Abstract
Background
Recent publications have reported conflicting findings regarding associations of blood donor demographics and mortality of transfused patients. We hypothesized that the analysis of additional donor characteristics and consideration of alternative outcomes might provide insight into these disparate results.
Study Design and Methods
We analyzed data from a retrospective cohort of transfused patients from the Recipient Epidemiology and Donor Evaluation Study (REDS-III). We used stratified Cox regression models to estimate associations between blood donor characteristics and hospital mortality and post-transfusion length of stay among patients transfused red blood cell (RBC) units. Donor characteristics evaluated included age, body mass index, hemoglobin levels, and smoking status. The statistical analyses were adjusted for recipient factors, including total number of transfusions.
Results
We studied 93,726 patients in 130,381 hospitalizations during which 428,461 RBC units were transfused. There were no associations between blood donor characteristics and hospital mortality. Receipt of RBC units from donors less than 20 years of age was associated with a shorter hospital length of stay (hazard ratio for discharge per transfused unit 1.03; 95% confidence interval, 1.02 to 1.04; p<0.001) but not for other donor characteristics.
Conclusion
We found no evidence of associations between blood donor factors and in-hospital mortality. Our finding of shorter hospital length of stay in patients transfused red cells from younger donors is intriguing but requires confirmation. Future collaborations are needed to develop a framework of appropriate methodologic approaches to be used in linked analyses across large cohorts.
Introduction
Blood donors are a demographically and genetically diverse group of individuals, and differences in donor characteristics may play a role in recipient outcomes related to transfused blood components. However, results of recent observational studies have been disparate, with some analyses finding that patients transfused with RBC units from female donors or specific donor age groups have increased mortality, while other studies finding no such associations.1–6 Differences in the studied sample size, proportions of missing data, and statistical methods may explain these variable results.7 However, there may be other blood donor characteristics, not previously accounted for, that have relevance to mortality of transfusion recipients.
In addition, patient outcomes other than mortality may be more sensitive to a deleterious transfusion effect or may be driven by other pathogenic mechanisms8; i.e., an adverse biologic effect related to a donor characteristic might not result in recipient mortality but could be associated with a morbid outcome (such as infection) that would extend the duration of hospitalization. Thus, as a potential measure of overall morbidity, we evaluated whether red cell exposures from donors with different characteristics were associated with changes in post transfusion hospital length of stay.
Sex-specific associations of anti-leukocyte antibodies are well-described in regard to the risk of transfusion-related acute lung injury.9,10 Donor sex and age have also been shown to modulate red blood cell susceptibility to in vitro hemolysis, which may have clinical relevance in vivo through inhibition of nitric oxide signaling and oxidative and inflammatory stress.11–14 In parallel, hemoglobin levels and body mass index (BMI) vary with donor sex and age, and differences in these characteristics have been associated with inflammatory markers in donors.15–17 Lastly, tobacco use in blood donors is similar or lower than in the general population where it is known to vary by age and sex.18–21 Tobacco use has been shown to cause elevated levels of hemoglobin and carboxyhemoglobin in blood donors which may adversely affect transfusion recipients.22–25 We hypothesized that one or more of these donor characteristics might be associated with transfusion recipient outcomes and could explain the disparate results of prior studies.26–29
In this study, we used data from a large research database linking blood donor, donation, and recipient characteristics in 12 hospitals to examine dose-related associations between donor age, BMI, hemoglobin level, and smoking status with hospital mortality and length of stay in transfused adults.
Methods
Data Sources
Analyses were based on a cohort of transfused patients from the Recipient Epidemiology Donor Evaluation Study-III (REDS-III) database, which includes data on patients who were transfused in 12 academic hospitals located in different geographic regions of the United States between 2013 and 2016.30,31 Data were structured to link information between donors and transfused hospitalized patients as previously described, allowing the analysis of association of donor characteristics such as donor age and BMI on the outcomes of patients transfused RBC units.6 Outcome data included in-hospital mortality and hospital length of stay.
Study design and participants
This retrospective cohort study was modelled after previous publications2,4,6 with adult and pediatric patients being followed from the time of first RBC transfusion during hospitalization until the occurrence of death or hospital discharge. We excluded recipients of autologous RBC transfusions. There were no other exclusions, and the patient cohort reflected differences in case mix between the 12 participating hospitals.
Outcomes and exposures
The primary outcome was in-hospital mortality, and the secondary outcome was post-transfusion hospital length of stay among patients who survived to discharge (time to hospital discharge). Patients were allowed to contribute multiple hospital episodes if they were transfused RBC units in more than one hospitalization. Transfusion of other blood components was not analyzed or included in the models.
The main exposures of interest were the number of RBC transfusions from strata of donor age, BMI, hemoglobin level, and by smoking status. These exposures were calculated time-dependently on a day-to-day basis, allowing patients to change exposure with additional transfusions. The reference group for each donor exposure category was an equal number of red blood cell units from all other donor exposure groups. Some donor characteristics (i.e. BMI, tobacco use and hemoglobin) were missing for RBC units imported from multiple geographic regions in the United States. Given likely differences in demographic characteristics, including the prevalence of tobacco use, for donors of imported units, we did not utilize multiple imputation for missing data.
Statistical analyses
We conducted separate analyses for the four donor exposures and two outcomes using stratified Cox proportional hazards regression models with time-dependent exposures, where we used time from first transfusion as the time-scale and stratified on calendar year, hospital and time-varying total number of red-cell units transfused. We conducted secondary analyses of donor age and hemoglobin levels, stratifying by donor sex, given known differences in hemoglobin levels by sex and the results of previous publications.2,3 Because the number of transfusions was one of the stratifying variables, comparisons were only done between patients who received the same number of transfusions. The data were set up for analysis using the Andersen-Gill counting process format using the publicly available Stratify macro.32,33 Analyses were adjusted for month of blood donation, recipient age (as a restricted cubic spline with 3 evenly placed knots), recipient sex (as a categorical term), ABO blood type (as a categorical term), and Charlson comorbidity index (categorized as 0, 1–2, 3–4, 5–6, 7–8, or ≥9).34 We calculated the association between receiving a RBC unit with a feature of interest, compared with receiving a RBC unit from all other reference groups, and the risk (hazard) of hospital death or prolonged length of stay, adjusting for all other covariates including the cumulative number of RBC units received. Because patients could contribute multiple transfusion episodes, confidence limits for the hazard ratios (HRs) were constructed using robust standard errors.
