancer Institute. 2016;108(8):djw036. doi: 10.1093/jnci/djw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 141.Hong T, Yan Y, Li J, Radovanovic I, Ma X, Shao YW, et al. High prevalence of KRAS/BRAF somatic mutations in brain and spinal cord arteriovenous malformations. Brain. 2019;142:23–34. doi: 10.1093/brain/awy307. [DOI] [PubMed] [Google Scholar]
- 142.Angelo LS, Talpaz M, Kurzrock R. Autocrine interleukin-6 production in renal cell carcinoma: evidence for the involvement of p53. Cancer Res. 2002;62:932–940. [PubMed] [Google Scholar]
- 143.Zhang T, Li H, Shi J, Li S, Li M, Zhang L, et al. p53 predominantly regulates IL-6 production and suppresses synovial inflammation in fibroblast-like synoviocytes and adjuvant-induced arthritis. Arthritis Res Ther. 2016;18:271. doi: 10.1186/s13075-016-1161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tsao H, Bevona C, Goggins W, Quinn T. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: a population-based estimate. Arch Dermatol. 2003;139:282–288. doi: 10.1001/archderm.139.3.282. [DOI] [PubMed] [Google Scholar]
- 145.Tiersten AD, Grin CM, Kopf AW, Gottlieb GJ, Bart RS, Rigel DS, et al. Prospective follow-up for malignant melanoma in patients with atypical-mole (dysplastic-nevus) syndrome. J Dermatol Surg Oncol. 1991;17:44–48. doi: 10.1111/j.1524-4725.1991.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 146.Hoeflich KP, Gray DC, Eby MT, Tien JY, Wong L, Bower J, et al. Oncogenic BRAF is required for tumor growth and maintenance in melanoma models. Cancer Res. 2006;66:999–1006. doi: 10.1158/0008-5472.CAN-05-2720. [DOI] [PubMed] [Google Scholar]
- 147.Kurzrock Razelle, Parulkar Rahul, Yeatman Timothy Joseph, El-Deiry Wafik S., Pluard Timothy J., Garner Chad, Reddy Sandeep K. Seventeen percent of NGS 50 gene panel variants are not expressed in RNAseq. Journal of Clinical Oncology. 2018;36(15_suppl):12118–12118. doi: 10.1200/JCO.2018.36.15_suppl.12118. [DOI] [Google Scholar]
- 148.Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Anandakrishnan R, Varghese RT, Kinney NA, Garner HR. Estimating the number of genetic mutations (hits) required for carcinogenesis based on the distribution of somatic mutations. PLoS Comput Biol. 2019;15:e1006881. doi: 10.1371/journal.pcbi.1006881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fernandez-Antoran D, Piedrafita G, Murai K, Ong SH, Herms A, Frezza C, Jones PH. Outcompeting p53-mutant cells in the normal esophagus by redox manipulation. Cell Stem Cell. 2019;25:329–341. doi: 10.1016/j.stem.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yokoyama A, Kakiuchi N, Yoshizato T, Nannya Y, Suzuki H, Takeuchi Y, et al. Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature. 2019;565:312–317. doi: 10.1038/s41586-018-0811-x. [DOI] [PubMed] [Google Scholar]
- 152.Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348:880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Buscarlet M, Provost S, Zada YF, Barhdadi A, Bourgoin V, Lépine G, et al. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood. 2017;130:753–762. doi: 10.1182/blood-2017-04-777029. [DOI] [PubMed] [Google Scholar]
- 154.Coombs CC, Zehir A, Devlin SM, Kishtagari A, Syed A, Jonsson P, et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017;21:374–382. doi: 10.1016/j.stem.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ptashkin RN, Mandelker DL, Coombs CC, Bolton K, Yelskaya Z, Hyman DM, et al. Prevalence of clonal hematopoiesis mutations in tumor-only clinical genomic profiling of solid tumors. JAMA Oncol. 2018;4:1589–1593. doi: 10.1001/jamaoncol.2018.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Coombs CC, Gillis NK, Tan X, Berg JS, Ball M, Balasis ME, et al. Identification of clonal hematopoiesis mutations in solid tumor patients undergoing unpaired next-generation sequencing assays. Clin Cancer Res. 2018;24:5918–5924. doi: 10.1158/1078-0432.CCR-18-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee-Six H, Olafsson S, Ellis P, Osborne RJ, Sanders MA, Moore L, et al. The landscape of somatic mutation in normal colorectal epithelial cells. Nature. 2019;574:532–537. doi: 10.1038/s41586-019-1672-7. [DOI] [PubMed] [Google Scholar]
- 158.Martincorena I. Somatic mutation and clonal expansions in human tissues. Genome Med. 2019;11:35. doi: 10.1186/s13073-019-0648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Corthay A. Does the immune system naturally protect against cancer? Front Immunol. 2014;5:197. doi: 10.3389/fimmu.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Marty R, Kaabinejadian S, Rossell D, Slifker MJ, van de Haar J, Engin HB, et al. MHC-I genotype restricts the oncogenic mutational landscape. Cell. 2017;171:1272–1283. doi: 10.1016/j.cell.2017.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Halaban R, Krauthammer M. RASopathy gene mutations in melanoma. J Invest Dermatol. 2016;136:1755–1759. doi: 10.1016/j.jid.2016.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hafner C, Groesser L. Mosaic RASopathies. Cell Cycle. 2013;12:43–50. doi: 10.4161/cc.23108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van Leeuwaarde RS, Ahmad S, Links TP, Giles RH. Von Hippel-Lindau syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al., editors. GeneReviews. Seattle: University of Washington; 2000.
