Abstract
Avelumab, an anti–programmed death‐ligand 1 monoclonal antibody approved for the treatment of metastatic Merkel cell carcinoma and platinum‐treated urothelial carcinoma, was initially approved with a 10 mg/kg weight‐based dose. We report pharmacokinetic (PK)/pharmacodynamic analyses for avelumab comparing weight‐based dosing and a flat 800 mg dose, developed using data from 1,827 patients enrolled in 3 clinical trials (NCT01772004, NCT01943461, and NCT02155647). PK metrics were simulated for weight‐based and flat‐dosing regimens and summarized by quartiles of weight. Derived exposure metrics were used in simulations of exposure‐safety (various tumors) and exposure‐efficacy (objective responses; Merkel cell or urothelial carcinoma). Flat dosing was predicted to provide similar exposure to weight‐based dosing, with slightly lower variability. Exposure‐safety and exposure‐efficacy simulations suggested similar benefit:risk profiles for the two dosing regimens. These pharmacometric analyses provided the basis for the US Food and Drug Administration approval of a flat dose of avelumab 800 mg every 2 weeks in approved indications.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THIS TOPIC?
☑ In recent years, dosing regimens of some anti–programmed cell death 1 and anti–programmed death‐ligand 1 antibodies have changed from weight‐based dosing to a flat dose based on pharmacokinetic modeling and clinical studies.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Does a flat dose of 800 mg avelumab provide a comparable predicted exposure and benefit:risk profile to the previously approved 10 mg/kg weight‐based dose?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Based on pharmacometric modeling, an 800 mg flat dose of avelumab was predicted to provide similar exposure with slightly lower variability compared with 10 mg/kg weight‐based dosing. Additionally, simulations of exposure‐efficacy and exposure‐safety suggest that the two dosing regimens have similar benefit:risk profiles.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ These analyses provided the basis for the US Food and Drug Administration approval of avelumab as an 800 mg flat dose every 2 weeks instead of weight‐based dosing across all approved indications. Changing to a flat dose provides several practical advantages, including ease of dose preparation, reduced chance of dosing errors, and minimized drug wastage.
Monoclonal antibodies that block the interaction between programmed death 1 (PD‐1) and its ligand (PD‐L1) are established treatments for various tumor types.1 By blocking the immune inhibitory effects induced by this molecular interaction, anti–PD‐1 or anti–PD‐L1 antibodies can reactivate and maintain antitumor immune responses, resulting in therapeutic efficacy.2 Within oncology, there has been a shift from traditional weight‐based dosing with cytotoxic agents to the use of a flat dose with monoclonal antibodies. Furthermore, the anti–PD‐1 antibodies nivolumab and pembrolizumab were initially approved with weight‐based dosing, but the dosing was changed to flat dosing based on exposure‐efficacy, exposure‐safety, and pharmacokinetic (PK) modeling studies, which showed a similar benefit:risk profile, in addition to subsequent clinical studies of pembrolizumab dosing.3, 4 Similarly, the anti–PD‐L1 antibody atezolizumab was administered via weight‐based dosing in early trials, but PK modeling led to flat dosing being selected for subsequent trials,5 which led to regulatory approvals. For monoclonal antibodies, flat‐based and weight‐based dosing regimens are generally associated with similar variability in exposure across a patient population, and flat dosing produces less variability when the power exponent defining the effect of weight on clearance (CL) is < 0.5. Importantly, flat dosing may provide several additional practical advantages, including ease of dose preparation, reduced chance of dosing errors, and minimized drug wastage.6
Avelumab is a human immunoglobulin G1 anti–PD‐L1 monoclonal antibody that has been approved in various countries for the treatment of patients with metastatic Merkel cell carcinoma (mMCC) and patients with advanced or metastatic urothelial carcinoma (UC) after disease progression on platinum therapy.7, 8 Unlike other approved anti–PD‐L1 or anti–PD‐1 antibodies, avelumab has a wild‐type Fc region and has been shown in preclinical studies to induce antitumor activity mediated by innate immune effector cells, in addition to reactivating adaptive immune responses.9, 10 In addition to approved indications, avelumab is in clinical development for the treatment of other cancers, including renal cell carcinoma, non‐small cell lung cancer, and gastric cancer.11
The first phase I trials of avelumab as monotherapy were JAVELIN Solid Tumor (NCT01772004), a large, global study,11, 12 and JAVELIN Solid Tumor JPN (NCT01943461), performed in Japan.13 Both studies included an initial dose‐escalation part. The approval of avelumab 10 mg/kg in advanced or metastatic UC was based on a pooled analysis of 2 dose‐expansion cohorts from JAVELIN Solid Tumor.14, 15 The approval of avelumab 10 mg/kg in patients with mMCC was based on results from JAVELIN Merkel 200 (NCT02155647, a separate single‐arm phase II study that included a cohort of 88 patients with stage IV chemotherapy‐refractory mMCC who received avelumab 10 mg/kg every 2 weeks (q2w)).16 Single‐dose PK for avelumab was reported from the dose‐escalation parts of JAVELIN Solid Tumor12 and JAVELIN Solid Tumor JPN.13 Population PK models have been developed that show a modest effect of body weight on CL17 and a potential shallow exposure‐response relationship in some tumor types.18, 19
Avelumab was initially approved with weight‐based dosing of 10 mg/kg given intravenously q2w, which has been administered in several clinical trials. In light of experiences with similar agents, we describe analyses supporting the use of a flat dose of 800 mg q2w for avelumab across approved indications. The 800 mg dose was selected based on previous studies showing that the median body weight for adults with various tumor types is ≈ 80 kg,3, 4, 20 which would require a dose of 800 mg with 10 mg/kg weight‐based dosing. The objectives of this analysis were to (i) compare avelumab exposure between weight‐based and flat‐dosing regimens using simulations based on previously developed population PK models, (ii) compare the simulated probability of experiencing an adverse event (AE) of special interest with weight‐based and flat‐dosing regimens, specifically immune‐related AEs (irAEs) and infusion‐related reactions (IRRs), and (iii) compare the simulated probability of objective response (OR) between weight‐based and flat‐dosing regimens in patients with mMCC or advanced/metastatic UC.
