Abstract
Despite decades of unequivocal evidence that waist circumference provides both independent and additive information to BMI for predicting morbidity and risk of death, this measurement is not routinely obtained in clinical practice. This Consensus Statement proposes that measurements of waist circumference afford practitioners with an important opportunity to improve the management and health of patients. We argue that BMI alone is not sufficient to properly assess or manage the cardiometabolic risk associated with increased adiposity in adults and provide a thorough review of the evidence that will empower health practitioners and professional societies to routinely include waist circumference in the evaluation and management of patients with overweight or obesity. We recommend that decreases in waist circumference are a critically important treatment target for reducing adverse health risks for both men and women. Moreover, we describe evidence that clinically relevant reductions in waist circumference can be achieved by routine, moderate-intensity exercise and/or dietary interventions. We identify gaps in the knowledge, including the refinement of waist circumference threshold values for a given BMI category, to optimize obesity risk stratification across age, sex and ethnicity. We recommend that health professionals are trained to properly perform this simple measurement and consider it as an important ‘vital sign’ in clinical practice.
Subject terms: Disease prevention, Obesity, Metabolic syndrome, Predictive markers
In this Consensus Statement, the International Atherosclerosis Society and International Chair on Cardiometabolic Risk Working Group on Visceral Obesity recommend that waist circumference be included routinely as a measurement in clinical practice. They summarize the evidence that waist circumference and BMI together can provide improved assessments of cardiometabolic risk compared with either measurement alone.
Introduction
The prevalence of adult overweight and obesity as defined using BMI has increased worldwide since the 1980s, with no country demonstrating any successful declines in the 33 years of recorded data1. Obesity is a major public health problem worldwide2 and reliance on measurements of BMI alone has proven inadequate to help clinicians assess and manage obesity-related health risk in their patients. For instance, although many individuals with overweight or obesity will develop cardiometabolic health complications such as type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) during their lifetimes, a sizeable minority will remain free of these chronic diseases, a phenomenon that has been described as metabolically healthy obesity (MHO).
The prevalence of MHO among adults varies greatly between studies owing to differences in age, ethnicity and environmental factors, as well as the lack of a universal definition of metabolic health and a universal classification system for obesity3. Furthermore, studies with long-term follow-up periods have generally found that MHO is often a temporary or transition state for most individuals with obesity. For example, in a study with a 20-year follow-up, approximately half of adults with MHO (defined in this study as having less than two cardiometabolic parameters that fall outside of healthy ranges) became metabolically unhealthy by the end of the study. Moreover, study participants with MHO were at increased risk of cardiovascular events after long-term follow-up4. Similarly, a study considering the full range of possible definitions for MHO suggested that the risk of a cardiovascular event associated with the MHO phenotype increased with longer follow-up times. Furthermore, similar CVD risk estimates were observed when MHO was defined by criteria other than the absence of the metabolic syndrome5. Despite the fact that the limitations of BMI as an index for obesity have been known for decades, several obesity guidelines worldwide remain steadfast in the recommendation that BMI alone be the measure to characterize obesity-related morbidity and risk of death6–9.
The failure of BMI to fully capture cardiometabolic risk is partially related to the fact that BMI in isolation is an insufficient biomarker of abdominal adiposity. Waist circumference is a simple method to assess abdominal adiposity that is easy to standardize and clinically apply. Waist circumference is strongly associated with all-cause10,11 and cardiovascular mortality12,13 with or without adjustment for BMI10,14. However, the full strength of the association between waist circumference with morbidity and mortality is realized only after adjustment for BMI10,15,16. Thus, waist circumference enables a further refinement of the adverse health risk characterized by BMI and this measurement should be included when stratifying obesity-related health risk. Indeed, resistance to the routine inclusion of waist circumference in clinical practice not only ignores the evidence of its utility, but fails to take advantage of opportunities to counsel patients regarding the higher-risk phenotype of obesity. In addition, the measurement of both BMI and waist circumference will provide unique opportunities to follow the utility of treatment and effectiveness of interventions designed to manage obesity and related metabolic disease.
In 2017, the International Atherosclerosis Society (IAS) and International Chair on Cardiometabolic Risk (ICCR) Working Group on Visceral Obesity convened in Prague, Czech Republic, to discuss the importance of abdominal obesity as a risk factor for premature atherosclerosis and CVD in adults (Supplementary Information). The group agreed to work on the development of consensus documents which would reflect the position of the two organizations. In this Consensus Statement, we summarize the evidence that BMI alone is not sufficient to properly assess, evaluate or manage the cardiometabolic risk associated with increased adiposity and recommend that waist circumference be adopted as a routine measurement in clinical practice alongside BMI to classify obesity.
Methodology
This Consensus Statement is designed to provide the consensus of the IAS and ICCR Working Group (Supplementary Information) on waist circumference as an anthropometric measure that improves patient management. The Consensus Statement was developed as follows. The first face-to-face meeting occurred on 24 April 2017 to review the high-quality evidence available and known to the subject experts. After discussion and deliberation amongst the experts regarding the context and quality of the evidence, an executive writing group (R.R., I.J.N., J.-P.D., J.S. and Y.M.) was appointed and tasked with writing the first draft. The draft was subsequently circulated to all authors for critical revision of intellectual content pertinent to each authors’ expertise. High-quality published literature that became available after the initial face-to-face meeting (through June 2019) was identified by all authors and reviewed by the executive writing group for inclusion in the manuscript. The first author coordinated the final preparation and submission of the Consensus Statement after the group achieved consensus and approved its content.
Historical perspective
The importance of body fat distribution as a risk factor for several diseases (for example, CVD, hypertension, stroke and T2DM) and mortality has been recognized for several decades. In 1956, Jean Vague was the first to show the importance of fat distribution in relation to various diseases, describing what he termed ‘android’ and ‘gynoid’ types of obesity17. These classifications were later interpreted by Ahmed Kissebah and colleagues as upper versus lower body fat accumulation as reflected by a high or low waist–hip circumference ratio (WHR), respectively18. The upper and lower body fat accumulation phenotypes were based on body morphology as assessed by external anthropometric measures such as skinfolds and circumferences.
The WHR increased in popularity when epidemiologists in the USA and Sweden showed that WHR, separately or in combination with BMI, was associated with increased risk of death, CVD and T2DM19–22, findings that were subsequently confirmed in many studies. However, later evidence indicated that, compared with the WHR, waist circumference alone was more strongly associated with the absolute amount of intra-abdominal or visceral fat, the fat depot that conveys the strongest health risk23,24. Furthermore, when a ratio such as WHR is used to follow changes in regional adipose depots, the utility of the ratio is limited when both the numerator and denominator values change in response to treatment. Consequently, the combination of WHR and BMI for assessing obesity risk were replaced by single threshold values for waist circumference alone25. The NIH was the first to use the threshold values for waist circumference (≥88 cm in women and ≥102 cm in men) as suggested by Michael Lean and colleagues, in combination with a classification of overall obesity as assessed by BMI25. Although the use of these specific waist circumference values to identify white adults with abdominal obesity remains a cornerstone of obesity guidelines worldwide, we present evidence to challenge the supportive rationale and provide evidence in support of alternative waist circumference values to be used in concert with BMI.
As an alternative to measurements of waist circumference, the WHR or waist–thigh circumference ratio, Margaret Ashwell and others proposed the waist–height ratio as a measure of abdominal obesity26,27. Compared with the previous measurements, the waist–height ratio shows similar and sometimes slightly stronger associations with the risk of CVD or T2DM28,29. An explanation for why adding height increases the prediction of disease risk might be because short stature is associated with increased risk of CVD30. In growing children and adolescents, the waist–height ratio could be more useful for the classification of abdominal obesity than waist circumference alone. However, in fully grown adults, the waist–height ratio is less useful as height is generally fixed and the value can only be altered by changes in waist circumference. Moreover, height is only marginally associated with waist circumference31. For the assessment of the effectiveness of lifestyle changes in adults, waist circumference might be preferred as a simple tool. Other alternatives to waist circumference have included the conicity index32 and the abdominal obesity index33, but they are, at best, only slightly better predictors of disease risk than waist circumference alone.
Prevalence of abdominal obesity
Despite a strong association between waist circumference and BMI at the population level, emerging evidence suggests that, across populations, waist circumference might be increasing beyond what is expected according to BMI. In other words, the phenotype of obesity might be changing over time to one that reflects an increase in abdominal adiposity34. For example, Ian Janssen and colleagues examined the changes in waist circumference for a given BMI over a 30-year period in a Canadian sample35. Notably, for a given BMI, Canadians had a larger waist circumference in 2007 compared with 1981. Specifically, the researchers observed a waist circumference that was greater by 1.1 cm in men and 4.9 cm in women for a BMI of 25 kg/m2 between 1981 and 2007. Similarly, Sandra Albrecht and colleagues examined the secular changes in waist circumference in the USA (1988–2007), England (1992–2008), China (1993–2011) and Mexico (1999–2012)36 and reported statistically significantly increased waist circumference values relative to BMI in all countries studied and in most subpopulations.
