Abstract
Background
The survival rate of osteosarcoma therapy still lags behind overall cancer therapies due to the intrinsic or acquired drug resistance. Developing novel drug delivery systems that may overcome drug resistance would greatly facilitate osteosarcoma therapy.
Methods
Poly(ethylene glycol) (PEG)-sheddable reduction-sensitive polyurethane (SS-PU-SS-PEG) was synthesized using a disulfide-containing polycaprolactone diol as the hydrophobic block and a cystamine-functionalized PEG as the hydrophilic block. SS-PU-SS-PEG micelles were then prepared to load the anti-tumor drug Doxorubicin (DOX) in order to achieve triggered intracellular drug delivery to improve the efficacy of osteosarcoma therapy.
Results
When DOX was used as a model drug, the drug-loaded SS-PU-SS-PEG micelles were about 82∼94 nm in diameter and exhibited good stability in phosphate buffer saline (PBS). The micelles could release about 80% DOX in a quantitative fashion within 5 hours under a reductive environment. The intracellular drug release of DOX-loaded SS-PU-SS-PEG micelles increased upon incubation with Saos-2 cells in vitro. The micelles had good biocompatibility. In vitro, DOX-loaded SS-PU-SS-PEG micelles showed significant antitumor activity toward Saos-2 cells, which was close to that of free DOX. In vivo, DOX-loaded SS-PU-SS-PEG micelles exhibited better antitumor activity than free DOX.
Conclusion
Findings from this study suggest that the SS-PU-SS-PEG micelles could achieve well-controlled triggered drug release in a reduction environment and could therefore improve the antitumor efficacy of osteosarcoma therapies.
Translation potential of this article
In this study we developed PEG-sheddable reduction-sensitive polyurethane micelles (SS-PU-SS-PEG), which were able to achieve well-controlled triggered release of anti-tumor drug Doxorubicin (DOX) in an intracellular reduction environment. DOX-loaded SS-PU-SS-PEG micelles markedly improved the antitumor efficacy in a Saos-2 cells-bearing xenograft tumor model. Therefore, such micelles might be used as a novel drug delivery system for osteosarcoma treatment.
Keywords: Intracellular drug delivery, Micelles, Osteosarcoma, Polyurethane, Reduction-sensitive
Introduction
Osteosarcoma is one of the most common primary malignant bone tumours and is most prevalent in teenagers and young adults. Thanks to the significant progress in the combined use of chemotherapy and surgery, prognosis of patients with osteosarcoma has been significantly improved in the past decade. However, the survival rate of patients with osteosarcoma still lags behind the overall survival rates of patients with cancer in that age group, mainly because of the intrinsic or acquired drug resistance [[1], [2], [3]]. Therefore, novel methods that interfere with both intrinsic and acquired drug resistance mechanisms may greatly facilitate osteosarcoma treatment [[4], [5], [6], [7], [8]].
Recently, a variety of nanoparticle-based drug delivery systems have been developed to achieve drug accumulation in tumour tissues, cells, or even nucleus of tumour cells to overcome drug resistance [[8], [9], [10], [11], [12]]. Some have been applied for treatment of osteosarcoma [[13], [14], [15]]. Importantly, stimuli-responsive (e.g., pH, redox, temperature, and light), triggered intracellular drug delivery systems have shown potential to reverse multidrug resistance to improve treatment efficacy [[16], [17], [18], [19]]. One of the common stimuli is the redox potential gradient, which exists between the mildly oxidative extracellular milieu and the reductive intracellular fluids within tumour cells. Specifically, the cytosolic glutathione concentration (~10 mM) in some tumour cells, such as osteosarcoma cells, may be several times higher than that in normal cells. In light of this, a number of redox-sensitive drug delivery systems have been developed, including introducing disulfide linkages into the backbone and side chains of biodegradable polymeric materials or using reducible crosslinkers, for efficient delivery of antitumour drugs into tumour cells [[20], [21], [22], [23], [24], [25], [26], [27]].
Polyurethanes (PUs) are a large family of synthetic biomaterials with an enormous diversity of chemical composition and have been widely used in medical devices [[28], [29], [30]]. In recent years, biodegradable PUs, especially stimuli-sensitive degradable PUs, have been explored for achieving intracellular triggered drug release [[31], [32], [33], [34], [35], [36]]. For example, paclitaxel (PTX)-loaded reduction-responsive PU micelles based on L-lysine diisocyanate, poly(ε-caprolactone), bis(2-hydroxyethyl)-disulfide, and poly(ethylene glycol) (PEG) exhibited effective growth inhibition of HepG2 cells [31]. However, PEG-based micelles may shield the particle surface and prolong circulation. PEG coating may also weaken the cellular interaction and block drug release. Therefore, approaches to shed off the “stealth” PEG in response to stimuli, such as pH or reduction, have shown great promise in intracellular drug delivery [37,38].
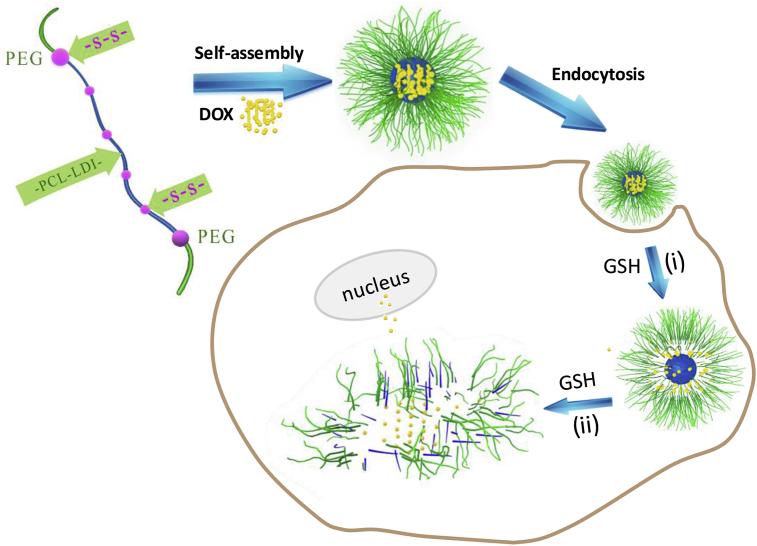
Doxorubicin (DOX), a chemotherapy medication for cancer treatment, has been widely used for treating osteosarcoma. However, the clinical outcomes of DOX are still not satisfactory because of the large dosage-associated adverse side effects and existence of drug resistance. In addition, the poor cellular uptake of DOX remains a problem, which prevents sufficient intracellular drug release to achieve effective antitumour activity [[39], [40], [41], [42], [43]]. In this study, we have developed a facile approach to prepare PEG-sheddable reduction-sensitive PU (SS-PU-SS-PEG micelles for triggered intracellular drug delivery for osteosarcoma therapy using DOX as a model drug (Scheme 1). The purposes and uniqueness of such an approach to improve the therapy efficacy of DOX to osteosarcoma are as follows. First, PU micelles are used to improve the water solubility of DOX and reduce its toxicity. Second, reduction-sensitive sheddable hydrophilic PEG is included to trigger fast drug delivery. Finally, the reduction-sensitive degradable hydrophobic block of PU further speeds up drug delivery. As such, the SS-PU-SS-PEG micelles may help achieve well-controllable, reduction-triggered drug release to improve the efficacy of drugs for treating tumours including osteosarcoma.