RESULTS
Comparison of avelumab exposure between weight‐based and flat dosing
Data were obtained from 1,827 patients enrolled in 3 trials of avelumab, comprising 1,688 patients from JAVELIN Solid Tumor, 51 patients from JAVELIN Solid Tumor JPN, and 88 patients from JAVELIN Merkel 200 part A. The median body weight among trial participants was 70.6 kg (range 30.4–204 kg; Figure S1). Avelumab was administered at doses of 1 mg/kg (n = 4), 3 mg/kg (n = 18), 10 mg/kg (n = 1,778), or 20 mg/kg (n = 27) q2w.
Simulations of exposure comparing weight‐based (10 mg/kg q2w) and flat dosing (800 mg q2w) after the first cycle and at steady state were performed using previously developed population PK models (see Methods). Using the first‐cycle model in the simulated population, the flat 800 mg dose resulted in slightly higher exposures than weight‐based dosing, with the median area under the curve (AUC) during the first dosing interval (AUC0–336 h) increasing by ≈ 12% (Figure 1). This increased exposure was expected because the median weight of sampled patients (70.6 kg) was lower than the weight used to determine the flat dose (80.0 kg). Differences in exposure were more pronounced within the lightest and heaviest weight quartiles (Figure 1 b) and extreme weight percentiles (2.5th and 97.5th percentiles; Figures S2 and S3). For weight‐based dosing (10 mg/kg), exposures were lowest in the lightest body weight quartile/percentile, and for flat dosing (800 mg), exposures were lowest in the heaviest body weight quartile/percentile. However, exposures in all weight groups showed considerable overlap, and exposures with flat dosing in all weight groups were lower than the median exposure observed following administration of avelumab 20 mg/kg q2w (Figure 1 b). Total variability for simulated AUC0–336 h values was slightly lower for flat dosing compared with weight‐based dosing (Table 1); the coefficients of variation for simulated AUC0–336 h values were 27.1% and 29.0%, respectively.
Figure 1.
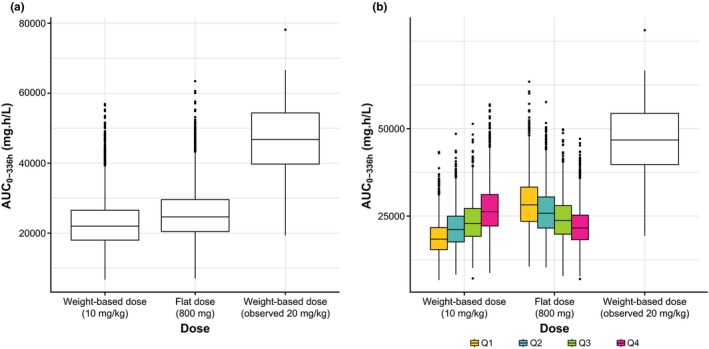
Simulated AUC0–336 h values for weight‐based (10 mg/kg q2w) and flat (800 mg q2w) dosing of avelumab using the first‐cycle population pharmacokinetic model. Box and whisker plots for (a) the entire population and (b) the population split by quartiles of weight; observed data with avelumab 20 mg/kg dosing are included for comparison purposes (n = 27). AUC0–336 h, area under the curve during the first dosing interval.
Table 1.
Simulated range of exposure (AUC0–336 h) and number of patients for weight‐based (10 mg/kg) and flat (800 mg) dosing of avelumab by quartiles of baseline body weight using the first‐cycle population pharmacokinetic model
| Weight quartiles | N | Median AUC0–336 h, mg/L*h (range) | |
|---|---|---|---|
| 10 mg/kg | 800 mg | ||
| Q1 (30.4 to ≤ 60.0 kg) | 415 | 19,102 (8,271–36,965) | 29,418 (14,191–54,763) |
| Q2 (60.0 to ≤ 70.6 kg) | 404 | 22,221 (9,057–35,556) | 27,170 (10,893–45,739) |
| Q3 (70.6 to ≤ 84.6 kg) | 435 | 24,720 (12,970–51,742) | 25,280 (13,627–51,103) |
| Q4 (84.4 to ≤ 204 kg) | 409 | 27,681 (7,438–44,938) | 22,583 (5,689–38,309) |
| Overall | 1,663 | 23,160 (7,438–51,742) | 25,913 (5,689–54,763) |
AUC0–336 h, area under the curve during the first dosing interval.
Simulations based on other exposure metrics (minimum and maximum serum concentrations (Ctrough and Cmax)) in the first‐cycle model and additional analyses using the steady‐state model resulted in findings consistent with those reported above for AUC0–336 h in the first‐cycle model (examples of results for Ctrough in the steady‐state model (Ctrough,ss) are shown in Figure S4).
Overall, population PK modeling and simulations suggested that exposure to avelumab was similar with 800 mg q2w flat dosing and 10 mg/kg q2w weight‐based dosing.
Exposure‐safety simulations
For a comparison of the simulated probability of experiencing AEs of special interest (irAEs and IRRs of any grade) between flat dosing and weight‐based dosing, simulated avelumab exposure metrics (AUC0–336 h, Ctrough, Cmax, AUCss, Ctrough,ss, and Cmax,ss) and safety data were analyzed in 1,712 patients with various tumors who had PK data collected in the aforementioned trials.