- 165.Yurgelun MB, Chenevix-Trench G, Lippman SM. Translating germline cancer risk into precision prevention. Cell. 2017;168:566–570. doi: 10.1016/j.cell.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 166.Brosseau JP, Liao CP, Wang Y, Ramani V, Vandergriff T, Lee M, et al. NF1 heterozygosity fosters de novo tumorigenesis but impairs malignant transformation. Nat Commun. 2018;9:5014. doi: 10.1038/s41467-018-07452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Freed D, Stevens EL, Pevsner J. Somatic mosaicism in the human genome. Genes (Basel) 2014;5:1064–1094. doi: 10.3390/genes5041064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Campbell IM, Shaw CA, Stankiewicz P, Lupski JR. Somatic mosaicism: implications for disease and transmission genetics. Trends Genet. 2015;31:382–392. doi: 10.1016/j.tig.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Thompson CA. FDA approves tocilizumab to treat rheumatoid arthritis. Am J Health Syst Pharm. 2010;67:254. doi: 10.2146/news100012. [DOI] [PubMed] [Google Scholar]
- 170.Goodman AM, Cohen PR, Li A, Hinds B, Kurzrock R. Schnitzler syndrome associated with MYD88 L265P mutation. JAAD Case Rep. 2019;5:312–316. doi: 10.1016/j.jdcr.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Leoni C, Onesimo R, Resta N, Patti ML, De Santis R, Bagnulo R, et al. Old treatments for new genetic conditions: sirolimus therapy in a child affected by mosaic overgrowth with fibroadipose hyperplasia. Clin Genet. 2019;96:102–103. doi: 10.1111/cge.13550. [DOI] [PubMed] [Google Scholar]
- 172.Jonquoy A, Mugniery E, Benoist-Lasselin C, Kaci N, Le Corre L, Barbault F, et al. A novel tyrosine kinase inhibitor restores chondrocyte differentiation and promotes bone growth in a gain-of-function Fgfr3 mouse model. Hum Mol Genet. 2012;21:841–851. doi: 10.1093/hmg/ddr514. [DOI] [PubMed] [Google Scholar]
- 173.Harbour JW. The genetics of uveal melanoma: an emerging framework for targeted therapy. Pigment Cell Melanoma Res. 2012;25:171–181. doi: 10.1111/j.1755-148X.2012.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen X, Wu Q, Depeille P, et al. RasGRP3 mediates MAPK pathway activation in GNAQ mutant uveal melanoma. Cancer Cell. 2017;31:685–696. doi: 10.1016/j.ccell.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Comi A. Current therapeutic options in Sturge-weber syndrome. Semin Pediatr Neurol. 2015;22:295–301. doi: 10.1016/j.spen.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Baldassari S, Ribierre T, Marsan E, Adle-Biassette H, Ferrand-Sorbets S, Bulteau C, et al. Dissecting the genetic basis of focal cortical dysplasia: a large cohort study. Acta Neuropathol. 2019;138:885–900. doi: 10.1007/s00401-019-02061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Muhlebner A, Bongaarts A, Sarnat HB, Scholl T, Aronica E. New insights into a spectrum of developmental malformations related to mTOR dysregulations: challenges and perspectives. J Anat. 2019;235:521–542. doi: 10.1111/joa.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 179.Bozic I, Antal T, Ohtsuki H, Carter H, Kim D, Chen S, et al. Accumulation of driver and passenger mutations during tumor progression. Proc Natl Acad Sci U S A. 2010;107:18545–18550. doi: 10.1073/pnas.1010978107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McFarland CD, Yaglom JA, Wojtkowiak JW, Scott JG, Morse DL, Sherman MY, Mirny LA. The damaging effect of passenger mutations on cancer progression. Cancer Res. 2017;77:4763–4772. doi: 10.1158/0008-5472.CAN-15-3283-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wodarz Dominik, Newell Alan C., Komarova Natalia L. Passenger mutations can accelerate tumour suppressor gene inactivation in cancer evolution. Journal of The Royal Society Interface. 