Scheme 1.
Schematic illustration of DOX-loaded SS-PU-SS-PEG micelles for triggered intracellular drug delivery for osteosarcoma therapy. As shown, PEG is reductively sheddable to trigger fast drug delivery and hydrophobic PU block is reductively degradable to further speed up the drug delivery.PEG = poly(ethylene glycol); SS-PU-SS-PEG = PEG-sheddable reduction-sensitive polyurethane; DOX = doxorubicin; PU = polyurethane; GSH = glutathione.
Materials and methods
Materials
ε-Caprolactone (ε-CL, 99%; Alfa Asear, Thermo Fisher Scientific, USA) and 2,2′-dithiodiethanol (98%; Alfa Aesar) were dried over CaH2 and vacuum distilled before use. L-Lysine diisocyanate (LDI, 99+%; Acros, Acros Organics, Belgium) and stannous octoate (96%; Alfa Aesar) were used as received. Poly(ethylene glycol) (mPEG-OH, 5 kDa; Alfa Aesar) was dried by azeotropic distillation from toluene. p-Nitrophenyl chloroformate (p-NPC, 97%; Alfa Aesar) and cystamine dihydrochloride (>98%; Alfa Aesar) were used as received. Toluene and N,N-dimethylformamide (DMF), purchased from Chinasun Specialty Products Co. Ltd, Changshu, China, were dried over CaH2 and vacuum distilled before use.
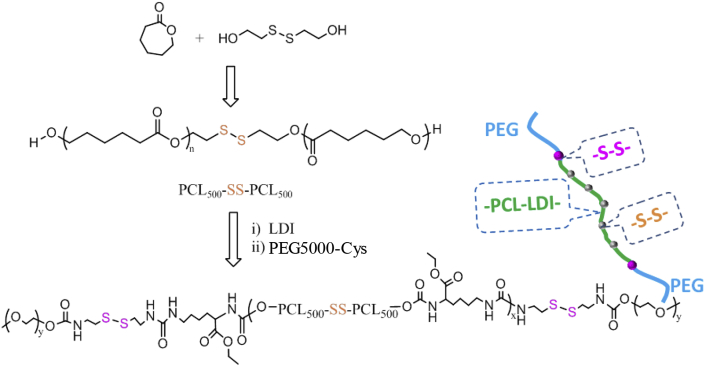
Synthesis of SS-PU-SS-PEG
SS-PU-SS-PEG was synthesised by a three-step polymerisation reaction as shown in Scheme 2. First, diol HO–polycaprolactone (PCL)–SS–PCL–OH was synthesised by ring-opening polymerisation of ε-CL with 2,2′-dithiodiethanol as an initiator, and the targeted molecular weight was set at about 1000 Da which was controlled by adjusting the ratio of ε-CL to 2,2′-dithiodiethanol. The diol HO–PCL–SS–PCL–OH (1 g, 1 mmol) was then dissolved in 15 mL dry DMF. After that, LDI (0.238 g, 1.05 mmol) was added to the solution together with 0.2 wt% stannous octoate as an initiator. The reaction was carried at 70 °C for 4 h under continuous stirring and then the solution was cooled to 0°C. After 0.5 g PEG-5000-Cys solution in DMF (5 mL) was added dropwise, the reaction was continued for 48 h at room temperature. Finally, the product was purified by precipitation in ether twice, filtered, and vacuum dried. 1-hydrogen nuclear magnetic resonance (1H NMR) spectra were recorded on a nuclear magnetic resonance system (INOVA 400 MHz, Varian Medical Systems, USA) using deuterated chloroform as a solvent.
Scheme 2.
Synthesis route of PEG-sheddable reduction-sensitive polyurethane.
The previously mentioned PEG-5000-Cys was synthesised by cystamine with p-NPC activated poly(ethylene glycol)-5000 (mPEG-5000-NPC) according to a previous study [44]. In this study, poly(ethylene glycol) (PEG)-unsheddable reduction-sensitive polyurethane (SS-PU-b-PEG was also synthesised as a control using amine-functionalized PEG5000-NH2 instead of PEG-5000-Cys.
Preparation and characterisations of SS-PU-SS-PEG micelles
SS-PU-SS-PEG micelles were prepared by the dialysis method. Typically, 3 mL of water was added dropwise into 1.0 mL of polymer solution in tetrahydrofuran (2 mg/mL) under stirring at room temperature followed by extensive dialysis against phosphate buffer (PB) (10 mM, pH7.4) with a molecular weight cutoff (MWCO) of 3500. The sizes of the self-assembled micelles were determined at 25 C by dynamic laser scattering (DLS) measurement (Zetasizer Nano ZS, Malvern Instruments, United Kingdom). The transmission electron microscopy (TEM) micrograph was tested by using a HT7700 TEM operated at an accelerating voltage of 120 kV. The critical micelle concentration (CMC) was determined using pyrene as a fluorescence probe. Size change of SS-PU-SS-PEG micelles in response to the intracellular reduction environment (10 mM dithiothreitol (DTT)) was tested by DLS measurement.
As controls, SS-PU-b-PEG micelles were also prepared and characterised in the same way.
DOX loading and reduction-triggered release of SS-PU-SS-PEG micelles
DOX-loaded SS-PU-SS-PEG micelles were also prepared by the dialysis method. In brief, 1.0 mL of water was added dropwise into the mixture of polymer solution (1 mL, 2 mg/mL) in tetrahydrofuran and DOX solution (calculated volume, 5 mg/mL) in dimethyl sulfoxide (DMSO) under stirring at room temperature, followed by 1 h ultrasonication and then by extensive dialysis against PB (10 mM, pH7.4) with a MWCO of 3500. The whole process was performed in the dark. The drug loading content (DLC) and the drug loading efficiency (DLE) were both determined by a fluorescence spectrophotometer with the excitation at 480 nm and emission at 560 nm. The DLC and DLE were determined according to the following formula:
The triggered drug release in response to the intracellular reduction environment was investigated at 37 °C in PB (10 mM, pH7.4) within 10 mM DTT. In brief, 1.0 mL of DOX-loaded micelles (0.1 mg/mL) were transferred into a dialysis tube with a MWCO of 12000-14000. Then the dialysis tube was immersed into 20 mL of corresponding buffer at 37 °C. At the desired time intervals, 6 mL of release media was taken out and replenished with 6 mL of fresh media. The amount of DOX released was determined also by the fluorescence spectrophotometer with excitation at 480 nm and emission at 560 nm.
Invitro cytotoxicity assay of SS-PU-SS-PEG micelles
The biocompatibility of the polyurethane micelles was tested by using cell counting Kit-8 (CCK-8) assay against C6 glioma cells or Saos-2 osteosarcoma cells. In the assay, the cells were seeded in 96-well plates at 1 × 104 per well in 100 μL of culture medium for 24 h. Then, the micelle solution was added with the final concentration from 0.2 to 1.0 mg/mL and incubated for another 24 h at 37 °C. The amount of viable cells was then determined by CCK-8 viability assay.