In the model, the simulated probability of irAEs across all patients with weight‐based dosing was 11.9%, which was the same as the observed rate with avelumab 10 mg/kg dosing across the clinical trials included. The simulated probability of experiencing an irAE based on AUC0–336 h showed a similar and overlapping distribution between flat and weight‐based dosing (Figure 2 a). The mean probability of irAEs was slightly higher for flat dosing compared with weight‐based dosing (12.6% vs. 11.4%, respectively), which can be attributed to the higher exposure of the flat‐dosing regimen resulting from the median weight of the sampled patients being < 80 kg. Across weight quartiles, there were opposing trends for the probabilities of irAEs in flat and weight‐based dose simulations (Figure 2 b). Specifically, in the lightest weight quartile, the mean probability of irAEs was numerically higher with flat dosing compared with weight‐based dosing (14.3% vs. 10.0%, respectively); however, this projected difference is not expected to be clinically meaningful because this rate is similar to or lower than rates of irAEs seen with other approved anti–PD‐1/PD‐L1 antibodies.21, 22 In contrast, in the heaviest weight quartile, the probability of irAEs was lower for flat dosing compared with weight‐based dosing.
Figure 2.
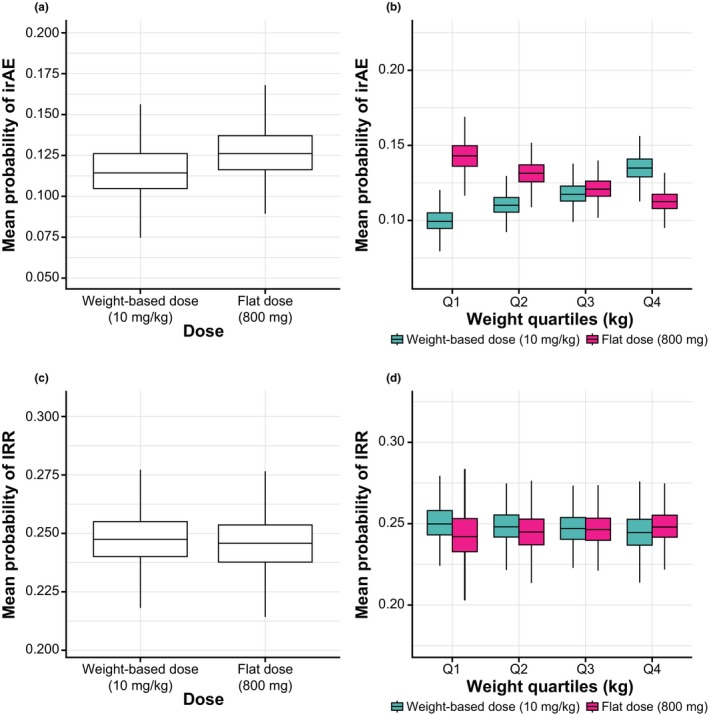
Mean probability of experiencing an irAE (upper panels) or IRR (lower panels) for weight‐based (10 mg/kg q2w) and flat (800 mg q2w) dosing with avelumab based on the first‐cycle population pharmacokinetic model. Box and whisker plots for (a) probability of irAEs based on AUC0–336 h in all patients; (b) probability of irAEs based on AUC0–336 h stratified by quartiles of weight; (c) probability of IRRs based on Cmax in all patients; and (d) probability of IRRs based on Cmax stratified by quartiles of weight. AUC0–336 h, area under the concentration curve during the first dosing interval; Cmax, maximum concentration; irAE, immune‐related adverse event; IRR, infusion‐related reaction.
In the model, the probability of experiencing an IRR with weight‐based avelumab dosing in the overall population was 24.7%, which was the same as the observed rate from clinical trials. Simulated probabilities of IRRs based on exposure (Cmax) were strongly concordant between weight‐based and flat dosing (Figure 2 c), with no trends seen in weight quartiles (Figure 2 d). This finding was expected because a previously developed exposure‐IRR model concluded that the probability of IRRs does not change with exposure (data not shown). Simulated probabilities of irAEs or IRRs based on other exposure metrics and using the steady‐state model resulted in findings consistent with those reported above (example of results for Ctrough,ss‐irAE and for Cmax,ss‐IRR are shown Figure S5 ).
Overall, these exposure‐based analyses suggest that the safety profile of flat dosing for avelumab is similar to that of weight‐based dosing.
Exposure‐efficacy simulations
For a comparison of the simulated probability of OR between flat dosing and weight‐based dosing, previously simulated avelumab exposure metrics (AUC0–336 h, Ctrough, and Ctrough,ss) and OR data were analyzed in 88 patients with mMCC from part A of JAVELIN Merkel 200 and 249 patients with advanced/metastatic UC from JAVELIN Solid Tumor. OR was chosen for efficacy simulations because it provided the primary basis for regulatory approval (primary end point) in both tumor types.
Simulated probabilities of achieving an OR with weight‐based dosing in the mMCC and advanced/metastatic UC populations were 31.8% and 17.3%, respectively, which matched the observed OR rates in clinical trials. In both mMCC and advanced/metastatic UC populations, the simulated probability of OR based on exposure (AUC0–336 h) was slightly higher with the 800 mg flat dose than weight‐based dosing with 10 mg/kg (Figure 3). For typical patients with mMCC or advanced or metastatic UC (see Methods), the estimated probability of OR was ≈ 5% higher and ≈ 3% higher with flat vs. weight‐based dosing, respectively. However, the two regimens showed an overlapping distribution of simulated ORs in the overall populations (Figure 3 a,c), suggesting that the probability of OR is similar within each tumor type.
Figure 3.