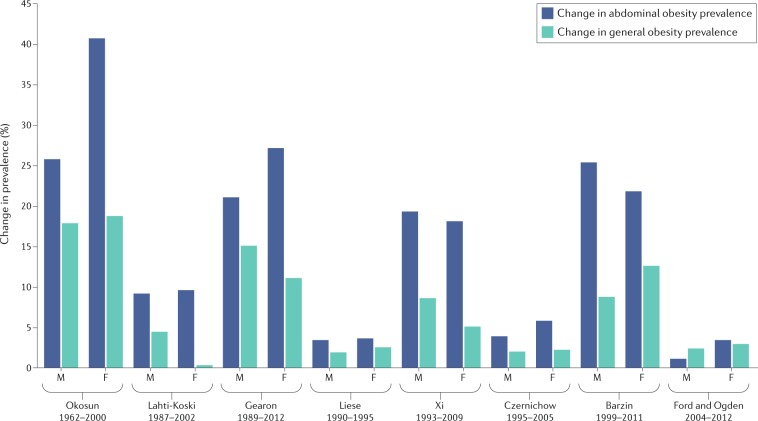
These observations are consistent with those of Tommy Visscher and colleagues, who performed an extensive review and concluded that the majority of the evidence suggests a trend in which the relative increases in waist circumference were larger than the relative increases in BMI37. This observation is seemingly independent of age, sex and ethnicity, as few groups failed to demonstrate the general trend of secular waist circumference increasing beyond that expected by BMI (Fig. 1). The failure of BMI to detect such an increase in abdominal obesity confirms the limitations of BMI alone to identify the phenotype of obesity that conveys the greatest health risk.
Fig. 1. The prevalence of abdominal obesity and obesity measured in different studies.
Changes in the prevalence of abdominal obesity (measured using waist circumference) and general obesity (measured using BMI) measured in different studies during the time period indicated on the x axis. General obesity was defined as BMI ≥30 kg/m2. Abdominal obesity was defined as waist circumference ≥88 cm and ≥102 cm for women and men, respectively. However, Xi et al. defined general obesity as BMI ≥28 kg/m2 and abdominal obesity as waist circumference ≥80 cm and ≥85 cm for Chinese women and men, respectively113. In addition, Barzin et al. defined general obesity as BMI ≥30 kg/m2 and abdominal obesity as waist circumference ≥91 cm and ≥89 cm for Iranian women and men, respectively114. Years given (for example, 1999–2011) indicate the years in which data were collected. F, female; M, male. Data are from refs37,113–121.
Conclusions and recommendations — prevalence of abdominal obesity
Although the prevalence of obesity measured by BMI might have plateaued in some countries, the prevalence of abdominal obesity as measured by waist circumference is generally increasing.
The lack of inclusion of waist circumference in global obesity surveillance might inadequately characterize the health risk associated with the global obesity prevalence, as it seems that the prevalence of abdominal obesity is increasing.
Current obesity prevalence trends based on BMI alone should be interpreted with caution. We recommend that serious consideration should be given to the inclusion of waist circumference in obesity surveillance studies.
Identifying the high-risk obesity phenotype
Waist circumference, BMI and health outcomes — categorical analysis
It is not surprising that waist circumference and BMI alone are positively associated with morbidity15 and mortality13 independent of age, sex and ethnicity, given the strong association between these anthropometric variables across cohorts. However, it is also well established that, for any given BMI, the variation in waist circumference is considerable, and, in any given BMI category, adults with higher waist circumference values are at increased adverse health risk compared with those with a lower waist circumference38–40. This observation is well illustrated by James Cerhan and colleagues, who pooled data from 11 prospective cohort studies with 650,386 white adults from the USA, Australia and Sweden aged 20–83 years11. In this study, the authors observed that waist circumference was positively associated with mortality within every BMI category examined, from 20 kg/m2 to 50 kg/m2. This finding is consistent with that of Ellen de Hollander and colleagues, who performed a meta-analysis involving over 58,000 predominantly white older adults from around the world and reported that the age-adjusted and smoking-adjusted mortality was substantially greater for those with an elevated waist circumference within normal weight, overweight and obese categories as defined by BMI41. The ability of waist circumference to add to the adverse health risk observed within a given BMI category provides the basis for the current classification system used to characterize obesity-related health risk8,42.
Waist circumference, BMI and health outcomes — continuous analysis
Despite the observation that the association between waist circumference and adverse health risk varies across BMI categories11, current obesity-risk classification systems recommend using the same waist circumference threshold values for all BMI categories42. We propose that important information about BMI and waist circumference is lost when they are converted from continuous to broad categorical variables and that this loss of information affects the manner in which BMI and waist circumference predict morbidity and mortality. Specifically, when BMI and waist circumference are considered as categorical variables in the same risk prediction model, they are both positively related to morbidity and mortality38. However, when BMI and waist circumference are considered as continuous variables in the same risk prediction model, risk prediction by waist circumference improves, whereas the association between BMI and adverse health risk is weakened10,43. The full strength of the association between waist circumference with morbidity and/or mortality is not fully realized until adjustment for BMI11,12,41.
Evidence in support of adjusting waist circumference for BMI comes from Janne Bigaard and colleagues who report that a strong association exists between waist circumference and all-cause mortality after adjustment for BMI43. For example, a 10% larger waist circumference corresponded to a 1.48 (95% CI 1.36–1.61) times higher mortality over the whole range of waist circumference in both men and women after adjustment for BMI. This observation was confirmed by Tobias Pischon and colleagues, who observed that the highest quintile of waist circumference (≥102.7 cm in men and ≥89.0 cm in women) was associated with an increased risk of all-cause death of 1.33 (95% CI 1.24–1.44) before BMI adjustment, with an increased risk of death of 2.05 (95% CI 1.80–2.33) after adjustment for BMI10.
Consistent with observations based on asymptomatic adults, Thais Coutinho and colleagues report similar observations for a cohort of 14,284 adults with CVD who were followed up for 2.3 years (5,696 deaths). The cohort was divided into tertiles for both waist circumference and BMI. In comparison with the lowest waist circumference tertile, a significant association with risk of death was observed for the highest tertile for waist circumference after adjustment for age, sex, smoking, diabetes mellitus, hypertension and BMI (HR 1.29, 95% CI 1.20–1.39). By contrast, after adjustment for age, sex, smoking, diabetes mellitus, hypertension and waist circumference, increasing tertiles of BMI were inversely associated with risk of death (HR 0.64, 95% CI 0.59–0.69)44.
The findings from this systematic review44 are partially confirmed by Diewertje Sluik and colleagues, who examined the relationships between waist circumference, BMI and survival in 5,435 individuals with T2DM over 4.6 years of follow-up (interquartile range 2.0–9.8 years)45. In this prospective cohort study, the cohort was divided into quintiles for both BMI and waist circumference. After adjustment for T2DM duration, insulin treatment, prevalent myocardial infarction, stroke, cancer, smoking status, smoking duration, educational level, physical activity, alcohol consumption and BMI, the HR for risk of death associated with the highest tertile was 2.11 (95% CI 1.23–3.61) in comparison with the lowest waist circumference quintile. By contrast, in comparison with the lowest quintile for BMI (adjusted for the same variables, with waist circumference replacing BMI), the HR for risk of death for the highest BMI quintile was 0.33 (95% CI 0.19–0.60). In summary, when associations between waist circumference and BMI with morbidity and mortality are considered in continuous models, for a given waist circumference, the higher the BMI the lower the adverse health risk.
Why the association between waist circumference and adverse health risk is increased following adjustment for BMI is not established. It is possible that the health protective effect of a larger BMI for a given waist circumference is explained by an increased accumulation of subcutaneous adipose tissue in the lower body46. For example, in a study of >2,000 older participants from the Health, Ageing and Body Composition study, Marieke Snijder and colleagues were among the first to report that thigh adipose tissue mass is negatively associated with glucose intolerance and dyslipidaemia, after accounting for abdominal adipose tissue mass47. This observation was confirmed by Sophie Eastwood and colleagues, who reported that in South Asian adults the protective effects of total subcutaneous adipose tissue for T2DM and HbA1c levels emerge only after accounting for visceral adipose tissue (VAT) accumulation48.
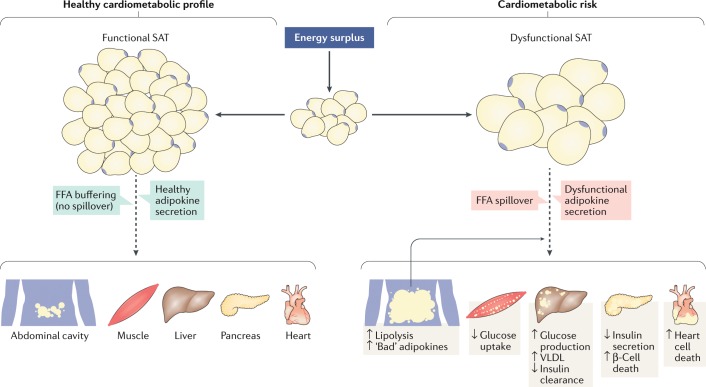
A causal mechanism has not been established that explains the attenuation in morbidity and mortality associated with increased lower body adiposity for a given level of abdominal obesity. We suggest that the increased capacity to store excess energy consumption in the gluteal–femoral subcutaneous adipocytes might protect against excess lipid deposition in VAT and ectopic depots such as the liver, the heart and the skeletal muscle (Fig. 2). Thus, for a given waist circumference, a larger BMI might represent a phenotype with elevations in lower body subcutaneous adipose tissue. Alternatively, adults with elevations in BMI for a given waist circumference could have decreased amounts of VAT. Excess lipid accumulation in VAT and ectopic depots is associated with increased cardiometabolic risk47–49. Moreover, VAT is an established marker of morbidity50,51 and mortality24,52. These findings provide a plausible mechanism by which lower values for BMI or hip circumference for a given waist circumference would increase adverse health risk.