Cellular uptake and intracellular release of SS-PU-SS-PEG micelles
The cellular uptake and intracellular release behaviours of DOX-loaded polyurethane micelles were observed by fluorescence microscopy. Saos-2 cells were seeded in a 24-well plate at 5 × 104 per well in 500 μL of culture medium for 24 h. Then, the micelle solution was added at the final concentration of 40 μg/mL and incubated for another 4 h at 37 °C. The culture medium was removed and washed by cold phosphate buffer saline (PBS) three times, and the cells were then fixed with 4% paraformaldehyde solution for 30 min at 4 C followed by washing with PBS for three times. Fluorescence images of cells were observed by fluorescence microscopy (EVOS f1, AMG EVOS, USA).
In vitro antitumour activity of DOX-loaded SS-PU-SS-PEG micelles
To evaluate the antitumour activity of DOX-loaded polyurethane micelles in vitro, Saos-2 osteosarcoma cells and C6 glioma cells were seeded in 96-well plates at 1 × 104 per well in 100 μL of culture medium for 24 h. The culture medium removed and replenished with 100 μL of medium containing the micelles with the final DOX concentration from 0.1 to 20 μg/mL and incubated for another 48 h. The amount of viable cells was then determined by CCK-8 viability assay.
In vivo antitumour activity of DOX-loaded SS-PU-SS-PEG micelles
Animals
Animal tests were performed to evaluate the antitumour activity of DOX-loaded micelles in vivo. All animal experiments were approved by the Institutional Animal Care and Use Committee of Soochow University and performed by the Suzhou Xinuosai Biotechnology Co. Ltd, Suzhou, China. Healthy male mice from the Institute of Cancer Research (ICR) (6- to 7-week-old, weight 19–21 g) and male BALB/c nude mice (6- to 7-week-old, weight 18–20 g) which were purchased from the animal centre were used as a tumour model. Saos-2 cells (5 × 106) in 0.1 mL of PBS were injected subcutaneously into the right rear flank area of male nude BALB/c mice of weight 20 g.
Blood circulation analysis
Blood circulation analysis was performed by measuring the remaining DOX content from the blood taken after injection at predefined time points. The ICR mice were randomly divided into four groups (n = 4). PBS, free DOX, DOX-loaded SS-PU-SS-PEG micelles, and DOX-loaded SS-PU-b-PEG micelles were administered intravenously via the tail vein (3 mg/kg DOX eq.). At predefined time points, blood samples (80 μL) were collected into the anticoagulant tube within heparin sodium and then mixed with acetonitrile, vortexed, and centrifuged to obtain plasma. The DOX contents were determined by fluorescence microscopy with excitation at 480 nm and emission at 560 nm.
In vivo antitumour efficacy study
When the volume of Saos-2 xenografts reached about 100 mm3, the mice were randomly divided into four groups (n = 5) and intravenously administrated with PBS, free DOX, DOX-loaded SS-PU-SS-PEG micelles, and DOX-loaded SS-PU-b-PEG micelles (3 mg/kg) at the 0, 2nd, and 4th day. After finishing drug injection, the tumour growth and body weight of the mice were checked at 0, 2nd, 4th, 8th, 12th, and 16th day. The tumour size was measured using a calliper. The tumour volume was calculated according to the equation:
| Volume = (tumour length × tumour width2) / 2 |
At the 16th day, the mice were sacrificed. The organs (heart, liver, spleen, lung, kidney etc.) were collected from each group and fixed with 10% formalin. Subsequently, immunohistochemical evaluations were performed.
Statistical analysis
All data were obtained at least in triplicate and presented as mean ± standard deviation. One-way analysis of variance together with Tukey's post hoc test was used to discern the statistical difference between groups. A probability value (p) of less than 0.05 is considered statistically significant.
Results and discussion
Synthesis of SS-PU-SS-PEG
SS-PU-SS-PEG was synthesised from polycondensation reaction between LDI and a disulfide-containing diol, HO–PCL–SS–PCL–OH, followed by conjugation with cystamine-functionalized PEG (PEG-5000-Cys) at both ends (Scheme 2).
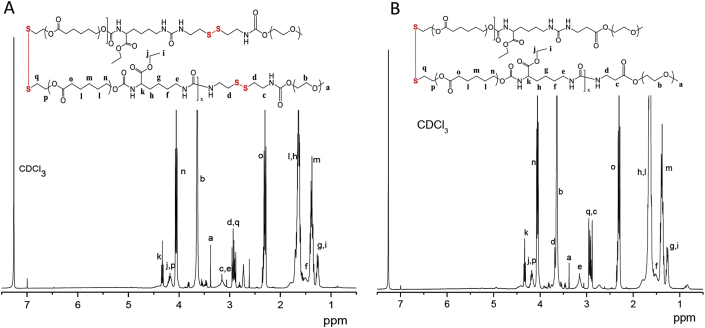
The disulfide-containing diol, HO–PCL–SS–PCL–OH, was synthesised by ring-opening polymerisation of ε-CL with 2,2′-dithiodiethanol as an initiator. The targeted molecular weight was about 1000 kDa, which was controlled by adjusting the ratio of ε-CL to 2,2′-dithiodiethanol. The polycondensation reaction between LDI and the disulfide-containing diol was carried out at a feed ratio of 1.05/1 in DMF using Sn(Oct)2 as a catalyst. 1H NMR spectrum showed clear signals which were assigned to LDI moieties, HO–PCL–SS–PCL–OH moieties, and PEG-5000-Cys moieties (Figure 1A). Gel permeation chromatography (GPC) measurements indicated that the SS-PU-SS-PEG copolymers had a unimodal distribution with polydispersity index of 1.18, which confirmed the successful synthesis of the copolymer SS-PU-SS-PEG. In this study, we also synthesised SS-PU-b-PEG copolymers as control, which were obtained by polycondensation reaction between LDI and a disulfide-containing diol, HO–PCL–SS–PCL–OH, followed by conjugation with amine-functionalized PEG (PEG5000-NH2) at both ends instead of cystamine-functionalized PEG (PEG-5000-Cys). The 1H NMR spectrum reveals the successful synthesis of SS-PU-b-PEG copolymers (Figure 1B). GPC measurements indicate that SS-PU-b-PEG had a unimodal distribution with a polydispersity index of 1.20, confirming successful synthesis of the copolymer.
Figure 1.
(A) The 1H NMR spectrum (400 MHz, CDCl3) of SS-PU-SS-PEG copolymer; (B) the 1H NMR spectrum (400 MHz, CDCl3) of SS-PU-b-PEG copolymer. SS-PU-SS-PEG = PEG-sheddable reduction-sensitive polyurethane; SS-PU-b-PEG = PEG-unsheddable reduction-sensitive polyurethane.