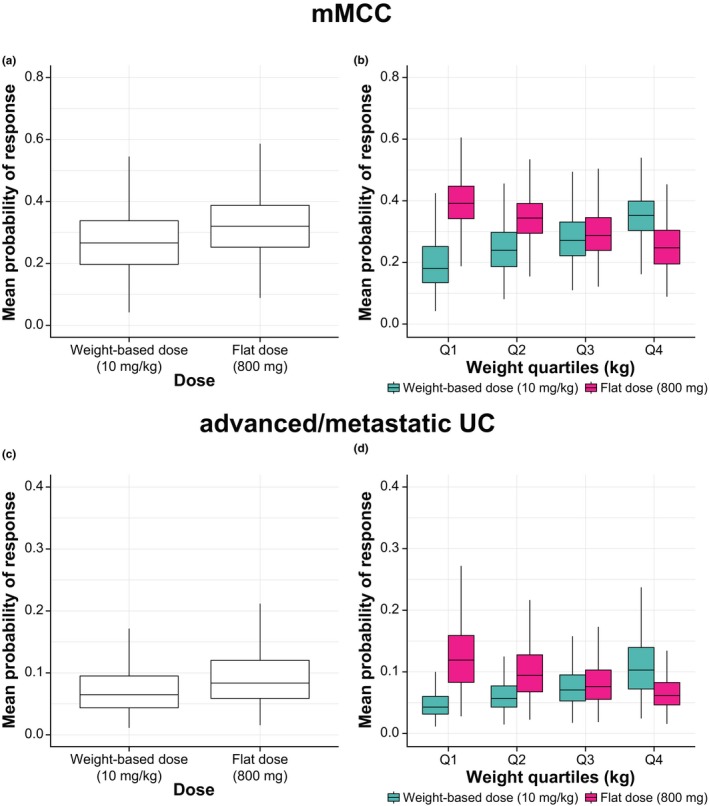
Mean probability of objective response in patients with mMCC (upper panels) or advanced/metastatic UC (lower panels) for weight‐based (10 mg/kg q2w) and flat (800 mg q2w) dosing with avelumab based on AUC0–336 h (first‐cycle population pharmacokinetic model). Box and whisker plots in (a) all patients with mMCC, (b) patients with mMCC stratified by quartiles of weight, (c) all patients with advanced/metastatic UC, and (d) patients with advanced/metastatic UC stratified by quartiles of weight. AUC0–336 h, area under the curve during the first dosing interval; Ctrough, minimum serum concentrations; mMCC, metastatic Merkel cell carcinoma; UC, urothelial carcinoma.
Across all weight quartiles for both tumor types, there was substantial overlap between the weight‐based and flat‐dose regimens but with opposing trends (Figure 3 b,d). Specifically, the probability of OR was highest for the heaviest weight quartile with weight‐based dosing and for the lowest weight quartile with flat dosing. Although the probability of OR was numerically decreased in the heaviest weight quartile with flat dosing, it was comparable to the OR probability with weight‐based dosing in the three lower weight quartiles (75% of the population). Simulated probabilities of OR for mMCC and advanced/metastatic UC based on other exposure metrics and using the steady‐state model (Ctrough and Ctrough,ss) showed consistent findings (examples of results for Ctrough,ss are shown in Figure S6).
Overall, these exposure‐efficacy simulations indicated that the probability of OR is likely to be similar with flat or weight‐based dosing in the populations examined.
DISCUSSION
Population PK modeling and simulation, based on a large patient data set, support the use of a flat avelumab dose of 800 mg q2w instead of the weight‐based 10 mg/kg q2w dose that was approved initially. Compared with weight‐based dosing, flat dosing provides similar predicted exposure with slightly less variability, providing the primary evidence that supports this change. The estimated power exponent for the effect of weight on CL in the avelumab first‐cycle population PK model was 0.324.17 Thus, our data are consistent with published data showing that flat dosing produces less variability in exposure when the estimated exponent defining the relationship is < 0.5.6 Although overall exposure was slightly increased with flat dosing, this was expected because the median weight of sampled patients (70.6 kg) was lower than the “standard” weight of 80 kg seen in other populations of patients with solid tumors, on which the flat dose was based. In comparison, in a real‐world population of ≈ 500 patients with mMCC enrolled in a global expanded access program for avelumab, the median body weight was 78 kg.23 Only minor differences in simulated exposure were seen between flat dosing and weight‐based dosing in quartiles of patients with lightest and heaviest body weights, but the distributions of exposure overlapped across all quartiles. Thus, the exposures expected with flat dosing of avelumab are within the exposure range of a weight‐based dose that has shown clinical activity and an acceptable safety profile. In addition, simulated exposure with the 800 mg dose was markedly lower than that observed with a 20 mg/kg dose, which has shown acceptable safety and tolerability in 2 phase I trials.12, 13
The preferred PK exposure metrics used to compare weight‐based and flat‐dosing regimens were simulated based on the population PK model developed using first‐cycle data. Avelumab displays a time‐dependent decrease in CL,17 which has also been reported for agents within the same class (nivolumab and pembrolizumab).24, 25 Thus, the results obtained with simulated PK exposures based on the first‐cycle model minimize the potential impact of time‐dependent changes in CL (also for subsequent exposure‐efficacy and exposure‐safety simulations).24 However, for completeness, PK exposures were also simulated based on the steady‐state model, which corroborated the results obtained with the first‐cycle model.