Fig. 2. Overview of potential role of functional and dysfunctional adipose tissue contributing to increased cardiometabolic risk.
The ability of subcutaneous adipose tissue (SAT) to expand through hyperplasia (generation of new fat cells) allows the safe storage of the excess energy from the diet into a properly expanding subcutaneous ‘metabolic sink’. When this process becomes saturated or in situations where adipose tissue has a limited ability to expand, there is a spillover of the excess energy, which must be stored in visceral adipose tissue as well as in normally lean organs such as the skeletal muscle, the liver, the pancreas and the heart, a process described as ectopic fat deposition. Visceral adiposity is associated with a hyperlipolytic state resistant to the effect of insulin along with an altered secretion of adipokines including inflammatory cytokines whereas a set of metabolic dysfunctions are specifically associated with increased skeletal muscle, liver, pancreas, and epicardial, pericardial and intra-myocardial fat. FFA, free fatty acid.
This notion is reinforced by Jennifer Kuk and colleagues who reported that BMI is an independent and positive correlate of VAT in adults before adjustment for waist circumference; however, BMI is negatively associated with VAT mass after adjustment for waist circumference53. This study also reported that, after adjustment for waist circumference, BMI was positively associated with lower body subcutaneous adipose tissue mass and skeletal muscle mass. These observations support the putative mechanism described above and, consequently, that the negative association commonly observed between BMI and morbidity and mortality after adjustment for waist circumference might be explained by a decreased deposition of lower body subcutaneous adipose tissue and muscle mass, an increased accumulation of visceral adiposity, or both.
In summary, the combination of BMI and waist circumference can identify the highest-risk phenotype of obesity far better than either measure alone. Although guidelines for the management of obesity from several professional societies recognize the importance of measuring waist circumference, in the context of risk stratification for future cardiometabolic morbidity and mortality, these guidelines limit the recommendation to measure waist circumference to adults defined by BMI to have overweight or obesity. On the basis of the observations described in this section, waist circumference could be just as important, if not more informative, in persons with lower BMI, where an elevated waist circumference is more likely to signify visceral adiposity and increased cardiometabolic risk. This observation is particularly true for older adults54.
Conclusions and recommendations — identifying the high-risk obesity phenotype
In categorical analyses, waist circumference is associated with health outcomes within all BMI categories independent of sex and age.
When BMI and waist circumference are considered as continuous variables in the same risk prediction model, waist circumference remains a positive predictor of risk of death, but BMI is unrelated or negatively related to this risk.
The strength of the association between waist circumference and morbidity and/or mortality is not fully realized until after adjustment for BMI.
The improved ability of waist circumference to predict health outcomes over BMI might be at least partially explained by the ability of waist circumference to identify adults with increased VAT mass.
We recommend that measurements of waist circumference and BMI should become a standard part of clinical encounters (that is, an accepted ‘vital sign’).
Importance in clinical settings
For practitioners, the decision to include a novel measure in clinical practice is driven in large part by two important, yet very different questions. The first centres on whether the measure or biomarker improves risk prediction in a specific population for a specific disease. For example, does the addition of a new risk factor improve the prognostic performance of an established risk prediction algorithm, such as the Pooled Cohort Equations (PCE) or Framingham Risk Score (FRS) in adults at risk of CVD? The second question is concerned with whether improvement in the new risk marker would lead to a corresponding reduction in risk of, for example, cardiovascular events. In many situations, even if a biomarker does not add to risk prediction, it can still serve as an excellent target for risk reduction. Here we consider the importance of waist circumference in clinical settings by addressing these two questions.
Risk prediction
The evaluation of the utility of any biomarker, such as waist circumference, for risk prediction requires a thorough understanding of the epidemiological context in which the risk assessment is evaluated. In addition, several statistical benchmarks need to be met in order for the biomarker to improve risk prediction beyond traditional measures. These criteria are especially important for waist circumference, as established sex-specific and ethnicity-specific differences exist in waist circumference threshold levels55,56. In 2009, the American Heart Association published a scientific statement on the required criteria for the evaluation of novel risk markers of CVD57, followed by recommendations for assessment of cardiovascular risk in asymptomatic adults in 2010 (ref.58). Novel biomarkers must at the very least have an independent statistical association with health risk, after accounting for established risk markers in the context of a multivariable epidemiological model. This characteristic alone is insufficient, however, as many novel biomarkers meet this minimum standard yet do not meaningfully improve risk prediction beyond traditional markers. More stringent benchmarks have therefore been developed to assess biomarker utility, which include calibration, discrimination58 and net reclassification improvement59. Therefore, to critically evaluate waist circumference as a novel biomarker for use in risk prediction algorithms, these stringent criteria need to be applied.
Numerous studies demonstrate a statistical association between waist circumference and mortality and morbidity in epidemiological cohorts. For example, a systematic review and meta-regression analysis of 18 studies comprising >680,000 European participants with up to 24 years of follow-up demonstrated that waist circumference was associated with increased all-cause death above values of 95 cm for men and 80 cm for women60. Notably, increased waist circumference above these thresholds was associated with increased relative risk of all-cause death, even among those with normal BMI (20.0–24.9 kg/m2)60. In the USA, prospective follow-up over 9 years of 14,699 black, white and mixed ethnicity participants in the Atherosclerosis Risk in Communities study showed that waist circumference was associated with increased risk of coronary heart disease (553 events; RR 1.37, 95% CI 1.21–1.56) but not with all-cause death61.
Despite the existence of a robust statistical association with all-cause death independent of BMI, there is no solid evidence that addition of waist circumference to standard cardiovascular risk models (such as FRS62 or PCE63) improves risk prediction using more stringent statistical benchmarks. For example, a study evaluating the utility of the PCE across WHO-defined classes of obesity42 in five large epidemiological cohorts comprised of ~25,000 individuals assessed whether risk discrimination of the PCE would be improved by including the obesity-specific measures BMI and waist circumference64. The researchers found that although each measure was individually associated (BMI: HR 1.04, 95% CI 1.02–1.07; waist circumference: HR 1.11, 95% CI 1.09–1.13 per 1 SD increase) with increased risk of atherosclerotic CVD, no significant improvement occurred in the c-statistic with the addition of either BMI or waist circumference to the other PCE variables64. Similarly, a pooled analysis of four French population studies including >20,000 participants assessed the utility of additional risk factors when added to the FRS for 10-year coronary heart disease risk prediction65. The researchers found that BMI (P = 0.03) but not waist circumference (P = 0.42) remained associated with risk of coronary heart disease when added to FRS, but the addition of either factor did not improve model discrimination65.
On the basis of these observations alone, one might conclude that the measure of waist circumference in clinical settings is not supported as risk prediction is not improved. However, Nancy Cook and others have demonstrated how difficult it is for the addition of any biomarker to substantially improve prognostic performance59,66–68. Indeed, Michael Pencina and colleagues estimated that the nonmodifiable risk factors of age, sex and ethnicity capture 63–80% of the prognostic performance of cardiovascular risk models, and that adding systolic blood pressure, plasma levels of non-HDL cholesterol, diabetes mellitus or smoking to a model with other risk factors increases prognostic performance as measured by the c-statistic by only 0.004–0.013 (ref.69). Furthermore, any additive value of waist circumference to risk prediction algorithms could be overwhelmed by more proximate, downstream causative risk factors such as elevated blood pressure and abnormal plasma concentrations of glucose. In other words, waist circumference might not improve prognostic performance as, independent of BMI, waist circumference is a principal driver of alterations in downstream cardiometabolic risk factors. A detailed discussion of the merits of different approaches (for example, c-statistic, net reclassification index and discrimination index) to determine the utility of novel biomarkers to improve risk prediction is beyond the scope of this article and the reader is encouraged to review recent critiques to gain insight on this important issue66,69.
Risk reduction
Whether the addition of waist circumference improves the prognostic performance of established risk algorithms is a clinically relevant question that remains to be answered; however, the effect of targeting waist circumference on morbidity and mortality is an entirely different issue of equal or greater clinical relevance. Several examples exist in the literature where a risk marker might improve risk prediction but modifying the marker clinically does not impact risk reduction. For example, a low level of HDL cholesterol is a central risk factor associated with the risk of coronary artery disease in multiple risk prediction algorithms, yet raising plasma levels of HDL cholesterol pharmacologically has not improved CVD outcomes70. Conversely, a risk factor might not meaningfully improve statistical risk prediction but can be an important modifiable target for risk reduction. Indeed, we argue that, at any BMI value, waist circumference is a major driver of the deterioration in cardiometabolic risk markers or factors and, consequently, that reducing waist circumference is a critical step towards reducing cardiometabolic disease risk.