The hospital length of stay (time to hospital discharge) within 90 days of transfusion was modeled for patients discharged alive using cause-specific proportional hazards regression with death as a competing risk. Hazard ratio estimates for length of stay denote likelihood of hospital discharge with increasing hazard ratio associated with shorter duration of hospitalization in patients who survived to discharge. In addition, we examined absolute lengths of stay in patients who survived to hospital discharge, stratifying by total number of transfusions and count of red cell exposures from donors aged less than 20, 20 to 39, or 40 years and older.
All statistical tests were two-sided and p-values < 0.05 were considered statistically significant. All data processing and statistical analyses were conducted using SAS statistical analysis software, version 9.4. The conduct of these analyses was approved by IRBs at all participating REDS-III hubs and by the REDS-III data coordinating center (RTI, Inc.).
Role of the funding source
The study sponsor had no role in study design, data collection, data analysis, data interpretation; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Recipient and donor characteristics are summarized in Table 1 and Table 2, respectively. Over the study period, 93,726 patients received at least 1 RBC transfusion during 130,381 hospitalizations. 276,496 unique blood donors donated 428,461 RBC units transfused to recipients in our cohort. There were minimal missing values for age and sex for either donors or recipients (<0.1%). However, for donor BMI, hemoglobin level, and self-reported smoking status, there was missing data for 26.1%, 8.8%, and 30.4% of units, respectively.
Table 1.
Characteristics of the Donor Matched Inpatient RBC transfusion recipients
| Subjects, No (%) | Encounters, No (%) | |
|---|---|---|
| All | 93,726 | 130,381 |
| Patient characteristics | ||
| Sex | ||
| Male | 45,360 (48.4) | 63,515 (48.7) |
| Female | 48,352 (51.6) | 66,866 (51.3) |
| Other | 14 (0.0) | 14 (0.0) |
| Median Age at study enrollment (IQR) | 64 (51,75) | 63 (50,74) |
| Race | ||
| White | 65,180 (69.5) | 89,585 (68.7) |
| Black | 13,763 (14.7) | 21,530 (16.5) |
| Asian | 2,481 (2.7) | 3,327 (2.6) |
| Missing/Other | 12,302 (13.1) | 15,946 (12.2) |
| Ethnicity | ||
| Hispanic | 6,368 (6.8) | 9,354 (7.2) |
| Non-Hispanic | 83,295 (88.9) | 115,770 (88.8) |
| Missing/Other | 4,063 (4.3) | 5,271 (4.0) |
| Body mass index at study entry (IQR) | 27.1 (23.2, 32.1) | 26.7 (22.9, 31.6) |
| Blood group | ||
| A | 33,884 (36.2) | 46,792 (35.9) |
| B | 12,502 (13.3) | 17,664 (13.6) |
| O | 40,596 (43.3) | 57,330 (44.0) |
| AB | 4,594 (4.9) | 6,361 (4.9) |
| Unknown/Untested | 2,150 (2.3) | 2,063 (1.6) |
| Rh(D) status | ||
| Rh(D) positive | 9,591 (84.9) | 111,478 (85.5) |
| Rh(D) negative | 12,099 (12.9) | 16,833 (12.9) |
| Unknown/Untested | 2,036 (2.2) | 2,084 (1.6) |
| Charlson Comorbidity Index (IQR) | 1 (1,1) | 1 (2,3) |
| Principal Diagnosis Category | ||
| Circulatory | 15,307 (16.6) | 18,513 (14.4) |
| Gastrointestinal | 10,812 (11.7) | 15,957 (12.4) |
| Blood Diseases | 4,289 (4.6) | 8,883 (6.9) |
| Neoplasms | 13,253 (14.3) | 17,373 (13.5) |
| Infectious | 6,615 (7.2) | 11,175 (8.7) |
| Musculoskeletal | 6,589 (7.1) | 7,468 (5.8) |
| Pulmonary | 3,016 (3.3) | 4,664 (3.6) |
| Injury/Poisoning | 15,717 (17.0) | 22,127 (17.2) |
| Obstetric | 2,522 (2.7) | 2,567 (2.0) |
| Congenital | 1,047 (1.1) | 1,301 (1.0) |
| Other | 12,436 (13.5) | 15,457 (12.1) |
| No primary DX Code | 788 (0.9) | 3,171 (2.5) |
Table 2.
Blood donor characteristics
| Units, No (%) | Donors, No (%) | |
|---|---|---|
| All | 428,461 | 276,496 |
| Sex | ||
| Male | 243,083 (56.7) | 146,695 (53.1) |
| Female | 185,273 (43.2) | 129,683 (46.9) |
| Other | 105 (0.0) | 91 (0.0) |
| Donor age, years | ||
| <20 | 53,235 (12.4) | 44,989 (16.3) |
| 20 – 29 | 52,991 (12.4) | 39,842 (14.4) |
| 30 – 39 | 48,492 (11.3) | 33,349 (12.1) |
| 40 – 49 | 65,619 (15.3) | 41,645 (15.1) |
| 50 – 59 | 104,188 (24.3) | 60,212 (21.8) |
| 60 – 69 | 76,591 (17.9) | 41,648 (15.1) |
| 70+ | 27,154 (6.3) | 14,607 (5.3) |
| Body mass index at study entry | ||
| Underweight/normal (<18.5–24.9) | 96,264 (22.5) | 64,170 (23.2) |
| Overweight (25.0–29.9) | 122,855 (28.7) | 71,800 (26.0) |
| Obesity Moderate (30.0–34.9) | 63,488 (14.8) | 36,079 (13.1) |
| Obesity Severe/Morbid (≥35.0) | 34,099 (8.0) | 20,003 (7.2) |
| Missing | 111,755 (26.1) | 84,417 (30.5) |
| Blood group | ||
| A | 144,757 (33.8) | 97,819 (35.4) |
| B | 49,061 (11.5) | 32,594 (11.8) |
| AB | 13,076 (3.1) | 8,943 (3.2) |
| O | 221,562 (51.7) | 137,108 (49.6) |
| Rh(D) status | ||
| Rh(D) positive | 338,528 (79.0) | 224,477 (81.2) |
| Rh(D) negative | 89,928 (21.0) | 51,987 (18.8) |
| Donor hemoglobin level (g/dL) | ||
| 12.5–14 | 140,355 (32.8) | 93,818 (33.9) |
| 14–15 | 107,926 (25.2) | 69,251 (25.1) |
| 15–16 | 84,057 (19.6) | 53,798 (19.5) |
| 16–17 | 42,392 (9.9) | 27,627 (10.0) |
| 17+ | 15,882 (3.7) | 10,783 (3.9) |
| Missing | 37,849 (8.8) | 21,192 (7.7) |
| Tobacco use in the 30 days prior to donation | ||
| Yes | 26,358 (6.2) | 17,681 (6.4) |
| No | 271,673 (63.4) | 164,013 (59.3) |
| Unknown | 130,430 (30.4) | 94,775 (34.3) |
Baseline recipient characteristics are presented in Table 1. The median recipient age was 64 years [interquartile range (IQR), 51–75 years], and 51.6% of recipients (n=48,352) were female. The median Charlson comorbidity index was 1 [IQR 1–1]. Common principal diagnoses were injury/poisoning, circulatory system, neoplasms, and digestive system disorders representing 59.2% of patients in the cohort. The median number of RBC transfusions was 2 [IQR 1–5]. The median length of stay was 6 [IQR 4–12] days from the time of first transfusion. Death occurred in 9,314 (7.1%) of transfused recipients’ hospitalizations. The total number of transfused units decreased annually over the study period, and there was minor seasonal variation in the number of transfused units by month of donation (Appendix Table 1). In addition, there was only minor variation in overall hospital lengths of stay following transfusion when examined by month of blood donation (Appendix Table 2).