Exposure‐safety and exposure‐efficacy simulations provided additional support for a change in dosing regimen and indicated that the benefit:risk profile for the 800 mg flat dose is likely to be similar to that of the 10 mg/kg weight‐based dose. Although there was a slight increase in the predicted probability of experiencing an irAE with flat dosing, the expected incidence remains consistent with safety profiles of other approved anti–PD‐1/PD‐L1 antibodies.26, 27, 28, 29 In addition, the probability of experiencing an AE of special interest was similar between flat dosing and weight‐based dosing. In an analysis of data derived from populations of patients with mMCC or advanced/metastatic UC, the probability of achieving an OR was similar with both dosing regimens. Thus, although minor differences between dosing regimens were observed in body weight quartiles in exposure‐safety and exposure‐efficacy simulations, these were not considered to be clinically significant based on the small numerical differences observed, and probabilities of AEs and OR were similar when dosing regimens were compared in the overall populations. Limitations of the exposure‐efficacy models include the absence of data from multiple dose levels, the assumption of linearity for logistic regression models, and the imbalance of covariates across the exposure quartiles. These could affect the observed exposure‐efficacy relationship, which may seem steeper than expected. Nonetheless, these exposure‐efficacy simulations provide additional support for a flat dosing regimen.
The potential interchangeability between flat dosing and weight‐based dosing based on pharmacometric modeling, which is reported here for the first time for avelumab, is generally consistent with previously reported analyses of other monoclonal antibodies,6 including those targeting PD‐1/PD‐L1 (e.g., nivolumab,3 pembrolizumab,4 atezolizumab,5 and durvalumab30). In general, use of flat dosing is expected to provide practical benefits for administration. In particular, avelumab is available in vials containing 200 mg of drug in a 10 mL volume; thus, administering the 800 mg dose will require 4 vials with no resulting waste.
In conclusion, a flat dose of avelumab 800 mg q2w is likely to provide a similar benefit:risk profile to that of avelumab 10 mg/kg q2w seen in clinical trials, and this regimen has been adopted for new clinical trials of avelumab. The analyses reported provided the basis for the US Food and Drug Administration approval of avelumab 800 mg for all approved indications7 and further demonstrate the value of pharmacometric modeling and simulation in the absence of newly generated clinical data to assess clinically untested dosing regimens.
METHODS
Clinical studies
Data for PK analyses were obtained from 3 clinical trials: JAVELIN Solid Tumor (NCT01772004), JAVELIN Solid Tumor JPN (NCT01943461), and JAVELIN Merkel 200 (NCT02155647). Data cutoff dates are shown in Table S1.
JAVELIN Solid Tumor is a phase I, international, open‐label trial of avelumab in patients with advanced or metastatic solid tumors. In the initial dose‐escalation part (phase Ia), patients with various tumors received avelumab at 1, 3, 10, or 20 mg/kg q2w via a 1‐hour intravenous infusion.12 In the dose‐expansion part (phase Ib), patients were enrolled into tumor‐specific cohorts and received avelumab at 10 mg/kg q2w.11, 31
JAVELIN Solid Tumor JPN is a phase I, open‐label, multicenter trial of avelumab in Japanese patients with various advanced or metastatic solid tumors. In the initial dose‐escalation part, patients received avelumab at 3, 10, or 20 mg/kg q2w. In the dose‐expansion part, patients with advanced/metastatic gastric or gastroesophageal cancer received avelumab at 10 mg/kg q2w.13
JAVELIN Merkel 200 is an international, open‐label, phase II trial evaluating the efficacy and safety of avelumab at 10 mg/kg q2w in patients with mMCC; patients included in the analysis population (from part A of the trial) received ≥ 1 prior line of chemotherapy.16
All trials were conducted in accordance with international standards of Good Clinical Practice. Study protocols were approved by institutional review boards or ethics committees at each center. All patients provided written informed consent.
Simulations of exposure
Previous population PK models were developed to describe avelumab PK after single‐dose infusion and multiple‐dose infusions,17, 32 which are described briefly below. These models were based on PK data collected from 1,827 patients enrolled in the 3 trials described above.
In the first population PK analysis, first‐cycle concentrations of avelumab over time (single infusion) were adequately described by a two‐compartmental linear model (the first‐cycle model). Covariate effects included in the final model, which had a significant effect on CL but were not considered clinically meaningful, were baseline body weight, baseline C‐reactive protein level, baseline albumin level, baseline tumor burden, tumor type (squamous cell carcinoma of the head and neck or mMCC), age, sex, race (black), estimated glomerular filtration rate (eGFR), Eastern Cooperative Oncology Group performance status (score of 0), baseline platelet count, baseline aspartate aminotransferase concentration, and previous use of biologics. For volume of distribution of the central compartment, baseline weight was of potential clinical importance, and sex, baseline albumin level, tumor size, and previous use of biologics produced small but statistically significant effects. The first‐cycle model was developed to minimize the limitations of the assumed nonindependence between exposure and response/post‐treatment effects.24
In the second population PK analysis, avelumab concentrations over time after multiple infusions were adequately described by a 2‐compartmental linear model incorporating time‐varying CL (the steady‐state model). In the final reduced steady‐state model for CL, baseline weight and baseline C‐reactive protein concentration produced a statistically significant effect that was considered unlikely to be clinically relevant. Baseline albumin level, antidrug antibodies (ever), sex, race (black), age, eGFR, tumor burden, baseline platelet count, aspartate aminotransferase concentration, no concomitant opioid use, and a history of biologics use also had a statistically significant influence on CL but were judged unlikely to be clinically significant. For volume of distribution of the central compartment, only weight was both statistically significant and considered possibly clinically relevant; other statistically significant (but not clinically meaningful) predictors were sex, albumin concentration, and a history of biologics use. Significant decreases in CL over time were seen with mMCC and squamous cell carcinoma of the head and neck tumor types.
PK exposure metrics, specifically Ctrough, Cmax, and AUC after a single dose (first‐cycle model) and multiple doses (steady‐state model), were simulated for both weight‐based and flat dosing.