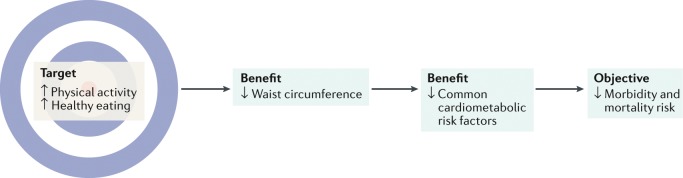
As we described earlier, waist circumference is well established as an independent predictor of morbidity and mortality, and the full strength of waist circumference is realized after controlling for BMI. We suggest that the association between waist circumference and hard clinical end points is explained in large measure by the association between changes in waist circumference and corresponding cardiometabolic risk factors. For example, evidence from randomized controlled trials (RCTs) has consistently revealed that, independent of sex and age, lifestyle-induced reductions in waist circumference are associated with improvements in cardiometabolic risk factors with or without corresponding weight loss71–76. These observations remain consistent regardless of whether the reduction in waist circumference is induced by energy restriction (that is, caloric restriction)73,75,77 or an increase in energy expenditure (that is, exercise)71,73–75. We have previously argued that the conduit between change in waist circumference and cardiometabolic risk is visceral adiposity, which is a strong marker of cardiometabolic risk24. Taken together, these observations highlight the critical role of waist circumference reduction through lifestyle behaviours in downstream reduction in morbidity and mortality (Fig. 3).
Fig. 3. Waist circumference is a modifiable risk factor that can indicate cardiometabolic risk, morbidity and mortality.
An illustration of the important role that decreases in waist circumference have for linking improvements in lifestyle behaviours with downstream reductions in the risk of morbidity and mortality. The benefits associated with reductions in waist circumference might be observed with or without a change in BMI.
In summary, whether waist circumference adds to the prognostic performance of cardiovascular risk models awaits definitive evidence. However, waist circumference is now clearly established as a key driver of altered levels of cardiometabolic risk factors and markers. Consequently, reducing waist circumference is a critical step in cardiometabolic risk reduction, as it offers a pragmatic and simple target for managing patient risk.
Conclusions and recommendations — waist circumference and risk prediction
The combination of BMI and waist circumference identifies a high-risk obesity phenotype better than either measure alone.
We recommend that waist circumference should be measured in clinical practice as it is a key driver of risk; for example, many patients have altered CVD risk factors because they have abdominal obesity.
Waist circumference is a critical factor that can be used to measure the reduction in CVD risk after the adoption of healthy behaviours.
A highly responsive vital sign
Evidence from several reviews and meta-analyses confirm that, regardless of age and sex, a decrease in energy intake through diet or an increase in energy expenditure through exercise is associated with a substantial reduction in waist circumference78–87. For studies wherein the negative energy balance is induced by diet alone, evidence from RCTs suggest that waist circumference is reduced independent of diet composition and duration of treatment88. Whether a dose–response relationship exists between a negative energy balance induced by diet and waist circumference is unclear.
Although it is intuitive to suggest that increased amounts of exercise would be positively associated with corresponding reductions in waist circumference, to date this notion is not supported by evidence from RCTs71,74,89–91. For example, Robert Ross and colleagues conducted a large RCT whereby participants (n = 300) were assigned to either a control arm or an intervention arm with different exercise levels: low, defined as 180 kcal per session for women and 300 kcal per session for men; and high, defined as 360 kcal per session for women and 600 kcal per session for men74. A doubling of the energy expenditure induced by exercise did not result in a difference in waist circumference reduction between the exercise groups. However, all intervention groups significantly reduced waist circumference (~5 cm) compared with the control arm (P < 0.001)74. These findings are consistent with the findings of Christopher Slentz and colleagues, who reported no difference in waist circumference or VAT reduction between low-level (14 kcal/kg body weight per week, n = 46) and high-level (23 kcal/kg body weight per week, n = 42) exercise groups90,91. In addition, Timothy Church and colleagues conducted an RCT whereby participants were prescribed different levels of exercise (low, 4 kcal/kg body weight per week, n = 155; moderate, 8 kcal/kg body weight per week, n = 104; or high, 12 kcal/kg body weight per week, n = 103) that was matched for intensity (50% peak oxygen consumption (VO2peak))71. A significant reduction was observed in waist circumference across all exercise groups compared with the no-exercise controls, with no difference between the different prescribed levels71.
Few RCTs have examined the effects of exercise intensity on waist circumference74,90–92. A small trial conducted by Brian Irving and colleagues observed that a high-intensity exercise (over the lactate threshold 3 days per week and under the lactate threshold 2 days per week) group (n = 9) had significantly reduced waist circumference compared with a low-intensity (under the lactate threshold 5 days per week) group (n = 11)92. However, no significant differences were observed in VAT reduction by single slice CT between high-intensity and low-intensity groups. A large RCT conducted by Slentz and colleagues observed that an increase in exercise intensity from moderate (40–55% VO2peak, n = 40) to vigorous (65–80% VO2peak, n = 42) intensity was not associated with differences in waist circumference reduction90,91. However, the researchers did not fix the level of exercise between the intensity groups, which might explain their observations. Ross and colleagues controlled the amount of energy expenditure between moderate-intensity (50% VO2peak, n = 76) and high-intensity (75% VO2peak, n = 76) exercise groups74. Their observations are consistent with those of Slentz and colleagues, whereby differences in exercise intensity did not affect waist circumference reductions. These findings are consistent with a meta-analysis carried out in 2017 wherein no difference in waist circumference reduction was observed between high-intensity interval training and moderate-intensity exercise93. In summary, current evidence suggests that increasing the intensity of exercise interventions is not associated with a further decrease in waist circumference.
VAT mass is not routinely measured in clinical settings, so it is of interest whether reductions in waist circumference are associated with corresponding reductions in VAT. Although evidence from systematic reviews and meta-analyses demonstrate an association between reductions in waist circumference and VAT79,82,84,85,94, the shared variance is modest (~40%)75,95,96. Of note, to our knowledge every study that has reported a reduction in waist circumference has also reported a corresponding reduction in VAT. Thus, although it is reasonable to suggest that a reduction in waist circumference is associated with a reduction in VAT mass, a precise estimation of individual VAT reduction from waist circumference is not possible. Nonetheless, the corresponding reduction of VAT with waist circumference in a dose-dependent manner highlights the importance of routine measurement of waist circumference in clinical practice. Of particular interest to practitioners, several reviews have observed significant VAT reduction in response to exercise in the absence of weight loss80,85.
Conclusions and recommendations — changes in waist circumference in response to treatment
Exercise and/or diet consistent with guideline recommendations are associated with substantial reductions in waist circumference, independent of age, sex or ethnicity.
Available evidence from RCTs suggests that exercise is associated with substantial reductions in waist circumference, independent of the quantity or intensity of exercise.
Exercise-induced or diet-induced reductions in waist circumference are observed with or without weight loss.
We recommend that practitioners routinely measure waist circumference as it provides them with a simple anthropometric measure to determine the efficacy of lifestyle-based strategies designed to reduce abdominal obesity.
Measurement of waist circumference
The emergence of waist circumference as a strong independent marker of morbidity and mortality is striking given that there is no consensus regarding the optimal protocol for measurement of waist circumference. Moreover, the waist circumference protocols recommended by leading health authorities have no scientific rationale. In 2008, a panel of experts performed a systematic review of 120 studies to determine whether measurement protocol influenced the relationship between waist circumference, morbidity and mortality, and observed similar patterns of association between the outcomes and all waist circumference protocols across sample size, sex, age and ethnicity97. Upon careful review of the various protocols described within the literature, the panel recommended that the waist circumference protocol described by the WHO guidelines98 (the midpoint between the lower border of the rib cage and the iliac crest) and the NIH guidelines99 (the superior border of the iliac crest) are probably more reliable and feasible measures for both the practitioner and the general public. This conclusion was made as both waist circumference measurement protocols use bony landmarks to identify the proper waist circumference measurement location.
The expert panel recognized that differences might exist in absolute waist circumference measures due to the difference in protocols between the WHO and NIH methods. However, few studies have compared measures at the sites recommended by the WHO and NIH. Jack Wang and colleagues reported no difference between the iliac crest and midpoint protocols for men and an absolute difference of 1.8 cm for women100. These observations were confirmed by Caitlin Mason and Peter Katzmarzyk, who reported no difference between the iliac crest (NIH) and midpoint (WHO) protocols for men and an absolute difference of ~2 cm for women101. More importantly, Mason and Katzmarzyk reported that the prevalence estimates of abdominal obesity (here defined as waist circumference >88 cm for women and >102 cm for men) identified using the iliac crest and midpoint protocols were about 32% for both protocols in men and 47% and 41% for the iliac crest and midpoint protocols, respectively, in women101. Consequently, although adopting a standard approach to waist circumference measurement would add to the utility of waist circumference measures for obesity-related risk stratification, the prevalence estimates of abdominal obesity in predominantly white populations using the iliac crest or midpoint protocols do not seem to be materially different.
Of note, the observation that the NIH and WHO protocols do not substantially differ is not consistent with those made by Yumi Matsushita and colleagues, who sampled 940 Japanese adults and reported that the mean difference between the iliac crest and midpoint protocols for men was ~2 cm, whereas for women the difference was ~9 cm (refs102,103). However, the waist circumference measurements assessed at the two sites had a similar ability to screen for the metabolic syndrome, as defined by National Cholesterol Education Program, in a cohort of 1,140 Japanese adults102.