Preparation of SS-PU-SS-PEG micelles
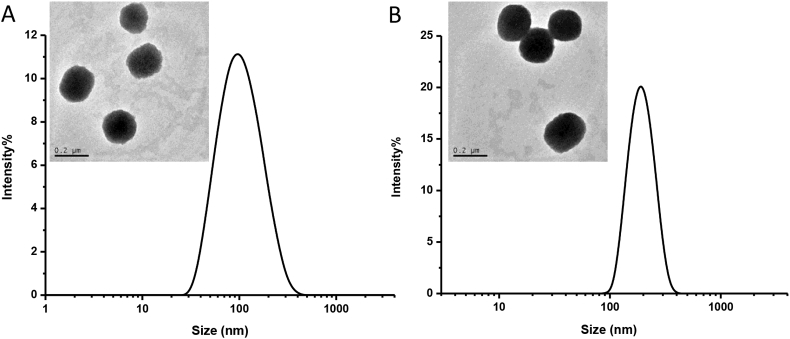
SS-PU-SS-PEG micelles were successfully prepared via solvent exchange method. DLS) measurements showed that the micelles had a narrow size of at about 115 nm (Table 1). This was further confirmed by TEM observation (Figure 2A). The CMC value was about 10.2 mg/L as measured using pyrene as a fluorescence probe. As control, SS-PU-b-PEG micelles could also be formed with the size of about 150 nm (Table 1 & Figure 2B). Although there was some difference in the micelle diameter and CMC between SS-PU-SS-PEG and SS-PU-b-PEG, they were still in the same order of magnitude. This difference might be due to the following reasons. First, the molecular structures of both copolymers were slightly different. SS-PU-SS-PEG had a hydrophobic cystamine segment (Figure 1). Second, the synthesis of polyurethane is not a “living” polymerisation reaction, so the molecular weight and the ratio of hydrophilic block to hydrophobic block might slightly differ. Finally, the procedure of micelle preparation and measurement methods also caused certain level of disturbance.
Table 1.
Characteristics of polyurethane micelles.
| Polymer | Micelle diameter (nm) | PDI | CMC (mg/L) |
|---|---|---|---|
| SS-PU-SS-PEG | 115.1 ± 7.3 | 0.251 | 10.2 |
| SS-PU-b-PEG | 156.4 ± 18.2 | 0.163 | 1.83 |
PDI = polydispersity index; CMC = critical micelle concentration; SS-PU-SS-PEG = PEG-sheddable reduction-sensitive polyurethane; SS-PU-b-PEG = PEG-unsheddable reduction-sensitive polyurethane.
Figure 2.
(A) Size distributions of SS-PU-SS-PEG micelles determined by DLS; (B) size distributions of SS-PU-b-PEG micelles determined by TEM. DLS = dynamic laser scattering; TEM = transmission electron microscopy; SS-PU-SS-PEG = PEG-sheddable reduction-sensitive polyurethane; SS-PU-b-PEG = PEG-unsheddable reduction-responsive polyurethane.
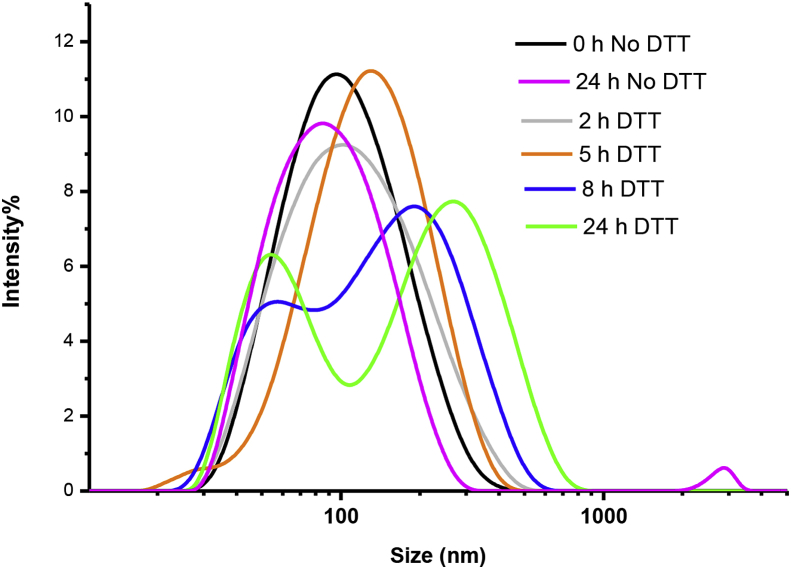
Then, the size change of SS-PU-SS-PEG micelles in response to 10 mM DTT was also followed by DLS measurement. The results show that micelles underwent rapid and remarkable swelling, during which their average size increased from approximately 100 to about 170 nm in 5 h. However, the size distribution curve started to split into two peaks after 8 h, indicating that the micelles were responsive to the reductive environment (Figure 3).
Figure 3.
Changes of size distribution of SS-PU-SS-PEG micelles in the presence of 10 mM DTT at 37 °C for 24 h. DTT = dithiothreitol; SS-PU-SS-PEG = PEG-sheddable reduction-sensitive polyurethane
DOX loading and reduction-triggered release of SS-PU-SS-PEG micelles
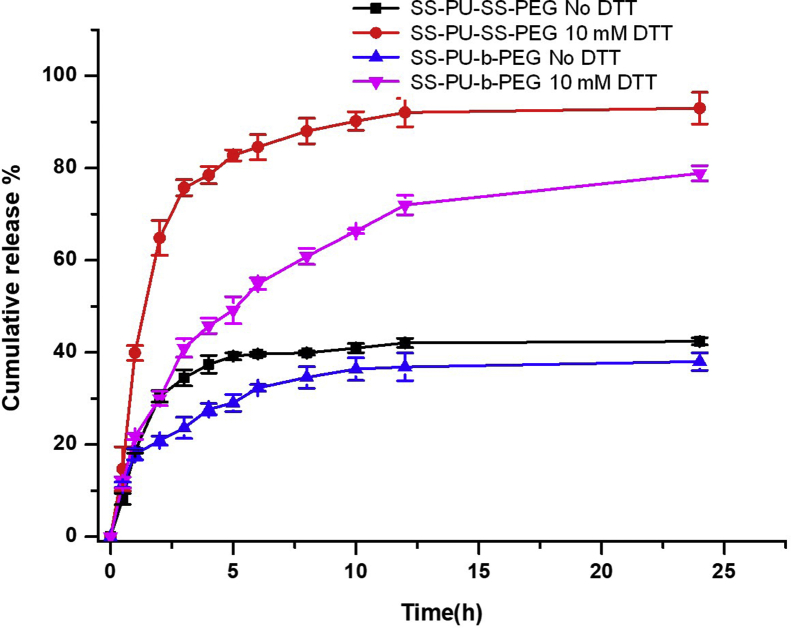
The DLC and DLE of micelles were tested using DOX as the model drug. SS-PU-SS-PEG micelles had DLCs of 3.75–7.80 wt% and DLEs of 52–75% (Table 2). The sizes of DOX-loaded micelles slightly decreased while maintaining low PDIs. The in vitro triggered release study of SS-PU-SS-PEG micelles and SS-PU-b-PEG micelles was performed at 37 C in responsive to the application of 10 mM DTT. The results revealed that under a reductive environment, SS-PU-SS-PEG micelles could release the majority of DOX in a quantitative fashion within 5 h (nearly 80%). In contrast, only minimal drug release (nearly 40%) was achieved under nonreductive environment even after 24 h (Figure 4). In SS-PU-b-PEG micelles, only 49.2% of DOX was released within 5 h under the same reductive environment, and only minimal drug release (nearly 40%) was achieved under nonreductive environment even after 24 h. These results clearly show that SS-PU-SS-PEG micelles had much faster release rate than that of SS-PU-b-PEG micelles under a reductive condition of 10 mM DTT.