The median donor age was 49 years [IQR 30–59], and 46.9% (n = 129,683) of donors were female. At the time of donation, 20.2% (n=56,082) of donors were obese (BMI ≥ 30). Donor hemoglobin levels were greater than or equal to 17 g/dL in 3.9% of blood donors (n=10,783) and 3.7% (n=15,882) donations. A total of 6.4% (n=17,681) of blood donors reported tobacco use within 30 days prior to donation representing 6.2% (n=26,358) of transfused units.
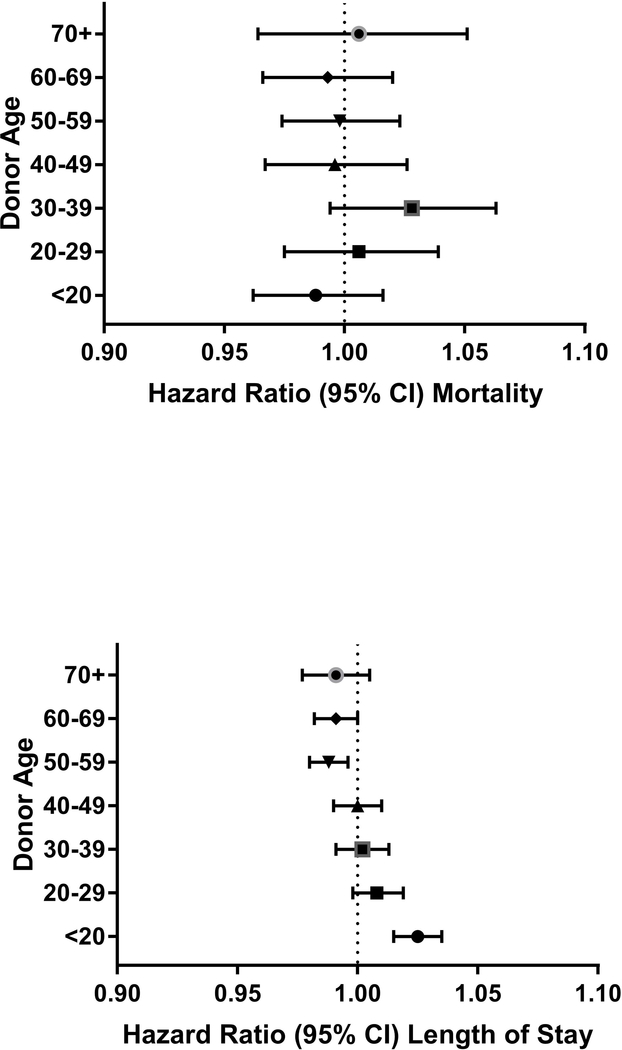
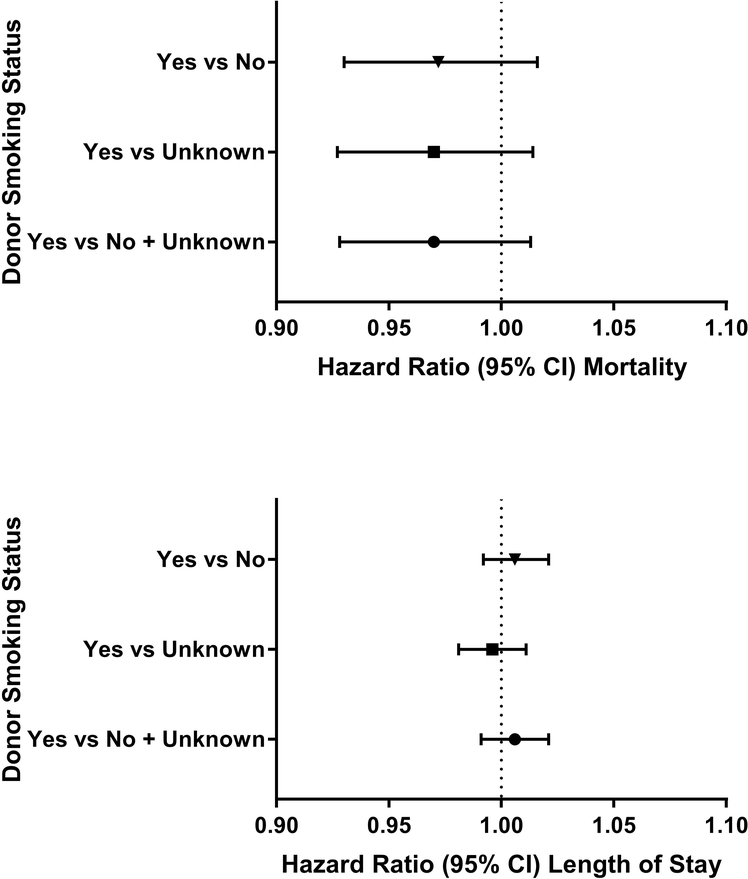
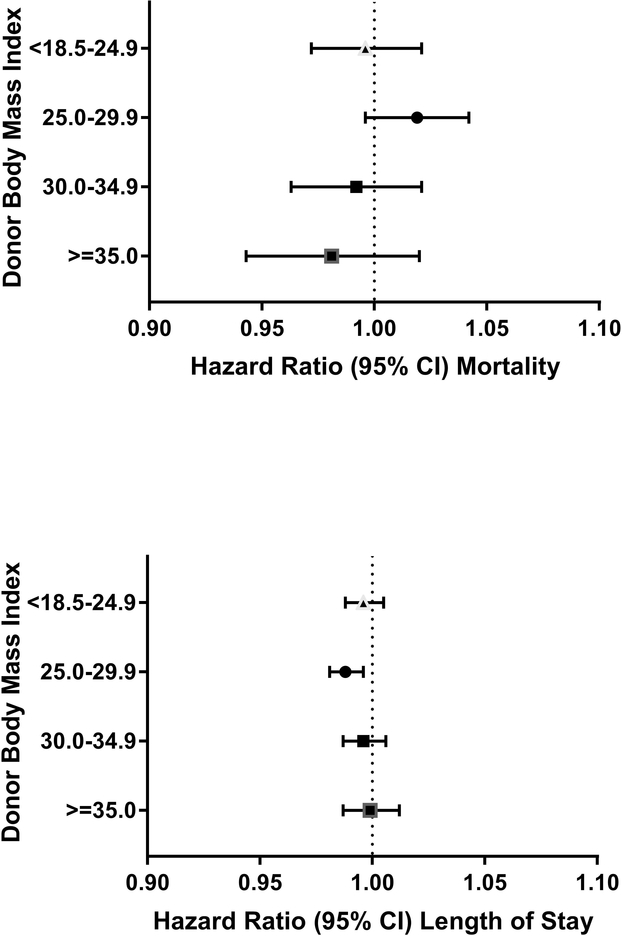
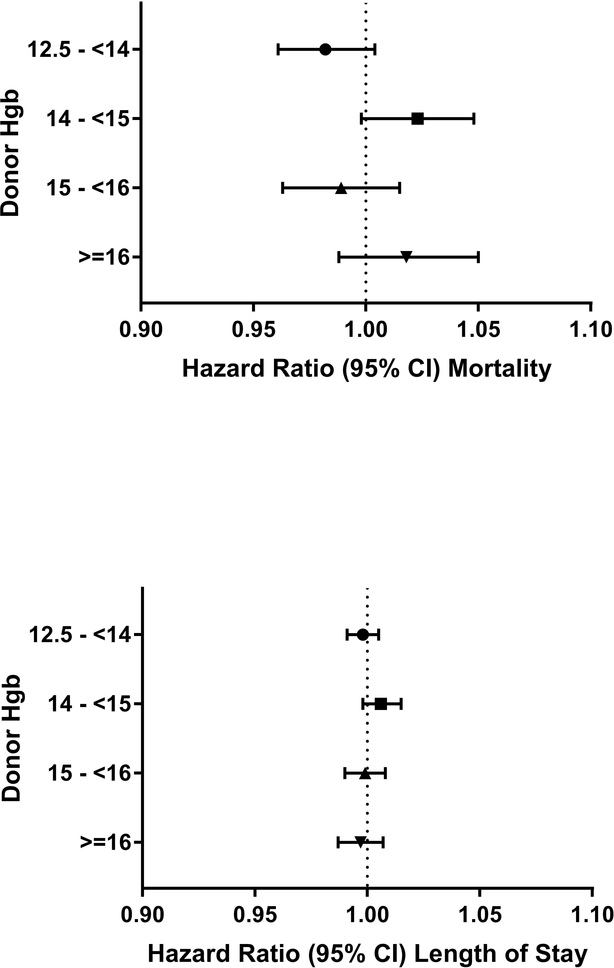
Increasing Charlson comorbidity index was associated with increased hospital mortality in regression analyses (Appendix Table 3). Donor age, BMI, hemoglobin levels (overall and stratified by donor sex), and smoking status were not associated with in-hospital mortality, p>0.05 for all associations (Figures 1–4; Appendix Figure 1).
Figure 1:
Forest plots of hazard ratios of hospital mortality and length of stay per red cell unit transfused for groups of donor age (relative to the reference group of all other ages)
Hazard ratio estimates for length of stay denote likelihood of hospital discharge with increasing hazard ratio associated with shorter duration of hospitalization in patients who survived to discharge.
Figure 4:
Forest plots of hazard ratios of hospital mortality and length of stay per red cell unit transfused for donor smoking status (Yes, No, and unknown smoking status in 30 days prior to blood donation)