The methodology for simulations using the first‐cycle and steady‐state models was similar. Two hundred sets of model parameters were sampled from the first‐cycle and steady‐state models. For each sampled parameter set, 500 sets of interpatient variability parameters were sampled from the variance‐covariance matrix, resulting in 100,000 sets of parameter estimates. A total of 100,000 simulated patients were then resampled from the PK data set (n = 1,827), with all covariates present in the final model retained at the patient level and randomly assigned to the parameter estimates. Thereafter, 10,000 patients were randomly sampled from the pool of 100,000 patients for both dosing regimens (10 mg/kg q2w and 800 mg q2w).
For the first‐cycle model, a closed‐form solution for a linear 2‐compartment model was used to calculate AUC0–τ (using the linear trapezoidal rule (τ = 336 h)), Ctrough (minimum concentration over the dosing interval (τ = 336 h), assumed to occur at 335.5 h after the start of infusion), and Cmax (maximum concentration over the dosing interval (τ = 336 h), assumed to occur at 1 h after the start of infusion). For the steady‐state model, a “skeleton” data set was constructed to reflect dosing with 10 mg/kg or 800 mg q2w for 28 weeks. Using this data set, simulations were performed in NONMEM. Metrics calculated for the steady‐state model were the same as those calculated for the first‐cycle model, with an assumed steady state occurring between weeks 26 and 28 (τ = 336 h). These metrics were used to compare dosing regimens by quartiles of weight, defined as 30.4 to ≤ 60 kg, > 60 to ≤ 70.6 kg, > 70.6 to ≤ 84.6 kg, and > 84.6 to ≤ 204 kg. To assess the variability of derived PK metrics, coefficients of variation were calculated as a ratio of SD to mean. Observed data for avelumab 20 mg/kg were included as a reference.
Exposure‐safety simulations
Simulated avelumab exposure metrics from the first‐cycle and steady‐state models were used to compare the probability of experiencing AEs of special interest (irAEs or IRRs of any grade) with weight‐based and flat dosing. AUC and Ctrough metrics were used to evaluate the probability of irAEs, whereas Cmax was used to evaluate IRRs. The univariate models that were used to perform the exposure‐irAE and exposure‐IRR simulations suggested that higher avelumab exposure was associated with a modest increase in the incidence of irAEs but not IRRs (data not shown). Because these models are univariate, the simulations represent worst‐case scenarios and estimate the effect of exposure on the probability of AEs.
Exposure‐efficacy simulations
Simulated avelumab exposure metrics were used to compare the simulated probability of OR with weight‐based or flat dosing in patients with mMCC or advanced/metastatic UC. Data from JAVELIN Solid Tumor JPN were not included in this analysis because no patients with mMCC or UC were enrolled. The multivariable logistic regression models used for these simulations were developed for both tumor subgroups.18 Briefly, covariates included in the final model for mMCC were PD‐L1 status (< 5%/≥ 5%), albumin concentration, tumor burden at baseline, number of prior anticancer treatments (≤ 1/> 1), and lymph node disease only at baseline (yes/no). Covariates included in the final model for advanced/metastatic UC were race, sex, age, PD‐L1 status (< 5%/≥ 5%), visceral metastasis (yes/no), tumor subsite (upper/lower tract), number of prior anticancer treatments or nontarget lesions, lactate dehydrogenase or hemoglobin concentration, eGFR, and concomitant steroids (yes/no).
For efficacy simulations in each tumor subgroup, 400 sets of vector parameter estimates were sampled from multivariate normal distributions. For each of the 400 parameter sets, 5,000 simulated patients were assigned from the simulated exposure data set (as described previously under “simulations of exposure”). The mean predicted probability of response across simulated patients was obtained for each of the 400 sets of logistic regression model parameter estimates. For simulation purposes, a typical patient in each tumor population was defined as an individual with median values for continuous covariates and the most common category for categorical covariates. A typical patient with mMCC had an albumin concentration of 40.5 g/L, a tumor burden at baseline of 60 mm, receipt of 1 prior anticancer therapy, tumor PD‐L1 expression of < 5%, and no positive lymph nodes at baseline. A typical patient with advanced or metastatic UC was a white man aged 68 years with visceral metastasis, a lower‐tract tumor subsite, tumor PD‐L1 expression of < 5%, receipt of > 1 prior anticancer therapy, 4 nontarget lesions, lactate dehydrogenase concentration of 206.9 UI/L, hemoglobin concentration of 7.1 mmol/L, eGFR of 65.9 mL/min/1.73 m2, and no concomitant corticosteroid use.
Funding
This work was supported by Merck KGaA, Darmstadt, Germany, and its subsidiary, EMD Serono, as part of an alliance with Pfizer Inc.
Conflict of Interest
A.M.N. and A.K. are employees of Merck KGaA. J.J.W. and J.R.W. were employed as consultants by Merck KGaA when analyses were performed. H.D. and B.N. are employees of EMD Serono, a business of Merck KGaA. S.B. and C.L.B. are employees of Pfizer Inc. P.G. is an employee of Merck Serono SA, Lausanne, Switzerland, an affiliate of Merck KGaA.
Author Contributions
All authors wrote the manuscript. All authors designed the research. A.M.N., J.J.W., and A.K. performed the research. All authors analyzed the data.
Supporting information
Table S1. Data cutoff dates for trials providing data for pharmacokinetic analyses. UC, urothelial carcinoma.
Figure S1. Weight distribution for the study population. Solid vertical lines represent the 25th percentile (60.0 kg), median (70.6 kg), and 75th percentile (84.6 kg) of weight.
Figure S2. Simulated exposure values for weight‐based (10 mg/kg q2w) and flat (800 mg q2w) dosing of avelumab by extremes of weight using the first‐cycle population pharmacokinetic model. (a) AUC0–336 h, (b) Ctrough, and (c) Cmax. AUC, area under the curve; Ctrough, trough concentration after first dose; Cmax, maximum concentration after first dose; q2w, every 2 weeks.