Table 2.
Characteristics of DOX-loaded SS-PU-SS-PEG and SS-PU-b-PEG micelles.
| Polymer | Theoretical DLC (wt.%) | Actual DLC (wt.%) | Actual DLE (%) | Micelle size (nm) | PDI |
|---|---|---|---|---|---|
| SS-PU-SS-PEG | 5 | 3.75 | 75 | 94.3 ± 2.6 | 0.268 |
| 10 | 6.30 | 63 | 82.0 ± 3.5 | 0.272 | |
| 15 | 7.80 | 52 | 83.0 ± 4.2 | 0.239 | |
| SS-PU-b-PEG | 5 | 4.05 | 81 | 109.1 ± 3.0 | 0.201 |
| 10 | 6.60 | 66 | 121.6 ± 6.7 | 0.116 | |
| 15 | 6.45 | 43 | 112.6 ± 7.2 | 0.138 |
DLC = drug loading content; DLE = drug loading efficiency; SS-PU-SS-PEG = PEG-sheddable reduction-sensitive polyurethane; SS-PU-b-PEG = PEG-unsheddable reduction-sensitive polyurethane; DOX = doxorubicin; PDI = polydispersity index.
Figure 4.
In vitro DOX release profiles of DOX-loaded SS-PU-SS-PEG and SS-PU-b-PEG micelles in the presence and absence of 10 mM DTT at 37 C in PBS for 24 h. Error bars represent standard deviation for n = 3. PBS; DOX = doxorubicin; DTT = dithiothreitol; SS-PU-SS-PEG = PEG-sheddable reduction-sensitive polyurethane; SS-PU-b-PEG = PEG-unsheddable reduction-sensitive polyurethane.
In vitro antitumour activity and intracellular release behaviours of DOX-loaded SS-PU-SS-PEG micelles
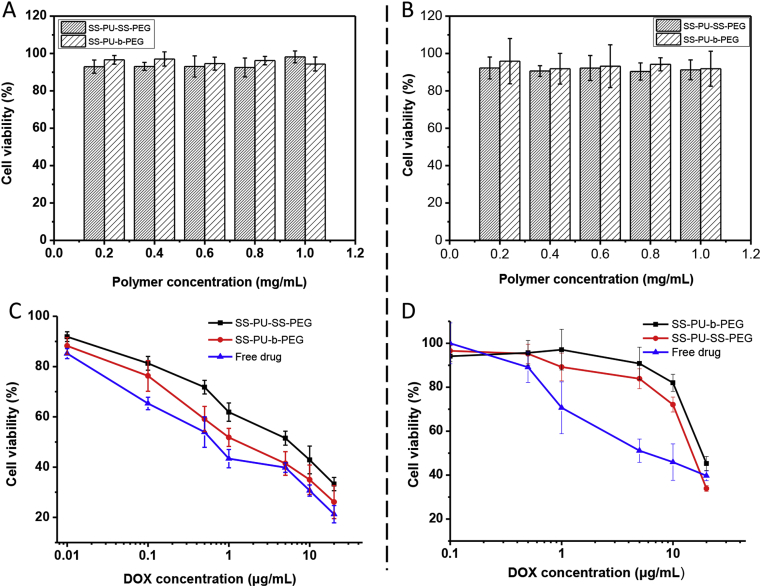
The biocompatibility of the drug carriers is a prerequisite for drug delivery application. In this study, the biocompatibility of SS-PU-SS-PEG micelles was evaluated by the CCK-8 assay after incubation with C6 cells or Saos-2 cells for 48 h. Both SS-PU-SS-PEG micelle and SS-PU-b-PEG micelle solutions did not show obvious cytotoxicity to C6 cells or Saos-2 cells (Figure 5A and B).
Figure 5.
(A) Cytotoxicity of micelles against C6 cells after 48 h of incubation; (B) cytotoxicity of micelles against Saos-2 cells after 48 h of incubation. (C) Antitumour activity of DOX-loaded SS-PU-SS-PEG micelles and SS-PU-b-PEG micelles as a function of drug concentration against C6 cells; (D) antitumour activity of DOX-loaded SS-PU-SS-PEG micelles and SS-PU-b-PEG micelles as a function of drug concentration against Saos-2 cells. DOX = doxorubicin; SS-PU-SS-PEG = PEG-sheddable reduction-sensitive polyurethane; SS-PU-b-PEG = PEG-unsheddable reduction-sensitive polyurethane.
In both of the cell lines, the cell viability remained higher than 90% even when the concentration was as high as 1.0 mg/mL. Therefore, both SS-PU-SS-PEG and SS-PU-b-PEG micelles could be considered as ideal candidates for drug carriers. The antitumour activity of micelles was also investigated via the CCK-8 assay in C6 cells or Saos-2 cells (Figure 5C and D). The results revealed that both the DOX-loaded micelles showed dose-dependent inhibition of C6 cells or Saos-2 cells with the half maximal inhibitory concentration (IC50) ranging from 1.4 to 18.0 μg/mL, while at the same time, both the micelles showed lower cytotoxicity than free DOX. This was likely because of the increased DOX accumulation in the nucleus of cells treated with free DOX than those treated with DOX-loaded micelles after incubation (Figure 6), which is in line with the results from previous studies [36,37,45,46].
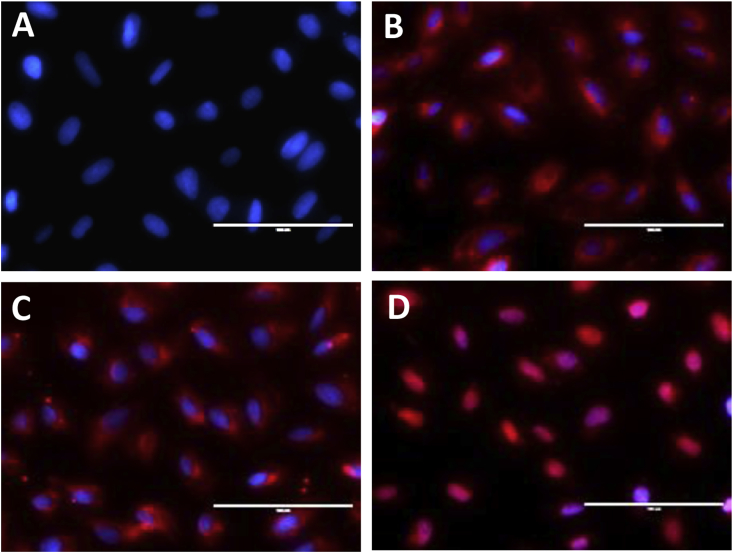
Figure 6.