We did, however, observe an association of donor age with prolonged hospital length of stay (Figure 1). The transfusion of each RBC unit from donors aged less than 20 years, was associated with a 3% shorter length of stay compared with a recipient receiving a RBC unit from all other donor ages (HR, 1.03; 95% CI 1.02 to 1.04; P<0.001). In contrast, transfusion of each RBC unit from donors aged 50–60 and 60–70 years was associated with prolonged lengths of stay (HR 0.99; 95% CI 0.98 to 1.00 for both age groups, with p=0.004 and 0.04, respectively). These findings persisted when stratifying by donor sex (Appendix Figure 2), with decreased hospital length of stay associated with RBC units from both male and female donors aged less than 20 and with prolonged length of stay associated with transfusion from female donors greater than 70 and from male donors 50–70 years old.
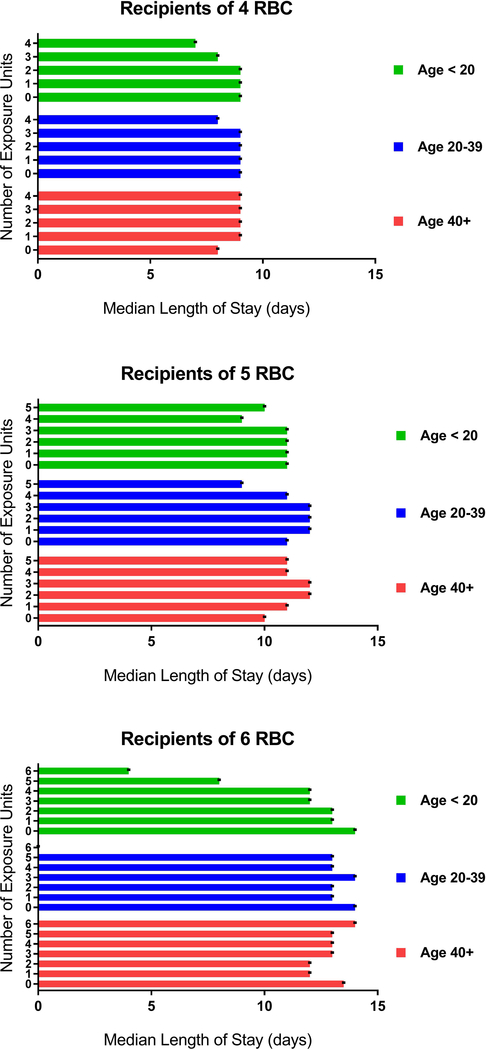
Figure 5 and Appendix Table 4 show median lengths of stay in days in relation to the number of RBC transfusions per hospitalization and exposures to groupings of donor age (< 20, 20–39, or >40 years). Overall, there was a dose-related increase in length of stay for each incremental red cell unit transfused, but this increase was attenuated in patients receiving more units from donors aged under 20. For example, recipients of 4 red cell units only from donors under age 20 had a 2-day shorter length of stay after transfusion (7 days [IQR 5–11]) compared to recipients who received their 4 units only from donors greater than the 40 years of age (9 days [IQR 6–15]). While the total number of units transfused from the three donor age groups was evenly distributed across the recipient cohort, the proportion of recipients who received blood only from young donors decreased with increased transfusion intensity. For example, in recipients of 4 red cell units during a hospitalization, transfusion events where all 4 units were derived from donors less than 20 years represented only 0.4% of units transfused.