Several investigations have evaluated the relationship between self-measured and technician-measured waist circumference104–108. Instructions for self-measurement of waist circumference are often provided in point form through simple surveys108. Good agreement between self-measured and technician-measured waist circumference is observed, with strong correlation coefficients ranging between 0.8 and 0.9 for both men and women. However, both men and women tend to underestimate their waist circumference measures compared with the technician-measured values, with differences ranging between about 1 cm and 3 cm. Moreover, high BMI and large baseline waist circumference are associated with a larger degree of under-reporting105,107. Overall these observations are encouraging and suggest that self-measures of waist circumference can be obtained in a straightforward manner and are in good agreement with technician-measured values.
Instructional videos that provide a detailed illustration of the step-by-step procedures for both technician-measurement and self-measurement of waist circumference can be freely accessed at myhealthywaist (http://www.myhealthywaist.org/evaluating-cmr/clinical-tools/waist-circumference-measurement-guidelines/index.html).
Conclusions and recommendations — measurement of waist circumference
Currently, no consensus exists on the optimal protocol for measurement of waist circumference and little scientific rationale is provided for any of the waist circumference protocols recommended by leading health authorities.
The waist circumference measurement protocol has no substantial influence on the association between waist circumference, all-cause mortality and CVD-related mortality, CVD and T2DM.
Absolute differences in waist circumference obtained by the two most often used protocols, iliac crest (NIH) and midpoint between the last rib and iliac crest (WHO), are generally small for adult men but are much larger for women.
The classification of abdominal obesity might differ depending on the waist circumference protocol.
We recommend that waist circumference measurements are obtained at the level of the iliac crest or the midpoint between the last rib and iliac crest. The protocol selected to measure waist circumference should be used consistently.
Self-measures of waist circumference can be obtained in a straightforward manner and are in good agreement with technician-measured values.
Threshold values to estimate risk
Current guidelines for identifying obesity indicate that adverse health risk increases when moving from normal weight to obese BMI categories. Moreover, within each BMI category, individuals with high waist circumference values are at increased risk of adverse health outcomes compared with those with normal waist circumference values109. For example, a single waist circumference threshold for white adults (men >102 cm; women >88 cm) is currently used to denote a high waist circumference, regardless of BMI category. Of note, these sex-specific thresholds were originally developed using cross-sectional data in white adults, among whom a waist circumference of 102 cm in men and 88 cm in women corresponded to a BMI of 30.0 kg/m2, which is the BMI threshold for obesity109. Thus, these waist circumference threshold values were designed to be used in place of BMI as an alternative way to identify obesity and consequently were not developed based on the relationship between waist circumference and adverse health risk.
In order to address this limitation, Christopher Ardern and colleagues developed and cross-validated waist circumference thresholds within BMI categories in relation to estimated risk of future CVD (using FRS)110. The utility of the derived values was compared with the single waist circumference thresholds (women >88 cm; men >102 cm) recommended by leading health authorities. The results of their study revealed that the current recommendations that use a single waist circumference threshold across all BMI categories are insufficient to identify those at increased health risk. In both sexes, the use of BMI category-specific waist circumference thresholds improved the identification of individuals at a high risk of future coronary events, leading the authors to propose BMI-specific waist circumference values (Table 1). In 2009, Harpreet Bajaj and colleagues compared the prognostic performance of the Ardern waist circumference values (Table 1) with the traditional waist circumference values (men >102 cm; women >88 cm) for all-cause mortality in a large cohort of 5,453 predominantly white adults with high cardiometabolic risk111. For both men and women, the Ardern waist circumference values substantially improved predictions of mortality compared with the traditional values. These observations are promising and support, at least for white adults, the clinical utility of the BMI category-specific waist circumference thresholds given in Table 1.
Table 1.
Waist circumference thresholds
| BMI category (kg/m2) | Waist circumference (cm)a | |
|---|---|---|
| Women | Men | |
| Normal weight (18.5–24.9) | ≥80 | ≥90 |
| Overweight (25–29.9) | ≥90 | ≥100 |
| Obese I (30–34.9 ) | ≥105 | ≥110 |
| Obese II and III (≥35 ) | ≥115 | ≥125 |
Table provides waist circumference thresholds stratified by BMI for white individuals; individuals with measurements higher than these values have a high risk of future coronary events (based on 10-year risk of coronary events or the presence of diabetes mellitus). aWaist circumference threshold indicating increased health risk within each BMI category. Data were originally presented in ref.110.
Of note, BMI-specific waist circumference thresholds have been developed in African American and white men and women112. Similar to previous research, the optimal waist circumference thresholds increased across BMI categories in both ethnic groups and were higher in men than in women. However, no evidence of differences in waist circumference occurred between ethnicities within each sex112.
Pischon and colleagues investigated the associations between BMI, waist circumference and risk of death among 359,387 adults from nine countries in the European Prospective Investigation into Cancer and Nutrition cohort10. In this study, the authors confirmed that, for a given BMI in men and women, the risk of death increased by 17% in men and 13% in women for every 5 cm increase in waist circumference. Although the waist circumference values that optimized prediction of the risk of death for any given BMI value were not reported, the findings reinforce the notion that waist circumference thresholds increase across BMI categories and that the combination of waist circumference and BMI provide improved predictions of health risk than either anthropometric measure alone.
Ethnicity-specific values for waist circumference that have been optimized for the identification of adults with elevated CVD risk have been developed (Table 2). With few exceptions, the values presented in Table 2 were derived using cross-sectional data and were not considered in association with BMI. The range in high-risk waist circumference values for both adult men (80–98 cm) and women (80–96 cm) varies considerably across ethnicities, which confirms the need for ethnicity-specific waist circumference values. Prospective studies using representative populations are required to firmly establish ethnicity-specific and BMI category-specific waist circumference threshold values that distinguish adults at increased health risk.
Table 2.
Ethnicity-specific thresholds
| Ethnic group | Waist circumference (cm)a | Ref. |
|---|---|---|
| Japaneseb | ||
| Men | ≥85 | 122 |
| Women | ≥90 | 122 |
| Jordanian | ||
| Men | ≥98 | 123 |
| Women | ≥96 | 123 |
| Chinese | ||
| Men | ≥80 | 124 |
| Women | ≥80 | 124 |
| Korean | ||
| Men | ≥90 | 125 |
| Women | ≥85 | 125 |
| Tunisian | ||
| Men | ≥85 | 126 |
| Women | ≥85 | 126 |
| Iranian | ||
| Men | ≥89 | 127 |
| Women | ≥91 | 127 |
| Asian Indian | ||
| Men | ≥90 | 128 |
| Women | ≥80 | 128 |
aWaist circumference values for adults above which cardiometabolic risk is elevated. bJapanese waist circumference values are thresholds above which visceral adipose tissue volume is >100 cm3.
As noted above, the ethnicity-specific waist circumference values in Table 2 were optimized for the identification of adults with elevated CVD risk. The Japanese waist circumference values, however, were optimized for identification of men and women with CT-measured VAT values >100 cm3 at the level of the umbilicus (navel)112. The rationale for using VAT as the outcome was that cardiometabolic risk was found to increase substantially at this VAT level for adult Japanese men and women56. Accordingly, Japanese threshold values for waist circumference were established at 85 cm in men and 90 cm in women, which corresponded to the VAT threshold of 100 cm3 (ref.112).
Conclusions and recommendations — values of waist circumference to estimate health risk
From the evidence available, we question the rationale behind current guidelines recommending that a single waist circumference threshold for white adults (men >102 cm; women >88 cm) be used to denote a high waist circumference, regardless of BMI category.
We recommend that prospective studies using representative populations are carried out to address the need for BMI category-specific waist circumference thresholds across different ethnicities (such as those proposed in Table 1 for white adults). This recommendation does not, however, diminish the importance of measuring waist circumference to follow changes over time and, hence, the utility of strategies designed to reduce abdominal obesity and associated health risk.
Conclusions
The main recommendation of this Consensus Statement is that waist circumference should be routinely measured in clinical practice, as it can provide additional information for guiding patient management. Indeed, decades of research have produced unequivocal evidence that waist circumference provides both independent and additive information to BMI for morbidity and mortality prediction. On the basis of these observations, not including waist circumference measurement in routine clinical practice fails to provide an optimal approach for stratifying patients according to risk. The measurement of waist circumference in clinical settings is both important and feasible. Self-measurement of waist circumference is easily obtained and in good agreement with technician-measured waist circumference. Numerous epidemiological studies and RCTs have now demonstrated that reductions in waist circumference can be achieved by routine, moderate-intensity exercise and/or diet changes.
Gaps in our knowledge still remain, and refinement of waist circumference threshold values for a given BMI category across different ages, by sex and by ethnicity will require further investigation. To address this need, we recommend that prospective studies be carried out in the relevant populations. Despite these gaps in our knowledge, overwhelming evidence presented here suggests that the measurement of waist circumference improves patient management and that its omission from routine clinical practice for the majority of patients is no longer acceptable. Accordingly, the inclusion of waist circumference measurement in routine practice affords practitioners with an important opportunity to improve the care and health of patients. Health professionals should be trained to properly perform this simple measurement and should consider it as an important vital sign to assess and identify, as an important treatment target in clinical practice.