(A)Fluorescence observation of Saos-2 cells incubated with DOX-loaded micelles (DOX concentration, 20 μg/mL) for 4 h for control; (B) fluorescence observation of Saos-2 cells incubated with DOX-loaded micelles (DOX concentration, 20 μg/mL) for 4 h for DOX-loaded SS-PU-b-PEG micelles; (C) fluorescence observation of Saos-2 cells incubated with DOX-loaded micelles (DOX concentration, 20 μg/mL) for 4 h for DOX-loaded SS-PU-SS-PEG micelles; (D) fluorescence observation of Saos-2 cells incubated with DOX-loaded micelles (DOX concentration, 20 μg/mL) for 4 h for free DOX. DOX = doxorubicin; SS-PU-SS-PEG = PEG-sheddable reduction-sensitive polyurethane; SS-PU-b-PEG = PEG-unsheddable reduction-sensitive polyurethane.
The intracellular release behaviour of DOX-loaded SS-PU-SS-PEG micelles was examined in Saos-2 cells by fluorescence microscopy. After 4 h of incubation with DOX-loaded SS-PU-SS-PEG micelles or DOX-loaded SS-PU-b-PEG micelles, Saos-2 cells showed strong DOX fluorescence in the cytoplasm both in the two groups, indicating efficient internalisation and rapid drug release in the cells. However, free DOX was more easily delivered into the nuclei of Saos-2 cells because of the fast cell uptake of free DOX (Figure 6).
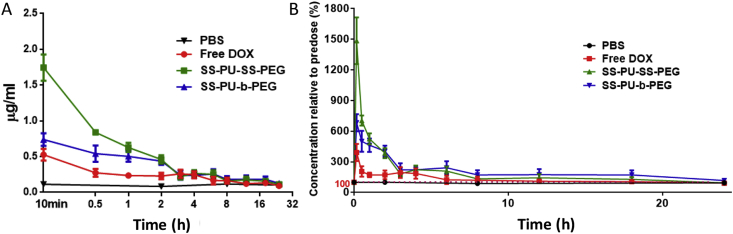
Blood circulation and in vivo antitumour efficacy of SS-PU-SS-PEG micelles
The in vivo blood circulation studies of SS-PU-SS-PEG micelles were carried out in healthy male ICR mice. The mice were intravenously injected with a single injection of free DOX, DOX-loaded SS-PU-SS-PEG, and DOX-loaded SS-PU-b-PEG micelles at a dose of 3.0 mg/kg (calculated DOX content). As shown in Figure 7, free DOX was rapidly cleared from circulation, while DOX-loaded SS-PU-SS-PEG micelles exhibited slower blood clearance and maintained higher DOX level in the bloodstream. In addition, the plasma area under the concentration time curve of DOX-loaded SS-PU-SS-PEG micelles and DOX-loaded SS-PU-b-PEG micelles was about 2 times higher than that of free DOX.
Figure 7.
(A) Time-dependent profile of DOX concentration in blood after intravenous administration to ICR mice as at a DOX dosage of 3.0 mg/kg; (B) Time-dependent profile of DOX concentration relative to predose (%) after intravenous administration to ICR mice as at a DOX dosage of 3.0 mg/kg. DOX = doxorubicin; ICR = Institute of Cancer Research.
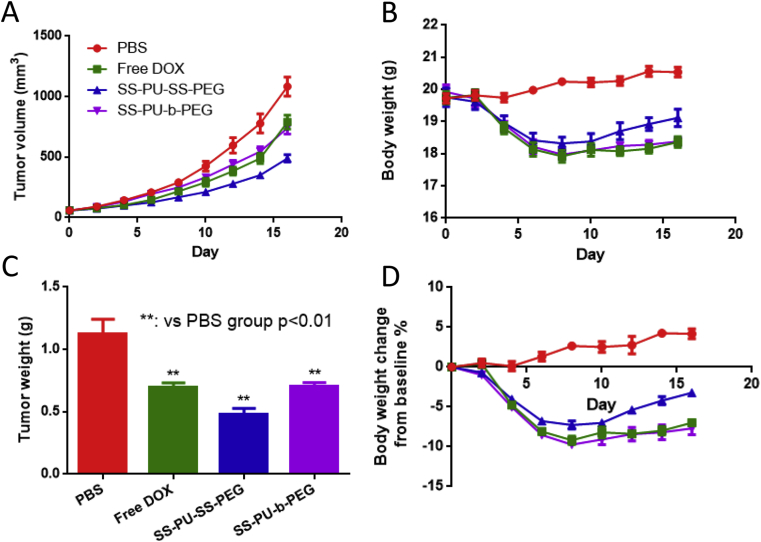
After that, in vivo antitumour efficacy was investigated using nude mice bearing Saos-2 tumours as the xenograft tumour model. Saos-2 tumour–bearing nude mice were intravenously injected with PBS, free DOX, DOX-loaded SS-PU-SS-PEG, and DOX-loaded SS-PU-b-PEG micelles with a DOX concentration of 3.0 mg/kg. As shown in Figure 8, rapid tumour growth was observed in mice that were treated with PBS. Free DOX exhibited considerable tumour inhibiting capacity. Apparently, the DOX-loaded SS-PU-SS-PEG micelles exhibited better antitumour activity than free DOX and saline both with a statistically significant difference (p < 0.01), while the DOX-loaded SS-PU-b-PEG micelles exhibited the same antitumour activity as free DOX group. After the mice were sacrificed at the 16th day, the tumour tissues were dissected. The tumour growth inhibition rate (TIR) of each group was calculated as shown in Table 3. According to the volume of tumour tissue, the TIR of the mice injected with DOX-loaded SS-PU-SS-PEG micelles was 54.8%, which was much higher than free DOX (27.1%) and DOX-loaded SS-PU-b-PEG micelles (31.6%). According to the weight of tumour tissue, the TIR of the mice injected with DOX-loaded SS-PU-SS-PEG micelles was 56.9%, which was much higher than free DOX (37.8%) and DOX-loaded SS-PU-b-PEG micelles (37.1%). All the results suggest significantly improved anticancer efficacy in osteosarcoma. In addition, after the mice were injected with free DOX, DOX-loaded SS-PU-SS-PEG micelles and DOX-loaded SS-PU-b-PEG micelles, continued decline of body weight happened (<10%). However, after treatments for 8 days, the body weight of the group injected with DOX-loaded SS-PU-SS-PEG micelles started to increase, whereas the body weight of the groups injected with free DOX and DOX-loaded SS-PU-b-PEG micelles remained almost unchanged.
Figure 8.
(A) The tumor volume growth curve in in vivo anti-tumor assay; (B) time course of changes in the body weight in in vivo anti-tumor assay; (C) the tumor weight after sacrifice in in vivo anti-tumor assay; (D) time course of change ratios in the body weight in in vivo anti-tumor assay. The arrows represent the day on which the intravenous administration was performed.
Table 3.
Tumour inhibition rates of various treatments.
| Group | Tumour volume (mm3) | TIRV% | Tumour weight (g) | TIRW% |
|---|---|---|---|---|
| PBS | 1083.05 ± 78.36 | — | 1.1223 ± 0.1196 | — |
| Free DOX | 789.71 ± 57.78* | 27.1 | 0.6981 ± 0.0339** | 37.8 |
| SS-PU-SS-PEG | 489.17 ± 30.51**## | 54.8 | 0.4833 ± 0.0442** | 56.9 |
| SS-PU-b-PEG | 740.83 ± 49.60** | 31.6 | 0.7059 ± 0.0284** | 37.1 |
TIR = tumour inhibition growth rate; SS-PU-SS-PEG = PEG-sheddable reduction-sensitive polyurethane; SS-PU-b-PEG = PEG-unsheddable reduction-sensitive polyurethane; DOX = doxorubicin.