Figure S3. Simulated exposure values for weight‐based (10 mg/kg q2w) and flat (800 mg q2w) dosing of avelumab by extremes of weight using the steady‐state population pharmacokinetic model. (a) AUCss, (b) Ctrough,ss, and (c) Cmax,ss. AUCss, steady‐state area under the curve; Ctrough,ss, steady‐state trough concentration; Cmax,ss, steady‐state maximum concentration; q2w, every 2 weeks.
Figure S4. Simulated Ctrough,ss values for weight‐based (10 mg/kg q2w) and flat (800 mg q2w) dosing of avelumab using the steady‐state population pharmacokinetic model. Box and whisker plots for (a) the entire population and (b) the population split by quartiles of weight; observed data with avelumab 20 mg/kg dosing is included for comparison purposes (n = 27). Ctrough,ss, steady‐state trough concentration; q2w, every 2 weeks.
Figure S5. Mean probability of experiencing an irAE (upper panels) or IRR (lower panels) for weight‐based (10 mg/kg q2w) and flat (800 mg q2w) dosing with avelumab based on the steady‐state population pharmacokinetic model. Box and whisker plots for (a) probability of irAEs based on Ctrough,ss in all patients; (b) probability of irAEs based on Ctrough,ss stratified by quartiles of weight; (c) box and whisker plots for probability of IRRs based on Cmax.ss in all patients; and (d) probability of IRRs based on Cmax.ss stratified by quartiles of weight. Cmax,ss, steady‐state maximum concentration; Ctrough,ss, steady‐state trough concentration; irAE, immune‐related adverse event; IRR, infusion‐related reaction; q2w, every 2 weeks.
Figure S6. Mean probability of objective response in patients with mMCC (upper panels) or advanced/metastatic UC (lower panels) for weight‐based (10 mg/kg q2w) and flat (800 mg q2w) dosing with avelumab based on Ctrough,ss (steady‐state population pharmacokinetic model). Box and whisker plots in (a) all patients with mMCC, (b) patients with mMCC stratified by quartiles of weight, (c) all patients with advanced/metastatic UC, and (d) in patients with advanced/metastatic UC stratified by quartiles of weight. Ctrough,ss, steady‐state trough concentration; mMCC, metastatic Merkel cell carcinoma; q2w, every 2 weeks; UC, urothelial carcinoma.
Acknowledgments
We thank the patients, their families, the investigators, coinvestigators, and study teams who participated in the clinical trials that provided the data.
References
- 1. Balar, A.V. & Weber, J.S. PD‐1 and PD‐L1 antibodies in cancer: current status and future directions. Cancer Immunol. Immunother. 66, 551–564 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hargadon, K.M. , Johnson, C.E. & Williams, C.J. Immune checkpoint blockade therapy for cancer: an overview of FDA‐approved immune checkpoint inhibitors. Int. Immunopharmacol. 62, 29–39 (2018). [DOI] [PubMed] [Google Scholar]
- 3. Zhao, X. et al. Assessment of nivolumab benefit‐risk profile of a 240‐mg flat dose relative to a 3‐mg/kg dosing regimen in patients with advanced tumors. Ann. Oncol. 28, 2002–2008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freshwater, T. et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J. Immunother. Cancer. 5, 43 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stroh, M. et al. Clinical pharmacokinetics and pharmacodynamics of atezolizumab in metastatic urothelial carcinoma. Clin. Pharmacol. Ther. 102, 305–312 (2017). [DOI] [PubMed] [Google Scholar]
- 6. Wang, D.D. et al. Fixed dosing versus body size‐based dosing of monoclonal antibodies in adult clinical trials. J. Clin. Pharmacol. 49, 1012–1024 (2009). [DOI] [PubMed] [Google Scholar]
- 7. Bavencio (avelumab) [package insert] (EMD Serono, Rockland, MD, 2019). [Google Scholar]
- 8. Bavencio (avelumab) [summary of product characteristics] (Merck KGaA, Darmstadt, Germany, 2019). [Google Scholar]
- 9. Boyerinas, B. et al. Antibody‐dependent cellular cytotoxicity activity of a novel anti‐PD‐L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol. Res. 3, 1148–1157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vandeveer, A.J. et al. Systemic immunotherapy of non‐muscle invasive mouse bladder cancer with avelumab, an anti‐PD‐L1 immune checkpoint inhibitor. Cancer Immunol. Res. 4, 452–462 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chin, K. , Chand, V.K. & Nuyten, D.S.A. Avelumab: clinical trial innovation and collaboration to advance anti‐PD‐L1 immunotherapy. Ann. Oncol. 28, 1658–1666 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heery, C.R. et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose‐escalation trial. Lancet Oncol. 18, 587–598 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doi, T. et al. Phase 1 trial of avelumab (anti‐PD‐L1) in Japanese patients with advanced solid tumors, including dose expansion in patients with gastric or gastroesophageal junction cancer: the JAVELIN Solid Tumor JPN trial. Gastric Cancer 22, 817–827 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Apolo, A.B. et al. Avelumab, an anti‐programmed death‐ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J. Clin. Oncol. 35, 2117–2124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel, M.R. et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open‐label, phase 1 trial. Lancet Oncol. 19, 51–64 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaufman, H.L. et al. Avelumab in patients with chemotherapy‐refractory metastatic Merkel cell carcinoma: a multicentre, single‐group, open‐label, phase 2 trial. Lancet Oncol. 17, 1374–1385 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilkins, J.J. et al. Time‐varying clearance and impact of disease state on the pharmacokinetics of avelumab in Merkel cell carcinoma and urothelial carcinoma. CPT Pharmacometrics Syst. Pharmacol. 8, 415–427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vugmeyster, Y. et al. Exposure–response analysis of avelumab in patients with advanced urothelial carcinoma via a full‐model approach. J. Pharmacokinet. Pharmacodyn. 45, S129 (Abstract W‐088) (2018). [Google Scholar]