Figure 5:
Median length of hospital stay after transfusion relative to number of donor age exposures by strata of RBC transfusion
Overweight donor status (BMI 25.0–29.9) was also associated with prolonged length of stay (HR, 0.99; 95% CI 0.98 to 1.00; P=0.002; Figure 2); however, a graded response was not apparent, as there was no effect for RBCs transfused from obese (BMI > 30) blood donors (HR, 1.00; 95% CI 0.99 to 1.01; P=0.92). Lastly, hazard ratios for length of stay were not statistically significant for groupings of donor hemoglobin levels or for positive smoking status (HR, 1.00; 95% CI 0.99 to 1.02; P=0.42; Figures 3 and 4).
Figure 2:
Forest plots of hazard ratios of hospital mortality and length of stay per red cell unit transfused for donor body mass index (relative to the reference group of all other BMI)
Figure 3:
Hazard ratios per red cell unit transfused for hospital mortality and length of stay for hemoglobin levels (relative to the reference group of all other hemoglobin levels)
Discussion
In contrast to two prior studies but consistent with a larger study, we found no association between blood donor age and mortality in patients transfused red cells.2–4 Nor did we identify an association between transfused patient mortality and other blood donor characteristics – BMI, hemoglobin level, or smoking status – hypothesized to impact transfusion recipient outcomes. We did however find that red cell transfusions from younger blood donors were associated with decreased post transfusion length of stay in patients who survived to hospital discharge. There were no other consistent associations between other donor characteristics and hospital length of stay.
Animal investigations suggesting improved cognitive function and synaptic plasticity in mice exposed to young blood served as the impetus for the conduct of observational studies of donor-recipient outcomes35. However, recent epidemiologic studies have found that red cell transfusions from younger donors are associated with increased mortality.2,3 While there are known to be in vitro differences in red blood cells based upon storage duration, methods of blood collection or processing and donor gender, it remains unclear why one might expect harmful biologic effects related to donor age.14,36,37 One potential factor is that inflammatory markers are known to vary with age.38
In this study, we hypothesized that patient outcomes other than mortality might be more sensitive to a potential donor effect and could explain discrepant findings of prior studies. Such morbidity outcomes might also provide insight into the pathogenesis of such a donor effect. We postulated that red cell exposures from donors with different characteristics would be associated with changes in hospital length of stay.
We found that RBC transfusions from donors under 20 years of age were associated with shorter time to hospital discharge, a beneficial effect which conflicts with results from prior mortality studies, but which could be consistent with results of animal studies. However, this result clearly needs corroboration in other cohorts and further evidence to support a potential biological basis. The effect we observed was small and only became clinically apparent with transfusion of four or more units from young donors. We are confident in the statistical approach utilized which controlled for indication bias by total number of transfusions received, severity of illness and the competing risk of death. Nevertheless, there are limitations to hospital length of stay outcome and potential confounding may remain.
In parallel with donor age, we assessed other donor characteristics that might impact transfusion recipient outcomes but found no association with either mortality or length of hospital stay. We hypothesized that extremes in donor hemoglobin levels could reflect an uncharacterized donor condition and impact transfusion recipient outcomes. For example, tobacco use has been associated with polycythemia and increased hemolysis of red blood cells, and a pilot study found smaller hemoglobin increments in recipients transfused cotinine-positive red cells donated from smokers. 25,39 On the other hand, repeated blood donation has been associated with lower hemoglobin levels and in cases of donor iron-deficiency, reduced post-transfusion RBC recovery in mouse studies, suggesting in-vivo hemolysis.40 Lastly, obesity-associated inflammation has been linked to iron deficiency and elevated hepcidin concentrations15,41,42. However, we saw no association between donor hemoglobin levels, obesity, or self-reported smoking status and hospital mortality or length of stay. Additional studies may be needed to directly assess smoking exposure through the measurement of cotinine levels in donated red cell units as well as the impact of smoking and other donor factors on red cell recovery and survival studies.43,44 However, our findings are reassuring that donor tobacco use does not appear to have an effect on recipient outcomes.
Given the complexity of large-scale linked donor-recipient analyses and inconsistent findings from other studies exploring these same issues, a discussion regarding methodologic approach is necessary. We assumed random allocation of red cell units with regard to donor characteristics (e.g. age, BMI, hemoglobin level, smoking) to transfused patients beyond ABO/Rh compatibility. In parallel with prior studies, we treated recipient’s blood product exposure as time-dependent throughout hospitalization, allowing patient’s exposure status to change as they received additional transfusions.2–4 Thus, patients receiving red-cells representative of more than one type of donor characteristic did not require their exclusion or separate categorization. Censoring of recipients who receive blood from more than one donor category results in analysis of a cohort who received fewer red cell units and thus may be influenced by differences in severity of illness and mortality risk.7
While our approach may result in a mixture of donor effects, analysis of time-dependent exposures without exclusions limits bias from informative censoring. However, subgroup analyses of infrequent transfusion events such as receipt of multiple units from a less common donor characteristic may result in false-positive findings (Type I error) due to small sample size. Differences in blood collection and processing may also be relevant to patient outcomes and be confounding factors in the evaluation of donor characteristics, and these analyses are similarly prone to the same methodologic challenges.37
As we recognize challenges of linked donor-recipient analyses, randomized clinical trials have been considered or are currently underway to overcome these limitations.45 In parallel, incorporation of more granular data and replication across observational cohorts may be beneficial to account for indication bias, confounding and recipient severity of illness.3 Recent studies have utilized daily hemoglobin and creatinine levels as time-varying covariates to account for patient disease severity, and these measures could also be considered as morbidity outcomes for donor effects.3 Collaboration by investigators to develop common analytic approaches and even re-analyze data using multiple statistical models may resolve some of the differences of prior studies. For example, the NHLBI REDS-III dataset that serves as the basis for this study is anticipated to become publicly available for analysis via the NHLBI Biologic Specimen and Data Repository Information Coordinator Center (BioLINCC), and the establishment of an international collaboration of experts to address methodologic issues is being planned under the auspices of the REDS programs.