Note: *, compared with PBS group, p < 0.05; **, compared with PBS group, p < 0.01; ##, compared with free DOX group, p < 0.01.
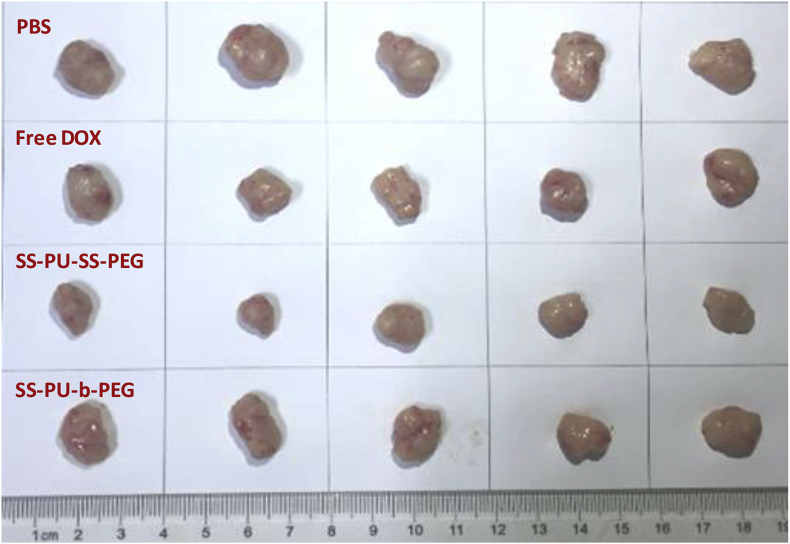
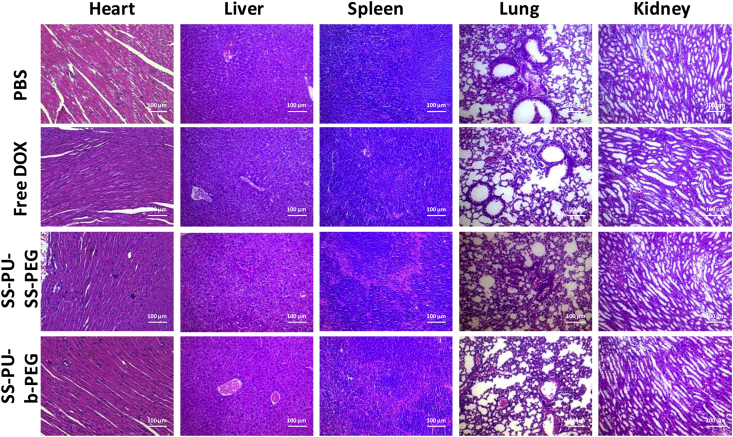
All the tumour tissues after sacrifice in each group are shown in Figure 9. It is obviously found that the tumour diameter and volume of SS-PU-SS-PEG micelle–treated group were smaller than those of the other groups. The immunohistochemical evaluation of heart, liver, spleen, lung, and kidney sections in different groups after 16 days of treatment indicates that both DOX-loaded SS-PU-SS-PEG micelles and DOX-loaded SS-PU-b-PEG micelles did not cause apparent damage to these organs (Figure 10).
Figure 9.
Images of Saos-2 graft tumours after sacrifice with the 16-day treatment with PBS, free DOX, DOX-loaded SS-PU-SS-PEG and DOX-loaded SS-PU-b-PEG micelles, respectively. DOX concentration was kept at 3.0 mg/kg. PBS; SS-PU-SS-PEG = PEG-sheddable reduction-sensitive polyurethane; SS-PU-b-PEG = PEG-unsheddable reduction-responsive polyurethane; DOX = doxorubicin.
Figure 10.
Histopathological analysis of heart, liver, spleen, lung, kidney sections stained with H&E. Images were obtained under an inverted microscope using a 20× magnification.
Conclusions
In this study, we have successfully synthesised SS-PU-SS-PEG copolymer, which was further used to form drug-loaded micelles for triggered intracellular drug delivery to improve the treatment efficacy of osteosarcoma. The SS-PU-SS-PEG micelles exhibited good stability in PBS and showed good biocompatibility. In vitro studies revealed drug-loaded SS-PU-SS-PEG micelles steadily released the model drug DOX under a reductive environment, and nearly 80% of DOX was released within 5 h. In vitro, DOX-loaded SS-PU-SS-PEG micelles displayed significant antitumour activity, which was comparable with that of free DOX, against Saos-2 cells. In Saos-2 cell–bearing xenograft tumour models, the SS-PU-SS-PEG DOX-loaded micelles exhibited enhanced antitumour activity compared with that of SS-PU-b-PEG micelle counterpart and free DOX. Together, findings from this study suggest that the SS-PU-SS-PEG micelles can achieve well-controllable triggered release under a reductive environment and can therefore improve the antitumour efficacy of drugs, implying good promise for osteosarcoma treatment.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (51303120, 31400826, and 81871805), Natural Science Foundation of Jiangsu Province (BK20130335), Xuzhou Basic Research Program of Jiangsu Province (KC16SG256), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Contributor Information
Caihong Zhu, Email: zhucaihong@suda.edu.cn.
Yuling Li, Email: ylli19722@163.com.
Bin Li, Email: binli@suda.edu.cn.