2018;15(143):20170967. doi: 10.1098/rsif.2017.0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Reeves MQ, Kandyba E, Harris S, et al. Multicolour lineage tracing reveals clonal dynamics of squamous carcinoma evolution from initiation to metastasis. Nat Cell Biol. 2018;20:699–709. doi: 10.1038/s41556-018-0109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hess JM, Bernards A, Kim J, Miller M, Taylor-Weiner A, Haradhvala NJ, et al. Passenger hotspot mutations in cancer. Cancer Cell. 2019;36:288–301. doi: 10.1016/j.ccell.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Aynardi J, Manur R, Hess PR, Chekol S, Morrissette JJD, Babushok D, et al. JAK2 V617F-positive acute myeloid leukaemia (AML): a comparison between de novo AML and secondary AML transformed from an underlying myeloproliferative neoplasm. A study from the bone marrow pathology group. Br J Haematol. 2018;182:78–85. doi: 10.1111/bjh.15276. [DOI] [PubMed] [Google Scholar]
- 185.Gaymes TJ, Mohamedali A, Eiliazadeh AL, Darling D, Mufti GJ. FLT3 and JAK2 mutations in acute myeloid leukemia promote interchromosomal homologous recombination and the potential for copy neutral loss of heterozygosity. Cancer Res. 2017;77:1697–1708. doi: 10.1158/0008-5472.CAN-16-1678. [DOI] [PubMed] [Google Scholar]
- 186.Patton EE, Widlund HR, Kutok JL, Kopani KR, Amatruda JF, Murphey RD, et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol. 2005;15:249–254. doi: 10.1016/j.cub.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 187.Kato S, Kurzrock R. An avatar for precision cancer therapy. Nat Biotechnol. 2018;36:1053–1055. doi: 10.1038/nbt.4293. [DOI] [PubMed] [Google Scholar]
- 188.Schöffski P, Sufliarsky J, Gelderblom H, Blay JY, Strauss SJ, Stacchiotti S, et al. Crizotinib in patients with advanced, inoperable inflammatory myofibroblastic tumours with and without anaplastic lymphoma kinase gene alterations (European Organisation for Research and Treatment of Cancer 90101 CREATE): a multicentre, single-drug, prospective, non-randomised phase 2 trial. Lancet Respir Med. 2018;6:431–441. doi: 10.1016/S2213-2600(18)30116-4. [DOI] [PubMed] [Google Scholar]
- 189.Burns K, Martinon F, Esslinger C, Pahl H, Schneider P, Bodmer JL, et al. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273:12203–12209. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- 190.Py C, Christinat Y, Kreutzfeldt M, McKee TA, Dietrich P-Y, Tsantoulis P. Response of NF1-mutated melanoma to an MEK inhibitor. JCO Precision Oncol 2018. https://ascopubs.org/doi/full/10.1200/PO.18.00028. Accessed 16 Dec 2019. [DOI] [PubMed]
- 191.NF1 mutations prevalent but not clinically relevant. Cancer Discov. 2015. doi: 10.1158/2159-8290.CD-NB2015-119. [DOI] [PubMed]
- 192.Krauthammer M, Kong Y, Bacchiocchi A, Evans P, Pornputtapong N, Wu C, et al. Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat Genet. 2015;47:996–1002. doi: 10.1038/ng.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Helsten T, Schwaederle M, Kurzrock R. Fibroblast growth factor receptor signaling in hereditary and neoplastic disease: biologic and clinical implications. Cancer Metastasis Rev. 2015;34:479–496. doi: 10.1007/s10555-015-9579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Barbany G, Arthur C, Lieden A, Nordenskjöld M, Rosenquist R, Tesi B, et al. Cell-free tumour DNA testing for early detection of cancer—a potential future tool. J Intern Med. 2019;286:118–136. doi: 10.1111/joim.12897. [DOI] [PubMed] [Google Scholar]
- 195.Shatsky R, Parker BA, Bui NQ, Helsten T, Schwab RB, Boles SG, Kurzrock R. Next-generation sequencing of tissue and circulating tumor DNA: The UC San Diego Moores Center for personalized cancer therapy experience with breast malignancies. Mol Cancer Ther. 2019;18:1001–1011. doi: 10.1158/1535-7163.MCT-17-1038. [DOI] [PubMed] [Google Scholar]