Some limitations need to be emphasized. We examined ranges of donor hemoglobin levels; however, assessment of prior donation frequency or ferritin status may be beneficial in regard to evaluating whether red cell fragility or propensity for hemolysis could influence recipient outcomes.40,48 In addition, a significant proportion of donor BMI and smoking status data was missing for imported RBC units, and these units were excluded from those analyses. Given possible differences in these parameters related to geographic region, multiple imputation was not utilized. However, since import status is not taken into consideration in blood allocation, imported units are likely randomly distributed to recipients. Hence, we believe that exclusion of these data is unlikely to have influenced our results. For the BMI and smoking status data that were available for analysis, we found no difference in outcomes.
We chose hospital length of stay as an outcome that might encompass morbidity effects related to a donor characteristic; however non-clinical factors are also known to influence length of stay.46 In addition, length of stay provides little insight into a biologic mechanism of donor factors on adverse patient outcomes. We attempted to control for other factors that might be related to both donor factor distribution and patient survival (e.g., hospital, comorbidity index and number of transfusions) to ensure that recipient confounders would be accounted for.4,7 However, incorporation of additional clinical details including diagnosis and procedure codes and laboratory results may better account for recipient confounding and provide more evidence of any biologic effect related to donor characteristics. We also accounted for conceivable associations between seasonality of blood donor age and recipient outcomes. There is known to be seasonal variation of patient mortality, and donor age may vary by month due to timing of school blood drives.47 However, accounting for calendar month did not impact our findings. Our study only examined short-term outcomes during hospitalization; however, a recent study using the same analytic approach found no differences when examining associations between donor exposures and short-term and long-term outcomes.6
In conclusion, our findings are reassuring as we did not find an association between blood donor characteristics including age (overall and independently by gender), hemoglobin level, obesity and self-reported smoking status and transfusion recipient mortality. The association between shorter hospital length of stay and younger donor age is intriguing but needs to be interpreted cautiously at this time given the non-specificity of this outcome, the small effect size, and the lack of consistency between results obtained from different studies. Discrepancies in findings may also be the result of the disparate statistical methods that have been used to analyze large linked donor-component-recipient databases, as well as the nature and quality of the data being analyzed. Future research should occur across cohorts with collaborative refinement of statistical approaches.
Supplementary Material
Appendix Figure 1: Hazard ratios per red cell unit transfused for hospital mortality and length of stay for hemoglobin levels by donor sex (relative to the reference group of all others)
Appendix Figure 2: Hazard ratios per red cell unit transfused for hospital mortality and length of stay for groups of donor age by donor sex (relative to the reference group of all others)
Acknowledgements
The NHLBI Recipient Epidemiology Donor Evaluation Study-III (REDS-III), domestic component, is the responsibility of the following persons:
Hubs:
A.E. Mast and J.L. Gottschall, Versity Incorporated, Milwaukee, WI
D.J. Triulzi and J.E. Kiss, The Institute for Transfusion Medicine (ITXM), Pittsburgh, PA
E.L. Murphy and E. St. Lezin, University of California, San Francisco (UCSF), San Francisco, CA
E.L. Snyder, Yale University School of Medicine, New Haven, CT
R.G. Cable, American Red Cross Blood Services, Farmington, CT
Data coordinating center:
D.J. Brambilla and M.T. Sullivan, RTI International, Rockville, MD
Central laboratory:
M.P. Busch and P.J. Norris, Vitalant Research Institute, San Francisco, CA
Publication committee chairman:
R.Y. Dodd, American Red Cross, Holland Laboratory, Rockville, MD
Steering committee chairman:
S.H. Kleinman, University of British Columbia, Victoria, BC, Canada
National Heart, Lung, and Blood Institute, National Institutes of Health:
S.A. Glynn and K. Malkin
Funding: National Institutes of Health
Financial Support: This work was supported by research support from the National Heart, Lung, and Blood Institute (NHLBI Contracts HHSN2682011000002I, HHSN2682011000003I, HHSN2682011000004I, HHSN2682011000005I, and HHSN268201100006I for the Recipient Epidemiology and Donor Evaluation Study-III) and R01HL126130.
Footnotes
Conflict of Interest Disclosures: No author reports any relevant conflicts of interest.
Ethics Committee Approval: All participating sites received IRB approval prior to performing the study.