References
- 1.Whelan J., McTiernan A., Cooper N., Wong Y.K., Francis M., Vernon S. Int J Cancer. 2012;131:E508–E517. doi: 10.1002/ijc.26426. [DOI] [PubMed] [Google Scholar]
- 2.He H.T., Ni J.D., Huang J. Oncol Lett. 2014;7:1352–1362. doi: 10.3892/ol.2014.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritter J., Bielack S.S. Ann Oncol. 2010;21:320–325. doi: 10.1093/annonc/mdq276. [DOI] [PubMed] [Google Scholar]
- 4.Luetke A., Meyers P.A., Lewis I., Juergens H. Cancer Treat Rev. 2014;40:523–532. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Deng C.J., Chang J., Wu C.T. J Orthop Transl. 2019;17:15–25. doi: 10.1016/j.jot.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H., Shen J., Choy E., Hornicek F.J., Duan Z.F. Cancer Treat Rev. 2016;43:8–18. doi: 10.1016/j.ctrv.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen R.L., Wang G.Y., Zheng Y., Hua Y.Q., Cai Z.D. J Cell Mol Med. 2019;23:2280–2292. doi: 10.1111/jcmm.14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapse-Mistry S., Govender T., Srivastava R., Yergeri M. Front Pharmacol. 2014;5:159. doi: 10.3389/fphar.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruvinov E., Re'em T.T., Witte F., Cohen S. J Orthop Transl. 2019;16:40–52. doi: 10.1016/j.jot.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S.P., Xu Y.Q., Chan H.F., Kim H.W., Wang Y.T., Leong K.W. J Control Release. 2016;240:454–464. doi: 10.1016/j.jconrel.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y.H., Cole S.P.C., Cai T.G., Cai Y. Oncol Lett. 2016;12:11–15. doi: 10.3892/ol.2016.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou L., Wang H., Li Y.P. Theranostics. 2018;8:1059–1074. doi: 10.7150/thno.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Fernandez Y., Imbuluzqueta E., Patino-Garcia A., Blanco-Prieto M.J. Curr Pharmaceut Des. 2015;21:6104–6124. doi: 10.2174/1381612821666151027152534. [DOI] [PubMed] [Google Scholar]
- 14.Susa M., Iyer A.K., Ryu K., Hornicek F.J., Mankin H., Amiji M.M. BMC Canc. 2009;9:399. doi: 10.1186/1471-2407-9-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han X.G., Yang S.B., Mo H.M., Wang M.Q., Zhou F., Li H.J. Adv Funct Mater. 2019;29 [Google Scholar]
- 16.Li R., Xie Y. J Control Release. 2017;251:49–67. doi: 10.1016/j.jconrel.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Yin Q., Shen J.N., Zhang Z.W., Yu H.J., Li Y.P. Adv Drug Deliv Rev. 2013;65:1699–1715. doi: 10.1016/j.addr.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Feng L.Z., Li K.Y., Shi X.Z., Gao M., Liu J., Liu Z. Adv Healthc Mater. 2014;3:1261–1271. doi: 10.1002/adhm.201300549. [DOI] [PubMed] [Google Scholar]
- 19.Lim E.K., Chung B.H., Chung S.J. Curr Drug Targets. 2018;19:300–317. doi: 10.2174/1389450117666160602202339. [DOI] [PubMed] [Google Scholar]
- 20.Yang B., Ai X.Y., Shang L., Zhong L., Ji B., Wu C.N. J Nanosci Nanotechnol. 2016;16:8424–8430. [Google Scholar]
- 21.Cheng R., Feng F., Meng F.H., Deng C., Feijen J., Zhong Z.Y. J Control Release. 2011;152:2–12. doi: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Han L., Zhang X.Y., Wang Y.L., Li X., Yang X.H., Huang M. J Control Release. 2017;259:40–52. doi: 10.1016/j.jconrel.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Sun H.L., Meng F.H., Cheng R., Deng C., Zhong Z.Y. Expert Opin Drug Deliv. 2013;10:1109–1122. doi: 10.1517/17425247.2013.783009. [DOI] [PubMed] [Google Scholar]
- 24.Jiang M.J., Zhang R.S., Wang Y.L., Jing W.N., Liu Y., Ma Y. Mol Pharm. 2017;14:3628–3635. doi: 10.1021/acs.molpharmaceut.7b00381. [DOI] [PubMed] [Google Scholar]
- 25.Li J., Xu R.T., Lu X., He J., Jin S.D. Int J Nanomed. 2017;12:8043–8056. doi: 10.2147/IJN.S148273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang L.Y., Wang Y.C., Li Y., Du J.Z., Wang J. Bioconjug Chem. 2009;20:1095–1099. doi: 10.1021/bc900144m. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y.C., Wang F., Sun T.M., Wang J. Bioconjug Chem. 2011;22:1939–1945. doi: 10.1021/bc200139n. [DOI] [PubMed] [Google Scholar]
- 28.Hearon K., Wierzbicki M.A., Nash L.D., Landsman T.L., Laramy C., Lonnecker A.T. Adv Healthc Mater. 2015;4:1386–1398. doi: 10.1002/adhm.201500156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung K.C., Tseng C.S., Hsu S.H. Adv Healthc Mater. 2014;3:1578–1587. doi: 10.1002/adhm.201400018. [DOI] [PubMed] [Google Scholar]
- 30.Kucinska-Lipka J., Gubanska I., Janik H. Polimery. 2013;58:678–684. [Google Scholar]
- 31.He X.L., Ding M.M., Li J.H., Tan H., Fu Q., Li L. RSC Adv. 2014;4:24736–24746. [Google Scholar]
- 32.Pardini F., Faccia P., Amalvy J. J Drug Deliv Sci Technol. 2015;30:199–208. [Google Scholar]
- 33.Huang F., Cheng R., Meng F., Deng C., Zhong Z. Biomacromolecules. 2015;16:2228–2236. doi: 10.1021/acs.biomac.5b00625. [DOI] [PubMed] [Google Scholar]
- 34.Yu S.J., He C.L., Ding J.X., Cheng Y.L., Song W.T., Zhuang X.L. Soft Matter. 2013;9:2637–2645. [Google Scholar]
- 35.Zhang P., Hu J.Y., Bu L.R., Zhang H.N., Du B.X., Zhu C.H. Polymers. 2019:11. doi: 10.3390/polym11020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bu L.R., Zhang H.N., Xu K., Du B.X., Zhu C.H., Li Y.L. Drug Deliv. 2019;26:300–308. doi: 10.1080/10717544.2019.1580323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Y.Q., Wang X.X., Zhang J., Meng F.H., Deng C., Cheng R. J Control Release. 2017;250:9–19. doi: 10.1016/j.jconrel.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Wang W., Sun H.L., Meng F.H., Ma S.B., Liu H.Y., Zhong Z.Y. Soft Matter. 2012;8:3949–3956. [Google Scholar]
- 39.Gonzalez-Fernandez Y., Imbuluzqueta E., Zalacain M., Mollinedo F., Patino-Garcia A., Blanco-Prieto M.J. Cancer Lett. 2017;388:262–268. doi: 10.1016/j.canlet.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Mealey K.L., Barhoumi R., Rogers K., Kochevar D.T. Cancer Lett. 1998;126:187–192. doi: 10.1016/s0304-3835(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 41.Susa M., Iyer A.K., Ryu K., Hornicek F.J., Mankin H., Amiji M.M. BMC Canc. 2009;9:399–411. doi: 10.1186/1471-2407-9-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baranski Z., de Jong Y., Ilkova T., Peterse E.F.P., Cleton-Jansen A.M., van de Water B. Oncotarget. 2015;6:36113–36125. doi: 10.18632/oncotarget.5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cagliero E., Ferracini R., Morello E., Scotlandi K., Manara M.C., Buracco P. Oncol Rep. 2004;12:1023–1031. doi: 10.3892/or.12.5.1023. [DOI] [PubMed] [Google Scholar]
- 44.Zhu C., Zheng M., Meng F., Mickler F.M., Ruthardt N., Zhu X. Biomacromolecules. 2012;13:769–778. doi: 10.1021/bm201693j. [DOI] [PubMed] [Google Scholar]
- 45.Wang W., Wang B., Liu S., Shang X., Yan X., Liu Z. ACS Appl Mater Interfaces. 2017;9:15986–15994. doi: 10.1021/acsami.7b03317. [DOI] [PubMed] [Google Scholar]
- 46.Low S.A., Yang J., Kopecek J. Bioconjug Chem. 2014;25:2012–2020. doi: 10.1021/bc500392x. [DOI] [PMC free article] [PubMed] [Google Scholar]