Supplementary information
Acknowledgements
The authors acknowledge the financial support of the IAS and the ICCR, an independent academic organization based at Université Laval, Québec, Canada, who were responsible for coordinating the production of our report. No funding or honorarium was provided by either the IAS or the ICCR to the members of the writing group for the production of this article. The scientific director of the ICCR (J.-P.D.) is funded by a Foundation Grant (Funding Reference Number FDN-167278) from the Canadian Institutes of Health Research.
Glossary
- Calibration
The ability to correctly predict the proportion of participants in a given group who will experience an event.
- Discrimination
The probability of a diagnostic test or risk prediction instrument to distinguish between higher and lower risk.
- Net reclassification improvement
The relative increase in the predicted probabilities for individuals who experience events and the decrease for individuals who do not.
- C-statistic
A measure of goodness-of-fit for binary outcomes in a logistic regression model.
- VO2peak
The highest value of VO2 (that is, oxygen consumption) attained during an incremental or other high-intensity exercise test.
- Lactate threshold
The exercise intensity at which the blood concentration of lactate and/or lactic acid begins to exponentially increase.
- Iliac crest
The superior border of the wing of the ilium.
Author contributions
R.R., I.J.N. and J.-P.D. researched data for the article. R.R., I.J.N., S.Y., I.S., J.S., F.B.H., Y.M. and J.-P.D. made a substantial contribution to discussion of the content. R.R., I.J.N. and J.-P.D. wrote the article. R.R., I.J.N., I.S., J.S., P.M., R.D.S., B.A., A.C., F.B.H., B.A.G., A.Z., P.B., J.-C.F., R.H.E., Y.M. and J.-P.D. reviewed and/or edited the manuscript before submission.
Competing interests
I.J.N. reports receiving fees for consulting and serving on the advisory board from Boehringer Ingelheim/Lilly Alliance and AMRA Medical and a research grant from Novo Nordisk. F.B.H. reports receiving speaker fees from Metagenics and Standard Process and a research grant from California Walnut Commission. R.D.S. reports receiving consulting and speaker fess from Amgen, Astra Zeneca, Akcea, Biolab, Esperion, Kowa, Merck, MSD, Novo Nordisk, Sanofi Regeneron, Akcea, Kowa and Esperion. S.Y. reports grants and personal fees from Kowa Company, Ltd., Otsuka Pharmaceutical Co., Ltd., Shionogi & Co., Ltd., Bayer Yakuhin, Ltd., MSD K.K., Takeda Pharmaceutical Company, Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Astellas Pharma Inc., Daiichi-Sankyo Company, Ltd., Astra Zeneka K.K. and Kaken Pharmaceutical Co., Ltd.; grants from Kyowa Medex Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., National Institute of Biomedical Innovation, Hayashibara Co., Ltd., Teijin Pharma Limited, Kissei and Mochida Pharmaceutical Company, Ltd.; and personal fees from Ono Pharmaceutical Company, Ltd., Skylight Biotec, Inc., Pfizer, Astellas Amgen, Sanofi and Aegerion. S.Y. also has patents issued with Fujirebio and Kyowa Medex Co., Ltd. issued. R.H.E. reports his role as a scientific adviser for PROMINENT (Kowa Company Ltd.) and being on the advisory committees for Novo Nordisk and Sanofi/Regeneron. The remaining authors declare no competing interests.
Footnotes
Peer review information
Nature Reviews Endocrinology thanks R. Kelishadi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Measurement guidelines for waist circumference: http://www.myhealthywaist.org/evaluating-cmr/clinical-tools/waist-circumference-measurement-guidelines/index.html
Supplementary information
Supplementary information is available for this paper at 10.1038/s41574-019-0310-7.
References
- 1.Ng M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afshin A, Forouzanfar MH, Reitsma MB, Sur P. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips CM. Metabolically healthy obesity across the life course: epidemiology, determinants, and implications. Ann. N. Y. Acad. Sci. 2017;1391:85–100. doi: 10.1111/nyas.13230. [DOI] [PubMed] [Google Scholar]
- 4.Bell JA, et al. The natural course of healthy obesity over 20 years. J. Am. Coll. Cardiol. 2015;65:101–102. doi: 10.1016/j.jacc.2014.09.077. [DOI] [PubMed] [Google Scholar]
- 5.Eckel N, Meidtner K, Kalle-Uhlmann T, Stefan N, Schulze MB. Metabolically healthy obesity and cardiovascular events: a systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2016;23:956–966. doi: 10.1177/2047487315623884. [DOI] [PubMed] [Google Scholar]
- 6.Brauer P, et al. Recommendations for prevention of weight gain and use of behavioural and pharmacologic interventions to manage overweight and obesity in adults in primary care. CMAJ. 2015;187:184–195. doi: 10.1503/cmaj.140887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garvey WT, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pract. 2016;22(Suppl. 3):1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 8.Jensen MD, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity society. Circulation. 2014;129:S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsigos C, et al. Management of obesity in adults: European clinical practice guidelines. Obes. Facts. 2008;1:106–116. doi: 10.1159/000126822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pischon T, Boeing H, Hoffmann K, Bergmann M. General and abdominal adiposity and risk of death in Europe. N. Engl. J. Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 11.Cerhan JR, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin. Proc. 2014;89:335–345. doi: 10.1016/j.mayocp.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 13.Song X, et al. Comparison of various surrogate obesity indicators as predictors of cardiovascular mortality in four European populations. Eur. J. Clin. Nutr. 2013;67:1298–1302. doi: 10.1038/ejcn.2013.203. [DOI] [PubMed] [Google Scholar]
- 14.Seidell JC. Waist circumference and waist/hip ratio in relation to all-cause mortality, cancer and sleep apnea. Eur. J. Clin. Nutr. 2010;64:35–41. doi: 10.1038/ejcn.2009.71. [DOI] [PubMed] [Google Scholar]
- 15.Snijder MB, et al. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn study. Am. J. Clin. Nutr. 2003;77:1192–1197. doi: 10.1093/ajcn/77.5.1192. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs EJ, et al. Waist circumference and all-cause mortality in a large US cohort. Arch. Intern. Med. 2010;170:1293–1301. doi: 10.1001/archinternmed.2010.201. [DOI] [PubMed] [Google Scholar]
- 17.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am. J. Clin. Nutr. 1956;4:20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- 18.Kissebah AH, et al. Relation of body fat distribution to metabolic complications of obesity. J. Clin. Endocrinol. Metab. 1982;54:254–260. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 19.Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women: importance of regional adipose tissue distribution. J. Clin. Invest. 1983;72:1150–1162. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartz AJ, Rupley DC, Jr., Kalkhoff RD, Rimm AA. Relationship of obesity to diabetes: influence of obesity level and body fat distribution. Prev. Med. 1983;12:351–357. doi: 10.1016/0091-7435(83)90244-x. [DOI] [PubMed] [Google Scholar]
- 21.Larsson B, et al. Br. Med. J. (Clin. Res. Ed.) 1984. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913; pp. 1401–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohlson LO, et al. The influence of body fat distribution on the incidence of diabetes mellitus: 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34:1055–1058. doi: 10.2337/diab.34.10.1055. [DOI] [PubMed] [Google Scholar]
- 23.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int. J. Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- 24.Neeland IJ, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7:715–725. doi: 10.1016/S2213-8587(19)30084-1. [DOI] [PubMed] [Google Scholar]
- 25.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158–161. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh SD, Yoshinaga H. Waist/height ratio as a simple and useful predictor of coronary heart disease risk factors in women. Intern. Med. 1995;34:1147–1152. doi: 10.2169/internalmedicine.34.1147. [DOI] [PubMed] [Google Scholar]
- 27.Ashwell M, Lejeune S, McPherson K. Ratio of waist circumference to height may be better indicator of need for weight management. BMJ. 1996;312:377. doi: 10.1136/bmj.312.7027.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutr. Res. Rev. 2010;23:247–269. doi: 10.1017/S0954422410000144. [DOI] [PubMed] [Google Scholar]
- 29.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes. Rev. 2012;13:275–286. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 30.Paajanen TA, Oksala NK, Kuukasjarvi P, Karhunen PJ. Short stature is associated with coronary heart disease: a systematic review of the literature and a meta-analysis. Eur. Heart J. 2010;31:1802–1809. doi: 10.1093/eurheartj/ehq155. [DOI] [PubMed] [Google Scholar]
- 31.Han TS, et al. The influences of height and age on waist circumference as an index of adiposity in adults. Int. J. Obes. Relat. Metab. Disord. 1997;21:83–89. doi: 10.1038/sj.ijo.0800371. [DOI] [PubMed] [Google Scholar]
- 32.Valdez R, Seidell JC, Ahn YI, Weiss KM. A new index of abdominal adiposity as an indicator of risk for cardiovascular disease. A cross-population study. Int. J. Obes. Relat. Metab. Disord. 1993;17:77–82. [PubMed] [Google Scholar]