- 196.Kato S, Okamura R, Baumgartner JM, Patel H, Leichman L, Kelly K, et al. Analysis of circulating tumor DNA and clinical correlates in patients with esophageal, gastroesophageal junction, and gastric adenocarcinoma. Clin Cancer Res. 2018;24:6248–6256. doi: 10.1158/1078-0432.CCR-18-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kato S, Schwaederle MC, Fanta PT, Okamura R, Leichman L, Lippman SM, et al. Genomic assessment of blood-derived circulating tumor DNA in patients with colorectal cancers: correlation with tissue sequencing, therapeutic response, and survival. JCO Precis Oncol. 2019;3 10.1200/PO.18.00158. [DOI] [PMC free article] [PubMed]
- 198.Fiala C, Kulasingam V, Diamandis EP. Circulating tumor DNA for early cancer detection. J Appl Lab Med. 2018;3:300–313. doi: 10.1373/jalm.2018.026393. [DOI] [PubMed] [Google Scholar]
- 199.Zhang L, Liang Y, Li S, Zeng F, Meng Y, Chen Z, et al. The interplay of circulating tumor DNA and chromatin modification, therapeutic resistance, and metastasis. Mol Cancer. 2019;18:36. doi: 10.1186/s12943-019-0989-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Elazezy M, Joosse SA. Techniques of using circulating tumor DNA as a liquid biopsy component in cancer management. Comput Struct Biotechnol J. 2018;16:370–378. doi: 10.1016/j.csbj.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dharajiya NG, Grosu DS, Farkas DH, McCullough RM, Almasri E, Sun Y, et al. Incidental detection of maternal neoplasia in noninvasive prenatal testing. Clin Chem. 2018;64:329–335. doi: 10.1373/clinchem.2017.277517. [DOI] [PubMed] [Google Scholar]
- 202.Heitzer E, Perakis S, Geigl JB, Speicher MR. The potential of liquid biopsies for the early detection of cancer. NPJ Precis Oncol. 2017;1:36. doi: 10.1038/s41698-017-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, Ebert BL. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhao H, Zhang X, Han Z, Wang Y. Circulating anti-p16a IgG autoantibodies as a potential prognostic biomarker for non-small cell lung cancer. FEBS Open Bio. 2018;8:1875–1881. doi: 10.1002/2211-5463.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Djureinovic D, Dodig-Crnković T, Hellström C, Holgersson G, Bergqvist M, Mattsson JSM, et al. Detection of autoantibodies against cancer-testis antigens in non-small cell lung cancer. Lung Cancer. 2018;125:157–163. doi: 10.1016/j.lungcan.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 206.Xu L, Lee JR, Hao S, Ling XB, Brooks JD, Wang SX, Gambhir SS. Improved detection of prostate cancer using a magneto-nanosensor assay for serum circulating autoantibodies. PLoS One. 2019;14:e0221051. doi: 10.1371/journal.pone.0221051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhao H, Zhang X, Han Z, Xie W, Yang W, Wei J. Alteration of circulating natural autoantibodies to CD25-derived peptide antigens and FOXP3 in non-small cell lung cancer. Sci Rep. 2018;8:9847. doi: 10.1038/s41598-018-28277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Van Hoesen K, Meynier S, Ribaux P, Petignat P, Delie F, Cohen M. Circulating GRP78 antibodies from ovarian cancer patients: a promising tool for cancer cell targeting drug delivery system? Oncotarget. 2017;8:107176–107187. doi: 10.18632/oncotarget.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hochst B, Diehl L. Antigen shedding into the circulation contributes to tumor immune escape. Oncoimmunology. 2012;1:1620–1622. doi: 10.4161/onci.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mather Q, Priego J, Ward K, Kundan V, Tran D, Dwivedi A, Bryan BA. A novel protein expression signature differentiates benign lipomas from well-differentiated liposarcomas. Mol Clin Oncol. 2017;7:315–321. doi: 10.3892/mco.2017.