References
- 1.Caram-Deelder C, Kreuger AL, Evers D, de Vooght KMK, de Kerkhof DV, Visser O, Pequeriaux NCV, Hudig F, Zwaginga JJ, van der Bom JG, Middelburg RA. Association of Blood Transfusion From Female Donors With and Without a History of Pregnancy With Mortality Among Male and Female Transfusion Recipients. JAMA 2017;318: 1471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chasse M, Tinmouth A, English SW, Acker JP, Wilson K, Knoll G, Shehata N, van Walraven C, Forster AJ, Ramsay T, McIntyre LA, Fergusson DA. Association of Blood Donor Age and Sex With Recipient Survival After Red Blood Cell Transfusion. JAMA Intern Med 2016;176: 1307–14. [DOI] [PubMed] [Google Scholar]
- 3.Heddle NM, Cook RJ, Liu Y, Zeller M, Barty R, Acker JP, Eikelboom J, Arnold DM. The association between blood donor sex and age and transfusion recipient mortality: an exploratory analysis. Transfusion 2019;59: 482–491. [DOI] [PubMed] [Google Scholar]
- 4.Edgren G, Ullum H, Rostgaard K, Erikstrup C, Sartipy U, Holzmann MJ, Nyren O, Hjalgrim H. Association of Donor Age and Sex With Survival of Patients Receiving Transfusions. JAMA Intern Med 2017;177: 854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasan SK, Chiesa F, Rostgaard K, Magnusson PK, Halmin M, Nielsen KR, Titlestad KE, Hjalgrim H, Edgren G. Lack of association between blood donor age and survival of transfused patients. Blood 2016;127: 658–61. [DOI] [PubMed] [Google Scholar]
- 6.Edgren G, Murphy EL, Brambilla DJ, Westlake M, Rostgaard K, Lee C, Cable RG, Triulzi D, Bruhn R, St Lezin EM, Erikstrup C, Ullum H, Glynn SA, Kleinman S, Hjalgrim H, Roubinian NH, Group NREaDES-IR-I. Association of Blood Donor Sex and Prior Pregnancy With Mortality Among Red Blood Cell Transfusion Recipients. JAMA 2019;321: 2183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edgren G, Rostgaard K, Hjalgrim H. Methodological challenges in observational transfusion research: lessons learned from the Scandinavian Donations and Transfusions (SCANDAT) database ISBT Science Series 2017;12: 191–5. [Google Scholar]
- 8.Sawamura Y, Ohto H, Ikeda K, Kanno T, Suzuki Y, Gonda K, Tasaki T, Nollet KE, Takahashi H, Aota S. Impact of prestorage leucoreduction of autologous whole blood on length of hospital stay with a subgroup analysis in bilateral hip arthroplasty. Vox Sang 2018;113: 584–93. [DOI] [PubMed] [Google Scholar]
- 9.Andrews AT, Zmijewski CM, Bowman HS, Reihart JK. Transfusion reaction with pulmonary infiltration associated with HL-A-specific leukocyte antibodies. Am J Clin Pathol 1976;66: 483–7. [DOI] [PubMed] [Google Scholar]
- 10.Triulzi DJ, Kleinman S, Kakaiya RM, Busch MP, Norris PJ, Steele WR, Glynn SA, Hillyer CD, Carey P, Gottschall JL, Murphy EL, Rios JA, Ness PM, Wright DJ, Carrick D, Schreiber GB. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion 2009;49: 1825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanias T, Lanteri MC, Page GP, Guo Y, Endres SM, Stone M, Keating S, Mast AE, Cable RG, Triulzi DJ, Kiss JE, Murphy EL, Kleinman S, Busch MP, Gladwin MT. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv 2017;1: 1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raslan R, Shah BN, Zhang X, Kanias T, Han J, Machado RF, Gladwin MT, Gordeuk VR, Saraf SL. Hemolysis and hemolysis-related complications in females vs. males with sickle cell disease. Am J Hematol 2018;93: E376–E80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanias T, Sinchar D, Osei-Hwedieh D, Baust JJ, Jordan A, Zimring JC, Waterman HR, de Wolski KS, Acker JP, Gladwin MT. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. Transfusion 2016;56: 2571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanias T, Gladwin MT. Nitric oxide, hemolysis, and the red blood cell storage lesion: interactions between transfusion, donor, and recipient. Transfusion 2012;52: 1388–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorensen CJ, Pedersen OB, Petersen MS, Sorensen E, Kotze S, Thorner LW, Hjalgrim H, Rigas AS, Moller B, Rostgaard K, Riiskjaer M, Ullum H, Erikstrup C. Combined oral contraception and obesity are strong predictors of low-grade inflammation in healthy individuals: results from the Danish Blood Donor Study (DBDS). PLoS One 2014;9: e88196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotzé SR, Pedersen OB, Petersen MS, Sørensen E, Thørner LW, Sørensen CJ, Rigas AS, Hjalgrim H, Rostgaard K, Ullum H, Erikstrup C. Low-grade inflammation is associated with lower haemoglobin levels in healthy individuals: results from the Danish blood donor study. Vox Sang 2016;111: 144–50. [DOI] [PubMed] [Google Scholar]
- 17.Enjeti AK, Ariyarajah A, D’Crus A, Seldon M, Lincz LF. Circulating microvesicle number, function and small RNA content vary with age, gender, smoking status, lipid and hormone profiles. Thromb Res 2017;156: 65–72. [DOI] [PubMed] [Google Scholar]
- 18.Kasza KA, Ambrose BK, Conway KP, Borek N, Taylor K, Goniewicz ML, Cummings KM, Sharma E, Pearson JL, Green VR, Kaufman AR, Bansal-Travers M, Travers MJ, Kwan J, Tworek C, Cheng YC, Yang L, Pharris-Ciurej N, van Bemmel DM, Backinger CL, Compton WM, Hyland AJ. Tobacco-Product Use by Adults and Youths in the United States in 2013 and 2014. N Engl J Med 2017;376: 342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mast AE, Steele WR, Johnson B, Wright DJ, Cable RG, Carey P, Gottschall JL, Kiss JE, Simon TL, Murphy EL, Investigators NREDS-IR-I. Population-based screening for anemia using first-time blood donors. Am J Hematol 2012;87: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotzé SR, Pedersen OB, Petersen MS, Sørensen E, Thørner LW, Sørensen CJ, Rigas AS, Hjalgrim H, Rostgaard K, Ullum H, Erikstrup C. Predictors of hemoglobin in Danish blood donors: results from the Danish Blood Donor Study. Transfusion 2015;55: 1303–11. [DOI] [PubMed] [Google Scholar]
- 21.Wiencek JR, Gehrie EA, Keiser AM, Szklarski PC, Johnson-Davis KL, Booth GS. Detection of Nicotine and Nicotine Metabolites in Units of Banked Blood. Am J Clin Pathol 2019;151: 516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aberg AM, Sojka BN, Winso O, Abrahamsson P, Johansson G, Larsson JE. Carbon monoxide concentration in donated blood: relation to cigarette smoking and other sources. Transfusion 2009;49: 347–53. [DOI] [PubMed] [Google Scholar]