- 33.Amankwah N, et al. Abdominal obesity index as an alternative central obesity measurement during a physical examination. Open. Nutr. J. 2018;12:21–29. [Google Scholar]
- 34.Walls HL, et al. Trends in BMI of urban Australian adults, 1980–2000. Public. Health Nutr. 2010;13:631–638. doi: 10.1017/S1368980009991455. [DOI] [PubMed] [Google Scholar]
- 35.Janssen I, Shields M, Craig CL, Tremblay MS. Changes in the obesity phenotype within Canadian children and adults, 1981 to 2007–2009. Obesity. 2012;20:916–919. doi: 10.1038/oby.2011.122. [DOI] [PubMed] [Google Scholar]
- 36.Albrecht SS, Gordon-Larsen P, Stern D, Popkin BM. Is waist circumference per body mass index rising differentially across the United States, England, China and Mexico? Eur. J. Clin. Nutr. 2015;69:1306–1312. doi: 10.1038/ejcn.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visscher TL, Heitmann BL, Rissanen A, Lahti-Koski M, Lissner L. A break in the obesity epidemic? Explained by biases or misinterpretation of the data? Int. J. Obes. 2015;39:189–198. doi: 10.1038/ijo.2014.98. [DOI] [PubMed] [Google Scholar]
- 38.Rexrode KM, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 39.Despres JP. Excess visceral adipose tissue/ectopic fat the missing link in the obesity paradox? J. Am. Coll. Cardiol. 2011;57:1887–1889. doi: 10.1016/j.jacc.2010.10.063. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, et al. Abdominal adiposity and mortality in Chinese women. Arch. Intern. Med. 2007;167:886–892. doi: 10.1001/archinte.167.9.886. [DOI] [PubMed] [Google Scholar]
- 41.de Hollander EL, et al. The association between waist circumference and risk of mortality considering body mass index in 65- to 74-year-olds: a meta-analysis of 29 cohorts involving more than 58,000 elderly persons. Int. J. Epidemiol. 2012;41:805–817. doi: 10.1093/ije/dys008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organisation. Obesity: preventing and managing the global epidemic: report of a WHO consultation (World Health Organisation Technical Report Series 894) (WHO, 2000). [PubMed]
- 43.Bigaard J, et al. Waist circumference, BMI, smoking, and mortality in middle-aged men and women. Obes. Res. 2003;11:895–903. doi: 10.1038/oby.2003.123. [DOI] [PubMed] [Google Scholar]
- 44.Coutinho T, et al. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J. Am. Coll. Cardiol. 2011;57:1877–1886. doi: 10.1016/j.jacc.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 45.Sluik D, et al. Associations between general and abdominal adiposity and mortality in individuals with diabetes mellitus. Am. J. Epidemiol. 2011;174:22–34. doi: 10.1093/aje/kwr048. [DOI] [PubMed] [Google Scholar]
- 46.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 47.Snijder MB, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The health ABC study. Diabetologia. 2005;48:301–308. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 48.Eastwood SV, et al. Thigh fat and muscle each contribute to excess cardiometabolic risk in South Asians, independent of visceral adipose tissue. Obesity. 2014;22:2071–2079. doi: 10.1002/oby.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr. Rev. 2002;23:201–229. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 50.Despres JP, et al. The insulin resistance-dyslipidemic syndrome: contribution of visceral obesity and therapeutic implications. Int. J. Obes. Relat. Metab. Disord. 1995;19(Suppl. 1):S76–S86. [PubMed] [Google Scholar]
- 51.Nguyen-Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am. J. Physiol. Endocrinol. Metab. 2003;284:E1065–E1071. doi: 10.1152/ajpendo.00442.2002. [DOI] [PubMed] [Google Scholar]
- 52.Kuk JL, et al. Visceral fat is an independent predictor of all-cause mortality in men. Obesity. 2006;14:336–341. doi: 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- 53.Kuk JL, Janiszewski PM, Ross R. Body mass index and hip and thigh circumferences are negatively associated with visceral adipose tissue after control for waist circumference. Am. J. Clin. Nutr. 2007;85:1540–1544. doi: 10.1093/ajcn/85.6.1540. [DOI] [PubMed] [Google Scholar]
- 54.Janssen I, Katzmarzyk PT, Ross R. Body mass index is inversely related to mortality in older people after adjustment for waist circumference. J. Am. Geriatr. Soc. 2005;53:2112–2118. doi: 10.1111/j.1532-5415.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 55.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome: a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 56.Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a global public health problem and a new definition. J. Atheroscler. Thromb. 2005;12:295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- 57.Hlatky MA, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenland P, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2010;122:2748–2764. doi: 10.1161/CIR.0b013e3182051bab. [DOI] [PubMed] [Google Scholar]
- 59.Pencina MJ, D Agostino RB, Pencina KM, Janssens AC, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am. J. Epidemiol. 2012;176:473–481. doi: 10.1093/aje/kws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carmienke S, et al. General and abdominal obesity parameters and their combination in relation to mortality: a systematic review and meta-regression analysis. Eur. J. Clin. Nutr. 2013;67:573–585. doi: 10.1038/ejcn.2013.61. [DOI] [PubMed] [Google Scholar]
- 61.Hong Y, et al. Metabolic syndrome, its preeminent clusters, incident coronary heart disease and all-cause mortality: results of prospective analysis for the atherosclerosis risk in communities study. J. Intern. Med. 2007;262:113–122. doi: 10.1111/j.1365-2796.2007.01781.x. [DOI] [PubMed] [Google Scholar]
- 62.Wilson PW, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 63.Goff DC, Jr., Lloyd-Jones DM, Bennett G, Coady S. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 64.Khera R, et al. Accuracy of the pooled cohort equation to estimate atherosclerotic cardiovascular disease risk events by obesity class: a pooled assessment of five longitudinal cohort studies. J. Am. Coll. Cardiol. 2018 doi: 10.1016/S0735-1097(18)32279-4. [DOI] [Google Scholar]
- 65.Empana JP, et al. Predicting CHD risk in France: a pooled analysis of the D.E.S.I.R., three city, PRIME, and SU.VI.MAX studies. Eur. J. Cardiovasc. Prev. Rehabil. 2011;18:175–185. doi: 10.1177/1741826710389354. [DOI] [PubMed] [Google Scholar]
- 66.Cook NR. Methods for evaluating novel biomarkers: a new paradigm. Int. J. Clin. Pract. 2010;64:1723–1727. doi: 10.1111/j.1742-1241.2010.02469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 68.Pencina MJ, D. Agostino RB, Sr. D. Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat. Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 69.Pencina MJ, et al. Quantifying importance of major risk factors for coronary heart disease. Circulation. 2018;139:1603–1611. doi: 10.1161/CIRCULATIONAHA.117.031855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lincoff AM, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N. Engl. J. Med. 2017;376:1933–1942. doi: 10.1056/NEJMoa1609581. [DOI] [PubMed] [Google Scholar]
- 71.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 72.O'Donovan G, et al. Changes in cardiorespiratory fitness and coronary heart disease risk factors following 24 wk of moderate- or high-intensity exercise of equal energy cost. J. Appl. Physiol. 2005;98:1619–1625. doi: 10.1152/japplphysiol.01310.2004. [DOI] [PubMed] [Google Scholar]
- 73.Ross R, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men: a randomized, controlled trial. Ann. Intern. Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 74.Ross R, Hudson R, Stotz PJ, Lam M. Effects of exercise amount and intensity on abdominal obesity and glucose tolerance in obese adults: a randomized trial. Ann. Intern. Med. 2015;162:325–334. doi: 10.7326/M14-1189. [DOI] [PubMed] [Google Scholar]
- 75.Ross R, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes. Res. 2004;12:789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 76.Short KR, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52:1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- 77.Weiss EP, et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am. J. Clin. Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int. J. Obes. 2008;32:619–628. doi: 10.1038/sj.ijo.0803761. [DOI] [PubMed] [Google Scholar]
- 79.Hammond, B. P., Brennan, A. M. & Ross, R. in Body Composition: Health and Performance in Exercise and Sport (ed. Lukaski, H. C.) 109–128 (CRC Press, Taylor and Francis Group, 2017).
- 80.Kay SJ, Fiatarone Singh MA. The influence of physical activity on abdominal fat: a systematic review of the literature. Obes. Rev. 2006;7:183–200. doi: 10.1111/j.1467-789X.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- 81.Merlotti C, Ceriani V, Morabito A, Pontiroli AE. Subcutaneous fat loss is greater than visceral fat loss with diet and exercise, weight-loss promoting drugs and bariatric surgery: a critical review and meta-analysis. Int. J. Obes. 2017;41:672–682. doi: 10.1038/ijo.2017.31. [DOI] [PubMed] [Google Scholar]
- 82.Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I. A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. Int. J. Obes. 2007;31:1786–1797. doi: 10.1038/sj.ijo.0803683. [DOI] [PubMed] [Google Scholar]
- 83.O'Neill, T., Shalev-Goldman, E. & Ross, R. in Exercise Therapy in Adult Individuals with Obesity (ed. Hansen, D.) 43-72 (Nova Science Publishers, 2013).