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang QX, Chen ED, Cai YF, Li Q, Jin YX, Jin WX, et al. A panel of four genes accurately differentiates benign from malignant thyroid nodules. J Exp Clin Cancer Res. 2016;35:169. doi: 10.1186/s13046-016-0447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Prasad NB, Somervell H, Tufano RP, Dackiw AP, Marohn MR, Califano JA, et al. Identification of genes differentially expressed in benign versus malignant thyroid tumors. Clin Cancer Res. 2008;14:3327–3337. doi: 10.1158/1078-0432.CCR-07-4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kukreti S, Cerussi AE, Tanamai W, Hsiang D, Tromberg BJ, Gratton E. Characterization of metabolic differences between benign and malignant tumors: high-spectral-resolution diffuse optical spectroscopy. Radiology. 2010;254:277–284. doi: 10.1148/radiol.09082134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mazzanti C, Zeiger MA, Costouros NG, Umbricht C, Westra WH, Smith D, et al. Using gene expression profiling to differentiate benign versus malignant thyroid tumors. Cancer Res. 2004;64:2898–2903. doi: 10.1158/0008-5472.CAN-03-3811. [DOI] [PubMed] [Google Scholar]
- 215.Sina AA, Carrascosa LG, Liang Z, Grewal YS, Wardiana A, Shiddiky MJA, et al. Epigenetically reprogrammed methylation landscape drives the DNA self-assembly and serves as a universal cancer biomarker. Nat Commun. 2018;9:4915. doi: 10.1038/s41467-018-07214-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kurzrock R, Gutterman JU, Talpaz M. The molecular genetics of Philadelphia chromosome-positive leukemias. N Engl J Med. 1988;319:990–998. doi: 10.1056/NEJM198810133191506. [DOI] [PubMed] [Google Scholar]
- 217.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 218.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 219.Schwaederle M, Parker BA, Schwab RB, Daniels GA, Piccioni DE, Kesari S, et al. Precision oncology: The UC San Diego Moores Cancer Center PREDICT Experience. Mol Cancer Ther. 2016;15:743–752. doi: 10.1158/1535-7163.MCT-15-0795. [DOI] [PubMed] [Google Scholar]
- 220.Schwaederle M, Zhao M, Lee JJ, Eggermont AM, Schilsky RL, Mendelsohn J, et al. Impact of precision medicine in diverse cancers: a meta-analysis of phase II clinical trials. J Clin Oncol. 2015;33:3817–3825. doi: 10.1200/JCO.2015.61.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Schwaederle M, Zhao M, Lee JJ, Lazar V, Leyland-Jones B, Schilsky RL, et al. Association of biomarker-based treatment strategies with response rates and progression-free survival in refractory malignant neoplasms: a meta-analysis. JAMA Oncol. 2016;2:1452–1459. doi: 10.1001/jamaoncol.2016.2129. [DOI] [PubMed] [Google Scholar]
- 222.Fontes Jardim Denis L., Schwaederle Maria, Wei Caimiao, Lee J. Jack, Hong David S., Eggermont Alexander M., Schilsky Richard L., Mendelsohn John, Lazar Vladimir, Kurzrock Razelle. Impact of a Biomarker-Based Strategy on Oncology Drug Development: A Meta-analysis of Clinical Trials Leading to FDA Approval. Journal of the National Cancer Institute. 2015;107(11):djv253. doi: 10.1093/jnci/djv253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sicklick JK, Kato S, Okamura R, Schwaederle M, Hahn ME, Williams CB, et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med. 2019;25:744–750. doi: 10.1038/s41591-019-0407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rodon J, Soria JC, Berger R, Miller WH, Rubin E, Kugel A, et al. Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial. Nat Med. 2019;25:751–758. doi: 10.1038/s41591-019-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rothwell DG, Ayub M, Cook N, et al. Utility of ctDNA to support patient selection for early phase clinical trials: the TARGET study. Nat Med. 2019;25:738–743. doi: 10.1038/s41591-019-0380-z. [DOI] [PubMed] [Google Scholar]
- 226.Janku F, Hong DS, Fu S, et al. Assessing PIK3CA and PTEN in early-phase trials with PI3K/AKT/mTOR inhibitors. Cell Rep. 2014;6:377–387. doi: 10.1016/j.celrep.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. 2018;36:536–542. doi: 10.1200/JCO.2017.75.3780. [DOI] [PubMed] [Google Scholar]
- 228.Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]