- 19. Gulley, J.L. et al. Exposure‐response and PD‐L1 expression analysis of second‐line avelumab in patients with advanced NSCLC: data from the JAVELIN Solid Tumor trial. J. Clin. Oncol. 35, 9086–9086 (2017). [Google Scholar]
- 20. Feng, Y. et al. Model‐based clinical pharmacology profiling of ipilimumab in patients with advanced melanoma. Br. J. Clin. Pharmacol. 78, 106–117 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun, X. et al. Immune‐related adverse events associated with programmed cell death protein‐1 and programmed cell death ligand 1 inhibitors for non‐small cell lung cancer: a PRISMA systematic review and meta‐analysis. BMC Cancer 19, 558 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang, P.‐F. et al. Immune‐related adverse events associated with anti‐PD‐1/PD‐L1 treatment for malignancies: a meta‐analysis. Front. Pharmacol. 8, 730 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walker, J. et al. Second‐line avelumab treatment of patients (pts) with metastatic Merkel cell carcinoma (mMCC): experience from a global expanded access program (EAP). J. Clin. Oncol. 36 (15 suppl.), 9537–9537 (2018). [Google Scholar]
- 24. Liu, C. et al. Association of time‐varying clearance of nivolumab with disease dynamics and its implications on exposure response analysis. Clin. Pharmacol. Ther. 101, 657–666 (2017). [DOI] [PubMed] [Google Scholar]
- 25. Li, H. et al. Time dependent pharmacokinetics of pembrolizumab in patients with solid tumor and its correlation with best overall response. J. Pharmacokinet. Pharmacodyn. 44, 403–414 (2017). [DOI] [PubMed] [Google Scholar]
- 26. Sharma, P. et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single‐arm, phase 2 trial. Lancet Oncol. 18, 312–322 (2017). [DOI] [PubMed] [Google Scholar]
- 27. Bellmunt, J. et al. Pembrolizumab as second‐line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 376, 1015–1026 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Powles, T. et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open‐label study. JAMA Oncol. 3, e172411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenberg, J.E. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: a single‐arm, multicentre, phase 2 trial. Lancet 387, 1909–1920 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baverel, P.G. et al. Population pharmacokinetics of durvalumab in cancer patients and association with longitudinal biomarkers of disease status. Clin. Pharmacol. Ther. 103, 631–642 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kelly, K. et al. Safety profile of avelumab in patients with advanced solid tumors: A pooled analysis of data from the phase 1 JAVELIN Solid Tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer 124, 2010–2017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilkins, J. et al. Clearance over time and effect of response in the pharmacokinetics of avelumab. J. Pharmacokinet. Pharmacodyn. 44, S132–S133 (Abstract W‐079) (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Data cutoff dates for trials providing data for pharmacokinetic analyses. UC, urothelial carcinoma.
Figure S1. Weight distribution for the study population. Solid vertical lines represent the 25th percentile (60.0 kg), median (70.6 kg), and 75th percentile (84.6 kg) of weight.
Figure S2. Simulated exposure values for weight‐based (10 mg/kg q2w) and flat (800 mg q2w) dosing of avelumab by extremes of weight using the first‐cycle population pharmacokinetic model. (a) AUC0–336 h, (b) Ctrough, and (c) Cmax. AUC, area under the curve; Ctrough, trough concentration after first dose; Cmax, maximum concentration after first dose; q2w, every 2 weeks.
Figure S3. Simulated exposure values for weight‐based (10 mg/kg q2w) and flat (800 mg q2w) dosing of avelumab by extremes of weight using the steady‐state population pharmacokinetic model. (a) AUCss, (b) Ctrough,ss, and (c) Cmax,ss. AUCss, steady‐state area under the curve; Ctrough,ss, steady‐state trough concentration; Cmax,ss, steady‐state maximum concentration; q2w, every 2 weeks.
Figure S4. Simulated Ctrough,ss values for weight‐based (10 mg/kg q2w) and flat (800 mg q2w) dosing of avelumab using the steady‐state population pharmacokinetic model. Box and whisker plots for (a) the entire population and (b) the population split by quartiles of weight; observed data with avelumab 20 mg/kg dosing is included for comparison purposes (n = 27). Ctrough,ss, steady‐state trough concentration; q2w, every 2 weeks.
Figure S5. Mean probability of experiencing an irAE (upper panels) or IRR (lower panels) for weight‐based (10 mg/kg q2w) and flat (800 mg q2w) dosing with avelumab based on the steady‐state population pharmacokinetic model. Box and whisker plots for (a) probability of irAEs based on Ctrough,ss in all patients; (b) probability of irAEs based on Ctrough,ss stratified by quartiles of weight; (c) box and whisker plots for probability of IRRs based on Cmax.ss in all patients; and (d) probability of IRRs based on Cmax.ss stratified by quartiles of weight. Cmax,ss, steady‐state maximum concentration; Ctrough,ss, steady‐state trough concentration; irAE, immune‐related adverse event; IRR, infusion‐related reaction; q2w, every 2 weeks.
Figure S6. Mean probability of objective response in patients with mMCC (upper panels) or advanced/metastatic UC (lower panels) for weight‐based (10 mg/kg q2w) and flat (800 mg q2w) dosing with avelumab based on Ctrough,ss (steady‐state population pharmacokinetic model). Box and whisker plots in (a) all patients with mMCC, (b) patients with mMCC stratified by quartiles of weight, (c) all patients with advanced/metastatic UC, and (d) in patients with advanced/metastatic UC stratified by quartiles of weight. Ctrough,ss, steady‐state trough concentration; mMCC, metastatic Merkel cell carcinoma; q2w, every 2 weeks; UC, urothelial carcinoma.