- 84.Sabag A, et al. Exercise and ectopic fat in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab. 2017;43:195–210. doi: 10.1016/j.diabet.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 85.Verheggen RJ, et al. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes. Rev. 2016;17:664–690. doi: 10.1111/obr.12406. [DOI] [PubMed] [Google Scholar]
- 86.Santos FL, Esteves SS, da Costa Pereira A, Yancy WS, Jr., Nunes JP. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes. Rev. 2012;13:1048–1066. doi: 10.1111/j.1467-789X.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- 87.Gepner Y, et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools: CENTRAL magnetic resonance imaging randomized controlled trial. Circulation. 2018;137:1143–1157. doi: 10.1161/CIRCULATIONAHA.117.030501. [DOI] [PubMed] [Google Scholar]
- 88.Sacks FM, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keating SE, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J. Hepatol. 2015;63:174–182. doi: 10.1016/j.jhep.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 90.Slentz CA, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity. STRRIDE: a randomized controlled study. Arch. Intern. Med. 2004;164:31–39. doi: 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- 91.Slentz CA, et al. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J. Appl. Physiol. 2005;99:1613–1618. doi: 10.1152/japplphysiol.00124.2005. [DOI] [PubMed] [Google Scholar]
- 92.Irving BA, et al. Effect of exercise training intensity on abdominal visceral fat and body composition. Med. Sci. Sports Exerc. 2008;40:1863–1872. doi: 10.1249/MSS.0b013e3181801d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wewege M, van den Berg R, Ward RE, Keech A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and meta-analysis. Obes. Rev. 2017;18:635–646. doi: 10.1111/obr.12532. [DOI] [PubMed] [Google Scholar]
- 94.Vissers D, et al. The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PLoS One. 2013;8:e56415. doi: 10.1371/journal.pone.0056415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Janiszewski PM, Ross R. Physical activity in the treatment of obesity: beyond body weight reduction. Appl. Physiol. Nutr. Metab. 2007;32:512–522. doi: 10.1139/H07-018. [DOI] [PubMed] [Google Scholar]
- 96.Kuk JL, Lee S, Heymsfield SB, Ross R. Waist circumference and abdominal adipose tissue distribution: influence of age and sex. Am. J. Clin. Nutr. 2005;81:1330–1334. doi: 10.1093/ajcn/81.6.1330. [DOI] [PubMed] [Google Scholar]
- 97.Ross R, et al. Does the relationship between waist circumference, morbidity and mortality depend on measurement protocol for waist circumference? Obes. Rev. 2008;9:312–325. doi: 10.1111/j.1467-789X.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- 98.World Health Organisation. Physical status: the use and interpretation of anthropometry: report of a WHO Expert Committee (WHO, 1995). [PubMed]
- 99.NHLBI Obesity Education Initiative. The practical guide to the identification, evaluation and treatment of overweight and obesity in adults (NIH, 2000).
- 100.Wang J, et al. Comparisons of waist circumferences measured at 4 sites. Am. J. Clin. Nutr. 2003;77:379–384. doi: 10.1093/ajcn/77.2.379. [DOI] [PubMed] [Google Scholar]
- 101.Mason C, Katzmarzyk PT. Variability in waist circumference measurements according to anatomic measurement site. Obesity. 2009;17:1789–1795. doi: 10.1038/oby.2009.87. [DOI] [PubMed] [Google Scholar]
- 102.Matsushita Y, Tomita K, Yokoyama T, Mizoue T. Optimal waist circumference measurement site for assessing the metabolic syndrome. Diabetes Care. 2009;32:e70. doi: 10.2337/dc09-0190. [DOI] [PubMed] [Google Scholar]
- 103.Matsushita Y, Tomita K, Yokoyama T, Mizoue T. Relations between waist circumference at four sites and metabolic risk factors. Obesity. 2010;18:2374–2378. doi: 10.1038/oby.2010.33. [DOI] [PubMed] [Google Scholar]
- 104.Pendergast K, et al. Impact of waist circumference difference on health-care cost among overweight and obese subjects: the PROCEED cohort. Value Health. 2010;13:402–410. doi: 10.1111/j.1524-4733.2009.00690.x. [DOI] [PubMed] [Google Scholar]
- 105.Spencer EA, Roddam AW, Key TJ. Accuracy of self-reported waist and hip measurements in 4492 EPIC-Oxford participants. Public Health Nutr. 2004;7:723–727. doi: 10.1079/phn2004600. [DOI] [PubMed] [Google Scholar]
- 106.Roberts CA, Wilder LB, Jackson RT, Moy TF, Becker DM. Accuracy of self-measurement of waist and hip circumference in men and women. J. Am. Diet. Assoc. 1997;97:534–536. doi: 10.1016/S0002-8223(97)00137-5. [DOI] [PubMed] [Google Scholar]
- 107.Bigaard J, Spanggaard I, Thomsen BL, Overvad K, Tjonneland A. Self-reported and technician-measured waist circumferences differ in middle-aged men and women. J. Nutr. 2005;135:2263–2270. doi: 10.1093/jn/135.9.2263. [DOI] [PubMed] [Google Scholar]
- 108.Wolf AM, et al. PROCEED: prospective obesity cohort of economic evaluation and determinants: baseline health and healthcare utilization of the US sample. Diabetes Obes. Metab. 2008;10:1248–1260. doi: 10.1111/j.1463-1326.2008.00895.x. [DOI] [PubMed] [Google Scholar]
- 109.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch. Intern. Med. 2002;162:2074–2079. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- 110.Ardern CI, Janssen I, Ross R, Katzmarzyk PT. Development of health-related waist circumference thresholds within BMI categories. Obes. Res. 2004;12:1094–1103. doi: 10.1038/oby.2004.137. [DOI] [PubMed] [Google Scholar]
- 111.Bajaj HS, Brennan DM, Hoogwerf BJ, Doshi KB, Kashyap SR. Clinical utility of waist circumference in predicting all-cause mortality in a preventive cardiology clinic population: a PreCIS database study. Obesity. 2009;17:1615–1620. doi: 10.1038/oby.2009.44. [DOI] [PubMed] [Google Scholar]
- 112.Staiano AE, Bouchard C, Katzmarzyk PT. BMI-specific waist circumference thresholds to discriminate elevated cardiometabolic risk in white and African American adults. Obes. Facts. 2013;6:317–324. doi: 10.1159/000354712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xi B, et al. Secular trends in the prevalence of general and abdominal obesity among Chinese adults, 1993–2009. Obes. Rev. 2012;13:287–296. doi: 10.1111/j.1467-789X.2011.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barzin M, et al. Rising trends of obesity and abdominal obesity in 10 years of follow-up among Tehranian adults: Tehran lipid and glucose study (TLGS) Public Health Nutr. 2015;18:2981–2989. doi: 10.1017/S1368980015000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lahti-Koski M, Harald K, Mannisto S, Laatikainen T, Jousilahti P. Fifteen-year changes in body mass index and waist circumference in Finnish adults. Eur. J. Cardiovasc. Prev. Rehabil. 2007;14:398–404. doi: 10.1097/HJR.0b013e32800fef1f. [DOI] [PubMed] [Google Scholar]
- 116.Liese AD, Doring A, Hense HW, Keil U. Five year changes in waist circumference, body mass index and obesity in Augsburg, Germany. Eur. J. Nutr. 2001;40:282–288. doi: 10.1007/s394-001-8357-0. [DOI] [PubMed] [Google Scholar]
- 117.Czernichow S, et al. Trends in the prevalence of obesity in employed adults in central-western France: a population-based study, 1995–2005. Prev. Med. 2009;48:262–266. doi: 10.1016/j.ypmed.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 118.Ford ES, Maynard LM, Li C. Trends in mean waist circumference and abdominal obesity among US adults, 1999–2012. JAMA. 2014;312:1151–1153. doi: 10.1001/jama.2014.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gearon E, Tanamas SK, Stevenson C, Loh VHY, Peeters A. Changes in waist circumference independent of weight: implications for population level monitoring of obesity. Prev. Med. 2018;111:378–383. doi: 10.1016/j.ypmed.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 121.Okosun IS, et al. Abdominal adiposity in U.S. adults: prevalence and trends, 1960–2000. Prev. Med. 2004;39:197–206. doi: 10.1016/j.ypmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 122.Examination Committee of Criteria for 'Obesity Disease' in Japan & Japan Society for the Study of Obesity New criteria for 'obesity disease' in Japan. Circ. J. 2002;66:987–992. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 123.Al-Odat AZ, Ahmad MN, Haddad FH. References of anthropometric indices of central obesity and metabolic syndrome in Jordanian men and women. Diabetes Metab. Syndr. 2012;6:15–21. doi: 10.1016/j.dsx.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 124.Wildman RP, Gu D, Reynolds K, Duan X, He J. Appropriate body mass index and waist circumference cutoffs for categorization of overweight and central adiposity among Chinese adults. Am. J. Clin. Nutr. 2004;80:1129–1136. doi: 10.1093/ajcn/80.5.1129. [DOI] [PubMed] [Google Scholar]
- 125.Yoon YS, Oh SW. Optimal waist circumference cutoff values for the diagnosis of abdominal obesity in Korean adults. Endocrinol. Metab. 2014;29:418–426. doi: 10.3803/EnM.2014.29.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bouguerra R, et al. Waist circumference cut-off points for identification of abdominal obesity among the Tunisian adult population. Diabetes Obes. Metab. 2007;9:859–868. doi: 10.1111/j.1463-1326.2006.00667.x. [DOI] [PubMed] [Google Scholar]
- 127.Delavari A, Forouzanfar MH, Alikhani S, Sharifian A, Kelishadi R. First nationwide study of the prevalence of the metabolic syndrome and optimal cutoff points of waist circumference in the Middle East: the national survey of risk factors for noncommunicable diseases of Iran. Diabetes Care. 2009;32:1092–1097. doi: 10.2337/dc08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Misra A, et al. Waist circumference cutoff points and action levels for Asian Indians for identification of abdominal obesity. Int. J. Obes. 2006;30:106–111. doi: 10.1038/sj.ijo.0803111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.