- 23.Boehm RE, Arbo BD, Leal D, Hansen AW, Pulcinelli RR, Thiesen FV, Balsan AM, Onsten TGH, Gomez R. Smoking fewer than 20 cigarettes per day and remaining abstinent for more than 12 hours reduces carboxyhemoglobin levels in packed red blood cells for transfusion. PLoS One 2018;13: e0204102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masilamani V, AlZahrani K, Devanesan S, AlQahtani H, AlSalhi MS. Smoking Induced Hemolysis: Spectral and microscopic investigations. Sci Rep 2016;6: 21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asgary S, Naderi G, Ghannady A. Effects of cigarette smoke, nicotine and cotinine on red blood cell hemolysis and their -SH capacity. Exp Clin Cardiol 2005;10: 116–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy EL, Schlumpf K, Wright DJ, Cable R, Roback J, Sacher R, Busch MP, II NREDS. BMI and obesity in US blood donors: a potential public health role for the blood centre. Public Health Nutr 2012;15: 964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Hurk K, Zalpuri S, Prinsze FJ, Merz EM, de Kort WLAM. Associations of health status with subsequent blood donor behavior-An alternative perspective on the Healthy Donor Effect from Donor InSight. PLoS One 2017;12: e0186662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shields M, Carroll MD, Ogden CL. Adult obesity prevalence in Canada and the United States. NCHS Data Brief 2011: 1–8. [PubMed] [Google Scholar]
- 29.Kaspersen KA, Pedersen OB, Petersen MS, Hjalgrim H, Rostgaard K, Møller BK, Juul-Sørensen C, Kotzé S, Dinh KM, Erikstrup LT, Sørensen E, Thørner LW, Burgdorf KS, Ullum H, Erikstrup C. Obesity and risk of infection: results from the Danish Blood Donor Study. Epidemiology 2015;26: 580–9. [DOI] [PubMed] [Google Scholar]
- 30.Karafin MS, Bruhn R, Westlake M, Sullivan MT, Bialkowski W, Edgren G, Roubinian NH, Hauser RG, Kor DJ, Fleischmann D, Gottschall JL, Murphy EL, Triulzi DJ, National Heart L, Blood Institute Recipient E, Donor Evaluation S III, Demographic and epidemiologic characterization of transfusion recipients from four US regions: evidence from the REDS-III recipient database. Transfusion 2017;57: 2903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleinman S, Busch MP, Murphy EL, Shan H, Ness P, Glynn SA, National Heart Ln, and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III). The National Heart, Lung, and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III): a research program striving to improve blood donor and transfusion recipient outcomes. Transfusion 2014;54: 942–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rostgaard K Methods for stratification of person-time and events - a prerequisite for Poisson regression and SIR estimation. Epidemiol Perspect Innov 2008;5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen PK, Gill RD. Cox’s Regression Model for Counting Processes: A Large Sample Study. The Annals of Statistics 1982;10: 1100–20. [Google Scholar]
- 34.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004;57: 1288–94. [DOI] [PubMed] [Google Scholar]
- 35.Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, Wabl R, Udeochu J, Wheatley EG, Zou B, Simmons DA, Xie XS, Longo FM, Wyss-Coray T. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med 2014;20: 659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ning S, Heddle NM, Acker JP. Exploring donor and product factors and their impact on red cell post-transfusion outcomes. Transfus Med Rev 2018;32: 28–35. [DOI] [PubMed] [Google Scholar]
- 37.Heddle NM, Arnold DM, Acker JP, Liu Y, Barty RL, Eikelboom JW, Webert KE, Hsia CC, O’Brien SF, Cook RJ. Red blood cell processing methods and in-hospital mortality: a transfusion registry cohort study. Lancet Haematol 2016;3: e246–54. [DOI] [PubMed] [Google Scholar]
- 38.Ferrucci L, Semba RD, Guralnik JM, Ershler WB, Bandinelli S, Patel KV, Sun K, Woodman RC, Andrews NC, Cotter RJ, Ganz T, Nemeth E, Longo DL. Proinflammatory state, hepcidin, and anemia in older persons. Blood 2010;115: 3810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeSimone RA, Hayden JA, Mazur CA, Vasovic LV, Sachais BS, Zhao Z, Goel R, Hsu YS, Racine-Brzostek SE, Cushing MM. Red blood cells donated by smokers: A pilot investigation of recipient transfusion outcomes. Transfusion 2019;59: 2537–2543. [DOI] [PubMed] [Google Scholar]
- 40.Bandyopadhyay S, Brittenham GM, Francis RO, Zimring JC, Hod EA, Spitalnik SL. Iron-deficient erythropoiesis in blood donors and red blood cell recovery after transfusion: initial studies with a mouse model. Blood Transfus 2017;15: 158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng HL, Bryant CE, Rooney KB, Steinbeck KS, Griffin HJ, Petocz P, O’Connor HT. Iron, hepcidin and inflammatory status of young healthy overweight and obese women in Australia. PLoS One 2013;8: e68675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aigner E, Feldman A, Datz C. Obesity as an emerging risk factor for iron deficiency. Nutrients 2014;6: 3587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Symvoulakis EK, Vardavas CI, Fountouli P, Stavroulaki A, Antoniou KM, Duijker G, Tzatzarakis MN, Sfiridaki K, Bolonaki E, Alegakis T, Tsatsakis AM. Time interval from cigarette smoke exposure to blood donation and markers of inflammation: should a smoking cut-off be designated? Xenobiotica 2010;40: 613–20. [DOI] [PubMed] [Google Scholar]
- 44.Nalbant D, Cancelas JA, Mock DM, Kyosseva SV, Schmidt RL, Cress GA, Zimmerman MB, Strauss RG, Widness JA. In premature infants there is no decrease in 24-hour posttransfusion allogeneic red blood cell recovery after 42 days of storage. Transfusion 2018;58: 352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.(ClinicalTrials.gov, ID: ) [monograph on the internet]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03344887
- 46.Tiessen J, Kambara H, Sakai T, Kato K, Yamauchi K, McMillan C. What causes international variations in length of stay: a comparative analysis for two inpatient conditions in Japanese and Canadian hospitals. Health Serv Manage Res 2013;26: 86–94. [DOI] [PubMed] [Google Scholar]
- 47.Parks RM, Bennett JE, Foreman KJ, Toumi R, Ezzati M. National and regional seasonal dynamics of all-cause and cause-specific mortality in the USA from 1980 to 2016. Elife 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanias T, Stone M, Page GP, Guo Y, Endres-Dighe SM, Lanteri MC, Spencer BR, Cable RG, Triulzi DJ, Kiss JE, Murphy EL, Kleinman S, Gladwin MT, Busch MP, Mast AE, Program NREDESR-I. Frequent blood donations alter susceptibility of red blood cells to storage- and stress-induced hemolysis. Transfusion 2019;59: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Figure 1: Hazard ratios per red cell unit transfused for hospital mortality and length of stay for hemoglobin levels by donor sex (relative to the reference group of all others)
Appendix Figure 2: Hazard ratios per red cell unit transfused for hospital mortality and length of stay for groups of donor age by donor sex (relative to the reference group of all others)